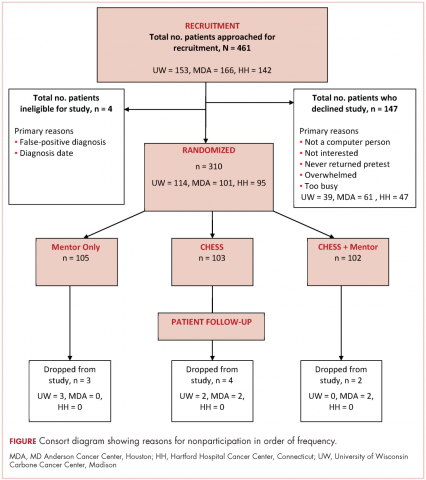
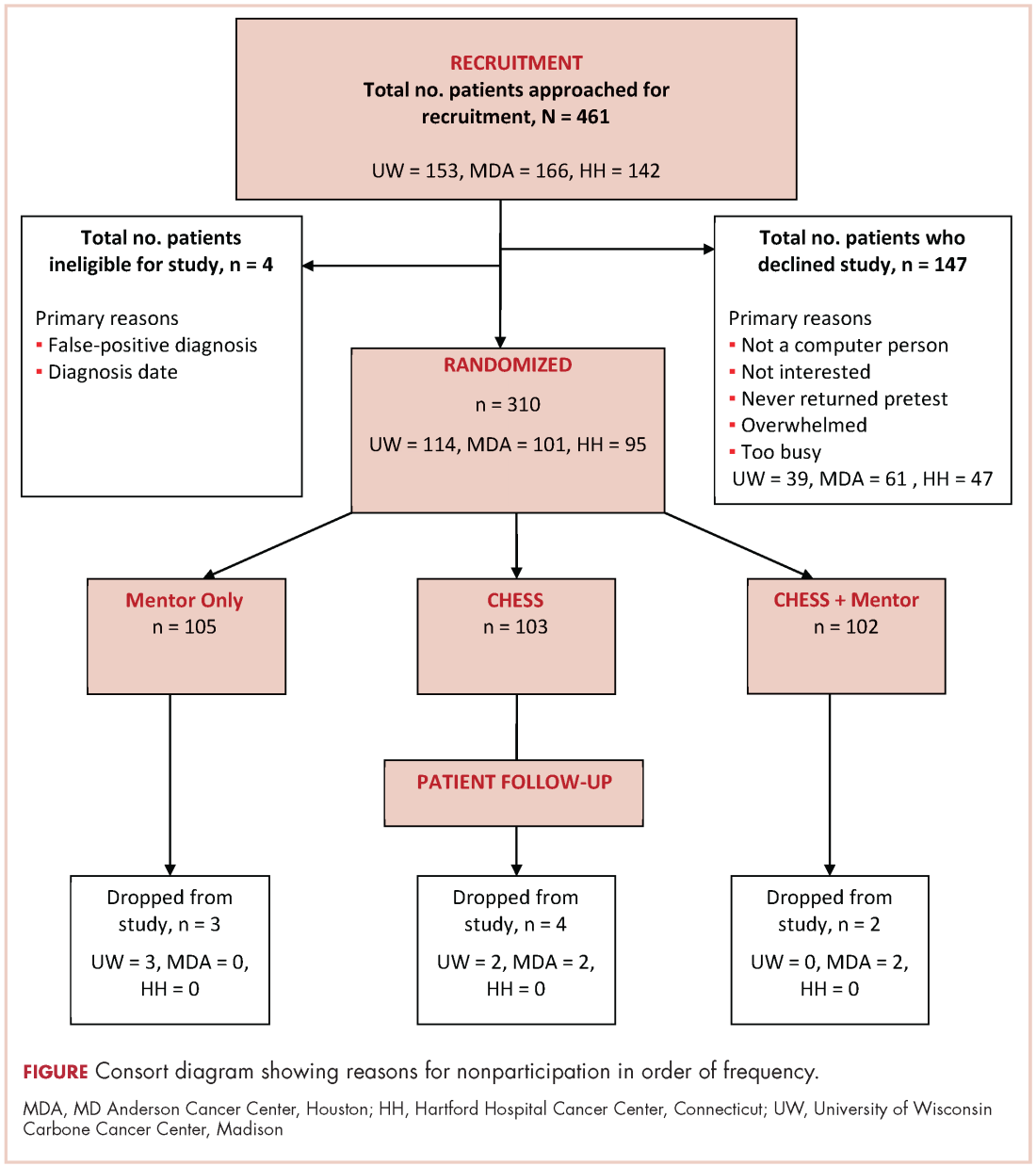
User login
Supportive medications and interventions received by prostate cancer survivors: results from the PiCTure study
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
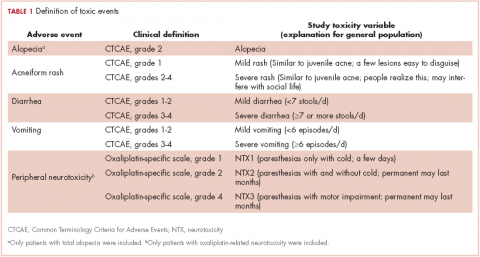
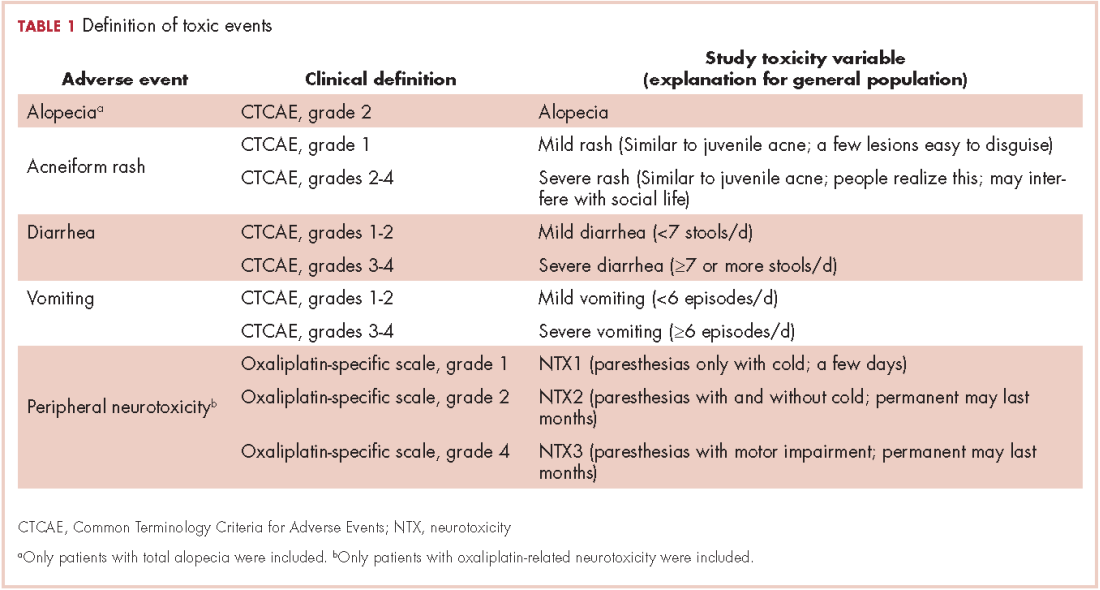
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
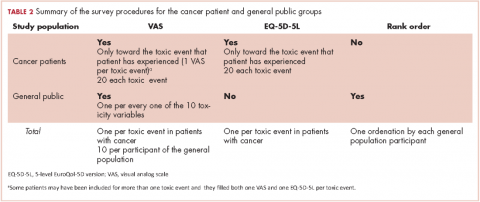
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
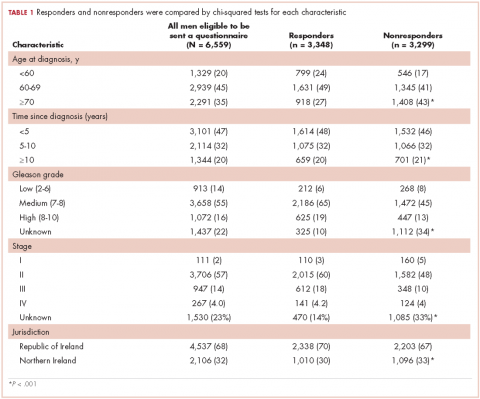
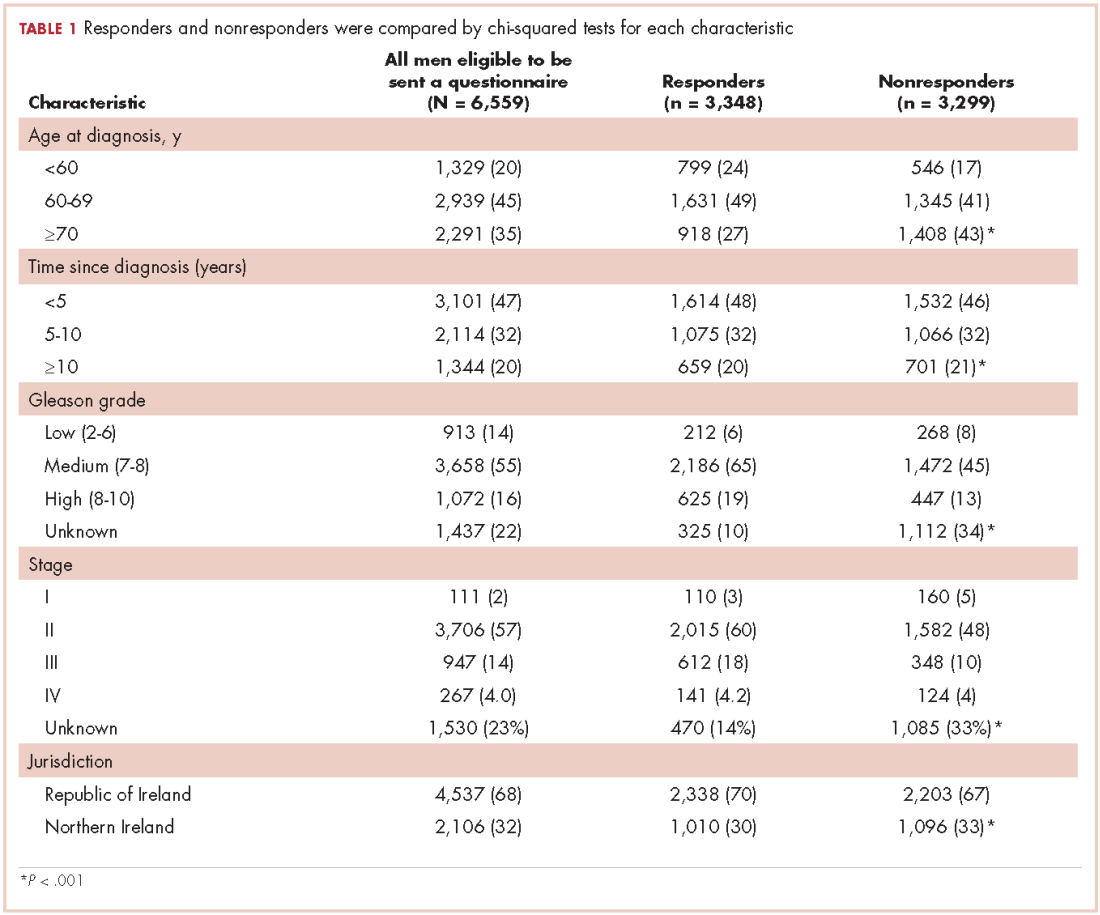
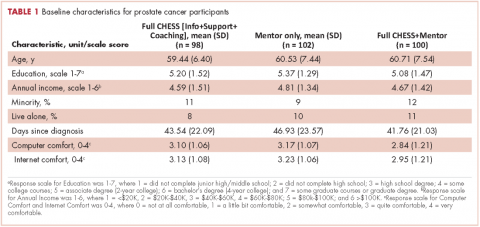
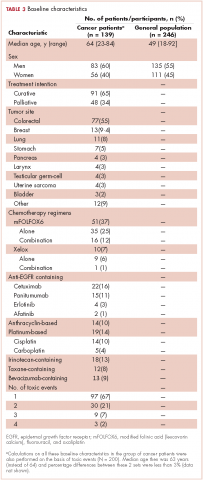
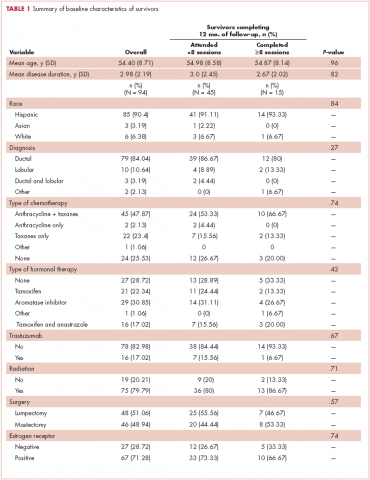
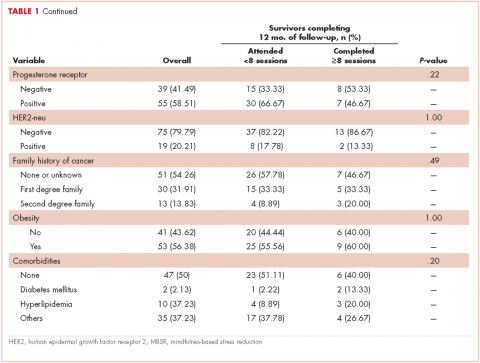
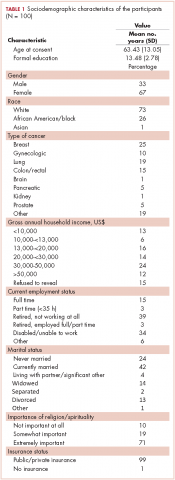
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
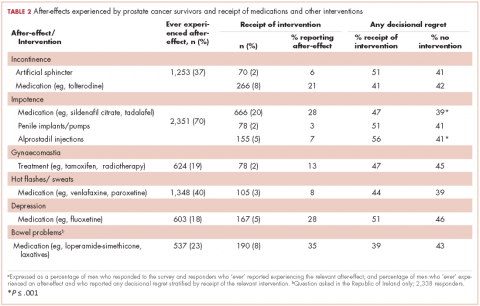
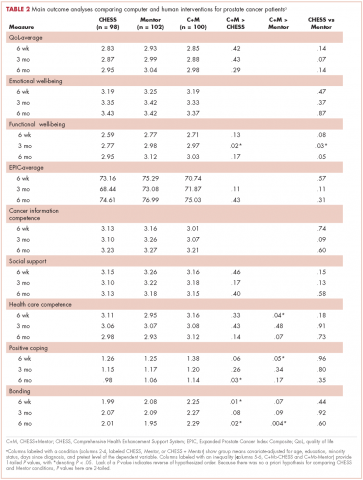
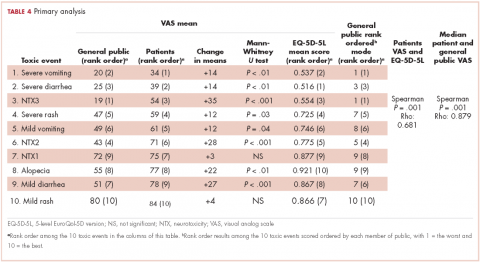
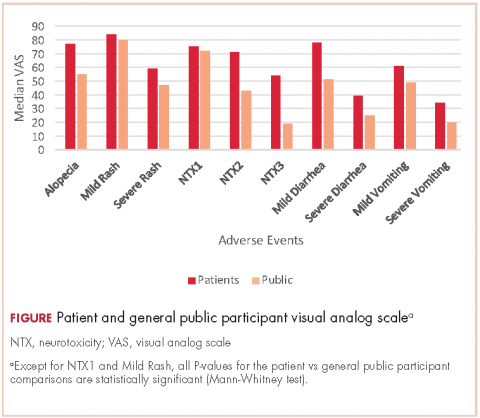
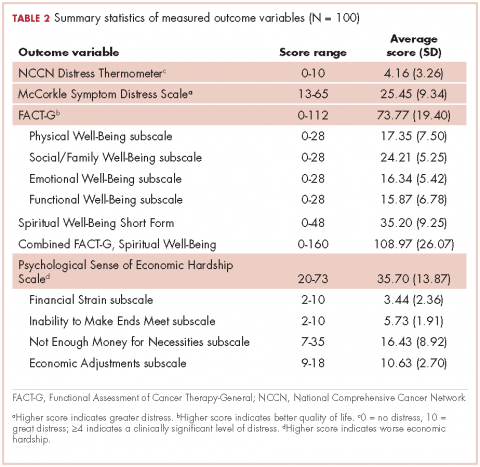
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
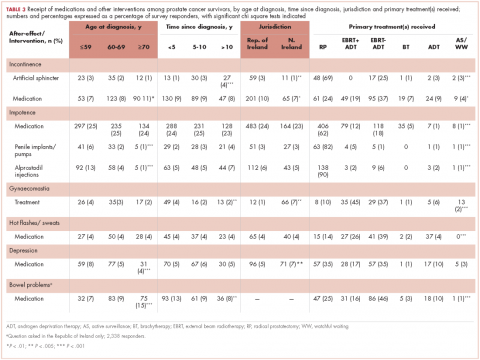
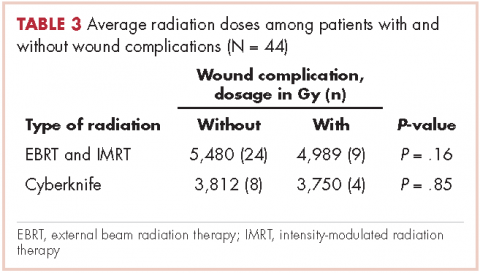
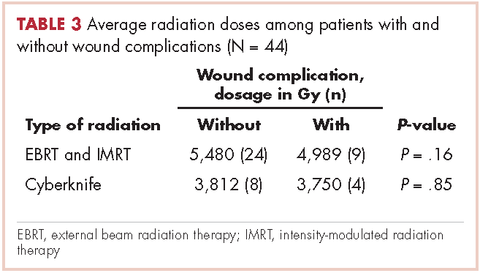
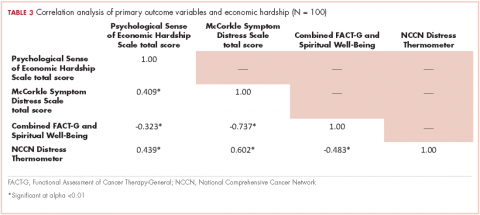
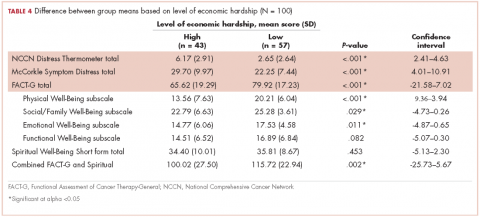
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
Prostate cancer treatments are associated with various physical after-effects, including urinary, sexual, and bowel symptoms.1 These after-effects can have an impact on survivors’ health-related quality of life (HRQoL).2 Pharmaceutical and surgical interventions are available to manage or ameliorate many of these after-effects (eg, sildenafil citrate taken during and after radiotherapy improves sexual function),3 and their receipt has a positive impact on HRQoL.4
However, studies of clinicians suggest that such interventions may not be used widely.5,6 Patient-reported data on this topic is lacking. Therefore, we investigated the use of supportive medications and interventions in this population-based study of prostate cancer survivors.
Methods
The PiCTure (Prostate Cancer Treatment, Your Experience) study methods have been described elsewhere.7 Briefly, 6,559 prostate cancer survivors 2-15 years after diagnosis (diagnosed during January 1, 1995-March 31, 2010, and alive in November 2011), identified from population-based cancer registries in the Republic of Ireland and Northern Ireland, were invited to complete a postal survey. Information was sought on after-effects (incontinence, impotence, gynaecomastia, hot flashes/sweats, bowel problems, depression) that had been experienced at any time after treatment. For each after-effect, men were asked if they had received any medication or interventions to alleviate symptoms, and, if so, what they had received; examples of common interventions were provided. Men were also asked if they had been told they may become infertile and, if so, whether they had preserved their sperm. The Decisional Regret Scale8 was used to measure survivors’ regret over their entire treatment experience. This 5-item scale, rated on a 5-point Likert scale from 1 (strongly agree) to 5 (strongly disagree) was summed and standardized to a value of 0-100, with higher scores reflecting higher levels of decisional regret. 8 This scale has good psychometric properties8 and strong reliability in our sample (Cronbach’s alpha = 0.85). Responders were categorized as having any regret (score ≥1) or no regret (score = 0).
The number of men who reported receiving an intervention was expressed as a percentage of survey responders and of men who reported ever having the relevant after-effect. Chi-square tests were used to investigate variations in receipt by: age at diagnosis (≤59, 60-69, ≥70 years); time since diagnosis (≤5, 5-10, >10 years); jurisdiction (Republic of Ireland, or Northern Ireland); and primary treatment(s) received (radical prostatectomy [RP], external beam radiotherapy [EBRT] with androgen deprivation therapy [ADT], EBRT without ADT, brachytherapy, ADT [without other therapies], and active surveillance/watchful waiting). Among survivors who ever experienced an after-effect, chi-square tests were used to investigate whether the percentage who reported decisional regret differed depending on whether or not they received the relevant supportive intervention.
Ethics approval was from the Irish College of General Practitioners (Republic of Ireland) and the Office for Research Ethics Committee Northern Ireland.
Results
In all, 3,348 survivors participated in the survey (adjusted response rate, 54%). Compared with nonresponders, responders were more often from the Republic of Ireland (P = .007), <70 years at diagnosis (P < .001), 5-10 years post diagnosis (P < .001), with low or medium Gleason grade (Gleason scores of ≤6 [good prognosis] and 7, respectively; P < .001), and clinical stage II-IV (P < .001; Table 1).
Impotence (70%) was the most commonly reported after-effect, followed by hot flashes/sweats (40%), incontinence (37%), bowel problems (23%), gynaecomastia (19%), and depression (18%; Table 2).
Of responders, 2% received an artificial sphincter, representing 6% of men who ever experienced incontinence post diagnosis (Table 2). This percentage was significantly higher in participants diagnosed longer ago, from the Republic of Ireland, and who received RP (Table 3).
Incontinence medication was received by 8% of participants (21% of those who experienced incontinence). Use varied significantly by age, jurisdiction, and treatment. For impotence, medications were more commonly used (20% of participants; 28% with impotence) than were injections (5% and 7%, respectively) or penile implants/pumps (2% and 3%, respectively). Use of all 3 types of intervention was highest in men who had RP; injections and implants/pumps were significantly more common among younger men. Of those experiencing gynaecomastia, 13% received interventions; receipt was highest in men who had EBRT with ADT, were <5 years post diagnosis and from Northern Ireland. For hot flashes/sweats, 3% of participants (8% who experienced symptoms) received mediations; this was higher in men who had EBRT. Of those who reported depression, 28% received medication; receipt was highest in younger men and in Northern Ireland. Medication for bowel problems was used by 35% of men who experienced these; use was highest in older men, those diagnosed more recently, and those who had EBRT. Sixty percent of men reported having been told they would become infertile; 11 (0.3% of participants) preserved their sperm, 7 from the Republic of Ireland and 4 from Northern Ireland.
A total of 35.6% of survivors reported any decisional regret. Among survivors who ever had an after-effect, a higher percentage of those who used a supportive intervention reported decisional regret compared with those who did not; this was only statistically significant for those using medication or alprostadil injections for impotence (Table 2).
Discussion
This study documents, for the first time, population-based data on patient-reported use of supportive medications and interventions to alleviate adverse effects of prostate cancer and its treatment. Among survivors who experienced after-effects, use was highest for bowel problems, impotence, and depression, but even for those, only 28%-35% of men took medication. Although it is possible that some survivors declined medications or other interventions, these low levels of use strongly suggest that not all survivors who might benefit from supports receive them.
There was little evidence that utilisation was higher in survivors diagnosed more recently. This suggests that, although the number of prostate cancer survivors has grown, and there is greater focus on survivorship issues in clinical practice, this has not translated into more men receiving support to manage after-effects. Care is needed to ensure that the newer models of post-cancer follow-up being considered or adopted in many settings,9 do not exacerbate this issue.
As expected, patterns of utilisation varied by treatment(s) received. Higher use of surgical and pharmaceutical interventions to alleviate incontinence among survivors in the Republic of Ireland than in Northern Ireland is likely owing to the higher rate of radical prostatectomy in the Republic of Ireland, whereas greater use of treatments for gynaecomastia in Northern Ireland reflects higher use of hormone therapy there.10 Other variations in intervention use were more surprising. Younger men were significantly more likely to report using supportive interventions for depression and impotence, the latter finding being consistent with findings in a Swedish population-based study.11 Older men were significantly more likely to report interventions for incontinence and bowel problems. Although those trends could be explained by differences in treatment receipt by age, it is possible that men of different ages may be more likely to seek, or be offered, help for certain types of after-effects. With the exception of interventions for bowel problems, a higher percentage of men who received intervention(s) for an after-effect reported decisional regret. There are a number of possible explanations: these men may have experienced more severe after-effects, which required interventions; they may have been less satisfied with their posttreatment function and/or more proactive about recovering or treating their after-effects. This requires further investigation.
This is a large, international, population-based study, the first such study to describe patient-reported use of supportive care following a range of prostate cancer treatments. Although this study is novel, there are a number of limitations. It is a cross-sectional, descriptive study. We did not ask survivors whether the supportive interventions received matched their needs and wants, and whether they were satisfied with the supportive care received. Furthermore, although the response rate is comparable with other similar studies,12,13 it is possible that the supportive care of nonresponders was different to that of responders.
Our study included men from 2 jurisdictions with separate health care systems, suggesting that low use of supportive interventions may be common across systems. There is a need for further research into patient and health care system factors associated with the receipt of supportive interventions and how satisfied men are with these, in this and other health care settings. Presently, it is clear that more needs to be done in the clinical setting to support prostate cancer survivors manage treatment after-effects; this in turn could improve survivors’ HRQoL.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
1. Drummond FJ, Kinnear H, O’Leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015;9(2):361-72.
2. Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009; 339:b4817.
3. Zelefsky MJ, Shasha D, Branco RD, et al. Prophylactic sildenafil citrate improves select aspects of sexual function in men treated with radiotherapy for prostate cancer. J Urol. 2014;192(3):868-874.
4. Haab F, Trockman BA, Zimmern PE, Leach GE. Quality of life and continence assessment of the artificial urinary sphincter in men with minimum 3.5 years of follow-up. J Urol. 1997;158(2):435-439.
5. Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103(2):237-241.
6. Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68(1):126-131,
7. Drummond FJ, Kinnear H, Donnelly C, et al. Establishing a population-based patient reported outcomes study (PROMs) using national cancer registries across two jurisdictions: Prostate Cancer Treatment, your experience (PiCTure) Study. BMJ Open 2015;5:e006851.
8. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-92.
9. Howell D, Hack TF, Oliver et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
10. Donnelly DW, Gavin AT, Comber H. Cancer in Ireland 1994-2004. A comprehensive report. Northern Ireland Cancer Registry/National Cancer Registry, Ireland, 2009.
11. Plym A, Folkvaljon Y, Garmo H, et al. Drug prescription for erectile dysfunction before and after diagnosis of localized prostate cancer. J Sex Med. 2014;11(8):2100-2108.
12. Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage. 2005;30(5):474-484.
13. Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1-5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3(4). pii: e002317.
Differences in psychosocial stressors between black and white cancer patients
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
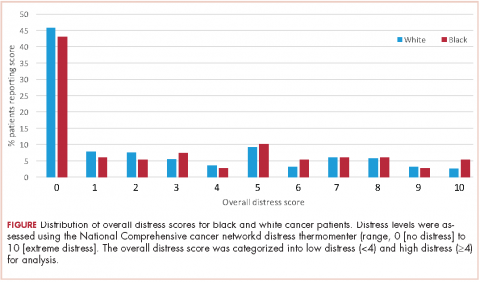
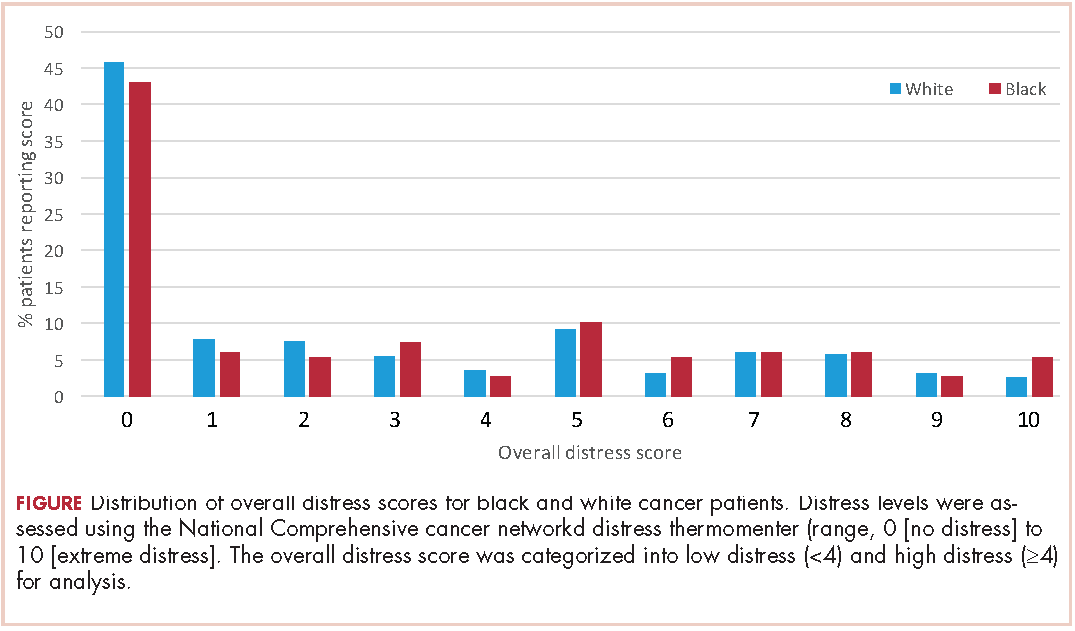
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
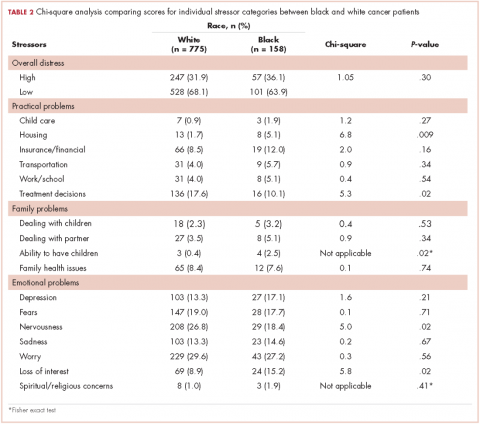
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
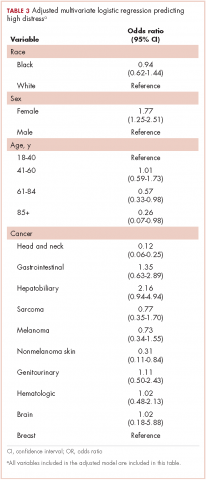
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
For patients with cancer, acknowledgment of mental and emotional distress is critically important when developing and implementing a treatment plan. The psychosocial distress associated with cancer diagnosis and treatment can have an impact on a patient’s quality of life, influence a patient’s ability to adhere to treatment regimens, and increase cost of care.1-4 Rates of depression have been reported to range from 8%-36%, with a 29% risk of anxiety in cancer patients.5, 6 Emotional distress is linked to increased hopelessness about their cancer diagnosis, increased issues with chronic pain, and negative treatment outcomes.7 Timely screening of psychosocial distress at the first clinical visit enables providers to make appropriate referrals to resources early in their course of treatment; however, referrals to psychosocial interventions remain infrequent nationwide in the United States.8
There is some evidence of a differential impact of cancer on mental health diagnoses between racial/ethnic groups; however, results are not entirely consistent across studies. Using the Kessler Pyschological Distress Scale (K6) score, Alcala and colleagues found that cancer was more detrimental to mental health for black patients than for non-Hispanic white patients.9 Black breast cancer survivors have also been shown to be more likely to stop working during the early phases of their treatment, indicating that they and their physicians need to take steps to minimize long-term employment consequences.10 However, in a study of women with breast cancer, black women reported fewer depressive symptoms than did non-Hispanic whites.11
The American College of Surgeons’ Commission on Cancer (ACS CoC) developed a set of Continuum of Care standards in 2012, including the implementation of psychosocial distress screening for patients with cancer. Since 2015, all accredited cancer programs are now required to evaluate these patients for signs of distress during at least 1 pivotal physician visit.12 The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology has developed a tool that provides a mechanism for meeting the requirements of the ACS CoC accreditation requirements. The NCCN defines distress in cancer as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms and its treatment.”13 The recommendation of the NCCN is to provide a brief screening for psychosocial distress to identify individuals in need of additional support and to provide referrals for patients at high risk of psychosocial distress. The NCCN Distress Thermometer screening tool has been widely accepted as an effective method of identifying and characterizing distress. The NCCN tool provides a visual analogue scale for patients to rate their current distress on a scale of 1-10, as well as a problem checklist. The problem checklist includes 22 stressors addressing the practical, spiritual/religious, emotional, and physical concerns of patients. Although the NCCN tool is used widely, differences in distress scores between black and white cancer patients have not been previously described. The purpose of the study was to compare the global distress screening scores of black and white patients at an academic comprehensive cancer center in the Midwest. A second objective was to examine the distribution of individual stressors between black and white women.
Methods
Study sample
The study included all cancer patients from a cancer center in the Midwest who completed the NCCN distress thermometer during January 1, 2015-February 19, 2016. The patient population for this cancer center was primarily non-Hispanic white and non-Hispanic black, therefore, only patients identifying as non-Hispanic white and non-Hispanic black are included in this analysis. As part of routine clinical care, patients are asked to complete the NCCN distress thermometer at their first visit to the center. All patients in this analytic sample were newly diagnosed patients. Some patients also completed the NCCN screening tool at additional appointments; therefore, for patients with more than 1 completed tool, only the first distress screening was used in this analysis. Overall scores and individual stressor scores were entered into the electronic medical record by clinic staff at the time the patients were roomed for their visit. Patient demographics were collected through a reporting mechanism within the electronic medical record that allows for monitoring of the psychosocial screening process.
Variables
Race was assessed through self-report and classified as non-Hispanic white and non-Hispanic black. There were not enough patients of any other racial/ethnic group to be included in this analysis. Age was categorized as 18-40 years, 41-60 years, 61-84 years, and 85 years and older. Cancer type was grouped as follows: head and neck cancer, gastrointestinal cancer (esophagus, stomach, small intestine, colon, rectum, anus), hepatobiliary (liver, gallbladder, pancreas), sarcoma (bone and soft tissue), melanoma, nonmelanoma skin cancer, breast cancer, genitourinary (prostate, kidney, bladder), hematologic, and brain.
Two primary outcomes were assessed: overall distress, and each individual problem indicator. Overall distress was assessed using the thermometer visual analog rating (the thermometer rating of the NCCN screening tool) where possible values range from 0 (no distress) to 10 (extreme distress). The overall distress score was categorized into low distress (<4) and high distress (≥4) for analysis. The response options for individual stressors on the problem list are Yes or No for each of 17 discrete stressors: child care, housing, insurance/financial, transportation, work/school, treatment decisions, dealing with children, dealing with partner, ability to have children, family health issues, depression, fears, nervousness, sadness, worry, loss of interest, and spiritual/religious concerns. Physical complaints were not assessed in this study. Comparisons were made between white and black patients on overall distress score as well as for each individual psychosocial stressor.
Data analysis
Descriptive statistics (counts and proportions or means and standard deviations) were calculated stratified by race. Categorical variables were compared by race using chi-square or Fisher exact test. Logistic regression was used to predict high distress by race adjusting for sex, age, and cancer type. All analyses were conducted using SAS 9.4 (Cary, NJ).
This study was reviewed and approved by the Saint Louis University Institutional Review Board (protocol number 26269).
Results
A total of 933 patients with cancer completed the NCCN distress screening tool. Of that total, 45 patients did not complete the overall distress score thermometer, but did complete the checklist of individual stressors. Those 45 patients were excluded from the logistic regression analysis for overall distress score, but included on comparisons of individual stressors. The distribution of overall distress scores by race can be seen in the Figure.
Briefly, the full sample was 16.9% black and 38.8% female. In all, 32.6% of the sample indicated high distress on the distress thermometer at their first visit. Demographics for the participants stratified by race are reported in Table 1 (see PDF).There was no statistically significant difference in the gender or age distribution between black and white patients. Cancer distribution did vary by race. Black patients were proportionally more represented in gastrointestinal cancers, hepatobiliary cancers, sarcomas, breast cancer, and genitourinary cancers. White patients were proportionally more represented in melanoma, nonmelanoma skin cancers, and hematologic cancers.
Table 2 presents bivariate comparisons on overall distress and individual stressors between black and white patients. There was no difference in the high distress between black and white patients in bivariate analysis (31.9% and 36.1%, respectively, P = .30). However, there were differences in the individual stressors identified for each racial group (Table 2). White patients, compared with black patients, more frequently identified treatment decisions (17.6% vs 10.1%, P = .02) and nervousness (26.8% vs 18.4%, P = .02) as sources of distress. Black patients, compared with white patients, more frequently identified housing (5.1% vs 1.7%, P = .009), the ability to have children (2.5% vs 0.4%, P =.02), and loss of interest (15.2% vs 8.9%, P = .02) as sources of distress. Distress scores did not differ between black and white patients for child care, insurance or financial issues, transportation, work or school, dealing with children, dealing with partners, family health issues, depression, fears, sadness, worry, or spiritual or religious concerns.
Table 3 presents the results from the logistic analysis predicting high distress. In adjusted analysis, black race did not predict high distress (OR, 0.94; 95% confidence interval [CI], 0.62-1.44). High distress was associated with sex, age, and some cancer categories. Women had 77% higher odds of high distress compared with men (OR, 1.77; 95% CI, 1.25-2.51).
Compared with patients aged 18-44 years, patients aged 61-84 had 43% lower odds of high distress (OR, 0.57; 95% CI, 0.33-0.98), and patients aged 85 and older had 74% lower odds of high distress (OR, 0.26; 95% CI, 0.07-0.98). There was no statistically significant difference between patients aged 18-40 and those aged 41-60 for high distress (OR, 1.01; 95% CI, 0.59-1.73).
Discussion
Management of patients with cancer continues to evolve. Although a tremendous amount of importance is still placed on the pathophysiology of cancer and its prescribed treatments, more emphasis is being assigned to the physical and psychosocial effects of cancer on these patients. In 2008, the Institute of Medicine published a report that examined the psychosocial health of patients with cancer.14 The report recommended that all cancer care should ensure the provision of appropriate psychosocial health services by facilitating effective communication between patients and care providers, identifying each patient’s psychosocial health needs, coordinating referrals for psychosocial services and monitoring efficacy of psychosocial interventions. The inclusion of psychosocial distress screening in all cancer programs accredited by the ACS CoC helped to prioritize the identification and treatment of psychosocial issues for all cancer patients.
The present study is the first of its kind to compare the individual stressors identified through psychosocial distress screening between black and white cancer patients. In our sample, 304 of 933 patients (32%) reported high distress, with a total score of ≥4. Previous research on overall distress difference across race/ethnicity is mixed. VanHoose and colleagues found no difference in overall distress between racial groups,15 Alcala and colleagues found higher overall distress in black patients with cancer compared with white patients with cancer,9 and Culver and colleagues found black women with breast cancer had lower overall distress compared with white women.11 We found no difference in the presence of high distress between black and white patients at our cancer center in either crude or adjusted analysis. Differences in overall distress across studies may be owing to the timing of screening. Given that overall distress may vary across time16,17 and there is no current information on whether temporal variations in distress differ by race, it is possible that the time of distress assessment may influence demonstrated differences between racial groups. For example, if different stressors affect black and white women differentially, and those stressors are associated with different points across the cancer continuum, then we might see that the magnitude of racial differences in overall stress are time dependent. Alcala and colleagues examined any cancer diagnosis across the lifespan, whereas Culver and colleagues examined multiple time points across treatment for a small group of breast cancer patients. Badr and colleagues, in a sample of head and neck cancer patients, found that distress increased across the course of treatment;18 however they did not examine variations in type of stressors related to overall distress, nor did they examine racial differences in distress. Differences in results may also be the result of differences in measurement of distress. Culver and colleagues did not examine distress using the NCCN distress thermometer, rather psychological distress was measured by a scale rating a series of “mood-descriptive adjectives” (p. 497).11 Alcala used the K-6 as a measure of psychological distress;9 therefore, demonstrated differences in overall distress between white and black women may vary across studies because of differences in measurement of the underlying distress variable. The lack of racial differences in overall distress in our study is consistent with the findings of VanHoose and colleagues,15 who also examined distress near the start of treatment and also used the NCCN distress thermometer as the measure of psychosocial distress.
We did find differences in the individual stressors between racial groups, indicating that the source of distress does vary between black and white cancer patients. Black patients more frequently reported distress secondary to housing, loss of interest and their ability to have children than did white patients. By comparison, white patients more frequently reported distress secondary to nervousness and treatment decisions than black patients. Identified differences in individual stressors may be attributable to sociocultural differences or differences in external support. It is also possible that black patients are more likely to willingly report distress related to nonpsychological factors, whereas white patients are more willing to report factors, such as nervousness, that are related to psychological disorders. Although it has been suggested that black cancer patients have more concerns about finances and work than do white cancer patients,19 we did not identify a statistically significant difference in child care, insurance or financial issues, transportation, work, or school between these 2 cohorts. This may be because the psychosocial distress screening score included in this study was performed at the time of initial diagnosis, and not further into their prescribed treatment at which point the financial worries may be more realized. Psychosocial screening scores obtained at subsequent visits were not included in the analysis because they are not routinely collected as part of clinical care in the center where this study took place. Furthermore, it is impossible to identify where a specific patient is in their treatment regimen based on their demographic data or subsequent distress scores in our data extraction tool. Further investigation into the sources of distress at different time points along the continuum of care may shed more light on this topic.
Limitations
There are several limitations to this study. First, the method of data extraction from an electronic medical record report limited the capacity to explore possible differences between the patients in our sample, such as insurance status, level of education, available social support, current employment status, stage of disease, overall prognosis and prescribed treatment regimen.
Second, there were likely patients who either did not complete a psychosocial distress screening tool or whose data were not entered into the electronic medical record for inclusion in the analysis. The present study period took place during the implementation of the NCCN tool at the center. Although the policy was to screen all new patients as part of routine care; not all patients seen at the center received the NCCN screening tool at their first visit. Owing to the mechanisms for data entry and abstraction, only information from the patients who had a completed form was able to be accessed for this study, thus a statistical comparison between those who did and did not receive the NCCN tool cannot be made. During the timeframe for this study, the head and neck, breast, genitourinary, and hematologic services completed proportionally more NCCN screening of new patients than other services in the center. This is reflected in the distributional breakdown of cancer in the overall sample of this study. It is possible that the results are more representative of differences between black and white cancer patients in the services that were more likely to properly implement NCCN screening.
Third, our patient population was derived from an urban, academic medical center and the results may not be generalizable to other patient populations.
Fourth, the NCCN distress thermometer is a single-item rating of overall global distress that is not intended to be a diagnostic indicator of psychological comorbidity and, therefore, does not distinguish between common psychological diagnoses such as depression or anxiety. However, the usefulness of the tool is to provide an impetus for referral to services that may then encompass the evaluation and diagnosis of particular psychological conditions. Further, the distress thermometer tool is designed to identify stress relating to the social aspects of cancer diagnosis and treatment and is not limited to psychological distress alone.
Strengths
Despite the limitations, there are also significant strengths to this study. The NCCN tool is a widely accepted measure for the assessment of psychosocial distress in patients with cancer. The measure is a common and routine clinical instrument,20 and has also been used widely in research.18,21-24 Given the urban, academic environment of our clinical practice, our population is more racially diverse than other settings, allowing for initial examination of disparities between white and black cancer patients.
Clinical implications
Understanding differences in common psychosocial stressor between black and white cancer patients may allow for clinicians to strategically look for different types of stressors in order to facilitate faster referrals to appropriate services. It has been established in the literature that distress is correlated to cancer-related outcomes and distress screening is now considered standard of care when treating cancer patients. Identifying differences in psychosocial stressors among black and white cancer patients is paramount to ensuring that the appropriate resources are available to assist them through their cancer journey. The differences in type of stressor, may indicate fundamental differences in the way patients perceive their disease or the social and cultural implication of a cancer diagnosis. In this study, white patients were more likely to find distress in the psychological realm (nervousness, decision-making), whereas black patients were more likely to be distressed about social issues (housing, ability to have children, and loss of interest). The referral needs of patients may be quite different, even with similar levels of overall distress. More research is necessary to further characterize sources of distress for cancer patients, how this distress impacts a patient’s physical and emotional well-being and how health care providers can better identify these issues and make the necessary referrals to support the whole patient.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
1. Holland JC, Reznik I. Pathways for psychosocial care of cancer survivors. Cancer. 2005;104(11 Suppl):2624-2637.
2. Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer L, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481-491.
3. Carlson LE, Bultz BD. Efficacy and medical cost offset of psychosocial interventions in cancer care: making the case for economic analyses. Psychooncology. 2004;13(12):837-849.
4. Holland J, Bultz BD. The NCCN Guideline for distress management: a case for making distress the sixth vital sign. J Natl Compr Canc Netw. 2007;5(1):3-7.
5. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130.
6. Sharp L, Carsin AE , Timmons A. Associations between cancer-related financial stress and strain and psychological well-being among individuals living with cancer. Psychooncology. 2013;22(4):745-755.
7. Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232-243.
8. Holland JC. Preliminary guidelines for the treatment of distress. Oncology. 1997;11(11A):109-114.
9. Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930.
10. Bradley CJ, Wilk A. Racial differences in quality of life and employment outcomes in insured women with breast cancer. J Cancer Surviv. 2014;8(1):49-59.
11. Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing African Americans, Hispanics and non-Hispanic whites. Psychooncology. 2002;11(6):495-504.
12. American College of Surgeons Commission on Cancer. ACSCC website. Cancer program standards: ensuring patient-centered care. 2016 edition. https://www.facs.org/quality-programs/cancer/coc/standards. Posted 2016. Accessed August 30, 2017.
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management. National Comprehensive Cancer Network, 2014.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Accessed August 30, 2017.
14. Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: The National Academies Press; 2008. https://doi.org/10.17226/11993. Accessed August 30, 2017.
15. VanHoose L, Black LL, Doty K, et al. An analysis of the distress thermometer problem list and distress in patients with cancer. Support Care Cancer. 2015;23(5):1225-1232.
16. Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psychooncology. 2008;17(6):538-547.
17. Enns A, Waller A, Groff SL, Bultz BD, Fung T, Carlson LE. Risk factors for continuous distress over a 12-month period in newly diagnosed cancer outpatients. J Psychosoc Oncol. 2013;31(5):489-506.
18. Badr H, Gupta V, Sikora A, Posner M. Psychological distress in patients and caregivers over the course of radiotherapy for head and neck cancer. Oral Oncol. 2014;50(10):1005-1011.
19. Wang X, Cosby LG, Harris MG, Liu T. Major concerns and needs of breast cancer patients. Cancer Nurs. 1999;22(2):157-163.
20. Dabrowski M, Boucher K, Ward JH, et al. Clinical experience with the NCCN distress thermometer in breast cancer patients. J Natl Compr Canc Netw. 2007;5(1):104-11.
21. Buchmann L, Conlee J, Hunt J, Agarwal J, White S. Psychosocial distress in prevalent in head and neck cancer patients. Laryngoscope. 2013;123(6):1424-1429.
22. Agarwal J, Powers K, Pappas L, et al. Correlates of elevated distress thermometer scores in breast cancer patients. Support Care Cancer. 2013;21(8):2125-2136.
23. Johnson R, Gold MA, Wythe KF. Distress in women with gynecologic cancer. Psychooncology. 2010;19(6):665-668.
24. Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psychooncology. 2011;20(6):594-600.
The impact of combining human and online supportive resources for prostate cancer patients
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
Prostate cancer is the most common cancer among men and the second leading cause of cancer-related death in men. 1 Treatment choices for prostate cancer are perhaps more varied than for many other cancers, with surgery, external beam radiation therapy, and brachytherapy all widely used, a number of adjuvant and nonstandard therapy options available, as well as the possibility of not immediately treating the cancer – the “active surveillance” option.
Biochemical failure rates do not differ between the 3 main treatments,2 but each exposes patients to the risk of side effects, including impotence, incontinence, rectal injury, and operative mortality. Recovery can be gradual and will not always involve a return to baseline functioning.3 Quality-of-life comparisons observed covariate-controlled decreases in varying specific aspects of quality of life for each of the treatments.4
Surgery, brachytherapy, and external beam radiation therapy have each shown advantages over other treatments on at least some specific aspect, but disadvantages on others.4 Ongoing surveillance of a cancer left in place has become a more common option in part because of the disadvantages of traditional treatment and because of the growing recognition that sensitive diagnosis techniques often locate cancers that might not be life threatening. Recent reviews and reasonably long-term trials portray active surveillance as a valid alternative to surgery and radiation in many cases, with little difference in life expectancy and cancer-related quality of life, and possibly some reduction in health system cost.5-7
Prostate cancer patients cope with these uncertainties and decisions in many ways,8 often using multiple coping behaviors,9 but coping almost always includes seeking information and social support, as well as active problem-solving, to make informed treatment decisions consistent with their values.
Unfortunately, prostate patients often do not receive or use needed information. McGregor10 reported that patients were aware of their incomplete understanding of their disease and treatment options. Findings from several studies suggest that patients often perceive that clinicians inform them about the disease and treatment options but then send them home unprepared to deal with such things as incontinence or difficulties with sexual functioning.11
Similarly, previous research demonstrates the benefits of social support for prostate cancer patients who receive it, but also that overall they are underserved.12,13 Male cancer patients are generally far less likely to seek support and health information than are female patients. And when patients with prostate cancer do participate in online cancer support groups, they are more likely to exchange information, whereas breast cancer patients provide support for each other.14
Mentoring
Some responses to these knowledge and support gaps pair newly diagnosed patients with survivors willing to be a guide, coach, and a source of information, as in the American Cancer Society’s (ACS’s) Man-to-Man support groups.15 Peer mentors may have a sophisticated level of understanding from their own experiences with medical literature and the health care system, but this cannot be assumed. Another mentoring model is expert-based, exemplified by the National Cancer Institute’s (NCI’s) cancer information specialist at the Cancer Information Service (CIS) and a similar system at the ACS. These telephone services allow for responsiveness to the caller’s needs, existing knowledge, and the caller’s readiness for information. The CIS specialist can also introduce important information the caller might not have known to ask about.16
However, not all problems presented by callers can be solved in a single conversation. Callers are encouraged to call back with additional questions or when their situation changes, but speaking with the same specialist is not facilitated, so it is hard for a second call to build upon the first. Combining the expertise of the cancer information specialist with the ongoing and proactive contact and support typical of the lay guide/mentor/navigator could be more effective. Here a CIS-trained information specialist called prostate patients multiple times over the intervention period to help them deal with information seeking and interpretation. In a previous study with breast cancer patients, a mentor of this sort improved patient information competence and emotional processing.17
Interactive resources
Online resources allow cancer patients self-paced and self-directed access to information and support anonymously and at any time. However, this can be more complicated than it might at first seem. With the complexities of the prostate cancer diagnosis, the multiple treatment options, and the uncertain but potentially serious effects of the treatments themselves, the amount of potentially relevant information is quite large. Then, because individuals will value differentially the attributes of treatments, their consequences, or even notions of risk and gain, a system must be able to respond appropriately to a range of very different people. Beyond this, as prostate cancer patients move from the shock of a cancer diagnosis to the problems of interpreting its details, to making treatment decisions, to dealing with problems of recovery, and then re-establishing what is a “new normal” for them, an individual’s demands on a system vary as well. Comprehensive and integrated systems of services meet the varying needs of their users at different times and different situations.18,19 The systems approach not only makes it far easier for users to find what they need, it may also encourage them to see connections between physical, emotional, and social aspects of their illness. Versions of the system used in the present study – CHESS, or Comprehensive Health Enhancement Support System – have been effective supporting patients with AIDS and breast and lung cancers, and teens with asthma.16,20
Study goals and hypotheses
Given the success of the 2 aforementioned approaches, we wanted to compare how CHESS and ongoing contact with a human cancer information mentor in patients with prostate cancer would affect both several general aspects of quality of life and 1 specific to prostate cancer. We also examined differences in the patients’ information competence, quality of life, and social support. There was no a priori expectation that one intervention would be superior to the other, but any differences found could be important to policy decisions, given their quite-different cost and scalability.
More importantly, the primary hypothesis of the study was that patients with access to both CHESS and a mentor would experience substantially better outcomes than those with access to either intervention alone, because each had the potential to enhance the other’s benefits. For example, a patient could read CHESS material and come to the mentor much better prepared. By referring the user to specific parts of CHESS for basic information, the mentor could use calls to address more complex issues, or help interpret and evaluate difficult issues. In addition, because CHESS provides the mentor information about changes in the patient’s treatments, symptoms, and CHESS use, in the combined condition the cancer information mentor can know much more about the patient than when working alone. We also expected that the mentor would stimulate the kind of diverse use of CHESS services we have found to be most effective
Because both mentoring and CHESS have consistently produced positive quality of life effects on their own, compared to controls, there is no reasonable expectation that negative effects of a combined condition could occur and should be tested for. Thus, the study was powered for 1-tailed significance in the comparison between the combined condition and either intervention alone, a procedure used consistently in previous studies of CHESS components or combined conditions. However, since the research question comparing the 2 interventions alone had no such strong history it was tested 2-tailed.
Methods
Recruitment
Study recruitment was conducted from January 1, 2007 to September 30, 2008 at the University of Wisconsin’s Paul P Carbone Comprehensive Cancer Center in Madison, Hartford Hospital’s Helen and Harry Gray Cancer Center in Hartford, Connecticut, and The University of Texas MD Anderson Cancer Center in Houston.
A total of 461 patients were invited to participate in the study. Of those patients, 147 declined to participate, 4 were excluded, and 310 were randomized to access to CHESS only, access to a human mentor only, or access to CHESS and a mentor (CHESS+Mentor) during the 6-month intervention period, which provided adequate power (>.80) for effects of moderate size (Figure 1). Randomization was done with a computer-generated list that site study managers accessed on a patient-by-patient basis, with experimental conditions blocked within sites.
Recruitment was done by posting brochures about the study at the relevant locations and devising standardized recruitment scripts for clinical staff to use when talking to patients about the study. Staff at all sites invited patients they thought might be eligible to learn more about the study. As appropriate, staff members then reviewed informed consent and HIPAA information, explained the interventions, answered patient questions, obtained written consent, collected complete patient contact and computer access information, and provided patients the baseline questionnaires.
The standard inclusion criteria were: men older than 17 years, being able to read and understand English, and being within 2 months of a diagnosis of primary prostate cancer (stage 1 or 2) at the time of recruitment. Despite the 2-month window, few men had begun treatment before pretest. Only 9 of the 310 participants reported having already had surgery (7 prostatectomies, 2 implants), so participants may be fairly characterized as beginning the study in time to benefit from interventions during most stages of their experience with prostate cancer.
Interventions
To provide an equal baseline, all of the participants were given access to the Internet, which is becoming a de facto standard for information access. Internet access charges were paid for all participants during the 6-month intervention period, and computers were loaned to those who did not have a personal computer. All of the participants were offered training on using the computer, particularly with Google search procedures so that they could access resources on prostate cancer.
Participants assigned to the CHESS or CHESS+Mentor conditions were also offered training in using CHESS (basically a guided tour), which typically took about 30 minutes on the telephone but was occasionally done in-person.
CHESS intervention. In creating CHESS for prostate cancer patients, a combination of patient needs assessments, focus groups with patients and family members, and clinician expertise helped us identify the needs, coping mechanisms, and relevant medical information to help patients respond to the disease. An article describing development of the CHESS Prostate Cancer Module22 presents how those different services address patient needs for information, communication, and support, or build skills.
Most of these services were present in CHESS for other diseases, but several were newly created to meet needs of prostate cancer patients and partners, such as a decision map tool and a module on managing sexual problems.22 Also, patients expressed frustration at being overwhelmed by the volume of information and said they would prefer to focus only on what was most relevant, so we created an alternative navigation structure on the CHESS homepage. Using terms suggested by focus groups of prostate cancer survivors and their spouses, we devised a navigation structure called Step-by-Step that identified 6 typical sequential steps of men’s experience with prostate cancer. Clicking on a step would take a patient to a menu focused on actions and considerations specific to that disease step, links to information most relevant at that step, and suggested questions to ask oneself and one’s doctor.
Mentor intervention. The cancer information mentor who made most of the calls to patients was an experienced information specialist with the Cancer Information Service and had served as the expert for the CHESS Ask an Expert service for 6 years. She was highly knowledgeable about prostate cancer and patient information needs. Her additional training for this study focused on taking advantage of repeated contacts with the participants and how to set limits to avoid any semblance of psychological counseling. At recruitment, we made clear that a male mentor was also available if the participant would prefer to discuss sensitive topics with another man. The male mentor was experienced in the Man-to-Man program and received additional training for this role, but he was used for only 1% of all contacts.
During calls, the mentor had Internet access to a range of NCI, ACS, and other resources. She could help interpret information the participant already possessed as well as refer him to other public resources, including those on the Internet. CHESS software designers created an additional interface for the mentor that handled call scheduling and allowed her to record the topics of conversations, her responses and recommendations, and her overall ratings of patient preparedness and satisfaction. Using this interface allowed the mentor to quickly review a participant’s status and focus the conversation on issues raised by past conversations or scheduled treatment events. The mentor calls were audiorecorded and reviewed frequently by the project director during the early months of intervention and less frequently thereafter to ensure adherence to the protocol.
The mentor telephoned weekly during the first month of intervention, then twice during the second month, and once a month during the final 4 months of the intervention (ie, 10 scheduled calls, though patients could also initiate additional calls). Calls were scheduled through a combination of telephone contact and e-mail according to the patient’s preference. Call length ranged from 5 minutes to an hour, with the average about 12 minutes (the first call tended to be considerably longer, and was scheduled for 45 minutes). About 10%-15% of participants in the Mentor conditions initiated calls to the mentor to obtain additional support, and about 15% of scheduled calls in fact took place as e-mail exchanges. A few calls were missed because of scheduling difficulties, and some participants stopped scheduling the last few calls, but the average number of full calls or e-mails was 6.41 per participant.
CHESS+Mentor intervention. For the CHESS+Mentor condition, the interactions and resources used were similar to those of the Mentor-only condition, but the interface also provided the mentor with a summary of the participant’s recent CHESS use and any concerns reported to CHESS, which helped the mentor assess knowledge and make tailored recommendations. The mentor could also refer participants to specific resources within CHESS, aided by knowledge of what parts of CHESS had or had not been used.
Assessment methods
Patients were given surveys at the baseline visit to complete and mail back to research staff before randomization. Follow-up surveys were mailed to patients at 2, 6, 12, and 24 weeks post intervention access, and patients returned the surveys by mail. Patient withdrawal rates were about 3%.
Measures
Outcomes. This study included 4 measures of quality of life (an average of relevant portions of the World Health Organization’s Quality of Life (WHOQOL) measure, Emotional and Functional Well-being, and a prostate-cancer specific index, the Expanded Prostate Cancer Index Composite (EPIC). We also tested group differences on 5 more specific outcomes that were likely to be proximal rather than distal effects of the interventions: Cancer Information Competence, Health care Competence, Social Support, Bonding (with other patients), and Positive Coping.
Quality of life. Quality of life was measured by combining the psychological, social, and overall dimensions of the WHOQOL measures.23 Each of the 11 items was assessed with a 5-point scale, and the mean of those answers was the overall score.
Emotional well-being. Respondents answered 6 items of the Functional Assessment of Cancer Therapy – Prostate
Functional well-being. Respondents indicated how often they experienced each of the seven functional well-being subscale items of the FACT-Prostate.24
Prostate cancer patient functioning. We used the EPIC to measure of 3 of 4 domains of prostate cancer patient functioning: urinary, bowel, and sexual (omitting hormonal).25 The EPIC measures frequency and subjective degree of being a problem of several aspects in each domain. We then summed scores across the domains and transformed linearly to a 0-100 scale, with higher scores representing better functioning.
Cancer information competence. Five cancer information competence items, measured on a 5-point scale, assessed a participant’s perception about whether he could find and effectively and use health information, and were summed to create a single score.20
Social support. Six 5-point social support items assessed the informational and emotional support provided by friends, family, coworkers, and others, and were summed to create a single score.20
Health care competence. Five 5-point health care competence items assessed a patient’s comfort and activation level dealing with physicians and health care situations, and were summed to create a single score.20
Positive coping. Coping strategies were measured with the Brief Cope, a shorter version of the original 60-item COPE scale.26 Positive coping strategy, a predictor of positive adaptation in numerous coping contexts, was measured with the mean score of 4 scales (8 items in all): active coping, planning, positive reframing, and humor.
Bonding. Bonding with other prostate cancer patients was measured with five 5-point items about how frequently participants connected with and got information and support from other men with prostate cancer.27
User vs nonuser. Intent-to-treat analyses compared the assigned conditions. However, because CHESS use was self-selected and available at any time whereas mentor calls were scheduled and initiated by another person, the proportion actually using the interventions was quite different.
Since a participant assigned access to CHESS had to select the URL, even a single entry to the system was counted as use. Of 198 participants assigned to either the CHESS or CHESS+Mentor conditions, 43 (22%) never logged in and were classified as nonusers.
Because the mentor scheduled calls and attempted repeatedly to complete scheduled calls, the patient was in a reactive position, and the decision not to use the mentor’s services could come at the earliest at the end of a first completed call. However, after examining call notes and consulting with the mentors, it was clear that opting not to receive mentoring typically occurred at the second call. Furthermore, much (though not all) of the first call was typically taken up with getting acquainted and scheduling issues, so that defining “nonuse” as 2 or fewer completed calls was most faithful to what actually happened. Of 202 participants assigned access to a mentor, 16 (8%) were thus defined as nonusers.
Results
Overall, the participants were about 60 years of age and had some college education and middle-class incomes (Table 1). Only about 10% were minorities or lived alone, and their comfort using computers and the Internet was at or above the “quite comfortable” level. None of groups differed significantly from any other.
The 2 primary hypotheses of the study were that participants in the combined condition would manifest higher outcome scores than those with either intervention alone. Table 2 displays group means at 3 posttest intervals, controlling for theoretically chosen covariates (age, education, and minority status) and pretest levels of the dependent variable. The table also summarizes tests examining the hypotheses and the comparison of CHESS and Mentor conditions. The 4 quality-of-life scores appear first, followed by 5 more specific outcomes that are perhaps more proximal effects of these interventions.
The combined condition scored significantly higher than the CHESS-only condition on functional well-being at 3 months, on positive coping at 6 months, and on bonding at both 6 weeks and 6 months. The combined condition scored significantly higher than Mentor-only on health care competence and positive coping at 6 weeks, and on bonding at 6 months. This represents partial but scattered support for the hypotheses. And some comparisons of the combined condition with the Mentor-only condition showed reversals of the predicted relationship (although only cancer information competence at 3 months would have reached statistical significance in a 2-tailed test).
No directional hypotheses were made for the comparison of the 2 interventions (see Table 2 for the results of 2-tailed tests). Participants in the Mentor condition reported significantly higher functional well-being at 3 months, although there were 5 other comparisons in which the Mentor group scored higher at P < .10, and higher than the CHESS group on 22 of the 27 comparisons. Thus, it seemed that the Mentor condition alone might have been a somewhat stronger intervention than CHESS alone.
Discussion
We used a randomized control design to test whether combining computer-based and human interventions would provide greater benefits to prostate cancer patients than either alone, as previous research had shown for breast cancer patients.18 The computer-based resource was CHESS, a repeatedly evaluated integrated system combining information, social support, and interactive tools to help patients manage their response to disease. The human cancer information mentor intervention combined the expertise of NCI’s Cancer Information Service with the repeated contact more typical of peer mentoring. Previous research with breast cancer patients had shown both interventions to provide greater information, support, and quality-of-life benefits than Internet access alone.14 This study also compared outcomes obtained by the separate CHESS and Mentor conditions, but without predicting a direction of difference.
Tests at 6 weeks, 3 months and 6 months after intervention found instances in which prostate cancer patients assigned to the combined CHESS+Mentor condition experienced more positive quality of life or other outcomes than those assigned to CHESS or Mentor alone, but those differences were scattered rather than consistent. In the direct comparisons of the separate CHESS and Mentor conditions, significance was even rarer, but outcome scores tended to be higher in the Mentor condition than in the CHESS condition.
We noted that differential uptake of the 2 interventions (92% for Mentor vs 78% for CHESS) made interpreting the intent-to-treat analyses problematic, as the mentor’s control of the call schedule meant that far more patients in that condition actually received at least some intervention than in the CHESS condition, where patients used or did not use CHESS entirely at their own volition. This could have biased results in several ways, such as by underestimating the efficacy of the CHESS condition alone and thus inflating the contrast between CHESS alone and CHESS+Mentor. Or the combined condition might have been less different than the Mentor-only condition than intended, thus making for a conservative test of that comparison. However, post hoc analyses of only those participants who had actually used their assigned interventions (and this led to some reclassification of those originally assigned to the CHESS+Mentor condition) produced results that were little different than the intent-to-treat analysis.
Thus, although the combined condition produced some small advantages over either intervention alone, these advantages did not live up to expectations or to previous experience with breast cancer patients.17 We expected the mentor to be able to reinforce and help interpret what the participants learned from CHESS and their clinicians, and also to advise and direct these patients to be much more effective users of CHESS and other resources. Similarly, we expected that CHESS would make patients much better prepared for mentoring, so that instead of dealing with routine information matters, the mentor could go into greater detail or deal with more complex issues. Their combined effect should have been much larger than each alone, and that was not the case. Perhaps from the prostate cancer patients’ perspective, the 2 interventions seemed to offer similar resources, and a patient benefitted from one or the other but expected no additional gain from attending to both.
The 2 interventions themselves seemed nearly equally effective. The Mentor intervention was significantly stronger than CHESS in only 1 of 27 tests in the intent-to-treat analysis and 2 in the analysis limited to intervention users.
These results for prostate cancer patients are somewhat weaker than those previously reported with breast cancer patients.17 It is possible that prostate cancer patients (or men in general) are less inclined to seek health information, support, and health self-management than breast cancer patients (or women in general), perhaps becaus
Although these interventions were experienced by prostate cancer patients in their homes in natural and familiar ways, any experimental manipulation must acknowledge possible problems with external validity. More important here, our recruitment procedures may have produced self-selection to enter or not enter the study in 2 ways that limit its applicability. First, although we thought that offering Internet access to all participants would make participation more likely, the most frequent reason men gave in declining to join the study was “not a computer person.” Our participants were certainly very comfortable with computers and the Internet, and most used them frequently even before the study. Second, it seems that, except for their prostate cancer, our sample was healthy in other respects, as indicated by the low number of other health care visits or surgeries/hospitalization they reported (and “overwhelmed” and “too busy,” 2 common reasons for declining study participations could also be coming from men with more comorbidities). Thus, our sample was probably more computer literate and healthier than the general population of prostate cancer patients.
Nonetheless, for policymakers deciding what information and support interventions to put in place for prostate cancer patients (or more generally for other cancer patients as well), these results have 2 implications. First, since the combination of the mentor and CHESS produced only small advantages over either alone, the extra effort of doing both seems clearly unwarranted for prostate cancer patients. The somewhat larger advantage of the combined intervention shown for breast cancer patients in previous studiesmight warrant using the combination in some circumstances, but even that is not clear-cut.
Finding that CHESS and the cancer information mentor separately provided essentially equal benefits might seem to suggest that they can be regarded as alternatives. However, computer-based services can be provided much more cheaply and scaled up far more readily than services dependent on one-on-one contacts by a highly trained professional. This may direct health care decision makers first toward computer-based services.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277-300.
2. Cozzarini C. Low-dose rate brachytherapy, radical prostatectomy, or external-beam radiation therapy for localized prostate carcinoma: The growing dilemma. European Urology. 2011;60(5):894-896.
3. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
4. Ferrer F, Guedea F, Pardo Y, et al. Quality of life impact of treatments for localized prostate cancer. Radiother Oncol. 2013;108(2):306-313.
5. Cooperberg, MR, Carroll, PR, Klotz, L. Active Surveillance for prostate cancer: progress and promise. J Clin Onc. 2011;29:3669-3676.
6. Hamdy, FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415-1424.
7. Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425-37.
8. Lavery JF, Clarke VA. Prostate cancer: patients’ spouses’ coping and marital adjustment. Psychol Health Med. 1999;4(3):289-302.
9. Folkman S, Lazarus R. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J Pers Soc Psycol. 1985;48:150-170.
10. McGregor S. What information patients with localized prostate cancer hear and understand. Patient Educ Couns. 2003;49:273-278.
11. Steginga SK, Occhipinti S, Dunn J, Gardiner RA, Heathcote P, Yaxley J. (2001) The supportive care needs of men with prostate cancer (2000). Psychooncology. 2001;10(1):66-75.
12. Gregoire I, Kalogeropoulos D, Corcos J. The effectiveness of a professionally led support group for men with prostate cancer. Urologic Nurs. 1997;17(2):58-66.
13. Katz D, Koppie T, Wu D, et al. Sociodemographic characteristics and health related quality of life in men attending prostate cancer support groups. J Urol. 2002;168:2092-2096.
14. Klemm P, Hurst M, Dearholt S, Trone S. Gender differences on Internet cancer support groups. Comput Nurs. 1999;17(2):65-72.
15. Gray R, Fitch M, Phillips C, Labrecque M, Fergus K. Managing the impact of illness: the experiences of men with prostate cancer and their spouses. J Health Psychol. 2000;5(4):531-548.
16. Thomsen CA, Ter Maat J. Evaluating the Cancer Information Service: a model for health communications. Part 1. J Health Commun. 1998;3(suppl.):1-13.
17. Hawkins RP, Pingree S, Baker TB, et al. Integrating eHealth with human services for breast cancer patients. Transl Behav Med. 2011;1(1):146-154.
18. Strecher V. Internet methods for delivering behavioral and health-related interventions. Ann Rev Clin Psychol. 2007;(3):53-76.
19. Gustafson DH, Hawkins RP, McTavish F, et al. Internet-based interactive support for cancer patients: Are integrated systems better? J Commun. 2008;58(2):238-257.
20. Gustafson DH, Hawkins RP, Boberg EW, et al. CHESS: Ten years of research and development in consumer health informatics for broad populations, including the underserved. Int J Med Inform. 2002;65(3):169-177.
21. Han JY, Hawkins RP, Shaw B, Pingree S, McTavish F, Gustafson D. Unraveling uses and effects of an interactive health communication system. J Broadcast Electron Media. 2009;53(1):1-22.
22. Van Bogaert D, Hawkins RP, Pingree S, Jarrard D. The development of an eHealth tool suite for prostate cancer patients and their partners. J Support Oncol. 2012;10(5):202-208.
23. The WHOQOL Group. Development of the WHOQOL: Rationale and current status. Int J Ment Health. 1994;23:24-56.
24. Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920-928.
25. Wei JT, Dunn R, Litwin M, Sandler H, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
26. Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med. 1997;4: 91-100.
27. Gustafson D, McTavish F, Stengle W, et al. Use and impact of eHealth System by low-income women with breast cancer. J Health Commun. 2005;10(suppl 1):219-234.
The effect of centralizing breast cancer care in an urban public hospital
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
When cancer care is centralized in a comprehensive fashion, the quality of care and the outcomes improve.1,2 Unfortunately, because of the medical insurance structure in New York City, most patients of lower socioeconomic status do not receive their cancer care in such dedicated cancer centers. In New York City, the majority of the underserved vulnerable populations – that is, those without health insurance – receive their care from the public hospital system known as NYC Health and Hospitals. Cancer care in this system is not centralized and may result in fragmented implementation of various modalities of treatment. In addition, because there is no centralized care, needs such as early screening and prevention programs are often not addressed. This problem was evident in Queens in 2000 and before when many patients with late-stage cancers were presenting for cancer care. Queens, which is one of the 5 boroughs of New York City, has more than 2.3 million residents. It has 2 public hospitals, Elmhurst Hospital Center and Queens Hospital Center (QHC). In 2001, the plan was devised for the establishment of a cancer center at QHC, mainly because of the high rate of late-stage cancers that were being seen at presentation and recognition of the need for more comprehensive care. In 2002, the Queens Cancer Center (QCC) began to see patients. QCC is a single facility that provides medical, surgical, radiation, gynecologic, and urologic oncology all in one area of the QHC.
This study is an investigation of the possible impact on care for breast cancer patients of low socioeconomic status who were treated at a comprehensive cancer center, with specific consideration of the change or improvement in treatment modalities and outcomes. Data on treatment modalities and outcomes of cancer patients who were treated at the QHC during 2000, before the QCC was set up, were compared with data of patients treated during 2008 (2008 was selected because we have 5-year survival data for those patients). The public hospital system treats all patients regardless of their ability to pay, so the majority of patients in the system are of lower socioeconomic status. In addition, 92% of the patients seen QHC are from a minority population. These are the populations that tend to have a worse prognosis and often are not given optimal treatment.3 The payer mix of patients in the public hospital system is different than that of private hospitals. Most of the patients present at the hospital with no insurance and if they are diagnosed with cancer they may be converted to emergency Medicaid. About 10% of patients will not be converted because of their document status.
Patients and methods
We used the Queens Hospital Tumor Registry to identify the patients who had been diagnosed with and treated for breast cancer in 2000 and 2008. The electronic medical records were reviewed, and in the case of the 2000-year patients, the written charts were also reviewed. The study was approved by the Mount Sinai institutional review board. It was not necessary to obtain patient consent because it was a retrospective study.
Only patients diagnosed with stage 0, I, II, or III breast cancer who received their treatment at QHC were included in the study. Patients who were seen in consultation at QHC but not treated there were excluded. Statistics were done using the 2x2 chi-squared SPSS analysis; a P value of .05 was considered significant. The survival data was analyzed using SAS.
Results
There were 24 evaluable patients in 2000 and 78 evaluable patients in 2008 who had stage 0, I, II, or III primary breast cancer and were treated at QHC. The average age of the patients in 2000 was 53.5 years and 54.7 years in 2008. The mean age for both groups was 55 years. The patients were ethnically diverse in both groups with 46% black, 17% Hispanic, 25% ethnic Asian Indian, and 6% white (Figure 1).
The payer mix in 2000 was 9 patients (37.5%) self-pay, 7 (29%) Medicaid, and 8 (33%) Medicare. In 2008, 11 patients (14%) were self-pay, 46 (59%) Medicaid, 11 (14%) Medicare, and 10 (13%) were private insurance. In 2000, there were 3 (12%) patients with stage 0 disease, 5 (21%) with stage I; 9 (37.5%) with stage II, and 7 (29%) with stage III. In 2008 there were 28 (36%) patients with stage 0 disease, 15 (19%) with stage I, 17 (22%) with stage II, and 18 (23%) with stage III (Figure 2).
None of those values are statistically different. In 2000, 2 of the 24 patients had lumpectomies (partial mastectomy) and the rest had mastectomies. In 2008, 39 (50%) patients had mastectomy and 39 (50%) had lumpectomies (Figure 3). This was a statistically significant difference.
Radiation was given to both patients with lumpectomy in the 2000 group. In the 2008 group, all patients with lumpectomies were evaluated for radiation, and 6 of them did not receive radiation for the following reasons: 3 had very small foci of ductal carcinoma in situ (DCIS) and were treated with hormone therapy and no radiation; 1 patient had a lumpectomy for stage 1 cancer and also did not get radiation therapy because of a low oncotype and very small lesion; 2 patients were older than 70 years and had DCIS and were treated with tamoxifen alone as per NCCN Guidelines for women in that age group. The rest of the patients with lumpectomies received postoperative radiation.
Hormone and HER2 (human epidermal growth factor receptor 2) status was obtained on all patients. For the 2000 patients, 71% had 1 hormone receptor–positive (estrogen receptor [ER] or progesterone receptor [PR]), 21% were triple negative (ER-PR and HER2-neu), and 42% had HER2-neu–positive tumors. For the 2008, patients 65% were positive for 1 hormone receptor (ER or PR), 28% were triple negative (ER-PR and HER2-neu), and 7% had HER2-neu-positive tumors.
All patients were offered chemotherapy and hormone therapy if appropriate, as per NCCN guidelines. If a patient’s tumor was found to be HER2-positive, then the chemotherapy regimen would include the use of trastuzumab in both groups.
The 5-year survival for the 2008 stage III patients was 73.7%, compared with 14.2% for the 2000 stage III patients. The only deaths in the 2008 group were in patients with stage III disease. In the 2000 group, 4 of the 5 patients with stage III cancer died, and 33% of patients with stage I or II either died or were lost to follow-up before 5 years. This survival difference is significant by a chi-square and Wilcoxon analysis, with a P value of .01.
In 2000, 86% of patients with cancer were termed self-pay, that is, they had no insurance and they were not converted to emergency Medicaid. In 2008, 16% of patients were self-pay, and the rest were converted to Medicaid. In 2000, fewer than 2% of patients had commercial insurance, compared with 9% in 2008.
Discussion
There have been numerous studies reporting on disparities in the treatment of patients with breast cancer based on race or socioeconomic status.4-18 Many studies have shown inferior survival for black women with breast cancer, but it is not entirely clear whether these differences are the result of the quality of medical care received or biologic differences.14,19 A moderately large study from a metropolitan medical center in Detroit showed no difference in survival in their patients based on race when all of the patients received equal treatments.15 A meta-analysis of survival in black and white breast cancer patients showed that the black women had significantly poorer outcomes.19
Findings from a recent study showed that patients of lower socioeconomic status are more likely to undergo mastectomy than breast conserving therapy.20 The study, which identified 727,927 patients with early-stage breast cancer during 1998-2011, found that the rate of breast conservation increased from 54% to 59% during that time period and that there were significant barriers to women receiving breast-conserving therapy based on their type of insurance and having a lower socioeconomic status.20
The treatment of breast cancer is best delivered in a multimodality setting, but many inner-city public hospitals do not have such a facility for their patients. QHC is the only public hospital in New York City that has established a comprehensive cancer center. The patient population of QHC is overwhelmingly of minority origin (only 5% of patients are white). In addition, it is a safety net hospital, so no patient is turned away because they cannot pay, and most patients are of lower socioeconomic status and do not have insurance. The purpose of the cancer center was to provide a single site at which our patients could receive all their treatment. It was to ensure that our patients had easy access to care and treatment during all phases of their disease trajectory and did not “fall through the cracks” of the system. Those goals were addressed by having all of the center’s physicians in one place. Physicians involved in care included medical, surgical, and radiation oncologists, a gynecologic oncologist, a genitourinary oncologist, and a thoracic surgery oncologist. The support groups organized for the cancer patients included 3 oncology social workers, an oncology navigator, a nutritionist, a pastoral care supporter, and an oncology psychologist, all located in the same area. All of the clerical and financial aspects of care were also placed within the center. This made the experience as seamless as possible for both the patients and the treating physicians. A “survivors clinic” was established so the cancer patients could be seen by integrated primary care providers to address all noncancer-related health issues such as hypertension, diabetes, or heart disease. Finally, a robust clinical oncology research team was established in the same location. The research included several protocols for new drug treatments for breast cancer from pharmaceutical companies as well as the multi-institutional oncology groups.
Part of the mission of the cancer center was to reach out into the community of Queens to provide education about early detection, cancer prevention, and other public health issues such as tobacco cessation. We established a close working relationship with the Queens Public Library System to connect with their users and dispense information about cancer care and early detection. The Queens Library system is the largest in the United States, and everyone who lives in Queens has easy access to one of its 63 branch libraries. We arranged several lectures about breast cancer awareness in some of the branch libraries. We also procured a mobile mammogram unit for free screening events at the lectures, especially in neighborhoods with a large number residents who were of lower socioeconomic status.
To study the possible effect of these changes on our patients with breast cancer, we compared 2 groups of patients. One group was from the year 2000, a year before the cancer center was opened. The other was from the year 2008, the last year we could get real 5-year survival statistics. We explored how establishing the cancer center might have changed the patients’ stage at diagnosis, care, treatment modalities such as type of surgery, and outcomes. It is difficult to compare these 2 groups because of differences in the patients’ cancers, such as their receptor status, as well as differences in treatment options between the two time periods. However, we had no other way to compare the data to see if there were any trends.
There was a migration to earlier-stage cancer at diagnosis during the 6-year period after the cancer center was opened. It is likely that the educational sessions that were done in the community contributed to this migration. We also saw an increase in the number of mammograms done, from 6,300 in 2000 to 8,800 in 2008. This increase in screening also could account for more patients being identified with earlier-stage disease and might be attributable to the community education through the outreach programs.
As a quality control method, the cancer center has been evaluated by the Commission on Cancer every 3 years. At the 2013 evaluation, we received the Gold Commendation – the highest possible recognition for having 8 out of 8 commendations – and a 3-year accreditation.
There was a notable increase in the use of lumpectomy over mastectomy after the establishment of the cancer center, possibly due to the addition of 2 surgical oncologists to the cancer center’s care team. The integration of multimodiality care for each patient may also have increased the use of breast-conserving surgery.
There was a significant increase from 2000 to 2008 in the survival of patients treated for stage III breast cancer. New drugs and new patterns of adjuvant care might have been partly responsible for that change. The establishment of the comprehensive cancer center with access to new protocols ensured that patients received state-of-the-art cancer treatment. Moreover, the facility addressed all aspects of patient care throughout the disease trajectory by including designated social workers, psychologists, a nutritionist, pastoral care, and patient and survivor support groups to ensure that patients would keep coming to the center for their therapy, with no delays and very little loss to follow-up.
Most patients without insurance were able to acquire emergency Medicaid through the cancer center. This was done by having 2 financial counselors who met with every patient and who could facilitate access to Medicaid as needed. As a result of that, the percentage of patients with no coverage went from 86% in 2000 to 16% in 2008. Before this system was set up, patients who were designated self-pay would pay a fee as low as $15 for each visit and received thousands of dollars’ worth of care. Thus, by forming a cancer center and facilitating patient access to Medicaid, we were able to save money for this public institution because of the gain in revenue from Medicaid.
Our findings suggest that the development of comprehensive cancer centers within inner-city health systems can ensure better treatment for patients of lower socioeconomic status. We present evidence that this may result in increased survival, more sophisticated surgical options, and better patient quality of life. Moreover, this can be achieved while effectively increasing revenue for the public hospitals. Correcting the inequality of access to care and better therapeutic options by setting up comprehensive cancer centers could contribute to improved parity of outcomes for underserved populations.
The author acknowledges the statistical help of Brian Altonen, MPH.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
1. Kesson EM, Allardice GM, George WD, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
2. Vrijens F, Stordeur S, Beirens K, Devriese S, Van Eycken E, Vlayen J. Effect of hospital volume on processes of care and 5-year survival after breast cancer: a population-based study on 25000 women. Breast. 2012;21(3):261-266.
3. Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490-496.
4. Wheeler SB, Hayes-Reeder KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18:986-993.
5. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78-93.
6. Chen F, Puig M, Yermilov I, et al. Using breast cancer quality indicators in a vulnerable population. Cancer. 2011;117:3311-3321.
7. Banerjee M, George J, Yee C, Hryniuk W, Schwartz K. Disentangling the effects of race on breast cancer treatment. Cancer. 2007;110:2169-2177.
8. Freedman RA, He Y, Winer EP, Keating NL. Trends in racial and age disparities in definitive local therapy of early-stage breast cancer. J Clin Oncol. 2009;27:713-719.
9. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008;100:1717-1723.
19. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: Racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24:1357-1362.
11. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18:121-131.
12. Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9-31.
13. Naik AM, Joseph K, Harris M, Davis C, Shapiro R, Hiotis KL. Indigent breast cancer patients among all racial and ethnic groups present with more advanced disease compared with nationally reported date. Am J Surg. 2003;186:400-403.
14. Hersman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of southwest oncology studies S8814/S8897. J Clin Oncol. 2009;27: 2157-2162.
15
16. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94:471-473.
17. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256-1264.
18. Cross C, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the US. Cancer. 2002;95:1988-1999.
19. Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: Ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342-1349.
20. Lautner M, Lin H, Shen Y, et al. Disparities in the use of breast-conserving therapy among patients with early-stage breast cancer. JAMA. 2015;150:778-786.
Adverse events from systemic treatment of cancer and patient-reported quality of life
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
Adverse events (AEs) from systemic treatment of cancer have a negative impact on patient quality of life (QoL). The extent of this impact is difficult to ascertain, particularly in patients undergoing palliative treatment because of variations in QoL resulting from antitumor effect.1 Patient-reported outcomes (PROs) are the best tool for elicitation of patient preferences, therefore helping cancer patients, oncologists, and health care managers to make better choices. Indeed, analysis of self-reported QoL during cancer chemotherapy provides new insights that are missed by other efficacy outcomes,2 although patient-reported AEs correlate well with AEs reported by clinicians.3 Self-reported symptoms provide better control during cancer treatment.4 However, there are other instruments to measure the impact of treatments on QoL that are based on preferences of members of the general public. Use of that strategy has been strongly debated. The most obvious problem is the difficulty that persons from the general public may have in putting themselves in the patient position.5 In addition, there is evidence that compared with the general public, patients adapt to their illness5,6 and then tend to downplay severity when rating values of health states.7 Therefore, a systematic discrepancy is observed between actual patients and the general public. It is not clear if it reflects the inability of members of the general public to fully grasp the relative severity of health problems or to the adaptation process of patients. This fact may obscure a negative impact on QoL which, in turn, could be detected using the general public as a surrogate. A combination of both approaches has been recommended for rating QoL when the ultimate purpose is making decisions on resource allocation.5 This debate is prolonging in time and it is far from over.8,9
Based on this background, this study investigates the impact of AEs on QoL of cancer patients from the perspective of cancer patients who had experienced the AEs of interest (ex post population) and the perspective of members of the general public. The second group comprised participants imagining themselves as hypothetical cancer patients experiencing the AEs (ex ante population). Previous studies with this dual approach allowing for comparisons between these two populations are small or centered on a few AEs.10 Therefore, a large and comprehensive study on the impact of AEs on QoL is lacking. Supported by previous literature, the investigational hypothesis was that ex post impact would be significantly lower than that imagined in an ex ante setting. The secondary objective is to study the potential use of the EuroQol (EQ-5D) instrument for health-related QoL in the measurement of the impact of AEs in cancer patients. This generic instrument is based on interviews with members of the general public. We tried to investigate to what extent those values relate to the cancer patients’ evaluation of their own health during treatment. The ultimate goal of the study is to assist in increasing the utility that patients derive from the benefits associated with cancer treatment.
Methods
Selection of AEs
Five AEs related to systemic treatment of cancer – alopecia, acneiform rash, oxaliplatin-associated peripheral neuropathy, diarrhea, and vomiting – were selected for the study. Investigators set up different relevant cut-off points for severity, resulting in 10 toxic events that were ad hoc defined as the variables for the study (Table 1). We used the Common Terminology Criteria for Adverse Events (CTCAE, version 4) to classify alopecia, acneiform rash, diarrhea, and vomiting. For oxaliplatin-associated peripheral neuropathy, we adapted Misset’s oxaliplatin-specific scale11 (range, grade 1-4; Table 1) in which grade 1 (neurotoxicity [NTX] 1) = paresthesias only with cold lasting a few days; grade 2 [NTX2] = paresthesias with and without cold that may last months; and grade 4 [NTX3] = paresthesias with functional consequence).
Participants
Two populations were included in the study: cancer patients who had experienced a particular AE and received treatment at the medical oncology departments of Hospital Santa Tecla and Hospital del Vendrell in Tarragona, Spain; and participants from the general public who received care at the Primary Health Care Center-Llevant in the same city.
Cancer patients. These participants had to be 18 years or older and had to have experienced 1 of the 10 toxic events in the 5 years before inclusion in the study; the treatment setting could be either curative attempt (adjuvant, neoadjuvant) or palliative, and patients with ongoing treatment should have received almost 3 months of treatment. Patients were excluded if they had an ECOG PS grade of 3 or more (Eastern Cooperative Oncology Group Performance Status; range, 0-5, where 0 = fully active, 3 = capable of limited self-care; confined to bed or chair more than 50% of waking hours, and 5 = dead). A particular patient with cancer could be included because of more than 1 study toxic event (eg, alopecia and severe vomiting or NTX1 and NTX2) but had to complete separate questionnaires for the different toxic events.
General public group. Participants in this group were selected from the records of general practitioner consultations at the aforementioned primary health care center. They had to be 18 years or older and could not have a history of cancer or symptomatic/severe chronic diseases (eg, they could have hypertension or diabetes without chronic target organ involvement, or they could be patients with either acute nonserious illness or nonserious injuries).
Survey procedures
Cancer patients. Participants in this group filled in 2 questionnaires provided by a medical oncologist in a face-to-face interview: the 5-dimension, 5-level EuroQol (EQ-5D-5L) questionnaire in reference to the days when patients were suffering the toxic event; and a visual analog scale (VAS) answering the question: How do you feel that this AE has impacted on your QoL the days you have experienced it?
VAS scores ranged from 0 (the poorest QoL, the highest impact) to 100 (the better QoL, the lower impact). The EQ-5D-5L has 5 dimensions (Mobility, Self-care, Daily life activities/social performance, Discomfort/pain, and Anxiety/depression) with 5 level response options each (No problem at all, Light problem, Moderate problem, Severe problem, Extreme problem/unable).12 The combination of 5 answers is converted to a single score, which is different for different countries; in the validated version for Spanish population, the score ranges from -0.654 (the worst health state) to 1.000 (the best health state). Patients were asked to make an effort to separate and encapsulate the impact of every adverse event and separate it from others they may have experienced during the same period. Table 2 summarizes survey procedures.
General public group. Two internal medicine residents who administered the questionnaire to the participants of the public group were well trained to carefully explain what each of the 10 the toxic events meant. Some details on these explanations are shown in Table 1, and the full set of explanations is shown in Appendix 1 (online only). Participants in the general public group were asked in a face-to-face interview to imagine they were cancer patients and envision how these toxic events would impact on their QoL if they were undergoing systemic treatment of cancer. They were asked to rate the imagined impact with the VAS (1 VAS/every toxic event = 10 VAS/participant). Then, they were presented with 10 cards, each with the name of 1 of the 10 toxic events (Appendix 2 [online only]), to show them the order of the impact on QoL based on their scores (respecting ties). The participants were asked if they agreed with the order, and if they did not, they were invited to change the scores. Therefore, results in the general public group also show the rank-order of the study toxic events.
Statistical analysis
We calculated the sample size as follows:
Primary outcomes were VAS score in cancer patients and VAS score in the general public. Primary analyses were comparison between VAS in both populations. Secondary outcomes were EQ-5D-5L score in cancer patients, and intra-participant rank-order in the general public group. Secondary analyses were correlation between VAS score and EQ-5D-5L in cancer patients and descriptive analysis of rank-ordered data in the general public group.
It was planned to compare means of quantitative variables with the Mann-Whitney U test and to assess correlation between quantitative variables with the Spearman rho test. All tests for contrast were nonparametric because a normal distribution was not expected from quoted scores with some ceiling or floor effect. A hierarchical generalized cluster analysis was planned to study clusters of variables grouped by VAS score in the public group.
Ethics
The study was conducted in accordance with the Declaration of Helsinki version Fortaleza 2013 and was approved by the institutional review board of the participant institutions. All of the patients provided written informed consent before study entry. Data of the participants of the general public were anonymous, so those participants were asked to provide only oral assent, with the permission of the review board.
Results
Between December 1, 2013 and January 31, 2015, a total of 250 participants of the general public and 139 cancer patients were included in the study. Four participants of the general public had incompletely filled the questionnaire and were excluded from the study, resulting in 246 participants with complete data available. There were no losses in the patient group, of whom 79 (57%) were currently on treatment and 118 (85%) had received the treatment in the previous 2 years. The total number of study toxic events in the 139 cancer patients was 200 (20 by each of the 10 study toxicity variables). Of those, 42 patients (30%) experienced (and were included in the study for) more than 1 toxic event.
Of the 139 patients, 91 (65%) received the treatment with curative intent. The most frequent diagnosis was colorectal cancer in 77 patients (55%), followed by breast cancer (13 patients, 9.4%), and lung cancer (11 patients, 7.9%). Systemic treatment of cancer was one of these options: chemotherapy alone, anti-EGFR [epidermal growth factor receptor] alone, chemotherapy plus anti-EGFR, or chemotherapy plus other biologics. The chemotherapy regimen most frequently administered was mFOLFOX6 [modified leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] which, alone or in combination, was administered to 51 patients (37%). An anti-EGFR agent was administered to 22 patients (16%): cetuximab (15 patients), panitumumab (4 patients), erlotinib (2 patients) and afatinib (1 patient). The baseline characteristics of the patients and participants in the study are shown in Table 3.
For all 10 toxicity outcomes, the mean VAS score from the general public was numerically lower (lower QoL, more impact) than that resulted from the cancer patients who had actually experienced the toxic event of interest (Table 4 and Figure).
Taking off 2 mild effects (NTX1 and mild rash), for the 8 remaining toxic events, this difference was statistically significant (Mann-Whitney U test; P < .01 for severe vomiting, severe diarrhea, and alopecia; P < .001 for NTX2, NTX3 and mild diarrhea; P = .03 for severe rash; P = .04 for mild vomiting). Severe vomiting resulted in the worst VAS score for cancer patients (median VAS, 34) and NTX3 had the worst VAS score for the general public participants (median, VAS 19). Table 4 summarizes the 4 sets of results (patient and public VAS, and patient EQ-5D-5L and public rank-order). Regarding the results of the esthetic toxicities compared with each other, impact from severe rash was considered higher than that from alopecia for both populations, patients (mean VAS, 59 [rash] vs 77 [alopecia]; mean EQ-5D-5L score, 0.725/rank order 4 vs 0.921/10) and the general public (mean VAS, 47 vs 55; EQ-5D-5L, rank order 5 vs 9). In the group of patients, linear correlation between VAS and EQ-5D-5L score was assessed resulting in a significant positive correlation (Spearman P = .001) with a correlation coefficient Rho 0.681 (Appendix 3 [online only]). Also, a positive linear correlation was observed between the 10 means of the cancer patients’ VAS and the 10 means of the general public participants’ VAS (Spearman P = .001; coefficient Rho 0.879). Both ceiling and floor effect were observed for VAS in the 2 populations, but only ceiling effect for EQ-5D-5L in the patient population. The most important floor effect was for NTX3, with 66 participants (27%) of the general public group scoring VAS 0 (see Appendix 4 [online only] for the frequencies of answers for every level of the 5 dimensions of the EQ-5D-5L). An analysis of the results, considered as an intraparticipant rank-ordered evaluation, was performed in the general public group. Fourteen participants of that group (5.7%) changed their scores after they were presented with the order shown in cards. Mode of the ranks show that NTX3 and severe vomiting were the worst-scored toxic events. The most frequent rank-order for alopecia and severe rash were (from best to worst) the second and the fourth, respectively.
Discussion
The findings in this study show that impact on QoL imagined by members of the general public is higher than that declared by cancer patients who have experienced the AEs. It is worth noting that that result was observed for all 10 toxic events, thus confirming the investigational hypothesis of the study. However, the graph shows a strong parallel between the 2 groups, which means that both populations similarly perceive upward and downward variations in the impact resulting from the different toxic events (Figure).
Three previous studies have addressed the comparison of the impact of different AEs in these 2 populations and findings from all 3 showed the same systematic difference between patients and the general public participants. The first, Calhoun and colleagues used time to trade-off (TTO, a measure of the QoL a person or groups is experiencing) to compare therapies for ovarian cancer in patients and the general population (n = 39 for each group). The results showed that cancer patients valued more the health status associated with toxicity than did the general public participants.13 The design of the second study, by Havrilesky and colleagues, was similar to that of the present study, and they compared toxic events one by one, using VAS and TTO in 13 ovarian cancer patients and 37 women of the general public.14 The investigators found the same results as we did on the parallel of the 2 groups and also a very similar order of the toxic events. Indeed, alopecia was the less bothersome, whereas both motor neuropathy and severe vomiting were among the worst toxic events. Therefore, our results correlate perfectly with theirs. Best and colleagues found that health states values associated with oxaliplatin-related peripheral neuropathy were lower in the general public population compared with those of cancer patients.15 Besides adaptive behavior of the patients, all these results may be explained by an established awareness cancer patients have of the dual outcome of cancer treatments (AEs and benefits from the treatment).9 This awareness is absent in the general public participants, who can only envision the negative outcomes and who do not realize the importance of the benefits.9 Findings from previous studies conducted in several tumor types such as breast cancer16-18 non–small-cell lung cancer,19 thyroid cancer,20 and renal cancer21 have shown that patients are willing to trade-off AEs for treatment benefits.
Alopecia has been considered as one of the most distressing and troublesome AEs of cancer therapy.22 However, in the present study, alopecia was rated inside the range of mild toxic events as it is in the study by Havrilesky and colleagues.14 Our results show that alopecia was placed as the first less damaging toxic event when assessed with the EQ-5D-5L, the second less damaging when assessed with a rank-order system, and the third less damaging when assessed the VAS. This could be related to current fashion trends that promote shaving one’s hair, which minimizes the social stigma of alopecia and its association with cancer treatment.
The other esthetic event we analyzed was acneiform rash associated with anti-EGFR agents. Our results show that severe rash was rated as clearly worse than alopecia by the 2 populations, irrespective of the measuring instrument (VAS, EQ-5D-5L, or ordinal assessment). To our knowledge, the present study is the first to demonstrate the relative impact of total alopecia and severe rash on patient QoL. This result is even more significant considering that we included grade 2 acneiform rash inside the Severe Rash toxic event. Our results show that the worst AEs for both populations were severe vomiting and neurotoxicity with functional impairment. The high impact of severe vomiting, the quintessentially chemotherapy-induced AE, was to be expected because it is strongly supported by a number of previous reports,14,23 as is also true for peripheral motor neuropathy.14,15,24
EQ-5D is a powerful instrument for measuring health status25 and is widely used to describe and evaluate patient health.26 Our results from the 5 dimensions represented by a single score were well correlated with the results of the VAS. However, whereas median VAS scores were evenly distributed in the 0-100 range of the VAS, median EQ-5D-5L scores were distributed mainly in the 0.5-1.0 range (full range, -0.654-1.000, for Spanish population).
The final single score of the original EQ-5D is based on responses from the general public, and we have shown that its use is a valid option when the objective is the evaluation of AEs in patients with cancer. Management of AEs is of the utmost importance in this era of personalized cancer medicine. Basch and colleagues recently reported that an intensive web-based follow-up of AEs during chemotherapy improved overall survival compared with standard follow-up.27 The results of our study show that patients have strongly defined preferences regarding AEs. Therefore, therapeutic strategies with a personalized approach in managing AEs would be associated with increased effectiveness.
There are some limitations in the present study. First, we modified slightly the EQ-5D-5L questionnaire by asking patients to recall and rate the days they experienced the adverse event instead of asking for “today’s feelings.” It is not known how this modification affects internal validity of this study. Second, we asked patients to isolate the toxic event to rate it independently from the other toxic events. We believe that this request may have been difficult for some patients to do because they might have experienced more than 1 toxic event concurrently. Third, using VAS to assess health status may be a weakness because it has been considered to be too straightforward an instrument. Likewise, there are some strengths of the study: it was performed in a face-to-face manner; it displayed cardinal and ordinal results for participants in the public group; and the results are the same as those in a previous study.14
In conclusion, patients with cancer who have experienced AEs perceive a lower impact on their QoL compared with that envisioned by participants from the general public. The EQ-5D-5L is a useful tool for evaluating cancer-therapy–related AEs. The impact of alopecia on QoL was notably low and even lower than that of severe rash. Further investigation on this issue should focus on patients’ and oncologists’ shared choices, which increasingly will be driven by patient preferences.
The Oncologic Association Dr Amadeu Pelegrí (AODAP), a charitable organization led by cancer patients and based in Salou, Spain, provided the financial support needed to conduct this study (www.aodapelegri.com).
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
1. Mazzotti E, Antonini Cappellini GC, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553-2557.
2. Gunnars B, Nygren P, Glimelius B, SBU-group. Swedish Council of Technology Assessment in Health Care. Assessment of quality of life during chemotherapy. Acta Oncol. 2001;40(2-3):175-184.
3. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015;1(8):1051-1059.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Menzel P, Dolan P, Richardson J, Olsen JA. The role of adaptation to disability and disease in health state valuation: a preliminary normative analysis. Soc Sci Med. 2002;55(12):2149-2158.
6. McTaggart-Cowan H, Tsuchiya A, O’Cathain A, Brazier J. Understanding the effect of disease adaptation information on general population values for hypothetical health states. Soc Sci Med. 2011;72(11): 1904-1912.
7. Ubel PA, Loewenstein G, Schwarz N, Smith D. (2005). Misimagining the unimaginable: the disability paradox and health care decision making. Health Psychol. 2005;24(4 Suppl):S57-62.
8. Brazier J, Akehurst R, Brennan A, et al. Should patients have a greater role in valuing health states? Appl Health Econ Health Policy. 2005;4(4):201-208.
9. Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Qual Life Res. 2003;12(6), 599-607.
10. Shabaruddin FH, Chen LC, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics. 2013;31(4):277-288.
11. Misset JL. Oxaliplatin in practice. Br J Cancer. 1998;77 Suppl 4:4-7.
12. EQ-5D website. About the EQ-5D-5L. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Last updated April 18, 2017. Accessed October 18, 2017.
13. Calhoun EA, Fishman DA, Lurain JR, Welshman EE, Bennett CL. A comparison of ovarian cancer treatments: analysis of utility assessments of ovarian cancer patients, at-risk population, general population, and physicians. Gynecol Oncol. 2004;93(1):164-169.
14. Havrilesky LJ, Broadwater G, Davis DM, et al. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216-220.
15. Best JH, Garrison LP, Hollingworth W, Ramsey SD, Veenstra DL. Preferences values associated with stage III colon cancer and adjuvant chemotherapy. Qual Life Res. 2010;19(3):391-400.
16. Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C. Patient preferences for chemotherapies used in breast cancer. Int J Womens Health. 2012;4:279-287.
17. Beusterien K, Grinspan J, Kuchuk I, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. The Oncologist 2014;19(2):127-134.
18. Kuchuk I, Bouganim N, Beusterien K, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat. 2013;142(1):101-107.
19. Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB. Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer. 2012;77(1):224-231.
20. Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive Iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015:438235.
21. Wong MK, Mohamed AF, Hauber AB, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139-1148.
22. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317-328.
23. Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother. 2013;14(6):757-766.
24. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882.
25. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337-343.
26. Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ.2003; 4(3):222-231.
27. Basch E, Deal AM, Dueck AC, et al. Survival results of a trial assessing patient-reported outcomes for symprom monitoring during routine cancer treatment. JAMA 2017. doi:10.1001/jama.2017.7156
Onodera’s Prognostic Nutritional Index in soft tissue sarcoma patients as a predictor of wound complications
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
Wound complications after pre- or post-operative radiation for soft tissue sarcomas are well established.1 The ability to predict who will have a wound complication remains difficult. Some studies have looked at risk factors such as smoking, and the preoperative nutritional status of patients has been identified as a risk factor for wound complication in patients with elective orthopedic surgical procedures.2 One validated method of measuring preoperative nutritional status in patients with gastrointestinal malignant tumors has been with Onodera’s Prognostic Nutritional Index (OPNI). It uses the patient’s preoperative albumin (g/dL) and absolute lymphocyte values (per mm3). The prognostic value of the OPNI has been demonstrated in patients with colorectal, esophageal, and gastric cancers, and has been shown to be prognostic for postoperative wound healing and overall prognosis.3-5 In this study, we investigate the significance of preoperative nutritional status, measured by OPNI, as a predictor of wound complications in patients treated with pre- or postoperative radiation for soft tissue sarcoma.
Methods
After receiving Institutional Review Board approval for the study, we conducted a retrospective review of consecutive patients treated during July 2012-April 2016 for a soft tissue sarcoma by the orthopedic oncology division at Cooper University Hospital in Camden, New Jersey. Inclusion criteria were patients with biopsy-proven soft tissue sarcoma, who were older than 18 years, had received pre- or postoperative radiation, and who had a recorded preoperative albumin and total lymphocyte count. A minimum follow-up of 3 months was required to assess for postoperative wound complications. Exclusion criteria included patients who had a bone sarcoma, had not received radiation therapy, or had a missing preoperative albumin or total lymphocyte count.
All of the surgeries were performed by 2 fellowshiptrained orthopedic oncologists. Patients received either pre- or postoperative radiation therapy by multiple radiation oncologists.
The OPNI was calculated based on the published formula OPNI = (10*albumin level [g/dL]) + (0.005*total lymphocyte count [per mm3]). The albumin level and total lymphocyte counts closest to the index operation were chosen.
Demographic information including gender, age at diagnosis, height, and weight were recorded. Data related to the patients’ pathologic diagnosis, stage at presentation, radiation therapy, and surgical resection were collected. A minor wound complication was defined as a wound problem that did not require operative intervention. Major wound complication was defined as a complication requiring operative intervention with or without flap reconstruction. Wound complications occurring within the 3-month postoperative period were considered.
Univariate and multiple variable analysis was performed. A P value <.05 was considered significant. A receiver operating curve as well as recursive partitioning was performed for OPNI and age to determine the best cut-off point to use in the analysis. The Sobel test was used to evaluate mediation. All statistical analysis was performed using SAS v9.4 and JMP10. (SAS Institute, Cary, NC).
Results
In all, 44 patients (28 men, 16 women) were included in the study. Their mean age was 61.2 years (range, 19-94). The average size of the tumors was 8.5 cm in greatest dimension (range, 1.2-27.4 cm), and all of the patients had nonmetastatic disease at the time of surgical resection; 37 patients had R0 resections, and 7 patients had a positive margin from an outside hospital, but obtained R0 resections on a subsequent resection (Table 1 and Table 2).
In all, 30 patients received preoperative radiation, 14 patients received postoperative radiation, 32 patients received external beam radiation, 8 received Cyberknife treatment, and information for 4 patients was not unavailable. Mean preoperative external beam radiation and Cyberknife dose was 4,931 Gy and 3,750 Gy, respectively. Mean postoperative external beam and Cyberknife radiation dose was 6,077 Gy and 4,000 Gy, respectively. When evaluating radiation dose delivered between those who had wound complications and those who did not, there was no significant difference (Table 3).
Of the total, 13 patients had a wound complication (30%). Ten patients had preoperative radiation, and 3 had postoperative radiation. Ten patients had major wound complications requiring a combined 27 surgeries. Three patients had minor wound complications, which resolved with conservative management. One patient had a major wound complication in the group that had an initial R1 resection.
The OPNI was calculated based on the aforementioned formula. When the univariate analysis was performed, only age and OPNI were statistically significant. Patients older than 72.6 years had a 6.8 times higher risk of a wound complication (P = .01; 95% confidence interval [CI], 1.6-28.7). When the OPNI value of 45.4 was used as the threshold, a patient with a preoperative OPNI value of <45.4 had a 7.5 times increased risk of developing a wound complication (P = .005; 95% CI, 1.8-31.0).
When the receiver operating curve and recursive partitioning was performed, an OPNI value of 45.4 showed a sensitivity of 62% and specificity of 82% in predicting wound complications (Figure 1).
When a multiple variable analysis was performed, OPNI and age were not statistically significant (P = .06 and P = .11, respectively). A test for mediation was performed, and the OPNI seemed to mediate the effect age has on wound complications, accounting for 36% of the total effect (Sobel test statistic, 1.79; P = .07).
Discussion
Wound complications after pre- and postoperative radiation for soft tissue sarcomas are well known. The best study to date to demonstrate that relationship was a randomized controlled trial performed in Canada, which showed that preoperative radiation resulted in 37% wound complications, compared with 17% for postoperative radiation.6 In that study, of the wound complications in both radiation types, more than 50%-60% required a secondary surgical procedure, designating it as a major wound complication. Other variables that have been shown to contribute to wound complications include being older than 40 years and/or having large tumors, diabetes, peripheral vascular disease, and begin a smoker.7-10
In our study, we applied OPNI to orthopedic oncology and showed that the patient’s age and preoperative nutritional status were significant predictors of developing a wound complication. An OPNI of <45.4 increased the chance of a wound complication by 7.5 times. Being older than 73 years increased the risk of a wound complication by 6.8 times. Most of these wound complications were major and required surgical intervention.
In general surgical oncology, the evaluation of nutritional status has had a significant impact on the care of patients, especially for those patients undergoing gastrointestinal surgery. The OPNI was initially designed to assess the nutritional and immunological statuses of patients undergoing gastrointestinal surgery.11 Preoperative OPNI has been shown to be a good predictor of postoperative complications and survival in patients with colorectal cancer, malignant mesothelioma, hepatocellular carcinoma and in patients who undergo total gastrectomy.12-15 Chen and colleagues evaluated the significance of OPNI in patients with colorectal cancer. They found an optimal cut-off value of 45. An OPNI value <45 has a sensitivity and specificity of 85% and 69%, respectively, in predicting 5-year overall survival.16 Hong and colleagues noted that an OPNI cut-off value of 52.6 as a predictor of overall survival.17
Poor preoperative nutritional status has been shown to have a negative impact on wound healing. In patients who underwent emergency laparotomy, a low OPNI had significantly higher rates of wound dehiscence and infection.18 This happens because protein deficiency leads to decreased wound tensile strength, decreased T-cell function, decreased phagocytic activity, which ultimately diminish the patient’s ability to heal and defend against wound infections.19-21
In soft tissue sarcoma patients, poor preoperative nutritional status is further compromised by radiation therapy to the wound. Gu and colleagues showed that radiation to wounds in mice showed early inhibition of the inflammatory phase, injury and inhibition of fibroblasts, and collagen formation, and then prolonged re-epithelialization.22 This “double hit” with radiation onto host tissue that is already nutritionally compromised could be an important cause of why wound complications occur at such high rates in our soft tissue sarcoma patients.
There are several limitations to this study. First, the study has a small sample size, which was a direct result of the number of patients who were excluded because an OPNI value could not be calculated for them. Second, we could not determine if the OPNI was more valuable in patients who underwent pre- or postoperative radiation. This study did not look at other nutritional indices such as prealbumin and vitamin levels. Third, the radiation was provided by different providers, so technique was variable, but the patients received nearly equivalent doses and variability in technique is likely limited. Fourth, we were not able to meaningfully analyze the role of chemotherapy in this patient population because there was a significant heterogeneity of patients receiving pre- and postoperative chemotherapy.
Our findings strongly suggest that a preoperative OPNI of <45.4 and being older than 73 years are strong predictors of patients who will experience a wound complication after radiation therapy for soft tissue sarcomas. This study has led us to start measuring preoperative albumin levels and assess complete metabolic panels. Our goal is to identify patients who are at high risk of wound complication and perform interventions to improve nutrition, then to study whether the interventions help lower the rates of wound complications.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
1. Ormsby MV, Hilaris BS, Nori D, Brennan MF. Wound complications of adjuvant radiation therapy in patients with soft-tissue sarcomas. Ann Surg. 1989;210(1):93-99.
2. Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients: relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321-325.
3. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28(4):396-400.
4. Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42(6):532-535.
5. Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40(5):440-443.
6. O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359(9325):2235-2241.
7. Peat BG, Bell RS, Davis A, et al. Wound-healing complications after soft-tissue sarcoma surgery. Plast Reconstr Surg. 1994;93(5):980-987.
8. Kunisada T, Ngan SY, Powell G, Choong PF. Wound complications following pre-operative radiotherapy for soft tissue sarcoma. Eur J Surg Oncol. 2002;28(1):75-79.
9. Saddegh MK, Bauer HC. Wound complication in surgery of soft tissue sarcoma: analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res. 1993;289:247-253.
10. Tseng JF, Ballo MT, Langstein HN, et al. The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol. 2006;13(9):1209-1215.
11. Smale BF, Mullen JL, Buzby GP, Rosato EF. The efficacy of nutritional assessment and support in cancer surgery. Cancer. 1981;47(10):2375-2381.
12. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688-2692.
13. Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
14. Pinato DJ, North BV, Sharma R. A novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI). Brit J Cancer. 2012;106(8):1439-1445.
15. Yao ZH, Tian GY, Wan YY, et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J Cancer Res Clin Oncol. 2013;139(12):2117-2123.
16. Jian-Hui C, Iskandar EA, Cai Sh I, et al. Significance of Onodera’s prognostic nutritional index in patients with colorectal cancer: a large cohort study in a single Chinese institution. Tumour Biol. 2016;37(3):3277-3283.
17. Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-9337.
18. Mohil RS, Agarwal A, Singh N, Arora J, Bhatnagar D. Does nutritional status play a role in patients undergoing emergency laparotomy? E Spen Eur E J Clin Nutr Metab. 2008;3(5):e226-e231.
19. Kay SP, Moreland JR, Schmitter E. Nutritional status and wound healing in lower extremity amputations. Clin Orthop Relat Res. 1987;(217):253-256.
20. Dickhaut SC, DeLee JC, Page CP. Nutritional status: importance in predicting wound-healing after amputation. J Bone Joint Surg Am. 1984;66(1):71-75.
21. Casey J, Flinn WR, Yao JS, Fahey V, Pawlowski J, Bergan JJ. Correlation of immune and nutritional status with wound complications in patients undergoing vascular operations. Surgery. 1983;93(6):822-827.
22. Gu Q, Wang D, Cui C, Gao Y, Xia G, Cui X. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol. 1998;17(2):117-123.
Bone remodeling associated with CTLA-4 inhibition: an unreported side effect
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Assessing a multidisciplinary survivorship program in a group of predominantly Hispanic women with breast cancer
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
Breast cancer survivors comprise the most prevalent cancer survivor population in the United States.1 The number of breast cancer survivors is increasing because of early detection and diagnosis, and advances in treatment have resulted in increased life expectancy. Therefore, greater attention is needed to improve the long-term quality of life of these survivors and to help them re-adjust to normal life. For many women, although the medical treatment may have been completed, the recovery process may have not.2 The prevalence of long-term mental and physical illness is significant among many breast cancer survivors. Long-term mental consequences may include memory problems, anxiety, depression, and fear of recurrence3, and long-term physical consequences may include pain, fatigue, and lymphedema, among others.4
El Paso, Texas, is the fourth most populous city in Texas and has a Hispanic majority. This provides an opportunity to conduct clinical research targeting participants of Hispanic descent. Several studies have noted the influence of race/ethnicity on the psychosocial function of breast cancer survivors.5,6 We have previously reported that Hispanic breast cancer survivors might experience decreased mental and physical health-related quality of life (QoL) which limit their normal social functioning.6Other studies have similarly reported poor outcomes of breast cancer survivors and higher rates of fatigue and depression among Hispanic patients.7 However, there is a paucity of research addressing specific interventions needed to improve these outcomes and provide better QoL for breast cancer survivors.8,9 In addition, a few survivorship care interventions have focused on minorities. We sought to assess whether a multidisciplinary cancer survivorship program in a primarily Hispanic populated area would lead to improved QoL and reduce anxiety and depressive symptoms among breast cancer survivors.
Methods
After obtaining Institutional Review Board approval, we recruited consecutive patients who were treated at our institution during October 2013-October 2014 and obtained informed consent from them. The participants were within the first 5 years after diagnosis with stages I-III breast cancer and had completed surgery, chemotherapy, and/or radiation therapy. We sought to determine whether breast cancer survivors would benefit from this intervention as determined by improvement of performance at 12 months compared with baseline based on the following self-reported validated questionnaires: Patient Health Questionnaire-9 (PHQ-9) for depression; General Anxiety Disorder-7 (GAD-7); and Short-Form Health Survey-36 (SF-36, version 2) for patient quality of life. The participants were enrolled in a comprehensive survivorship program staffed by an oncologist, an oncology nurse practitioner, a nutritionist, and a certified clinical psychologist who had trained in mindfulness-based stress reduction (MBSR).
Interventions
The participants received a one-on-one individual psychological consultation visit every 3 months for 20-45 minutes during which the psychologist addressed each patient’s emotional and psychological issues in depth, discussed relaxation techniques, and provided psychosocial counselling. In addition, all participants were asked to attend an 8-week-course (in Spanish or English) using MBSR, an interventional program in which participants receive training to promote reduction of stress by self-regulating mindfulness practice.3,10 Our institution’s MBSR program consists of a weekly 2-hour class for 8 sessions or more. The program is provided 3 times a year, in English and Spanish. It includes the following components:
- Learning various mindfulness meditation techniques (eg, body scans, awareness of breathing, sitting/walking meditations);
- Practicing the mindfulness techniques in class; and
- Practicing techniques at home through audiorecordings of mindfulness meditation exercises and daily diary writing.
Participants were provided with a workbook on MBSR in their preferred language.11 In addition to the psychological component, they were also provided with oncologic evaluations by an oncology nurse practitioner. The nurse practitioner met with participants every 3 months and provided each one with a personalized summary of all the treatments received and routine oncology follow-up care in consultation with the patients’ regular oncologists. This care also addresses the long-term sequelae of treatment, including arthritis and osteoporosis, referrals to receive screening for other cancers (eg, cervical and colon cancer), and genetic counselling as appropriate. In addition, a nutritionist provided general dietary advice in individual and group sessions every 3 months.
The self-administered questionnaires, PHQ-9, GAD-7, and SF-36, were completed at baseline, and every 3 months for 12 months. The scores were reviewed by the psychologist and the oncologist. The PHQ-9 was used to initially screen survivors for depression and monitor their improvement after the intervention. The PHQ-9 is a reliable and validated self-administered depression module.12 The PQH-9 exclusively focuses on the 9 diagnostic criteria for DSM-IV depression disorder and it can be used as a useful measure for monitoring outcomes of depression therapy. A score of 5-14 suggests mild-moderate depression, and a score of >15 suggests severe depression
The survivors were screened for anxiety using the GAD-7, a brief 7-item self-report scale to identify probable cases of anxiety disorder that has been shown to be an efficient tool for screening and assessing the severity of anxiety.13 For GAD-7, a score of 5 or higher is suggestive of anxiety. Scores of 5, 10, and 15 represent cut-off points for mild, moderate, and severe anxiety, respectively.
Survivor QoL was evaluated using the SF-36 questionnaire, a multipurpose survey containing 36 questions. It ranges from 0-100 and a score that is <50.0 is considered low. The lower the score, the worse the mental or physical function.14 The SF-36 yields a patient profile of 8 health domains – vitality, physical functioning, bodily pain, general health perceptions, physical, emotional, and social role functioning; and mental health.15,16 A score of 50.0 on either the Physical Component Summary (PCS – vitality, physical functioning, bodily pain, general health perceptions, physical role functioning) or Mental Component Summary (MCS – emotional and social role functioning, and mental health) is consistent with the US norm.
Statistical analysis
In this study, the primary objective was to use the MBSR survivorship program to improve the survivors’ outcomes at 12 months compared with baseline using the following measures: PHQ-9 for depression, GAD-7 for anxiety, and SF-36 for QoL using the PCS and MCS. Quantitative data were described using the mean and standard deviation, and categorical data were described using frequency and percentage. The outcome measures were compared between patients who completed 12-month follow-up and those who did not, using unpaired t test. The change in outcome measures at 12 months from baseline was evaluated using paired t test. The effect of intervention was summarized using relative percentage change. The “dose” of the intervention was categorized the number of MBSR sessions – ≤4 sessions, 5-7 sessions, or ≤8 sessions. The change in outcome measures were compared among three groups using 1-way analysis of variance (ANOVA) followed by post hoc multiple comparison using the Bonferroni adjustment. In addition, the effect of intervention on each outcome was evaluated by important baseline characteristics of patients. In each subgroup, the changes were compared with baseline measures using the paired t test, whereas changes in outcome between groups were compared using the unpaired t test. Statistical analyses were conducted using SAS 9.3. P-values less than 5% were considered to be significant.
Results
A total of 94 survivors of breast cancer were included in this study and 60 (63.8%) completed the 12 months of follow-up. The average age of the participants was 54.4 years (SD, 8.7), and 90.4% were Hispanic (Table 1). Tumor characteristics were as follows: invasive ductal carcinoma (84.04%), estrogen receptor–positive (ER-, 71.28%), progesterone receptor–positive (PR-, 58.51%), and HER2-neu–positive (20%). In regard to therapy received, 48% of the participants had received anthracycline- and taxane-based adjuvant chemotherapy and 23%, nonanthracycline-based chemotherapy; 71% had received anti-estrogen (hormonal) therapy and 80%, radiation therapy. In regard to surgery, half of the participants had a lumpectomy, and half, a mastectomy. The trends in the outcome measures over the follow-up period are show in the Figure 1.
The effect of survivorship program intervention on SF-36 (PCS and MCS), anxiety (GAD-7), and (PHQ-9) at 12 months are shown in Table 2, which also includes the 12-month effects on the body-mass index (BMI). The P-values correspond to the comparison of mean change in scores between baseline and 12-month follow-up. Significant improvement from baseline was observed for PHQ-9 (P = .0031) and GAD-7 (P = .0027). There was a significant trend toward improvement (14%) relative to baseline in the SF-36 MCS at 12 months (P = .097). Although the SF-36 PCS improved numerically, it did not reach to a statistical significance level (P = .896). The BMI at 12 months was found to be statistically significantly increased compared with baseline (P = .0007).
The effect of the number of MBSR sessions attended on the outcome measures is summarized in Table 3. There were significant improvements in the 12-month MCS scores for patients who completed 5-7 sessions of MBSR or ≥8 sessions, compared with patients who completed ≤4 sessions of MBSR. There was an improvement observed in PCS scores only among patients who received at least 8 sessions of MBSR. There was a marked improvement observed in GAD-7 and PHQ-9 among patients who received ≥8 sessions. There was no statistically significant change in the GAD-7 or PHQ-9 scores between patients who received ≤4 sessions and 5-7 sessions. No significant association was obtained between number of MBSR sessions attended and BMI.
The effect of survivorship program intervention on all outcomes according to important baseline cofactors is shown in Table 4. As such, there were no significant differences in changes in the outcome measures after intervention according to any considered baseline characteristics. However, the effect of survivorship program intervention was more pronounced in patients who were ≥3 years away from their initial diagnosis and who had attended a minimum of 80% of the 3-monthly visits and received a minimum of 8 MBSR sessions.
The mean baseline PCS and MCS scores of the SF-36 were 43.7 and 45.8, respectively, indicating that the participants’ scores were significantly less than half the standard deviation below the US norm (50.0; SD, 10). The SF-36 health-related QoL categories showed that, on an average, scores improved by more than 4 units for emotional and physical role functions, vitality, and mental health compared with baseline. In addition, scores improved by about 2 units for general health and social functioning compared with baseline data. In all, 65% of survivors had difficulty preforming work at baseline, but that dropped to 55% after enrollment in the program; and 60% had originally reduced the amount of time spent on work, but that increased to 50% after the intervention. Also of note is that 70% of survivors reported accomplishing less than they would like to have (role physical) before the intervention, but that was reduced to 57% after the intervention. Similarly, 77% of survivors felt worn out at baseline, compared with 65% at the 12-month follow-up; and 88% felt tired at baseline, but that percentage was reduced to 68% after the intervention. Before the intervention, 60% of the participants reported that they had been very nervous, and 45% said they had been so down in the dumps that nothing could cheer them up, but those percentages were reduced to 43% and 32%, respectively, after intervention. Before intervention, 63% of the women said they felt depressed and that was reduced to 50% after the intervention.
Discussion
In this study, we showed that a group of predominately Hispanic breast cancer survivors benefited from participating in a multidisciplinary cancer survivorship program that emphasized in-depth psychological care and MBSR. They also benefited from an education effort that included providing survivors with personalized summaries of their treatment and oncology survivorship care, addressing potential long-term side effects of treatment, referral for genetic counselling and screening for other cancers as appropriate, as well dietary advice. We found significant improvement compared with baseline in both mental and physical determinants of the patient-reported outcomes, including anxiety (GAD-7), depression (PHQ-9), and HR-QoL (PCS) and (MCS). Survivors demonstrated significant improvement on the MCS and PHQ-9 if they attended 5 or more sessions of the 8-week MBSR course, and attending 8 sessions was associated with significant improvement in GAD-7 and PCS. This might suggest that survivors who are more motivated do benefit the most from such program.
To our knowledge, this study is the first to address the benefit of the MBSR intervention in Hispanic breast cancer survivors. In a randomized controlled trial that included breast cancer survivors with stages 0-III breast cancer who completed surgery, adjunctive radiation, and/or chemotherapy, MBSR was shown to reduce the symptoms of depression and anxiety and increase energy and physical functioning compared with participants who received “usual care”.3 Furthermore, Bower and colleagues have reported improvements in sleep, fatigue, and pro-inflammatory signaling in younger survivors of breast cancer.17 A similar standardized MBSR program was tested on Danish women who had been treated for stage I-III breast cancer18 and the results showed reduced levels of anxiety and depression at the 12-month follow-up. A similar study by Hoffman and colleagues19 reported improved mood, breast- and endocrine-related quality of life, and well-being with MBSR compared with standard care in women with stage 0-III breast cancer.
Several theories have been suggested to explain how MBSR reduces symptoms of depression, anxiety, and fear of recurrence in breast cancer survivors, one of which is that it provides supportive interaction between group members to practice meditation and apply mindfulness in daily situations.3 In addition, evidence is beginning to emerge that stress-reducing interventions such as MBSR may improve telomere length (TL) and telomerase activity (TA), the markers for cellular aging, psychological stress, and disease risk.20-24 Lengacher and colleagues conducted a randomized controlled study to investigate the effects of MBSR on TL and TA in women with breast cancer, and suggested that MBSR increases telomere length and telomerase activity.25 The 142 patients with stages 0-III breast cancer had completed adjuvant treatment with radiation and/or chemotherapy at least 2 weeks before enrollment and within 2 years of completion of treatment with lumpectomy and/or mastectomy. They were randomly assigned to either a 6-week MBSR for breast cancer program or usual care.25 Assessments of TA and TL were obtained along with psychological measurements at baseline, 6 weeks, and 12 weeks after the patients had completed the MBSR program. The mean age of the participants was 55.3 years; 72% were non-Hispanic white; 78% had stage I or II cancer; and 36% received both chemotherapy and radiation. In analyses adjusted for baseline TA and psychological status, TA increased steadily by about 17% over 12 weeks in the MBSR group, compared with about 3% (P < .01) in the control group. No difference was observed for TL (P = .92). The authors concluded that the data provide preliminary evidence that MBSR increases TA in peripheral blood mononuclear cells from breast cancer patients and have implications for understanding how MBSR may extend cell longevity at the cellular level.
In another study among healthy volunteers who were randomly assigned to a 3-month meditation retreat or a control group, the 30 participants in the meditation group had higher TA compared with controls.20 In a nonrandomized study among prostate cancer patients, TA increased and psychological stress decreased following a stress-reducing, lifestyle-modification program.21 The results of another intervention study among overweight women showed improvement in distress, eating behavior, and metabolic health in women participating in a MBSR program, all of which correlated with increases in TA.22 Most recently, researchers explored the impact on TA of a Kirtan Kriya yogic meditation intervention compared with exposure to relaxing music in 39 dementia family caregivers. The yogic-meditation intervention group had a 43% increase in TA after the 8-week intervention period compared with 3.7% the music group (P < .05).23 Finally, among 22 patients with cervical cancer who were randomized to a psychosocial telephone counseling intervention,24 investigators found a significant association between increased TL and changes in psychological distress.20 Findings from other studies have assessed interventions to improve outcome of breast cancer survivors, such as the Taking CHARGE self-management intervention that is designed to facilitate the transition to survivorship after breast cancer treatment.8 Another intervention using home-based physical activity was shown in a randomized controlled trial to improve self-reported physical activity, body-mass index, and health-related QoL.9 Findings from another study suggested that a combined exercise and psychological counselling program might improve QoL more than a single entity intervention.26 As noted previously, these studies did not focus on minority breast cancer survivors’ population, and it is not clear if they are generalizable to Hispanics.
In addition to the MBSR component, our program has also included one-on-one psychological assessment for long-term treatment complications and provided participants with appropriate care and follow-up plans, adding the benefits of self-awareness and self-attention for the survivors, which can effectively reduce the fear of recurrence.3 Furthermore, we included dietary consults based on general cancer survivor guidelines recommending a high fruit and vegetable diet that is low in fat and sugar.27 Healthier dietary lifestyle has been reported to improve breast cancer prognosis, metabolic disease, and cardiovascular outcomes among Hispanic breast cancer survivors.28
Our study has some limitations, including a relatively small sample size. It did not include an exercise program, which would have been helpful in addressing the issue of overweight and obesity we encountered in the most of the Hispanic breast cancer survivors (baseline average BMI, 31.32 kg/m2; obesity range, >30 kg/m2). Because of the small sample size and nonrandomized design of the study, it is hard to evaluate the confounding effect of time on intervention effect. However, a subgroup analysis by MBSR number of sessions showed that the survivors who completed the full course of MBSR sessions (8 sessions) achieved superior benefit, compared with those who did not complete the full course, which indicates that the intervention did weigh in regardless of time. Despite these limitations, the participants in this interventional program showed improved outcomes, including less anxiety and depression and improved MCS score of the SF-36. A larger and longer follow-up prospective, randomized study is needed to validate the findings of this study. Implementing cancer survivorship as an integral component of cancer care during and after treatment is essential to improve the quality of life of cancer survivors and empower them in their transition from cancer treatment to survivorship.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
1. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012 [published correction in CA Cancer J Clin. 2012;62(5):348].CA Cancer J Clin. 2012;62(4):220-241.
2. Williams F, Jeanetta SC. Lived experiences of breast cancer survivors after diagnosis, treatment and beyond: qualitative study. Health Expect. 2016;19(3):631-642.
3. Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261-1272.
4. Feiten S, Dünnebacke J, Friesenhahn V, et al. Follow-up reality for breast cancer patients - standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76(5):557-563.
5. Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007;106(1):85-95.
6. Nahleh ZA, Dwivedi A, Khang T, et al. Decreased health related quality of life among hispanic breast cancer survivors. http://medcraveonline.com/MOJWH/MOJWH-01-00016.php. Published January 28, 2016. Accessed July 25, 2017.
7. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32(2):250-254.
8. Cimprich B, Janz NK, Northouse L, Wren PA, Given B, Given CW. Taking CHARGE: a self-management program for women following breast cancer treatment. Psychooncology. 2005;14(9):704-717.
9. Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2258-5. Published 2016. Accessed July 25, 2017.
10. Huang J, Shi L. The effectiveness of mindfulness-based stress reduction (MBSR) for survivors of breast cancer: study protocol for a randomized controlled trial. Trials. 2016;17(1):209.
11. Stahl B and Goldstein E, A mindfulness-based stress reduction workbook. 2010: New Harbinger Publications.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613.
13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
14. Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):AS264-279.
15. Gandek B, Sinclair SJ, Kosinski M, Ware JE Jr. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev. 2004;25(4):5-25.
16. Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF-36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307(6901):448-449.
17. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121(8):1231-1240.
18. Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49(6):1365-1373.
19. Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335-1342.
20. Jacobs TL, Epel ES, Lin J, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664-681.
21. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048-1057.
22. Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917-928.
23. Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57-65.
24. Biegler KA, Anderson AK, Wenzel LB, Osann K, Nelson EL. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila). 2012;5(10):1173-1182.
25. Lengacher CA, Reich RR, Kip KE. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol Res Nurs. 2014;16(4):438-447.
26. Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. J Support Oncol. 2012;10(5):188-194.
27. Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
28. Greenlee H, Gaffney AO, Aycinena AC, et al. Cocinar para su salud!: randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709-723.e3.
Perceived financial hardship among patients with advanced cancer
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
The American Cancer Society has identified a disparity in cancer death rates, noting that persons with lower socioeconomic status have higher rates of mortality.1 This is attributed to many factors, but it is largely owing to the higher burden of disease among lower-income individuals.1 A component of this disease burden is measured by assessing the patient-reported outcome of cancer-related distress. The National Comprehensive Cancer Network (NCCN) Distress Management Guidelines have defined distress as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social and/or spiritual nature that may interfere with the ability to cope with cancer, its physical symptoms and its treatment.”2
Financial hardship related to cancer diagnosis and treatment is increasingly being recognized as an important component of disease burden and distress. The advancements in costly cancer treatments have produced burdensome direct medical costs as well as numerous indirect costs that contribute to perceived financial hardship.3,4 These indirect costs include nonmedical expenses such as increased transportation needs or childcare, loss of earnings, or loss of household income due to caregiving needs.3 Moreover, indirect costs are often managed by patients and families through their use of savings, borrowing, reducing leisure activities, and selling possessions.3 Even though efforts to increase health coverage, such as the Affordable Care Act, have reduced the rates of individuals who are uninsured, persons with cancer who have insurance also face challenges because they cannot afford copays, monthly premiums, deductibles, and other high out-of-pocket expenses related to cancer treatment that are not covered by their insurance such as out-of-network services or providers.5-7
Thus, financial hardship may have an impact on several areas of a patient’s life and well-being, but the effects are commonly undetected.8-10 Research has established that financial strain can influence treatment choices and adherence to therapy.11 Furthermore, the effects of financial strain have been identified across the cancer care continuum, from diagnosis through survivorship, suggesting a bidirectional relationship between financial strain and well-being.11 Financial strain may reduce patient quality of life and worsen symptom burden because of the patient’s inability to access needed care, poor social supports, and/or increased stress.11-12 These worsening outcomes may also increase the use of financial reserves and affect their ability to work.7,11 Financial difficulties may also be associated with anxiety and depression, leading to worse quality of life and greater distress and symptom burden.12 Identifying groups at high risk for financial strain is crucial to ensure that resources are available to assist these populations.13 This burden can be even more pronounced in minority and underserved patients with cancer.7 Patients with advanced cancer are especially vulnerable to the burden of increased costs because of the use of expensive targeted therapies; their improved survival, which extends the time of expenditure; and increased use of financial reserves.9 Financial hardship in patients with advanced cancer is not well understood or characterized,9 which is why this study aimed to better quantify distress in advanced stage cancers by describing :
▪ A cohort of patients with advanced cancer and their levels of quality of life, symptom distress, cancer-related distress and perceived financial hardship;
▪ The relationship between perceived financial hardship, quality of life, symptom distress and overall cancer-related distress; and
▪ Quality of life, symptom distress, and overall cancer-related distress according to level of perceived financial hardship.
Methods
This study is a cross-sectional, descriptive, comparative study of distress, including perceived financial hardship, among patients with advanced cancer who were receiving palliative care treatment in two outpatient medical oncology clinics in Western Pennsylvania. The data were collected during May 2013-November 2014. The study protocol was approved by the Institutional Review Board at the University of Pittsburgh. Eligible participants had to be 18 years or older and have an advanced solid tumor of any kind, with a prognosis of 1 year or less confirmed by a physician or clinic nurse practitioner/physician assistant, and be able to read and understand English at the fourth-grade level. The sample was recruited from two clinics at the University of Pittsburgh Cancer Institute, a National Cancer Institute-designated Comprehensive Cancer Program.
Measurements
Sociodemographic factors. These were measured using an investigator-derived Sociodemographic Questionnaire, a 12-item form that includes variables such as age, race, marital status, cancer type, religion and spirituality, employment status, years of education, health insurance status, and income level.
Cancer-related distress. The NCCN Distress Thermometer is a self-report visual analog scale (0, no distress; 10, great distress) formed in the shape of a thermometer combined with a problem list that is often used in outpatient cancer settings for reporting of cancer-related distress.14-16 The sensitivity, specificity and convergent validity with the Brief Symptom Inventory and the Hospital Anxiety and Depression Scale have been established and appropriate cut-off score of the distress thermometer identified.14-16 A score of 4 or above indicates a clinically significant level of distress.14-16
Symptom distress. The McCorkle Symptom Distress Scale was developed in 1977 based on interviews that focused on the symptom experiences of patients. Psychometric testing among patients with cancer using the modified Symptom Distress Scale revealed high reliability (Cronbach alpha, 0.97).17 The instrument is a 13-item Likert scale (1-5) assessing the severity of distress experienced by a symptom. Total scores range from 13 to 65, where a higher score indicates greater distress. Moderate distress is indicated with a score of 25-33, and a score above 33 indicates severe distress, identifying the need for immediate intervention.17
Quality of life and spiritual well-being. The Functional Assessment of Cancer Therapy (FACT-G) is used to assess general cancer-related quality of life. It has four subscales: physical, emotional, social and family, and functional well-being, with a total score that ranges from 0-112, where higher scores show higher quality of life. The Spiritual Distress Well-Being questionnaire was used alongside the valid FACT-G assessment.18,19 The Spiritual Well-Being Short Form was developed with an ethnically diverse population and adds 12 items to the FACT-G. The items do not necessarily assume a faith in God, allowing a wide flexibility in application and tapping into issues such as faith, meaning, and finding peace and comfort despite advanced illness. Higher scores on the Spiritual Well-Being subscore (range, 0-48) are correlated with higher scores of quality of life. The possible scores for the combined FACT-G and Spiritual Well-Being assessment range from 0-160, with higher scores showing higher quality of life.
Economic hardship. Perceived financial hardship was measured using Barrera and colleagues’ Psychological Sense of Economic Hardship Scale. 20 The scale consists of 20-items broken down into 4 subscales: financial strain, inability to make ends meet, not enough money for necessities, and economic adjustments.20 Economic adjustments in the 3 months before administration of the questionnaire were assessed with 9 Yes or No items, such as added another job, received government assistance, or sold possessions to increase income. The subscale of not enough money for necessities was assessed with seven 5-point scale items in which respondents noted whether they felt they had enough money for housing, clothing, home furnishings, and a car over the previous 3 months. Inability to make ends meet included two 5-point scale items that assessed the difficulty in meeting financial demands in the previous 3 months. Financial strain consisted of two 5-point scale items concerned with expecting financial hardships in the coming 3 months. Scores can range from 20-73, with a higher score indicating worse economic hardship.
Data collection and analysis
In-person data collection occurred in the clinical waiting area before the clinician visit or in the treatment room with the patient using a consecutive, convenience sample. The nursing staff checked the clinic lists daily for possible patient participants. Patients with metastatic cancer were identified and then approached for consent. After we had received the patient’s consent, the administration of the instruments took about 20 minutes to complete. The data were then entered and verified in REDCap (Research Electronic Data Capture), which is hosted at the University of Pittsburgh.21The levels of symptom distress, quality of life, perceived financial hardship, and cancer-related distress were described through continuously measured variables. Descriptive statistics, measures of central tendency (mean and median), and dispersion (standard deviation and range), were obtained for the subscales and total scores. Correlation analysis was used to describe the relationship between perceived financial hardship and quality of life, symptom distress, and cancer-related distress. These primary outcome variables were further explored according to the level of dichotomized perceived financial hardship using mean score as the cut point. Independent sample t tests were used to compare patients experiencing high perceived financial hardship with those experiencing low perceived financial hardship.
Results
In all, 100 patients participated in the study. Any missing data points were replaced with the mean score for that variable, although this was minimal in this study. Most of the participants were women (67%), and the average age of the participants was 63.43 years (SD, 13.05; Table 1). Of the total number of participants, 73% were white, 26% were black, and 1% were Asian. Most of the participants were either retired and not working (39%) or disabled or unable to work (34%). Almost all of the participants had some form of insurance, with 99% having either private or public health insurance. A variety of cancer types were represented in this patient population, with higher percentages of breast (25%), gynecologic (10%), lung (19%), and colon/rectal cancer (15%). Of the total number of participants, 35% had annual household incomes below $20,000, and 50% had annual household incomes of more than $20,000. On average, participants had 13.48 years (SD, 2.78) of formal education.
Descriptive statistics for the primary outcome variables can be found in Table 2. The average score for cancer-related distress based on the NCCN Distress Thermometer tool was 4.16 (SD, 3.26). The average score for the McCorkle Symptom Distress measurement was 25.45 (SD, 9.34). For quality of life, the average FACT-G total score was 73.77 (SD, 19.40). Of the FACT-G subscale average scores, physical well-being was 17.35 (SD, 7.50), social/family well-being 24.21 (SD, 5.25), emotional well-being 16.34 (SD, 5.42), and functional well-being 15.87 (SD, 6.78). Participants’ average score for the spiritual well-being measure was 35.20 (SD, 9.25) and the combined FACT-G and spiritual well-being average score was 108.97 (SD, 26.07). The total average score for perceived financial hardship was 35.70 (SD, 13.87), with subscale average scores of 3.44 (SD, 2.36) for financial strain, 5.73 (SD, 1.91) for inability to make ends meet, 16.43 (SD, 8.92) for not enough money for necessities, and 10.63 (SD, 2.70) for economic adjustments.
We conducted a bivariate correlation analysis to assess the relationship between perceived financial hardship and three other primary outcome variables (Table 3). These analyses showed significant low to moderate correlations with overall cancer-related distress (r, 0.439; P < .001), symptom distress (r, 0.409; P < .001) and overall quality of life scores (FACT-G and spiritual well-being combined score: r, -0.323; P < .001).
Forty-three participants reporting high perceived financial hardship experienced worse quality of life overall (FACT-G and spiritual well-being; P = .002), worse FACT-G total scores (P < .001), worse physical well-being (P < .001), worse social/family well-being (P = .029), worse emotional well-being, and no significant difference for functional (P = .082) or spiritual well-being (P = .453), compared with those with lower economic hardship. In overall cancer-related distress, participants with higher perceived financial hardship reported higher levels of cancer-related distress (P < .001) than those with lower perceived financial hardship. For those participants reporting higher perceived financial hardship there was also worse symptom distress (P < .001), compared with those with lower economic hardship (Table 4).
Discussion
Overall, this report provides data to illuminate our understanding of disparities in well-being that may be present in patients with advanced cancer. Our analysis found that patients with advanced cancer who have higher perceived financial hardship have significantly higher overall cancer-related distress, symptom distress, and poorer overall quality of life. In this study’s population of patients with advanced cancer, the most notable areas of economic hardship identified by participants were: not having enough money for necessities in the 3 months before the survey and the inability to make ends meet during the same time span, with difficulty paying bills and not having enough money left at the end of the month being most noteworthy among this study’s patient population. Financial strain and making economic adjustment were not as notable in the category of perceived financial hardship.
In regard to not having enough money, participants most commonly cited not being able to afford everyday necessities such as food, clothing, medical care, or a home, as well as leisure and recreational activities. These findings are further supported with the positive, moderate associations between perceived financial hardship and symptom distress and overall cancer-related distress found in this cohort of patients with advanced cancer and the negative, moderately associated relationship between perceived financial hardship and overall quality of life in this study’s sample.
Although these findings have been confirmed in the literature on cancer-related distress, our findings add to our knowledge on both economic and cancer-related distress exclusively in patients with advanced cancer.9,22 The broader cancer-related distress literature has also found an association between being younger and having a lower household income as risk factors for increased financial hardship; however, the perception of financial strain and magnitude was a more significant predictor of quality of life and perception of overall well-being.6,8-9,12,22-23 Furthermore, patients with cancer who noted having higher financial distress typically reported decreased satisfaction with cancer care which also influenced their adherence to treatment and quality of life.24
Our work now adds the important element of perceived financial hardship to the advanced cancer-related distress puzzle. We should consider integrating a financial distress assessment into routine cancer care, particularly with patients and families with advanced cancer, to proactively and routinely assess and intervene with available distress mitigating resources. Therefore, understanding the patients most likely to experience financial distress will help personalize supportive therapy.
This study’s results as well as the existing literature describing financial distress support the use of comprehensive screening instruments to capture elements of financial burden beyond out-of-pocket costs.8,25 This screening is particularly relevant because we are increasingly recognizing that gross annual household income does not always reflect financial hardship or distress. The instrument we used for this analysis, the Psychological Sense of Economic Hardship, provides a broad view of financial toxicity including the specific components of financial strain, the inability to make ends meet, not having enough money for necessities, and economic adjustments experienced by patients with advanced cancer.20 Another measure to evaluate financial toxicity among patients with cancer includes the Comprehensive Score for Financial Toxicity (COST), which is a widely used patient-reported outcome measure. It was developed with input from both patients and oncology experts.25 Use of a financial toxicity assessment tool adds to our understanding of the economic financial burden experienced by patients with cancer, specifically those with advanced cancer.
Tucker-Seeley and Yabroff have identified several areas in which the research agenda for financial toxicity should focus, including: documentation of the socioeconomic context among patients across all areas of the cancer care continuum, further identification and characterization of at risk populations to address health disparities, and the inclusion of cost discussions in the health care context.26 Furthermore, research is needed to identify key areas to target for interventions addressing financial toxicity, such as addressing lack of financial resources to cover the cost of cancer care, focusing on managing or preventing the distress that results from a lack of financial resources, or addressing coping behaviors used by families to manage the financial burden of cancer care.26 Although cost discussions between health care providers and patients have been identified as important in reducing the financial burden of cancer care, the content, timing, and goals of those discussions still need to be better articulated for different patient populations, including patients with advanced cancer.3,27-28 In addition, resources such as social workers, patient navigators, or financial counselors have been identified as effective in assisting patients with financial planning and accessing community resources to address financial burden and assistance.4
Design considerations
This study has limitations that need to be noted. Its cross-sectional design does not allow for the analysis of causal inferences. In addition, certain groups were underrepresented in this study’s sample, including uninsured patients, men, and some minority groups, which may have underestimated the amount of financial burden experienced by patients with advanced cancer. The lack of representativeness of uninsured individuals may be a result of the eligibility of persons with advanced cancer for Medicaid. However, a strength of this study is its ability to increase the representativeness of African American/black patients in the study of advanced cancer and financial hardship. In our study, just over a quarter of the participants (26 of 100; 26%) were black/African American, compared with the US Census Bureau’s national census level of 13.3% and 13.4% in Allegheny County, Pennsylvania .29
The lack of employed participants in this study could be because many were not able to work because of the advanced stage of their disease. The low level of partnered status is a limitation, although one study site was a low-income hospital where one generally tends to see higher levels of unpartnered status. This study did not control for demographic information such as gender or age, thus, the relationships between the primary outcome variables and financial hardship may be overestimated. Moreover, this analysis of financial distress is limited to the context of the United States due to our lack of universal health care and unique payment system. Although we included only patients who were in the palliative phase of cancer treatment, no medical record review was conducted to determine previous cancer history and treatments, which might have provided more insight into other financial loss or cost of cancer treatment. Furthermore, we note that it can be difficult to prognosticate with accuracy and identify that some patients with advanced cancer may have been excluded from the study due to the inclusion criteria of less than 1 year of survival.
Conclusion
Perceived financial hardship is an important assessment of the burden placed on patients due to the cost of disease; and is a good start in assessing indirect costs that patients take on when coping with advanced stages of cancer and can shed light on an aspect of distress experienced by this patient population that is not commonly addressed. Subjective measures of perceived financial hardship complement objective measures that are commonly indicative of economic resources and can further our understanding of the impact of financial distress experienced by patients with cancer. Further study of financial impacts of advanced cancer as well as predictors of financial distress are essential to the early identification of financial hardship and the development of interventions to support those at high risk or experiencing financial distress.
Acknowledgments
The authors acknowledge the patients and staff at the UPMC Mercy Cancer Center in Pittsburgh, Pennsylvania, who made this study possible, and Peggy Tate for her role in data collection. They also recognize the support of the Robert Wood Johnson Foundation through the Future Nursing Scholars program. They would also like to acknowledge that permission was granted for the use of the Psychological Sense of Economic Hardship study instrument.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf. Atlanta, GA: American Cancer Society. 2015. Accessed January 16, 2016.
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology distress management version 1.2014. http://williams.medicine.wisc.edu/distress.pdf. Updated May 2014. Accessed January 16, 2016.
3. de Souza JA, Wong Y-N. Financial distress in cancer patients. J Med Person. 2014;11(2):13-15.
4. Mcdougall JA, Ramsey SD, Hutchinson F, Shih Y-CT. Financial toxicity: a growing concern among cancer patients in the United States. ISPOR Connect. 2014;20(2):10-11.
5. Sharpe K, Shaw B, Seiler MB. Practical solutions when facing cost sharing: the American Cancer Society’s health insurance assistance service. Am J Manag Care. 2016;22(4):92-94.
6. Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer : a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608-1614.
7. Meneses K, Azuero A, Hassey L, Mcnees P, Pisu M. Does economic burden influence quality of life in breast cancer survivors? Gynecol Oncol. 2012;124(3):437-443.
8. Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211-232.
9. Delgado-Guay M, Ferrer J, Rieber AG, et al. Financial distress and its associations with physical and emotional symptoms and quality of life among advanced cancer patients. Oncologist. 2015;20:1092-1098.
10. Kale HP, Carroll N V. Self-reported financial burden of cancer care and its effect on physical and mental health-related quality of life among US cancer survivors. Cancer. 2016;122:1283-1289.
11. Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732-1740.
12. Fenn KM, Evans SB, Mccorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Onocol Pract. 2014;10(5):332-339.
13. Azzani M, Roslani AC, Su TT. The perceived cancer-related financial hardship among patients and their families: a systematic review. Support Cancer Care. 2015;23:889-898.
14. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients a multicenter evaluation of the distress thermometer. Cancer. 2005;103:1494-1502.
15. Ransom S, Jacobsen PB, Booth-Jones M. Validation of the distress thermometer with bone marrow. Psychooncology. 2006;15:604-612.
16. Vodermaier A, Linden W, Siu C. Screening for emotional distress in cancer patients: a systematic review of assessment instruments. J Natl Cancer Inst. 2009;101:1464-1488.
17. McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17(7):431–8.
18. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570-579.
19. Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy – Spiritual Well-being Scale (FACIT-Sp). Ann Behav Med. 2002;24:49–58.
20. Barrera M, Caples H, Tein J. The psychological sense of economic hardship: measurement models, validity, and cross-ethnic equivalence for urban families. Am J Community Psychol. 2001;29:493-517.
21. Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381.
22. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population-based assessment of cancer survivors’ financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145-152.
23. Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710-3717.
24. Chino F, Peppercorn J, Taylor Jr. DH, et al. Self-reported financial burden and satisfaction with care among patients with cancer. Oncologist. 2014;19:414-420.
25. De Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient-reported outcome in cancer. Cancer. 2014;120:3245-3253.
26. Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care : advancing the research agenda. J Natl Cancer Inst. 2016;108(5):1-3.
27. Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract. 2014;10(3):162-168.
28. Irwin B, Kimmick G, Altomare I, et al. Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist. 2014;19:1135-1140.
29. US Census Bureau. United States. https://www.census.gov/quickfacts/. 2015.