User login
Why Required Pediatric Hospital Medicine Fellowships Are Unnecessary
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
The Joint Council of Pediatric Hospital Medicine (JCPHM), successor to the Strategic Planning (STP) Committee, recently recommended submitting a petition for two-year pediatric hospital medicine (PHM) fellowship certification to the American Board of Pediatrics (ABP), which was completed in 2014. In December 2015, the ABP Board of Directors voted to (1) approve the proposal for a two-year PHM fellowship incorporating scholarly activity with the provision that entrustable professional activities (EPAs) be used as the framework for assessing competencies and (2) not require those who achieve and maintain PHM certification to maintain general pediatrics certification. The proposal for certification of a two-year PHM fellowship will now be submitted to the American Board of Medical Specialties (ABMS). Concerns regarding the formal certification of PHM as an ABMS-recognized subspecialty have been raised by many stakeholders, including community pediatric hospitalists, pediatric residency program directors, and med-peds physicians.
We feel that the “first, do no harm” guiding principle seems to have been forgotten by the ABP as it attempts to formalize the training of pediatric hospitalists. In December 2015, the ABP voted in favor of a two-year ACGME-accredited PHM fellowship. The intent was to “assure the best care of hospitalized children,” “assure the public,” “accelerate improvements and innovation in quality improvement,” and “raise the level of care of all hospitalized children by establishing best practices in clinical care.” To be clear, these goals are shared by all of us (although there is no indication that the public is seeking additional assurance). Prior to launching broad-scale, time-intensive, and financially costly initiatives, we should ensure that our efforts would achieve—rather than obstruct—their intended aims. In addition to a lack of evidence supporting that subspecialty certification will advance our path toward achieving these goals, there are numerous reasons a required PHM fellowship is unnecessary and potentially even harmful to the hospitalist workforce. The negative unintended consequences need to be weighed heavily.
We have found no data to support that children would receive inferior inpatient care from pediatric hospitalists due to lack of formal certification. Hospital medicine physicians are paving the way in quality improvement, high-value care, medical education, palliative care, and global health, supported in part through training in various non-accredited hospital medicine fellowships. There is nothing stopping pediatric hospitalists from establishing and disseminating best practices in clinical care. Hospitalists are already making strides in providing high-quality care at low costs, as demonstrated by the abundant PHM scholarly work described in the ABP application to the ABMS. The alleged problem of needing to build trust within the community is yet to be demonstrated, as we have leaders at local, regional, and national levels. The chief medical officer of the Centers for Medicare & Medicaid Services is a hospitalist as is our surgeon general. Hospital medicine is the fastest-growing specialty in the history of medicine,1 and we should seek to propel rather than fetter our future colleagues.
Below are our reasons for opposing this formal certification.
We already have a fellowship system.
As we all know, advanced training opportunities already exist for those interested in pursuing extra research and quality improvement training. Similar to other pediatric subspecialty fellowships, these PHM fellowships are undersubscribed (20% of PHM fellowships did not fill in 2016),2 with the majority of graduating pediatric residents transitioning to hospitalists opting not to pursue fellowship training. We should continue to let graduating pediatric residents vote with their feet without the undue influence of subspecialty certification.
Subspecialization has opportunity costs that may reduce the PHM pipeline.
Even if we assume an adequate number of fellowship programs could be developed and funded, our fear is that the decision to turn PHM into an accredited subspecialty could paradoxically reduce the pipeline of inpatient providers. Residency is already a three- to four-year endeavor (pediatrics and med-peds) that is poorly compensated and time-intensive. In the absence of evidence supporting the value of additional training, tacking on another two years seems unreasonable in the face of the student loan debt crisis, reduced compensation, and lost time for career advancement. These are significant opportunity costs. While most specialties lead to a significant pay raise to compensate for the added training time, pediatrics remains the lowest-paid physician specialty.3 Should PHM follow the trend of most pediatric subspecialties, pursuit of fellowship training would be a negative financial decision for residency graduates.4 For the health system, increasing debt-to-income ratios runs the risk of creating a medical education bubble market.5
More than 25% of med-peds graduates pursue careers in hospital medicine, a percentage that continues to grow, accounting for more than 100 new hospitalists per year.6 As a result, med-peds-trained hospitalists constitute more than 10% of the pediatric hospitalist workforce.6 Requiring PHM fellowship training may reduce this crucial pipeline of practitioners. In a 2014 unpublished survey of 225 med-peds practitioners, 78% of residents and 96% of attendings responded that they would not consider pursuing an ACGME-accredited PHM fellowship.7 This is compounded by a lack of parity with the practice of adult hospital medicine both in compensation and required training and is heightened by the fact that the training in question does not incorporate care for adult patients. There is clear consensus by 96% of med-peds hospitalists that the creation of an ACGME-certified PHM subspecialty will negatively affect the likelihood of med-peds providers pursuing PHM.7
Certification will pose a potential risk to specific patient populations.
We are also concerned that a reduced PHM workforce could disproportionately impact young adults with special healthcare needs and those children cared for in rural or community-based hospitals. Med-peds training equips providers to care for children with chronic diseases that then transition into adulthood; more than 25% provide care for young adults with special healthcare needs.6 With the increasing number of children with chronic health conditions surviving into adulthood,8 med-peds hospitalists serve essential roles in providing care and coordination for this vulnerable population. Furthermore, hospital medicine groups in medical systems that cannot support a full-time categorical pediatric hospitalist tend to employ med-peds physicians or family practitioners. Concerns with PHM certification are thus extended to those family medicine physicians who practice PHM.
Pediatric residency trains pediatricians in inpatient care.
We feel that the decision to move forward on PHM subspecialty certification calls into question the value of pediatric residency training. There is no evidence that clinical inpatient training in pediatrics residency is inadequate. If one leaves residency trained to do anything, it is practicing hospital medicine. A significant portion of residency takes place inpatient, both on wards and in the intensive care units. The 2009 ABP Foundation–funded study of PHM reported that 94% of pediatric hospitalist respondents rated their training in general clinical skills during residency as fully adequate, 85% rated their training in communication skills as fully adequate, and 73% did not believe any additional training beyond residency should be required.9 With respect to med-peds graduates, more than 90% feel equipped to care for children and adults upon residency completion.10 If the ABMS carries forward with this decision, the only clinical work one would be “certified” to do after residency is primary care. However, after completion of residency training, most of us feel at least as comfortable, if not more comfortable, caring for children in the inpatient setting.
Primary care should require subspecialty certification as well.
Furthermore, the decision to create a certified subspecialty begs the question as to why fellowship should not be mandated for those entering the field of primary care. Does the field of primary care not require research to move it forward? Does the field of primary care not require providers who can adeptly apply quality improvement methodologies to improve primary-care delivery? Does the public not require the same type of assurance? By these measures, primary care should require subspecialty certification as well. These arguments could easily be construed as an indictment of residency training.
The target should be residency training.
The PHM ABMS application describes a clinical curriculum consisting of eight core clinical rotations in various settings. That small number emphasizes the fact that extra clinical training is really not needed and that we do not require a complete overhaul of the current training system. The skills in question for the accredited PHM fellowship include communication, negotiation, leadership, quality improvement, pain management, sedation, procedures, transport, billing/coding, autonomous decision making, and scholarly practice. Are most of these not skills that we should foster in all practicing pediatricians? If graduating pediatric residents lack competence in core pediatric skills (e.g., communication, pain management, autonomous decision making), we should target improvements in residency education rather than require years of further training. Pediatrics residency training already requires training in quality improvement and is incorporating “tracks” that target areas of perceived deficiency. Those physicians who actually require specialized hospital-based skills (e.g., sedation, procedures, and transport) could receive core training during residency (e.g., through PHM tracks or electives) and further hone these skills through faculty development efforts. While non-PhD researchers may benefit from additional training in research methodologies, this training comes at the expense of time spent caring for patients on the wards and should not be required training for the majority of pediatric hospitalists pursuing purely clinical roles.
Broad-based support for a PHM subspecialty has not been demonstrated.
While approximately 40 pediatric hospitalists originated the PHM certification petition, we have not seen clear support for subspecialty certification from the community. PHM certification runs the risk of alienating the general pediatrics community, as many outpatient pediatricians continue to care for their patients in the inpatient setting. Furthermore, at tertiary-care medical centers, pediatric subspecialists often serve as hospitalists, yet this stakeholder group has not entered into this conversation. Importantly, the Association of Pediatric Program Directors (APPD) did not endorse this proposal. Many of the APPD members were quite concerned about the harm this certification could cause. While the APA Board and the AAP Board of Directors support PHM subspecialty certification, it is not clear that the rank-and-file members do. The Society of Hospital Medicine did not support or oppose certification. In an era of controversy surrounding certification requirements, prior to making a decision that will alter the direction of an entire field and impact all future residency graduates interested in entering that field, we should ensure there is broad-based support for this decision.
An alternative path has already been established and validated.
A more prudent, cost-effective, and universally acceptable approach would be to follow in the footsteps of the American Board of Internal Medicine (ABIM) and American Board of Family Medicine (ABFM) in establishing a Focused Practice in Pediatric Hospital Medicine program. This approach respects the unique body of knowledge required of those who care for hospitalized children while maintaining the required flexibility to nurture and help to mature existing training pipelines. Core hospital medicine skills should be further honed through residency curricular changes and faculty development efforts, while hospital-based physicians interested in developing niche skills could still do so via already existing fellowships.
When it comes to pediatric hospital medicine, first, do no harm.
Pediatric hospitalists are inpatient generalists by training and clinical approach. Our practices vary from large academic medical centers with every imaginable subspecialty consult service available to remote rural settings that require hospitalists to possess unique and specific skills. Some pediatric hospitalists participate in newborn care, some perform sedations, and some perform a variety of diagnostic and therapeutic procedures. The current system is meeting the needs of the vast majority of our PHM community. Changes to the residency curriculum that are already under way can address any clinical and quality improvement gaps. More than enough PHM fellowships are available to those who choose to pursue them. The public is not requesting reassurance, and the field is already advancing at a rapid rate both clinically and scholarly. Subspecialty recognition is not necessary and will likely lead to negative unintended consequences. Given the financial constraints on our current system and the need for pediatric hospitalists to be stewards of high-value care, we should make collective decisions that will clearly benefit our patients and health system. As medical professionals, our priority should always be first, do no harm.
Weijen W. Chang, MD, is chief of the Division of Pediatric Hospital Medicine at Baystate Children’s Hospital and associate professor of pediatrics at the University of Massachusetts Medical School.
Leonard Samuel Feldman, MD, is director of the Medicine-Pediatrics Urban Health Residency Program and associate professor of medicine and pediatrics at Johns Hopkins School of Medicine.
Bradley Monash, MD, is associate chief of medicine at University of California, San Francisco and assistant clinical professor of medicine and pediatrics at UCSF School of Medicine.
Archna Eniasivam, MD, is assistant clinical professor of medicine at UCSF School of Medicine.
References
- Chen C, Eagle S. “Should Pediatric HM Pursue Subspecialty Certification, Required Fellowship Training?” The Hospitalist. July 31, 2012
- Results and Data: Specialties Matching Service 2016 Appointment Year. National Resident Matching Program website. Accessed May 15, 2016.
- Medscape Pediatrician Compensation Report 2015. Medscape website. Accessed April 29, 2016.
- Rochlin JM, Simon HK. Does fellowship pay: what is the long-term financial impact of subspecialty training in pediatrics? Pediatrics. 2001;127(2):254-260.
- Asch DA, Nicholson S, Vujicic M. Are we in a medical education bubble market? N Engl J Med. 2013;369(21):1973-1975.
- O’Toole JK, Friedland AR, Gonzaga AM, et al. The practice patterns of recently graduated internal medicine-pediatric hospitalists. Hosp Pediatr. 2015;5(6):309-314.
- Society of Hospital Medicine: Survey of Med-Peds Physicians about PHM Certification. May 2014 (unpublished).
- Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
- Freed GL, Dunham KM, Research Advisory Committee of the American Board of P. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186.
- Donnelly MJ, Lubrano L, Radabaugh CL, Lukela MP, Friedland AR, Ruch-Ross HS. The med-peds hospitalist workforce: results from the American Academy of Pediatrics Workforce Survey. Hosp Pediatr. 2015;5(11):574-579.
HHS Awards Grants to Improve Rural Patient Care
The Health Resources and Services Administration has awarded more than $16 million to improve access to quality healthcare in rural communities. The awards include funding to expand use of telehealth technology for veterans and other patients.
Administered by the Federal Office of Rural Health Policy (FORHP), the awards will support 60 rural communities in 32 states, along with 7 Rural Health Research Centers.
Related: Shared Medical Appointments for Glycemic Management in Rural Veterans
Flex Rural Veterans Health Access Program will receive 3 awards of $300,000 for 3 years to use telehealth and health information technology to bring mental health and other health services to veterans in rural areas. The program began 3 years ago in collaboration with the VA Office of Rural Health, and has helped test the effectiveness of community partnerships that can be replicated in other remote areas.
Telehealth Network Grant Program will receive approximately $300,000 annually for 21 community health organizations for up to 3 years to help build sustainable telehealth programs and networks in medically underserved areas. The program particularly encourages teleconnections to School Based Health Centers; all networks receiving the award include at least 1.
Related: Telehealth for Native Americans With PTSD
Seven Rural Health Research Centers will receive $700,000 annually for 4 years to conduct rural-focused health services research that helps health care providers and decision makers better understand the challenges faced by rural communities.
Another $4,065,624 will go to 21 member organizations of the Small Health Care Provider Quality Improvement project over 3 years. The organizations will use the money to improve the quality of care for populations with high rates of chronic conditions. The program focus on supporting rural primary care providers as they implement quality improvement activities and furthering coordination of care using evidence-based treatment.
Related: Clinical Video Telehealth for Gait and Balance
“These grants encourage and support collaboration at the community level, expanding and strengthening the safety net with networks of care in rural areas,” says FORHP Associate Administrator Tom Morris. “Collaboration among different providers of health and social services within a community means shared resources, shared expertise, and shared innovations.”
The Health Resources and Services Administration has awarded more than $16 million to improve access to quality healthcare in rural communities. The awards include funding to expand use of telehealth technology for veterans and other patients.
Administered by the Federal Office of Rural Health Policy (FORHP), the awards will support 60 rural communities in 32 states, along with 7 Rural Health Research Centers.
Related: Shared Medical Appointments for Glycemic Management in Rural Veterans
Flex Rural Veterans Health Access Program will receive 3 awards of $300,000 for 3 years to use telehealth and health information technology to bring mental health and other health services to veterans in rural areas. The program began 3 years ago in collaboration with the VA Office of Rural Health, and has helped test the effectiveness of community partnerships that can be replicated in other remote areas.
Telehealth Network Grant Program will receive approximately $300,000 annually for 21 community health organizations for up to 3 years to help build sustainable telehealth programs and networks in medically underserved areas. The program particularly encourages teleconnections to School Based Health Centers; all networks receiving the award include at least 1.
Related: Telehealth for Native Americans With PTSD
Seven Rural Health Research Centers will receive $700,000 annually for 4 years to conduct rural-focused health services research that helps health care providers and decision makers better understand the challenges faced by rural communities.
Another $4,065,624 will go to 21 member organizations of the Small Health Care Provider Quality Improvement project over 3 years. The organizations will use the money to improve the quality of care for populations with high rates of chronic conditions. The program focus on supporting rural primary care providers as they implement quality improvement activities and furthering coordination of care using evidence-based treatment.
Related: Clinical Video Telehealth for Gait and Balance
“These grants encourage and support collaboration at the community level, expanding and strengthening the safety net with networks of care in rural areas,” says FORHP Associate Administrator Tom Morris. “Collaboration among different providers of health and social services within a community means shared resources, shared expertise, and shared innovations.”
The Health Resources and Services Administration has awarded more than $16 million to improve access to quality healthcare in rural communities. The awards include funding to expand use of telehealth technology for veterans and other patients.
Administered by the Federal Office of Rural Health Policy (FORHP), the awards will support 60 rural communities in 32 states, along with 7 Rural Health Research Centers.
Related: Shared Medical Appointments for Glycemic Management in Rural Veterans
Flex Rural Veterans Health Access Program will receive 3 awards of $300,000 for 3 years to use telehealth and health information technology to bring mental health and other health services to veterans in rural areas. The program began 3 years ago in collaboration with the VA Office of Rural Health, and has helped test the effectiveness of community partnerships that can be replicated in other remote areas.
Telehealth Network Grant Program will receive approximately $300,000 annually for 21 community health organizations for up to 3 years to help build sustainable telehealth programs and networks in medically underserved areas. The program particularly encourages teleconnections to School Based Health Centers; all networks receiving the award include at least 1.
Related: Telehealth for Native Americans With PTSD
Seven Rural Health Research Centers will receive $700,000 annually for 4 years to conduct rural-focused health services research that helps health care providers and decision makers better understand the challenges faced by rural communities.
Another $4,065,624 will go to 21 member organizations of the Small Health Care Provider Quality Improvement project over 3 years. The organizations will use the money to improve the quality of care for populations with high rates of chronic conditions. The program focus on supporting rural primary care providers as they implement quality improvement activities and furthering coordination of care using evidence-based treatment.
Related: Clinical Video Telehealth for Gait and Balance
“These grants encourage and support collaboration at the community level, expanding and strengthening the safety net with networks of care in rural areas,” says FORHP Associate Administrator Tom Morris. “Collaboration among different providers of health and social services within a community means shared resources, shared expertise, and shared innovations.”
Palliative Care May Improve End-of-Life Care for Patients with ESRD, Cardiopulmonary Failure, Frailty
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Clinical Question: Is there a difference in family-rated quality of care for patients dying with different serious illnesses?
Background: End-of-life care has focused largely on cancer patients. However, other conditions lead to more deaths than cancer in the United States.
Study Design: A retrospective cross-sectional study.
Setting: 146 inpatient Veterans Affairs (VA) facilities.
Synopsis: This study included 57,753 patients who died in inpatient facilities with a diagnosis of cancer, dementia, end-stage renal disease (ESRD), cardiopulmonary failure (heart failure or chronic obstructive pulmonary disease), or frailty. Measures included palliative care consultations, do-not-resuscitate (DNR) orders, death in inpatient hospice, death in the intensive care unit (ICU), and family-reported quality of end-of-life care. Palliative care consultations were given to 73.5% of patients with cancer and 61.4% of patients with dementia, which was significantly more than patients with other diagnoses (P < .001).
Approximately one-third of patients with diagnoses other than cancer or dementia died in the ICU, which was more than double the rate among patients with cancer or dementia (P < .001). Rates of excellent quality of end-of-life care were similar for patients with cancer and dementia (59.2% and 59.3%) but lower for other conditions (P = 0.02 when compared with cancer patient). This was mediated by palliative care consultation, setting of death, and DNR status. Difficulty defining frailty and restriction to only the VA system are limitations of this study.
Bottom Line: Increasing access to palliative care, goals-of-care discussions, and preferred setting of death may improve overall quality of end-of-life care.
Citation: Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176(8):1095-1102. doi:10.1001/jamainternmed.2016.1200.
Patients’ Out-of-Pocket Spending Increasing
Clinical Question: How much are insured nonelderly adult patients paying out of pocket for inpatient care, and does that amount vary over time or by patient characteristics, region, or type of insurance?
Background: Prior estimates have been based on patient-reported survey data. This is the first study to find nationwide out-of-pocket expenditure for inpatient hospitalizations.
Study Design: Retrospective analysis.
Setting: Medical claims data from Aetna, UnitedHealthcare, and Humana including 7.3 million hospitalizations from 2009 to 2013.
Synopsis: Authors used the Health Care Cost Institute (HCCI) database and studied inpatient hospitalization for ages 18–64. The adjusted total cost sharing per inpatient hospitalization increased by 37% (from $738 in 2009 to $1,013 in 2013). Both the mean amount of coinsurance and deductibles increased during this period by 33% (from $518 to $688) and 86% (from $145 to $270), respectively. The mean copayment decreased by 27% (from $75 to $55).
Increase in cost sharing was lowest in individual-market and consumer-directed health plans, although both had highest cost sharing.
Total cost sharing increased in every state. The largest increases were seen in Georgia, Louisiana, and Colorado. In 2013, the states with the highest cost sharing were Utah, Alaska, and Oregon.
Acute myocardial infarction and acute appendicitis saw maximum rise in out-of-pocket spending; both surpassed $1,500 in 2013. Cost sharing associated with procedures was lower.
Bottom Line: Even after adjusting for inflation and case-mix differences, the total cost sharing per inpatient hospitalization increased between 2009 and 2013. Policymakers and patients need to pay attention to these trends.
Citation: Adrion ER, Ryan AM, Seltzer AC, Chen LM, Ayanian JZ, Nallamothu BK. Out-of-pocket spending for hospitalizations among nonelderly adults. JAMA Intern Med. 2016;176(9)1325-1332.
Short Take
Aspirin Is Being Used Instead of Anticoagulation in Afib
Despite recommendations to anticoagulate patients with CHADS2 /CHA2DS2-VASc scores of ≥2, more than one-third of the patients in a large population of cardiology outpatients were treated with aspirin alone.
Citation: Hsu JC, Maddox TM, Kennedy K, et al. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J Am Coll Cardiol. 2016;67(25):2913-2923.
Clinical Question: How much are insured nonelderly adult patients paying out of pocket for inpatient care, and does that amount vary over time or by patient characteristics, region, or type of insurance?
Background: Prior estimates have been based on patient-reported survey data. This is the first study to find nationwide out-of-pocket expenditure for inpatient hospitalizations.
Study Design: Retrospective analysis.
Setting: Medical claims data from Aetna, UnitedHealthcare, and Humana including 7.3 million hospitalizations from 2009 to 2013.
Synopsis: Authors used the Health Care Cost Institute (HCCI) database and studied inpatient hospitalization for ages 18–64. The adjusted total cost sharing per inpatient hospitalization increased by 37% (from $738 in 2009 to $1,013 in 2013). Both the mean amount of coinsurance and deductibles increased during this period by 33% (from $518 to $688) and 86% (from $145 to $270), respectively. The mean copayment decreased by 27% (from $75 to $55).
Increase in cost sharing was lowest in individual-market and consumer-directed health plans, although both had highest cost sharing.
Total cost sharing increased in every state. The largest increases were seen in Georgia, Louisiana, and Colorado. In 2013, the states with the highest cost sharing were Utah, Alaska, and Oregon.
Acute myocardial infarction and acute appendicitis saw maximum rise in out-of-pocket spending; both surpassed $1,500 in 2013. Cost sharing associated with procedures was lower.
Bottom Line: Even after adjusting for inflation and case-mix differences, the total cost sharing per inpatient hospitalization increased between 2009 and 2013. Policymakers and patients need to pay attention to these trends.
Citation: Adrion ER, Ryan AM, Seltzer AC, Chen LM, Ayanian JZ, Nallamothu BK. Out-of-pocket spending for hospitalizations among nonelderly adults. JAMA Intern Med. 2016;176(9)1325-1332.
Short Take
Aspirin Is Being Used Instead of Anticoagulation in Afib
Despite recommendations to anticoagulate patients with CHADS2 /CHA2DS2-VASc scores of ≥2, more than one-third of the patients in a large population of cardiology outpatients were treated with aspirin alone.
Citation: Hsu JC, Maddox TM, Kennedy K, et al. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J Am Coll Cardiol. 2016;67(25):2913-2923.
Clinical Question: How much are insured nonelderly adult patients paying out of pocket for inpatient care, and does that amount vary over time or by patient characteristics, region, or type of insurance?
Background: Prior estimates have been based on patient-reported survey data. This is the first study to find nationwide out-of-pocket expenditure for inpatient hospitalizations.
Study Design: Retrospective analysis.
Setting: Medical claims data from Aetna, UnitedHealthcare, and Humana including 7.3 million hospitalizations from 2009 to 2013.
Synopsis: Authors used the Health Care Cost Institute (HCCI) database and studied inpatient hospitalization for ages 18–64. The adjusted total cost sharing per inpatient hospitalization increased by 37% (from $738 in 2009 to $1,013 in 2013). Both the mean amount of coinsurance and deductibles increased during this period by 33% (from $518 to $688) and 86% (from $145 to $270), respectively. The mean copayment decreased by 27% (from $75 to $55).
Increase in cost sharing was lowest in individual-market and consumer-directed health plans, although both had highest cost sharing.
Total cost sharing increased in every state. The largest increases were seen in Georgia, Louisiana, and Colorado. In 2013, the states with the highest cost sharing were Utah, Alaska, and Oregon.
Acute myocardial infarction and acute appendicitis saw maximum rise in out-of-pocket spending; both surpassed $1,500 in 2013. Cost sharing associated with procedures was lower.
Bottom Line: Even after adjusting for inflation and case-mix differences, the total cost sharing per inpatient hospitalization increased between 2009 and 2013. Policymakers and patients need to pay attention to these trends.
Citation: Adrion ER, Ryan AM, Seltzer AC, Chen LM, Ayanian JZ, Nallamothu BK. Out-of-pocket spending for hospitalizations among nonelderly adults. JAMA Intern Med. 2016;176(9)1325-1332.
Short Take
Aspirin Is Being Used Instead of Anticoagulation in Afib
Despite recommendations to anticoagulate patients with CHADS2 /CHA2DS2-VASc scores of ≥2, more than one-third of the patients in a large population of cardiology outpatients were treated with aspirin alone.
Citation: Hsu JC, Maddox TM, Kennedy K, et al. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J Am Coll Cardiol. 2016;67(25):2913-2923.
How Should Hospitalists Manage Elderly Patients with Dysphagia?
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
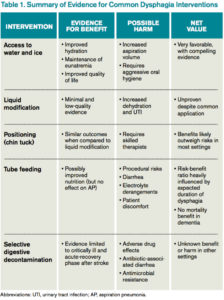
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.
The Case
A 74-year-old man with Alzheimer’s dementia presents with urinary tract infection (UTI), hypovolemia, and hypernatremia. He also has chronic dysphagia with a history of aspiration pneumonia and has been on thickened liquids at home for the past five months. As his infection is treated, he improves and requests water to drink.
Background
The diagnosis of dysphagia is clinical, and assessments from patients and family are often sufficient. The optimal test to assess the severity of dysphagia is a bedside swallow evaluation using small amounts of water.1 Video-assisted fluoroscopic examinations can identify problem areas within the oropharynx and esophagus and may help determine the etiology of dysphagia.
What evidence supports various treatment options for dysphagia?
Access to Water
Water is a thin liquid with low viscosity, which allows for rapid transit through the oropharynx. In debilitated and elderly patients, thin liquids easily reach the epiglottis and enter the trachea before pharyngeal muscles compensate. As such, access to water and other thin liquids is often restricted in patients suspected to have dysphagia.4
However, allowing access to water improves patient satisfaction, reduces the development of dehydration, and does not increase the incidence of AP. Bedside therapy interventions such as correct positioning and chin-tuck and sipping technique as well as attention to oral hygiene are recommended prior to more noxious options such as thickened liquids.1 The Frazier water protocol may help provide logistical guidance for facilities interested in improving access to water for patients with dysphagia.
Liquid Modification
Many clinicians manage dysphagia through restricting access to all thin liquids. In the hospital setting where video fluoroscopy and speech therapy are readily available, clinicians frequently employ the use of modified diets with thickened liquids in order to minimize the risk of aspiration despite the lack of high-quality evidence supporting liquid modification.2 Patients associate thickened liquids and restricted diets with a reduction in quality of life. Compliance studies have shown that only a minority of patients are compliant with thickened liquids at five days. In addition, thickening liquids has not been shown to decrease the risk of AP nor improve nutritional status, and it may actually cause harm by increasing the risk of dehydration and UTI.4
Tube Feeding
In patients with severe dysphagia in whom conservative management is not feasible or has failed, maintaining adequate nutrition can be a challenge. There are encouraging data with nutritionally enriching and modifying the texture of solid foods.1 Alternative methods of enteral nutrition delivery are often also considered. The most common vehicles of delivery are nasogastric tubes, post-pyloric feeding tubes, and percutaneous endoscopic gastrostomy (PEG) tubes. In theory, bypassing the pharynx and esophagus could result in fewer aspiration events and less AP.3 However, nasogastric, post-pyloric, or PEG feeding does not decrease the risk of AP. For patients with advanced dementia, there have been no randomized trials demonstrating an improvement in mortality with tube feeds.4 Tube feeding also carries with it a slight procedural risk and a high incidence of associated diarrhea, plus is associated with electrolyte derangements such as hypernatremia. The decision to pursue tube feeding should be weighed heavily in every patient and is highly influenced by the etiology and anticipated duration of dysphagia.
Selective Digestive Decontamination
Selective digestive decontamination (SDD) is a protocol-based treatment that aims to eradicate potentially pathogenic gut flora, particularly aerobic gram-negatives, in critically ill patients to reduce the impact of aspiration events. The utilization of SDD and the available literature center firmly on critically ill and ventilated patients. Subsequent studies have demonstrated recolonization after protocol cessation, and long-term effects are currently undefined.5 Until it can be studied in broader populations and proven to have clinical benefit, employing SSD in non-critically ill patients with dysphagia remains unsupported.
Multimodal Approach
Many rehabilitation centers incorporate a therapist-driven swallowing treatment program. Evidence suggests patient and family counseling alone may not be effective, so these programs variably incorporate diet/liquid modification, strengthening exercises, sensory processing techniques, and even neuromuscular electrical stimulation for muscle building.1 Accordingly, these programs are resource-intensive.
Management
Dysphagia remains a significant clinical problem for hospitalized patients. The existing literature and practice guidelines generally support a “less is more” approach. Though liquid/diet modification is common practice, it is not based in solid evidence and may contribute to unnecessary tube feeding. The best current evidence supports allowing access to water and ice chips. The ideal management plan for each patient will differ and should incorporate patient and family preferences in a multidisciplinary approach.
Back to the Case
Our patient requests water. He coughs after drinking during a bedside swallow evaluation. The risks of potential aspiration and AP are explained, and he expresses his understanding. He reiterates his choice to be allowed access to water as it is important to his quality of life. The speech therapy team is consulted and provides instruction on chin-tuck positioning, oral care, and timing water between meals rather than while eating food. He does well for the remainder of the hospital stay, and by time of discharge, his electrolytes are corrected, and he is much more comfortable being allowed to drink water. He is discharged home and encouraged to continue with these conservative measures.
Bottom Line
Evidence to support many common interventions for dysphagia is lacking; patients with dysphagia are best managed via a multidisciplinary, multimodal approach that provides access to water whenever possible. TH
Vijay G. Paryani, MD, is an internal medicine resident in the department of internal medicine at the University of Kentucky. Joseph R. Sweigart, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky. Laura C. Fanucchi, MD, is a hospitalist and assistant professor of hospital medicine in the division of hospital medicine at the University of Kentucky.
References
- Karagiannis MJ, Chivers L, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatr. 2011;11(2):9.
- Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomized controlled trials. Age Ageing. 2008;37(3):258-264.
- Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671.
- Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022.
- Gosney M, Martin MV, Wright AE. The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 2006;35(1):42-47.
Updated Guideline for Acute Diarrheal Infection
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Clinical Question: What are current recommendations for diagnosis, management, and prevention of acute gastrointestinal infection in immune-competent adults?
Background: Acute diarrheal infection is a leading cause of healthcare visits and lost quality of life. The Centers for Disease Control and Prevention estimates there are 47.8 million cases annually, with a healthcare economy burden of $150 million.
Study Design: American College of Gastroenterology (ACG) practice guideline.
Setting: Expert panel.
Synopsis: Stool diagnostic studies may be used for dysentery with moderate-severe disease and symptoms lasting more than seven days (strong recommendation, low level of evidence). Traditional diagnostic methods in most cases fail to reveal etiology (strong recommendation, low level of evidence). Treatment with probiotics or prebiotics is not recommended (strong recommendation, moderate level of evidence). Bismuth subsalicylates may be considered for prophylaxis against traveler’s diarrhea (strong recommendation, high level of evidence). Short-term antibiotic chemoprophylaxis also may be considered for high-risk groups (strong recommendation, high level of evidence). Empiric antimicrobial therapy is not recommended except in cases of traveler’s diarrhea (strong recommendation, high level of evidence). Loperamide may be used as an adjunct to antibiotics for traveler’s diarrhea (strong recommendation, moderate level of evidence).
Bottom Line: ACG acute diarrheal illness guidelines have been updated. Few recommendations are strong, and very few have high levels of evidence.
Citation: Riddle MS, DuPont HL, Conner BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622.
Risk-Assessment Models Are Unreliable Predictors of Venous Thromboembolism
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Clinical Question: Do risk-assessment models (RAMs) accurately predict which hospitalized medical patients are at risk for venous thromboembolism (VTE)?
Background: Predicting which patients are at high risk for VTE is important. Several models exist, but limited data support their generalizability and accuracy in medical inpatients.
Study Design: Retrospective cohort.
Setting: Hospitals participating in the Michigan Hospital Medicine Safety Consortium (MHMSC).
Synopsis: Data collected through MHMSC for selected medical patients were used in the Kucher, Padua, predictive IMPROVE, and Intermountain DVT risk-assessment models. Patients were classified as “low risk” or “at risk” based on each RAM. Follow-up data came from chart extraction (100% of patients) and 90-day post-discharge telephone calls (58% of patients). The primary outcome was image-confirmed hospital associated VTE, including proximal upper- or proximal lower-extremity DVT or pulmonary embolism. These RAMs classified less than 20% of patients as “at risk.” The incidence of VTE was less than 1%. In this external validation study, the Kucher RAM was the least discriminate and the Intermountain was the best, but none yielded results equivalent to the original studies.
This study was limited by the retrospective design, subjectivity of some risk factors (such as immobility), and inability to obtain 90-day telephone follow-up in all patients. Lastly, the binary approach (“at risk” versus “low risk”) may not align with the original derivation studies in which each factor was evaluated independently.
Bottom Line: The incidence of VTE is low in medical inpatients, and current RAMs may not accurately identify at-risk patients.
Citation: Greene MT, Spyropoulos AC, Chopra V, et al. Validation of risk assessment models of venous thromboembolism in hospitalized medical patients. Am J Med. 2016;129(9):1001.e9-1001.e18. doi:10.1016/j.amjmed.2016.03.031.
Veteran Perceptions, Interest, and Use of Complementary and Alternative Medicine
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
Complementary and alternative medicine (CAM) are health and wellness practices that are outside conventional allopathic medicine. In the U.S., the popularity of CAM has grown, and patients often use CAM to treat pain, insomnia, anxiety, and depression.1-5 Veterans also have been increasingly adding CAM to conventional medicine, although limited studies exist on veteran use and attitudes toward CAM.6-8
Recently, the VA has increased its CAM services, offering different treatments at various VA facilities where CAM is most commonly used to treat anxiety, posttraumatic stress disorder (PTSD), depression, and back pain.9 Some veterans also seek CAM services outside the VA.6,8 Across studies of veterans and the broader population, having more years of education and higher income and being middle-aged, female, and white were associated with greater CAM use.1,3,6-8
Some CAM practices, such as acupuncture, require a practitioner’s regular and direct involvement. Other, independent CAM practices can be taught in classes, individual sessions, or through self-instructional multimedia. Once learned, these practices can be done independently, allowing for easier and less costly access. Independent CAM practices, such as yoga, meditation, breathing exercises, qigong, and tai chi promote general wellness or treat a particular ailment.
Although results have been mixed, several studies support independent CAM practices for treatment and symptom relief. For example, yoga improves symptoms in neurologic and psychiatric disorders, lessens pain, and helps decrease anxiety and depression and improve self-efficacy.10-13 Qigong can improve hypertensionand self-efficacy.14,15
This study examines veterans’ attitudes and beliefs about CAM, which can affect their interest and use of CAM services within and outside the VA. The focus is exclusively on independent CAM practices. At the time of the study, the availability of more direct CAM practices, such as acupuncture, was limited at many VA sites, and independently practiced techniques often require fewer resources and, therefore, could be adapted more easily. Subsequent references to CAM in this study refer only to independent CAM practices.
The current study surveyed veterans in New Jersey in multiple VA clinics and non-VA peer-counseling settings as part of an implementation study of a veteran-centric DVD called the STAR (Simple Tools to Aid and Restore) Well-Kit (SWK), which serves as a veteran introduction to CAM.16 Before watching the DVD, veterans were asked to fill out a baseline survey about their knowledge, attitudes, beliefs, and experiences with CAM as well as answer screening and demographic questions.
The authors describe the findings of the baseline survey to inform how to best implement CAM more broadly throughout VA. They expected that knowledge, attitudes, beliefs, and experiences with CAM would vary by clinical setting and respondent characteristics and hypothesized that psychological factors would be related to interest in CAM. Finally, barriers and facilitators of use of CAM are reported to inform policies to promote veteran access to CAM.
Methods
This cross-sectional analysis of the baseline SWK surveys had no inclusion or exclusion criteria because participation was anonymous. Recipients received a packet that instructed them to complete a previewing survey, watch the DVD, and complete a postviewing survey about the DVD. Surveys were returned in person or by postage-paid envelopes. No follow-up reminders were provided. This study examines data from only the previewing survey, and all further references to the veteran presurvey refers to it as the survey.
Study sites were the outpatient services of the VA New Jersey Health Care System (VANJHCS) and a non-VHA New Jersey veteran peer-counseling office. VANJHCS, which enrolls patients from northern and central New Jersey, offers health care services at 2 campuses and 9 outpatient clinics. Waivers of informed consent were approved by the VANJHCS Institutional Review Board and Research and Development Committee given the anonymous and low-risk nature of the research.
Participant Recruitment
The survey was distributed at 4 settings selected with a focus on ambulatory services and a goal of ensuring participant diversity in age, deployment experience, and mental and physical health conditions. At 3 settings, surveys were distributed using 3 methods: by a researcher; left for pickup in waiting rooms; or by selected health care providers at their discretion in the context of routine clinical visits. The VANJHCS settings were outpatient mental-health clinics, outpatient primary-care settings, and outpatient transition-unit clinics for recent combat veterans. The fourth setting was a community veteran peer-support organization staffed by veterans and included events held at the organization’s offices, veteran informational and health fairs in the community, and outreach events at college campuses. In this setting, veteran peers distributed the SWK at their discretion; they were given suggested talking points for distribution.
Survey Data Collection
Veterans filled out baseline surveys before viewing the SWK DVD. The surveys were anonymous but coded with a number to allow for tracking by setting and dissemination method. The surveys asked for demographic and health information and experience with and interest in CAM techniques. To minimize respondent burden, the authors focused on the most critical domains as summarized in the background section (demographics; health status and symptoms, including pain; self-efficacy; mental health conditions; knowledge, attitudes, and beliefs about CAM).
Demographic Information
Age range was assessed to avoid collecting identifying information. The questionnaire also included gender, military era/deployment, employment status, and race and ethnicity.
- Self-Rated Health (SF1). Self-rated health was assessed with a widely used single-item question that correlates highly with actual overall health and with function and quality of life.17,18 Respondents were asked to rate their health as excellent (5), very good, good, fair, or poor (1).
- Pain Screen (PEG 3-item scale). This 3-item screen has shown reliability and validity and is comparable to longer pain questionnaires.19 Respondents were asked to rate 3 measures of their pain and its consequences on a scale of 0 (no pain or no interference from pain) to 10 (worst pain or interference). Responses were averaged to determine pain score.
- PTSD Screen. This 4-item PTSD screen was developed for primary care and is widely used in VA settings.20 For each item, respondents were asked to check off whether they have had specific PTSD symptoms within the past month. The screen was considered positive with 3 of 4 affirmative responses.
- Anxiety and Depression Screen (PHQ-4). This 4-item scale combines the brief 2-item scales for screening anxiety and depression in primary care.21 For each depression or anxiety symptom, respondents selected from “not at all,” (1) “several days ”(2), “more days than not,” (3) and “nearly every day.” (4) For each 2-item screen, a sum of 5 or more indicated a positive screen.
- Self-Efficacy for Health Management (modified). The original 6-item self-efficacy screen was developed to test self-efficacy in managing chronic disease.22 Since not all participants in the current study were expected to have a chronic disease, the questions were modified to address more general self-efficacy for health management. Although the scale had not been adapted in this way or validated with this change, other authors have similarly adapted it to address specific chronic diseases with satisfactory results.23,24 For each item, respondents were asked to rate their confidence in their ability to manage aspects of their health on a scale of 1 (not at all confident) to 10 (very confident). Participants could also check “not applicable” for items that did not apply to their health concerns, and these items were not counted in the average score.
- Familiarity With and Interest in CAM. The authors developed a checklist to assess whether participants had heard of, tried, or were practicing the 4 CAM techniques featured in the SWK and to gauge their interest in learning about them (ie, meditation/guided imagery, breathing exercises, yoga, tai chi or qigong). For each technique, respondents selected that they have “never heard of,” “heard of but never tried,” “have done this in the past,” or “are currently doing.” For some analyses, the first 2 and last 2 options were combined to determine whether respondents had done each practice. They were also asked to check off whether they would like to learn more about the practice and whether they would like to try it with an instructor and/or try it on their own. For some analyses, each technique was looked at separately, whereas for others, the 4 techniques were combined to determine whether they had tried or were currently doing any of them.
- Barriers to Practice. The authors developed a checklist of 10 barriers to practicing CAM techniques based on research but with adjustments to the specific practices and population under investigation.25 The checklist included an open-end response to allow respondents to add barriers. The barrier list was a checklist and not a validated scale.
- Perceived Benefits of CAM. The authors developed 2 questions to assess the perceived benefits of these techniques on functionality and overall wellness, rated on a Likert scale from 1 (no benefits) to 10 (very much).
Statistical Analysis
Survey instruments were scored according to generally accepted and published practices. Item-level analysis was performed to identify missing responses and describe the sample. Summary statistics were reported. Pearson product moment correlation was used to detect associations between continuous variables. Analysis of variance (ANOVA) was used to detect associations between dichotomous and continuous variables. Chi-square tests were used to detect associations between categorical variables, specifically looking at clinically meaningful differences between veterans who had experience with or interest in trying independent CAM practices and those who did not. Linear regression analysis was used to determine significant associations between participant characteristics and the belief that independent CAM practices would be helpful with daily function.
Results
The response rate for returning surveys was low (n = 134; 18.2%). Surveys distributed by peers in the community setting had the highest response rate (38%), followed by surveys distributed in primary care (23%).
Due to the anonymous nature of the survey, information on nonresponder characteristics was not available. Respondents covered a range of ages, with 64% of respondents aged ≥ 50 years. Respondents were men (88%) and white (49%) or African American (40%). Fifty-five percent screened positive for at least 1 mental health condition (PTSD, depression, or anxiety). The average self-rated health was 2.9 on a scale of 1 (poor health) to 5 (excellent health). Gender, age range, race, and deployment status were comparable with New Jersey VA veteran demographics.26
Table 1 shows veteran experience and interest in CAM practices. More than half of veterans who returned the survey reported doing either a CAM practice or having done 1 (n = 82; 61%). Many also reported interest in trying at least 1 practice (n = 73; 55%) or learning more about at least 1 practice (n = 71; 53%) either on their own or with an instructor. More veterans indicated they would prefer to try the techniques with an instructor (n = 61; 46%) rather than on their own (n = 26; 19%). Chi-square testing showed that interest and experience with CAM were not significantly associated with specific demographic characteristics.
Several barriers to CAM practice were frequently cited (Table 2). The 2 most commonly endorsed barriers were veterans who wanted to try the techniques but needed more guidance (n = 62; 46%) and heard of CAM but never thought to try them (n = 43; 32%). Only a small percentage of veterans indicated that they did not think the practice would help (n = 13; 10%) or were concerned that it might hurt them (n = 11; 8%).
There were several significant bivariate associations (Table 3), although overall r2 values were low. More severe pain was associated with a weaker belief that the techniques could benefit overall wellness (r2 = – .19; P = .04) and help daily functioning (r2= – .27; P < .01). Higher health-related self-efficacy was associated with a stronger belief in the techniques’ effectiveness for overall wellness (r2= .30; P < .01) and daily function (r2 = .35; P < .01). Higher self-rated health was associated with stronger belief in effectiveness for overall wellness (r2 = .20; P = .02) and daily function (r2= .23; P < .01). One-way ANOVAs found no significant associations between belief in the techniques’ effectiveness for wellness or for daily activities (for which statistics are presented here) and positive screens for PTSD (F1,116 = 3.04; P = .08), depression (F1,116 = 2.06; P = .15), anxiety (F1,122 = 1.41; P = .23), or any of the 3 combined (F1,116 = 3.74; P = .06). None of the health factors was associated with veteran interest in trying a technique or with a history of trying at least 1 technique.
Of the multivariate linear regression models examining associations between veteran characteristics and responses to CAM, only 1 was significant (Table 4). Of all the factors in the model, only self-efficacy was significantly associated with the belief that CAM can improve daily function. Pain moderated this relationship; those with higher pain levels believed CAM could help with daily function only if they also had high self-efficacy (interaction term β = 0.27; SE = 0.03). For example, veterans with no pain (pain score 1 on a scale of 1-10) had a β = .07 (SE .13, P = .28), whereas those with the highest pain level (10) had a β = .92 (SE = .24, P = .001).
Discussion
The authors report 3 main findings from this study: Personal characteristics are not associated with experience, interest in, or belief in the efficacy of CAM; despite a large proportion reporting experience with CAM, veterans reported several barriers to using CAM; and the level of pain reported moderated the relationship between health-related self-efficacy and the belief that CAM will help with daily function.
Determining which personal characteristics are associated with CAM perceptions may indicate who is willing to try CAM techniques and who may require additional education or support. Although the authors hypothesized a difference in experience, interest, and belief of efficacy according to patient characteristics, these differences were not demonstrated. Some published research supports an association between white race, female sex, and middle or younger age and use of CAM, but this sample of veterans did not confirm these associations.1,3,6-8
The lack of associations may be related to selection bias, reflected in the relatively high report of baseline use of CAM. Nevertheless, this finding implies that clinicians should not make assumptions about an individual’s experience with CAM or interest in trying a modality. From a policy perspective, the VHA should consider a broad-based approach targeting a general audience or multiple segmented audiences to increase awareness and a trial of CAM for veterans.
Barriers should be considered when introducing CAM into routine clinical care. The current study revealed several important barriers to veterans accessing or trying CAM techniques, including need for guidance, the lack of awareness or access, and cost. The VHA services are often provided at low or no cost to eligible veterans, likely mitigating the cost barrier to a great extent. However, being able to easily access instruction in CAM modalities in a timely manner may be just as important. The authors detected a preference among respondents for classes to learn CAM (46%) vs independently (19%), supported by the commonly endorsed barrier to trying CAM of “I want in-person instruction but can’t find it.” Offering CAM modalities that can be taught in a group or individual setting and later practiced independently may be an appropriate approach to introduce CAM techniques to the largest number of people and encourage uptake. This approach can maximize access while satisfying many veterans’ preferences for in-person instruction. This leverage of skilled practitioner time could be extended for some modalities through remote telehealth participation or on-demand instruction, such as online videos or DVDs, including the SWK.
Chronic pain can be a challenge for patients and clinicians to manage, so the role of CAM in pain management is growing.2,27 The study’s findings suggest that motivating veterans with chronic pain to try CAM may take extra effort by the clinician. Multivariate linear regression modeling showed that respondents with higher pain levels believed CAM could help with their function only if they also had high health-related self-efficacy, whereas those with low pain scores reported this belief even with low self-efficacy. Thus, strong self-efficacy may overpower doubts about CAM that accompany having pain. Conversely, high reported pain levels may reduce self-efficacy and lead to doubts about the benefit of CAM.
In one study, the belief that lifestyle contributes to illness predicted CAM use, which is similar to this study’s finding that health-related self-efficacy predicted CAM use.8 Several other studies examined CAM use and self-efficacy, although usually not self-efficacy for general health management. To promote experimentation with CAM, patients with chronic pain may require interventions targeted to increase self-efficacy related to CAM.
Of the 733 surveys distributed to veterans, 134 (18%) responses were received. More than 60% of the respondents had tried a CAM technique, higher rates than reported by most other CAM utilization studies: U.S. prevalence studies range from 29% to 42% of respondents having tried some CAM technique, and studies of veterans or military personnel range from 37% to 50% having tried CAM.3,5-7 Because these studies asked about CAM generally or about specific practices that do not fully overlap with the independent CAM practices evaluated in the current study, it is difficult to assess how the experiences of the current sample compare with those populations.
Another study asked veterans with multiple sclerosis whether they were interested in trying CAM practices, and 40% responded “yes,” which is similar to 55% in the current study.6 It is possible the rate of experience with CAM is higher in the current study due to self-selection of respondents who were interested in the SWK. Another factor may be that some veterans were recruited from VA mental health clinics where independent CAM practices are more frequently offered.9 It is also possible that there are regional differences in CAM use; this study took place at a single facility in the northeast U.S., although subsequent phases of the SWK project involved more widespread national dissemination, to be reported in the future.
Limitations
Self-selection and low response rate are limitations in this study. Despite the low response rate, the demographic information of the sample generally resembles the population of veterans at VANJHCS for age, sex, era, health status, and presence of mental health problems.24 Of note, the authors received responses from a wide range of veterans in terms of age, military era, and care setting, including some veterans who do not use the VA. However, data are lacking for nonresponders, and the possibility remains that survey respondents self-selected and were more interested in or experienced with CAM than were nonrespondents. Regardless, many findings, including barriers to CAM and the interaction of pain and self-efficacy, are internally valid and are important to consider even if the sample is not representative of the veteran population.
Conclusion
No studies have focused on veteran use of independent CAM practices as defined for this study. These techniques (eg, meditation, qigong) may promote wellness and relieve common symptoms in veterans. The authors’ results suggest that a broad interest in independent CAM practices among veterans exists. The VA and other health care settings should consider implementing classes in these modalities, especially as their reach may be greater than other CAM modalities requiring one-on-one practitioner-patient interaction. Even with broader availability, patients with chronic pain may require extra attention and context to improve or overcome low health-related self-efficacy, maximizing their likelihood of engaging in CAM. This possibility needs to be explored.
Acknowledgments
Funding for this research was provided by the Veterans Affairs Office of Patient Centered Care and Cultural Transformation, which was not involved in the study design or production of the manuscript. The authors also acknowledge the work of Anna Rusiewicz, PhD, in developing the STAR Well-Kit that was disseminated during this study.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.
1. Eisenberg DM, Kessler RC, Van Rompay MI, et al. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Ann Intern Med. 2001;135(5):344-351.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;(12):1-23.
3. Frass M, Strassl RP, Friehs H, Müllner M, Kundi M, Kaye AD. Use and acceptance of complementary and alternative medicine among the general population and medical personnel: a systematic review. Ochsner J. 2012;12(1):45-56.
4. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252.
5. Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
6. Campbell DG, Turner AP, Williams RM, et al. Complementary and alternative medicine use in veterans with multiple sclerosis: prevalence and demographic associations. J Rehabil Res Dev. 2006;43(1):99-110.
7. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704.
8. McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med. 2006;6:34.
9. Ezeji-Okoye SC, Kotar TM, Smeeding SJ, Durfee JM. State of care: complementary and alternative medicine in Veterans Health Administration—2011 survey results. Fed Pract. 2013;30(11):14-19.
10. Cabral P, Meyer HB, Ames D. Effectiveness of yoga therapy as a complementary treatment for major psychiatric disorders: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(4).
11. Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012;13(1):1-9.
12. Lee SW, Mancuso CA, Charlson ME. Prospective study of new participants in a community-based mind-body training program. J Gen Intern Med. 2004;19(7):760-765.
13. Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677-687.
14. Lee MS, Lee MS, Kim HJ, Choi ES. Effects of qigong on blood pressure, high-density lipoprotein cholesterol and other lipid levels in essential hypertension patients. Int J Neurosci. 2004;114(7):777-786.
15. Lee MS, Lim HJ, Lee MS. Impact of qigong exercise on self-efficacy and other cognitive perceptual variables in patients with essential hypertension. J Altern Complement Med. 2004;10(4):675-680.
16. U.S. Department of Veterans Affairs, War Related Illness & Injury Study Center. STAR Well-Kit. http://www.warrelatedillness.va.gov/WARRELATEDILLNESS/education/STAR/index.asp. Updated September 18, 2015. Accessed July 6, 2016.
17. Benyamini Y, Idler EL, Leventhal H, Leventhal EA. Positive affect and function as influences on self-assessments of health: expanding our view beyond illness and disability. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):P107-P116.
18. Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995;50(6):S344-S353.
19. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738.
20. Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9(1):9-14.
21. Kroenke K. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-621.
22. Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256-262.
23. Kim MT, Han HR, Song HJ, et al. A community-based, culturally tailored behavioral intervention for Korean Americans with type 2 diabetes. Diabetes Educ. 2009;35(6):986-994.
24. Webel AR, Okonsky J. Psychometric properties of a Symptom Management Self-Efficacy Scale for women living with HIV/AIDS. J Pain Symptom Manage. 2011;41(3):549-557.
25. Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
26. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. http://www.va.gov/vetdata. Updated June 3, 2016. Accessed July 6, 2016.
27. Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299-309.
Acute HIV Causes Transient Neurologic Findings
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.
Clinical Question: How common are neurologic findings in acute HIV infection?
Background: The incidence of neurologic findings with acute HIV is unknown.
Study Design: Cohort study.
Setting: Bangkok, Thailand.
Synopsis: In this study, 134 patients were identified after presenting for voluntary HIV testing. Five others were enrolled through an ongoing local study. All 139 participants underwent structured neurologic evaluations at enrollment (median of 19 days after presumed exposure), then at four and 12 weeks. Combination antiretroviral therapy (cART) was initiated immediately after initial evaluation.
The cohort was 93% male. Mean age was younger than 30 years. Fifty-three percent of participants experienced some neurologic finding within 12 weeks of diagnosis. One-third (33%) were cognitive symptoms, predominantly problems of concentration (24% of patients) and memory (16% of patients). One-third (34%) were motor findings, and 11% were neuropathy. Forty-nine percent of the neurologic issues were present at diagnosis. Symptoms were mostly mild, although one patient developed fulminant Guillain-Barré syndrome. Patients with neurologic findings had higher viral loads at diagnosis (mean plasma log10 HIV RNA 5.9 versus 5.4; P = 0.006). Participants with and without neurologic findings had similar cerebral spinal fluid viral loads (mean log10 HIV RNA 3.7 versus 3.1, P = 0.14) and serum CD4 counts (339 versus 381 cells/mm3; P = 0.46). Neurologic findings resolved within one month of cART treatment in 90% of patients. Study limitations include lack of a control cohort and potential confounding from illicit drug use among participants.
Bottom Line: Acute HIV infection commonly causes mild neurologic problems, which remit with treatment.
Citation: Hellmuth J, Fletcher JL, Valcour V, et al. Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology. 2016;87(2):148-154.
Two-Minute Screen Effective for Post-Op Delirium
Clinical Question: Is the 10-point cognitive screener (10-CS) effective in screening for delirium in older adults with hip fracture?
Background: Delirium in elderly hip fracture patients has been established as a significant comorbidity. There is, however, no agreement on the most appropriate and practical screening tool. Commonly used screening methods, which focus on the detection of cognitive impairment as a surrogate, are time-consuming, insensitive for mild impairment, and limited in their application to patients with impaired dexterity and poor education.
Study Design: Prospective cohort study.
Setting: Tertiary referral hospital in São Paulo, Brazil.
Synopsis: In the study, 147 consecutive hip fracture patients over age 60 were screened using the 10-CS. This test stratifies patients into three categories: normal, possible, and probable cognitive impairment. Development of in-hospital delirium was evaluated by daily Confusion Assessment Method testing administered by a geriatrician. Patients categorized as probable cognitive impairment were more likely to develop delirium (hazard ratio, 7.48; 95% CI, 2.2–25.4).
Hospitalists involved in perioperative care should consider using this simple screening tool. With an area under ROC curve of 0.83 (95% CI, 0.76–0.89), it effectively detects delirium in this high-risk population. Independently, patients who developed delirium had a longer length of stay (median 11.0 versus 7.0; P < 0.001). This serves as a reminder of the importance of screening and preventing delirium in this population.
Bottom Line: The 10-CS tool is practical in its application and effective in identifying elderly hip fracture patients at risk for delirium.
Citation: Fortes-Filho SQ, Apolinario D, Melo JA, Suzuki I, Sitta MD, Garcez-Leme LE. Predicting delirium after hip fracture with a 2-min cognitive screen: prospective cohort study [published online ahead of print May 17, 2016]. Age Ageing. pii:afw084.
Clinical Question: Is the 10-point cognitive screener (10-CS) effective in screening for delirium in older adults with hip fracture?
Background: Delirium in elderly hip fracture patients has been established as a significant comorbidity. There is, however, no agreement on the most appropriate and practical screening tool. Commonly used screening methods, which focus on the detection of cognitive impairment as a surrogate, are time-consuming, insensitive for mild impairment, and limited in their application to patients with impaired dexterity and poor education.
Study Design: Prospective cohort study.
Setting: Tertiary referral hospital in São Paulo, Brazil.
Synopsis: In the study, 147 consecutive hip fracture patients over age 60 were screened using the 10-CS. This test stratifies patients into three categories: normal, possible, and probable cognitive impairment. Development of in-hospital delirium was evaluated by daily Confusion Assessment Method testing administered by a geriatrician. Patients categorized as probable cognitive impairment were more likely to develop delirium (hazard ratio, 7.48; 95% CI, 2.2–25.4).
Hospitalists involved in perioperative care should consider using this simple screening tool. With an area under ROC curve of 0.83 (95% CI, 0.76–0.89), it effectively detects delirium in this high-risk population. Independently, patients who developed delirium had a longer length of stay (median 11.0 versus 7.0; P < 0.001). This serves as a reminder of the importance of screening and preventing delirium in this population.
Bottom Line: The 10-CS tool is practical in its application and effective in identifying elderly hip fracture patients at risk for delirium.
Citation: Fortes-Filho SQ, Apolinario D, Melo JA, Suzuki I, Sitta MD, Garcez-Leme LE. Predicting delirium after hip fracture with a 2-min cognitive screen: prospective cohort study [published online ahead of print May 17, 2016]. Age Ageing. pii:afw084.
Clinical Question: Is the 10-point cognitive screener (10-CS) effective in screening for delirium in older adults with hip fracture?
Background: Delirium in elderly hip fracture patients has been established as a significant comorbidity. There is, however, no agreement on the most appropriate and practical screening tool. Commonly used screening methods, which focus on the detection of cognitive impairment as a surrogate, are time-consuming, insensitive for mild impairment, and limited in their application to patients with impaired dexterity and poor education.
Study Design: Prospective cohort study.
Setting: Tertiary referral hospital in São Paulo, Brazil.
Synopsis: In the study, 147 consecutive hip fracture patients over age 60 were screened using the 10-CS. This test stratifies patients into three categories: normal, possible, and probable cognitive impairment. Development of in-hospital delirium was evaluated by daily Confusion Assessment Method testing administered by a geriatrician. Patients categorized as probable cognitive impairment were more likely to develop delirium (hazard ratio, 7.48; 95% CI, 2.2–25.4).
Hospitalists involved in perioperative care should consider using this simple screening tool. With an area under ROC curve of 0.83 (95% CI, 0.76–0.89), it effectively detects delirium in this high-risk population. Independently, patients who developed delirium had a longer length of stay (median 11.0 versus 7.0; P < 0.001). This serves as a reminder of the importance of screening and preventing delirium in this population.
Bottom Line: The 10-CS tool is practical in its application and effective in identifying elderly hip fracture patients at risk for delirium.
Citation: Fortes-Filho SQ, Apolinario D, Melo JA, Suzuki I, Sitta MD, Garcez-Leme LE. Predicting delirium after hip fracture with a 2-min cognitive screen: prospective cohort study [published online ahead of print May 17, 2016]. Age Ageing. pii:afw084.