User login
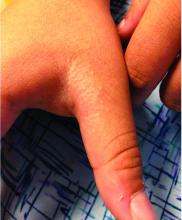
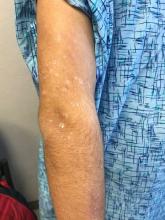
Rash on hands and feet
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
A 9-year-old healthy Kuwaiti male with no significant past medical history presents with a rash on his hands and feet that has been present for 3 years.
His mother reports that he has been seen by dermatologists in various countries and was last seen by a dermatologist in Kuwait 3 years ago. At that time, he was told that it was dryness and advised to not shower daily. Since then he has been taking showers three times weekly and using Cetaphil once weekly without improvement. He was seen by his pediatrician 6 months ago, diagnosed with xerosis, and was given hydrocortisone 2.5% to use twice daily, again without any improvement.
The rash is not itchy, and he has no oral lesions or nail involvement. Exam revealed lichenoid papules on bilateral dorsal hands and feet, bilateral upper arms, bilateral axilla, lower abdomen, and left upper chest.
Make the Diagnosis
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
A skin biopsy of one of the lesions showed granulomatous inflammation composed of lymphocytes, macrophages, and giant cells around hair follicles with negative mycobacterium stains and fungal stains, consistent with granulomatous periorificial dermatitis. Tissue cultures from a skin biopsy for aerobic bacteria, mycobacteria, and fungus all were negative.
The patient initially was treated with erythromycin, but after 2 weeks, he reported abdominal pain and nausea and was unable to tolerate the medication. He was switched to clarithromycin, which he took for 6 weeks with clearance of the lesions.
A year later, some of the lesions recurred. He was treated again with clarithromycin and the lesions resolved.
Childhood granulomatous periorificial dermatitis (CGPD) is a benign skin eruption that occurs in prepubertal children. It also has been called facial Afro-Caribbean childhood eruption (FACE), and it tends to occur most commonly in children of darker skin types.1 but there are some cases reported of extra facial involvement.2 The lesions usually are not symptomatic, and they are more common in boys. The cause of this condition is not known, but possible triggers could include prior exposure to topical and systemic corticosteroids, as well as exposure to certain allergens such as formaldehyde.1
In histopathology, the lesions are characterized by granulomatous infiltrates around the hair follicles and the upper dermis. The granulomas are formed of macrophages, lymphocytes, and giant cell, as were seen in our patient.3
Several conditions can look very similar to CGPD; these include sarcoidosis, lupus miliaris disseminatus faciei (LMDF), and granulomatous rosacea.
Sarcoidosis is a rare condition in children, and the lesions can be similar to the ones seen in our patient. Patients with sarcoidosis usually present with other systemic symptoms including fever, weight loss, respiratory symptoms, and fatigue; none of these were seen in our patient. Under the microscope, the lesions are characterized by “naked granulomas” instead of the inflammatory granulomas seen on our patient.
Lupus miliaris disseminatus faciei is a rare inflammatory skin condition commonly seen in young adults and is thought to be a variant of rosacea. It is characterized by skin-color to pink to yellow dome-shaped papules on the central face. Histologically, the lesions present as dermal epithelioid cell granulomas with central necrosis and surrounding lymphocytic infiltrate with multinucleate giant cells.4
Granulomatous rosacea and CGPD are considered two separate entities. Granulomatous rosacea tends to have a more chronic course, is not that common in children, and clinically presents with pustules, papules, and cysts around the eyes and cheeks.
Infectious processes like tuberculosis and fungal infections were ruled out in our patient with cultures and histopathology. Allergic contact dermatitis on the face can present with skin-color to pink papules, but they usually are very pruritic and improve with topical corticosteroids, while these medications can worsen CGPD.
CGPD can be a self-limiting condition. When mild, it can be treated with topical metronidazole, topical erythromycin, topical clindamycin solution, or pimecrolimus. Our patient failed treatment with pimecrolimus. For severe presentations, oral tetracyclines, erythromycin, and other macrolides, metronidazole, and oral isotretinoin can help clear the lesions.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Ann Dermatol. 2011 Aug;23(3):386-8.
2. Int J Dermatol. 2007 Feb;46(2):143-5.
3. J Cutan Med Surg. 2009 Feb 28;13(2):115-8.
4. An Bras Dermatol. 2017 Nov-Dec;92(6):851-3.
5. Indian Dermatol Online J. 2018 Jan-Feb; 9(1):68-70.
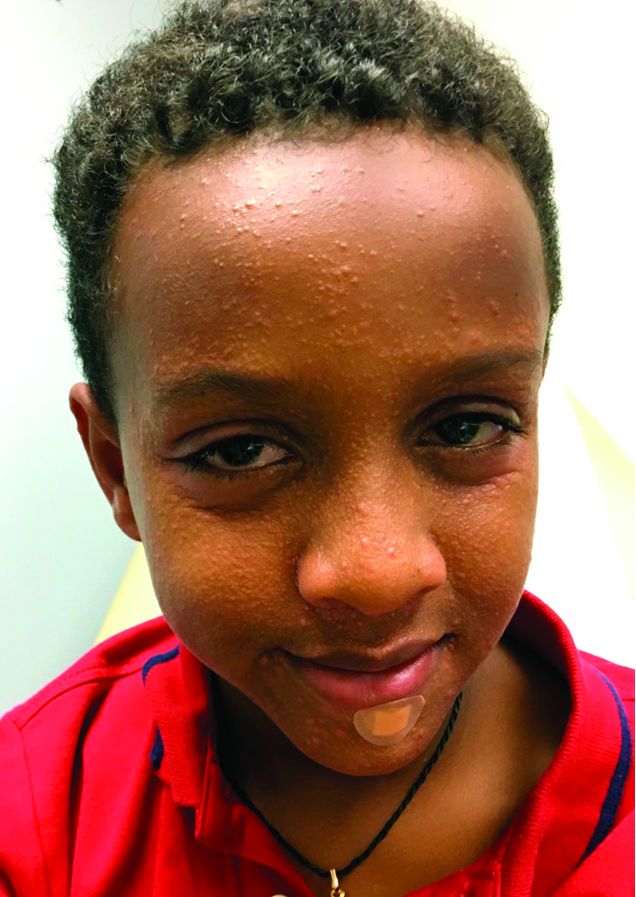
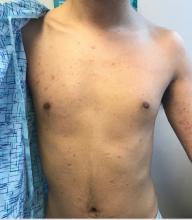
An 8-year-old African American male presented to our pediatric dermatology clinic for evaluation of a 3-month history of flesh-colored bumps on the face. According to the patient's mother, the lesions started with small pimple-like lesions around the nose and then spread to the whole face. Some lesions were crusty and somewhat itchy. He was treated with cephalexin and pimecrolimus with no improvement. The mother was very concerned because the lesions were close to the eyes and spreading.
He had no fevers, arthritis, or upper respiratory or gastrointestinal symptoms. He recently came back from a trip to Africa to visit his family. No other family members were affected. He used some new soaps, sunscreens, and moisturizers while he was in Africa.
On physical examination, the boy was in no acute distress. He had multiple flesh-colored papules on the face, especially around the eyes, nose, and mouth, where some lesions appeared crusted. There were no other skin lesions elsewhere on his body. There was no lymphadenopathy or hepatosplenomegaly.
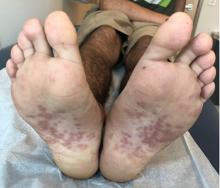
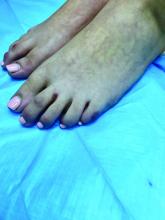
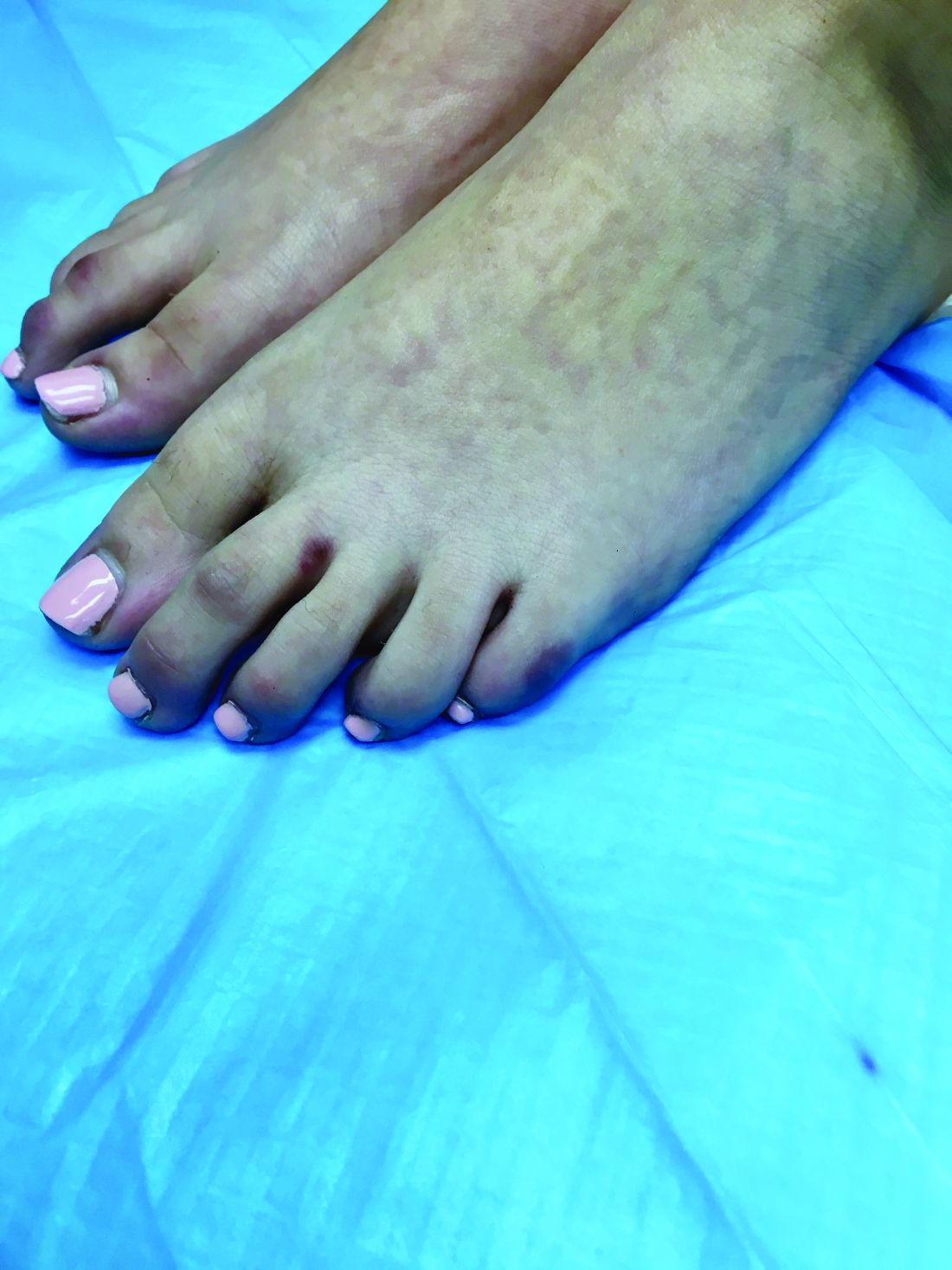
Pink scaly rash on torso and extremities
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
Tumor necrosis factor–alpha (TNF-alpha) inhibitors are therapeutic agents used to treat a variety of inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease, as well as psoriasis of the skin (PSO) and psoriatic arthritis. In a 2017 systematic review, there were 216 reported cases of new-onset TNF-alpha inhibitor–induced psoriasis, with an estimated rate of 1 per 1,000. The cases thus far have had a wide range of presentations, the most common being plaque psoriasis, scalp psoriasis, as well as palmoplantar pustular psoriasis.1
A retrospective chart review study at Mayo clinic published in 2017 evaluated children younger than 19 years seen in 2003-2015 who developed new-onset or recurrent PSO with a history of inflammatory bowel disease being treated with anti-TNF-alpha therapy. The review showed variable latency in the development of PSO in these patients, although it typically occurred during inflammatory bowel disease remission.2 It is unclear whether there is an association between a personal or family history of psoriasis and development of these lesions.
TNF-alpha, interleukin (IL)–17) and interferon-alpha (IFN-alpha) are main cytokines that contribute to the development of psoriasis. The mechanism of action for paradoxical PSO/psoriasis in patients treated with anti-TNF is not clearly understood; however, many hypotheses are based on an imbalance between TNF-alpha and interferon-alpha – more specifically, an increased production of interferon-alpha. TNF-alpha inhibits the activity of plasmacytoid dendritic cells which are key producers of IFN-alpha. Because of this blockade, there is unopposed IFN-alpha production. Interferon-alpha allows for the expression of chemokines such as CXCR3, which favor T cells homing to the skin. IFN-alpha also stimulates and activates T cells to produce TNF-alpha and IL-17, which in turn sustains inflammatory mechanisms and allows for the development of psoriatic lesions.3
There are no universal management guidelines. Most of these patients’ treatment plans mirror standard psoriasis therapies while the main question remains the decision to continue the same anti-TNF therapy, change anti-TNF agents, or entirely switch classes of biologic or other systemic therapy. This decision in management requires several considerations: treatability of TNF-alpha inhibitor-induced psoriasis, the severity of background disease (i.e., rheumatoid arthritis, inflammatory bowel disease, other systemic condition), and whether the underlying disease is well controlled on current therapy, as well as the consideration of possible loss in efficacy if a drug is discontinued and then restarted at a later date.4
A reasonable initial approach in patients with well-controlled underlying disease and mild skin eruption is to continue anti-TNF therapy and manage skin topically with topical corticosteroids and/or phototherapy. In patients that either do not have well-controlled underlying disease or moderate skin involvement, changing to an alternative anti-TNF or other agent may be reasonable, and requires coordinated care with involved specialists. In the 2017 pediatric review mentioned previously, nearly half of the patients required a change in their initial anti-TNF-alpha agent despite conventional skin-directed therapies, and one-third of patients discontinued all anti-TNF-alpha therapy because of PSO.2
The psoriasiform papulosquamous features of this case along with the history suggests the diagnosis. Pityriasis rosea would be highly atypical on the feet and with the duration of findings. Lichen planus and atopic dermatitis morphology are inconsistent with this eruption, and coxsackie viral infection would have a shorter course.
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Email them at [email protected].
References
1. J Am Acad Dermatol. 2017 Feb;76(2):334-41.
2. Pediatr Dermatol. 2017 May;34(3):253-60.
3. RMD Open. 2016 Jul 15;2(2):e000239.
4. J Psoriasis Psoriatic Arthritis. 2019 Apr;4(2):70-80.
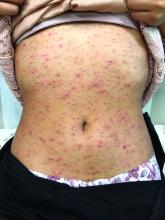
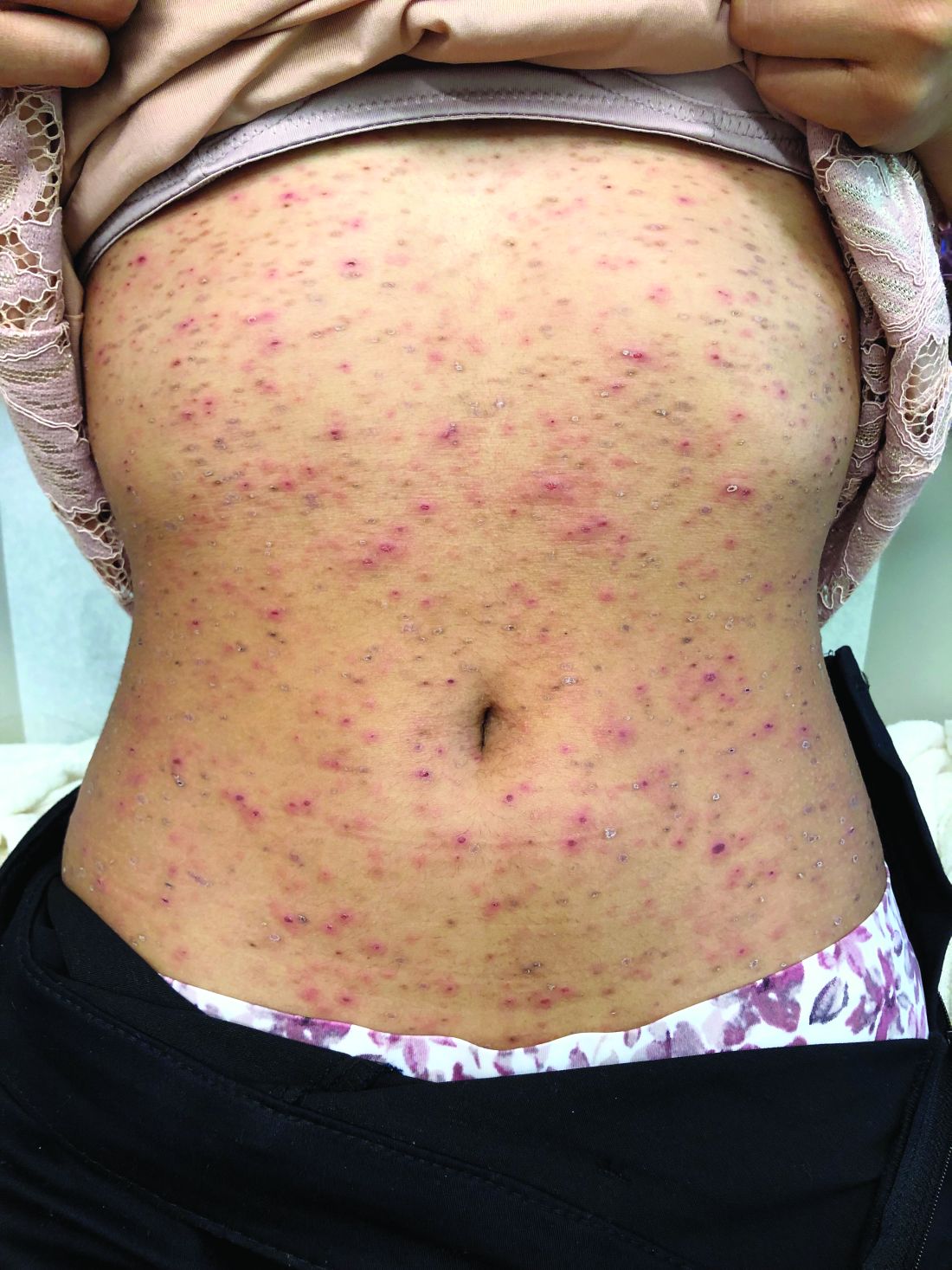
A 17-year-old male with a history of Crohn's disease, well controlled on infliximab, is seen for evaluation of a pink scaly rash on the torso, upper extremities, and lower extremities. The rash began 5 months previously and has been mostly asymptomatic. The patient denies pruritus or pain at the affected areas. There is no fever or drainage from any of the sites. The patient has not undergone any treatments. He does not have a personal or family history of chronic skin conditions.
On physical exam, he is noted to have numerous pink papules and plaques with overlying scale on his trunk, as well as the dorsal aspects of bilateral hands and the plantar surfaces of bilateral feet. His skin is otherwise unremarkable.
Pruritic, pink to violaceous, scaly papules
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A skin biopsy of one of the lesions was consistent with pityriasis lichenoides et varioliformis acuta (PLEVA).
The patient was diagnosed with PLEVA, also known as Mucha-Habermann disease. The true incidence of the condition is not known.
The typical presentation is an abrupt onset of pink to violaceous, scaly papules and plaques that later develop violaceous or necrotic centers, like the ones seen in our patient. The lesions more typically occur on the trunk and proximal extremities, but they may present in any other part of the body, rarely in the mucosa.1 Some patients can develop the febrile, more severe form of PLEVA called febrile, ulceronecrotic Mucha-Habermann disease (FUMHD), which potentially can be life threatening.
Patients with PLEVA can complain of pruritus or a burning sensation, and in some cases can have associated arthralgia and edema. The more severe form FUMHD is characterized by persistent high fevers with associated internal organ involvement such as cardiomyopathy, small vessel vasculitis, abdominal pain, arthritis, pneumonitis, and hematologic abnormalities.2 Mucosal involvement is a common finding in patients with FUMHD.
The pathogenesis of PLEVA is not very well understood. Some theories include a T-cell dyscrasia and an atypical immune response to an infection or vaccination.3,4
The differential diagnosis of PLEVA includes varicella, pityriasis lichenoides chronica (PLC), lymphomatoid papulosis (LyP), disseminated herpes simplex infection, Gianotti-Crosti syndrome, and Langerhans cell histiocytosis.
Patients with varicella also present with lesions in different stages, similar to PLEVA, but the classic lesions are usually vesicular and described as dewdrops on a rose petal. The course of varicella is 1-2 weeks, compared with PLEVA where the lesions can be present for months to years.
Patients with PLC can have similar lesions to PLEVA, but the lesions rarely are necrotic. Some consider these two entities a spectrum of the same condition.5
LyP is a rare condition in children, and it is characterized by crops of pink papules and nodules that resolve within weeks. A skin biopsy may help distinguish between the two conditions because LyP lesions are characterized by atypical lymphocytes that are CD30 positive.
Children with Gianotti-Crosti syndrome present with papules and papulovesicles on the face, arms, buttocks, and legs, after an upper respiratory or GI infection. Sometimes the lesions may be hemorrhagic. Lesions resolve within weeks to months.
Hemorrhagic-crusted papules on a seborrheic and intertriginous distribution characterize Langerhans cell histiocytosis. These patients may present hepatosplenomegaly and lymphadenopathy – neither of which were present on our patient.
Children with mild PLEVA disease and who are not symptomatic may be followed without intervention. In those with more severe disease and who are symptomatic can be treated with tetracyclines such as minocycline or doxycycline or erythromycin for about 3 months.6,7 Phototherapy also is recommended as a first-line therapy. In cases that do not respond to oral antibiotics and light therapy, methotrexate can be an alternative.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. J Drugs Dermatol. 2019 Jul 1;18(7):690-1.
2. Pediatr Dermatol. 1991 Mar;8(1):51-7.
3. Arch Dermatol. 2000 Dec;136(12):1483-6.
4. Actas Dermosifiliogr. 2018 Sep;109(7):e6-10.
5. Pediatr Dermatol. 2018 Mar;35(2):213-9.
6. Pediatr Dermatol. 2012 Nov-Dec;29(6):719-24.
7. J Eur Acad Dermatol Venereol. 2019 Jul 18. doi: 10.1111/jdv.15813.
A healthy 14-year-old female was referred urgently by her pediatrician to our pediatric dermatology clinic for evaluation of a rash. The rash had been present for 4 weeks on her torso and proximal extremities, and had been spreading. She had been very itchy. She denied any fevers, chills, joint pain, oral or genital lesions.
She was visiting some family members in Washington State during the summer. The rash started 1 month after this visit.
The adolescent had been treated with acyclovir, trimethoprim/sulfamethoxazole, and intramuscular triamcinolone without improvement. She had been taking children's multivitamins occasionally. Her vaccinations were up-to-date. She denied any history of varicella or herpes infection. Her mom has a history of cold sores. The teen is not sexually active.
On physical examination, the girl was not in acute distress. Her vital signs were stable. She was not febrile. She had pink, scaly, and hyperpigmented papules and plaques, some of which were crusted with violaceous centers on the trunk and proximal extremities. There were no lesions on the mouth, palms, or soles. She had no lymphadenopathy or hepatosplenomegaly.
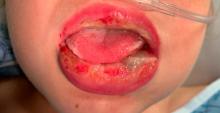
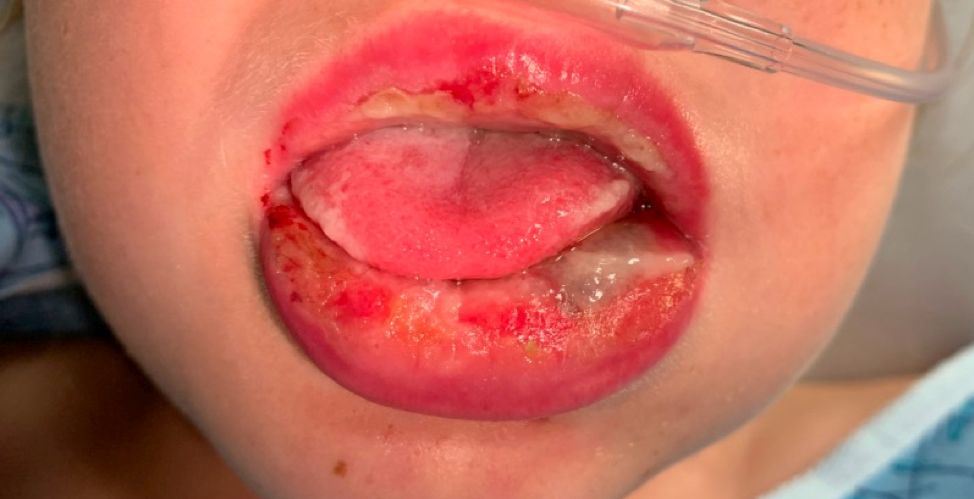
Pneumonia with tender, dry, crusted lips
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
Mycoplasma pneumoniae infection commonly manifests as an upper or lower respiratory tract infection with associated fever, dyspnea, cough, and coryza. However, patients can present with extrapulmonary complications with dermatologic findings including mucocutaneous eruptions. M. pneumoniae–associated mucocutaneous disease has prominent mucositis and typically sparse cutaneous involvement. The mucositis usually involves the lips and oral mucosa, eye conjunctivae, and nasal mucosa and can involve urogenital lesions. It predominantly is observed in children and adolescents. This condition is essentially a subtype of Stevens-Johnson syndrome, with a specific infection-associated etiology, and has been called “Mycoplasma pneumoniae–induced rash and mucositis,” shortened to “MIRM.”
Severe reactive mucocutaneous eruptions include erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). While there has been semantic confusion over the years, there are some distinctive characteristics.
EM is characterized by typical three-ringed target papules that are predominantly acral in location and often without mucosal involvement. The lesions are “multiforme” in that they can appear polymorphous and evolve during an episode, with erythematous macules progressing to edematous papules, sometimes with a halo of pallor and concentric “target-like” appearance. Lesions of EM are fixed, meaning individual lesions last 7-10 days, unlike urticarial lesions that last hours. EM classically is associated with herpes simplex virus infections which usually precede its development.
SJS and TEN display atypical macules and papules which develop into erythematous vesicles, bullae, and potentially extensive desquamation, usually presenting with fever and systemic symptoms, with multiple mucosal sites involved. SJS usually is defined by having bullae restricted to less than 10% of body surface area (BSA), TEN as greater than 30% BSA, and “overlap SJS-TEN” as 20%-30% skin detachment.1 SJS and TEN commonly are induced by medications and on a spectrum of drug hypersensitivity–induced epidermal necrolysis.
MIRM has been highlighted as a distinct, common condition, usually mucous-membrane predominant with involvement of two or more mucosal sites, less than 10% total BSA, the presence of few vesiculobullous lesions or scattered atypical targets with or without targetoid lesions (without rash is called MIRM sine rash), and clinical and laboratory evidence of atypical pneumonia.2 Other infections can cause similar eruptions (for example, Chlamydia pneumoniae), and a recent proposal by the Pediatric Dermatology Research Alliance has suggested the term “Reactive Infectious Mucocutaneous Eruption” (RIME) to include MIRM and other infection-induced reactions.
Laboratory diagnosis of M. pneumoniae is via serology or polymerase chain reaction. Antibody titers begin to rise approximately 7-9 days after infection and peak at 3-4 weeks. Enzyme immunoassay is more sensitive in detecting acute infection than culture and has sensitivity comparable to the polymerase chain reaction if there has been sufficient time to develop an antibody response.
The differential diagnosis between RIME/MIRM, SJS, and TEN may be difficult to distinguish in the first few days of presentation, and consideration of infections and possible medication causes is important. DRESS syndrome (drug reaction with eosinophilia and systemic symptoms) also is in the differential diagnosis. DRESS usually has a long latency (2-8 weeks) between drug exposure and disease onset.
Treatment of RIME/MIRM is supportive care and treatment of any underlying infection. Steroids and intravenous immune globulin (IVIG) have been used to treat reactive mucositis, as well as cyclosporine and biologic agents (such as etanercept), in an attempt to minimize the extent and duration of mucous membrane vesiculation and denudation. While these drugs may help shorten the duration of the disease course, controlled trials are lacking and there is little comparative literature on efficacy or safety of these agents.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. They said they have no financial disclosures. Email Dr. Eichenfield and Dr. Bhatti at [email protected].
References
1. Arch Dermatol. 1993 Jan;129(1):92-6.
2. J Am Acad Dermatol. 2015 Feb;72(2):239-45.
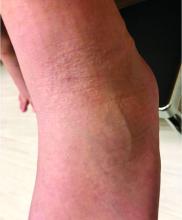
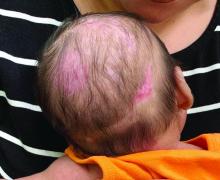
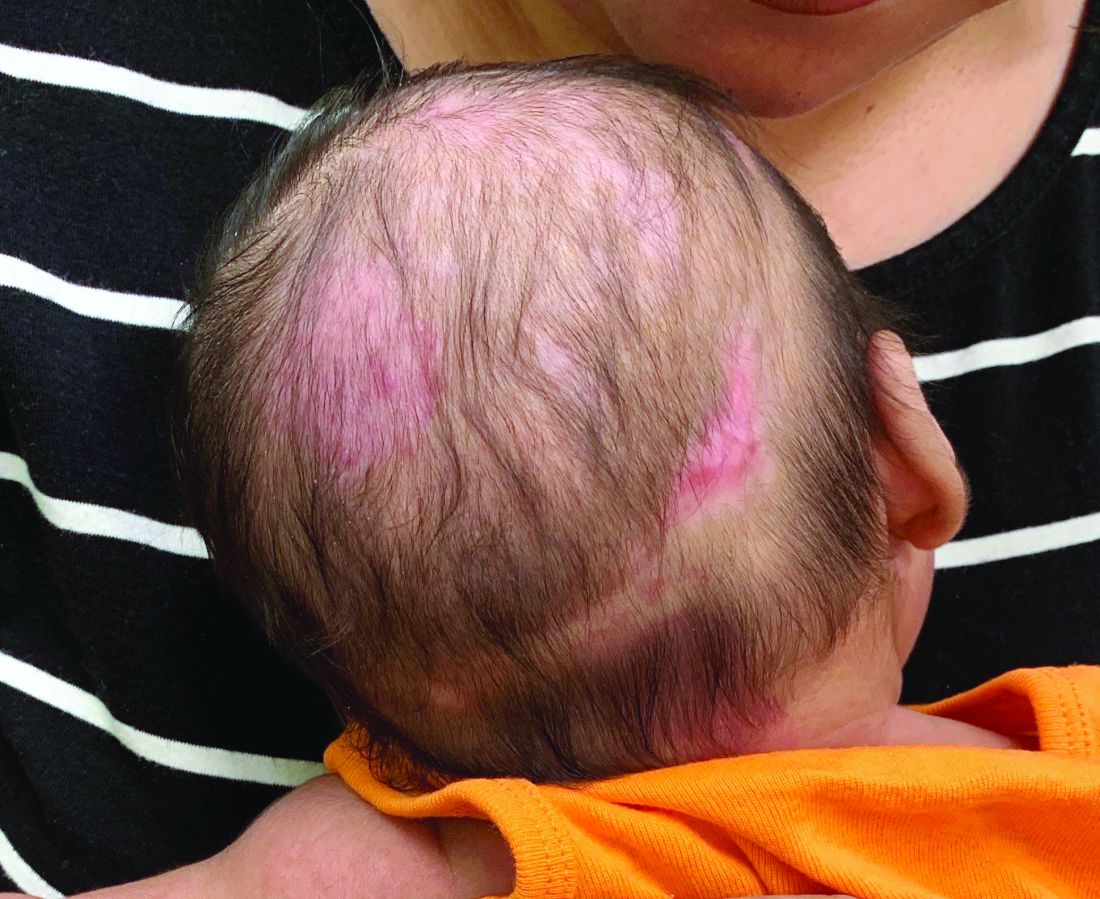
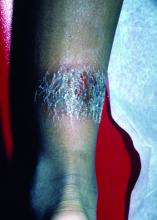
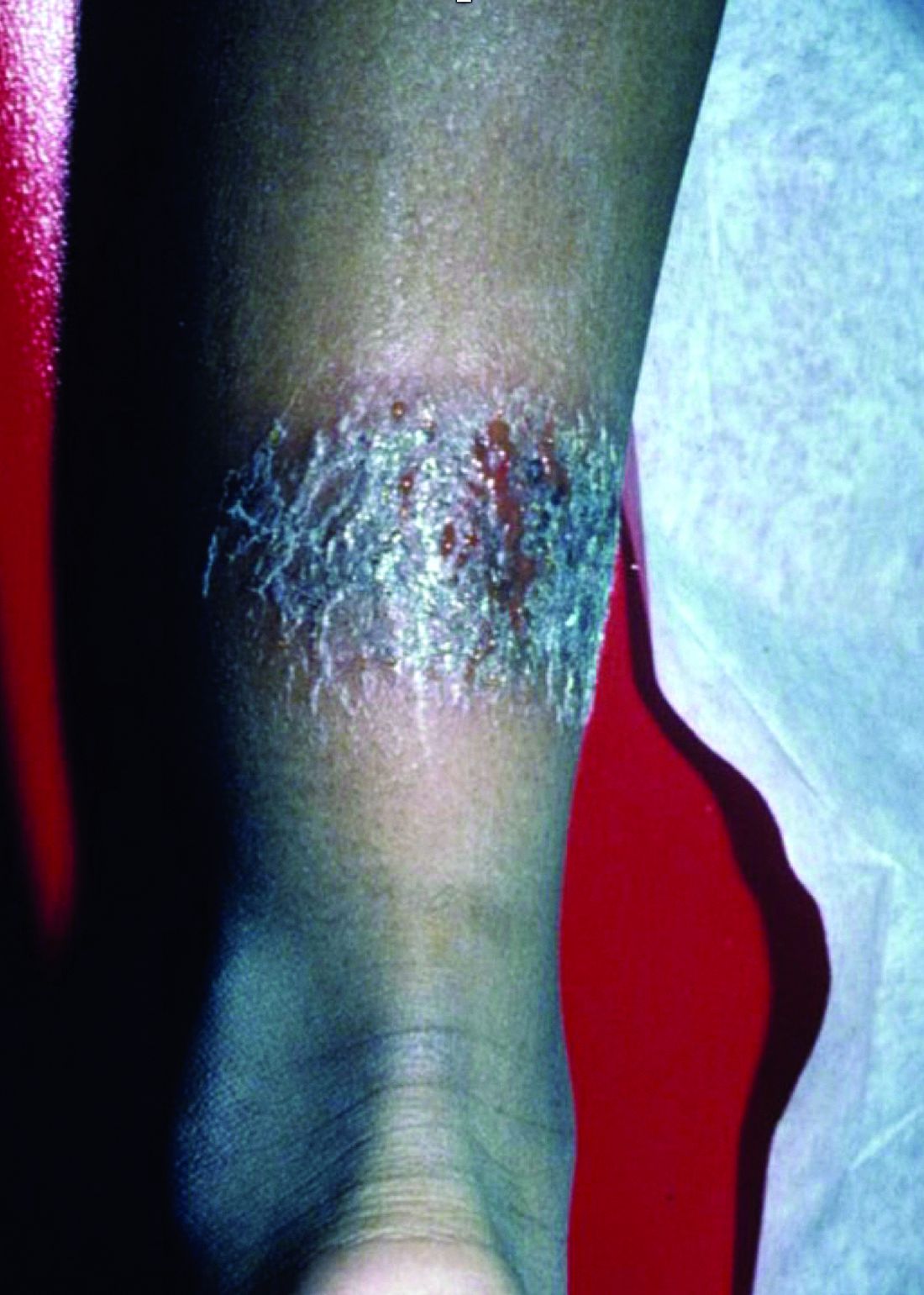
A 2-month-old infant with a scalp rash that appeared after birth
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
A 2-month-old male is referred to our pediatric dermatology clinic for evaluation of persistent seborrheic dermatitis. The mother reports that he presented with a rash on his scalp a few days after birth. She has been treating the crusted areas with clotrimazole and hydrocortisone and has noted improvement on the crusting, but now is worried that there is some scarring. The affected areas are not bleeding or tender. There are no other rashes elsewhere in the body.
He was born at 36 weeks from a 35-year-old gravida 1 para 0 woman with adequate prenatal care. The mother was diagnosed with preeclampsia and was induced. She had a prolonged labor and had premature rupture of membranes. The baby was delivered via cesarean section because of failure to progress and fetal distress; forceps, vacuum, and a scalp probe were not used during delivery. He was admitted to the neonatal unit for 5 days for sepsis work-up and respiratory distress. No intubation was needed.
Besides the preeclampsia, the mother denied any other medical conditions and was not taking any medications. He has met all developmental milestones for his age. He has no history of seizures.
On physical exam, there are semicircular patches of alopecia on the scalp. Some areas have pink, rubbery plaques with loss of hair follicles. On the frontal scalp, there are waxy plaques.
There is a blanchable violaceous patch on the occiput and there are some erythematous papules on the cheeks.
A 3-year-old is brought to the clinic for evaluation of a localized, scaling inflamed lesion on the left leg
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
Nummular dermatitis, or nummular eczema, is an inflammatory skin condition that is considered to be a distinctive form of idiopathic eczema, while the term also is used to describe lesional morphology associated with other conditions.
The term nummular derives from the Latin word for “coin,” as lesions are commonly annular plaques. Lesions of nummular dermatitis can be single or multiple. The typical distribution involves the extremities and, although less common, it can affect the trunk as well.
Nummular dermatitis may be associated with atopic dermatitis, or it can be an isolated condition.1 While the pathogenesis is uncertain, instigating factors include xerotic skin, insect bites, or scratches or scrapes.1Staphylococcus infection or colonization, contact allergies to metals such as nickel and less commonly mercury, sensitivity to formaldehyde or medicines such as neomycin, and sensitization to an environmental aeroallergen (such as Candida albicans, dust mites) are considered risk factors.2
The diagnosis of nummular dermatitis is clinical. Laboratory testing and/or biopsy generally are not necessary, although a bacterial culture can be considered in patients with exudative and/or crusted lesions to rule out impetigo as a primary process of secondary infection. In some cases, patch testing for allergic contact dermatitis may be useful.
The differential diagnosis of nummular dermatitis includes tinea corporis (ringworm), atopic dermatitis, allergic contact dermatitis, impetigo, and psoriasis. Tinea corporis usually presents as annular lesions with a distinct peripheral scaling, rather than the diffuse induration of nummular dermatitis. Potassium hydroxide preparation or fungal culture can identify tinea species. Nummular dermatitis may be seen in patients with atopic dermatitis, who should have typical history, morphology, and course consistent with standard diagnostic criteria. Allergic contact dermatitis can present with regional, localized eczematous plaques in areas exposed to contact allergens. Patterns of lesions in areas of contact and worsening with repeat exposures can be clues to this diagnosis. Impetigo can present with honey-colored crusted lesions and/or superficial erosions, or purulent pyoderma. Lesions can be single or multiple and generally appear less inflammatory than nummular dermatitis. Psoriasis lesions may be annular, are more common on extensor surfaces, and usually have more prominent overlying pinkish, silvery white or micaceous scale.
Management of nummular dermatitis requires strong anti-inflammatory medications, usually mid-potency or higher topical corticosteroids, along with moisturizers and limiting exposure to skin irritants. “Wet wraps,” with application of topical corticosteroids to wet skin with occlusive wet dressings can enhance response. Transition from higher strength topical corticosteroids to lower strength agents used intermittently can help achieve remission or cure. Management practices include less frequent bathing with lukewarm water, using hypoallergenic cleansers and detergents, and applying moisturizers frequently. If plaques do recur, they tend to do so in the same location and in some patients resolution may result in hyper or hypopigmentation. Refractory disease may be managed with intralesional steroid injections, or systemic medications such as methotrexate.3
Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital–San Diego and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither Dr. Tracy nor Dr. Eichenfield have any relevant financial disclosures. Email them at [email protected].
References
1. Pediatr Dermatol. 2012 Sep-Oct;29(5):580-3.
2. American Academy of Dermatology. Nummular Dermatitis Overview
3. Pediatr Dermatol. 2018 Sep;35(5):611-5.
On physical exam, he is noted to have a localized eczematous plaque with erythema and edema. Also, he is noted to have diffuse, fine xerosis of the bilateral lower extremities. His skin is otherwise nonremarkable.
A healthy 8-year-old boy presents with several skin-colored, round 1-3 mm papules on the nose, forehead, and cheeks
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A shave biopsy of one of the lesions was performed that showed a proliferation of nests of basaloid cells on the dermis with palisading and rare vacuolated clear cell change. A rare ductal structure with luminal proteinaceous contents was noted. The findings were consistent with a trichoepithelioma.
Trichoepitheliomas are rare, benign, adnexal skin tumors that can start in early childhood or during puberty. The lesions are most commonly seen in girls as skin color papules on the face, and sometimes on the trunk and the neck. Trichoepitheliomas can appear as a benign single lesion nonfamilial form or as a familial form with multiple lesions.1 Brooke-Spiegler syndrome (BSS) is a rare autosomal dominant condition where affected individuals have multiple trichoepitheliomas, cylindromas, and spiradenomas. Depending on the predominant type of lesion, phenotypic variants include multiple familial trichoepithelioma type 1 and familial cylindromatosis.2 BSS is caused by mutations within CYLD, a tumor-suppressor gene located on chromosome 16q12-q13.3 Our patient presented only with trichoepitheliomas with no other lesions on the scalp, neck, or torso.
Multiple trichoepitheliomas also can be seen in other syndromes including Rombo syndrome, which is characterized by basal cell carcinomas, milia, hypotrichosis, distal vasodilation, and atrophoderma vermiculata; none seen in our patient. Bazex-Dupré-Christol syndrome is an X-linked dominant condition in which affected individuals can present with multiple trichoepitheliomas, as well as milia, hypotrichosis, follicular atrophoderma, and basal cell carcinomas.
The differential diagnosis of skin color papules on the central face on a child should include acne, flat warts, and angiofibromas seen in tuberous sclerosis. Our patient’s lesions were monomorphous, and there were no comedones, pustules, or inflammatory papules characteristic of acne.
He had warts on his hands which could make it suspicious for the face lesions to be verrucous in nature. Flat warts also present as skin color papules, but characteristically are flat, not round and shiny as our patient’s lesions were. Angiofibromas, as seen in individuals with tuberous sclerosis, also can start at an early age in the same location as trichoepitheliomas in BSS, but clinically the lesions are pinker and redder rather than the skin-color, round shape papules characteristic of trichoepitheliomas. Patients may have other findings suggestive of tuberous sclerosis including confetti hypopigmentation, ash leaf spots, shagreen patch, and a history of seizures or developmental delay – none of which were present in our patient. Children with basal cell nevus syndrome can present with skin color to shiny telangiectatic papules (basal cell carcinomas) that can be single or multiple on the face, chest, and back. The lesions usually are not seen in clusters around the nose and central face as seen in patients with BSS. Patients with basal cell nevus syndrome can develop jaw bone cysts, brain tumors (medulloblastoma), and fibromas on the heart or ovaries, palmar pits and be macrocephalic.4
Trichoepitheliomas usually are treated surgically but other nonsurgical removing techniques include laser resurfacing, curettage, and electrocautery.5 Malignant transformation can occur in 5%-10% of the individuals and should be managed by a multidisciplinary team. Topical treatment with sirolimus previously has been reported to be effective in young patients.6
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. She said she had no relevant financial disclosures. Email Dr. Matiz at [email protected].
References
1. Acta Dermatovenerol Croat. 2018 Jun;26(2):162-5.
2. Eur J Med Genet. 2015;58(5):271-8.
3. Am J Dermatopathol. 2014;36(11):868-74.
4. Int J Dermatol. 2016 Apr;55(4):367-75.
5. Int J Dermatol. 2007;46(6):583-6.
6. Dermatol Ther. 2017 Mar. doi: 10.1111/dth.12458.
A white 8-year-old boy comes to our pediatric dermatology clinic with his mother for evaluation of acne. The lesions started about a year ago on his nose and now have spread to his cheeks. The bumps are not symptomatic. He has been applying over the counter salicylic acid and benzoyl peroxide gels with no help. The mother reports he has been growing well, denies any growth spurt, no axillary or genital hair or body odor noted.
None of the family members have a history of acne. The mother cannot recall any family members with similar lesions on the face. He has had some warts on his fingers for years and has been treated with over the counter salicylic acid. There is no family history of skin cancer.
On physical exam, he is a healthy young boy with several skin color, round papules 1-3 mm on the nose, forehead, and cheeks. There are no lesions on the scalp. He has abundant brown hair. He has few verrucous papules on the fingers. Axillary and genital hair is not noted. There is no body odor and he is Tanner stage I.
A 5-year-old boy with a papular rash on his arm
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
What is your diagnosis?
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
A skin biopsy of one of the lesions on the right toe showed dermal edema with an associated lymphohistiocytic infiltrate. There are scattered areas of perieccrine involvement and areas of vasculitis. Laboratory work up showed a normal complete blood count, a negative antinuclear antibodies (ANA) titer, a negative double-stranded DNA, normal levels of inflammatory markers, and negative cryoglobulins and cold agglutinins. The patient was diagnosed with pernio. The lesions improved within several weeks. She now wears thicker socks when she is ice skating.
Children, women, and the elderly are at a higher risk.1 This condition is frequently described in Northwestern Europe and the United Kingdom, especially in those living in houses without central heating.2
Clinically, the lesions appear a few hours or days after cold exposure on the toes, fingers, and in some unusual cases on the nose and the ears. The lesions present as erythematous to violaceous macules, papules, or nodules that in severe cases may blister and ulcerate. The lesions may be asymptomatic, pruritic, or tender. In children, pernio can be associated with the presence of cryoglobulins, cold agglutinins, anorexia nervosa, and genetic interferonopathy; it may precede the diagnosis of chronic myelomonocytic leukemia and may occur as a presenting sign of a blast crisis in acute lymphoblastic leukemia.3,4 The skin lesions usually resolve within days to a few weeks. Histopathologic analysis shows dermal edema with associated superficial and deep lymphohistiocytic infiltrate and perieccrine involvement.
The differential diagnosis of pernio includes other cold-induced syndromes such as Raynaud’s syndrome, cold panniculitis, cold urticaria, livedo reticularis, acrocyanosis, and chilblain lupus. In chilblain lupus (a form of chronic cutaneous lupus), the lesions may be very similar to pernio but the histopathology is consistent with changes of discoid lupus. Lesions of idiopathic palmoplantar hidradenitis present as erythematous tender nodules on the palms and the soles.5 The lesions can be triggered by vigorous physical activity, exposure to moisture, and excessive sweating. White, blue, and red discoloration of the fingers is seen in Raynaud’s phenomenon rather than the fixed erythematous to violaceous macules, papules, or nodules seen in pernio. Patients with erythromelalgia present with red painful palms and soles triggered by heat and, in contrast to pernio, relieved by cooling. Sweet syndrome, a febrile neutrophilic dermatoses, is characterized by tender erythematous papules and plaques with associated systemic symptoms. These patients may have an associated internal malignancy or infection, or the disorder may be triggered by medications or pregnancy.
Our patient had no systemic symptoms, and the pathology didn’t show any neutrophils. When the diagnosis is in doubt, a skin biopsy may help elucidate the diagnosis.
Once the diagnosis of pernio is made, it is recommended to order a complete blood count to rule out blood malignancies and cryoproteins.
Treatment of this condition consists of rewarming the extremity. If rewarming does not improve the patient’s symptoms, systemic treatment with nifedipine may be warranted.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Dr. Matiz said she had no relevant financial disclosures. Email her at [email protected].
References
1. Pediatrics. 2005 Sep;116(3):e472-5.
2. Mayo Clin Proc. 2014 Feb;89(2):207-15.
3. Pediatr Dermatol. 2018 Jan;35(1):e74-5.
4. Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
5. Eur J Pediatr. 2001 Mar;160(3):189-91.
An 8-year-old girl comes to our pediatric dermatology clinic in the company of her mother for evaluation of painless purple spots on her toes. The lesions have been present for about 2 weeks. She has not been treated with any medications or creams. She denies any fevers, weight loss, mouth ulcers, sun sensitivity, joint pain, or any other symptoms. The patient has been a very healthy girl with occasional colds and no recent illnesses. The girl has never been admitted to the hospital. All her vaccinations are up to date. She takes no chronic medications. She lives in San Diego with her parents and two siblings. The girl recently started practicing ice-skating several times a week. There is no family history of any chronic medical conditions. She has no pets.