User login
FDA Regulation of Predictive Clinical Decision-Support Tools: What Does It Mean for Hospitals?
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
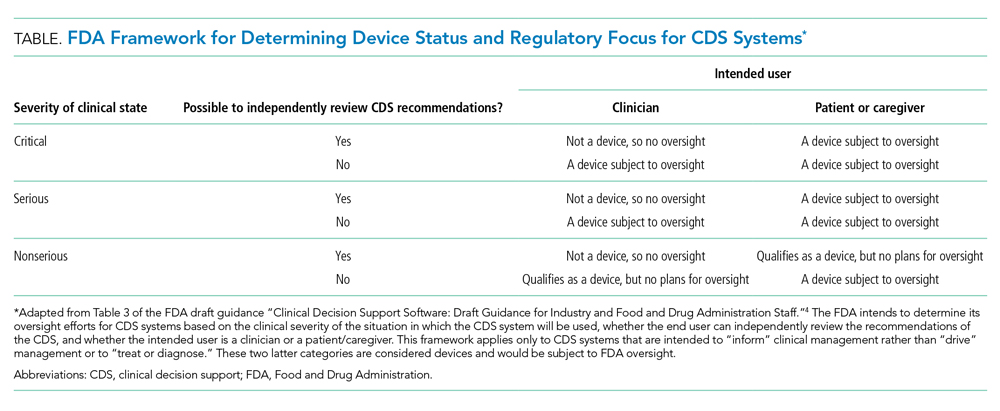
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.
Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.

Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
Recent experiences in the transportation industry highlight the importance of getting right the regulation of decision-support systems in high-stakes environments. Two tragic plane crashes resulted in 346 deaths and were deemed, in part, to be related to a cockpit alert system that overwhelmed pilots with multiple notifications.1 Similarly, a driverless car struck and killed a pedestrian in the street, in part because the car was not programmed to look for humans outside of a crosswalk.2 These two bellwether events offer poignant lessons for the healthcare industry in which human lives also depend on decision-support systems.
Clinical decision-support (CDS) systems are computerized applications, often embedded in an electronic health record (EHR), that provide information to clinicians to inform care. Although CDS systems have been used for many years,3 they have never been subjected to any enforcement of formal testing requirements. However, a draft guidance document released in 2019 from the Food and Drug Administration (FDA) outlined new directions for the regulation of CDS systems.4 Although the FDA has thus far focused regulatory efforts on predictive systems developed by private manufacturers,5,6 this new document provides examples of software that would require regulation for CDS systems that hospitals are already using. Thus, this new guidance raises critical questions—will hospitals themselves be evaluated like private manufacturers, be exempted from federal regulation, or require their own specialized regulation? The FDA has not yet clarified its approach to hospitals or hospital-developed CDS systems, which leaves open numerous possibilities in a rapidly evolving regulatory environment.
Although the FDA has officially regulated CDS systems under section 201(h) of the Federal Food, Drug, and Cosmetic Act (1938), only recently has the FDA begun to sketch the shape of its regulatory efforts. This trend to actually regulate CDS systems began with the 21st Century Cures Act (2016) that amended the definition of software systems that qualify as medical devices and outlined criteria under which a system may be exempt from FDA oversight. For example, regulation would not apply to systems that support “population health” or a “healthy lifestyle” or to ones that qualify as “electronic patient records” as long as they do not “interpret or analyze” data within them.7 Following the rapid proliferation of many machine learning and other predictive technologies with medical applications, the FDA began the voluntary Digital Health Software Precertification (Pre-Cert) Program in 2017. Through this program, the FDA selected nine companies from more than 100 applicants and certified them across five domains of excellence. Notably, the Pre-Cert Program currently allows for certification of software manufacturers themselves and does not approve or test actual software devices directly. This regulatory pathway will eventually allow manufacturers to apply under a modified premarket review process for individual software as a medical device (SaMD) that use artificial intelligence (AI) and machine learning. In the meantime, however, many hospitals have developed and deployed their own predictive CDS systems that cross the boundaries into the FDA’s purview and, indeed, do “interpret or analyze” data for real-time EHR alerts, population health management, and other applications.
Regulatory oversight for hospitals could provide quality or safety standards where currently there are none. However, such regulations could also interfere with existing local care practices, hinder rapid development of new CDS systems, and may be perceived as interfering in hospital operations. With the current enthusiasm for AI-based technologies and the concurrent lack of evidence to suggest their effectiveness in practice, regulation could also prompt necessary scrutiny of potential harms of CDS systems, an area with even less evidence. At the same time, CDS developers—private or hospital based—may be able to avoid regulation for some devices with well-placed disclaimers about the intended use of the CDS, one of the FDA criteria for determining the degree of oversight. If the FDA were to regulate hospitals or hospital-developed CDS systems, there are several unanswered questions to consider so that such regulations have their intended impact.
First, does the FDA intend to regulate hospitals and hospital-developed software at all? The framework for determining whether a CDS system will be regulated depends on the severity of the clinical scenario, the ability to independently evaluate the model output, and the intended user (Table). Notably, many types of CDS systems that would require regulation under this framework are already commonplace. For example, the FDA intends to regulate software that “identifies patients who may exhibit signs of opioid addiction,” a scenario similar to prediction models already developed at academic hospitals.8 The FDA also plans to regulate a software device even if it is not a CDS system if it is “intended to generate an alarm or an alert to notify a caregiver of a life-threatening condition, such as stroke, and the caregiver relies primarily on this alarm or alert to make a treatment decision.” Although there are no published reports of stroke-specific early warning systems in use, analogous nonspecific and sepsis-specific early warning systems to prompt urgent clinical care have been deployed by hospitals directly9 and developed for embedding in commercial EHRs.10 Hospitals need clarification on the FDA’s regulatory intentions for such CDS systems. FDA regulation of hospitals and hospital-developed CDS systems would fill a critical oversight need and potentially strengthen processes to improve safety and effectiveness. But burdensome regulations may also restrain hospitals from tackling complex problems in medicine for which they are uniquely suited.

Such a regulatory environment may be especially prohibitive for safety-net hospitals that could find themselves at a disadvantage in developing their own CDS systems relative to large academic medical centers that are typically endowed with greater resources. Additionally, CDS systems developed at academic medical centers may not generalize well to populations in the community setting, which could further deepen disparities in access to cutting-edge technologies. For example, racial bias in treatment and referral patterns could bias training labels for CDS systems focused on population health management.11 Similarly, the composition of patient skin color in one population may distort predictions of a model in another with a different distribution of skin color, even when the primary outcome of a prediction model is gender.12 Additional regulatory steps may apply for models that are adapted to new populations or recalibrated across locations and time.13 Until there is more data on the clinical impact of such CDS systems, it is unknown how potential differences in evaluation and approval would actually affect clinical outcomes.
Second, would hospitals be eligible for the Pre-Cert program, and if so, would they be held to the same standards as a private technology manufacturer? The domains of excellence required for precertification approval such as “patient safety,” “clinical responsibility,” and “proactive culture” are aligned with the efforts of hospitals that are already overseen and accredited by organizations like the Joint Commission on Accreditation of Healthcare Organizations and the American Nurses Credentialing Center. There is limited motivation for the FDA to be in the business of regulating these aspects of hospital functions. However, while domains like “product quality” and “cybersecurity” may be less familiar to some hospitals, these existing credentialing bodies may be better suited than the FDA to set and enforce standards for hospitals. In contrast, private manufacturers may have deep expertise in these latter domains. Therefore, as with public-private partnerships for the development of predictive radiology applications,14 synergies between hospitals and manufacturers may also prove useful for obtaining approvals in a competitive marketplace. Simultaneously, such collaborations would continue to raise questions about conflicts of interest and data privacy.
Finally, regardless of how the FDA will regulate hospitals, what will become of predictive CDS systems that fall outside of the FDA’s scope? Hospitals will continue to find themselves in the position of self-regulation without clear guidance. Although the FDA suggests that developers of unregulated CDS systems still follow best practices for software validation and cybersecurity, existing guidance documents in these domains do not cover the full range of concerns relevant to the development, deployment, and oversight of AI-based CDS systems in the clinical domain. Nor do most hospitals have the infrastructure or expertise to oversee their own CDS systems. Disparate recommendations for development, training, and oversight of AI-based medical systems have emerged but have yet to be endorsed by a federal regulatory body or become part of the hospital accreditation process.15 Optimal local oversight would require a collaboration between clinical experts, hospital operations leaders, statisticians, data scientists, and ethics experts to ensure effectiveness, safety, and fairness.
Hospitals will remain at the forefront of developing and implementing predictive CDS systems. The proposed FDA regulatory framework would mark an important step toward realizing benefit from such systems, but the FDA needs to clarify the requirements for hospitals and hospital-developed CDS systems to ensure reasonable standards that account for their differences from private software manufacturers. Should the FDA choose to focus regulation on private manufacturers only, hospitals leaders may both feel more empowered to develop their own local CDS tools and feel more comfortable buying CDS systems from vendors that have been precertified. This strategy would provide an optimal balance of assurance and flexibility while maintaining quality standards that ultimately improve patient care.
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
1. Sumwalt RL III, Landsbert B, Homendy J. Assumptions Used in the Safety Assessment Process and the Effects of Multiple Alerts and Indications on Pilot Performance. National Transportation Safety Board; 2019. https://www.ntsb.gov/investigations/AccidentReports/Reports/ASR1901.pdf
2. Becic E, Zych N, Ivarsson J. Vehicle Automation Report. National Transportation Safety Board; 2019. https://dms.ntsb.gov/public/62500-62999/62978/629713.pdf
3. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. https://doi.org/10.1038/s41746-020-0221-y
4. Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed October 15, 2019. https://www.fda.gov/media/109618/download
5. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2410. https://doi.org/10.1001/jama.2016.17216
6. Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digital Medicine. 2018;1(1):39. https://doi.org/10.1038/s41746-018-0040-6
7. Changes to Existing Medical Software Policies Resulting from Section 3060 of the 21st Century Cures Act: Guidance for Industry and Food and Drug Administration Staff. Food and Drug Administration. September 27, 2019. Accessed March 18, 2020. https://www.fda.gov/media/109622/download
8. Lo-Ciganic W-H, Huang JL, Zhang HH, et al. Evaluation of machine-learning algorithms for predicting opioid overdose risk among Medicare beneficiaries with opioid prescriptions. JAMA Netw Open. 2019;2(3):e190968. https://doi.org/10.1001/jamanetworkopen.2019.0968
9. Smith MEB, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: a systematic review. Ann Am Thorac Soc. 2014;11(9):1454-1465. https://doi.org/10.1513/annalsats.201403-102oc
10. WAVE Clinical Platform 510(k) Premarket Notification. Food and Drug Administration. January 4, 2018. Accessed March 3, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K171056
11. Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447-453. https://doi.org/10.1126/science.aax2342
12. Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. Proc Machine Learning Res. 2018;81:1-15.
13. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD). Food and Drug Administration. April 2, 2019. Accessed April 6, 2020. https://www.regulations.gov/contentStreamer?documentId=FDA-2019-N-1185-0001&attachmentNumber=1&contentType=pdf
14. Allen B. The role of the FDA in ensuring the safety and efficacy of artificial intelligence software and devices. J Am Coll Radiol. 2019;16(2):208-210. https://doi.org/10.1016/j.jacr.2018.09.007
15. Reddy S, Allan S, Coghlan S, Cooper P. A governance model for the application of AI in health care. J Am Med Inform Assoc. 2019. https://doi.org/10.1093/jamia/ocz192
© 2020 Society of Hospital Medicine
Multiplying the Impact of Opioid Settlement Funds by Investing in Primary Prevention
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
There is growing momentum to hold drug manufacturers accountable for the more than 400,000 US opioid overdose deaths that have occurred since 1999.1 As state lawsuits against pharmaceutical manufacturers and distributors wind their way through the legal system, hospitals—which may benefit from settlement funds—have been paying close attention. Recently, former Governor John Kasich (R-Ohio), West Virginia University president E. Gordon Gee, and America’s Essential Hospitals argued that adequately compensating hospitals for the costs of being on the crisis’ “front lines” requires prioritizing them as settlement fund recipients.2
Hospitals should be laying the groundwork for how settlement funds might be used. They may consider enhancing some of the most promising, evidence-based services for individuals with opioid use disorders (OUDs), including improving treatment for commonly associated health conditions such as HIV and hepatitis C virus (HCV); expanding ambulatory long-term antibiotic treatment for endocarditis and other intravenous drug use–associated infections; more broadly adopting harm-reduction practices such as naloxone coprescribing; and applying best practices to caring for substance-exposed infants. They could also develop clinical services not already provided, including creating programs for OUD management during pregnancy and initiating medication for OUD in inpatient, emergency department, and ambulatory settings. In short, hospitals play a critical role in engaging people with OUD in treatment at every possible opportunity.3
When considering how to most effectively use opioid settlement funding, hospitals may consider adding or expanding these much-needed clinical services to address opioid-related harms; however, their efforts should not stop there. Investments made outside hospital walls could have a significant effect on the public’s health, especially if they target social determinants of health. By tackling factors in the pathway to developing OUD, such as lack of meaningful employment, affordable housing, and mental health care, hospitals can move beyond treating the downstream consequences of addiction and toward preventing community-level opioid-related harms. To accomplish this daunting goal, hospitals will need to strengthen existing relationships with community partners and build new ones. Yet in a 2015 study, only 54% of nonprofit hospitals proposed a strategy to address the overdose crisis that involved community partnering.4
In this Perspective, we describe the following three strategies hospitals can use to multiply the reach of their opioid settlement funding by addressing root causes of opioid use through primary prevention: (1) supporting economic opportunities in their communities, (2) expanding affordable housing options in surrounding neighborhoods, and (3) building capacity in ambulatory practices and pharmacies to prevent OUD (Table).

SUPPORTING ECONOMIC OPPORTUNITY IN THEIR COMMUNITIES
Lack of economic opportunity is one of many root causes of opioid use. For example, a recent study found that automotive assembly plant closures were associated with increases in opioid overdose mortality.5 To tackle this complex issue, hospitals can play a crucial role in expanding employment and career advancement options for members of their local communities. Specifically, hospitals can do the following:
- Create jobs within the healthcare system and preferentially recruit and hire from surrounding neighborhoods
- Establish structured career development programs to build skills among entry-level healthcare employees
- Award contracts of varying sizes to locally owned businesses
- Employ individuals with lived experience with substance use disorders, such as peer recovery coaches6
To illustrate how health systems are investing in enhancing career opportunities for members of their communities, hundreds of institutions have implemented “School at Work,” a 6-month career development program for entry-level healthcare employees.7 The hospitals’ Human Resources department trains participants in communication skills, reading and writing, patient safety and satisfaction, medical terminology, and strategies for success and career advancement. Evaluations of this program have demonstrated improved employee outcomes and a favorable return on investment for hospitals.8
As “anchor institutions” and large employers in many communities, hospitals can simultaneously enhance their own workforce and offer employment opportunities that can help break the cycle of addiction that commonly traps individuals and families in communities affected by the overdose crisis.
EXPANDING AFFORDABLE HOUSING OPTIONS
Hospitals are increasingly supporting interventions that fall outside their traditional purview as they seek to improve population health, such as developing safe green outdoor spaces and increasing access to healthy food options by supporting local farmers markets and grocers.9 Stable, decent, and affordable housing is critically important to health and well-being,10 and there is a well-documented association of opioid use disorder and opioid misuse with housing instability.11 Given evidence of improved outcomes with hospital-led housing interventions,12 a growing number of hospitals are partnering with housing authorities and community groups to help do the following13:
- Contribute to supportive housing options
- Provide environmental health assessments, repairs, and renovations
- Buy or develop affordable housing units
Boston Medical Center, where one in four inpatients are experiencing homelessnes and one in three pediatric emergency department patients are housing insecure, provides an example of how a hospital has invested in housing.14 In 2017, the hospital began a 5-year, $6.5 million investment in community partnerships in surrounding neighborhoods. Instead of building housing units or acting as a landlord, the hospital chose to invest funding in creative ways to increase the pool of affordable housing. It invested $1 million to rehabilitate permanent, supportive housing units for individuals with mental health conditions in a nearby Boston neighborhood and in a housing stabilization program for people with complex medical issues including substance use disorder. It provided resources to a homeless shelter near the hospital and to the Boston Health Care for the Homeless Program, which provides healthcare to individuals with housing instability. It also funded a community wellness advocate based at the hospital, who received training in substance use disorders and served as a liaison between the hospital and the Boston Housing Authority.
Housing instability is just one of the social determinants of health that hospitals have the capacity to address as they consider where to invest their opioid settlement funds.
BUILDING PREVENTION CAPACITY IN THE COMMUNITY
Finally, hospitals can partner with community ambulatory practices and pharmacies to prevent the progression to problematic opioid use and OUD. Specifically, hospitals can do as follows:
- Provide evidence-based training to community providers on safe prescribing practices for acute and chronic pain management, as well as postoperative, postprocedural, and postpartum pain management
- Support ambulatory providers in expanding office-based mental health treatment through direct care via telemedicine and in building mental health treatment capacity through consultation, continuing medical education, and telementorship (eg, Project ECHO15)
- Support ambulatory providers to implement risk reduction strategies to prevent initiation of problematic opioid use, particularly among adolescents and young adults
- Partner with local pharmacies to promote point-of-prescription counseling on the risks and benefits of opioids
Hospitals bring key strengths and resources to these prevention-oriented partnerships. First, they may have resources available for clinical research, implementation support, program evaluation, and quality improvement, bringing such expertise to partnerships with ambulatory practices and pharmacies. They likely have specific expertise among their staff, including areas such as pain management, obstetric care, pediatrics, and adolescent medicine, and can provide specialists for consultation services or telementoring initiatives. They also can organize continuing medical education and can offer in-service training at local practices and pharmacies.
Project ECHO is one example of telementoring to build capacity among community providers to manage chronic pain and address addiction and other related harms.16 The Project ECHO model includes virtual sessions with didactic content and case presentations during which specialists mentor community clinicians. Specific to primary prevention, telementoring has been shown to improve access to evidence-based treatment of chronic pain and mental health conditions,17,18 which could prevent the development of OUD. By equipping community clinicians with tools to prevent the development of problematic opioid use, hospitals can help reduce the downstream burden of OUD and its associated morbidity, mortality, and costs.
CONCLUSION
The opioid crisis has devastated families, reduced life expectancy in certain communities,19 and had a substantial financial impact on hospitals—resulting in an estimated $11 billion in costs to US hospitals each year.20 This ongoing crisis is only going to be compounded by the recent emergence of the SARS-CoV-2 virus. Hospital resources are being strained in unprecedented ways, which has required unprecedented responses in order to continue to serve their communities. Supporting economic opportunity, stable housing, and mental health treatment will be challenging in this new environment but has never been more urgently needed. If opioid settlement funds are targeted to US hospitals, they should be held accountable for where funds are spent because they have a unique opportunity to focus on primary prevention in their communities—confronting OUD before it begins.21 However, if hospitals use opioid settlement funding only to continue to provide services already offered, or fail to make bold investments in their communities, this public health crisis will continue to strain the resources of those providing clinical care on the front lines.
Acknowledgment
The authors wish to thank Hilary Peterson of the RAND Corporation for preparing the paper for submission. She was not compensated for her contribution.
Disclosures
The authors report being supported by grants from the National Institute on Drug Abuse of the National Institutes of Health under awards R21DA045212 (Dr Faherty), K23DA045085 (Dr Hadland), L40DA042434 (Dr Hadland), K23DA038720 (Dr Patrick), R01DA045729 (Dr Patrick), and P50DA046351 (Dr Stein). Dr Hadland also reports grant support from the Thrasher Research Fund and the Academic Pediatric Association. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
1. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427. http://doi.org/10.15585/mmwr.mm675152e1
2. Kasich J, Gee EG. Don’t forget our frontline caregivers in the opioid epidemic. New York Times. Published September 18, 2019. Accessed December 16, 2019. https://www.nytimes.com/2019/09/17/opinion/opioid-settlement-hospitals.html
3. Englander H, Priest KC, Snyder H, Martin M, Calcaterra S, Gregg J. A call to action: hospitalists’ role in addressing substance use disorder. J Hosp Med. 2020;15(3):184-187. https://doi.org/10.12788/jhm.3311
4. Franz B, Cronin CE, Wainwright A, Pagan JA. Measuring efforts of nonprofit hospitals to address opioid abuse after the Affordable Care Act. J Prim Care Communit. 2019;10:2150132719863611. https://doi.org/10.1177/2150132719863611
5. Venkataramani AS, Bair EF, O’Brien RL, Tsai ALC. Association between automotive assembly plant closures and opioid overdose mortality in the United States a difference-in-differences analysis. JAMA Intern Med. 2020;180(2):254-262. https://doi.org/10.1001/jamainternmed.2019.5686
6. Englander H, Gregg J, Gullickson J, et al. Recommendations for integrating peer mentors in hospital-based addiction care. Subst Abus. 2019:1-6. https://doi.org/10.1080/08897077.2019.1635968
7. Geisinger investing in employees’ careers with School at Work program. News Release. Geisinger; November 5, 2018. Updated November 5, 2018. Accessed February 17, 2020. https://www.geisinger.org/about-geisinger/news-and-media/news-releases/2018/11/19/17/31/geisinger-investing-in-employees-careers-with-school-at-work-program
8. Jackson A, Brasfield-Gorrigan H. Investing in the Future of the Healthcare Workforce: An Analysis of the Business Impact of Select Employee Development Programs at TriHealth in 2013. TriHealth. March 30, 2015. Accessed 20 April 2020. http://www.catalystlearning.com/Portals/0/Documents/TriHealth%20RoI%20Study%20Updated%20Version.pdf
9. Roy B, Stanojevich J, Stange P, Jiwani N, King R, Koo D. Development of the Community Health Improvement Navigator Database of Interventions. MMWR Suppl. 2016;65:1-9. http://doi.org/10.15585/mmwr.su6502a1
10. Sandel M, Desmond M. Investing in housing for health improves both mission and margin. JAMA. 2017;318(23):2291-2292. https://doi.org/10.1001/jama.2017.15771
11. Vijayaraghavan M, Penko J, Bangsberg DR, Miaskowski C, Kushel MB. Opioid analgesic misuse in a community-based cohort of HIV-infected indigent adults. JAMA Intern Med. 2013;173(3):235-237. https://doi.org/10.1001/jamainternmed.2013.1576
12. Sadowski LS, Kee RA, VanderWeele TJ, Buchanan D. Effect of a housing and case management program on emergency department visits and hospitalizations among chronically ill homeless adults a randomized trial. JAMA. 2009;301(17):1771-1778. https://doi.org/10.1001/jama.2009.561
13. Health Research & Educational Trust. Social Determinants of Health Series: Housing and the Role of Hospitals. American Hospital Association. August 2017. Accessed December 16, 2019. https://www.aha.org/ahahret-guides/2017-08-22-social-determinants-health-series-housing-and-role-hospitals
14. Boston Medical Center to Invest $6.5 Million in Affordable Housing to Improve Community Health and Patient Outcomes, Reduce Medical Costs. Press release. Boston Medical Center; December 7, 2017. Accessed March 4, 2020. https://www.bmc.org/news/press-releases/2017/12/07/boston-medical-center-invest-65-million-affordable-housing-improve
15. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199-2207. https://doi.org/10.1056/nejmoa1009370
16. Chronic Pain and Opioid Management. Project ECHO. Accessed February 16, 2020. https://echo.unm.edu/teleecho-programs/chronic-pain
17. Anderson D, Zlateva I, Davis B, et al. Improving pain care with Project ECHO in community health centers. Pain Med. 2017;18(10):1882-1889. https://doi.org/10.1093/pm/pnx187
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100. https://doi.org/10.1111/pme.12715
19. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322(20):1996-2016. https://doi.org/10.1001/jama.2019.16932
20. Opioid Overdoses Costing US Hospitals an Estimated $11 Billion Annually. Press Release. Premier; January 3, 2019. Accessed March 4, 2020. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually
21. Butler JC. 2017 ASTHO president’s challenge: public health approaches to preventing substance misuse and addiction. J Public Health Manag Pract. 2017;23(5):531-536. https://doi.org/10.1097/phh.0000000000000631
© 2020 Society of Hospital Medicine
Immigrant Physicians Fill a Critical Need in COVID-19 Response
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
Immigrant physicians and international medical graduates (IMGs) have for decades been very important to the healthcare delivery in the United States. For many currently serving on the front lines, the path has been full of challenges and uncertainties, now acutely worsened by the pandemic at hand. Manpreet Malik, MD, is one of those hospitalists. He grew up in a small city in India. He completed medical school in South India where he met students from all over the world and learned to speak a new language to serve local patients. The multicultural experience inspired him to pursue residency in the United States. Manpreet obtained a J-1 visa for residency and subsequently applied for a J-1 waiver for his first hospitalist job in 2013. Then his employer, a nonprofit organization, applied for H-1B and permanent resident status. He continues on an H-1B status but awaits his green card 7 years later. His wife, a dentist, is also an H-1B visa holder and they have two children. While they have assimilated into American society and flourished professionally, a sense of security eludes them. The COVID-19 pandemic has amplified this for their family. Like many other families, they are both in high-risk occupations and worry about the future, including what would happen if either or both of them contracted the virus. Their carefully planned life feels like a wobbly house of cards.
Immigrant healthcare workers are on the front lines in the fight against COVID-19 in the United States, accounting for 16.4% of healthcare workers amid this pandemic.1 Of physicians in the United States, 29% are not born in the United States,and of the practicing hospitalists, 32% are IMGs.1,2 IMGs are physicians who have graduated from medical schools outside of the United States and Canada who lack accreditation by the Liaison Committee on Medical Education.3 IMGs are a heterogeneous group with widely varying cultural, educational, and linguistic backgrounds with around 12,000 IMGs applying yearly for US residency positions.4 IMG hospitalists are uniquely positioned at the front lines facing arguably more risks with less recognition.5 The top five countries sending physicians to the United States are India, China, the Philippines, South Korea, and Pakistan.6 Yet many of these doctors—more than a third of those practicing in this country who graduated from international medical schools—have visa restrictions that limit their ability to work in communities with the greatest need.7 Another group of approximately 65,000 IMGs currently living in the United States are not licensed; they have not passed the board exam because they haven’t matched into a residency program to be eligible to take it.8 Many are working other jobs such as medical research, even though they could be deployed to serve as scribes or work in triage via telemedicine if their visas permitted.
During the COVID-19 pandemic, immigrant doctors are putting their lives on the line daily to care for patients. Immigrant doctors on visas are not eligible for Medicaid or Social Security benefits. Further, their partners and children are often dependent on them for legal resident status in the United States because of employer-based visa sponsorship. As the primary visa holder, if a non–US-born physician in the United States gets severely ill while fighting the virus, or gets disabled, they may have no benefits to fall back on. These physicians have houses, families, and children who are American citizens, and they are contributing members of society. Physicians on visas pay taxes the same way US citizens do. If their health or employment is jeopardized, their families would be unable to stay in the US legally, becoming undocumented and risking deportation. These physicians, who are fighting COVID-19 today, are helpless to provide a stable structure for their own loved ones.
With the COVID-19 pandemic unfolding, there is a risk of more physician shortages. The US healthcare workforce relies on immigrant physicians to help provide high-quality and accessible patient care. There are challenges for IMGs for getting into residency programs, and this limits the potential workforce during COVID-19. This year, according to the National Resident Matching Program, 4,222 non–US-born IMGs are due to start their US residency training on July 1.9 These doctors have the opportunity to serve across the country during this pandemic. According to data from the matching program, IMGs make up a large proportion of the workforce, obtaining 23% of the total number of US residency positions filled, and are in many leading academic institutions. These doctors, many of whom are waiting for their visas to be processed, need to be admitted in order to provide the care that Americans need during this pandemic. A similar number of IMGs will be completing their specialty training and are due to become attending physicians in their chosen field, including areas with critical shortages in this pandemic, such as critical care medicine. These skilled physicians depend on the processing of visa extensions or green cards in order to remain in the United States. Subspecialties like internal medicine and family medicine have a large proportion of actively practicing IMGs,7 and therefore provide primary care and inpatient care across the nation, especially in underserved areas. However, the geographic location of their practice is limited to the place that sponsored their visa. So a physician in rural Minnesota, where the outbreak of COVID-19 is not severe, cannot travel to hot spots such as New York or Detroit to provide care, even if they have a desire to serve.
For IMGs, the process of obtaining legal status in the US and pertinent immigration policies includes utilizing the H-1B visa program for highly skilled workers10 or J-1 visas for residencies.11 H-1B visas are usually granted for sponsored positions in underserved or rural areas for at least 3 years, and the healthcare sector must compete with other industries, such as tech, engineering, and other specialty occupations. Physicians working on H-1B visas may apply for permanent work permits, though there is an annual cap for each country and candidates may wait decades to receive one. As a J-1 visa (cultural exchange program) holder, physicians are required to practice in their home country for 2 years prior to working again in the United States. This requirement could be waived by turning to the Conrad 30 Waiver Program12 or J-1 waivers if they agreed to work in an underserved area in the United States. A limited number of J-1 waivers for each state are dispensed on a first-come, first-served basis (30 IMGs per state per year). This program currently is only authorized through the end of 2020, although legislation has been introduced to extend it, which could expand the slots.13 Applying for a J-1 waiver thus becomes a race against time with high-stakes suspense and anxiety for many IMGs. Most, regardless of visa status, dream of a stable and secure life, with permanent resident status as they serve their communities. For some, however, the endgame could mean deportation and the premature demise of dreams.
Permanent resident status is allotted by country, and there is a long wait for green cards. Three-quarters of skilled workers waiting for green cards are from India. That translates to more than 700,000 people, of which approximately 200,000 are expected to die of old age before being granted green cards.14,15 In the meantime, while they live with restrictions on both their employment and mobility, many physicians are doing essential medical work in underserved and rural areas throughout the United States.
We urge immigration reform to increase the physician workforce by providing immigrant doctors and IMGs with more flexibility to travel to areas where they are needed the most during this pandemic. There should be a blanket extension of visa deadlines. IMGs on J-1 student visas and H-1B specialty work visas should be exempt from any future immigration bans or limitations during the COVID-19 pandemic. The time is right for accelerating permanent resident status for these highly skilled IMGs. Green cards soon after finishing residency or fellowship training or satisfying a condition of initial visa approval should be the norm instead of a stressful unending wait. Clinicians who serve in underserved communities should be incentivized, and this should include health benefits. Restrictions related to primary and secondary work sites, as well as number of J-1 waivers, should also be relaxed. This flexibility would allow immigrant physicians to care at a variety of locations or by means of telemedicine.
A physician’s role is to heal and to serve their patients, regardless of their own origin. We are the voices of America’s immigrant physicians, particularly hospitalists, serving as frontline workers in our nation’s response to the COVID-19 crisis. The battle against COVID-19 has strained many of our resources, including the need for physicians. Uncertainty and chaos reign professionally and personally for many healthcare workers across America, and more challenges lie ahead for the foreseeable future. Healthcare workers are the unselfish and unwavering wall that stands between COVID-19 and more lives lost in our country. Every effort should be made to preserve and strengthen the healthcare workforce. Immigrant hospitalists, shackled by visa restrictions, could play an even bigger role if their obstacles were removed. It is time to provide them with the sense of security they deserve and rebuild the house of cards into something with a stronger foundation and more stability for our future.
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
1. New American Economy Research Fund. Immigration and Covid-19. March 26, 2020. Accessed May 5, 2020. https://research.newamericaneconomy.org/report/immigration-and-covid-19/
2. Compensation and Career Survey. Today’s Hospitalist. November 1, 2008. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/16_salary_survey/index.php
3. Rao NR. “A little more than kin, and less than kind”: US immigration policy on international medical graduates. Virtual Mentor. 2012;14(4):329-337. https://doi.org/10.1001/virtualmentor.2012.14.4.pfor1-1204
4. ECFMG Fact Card: Summary Data Related to ECFMG Certification. Educational Commission for Foreign Medical Graduates (ECFMG). March 20, 2019. Accessed April 22, 2020. https://www.ecfmg.org/forms/factcard.pdf
5. Compensation and Career Survey. Today’s Hospitalist. November 1, 2016. Accessed May 29, 2020. https://www.todayshospitalist.com/survey/08_salary_survey/index.php
6. Harker YS. In rural towns, immigrant doctors fill a critical need. Health Affairs. 2018;37(1):161-164. https://doi.org/10.1377/hlthaff.2017.1094
7. Ahmed AA, Hwang WT, Thomas CR Jr, Deville C Jr. International medical graduates in the US physician workforce and graduate medical education: current and historical trends. J Grad Med Educ. 2018;10(2):214‐218. https://doi.org/10.4300/jgme-d-17-00580.1
8. Peters J. Highly trained and educated, some foreign-born doctors still can’t practice medicine in the US. Public Radio International. March 28, 2018. Accessed April 22, 2020. https://www.pri.org/stories/2018-03-26/highly-trained-and-educated-some-foreign-born-doctors-still-can-t-practice
9. Results and Data: 2020 Main Residency Match. National Resident Matching Program. 2020. Accessed May 15, 2020. http://www.nrmp.org/main-residency-match-data/
10. H-1B Specialty Occupations, DOD Cooperative Research and Development Project Workers, and Fashion Models. U.S. Citizenship and Immigration Services. March 27, 2020. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/temporary-workers/h-1b-specialty-occupations-dod-cooperative-research-and-development-project-workers-and-fashion-models
11. J-1 Visa Sponsorship Fact Sheet. Educational Commission for Foreign Medical Graduates (ECFMG). May 2017. Accessed April 22, 2020. https://www.ecfmg.org/evsp/j1fact.pdf
12. Conrad 30 Waiver Program. U.S. Citizenship and Immigration Services. August 25, 2011. Accessed April 22, 2020. https://www.uscis.gov/working-united-states/students-and-exchange-visitors/conrad-30-waiver-program
13. Conrad State 30 and Physician Access Reauthorization Act, S 948, 116th Congress (2019). Accessed April 22, 2020. https://www.congress.gov/bill/116thcongress/senate-bill/948/text
14. Bhattacharya A. For over 200,000 Indians, the wait for a green card is longer than their lifetimes. Quartz India. March 31, 2020. Accessed April 22, 2020. https://qz.com/india/1828970/over-200000-indians-could-die-waiting-for-a-us-green-card/
15. Bier DJ. Immigration Research and Policy Brief: Backlog for Skilled Immigrants Tops 1 Million: Over 200,000 Indians Could Die of Old Age While Awaiting Green Cards. Cato Institute: Immigration Research and Policy Brief, No. 18. March 30, 2020. Accessed April 26, 2020. https://www.cato.org/sites/cato.org/files/2020-03/irpb-18-updated.pdf
© 2020 Society of Hospital Medicine
Collateral Damage: How COVID-19 Is Adversely Impacting Women Physicians
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
The coronavirus disease of 2019 (COVID-19) pandemic has affected every facet of our work and personal lives. While many hope we will return to “normal” with the pandemic’s passing, there is reason to believe medicine, and society, will experience irrevocable changes. Although the number of women pursuing and practicing medicine has increased, inequities remain in compensation, academic rank, and leadership positions.1,2 Within the workplace, women are more likely to be in frontline clinical positions, are more likely to be integral in promoting positive interpersonal relationships and collaborative work environments, and often are less represented in the high-level, decision-making roles in leadership or administration.3,4 These well-described issues may be exacerbated during this pandemic crisis. We describe how the current COVID-19 pandemic may intensify workplace inequities for women, and propose solutions for hospitalist groups, leaders, and administrators to ensure female hospitalists continue to prosper and thrive in these tenuous times.
HOW THE PANDEMIC MAY EXACERBATE EXISTING INEQUITIES
Increasing Demands at Home
Female physicians are more likely to have partners who are employed full-time and report spending more time on household activities including cleaning, cooking, and the care of children, compared with their male counterparts.5 With school and daycare closings, as well as stay-at-home orders in many US states, there has been an increase in household responsibilities and care needs for children remaining at home with a marked decrease in options for stable or emergency childcare.6 As compared with primary care and subspecialty colleagues who can provide a large percentage of their care through telemedicine, this is not the case for hospitalists who must be physically present to care for their patients. Therefore, hospitalists are unable to clinically “work from home” in the same way as many of their colleagues in other specialties. Increased childcare and schooling obligations, coupled with disproportionate household responsibilities and an inability to work from home, will likely result in female hospitalists struggling to meet family needs while pandemic-related work responsibilities are ramping up.7 In addition, women who are involved with administrative, leadership, or research activities may struggle to execute their responsibilities as a result of increased domestic duties.
Many hospitalists are also concerned about contracting COVID-19 and exposing their families to the illness given the high infection rate among healthcare workers and the shortage of personal protective equipment (PPE).8,9 Institutions and national organizations, including the Society of Hospital Medicine, have partnered with industry to provide discounted or complimentary hotel rooms for members to aid self-isolation while providing clinical care.10 One famous photo in popular and social media showed a pulmonary and critical care physician in a tent in his garage in order to self-isolate from his family.11 However, since women are often the primary caregivers for their children or other family members and may also be responsible for other important household activities, they may be unable or unwilling to remove themselves from their children and families. As a result, female hospitalists may encounter feelings of guilt or inadequacy if they’re unable to isolate in the same manner as male colleagues.8
Exaggerating Leadership Gap
One of the keys to a robust response to this pandemic is strong, thoughtful, and strategic leadership.12 Institutional, regional, and national leaders are at the forefront of designing the solutions to the many problems the COVID-19 pandemic has created. The paucity of women at high-level leadership positions in institutions across the United States, including university-based, community, public, and private institutions, means that there is a lack of female representation when institutional policy is being discussed and decided.4 This lack of representation may lead to policies and procedures that negatively affect female hospitalists or, at best, fail to consider the needs of or support female physicians. For example, leaders of a hospital medicine group may create mandatory “backup” coverage for night and weekend shifts for their group during surge periods of the pandemic without considering implications for childcare. Finding weekday, daytime coverage is challenging for many during this time when daycares and school are closed, and finding coverage during weekend or overnight hours will be even more challenging. With increased risks for older adults with high-risk medical conditions, grandparents or other friends or family members that previously would have assisted with childcare may no longer be an option. If a female hospitalist is not a member of the leadership group that helped design this coverage structure, there could be a lack of recognition of the undue strain this coverage model could create for women in the group. Even if not intentional, such policies may hinder women’s career stability and opportunities for further advancement, as well as their ability to adequately provide care for their families. Having women as a part of the leadership group that creates policies and schedules and makes pivotal decisions is imperative, especially regarding topics of providing access and compensation for “emergency childcare,” hazard pay, shift length, work conditions, job security, sick leave, workers compensation, advancement opportunities, and hiring practices.
Compensation
The gender pay gap in medicine has been consistently demonstrated among many specialties.13,14 The reasons for this inequity are multifactorial, and the COVID-19 pandemic has the potential to further widen this gap. With the unequal burden of unpaid care provided by women and their higher prevalence as frontline workers, they are at greater risk of needing to take unpaid leave to care for a sick family member or themselves.6,7 Similarly, without hazard pay, those with direct clinical responsibilities bear the risk of illness for themselves and their families without adequate compensation.
Impact on Physical and Mental Health
The overall well-being of the hospitalist workforce is critical to continue to provide the highest level of care for our patients. With higher workloads at home and at work, female hospitalists are at risk for increased burnout. Burnout has been linked to many negative outcomes including poor performance, depression, suicide, and leaving the profession.15 Burnout is documented to be higher in female physicians with several contributing factors that are aggravated by gender inequities, including having children at home, gender bias, and real or perceived lack of fairness in promotion and compensation.16 The COVID-19 pandemic has amplified the stress of having children in the home, as well as concerns around fair compensation as described above. The consequences of this have yet to be fully realized but may be dire.
PROPOSED RECOMMENDATIONS
We propose the following recommendations to help mitigate the effects of this epidemic and to continue to move our field forward on our path to equity.
1. Closely monitor the direct and indirect effects of COVID-19 on female hospitalists. While there has been a recent increase in scholarship on the pre–COVID-19 state of gender disparities, there is still much that is unknown. As we experience this upheaval in the way our institutions function, it is even more imperative to track gender deaggregated key indicators of wellness, burnout, and productivity. This includes the use of burnout inventories, salary equity reviews, procedures that track progress toward promotion, and even focus groups of female hospitalists.
2. Inquire about the needs of women in your organization and secure the support they need. This may take the form of including women on key task forces that address personal protective equipment allocation, design new processes, and prepare for surge capacity, as well as providing wellness initiatives, fostering collaborative social networks, or connecting them with emergency childcare resources.
3. Provide a mechanism to account for lack of academic productivity during this time. This period of decreased academic productivity may disproportionately derail progress toward promotion for women. Academic institutions should consider extending deadlines for promotion or tenure, as well as increasing flexibility in metrics used to determine appropriate progress in annual performance reviews.
4. Recognize and reward increased efforts in the areas of clinical or administrative contribution. In this time of crisis, women may be stepping up and leading efforts without titles or positions in ways that are significant and meaningful for their group or organization. Recognizing the ways women are contributing in a tangible and explicit way can provide an avenue for fair compensation, recognition, and career advancement. Female hospitalists should also “manage up” by speaking up and ensuring that leaders are aware of contributions. Amplification is another powerful technique whereby unrecognized contributions can be called out by other women or men.17
5. Support diversity, inclusion, and equity efforts. Keeping equity targets at the top of priority lists for goals moving forward will be imperative. Many institutions struggled to support strong diversity, inclusion, and equity efforts prior to COVID-19; however, the pandemic has highlighted the stark racial and socioeconomic disparities that exist in healthcare.18,19 As healthcare institutions and providers work to mitigate these disparities for patients, there would be no better time to look internally at how they pay, support, and promote their own employees. This would include actively identifying and mitigating any disparities that exist for employees by gender, race, religion, sexual orientation, ethnicity, age, or disability status.
6. Advocate for fair compensation for providers caring for COVID-19 patients. Frontline clinicians are bearing significant risks and increased workload during this crisis and should be compensated accordingly. Hazard pay, paid sick leave, medical and supplemental life insurance, and strong workers’ compensation protections for hospitalists who become ill at work are important for all clinicians, including women. Other long-term plans should include institutional interventions such as salary corrections and ongoing monitoring.20
SUMMARY
The COVID-19 pandemic will have long-term effects that are yet to be realized, including potentially widening gender disparities in medicine. With the current health and economic crises facing our institutions and nations, it can be tempting for diversity, equity, and inclusion initiatives to fall by the wayside. However, it is imperative that hospitalists, leaders, and institutions monitor the effects of the COVID-19 pandemic on women and proactively work to mitigate worsening disparities. Without this focus there is a risk that the recent gains in equity and advancement for women may be lost.
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
1. Association of American Medical Colleges. Table 13: US medical school faculty by sex, rank, and department, 2017-2018. December 31, 2019. Accessed January 16, 2020. https://www.aamc.org/download/486102/data/17table13.pdf
2. Spector ND, Asante PA, Marcelin JR, et al. Women in pediatrics: progress, barriers, and opportunities for equity, diversity, and inclusion. Pediatrics. 2019;144(5):e20192149. https://doi.org/10.1542/peds.2019-2149
3. Rouse LP, Nagy-Agren S, Gebhard RE, Bernstein WK. Women physicians: gender and the medical workplace. J Womens Health (Larchmt). 2020;29(3):297‐309. https://doi.org/10.1089/jwh.2018.7290
4. Burden M, Frank MG, Keniston A, et al. Gender disparities in leadership and scholarly productivity of academic hospitalists. J Hosp Med. 2015;10(8):481-485. https://doi.org/10.1002/jhm.2340
5. Starmer AJ, Frintner MP, Matos K, Somberg C, Freed G, Byrne BJ. Gender discrepancies related to pediatrician work-life balance and household responsibilities. Pediatrics. 2019;144(4):e20182926. https://doi.org/10.1542/peds.2018-2926
6. Alon TM, Doepke M, Olmstead-Rumsey J, Tertilt Ml. The impact of COVID-19 on gender equality. NBER Working Paper Series. 2020. https://doi.org/10.3386/w26947
7. Addati L, Cattaneo U, Esquivel V, Valarino I. Care work and care jobs for the future of decent work. Geneva: International Labour Office; 2018.
8. Maguire P. Should you steer clear of your own family? Hospitalists weigh living in isolation. Today’s Hospitalist. May 2020. Accessed May 4, 2020. https://www.todayshospitalist.com/treating-covid-patients/
9. Burrer SL, de Perio MA, Hughes MM, et al. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477-481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
10. SHM Teams Up with Hilton and American Express to Provide Hotel Rooms for Members. SHM. April 13, 2020. Accessed May 7, 2020. https://www.hospitalmedicine.org/about/press-releases/SHM-One-Million-Beds-Hilton-AMEX/
11. Fichtel C, Kaufman S. Fearing COVID-19 spread to families, health care workers self-isolate at home. NBC News. March 31, 2020. Accessed May 7, 2020. https://www.nbcnews.com/health/health-news/fearing-covid-19-spread-families-health-care-workers-self-isolate-n1171726
12. Meier KA, Jerardi KE, Statile AM, Shah SS. Pediatric hospital medicine management, staffing, and well-being in the face of COVID-19. J Hosp Med. 2020;15(5):308‐310. https://doi.org/10.12788/jhm.3435
13. Frintner MP, Sisk B, Byrne BJ, Freed GL, Starmer AJ, Olson LM. Gender differences in earnings of early- and midcareer pediatricians. Pediatrics. 2019;144(4):e20183955. https://doi.org/10.1542/peds.2018-3955
14. Read S, Butkus R, Weissman A, Moyer DV. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169(9):658-661. https://doi.org/10.7326/m18-0693
15. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516‐529. https://doi.org/10.1111/joim.12752
16. Templeton K, Halpern L, Jumper C, Carroll RG. Leading and sustaining curricular change: workshop proceedings from the 2018 Sex and Gender Health Education Summit. J Womens Health (Larchmt). 2019;28(12):1743-1747. https://doi.org/10.1089/jwh.2018.7387
17. Eilperin J. White House women want to be in the room where it happens. The Washington Post. September 13, 2016. Accessed April 24, 2020. https://www.washingtonpost.com/news/powerpost/wp/2016/09/13/white-house-women-are-now-in-the-room-where-it-happens/
18. Choo EK. COVID-19 fault lines. Lancet. 2020;395(10233):1333. https://doi.org/10.1016/s0140-6736(20)30812-6
19. Núñez A, Madison M, Schiavo R, Elk R, Prigerson HG. Responding to healthcare disparities and challenges with access to care during COVID-19. Health Equity. 2020;4(1):117-128. https://doi.org/10.1089/heq.2020.29000.rtl
20. Paturel A. Closing the gender pay gap in medicine. AAMC News. April 16, 2019. Accessed April 21, 2020. https://www.aamc.org/news-insights/closing-gender-pay-gap-medicine
© 2020 Society of Hospital Medicine
Truth in Tension: Reflections on Racism in Medicine
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
Core values should reflect our fundamental beliefs and serve as the building blocks of our behaviors and actions. Health systems across the United States define themselves by a myriad of guiding principles, which include patient-centeredness, dignity, respect, safety, and teamwork. On the surface, medicine’s ties to such altruistic values make intuitive sense. However, as Black physicians, we are in a state of cognitive dissonance as we wrestle with healthcare’s real identity and the principles it espouses. We know that within this psychological tension lies the truth: the US healthcare system was not designed to live up to these ideals. This truth is most evident in health inequities that exist among Black people and other marginalized communities of color. It is also the undeniable reality of Black physicians whose professional role is juxtaposed with recurring experiences that signal to us that we do not belong.
SYSTEMIC RACISM, MISTRUST, AND HEALTH INEQUITIES
Racism in healthcare, laid bare by the well-documented exploitation of Black people by the medical community, adds to the not-so-subtle ways we are told our lives don’t matter.1 This mistreatment has resulted in a deep mistrust of healthcare providers that is legitimate and real. The 40-year Tuskegee Syphilis Study is infamous for breaking trust via the deception of hundreds of Black men. The study participants with syphilis were denied treatment despite a known and available cure; an act both unconscionable and inhumane. As recently as the 1990s, a study sought to identify a genetic origin for aggressive behavior; however, enrollment was restricted to Black and Latino boys, and families were incentivized with money. Furthermore, the children were taken off all medications, kept overnight without their parents, deprived of water, subjected to hourly blood draws, and given fenfluramine, a drug known to be associated with precipitating aggressive behavior.1 The study design perpetuated the stereotype of Black males as perpetrators of violence—a distorted and biased perception that continues to cost Black people their lives. This sobering example illustrates that even in the era of institutional review boards, the welfare and protection of Black people who participate in research is by no means guaranteed.
The very notion of social determinants of health exposes the underbelly of institutional racism and its pervasiveness in our healthcare system. As Black physicians, we see the flawed healthcare system’s disproportionate and devastating effects on patients who look like us: we have first-hand accounts as patients ourselves, and we have traversed the experiences endured by our loved ones. Broken trust and fractured care contribute to disparate rates of morbidity and mortality in Black men and women with cardiovascular disease, stroke, and diabetes.2 Black mothers have the highest rates of premature births and are three times more likely than White women to die from pregnancy-related complications.3 Black infants are two times more likely to die before their first birthday than are White infants.4 Children and adolescents from poor, predominantly Black and Latinx neighborhoods spend significantly more days in the hospital for various acute and chronic diagnoses than their counterparts from affluent, predominantly White neighborhoods.5 Not surprisingly, the COVID-19 pandemic’s effects on the Black community read like lines memorized from the same old, tired, script6: staggering mortality rates, extreme poverty, food insecurity, alarming education inequities, and a widening digital divide. And, as Black pediatricians, we hold our breath as we wait until the coast is clear to fully assess the overwhelming damage to our children caused by the pandemic’s tsunami.
ACADEMIC MEDICINE AND OUR INVISIBLE WOUNDS
In our roles as doctors, we experience first-hand the ills of academic medicine, an environment that poses significant challenges for those of us who are underrepresented in medicine (UIM). Despite an acute awareness of the need for Black physicians, little has changed over the past few decades. As of 2018, the percentages of Black or African American students who applied and were accepted to US medical schools were 8.4% and 7%, respectively.7 Diversity gains in the acceptance and matriculation rates of medical students were noted across multiple demographic groups over the past 40 years; however, Black applicants were the exception. In fact, the number of Black men enrolled in medical schools is currently less than it was in 1978, a dismal statistic that underscores this issue.8 Only 5% of US physicians identify as Black or African American.7 Furthermore, in academia, while 64% of faculty are White, only 3.6% are Black or African American.7 But there is more to it than just the numbers. Diversity means nothing without an inclusive environment. As Black physicians, we understand the power of visibility, and our strong desire to cultivate a safe and inclusive environment for students, trainees, and other faculty is a large part of why we remain in academia. Nevertheless, the experience in academic medicine for Black physicians and other UIMs is commonly one of isolation.
Lack of inclusivity and feelings of isolation are common themes among Black physicians in academia.9 They are intensified by microaggressions,10 shards of glass that slowly cut at our self-concept, confidence, and resolve. We nurse the wounds from the ones hurled at our Black patients as well as the ones directed our way. They are the microassaults from the mother who requests that a different physician care for her child; the father who proudly displays a swastika tattoo as you examine his newborn infant in the nursery; or the directive to empty out the garbage when you walk into a patient’s room. They are the microinsults from colleagues that convey our inferiority and associate our advancement with handouts because of our race; questions like, “How did you get that role?” and backhanded compliments such as, “You are so articulate,” as we exceed their mediocre expectations. They are the microinvalidations, for example being constantly confused with the few other Black physicians in the hospital, which sends the message that we are invisible. Likewise, our minority tax9—an underappreciated list of service-oriented expectations and responsibilities related to our UIM status—is paid in full via the call to put our “otherness” on display for the sake of diversity and when we speak out against racism and bias because no one else will. There are limited opportunities to establish strong relationships with Black physician mentors, who are more likely to understand the needs and identify with the differential experiences of Black physician mentees. Examples of authentic and effective cross-race mentorship relationships built on trust and psychological safety are scarce, and their rarity exacerbates feelings of isolation and disillusionment among Black physicians. And rare sponsorship—in the form of high visibility recognition or career advancing opportunities—is conflated with veiled tokenism. This atmosphere breeds hypervigilance for Black physicians in academia. The weight of our actions and performance being judged not on an individual level, but rather as a reflection of our entire race, is a heavy load to bear.
A CRITICAL JUNCTURE
Our country is at a crossroads, with resounding calls to dismantle systemic racism in all its forms. The call is greatest for those of us who fight to heal our patients yet work in a healthcare system that perpetuates inequity. Radical steps are needed to rebuild the system and include:
- Working relentlessly towards health equity in all phases and facets of patient care. This must involve mandating data transparency, defining clear measures, and implementing processes that make equitable practices the default.
- Moving beyond one-dimensional diversity initiatives that focus on recruitment, and investing in strategies that promote the inclusion, retention, and advancement of UIM faculty along leadership and academic ranks.
- Establishing specific experiences, opportunities, and support structures for UIMs that include Black students, trainees, and faculty to combat isolation and foster inclusivity.
- Developing and implementing comprehensive trainee and faculty education focused on implicit bias in general, and structural racism, medical mistrust, and racial bias in healthcare in particular.
- Cultivating an antiracist environment in which true and authentic allyship is widespread and macro- and microaggressions are not silently endured by UIMs but are immediately and effectively addressed by all.
We must reconcile the dissonance that currently exists in our healthcare system between lofty ideals of racial equity and opportunity with actual practice—and as a result, honor the dignity and worth of the people who experience and work in it.
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
1. Washington HA. Medical Apartheid: The Dark History Of Medical Experimentation On Black Americans From Colonial Times to the Present. Doubleday Books; 2006.
2. Calvin R, Winters K, Wyatt SB, Williams DR, Henderson FC, Walker ER. Racism and cardiovascular disease in African Americans. Am J Med Sci. 2003;325(6):315-331. https://doi.org/10.1097/00000441-200306000-00003
3. Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68(18):423. https://doi.org/10.15585/mmwr.mm6818e1
4. Centers for Disease Control and Prevention. Reproductive Health. Maternal and Infant Health. Infant Mortality Rates by Race and Ethnicity, 2016. Accessed June 6, 2020. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm
5. Beck AF, Anderson KL, Rich K, et al. Cooling the hot spots where child hospitalization rates are high: a neighborhood approach to population health. Health Aff. 2019;38(9):1433-1441. https://doi.org/10.1377/hlthaff.2018.05496
6. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. https://doi.org/10.1001/jama.2020.6548
7. Diversity in Medicine: Facts and Figures 2019. Association of American Medical Colleges. Accessed June 6, 2020. https://www.aamc.org/data-reports/workforce/report/diversity-medicine-facts-and-figures-2019
8. Altering the Course: Black Males in Medicine. Association of American Medical Colleges; 2015.
9. Campbell KM, Rodríguez JE. Addressing the minority tax: perspectives from two diversity leaders on building minority faculty success in academic medicine. Acad Med. 2019;94(12):1854-1857. https://doi.org/10.1097/ACM.0000000000002839
10. Freeman L, Stewart H. Microaggressions in clinical medicine. Kennedy Inst Ethics J. 2018;28(4):411-449. https://doi.org/10.1353/ken.2018.0024
© 2020 Society of Hospital Medicine
Communicating Effectively With Hospitalized Patients and Families During the COVID-19 Pandemic
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
For parents of children with medical complexity (CMC), bringing a child to the hospital for needed expertise, equipment, and support is necessarily accompanied by a loss of power, freedom, and control. Two of our authors (K.L., P.M.) are parents of CMC—patients affectionately known as “frequent flyers” at their local hospitals. When health needs present, these experienced parents quickly identify what can be managed at home and what needs a higher level of care. The autonomy and security that accompany this parental expertise have been mitigated by, and in some cases even lost in, the COVID-19 pandemic. In particular, one of the most obvious changes to patients’ and families’ roles in inpatient care has been in communication practices, including changes to patient- and family-centered rounding that result from current isolation procedures and visitation policies. Over the past few months, we’ve learned a tremendous amount from providers and caregivers of hospitalized patients; in this article, we share some of what they’ve taught us.
Before we continue, we take a humble pause. The process of writing this piece spanned weeks during which certain areas of the world were overwhelmed. Our perspective has been informed by others who shared their experiences, and as a result, our health systems are more prepared. We offer this perspective recognizing the importance of learning from others and feeling a sense of gratitude to the providers and patients on the front lines.
CHANGING CIRCUMSTANCES OF CARE
As a group of parents, nurses, physicians, educators, and researchers who have spent the last 10 years studying how to communicate more effectively in the healthcare setting,1,2 we find ourselves in uncharted territory. Even now, we are engaged in an ongoing mentored implementation program examining the effects of a communication bundle on patient- and family- centered rounds (PFCRs) at 21 teaching hospitals across North America (the SHM I-PASS SCORE Study).3 COVID-19 has put that study on hold, and we have taken a step back to reassess the most basic communication needs of patients and families under any circumstance.
Even among our study group, our family advisors have also been on the front lines as patients and caregivers. One author (P.M.), shared a recent experience that she and her son, John Michael had:
“My son [who has autoimmune hepatitis and associated conditions] began coughing and had an intense sinus headache. As his symptoms continued, our concern steadily grew: Could we push through at home or would we have to go in [to the hospital] to seek care? My mind raced. We faced this decision many times, but never with the overwhelming threat of COVID-19 in the equation. My son, who is able to recognize troublesome symptoms, was afraid his sinuses were infected and decided that we should go in. My heart sank.”
Now, amid the COVID-19 pandemic, we have heard that patients like John Michael, who are accustomed to the healthcare setting, are “terrified with this additional concern of just being safe in the hospital,” reported a member of our Family Advisory Council. One of our members added, “We recognize this extends to the providers as well, who maintain great care despite their own family and personal safety concerns.” Although families affirmed the necessity of the enhanced isolation procedures and strict visitation policies, they also highlighted the effects of these changes on usual communication practices, including PFCRs.
CORE VALUES DURING COVID-19
In response to these sentiments, we reached out to all of our family advisors, as well as other team members, for suggestions on how healthcare teams could help patients and families best manage their hospital experiences in the setting of COVID-19. Additionally, we asked our physician and nursing colleagues across health systems about current inpatient unit adaptations. Their suggestions and adaptations reinforced and directly aligned with some of the core values of family engagement and patient- and family-centered care,4 namely, (1) prioritizing communication, (2) maintaining active engagement with patients and families, and (3) enhancing communication with technology.
Prioritizing Communication
Timely and clear communication can help providers manage the expectations of patients and families, build patient and family feelings of confidence, and reduce their feelings of anxiety and vulnerability. Almost universally, families acknowledged the importance of infection control and physical distancing measures while fearing that decreased entry into rooms would lead to decreased communication. “Since COVID-19 is contagious, families will want to see every precaution taken … but in a way that doesn’t cut off communication and leave an already sick and scared child and their family feeling emotionally isolated in a scary situation,” an Advisory Council member recounted. Importantly, one parent shared that hearing about personal protective equipment conservation could amplify stress because of fear their child wouldn’t be protected. These perspectives remind us that families may be experiencing heightened sensitivity and vulnerability during this pandemic.
Maintaining Active Engagement With Patients and Families
PFCRs continue to be an ideal setting for providers, patients, and families to communicate and build shared understanding, as well as build rapport and connection through human interactions. Maintaining rounding structures, when possible, reinforces familiarity with roles and expectations, among both patients who have been hospitalized in the past and those hospitalized for the first time. Adapting rounds may be as simple as opening the door during walk-rounds to invite caregiver participation while being aware of distancing. With large rounding teams, more substantial workflow changes may be necessary.
Beyond PFCRs, patients and family members can be further engaged through tasks/responsibilities for the time in between rounding communication. Examples include recording patient symptoms (eg, work of breathing) or actions (eg, how much water their child drinks). By doing this, patients and caregivers who feel helpless and anxious may be given a greater sense of control while at the same time making helpful contributions to medical care.
Parents also expressed value in reinforcing the message that patients and families are experts about themselves/their loved ones. Healthcare teams can invite their insights, questions, and concerns to show respect for their expertise and value. This builds trust and leads to a feeling of togetherness and teamwork. Across the board, families stressed the value of family engagement and communication in ideal conditions, and even more so in this time of upheaval.
Enhancing Communication With Technology
Many hospitals are leveraging technology to promote communication by integrating workstations on wheels & tablets with video-conferencing software (eg, Zoom, Skype) and even by adding communication via email and phone. While fewer team members are entering rooms, rounding teams are still including the voices of pharmacists, nutritionists, social workers, primary care physicians, and caregivers who are unable to be at the bedside.
These alternative communication methods may actually provide patients with more comfortable avenues for participating in their own care even beyond the pandemic. Children, in particular, may have strong opinions about their care but may not be comfortable speaking up in front of providers whom they don’t know very well. Telehealth, whiteboards, email, and limiting the number of providers in the room might actually create a more approachable environment for these patients even under routine conditions.
CONCLUSION
Patients, families, nurses, physicians, and other team members all feel the current stress on our healthcare system. As we continue to change workflows, alignment with principles of family engagement and patient- and family-centered care4 remain a priority for all involved. Prioritizing effective communication, maintaining engagement with patients and families, and using technology in new ways will all help us maintain high standards of care in both typical and completely atypical settings, such as during this pandemic. Nothing captures the benefits of effective communication better than P.M.’s description of John Michael’s experience during his hospitalization:
“Although usually an expedited triage patient, we spent hours in the ER among other ill and anxious patients. Ultimately, John Michael tested positive for influenza A. We spent 5 days in the hospital on droplet protection.
“The staff was amazing! The doctors and nurses communicated with us every step of the way. They made us aware of extra precautions and explained limitations, like not being able to go in the nutrition room or only having the doctors come in once midday. Whenever they did use [personal protective equipment] and come in, the nurses and team kept a safe distance but made sure to connect with John Michael, talking about what was on TV, what his favorite teams are, asking about his sisters, and always asking if we needed anything or if there was anything they could do. I am grateful for the kind, compassionate, and professional people who continue to care for our children under the intense danger and overwhelming magnitude of COVID-19.”
Disclosures
Dr Landrigan has served as a paid consultant to the Midwest Lighting Institute to help study the effect of blue light on health care provider performance and safety. He has consulted with and holds equity in the I-PASS Institute, which seeks to train institutions in best handoff practices and aid in their implementation. Dr Landrigan has received consulting fees from the Missouri Hospital Association/Executive Speakers Bureau for consulting on I-PASS. In addition, he has received monetary awards, honoraria, and travel reimbursement from multiple academic and professional organizations for teaching and consulting on sleep deprivation, physician performance, handoffs, and safety and has served as an expert witness in cases regarding patient safety and sleep deprivation. Drs Spector and Baird have also consulted with and hold equity in the I-PASS Institute. Dr Baird has consulted with the I-PASS Patient Safety Institute. Dr Patel holds equity/stock options in and has consulted for the I-PASS Patient Safety Institute. Dr Rosenbluth previously consulted with the I-PASS Patient Safety Institute, but not within the past 36 months. The other authors have no conflicts of interest or external support other than the existing PCORI funding for the Society of Hospital Medicine I-PASS SCORE study.
Disclaimer
The I-PASS Patient Safety Institute did not provide support to any authors for this work.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
1. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/nejmsa1405556.
2. Khan A, Spector ND, Baird JD, et al. Patient safety after implementation of a coproduced family centered communication programme: multicenter before and after intervention study. BMJ. 2018;363:k4764. https://doi.org/10.1136/bmj.k4764.
3. Patient-Centered Outcomes Research Institute. Helping Children’s Hospitals Use a Program to Improve Communication with Families. December 27, 2019. https://www.pcori.org/research-results/2018/helping-childrens-hospitals-use-program-improve-communication-families. Accessed March 26, 2020.
4. Institute for Patient- and Family-Centered Care (IPFCC). PFCC and COVID-19. https://www.ipfcc.org/bestpractices/covid-19/index.html. Accessed April 10, 2020.
© 2020 Society of Hospital Medicine
Trust in Public Health Is Essential Amid the COVID-19 Pandemic
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
The visibility of public health—both as a science and a government responsibility—has increased dramatically with the COVID-19 pandemic. Public health science, surveillance, and emergency interventions are saving lives across the globe. Public health leaders are advising local, state, national, and international policymakers and have a consistent and strong voice in the media. We describe here the trust challenges facing public health in this moment of crisis, as well as the strategies necessary to maintain and increase that trust.
In the United States, public opinion data suggest that, while trust in science and government is relatively low and has been declining in recent years, trust in public health is high.1,2 In a survey released in April, 2020, the most trusted groups “to do the right thing” on COVID-19 were doctors, hospitals, scientists, researchers, and the Centers for Disease Control and Prevention (CDC).3 Trust in state government was the next highest. Some governors have been particularly strong in supporting public health messages. For example, Governor Gretchen Whitmer in Michigan has repeatedly stated that her decisions are based on science and public health4; Michiganders reported trust in state government at 79%, compared with trust in the White House at 54%.3 In Ohio, where Governor Mike DeWine has stood with his director of public health, Amy Acton, MD, MPH, in his pandemic response, trust in state government was 80%, compared with trust in the White House at 62%.3
Until there is an effective vaccine with high levels of uptake, COVID-19 prevention and control efforts are going to primarily rely on intrusive and challenging public health interventions such as school/business closures, stay-at-home orders, crowd limits, and travel restrictions. Maintaining trust in and support for both public health interventions and leaders requires intentional strategies that are sophisticated and deploy effective social marketing and risk communication strategies.
CHALLENGES TO MAINTAINING TRUST IN PUBLIC HEALTH
Early in the trajectory of COVID-19, Americans were almost uniform in their support for stay-at-home orders.5 Later, as the economic and social impact of self-quarantine, business, and school closures deepened, backlash began to increase.6 As recent protests against stay-at-home orders and other COVID-19-interventions reveal, many people do not understand the breadth of government’s duty to protect the public’s health and welfare. In fact, the US Constitution gives states a significant amount of power to protect the health, safety, and welfare of their populations, including “police powers” that generally fall into three categories: (a) protecting people who cannot protect themselves, (b) protecting people from others, and (c) protecting people from themselves.7,8 Current executive orders and other government actions designed to combat COVID-19 represent the use of police powers in all three of these areas.
It is exceedingly difficult for governments to design effective pandemic interventions—including executive orders and laws based on “police power”—that protect the public’s health without negatively affecting the economy, healthcare system, schools, and the financial and psychosocial welfare of citizens.
To compound this challenge, while local, state, and federal governments have the authority to act strongly and swiftly in a public crisis, American’s passionate political and philosophical attachments to freedom and self-determination and their skepticism about government interference cannot be dismissed. “Life, liberty, and the pursuit of happiness” is more than a line in the Declaration of Independence—it reflects a strong set of American values that make the case for action that is collectively based while honoring individual interests. Although Americans have a deep-seated belief in individual freedoms, public health relies on collective action for success. Public health leaders must understand this tension and effectively articulate why and when collective action is necessary while also articulating a path to move from a uniform, state-imposed emergency response to one that relies on responsible individual actions.
The federal government’s conflicting messages on science and the public health are also an enormous threat to public health. When the White House’s top trade adviser publicly criticizes the response of the CDC, the CDC guidance appears politicized, which erodes public trust.
Unfortunately, public health in the United States has generally struggled to make a clear and compelling case for prevention and nonmedical approaches to health and well-being. As the saying goes, “Public health is invisible when it is most effective.” Public health leaders are trained in epidemiology and other sciences, in community-based partnerships, and sometimes medicine. However, few public health leaders have been trained in advocacy communication.
STRATEGIES TO STRENGTHEN TRUST IN PUBLIC HEALTH
Government leaders and their partners can better balance the health, economic, and other needs of the population if they effectively communicate the rationale and need for population-based public health interventions in ways that are based on communication science and are politically savvy. A civics lesson from public health officials about constitutional law and the role of police power in combating COVID-19 is not likely to be effective. However, sophisticated messaging tailored to different audiences about the government’s role in protecting the health of everyone could be.
While much is still unknown regarding COVID-19, the evidence is clear that nonpharmaceutical interventions like self-quarantine and isolation, physical distancing, business and school closures, and other core public health strategies are effective in reducing community spread and can flatten the infectious-disease epidemiologic curve.9,10 Countries such as South Korea, New Zealand, Australia, and Germany—countries that have taken strong public-health approaches on social distancing and stay-at-home orders along with extensive testing and contact tracing—have demonstrated reduced rates of severe morbidity and mortality from COVID-19. Vietnam, a developing country of 96 million people that borders China, has reported zero deaths from COVID-19 to date because of both swift public health actions and strong communication strategies.11
Public health communication efforts regarding COVID-19 should be based on risk and crisis communication science and on best practices for social marketing that rallies people around shared values.12,13 For example, communications from Dr Acton have attempted to “inspire” rather than “order” people to physically isolate by appealing to widely shared core values.14 This includes acknowledging the hardships people are experiencing, emphasizing the important historic role that everyone is playing in their sacrifices, promoting determination rather than fear, and declaring that “not all heroes wear capes.” Best practices in communication also include segmenting audiences for the design and testing of different communication approaches.12
Public health leaders can also learn from the extensive research from other fields in how to build trust. Consumer product research emphasizes the importance of transparency in sharing known and unknown risks and admitting error when errors are made.15
Engagement of the public in policy decision-making is also essential in situations of uncertainty. Since much is unknown about COVID-19, policy guidance about mitigation and prevention strategies has changed in real time. Changing messages on the importance of face masks is an example of the trust challenge for public health. In the initial stages of the pandemic, the CDC discouraged the use of face masks. As more data became available, the CDC changed its guidance. Such changed guidance can undermine the entire public health message on protective factors. Acknowledging uncertainty and engaging the public in decision-making through a process of reflexive learning can build public trust in a time of uncertainty.16
COVID-19 has also reaffirmed and illuminated that the public health and healthcare delivery systems are intertwined. Failure to “flatten the curve” results in an overrun healthcare system, enormous costs, and significant mortality. However, public health efforts that successfully slow and limit community spread also produce significant financial losses for healthcare systems because the use of all types of nonemergent care greatly decreases. Public health and healthcare system leaders must partner in the strategic design and reinforcement of messages to build strong and lasting trust in the ongoing public health interventions and mandates that are going to be with us for the unforeseen future.
Finally, maintaining trust in the face of political attacks on our agencies of public health requires the healthcare community speak out in unity—endorsing science-based recommendations and supporting the CDC, the World Health Organization, and local public health.
CONCLUSION
Public health is at an unprecedented and crucial moment in this global pandemic, with growing societal understanding of the role that public health plays in our lives. Public health leaders have a unique opportunity to build on that understanding, strengthen trust, and increase funding and support for core public health services.
Balancing risks and benefits in the face of great uncertainty is never easy. With COVID-19, the horrific number of deaths and speed of community spread has led to a strong and essential public health emergency response throughout most of the country. Keeping the public committed to the important and ongoing measures necessary to ensure that prevention/control efforts are effective and that as few lives as possible are lost will require strengthening the widespread and deep trust in the science and practice of public health.
Disclosures
The authors have nothing to disclose.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
1. Pew Research Center. Trust and Distrust in America. July 2019. https://www.people-press.org/wp-content/uploads/sites/4/2019/07/pew-research-center_trust-distrust-in-america-report_2019-07-22-1.pdf. Accessed May 24, 2020.
2. Kirzinger A, Kearney A, Hamel L, Brodie M. KFF Health Tracking Poll – Early April 2020: The Impact of Coronavirus on Life in America. Kaiser Family Foundation. April 2, 2020. https://www.kff.org/health-reform/report/kff-health-tracking-poll-early-april-2020/. Accessed May 24, 2020.
3. Lazer D, Baum MA, Ognyanova K, Della Volpe J. The State of the Nation: A 50-State COVID-19 Survey. April 30, 2020. http://www.kateto.net/COVID19%20CONSORTIUM%20REPORT%20April%202020.pdf. Accessed May 24, 2020
4. Whitmer G. I have made gut-wrenching choices to keep people safe. New York Times. April 21, 2020. https://www.nytimes.com/2020/04/21/opinion/gretchen-whitmer-coronavirus-michigan.html. Accessed May 24, 2020.
5. Kluch S. The compliance curve: Will people stay home much longer? Gallup Blog. April 29, 2020. https://news.gallup.com/opinion/gallup/309491/compliance-curve-americans-stay-home-covid.aspx. Accessed May 24, 2020.
6. Deutsch J, Wheaton S. Public health experts are now the bad guys. Politico. April 21, 2020. https://www.politico.com/news/2020/04/21/public-health-experts-are-now-the-bad-guys-198174. Accessed May 24, 2020.
7. Galva JE, Atchinson C, Levey S. Public health strategy and the police powers of the state. Public Health Rep. 2005;120(Suppl 1):20-27. https://doi.org/10.1177/00333549051200s106.
8. Gostin LO. Public health law in a new century: part III: public health regulation: a systematic evaluation. JAMA. 2000;283(23):3118-3122. https://doi.org/10.1001/jama.283.23.3118.
9. Smith SMS, Sonego S, Wallen G, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology. 2015;20(6):896-903. https://doi.org/10.1111/resp.12541.
10. Harris JE. The coronavirus epidemic curve is already flattening in New York City. National Bureau of Economic Research. April 2020. https://www.nber.org/papers/w26917. Accessed May 24, 2020.
11. La VP, Pham TH, Ho MT, et al. Policy response, social media and scientific journals for the sustainability of the public health system amid the COVID-19 outbreak: the Vietnam lessons. Sustainability. 2020;12(7):2931. https://doi.org/10.3390/su12072931.
12. Glik DC. Risk communication for public health emergencies. Annu Rev Public Health. 2007;28:33-54. https://doi.org/10.1146/annurev.publhealth.28.021406.144123.
13. MacDonald L, Cairns G, Angus K, Stead M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease. Stockholm: ECDC; 2012. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/Social-marketing-prevention-control-of-communicable-disease.pdf. Accessed May 8, 2020.
14. Dosani S and Westbrook A. The leader we wish we all had: A look at the style of Dr Amy Acton, who has earned praise for her daily briefings on the pandemic. New York Times. May 5, 2020. https://www.nytimes.com/2020/05/05/opinion/coronavirus-ohio-amy-acton.html.
15. Snyder L. An anniversary review and critique: the Tylenol crisis. Public Relations Rev. 1983;9(3):24-34. https://doi.org/10.1016/S0363-8111(83)80182-9.
16. Millar H, Davidson A, White LA. Puzzling publics: the role of reflexive learning in universal pre-kindergarten (UPK) policy formulation in Canada and the US. Public Policy Adm. 2020;35(3):312-336. https://doi.org/10.1177/0952076719889100.
© 2020 Society of Hospital Medicine
Empiric Therapies for COVID-19: Destined to Fail by Ignoring the Lessons of History
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
Manifestations of disease, as perceived by physicians, can shape conceptual views and favor specific therapeutic actions. Historically, these factors appear to have an outsized influence on medical thinking in general. Disease concepts derived from empirical observations during pandemics impose a trade-off. We obtain unparalleled insight into medical thought and practice, but risk incurring the cost of unfortunate mistakes. The psychologist and Nobel Prize winner in economics Daniel Kahneman describes two mental systems that shape our judgments and decision-making in his book, Thinking, Fast and Slow: System One is intuitive, emotional, and fast, whereas System Two is deliberative and logical and has slower onset.1 If we extrapolate these observations to clinical medicine, we often rely on either System One or System Two depending on particular situations. Errors can emerge when we default to fast and emotional responses in situations that instead require more deliberate and logical assessments. These include instances in which the desire to help—our humanitarian role as physicians, associated with an “adrenaline rush”—results from attempts to relieve human suffering. As mercenaries of misfortune, it is inevitable we engage medical interventions based on an incomplete understanding of the pathophysiology—in other words, without understanding the full risks and benefits.
During the ongoing COVID-19 pandemic, members of the medical community continue to provide care with the utmost nobility, empathy, and desire for action amid uncertainty. However, as the number of cases continues to increase worldwide, we urge caution in evaluating the current state of scientific understanding, our approaches to treatment, and the safety of empiric medical interventions targeting COVID-19. We are concerned that the extensive history of unintended adverse consequences of therapies for emerging infectious diseases in the past is being ignored in the development of approaches to COVID-19 treatment. It is likely harms will emerge from current empiric therapies for COVID-19 given what can be learned from history.
HISTORICAL EXAMPLES OF UNINTENDED ADVERSE CONSEQUENCES
Whereas influenza can be treated with neuraminidase inhibitors,2 there are currently no established effective antiviral therapies for COVID-19, which is similar to two other coronavirus diseases from the 21st century, SARS (Severe Acute Respiratory Syndrome) in 2003 and MERS (Middle-Eastern Respiratory Syndrome) in 2012.3 Even in times of global pandemic, we need to consider potential harms and adverse consequences of novel treatments and show justifiable ratio of risk versus benefit. With the absence of proven COVID-19 therapy and the desire to fulfill our oath of primum non nocere (first, do no harm) in mind, we review selected unintended adverse events of developing therapies for infectious diseases.
Two types of error in our decision-making strategies are errors of omission and errors of commission.4 Errors of omission, defined as instances in which a medical intervention was not carried out when there was a clear indication to do so, are less conspicuous in the history of infectious disease therapeutics. Errors of commission, in contrast, have become a more concerning component of our approach to COVID-19 therapy, perhaps prompted by our desire to act. Errors of commission are defined as instances in which a specific medical intervention that should have been avoided was instead performed. We will discuss historical examples of errors of commission to highlight parallels with the current pandemic (Appendix Figure).
During influenza epidemics in the 18th century, some physicians advocated the use of therapeutic lancet phlebotomies, while others recommended indiscriminate use of opium, which led to high rates of addiction.5 Neither intervention was supported by a reassuring body of evidence. Many recommended mercury-based preparations during major outbreaks of syphilis in medieval protestant Europe. Because of accumulated mercurial toxicity, many persons suffered long-term sequelae including chronic kidney injury and peripheral neuropathy.6 After the discovery of the tuberculous bacillus, Robert Koch attempted the inoculation of tuberculin as a curative intervention for tuberculosis.7 Under pressure from the king of Prussia to present his findings at the International Medical Meeting in Berlin, Germany, in 1890, Koch conducted a poorly executed clinical trial. Rudolf Virchow then demonstrated endobronchial spread of the infection with resultant clinical worsening in those who received Koch’s tuberculin. In 1905, Harold Wolfersan Thomas at the Liverpool School of Tropical Medicine treated cases of African trypanosomiasis with the arsenical drug Atoxyl (arsanilic acid), which demonstrated some efficacy but also caused optic nerve atrophy leading to blindness.8
There have also been errors of commission in the development of vaccines. One such event, known as the Cutter incident, followed from an incompletely inactivated batch of polio vaccine that caused 40,000 cases of abortive poliomyelitis and many cases of paralysis and death.9 In the early phases of the development of the yellow fever vaccine, Hideyo Noguchi tried to develop a vaccine based on the erroneous assumption that yellow fever was caused by Leptospira icteroides.10 In 1976, an error of commission occurred in response to an outbreak of a few dozen cases of Influenza A/H1N1 in Fort Dix, New Jersey: The accelerated implementation of a swine influenza–vaccination program led to many cases of Guillian-Barré Syndrome among recipients.11 Immunization experts defended this decision to vaccinate by arguing that “when lives are at risk, it’s better to err on the side of overreaction over underreaction.”11 However, this is a risk-perception versus risk-management concept that drives potential errors of commission.
A more recent error of commission involved the use of drotrecogin alfa (activated protein C) in the treatment of sepsis. This drug became the first and only Food and Drug Administration–approved drug for sepsis treatment. The approval process of this medication relied on one clinical trial, which was terminated early because of perceived overwhelming evidence of efficacy. Despite the initial high medical and financial expectations, Eli Lilly (Indianapolis) withdrew the drug when a larger, international clinical trial (PROWESS-SHOCK) did not show a similar benefit.12
THE COVID-19 ERA
The gravity of the COVID-19 pandemic has motivated the repurposing of previously available therapies. This explains the use of medications like hydroxychloroquine, interleukin-6 (IL-6) receptor antagonists, and remdesivir.13-15
Despite early authorization of emergency use for hydroxychloroquine by the FDA based on limited and poor-quality evidence,16 this drug has yet to demonstrate treatment efficacy for COVID-19. On the contrary, other, controlled, retrospective studies have shown that hydroxychloroquine might actually increase mortality, possibly through prolongation of the QT-interval.16,17 Also, diversion of this drug to treat COVID-19 raises the concern of hydroxychloroquine shortages for treatment of patients with autoimmune disease, in whom the drug has proven benefit. We question the hasty FDA authorization for emergency use of hydroxychloroquine for COVID-19.
There is also great enthusiasm among the medical community to administer IL-6 receptor antagonists as a COVID-19 treatment. The rationale for this approach includes observations in case series in which IL-6 levels correlated with adverse clinical outcomes.13 IL-6 antagonists have a proven track record of improving the outcome in autoimmune diseases. However, we must avoid the logical trap of post hoc, ergo propter hoc (after this, therefore because of this) dictum from which one would assume that, based on those observations of high IL-6 levels and adverse outcomes, lowering IL-6 levels will necessarily improve outcomes in COVID-19. The supposed role of IL-6 in causing COVID-19 is based on scant preliminary observations and on the yet unproven assumption that IL-6 association with disease severity is a cause-effect relationship and not an association separate from pathogenesis. Moreover, there is sufficient scientific evidence that, in the case of severe influenza infections, IL-6 limits inflammation and protects against severe and potentially life-threatening lung injury. The road ahead for IL-6 inhibition to treat COVID-19 is perilous and should be entered cautiously. One immediate concern of administering IL-6 receptor antagonists in this patient population is the potential reactivation of latent tuberculosis infection and hepatitis B, colonic perforation, and increased rate of infections in general.
The greatest hope at this early stage of the COVID-19 pandemic may be remdesivir, which is a direct-acting antiviral. Here again, initial case series in prestigious medical journals signaled the possibility of a morbidity and mortality benefit.14 Despite these encouraging signs, a recent clinical trial from China that was limited by incomplete patient enrollment demonstrated a lack of efficacy of remdesivir in accelerating clinical improvement or limiting mortality.18 In spite of these negative results, preliminary data from the Adaptive COVID-19 Treatment Trial (ACTT) has revealed a nonsignificant signal of reduced mortality and shorter time to recovery in the remdesivir group. In response to these reports, the FDA has now issued emergency use authorization of remdesivir for treating COVID-19. Given the precedence of conflicting study data in therapeutic development for infectious diseases, we urge caution in drawing interpretations of benefit based on these early reports. Early termination of clinical studies is often associated with a 30% overestimation of clinical benefit.19 Furthermore, the availability of remdesivir is limited, which raises substantial ethical concerns on the preferential allocation of the drug to selected populations in high-income countries. At the time of this report, uncertainty regarding the risk-benefit balance of remdesivir and other COVID-19 treatments should be emphasized among decision makers.
CONCLUSION
Errors of commission present particular concerns for risk in treating COVID-19 patients and suggest that sometimes inaction is preferable to action. With many pandemics, there is a history of repeating mistakes, and we believe this can be curtailed by heeding the lessons of history. In the end, we may learn that avoiding therapeutic interventions that are poorly supported may prove to be one of the most important legacies of the COVID-19 pandemic.
Disclosures
The authors reported having nothing to disclose.
Funding
Dr Shapiro is supported by The Emily Foundation, Boston, Massachusetts. For all other authors, no financial support was declared.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
1. Kahneman D. Thinking, Fast and Slow. New York: Farrar, Straus and Giroux; 2011.
2. Boikos C, Caya C, Doll MK, et al. Safety and effectiveness of neuraminidase inhibitors in situations of pandemic and/or novel/variant influenza: a systematic review of the literature, 2009-15. J Antimicrob Chemother. 2017;72(6):1556-1573. https://doi.org/10.1093/jac/dkx013.
3. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-534. https://doi.org/10.1038/nrmicro.2016.81.
4. Grober ED, Bohnen JM. Defining medical error. Can J Surg. 2005;48(1):39-44.
5. Schofield AT. Opium in influenza. Lancet. 1894;143(3676):372. https://doi.org/10.1016/S0140-6736(01)66349-9.
6. Abraham JJ. Some account of the history of the treatment of syphilis. Br J Vener Dis. 1948;24(4):153-161. https://doi.org/10.1136/sti.24.4.153.
7. Gradmann C. Laboratory Disease: Robert Koch’s Medical Bacteriology. Baltimore, MD: Johns Hopkins University Press; 2009. .
8. Steverding D. The history of African trypanosomiasis. Parasit Vectors. 2008;1(1):3. https://doi.org/10.1186/1756-3305-1-3
9. Offit PA. The Cutter incident, 50 years later. N Engl J Med. 2005;352(14):1411-1412. https://doi.org/10.1056/nejmp048180.
10. Frierson JG. The yellow fever vaccine: a history. Yale J Biol Med. 2010;83(2):77-85.
11. Sencer DJ, Millar JD. Reflections on the 1976 swine flu vaccination program. Emerg Infect Dis. 2006;12(1):29-33. https://doi.org/10.3201/eid1201.051007.
12. Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055-2064. https://doi.org/10.1056/nejmoa1202290.
13. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab [online first]. ChinaXiv. 2020. https://doi.org/10.1073/pnas.2005615117.
14. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19 [online first]. N Engl J Med. 2020. https://doi.org/10.1056/nejmoa2007016.
15. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [online first]. Int J Antimicrob Agents. 2020. https://doi.org/10.1016/j.ijantimicag.2020.105949.
16. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [preprint]. medRxiv. 2020. https://doi.org/10.1101/2020.04.16.20065920.
17. Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit [online first]. JAMA Cardiology. 2020. https://doi.org/10.1001/jamacardio.2020.1787.
18. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. https://doi.org/10.1016/S0140-6736(20)31022-9.
19. Bassler D, Briel M, Montori VM, et al; STOPIT-2 Study Group. Stopping randomized trials early for benefit and estimation of treatment effects, systematic review and meta-regression analysis. JAMA. 2010 Mar 24;303(12):1180-1187. https://doi.org/jama.2010.310.
© 2020 Society of Hospital Medicine