User login
Guidelines for AD management differ among health groups
Numerous differences exist between dermatology groups on treatment for atopic dermatitis (AD), report Girish C. Mohan and Dr. Peter A. Lio.
A clinical review of AD management guidelines from seven international dermatology organizations found that, although basic guidelines for first-line topical and systemic treatment are similar, “notable differences” were found on guidelines for adjunctive therapies such as diluted bleach baths, vitamin D, and environmental modifications.
“The juxtaposition of different guidelines can enhance individualization of treatment for a patient with AD by drawing from different disciplines with varying traditions and perspectives,” the investigators said in the report.
Read the full review in JAMA Dermatology.
Numerous differences exist between dermatology groups on treatment for atopic dermatitis (AD), report Girish C. Mohan and Dr. Peter A. Lio.
A clinical review of AD management guidelines from seven international dermatology organizations found that, although basic guidelines for first-line topical and systemic treatment are similar, “notable differences” were found on guidelines for adjunctive therapies such as diluted bleach baths, vitamin D, and environmental modifications.
“The juxtaposition of different guidelines can enhance individualization of treatment for a patient with AD by drawing from different disciplines with varying traditions and perspectives,” the investigators said in the report.
Read the full review in JAMA Dermatology.
Numerous differences exist between dermatology groups on treatment for atopic dermatitis (AD), report Girish C. Mohan and Dr. Peter A. Lio.
A clinical review of AD management guidelines from seven international dermatology organizations found that, although basic guidelines for first-line topical and systemic treatment are similar, “notable differences” were found on guidelines for adjunctive therapies such as diluted bleach baths, vitamin D, and environmental modifications.
“The juxtaposition of different guidelines can enhance individualization of treatment for a patient with AD by drawing from different disciplines with varying traditions and perspectives,” the investigators said in the report.
Read the full review in JAMA Dermatology.
Mandatory prescriber training now available for flibanserin
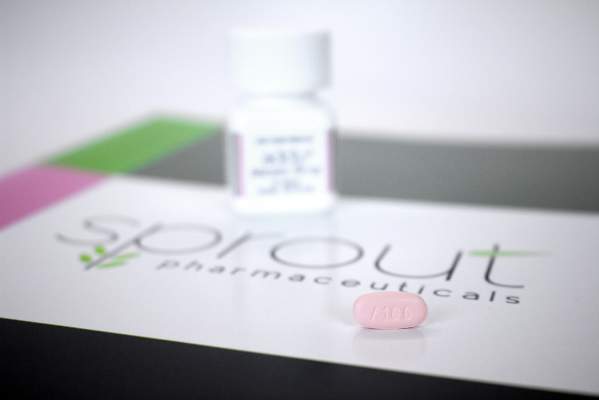
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
Physicians can now complete the training and paperwork required to prescribe flibanserin (Addyi, Sprout Pharmaceuticals), a new centrally acting, nonhormonal daily medication that treats female hypoactive sexual desire disorder.
The Food and Drug Administration’s August 2015 approval of flibanserin came with a required REMS(Risk Evaluation and Mitigation Strategy ) to address safety concerns.
Flibanserin, which the FDA had twice previously declined to approve, has an increased risk for syncope and hypotension with alcohol and moderate or strong CYP3A4 inhibitors, such as proton pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, and antifungals. Flibanserin taken alone also caused hypotension and syncope in a few patients during clinical trials.
The REMS addresses these risks by requiring all prescribers to complete training and a knowledge assessment about flibanserin’s risks and to enroll in a REMS certification program for the drug. Prescribers must also review a patient-provider agreement form with patients and have both parties sign before prescribing flibanserin.
Outpatient pharmacies must designate a representative to complete training and knowledge assessment, train their staff, and counsel every patient receiving flibanserin to abstain from alcohol. Inpatient pharmacies have similar training requirements and may not dispense flibanserin for outpatient use.
Flibanserin is approved for treatment of acquired, generalized hypoactive sexual desire disorder in premenopausal women only. It is a medication that is meant to be taken on a chronic basis, acting as a mixed agonist/antagonist for dopamine and serotonin receptors. In clinical trials, it showed a statistically significant, but modest improvement in reported sexual desire and the number of sexually satisfying events per month.
The certification materials are available online at www.Addyi.com. To complete the certification process, prescribers and pharmacists should fax the completed knowledge assessment and enrollment forms to 844-694-3373 or email scanned copies to [email protected].
On Twitter @karioakes
MedPAC to look at physician prescribing tools as a way to control drug spending
WASHINGTON – The Medicare Payment Advisory Commission is going to look at physician prescribing tools as part of a broader examination of how to rein in Medicare drug spending.
Members acknowledged during the Sept. 11, 2015, meeting that when it comes to the prices of drugs in the Medicare programs, the tools are limited to keep the prices low. Between statutory requirements for coverage of drugs in protected classes and a prohibition against the secretary of Health and Human Services negotiating prices for the Part D prescription drug benefit and other statutory requirements, even for intermediaries such as plan providers and hospital groups, leverage in price negotiations is very limited.
However, commission member Dr. Craig Samitt, former partner at Oliver Wyman of Paradise Valley, Ariz., suggested that the focus should be more on what leverage providers might have when it comes to utilization.
“So if we feel that neither CMS nor the intermediaries have sufficient leverage, well then who has significant leverage? The prescribing clinician,” Dr. Samitt said. “How well have we aligned interests around utilization in particular, not so much price, with the clinicians?”
Dr. Samitt noted that on the commercial side, there is a focus on utilization as a more effective driver of price, rather than simply targeting price first in the negotiation process, and suggested there might be room in Medicare for that kind of focus.
He also suggested that perhaps including drug utilization within the context of accountable care organizations could result in “additional focus on more effective prescribing patterns.”
The conversation occurred against a backdrop of examination of drug spending in general. MedPAC staff noted that Medicare is becoming a more prominent payer for drugs in the wake of Part D’s launch.
MedPAC staff estimates that in 2013, retail drugs made up 13% of Medicare spending, versus 9% of national health expenditures. Additionally, of the $574 billion spent by Medicare in that year, 19% was drugs and pharmacy, with the majority of drug spending (57%) coming from Part D.
The discussion was just the first on the subject as the group will look at other aspects of drug pricing and spending in future meetings. A specific timetable for offering policy recommendations was not discussed.
Dr. William Hall, professor at the University of Rochester (N.Y.) School of Medicine, added that it is not the price of the drug per se, but its value that needs to be focused on. He noted that the prices of the latest hepatitis C drugs might be high, but the value they have to the health care system is much greater and needs to be taken into consideration.
“One of the big differences from 2004 is we know a great deal more about the efficacy of drugs,” Dr. Hall said, suggesting that more needs to be done to educate clinicians on the proper use of medications as part of finding the right way to use physician prescribing patterns as leverage in price negotiations.
WASHINGTON – The Medicare Payment Advisory Commission is going to look at physician prescribing tools as part of a broader examination of how to rein in Medicare drug spending.
Members acknowledged during the Sept. 11, 2015, meeting that when it comes to the prices of drugs in the Medicare programs, the tools are limited to keep the prices low. Between statutory requirements for coverage of drugs in protected classes and a prohibition against the secretary of Health and Human Services negotiating prices for the Part D prescription drug benefit and other statutory requirements, even for intermediaries such as plan providers and hospital groups, leverage in price negotiations is very limited.
However, commission member Dr. Craig Samitt, former partner at Oliver Wyman of Paradise Valley, Ariz., suggested that the focus should be more on what leverage providers might have when it comes to utilization.
“So if we feel that neither CMS nor the intermediaries have sufficient leverage, well then who has significant leverage? The prescribing clinician,” Dr. Samitt said. “How well have we aligned interests around utilization in particular, not so much price, with the clinicians?”
Dr. Samitt noted that on the commercial side, there is a focus on utilization as a more effective driver of price, rather than simply targeting price first in the negotiation process, and suggested there might be room in Medicare for that kind of focus.
He also suggested that perhaps including drug utilization within the context of accountable care organizations could result in “additional focus on more effective prescribing patterns.”
The conversation occurred against a backdrop of examination of drug spending in general. MedPAC staff noted that Medicare is becoming a more prominent payer for drugs in the wake of Part D’s launch.
MedPAC staff estimates that in 2013, retail drugs made up 13% of Medicare spending, versus 9% of national health expenditures. Additionally, of the $574 billion spent by Medicare in that year, 19% was drugs and pharmacy, with the majority of drug spending (57%) coming from Part D.
The discussion was just the first on the subject as the group will look at other aspects of drug pricing and spending in future meetings. A specific timetable for offering policy recommendations was not discussed.
Dr. William Hall, professor at the University of Rochester (N.Y.) School of Medicine, added that it is not the price of the drug per se, but its value that needs to be focused on. He noted that the prices of the latest hepatitis C drugs might be high, but the value they have to the health care system is much greater and needs to be taken into consideration.
“One of the big differences from 2004 is we know a great deal more about the efficacy of drugs,” Dr. Hall said, suggesting that more needs to be done to educate clinicians on the proper use of medications as part of finding the right way to use physician prescribing patterns as leverage in price negotiations.
WASHINGTON – The Medicare Payment Advisory Commission is going to look at physician prescribing tools as part of a broader examination of how to rein in Medicare drug spending.
Members acknowledged during the Sept. 11, 2015, meeting that when it comes to the prices of drugs in the Medicare programs, the tools are limited to keep the prices low. Between statutory requirements for coverage of drugs in protected classes and a prohibition against the secretary of Health and Human Services negotiating prices for the Part D prescription drug benefit and other statutory requirements, even for intermediaries such as plan providers and hospital groups, leverage in price negotiations is very limited.
However, commission member Dr. Craig Samitt, former partner at Oliver Wyman of Paradise Valley, Ariz., suggested that the focus should be more on what leverage providers might have when it comes to utilization.
“So if we feel that neither CMS nor the intermediaries have sufficient leverage, well then who has significant leverage? The prescribing clinician,” Dr. Samitt said. “How well have we aligned interests around utilization in particular, not so much price, with the clinicians?”
Dr. Samitt noted that on the commercial side, there is a focus on utilization as a more effective driver of price, rather than simply targeting price first in the negotiation process, and suggested there might be room in Medicare for that kind of focus.
He also suggested that perhaps including drug utilization within the context of accountable care organizations could result in “additional focus on more effective prescribing patterns.”
The conversation occurred against a backdrop of examination of drug spending in general. MedPAC staff noted that Medicare is becoming a more prominent payer for drugs in the wake of Part D’s launch.
MedPAC staff estimates that in 2013, retail drugs made up 13% of Medicare spending, versus 9% of national health expenditures. Additionally, of the $574 billion spent by Medicare in that year, 19% was drugs and pharmacy, with the majority of drug spending (57%) coming from Part D.
The discussion was just the first on the subject as the group will look at other aspects of drug pricing and spending in future meetings. A specific timetable for offering policy recommendations was not discussed.
Dr. William Hall, professor at the University of Rochester (N.Y.) School of Medicine, added that it is not the price of the drug per se, but its value that needs to be focused on. He noted that the prices of the latest hepatitis C drugs might be high, but the value they have to the health care system is much greater and needs to be taken into consideration.
“One of the big differences from 2004 is we know a great deal more about the efficacy of drugs,” Dr. Hall said, suggesting that more needs to be done to educate clinicians on the proper use of medications as part of finding the right way to use physician prescribing patterns as leverage in price negotiations.
AT A MEETING OF THE MEDICARE PAYMENT ADVISORY COMMISSION
H. pylori resistance highlights need for guided therapy
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Antimicrobial-resistant strains of H. pylori are increasing in prevalence in the United States. In the study described here, only half of H. pylori strains were susceptible to commonly used antibiotics and approximately one in three were resistant to at least one antibiotic, according to a single-center study of U.S. veterans. The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. Patients were mostly male and had an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline.

|
Dr. Nimish Vakil |
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers.
The study mirrors findings in Europe where similar rates of resistance have been reported. European studies have also shown that levofloxacin resistance rises rapidly when it becomes widely used in the community, The study described here is not population based and consists mostly of male subjects and therefore may not be generalizable to the rest to the rest of the United States. As culture and antimicrobial sensitivity testing is not available to most gastroenterologists, the initial treatment chosen should reflect resistance data in the community. Given the rising rates of resistance, it is important that eradication be confirmed 4 weeks or more after eradication therapy ends using a stool antigen test or a breath test. Clinicians should be prepared to re-treat patients if necessary.
Dr. Nimish Vakil, AGAF, is clinical professor of medicine at the University of Wisconsin School of Medicine and Public Health in Madison. He has no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Only half of Helicobacter pylori strains were pansusceptible, and almost one in three was resistant to at least one antibiotic, according to a single-center study of U.S. veterans published in Clinical Gastroenterology and Hepatology.
The analysis is the first published report of H. pylori resistance in more than a decade, said Dr. Seiji Shiota at the Michael E. DeBakey Veterans Affairs Medical Center and the Baylor College of Medicine, Houston, and his associates. “Clarithromycin, metronidazole, and levofloxacin resistances were all high among untreated patients, suggesting that they all should be avoided as components of empiric triple therapy [consisting of a] proton pump inhibitor, amoxicillin, plus a third antibiotic,” said the researchers. “The four-drug concomitant therapy and bismuth quadruple therapy, or antibiotic susceptibility–guided therapy, are likely be the best strategies locally and are recommended for previously untreated patients with H. pylori infection.”
The study assessed 656 gastric biopsies randomly selected from a cohort of 1,559 patients who underwent esophagogastroduodenoscopy at the Houston VA Medical Center between 2009 and 2013. About 90% of patients were male, and patients ranged in age from 40 to 79 years old, with an average age of 60 years. The researchers cultured tissue samples and used the E test to assess minimum inhibitory concentrations for amoxicillin, clarithromycin, metronidazole, levofloxacin, and tetracycline. (Clin Gastroenterol Hepatol. 2015 Feb 11. pii: S1542-3565(15)00122-6).
A total of 135 (20.6%) of the biopsies cultured H. pylori, of which half (65 strains) were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin (95% confidence interval, 23%-39%), 20% were resistant to metronidazole (95% CI, 13%-27%), 16% were resistant to clarithromycin (95% CI, 10%-23%), 0.8% were resistant to tetracycline (95% CI, 0%-2%), and none were resistant to amoxicillin, said the researchers. The extent of levofloxacin resistance was a “new and concerning finding” that was linked in the multivariable analysis with past fluoroquinolone treatment, reflecting the rising use of fluoroquinolones in community practice, they said. “Levofloxacin has been recommended as a rescue drug to eradicate H. pylori in patients who fail first-line therapy,” they added. “Locally, it would seem to be a poor choice on the basis of the high resistance rate (31.9%), which is higher than the 10% limit suggested as a cutoff for use of fluoroquinolone-containing triple therapy for H. pylori.”
Clarithromycin resistance also rose during the study period, probably because of the rising use of macrolides in respiratory and otorhinolaryngology, the investigators noted. Patients who had been treated before for helicobacteriosis were significantly more likely to have clarithromycin-resistant H. pylori infections even after accounting for demographic factors, smoking status, gastroesophageal reflux disease, and past use of macrolides and fluoroquinolones, they said. Based on that result, patients with a history of prior helicobacteriosis should not receive clarithromycin as part of triple therapy, they emphasized.
Resistance to metronidazole also remained high, but only 1.8% of isolates were resistant to both metronidazole and clarithromycin, making combination therapy with a proton pump inhibitor, clarithromycin, metronidazole, and amoxicillin “an excellent choice as an empiric therapy,” added Dr. Shiota and his associates. Furthermore, the study might have overestimated the rate of metronidazole resistance because the E test yielded significantly higher minimum inhibitory concentration values than did agar dilution, they noted. The study cohort also was demographically dissimilar to that of the United States and might have reflected selection bias, because patients with a history of helicobacteriosis would be more likely to be referred for endoscopy, they said.
The National Institutes of Health and the Veterans Affairs Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Because H. pylori showed high rates of resistance to clarithromycin, metronidazole, and levofloxacin, they should be excluded from triple therapy regimens for helicobacteriosis.
Major finding: Half of strains were susceptible to all five antibiotics tested, 31% were resistant to levofloxacin, 20% were resistant to metronidazole, 16% were resistant to clarithromycin, 0.8% were resistant to tetracycline, and none were resistant to amoxicillin.
Data source: Analysis of gastric biopsies from 656 U.S. veterans who underwent esophagogastroduodenoscopy in Texas between 2009 and 2013.
Disclosures: The National Institutes of Health and the VA Health Services Research & Development Center for Innovations in Quality, Effectiveness, and Safety supported the study. The researchers reported having no conflicts of interest.
Online resource launched to prevent inpatient hospital falls
An online resource guide offers 21 targeted solutions for reducing the rate of falls in hospitals and urgent care settings, The Joint Commission Center for Transforming Healthcare announced in a statement.
Using the fall prevention methodology of the Targeted Solutions Tool, developed in collaboration with seven hospitals and five health care organizations, a typical 200-bed hospital could potentially reduce the number of patients injured from a fall from 117 to 45, avoiding approximately $1 million in costs annually, the agency claims.
Some of the recommendations for reducing in-hospital falls include:
• Creating awareness among staff.
• Using a validated fall risk assessment tool.
• Engaging patients and their families in the fall safety program.
• Hourly rounding with scheduled restroom use for patients.
• Engaging all hospital staff and patients to ensure no patient walks without assistance.
“Hundreds of thousands of patients fall in hospitals every year and many of these falls result in moderate to severe injuries that can prolong hospital stays and require the patient to undergo additional treatment,” Dr. Erin DuPree, vice president and chief medical officer of the Joint Commission Center for Transforming Healthcare, said in a statement.
The Joint Commission Center for Transforming Healthcare was created in 2008 as a nonprofit affiliate of The Joint Commission.
Check out the online resource here.
An online resource guide offers 21 targeted solutions for reducing the rate of falls in hospitals and urgent care settings, The Joint Commission Center for Transforming Healthcare announced in a statement.
Using the fall prevention methodology of the Targeted Solutions Tool, developed in collaboration with seven hospitals and five health care organizations, a typical 200-bed hospital could potentially reduce the number of patients injured from a fall from 117 to 45, avoiding approximately $1 million in costs annually, the agency claims.
Some of the recommendations for reducing in-hospital falls include:
• Creating awareness among staff.
• Using a validated fall risk assessment tool.
• Engaging patients and their families in the fall safety program.
• Hourly rounding with scheduled restroom use for patients.
• Engaging all hospital staff and patients to ensure no patient walks without assistance.
“Hundreds of thousands of patients fall in hospitals every year and many of these falls result in moderate to severe injuries that can prolong hospital stays and require the patient to undergo additional treatment,” Dr. Erin DuPree, vice president and chief medical officer of the Joint Commission Center for Transforming Healthcare, said in a statement.
The Joint Commission Center for Transforming Healthcare was created in 2008 as a nonprofit affiliate of The Joint Commission.
Check out the online resource here.
An online resource guide offers 21 targeted solutions for reducing the rate of falls in hospitals and urgent care settings, The Joint Commission Center for Transforming Healthcare announced in a statement.
Using the fall prevention methodology of the Targeted Solutions Tool, developed in collaboration with seven hospitals and five health care organizations, a typical 200-bed hospital could potentially reduce the number of patients injured from a fall from 117 to 45, avoiding approximately $1 million in costs annually, the agency claims.
Some of the recommendations for reducing in-hospital falls include:
• Creating awareness among staff.
• Using a validated fall risk assessment tool.
• Engaging patients and their families in the fall safety program.
• Hourly rounding with scheduled restroom use for patients.
• Engaging all hospital staff and patients to ensure no patient walks without assistance.
“Hundreds of thousands of patients fall in hospitals every year and many of these falls result in moderate to severe injuries that can prolong hospital stays and require the patient to undergo additional treatment,” Dr. Erin DuPree, vice president and chief medical officer of the Joint Commission Center for Transforming Healthcare, said in a statement.
The Joint Commission Center for Transforming Healthcare was created in 2008 as a nonprofit affiliate of The Joint Commission.
Check out the online resource here.
ACIP releases 2015-2016 flu vaccine recommendations
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
Influenza vaccination is recommended for all patients aged 6 months and older, as long as they don’t have a contraindication, according to a report Aug. 7 in Morbidity and Mortality Weekly Report.
Trivalent influenza vaccines for the 2015-2016 season will contain hemagglutinin (HA) derived from an H1N1-like virus, an H3N2-like virus, and a B/Phuket/3073/2013-like (Yamagata lineage) virus. Quadrivalent vaccines will contain those components, as well as a B/Brisbane/60/2008-like (Victoria lineage) virus, the same virus recommended for quadrivalent formulations in the 2013-14 and 2014-15 seasons, ACIP said in a statement.
New FDA-approved vaccines include Afluria, the Fluzone Intradermal Quadrivalent vaccine (both approved in 2014 for adults aged 18-64 years), and an expanded age indication for Flublok, which is now indicated for adults aged 18 years and older.
The live attenuated influenza vaccine (LAIV) should not be used in certain populations, including those aged less than 2 years or greater than 49 years; children aged 2-17 years taking aspirin; pateints with severe allergic reactions to the vaccine; pregnant women; and those with egg allergies, among others. Either the LAIV or the inactivated influenza vaccine (IIV) is appropriate for administration in healthy children aged 2-8 years, ACIP said.
For a detailed explanation of the recommendations, see MMWR.
NICE recommends empagliflozin in combo therapy for type 2 diabetes
The National Institute for Health and Care Excellence (NICE) has issued guidance on the clinical and cost-effectiveness of empagliflozin in combination therapy for treatment of type 2 diabetes.
The guideline, released in March, is for advanced-practice nurses, nurses, physician assistants, and physicians, according to a summary by the National Guideline Clearinghouse (NGC).
The summary lists recommendations by NICE for treatment of type 2 diabetes as follows:
• Empagliflozin in a dual-therapy regimen in combination with metformin, only if a sulfonylurea is contraindicated or not tolerated, or if the person is at significant risk of hypoglycemia or its consequences.
• Empagliflozin in a triple-therapy regimen, in combination with metformin and a sulfonylurea or metformin and a thiazolidinedione.
• Empagliflozin in combination with insulin with or without other antidiabetic drugs.
According to the summary, the most commonly reported adverse reactions for empagliflozin are hypoglycemia in combination with insulin or a sulfonylurea, vulvovaginal candidiasis, urinary tract infection, and polyuria or pollakiuria.
As for the cost-effectiveness, an appraisal committee independent of NICE “concluded that the very small differences in costs and quality-adjusted life years between empagliflozin (10 mg and 25 mg) and its key comparators showed that empagliflozin was a cost-effective use of National Health Service resources as dual therapy in combination with metformin, triple therapy in combination with metformin and either a sulfonylurea or a thiazolidinedione, and as an add-on treatment to insulin.”
The National Institute for Health and Care Excellence (NICE) has issued guidance on the clinical and cost-effectiveness of empagliflozin in combination therapy for treatment of type 2 diabetes.
The guideline, released in March, is for advanced-practice nurses, nurses, physician assistants, and physicians, according to a summary by the National Guideline Clearinghouse (NGC).
The summary lists recommendations by NICE for treatment of type 2 diabetes as follows:
• Empagliflozin in a dual-therapy regimen in combination with metformin, only if a sulfonylurea is contraindicated or not tolerated, or if the person is at significant risk of hypoglycemia or its consequences.
• Empagliflozin in a triple-therapy regimen, in combination with metformin and a sulfonylurea or metformin and a thiazolidinedione.
• Empagliflozin in combination with insulin with or without other antidiabetic drugs.
According to the summary, the most commonly reported adverse reactions for empagliflozin are hypoglycemia in combination with insulin or a sulfonylurea, vulvovaginal candidiasis, urinary tract infection, and polyuria or pollakiuria.
As for the cost-effectiveness, an appraisal committee independent of NICE “concluded that the very small differences in costs and quality-adjusted life years between empagliflozin (10 mg and 25 mg) and its key comparators showed that empagliflozin was a cost-effective use of National Health Service resources as dual therapy in combination with metformin, triple therapy in combination with metformin and either a sulfonylurea or a thiazolidinedione, and as an add-on treatment to insulin.”
The National Institute for Health and Care Excellence (NICE) has issued guidance on the clinical and cost-effectiveness of empagliflozin in combination therapy for treatment of type 2 diabetes.
The guideline, released in March, is for advanced-practice nurses, nurses, physician assistants, and physicians, according to a summary by the National Guideline Clearinghouse (NGC).
The summary lists recommendations by NICE for treatment of type 2 diabetes as follows:
• Empagliflozin in a dual-therapy regimen in combination with metformin, only if a sulfonylurea is contraindicated or not tolerated, or if the person is at significant risk of hypoglycemia or its consequences.
• Empagliflozin in a triple-therapy regimen, in combination with metformin and a sulfonylurea or metformin and a thiazolidinedione.
• Empagliflozin in combination with insulin with or without other antidiabetic drugs.
According to the summary, the most commonly reported adverse reactions for empagliflozin are hypoglycemia in combination with insulin or a sulfonylurea, vulvovaginal candidiasis, urinary tract infection, and polyuria or pollakiuria.
As for the cost-effectiveness, an appraisal committee independent of NICE “concluded that the very small differences in costs and quality-adjusted life years between empagliflozin (10 mg and 25 mg) and its key comparators showed that empagliflozin was a cost-effective use of National Health Service resources as dual therapy in combination with metformin, triple therapy in combination with metformin and either a sulfonylurea or a thiazolidinedione, and as an add-on treatment to insulin.”
NICE releases guidelines on medicine optimization
Effective systems and processes are a key part of minimizing the risk of preventable medicine-related problems and ensuring best possible outcomes for care, according to newly released clinical guidelines from the Medicines Prescribing Centre of the U.K.-based National Institute for Health and Care Excellence (NICE).
The guidelines, developed by a multidisciplinary Guideline Development Group (GDG) of NICE staff, health professionals, and lay members, were designed to ensure that National Health Service patients get the best possible outcomes from their medicines.
“Relevant information about medicines should be shared with patients, and their family members or carers, where appropriate, and between health and social care practitioners when a person moves from one care setting to another, to support high-quality care,” wrote the authors of the guidelines, led by Dr. Weeliat Chong, chair of the GDG.
The report identified four key recommendations as priorities for implementation:
• Consider using multiple methods (such as health record review, patient surveys and direct observation of medicines administration) to identify medicine-related patient safety incidents
• Organizations should ensure that medicines reconciliation (i.e., making sure medicines prescribed on admission correspond to those that the patient was taking before admission) is carried out by a trained and competent health professional with effective communication skills, technical knowledge of processes for managing medicines, and therapeutic knowledge of medicines use.
• Health and social care practitioners should share relevant information about the [patients] and their medicines via medicines-related communication systems when a person transfers from one care setting to another.
• Consider sending the patient’s medicines discharge information to [his or her] nominated community pharmacy, when possible and in agreement with the patient.
Click here for the full report.
Effective systems and processes are a key part of minimizing the risk of preventable medicine-related problems and ensuring best possible outcomes for care, according to newly released clinical guidelines from the Medicines Prescribing Centre of the U.K.-based National Institute for Health and Care Excellence (NICE).
The guidelines, developed by a multidisciplinary Guideline Development Group (GDG) of NICE staff, health professionals, and lay members, were designed to ensure that National Health Service patients get the best possible outcomes from their medicines.
“Relevant information about medicines should be shared with patients, and their family members or carers, where appropriate, and between health and social care practitioners when a person moves from one care setting to another, to support high-quality care,” wrote the authors of the guidelines, led by Dr. Weeliat Chong, chair of the GDG.
The report identified four key recommendations as priorities for implementation:
• Consider using multiple methods (such as health record review, patient surveys and direct observation of medicines administration) to identify medicine-related patient safety incidents
• Organizations should ensure that medicines reconciliation (i.e., making sure medicines prescribed on admission correspond to those that the patient was taking before admission) is carried out by a trained and competent health professional with effective communication skills, technical knowledge of processes for managing medicines, and therapeutic knowledge of medicines use.
• Health and social care practitioners should share relevant information about the [patients] and their medicines via medicines-related communication systems when a person transfers from one care setting to another.
• Consider sending the patient’s medicines discharge information to [his or her] nominated community pharmacy, when possible and in agreement with the patient.
Click here for the full report.
Effective systems and processes are a key part of minimizing the risk of preventable medicine-related problems and ensuring best possible outcomes for care, according to newly released clinical guidelines from the Medicines Prescribing Centre of the U.K.-based National Institute for Health and Care Excellence (NICE).
The guidelines, developed by a multidisciplinary Guideline Development Group (GDG) of NICE staff, health professionals, and lay members, were designed to ensure that National Health Service patients get the best possible outcomes from their medicines.
“Relevant information about medicines should be shared with patients, and their family members or carers, where appropriate, and between health and social care practitioners when a person moves from one care setting to another, to support high-quality care,” wrote the authors of the guidelines, led by Dr. Weeliat Chong, chair of the GDG.
The report identified four key recommendations as priorities for implementation:
• Consider using multiple methods (such as health record review, patient surveys and direct observation of medicines administration) to identify medicine-related patient safety incidents
• Organizations should ensure that medicines reconciliation (i.e., making sure medicines prescribed on admission correspond to those that the patient was taking before admission) is carried out by a trained and competent health professional with effective communication skills, technical knowledge of processes for managing medicines, and therapeutic knowledge of medicines use.
• Health and social care practitioners should share relevant information about the [patients] and their medicines via medicines-related communication systems when a person transfers from one care setting to another.
• Consider sending the patient’s medicines discharge information to [his or her] nominated community pharmacy, when possible and in agreement with the patient.
Click here for the full report.
NICE advises on how to maintain, achieve healthy weight
The National Institute for Health and Care Excellence (NICE) has issued a new guideline on maintaining a healthy weight and preventing excess weight gain.
This guideline replaces section 1.1.1 of NICE’s guideline on obesity, CG43 (2006).
The guideline, for those who educate people on how to maintain a healthy weight or prevent excess weight gain, comprises the following recommendations:
• Encourage people to make changes in line with existing advice.
• Encourage physical activity habits to avoid low energy expenditure.
• Encourage dietary habits that reduce the risk of excess energy intake.
• Provide further advice for parents and carers of children and young people.
• Encourage adults to limit the amount of alcohol they drink.
• Encourage self-monitoring.
• Clearly communicate the benefits of maintaining a healthy weight.
• Clearly communicate the benefits of gradual improvements to physical activity and dietary habits.
• Tailor messages for specific groups.
• Ensure activities are integrated with the local strategic approach to obesity.
The National Institute for Health and Care Excellence (NICE) has issued a new guideline on maintaining a healthy weight and preventing excess weight gain.
This guideline replaces section 1.1.1 of NICE’s guideline on obesity, CG43 (2006).
The guideline, for those who educate people on how to maintain a healthy weight or prevent excess weight gain, comprises the following recommendations:
• Encourage people to make changes in line with existing advice.
• Encourage physical activity habits to avoid low energy expenditure.
• Encourage dietary habits that reduce the risk of excess energy intake.
• Provide further advice for parents and carers of children and young people.
• Encourage adults to limit the amount of alcohol they drink.
• Encourage self-monitoring.
• Clearly communicate the benefits of maintaining a healthy weight.
• Clearly communicate the benefits of gradual improvements to physical activity and dietary habits.
• Tailor messages for specific groups.
• Ensure activities are integrated with the local strategic approach to obesity.
The National Institute for Health and Care Excellence (NICE) has issued a new guideline on maintaining a healthy weight and preventing excess weight gain.
This guideline replaces section 1.1.1 of NICE’s guideline on obesity, CG43 (2006).
The guideline, for those who educate people on how to maintain a healthy weight or prevent excess weight gain, comprises the following recommendations:
• Encourage people to make changes in line with existing advice.
• Encourage physical activity habits to avoid low energy expenditure.
• Encourage dietary habits that reduce the risk of excess energy intake.
• Provide further advice for parents and carers of children and young people.
• Encourage adults to limit the amount of alcohol they drink.
• Encourage self-monitoring.
• Clearly communicate the benefits of maintaining a healthy weight.
• Clearly communicate the benefits of gradual improvements to physical activity and dietary habits.
• Tailor messages for specific groups.
• Ensure activities are integrated with the local strategic approach to obesity.
NICE recommends rivaroxaban for acute coronary syndrome
Rivaroxaban has been recommended by the U.K. National Institute for Health and Care Excellence (NICE) as a treatment option for prevention of blood clots in adults who have had acute coronary syndrome with elevated cardiac biomarkers, the agency announced in a statement.
NICE officials recommended rivaroxaban (Xarelto), in combination with aspirin plus clopidogrel or aspirin alone, as an option for preventing atherothrombotic events in patients who have had a heart attack. Assessment of clinical-effectiveness evidence was based on data from an international, multicenter, randomized controlled trial. An independent appraisal committee considered clinical and cost-effectiveness evidence before making the recommendation.
Rivaroxaban, manufactured by Bayer and marketed by Janssen Pharmaceuticals, is an orally active direct factor Xa inhibitor.
Clinicians should regularly reassess the benefits and risks of continuing treatment with rivaroxaban, the agency recommended, and a decision on continuation of treatment should be made no later than 12 months after starting treatment.
Read the full guideline statement here.
Rivaroxaban has been recommended by the U.K. National Institute for Health and Care Excellence (NICE) as a treatment option for prevention of blood clots in adults who have had acute coronary syndrome with elevated cardiac biomarkers, the agency announced in a statement.
NICE officials recommended rivaroxaban (Xarelto), in combination with aspirin plus clopidogrel or aspirin alone, as an option for preventing atherothrombotic events in patients who have had a heart attack. Assessment of clinical-effectiveness evidence was based on data from an international, multicenter, randomized controlled trial. An independent appraisal committee considered clinical and cost-effectiveness evidence before making the recommendation.
Rivaroxaban, manufactured by Bayer and marketed by Janssen Pharmaceuticals, is an orally active direct factor Xa inhibitor.
Clinicians should regularly reassess the benefits and risks of continuing treatment with rivaroxaban, the agency recommended, and a decision on continuation of treatment should be made no later than 12 months after starting treatment.
Read the full guideline statement here.
Rivaroxaban has been recommended by the U.K. National Institute for Health and Care Excellence (NICE) as a treatment option for prevention of blood clots in adults who have had acute coronary syndrome with elevated cardiac biomarkers, the agency announced in a statement.
NICE officials recommended rivaroxaban (Xarelto), in combination with aspirin plus clopidogrel or aspirin alone, as an option for preventing atherothrombotic events in patients who have had a heart attack. Assessment of clinical-effectiveness evidence was based on data from an international, multicenter, randomized controlled trial. An independent appraisal committee considered clinical and cost-effectiveness evidence before making the recommendation.
Rivaroxaban, manufactured by Bayer and marketed by Janssen Pharmaceuticals, is an orally active direct factor Xa inhibitor.
Clinicians should regularly reassess the benefits and risks of continuing treatment with rivaroxaban, the agency recommended, and a decision on continuation of treatment should be made no later than 12 months after starting treatment.
Read the full guideline statement here.