User login
The latest recommendations from the USPSTF
Recently, the US Preventive Services Task Force (USPSTF) finalized 7 recommendations on 5 topics and posted draft recommendations on an additional 10 topics. It also implemented new procedures that include posting draft recommendations for public comment (see “A new review process for the USPSTF”). This article reviews the USPSTF activity in 2011, as well as cervical cancer screening recommendations issued earlier this year.
In response to the adverse publicity from the 2009 mammogram recommendations and the increased scrutiny brought on by the affordable care act—which mandates that A and B recommendations from the US Preventive Services Task force are covered preventive services provided at no charge to the patient—the USPSTF developed and implemented a new review procedure. This is intended to increase stakeholder involvement at all steps in the process.
Last year, the USPSTF completed its rollout of this new online review process. The USPSTF now posts all draft recommendations and the evidence report supporting them on its Web site for public comment. final recommendations are posted months later after consideration of the public input. The final recommendations for the 10 topics with draft recommendations posted in 2011 are expected to be released this year.
Potential for confusion. The new process may cause confusion for family physicians. Draft recommendations will receive press coverage and may differ from the final recommendations, as happened with cervical cancer screening recommendations. Physicians will need to familiarize themselves with the process and look for final recommendations on the USPSTF Web site at http://www.uspreventiveservicestaskforce.org/recommendations.htm.
2012 recommendations
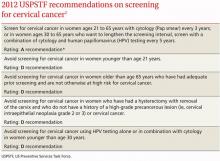
Screening for cervical cancer
The USPSTF released its new recommendations on screening for cervical cancer in March (TABLE 1).1 The final document varied from the 2011 draft recommendations in 2 areas: the roles of human papillomavirus (HPV) testing and sexual history.
- The draft issued an I statement (insufficient evidence) for the role of HPV testing. Subsequently, based on stakeholder and public comment (as well as a review of 2 large recently published studies), the USPSTF gave an A recommendation to the use of HPV testing in conjunction with cervical cytology as an option for women ages 30 years and older who want to increase the interval between screening to 5 years.2,3
- The draft stated that the age at which screening should be initiated depends on a patient’s sexual history. The final recommendations state that screening should not begin until age 21, regardless of sexual history.
TABLE 1
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
These new recommendations balance the proven benefits of cervical cytology with the harms from overscreening and are now essentially the same as those of other organizations, including the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology. They differ in minor ways from those of the American Congress of Obstetricians and Gynecologists, and the American Academy of Family Physicians is assessing whether to endorse them.
Importantly, the new recommendations identify individuals for whom cervical cytology should be avoided—women younger than age 21, most women older than age 65, and those who have had a hysterectomy with removal of the cervix. A decision to stop screening after the 65th birthday depends on whether the patient has had adequate screening yielding normal findings: This is defined by the USPSTF as 3 consecutive negative cytology results (or 2 consecutive negative co-test results with cytology and HIV testing) within 10 years of the proposed time of cessation, with the most recent test having been performed within 5 years. Avoiding cytology testing after hysterectomy is contingent on the procedure having been performed for an indication other than a high-grade precancerous lesion or cervical cancer. In addition, the recommendations advise against HPV testing in women younger than age 30, as it offers little advantage and leads to much overdiagnosis.
Liquid vs conventional cytology. As a minor point, the USPSTF says the evidence clearly shows that liquid cytology offers no advantage over conventional cytology. But it recognizes that the screening method used is often not determined by the physician.
Recommendations finalized in 2011
TABLE 2 summarizes recommendations completed by the USPSTF last year.
Neonatal gonococcal eye infection prevention
The recommendation to use topical medication (erythromycin ointment) to prevent neonatal gonococcal eye infection is an update and reaffirmation of a previous recommendation. Blindness due to this disease has become rare in the United States because of the routine use of a neonatal topical antibiotic, and there is good evidence that it causes no significant harm. Its use continues to be recommended for all newborns.4
TABLE 2
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
Vision screening for children
Vision screening for preschool children can detect visual acuity problems such as amblyopia and refractive errors. A variety of screening tests are available, including visual acuity, stereoacuity, cover-uncover, Hirschberg light reflex, and auto-refractor tests (automated optical instruments that detect refractive errors). The most benefit is obtained by discovering and correcting amblyopia.
There is no evidence that detecting problems before age 3 years leads to better outcomes than detection between 3 and 5 years of age. Testing is more difficult in younger children and can yield inconclusive or false-positive results more frequently. This led the USPSTF to reaffirm vision testing once for children ages 3 to 5 years, and to state that the evidence is insufficient to make a recommendation for younger children.5
Screening for osteoporosis
The recommendations indicate that all women ages 65 and older should undergo screening, although the optimal frequency of screening is not known. The clinical discussion accompanying the recommendation indicates there is reason to believe that screening men may reduce morbidity and mortality, but that sufficient evidence for or against this is lacking.6
Screening can be done with dual-energy x-ray absorptiometry (DEXA) of the hip and lumbar spine, or quantitative ultrasonography of the calcaneus. DEXA is most commonly used, and is the basis for most treatment recommendations.
The recommendation to screen some women younger than 65 years, based on risk, is somewhat complex. The USPSTF recommends screening younger women if their 10-year risk of fracture is comparable to that of a 65-year-old white woman with no additional risk factors (a risk of 9.3% over 10 years). To calculate that risk, the USPSTF recommends using the FRAX (Fracture Risk Assessment) tool developed by the World Health Organization Collaborating Centre for Metabolic Bone Diseases, Sheffield, United Kingdom, which is available free to clinicians and the public (www.shef.ac.uk/FRAX/).
Screening for testicular cancer
The recommendation against screening for testicular cancer may surprise many physicians, even though it is a reaffirmation of a previous recommendation. Testicular cancer is uncommon (5 cases per 100,000 males per year) and treatment is successful in a large proportion of patients, regardless of the stage at which it is discovered. Patients or their partners discover these tumors in time for a cure and there is no evidence physician exams improve outcomes. Physician discovery of incidental and inconsequential findings such as spermatoceles and varicoceles can lead to unnecessary testing and follow-up.7
Screening for bladder cancer
The USPSTF issued an I statement for bladder cancer screening because there is little evidence regarding the diagnostic accuracy of available tests (urinalysis for microscopic hematuria, urine cytology, or tests for urine biomarkers) in detecting bladder cancer in asymptomatic patients. In addition, there is no evidence regarding the potential benefits of detecting asymptomatic bladder cancer.8
Current draft recommendations
The USPSTF posts recommendations on its Web site for public comment for 30 days. To see current draft recommendations, go to http://www.uspreventiveservicestaskforce.org/tfcomment.htm.
1. USPSTF. Screening for cervical cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm. Accessed March 10, 2012.
2. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88.
3. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
4. USPSTF. Ocular prophylaxis for gonococcal ophthalmia neonatorum. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgononew.htm. Accessed March 10, 2012.
5. USPSTF. Screening for vision impairment in children ages 1 to 5 years. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsvsch.htm. Accessed March 10, 2012.
6. USPSTF. Screening for osteoporosis. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsoste.htm. Accessed March 10, 2012.
7. USPSTF. Screening for testicular cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstest.htm. Accessed March 10, 2012.
8. USPSTF. Screening for bladder cancer in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsblad.htm. Accessed March 10, 2012.
Recently, the US Preventive Services Task Force (USPSTF) finalized 7 recommendations on 5 topics and posted draft recommendations on an additional 10 topics. It also implemented new procedures that include posting draft recommendations for public comment (see “A new review process for the USPSTF”). This article reviews the USPSTF activity in 2011, as well as cervical cancer screening recommendations issued earlier this year.
In response to the adverse publicity from the 2009 mammogram recommendations and the increased scrutiny brought on by the affordable care act—which mandates that A and B recommendations from the US Preventive Services Task force are covered preventive services provided at no charge to the patient—the USPSTF developed and implemented a new review procedure. This is intended to increase stakeholder involvement at all steps in the process.
Last year, the USPSTF completed its rollout of this new online review process. The USPSTF now posts all draft recommendations and the evidence report supporting them on its Web site for public comment. final recommendations are posted months later after consideration of the public input. The final recommendations for the 10 topics with draft recommendations posted in 2011 are expected to be released this year.
Potential for confusion. The new process may cause confusion for family physicians. Draft recommendations will receive press coverage and may differ from the final recommendations, as happened with cervical cancer screening recommendations. Physicians will need to familiarize themselves with the process and look for final recommendations on the USPSTF Web site at http://www.uspreventiveservicestaskforce.org/recommendations.htm.
2012 recommendations
Screening for cervical cancer
The USPSTF released its new recommendations on screening for cervical cancer in March (TABLE 1).1 The final document varied from the 2011 draft recommendations in 2 areas: the roles of human papillomavirus (HPV) testing and sexual history.
- The draft issued an I statement (insufficient evidence) for the role of HPV testing. Subsequently, based on stakeholder and public comment (as well as a review of 2 large recently published studies), the USPSTF gave an A recommendation to the use of HPV testing in conjunction with cervical cytology as an option for women ages 30 years and older who want to increase the interval between screening to 5 years.2,3
- The draft stated that the age at which screening should be initiated depends on a patient’s sexual history. The final recommendations state that screening should not begin until age 21, regardless of sexual history.
TABLE 1
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
These new recommendations balance the proven benefits of cervical cytology with the harms from overscreening and are now essentially the same as those of other organizations, including the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology. They differ in minor ways from those of the American Congress of Obstetricians and Gynecologists, and the American Academy of Family Physicians is assessing whether to endorse them.
Importantly, the new recommendations identify individuals for whom cervical cytology should be avoided—women younger than age 21, most women older than age 65, and those who have had a hysterectomy with removal of the cervix. A decision to stop screening after the 65th birthday depends on whether the patient has had adequate screening yielding normal findings: This is defined by the USPSTF as 3 consecutive negative cytology results (or 2 consecutive negative co-test results with cytology and HIV testing) within 10 years of the proposed time of cessation, with the most recent test having been performed within 5 years. Avoiding cytology testing after hysterectomy is contingent on the procedure having been performed for an indication other than a high-grade precancerous lesion or cervical cancer. In addition, the recommendations advise against HPV testing in women younger than age 30, as it offers little advantage and leads to much overdiagnosis.
Liquid vs conventional cytology. As a minor point, the USPSTF says the evidence clearly shows that liquid cytology offers no advantage over conventional cytology. But it recognizes that the screening method used is often not determined by the physician.
Recommendations finalized in 2011
TABLE 2 summarizes recommendations completed by the USPSTF last year.
Neonatal gonococcal eye infection prevention
The recommendation to use topical medication (erythromycin ointment) to prevent neonatal gonococcal eye infection is an update and reaffirmation of a previous recommendation. Blindness due to this disease has become rare in the United States because of the routine use of a neonatal topical antibiotic, and there is good evidence that it causes no significant harm. Its use continues to be recommended for all newborns.4
TABLE 2
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
Vision screening for children
Vision screening for preschool children can detect visual acuity problems such as amblyopia and refractive errors. A variety of screening tests are available, including visual acuity, stereoacuity, cover-uncover, Hirschberg light reflex, and auto-refractor tests (automated optical instruments that detect refractive errors). The most benefit is obtained by discovering and correcting amblyopia.
There is no evidence that detecting problems before age 3 years leads to better outcomes than detection between 3 and 5 years of age. Testing is more difficult in younger children and can yield inconclusive or false-positive results more frequently. This led the USPSTF to reaffirm vision testing once for children ages 3 to 5 years, and to state that the evidence is insufficient to make a recommendation for younger children.5
Screening for osteoporosis
The recommendations indicate that all women ages 65 and older should undergo screening, although the optimal frequency of screening is not known. The clinical discussion accompanying the recommendation indicates there is reason to believe that screening men may reduce morbidity and mortality, but that sufficient evidence for or against this is lacking.6
Screening can be done with dual-energy x-ray absorptiometry (DEXA) of the hip and lumbar spine, or quantitative ultrasonography of the calcaneus. DEXA is most commonly used, and is the basis for most treatment recommendations.
The recommendation to screen some women younger than 65 years, based on risk, is somewhat complex. The USPSTF recommends screening younger women if their 10-year risk of fracture is comparable to that of a 65-year-old white woman with no additional risk factors (a risk of 9.3% over 10 years). To calculate that risk, the USPSTF recommends using the FRAX (Fracture Risk Assessment) tool developed by the World Health Organization Collaborating Centre for Metabolic Bone Diseases, Sheffield, United Kingdom, which is available free to clinicians and the public (www.shef.ac.uk/FRAX/).
Screening for testicular cancer
The recommendation against screening for testicular cancer may surprise many physicians, even though it is a reaffirmation of a previous recommendation. Testicular cancer is uncommon (5 cases per 100,000 males per year) and treatment is successful in a large proportion of patients, regardless of the stage at which it is discovered. Patients or their partners discover these tumors in time for a cure and there is no evidence physician exams improve outcomes. Physician discovery of incidental and inconsequential findings such as spermatoceles and varicoceles can lead to unnecessary testing and follow-up.7
Screening for bladder cancer
The USPSTF issued an I statement for bladder cancer screening because there is little evidence regarding the diagnostic accuracy of available tests (urinalysis for microscopic hematuria, urine cytology, or tests for urine biomarkers) in detecting bladder cancer in asymptomatic patients. In addition, there is no evidence regarding the potential benefits of detecting asymptomatic bladder cancer.8
Current draft recommendations
The USPSTF posts recommendations on its Web site for public comment for 30 days. To see current draft recommendations, go to http://www.uspreventiveservicestaskforce.org/tfcomment.htm.
Recently, the US Preventive Services Task Force (USPSTF) finalized 7 recommendations on 5 topics and posted draft recommendations on an additional 10 topics. It also implemented new procedures that include posting draft recommendations for public comment (see “A new review process for the USPSTF”). This article reviews the USPSTF activity in 2011, as well as cervical cancer screening recommendations issued earlier this year.
In response to the adverse publicity from the 2009 mammogram recommendations and the increased scrutiny brought on by the affordable care act—which mandates that A and B recommendations from the US Preventive Services Task force are covered preventive services provided at no charge to the patient—the USPSTF developed and implemented a new review procedure. This is intended to increase stakeholder involvement at all steps in the process.
Last year, the USPSTF completed its rollout of this new online review process. The USPSTF now posts all draft recommendations and the evidence report supporting them on its Web site for public comment. final recommendations are posted months later after consideration of the public input. The final recommendations for the 10 topics with draft recommendations posted in 2011 are expected to be released this year.
Potential for confusion. The new process may cause confusion for family physicians. Draft recommendations will receive press coverage and may differ from the final recommendations, as happened with cervical cancer screening recommendations. Physicians will need to familiarize themselves with the process and look for final recommendations on the USPSTF Web site at http://www.uspreventiveservicestaskforce.org/recommendations.htm.
2012 recommendations
Screening for cervical cancer
The USPSTF released its new recommendations on screening for cervical cancer in March (TABLE 1).1 The final document varied from the 2011 draft recommendations in 2 areas: the roles of human papillomavirus (HPV) testing and sexual history.
- The draft issued an I statement (insufficient evidence) for the role of HPV testing. Subsequently, based on stakeholder and public comment (as well as a review of 2 large recently published studies), the USPSTF gave an A recommendation to the use of HPV testing in conjunction with cervical cytology as an option for women ages 30 years and older who want to increase the interval between screening to 5 years.2,3
- The draft stated that the age at which screening should be initiated depends on a patient’s sexual history. The final recommendations state that screening should not begin until age 21, regardless of sexual history.
TABLE 1
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
These new recommendations balance the proven benefits of cervical cytology with the harms from overscreening and are now essentially the same as those of other organizations, including the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology. They differ in minor ways from those of the American Congress of Obstetricians and Gynecologists, and the American Academy of Family Physicians is assessing whether to endorse them.
Importantly, the new recommendations identify individuals for whom cervical cytology should be avoided—women younger than age 21, most women older than age 65, and those who have had a hysterectomy with removal of the cervix. A decision to stop screening after the 65th birthday depends on whether the patient has had adequate screening yielding normal findings: This is defined by the USPSTF as 3 consecutive negative cytology results (or 2 consecutive negative co-test results with cytology and HIV testing) within 10 years of the proposed time of cessation, with the most recent test having been performed within 5 years. Avoiding cytology testing after hysterectomy is contingent on the procedure having been performed for an indication other than a high-grade precancerous lesion or cervical cancer. In addition, the recommendations advise against HPV testing in women younger than age 30, as it offers little advantage and leads to much overdiagnosis.
Liquid vs conventional cytology. As a minor point, the USPSTF says the evidence clearly shows that liquid cytology offers no advantage over conventional cytology. But it recognizes that the screening method used is often not determined by the physician.
Recommendations finalized in 2011
TABLE 2 summarizes recommendations completed by the USPSTF last year.
Neonatal gonococcal eye infection prevention
The recommendation to use topical medication (erythromycin ointment) to prevent neonatal gonococcal eye infection is an update and reaffirmation of a previous recommendation. Blindness due to this disease has become rare in the United States because of the routine use of a neonatal topical antibiotic, and there is good evidence that it causes no significant harm. Its use continues to be recommended for all newborns.4
TABLE 2
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
Vision screening for children
Vision screening for preschool children can detect visual acuity problems such as amblyopia and refractive errors. A variety of screening tests are available, including visual acuity, stereoacuity, cover-uncover, Hirschberg light reflex, and auto-refractor tests (automated optical instruments that detect refractive errors). The most benefit is obtained by discovering and correcting amblyopia.
There is no evidence that detecting problems before age 3 years leads to better outcomes than detection between 3 and 5 years of age. Testing is more difficult in younger children and can yield inconclusive or false-positive results more frequently. This led the USPSTF to reaffirm vision testing once for children ages 3 to 5 years, and to state that the evidence is insufficient to make a recommendation for younger children.5
Screening for osteoporosis
The recommendations indicate that all women ages 65 and older should undergo screening, although the optimal frequency of screening is not known. The clinical discussion accompanying the recommendation indicates there is reason to believe that screening men may reduce morbidity and mortality, but that sufficient evidence for or against this is lacking.6
Screening can be done with dual-energy x-ray absorptiometry (DEXA) of the hip and lumbar spine, or quantitative ultrasonography of the calcaneus. DEXA is most commonly used, and is the basis for most treatment recommendations.
The recommendation to screen some women younger than 65 years, based on risk, is somewhat complex. The USPSTF recommends screening younger women if their 10-year risk of fracture is comparable to that of a 65-year-old white woman with no additional risk factors (a risk of 9.3% over 10 years). To calculate that risk, the USPSTF recommends using the FRAX (Fracture Risk Assessment) tool developed by the World Health Organization Collaborating Centre for Metabolic Bone Diseases, Sheffield, United Kingdom, which is available free to clinicians and the public (www.shef.ac.uk/FRAX/).
Screening for testicular cancer
The recommendation against screening for testicular cancer may surprise many physicians, even though it is a reaffirmation of a previous recommendation. Testicular cancer is uncommon (5 cases per 100,000 males per year) and treatment is successful in a large proportion of patients, regardless of the stage at which it is discovered. Patients or their partners discover these tumors in time for a cure and there is no evidence physician exams improve outcomes. Physician discovery of incidental and inconsequential findings such as spermatoceles and varicoceles can lead to unnecessary testing and follow-up.7
Screening for bladder cancer
The USPSTF issued an I statement for bladder cancer screening because there is little evidence regarding the diagnostic accuracy of available tests (urinalysis for microscopic hematuria, urine cytology, or tests for urine biomarkers) in detecting bladder cancer in asymptomatic patients. In addition, there is no evidence regarding the potential benefits of detecting asymptomatic bladder cancer.8
Current draft recommendations
The USPSTF posts recommendations on its Web site for public comment for 30 days. To see current draft recommendations, go to http://www.uspreventiveservicestaskforce.org/tfcomment.htm.
1. USPSTF. Screening for cervical cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm. Accessed March 10, 2012.
2. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88.
3. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
4. USPSTF. Ocular prophylaxis for gonococcal ophthalmia neonatorum. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgononew.htm. Accessed March 10, 2012.
5. USPSTF. Screening for vision impairment in children ages 1 to 5 years. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsvsch.htm. Accessed March 10, 2012.
6. USPSTF. Screening for osteoporosis. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsoste.htm. Accessed March 10, 2012.
7. USPSTF. Screening for testicular cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstest.htm. Accessed March 10, 2012.
8. USPSTF. Screening for bladder cancer in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsblad.htm. Accessed March 10, 2012.
1. USPSTF. Screening for cervical cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm. Accessed March 10, 2012.
2. Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88.
3. Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-672.
4. USPSTF. Ocular prophylaxis for gonococcal ophthalmia neonatorum. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgononew.htm. Accessed March 10, 2012.
5. USPSTF. Screening for vision impairment in children ages 1 to 5 years. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsvsch.htm. Accessed March 10, 2012.
6. USPSTF. Screening for osteoporosis. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsoste.htm. Accessed March 10, 2012.
7. USPSTF. Screening for testicular cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspstest.htm. Accessed March 10, 2012.
8. USPSTF. Screening for bladder cancer in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsblad.htm. Accessed March 10, 2012.
ACIP immunization update
In February, the Centers for Disease Control and Prevention (CDC) published the 2012 immunization schedules for infants and children, adolescents, and adults.1,2 The schedules, which are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm, are updated annually and incorporate additions and changes recommended by the Advisory Committee on Immunization Practices (ACIP) over the past year. While there were no major advances in new vaccines in 2011, there were a number of new indications for existing ones.
Human papillomavirus vaccine for males
Quadrivalent vaccine against human papillomavirus is now recommended for routine use for males ages 11 to 12 years to prevent genital warts and anal intraepithelial neoplasia.3,4 Catch-up vaccination is also recommended for males ages 13 to 21 who have not received it. In addition, routine use is recommended for males ages 22 to 26 years who have sex with men or are HIV positive or immuno-compromised.
Tetanus toxoid, reduced strength diphtheria toxoid, and acellular pertussis (Tdap)
Indications for the routine use of Tdap were expanded to include children ages 7 to 10 years, pregnant women, and adults age 65 and older who have contact with infants.5,6 Children ages 7 to 10 years who have not had the full series of DTaP should receive Td/Tdap according to the catch-up schedule,1 with one of the doses being Tdap. Adults older than 65 who have never received Tdap and who have close contact with infants should receive one dose. No minimum interval is required between receipt of the Td and Tdap vaccines. Other older adults who ask for Tdap vaccination should receive it. Use of Tdap in those ages 7 to 10 years or 65 and older is off label.5
Pregnant women who have not received Tdap should receive 1 dose after week 20 of pregnancy, although receiving it earlier is not contraindicated if tetanus toxoid is needed for tetanus prevention following a wound.6
Hepatitis B virus (HBV) vaccine
Added to the list of high-risk adults who should receive HBV vaccine routinely are those ages 19 through 59 years with diabetes.7 Vaccinate as soon as possible after the diabetes diagnosis is confirmed. The decision as to whether to vaccinate patients ≥60 years with diabetes should be based on the likelihood that they will become infected. Considerations include the risks associated with an increased need for help with blood-glucose monitoring in long-term care facilities, the likelihood that the patient will experience chronic sequelae if infected, and the likelihood that the patient will mount a proper immune response to the vaccine.7 (The more frail patients are, the less likely they are to achieve adequate immunity.7)
Meningococcal conjugate vaccine, quadrivalent (MCV4)
An MCV4 vaccine (Menactra) has now been licensed for use in children as young as 9 months.8 At this time, however, neither Menactra nor its competitor, Menveo (licensed for use in those 2 years and older), is recommended for routine administration until the age of 11 to 12 years. Infants and children ages 9 through 23 months with complement deficiencies, or who will be traveling to countries with endemic high levels of meningococcus, should be vaccinated with 2 doses of Menactra 3 months apart, and with a booster dose after 3 years if risk persists. The recommendations regarding the use of MCV4 in those ≤2 years with high-risk conditions are listed in TABLE 1.
In February 2012, the CDC announced results of the 2010 National Health Interview Survey. Increases in immunization coverage occurred only with Tdap vaccination for individuals 19 to 64 years of age (from 6.6% to 8.2%), herpes zoster vaccination among those ≥60 years (from 10% to 14.4%), and ≥1 dose of HPV vaccination for women 19 to 26 years (from 17.1% to 20.7%). Rates of immunization were unchanged for other vaccines. The CDC said a substantial improvement in coverage is needed to reduce vaccine-preventable diseases among adults.
Source: CDC. Adult vaccination coverage—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66-72.
Another change regarding the use of MCV4 is a recommended booster dose for those age 16 and older who were first vaccinated at age 11 or 12 years.9 For those vaccinated at ages 13 to 15, a booster should be received at ages 16 to 18. No booster is needed if the first MCV4 dose is received at or after age 16. Recommendations for MCV4 use and booster doses for those 2 years and older are listed in TABLE 2.
TABLE 1
Recommended Menactra schedule for young children at high risk for invasive meningococcal disease8
| Risk group | Primary vaccination series | Booster dose, if child remains at increased risk |
|---|---|---|
| Children ages 9-23 months at high risk for invasive meningococcal disease,* except those with functional or anatomic asplenia | 2 doses, 3 months apart Catch-up dose at earliest opportunity if dose 2 is not given on schedule | Initial booster 3 years after completing primary series At 5-year intervals after initial booster |
| Children with functional or anatomic asplenia at high risk for invasive meningococcal disease | 2 doses, 2 months apart, starting at age 2 years and ≥4 weeks after completing the PCV13 vaccine series | |
| PCV, pneumococcal conjugate vaccine. *Children who have persistent complement component deficiencies (eg, C5–C9, properdin, factor H, or factor D); those traveling to (or residents of) countries where meningococcal disease is hyperendemic or epidemic; or those who are in a defined risk group during a community or institutional meningococcal outbreak. | ||
TABLE 2
Recommended schedule for meningococcal conjugate vaccine in those ≥2 years, according to risk9
| Risk group | Primary vaccination series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at 11 or 12 years | At age 16 years, if primary dose given at age 11 or 12 years Age 16-18 years, if primary dose given at age 13-15 years No booster needed if primary dose given on or after age 16 years |
| Individuals ages 11-18 years infected with HIV | 2 doses, 2 months apart | |
| Individuals ages 2-55 years with persistent complement component deficiency (eg, C5–C9, properdin, or factor D) or functional or anatomical asplenia | 2 doses, 2 months apart | Every 5 years At the earliest opportunity if only 1 primary dose; every 5 years thereafter |
| Individuals ages 2-55 years with prolonged increased risk for exposure, such as microbiologists routinely working with Neisseria meningitides, and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose | After 3 years, if primary dose given at age 2-6 years After 5 years, if primary dose given at ≥7 years and the individual remains at risk Every 5 years thereafter, as long as the risk persists |
| HIV, human immunodeficiency virus. | ||
Herpes zoster vaccine
The herpes zoster vaccine was initially licensed for those 60 years and older. Last year the FDA approved lowering the age to 50 years and older. At this time, however, the ACIP continues to recommend that the vaccine be used routinely starting at age 60 years. The age was not lowered because of a concern about vaccine supply and the uncertainty about the possible need for a booster dose if administered at age 50.10
Influenza vaccine
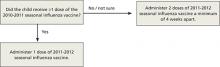
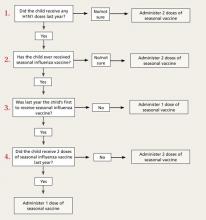
As described in a previous Practice Alert,11 a history of egg allergy is no longer a strict contraindication for receipt of the influenza vaccine. The other major adjustment is a simplified recommendation on how to determine the required number of doses for a child younger than 9 years. If the child received 1 or both doses of the 2010-2011 vaccine, give just a single dose of the 2011-2012 vaccine. If the history is uncertain, give 2 doses of the new vaccine at least 4 weeks apart.12
1. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(5):1-4.
2. CDC. Recommended adult immunization schedule—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(4):1-7.
3. Campos-Outcalt D. Human papilloma virus: Vaccine is now routinely indicated for males. J Fam Pract. 2012;61:38-40.
4. CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705-1708.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:13-15.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. CDC. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
8. CDC. Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391-1392.
9. CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
10. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528-
11. Campos-Outcalt D. Ready for flu season? The 2011-2012 ACIP recommendations. J Fam Pract. 2011;60:543-544.
12. CDC. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128-1132.
In February, the Centers for Disease Control and Prevention (CDC) published the 2012 immunization schedules for infants and children, adolescents, and adults.1,2 The schedules, which are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm, are updated annually and incorporate additions and changes recommended by the Advisory Committee on Immunization Practices (ACIP) over the past year. While there were no major advances in new vaccines in 2011, there were a number of new indications for existing ones.
Human papillomavirus vaccine for males
Quadrivalent vaccine against human papillomavirus is now recommended for routine use for males ages 11 to 12 years to prevent genital warts and anal intraepithelial neoplasia.3,4 Catch-up vaccination is also recommended for males ages 13 to 21 who have not received it. In addition, routine use is recommended for males ages 22 to 26 years who have sex with men or are HIV positive or immuno-compromised.
Tetanus toxoid, reduced strength diphtheria toxoid, and acellular pertussis (Tdap)
Indications for the routine use of Tdap were expanded to include children ages 7 to 10 years, pregnant women, and adults age 65 and older who have contact with infants.5,6 Children ages 7 to 10 years who have not had the full series of DTaP should receive Td/Tdap according to the catch-up schedule,1 with one of the doses being Tdap. Adults older than 65 who have never received Tdap and who have close contact with infants should receive one dose. No minimum interval is required between receipt of the Td and Tdap vaccines. Other older adults who ask for Tdap vaccination should receive it. Use of Tdap in those ages 7 to 10 years or 65 and older is off label.5
Pregnant women who have not received Tdap should receive 1 dose after week 20 of pregnancy, although receiving it earlier is not contraindicated if tetanus toxoid is needed for tetanus prevention following a wound.6
Hepatitis B virus (HBV) vaccine
Added to the list of high-risk adults who should receive HBV vaccine routinely are those ages 19 through 59 years with diabetes.7 Vaccinate as soon as possible after the diabetes diagnosis is confirmed. The decision as to whether to vaccinate patients ≥60 years with diabetes should be based on the likelihood that they will become infected. Considerations include the risks associated with an increased need for help with blood-glucose monitoring in long-term care facilities, the likelihood that the patient will experience chronic sequelae if infected, and the likelihood that the patient will mount a proper immune response to the vaccine.7 (The more frail patients are, the less likely they are to achieve adequate immunity.7)
Meningococcal conjugate vaccine, quadrivalent (MCV4)
An MCV4 vaccine (Menactra) has now been licensed for use in children as young as 9 months.8 At this time, however, neither Menactra nor its competitor, Menveo (licensed for use in those 2 years and older), is recommended for routine administration until the age of 11 to 12 years. Infants and children ages 9 through 23 months with complement deficiencies, or who will be traveling to countries with endemic high levels of meningococcus, should be vaccinated with 2 doses of Menactra 3 months apart, and with a booster dose after 3 years if risk persists. The recommendations regarding the use of MCV4 in those ≤2 years with high-risk conditions are listed in TABLE 1.
In February 2012, the CDC announced results of the 2010 National Health Interview Survey. Increases in immunization coverage occurred only with Tdap vaccination for individuals 19 to 64 years of age (from 6.6% to 8.2%), herpes zoster vaccination among those ≥60 years (from 10% to 14.4%), and ≥1 dose of HPV vaccination for women 19 to 26 years (from 17.1% to 20.7%). Rates of immunization were unchanged for other vaccines. The CDC said a substantial improvement in coverage is needed to reduce vaccine-preventable diseases among adults.
Source: CDC. Adult vaccination coverage—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66-72.
Another change regarding the use of MCV4 is a recommended booster dose for those age 16 and older who were first vaccinated at age 11 or 12 years.9 For those vaccinated at ages 13 to 15, a booster should be received at ages 16 to 18. No booster is needed if the first MCV4 dose is received at or after age 16. Recommendations for MCV4 use and booster doses for those 2 years and older are listed in TABLE 2.
TABLE 1
Recommended Menactra schedule for young children at high risk for invasive meningococcal disease8
| Risk group | Primary vaccination series | Booster dose, if child remains at increased risk |
|---|---|---|
| Children ages 9-23 months at high risk for invasive meningococcal disease,* except those with functional or anatomic asplenia | 2 doses, 3 months apart Catch-up dose at earliest opportunity if dose 2 is not given on schedule | Initial booster 3 years after completing primary series At 5-year intervals after initial booster |
| Children with functional or anatomic asplenia at high risk for invasive meningococcal disease | 2 doses, 2 months apart, starting at age 2 years and ≥4 weeks after completing the PCV13 vaccine series | |
| PCV, pneumococcal conjugate vaccine. *Children who have persistent complement component deficiencies (eg, C5–C9, properdin, factor H, or factor D); those traveling to (or residents of) countries where meningococcal disease is hyperendemic or epidemic; or those who are in a defined risk group during a community or institutional meningococcal outbreak. | ||
TABLE 2
Recommended schedule for meningococcal conjugate vaccine in those ≥2 years, according to risk9
| Risk group | Primary vaccination series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at 11 or 12 years | At age 16 years, if primary dose given at age 11 or 12 years Age 16-18 years, if primary dose given at age 13-15 years No booster needed if primary dose given on or after age 16 years |
| Individuals ages 11-18 years infected with HIV | 2 doses, 2 months apart | |
| Individuals ages 2-55 years with persistent complement component deficiency (eg, C5–C9, properdin, or factor D) or functional or anatomical asplenia | 2 doses, 2 months apart | Every 5 years At the earliest opportunity if only 1 primary dose; every 5 years thereafter |
| Individuals ages 2-55 years with prolonged increased risk for exposure, such as microbiologists routinely working with Neisseria meningitides, and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose | After 3 years, if primary dose given at age 2-6 years After 5 years, if primary dose given at ≥7 years and the individual remains at risk Every 5 years thereafter, as long as the risk persists |
| HIV, human immunodeficiency virus. | ||
Herpes zoster vaccine
The herpes zoster vaccine was initially licensed for those 60 years and older. Last year the FDA approved lowering the age to 50 years and older. At this time, however, the ACIP continues to recommend that the vaccine be used routinely starting at age 60 years. The age was not lowered because of a concern about vaccine supply and the uncertainty about the possible need for a booster dose if administered at age 50.10
Influenza vaccine
As described in a previous Practice Alert,11 a history of egg allergy is no longer a strict contraindication for receipt of the influenza vaccine. The other major adjustment is a simplified recommendation on how to determine the required number of doses for a child younger than 9 years. If the child received 1 or both doses of the 2010-2011 vaccine, give just a single dose of the 2011-2012 vaccine. If the history is uncertain, give 2 doses of the new vaccine at least 4 weeks apart.12
In February, the Centers for Disease Control and Prevention (CDC) published the 2012 immunization schedules for infants and children, adolescents, and adults.1,2 The schedules, which are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm, are updated annually and incorporate additions and changes recommended by the Advisory Committee on Immunization Practices (ACIP) over the past year. While there were no major advances in new vaccines in 2011, there were a number of new indications for existing ones.
Human papillomavirus vaccine for males
Quadrivalent vaccine against human papillomavirus is now recommended for routine use for males ages 11 to 12 years to prevent genital warts and anal intraepithelial neoplasia.3,4 Catch-up vaccination is also recommended for males ages 13 to 21 who have not received it. In addition, routine use is recommended for males ages 22 to 26 years who have sex with men or are HIV positive or immuno-compromised.
Tetanus toxoid, reduced strength diphtheria toxoid, and acellular pertussis (Tdap)
Indications for the routine use of Tdap were expanded to include children ages 7 to 10 years, pregnant women, and adults age 65 and older who have contact with infants.5,6 Children ages 7 to 10 years who have not had the full series of DTaP should receive Td/Tdap according to the catch-up schedule,1 with one of the doses being Tdap. Adults older than 65 who have never received Tdap and who have close contact with infants should receive one dose. No minimum interval is required between receipt of the Td and Tdap vaccines. Other older adults who ask for Tdap vaccination should receive it. Use of Tdap in those ages 7 to 10 years or 65 and older is off label.5
Pregnant women who have not received Tdap should receive 1 dose after week 20 of pregnancy, although receiving it earlier is not contraindicated if tetanus toxoid is needed for tetanus prevention following a wound.6
Hepatitis B virus (HBV) vaccine
Added to the list of high-risk adults who should receive HBV vaccine routinely are those ages 19 through 59 years with diabetes.7 Vaccinate as soon as possible after the diabetes diagnosis is confirmed. The decision as to whether to vaccinate patients ≥60 years with diabetes should be based on the likelihood that they will become infected. Considerations include the risks associated with an increased need for help with blood-glucose monitoring in long-term care facilities, the likelihood that the patient will experience chronic sequelae if infected, and the likelihood that the patient will mount a proper immune response to the vaccine.7 (The more frail patients are, the less likely they are to achieve adequate immunity.7)
Meningococcal conjugate vaccine, quadrivalent (MCV4)
An MCV4 vaccine (Menactra) has now been licensed for use in children as young as 9 months.8 At this time, however, neither Menactra nor its competitor, Menveo (licensed for use in those 2 years and older), is recommended for routine administration until the age of 11 to 12 years. Infants and children ages 9 through 23 months with complement deficiencies, or who will be traveling to countries with endemic high levels of meningococcus, should be vaccinated with 2 doses of Menactra 3 months apart, and with a booster dose after 3 years if risk persists. The recommendations regarding the use of MCV4 in those ≤2 years with high-risk conditions are listed in TABLE 1.
In February 2012, the CDC announced results of the 2010 National Health Interview Survey. Increases in immunization coverage occurred only with Tdap vaccination for individuals 19 to 64 years of age (from 6.6% to 8.2%), herpes zoster vaccination among those ≥60 years (from 10% to 14.4%), and ≥1 dose of HPV vaccination for women 19 to 26 years (from 17.1% to 20.7%). Rates of immunization were unchanged for other vaccines. The CDC said a substantial improvement in coverage is needed to reduce vaccine-preventable diseases among adults.
Source: CDC. Adult vaccination coverage—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66-72.
Another change regarding the use of MCV4 is a recommended booster dose for those age 16 and older who were first vaccinated at age 11 or 12 years.9 For those vaccinated at ages 13 to 15, a booster should be received at ages 16 to 18. No booster is needed if the first MCV4 dose is received at or after age 16. Recommendations for MCV4 use and booster doses for those 2 years and older are listed in TABLE 2.
TABLE 1
Recommended Menactra schedule for young children at high risk for invasive meningococcal disease8
| Risk group | Primary vaccination series | Booster dose, if child remains at increased risk |
|---|---|---|
| Children ages 9-23 months at high risk for invasive meningococcal disease,* except those with functional or anatomic asplenia | 2 doses, 3 months apart Catch-up dose at earliest opportunity if dose 2 is not given on schedule | Initial booster 3 years after completing primary series At 5-year intervals after initial booster |
| Children with functional or anatomic asplenia at high risk for invasive meningococcal disease | 2 doses, 2 months apart, starting at age 2 years and ≥4 weeks after completing the PCV13 vaccine series | |
| PCV, pneumococcal conjugate vaccine. *Children who have persistent complement component deficiencies (eg, C5–C9, properdin, factor H, or factor D); those traveling to (or residents of) countries where meningococcal disease is hyperendemic or epidemic; or those who are in a defined risk group during a community or institutional meningococcal outbreak. | ||
TABLE 2
Recommended schedule for meningococcal conjugate vaccine in those ≥2 years, according to risk9
| Risk group | Primary vaccination series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at 11 or 12 years | At age 16 years, if primary dose given at age 11 or 12 years Age 16-18 years, if primary dose given at age 13-15 years No booster needed if primary dose given on or after age 16 years |
| Individuals ages 11-18 years infected with HIV | 2 doses, 2 months apart | |
| Individuals ages 2-55 years with persistent complement component deficiency (eg, C5–C9, properdin, or factor D) or functional or anatomical asplenia | 2 doses, 2 months apart | Every 5 years At the earliest opportunity if only 1 primary dose; every 5 years thereafter |
| Individuals ages 2-55 years with prolonged increased risk for exposure, such as microbiologists routinely working with Neisseria meningitides, and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose | After 3 years, if primary dose given at age 2-6 years After 5 years, if primary dose given at ≥7 years and the individual remains at risk Every 5 years thereafter, as long as the risk persists |
| HIV, human immunodeficiency virus. | ||
Herpes zoster vaccine
The herpes zoster vaccine was initially licensed for those 60 years and older. Last year the FDA approved lowering the age to 50 years and older. At this time, however, the ACIP continues to recommend that the vaccine be used routinely starting at age 60 years. The age was not lowered because of a concern about vaccine supply and the uncertainty about the possible need for a booster dose if administered at age 50.10
Influenza vaccine
As described in a previous Practice Alert,11 a history of egg allergy is no longer a strict contraindication for receipt of the influenza vaccine. The other major adjustment is a simplified recommendation on how to determine the required number of doses for a child younger than 9 years. If the child received 1 or both doses of the 2010-2011 vaccine, give just a single dose of the 2011-2012 vaccine. If the history is uncertain, give 2 doses of the new vaccine at least 4 weeks apart.12
1. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(5):1-4.
2. CDC. Recommended adult immunization schedule—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(4):1-7.
3. Campos-Outcalt D. Human papilloma virus: Vaccine is now routinely indicated for males. J Fam Pract. 2012;61:38-40.
4. CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705-1708.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:13-15.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. CDC. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
8. CDC. Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391-1392.
9. CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
10. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528-
11. Campos-Outcalt D. Ready for flu season? The 2011-2012 ACIP recommendations. J Fam Pract. 2011;60:543-544.
12. CDC. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128-1132.
1. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(5):1-4.
2. CDC. Recommended adult immunization schedule—United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(4):1-7.
3. Campos-Outcalt D. Human papilloma virus: Vaccine is now routinely indicated for males. J Fam Pract. 2012;61:38-40.
4. CDC. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705-1708.
5. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:13-15.
6. CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424-1426.
7. CDC. Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2011;60:1709-1711.
8. CDC. Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391-1392.
9. CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
10. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528-
11. Campos-Outcalt D. Ready for flu season? The 2011-2012 ACIP recommendations. J Fam Pract. 2011;60:543-544.
12. CDC. Prevention and control of influenza with vaccines: recommendations of the advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128-1132.
HPV vaccine is now routinely indicated for males
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
At its October 2011 meeting, the Advisory Committee on Immunization Practices (ACIP) recommended to the CDC that quadrivalent human papilloma virus vaccine (HPV4, Gardasil) be routinely given to all males ages 11 to 21 and to men ages 22 to 26 who have sex with men or who are HIV positive, if they have not been previously vaccinated. This replaces a 2009 recommendation that stated HPV4 vaccine could be used in males to prevent genital warts, but stopped short of advocating routine use for all males.1
There were 3 reasons the previous recommendation did not include HPV4 for routine vaccination of males:
- The vaccine had been shown to be effective only for prevention of genital warts.
- The cost effectiveness of the vaccine for use in boys was poor and, in modeling, it yielded less benefit as more females were vaccinated.
- It was thought that a more effective approach to preventing HPV disease would be to emphasize high rates of vaccination of females.
The new recommendation takes into account recent evidence demonstrating that the vaccine prevents anal intraepithelial neoplasia (AIN) in males, in addition to genital warts. Moreover, vaccination rates in females remain low, which makes vaccinating males more cost effective and additionally protective for females.
Female vaccination rates lower than expected
Despite its effectiveness and safety record, HPV vaccination has had a slow rate of acceptance among females ages 13 to 17 years. Coverage for this group documented in the last national vaccine survey was 48.7% for one dose and 32% for the recommended 3 doses.2
The vaccine is effective in preventing cervical intraepithelial neoplasia (TABLE 1),3 condyloma, and vaginal intraepithelial neoplasia in women ~15 to 26 years of age. Large studies of vaccine safety have documented no serious adverse reactions, other than syncope, which could occur as frequently as 17.9/10,000 females and 12.5/10,000 males.4 Another study that involved post-licensure safety data from >600,000 HPV4 doses found no increased risk for a variety of outcomes, including Guillain-Barré syndrome, stroke, venous thromboembolism, appendicitis, seizures, syncope, allergic reactions, and anaphylaxis.5,6
TABLE 1
HPV vaccine efficacy against HPV type-related CIN2+ in females ages ~15 to 26 years3
| Vaccine/HPV type | Vaccine | Placebo | Efficacy | |||
|---|---|---|---|---|---|---|
| N | CIN cases | N | CIN cases | % | CI* | |
| Bivalent HPV 16/18 HPV 16 HPV 18 | 7344 6303 6794 | 4 2 2 | 7312 6165 6746 | 56 46 15 | 93 96 87 | 80-98 83-100 40-99 |
| Quadrivalent HPV 16/18 HPV 16 HPV 18 | 7738 6647 7382 | 2 2 0 | 7714 6455 7316 | 100 81 29 | 98 98 100 | 93-100 91-100 87-100 |
| CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. *Confidence interval for bivalent results was 96.1%, and for quadrivalent results was 95%. | ||||||
HPV-associated disease in males
HPV causes anal, penile, and oropharyngeal cancers in males, with about 7500 cancers occurring each year in the United States.3 In addition, about 1% of sexually active males in America have genital warts at any one time.7 HPV types 6 and 11 cause about 90% of cases.1
The HPV4 vaccine—when all 3 doses are given—is 89.3% effective in preventing genital warts related to HPV types 6 and 11. Even a single dose is 68.1% effective (95% CI, 48.8–80.7).1 New evidence shows that HPV4 prevents AIN, which can lead to anal cancer.8 Effectiveness in preventing AIN 2/3 is 74.9% (95% CI, 8.8–95.4) in those completing 3 doses before onset of infection with one of the HPV types contained in vaccine. Notably, these results were obtained in a subgroup analysis of men who have sex with men. And although the reduction in AIN is expected to lower the incidence of anal cancer, ongoing studies require time to confirm this. If such a reduction is confirmed (and vaccination is started at age 12 in the general male population), the number-needed-to-vaccinate to prevent one case of genital warts would be 18, and to prevent one case of anal cancer, 1581.6
No studies have evaluated efficacy of HPV4 in preventing penile or oropharyngeal cancers.
Men who have sex with men at high risk
Men who have sex with men have higher rates of AIN, anal cancers, and genital warts than the general male population.3 Those who are additionally HIV positive have higher rates of genital warts, which are also more difficult to treat.3 AIN is also more common in HIV-infected males.3 The HPV4 vaccine is immunogenic in those who are HIV infected, although the resulting antibody titers are lower than in other populations.
A look at the 2 HPV vaccines
Two HPV vaccines are available (TABLE 2).3 HPV4 vaccine protects against HPV 6, 11, 16, and 18. Bivalent (HPV2, Cervarix) vaccine contains antigens from HPV 16 and 18. Both vaccines are approved for use in females for the prevention of cervical cancer; HPV4 is preferred if protection against genital warts is also desired. Only HPV4 has been licensed for use in males.
TABLE 2
A look at the human papillomavirus vaccines3
| Quadrivalent (Gardasil) | Bivalent (Cervarix) | |
|---|---|---|
| Manufacturer/VLP types | Merck/6, 11, 16, 18 | GlaxoSmithKline/16, 18 |
| Date of US licensure | 2006, females 2009, males | 2009, females |
| Dose of protein | 20/40/40/20 μg | 20/20 μg |
| Producer cells | Saccharomyces cerevisiae (yeast) | Baculovirus-infected Trichoplusia ni (insect cell line) |
| Adjuvant | AAHS: 225 μg amorphous aluminum hydroxyphosphate sulfate | AS04: 500 μg aluminum hydroxide; 50 μg 3-O-desacyl-4’-monophosphoryl lipid A |
| Schedule (IM) | 3-dose series | 3-dose series |
| VLP, virus-like particle; IM, intramuscular. | ||
HPV vaccine is effective, but costly
A major consideration with HPV vaccines is their cost. With 3 doses required and each dose costing about $130,9 cost effectiveness is poor when preventing uncommon diseases such as cervical and anal cancer, and a relatively benign disease such as genital warts. Male vaccination at age 12 years, when added to a female vaccination program, costs about $20,000 to $40,000 per quality-adjusted life year (QALY) if all potential HPV morbidity is included, not just that which has been proven to be prevented by the vaccine (assuming oral and penile cancer will also be prevented). Counting only HPV disease demonstrated to be prevented by the vaccine, the result is $75,000 to $250,000+ per QALY.6 Vaccinating males older than 21 years results in a cost per QALY 2 to 4 times that of vaccinating males younger than 18 years.10
A final decision. After considering these factors, ACIP approved a set of recommendations at its October 2011 meeting that will become official once they are published in the Morbidity and Mortality Weekly Report. (See “ACIP recommendations for HPV vaccine use in males”.)
- Routinely vaccinate males ages 11 to 12 years with 3 doses of HPV4. The vaccination series can be started at 9 years of age. (A recommendation)
- Vaccinate males, ages 13 to 21 years, who have not been vaccinated previously or who have not completed the 3-dose series. (A recommendation)
- Consider vaccinating males ages 22 to 26 years. (B recommendation)
- Vaccinate men ages 22 to 26 years of age who have sex with men and those in this age group who are HIV positive, if they have not been previously vaccinated. (A recommendation)
Levels of recommendation
A: Applies to all individuals in an age- or risk factor-based group.
B: Defers to clinician judgment in determining benefit for individuals.
Source: ACIP meeting; October 25, 2011; Atlanta, Ga.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
1. CDC. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:630-632.
2. CDC. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123.
3. Markowitz L. HPV vaccine for males: background and review of data. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/04-HPV-Markowitz.pdf. Accessed December 2, 2011.
4. Gee J. Safety of quadrivalent human papilloma virus (HPV4) vaccine. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/downloads/ mtg-slides-oct11/02-HPV-Gee.pdf. Accessed December 2, 2011.
5. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279-8284.
6. Dunne EF. HPV vaccine considerations for males. Presented at: ACIP meeting; October 25, 2011; Atlanta, GA. http://www.cdc. gov/vaccines/recs/acip/downloads/mtg-slides-oct11/05-HPVDunne.pdf. Accessed December 2, 2011.
7. CDC. HPV and men—fact sheet. http://www.cdc.gov/std/hpv/std/hpv/stdfact-hpv-and-men.htm. Accessed December 19, 2011.
8. Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
9. CDC. Sexually transmitted diseases (STDs): HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Accessed December 2, 2011.
10. Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presented at: ACIP meeting; June 22, 2011; Atlanta, GA. http://www.cdc.gov/vaccines/recs/acip/down-loads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed December 2, 2011.
CDC update on gonorrhea: Expand treatment to limit resistance
Public health efforts have decreased the incidence of gonorrhea over the past several decades, but this progress is threatened by emergent bacteria resistance to the few remaining antibiotics available to treat it.
Gonococcal resistance to penicillin and tetracycline began in the 1970s and was widespread by the 1980s. Resistance to fluoroquinolones developed during the last decade and led the Centers for Disease Control and Prevention (CDC) in 2007 to stop recommending this class of antibiotics for treatment of gonorrhea.1 (See “The decline of gonorrhea: A success story now threatened by antibiotic resistance”.)
Given the speed with which gonococci developed resistance to fluoroquinolones, the CDC sees as inevitable the eventual development of resistance to cephalosporins—the currently favored agents for gonorrhea.2 This is the main reason behind the new recommendations for treating all cases of gonorrhea with both a cephalosporin and azithromycin, whether or not co-infection with Chlamydia trachomatis is documented or suspected.3
The reported rate of gonorrhea rose steadily from the early 1960s until the mid-1970s, when it was close to 500 cases per 100,000. With implementation of the national gonorrhea control program, the annual rate began to fall, and by the mid-1990s it had declined by 74%. Between 1996 and 2006, the rate remained at about 115 cases per 100,000. Between 2006 and 2009, it decreased to the lowest rate since national reporting began, but increased 2.8% between 2009 and 2010 (FIGURE 1).
The highest rates of gonorrhea are in the South (FIGURE 2) and in women ages 15 to 24 and men ages 20 to 24 (FIGURE 3). Rates are highest among blacks (432.5 cases per 100,000), followed by American Indians/Alaska natives (105.7) and Hispanics (49.9). Between 2009 and 2010, gonorrhea rates increased among American Indians/Alaska natives (21.5%), Asians/Pacific Islanders (13.1%), Hispanics (11.9%), whites (9.0%), and blacks (0.3%).
Recent trends in Gonococcus susceptibility to cephalosporins have the CDC concerned. While cephalosporin resistance remains rare, the proportion of Gonococcus isolates that have shown elevated minimum inhibitory concentrations to cephalosporins has increased.
Gonorrhea control depends in part on appropriate screening of individuals at risk (TABLE). Risk factors for gonorrhea include a history of previous gonorrhea infection, other sexually transmitted infections, new or multiple sexual partners, inconsistent condom use, sex work, and drug use. Risk factors for pregnant women are the same as for nonpregnant women. Prevalence of gonorrhea infection varies widely among communities and patient populations.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 1
A decline in gonorrhea that began in the mid-1970s*
*The initiation of a national gonorrhea control program reaped immediate benefits that have continued through the present.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates, United States, 1941-2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 2
Gonorrhea prevalence, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by state, United States and outlying areas, 2010. Available at: http://www.cdc.gov/std/10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 3
Gonorrhea prevalence by age and sex, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by age and sex, United States, 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
Augment therapy, follow up thoroughly
Family physicians can assist with public health efforts to control gonorrhea and delay the development of cephalosporin resistance by screening for and detecting the infection, diagnosing those with symptoms, and treating according to newer recommendations. It’s also essential to report cases to local public health departments, assist with finding and treating sexual contacts of infected individuals, and immediately report suspected treatment failures.
A 2-drug regimen is imperative. The latest recommendation for treating gonorrhea is ceftriaxone 250 mg IM in a single dose and azithromycin 1 g orally in a single dose. Until 2010, the dose of ceftriaxone had been 125 mg. This dual drug regimen is recommended for several reasons: As with using multiple drugs to treat tuberculosis, it is hoped dual drug therapy will slow development of resistance to both cephalosporins and azithromycin; co-infection with C trachomatis remains a significant problem; and the combination may be more effective against pharyngeal gonorrhea, which is hard to detect.
Alternative regimens. Cefixime 400 mg orally as a single dose is an option in lieu of ceftriaxone, but is not preferred because of the higher number of reported failures of treatment with oral cephalosporins and less efficacy against pharyngeal disease.3 Other injectable cephalosporins are also an option, but less is known about their effectiveness in treating pharyngeal infection. Injectable options include ceftizoxime 500 mg IM, cefoxitin 2 g IM with probenecid 1 g orally, and cefotaxime 500 mg IM.
Regardless of the cephalosporin chosen, always administer azithromycin. If necessary, an alternative to azithromycin is doxycycline 100 mg orally twice a day. But doxycycline is not preferred because it has a multiple daily dosing requirement and higher levels of gonococcal resistance than is seen with azithromycin.
Necessary follow-up. Although routine testing for cure is not advocated for those treated with a recommended antibiotic regimen, a gonococcal culture and testing for antibiotic susceptibility should be done for any patient whose symptoms persist after treatment. Rapid tests using nucleic acid amplification are unsuitable for testing antibiotic susceptibility. The CDC does recommend retesting patients 3 months after treatment is completed because of a high prevalence of reinfection.3 If cephalosporin resistance becomes prevalent, routine tests of cure might become a recommended standard.
Report all patients with gonorrhea to the local public health department so that sexual contacts within the past 60 days can be notified, tested, and treated presumptively with the dual drug regimen. Recommend simultaneous treatment for all current sex partners, and discourage sexual intercourse until symptoms have resolved. Promptly report any patient with suspected treatment failure to the local health department, and consult the local or state health department for recommendations on subsequent treatment regimens.
The US Preventive Services Task Force (USPSTF) recommends routine screening for asymptomatic infection in women at risk, as per the details in the TABLE.4 While the USPSTF found insufficient evidence to recommend screening of high-risk men, physicians might still consider screening men who have sex with multiple male partners.
TABLE
USPSTF recommendations on screening for gonorrhea4
|
|
|
|
| USPSTF, US Preventive Services Task Force. *For more on the USPSTF’s grade definitions, see: http://www.uspreventiveservicestaskforce.org/uspstf/gradespre.htm#brec. |
Doing our best in the face of uncertainty
Although evidence is lacking that dual drug therapy will delay the progression of resistance, the strategy makes empirical sense. If gonorrhea develops resistance to cephalosporins, it will seriously challenge public health efforts to control this infection. Family physicians have an important role in controlling this sexually transmitted infection and helping to prevent drug resistance.
1. Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted disease treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
2. Centers for Disease Control and Prevention. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
3. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
4. US Preventive Services Task Force. Screening for gonorrhea. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgono.htm. Accessed September 26, 2011.
Public health efforts have decreased the incidence of gonorrhea over the past several decades, but this progress is threatened by emergent bacteria resistance to the few remaining antibiotics available to treat it.
Gonococcal resistance to penicillin and tetracycline began in the 1970s and was widespread by the 1980s. Resistance to fluoroquinolones developed during the last decade and led the Centers for Disease Control and Prevention (CDC) in 2007 to stop recommending this class of antibiotics for treatment of gonorrhea.1 (See “The decline of gonorrhea: A success story now threatened by antibiotic resistance”.)
Given the speed with which gonococci developed resistance to fluoroquinolones, the CDC sees as inevitable the eventual development of resistance to cephalosporins—the currently favored agents for gonorrhea.2 This is the main reason behind the new recommendations for treating all cases of gonorrhea with both a cephalosporin and azithromycin, whether or not co-infection with Chlamydia trachomatis is documented or suspected.3
The reported rate of gonorrhea rose steadily from the early 1960s until the mid-1970s, when it was close to 500 cases per 100,000. With implementation of the national gonorrhea control program, the annual rate began to fall, and by the mid-1990s it had declined by 74%. Between 1996 and 2006, the rate remained at about 115 cases per 100,000. Between 2006 and 2009, it decreased to the lowest rate since national reporting began, but increased 2.8% between 2009 and 2010 (FIGURE 1).
The highest rates of gonorrhea are in the South (FIGURE 2) and in women ages 15 to 24 and men ages 20 to 24 (FIGURE 3). Rates are highest among blacks (432.5 cases per 100,000), followed by American Indians/Alaska natives (105.7) and Hispanics (49.9). Between 2009 and 2010, gonorrhea rates increased among American Indians/Alaska natives (21.5%), Asians/Pacific Islanders (13.1%), Hispanics (11.9%), whites (9.0%), and blacks (0.3%).
Recent trends in Gonococcus susceptibility to cephalosporins have the CDC concerned. While cephalosporin resistance remains rare, the proportion of Gonococcus isolates that have shown elevated minimum inhibitory concentrations to cephalosporins has increased.
Gonorrhea control depends in part on appropriate screening of individuals at risk (TABLE). Risk factors for gonorrhea include a history of previous gonorrhea infection, other sexually transmitted infections, new or multiple sexual partners, inconsistent condom use, sex work, and drug use. Risk factors for pregnant women are the same as for nonpregnant women. Prevalence of gonorrhea infection varies widely among communities and patient populations.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 1
A decline in gonorrhea that began in the mid-1970s*
*The initiation of a national gonorrhea control program reaped immediate benefits that have continued through the present.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates, United States, 1941-2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 2
Gonorrhea prevalence, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by state, United States and outlying areas, 2010. Available at: http://www.cdc.gov/std/10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 3
Gonorrhea prevalence by age and sex, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by age and sex, United States, 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
Augment therapy, follow up thoroughly
Family physicians can assist with public health efforts to control gonorrhea and delay the development of cephalosporin resistance by screening for and detecting the infection, diagnosing those with symptoms, and treating according to newer recommendations. It’s also essential to report cases to local public health departments, assist with finding and treating sexual contacts of infected individuals, and immediately report suspected treatment failures.
A 2-drug regimen is imperative. The latest recommendation for treating gonorrhea is ceftriaxone 250 mg IM in a single dose and azithromycin 1 g orally in a single dose. Until 2010, the dose of ceftriaxone had been 125 mg. This dual drug regimen is recommended for several reasons: As with using multiple drugs to treat tuberculosis, it is hoped dual drug therapy will slow development of resistance to both cephalosporins and azithromycin; co-infection with C trachomatis remains a significant problem; and the combination may be more effective against pharyngeal gonorrhea, which is hard to detect.
Alternative regimens. Cefixime 400 mg orally as a single dose is an option in lieu of ceftriaxone, but is not preferred because of the higher number of reported failures of treatment with oral cephalosporins and less efficacy against pharyngeal disease.3 Other injectable cephalosporins are also an option, but less is known about their effectiveness in treating pharyngeal infection. Injectable options include ceftizoxime 500 mg IM, cefoxitin 2 g IM with probenecid 1 g orally, and cefotaxime 500 mg IM.
Regardless of the cephalosporin chosen, always administer azithromycin. If necessary, an alternative to azithromycin is doxycycline 100 mg orally twice a day. But doxycycline is not preferred because it has a multiple daily dosing requirement and higher levels of gonococcal resistance than is seen with azithromycin.
Necessary follow-up. Although routine testing for cure is not advocated for those treated with a recommended antibiotic regimen, a gonococcal culture and testing for antibiotic susceptibility should be done for any patient whose symptoms persist after treatment. Rapid tests using nucleic acid amplification are unsuitable for testing antibiotic susceptibility. The CDC does recommend retesting patients 3 months after treatment is completed because of a high prevalence of reinfection.3 If cephalosporin resistance becomes prevalent, routine tests of cure might become a recommended standard.
Report all patients with gonorrhea to the local public health department so that sexual contacts within the past 60 days can be notified, tested, and treated presumptively with the dual drug regimen. Recommend simultaneous treatment for all current sex partners, and discourage sexual intercourse until symptoms have resolved. Promptly report any patient with suspected treatment failure to the local health department, and consult the local or state health department for recommendations on subsequent treatment regimens.
The US Preventive Services Task Force (USPSTF) recommends routine screening for asymptomatic infection in women at risk, as per the details in the TABLE.4 While the USPSTF found insufficient evidence to recommend screening of high-risk men, physicians might still consider screening men who have sex with multiple male partners.
TABLE
USPSTF recommendations on screening for gonorrhea4
|
|
|
|
| USPSTF, US Preventive Services Task Force. *For more on the USPSTF’s grade definitions, see: http://www.uspreventiveservicestaskforce.org/uspstf/gradespre.htm#brec. |
Doing our best in the face of uncertainty
Although evidence is lacking that dual drug therapy will delay the progression of resistance, the strategy makes empirical sense. If gonorrhea develops resistance to cephalosporins, it will seriously challenge public health efforts to control this infection. Family physicians have an important role in controlling this sexually transmitted infection and helping to prevent drug resistance.
Public health efforts have decreased the incidence of gonorrhea over the past several decades, but this progress is threatened by emergent bacteria resistance to the few remaining antibiotics available to treat it.
Gonococcal resistance to penicillin and tetracycline began in the 1970s and was widespread by the 1980s. Resistance to fluoroquinolones developed during the last decade and led the Centers for Disease Control and Prevention (CDC) in 2007 to stop recommending this class of antibiotics for treatment of gonorrhea.1 (See “The decline of gonorrhea: A success story now threatened by antibiotic resistance”.)
Given the speed with which gonococci developed resistance to fluoroquinolones, the CDC sees as inevitable the eventual development of resistance to cephalosporins—the currently favored agents for gonorrhea.2 This is the main reason behind the new recommendations for treating all cases of gonorrhea with both a cephalosporin and azithromycin, whether or not co-infection with Chlamydia trachomatis is documented or suspected.3
The reported rate of gonorrhea rose steadily from the early 1960s until the mid-1970s, when it was close to 500 cases per 100,000. With implementation of the national gonorrhea control program, the annual rate began to fall, and by the mid-1990s it had declined by 74%. Between 1996 and 2006, the rate remained at about 115 cases per 100,000. Between 2006 and 2009, it decreased to the lowest rate since national reporting began, but increased 2.8% between 2009 and 2010 (FIGURE 1).
The highest rates of gonorrhea are in the South (FIGURE 2) and in women ages 15 to 24 and men ages 20 to 24 (FIGURE 3). Rates are highest among blacks (432.5 cases per 100,000), followed by American Indians/Alaska natives (105.7) and Hispanics (49.9). Between 2009 and 2010, gonorrhea rates increased among American Indians/Alaska natives (21.5%), Asians/Pacific Islanders (13.1%), Hispanics (11.9%), whites (9.0%), and blacks (0.3%).
Recent trends in Gonococcus susceptibility to cephalosporins have the CDC concerned. While cephalosporin resistance remains rare, the proportion of Gonococcus isolates that have shown elevated minimum inhibitory concentrations to cephalosporins has increased.
Gonorrhea control depends in part on appropriate screening of individuals at risk (TABLE). Risk factors for gonorrhea include a history of previous gonorrhea infection, other sexually transmitted infections, new or multiple sexual partners, inconsistent condom use, sex work, and drug use. Risk factors for pregnant women are the same as for nonpregnant women. Prevalence of gonorrhea infection varies widely among communities and patient populations.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 1
A decline in gonorrhea that began in the mid-1970s*
*The initiation of a national gonorrhea control program reaped immediate benefits that have continued through the present.
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates, United States, 1941-2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 2
Gonorrhea prevalence, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by state, United States and outlying areas, 2010. Available at: http://www.cdc.gov/std/10/surv2010.pdf. Accessed November 17, 2011.
FIGURE 3
Gonorrhea prevalence by age and sex, 2010
Source: Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2010. Gonorrhea—rates by age and sex, United States, 2010. Available at: http://www.cdc.gov/std/stats10/surv2010.pdf. Accessed November 17, 2011.
Augment therapy, follow up thoroughly
Family physicians can assist with public health efforts to control gonorrhea and delay the development of cephalosporin resistance by screening for and detecting the infection, diagnosing those with symptoms, and treating according to newer recommendations. It’s also essential to report cases to local public health departments, assist with finding and treating sexual contacts of infected individuals, and immediately report suspected treatment failures.
A 2-drug regimen is imperative. The latest recommendation for treating gonorrhea is ceftriaxone 250 mg IM in a single dose and azithromycin 1 g orally in a single dose. Until 2010, the dose of ceftriaxone had been 125 mg. This dual drug regimen is recommended for several reasons: As with using multiple drugs to treat tuberculosis, it is hoped dual drug therapy will slow development of resistance to both cephalosporins and azithromycin; co-infection with C trachomatis remains a significant problem; and the combination may be more effective against pharyngeal gonorrhea, which is hard to detect.
Alternative regimens. Cefixime 400 mg orally as a single dose is an option in lieu of ceftriaxone, but is not preferred because of the higher number of reported failures of treatment with oral cephalosporins and less efficacy against pharyngeal disease.3 Other injectable cephalosporins are also an option, but less is known about their effectiveness in treating pharyngeal infection. Injectable options include ceftizoxime 500 mg IM, cefoxitin 2 g IM with probenecid 1 g orally, and cefotaxime 500 mg IM.
Regardless of the cephalosporin chosen, always administer azithromycin. If necessary, an alternative to azithromycin is doxycycline 100 mg orally twice a day. But doxycycline is not preferred because it has a multiple daily dosing requirement and higher levels of gonococcal resistance than is seen with azithromycin.
Necessary follow-up. Although routine testing for cure is not advocated for those treated with a recommended antibiotic regimen, a gonococcal culture and testing for antibiotic susceptibility should be done for any patient whose symptoms persist after treatment. Rapid tests using nucleic acid amplification are unsuitable for testing antibiotic susceptibility. The CDC does recommend retesting patients 3 months after treatment is completed because of a high prevalence of reinfection.3 If cephalosporin resistance becomes prevalent, routine tests of cure might become a recommended standard.
Report all patients with gonorrhea to the local public health department so that sexual contacts within the past 60 days can be notified, tested, and treated presumptively with the dual drug regimen. Recommend simultaneous treatment for all current sex partners, and discourage sexual intercourse until symptoms have resolved. Promptly report any patient with suspected treatment failure to the local health department, and consult the local or state health department for recommendations on subsequent treatment regimens.
The US Preventive Services Task Force (USPSTF) recommends routine screening for asymptomatic infection in women at risk, as per the details in the TABLE.4 While the USPSTF found insufficient evidence to recommend screening of high-risk men, physicians might still consider screening men who have sex with multiple male partners.
TABLE
USPSTF recommendations on screening for gonorrhea4
|
|
|
|
| USPSTF, US Preventive Services Task Force. *For more on the USPSTF’s grade definitions, see: http://www.uspreventiveservicestaskforce.org/uspstf/gradespre.htm#brec. |
Doing our best in the face of uncertainty
Although evidence is lacking that dual drug therapy will delay the progression of resistance, the strategy makes empirical sense. If gonorrhea develops resistance to cephalosporins, it will seriously challenge public health efforts to control this infection. Family physicians have an important role in controlling this sexually transmitted infection and helping to prevent drug resistance.
1. Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted disease treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
2. Centers for Disease Control and Prevention. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
3. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
4. US Preventive Services Task Force. Screening for gonorrhea. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgono.htm. Accessed September 26, 2011.
1. Centers for Disease Control and Prevention. Update to CDC’s sexually transmitted disease treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
2. Centers for Disease Control and Prevention. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60:873-877.
3. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
4. US Preventive Services Task Force. Screening for gonorrhea. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspsgono.htm. Accessed September 26, 2011.
Ready for flu season? The 2011-2012 ACIP recommendations
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
The Centers for Disease Control and Prevention (CDC) has released recommendations made by the Advisory Committee for Immunization Practices (ACIP) for using influenza vaccine for the upcoming influenza season.1 The recommendations, which are easier to follow than in past years, continue to advise that patients older than 6 months of age (without a contraindication) be vaccinated annually. But there are some newer recommendations, as well, and they are reviewed here.
2011-2012 vaccine choices include a new product
Although the virus strains in the 2011-2012 vaccines are the same as in 2010-20112—A/California/7/2009 (H1N1)-like, A/Perth/16/ 2009 (H3N2)-like, and B/Brisbane/60/2008-like antigens—individuals vaccinated last year should receive the vaccine again this year. Over the course of a year, antibodies that developed in response to an influenza vaccine decline, and it is believed that even if the vaccine strains have not changed, annual vaccination confers optimal protection.
Although all influenza vaccine products available in the United States contain the same virus strains, the products contain either killed virus (trivalent influenza vaccine [TIV]) or live virus (live attenuated influenza vaccine [LAIV]). The only LAIV vaccine available is the intranasally administered FluMist (MedImmune). It is licensed for use in those between the ages of 2 and 49 years who are healthy, nonpregnant, and without high-risk medical conditions. The CDC does not state a preference for LAIV or TIV in this age group.
A new intradermally administered TIV, Fluzone Intradermal (Sanofi Pasteur),3 was licensed in May 2011 for use in individuals ages 18 through 64 years. It contains less antigen than intramuscular TIV options and is administered in a smaller volume (0.1 rather than 0.5 mL). The preferred site of administration is over the deltoid muscle. Injection-site erythema, induration, swelling, and pruritus occur more frequently than with intramuscular vaccine, but usually these reactions are self-limited, resolving within 3 to 7 days.
Again this coming season, a higher antigen product, Fluzone High-Dose (Sanofi Pasteur), will be available for adults ages 65 and older.4 Fluzone High-Dose contains 4 times the amount of influenza antigen as other TIV options. Ongoing studies are comparing this product with others for effectiveness and rates of adverse reactions. At this time, however, ACIP has not identified a preferred TIV product for this age group.
Easier decision making with young children
How to determine the number of doses needed by a child younger than 9 years has been simplified. If the child received 1 or more doses of vaccine last season, only 1 dose is needed this year. If no vaccine was received last year or if that status is unknown, 2 doses are recommended (FIGURE).1
FIGURE
How many doses of flu vaccine for children 6 months through 8 years of age?1
Egg allergy does not necessarily prohibit vaccination
The last recommendation change this season is that a history of egg allergy is no longer an automatic contraindication to influenza vaccine.1 The only contraindication to receiving the vaccine is a prior severe allergic reaction to influenza vaccine. ACIP now states that individuals who have experienced only hives after exposure to egg should receive the vaccine, but only a TIV product and only from a health care provider who is familiar with the potential manifestations of egg allergy. Additionally, those who receive the vaccine should be observed for at least 30 minutes for signs of a reaction.
In the past, some providers have used a 2-step approach (giving a small proportion as a dose first; then, if no reaction occurs, administering the remaining portion). Others have recommended skin testing with vaccine before administration. Neither of these approaches is necessary, according to ACIP, which cites studies that showed skin prick testing with vaccine is poorly predictive of allergic reactions and administration of both full doses and 2-step doses have been well tolerated.1
Many people reporting egg allergy will not have a reaction to influenza vaccine.1 In addition, current influenza vaccine products contain very low levels of egg protein. Individuals more likely to have a serious reaction are those who have had severe reactions to egg—eg, angioedema, respiratory distress, light-headedness, or recurrent emesis, or who required epinephrine or other emergency medical interventions. Such people should be referred to a physician with expertise in the management of allergic conditions for further risk assessment.1 As another precaution, the ACIP recommendations state that all vaccines should be administered in settings where equipment is on hand for treatment of anaphylaxis and where providers are trained in the recognition and treatment of allergic reactions.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
1. Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011 [early release]. MMWR Morb Mortal Wkly Rep. 2011;60:1-5.
2. Centers for Disease Control and Prevention Update: influenza activity—United States, 2010–11 season, and composition of the 2011–12 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2011;60:705-712.
3. Johnson DR. Fluzone Intradermal (influenza virus vaccine): a new option for influenza prevention. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-5-flu-intradermal.pdf. Accessed July 27, 2011.
4. Johnson DR. Fluzone High-Dose vaccine: one year post-approval: experience during the 2010-2011 influenza season. Presented at: Meeting of the Advisory Committee on Immunization Practices (ACIP); June 22-23, 2011; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/11-4-flu-high-dose.pdf. Accessed July 27, 2011.
CVD prevention in women: A practice update
Nearly 3 out of 4 (71.9%) US women (and 72.6% of men) ages 60 to 79 years have cardiovascular disease (CVD)—the leading cause of death despite marked improvement in mortality rates in the last 4 decades. In that same age group, the prevalence of cerebral vascular disease is 8.2% in women and 7.2% in men.1
The age-adjusted death rate for all adults is 135.1 in 100,000 for coronary heart disease (CHD) and 44.1 in 100,000 for cerebral vascular disease. In 2007, CVD caused 34.5% of deaths in women and 32.7% of deaths in men.1
Evidence that CVD frequently manifests differently in women than in men led the American Heart Association (AHA) to issue recommendations for the prevention of CVD in women in 1999, and to follow with guidelines in 2004 and an update in 2007.2-4 However, the recommended interventions were, with a few exceptions, the same as the recommendations for men. But that’s changed.
The latest update of the guidelines, published earlier this year, focuses more on sex-based differences, with the addition of pregnancy complications as a major risk factor, for example. (See “AHA’s 2011 CVD guideline update: What’s new?”.) Highlights of the guidelines,5 including the recommended interventions for all women (TABLE 1) and a comparison of its recommendations with those of the US Preventive Services Task Force (USPSTF)6 (TABLE 2)—are detailed here.
The updated guidelines for prevention of CVD in women give more weight to conditions that increase risk for heart disease and stroke primarily or exclusively in women, including gestational diabetes and other complications of pregnancy, lupus, and rheumatoid arthritis. Some of the changes include:
- adding a history of preeclampsia, gestational diabetes, and pregnancy-induced hypertension as criteria for the "at risk" classification
- revising the criterion for "high risk" classification based on risk calculation to ≥10% 10-year predicted risk of CVD (it was previously ≥20%)
- addressing the challenges of diversity, including recommendations that providers develop cultural competence and become aware of, and take steps to reduce, CVD health disparities
- redefining the lowest risk category as "ideal cardiovascular health," for women who have ideal blood pressure, cholesterol, and fasting glucose levels, and adhere to optimal lifestyle/behavioral recommendations.
The AHA indicates that it has changed from evidence-based to effectiveness-based guidelines;5 however, the practical implications within the guidelines themselves are unclear.
TABLE 1
AHA recommends these interventions for all women5
| Avoid smoking (incorporates smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs) and environmental tobacco smoke |
| Exercise (≥150 minutes of moderate exercise or ≥75 minutes of vigorous exercise per week, with additional benefit gained by more time and higher-level exercise) |
| Consume a healthy diet, rich in fruits and vegetables; whole-grain, high-fiber foods; and fish (at least twice a week); limit intake of saturated fat, cholesterol, alcohol, sodium, and sugar and avoid trans-fatty acids |
| Control your weight (maintain a BMI of <25 kg/m2) |
| Keep blood pressure <120/mm Hg through diet, exercise, and weight control; take medication for BP ≥140/90 mm Hg (or ≥130/80 mm Hg for women with diabetes or chronic kidney disease) |
| Maintain healthy lipid levels (LDL-C <100 mg/dL, HDL-C >50 mg/dL, triglycerides <150 mg/dL, and non-HDL-C [total cholesterol minus HDL] <130 mg/dL) through lifestyle and diet; consider medication for hyperlipidemia based on CVD risk and lipid levels |
| BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. |
TABLE 2
CVD prevention in women: Comparing AHA1and USPSTF recommendations5,6
| AHA | USPSTF | |
|---|---|---|
| Screening for CVD risks | ||
| Hypertension | Implied, but no specific recommendation | Recommends screening for high BP in women ≥18 y |
| Lipid disorders | Implied, but no specific recommendation | Recommends screening women ≥20 y for lipid disorders if they are at increased risk for CHD (evidence is stronger for women ≥45 y) No recommendation for or against routine screening for lipid disorders in women who are not at increased risk for CHD |
| Obesity | Implied, but no specific recommendation | Recommends screening all adult patients for obesity |
| Diabetes | Implied, but no specific recommendation | Recommends screening for asymptomatic adults with sustained BP (treated or untreated) >135/80 mm Hg Insufficient evidence to assess the balance of benefits and harms of screening asymptomatic adults with BP ≤135/80 mm Hg |
| Tobacco use | Implied, but no specific recommendation | Recommends asking all adults about tobacco use and providing tobacco cessation interventions for those who use tobacco products |
| Nontraditional risk factors | The role that novel CVD risk biomarkers (hs-CRP and advanced lipid testing) and imaging technologies (coronary calcium scoring assessment) is not yet well defined | Insufficient evidence to assess the balance of benefits and harms of using nontraditional risk factors* to screen asymptomatic women with no history of CHD |
| Screening for CVD | ||
| Carotid artery stenosis | Not addressed, but implies it might be useful for classification | Recommends against screening for asymptomatic carotid artery stenosis in the general adult population |
| Peripheral artery disease | Not addressed, but implies it might be useful for classification | Recommends against routine screening for peripheral arterial disease |
| CHD or prediction of CHD | Not addressed, but implies it might be useful for classification | Recommends against routine screening with resting EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at low risk for CHD events Insufficient evidence to recommend for or against routine screening with EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at increased risk for CHD events |
| Behavioral counseling to reduce risk | ||
| To promote physical activity | Sets physical activity targets but does not address how to achieve them | Insufficient evidence to recommend for or against behavioral counseling in primary care settings to promote physical activity |
| To promote weight loss | Sets ideal weight targets but does not address how to achieve them | Recommends intensive counseling and behavioral interventions+ to promote sustained weight loss for obese adults Insufficient evidence to recommend for or against the use of moderate (monthly) or low-intensity (less than once a month) counseling together with behavioral interventions to promote sustained weight loss in obese adults Insufficient evidence to recommend for or against the use of counseling of any intensity and behavioral interventions to promote sustained weight loss in overweight adults |
| Tobacco use | Recommends smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs | Recommends tobacco cessation interventions for those who use tobacco products |
| Risk reduction interventions | ||
| Aspirin | Recommends the use of aspirin in women with CHD unless it is contraindicated Says use of aspirin is reasonable in women with diabetes, unless it is contraindicated If aspirin is indicated but not tolerated, clopidogrel should be substituted. Aspirin may be reasonable for women <65 years for stroke prevention, but is not recommended for MI prevention. Aspirin can be useful for women >65 years if BP is controlled; benefit for stroke and MI prevention is likely to outweigh risk of GI bleeding and hemorrhagic stroke | Recommends the use of aspirin for women ages 55 to 79 years when the potential benefit of a reduction in ischemic stroke outweighs the potential harm of an increased risk of GI hemorrhage Insufficient evidence to assess aspirin for cardiovascular disease prevention in women ≥80 years Recommends against the use of aspirin for stroke prevention in women ≤55 years |
| Beta-carotene | Should not be used for prevention of CVD | Recommends against the use of beta-carotene supplements, either alone or in combination, for the prevention of cancer or cardiovascular disease |
| Antioxidants and vitamins | Vitamins E, C, B6, B12, and folic acid should not be used for CVD prevention. | Insufficient evidence to recommend for or against the use of supplements of vitamins A, C, or E; multivitamins with folic acid; or antioxidant combinations for the prevention of cancer or cardiovascular disease |
| Hormonal therapy | Hormone therapy and selective estrogen-receptor modulators should not be used for CVD prevention. | Recommends against the routine use of combined estrogen and progestin for the prevention of chronic conditions in postmenopausal women Recommends against the routine use of unopposed estrogen for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy |
| †;Defined by the USPSTF as >1 individual or group session per month for ≥3 months. *Nontraditional risk factors included in this recommendation are high-sensitivity C-reactive protein, ankle-brachial index, leukocyte count, fasting blood glucose level, periodontal disease, carotid intima-media thickness, coronary artery calcification score on electron-beam computed tomography, homocysteine level, and lipoprotein(a) level. AHA, American Heart Association; BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; EBCT, electron-beam computed tomography; EKG, electrocardiography; ETT, exercise treadmill test; GI, gastrointestinal; hs-CRP, high-sensitivity C-reactive protein; MI, myocardial infarction; USPSTF, US Preventive Services Task Force. | ||
The AHA’s assessment of risk
The new guideline update recommends assessing each woman’s CVD risk and placing her into one of 3 risk groups—high risk, at risk, and ideal cardiovascular health (TABLE 3)—then using an algorithm to determine which preventive interventions to recommend based on her risk level.
This classification approach is challenging, for several reasons. It lumps women with markedly different risk profiles into the “at risk” group, a category that will likely apply to a high proportion of women. It also appears to encourage the use of diagnostic tests for subclinical vascular disease, for which there is no evidence of effectiveness. In addition, some of the terms used in the at-risk criteria, such as ”physical inactivity” and “poor diet,” are vague.
TABLE 3
Cardiovascular disease: How the AHA classifies women’s risk5
High risk ≥1 of the following: Documented CVD Diabetes Chronic or end-stage renal disease 10-year predicted risk of CVD ≥10%* |
At risk ≥1 of the following major risk factors: Smoking Hypertension (BP ≥120/80 mm Hg, or treated hypertension) Hyperlipidemia (total cholesterol ≥200 mg/dL, HDL cholesterol <50 mg/dL, or treated dyslipidemia) Obesity Poor diet Physical inactivity Premature CVD in a first-degree relative (<55 years for men and <65 for women) Metabolic syndrome Subclinical atherosclerosis Poor exercise tolerance on a treadmill test Systemic autoimmune disease A history of preeclampsia, gestational diabetes, or PIH |
Ideal cardiovascular health All of the following: Total cholesterol <200 mg/dL, untreated BP <120/80 mm Hg, untreated Fasting blood glucose <100 mg/dL, untreated BMI <25 mm/kg2 Nonsmoking Healthy diet (rich in fruits and vegetables; whole-grain, high-fiber foods; and fish, especially oily fish such as salmon and mackerel, at least twice a week; with limited intake of saturated fat, cholesterol, alcohol, sodium, and sugar; and avoidance of trans-fatty acids) Physical activity (≥150 minutes per week at moderate intensity or ≥75 minutes per week at vigorous intensity) |
| *Calculation tools can be found at http://hp2010.nhlbihin.net/atpiii/calculator.asp (for CHD) and at http://www.westernstroke.org/PersonalStrokeRisk1.xls (for stroke). AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein, PIH, pregnancy-induced hypertension. |
Some recommendations apply to all women, regardless of risk
The AHA recommendations for all women (TABLE 1) include smoking prevention or cessation, maintenance of optimal weight, regular physical activity, and a diet aimed at preventing CVD. The guidelines also emphasize that major CVD risks should be controlled, with either lifestyle and diet modifications (preferably) or pharmacotherapy. The aggressiveness of control targets depends on the level of risk and the presence of other risk factors.
The guidelines recommend against some interventions that are often used for CVD prevention, based on a high level of evidence that they are ineffective. These include estrogen or selective estrogen receptor modulators, antioxidant vitamins (vitamins E and C, and beta-carotene), folic acid with or without vitamins B6 and B12, and aspirin (for CHD prevention) for healthy women <65 years old.
The AHA does not take a position for or against several diagnostic and risk classification tools because of a lack of evidence of usefulness. These include CVD risk biomarkers such as high sensitivity C-reactive protein and imaging technologies such as coronary calcium scoring assessment.
AHA and USPSTF diverge, but not by much
Screening for conditions that increase CVD risk is not explicitly addressed in the AHA guidelines. Screening is implied by the proposed classification scheme, which includes the presence or absence of smoking, obesity, diabetes, hypertension, and dyslipidemia, but there is no guidance on when to start or stop screening for these conditions. The AHA and the USPSTF diverge on screening women for dyslipidemia, with the USPSTF recommending screening for lipid disorders only in women at increased risk for CHD.
The recommendations for optimal weight and activity levels in the AHA guidelines do not include advice on how to achieve them, nor do they call for an assessment of the effectiveness of behavioral counseling in the clinical setting. Because the USPSTF includes an assessment of, and recommendations for, asymptomatic patients in primary care settings, its recommendations do not address women with conditions such as established CVD, heart failure, or atrial fibrillation—which the AHA guidelines do.
Overall, the AHA and USPSTF agree more than they disagree, and each covers some areas that the other does not (TABLE 2). Family physicians can use the information provided by both entities to ensure that their female patients receive high-quality preventive care that will minimize their risk for CVD.
1. American Heart Association. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. Circulation. 1999;99:2480-2484.
3. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
4. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481-1501.
5. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243-1262.
6. United States Preventive Services Task Force. USPSTF A-Z guide. Available at: www.uspreventiveservicestaskforce.org/uspstopics.htm. Accessed June 7, 2011.
Nearly 3 out of 4 (71.9%) US women (and 72.6% of men) ages 60 to 79 years have cardiovascular disease (CVD)—the leading cause of death despite marked improvement in mortality rates in the last 4 decades. In that same age group, the prevalence of cerebral vascular disease is 8.2% in women and 7.2% in men.1
The age-adjusted death rate for all adults is 135.1 in 100,000 for coronary heart disease (CHD) and 44.1 in 100,000 for cerebral vascular disease. In 2007, CVD caused 34.5% of deaths in women and 32.7% of deaths in men.1
Evidence that CVD frequently manifests differently in women than in men led the American Heart Association (AHA) to issue recommendations for the prevention of CVD in women in 1999, and to follow with guidelines in 2004 and an update in 2007.2-4 However, the recommended interventions were, with a few exceptions, the same as the recommendations for men. But that’s changed.
The latest update of the guidelines, published earlier this year, focuses more on sex-based differences, with the addition of pregnancy complications as a major risk factor, for example. (See “AHA’s 2011 CVD guideline update: What’s new?”.) Highlights of the guidelines,5 including the recommended interventions for all women (TABLE 1) and a comparison of its recommendations with those of the US Preventive Services Task Force (USPSTF)6 (TABLE 2)—are detailed here.
The updated guidelines for prevention of CVD in women give more weight to conditions that increase risk for heart disease and stroke primarily or exclusively in women, including gestational diabetes and other complications of pregnancy, lupus, and rheumatoid arthritis. Some of the changes include:
- adding a history of preeclampsia, gestational diabetes, and pregnancy-induced hypertension as criteria for the "at risk" classification
- revising the criterion for "high risk" classification based on risk calculation to ≥10% 10-year predicted risk of CVD (it was previously ≥20%)
- addressing the challenges of diversity, including recommendations that providers develop cultural competence and become aware of, and take steps to reduce, CVD health disparities
- redefining the lowest risk category as "ideal cardiovascular health," for women who have ideal blood pressure, cholesterol, and fasting glucose levels, and adhere to optimal lifestyle/behavioral recommendations.
The AHA indicates that it has changed from evidence-based to effectiveness-based guidelines;5 however, the practical implications within the guidelines themselves are unclear.
TABLE 1
AHA recommends these interventions for all women5
| Avoid smoking (incorporates smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs) and environmental tobacco smoke |
| Exercise (≥150 minutes of moderate exercise or ≥75 minutes of vigorous exercise per week, with additional benefit gained by more time and higher-level exercise) |
| Consume a healthy diet, rich in fruits and vegetables; whole-grain, high-fiber foods; and fish (at least twice a week); limit intake of saturated fat, cholesterol, alcohol, sodium, and sugar and avoid trans-fatty acids |
| Control your weight (maintain a BMI of <25 kg/m2) |
| Keep blood pressure <120/mm Hg through diet, exercise, and weight control; take medication for BP ≥140/90 mm Hg (or ≥130/80 mm Hg for women with diabetes or chronic kidney disease) |
| Maintain healthy lipid levels (LDL-C <100 mg/dL, HDL-C >50 mg/dL, triglycerides <150 mg/dL, and non-HDL-C [total cholesterol minus HDL] <130 mg/dL) through lifestyle and diet; consider medication for hyperlipidemia based on CVD risk and lipid levels |
| BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. |
TABLE 2
CVD prevention in women: Comparing AHA1and USPSTF recommendations5,6
| AHA | USPSTF | |
|---|---|---|
| Screening for CVD risks | ||
| Hypertension | Implied, but no specific recommendation | Recommends screening for high BP in women ≥18 y |
| Lipid disorders | Implied, but no specific recommendation | Recommends screening women ≥20 y for lipid disorders if they are at increased risk for CHD (evidence is stronger for women ≥45 y) No recommendation for or against routine screening for lipid disorders in women who are not at increased risk for CHD |
| Obesity | Implied, but no specific recommendation | Recommends screening all adult patients for obesity |
| Diabetes | Implied, but no specific recommendation | Recommends screening for asymptomatic adults with sustained BP (treated or untreated) >135/80 mm Hg Insufficient evidence to assess the balance of benefits and harms of screening asymptomatic adults with BP ≤135/80 mm Hg |
| Tobacco use | Implied, but no specific recommendation | Recommends asking all adults about tobacco use and providing tobacco cessation interventions for those who use tobacco products |
| Nontraditional risk factors | The role that novel CVD risk biomarkers (hs-CRP and advanced lipid testing) and imaging technologies (coronary calcium scoring assessment) is not yet well defined | Insufficient evidence to assess the balance of benefits and harms of using nontraditional risk factors* to screen asymptomatic women with no history of CHD |
| Screening for CVD | ||
| Carotid artery stenosis | Not addressed, but implies it might be useful for classification | Recommends against screening for asymptomatic carotid artery stenosis in the general adult population |
| Peripheral artery disease | Not addressed, but implies it might be useful for classification | Recommends against routine screening for peripheral arterial disease |
| CHD or prediction of CHD | Not addressed, but implies it might be useful for classification | Recommends against routine screening with resting EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at low risk for CHD events Insufficient evidence to recommend for or against routine screening with EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at increased risk for CHD events |
| Behavioral counseling to reduce risk | ||
| To promote physical activity | Sets physical activity targets but does not address how to achieve them | Insufficient evidence to recommend for or against behavioral counseling in primary care settings to promote physical activity |
| To promote weight loss | Sets ideal weight targets but does not address how to achieve them | Recommends intensive counseling and behavioral interventions+ to promote sustained weight loss for obese adults Insufficient evidence to recommend for or against the use of moderate (monthly) or low-intensity (less than once a month) counseling together with behavioral interventions to promote sustained weight loss in obese adults Insufficient evidence to recommend for or against the use of counseling of any intensity and behavioral interventions to promote sustained weight loss in overweight adults |
| Tobacco use | Recommends smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs | Recommends tobacco cessation interventions for those who use tobacco products |
| Risk reduction interventions | ||
| Aspirin | Recommends the use of aspirin in women with CHD unless it is contraindicated Says use of aspirin is reasonable in women with diabetes, unless it is contraindicated If aspirin is indicated but not tolerated, clopidogrel should be substituted. Aspirin may be reasonable for women <65 years for stroke prevention, but is not recommended for MI prevention. Aspirin can be useful for women >65 years if BP is controlled; benefit for stroke and MI prevention is likely to outweigh risk of GI bleeding and hemorrhagic stroke | Recommends the use of aspirin for women ages 55 to 79 years when the potential benefit of a reduction in ischemic stroke outweighs the potential harm of an increased risk of GI hemorrhage Insufficient evidence to assess aspirin for cardiovascular disease prevention in women ≥80 years Recommends against the use of aspirin for stroke prevention in women ≤55 years |
| Beta-carotene | Should not be used for prevention of CVD | Recommends against the use of beta-carotene supplements, either alone or in combination, for the prevention of cancer or cardiovascular disease |
| Antioxidants and vitamins | Vitamins E, C, B6, B12, and folic acid should not be used for CVD prevention. | Insufficient evidence to recommend for or against the use of supplements of vitamins A, C, or E; multivitamins with folic acid; or antioxidant combinations for the prevention of cancer or cardiovascular disease |
| Hormonal therapy | Hormone therapy and selective estrogen-receptor modulators should not be used for CVD prevention. | Recommends against the routine use of combined estrogen and progestin for the prevention of chronic conditions in postmenopausal women Recommends against the routine use of unopposed estrogen for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy |
| †;Defined by the USPSTF as >1 individual or group session per month for ≥3 months. *Nontraditional risk factors included in this recommendation are high-sensitivity C-reactive protein, ankle-brachial index, leukocyte count, fasting blood glucose level, periodontal disease, carotid intima-media thickness, coronary artery calcification score on electron-beam computed tomography, homocysteine level, and lipoprotein(a) level. AHA, American Heart Association; BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; EBCT, electron-beam computed tomography; EKG, electrocardiography; ETT, exercise treadmill test; GI, gastrointestinal; hs-CRP, high-sensitivity C-reactive protein; MI, myocardial infarction; USPSTF, US Preventive Services Task Force. | ||
The AHA’s assessment of risk
The new guideline update recommends assessing each woman’s CVD risk and placing her into one of 3 risk groups—high risk, at risk, and ideal cardiovascular health (TABLE 3)—then using an algorithm to determine which preventive interventions to recommend based on her risk level.
This classification approach is challenging, for several reasons. It lumps women with markedly different risk profiles into the “at risk” group, a category that will likely apply to a high proportion of women. It also appears to encourage the use of diagnostic tests for subclinical vascular disease, for which there is no evidence of effectiveness. In addition, some of the terms used in the at-risk criteria, such as ”physical inactivity” and “poor diet,” are vague.
TABLE 3
Cardiovascular disease: How the AHA classifies women’s risk5
High risk ≥1 of the following: Documented CVD Diabetes Chronic or end-stage renal disease 10-year predicted risk of CVD ≥10%* |
At risk ≥1 of the following major risk factors: Smoking Hypertension (BP ≥120/80 mm Hg, or treated hypertension) Hyperlipidemia (total cholesterol ≥200 mg/dL, HDL cholesterol <50 mg/dL, or treated dyslipidemia) Obesity Poor diet Physical inactivity Premature CVD in a first-degree relative (<55 years for men and <65 for women) Metabolic syndrome Subclinical atherosclerosis Poor exercise tolerance on a treadmill test Systemic autoimmune disease A history of preeclampsia, gestational diabetes, or PIH |
Ideal cardiovascular health All of the following: Total cholesterol <200 mg/dL, untreated BP <120/80 mm Hg, untreated Fasting blood glucose <100 mg/dL, untreated BMI <25 mm/kg2 Nonsmoking Healthy diet (rich in fruits and vegetables; whole-grain, high-fiber foods; and fish, especially oily fish such as salmon and mackerel, at least twice a week; with limited intake of saturated fat, cholesterol, alcohol, sodium, and sugar; and avoidance of trans-fatty acids) Physical activity (≥150 minutes per week at moderate intensity or ≥75 minutes per week at vigorous intensity) |
| *Calculation tools can be found at http://hp2010.nhlbihin.net/atpiii/calculator.asp (for CHD) and at http://www.westernstroke.org/PersonalStrokeRisk1.xls (for stroke). AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein, PIH, pregnancy-induced hypertension. |
Some recommendations apply to all women, regardless of risk
The AHA recommendations for all women (TABLE 1) include smoking prevention or cessation, maintenance of optimal weight, regular physical activity, and a diet aimed at preventing CVD. The guidelines also emphasize that major CVD risks should be controlled, with either lifestyle and diet modifications (preferably) or pharmacotherapy. The aggressiveness of control targets depends on the level of risk and the presence of other risk factors.
The guidelines recommend against some interventions that are often used for CVD prevention, based on a high level of evidence that they are ineffective. These include estrogen or selective estrogen receptor modulators, antioxidant vitamins (vitamins E and C, and beta-carotene), folic acid with or without vitamins B6 and B12, and aspirin (for CHD prevention) for healthy women <65 years old.
The AHA does not take a position for or against several diagnostic and risk classification tools because of a lack of evidence of usefulness. These include CVD risk biomarkers such as high sensitivity C-reactive protein and imaging technologies such as coronary calcium scoring assessment.
AHA and USPSTF diverge, but not by much
Screening for conditions that increase CVD risk is not explicitly addressed in the AHA guidelines. Screening is implied by the proposed classification scheme, which includes the presence or absence of smoking, obesity, diabetes, hypertension, and dyslipidemia, but there is no guidance on when to start or stop screening for these conditions. The AHA and the USPSTF diverge on screening women for dyslipidemia, with the USPSTF recommending screening for lipid disorders only in women at increased risk for CHD.
The recommendations for optimal weight and activity levels in the AHA guidelines do not include advice on how to achieve them, nor do they call for an assessment of the effectiveness of behavioral counseling in the clinical setting. Because the USPSTF includes an assessment of, and recommendations for, asymptomatic patients in primary care settings, its recommendations do not address women with conditions such as established CVD, heart failure, or atrial fibrillation—which the AHA guidelines do.
Overall, the AHA and USPSTF agree more than they disagree, and each covers some areas that the other does not (TABLE 2). Family physicians can use the information provided by both entities to ensure that their female patients receive high-quality preventive care that will minimize their risk for CVD.
Nearly 3 out of 4 (71.9%) US women (and 72.6% of men) ages 60 to 79 years have cardiovascular disease (CVD)—the leading cause of death despite marked improvement in mortality rates in the last 4 decades. In that same age group, the prevalence of cerebral vascular disease is 8.2% in women and 7.2% in men.1
The age-adjusted death rate for all adults is 135.1 in 100,000 for coronary heart disease (CHD) and 44.1 in 100,000 for cerebral vascular disease. In 2007, CVD caused 34.5% of deaths in women and 32.7% of deaths in men.1
Evidence that CVD frequently manifests differently in women than in men led the American Heart Association (AHA) to issue recommendations for the prevention of CVD in women in 1999, and to follow with guidelines in 2004 and an update in 2007.2-4 However, the recommended interventions were, with a few exceptions, the same as the recommendations for men. But that’s changed.
The latest update of the guidelines, published earlier this year, focuses more on sex-based differences, with the addition of pregnancy complications as a major risk factor, for example. (See “AHA’s 2011 CVD guideline update: What’s new?”.) Highlights of the guidelines,5 including the recommended interventions for all women (TABLE 1) and a comparison of its recommendations with those of the US Preventive Services Task Force (USPSTF)6 (TABLE 2)—are detailed here.
The updated guidelines for prevention of CVD in women give more weight to conditions that increase risk for heart disease and stroke primarily or exclusively in women, including gestational diabetes and other complications of pregnancy, lupus, and rheumatoid arthritis. Some of the changes include:
- adding a history of preeclampsia, gestational diabetes, and pregnancy-induced hypertension as criteria for the "at risk" classification
- revising the criterion for "high risk" classification based on risk calculation to ≥10% 10-year predicted risk of CVD (it was previously ≥20%)
- addressing the challenges of diversity, including recommendations that providers develop cultural competence and become aware of, and take steps to reduce, CVD health disparities
- redefining the lowest risk category as "ideal cardiovascular health," for women who have ideal blood pressure, cholesterol, and fasting glucose levels, and adhere to optimal lifestyle/behavioral recommendations.
The AHA indicates that it has changed from evidence-based to effectiveness-based guidelines;5 however, the practical implications within the guidelines themselves are unclear.
TABLE 1
AHA recommends these interventions for all women5
| Avoid smoking (incorporates smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs) and environmental tobacco smoke |
| Exercise (≥150 minutes of moderate exercise or ≥75 minutes of vigorous exercise per week, with additional benefit gained by more time and higher-level exercise) |
| Consume a healthy diet, rich in fruits and vegetables; whole-grain, high-fiber foods; and fish (at least twice a week); limit intake of saturated fat, cholesterol, alcohol, sodium, and sugar and avoid trans-fatty acids |
| Control your weight (maintain a BMI of <25 kg/m2) |
| Keep blood pressure <120/mm Hg through diet, exercise, and weight control; take medication for BP ≥140/90 mm Hg (or ≥130/80 mm Hg for women with diabetes or chronic kidney disease) |
| Maintain healthy lipid levels (LDL-C <100 mg/dL, HDL-C >50 mg/dL, triglycerides <150 mg/dL, and non-HDL-C [total cholesterol minus HDL] <130 mg/dL) through lifestyle and diet; consider medication for hyperlipidemia based on CVD risk and lipid levels |
| BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. |
TABLE 2
CVD prevention in women: Comparing AHA1and USPSTF recommendations5,6
| AHA | USPSTF | |
|---|---|---|
| Screening for CVD risks | ||
| Hypertension | Implied, but no specific recommendation | Recommends screening for high BP in women ≥18 y |
| Lipid disorders | Implied, but no specific recommendation | Recommends screening women ≥20 y for lipid disorders if they are at increased risk for CHD (evidence is stronger for women ≥45 y) No recommendation for or against routine screening for lipid disorders in women who are not at increased risk for CHD |
| Obesity | Implied, but no specific recommendation | Recommends screening all adult patients for obesity |
| Diabetes | Implied, but no specific recommendation | Recommends screening for asymptomatic adults with sustained BP (treated or untreated) >135/80 mm Hg Insufficient evidence to assess the balance of benefits and harms of screening asymptomatic adults with BP ≤135/80 mm Hg |
| Tobacco use | Implied, but no specific recommendation | Recommends asking all adults about tobacco use and providing tobacco cessation interventions for those who use tobacco products |
| Nontraditional risk factors | The role that novel CVD risk biomarkers (hs-CRP and advanced lipid testing) and imaging technologies (coronary calcium scoring assessment) is not yet well defined | Insufficient evidence to assess the balance of benefits and harms of using nontraditional risk factors* to screen asymptomatic women with no history of CHD |
| Screening for CVD | ||
| Carotid artery stenosis | Not addressed, but implies it might be useful for classification | Recommends against screening for asymptomatic carotid artery stenosis in the general adult population |
| Peripheral artery disease | Not addressed, but implies it might be useful for classification | Recommends against routine screening for peripheral arterial disease |
| CHD or prediction of CHD | Not addressed, but implies it might be useful for classification | Recommends against routine screening with resting EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at low risk for CHD events Insufficient evidence to recommend for or against routine screening with EKG, ETT, or EBCT scanning for coronary calcium for the presence of severe carotid artery stenosis or the prediction of CHD events in adults at increased risk for CHD events |
| Behavioral counseling to reduce risk | ||
| To promote physical activity | Sets physical activity targets but does not address how to achieve them | Insufficient evidence to recommend for or against behavioral counseling in primary care settings to promote physical activity |
| To promote weight loss | Sets ideal weight targets but does not address how to achieve them | Recommends intensive counseling and behavioral interventions+ to promote sustained weight loss for obese adults Insufficient evidence to recommend for or against the use of moderate (monthly) or low-intensity (less than once a month) counseling together with behavioral interventions to promote sustained weight loss in obese adults Insufficient evidence to recommend for or against the use of counseling of any intensity and behavioral interventions to promote sustained weight loss in overweight adults |
| Tobacco use | Recommends smoking prevention and cessation advice and assistance, including nicotine replacement, pharmacotherapy, and formal smoking cessation programs | Recommends tobacco cessation interventions for those who use tobacco products |
| Risk reduction interventions | ||
| Aspirin | Recommends the use of aspirin in women with CHD unless it is contraindicated Says use of aspirin is reasonable in women with diabetes, unless it is contraindicated If aspirin is indicated but not tolerated, clopidogrel should be substituted. Aspirin may be reasonable for women <65 years for stroke prevention, but is not recommended for MI prevention. Aspirin can be useful for women >65 years if BP is controlled; benefit for stroke and MI prevention is likely to outweigh risk of GI bleeding and hemorrhagic stroke | Recommends the use of aspirin for women ages 55 to 79 years when the potential benefit of a reduction in ischemic stroke outweighs the potential harm of an increased risk of GI hemorrhage Insufficient evidence to assess aspirin for cardiovascular disease prevention in women ≥80 years Recommends against the use of aspirin for stroke prevention in women ≤55 years |
| Beta-carotene | Should not be used for prevention of CVD | Recommends against the use of beta-carotene supplements, either alone or in combination, for the prevention of cancer or cardiovascular disease |
| Antioxidants and vitamins | Vitamins E, C, B6, B12, and folic acid should not be used for CVD prevention. | Insufficient evidence to recommend for or against the use of supplements of vitamins A, C, or E; multivitamins with folic acid; or antioxidant combinations for the prevention of cancer or cardiovascular disease |
| Hormonal therapy | Hormone therapy and selective estrogen-receptor modulators should not be used for CVD prevention. | Recommends against the routine use of combined estrogen and progestin for the prevention of chronic conditions in postmenopausal women Recommends against the routine use of unopposed estrogen for the prevention of chronic conditions in postmenopausal women who have had a hysterectomy |
| †;Defined by the USPSTF as >1 individual or group session per month for ≥3 months. *Nontraditional risk factors included in this recommendation are high-sensitivity C-reactive protein, ankle-brachial index, leukocyte count, fasting blood glucose level, periodontal disease, carotid intima-media thickness, coronary artery calcification score on electron-beam computed tomography, homocysteine level, and lipoprotein(a) level. AHA, American Heart Association; BP, blood pressure; CHD, coronary heart disease; CVD, cardiovascular disease; EBCT, electron-beam computed tomography; EKG, electrocardiography; ETT, exercise treadmill test; GI, gastrointestinal; hs-CRP, high-sensitivity C-reactive protein; MI, myocardial infarction; USPSTF, US Preventive Services Task Force. | ||
The AHA’s assessment of risk
The new guideline update recommends assessing each woman’s CVD risk and placing her into one of 3 risk groups—high risk, at risk, and ideal cardiovascular health (TABLE 3)—then using an algorithm to determine which preventive interventions to recommend based on her risk level.
This classification approach is challenging, for several reasons. It lumps women with markedly different risk profiles into the “at risk” group, a category that will likely apply to a high proportion of women. It also appears to encourage the use of diagnostic tests for subclinical vascular disease, for which there is no evidence of effectiveness. In addition, some of the terms used in the at-risk criteria, such as ”physical inactivity” and “poor diet,” are vague.
TABLE 3
Cardiovascular disease: How the AHA classifies women’s risk5
High risk ≥1 of the following: Documented CVD Diabetes Chronic or end-stage renal disease 10-year predicted risk of CVD ≥10%* |
At risk ≥1 of the following major risk factors: Smoking Hypertension (BP ≥120/80 mm Hg, or treated hypertension) Hyperlipidemia (total cholesterol ≥200 mg/dL, HDL cholesterol <50 mg/dL, or treated dyslipidemia) Obesity Poor diet Physical inactivity Premature CVD in a first-degree relative (<55 years for men and <65 for women) Metabolic syndrome Subclinical atherosclerosis Poor exercise tolerance on a treadmill test Systemic autoimmune disease A history of preeclampsia, gestational diabetes, or PIH |
Ideal cardiovascular health All of the following: Total cholesterol <200 mg/dL, untreated BP <120/80 mm Hg, untreated Fasting blood glucose <100 mg/dL, untreated BMI <25 mm/kg2 Nonsmoking Healthy diet (rich in fruits and vegetables; whole-grain, high-fiber foods; and fish, especially oily fish such as salmon and mackerel, at least twice a week; with limited intake of saturated fat, cholesterol, alcohol, sodium, and sugar; and avoidance of trans-fatty acids) Physical activity (≥150 minutes per week at moderate intensity or ≥75 minutes per week at vigorous intensity) |
| *Calculation tools can be found at http://hp2010.nhlbihin.net/atpiii/calculator.asp (for CHD) and at http://www.westernstroke.org/PersonalStrokeRisk1.xls (for stroke). AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; HDL, high-density lipoprotein, PIH, pregnancy-induced hypertension. |
Some recommendations apply to all women, regardless of risk
The AHA recommendations for all women (TABLE 1) include smoking prevention or cessation, maintenance of optimal weight, regular physical activity, and a diet aimed at preventing CVD. The guidelines also emphasize that major CVD risks should be controlled, with either lifestyle and diet modifications (preferably) or pharmacotherapy. The aggressiveness of control targets depends on the level of risk and the presence of other risk factors.
The guidelines recommend against some interventions that are often used for CVD prevention, based on a high level of evidence that they are ineffective. These include estrogen or selective estrogen receptor modulators, antioxidant vitamins (vitamins E and C, and beta-carotene), folic acid with or without vitamins B6 and B12, and aspirin (for CHD prevention) for healthy women <65 years old.
The AHA does not take a position for or against several diagnostic and risk classification tools because of a lack of evidence of usefulness. These include CVD risk biomarkers such as high sensitivity C-reactive protein and imaging technologies such as coronary calcium scoring assessment.
AHA and USPSTF diverge, but not by much
Screening for conditions that increase CVD risk is not explicitly addressed in the AHA guidelines. Screening is implied by the proposed classification scheme, which includes the presence or absence of smoking, obesity, diabetes, hypertension, and dyslipidemia, but there is no guidance on when to start or stop screening for these conditions. The AHA and the USPSTF diverge on screening women for dyslipidemia, with the USPSTF recommending screening for lipid disorders only in women at increased risk for CHD.
The recommendations for optimal weight and activity levels in the AHA guidelines do not include advice on how to achieve them, nor do they call for an assessment of the effectiveness of behavioral counseling in the clinical setting. Because the USPSTF includes an assessment of, and recommendations for, asymptomatic patients in primary care settings, its recommendations do not address women with conditions such as established CVD, heart failure, or atrial fibrillation—which the AHA guidelines do.
Overall, the AHA and USPSTF agree more than they disagree, and each covers some areas that the other does not (TABLE 2). Family physicians can use the information provided by both entities to ensure that their female patients receive high-quality preventive care that will minimize their risk for CVD.
1. American Heart Association. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. Circulation. 1999;99:2480-2484.
3. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
4. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481-1501.
5. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243-1262.
6. United States Preventive Services Task Force. USPSTF A-Z guide. Available at: www.uspreventiveservicestaskforce.org/uspstopics.htm. Accessed June 7, 2011.
1. American Heart Association. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. Circulation. 1999;99:2480-2484.
3. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
4. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481-1501.
5. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123:1243-1262.
6. United States Preventive Services Task Force. USPSTF A-Z guide. Available at: www.uspreventiveservicestaskforce.org/uspstopics.htm. Accessed June 7, 2011.
ACIP immunization update
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009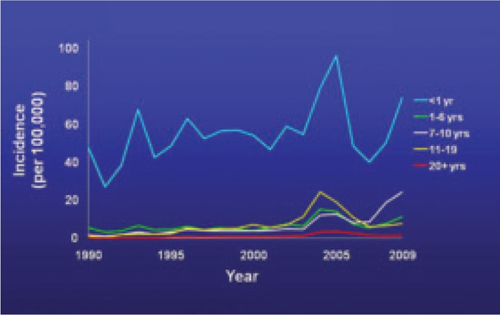
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
CDC update: Guidelines for treating STDs
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
ACIP update: 2 new recommendations for meningococcal vaccine
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
Flu season’s almost here: Are you ready?
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.