User login
Are you up to date with new immunization recommendations?
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.
Disaster medical response: Maximizing your effectiveness
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
In the aftermath of Hurricane Katrina, physicians and other health professionals volunteered for deployment to the affected area to provide medical services. The frustrating reality most of them encountered was the incapacity of those in charge to use the number of professional volunteers expressing interest.
Lesson: Build the infrastructure to support professional volunteerism
Untrained volunteers, though well intentioned, are often not that helpful. The immediate needs of a disaster area relate to public health and other safety issues. Until a proper infrastructure is re-established, general medical services cannot be provided. Physician services are most effectively provided in collaboration with, or as part of, an organized local response agency.
First things first
In addition to immediate loss of life and injuries caused by a disaster—natural or man-made (eg, war, terrorism)—mass disruption of the local infrastructure and relocation of a large segment of the population pose ongoing threats to health. The most crucial services to re-establish include adequate clean water, sanitation, food supplies, vector control (eg, insects and rodents), shelter, and immunizations. Also essential is establishing surveillance systems to rapidly assess needs and to detect disease trends.
Tasks that can wait
Contrary to what is commonly believed and stated in the press, rapid burial or cremation of cadavers is not an immediate need. Bodies almost never pose a serious public health threat. Moreover, rapid disposal of bodies can deprive families of knowing what happened to their relatives and cause psychological harm as well as legal and economic hardships.
Epidemics can occur but they usually result from respiratory or gastrointestinal pathogens caused by poor sanitation, inadequate water supplies, and overcrowding in inadequate shelters. Public health surveillance systems are important for detecting, tracking, and controlling such outbreaks.
Physicians as volunteers
Volunteer physicians are most effective following a disaster if they understand the importance of re-establishing the needed infrastructure, and if they arrive on scene as part of an organized response, having been trained in disaster medicine and public health. Disaster Medicine is becoming a recognized field of medicine with its own set of skills and an evolving literature base and training materials and courses.
After the immediate post-disaster period, it often takes a prolonged period of time to re-establish basic medical services. During this phase, volunteers continue to be needed but are harder to recruit. Mental health professionals are especially useful to assist with the posttraumatic stress and grief issues common after disasters.
If you would like to become part of organized disaster response team, you have several options.
If volunteering for deployment to other regions is not something you’re likely to do, but you live in an area vulnerable to, say, tornadoes or earthquakes, there is plenty you can do to prepare for disaster.
The AMA offers 3 courses in basic disaster response: Core Disaster Life Support (CDLS); Basic Disaster Life Support (BDLS); and Advanced Disaster Life Support (ADLS). Details are available at the AMA website: www.ama-assn.org/ama/pub/category/12606.html.
The CDC offers web-based training materials in the medical and public health response to an array of natural and man-made disasters (available on the Web at www.phppo.cdc.gov/phtn/default.asp).
You can also assist your local health department in planning for the most likely disasters in your area.
The Medical Reserve Corps
The Medical Reserve Corps (MRC) is a program started by the federal government after the terrorist attacks of September 11, 2001. It is part of the Citizen Corps, which is one component of the USA Freedom Corps (www.usafreedomcorps.gov). The purpose of the MRC is to organize local groups of medical and public health professionals to prepare for and respond to local and national emergency needs.
The Office of the Surgeon General coordinates the MRC program. This coordinating function includes recognizing and listing MRCs, offering technical assistance, serving as a clearinghouse of information for local MRCs, and offering training. Physicians interested in joining a local MRC can check on the MRC home page (www.medicalreservecorps.gov) to see if one has been organized their area. If no MRC exists in your area, you can help start one with the approval of the local Citizen Corps Council (www.citizencorps.gov/councils).
Since the MRC is a federal program—albeit relying on local organization and initiative—it is not clear how well local MRC units are fitting into the local, state, and national disaster relief infrastructure. Reportedly at least 20 MRC units assisted with relief efforts in Louisiana after Katrina. The MRC is intended to serve as a local resource and to augment the public health workforce should mass immunization or antibiotic distribution be needed.
Disaster Medical Assistance Teams
Disaster Medical Assistance Teams (DMATs) are part of the National Disaster Medical System, under the auspices of the Department of Homeland Security. The role of these teams is to provide medical care in a disaster area.
As stated in DMAT promotional material, “DMATs deploy to disaster sites with sufficient supplies and equipment to sustain themselves for a period of 72 hours while providing medical care at a fixed or temporary medical care site.” In incidents with large numbers of casualties, DMATs responsibilities include “triaging patients, providing high-quality medical care despite the adverse and austere environment often found at a disaster site, and preparing patients for evacuation.” DMATs may also provide primary medical care or may augment overloaded local health care staffs.
Under those unusual circumstances when victims of a disaster are evacuated to another location for their medical care, “DMATs may be activated to support patient reception and disposition of patients to hospitals. DMATs are designed to be a rapid-response element to supplement local medical care until other Federal or contract resources can be mobilized, or the situation is resolved.”
DMATs are organized by a local sponsor—a medical center, local public health agency, or a nonprofit organization. The responsibilities of the sponsor include recruiting DMAT team members, training, and organizing the dispatch of team members if called upon. Members of DMATs become temporary federal employees when deployed; this provides them liability protection through the Federal Tort Claims Act. In addition, professional licenses of federal employees are recognized by states, freeing DMAT team members from state licensing concerns.
To become a member of a DMAT, you must fill out a Federal Job Application form, be interviewed, and accepted as a team member. The NDMS has 10 regional offices (detailed at www.oep-ndms.dhhs.gov/region_1.html) where information can be found about existing DMAT teams and how to form a team. The DMAT home page is www.oep-ndms.dhhs.gov/dmat.html.
Search-and-rescue teams
Local fire departments and law enforcement departments frequently have search-and-rescue teams that can be called on to respond to disasters throughout the country. When these teams are deployed, they should take along medical personnel to attend to the needs of the responders. The medical professional should be prepared to screen responders and provide medical clearance before they deploy, provide urgent care medical services to responders, and ensure that measures are taken to prevent illness among team members.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Medicare prescription drug bill: Resources to inform and equip your patients
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
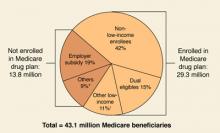
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
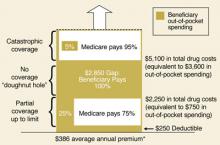
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
On November 15, 2005, enrollment opened for Medicare’s new prescription drug program. You have most likely been asked by patients for help in understanding the complexity of the options. No small task. Though there is a lot to disagree with in the way the Medicare drug benefit has been crafted, it’s what we now have and many beneficiaries can benefit from it. In addition to knowing which agencies in your communities serve seniors, you can better equip your elderly patients to make decisions by becoming familiar with web sites and other resources listed in this article.
Confusion reigns
By May 15, 2006, when the initial enrollment period ends, the government predicts that over 29 million of 43 million eligible beneficiaries will have signed up for the new benefit, with another 9 to 10 million beneficiaries maintaining their drug coverage through an employer-sponsored plan (FIGURE 1).
Whether these predictions come to pass is an open question. A Kaiser Family Foundation survey from late October 2005 found that 60% of senior citizens did not understand the benefit and 50% thought it would not help them. Of those surveyed, 43% did not know whether they would enroll, 37% said they would not enroll, and only 20% said they would definitely enroll.
One problem for seniors is that unlike the traditional Medicare program in which there are only 2 choices—whether to sign up for the traditional fee-for-service plan or a managed care plan—the new drug plan is administered by a large number of private plans that cover different medications and charge different prices for them. Seniors who said they understood the drug benefit (a minority) were more likely to view the program favorably. Perhaps most relevant for physicians is that seniors said they would likely turn to the Medicare program (33%), their personal doctor (32%), or their pharmacist (25%) for assistance.1
A quick review
Readers may recall that the prescription drug benefit portion of the Medicare Modernization Act of 2003 includes a premium (current national average of $32/month), an annual deductible, copays, and the infamous “donut hole” (FIGURE 2).2
With the program beginning in January 2006, however, there is more to keep in mind than the costs to beneficiaries: for example, who will be offering the benefit, what medications will be covered (formularies), and the effect on dual-eligibles (beneficiaries covered by both Medicare and Medicaid), so-called “Medigap” policyholders, and low-income beneficiaries.
FIGURE 1
Estimated Medicare prescription drug benefit participation, 2006
* “Others” not enrolled includes federal retirees with drug coverage through FEHBP or TRICARE, and those who lack drug coverage.
† “Other low-income” includes non-dual-eligibles with incomes <150% FPL.
Source: HHS OACT, MMA final rule, January 2005.
FIGURE 2
Standard Medicare prescription drug benefit, 2006
* Annual amount based on $32.20 national average beneficiary premium (CMS, August 2005)
Source: Kaiser Family Foundation illustration of standard Medicare drug benefit described in the Medicare Modernization Act of 2003
Who offers the plans
Medicare beneficiaries can obtain the drug benefit in either of 2 ways: through a stand-alone prescription drug plan (PDP) that covers only drugs (with the usual medical benefits obtained through the traditional Medicare program), or through a Medicare Advantage plan (MA) that is essentially a managed care plan, providing drug coverage and medical benefits in place of the traditional Medicare program.
The Bush administration has long promoted MA plans as a way to better control Medicare costs, even though the federal government currently spends more per MA beneficiary than for beneficiaries in traditional Medicare. MA plans can offer additional benefits and adjust premiums to attract customers—drug benefits for a lower premium, vision benefits, and dental benefits. However, there may be limits on using providers outside the MA plan’s network of physicians and hospitals.3
Drug plans must cover at least 2 drugs in each therapeutic class approved by Medicare, but they may use tiered cost-sharing (eg, generics and brand-name drugs in different tiers) and other management tools as long as they meet the minimum requirements of the overall bill. The decision by the White House and Congress to approve the privatization of the drug benefit has led to the current situation in which multiple private plans are competing for enrollment in each geographic region and offering different drugs for different prices. For instance, in my county, there are 7 MA plans and 43 PDP plans being offered. This makes the system overly complex and confusing. In addition, the Medicare Modernization Act allows plans to increase the copays or even end coverage of specific drugs with 60 days notice.
Dual eligibles and low-income beneficiaries
The Medicare Modernization Act provided additional assistance to persons of limited means—those currently covered by Medicare and Medicaid plans who receive their medications through Medicaid (the dual-eligibles) and those who have limited income and resources but are only covered by Medicare. The former group will automatically be enrolled into PDPs if they do not sign up on their own, and they will pay reduced fees for their medications. The states, in turn, will reimburse the federal government for the drug cost savings gained by their Medicaid programs.
Other low-income individuals may also be eligible for drug benefit subsidies based on their income and assets (TABLE). Clearly, the Medicare Modernization Act offers significant drug benefits to beneficiaries of limited means. The Centers for Medicare and Medicaid Services (CMS) projects that 10.9 million beneficiaries will receive low-income subsidies out of 14.5 million eligible.
TABLE
Medicare prescription drug benefit subsidies for low-income beneficiaries, 2006
| LOW-INCOME SUBSIDY LEVEL | PREMIUM | MONTHLY DEDUCTIBLE | ANNUAL COPAYMENTS |
|---|---|---|---|
| Full-benefit dual eligibles Income <100% of poverty ($9750 individual; $12,830/couple) | $0 | $0 | $1/generic, $3/brand-name; no copays after total drug spending reaches $5100 |
| Full-benefit dual eligibles Income ≥100% of poverty | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Institutionalized full-benefit dual eligibles | $0 | $0 | No copays |
| Individuals with income <135% of poverty ($12,920 individuals, $17,321/couple) and assets <$6000/individual; $9000/couple | $0 | $0 | $2/generic, $5/brand-name; no copays after total drug spending reaches $5100 |
| Individuals with income 135%–150% of poverty ($12,920–$14,355 individuals, $17,321–$19,245/couple) and assets <$10,000/individual, $20,000/couple | Sliding scale up to $32.20* | $50 | 15% of total costs up to $5100; $2/generic, $5/brand-name thereafter |
| Note: Poverty-level dollar amounts are for 2005. Additional assests of up to $1500/individual and $3000/couple for funeral or burial expenses are permitted. *$32.20 is the national monthly Part D base beneficiary premium for 2006. | |||
| Source: Kaiser Family Foundation summary of Medicare prescription drug benefit low-income subsidies in 2006. | |||
Medigap and employer-sponsored plans
Many current beneficiaries have Medigap insurance policies, which cover part or all of the financial holes in the traditional Medicare plan—eg, deductibles, copays, and other benefits such as drug coverage. Beginning in January 2006, new policies that include drug coverage can no longer be issued. Policyholders can keep their current Medigap policies that cover medications; however, these are generally not considered equivalent to the new coverage. In addition, Medicare will provide subsidies to employers to encourage them to continue any current retiree plans that provide drug coverage comparable to the new plans.4
Enrollment
While the new drug plans start on January 1, 2006, the initial enrollment period runs until May 15, 2006. Beneficiaries who enroll after that time and do not currently have drug coverage as good as the new Medicare drug benefit will pay a higher premium equal to 1% of the average monthly premium for each month they delay enrollment. Those who enroll may change plans one time between December 31, 2005 and May 15, 2006. After May 15, the next enrollment period will be from November 15 to December 31, 2006. Any enrollee can change plans during that time.
In order to assist beneficiaries in making a decision about whether to enroll in a Medicare drug plan and which to choose, the federal government, assisted by a number of medical organizations (such as the AAFP) and nonprofits like the local Area Agencies on Aging, is providing seniors with information in a variety of formats. Beneficiaries should all have received a booklet, “Medicare and You,” in October 2005. There is a 24-hour telephone help line, 1-800-MEDICARE, that has automated answers and can provide access to a real person.
Finally, there is the Internet: www.medicare.gov. While 3 of 4 seniors have never been online, this is the best method to locate available plans in your area, find out which specific medications are included in each plan, and try to compare costs.5 For many seniors, it will be worth asking family members, friends, or community agencies for help in navigating the web site and the information it contains.
CORRESPONDENCE
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
1. Kaiser Family Foundation news release. Available at: www.kff.org/kaiserpolls/med111005nr.cfm. Accessed on November 21, 2005.
2. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
3. Fuhrmans V, Lueck S. Insurers sweeten health plans for seniors. Wall Street Journal, November 8, 2005.
4. The Medicare Prescription Drug Benefit. Kaiser Family Foundation. Available at: www.kff.org/medicare/7044-02.cfm. Accessed on December 4, 2005.
5. Glendinning D. Patients look to doctors for help on Medicare drug plans. AMA News, December 5, 2005.
Pandemic influenza: How it would progress and what it would require of you
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
An influenza pandemic, or world-wide outbreak, advances through 3 periods—interpandemic, pandemic alert, and pandemic—and their respective phases defined by the World Health Organization (TABLE 1). Your responsibilities would be different in each of these periods (TABLE 2), requiring you to stay current on the progression of the disease and changing recommendations coming from the Centers for Disease Control and Prevention (CDC) and state and local public health departments.
A pandemic would be caused by the emergence of a new strain of influenza A. This strain could be the avian strain described in the May 2005 Practice Alert,1 “The growing threat of avian influenza,”or another novel strain.
This column describes the family physician’s role in a pandemic and includes advice on diagnosis, treatment, and prevention of disease transmission. It is based on recent recommendations from the CDC.2
Major differences between pandemic flu and a regular flu season
Vaccine shortage. Unless faster vaccine production methods are developed, there will probably be little to no vaccine initially, and once vaccine production commences the amount produced will not keep up with the need. This will necessitate prioritizing vaccine administration, forcing us to weigh societal infrastructure needs (fire-fighters, health care workers, police, etc) against those of individuals at high risk of complications.
In addition, 2 doses of vaccine 1 month apart will be needed for full protection. (Note: There is an approved provisional plan through the Advisory Committee on Immunization Practices [ACIP] and the National Vaccine Advisory Committee [NVAC] for vaccine prioritization.)
Antiviral shortage. There will also likely be a shortage of antiviral medication. Amantadine (Symmetrel) and rimantadine (Flumadine)—antivirals recommended for use against influenza A—have reduced efficacy against avian influenza, and the same may be true with any other novel strain.
Other antivirals if they are effective and available, will be used to treat acute infections and to prevent infection in those exposed and/or at high risk of complications and will be administered according to a prioritization schedule. Recommendations for prioritization of both vaccine and antivirals will come from ACIP/NVAC and the Secretary of the Department of Health and Human Services. The recommendations will be implemented by the CDC and state and local health departments, but may change as the pandemic evolves, depending on the number of people and age groups infected and the rates of morbidity and mortality.
Complicating factors. A common influenza strain could circulate at the same time as a pandemic strain, complicating the diagnostic and epidemiological picture. Office-based, rapid diagnostic tests cannot distinguish between influenza A strains. Finally, if pandemic flu exhibits the expected high rates of proliferation and mortality seen in past pandemics, our current hospital capacity will be strained and likely exceeded.
TABLE 1
WHO global pandemic phases
| INTERPANDEMIC PERIOD |
| Phase 1 No new influenza virus subtypes have been detected in humans. An influenza virus subtype that has caused human infection may exist in animals but the risk of human infection or disease is considered low. |
| Phase 2 No new influenza virus subtypes have been detected in humans. However, a circulating animal influenza virus subtype poses a substantial risk of human disease. |
| PANDEMIC ALERT PERIOD |
| Phase 3 Human infection with a new sub-type has occurred but no human-to-human spread has occurred, or at most there have been rare instances of spread to a close contact. |
| Phase 4 Small clusters with limited human-to-human transmission are detected, but spread is highly localized, suggesting that the virus is not well adapted to humans. |
| Phase 5 Larger clusters but human-to-human spread is still localized, suggesting the virus is becoming increasingly better adapted to humans but may not yet be fully transmissible. |
| PANDEMIC PERIOD |
| Phase 6 Transmission increases and is sustained in the general population. |
| POSTPANDEMIC PERIOD |
| Return to Phase 1 |
TABLE 2
Family physician responsibilities
| INTERPANDEMIC AND PANDEMIC ALERT PERIODS |
| Become familiar with case definitions |
| Know procedures for screening, infection control, and laboratory testing |
| Know antiviral regimens for Avian and other novel influenza viruses |
| Notify local public health authorities about suspected and confirmed novel influenza cases |
| Collect recommended specimens for diagnosis of novel influenza strains and have them forwarded to designated public health laboratories |
| PANDEMIC PERIOD |
| Regularly review updates on case definitions and recommendations for screening, laboratory testing and treatment |
| Report pandemic influenza cases as requested by the public health department |
| Collect specimens as requested by the public health department for ongoing surveillance and have them forwarded to designated public health laboratories |
| Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department |
Back to basics
Even with a limited supply of vaccine and antiviral medication, useful advice can still be given to individuals and the public to help them protect them and others from infection should a pandemic occur. People should be advised to:
- What hands frequently and thoroughly
- Avoid locations where infection is likely to occur
- Avoid close contact with those who have flu-like symptoms
- Cover coughs and sneezes with tissues, properly dispose of used tissue, and wash hands after handling waste
- Use infection control measures in the home if a household member is ill (TABLE 3)
- Possibly use masks. (No consensus exists on the use of masks by those infected or potentially exposed. Surgical masks may be useful for providers of patient care.)
Physicians can take measures to minimize the chance of spreading the virus in their clinics and to protect themselves and other staff (covered in a previous Practice Alert).3 Infection control guidelines can be implemented in hospitals and other health care facilities, as well as in schools and other high-risk settings.
TABLE 3
Infection control measures for patients cared for at home
| MANAGING THE PATIENT |
| Place the patient in a separate room or separate physically from other household members as much as possible |
| The patient should stay at home while most infectious (5 days after symptom onset) to avoid infecting others. If they have to leave the home they should strictly follow respiratory hygiene |
| Consider having the patient wear a surgical mask |
| ADVICE FOR OTHERS IN THE HOME |
| Non-household members should not enter the home |
| If non-household members need to enter the home they should avoid close contact with the patient |
| Limit the number of household members having contact with the patient Follow hand hygiene after contact with the patient or the patient environment and waste products. This includes hand washing with soap and water or use of an alcohol-based hand rub |
| Consider having direct caregivers wear a surgical mask |
| Wash dishes, utensils, and laundry in warm water and soap |
| Consider antiviral prophylaxis for household members, if it is available |
| Have household members seek care as soon as they develop symptoms of influenza |
Clinical guidelines: Pandemic alert
The recommended clinical approach to a patient suspected of having a novel flu strain will vary depending on the phase of the pandemic.
Through phase 5, in the pandemic alert period, acute febrile respiratory illness will be caused by a novel influenza virus only rarely. Suspect novel influenza only if the patient meets both clinical and epidemiologic criteria. The clinical criteria are fever plus 1 or more of the following: sore throat, cough, dyspnea.
Epidemiologic criteria include travel within the past 10 days to an area affected by highly pathogenic avian influenza out-breaks in poultry or where human cases of novel influenza have been confirmed; and either direct contact with poultry (touching birds or bird feces or surfaces contaminated by bird feces or eating uncooked poultry products) or close contact with a person with confirmed or suspected novel influenza. Occupational exposure through laboratory work with the novel influenza strain would also be considered an epidemiologic criterion, but this occurrence would be rare. Geographic areas affected by avian influenza can be found on the CDC web site (www.cdc.gov/flu/) and World Health Organization web site (www.who.int.en/).
6 Steps to proper management. Once a patient is suspected of having a novel influenza strain, take the following steps.
- Control spread of infection. Consider admitting the patient to a single-patient hospital room. If this is not possible, take precautions to control infection in the home (TABLE 3). Details of hospital infection control precautions can be found on the CDC influenza web site.
- Notify local or state public health departments. Report the suspicious case and ask for advice regarding collecting laboratory specimens and treatment options.
- Obtain clinical specimens requested by the public health department and arrange to have them transported to a designated public health laboratory. These will probably consist of a nasopharyngeal swab, nasal swab, throat swab, and an acute serum specimen (for comparison to a convalescent specimen 2 to 3 weeks later).
- Evaluate alternative diagnoses. Remember that a novel influenza infection can co-infect with a more common organism. Discontinue isolation and antiviral therapy prematurely only if an alternative diagnosis is confirmed with a high-predictive value test, the clinical course is explained by the alternative diagnosis, and the epidemiologic link to the novel influenza strain is not strong.
- Start antiviral treatment.
- Assist the public health department in locating potentially exposed contacts and providing antiviral prophylaxis if recommended.
Clinical guidelines: Pandemic period
During the pandemic period, managing suspected infection differs from the pandemic alert period in several respects.
- Suspected cases need only meet the clinical criteria: fever with sore throat, cough, or dyspnea. These criteria may be modified as the pandemic evolves.
- Hospitalize only those patients with severe complications who cannot be cared for at home.
- Submit clinical specimens to the designated lab only as requested by the public health department. Such monitoring will probably be needed only for a subset of patients to watch the epidemiology of the epidemic or to investigate unusual presentations or failures of preventive therapy.
- Report atypical cases, prophylaxis failures, and other abnormal cases to the public health department.
Pre-pandemic planning
If and when another influenza pandemic will occur is difficult to predict. To be prepared, follow sound public health practices: adhere to office infection control practices, insure that patients and staff are current on all immunizations—influenza and pneumococcal vaccines can probably limit the complications from a novel influenza pandemic—maintain a line of communication with the local public health department, report communicable diseases and suspicious presentations to the public health department, and participate in local emergency planning.
Family physicians who serve in leadership positions in hospitals and other health care facilities can also promote planning for a possible pandemic at these facilities, including how to manage a surge of critically ill patients.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The uninsured: You can make a difference
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
Family physicians have always done what they can to care for those whose means of obtaining health care are inadequate. Such generosity has enriched, and literally saved, many persons’ lives. Sadly, though, the number of US citizens unable to pay for health care is increasing, and individual physician goodwill will not solve the problem. A comprehensive solution is needed, and that depends on restoring health to the currently paralyzed national political process.
If you are inclined to become more involved in pursuing efforts to address the issue in political, social, and educational spheres, you will find help in this article, which summarizes the problem and reviews health policy options being considered and their implications for our patients. You will also find resources through which you can make your voice heard.
Scope of the problem
The US is the only country in the developed world without a national program for health insurance for all its citizens. Though we spend over $1.7 trillion on health care, the number of uninsured people in 2004 was about 46 million, almost 16% of the population. In 2000, 40 million people (14%) were uninsured.1 The premise of consumer-driven care is that patients who pay more of the true cost of their care will become more prudent purchasers of health services.
Critics argue that increasing out-of-pocket costs (deductibles and co-pays) acts as a deterrent to seeking appropriate care, particularly for the less well off; that wealthy and healthy people will purchase these policies, thus increasing the costs of caring for those left in the traditional insurance system; and that such a system preserves the inefficient and wasteful private insurance industry.
Government as the single payer, not employer, of health care. Proponents argue that administrative cost savings in such a system (no or minimal private health insurance) will more than cover the cost of care for the uninsured.
Web resources for information on the uninsured and other health policy topics:
Kaiser Family Foundation: www.kff.org
Cover the Uninsured campaign: www.covertheuninsured.org
Health policy for students and faculty: www.KaiserEdu.org
Physicians for a National Health Plan (single-payer advocates): www.pnhp.org
Opponents of this idea argue it will lead to long lines for specialty care (read: Britain and Canada) and stifle innovation. Considering the US spends almost 50% more of its GDP on health care than does Canada and almost double what Britain spends, it seems unlikely our experience will parallel those countries. Ironically, in a system such as Canada’s, where most care is delivered by private practice physicians, there is almost certainly less administrative burden on physicians than they currently experience in the US.
A system of health care vouchers as recently proposed by Emanuel and Fuchs. Their plan would preserve the private insurance system, phase out Medicare, Medicaid, and employer-based insurance, include Federal oversight of the benefit package and technology assessment, and be funded by an earmarked value-added tax. It attempts to achieve administrative savings and a more equitable system of care, while not challenging the current substantial role of the private insurance sector. Pay-for-performance: What can you expect?”, J Fam Pract 2005; 54[7]:609) exemplify this effort. Certainly, efforts at improving care quality are worthwhile as our health system has too much practice variability as well as over- and underutilization of care. However, it is difficult to believe that such efforts will control costs enough to allow for the expansion of health insurance rates.
Where to go from here
Given past failures at expanding health care to all, many observers are resigned to the incrementalist approach such as expanding small business insurance by pooling risk, perhaps controlling costs through consumer-driven care and pay-for-performance programs, and dealing more effectively with malpractice problems (high premiums and defensive medicine). Most would agree, however, that such efforts will not make a serious dent in the uninsured problem.
The continual increase in the number of uninsured in a country as wealthy as ours is a national tragedy and is tremendously frustrating, particularly for family physicians who constantly see the negative health effects. Physicians need to become better informed about health policy and its implications for our patients and more actively work in political, social, and educational spheres to help move the political process out of its current paralysis to address the uninsured problem.
For information on the uninsured and other health policy topics, and for adding your voice to the debate, see Web resources.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
1. Finkelstein J. 45.8 million now uninsured. AMA News, September 19 2005;1-2.
2. Rhoades J. The uninsured in America, 2004. Statistical Brief #83. Available at: www.meps.ahrq.gov/papers/st83/stat83.pdf.
3. Hoffman C, Wang M. Health Insurance Coverage in America. 2002 Update. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2002.
4. Finkelstein J. Many workers lack insurance, report shows. AMA News, May 16, 2005;5.-
5. Gabel J, Claxton G, Gil I, et al. Health benefits in 2004: four years of double-digit premium increases take their toll on coverage. Health Aff (Millwood) 2004;23:200-209.
6. Institute of Medicine. Care without Coverage: Too Little, Too Late. Washington, DC: National Academies Press; 2002.
7. Himmelstein D, Warren E, Thorn D, Woolhandler Sl. Health Aff (Millwood) 2005;W5 63-73.Feb 2, 2005.
8. Paying a premium: The added cost of care for the uninsured. A Report from Families USA. June 2005. Available at: www.familiesusa.org/site/PageServer?pagename=Paing_a_Premium_Findings. Accessed on October 5, 2005.
9. Mongan JJ, Lee TH. Do we really want broad access to health care? N Engl J Med 2005;352:1260-1263.
10. Emanuel EJ, Fuchs VR. Health care vouchers—a proposal for universal coverage. N Engl J Med 2005;352:1255-1260.
When, and when not, to use the interferon-gamma TB blood test
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Consider the following cases of patients needing testing for tuberculosis:
- A 55-year-old nurse returns to work after 10 years. Her pre-employment evaluation requires a test for tuberculosis.
- A 35-year-old woman has lupus. Her physician is considering placing her on prednisone and wants to know if she has latent TB infection.
- A homeless man presents to the clinic stating he has lost his TB medications that he has taken or the past 2 months. His diagnosis of TB was made in another city; he cannot remember its name. The physician wants to confirm the diagnosis of TB.
- A 5-year-old from Mexico with a history of BCG vaccination presents for a preschool health evaluation.
- A 35-year-old immigrant from Africa is pregnant and presents for prenatal care.
Patients 1 and 2 above are logical candidates for the newer interferon-gamma blood test to detect tuberculosis (TB). Patients 3, 4, and 5 are not; They should be evaluated with the conventional TB skin test.
The advantages and disadvantages of both kinds of testing are described in this review.
Is a better test at hand?
The TB skin test, using an intradermal injection of purified protein derivative, has been used to assist in the detection of active and latent TB for more than a century. However, this test has its problems: difficulty in accurately measuring and interpreting the reaction; low sensitivity in those with depressed cell-mediated immunity and those with early infections; lower specificity in those with a history of bacille Calmette-Guérin (BCG) vaccination and infection with other Mycobacteria; the need for 2 visits with-in 48 to 72 hours for test interpretation; and boosting of immune response caused by the TB skin test itself (see Boosting phenomena).
Those who work in TB control programs have sought better diagnostic tools. Two that are available today are the interferon-gamma blood tests QuantiFERON-TB (QFT) and QuantiFERON-TB GOLD (QFTG). The QFT and QFTG measure the release of interferon-gamma by sensitized lymphocytes when exposed to antigens of Mycobacterium tuberculosis.
The QFTG, licensed in 2004, is an improvement over the earlier QFT, licensed in 2001, which included antigens that M tuberculosis shares with other commonly encountered Mycobacteria. The QFTG is more specific to M tuberculosis, although it does cross-react with several relatively uncommon nontuberculous Mycobacteria. These tests have several advantages over the older skin test (TABLE 1).
In those who have had prior Mycobacteria infections or BCG vaccine, cell-mediated immunity can wane and a TB skin test can therefore be negative. However, the skin test can boost the person’s immunity, and a second skin test can then be positive as a result of the immune boost. This can cause someone who is actually a reactor (someone who reacts positively to a skin test because of prior Mycobacteria infection) to look like a converter (someone who has a negative skin test at a recent point in time followed by a positive skin test, indicated recent infection with Mycobacteria). It is recommended that adults who have not had a skin test within 12 months and who will need repeated skin tests should receive a 2-step skin test on initial evaluation. A 2-step skin test involves an intitial test followed by a second one 1–2 weeks later.
TABLE 1
Advantages/disadvantages of the QFTG
| Advantages of QFTG compared with the TB skin test |
|
| Disadvantages of QFTG |
|
Factors to keep in mind when considering the QFT
In spite of the theoretical advantages of the QFT, research on its use is at an early stage. It can be considered a testing option for persons identified in TABLE 2. It may ultimately prove to be the test of choice for patients who have previously received a BCG vaccine, and in other instances where specificity is the predominant consideration. However, the Centers for Disease Control and Prevention (CDC) does not recommend it over a TB skin test in any situation.
Whether a skin test or QFTG is used, testing is recommended only for those at high risk of having latent TB infection and for those at high risk of developing active TB disease, if infected. Targeted testing along with treatment of active and latent TB remains the basis of TB control activities in the US.
When QFTG is unwarranted. The QFTG appears to be less sensitive than a TB skin test in those with symptoms of active TB, with the exception of those who are HIV-positive.1 In addition, the QFTG is not recommended for use with patients who are being treated for active TB. Current information is inadequate to recommend any QFTG use in children and pregnant women.
Gray areas. While there is some indication that QFTG is more sensitive for detecting TB infection in those exposed to an infectious patient, it is unknown whether it will predict as well as a skin test which patients are at risk of developing active disease.2 Therefore, it is not clear at this time if all those who have a positive QFTG should be considered candidates for treatment of latent TB infection, or if this should be offered only to those who have both a positive QFTG and TB skin test.
Level of risk influences interpretation. The current CDC recommendations, which have not been updated since QFTG was licensed, state that low-risk patients with a positive QFT and negative TB skin test should not receive treatment for latent infection.3 However, clinical judgment and perceived risk should be the basis for deciding on treatment in those at increased risk who have a positive QFT and negative TB skin test.
It is also not clear what effect a recent TB skin test has on QFTG results, and performing a QFTG soon after a TB skin test is not recommended by the US Food and Drug Administration or the manufacturer.
How to interpret QFTG results. If the QFTG result is positive, the patient needs clinical evaluation and a chest x-ray to rule out active disease. The diagnosis and treatment of active and latent TB has been covered in a previous Practice Alert.4 If the QFTG result is negative, no further evaluation is indicated unless symptoms of TB exist. The QFTG can have an indeterminate result, in which case a skin test can be useful.
Weighing the cost. The cost of a QFTG (about $80–$100 per test) needs to be compared with cost of staff time to read and interpret a skin test and to follow up with patients who fail to return for a skin test measurement.
TABLE 2
Recommendations for use of the QFT
| SITUATIONS WHERE QFT IS RECOMMENDED AS A POSSIBLE DIAGNOSTIC TOOL |
| Persons at increased risk for latent TB infection |
|
| Persons at increased risk of TB infection if exposed |
|
| Persons with conditions that cause increased risk of TB disease if infected, including those with: |
|
| Persons at low risk of TB infection who require initial or periodic testing |
|
| SITUATIONS WHERE QFT IS NOT CURRENTLY RECOMMENDED |
|
| SITUATIONS WHERE QFT IS PROMISING BUT FUTURE VALUE IS UNCERTAIN |
|
Conclusion
The QFTG test is relatively new; as more evidence becomes available, its place among the tools available for the diagnosis of latent and active TB will clarify. Check with your state and local public health departments to find out the situations for which they are recommending this new diagnostic tool, as practice varies across the country.
CORRESPONDING AUTHOR
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
1. Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis 2004;4:761-776.
2. Richeldi L, Ewer K, Losi M, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med 2004;170:288-295.
3. Centers for Disease Control and Prevention. Guidelines for using QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep 2003;52(RR-2):15-18.
4. Campos-Outcalt D. Tuberculosis: old problem, new concerns. J Fam Pract 2003;52:792-798.
Pertussis: A disease re-emerges
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The incidence of pertussis in the United States declined dramatically after the introduction of pertussis vaccine in the 1940s. Before that, an average of 160,000 cases of pertussis (150/100,000 population) occurred each year, resulting in 5000 deaths. FIGURE 1 shows how pertussis incidence declined steadily through 3 decades to reach a low of 1010 cases in 1976.
While other vaccine-preventable diseases such as polio, measles, rubella, diphtheria, and tetanus have been eradicated or have declined to only a few cases each year, pertussis has made a slight comeback. The number of cases began increasing in the 1980s and reached a level of 7000 to 8000 cases annually between 1996 and 2000 (see insert in FIGURE 1). There were 11,647 cases in 2003.
While these numbers are small compared with all cases that occurred in the prevaccine era, the increase is cause for public health concern.
FIGURE 1
Number of reported pertussis cases, by year—1922–2000
Unique features of this rebound
The recent rise of pertussis displays several notable trends:
- Disease incidence now ebbs and flows in 3- to 4-year cycles
- The proportion of cases occurring among adolescents and young adults has increased. TABLE 1 shows the age breakdown of reported pertussis cases for 2001. Infants still account for the highest proportion of cases (29%) and the highest attack rates (55 cases per 100,000); but half of reported cases now appear in those age 10 years and older
- Nonimmunized or incompletely immunized infants are usually exposed to the disease by older household members, and not by same-age cohorts
- Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed. The incidence is probably much higher than the reported number of cases would indicate.
TABLE 1
Pertussis-related hospitalizations, complications, and deaths, by age group—United States, 1997–2000
| AGE GROUP | NO. WITH PERTUSSIS | HOSITALIZED NO. (%) | COMPLICATIONS | DEATHS NO. (%) | ||
|---|---|---|---|---|---|---|
| PNEUMONIA* NO. (%) | SEIZURES NO. (%) | ENCEPHALOPTHY NO. (%) | ||||
| < 6 mo | 7203 | 4543 (63.1) | 847 (11.8) | 103 (1.4) | 15 (0.2) | 56 (0.8) |
| 6–11 mo | 1073 | 301 (28.1) | 92 (8.6) | 7 (0.7) | 1 (0.1) | 1 (0.1) |
| 1–4 years | 3137 | 324 (10.3) | 168 (5.4) | 36 (1.2) | 3 (0.1) | 1 (<0.1) |
| 5–9 years | 2756 | 86 (3.1) | 68 (2.5) | 13 (0.5) | 0 | 2 (0.1) |
| 10–19 years | 8273 | 174 (2.1) | 155 (1.9) | 25 (0.3) | 4 (0.1) | 0 |
| ≥ 20 years | 5745 | 202 (3.5) | 147 (2.6) | 32 (0.6) | 3 (0.1) | 2 (<0.1) |
| Total | 28,187† | 5630 (20.0) | 1477 (5.2) | 216‡ (0.8) | 26 (0.1) | 62 (0.2) |
| *Radiographicaly confirmed. | ||||||
| †Excludes 92 (0.3%) persons of unknown age with pertussis. | ||||||
| ‡Excludes one person of unknown age with seizures. | ||||||
| Source: Pertussis—United States, 1997–2000. MMWR Morb Mortal Wkly Rpt 2002; 51:73–76. | ||||||
Why the increase in cases?
Several possible causes could account for the increased incidence in reported pertussis. For one, the efficacy of the vaccine wanes with time after vaccination. Adolescents and young adults are left susceptible because, until recently, no vaccine has been available for persons after their 7th birthday. This, however, has been true for decades and does not explain recent increases.
The Bordetella pertussis bacteria may have genetically drifted to become less susceptible to vaccine-induced antibodies, or the apparent increase in cases could actually be just an increase in case detection.
It is also possible that the recent emphasis on avoiding unnecessary antibiotic use for respiratory infections has had the unanticipated consequence of decreasing previously fortuitous treatment of undiagnosed pertussis among older age groups.
New tool in fight against pertussis now available
Two products with tetanus toxoid—reduced diphtheria toxoid and acellular pertussis vaccines adsorbed (Tdap)—have been licensed for active booster immunization against tetanus, diphtheria, and pertussis as a single dose. BOOSTRIX (GlaxoSmithKline) is for persons aged 10 to 18 years, and ADACEL (Sanofi Pasteur) is for those aged 11 to 64 years.
The Advisory Committee on Immunization Practice of the Centers for Disease Control and Prevention (CDC) has recommended Tdap for universal use among adolescents aged 11 to 12 years, and may in the future recommend its use periodically for all adults.
Rethink your approach to older patients
Clinical presentation of pertussis in an adolescent and adult is nonspecific. After an incubation period of 1 to 3 weeks, pertussis infection appears as a mild respiratory infection or the common cold.
After 1 to 2 weeks, the nonproductive cough can evolve into paroxysms of severe coughing (causing apnea), a posttussive inspiratory whoop, and vomiting. The inspiratory whoop and apnea are usually absent in previously immunized adults and adolescents. The cough gradually diminishes but can persist for up to 3 months.
Suspect pertussis in any adolescent or adult who has had a cough for 2 weeks or longer, even if the paroxysms and post tussive symptoms are absent.
Infants exhibit more severe symptoms and suffer higher rates of complications, including severe apnea, hospitalization, seizures, secondary pneumonia, and death (TABLE 1).
Required laboratory confirmation
For any patient with suspected pertussis, obtain an aspirate of the posterior nasopharyngeal region or swab it for culture. The specimen can be collected by inserting a Dacron nasopharyngeal swab into the nostril to reach the posterior nares and leaving it in place for 10 seconds (FIGURE 2). If the specimen cannot be streaked onto a special enriched culture medium, place it in a special transport medium and refrigerate it until sent to the laboratory. Local or state health departments can often assist with obtaining the transport medium.
Polymerase chain reaction can be used to make a presumptive diagnosis but should be followed by culture confirmation. Direct fluorescent antibody testing of nasopharyngeal specimens and serologic testing for antibodies are not currently recommended as diagnostic tests by the CDC.
FIGURE 2
To collect a Bordella pertussis specimen, a nasopharyngeal swab should be performed in both nares. Insert the swab gently through the nostril toward the posterior nasopharynx; leave it there 15 to 30 seconds, then rotate it and remove. The sample should be put in an appropriate transport medium or immediately onto agar. (Choose shipping conditions based on how long the sample will be in transit; the laboratory will provide specific swab and transport medium requirements). Cotton swabs are not recommended because cotton is harmful to B pertussis. Cultures are usually incubated 10 to 14 days, but results are often available in 7 to 10 days.
Four antibiotics available for treatment of pertussis
The CDC’s recommended medications, doses, and duration of treatment are listed in TABLE 2.
Treatment, if started within the first 2 to 3 weeks, can reduce symptoms and clear B pertussis from the nasopharynx, thus reducing transmission to others. The CDC recommends initiating treatment as soon as pertussis is suspected.
Erythromycin is the agent with the longest history of use and most evidence for effectiveness. Due to its duration of therapy, however, and to the incidence of gastrointestinal side effects, it is less attractive to patients and physicians than other options.
New outcome studies of other macrolides are promising. Most authorities believe azithromycin and clarithromycin are acceptable alternatives and should achieve the same results as erythromycin.
Trimethoprim-sulfamethoxazole can be used for those older than 2 months, for those allergic to, or intolerant of, macrolides. The dose for children is 8 mg/40 mg per day in 2 divided doses for 14 days; for adults it is 320 mg/1200 mg per day in 2 divided doses for 14 days.
TABLE 2
Antibiotic treatment and chemoprophylaxis for pertussis
| AGE | AZITHROMYCIN (5-DAY COURSE) | ERYTHROMYCIN (14-DAY COURSE) | CLARITHROMYCIN (7-DAY COURSE) |
|---|---|---|---|
| <1 month | Recommended in this age group. 10 mg/kg/d in a single dose for 5 days | Not preferred due to association with pyloric stenosis. If used dose is 40–50 mg/kg/d divided in 4 doses for 14 days | Not recommended |
| 1–5 months | 10 mg/kg/d in a single dose for 5 days | 40–50 mg/kg/day divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| ≥ 6 months | 10 mg/kg/d in a single dose on day 1 then 5 mg/kg/d on days 2–5 | 40–50 mg/kg/d divided in 4 doses for 14 days | 15 mg/kg/d in 2 divided doses (maximum 500 mg/dose for 7 days) |
| Adults | 500 mg single dose on day 1 then 250 mg on days 2–5 | 2 g/d in 4 divided doses for 14 days | 500 mg twice a day for 7 days |
TABLE 3
Important public health functions for physicians in control of pertussis
| Be aware of the local infectious disease epidemiology and know when pertussis is circulating and increasing. |
| Think of the potential for pertussis in adults with a cough of two weeks duration or greater. |
| Collect nasopharyngeal specimens for culture on all patients with suspected pertussis. |
| Begin treatment when pertussis is suspected. |
| Report suspect and confirmed pertussis to the local public health department. |
| When pertussis is highly suspected or confirmed, begin chemoprophylaxis for family members. |
| Insure that respiratory hygiene is practiced in the clinic waiting areas. |
| Implement systems to insure that all patients are vaccinated according to CDC recommendations. |
| Insure that all clinic staff who have been exposed are given chemoprophylaxis and that symptomatic staff are excluded from work until after 5 days of treatment or until 21 days after cough onset if treatment is refused. |
| Collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected. |
Preventing infection in family members and close contacts
Those with pertussis are most infectious in the first 3 weeks of symptoms. The CDC recommends initiating chemoprophylaxis for all household and close contacts of those in whom pertussis is highly suspected or confirmed, regardless of the contacts’ age and vaccination status.
Close contacts are those with direct face-to-face contact with a symptomatic patient, or those who share a confined space for a prolonged period with the patient. Chemoprophylaxis is especially important for high-risk contacts: those under 1 year of age and those with chronic conditions that make them susceptible to complications from pertussis (eg, immune deficiencies, chronic lung disease, or cystic fibrosis).
Chemoprophylaxis has limited benefit if started beyond 3 weeks after exposure. The same antibiotics, doses, and treatment durations are recommended for chemoprophylaxis as for treatment.
Completion of a 4-dose series of pertussis-containing vaccine is also recommended for close contacts. This recommendation has historically pertained only to those before their seventh birthday. With the licensure of Tdap for adolescents and adults, this recommendation may soon include contacts through age 18 and could be expanded to include adults through age 64 in the near future.
Preventing the spread of pertussis in your community
Schools, day care centers, and health care facilities are all potential foci of spread of infection. During outbreaks the local public health department may implement guidelines at schools and day care centers that refer symptomatic staff and students to a physician for evaluation.
If you examine such a patient, perform a nasopharyngeal culture and initiate treatment for those who are symptomatic and for all high-risk contacts. Symptomatic persons should not attend school until either pertussis is ruled out or they have completed 5 days of antibiotic therapy, regardless of their vaccination history. If they refuse treatment, they should be barred from attending school for 21 days from onset of cough.
In health care settings, staff should receive chemoprophylaxis if they have had close exposure to a person with confirmed pertussis, or have had contact with nasal, respiratory, or oral secretions of such a person. Staff members who refuse chemoprophylaxis should be closely observed for symptoms of pertussis; if they become symptomatic, they should be treated and allowed to return to work after 5 days of treatment.
Take-home message
Awareness of local infectious disease epidemiology and knowing when pertussis is circulating and increasing will ensure that you serve the most valuable public health role possible.
Consider pertussis when an adolescent or adult has had a cough for 2 weeks or longer, and collect nasopharyngeal specimens for culture on all patients with suspected pertussis.
Initiate treatment when pertussis is suspected, report suspected and confirmed pertussis to the local public health department, and begin chemoprophylaxis for family members and contacts as indicated.
Implement systems that insure all patients are vaccinated according to CDC recommendations. Institute policies and procedures to insure that respiratory hygiene is practiced in the clinic waiting areas and that staff practice infectious disease precautions and are managed appropriately if they are exposed.
Finally, collaborate with schools and local public health departments to evaluate symptomatic close contacts from schools and day care centers with outbreaks. This includes taking nasopharyngeal specimens for culture and initiating treatment if pertussis is suspected.
Some of these procedures may become unnecessary in the future if the new pertussis vaccine products for adolescents and adults are successful in turning pertussis into another member of an expanding list of rarely encountered, vaccine-preventable diseases.
Corresponding Author
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
Pay-for-performance: What can you expect?
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
Pay-for-performance programs (P4P) are spreading. Medicare has committed to a national P4P demonstration project, a large employer group has initiated its own program, and the American Medical Association (AMA) has published principles it will use to assess such programs. The American Academy of Family Physicians (AAFP) published its own criteria last year. What are the characteristics of P4P programs, private and public examples, and benefits and risks of their use?
How does it work?
Pay-for-performance refers to financial-incentive programs that pay bonuses to participants (physicians, physician groups, health plans, or hospitals) that make progress, or attain specific benchmarks, in quality and efficiency. Alternatively, P4P programs may create different tiers of providers based on quality standards, and then give patients financial incentives (such as lower co-payments) to use one tier instead of another. This latter mechanism is currently the subject of a nasty argument between the Barnes Jewish health system in St. Louis and United Healthcare.
Goals may be clinical or nonclinical. Clinical goals usually measure processes of care (eg, measurement of hemoglobin A1C and lipids in persons with diabetes, use of beta-blockers and aspirin after myocardial infarction, anti-inflammatory medications for chronic asthma, or appropriate cancer screening). However, of late there has been movement toward using intermediate out-come measures, such as control of hypertension and blood sugar, and long-term outcomes such as mortality, morbidity, and quality of life. Nonclinical goals include implementing such information technology as electronic health records, or improving access to care and patient satisfaction.
How prevalent is P4P? A national survey conducted by Med-Vantage, a health informatics company, in November 2004, identified 84 programs—covering 39 million beneficiaries—that had some P4P characteristics.
They found P4P programs expanding from primary care providers to specialist involvement, from HMOs to PPOs, and from annual bonuses to tiered fee schedules. They also reported an emphasis on using the National Commission for Quality Assurance (NCQA) measures as performance goals, rewarding information technology adoption, and increasing involvement of the Center for Medicare and Medicaid Services (CMS).
P4P programs surveyed reported quality improvement as the #1 reason for their programs, validity of the data as their #1 concern, and early provider involvement and use of standardized measures as the main recommendations for new programs.1
National programs and how they might affect you
MedPAC and providers stress information technology. The Medicare Payment Advisory Commission (MedPAC), which makes recommendations on provider payments to CMS, announced in its 2005 annual report that Medicare should begin paying all physicians differently based on how they perform. MedPAC envisions rewarding the use of information technology such as electronic health records first, and later adding measures for quality outcome.2
Almost simultaneously with this recommendation, CMS announced that 10 large physician group practices would participate in a new P4P Medicare demonstration project. These practices hope to improve quality and lower Medicare costs (by focusing on disease management strategies and information technology), and in return, CMS will return a portion of the savings to them. Initially, CMS will base the majority of bonus payments on financial savings rather than quality improvement; this has led to concern that costs are the primary driver of the program.3
Premier Hospital Quality Incentive focusing on 5 clinical areas. CMS also sponsors the Premier Hospital Quality Incentive Demonstration, a P4P program that tracks performance for 5 common clinical conditions at 270 participating hospitals. The program rewards high performers from a bonus pool of $7 million per year over a 3-year period. In May 2005, Mark McClellan, MD, PhD, the director of CMS, announced improvement in all 5 areas (acute myocardial infarction care, coronary artery bypass graft surgery, care for congestive heart failure, hip and knee replacement surgery, and pneumonia care) in the first year of the project.4
Bridge to Excellence encourages more patient involvement. A national private sector response to the P4P movement has been Bridge to Excellence (BTE), a nonprofit organization whose board represents employers, providers, and health plans (emphasis on the employers) with major funding from large companies. It was created in response to the Institute of Medicine’s 2001 report, Crossing the Quality Chasm, which included a recommendation to redesign the way providers are paid to encourage quality improvement (TABLE 1).
TABLE 1
Bridges to Excellence key principles
|
BTE has developed several P4P programs in cooperation with the NCQA. Physician Office Link pays physician’s offices up to $50 per year for each patient covered by a participating employer or plan. NCQA criteria include the use of clinical information systems, education to promote patient self-management, a quality improvement system, and programs to care for patients with chronic disease.
Diabetes Care Link rewards physicians who meet NCQA standards for its Diabetes Physician Recognition program with up to $80 for each patient with diabetes covered by the employer or health plan sponsor.
Cardiac Care Link rewards physicians who qualify for NCQA’s Heart/Stroke Recognition Program with up to $160 for each covered patient with cardiac disease. Physicians must submit data on blood pressure, lipid testing, antithrombotic use, and smoking cessation. Physicians qualify for the bonus based on a combination of process measures (performing tests/screenings) and outcome measures (eg, appropriate LDL level, aspirin use).5 The program started with about a dozen employers in just a few areas (Cincinnati, Massachusetts, and upstate New York). In March 2005, BTE announced that coalitions in 3 additional states (Illinois, Colorado, and Arkansas) are working with employers to license and launch BTE-related incentive projects later this year.6
NCQA. As the leader in accrediting managed care organizations, the nonprofit NCQA is often thought of as the expert in developing reliable performance measures. For almost 15 years, the NCQA has been refining its Health Plan Employer Data and Information Set (HEDIS) as a means for evaluating health plans. Many physicians have had their care reviewed as part of having contracts with managed care companies that apply for NCQA accreditation. The NCQA’s longstanding commitment to the development of reliable performance measures and the involvement of multiple health system stakeholders in its work has given them a great deal of national credibility.
Concerns
While embracing the quality improvement movement, major physician organizations have been cautious in their support of current P4P programs. Both the AAFP and AMA guidelines emphasize the need to focus on quality rather than cost reduction, involve physicians in program design, use evidence-based and statistically valid performance measures, reward both performance improvement and attainment of predetermined targets, and use new money for incentive payments rather than reducing existing payments to physicians (TABLE 2).7,8
TABLE 2
AMA principles to evaluate P4P programs
|
Benefits and risks
The hope is that P4P will change physician and systems behavior to improve quality and patient safety. It may be that such changes will also reduce costs, although it is certainly true that additional resources will be needed initially to help implement the technology expected to make such improvement more likely. Proponents hope that incentive payments and improved information systems will also lead to improved population management: caring for an entire practice, not just the patient who comes to the office. Disease registries and electronic health records are envisioned as 2 of the keys to making this happen.9
Why success will not be easy. One hurdle will be the difficulty of providing sufficient incentives to individual physicians or small group practices that deal with numerous insurers. One company’s P4P program may not matter much to a physician who cares for only a small number of that company’s patients. Groups of health plans and purchasers will need to cooperate in developing a common set of measures and incentives to use in a P4P program. Such cooperation will not be easy to accomplish.
Another challenge will be to identify the right number and type of measures to engage providers and actually improve care.
Then there are the financing issues—will additional money be made available for positive incentives, or will there be a revenue-neutral system in which some providers get more money while others less? This is an issue that greatly concerns the AAFP and AMA.
Finally, how will P4P affect physicians who care for the underserved and socioeconomically disadvantaged. These patients are often more difficult to care for than those with adequate healthcare coverage, and often require more intensive use of resources—all of which may limit the ability of their providers to qualify for incentive payments through P4P programs. This could lead to the unintended consequence of physicians reducing the number of underserved patients they care for.
One way to address this problem would be to improve risk-adjustment methods to compensate for the increased difficulty in providing high-quality care to certain kinds of patients. Development of such methods will likely be difficult to implement. Another way would be to evaluate providers based on their quality improvement over time rather than establishing minimum targets that have to be met to qualify for any incentive payments.
P4P is likely to expand
The recent entrance of CMS into P4P programs as well as the interest coming from large employers makes it likely that P4P will continue to expand. While paying more for higher-quality care makes sense and should save money in the long run, the constraint on resources currently available from the government and private insurers to reward higher performers as well as fund improvements necessary to ensure better care make it probable that there will be increased tension between P4P as a quality-improvement vs a cost-savings effort.
CORRESPONDING AUTHOR
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
1. Med-Vantage, Inc. Provider Pay-for-performance incentive programs: 2004 national study results. Available at: www.medvantageinc.com/Pdf/MV_2004_P4P_National_ Study_Results-Exec_Summary.pdf. Accessed on May 25, 2005.
2. Glendinning D. AMA: Medicare pay-for-performance must be voluntary and not punitive. AMNews, March 21, 2005.
3. Glendinning D. Medicare tests pay-for-performance. AMNews, February 21, 2005.
4. Centers for Medicare and Medicare Services. Medicare pay-for-performance demonstration shows significant quality of care improvement at participating hospitals. Medicare news release, May 3, 2005. Available at: www.cms.hhs.gov/media/press/release.asp?Counter= 1441. Accessed on May 25, 2005.
5. National Committee for Quality Assurance. Bridges to excellence. Available at: www.ncqa.org/Programs/bridgestoexcellence/index.htm. Accessed on May 25, 2005.
6. National Committee for Quality Assurance. Physicians, business, government and industry embrace common strategy to improve health care: pay-for-performance. Available at: www.ncqa.org/Communications/News/BTE_P4P2005.htm. Accessed on May 25, 2005.
7. American Academy of Family Physicians. Pay-for-per-formance. Available at: http://www.aafp.org/x30307.xml. Accessed on May 25, 2005.
8. American Medical Association. Quality, fairness must be paramount in pay-for-performance. Available at: www.ama-assn.org/ama/pub/category/14774.html. Accessed on May 25, 2005.
9. Epstein A, Lee T, Hamel M. Paying physicians for high-quality care. N Engl J Med 2004;350:406-410.
US Preventive Services Task Force: The gold standard of evidence-based prevention
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The United States Preventive Services Task Force (USPSTF) was first formed in 1984 to assist physicians in making decisions about which preventive services to offer patients. It consists of a 15-member panel of independent scientists picked for their expertise in primary care, clinical prevention, and evidence-based methodology. The first set of recommendations was published in 1989 as the Guide to Clinical Preventive Services, and was revised in 1996 in the second edition. Recommendations are now published on the USPSTF web site (www.ahrq.gov/ clinic/uspstfix.htm).
The USPSTF uses an explicit set of steps and criteria to judge the effectiveness, harms, costs and benefits of preventive interventions: screening, counseling, and chemoprevention. Topics are suggested by outside partners, including the American Academy of Family Physicians, and are then sent to one of 13 evidence-based practice centers, where an extensive review is conducted of the current scientific literature on the topic. The evidence report is then reviewed by the 15-member USPSTF and a recommendation is made using the rating system described in TABLE 1. The current members of the USPSTF arefound at www.ahrq.gov/clinic/uspstfab.htm# Members. The staff for the task force is provided by the Agency for Health Care Quality and Research (AHRQ), one of the agencies in the Public Health Service of the US Department of Health and Human Services.
In addition to listing the recommendations and the rationales behind them, the USPSTF web site also provides the evidence report and a description of recommendations on that topic made by other organizations, with a discussion of clinical implications of the recommendation. During 2004, the USPSTF made or updated 29 recommendations ( TABLE 2 ). There were 5 A recommendations, 4 B recommendations, no C recommendations, 11 recommendations against an intervention (D recommendation), and 9 instances of insufficient evidence to make a recommendation.
TABLE 1
Standard recommendation language, USPSTF
| RECOMMENDATION: |
| A Language: The USPSTF strongly recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found good evidence that [the service] improves important health outcomes and concludes that benefits substantially outweigh harms.) |
| RECOMMENDATION: |
| B Language: The USPSTF recommends that clinicians routinely provide [the service] to eligible patients. (The USPSTF found at least fair evidence that [the service] improves important health outcomes and concludes that benefits outweigh harms.) |
| RECOMMENDATION: |
| C Language: The USPSTF makes no recommendation for or against routine provision of [the service]. (The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation.) |
| RECOMMENDATION: |
| D Language: The USPSTF recommends against routinely providing [the service] to asymptomatic patients. (The USPSTF found at least fair evidence that [the service] is ineffective or that harms outweigh benefits.) |
| RECOMMENDATION: |
| I Language: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing [the service]. (Evidence that [the service] is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined.) |
TABLE 2
USPSTF recommendations made in 2004
| A Recommendation (strongly recommends) |
|
| B Recommendation (recommends) |
|
| D Recommendation (recommends against) |
|
| I Recommendation (insufficient evidence) |
|
Recommendations for 2005
So far in 2005, new recommendations have been added on 3 topics: abdominal aortic aneurisms, glaucoma, and herpes simplex.
Abdominal aortic aneurisms. The recommendations on screening for abdominal aortic aneurisms are contained in TABLE 3. Of special note is the recommendation to screen (using abdominal ultrasound) men over the age of 65 years who have ever smoked.
Glaucoma. The statement that evidence is insufficient to recommend for or against routinely screening for glaucoma reflects the uncertainty about the contribution of screening to improved outcomes, as well as the documented harms of treating elevated intraocular pressure, such as local eye irritation and an increased risk for cataracts.
Herpes simplex. The task force recommends against screening for herpes in pregnant women and asymptomatic adults and adolescents because of a lack of improved outcomes and documented potential harms.
TABLE 3
USPSTF 2005 recommendations for screening for abdominal aortic aneurisms
| The USPSTF recommends one-time screening for abdominal aortic aneurysm (AAA) by ultrasonography in men aged 65 to 75 who have ever smoked. |
| RATING: B RECOMMENDATION |
| Rationale: The USPSTF found good evidence that screening for AAA and surgical repair of large AAAs (5.5 cm or more) in men aged 65 to 75 who have ever smoked (current and former smokers) leads to decreased AAA-specific mortality. There is good evidence that abdominal ultrasonography, performed in a setting with adequate quality assurance (ie, in an accredited facility with credentialed technologists), is an accurate screening test for AAA. There is also good evidence of important harms of screening and early treatment, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. Based on the moderate magnitude of net benefit, the USPSTF concluded that the benefits of screening for AAA in men aged 65 to 75 who have ever smoked outweigh the harms. |
| The USPSTF makes no recommendation for or against screening for AAA in men aged 65 to 75 who have never smoked. |
| RATING: C RECOMMENDATION. |
| Rationale: The USPSTF found good evidence that screening for AAA in men aged 65 to 75 who have never smoked leads to decreased AAA-specific mortality. There is, however, a lower prevalence of large AAAs in men who have never smoked compared with men who have ever smoked; thus, the potential benefit from screening men who have never smoked is small. There is good evidence that screening and early treatment leads to important harms, including an increased number of surgeries with associated clinically-significant morbidity and mortality, and short-term psychological harms. The USPSTF concluded that the balance between the benefits and harms of screening for AAA is too close to make a general recommendation in this population. |
| The USPSTF recommends against routine screening for AAA in women. |
| RATING: D RECOMMENDATION. |
| Rationale: Because of the low prevalence of large AAAs in women, the number of AAA-related deaths that can be prevented by screening this population is small. There is good evidence that screening and early treatment result in important harms, including an increased number of surgeries with associated morbidity and mortality, and psychological harms. The USPSTF concluded that the harms of screening women for AAA outweigh the benefits. |
USPSTF The Gold Standard
The USPSTF offers busy practicing physicians a valuable set of resources to assist in staying current on the ever changing field of clinical prevention and to guide clinical practice. Their recommendations often are at odds with common beliefs. But over time, their methodology and resulting recommendations have become the gold standard for evidence-based prevention practice.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected]
The growing threat of avian influenza
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
In the first half of 2004, an estimated 200 million poultry birds across Asia died or were destroyed in an effort to control a widespread outbreak of avian influenza.1 Though such outbreaks have occurred before, this one, primarily in China and Southeast Asia, demonstrated increased pathogenicity in poultry, increased resistance to environmental controls, and an expanded range of mammalian hosts.
Lest anyone conclude this is a problem affecting only the birds and the people who raise them, clinical and laboratory evidence since 1997 point to a series of human cases of avian flu (TABLE 1), most of which were associated with outbreaks of the disease in poultry.2 The change in characteristics of the current outbreak in birds in Asia combined with increased knowledge of the characteristics of human influenza have many scientists and public health officials increasingly concerned that we may be watching the unfolding of the next great flu pandemic. In this article, I describe how this might happen and what we can attempt to prevent it.
TABLE 1
Avian influenza infection in humans
| Hong Kong (1997): | 18 people hospitalized with 6 deaths |
| China and Hong Kong (1999): | 2 cases in children who recovered |
| Virginia (2002): | 1 person with serologic evidence of avian flu |
| China and Hong Kong (2003): | 2 adults cases with one death |
| Netherlands (2003): | 89 human cases with 1 death; most cases were of conjunctivitis, some with flu symptoms. The antiviral drug oseltamivir was used to help control spread |
| Canada (2004): | Human infections among poultry workers consisting of eye infections |
| Asia (since January 2004): | 55 cases with 42 deaths in Vietnam, Thailand, and Cambodia |
Human influenza pandemics
A flu pandemic is a global outbreak of disease among people after the emergence of a new influenza A virus. Three great pandemics occurred in the 20th century, all spreading worldwide within 1 year.
- Spanish flu (1918–1919): 20 to 50 million people died, more than 500,000 in the US. Nearly half of those dying were young, healthy adults.
- Asian flu (1957–1958): Caused about 70,000 deaths in the US.
- Hong Kong flu (1968–1969): Caused about 34,000 deaths in the US.
Both the Asian and Hong Kong flus resulted from a mixing of a human and avian influenza virus; the Spanish flu may have resulted from a mutation in a purely avian virus.3
How avian influenza viruses spread
Avian influenza A viruses vary greatly, owing to the myriad combinations of their 15 hemagglutinins and 9 neuraminidases. The viruses are widespread in migratory birds and waterfowl and are usually of low pathogenicity. Water birds, in particular, act as hosts for influenza viruses, carrying them in their intestines and then shedding them.
Wild bird hosts do not usually get sick, but they can spread influenza to other birds. For instance, there have been 16 outbreaks of H5 and H7 influenza in US poultry since 1997. Usually, such events are from low-pathogenic avian viruses that cause little illness in affected chickens. When highly pathogenic viruses cause outbreaks, 90% to 100% of affected poultry can die. This has been happening in the current Asian outbreaks.
In the past, isolating poultry, culling (destroying) infected flocks, and vaccinating poultry eventually quelled the outbreaks. Such measures have not worked this time, and scientists think it likely that H5N1 infection among birds has become endemic to the region—ie, Cambodia, China, Indonesia, Malaysia, Thailand, and Vietnam.4
Rethinking how humans become infected
Since humans have distinct receptors for human viruses, as do birds for avian viruses, it was thought that an intermediate host—the pig, with both receptors—was necessary to allow the mixing of the viruses and subsequent human infection with avian influenza. However, recent outbreaks in poultry with proven spread of highly pathogenic virus from chickens directly to humans have challenged this theory (TABLE 1). While the mortality rate so far has been very high, this rate may turn out to be overstated as milder, nonfatal cases of human avian flu are discovered.
New research has raised additional concerns: ducks infected with H5N1 are now shedding virus for longer times while remaining asymptomatic; pigs in China and tigers in Thailand have been infected with H5 virus; and experiments with house cats in the Netherlands have demonstrated they could be infected with H5. These findings are troubling because the reassortment of avian viruses is more likely to occur when they are able to infect multiple species. Moreover, the infection in humans from Vietnam has shown resistance to the older antiviral drugs, amantadine and rimantadine, leaving oseltamivir and zanamivir as the antivirals likely effective against avian influenza A H5N1.4
Fear of human-to-human transmission
Direct spread of avian influenza from poultry to humans resulting in a high fatality rate is of course a major concern. What worries scientists and public health officials more, however, is the increasing risk of person-to-person transmission as a result of a change in the viral genome. Genomic variation could occur if avian virus genetic material mixes with that of a human virus in an intermediate host such as the pig or in a patient infected simultaneously with both avian and human influenza strains; or it could occur with spontaneous mutation of an avian virus. A recent report provided strong evidence of avian influenza that spread from the index patient to her mother and aunt (both the 11-year old girl and her mother died). No further spread occurred, suggesting the infection resulted from a purely avian virus with no human virus involved.5
How effective will preventive measures be?
The current danger to people from avian influenza has been recognized sooner than the threats that preceded previous influenza pandemics, which burst upon the world with little warning. Lessons from the severe acute respiratory syndrome (SARS) epidemic in 2003, advances in science and public health surveillance, and cooperation among countries and organizations such as the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) have allowed us to observe the emergence of avian influenza in rural Asia and consider its wider implications. CDC’s response has focused on enhanced surveillance and laboratory testing for human and avian influenza in the US and Asia and work on vaccine development with WHO and the National Institutes of Health (TABLE 2). CDC has not recommended avoiding travel to any of the involved countries, but has released guidelines for travel to affected areas (TABLE 3).6
In the event of an avian flu outbreak in humans, there will be questions about the best public health response, the use of antiviral agents, and infection control in health care settings. The SARS outbreak was substantially controlled through the use of the traditional public health measures of isolation (separating ill or infected people from others) and quarantine (separating people exposed to infected people from others in order to prevent the further spread of the infection). Whether such measures would be successful with a highly contagious viral infection like influenza is debatable. Stohr of WHO has outlined a research agenda that includes study of hospital infection control practices, vaccine clinical immunogenicity, early interventions such as use of antivirals or vaccine to slow the spread of an emerging pandemic virus, the role of animal and bird species in influenza virus development, and risk assessment.7
TABLE 2
CDC response to avian influenza
|
TABLE 3
General precautions for travel to countries with avian influenza outbreaks*
|
| * As of February 4, 2005 this was directed at travelers to Vietnam only; see reference #6 for complete details. |
Awareness the best defense now
If avian influenza makes the leap to person-to-person transmission, family physicians across the globe will be at the forefront of diagnosis and treatment. Though no immediate actions are necessary, we all must follow developments and support the work of health departments at improving their ability to monitor emerging outbreaks. The interconnectedness of global health is well exemplified in the concern about avian flu and the efforts to prevent an influenza pandemic.
Correspondence
Eric A. Henley, MD, MPH, Department of Family and Community Medicine, University of Illinois College of Medicine at Rockford, 1601 Parkview Avenue, Rockford, IL 61107-1897. E-mail: [email protected]
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.
1. Altman L. UN agency to urge vaccinations to health avian flu. New York Times, July 30, 2004.
2. World Health Organization. Disease surveillance. Cumulative number of confirmed cases of human avian influenza. WHO Communicable. Available at: www.who.int/csr/disease/avian_influenza/country/cas es_table_2005_02_02/en/. Accessed on April 10. 2005.
3. Monto A. The threat of an avian influenza pandemic. New Engl J Med 2005;352:323-325.
4. Centers for Disease Control and Prevention (CDC) Avian Influenza home page. Available at: www.cdc.gov/flu/avian/index.htm. Accessed on April 10, 2005.
5. Ungchusak K, Auewarakul P, Dowell S, et al. Probable person-person transmission of avian influenza (H5N1). New Engl J Med 2005;352:333-340.
6. CDC Asian Influenza, Vietnam, Notice to Travelers. Updated February 4, 2005. Available at: www.cdc.gov/travel/other/avian_flu_vietnam_2005_tra velers.htm. Accessed on April 10, 2005.
7. Stohr K. Avian influenza and pandemics. New Engl J Med 2005;352:405-407.