User login
Meningococcal vaccine: New product, new recommendations
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
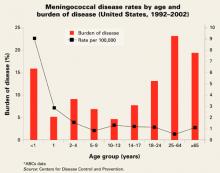
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
The Centers for Disease Control and Prevention (CDC) now recommends that all adolescents aged 11 to 12 years receive a quadrivalent, conjugate meningococcal vaccine (MCV-4). With time, universal administration of meningococcal vaccine for this age group will make moot the question of whether entering college freshmen should receive meningococcal vaccine. It should lead to a marked reduction in a potentially catastrophic disease and contribute to the continued decline in morbidity and mortality from bacterial meningitis in the United States, which has largely been due to advances in vaccine technology.
Some 1400 to 2800 cases of meningococcal infection occur in the US each year.1 The infection has a 10% to 14% fatality rate,1 and 11% to 19% of survivors are left with serious sequelae such as hearing loss, neurological problems, and mental deficits.
Risk of infection varies by age (FIGURE). At highest risk are those under 1 year, with rates of between 5–15/100,000.
Five serogroups of the bacteria are important causes of disease: A, B, C, Y, and W-135. In the US, most disease is caused by groups B, C, and Y; about a third by each.
FIGURE
Meningococcal disease rates by age and burden of disease (United States, 1992–2002)
New vaccine lasts longer
Fortunately, some meningococcal infections are vaccine-preventable. The new quadrivalent conjugate vaccine (Menactra) has been approved for persons aged 11–55 years. It offers protection against the same serogroups (A, C, Y, and W-135) as the older polysaccharide vaccine (Menomune). However, whereas immunity from the polysaccharide vaccine wanes after about 3 to 5 years, the newer vaccine is expected to provide much longer immunity (though experience with the conjugate product has not accumulated).
Who should receive the vaccine?
Until the cohort of current 11- to 12-year-olds being vaccinated reaches high school and college, the CDC recommends universal administration of the vaccine to those entering high school and to college freshmen living in dormitories. Those for whom the vaccine is recommended who have received the polysaccharide vaccine more than 3 years previously should be revaccinated with conjugate vaccine.
Meningococcal vaccine has also been recommended for those in high-risk groups: those with anatomical or functional asplenia, those with terminal complement component deficiency, laboratory and research personnel with potential exposure to meningococci, and travelers to areas with endemic meningococcal disease.2
Meningococcal vaccine is also used in the military and to control outbreaks, defined as 3 or more cases resulting in a case rate of 10/100,000 in a 3-month period. College freshmen who live in dormitories are at higher risk for meningococcal infection and the recommendation has been to discuss the risks and benefits of vaccine with those in this category.
Caveats
The new vaccine is contraindicated for those with allergy to latex since that substance is used in the vial stopper. Adverse reactions have been mild; redness, pain and swelling at the injection site, headache, and malaise. As with all new products, physicians should be alert for previously unreported adverse reactions and report suspected reactions to the Vaccine Adverse Event Reporting System (www.vaers.org/).
Unresolved issues
These new meningococcal vaccine recommendations leave several issues to be resolved with time.
Will there be enough vaccine? Since new vaccine recommendations for vaccination take time to become universal in actual practice, it is expected that the supply of the vaccine will keep up with demand.
What will be the fate of the old polysaccharide vaccine? The manufacturer intends for the new conjugate vaccine to replace the polysaccharide vaccine, especially if the license can be expanded to include other age groups. Until that occurs, the polysaccharide vaccine is the only product available for those at risk who are aged 2 to 10 years or aged more than 55 years.
Will a booster dose be needed? The full duration of protection from the new vaccine is not currently known but is expected to be at least 10 years. This will protect adolescents and young adults through the highest-risk periods. Whether a booster will eventually be recommended will depend on information gathered in the next several years.
Will the license for the new vaccine be extended to a larger age group? It is anticipated that with time the license for the conjugate vaccine will be expanded to include other age groups, particularly children under age 11.
What about children under age 2? There is currently no meningococcal vaccine proven effective for children in this age group. More than 50% of invasive meningococcal disease in this age group is caused by serogroup B. Neither meningococcal vaccine offers protection against this serogroup.
Chemoprophylaxis of contacts
The universal use of conjugate meningococcal vaccine should lead to a marked decrease in the number of meningococcal infections. However, physicians should keep in mind that close contacts of patients with meningococcal infections should be given one of the antibiotic regimens described in the TABLE within 24 hours of confirming the disease. Close contacts include household members, daycare center cohorts, and those directly exposed to the patients’ oral secretions through kissing, mouth-to-mouth resuscitation, and intubations.
Patients treated for meningococcemia with other than a third-generation cephalosporin should also be treated with one of the regimens in the TABLE since other antibiotics have not been shown to eradicate nasopharyngeal carriage.
An expected result of conjugate vaccine is to decrease the nasopharyngeal carriage of Neisseria meningitidis. It is unknown if close contacts who have been vaccinated will benefit from chemoprophylaxis.
TABLE
Schedule for administering chemoprophylaxis against meningococcal disease
| DRUG | AGE GROUP | DOSAGE | DURATION/ROUTE OF ADMINISTRATION* |
|---|---|---|---|
| Rifampin† | Children <1 mo | 5 mg/kg every 12 h | 2 days |
| Children ≥1 mo | 10 mg/kg every 12 h | 2 days | |
| Adults | 600 mg every 12 h | 2 days | |
| Ciprofloxacin§ | Adults | 500 mg | Single dose |
| Ceftriaxone | Children <15 yrs | 125 mg | Single intramuscular dose |
| Ceftriaxone | Adults | 250 mg | Single intramuscular dose |
| *Oral administration unless indicated otherwise. †Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives may be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. §Ciprofloxacin is not generally recommended for persons aged <18 years or for pregnant and lactating women because the drug causes cartilage damage for immature laboratory animals. However, ciprofloxacin can be used for chemoprophylaxis of children when no acceptable alternative therapy is available. | |||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
1. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. MMWR 2005; in press.
2. CDC. National Center for Infectious Diseases. Traveler’s Health web site. Health information for international travel: meningococcal disease. Available at: www.cdc.gov/travel/diseases/menin.htm. Map of African meningitis belt available at: www.cdc.gov/ travel/diseases/maps/menin_map.htm. Accessed on February 23, 2005.
Consumer-directed health care: One step forward, two steps back?
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
The way to manage rising health care costs—espoused by some analysts and the current administration—is to give consumers greater control over health care decisions through a concept termed consumer-directed health care (CDHC). Sounds good. But how would the likely implications really play out?
CDHC in a nutshell
Herzlinger, a CDHC proponent from the Harvard Business School, describes the concept’s key principles:
- Insurers and providers freely design and price their services to offer good value for the money.
- Consumers receive excellent information about the costs, quality, and scope of services, so they can make better health care purchasing decisions.
- Consumers buy health insurance plans, sometimes with employer funds, knowing their full costs so they can obtain good value for the money.1
In the US, CDHC combines a high-deductible health plan (HDHP) with a health savings account (HSA). In 2004, the consulting firm Mercer estimated that just 1% of all covered employees were in consumer-directed health plans, but 26% of all employers were likely to offer a CDHP within the next 2 years.
Recently, Humana introduced a health plan that could be combined with an HSA. UnitedHealth Group bought Definity Health, which specializes in HSAs. Blue Cross/Blue Shield announced it would offer HSA-compatible health plans nationwide by 2006, and Kaiser said it would do the same by 2005.2 The American Medical Association has made the provision of HSAs a key part of its health policy agenda.3
High-deductible health plans
An HDHP is a health insurance policy with a minimum deductible of $1050 for self or $2100 for family coverage. The minimum deductible amount will likely increase yearly. The policy’s annual out-of-pocket expenses, including deductibles and co-pays, cannot exceed $5000 for self or $10,000 for family coverage (Table 1).
The program may offer medical services through the variety of managed care options such as health maintenance organization (HMO), preferred provider organization (PPO), or point of service plans with in-network and out-of-network providers. Persons using in-net-work options save money by receiving price discounts on services.
Companies may offer HDHPs with no deductible for preventive services (eg, physicals, immunizations, screening tests, prenatal and well child care) and higher deductibles and co-pays for using out-of-network providers.4
TABLE 1
Allowable limits on HDHP and HSA accounts
| High-deductible health plan (HDHP) | |
| Minimum deductible: | |
| Individual | $1050 |
| Family | $2100 |
| Maximum out-of-pocket spending | |
| Individual | $5000 |
| Family | $10,000 |
| Health savings account (HSA) | |
| Maximum annual contribution | |
| Whichever is lesser: the HDHP deductible, or | |
| Individual | $2600 |
| Family | $5150 |
Health savings account: how it works
An HSA is a tax-exempt personal savings account used to pay for qualified medical expenses. Think of it as an IRA for health. Legislation to establish HSAs was included in the Medicare Prescription Drug Bill of 2003.
To set up an HSA, a consumer must have an HDHP, have no other health insurance, and be ineligible for Medicare. Individuals can sign up on their own through insurance companies (including the American Medical Association insurance company) or banks, or may be offered an HSA option through their employer. Contributions to the account can be made by individuals, by an employer, or both. If made by the individual, contributions are tax exempt; if by the employer, they are not taxable as income to the employee.
The maximum deposit that can be made in 2005 is the lesser of either the HDHP deductible or $2650 for the individual or $5250 for family coverage. These amounts will be indexed to inflation yearly. Individuals aged 55 to 65 can make catch-up contributions. Once eligible for Medicare, you can no longer contribute to an HSA.4,5
Funds in an HSA are usually controlled by the individual who sets up the account. Withdrawals are tax-exempt if used for qualified medical expenses. If used for other expenses, withdrawals are taxed and subject to a tax penalty. Monies in an HSA can accrue tax-exempt savings from investments (stocks, bonds, etc) and be rolled over each year with no maximum cap. Since the individual owns the account, it is portable. If a person moves, the account moves too. However, contributions to an HSA must stop if the person is no longer enrolled in an HDHP.
After age 65, a person may continue to use an HSA for medical expenses or to pay insurance premiums like Medicare Part B and Medicare HMOs, or the funds can be taxed and used for non-medical expenses. In addition to the usual services covered in a traditional health plan, the list of qualified medical expenses is quite extensive (Table 2). Cosmetic surgery is generally not a qualified expense. The general goal is to have enough funds in the HSA to cover all medical expenses before the deductible in the health insurance plan is met.4,5
TABLE 2
Qualified CDHC medical coverage beyond traditional services
| Certain alternative medicine therapies |
| Substance abuse therapy |
| Ambulance service |
| Medical equipment and home remodeling related to medical requirements |
| Reproductive health services |
| Vision, hearing aides, and dental care |
| Certain health insurance premium costs |
| Long-term care |
| Medications and home oxygen |
| Mental health services |
| Source: Internal Revenue Service Publication 502. Available at: http://www.irs.gov/publications/p502/ar02.html#d0e516. Accessed on February 1, 2005. |
Public opinion generally unfavorable
A recent survey by the Kaiser Family Foundation found that 73% of respondents with employer-sponsored insurance had an unfavorable view of a health plan that combined an HDHP with an HSA, and 78% said they would feel vulnerable to high medical bills with this type of coverage.6
Iimplications of cdhc
Advocates of CDHC believe the financial disincentives of co-pays and high deductibles will encourage consumers to reduce their use of marginal services and to seek lower-cost, higher quality providers. They cite early studies showing CDHC participants decreased their use of certain medical services while increasing their use of preventive services and maintaining a balance in their HSA from one year to the next.1
Opponents of CDHC emphasize research that shows patients who pay more of their health care bills consume less care, including essential care. The RAND study of the 1970s confirmed that greater cost-sharing by patients reduced the chance they would receive effective medical care. This was particularly so for low-income patients. A recent study showed that increased medication cost-sharing led patients to stop using important drugs like statins and ACE inhibitors.7
How accessible/usable are health data?
Herzlinger cites informed consumer choices as a strength of the CDHC concept. However, the amount of information on cost and quality of health care is limited, albeit growing. More worrisome perhaps, there is little evidence that most patients can use this kind of information to make good health care decisions.
Who would benefit, who would not?
Another concern is that HDHPs and HSAs will more likely appeal to healthier, well-off people who can take full advantage of the tax incentives and more readily fund their accounts. If a significant number of consumers in this group moves toward CDHC plans, it would leave more unhealthy people in the traditional insurance system. This, in turn, would lead insurers to increase premiums for those less healthy consumers, thus making their insurance more expensive and, ironically, increasing the numbers of uninsured. CDHC plans may also appeal to the uninsured and those who have difficulty paying the usual health insurance premiums. This group is likely to have more difficulty fully funding their HSAs and, consequently, they will need to pay more of their deductible costs out-of-pocket, which can create a disincentive to seek needed care.
In an alternative analysis of CDHC, Robinson sees HSA products representing an evolution from collective insurance in which those in good health help finance the care of unhealthy enrollees with high expenditures (traditional health insurance with its “use it or lose it” design) to one in which unspent balances are retained by healthy enrollees rather than diverted to pay for the care of others (an HSA account with its “use it or save it” design).
In this scenario, healthy (and often well-off) consumers are favored by low-premium, high-deductible products. The savings are also financially protected from chronically ill users who would pay more in deductibles and coinsurance. The negative consequence is further diminishment of the already fragile social pooling effect of the current health insurance system and the potential for increasing the plight of the uninsured and underinsured.8
While it is likely that CDHC will attract more participants, it remains to be seen whether the public will support the concept if reports start appearing of significant numbers of patients refusing recommended services when faced with high deductibles and large out-of-pocket costs. CDHC will likely look attractive to healthy and well-off consumers, but its ability to control costs and improve quality in our already stressed health care system is suspect.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
1. Herzlinger R, Parsa-Parsi R. Consumer-driven health care. JAMA 2004;292:1213-1220.
2. Vogt K. Doctors looking at details as interest in HSAs grows. American Medical News, December 20, 2004.
3. Health savings accounts at a glance American Medical Association web site. November 2004. Available at: www.ama-assn.org/ama1/pub/upload/mm/363/hsabrochure.pdf. Accessed on February 1, 2005.
4. High deductible health plans with health savings accounts Office of Personnel Management web site. Available at: www.opm.gov/hsa/faq.asp. Accessed February 1, 2005.
5. US Department of the Treasury Health savings accounts. Available at: http://www.ustreas.gov/offices/public-affairs/hsa/. Accessed on February 1, 2005.
6. Kaiser Family Foundation. Kaiser health poll report, September/October 2004. Available at www.kff.org/healthpollreport/Oct_2004/13.cfm. Accessed February 1, 2005.
7. Davis K. Consumer-directed health care: Will it improve health system performance? Health Services Research 2004;39:1219-1233.
8. Robinson J. Reinvention of health insurance in the consumer era. JAMA 2004;291:1880-1886.
Cause-of-death certification: Not as easy as it seems
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Think you know how to fill out a death certificate? It’s often not as easy as it seems. Try the following cases.
Case 1
A 68-year-old woman is admitted to the ICU because of acute chest pain. She has a history of type 2 diabetes, hypertension, obesity, and angina. Over the next 24 hours an acute myocardial infarction is confirmed. Heart failure develops but improves with medical management. The patient then experiences a pulmonary embolus, confirmed by ventilation-perfusion lung scan and blood gases; over the next 2 hours she becomes unresponsive and dies.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: pulmonary embolus due to acute myocardial infarction due to atherosclerotic heart disease.
Question: What should be listed as conditions contributing to death but not directly causing death? Answer: type 2 diabetes, obesity, hypertension, and congestive heart failure.
Case 2
A 78-year-old woman has left hemiparesis from a stroke 2 years earlier. She has been unable to care for herself and has lived in a nursing home. She has had an indwelling urinary catheter for the past 6 months. Because of fever, increased leukocyte count, and pyuria, she is admitted to the hospital and started on 2 antibiotics. Two days later, the blood culture result is positive for Pseudomonas aeruginosa resistant to the antibiotics being administered. Despite a change of antibiotics, hypotension ensues and the patient dies on hospital day 4.
Question: What should be written on the death certificate as the immediate and underlying cause of death? Answer: P aeruginosa sepsis, due to a urinary tract infection due to an indwelling catheter, due to left hemiparesis, due to an old cerebral infarction.
Question: What should be listed as conditions that contributed to the death but that did not directly cause the death? Answer: nothing.
If you were correct on both cases, congratulations. If you were not, this article offers basic advice that will help you provide accurate medical information on death certificates.
Death certificates are important official records used for personal, legal, and public health purposes, yet they are frequently filled out inaccurately. Physicians are responsible for determining the cause and manner of death, yet they are seldom formally trained for this responsibility in medical school or residency. The result is frequent and avoidable errors.
Who is responsible for what?
Registration of deaths is a state responsibility. The National Center for Health Statistics compiles data from all states to produce national vital statistics, and most states use death certificate forms that conform to a recommended national standard. Though funeral directors are responsible for filing the certificate with the state, physicians are responsible for completing the medical portion of the certificate.
With the medical information provided, trained coders classify the cause of death using standardized methodology.
Medical examiners or coroners are responsible for investigating and certifying the cause of any death that is unexpected, unexplained, or resulting from injury, poisoning, or a public health threat.
Physicians are additionally responsible for answering inquiries from the registrar (these inquiries can be reduced by accurately and completely filling in the medical information) and submitting a supplemental report when autopsy findings or other information indicates a cause of death different from that originally reported.
How to complete the medical portion of the death certificate
The Figure is a standard certificate of death. It may vary slightly state to state. Physicians are responsible for items 24 through 49. If the state requires a pronouncing physician (Table 1), the pronouncing and certifying physicians may be different, in which case the pronouncing physician completes items 24 through 31 and the certifying physician items 32 through 49. If the pronouncing physician is also the certifying physician, items 26 through 28 need not be completed. If the death is referred to the coroner or medical examiner, they complete items 24, 25, 29, 30, and 32 through 49.
FIGURE
US standard death certificate
TABLE 1
Definitions
| Immediate cause of death:The final disease or injury causing the death. |
| Intermediate cause of death: A disease or condition that preceded and caused the immediate cause of death. |
| Underlying cause of death: A disease or condition present before, and leading to, the intermediate or immediate cause of death. It can be present for years before the death. |
| Manner of death: The circumstances leading to death—accident, homicide, suicide, unknown or undetermined, and natural causes. |
| Medical examiner: A physician, acting in an official capacity within a particular jurisdiction, charged with the investigation and examination of persons dying suddenly, unexpectedly, or violently, or whose death resulted from, or presents, a potential public health hazard. The medical examiner is not always required to be a specialist in death investigation or pathology. Most systems employing physicians as part time medical examiners encourage them to obtain training for medical examiners such as that offered by the National Association of Medical Examiners. |
| Coroner: A coroner is a public official, appointed or elected, in a particular geographic jurisdiction, whose official duty is to make inquiry into deaths in certain categories. In some jurisdictions, the coroner is a physician, but in many localities, the coroner is not required to be a physician nor be trained in medicine. |
| Pronouncing physician: The one who determines the decedent is legally dead. Not all states require a death to be pronounced by a physician. |
| Certifying physician: The one who certifies the cause of death. |
The most challenging part
Item 32, the Cause of Death, is the most difficult item to complete accurately. It consists of two parts. Part I is a sequential list of conditions leading to the immediate cause of death and the time interval between their onset and the death. Part II is a list of other conditions contributing to the death but not directly causing death. Thinking about the death as a sequence of events and reconstructing this sequence helps classify correctly the various illnesses and conditions the decedent might have had.
Immediate cause of death. Part I, line a, is for the immediate cause of death (see Table 1). This should be a disease, complication, or injury that directly caused the death. A common error is to list a mechanism of death (for example, cardiac arrest) rather than a disease (myocardial infarction).
Specific terms are better than vague ones. For instance,“cerebral infarction” is better than “stroke.” “Escherichia coli sepsis” is better than just “sepsis.”
When cancer is the cause of death, list the primary site, cell type, cancer grade, and specific organ or lobe affected.
Avoid terms without medical meaning, such as old age or senescence.
If additional information is expected from an autopsy, it is acceptable to list the cause of death as pending. But an update to certificate will be required once the additional information is obtained. It is also acceptable to list a cause as unknown. This will not automatically forward the case to the medical examiner.
Intermediate/underlying causes. Lines b, c, and d are for intermediate and underlying causes. Each condition listed should cause the one above it. You should be able to proceed logically from the underlying cause through each intermediate cause by saying the phrase “due to” or “as a consequence of,” moving from the lower line up through line b. There may be several intermediate causes. For example, a death may be due to a pulmonary embolus, as a consequence of hip surgery, resulting from a injury from a fall, resulting from a cerebral infarction. The underlying cause is the cerebral infarction.
Marking time intervals. To the right of lines a through d is space to write the time interval between the condition listed (immediate, intermediate, or underlying cause of death) and the time of death. The more precise the time the better. But it is understood that times must occasionally be estimated, and terms such as “approximately” are acceptable. If the time cannot be estimated, insert the phrase “unknown duration.” Something should be listed on this line next to the immediate, intermediate, and underlying conditions listed. No lines should be left blank.
Other illnesses. Part II is where to list other significant illnesses or conditions that may have contributed to the death but were not the direct causes of it. More than one condition may be listed. Many patients have multiple conditions and there may be uncertainty as to direct and contributing causes of the death. The physician is only expected to make the best judgment possible as to the most likely causes and sequences. Coders referring to international standards and rules will use the information to make a final classification of the underlying cause.
Specific errors to avoid
Table 2 includes some points to remember to avoid making errors when filling out the death certificate medical information. By following these rules, studying the cases provided in the Physicians’ Handbook on Medical Certification and Death, and systematically thinking about the sequence of events that caused the death, physicians can improve on their accuracy when performing the important and under appreciated role of accurately certifying the medical cause of death.
TABLE 2
Important points to remember when completing medical information on a death certificate
| Do not use abbreviations. |
| Do not use numbers for months; spell out the month. |
| Use a 24-hour clock (1600, not 4 P.M.). |
| Do not alter the document or erase any part of it. |
| Print legibly using black ink. |
| Complete all required items, do not leave them blank. If necessary, write “unknown.” |
| Do not delay completing the certification. The burial or other disposition of the remains depends on the correct completion of the certificate and its acceptance by the state or local registrar. |
| Do not complete the medical information if another physician has more knowledge of the circumstances, unless they are unavailable. |
Useful resources
The Physicians’ Handbook on Medical Certification of Death, published by the Centers for Disease Control, National Center for Health Statistics, is available at www.cdc.gov/nchs/data/misc/hb_cod.pdf. It contains instructions on how to complete a death certificate and a series of useful examples that take about a half hour to review.
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
Acting on synergies between clinic and community strategies to improve preventive medicine
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
From their days in training through their years of practice, family physicians emphasize preventive medicine. They counsel patients on diet and exercise, safe sex, and substance abuse; screen for early detection of cancer (eg, cervical, breast, colon, prostate); and administer chemo- and immunoprophylaxis (daily aspirin, vaccinations).
Though physicians are accustomed to caring for individuals, adopting a population perspective—considering the practice’s patient panel or even the larger community—is a logical extension of one’s daily practice. The public’s health benefits from parallel efforts in risk assessment and community-wide prevention. And, as we propose here, awareness of the similarities between the 2 areas of endeavor strengthens both.
Assessing risk for the individual
Risk factors are characteristics of a person that increase the likelihood of becoming diseased. Obvious risk factors include physical traits and laboratory values such as obesity, high cholesterol levels, and high blood pressure, and behaviors such as smoking and binge drinking. Other risk factors include demographic traits (age, race/ethnicity, gender, income), environmental influences (occupation, geographic location), and system issues (insurance status, usual source of care). Though risk factors may not cause disease, their presence can increase the probability that disease will eventually develop.
The degree to which a risk factor may influence disease development can be calculated with 2 measures.
Absolute risk is the difference between the incidence of disease in the group exposed to a risk factor and the incidence in the group not exposed to the factor.
Relative risk is the extent to which persons exposed to a risk are likely to develop the disease compared with those not exposed (see Calculating relative risk). Relative risk is more useful for judging a factor’s strength of disease causality, but it does not necessarily indicate the magnitude of risk for a population. With an uncommon disease, for instance, the relative risk of disease from an exposure may be large but the absolute risk may be small.
Relative risk—the likelihood that those exposed to a risk factor for disease will become diseased, compared with those not exposed to the factor—is calculated as follows:
Relative risk = (a/a+b) / (c/c+d)
The incidence of disease among those exposed (a divided by a+b) divided by the indicence of disease among those not exposed (c divided by c+d).
For more on relative risk, see “Relative risks and odds ratios: What’s the difference?,” in the February 2004 JFP.
Assessing risk for a population
Just as absolute and relative risk help quantify an individual’s susceptibility to disease, the population attributable risk helps gauge the level of risk to a community. The calculation takes into account disease incidence as well as how often the population is exposed to a related risk factor. This measure can be particularly influential in health policy decisions such as how to spend scarce resources—illustrated in the following example.2
Individual and population risks are determined in part by the prevalence of a risk factor. Consider the risk of death in the case of hypertension. For an individual, the risk of death is greater with severe hypertension than with mild hypertension. However, mild hypertension is quite common; severe hypertension is not. Therefore, the population attributable risk of hypertension (the number of extra deaths in the population attributable to hypertension) is greater for those with mild hypertension, even though the risk to an individual is greatest when hypertension is severe. This suggests that more lives can be saved by treating lots of people who have mild hypertension than by treating the much smaller number of people with severe hypertension—a concept called the “prevention paradox,” and one that underlies a number of national prevention efforts.
Synergies between individual and community prevention
Individual and community prevention strategies both have merit. For example, public health smoking cessation campaigns have been effective at the population level. At the same time, physician efforts to promote smoking cessation through office counseling sessions are effective with individuals. Individual physician efforts become population efforts if thousands of physicians each day provide cessation counseling to their patients. Public health campaigns help accomplish this by reinforcing the importance of cessation efforts including those delivered by practicing physicians.
Another excellent example of this type of reinforcing synergy is the work of the past decade to address hyperlipidemia as a risk factor for heart disease. In this effort, physician screening and treatment of patients has been both promoted and complemented by national media campaigns, local health fairs, and ongoing research, to the point where many individuals now are familiar with cholesterol and want to know their own lipid values.
Drawing on both arenas to prioritize prevention strategies
The federal government has supported the development of several types of evidence-based prevention guidelines. Family physicians are familiar with the US Preventive Service Task Force Guide to Clinical Preventive Services,3 but may be less familiar with the more recently developed Guide to Community Preventive Services.4 For a comprehensive continuum of preventive care, both guides can be used in combination. Using both sets of recommendations can help physicians to prioritize prevention strategies for individuals, office patient populations, and communities.
The Community Guide is a federally sponsored initiative producing evidenced-based recommendations for health promotion and disease prevention from a population-based perspective sponsored by the Centers for Disease Control and Prevention (Table 1). Like the Guide to Clinical Preventive Services, the independent task force that oversees the Community Guide develops evidence-based reviews of potential interventions and translates the findings into recommendations that can be used by policy makers, public health entities, and health systems.
TABLE 1
Topics covered in the Guide to Community Preventive Services
|
Actionable recommendations for practitioners
Though the Community Guide takes a population-based approach to prevention (eg, recommendations for policy strategies, mass media campaigns, and school health programs), a number of its recommendations focus on the health care system and are directly applicable to practicing family physicians. For instance:
- Vaccination: a section on provider-based interventions such as reminder systems, standing orders for adults, and provider feedback strategies
- Diabetes: a section on disease management strategies
- Tobacco: a section on improving the delivery of cessation services
- Cancer: a section on improving screening for specific diseases.
The topic reviews grade the strength of each intervention (strong evidence, sufficient evidence, or insufficient evidence), provide a 1-page summary of the recommendations, and have links to longer papers that review the specific evidence.
Complementary web support
The website also includes an excellent 2-page summary of types of activities family doctors can undertake, with links to specific directions for implementing prevention activities. Included are patient and provider reminder systems, disease and case management, and use of standing orders.4
The recommendations in both the Clinical and Community Guides that address common conditions provide especially strong “roadmaps” for focusing on and addressing health promotion and disease prevention. By using both guides, physicians can develop a range of effective, evidence-based approaches for practice. For example, Table 2 presents evidence-based recommendations concerning smoking. These recommendations fall across a range of preventive interventions.
Producing successful initiatives
Using the guides as a reference, the family physician could initiate screening of patients for smoking status in the practice and then implement one or more of the recommended strategies listed in Table 2. Possibilities include adding a provider reminder system, delivering brief counseling to quit smoking, prescribing cessation medications, and coupling these efforts with advocacy for development of a media campaign in the community. Family physicians who undertake community prevention efforts outside the office setting are likely to improve their success by partnering with other community health and social service professionals to implement agreed upon prevention interventions.5
As more topics are developed for the Community Guide, family physicians will likely find more reasons to refer to it as a companion to the Clinical Guide. Using the 2 together will enhance the benefit gained from using each alone in a way that is analogous to the added health benefits obtained when the traditional health care system works more closely with the public health system.
TABLE 2
Intervention strategies in the clinical and community guides
| Intervention strategy recommendation | Where it’s found | Where it’s to be used |
|---|---|---|
| Screening patients for tobacco use | Clinical Guide | Office |
| Provider gives brief advise to quit to patients who use tobacco | Clinical Guide | Office |
| Provider counseling to patients on tobacco cessation | Clinical Guide | Office |
| Self-help education materials for patients who use tobacco | Clinical Guide | Office |
| Pharmacologic treatment for tobacco and dependence | Clinical Guide | Office |
| Provider reminder systems | Community Guide | Office |
| Multicomponent clinical program (provider reminder + education) | Community Guide | Office |
| Patient-oriented interventions (telephone support; sliding fee scale) | Community Guide | Office |
| Policies, regulations, and laws (smoking ban and restrictions) | Community Guide | Community |
| Mass media campaigns | Community Guide | Community |
| Sources: Adapted from USPSTF, Guide to Clinical Preventive Services, 19963; | ||
| USPSTF, Guide to Community Preventive Services, 2000.4 | ||
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.
1. Turabian JL, Perez-Franco B. Cual es el sentido de la educacion para la salud y las actividades “communitaria” en atencion primaria? Aten Primaria. 1998;22:662-666.
2. Fletcher R, Fletcher S, Wagner E. Clinical Epidemiology: The Essentials. Philadelphia, Pa: Williams and Wilkins; 1996.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. Available at: www.ahcpr.gov/clinic/cpsix.htm
4. US Preventive Services Task Force. Guide to Community Preventive Services. Available at: www.thecommunityguide.org.
5. Peters K, Elster A. Population based medicine: Roadmaps to clinical practice. Chicago, Ill: American Medical Association, 2002.
Influenza vaccine: New recommendations for infants and children aged 6 to 23 months
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
For the 2004–2005 influenza season, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends that influenza vaccine be given to all infants and children aged 6 to 23 months.1 It further recommends vaccination for members of households that have children aged <2 years and for out-of-home caregivers for these children.
These changes will make universal coverage more difficult to achieve. Family physicians will need to educate themselves, office staff, and parents and guardians about these recommendations. Be prepared to implement office protocols that identify and notify those who require vaccination, and address the concerns of parents and guardians regarding thimerosal and the addition of yet another vaccine to the child vaccine schedule.
Rationale for change
In 2002, ACIP began to encourage use of influenza vaccine for all children 6 to 23 month old. Before then, it had been recommended only for those in that age group with certain chronic medical conditions.
Still, the coverage level achieved in the 2002 to 2003 flu season for this age group was low—only 4.4 % were fully immunized with 2 doses.2 To increase coverage, the influenza vaccine was included in vaccines offered by the Vaccine for Children program in 2003 and was made part of the universal recommendations for the coming season.
The rationale for the new universal recommendation is the high rate of influenza-related hospitalizations among those aged 6 to 23 months, which varies from year to year and has been documented to be as high as 5/1000.3,4 While hospitalization rates are higher for infants aged 0 to 5 months, influenza vaccine is not approved for use in this age group.
Death from influenza in infants and children is not common. However, in the 2003 to 2004 influenza season, 58 influenza deaths among those aged <2 years were recorded.1 Added benefits from vaccinating infants and children may include decreased rates of otitis media.5,6
Practical aspects of vaccine administration
Some of the most important practical details involved with immunizing 6- to 23-month-olds include the following:
- The age group includes those who have completed 6 months of life (are now in their 7th month) up to the second birthday. Influenza vaccine is 65% to 90% effective in this age group.
- The dose of influenza vaccine for infants and children up to their 3rd birthday is 0.25 mL.
- The vaccine should be administered intramuscularly in the anterolateral thigh with a 1-inch needle.
- Two doses, 1 month apart, are recommended for infants or children aged <9 years receiving influenza vaccine for the first time. (Dose recommendations for all age groups are listed in Table 1.)
- Only 1 vaccine product has been approved for those younger than 4 years: FluZone, produced by Aventis Pasteur.
- The vaccine is made with killed virus and can be given simultaneously with other recommended vaccines.
- The live attenuated influenza vaccine, administered intranasally, is not approved for children before their 5th birthday.
- The vaccine should be stored at 2° to 8°C (35° to 46°F) and should not be frozen.
- Vaccine left over from last year should not be used this year.
- Contraindications to influenza vaccine are listed in Table 2.
TABLE 1
Inactivated influenza vaccine* dosage, by age group
| Age group† | Dose | No of doses | Route‡ |
|---|---|---|---|
| 6–35 mo | 0.25 mL | 1 or 2§ | Intramuscular |
| 3–8 y | 0.50 mL | 1 or 2§ | Intramuscular |
| 9 y | 0.50 mL | 1 | Intramuscular |
| *A 0.5-mL dose contains 15 mg each of A/Fujian/411/2002 (H3H2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. For the A/Fujian/411/2002 (H3H2)-like antigen, manufacturers may use the antigenically equivalent A/Wyoming/3/2003 (H3N2) virus, and for the B/Shanghai/361/2002-like antigen, manufacturers may use the antigenically equivalent B/Jilin/20/2003 virus or B/Jiangsu/10/2003 virus. Manufacturers include Aventis Pasteur, Inc (FluZone© split virus), and Chiron (Fluvirin™ purified surface antigen vaccine). FluZone is approved by the Food and Drug Administration for use among persons aged 6 months. Fluvirin is approved for use among persons aged 4 months. For further product information, call Aventis Pasteur at 800-822-2463 or Chiron at 800-200-4278. | |||
| † Because of their decreased potential for causing febrile reactions, only split-virus vaccines should be used for children aged <13 years. Whole-virus vaccine is not available in the United States. Split-virus vaccine might be labeled as split, subvirion, or purified surface antigen vaccine. Immunogenicity and side effects of split- and whole-virus vaccines are similar among adult when vaccines are administered in the recommended dosage. | |||
| ‡ For adults and older children, the recommended site of vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. | |||
| § Two doses administered at least 1 month apart are recommended for children aged <9 years who are receiving influenza vaccine for the first time. | |||
| Source: Harper et al 2004.1 | |||
TABLE 2
Contraindications to influenza vaccine
|
The thimerosal controversy
Family physicians are likely to encounter questions from parents or guardians about thimerosal in influenza vaccines. Thimerosal is a preservative containing mercury, which has been used in vaccines for more than 70 years. Because of the increasing number of recommended childhood vaccines and the resulting cumulative exposure to mercury, there has been a concerted effort since 1999 to reduce the content of thimerosal in vaccine products.
Almost all vaccines are now free of thimerosal. However, inactivated influenza vaccine distributed in multidose vials does contain thimerosal, 12.5 μg mercury/0.25 mL dose. Single-dose vials containing inactivated influenza vaccine do not contain thimerosal as a preservative, but this product still contains trace amounts of mercury, <0.5 μg/0.25 mL dose.
No evidence has shown conclusively that mercury-containing vaccines cause serious adverse effects. A recent Institute of Medicine report concludes that the weight of evidence supports a lack of causation between thimerosal and autism.7 Nevertheless, many parents remain concerned about mercury exposure from vaccines. Reassure those who are concerned that the cumulative exposure to mercury from all vaccines has decreased markedly and that influenza vaccine contains only low amounts of thimerosal. Those wishing to decrease this risk even further should be given the option of the single-dose vial product, if available.
Timing vaccination for optimal protection
The influenza season varies year to year but normally occurs between November and March. Vaccination for those at high risk of influenza complications, including those aged 6 to 23 months, should begin in September and October. Those who need 2 doses should receive the second by December if possible.
Because of the length of the influenza season and the fact that the inactivated influenza vaccine is not approved for use during the first 6 months of life, infants will become eligible for the vaccine at different points in the influenza season. Office procedures should be implemented to identify eligible infants as the season progresses and to notify parents or guardians of the opportunity to vaccinate their infant.
If an infant enters the 7th month of life late in the influenza season and vaccine is still available, vaccination should still be considered, not only to offer protection in the current year but also to reduce the number of doses needed the next year. In instances when only 1 dose of a recommended 2-dose schedule is completed, only 1 dose is needed the following year.
Vaccinate close contacts of infants and children
The new ACIP recommendations state that persons who can transmit influenza to those at high risk of complications should also be vaccinated. This includes household contacts of, and those who provide care to, children aged <2 years.
For infants in their first 6 months of life, preventing infection among close contacts is the major preventive intervention available. The Vaccine for Children program now includes influenza vaccine for members of households (those who are aged <18 years) where children aged <2 years live.
Vaccine complications and contraindications
Local reactions including redness, pain, and swelling are common after influenza vaccine administration. Generalized reactions including fever, malaise, and myalgia are less common and can start within 6 to 12 hours and last 1 to 2 days. Contraindications to vaccine administration are few (Table 2).
When chemoprophylaxis is an option
Only 2 options exist for chemoprophylaxis against influenza in children before their 13th birthday: amantadine and rimantadine. The dosage for each is 5 mg/kg/d, up to 150 mg, in 1 or 2 doses. Both are effective only against influenza A and are approved for use only after the 1st birthday. Still, this option should be kept in mind for unvaccinated children who are exposed to influenza.
A chemoprophylactic agent can be started at the same time the vaccine is administered. Since it takes 2 weeks to develop protective levels of antibodies, chemoprophylaxis should be continued for 2 weeks with those who need only 1 vaccine, and until 2 weeks after the 2nd vaccine for those who need 2 doses.
In early October, 1 of the 2 producers of inactivated influenza vaccine for the United States market, Chiron Corporation, had its license suspended by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom, where their plant is located. This will cause a shortage of vaccine, since Chiron was to have supplied about half of the 100 million doses planned for this country.
Chiron, however, was not a producer of vaccine for children aged <4 years. Based on this anticipated shortfall, the ACIP is recommending that the vaccine be given preferentially to those at high risk for influenza complications ( Table 3 ) and that those who are not in one of these categories forgo vaccination. Physicians who will not have an adequate supply of vaccine for their patient population are encouraged to have their priority patients seek vaccine at another location.
TABLE 3
Priority groups for influenza vaccination
| The following priority groups for vaccination with inactivated influenza vaccine this season are considered to be of equal importance. |
|
Correspondence
12229 S. Chinook, Phoenix, AZ 85044. E-mail: [email protected].
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
1. Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2004; 53(RR-6):1–40. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5306a1.htm. Accessed on October 19, 2004. (Note: Recommendations for prevention of influenza in all age groups are available in this article.)
2. CDC. Childhood Influenza—vaccination coverage—United States, 2002–2003 Influenza season. MMWR Morb Mortal Wkly Rep 2004;53:863-866.
3. Neuzil KM, Wright PF, Mitchel EF, Jr, Griffin MR. Burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr 2000;137:856-864.
4. Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med 2000;342:225-231.
5. Heikkinen T, Ruuskanen O, Waris M, Ziegler T, Arola M, Halonen P. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child 1991;145:445-448.
6. Clements DA, Langdon L, Bland C, Walter E. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med 1995;149:1113-1117.
7. Institute of Medicine. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004.
How the presidential candidates’ health care proposals contrast
As we go to press, national polls indicate a tight race in the 2004 presidential election between President George W. Bush and his Democratic challenger, Senator John Kerry.1 In polling done in late spring by the Kaiser Family Foundation, respondents said health care followed the economy, the war, and terrorism as the top concerns. Health care issues rated most important were costs and insurance, prescription drug costs, drug benefits for seniors, the uninsured, Medicare, bioterrorism, abortion, and health care quality; however, no single issue dominated.2
This article examines the health care proposals of President Bush and Senator Kerry in 4 main areas: health insurance coverage, malpractice reform, prescription drug costs, and stem cell research. The candidates’ platforms differ significantly in all areas.
Health insurance proposals
Senator Kerry’s proposal is by far the more expansive of the two:3
- Starting in 2006, enrolling uninsured children of families under 300% of the poverty line (about $56,000 for a family of 4) into Medicaid and the State-Children’s Health Insurance Program (SCHIP). Currently, this program covers children in families under 200% of the poverty level. The SCHIP is a federally funded program administered by the states that has helped decrease the percentage of uninsured children.
- Starting in 2007, enrolling uninsured parents in families under 200% of the poverty level (about $37,000 for a family of 4) into state Medicaid programs and the SCHIP. This is an innovative idea to expand the benefits of the SCHIP to adults as many parents of uninsured children are themselves uninsured. Several states have already begun experimenting with this strategy.
- Starting in 2008, enrolling single adults and childless couples in poverty into Medicaid. The poverty level for a single adult is $9310. • Allowing small businesses, workers from 55-to 64 years old, and workers between jobs to enroll new insurance pools with the same health plans offered by the Federal Employees Health Benefits Program (FEHB). This proposal would be financed by employer contributions and tax credits to employers and workers. The FEHB is often cited as an example of the kind of health insurance program to which all Americans should have access.
- A reinsurance proposal to make insurance more affordable. This fund would reimburse employer groups for an individual’s health care expenses in excess of $50,000. Eligible employer groups would have to provide insurance coverage to all of their workers, return health insurance savings to the workers in the form of reduced premiums, and introduce disease and care management programs into the worksite. Currently, while only a very small number of Americans have medical expenses in a given year that exceed this amount, their care accounts for a greater percentage of overall costs. This reinsurance proposal is designed to reduce the cost of employer funded health insurance by about 10%.
Commentary. Kenneth Thorpe, Chair of Health Policy at Emory University, estimates these proposals will provide health insurance for 27 million of the estimated 41 million Americans now unin-sured. He estimates the net costs of Kerry’s plan at $653 billion from 2005 to 2014. In Thorpe’s projections, the costs of the proposals are partially offset by savings in several areas—disease management programs, decreased reimbursements to hospitals for care of the uninsured (since there will be fewer uninsured persons), and savings due to expanded electronic filing and processing of claims.4 Kerry has proposed generating the revenue to fund this health care expansion by rolling back the tax cuts for taxpayers with incomes over $200,000. The nonpartisan Tax Policy Center estimates the revenue from rescinding these cuts at $632 billion over the same time period.5
President Bush proposes 3 strategies for reducing health insurance costs:6
- A refundable tax credit for those under 65 who lack employer-sponsored insurance and are not covered by Medicaid. The credit maximum is $1000 for a single adult with less than $15,000 in adjusted gross income (AGI) and phases out at $30,000. It is up to $3000 for 2 adults with 2 or more children with less than $25,000 in AGI and phases out at an AGI of $60,000. Because of the way in which the tax credit is calculated to grow between 2006 and 2014, it is likely that its economic value will decline over time, which will somewhat decrease the number of uninsured it covers.
- Individuals purchasing qualified high deductible policies (at least a $1000 deductible for a single person; $2000 for a family policy) will be able to take a deduction for the premium paid when the insurance is purchased in combination with a health savings account (HSA). Last year’s Medicare drug bill allowed people setting up these accounts to save taxes on their contributions. This new proposal adds to this tax advantage. While HSAs are available to all, the tax benefits make them more likely to be used by healthier, more affluent people and could wind up having the perverse effect of increasing premiums for those families who do not choose or cannot afford to have them.6
- Allowing association health plans (these permit small businesses to purchase health insurance through large purchasing pools, which may reduce costs) to be regulated under federal law rather than state insurance law. Through this means, the plans would be exempt from certain benefit mandates and other state regulations.
Commentary. Thorpe estimates the Bush proposal will initially cover an additional 2.4 million uninsured Americans, but this number will decrease to 2.1 million by 2014 due to the decreasing value of the refundable tax credits. He projects the total cost at $90.5 billion over the 10 years.7 The Table compares the Bush and Kerry health insurance proposals.
TABLE
Comparing costs assumed in the Bush and Kerry proposals for reforming health insurance
| Bush | Kerry* | |
|---|---|---|
| Federal cost of plan (billions) | ||
| 2005–2010 | $45.7 | 176.3 |
| 2005–2014 | $90.5 | $653.1 |
| Newly insured (millions) | ||
| 2008* | 2.4 | 26.7 |
| 2014* | 2.1 | 26.7 |
| Note: About 70% of the newly insured would claim the credit in the Bush plan expressed in 2003 figures. | ||
| *Net cost of plan including estimated federal savings. | ||
| Source: AAFP website: www.aafp.org/x22202.xml. | ||
Malpractice reform
President Bush made malpractice reform a priority issue in the current Congressional session. The key provision in his proposals is a $250,000 cap on non-economic damages, a position strongly supported by most national medical organizations (eg, AMA, AAFP). In the current Congress, a bill including this provision has passed the House but has failed to gather sufficient support for approval in the Senate.
Senator Kerry reflects the prevailing Democrat view opposing a cap on non-economic damages. Instead, he proposes prohibiting individuals from filing a suit unless a qualified specialist determines a reasonable claim exists and requiring mandatory sanctions for frivolous lawsuits.
Prescription drug costs
The Medicare prescription drug bill was reviewed in a recent issue of the JOURNAL OF FAMILY PRACTICE. Since the passage of that legislation, many Democrats continue to argue that not enough was done to provide recipients with affordable drug prices. A recent survey of Medicare recipients by the Kaiser Family Foundation showed 47% had unfavorable views of the law and 26% had favorable views, but a majority wanted Congress to fix the law, not repeal it. The survey found extensive support for allowing importation of drugs from Canada and allowing the government to negotiate the price of drugs with pharmaceutical companies.9
Senator Kerry has made these 2 proposals a prominent part of his campaign.
President Bush has resisted allowing importation of drugs from Canada on the basis of safe-ty concerns and has opposed having the government directly involved in negotiating Medicare drug prices saying that competition among private plans would lead to similar savings. As of September 2004, a handful of states had set up websites to allow residents to access information about buying medicines from Canada. The FDA has said this amounted to allowing importation of pharmaceuticals from other countries and was not allowed, but it had not moved to stop the activity.
Stem cell research
President Bush limited government funding of stem-cell research to those cell lines that were created before August 9, 2001. Research has continued in other countries and somewhat in this country through private funding, but many US medical organizations and scientists have argued that we are gradually losing ground in this field to foreign scientists.10
Senator Kerry has made the expansion of government funding of stem cell research a prominent part of his health care platform.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Nagourney A. Polls show tight race with a few gains for Kerry. New York Times, August 3, 2004.
2. Kaiser Family Foundation. Kaiser Health Poll Report. July/August 2004. Available at: www.kff.org/health-pollreport/currentedition/care/index.cfm. Accessed on September 2, 2004.
3. John Kerry. for President website. John Kerry’s plan to make health care affordable to every American. Available at: www.johnkerry.com/issues/health_care/health_care.html. Accessed on September 2, 2004.
4. Thorpe K. Federal costs and savings associated with Senator Kerry’s health care plan. August 23, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
5. Senator Kerry’s. Tax Proposals Tax Policy Center. July 2004. Available at: www.taxpolicycenter.org/publications/template.cfm?PubID=8820. Accessed on September 13, 2004.
6. GeorgeBush.com. Health Care issue brief. Available at: www.georgebush.com/HealthCare/Brief.aspx. Accessed on September 2, 2004.
7. Thorpe K. Federal costs and savings associated with President Bush’s health care plan. May 5, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
8. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
9. Kaiser Family Foundation. Views of the new Medicare drug law: A survey of people on Medicare. Available at: www.kff.org/medicare/pomr081004pkg.cfm. Accessed on September 13, 2004.
10. Daley G. Missed opportunities in embryonic stem-cell research. N Engl J Med 2004;351:627-628
As we go to press, national polls indicate a tight race in the 2004 presidential election between President George W. Bush and his Democratic challenger, Senator John Kerry.1 In polling done in late spring by the Kaiser Family Foundation, respondents said health care followed the economy, the war, and terrorism as the top concerns. Health care issues rated most important were costs and insurance, prescription drug costs, drug benefits for seniors, the uninsured, Medicare, bioterrorism, abortion, and health care quality; however, no single issue dominated.2
This article examines the health care proposals of President Bush and Senator Kerry in 4 main areas: health insurance coverage, malpractice reform, prescription drug costs, and stem cell research. The candidates’ platforms differ significantly in all areas.
Health insurance proposals
Senator Kerry’s proposal is by far the more expansive of the two:3
- Starting in 2006, enrolling uninsured children of families under 300% of the poverty line (about $56,000 for a family of 4) into Medicaid and the State-Children’s Health Insurance Program (SCHIP). Currently, this program covers children in families under 200% of the poverty level. The SCHIP is a federally funded program administered by the states that has helped decrease the percentage of uninsured children.
- Starting in 2007, enrolling uninsured parents in families under 200% of the poverty level (about $37,000 for a family of 4) into state Medicaid programs and the SCHIP. This is an innovative idea to expand the benefits of the SCHIP to adults as many parents of uninsured children are themselves uninsured. Several states have already begun experimenting with this strategy.
- Starting in 2008, enrolling single adults and childless couples in poverty into Medicaid. The poverty level for a single adult is $9310. • Allowing small businesses, workers from 55-to 64 years old, and workers between jobs to enroll new insurance pools with the same health plans offered by the Federal Employees Health Benefits Program (FEHB). This proposal would be financed by employer contributions and tax credits to employers and workers. The FEHB is often cited as an example of the kind of health insurance program to which all Americans should have access.
- A reinsurance proposal to make insurance more affordable. This fund would reimburse employer groups for an individual’s health care expenses in excess of $50,000. Eligible employer groups would have to provide insurance coverage to all of their workers, return health insurance savings to the workers in the form of reduced premiums, and introduce disease and care management programs into the worksite. Currently, while only a very small number of Americans have medical expenses in a given year that exceed this amount, their care accounts for a greater percentage of overall costs. This reinsurance proposal is designed to reduce the cost of employer funded health insurance by about 10%.
Commentary. Kenneth Thorpe, Chair of Health Policy at Emory University, estimates these proposals will provide health insurance for 27 million of the estimated 41 million Americans now unin-sured. He estimates the net costs of Kerry’s plan at $653 billion from 2005 to 2014. In Thorpe’s projections, the costs of the proposals are partially offset by savings in several areas—disease management programs, decreased reimbursements to hospitals for care of the uninsured (since there will be fewer uninsured persons), and savings due to expanded electronic filing and processing of claims.4 Kerry has proposed generating the revenue to fund this health care expansion by rolling back the tax cuts for taxpayers with incomes over $200,000. The nonpartisan Tax Policy Center estimates the revenue from rescinding these cuts at $632 billion over the same time period.5
President Bush proposes 3 strategies for reducing health insurance costs:6
- A refundable tax credit for those under 65 who lack employer-sponsored insurance and are not covered by Medicaid. The credit maximum is $1000 for a single adult with less than $15,000 in adjusted gross income (AGI) and phases out at $30,000. It is up to $3000 for 2 adults with 2 or more children with less than $25,000 in AGI and phases out at an AGI of $60,000. Because of the way in which the tax credit is calculated to grow between 2006 and 2014, it is likely that its economic value will decline over time, which will somewhat decrease the number of uninsured it covers.
- Individuals purchasing qualified high deductible policies (at least a $1000 deductible for a single person; $2000 for a family policy) will be able to take a deduction for the premium paid when the insurance is purchased in combination with a health savings account (HSA). Last year’s Medicare drug bill allowed people setting up these accounts to save taxes on their contributions. This new proposal adds to this tax advantage. While HSAs are available to all, the tax benefits make them more likely to be used by healthier, more affluent people and could wind up having the perverse effect of increasing premiums for those families who do not choose or cannot afford to have them.6
- Allowing association health plans (these permit small businesses to purchase health insurance through large purchasing pools, which may reduce costs) to be regulated under federal law rather than state insurance law. Through this means, the plans would be exempt from certain benefit mandates and other state regulations.
Commentary. Thorpe estimates the Bush proposal will initially cover an additional 2.4 million uninsured Americans, but this number will decrease to 2.1 million by 2014 due to the decreasing value of the refundable tax credits. He projects the total cost at $90.5 billion over the 10 years.7 The Table compares the Bush and Kerry health insurance proposals.
TABLE
Comparing costs assumed in the Bush and Kerry proposals for reforming health insurance
| Bush | Kerry* | |
|---|---|---|
| Federal cost of plan (billions) | ||
| 2005–2010 | $45.7 | 176.3 |
| 2005–2014 | $90.5 | $653.1 |
| Newly insured (millions) | ||
| 2008* | 2.4 | 26.7 |
| 2014* | 2.1 | 26.7 |
| Note: About 70% of the newly insured would claim the credit in the Bush plan expressed in 2003 figures. | ||
| *Net cost of plan including estimated federal savings. | ||
| Source: AAFP website: www.aafp.org/x22202.xml. | ||
Malpractice reform
President Bush made malpractice reform a priority issue in the current Congressional session. The key provision in his proposals is a $250,000 cap on non-economic damages, a position strongly supported by most national medical organizations (eg, AMA, AAFP). In the current Congress, a bill including this provision has passed the House but has failed to gather sufficient support for approval in the Senate.
Senator Kerry reflects the prevailing Democrat view opposing a cap on non-economic damages. Instead, he proposes prohibiting individuals from filing a suit unless a qualified specialist determines a reasonable claim exists and requiring mandatory sanctions for frivolous lawsuits.
Prescription drug costs
The Medicare prescription drug bill was reviewed in a recent issue of the JOURNAL OF FAMILY PRACTICE. Since the passage of that legislation, many Democrats continue to argue that not enough was done to provide recipients with affordable drug prices. A recent survey of Medicare recipients by the Kaiser Family Foundation showed 47% had unfavorable views of the law and 26% had favorable views, but a majority wanted Congress to fix the law, not repeal it. The survey found extensive support for allowing importation of drugs from Canada and allowing the government to negotiate the price of drugs with pharmaceutical companies.9
Senator Kerry has made these 2 proposals a prominent part of his campaign.
President Bush has resisted allowing importation of drugs from Canada on the basis of safe-ty concerns and has opposed having the government directly involved in negotiating Medicare drug prices saying that competition among private plans would lead to similar savings. As of September 2004, a handful of states had set up websites to allow residents to access information about buying medicines from Canada. The FDA has said this amounted to allowing importation of pharmaceuticals from other countries and was not allowed, but it had not moved to stop the activity.
Stem cell research
President Bush limited government funding of stem-cell research to those cell lines that were created before August 9, 2001. Research has continued in other countries and somewhat in this country through private funding, but many US medical organizations and scientists have argued that we are gradually losing ground in this field to foreign scientists.10
Senator Kerry has made the expansion of government funding of stem cell research a prominent part of his health care platform.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
As we go to press, national polls indicate a tight race in the 2004 presidential election between President George W. Bush and his Democratic challenger, Senator John Kerry.1 In polling done in late spring by the Kaiser Family Foundation, respondents said health care followed the economy, the war, and terrorism as the top concerns. Health care issues rated most important were costs and insurance, prescription drug costs, drug benefits for seniors, the uninsured, Medicare, bioterrorism, abortion, and health care quality; however, no single issue dominated.2
This article examines the health care proposals of President Bush and Senator Kerry in 4 main areas: health insurance coverage, malpractice reform, prescription drug costs, and stem cell research. The candidates’ platforms differ significantly in all areas.
Health insurance proposals
Senator Kerry’s proposal is by far the more expansive of the two:3
- Starting in 2006, enrolling uninsured children of families under 300% of the poverty line (about $56,000 for a family of 4) into Medicaid and the State-Children’s Health Insurance Program (SCHIP). Currently, this program covers children in families under 200% of the poverty level. The SCHIP is a federally funded program administered by the states that has helped decrease the percentage of uninsured children.
- Starting in 2007, enrolling uninsured parents in families under 200% of the poverty level (about $37,000 for a family of 4) into state Medicaid programs and the SCHIP. This is an innovative idea to expand the benefits of the SCHIP to adults as many parents of uninsured children are themselves uninsured. Several states have already begun experimenting with this strategy.
- Starting in 2008, enrolling single adults and childless couples in poverty into Medicaid. The poverty level for a single adult is $9310. • Allowing small businesses, workers from 55-to 64 years old, and workers between jobs to enroll new insurance pools with the same health plans offered by the Federal Employees Health Benefits Program (FEHB). This proposal would be financed by employer contributions and tax credits to employers and workers. The FEHB is often cited as an example of the kind of health insurance program to which all Americans should have access.
- A reinsurance proposal to make insurance more affordable. This fund would reimburse employer groups for an individual’s health care expenses in excess of $50,000. Eligible employer groups would have to provide insurance coverage to all of their workers, return health insurance savings to the workers in the form of reduced premiums, and introduce disease and care management programs into the worksite. Currently, while only a very small number of Americans have medical expenses in a given year that exceed this amount, their care accounts for a greater percentage of overall costs. This reinsurance proposal is designed to reduce the cost of employer funded health insurance by about 10%.
Commentary. Kenneth Thorpe, Chair of Health Policy at Emory University, estimates these proposals will provide health insurance for 27 million of the estimated 41 million Americans now unin-sured. He estimates the net costs of Kerry’s plan at $653 billion from 2005 to 2014. In Thorpe’s projections, the costs of the proposals are partially offset by savings in several areas—disease management programs, decreased reimbursements to hospitals for care of the uninsured (since there will be fewer uninsured persons), and savings due to expanded electronic filing and processing of claims.4 Kerry has proposed generating the revenue to fund this health care expansion by rolling back the tax cuts for taxpayers with incomes over $200,000. The nonpartisan Tax Policy Center estimates the revenue from rescinding these cuts at $632 billion over the same time period.5
President Bush proposes 3 strategies for reducing health insurance costs:6
- A refundable tax credit for those under 65 who lack employer-sponsored insurance and are not covered by Medicaid. The credit maximum is $1000 for a single adult with less than $15,000 in adjusted gross income (AGI) and phases out at $30,000. It is up to $3000 for 2 adults with 2 or more children with less than $25,000 in AGI and phases out at an AGI of $60,000. Because of the way in which the tax credit is calculated to grow between 2006 and 2014, it is likely that its economic value will decline over time, which will somewhat decrease the number of uninsured it covers.
- Individuals purchasing qualified high deductible policies (at least a $1000 deductible for a single person; $2000 for a family policy) will be able to take a deduction for the premium paid when the insurance is purchased in combination with a health savings account (HSA). Last year’s Medicare drug bill allowed people setting up these accounts to save taxes on their contributions. This new proposal adds to this tax advantage. While HSAs are available to all, the tax benefits make them more likely to be used by healthier, more affluent people and could wind up having the perverse effect of increasing premiums for those families who do not choose or cannot afford to have them.6
- Allowing association health plans (these permit small businesses to purchase health insurance through large purchasing pools, which may reduce costs) to be regulated under federal law rather than state insurance law. Through this means, the plans would be exempt from certain benefit mandates and other state regulations.
Commentary. Thorpe estimates the Bush proposal will initially cover an additional 2.4 million uninsured Americans, but this number will decrease to 2.1 million by 2014 due to the decreasing value of the refundable tax credits. He projects the total cost at $90.5 billion over the 10 years.7 The Table compares the Bush and Kerry health insurance proposals.
TABLE
Comparing costs assumed in the Bush and Kerry proposals for reforming health insurance
| Bush | Kerry* | |
|---|---|---|
| Federal cost of plan (billions) | ||
| 2005–2010 | $45.7 | 176.3 |
| 2005–2014 | $90.5 | $653.1 |
| Newly insured (millions) | ||
| 2008* | 2.4 | 26.7 |
| 2014* | 2.1 | 26.7 |
| Note: About 70% of the newly insured would claim the credit in the Bush plan expressed in 2003 figures. | ||
| *Net cost of plan including estimated federal savings. | ||
| Source: AAFP website: www.aafp.org/x22202.xml. | ||
Malpractice reform
President Bush made malpractice reform a priority issue in the current Congressional session. The key provision in his proposals is a $250,000 cap on non-economic damages, a position strongly supported by most national medical organizations (eg, AMA, AAFP). In the current Congress, a bill including this provision has passed the House but has failed to gather sufficient support for approval in the Senate.
Senator Kerry reflects the prevailing Democrat view opposing a cap on non-economic damages. Instead, he proposes prohibiting individuals from filing a suit unless a qualified specialist determines a reasonable claim exists and requiring mandatory sanctions for frivolous lawsuits.
Prescription drug costs
The Medicare prescription drug bill was reviewed in a recent issue of the JOURNAL OF FAMILY PRACTICE. Since the passage of that legislation, many Democrats continue to argue that not enough was done to provide recipients with affordable drug prices. A recent survey of Medicare recipients by the Kaiser Family Foundation showed 47% had unfavorable views of the law and 26% had favorable views, but a majority wanted Congress to fix the law, not repeal it. The survey found extensive support for allowing importation of drugs from Canada and allowing the government to negotiate the price of drugs with pharmaceutical companies.9
Senator Kerry has made these 2 proposals a prominent part of his campaign.
President Bush has resisted allowing importation of drugs from Canada on the basis of safe-ty concerns and has opposed having the government directly involved in negotiating Medicare drug prices saying that competition among private plans would lead to similar savings. As of September 2004, a handful of states had set up websites to allow residents to access information about buying medicines from Canada. The FDA has said this amounted to allowing importation of pharmaceuticals from other countries and was not allowed, but it had not moved to stop the activity.
Stem cell research
President Bush limited government funding of stem-cell research to those cell lines that were created before August 9, 2001. Research has continued in other countries and somewhat in this country through private funding, but many US medical organizations and scientists have argued that we are gradually losing ground in this field to foreign scientists.10
Senator Kerry has made the expansion of government funding of stem cell research a prominent part of his health care platform.
Correspondence
Eric Henley, MD, MPH, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Nagourney A. Polls show tight race with a few gains for Kerry. New York Times, August 3, 2004.
2. Kaiser Family Foundation. Kaiser Health Poll Report. July/August 2004. Available at: www.kff.org/health-pollreport/currentedition/care/index.cfm. Accessed on September 2, 2004.
3. John Kerry. for President website. John Kerry’s plan to make health care affordable to every American. Available at: www.johnkerry.com/issues/health_care/health_care.html. Accessed on September 2, 2004.
4. Thorpe K. Federal costs and savings associated with Senator Kerry’s health care plan. August 23, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
5. Senator Kerry’s. Tax Proposals Tax Policy Center. July 2004. Available at: www.taxpolicycenter.org/publications/template.cfm?PubID=8820. Accessed on September 13, 2004.
6. GeorgeBush.com. Health Care issue brief. Available at: www.georgebush.com/HealthCare/Brief.aspx. Accessed on September 2, 2004.
7. Thorpe K. Federal costs and savings associated with President Bush’s health care plan. May 5, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
8. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
9. Kaiser Family Foundation. Views of the new Medicare drug law: A survey of people on Medicare. Available at: www.kff.org/medicare/pomr081004pkg.cfm. Accessed on September 13, 2004.
10. Daley G. Missed opportunities in embryonic stem-cell research. N Engl J Med 2004;351:627-628
1. Nagourney A. Polls show tight race with a few gains for Kerry. New York Times, August 3, 2004.
2. Kaiser Family Foundation. Kaiser Health Poll Report. July/August 2004. Available at: www.kff.org/health-pollreport/currentedition/care/index.cfm. Accessed on September 2, 2004.
3. John Kerry. for President website. John Kerry’s plan to make health care affordable to every American. Available at: www.johnkerry.com/issues/health_care/health_care.html. Accessed on September 2, 2004.
4. Thorpe K. Federal costs and savings associated with Senator Kerry’s health care plan. August 23, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
5. Senator Kerry’s. Tax Proposals Tax Policy Center. July 2004. Available at: www.taxpolicycenter.org/publications/template.cfm?PubID=8820. Accessed on September 13, 2004.
6. GeorgeBush.com. Health Care issue brief. Available at: www.georgebush.com/HealthCare/Brief.aspx. Accessed on September 2, 2004.
7. Thorpe K. Federal costs and savings associated with President Bush’s health care plan. May 5, 2004. Available at: www.sph.emory.edu/hphpm.html. Accessed on September 13, 2004.
8. Henley E. What the new Medicare prescription drug bill may mean for providers and patients. J Fam Pract 2004;53:389-392.
9. Kaiser Family Foundation. Views of the new Medicare drug law: A survey of people on Medicare. Available at: www.kff.org/medicare/pomr081004pkg.cfm. Accessed on September 13, 2004.
10. Daley G. Missed opportunities in embryonic stem-cell research. N Engl J Med 2004;351:627-628
How should we manage Bell’s palsy?
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- Do steroids change the course of Bell’s palsy?
- What is the role of surgery for Bell’s palsy?
- Should antiviral therapy be initiated for all patients?
Recommendations for these management issues are found in the guideline that was funded and developed by the American Academy of Neurology. Their Quality Standards Subcommittee, Practice Committee, and Board of Directors approved the recommendations. The target audience is physicians.
Patients with Bell’s palsy are the target population of this guideline. The objective is to summarize evidence regarding effectiveness of steroids, acyclovir, or surgical facial nerve decompression for improved functional outcomes in facial nerve palsy (Bell’s palsy). The evidence categories for this guideline are therapeutic effectiveness and treatment. Outcomes considered are 1) relative rate and 95% confidence interval for goodreturn of facial function, and 2) relative rate and 95% confidence interval for completereturn of facial function. The rating scheme is updated to comply with the SORT taxonomy.1
Guideline relevance and limitations
Bell’s palsy results from damage to the 7th (facial) cranial nerve and affects 40,000 Americans each year. It is seen commonly in pregnant women and diabetics, as well as those with viral illnesses. Besides facial paralysis, other symptoms of Bell’s palsy may include pain, hypersensitivity to sound in the affected ear, and impairment of taste. The common cold sore viruses, herpes simplex virus, and other herpes viruses are the likely pathogens causing many cases of Bell’s palsy. The prognosis for Bell’s palsy is good and most patients get better within 2 weeks. Over 80% recover facial nerve function within 3 months.2
A lengthy bibliography accompanies the guideline. The guideline is weakened by lack of a cost-effectiveness analysis.
Guideline development and evidence review
The authors searched the National Library of Medicine’s Medline database from 1966 to June 2000. The resultant prospective studies for treatments with steroids, acyclovir, or surgery were screened for outcome evidence. There are 25 references. A meta-analysis of patient data and a systematic review of the evidence were performed. Quality and strength of evidence were weighted according to a rating scheme.
Grade B Recommendations
- Treatment with oral corticosteroids improves facial function.
- Treatment with acyclovir, combined with steroids, improves facial function.
Grade C Recommendations
- Facial nerve decompression does not improve facial function.
Source for this guideline
Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:830–836.
Other guidelines on bell’s palsy
- Assessment: Neurologic risk of immunization. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology.
This older guideline refers to a few cases of Bell’s palsy associated with the plasma-derived hepatitis B vaccine used from 1982 to 1988. Since then the recombinant product has replaced the plasma-derived vaccine.
Source: Fenichel GM. Assessment: Neurologic risk of immunization: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology1999; 52:1546–1552. [30 references]
CORRECTION
The photographs in the Figure appearing in last month’s Clinical Inquiry, “Do routine eye exams reduce occurrence of blindness from type 2 dia-betes?” (page 733), were transposed. They appear correctly below.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
1. Ebell M, Siwek J, Weiss BD et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
2. NINDS Bell’s Palsy Information Page National Institute of Neurological Disorders and Stroke.April 2003. Access at: www.ninds.nih.gov/health_and_medical/disorders/bells_doc.htm. Accessed on August 6, 2004.
How does HIPAA affect public health reporting?
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].
Since the Health Insurance Portability and Accountability Act (HIPAA) privacy rule was put into effect in April 2003, healthcare providers have sometimes been confused about what information they can legally disclose to public health agencies. A clear understanding of permissible disclosure will enable family physicians to continue their important role of providing individual patient information for the critical activities of disease surveillance, outbreak investigation, monitoring causes of death and birth complications, assuring health care services, conducting public health research, and formulating health policy.
HIPAA does not prohibit disclosure for public health purposes
The HIPAA is intended to protect the public from unauthorized access to, use of, and disclosure of individually identifiable health information. It places responsibility on health care providers to avoid using or disclosing protected health information (PHI) unless authorized by the person to whom it pertains, or unless the disclosure or use is required or permitted by regulation or law. Specifically excluded from the requirement for individual authorization are disclosures for public health activities. This means that sharing PHI for public health purposes is permitted as long as the agency to which the information is provided is legally authorized to collect and receive the information (see Lawful recipients of personal health information).
This specific exclusion was allowed because public health authorities have a legitimate need for PHI to ensure public health and safety, and because public health agencies have a track record of protecting the confidentiality of PHI. The HIPAA privacy rule attempts to strike a balance between individual privacy rights and the need for public protection.
Public health agencies included in this category include state, territorial, tribal, and local health departments, as well as federal health agencies such as the Centers for Disease Control and Prevention, the Food and Drug Administration, the National Institutes of Health, the Occupational Safety and Health Administration, the Substance Abuse and Mental Health Services Administration, and others. It also includes individuals and agencies working under a grant of authority from a public health agency.
Lawful disclosure: Examples
It’s instructive to consider how this public health HIPAA exception plays out in the daily practice of medicine. First, some definitions:
Protected Health Information.Individually identifiable health information transmitted electronically or any other way. It includes information about past, present, or anticipated mental or physical health, and the provision of or payment for health care.
Covered entities. These are the entities who must adhere to the HIPAA rules. Included are health care providers, health plans, and health care clearinghouses that transmit any health information in an electronic format
Personal Identifiers. Information that can be used to find the identity of an individual to link them to their PHI.
Scenario 1
A family physician’s patient dies at home. The physician is asked to fill out a death certificate, which contains PHI as defined by the HIPAA privacy rule. Is this permitted without family authorization?
Unauthorized disclosure is permitted. Vital statistics—required information on death and birth certificates—has not been changed by HIPAA. The information required on the death certificate can be provided without authorization.
Scenario 2
A patient is diagnosed with tuberculosis.This is a reportable disease per the state health code. Can the physician report the PHI requested on the disease reporting form?
Unauthorized disclosure is permitted. Each state health authority requires health care providers to report information about individuals who have contracted a disease of public health significance. Reportable disease lists differ by jurisdiction, and physicians should be aware of the diseases reportable in their areas and how the information is to be reported. Individual authorization for release of PHI in these disease reports is not required by HIPAA.
Scenario 3
A physician examines an infant who has unexplained injuries. Child abuse is suspected. Is child abuse reporting exempted from the privacy rule?
Unauthorized disclosure is permitted. Reporting of child abuse and neglect is exempted. This information may even be reported to a non-health agency, such as a child protective service, as long as the reportable information is required by law, and individual authorization is not required.
Scenario 4
A patient suffers what appears to be an adverse reaction to a medication. The FDA adverse event reporting form asks for PHI. Can a physician report PHI in this instance without patient authorization?
Unauthorized disclosure is permitted. Reporting of adverse events or reactions from drugs, food, biological products, and medical devices is still permitted without authorization.
Scenario 5
A patient is newly diagnosed with lung cancer. The state maintains a cancer registry and physicians are required to report PHI about patients with cancer. In this state the cancer registry is maintained by the university under contract with the State Health Department. Is reporting permitted without patient authorization?
Unauthorized disclosure is permitted. Cancer and immunization registry reporting of PHI is still permitted even if the entity responsible for the registry is not a public health agency, as long as it is under the authority of the agency to perform this public health function.
Scenario 6
A patient dies from meningitis and the local health department requests to view the hospital record to investigate cause of death. The cause turns out to be West Nile virus, which is not on the list of reportable diseases. Is the health department permitted to view the record and is authorization required?
Unauthorized disclosure is permitted. The privacy rule exception does not require a law or regulation specifically mandating disclosure. The health care provider can release requested information to a public health authority when the information is for the purpose of controlling disease, injury, or disability. The information released should be the minimum necessary for the stated public health purpose, and the provider can rely on the agency to determine what that information is. In this case, examination of the record is permitted and authorization is not required.
Scenario 7
An auditor from the Vaccine for Children program arrives at the office and requests to see patient records to audit adherence to the rules governing this program. Is the auditor allowed to exam records, and is authorization required?
Unauthorized disclosure is permitted. Patient records can be reviewed by staff of public health agencies authorized by law to collect PHI for program management purposes. No patient authorization is required.
Scenario 8
A local community agency is concerned about the potential health effects of groundwater contamination. They request information about all your patients who have contracted cancer within the past 5 years. What information can you provide them?
PHI disclosure requires patient authorization. This agency, unless under the authority of a public health agency to collect PHI, cannot obtain PHI without patient authorization. However, deidentified information could be provided. Deidentified data are not covered by HIPAA and do not require individual privacy protection or authorization for release. De-identification means removing 18 “identifiers” (Table) or enough information that allows a statistician to conclude that the chance of an individual being identified is remote.
TABLE
Individual identifiers to be removed from reports
| The following 18 identifiers of a person, or of relatives, employers, or household members of a person must be removed, and the covered entity must not have actual knowledge that the information could be used alone or in combination with other information to identify the individual, for the information to be considered de-identified and not protected health information. |
|---|
| • Names |
| • All geographic subdivisions smaller than a state, including county, city, street address, precinct, zip code (first 3 digits OK if geographic unit contains >20,000 persons), and their equivalent geocodes |
| • All elements of dates (except year) directly related to an individual; all ages >89 and all elements of dates (including year) indicative of such age (except for an aggregate into a single category of age >90) |
| • Telephone numbers |
| • Fax numbers |
| • Electronic mail addresses |
| • Social Security numbers |
| • Medical record numbers |
| • Health-plan beneficiary numbers |
| • Account numbers |
| • Certificate and license numbers |
| • Vehicle identifiers and serial numbers, including license plate numbers |
| • Medical device identifiers and serial numbers |
| • Internet universal resource locators (URLs) |
| • Internet protocol (IP) addresses |
| • Biometric identifiers, including fingerprints and voice prints |
| • Full-face photographic images and any comparable images |
| • Any other unique identifying number, characteristic, or code, except that covered identities may, under certain circumstances, assign a code or other means of record identification that allows de-identified information to be re-identified. |
| Source: “HIPAA privacy rule and public health,” Morbidity and Mortality Weekly Report, April 11, 2003; 52:1–12. |
Physician obligations with disclosure
Confirm the legitimacy of a request. Even though physicians can release PHI to public health agencies without a patient’s authorization, they have other obligations to meet. One of these is to ensure that the person or agency requesting PHI is a legitimate public health authority. If the request is made in person, some form of credentials or proof of government status should be provided. If the request is in writing, it should be on official letterhead. A person or agency acting under the authority of a pubic health agency should provide proof of this authority. If physicians have any doubt about the authenticity of a request, they should call the agency being represented and inquire.
Let patients know. The second obligation is to provide information about the disclosure to the individual whose PHI was released, if this information is requested, and to inform patients in statements about privacy practices that PHI information is released to public health agencies when required and permitted by law.
Other exceptions to HIPAA
HIPAA allows the legitimate use of PHI, without authorization, for the purpose of protecting the public under conditions involving law enforcement, court proceedings, worker’s compensation, and national security. These exceptions are outside the scope of this article.
Explain HIPAA to patients
The trend toward electronic medical records and the increasing public concern about privacy led to the enactment of the HIPAA privacy rule. A natural tension exists between individual rights and public protection, and the HIPAA privacy rule attempts to balance these competing concerns. For patients who are concerned about confidentiality, family physicians can explain the purpose of public health exceptions and give reassurance about how public health agencies have a good record of protecting individuals’ identity.
Correspondence
4001 North Third #415, Phoenix, AZ 85012. E-mail: [email protected].
Mad Cow disease: Dealing sensibly with a new concern
After a period out of the spotlight, Mad Cow disease is again causing a stir. Following the first documented case in this country on December 23, 2003,1 the US government is instituting new preventive measures, and patients may be asking for assurances of safe-ty (see “What to advise patients,” page 565).
Mad Cow’s connection to humans: vCJD
Mad Cow disease is the bovine form of transmissible spongiform encephalopathy (TSE), a disease that can also affect sheep, deer, goats, and humans (Table 1). The causative agent is thought to be an infective protein called a prion, discovered in 1997.
Bovine spongiform encephalopathy (BSE) was first identified in the United Kingdom in 1986 and caused a large outbreak in cattle, which peaked in 1993. Subsequently, it was discovered that BSE could rarely spread to humans, causing a variant of Creutzfeldt-Jakob disease (vCJD) that is universally fatal. As of December 2003, 153 cases of vCJD had been reported worldwide, most in the UK. Confirmation of either the classic or variant form requires pathology examination of brain tissue collected by biopsy or, if a patient has died, at autopsy.2
TABLE 1
Transmissible spongiform encephalopathies
| Species affected | Prion disease | Transmissible to humans? |
|---|---|---|
| Mink | Transmissible mink encephalopathy | No |
| Sheep and goats | Scrapie | Historically no; questionable in newly discovered atypical cases |
| Deer and elk | Chronic wasting disease | Possible (under investigation) |
| Cattle and bison | Bovine spongiform encephalopathy | Yes (variant CJD) |
| Humans | Creutzfeldt-jakob disease; variant CJD, Gerstmann-Straussler-Scheinker disease, Kuru, fatal familial insomnia | Through contaminated medical products, instruments, possibly blood |
Uniqueness of vCJD
In medical school, family physicians learned about classic CJD, which is endemic throughout the world and, in the US, causes an average of 1 death per million people per year. The epidemiology of vCJD and CJD are quite different (Table 2). Because vCJD is a new disease, its incubation period is unknown, but it is likely to be years or decades. In the UK it is thought that exposure to BSE-contaminated food from 1984–86 and the onset of vCJD cases in 1994–96 is consistent with such a long incubation period.
Since 1986, BSE has been identified in 20 European countries, Japan, Israel, Canada, and now the US. The main method of its spread through herds is believed to be the former practice of feeding cattle the meat and bone meal products that, at some point, were contaminated with BSE. In 1997, the US and Canada prohibited the feeding of ruminant meat and bone meal to other ruminants. It is thought that most cases of vCJD are transmitted to people when they eat beef products containing brain or spinal cord material contaminated with BSE.
Neuropathology. Variant CJD deposits plaques, vacuoles, and prion protein in the brain. To date, all persons with vCJD have had methio-nine homozygosity at the polymorphic codon 129 of the prion protein gene, suggesting that persons not carrying this genotype (who make 60% of the population) have increased resistance to the disease. In addition, vCJD and BSE are both dose-dependent infections, so both genetics and exposure may explain why so few human cases have occurred despite the widespread outbreak of BSE in the UK.
TABLE 2
Characteristics distinguishing vCJD from CJD
| Characteristic | UK vCJD | US classic CJD |
|---|---|---|
| Median age at death | 28 (range, 14–74) | 68 (range, 23–97)* |
| Median illness duration (mo) | 13–14 | 4–5 |
| Clinical presentation | Prominent psychiatric/behavioral symptoms; delayed neurologic signs | Dementia; early neurologic signs |
| Periodic sharp waves on EEG | Absent | Often present |
| “Pulvinar sign” on MRI† | Present in >75% of cases | Not reported |
| Presense of “florid plaques” on neuropathology | Present in great numbers | Rare or absent |
| Immunohistochemical analysis of brain tissue | Marked accumulation of PrPres | Variable accumulation |
| Presense of agent in lymphoid tissue | Readily detected | Not readily detected |
| Increased glycoform ratio on immunoblot analysis of PrPres | Present | Not present |
| Genotype at codon 129 of prion protein | Methionine/Methionine | Polymorphic |
| *Surveillance data 1997–2001. | ||
| † High signal in the posterior thalmus. | ||
| CJD; Creutzfeldt-Jakob disease; vCJD, variant CJD; EEG, electoencephalogram; MRI, magnetic resonance imaging; | ||
| PrPres, protease-resistant prion protein. | ||
| Source: Centers for Disease Control and Prevention, MMWR Morb Mortal Wkly Rep 2004; 52:1280–1285.1 | ||
Prevention measures have been updated
Before December 30, 2003, prevention measures in place to prevent BSE in this country were the following:
- Import restrictions on bovine-derived consumer products from high-risk BSE countries (initiated in 1989).
- Prohibition of the use of ruminant derived meat and bone meal in cattle feed (initiated in 1997).
- A surveillance system for BSE that involved annual testing of between 5000 and 20,000 cattle slaughtered for human consumption (out of about 35 million cattle slaughtered per year).
Since December 30, 2003, the US Department of Agriculture (USDA) and Food and Drug Administration (FDA) have added or proposed a number of additional provisions to prevent BSE:
- Defining high-risk materials banned for human consumption, including the entire verte-bral column.
- Banning the use of advanced meat recovery systems on vertebral columns. These systems use brushes and air to blast soft tissue off of bone and led to up to 30% of hamburger sampled to be contaminated with central nervous system tissue.
- Proposing an expanded annual surveillance to include about 200,000 high-risk cattle (sick, suspect, dead) and a random sample of 20,000 normal cattle over 30 months old.
But are these measures enough?
Concerns about these new measures center on the surveillance program. First, how long will it take the USDA to expand its testing? Second, will even this expanded testing be sufficient? Some scientists and consumer advocates propose adopting the policy of the European Union, which is to test all cattle over 30 months of age, since this age group can harbor BSE without being ill.
Other congressional proposals include ban-ning all high-risk meat products from all animal feeds and cosmetics, and creating a prion disease task force to coordinate surveillance and research for all prion diseases. Unfortunately, because we have been testing so few cattle for BSE, we don’t really know if there are more infected cattle in our food system. Interestingly, in Japan, where all cattle are tested for BSE after slaughter, 10 more infected animals were discovered, most of which lacked the characteristics that would put them at high risk.3
To date, the beef industry has supported the changes already put into effect, but not the additional ones noted above. Ironically, a number of small, upscale slaughterhouses have proposed testing all cattle they slaughter (mostly under 30 months old) so they may resume sales to Japan. The USDA has turned down their requests for the chemical reagents to run the BSE tests (the agency controls the sale of these kits), citing its concern that testing all cattle would give the impression it is necessary for the entire US herd—a proposition the USDA and many scientists believe is unnecessary. Thus, the controversy over BSE surveillance has now become an economic, political, and scientific issue.
What to advise patients
The risk of contracting vCJD from eating contaminated beef is extremely small.4
There has yet to be a case of BSE found in any native-born US cattle.
There is no association between BSE and milk or milk products.
If traveling to countries where BSE is endem-ic—ie, UK and Portugal—patients may avoid beef altogether or limit consumption to whole cuts, not ground beef or sausage.5
Avoid bovine-derived nutritional supplements, especially those containing bovine pitu-itary, thyroid, adrenal, thymus, or other organ tissue.
Avoid products containing bovine meat or bone meal, such as some types of garden fertilizers.
Deer and elk can develop chronic wasting disease (CWD), another form of TSE. States that have recorded CWD cases include Colorado, Illinois, Wisconsin, and Wyoming. CWD is not known to cause disease in humans, but the risk to hunters and those who eat the meat is unknown. Physicians may want to advise hunters to have deer and elk hunted in CWD areas tested and only CWD negative animals processed for meat. Guidelines for field dressing deer and elk to prevent possible contamination of meat are available at state Departments of Natural Resources.
Investigating suspected disease
Physicians who suspect a patient may have vCJD or CJD, or that a patient has died of such disease, should advocate for brain biopsy or autopsy. The National Prion Disease Pathology Surveillance Center at Case Western University (funded by the Centers for Disease Control and Prevention) provides diagnostic services free of charge to physicians and health departments (available at www.cjdsurveillance.com).
Federal agencies, Congress, and the public became more aware of BSE on December 23, 2003, when the US Department of Agriculture (USDA) diagnosed the disease in a dairy cow in Washington state.1 The cow, traced to a herd originating in Canada, was 6.5 years old and had been slaughtered on December 9. Whether the cow was a “downer” (nonambulatory) is still under investigation. Downer cows are automatically tested; however, it is possible this cow was tested as part of a routine surveillance system rather than because it was at high risk of disease. Regardless, the carcass was released for use as food while tissues considered more risky for BSE transmission (brain, spinal cord, and small intestine) were kept from the human food supply.
After the case was diagnosed, the USDA recalled all meat from cattle slaughtered at that plant the same day. Unfortunately about 30,000 pounds of potentially contaminated meat was never recovered and ended up on consumers’ plates.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC) Bovine spongiform encephalopathy in a dairy cow—Washington State, 2003. MMWR Morb Mortal Wkly Rep 2004;52:1280-1285.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5253a2.htm. Accessed on July 15, 2004.
2. CDC.BSE and CJD information and resources. Available at: www.cdc.gov/ncidod/diseases/cjd/cjd.html. Accessed on July 15, 2004.
3. Kaufman M. They’re not allowed to test for Mad Cow. Washington Post National Weekly, May 3–9, 2004;21.-
4. US Food and Drug Administration, Center for Food Safety and Applied Nutrition Commonly asked questions about BSE in products regulated by FDA’s Center for Food Safety and Applied Nutrition (CFSAN). Available at: www.cfsan.fda.gov/~comm/bsefaq.html. Accessed on July 15, 2004.
5. CDC. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. Available at: www.cdc.gov/travel/diseases/madcow.htm. Accessed on July 15, 2004.
After a period out of the spotlight, Mad Cow disease is again causing a stir. Following the first documented case in this country on December 23, 2003,1 the US government is instituting new preventive measures, and patients may be asking for assurances of safe-ty (see “What to advise patients,” page 565).
Mad Cow’s connection to humans: vCJD
Mad Cow disease is the bovine form of transmissible spongiform encephalopathy (TSE), a disease that can also affect sheep, deer, goats, and humans (Table 1). The causative agent is thought to be an infective protein called a prion, discovered in 1997.
Bovine spongiform encephalopathy (BSE) was first identified in the United Kingdom in 1986 and caused a large outbreak in cattle, which peaked in 1993. Subsequently, it was discovered that BSE could rarely spread to humans, causing a variant of Creutzfeldt-Jakob disease (vCJD) that is universally fatal. As of December 2003, 153 cases of vCJD had been reported worldwide, most in the UK. Confirmation of either the classic or variant form requires pathology examination of brain tissue collected by biopsy or, if a patient has died, at autopsy.2
TABLE 1
Transmissible spongiform encephalopathies
| Species affected | Prion disease | Transmissible to humans? |
|---|---|---|
| Mink | Transmissible mink encephalopathy | No |
| Sheep and goats | Scrapie | Historically no; questionable in newly discovered atypical cases |
| Deer and elk | Chronic wasting disease | Possible (under investigation) |
| Cattle and bison | Bovine spongiform encephalopathy | Yes (variant CJD) |
| Humans | Creutzfeldt-jakob disease; variant CJD, Gerstmann-Straussler-Scheinker disease, Kuru, fatal familial insomnia | Through contaminated medical products, instruments, possibly blood |
Uniqueness of vCJD
In medical school, family physicians learned about classic CJD, which is endemic throughout the world and, in the US, causes an average of 1 death per million people per year. The epidemiology of vCJD and CJD are quite different (Table 2). Because vCJD is a new disease, its incubation period is unknown, but it is likely to be years or decades. In the UK it is thought that exposure to BSE-contaminated food from 1984–86 and the onset of vCJD cases in 1994–96 is consistent with such a long incubation period.
Since 1986, BSE has been identified in 20 European countries, Japan, Israel, Canada, and now the US. The main method of its spread through herds is believed to be the former practice of feeding cattle the meat and bone meal products that, at some point, were contaminated with BSE. In 1997, the US and Canada prohibited the feeding of ruminant meat and bone meal to other ruminants. It is thought that most cases of vCJD are transmitted to people when they eat beef products containing brain or spinal cord material contaminated with BSE.
Neuropathology. Variant CJD deposits plaques, vacuoles, and prion protein in the brain. To date, all persons with vCJD have had methio-nine homozygosity at the polymorphic codon 129 of the prion protein gene, suggesting that persons not carrying this genotype (who make 60% of the population) have increased resistance to the disease. In addition, vCJD and BSE are both dose-dependent infections, so both genetics and exposure may explain why so few human cases have occurred despite the widespread outbreak of BSE in the UK.
TABLE 2
Characteristics distinguishing vCJD from CJD
| Characteristic | UK vCJD | US classic CJD |
|---|---|---|
| Median age at death | 28 (range, 14–74) | 68 (range, 23–97)* |
| Median illness duration (mo) | 13–14 | 4–5 |
| Clinical presentation | Prominent psychiatric/behavioral symptoms; delayed neurologic signs | Dementia; early neurologic signs |
| Periodic sharp waves on EEG | Absent | Often present |
| “Pulvinar sign” on MRI† | Present in >75% of cases | Not reported |
| Presense of “florid plaques” on neuropathology | Present in great numbers | Rare or absent |
| Immunohistochemical analysis of brain tissue | Marked accumulation of PrPres | Variable accumulation |
| Presense of agent in lymphoid tissue | Readily detected | Not readily detected |
| Increased glycoform ratio on immunoblot analysis of PrPres | Present | Not present |
| Genotype at codon 129 of prion protein | Methionine/Methionine | Polymorphic |
| *Surveillance data 1997–2001. | ||
| † High signal in the posterior thalmus. | ||
| CJD; Creutzfeldt-Jakob disease; vCJD, variant CJD; EEG, electoencephalogram; MRI, magnetic resonance imaging; | ||
| PrPres, protease-resistant prion protein. | ||
| Source: Centers for Disease Control and Prevention, MMWR Morb Mortal Wkly Rep 2004; 52:1280–1285.1 | ||
Prevention measures have been updated
Before December 30, 2003, prevention measures in place to prevent BSE in this country were the following:
- Import restrictions on bovine-derived consumer products from high-risk BSE countries (initiated in 1989).
- Prohibition of the use of ruminant derived meat and bone meal in cattle feed (initiated in 1997).
- A surveillance system for BSE that involved annual testing of between 5000 and 20,000 cattle slaughtered for human consumption (out of about 35 million cattle slaughtered per year).
Since December 30, 2003, the US Department of Agriculture (USDA) and Food and Drug Administration (FDA) have added or proposed a number of additional provisions to prevent BSE:
- Defining high-risk materials banned for human consumption, including the entire verte-bral column.
- Banning the use of advanced meat recovery systems on vertebral columns. These systems use brushes and air to blast soft tissue off of bone and led to up to 30% of hamburger sampled to be contaminated with central nervous system tissue.
- Proposing an expanded annual surveillance to include about 200,000 high-risk cattle (sick, suspect, dead) and a random sample of 20,000 normal cattle over 30 months old.
But are these measures enough?
Concerns about these new measures center on the surveillance program. First, how long will it take the USDA to expand its testing? Second, will even this expanded testing be sufficient? Some scientists and consumer advocates propose adopting the policy of the European Union, which is to test all cattle over 30 months of age, since this age group can harbor BSE without being ill.
Other congressional proposals include ban-ning all high-risk meat products from all animal feeds and cosmetics, and creating a prion disease task force to coordinate surveillance and research for all prion diseases. Unfortunately, because we have been testing so few cattle for BSE, we don’t really know if there are more infected cattle in our food system. Interestingly, in Japan, where all cattle are tested for BSE after slaughter, 10 more infected animals were discovered, most of which lacked the characteristics that would put them at high risk.3
To date, the beef industry has supported the changes already put into effect, but not the additional ones noted above. Ironically, a number of small, upscale slaughterhouses have proposed testing all cattle they slaughter (mostly under 30 months old) so they may resume sales to Japan. The USDA has turned down their requests for the chemical reagents to run the BSE tests (the agency controls the sale of these kits), citing its concern that testing all cattle would give the impression it is necessary for the entire US herd—a proposition the USDA and many scientists believe is unnecessary. Thus, the controversy over BSE surveillance has now become an economic, political, and scientific issue.
What to advise patients
The risk of contracting vCJD from eating contaminated beef is extremely small.4
There has yet to be a case of BSE found in any native-born US cattle.
There is no association between BSE and milk or milk products.
If traveling to countries where BSE is endem-ic—ie, UK and Portugal—patients may avoid beef altogether or limit consumption to whole cuts, not ground beef or sausage.5
Avoid bovine-derived nutritional supplements, especially those containing bovine pitu-itary, thyroid, adrenal, thymus, or other organ tissue.
Avoid products containing bovine meat or bone meal, such as some types of garden fertilizers.
Deer and elk can develop chronic wasting disease (CWD), another form of TSE. States that have recorded CWD cases include Colorado, Illinois, Wisconsin, and Wyoming. CWD is not known to cause disease in humans, but the risk to hunters and those who eat the meat is unknown. Physicians may want to advise hunters to have deer and elk hunted in CWD areas tested and only CWD negative animals processed for meat. Guidelines for field dressing deer and elk to prevent possible contamination of meat are available at state Departments of Natural Resources.
Investigating suspected disease
Physicians who suspect a patient may have vCJD or CJD, or that a patient has died of such disease, should advocate for brain biopsy or autopsy. The National Prion Disease Pathology Surveillance Center at Case Western University (funded by the Centers for Disease Control and Prevention) provides diagnostic services free of charge to physicians and health departments (available at www.cjdsurveillance.com).
Federal agencies, Congress, and the public became more aware of BSE on December 23, 2003, when the US Department of Agriculture (USDA) diagnosed the disease in a dairy cow in Washington state.1 The cow, traced to a herd originating in Canada, was 6.5 years old and had been slaughtered on December 9. Whether the cow was a “downer” (nonambulatory) is still under investigation. Downer cows are automatically tested; however, it is possible this cow was tested as part of a routine surveillance system rather than because it was at high risk of disease. Regardless, the carcass was released for use as food while tissues considered more risky for BSE transmission (brain, spinal cord, and small intestine) were kept from the human food supply.
After the case was diagnosed, the USDA recalled all meat from cattle slaughtered at that plant the same day. Unfortunately about 30,000 pounds of potentially contaminated meat was never recovered and ended up on consumers’ plates.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
After a period out of the spotlight, Mad Cow disease is again causing a stir. Following the first documented case in this country on December 23, 2003,1 the US government is instituting new preventive measures, and patients may be asking for assurances of safe-ty (see “What to advise patients,” page 565).
Mad Cow’s connection to humans: vCJD
Mad Cow disease is the bovine form of transmissible spongiform encephalopathy (TSE), a disease that can also affect sheep, deer, goats, and humans (Table 1). The causative agent is thought to be an infective protein called a prion, discovered in 1997.
Bovine spongiform encephalopathy (BSE) was first identified in the United Kingdom in 1986 and caused a large outbreak in cattle, which peaked in 1993. Subsequently, it was discovered that BSE could rarely spread to humans, causing a variant of Creutzfeldt-Jakob disease (vCJD) that is universally fatal. As of December 2003, 153 cases of vCJD had been reported worldwide, most in the UK. Confirmation of either the classic or variant form requires pathology examination of brain tissue collected by biopsy or, if a patient has died, at autopsy.2
TABLE 1
Transmissible spongiform encephalopathies
| Species affected | Prion disease | Transmissible to humans? |
|---|---|---|
| Mink | Transmissible mink encephalopathy | No |
| Sheep and goats | Scrapie | Historically no; questionable in newly discovered atypical cases |
| Deer and elk | Chronic wasting disease | Possible (under investigation) |
| Cattle and bison | Bovine spongiform encephalopathy | Yes (variant CJD) |
| Humans | Creutzfeldt-jakob disease; variant CJD, Gerstmann-Straussler-Scheinker disease, Kuru, fatal familial insomnia | Through contaminated medical products, instruments, possibly blood |
Uniqueness of vCJD
In medical school, family physicians learned about classic CJD, which is endemic throughout the world and, in the US, causes an average of 1 death per million people per year. The epidemiology of vCJD and CJD are quite different (Table 2). Because vCJD is a new disease, its incubation period is unknown, but it is likely to be years or decades. In the UK it is thought that exposure to BSE-contaminated food from 1984–86 and the onset of vCJD cases in 1994–96 is consistent with such a long incubation period.
Since 1986, BSE has been identified in 20 European countries, Japan, Israel, Canada, and now the US. The main method of its spread through herds is believed to be the former practice of feeding cattle the meat and bone meal products that, at some point, were contaminated with BSE. In 1997, the US and Canada prohibited the feeding of ruminant meat and bone meal to other ruminants. It is thought that most cases of vCJD are transmitted to people when they eat beef products containing brain or spinal cord material contaminated with BSE.
Neuropathology. Variant CJD deposits plaques, vacuoles, and prion protein in the brain. To date, all persons with vCJD have had methio-nine homozygosity at the polymorphic codon 129 of the prion protein gene, suggesting that persons not carrying this genotype (who make 60% of the population) have increased resistance to the disease. In addition, vCJD and BSE are both dose-dependent infections, so both genetics and exposure may explain why so few human cases have occurred despite the widespread outbreak of BSE in the UK.
TABLE 2
Characteristics distinguishing vCJD from CJD
| Characteristic | UK vCJD | US classic CJD |
|---|---|---|
| Median age at death | 28 (range, 14–74) | 68 (range, 23–97)* |
| Median illness duration (mo) | 13–14 | 4–5 |
| Clinical presentation | Prominent psychiatric/behavioral symptoms; delayed neurologic signs | Dementia; early neurologic signs |
| Periodic sharp waves on EEG | Absent | Often present |
| “Pulvinar sign” on MRI† | Present in >75% of cases | Not reported |
| Presense of “florid plaques” on neuropathology | Present in great numbers | Rare or absent |
| Immunohistochemical analysis of brain tissue | Marked accumulation of PrPres | Variable accumulation |
| Presense of agent in lymphoid tissue | Readily detected | Not readily detected |
| Increased glycoform ratio on immunoblot analysis of PrPres | Present | Not present |
| Genotype at codon 129 of prion protein | Methionine/Methionine | Polymorphic |
| *Surveillance data 1997–2001. | ||
| † High signal in the posterior thalmus. | ||
| CJD; Creutzfeldt-Jakob disease; vCJD, variant CJD; EEG, electoencephalogram; MRI, magnetic resonance imaging; | ||
| PrPres, protease-resistant prion protein. | ||
| Source: Centers for Disease Control and Prevention, MMWR Morb Mortal Wkly Rep 2004; 52:1280–1285.1 | ||
Prevention measures have been updated
Before December 30, 2003, prevention measures in place to prevent BSE in this country were the following:
- Import restrictions on bovine-derived consumer products from high-risk BSE countries (initiated in 1989).
- Prohibition of the use of ruminant derived meat and bone meal in cattle feed (initiated in 1997).
- A surveillance system for BSE that involved annual testing of between 5000 and 20,000 cattle slaughtered for human consumption (out of about 35 million cattle slaughtered per year).
Since December 30, 2003, the US Department of Agriculture (USDA) and Food and Drug Administration (FDA) have added or proposed a number of additional provisions to prevent BSE:
- Defining high-risk materials banned for human consumption, including the entire verte-bral column.
- Banning the use of advanced meat recovery systems on vertebral columns. These systems use brushes and air to blast soft tissue off of bone and led to up to 30% of hamburger sampled to be contaminated with central nervous system tissue.
- Proposing an expanded annual surveillance to include about 200,000 high-risk cattle (sick, suspect, dead) and a random sample of 20,000 normal cattle over 30 months old.
But are these measures enough?
Concerns about these new measures center on the surveillance program. First, how long will it take the USDA to expand its testing? Second, will even this expanded testing be sufficient? Some scientists and consumer advocates propose adopting the policy of the European Union, which is to test all cattle over 30 months of age, since this age group can harbor BSE without being ill.
Other congressional proposals include ban-ning all high-risk meat products from all animal feeds and cosmetics, and creating a prion disease task force to coordinate surveillance and research for all prion diseases. Unfortunately, because we have been testing so few cattle for BSE, we don’t really know if there are more infected cattle in our food system. Interestingly, in Japan, where all cattle are tested for BSE after slaughter, 10 more infected animals were discovered, most of which lacked the characteristics that would put them at high risk.3
To date, the beef industry has supported the changes already put into effect, but not the additional ones noted above. Ironically, a number of small, upscale slaughterhouses have proposed testing all cattle they slaughter (mostly under 30 months old) so they may resume sales to Japan. The USDA has turned down their requests for the chemical reagents to run the BSE tests (the agency controls the sale of these kits), citing its concern that testing all cattle would give the impression it is necessary for the entire US herd—a proposition the USDA and many scientists believe is unnecessary. Thus, the controversy over BSE surveillance has now become an economic, political, and scientific issue.
What to advise patients
The risk of contracting vCJD from eating contaminated beef is extremely small.4
There has yet to be a case of BSE found in any native-born US cattle.
There is no association between BSE and milk or milk products.
If traveling to countries where BSE is endem-ic—ie, UK and Portugal—patients may avoid beef altogether or limit consumption to whole cuts, not ground beef or sausage.5
Avoid bovine-derived nutritional supplements, especially those containing bovine pitu-itary, thyroid, adrenal, thymus, or other organ tissue.
Avoid products containing bovine meat or bone meal, such as some types of garden fertilizers.
Deer and elk can develop chronic wasting disease (CWD), another form of TSE. States that have recorded CWD cases include Colorado, Illinois, Wisconsin, and Wyoming. CWD is not known to cause disease in humans, but the risk to hunters and those who eat the meat is unknown. Physicians may want to advise hunters to have deer and elk hunted in CWD areas tested and only CWD negative animals processed for meat. Guidelines for field dressing deer and elk to prevent possible contamination of meat are available at state Departments of Natural Resources.
Investigating suspected disease
Physicians who suspect a patient may have vCJD or CJD, or that a patient has died of such disease, should advocate for brain biopsy or autopsy. The National Prion Disease Pathology Surveillance Center at Case Western University (funded by the Centers for Disease Control and Prevention) provides diagnostic services free of charge to physicians and health departments (available at www.cjdsurveillance.com).
Federal agencies, Congress, and the public became more aware of BSE on December 23, 2003, when the US Department of Agriculture (USDA) diagnosed the disease in a dairy cow in Washington state.1 The cow, traced to a herd originating in Canada, was 6.5 years old and had been slaughtered on December 9. Whether the cow was a “downer” (nonambulatory) is still under investigation. Downer cows are automatically tested; however, it is possible this cow was tested as part of a routine surveillance system rather than because it was at high risk of disease. Regardless, the carcass was released for use as food while tissues considered more risky for BSE transmission (brain, spinal cord, and small intestine) were kept from the human food supply.
After the case was diagnosed, the USDA recalled all meat from cattle slaughtered at that plant the same day. Unfortunately about 30,000 pounds of potentially contaminated meat was never recovered and ended up on consumers’ plates.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC) Bovine spongiform encephalopathy in a dairy cow—Washington State, 2003. MMWR Morb Mortal Wkly Rep 2004;52:1280-1285.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5253a2.htm. Accessed on July 15, 2004.
2. CDC.BSE and CJD information and resources. Available at: www.cdc.gov/ncidod/diseases/cjd/cjd.html. Accessed on July 15, 2004.
3. Kaufman M. They’re not allowed to test for Mad Cow. Washington Post National Weekly, May 3–9, 2004;21.-
4. US Food and Drug Administration, Center for Food Safety and Applied Nutrition Commonly asked questions about BSE in products regulated by FDA’s Center for Food Safety and Applied Nutrition (CFSAN). Available at: www.cfsan.fda.gov/~comm/bsefaq.html. Accessed on July 15, 2004.
5. CDC. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. Available at: www.cdc.gov/travel/diseases/madcow.htm. Accessed on July 15, 2004.
1. Centers for Disease Control and Prevention (CDC) Bovine spongiform encephalopathy in a dairy cow—Washington State, 2003. MMWR Morb Mortal Wkly Rep 2004;52:1280-1285.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5253a2.htm. Accessed on July 15, 2004.
2. CDC.BSE and CJD information and resources. Available at: www.cdc.gov/ncidod/diseases/cjd/cjd.html. Accessed on July 15, 2004.
3. Kaufman M. They’re not allowed to test for Mad Cow. Washington Post National Weekly, May 3–9, 2004;21.-
4. US Food and Drug Administration, Center for Food Safety and Applied Nutrition Commonly asked questions about BSE in products regulated by FDA’s Center for Food Safety and Applied Nutrition (CFSAN). Available at: www.cfsan.fda.gov/~comm/bsefaq.html. Accessed on July 15, 2004.
5. CDC. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. Available at: www.cdc.gov/travel/diseases/madcow.htm. Accessed on July 15, 2004.
How should we manage newly diagnosed atrial fibrillation?
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- What is the primary treatment goal of cardiac medication?
- What is the role of digoxin?
- When should medications be used to maintain sinus rhythm after cardioversion?
- Should all patients be anticoagulated with warfarin?
- What are the contraindications to warfarin therapy?
Recommendations for these management issues are found in the guideline developed in a joint effort of the American College of Physicians Clinical Efficacy Assessment Subcommittee and the American Academy of Family Physicians Commission on Clinical Policies and Research. It was funded by both organizations and approved by their Boards before publication. The target audience is internists and family physicians.
The target patients are adults with newly diagnosed atrial fibrillation. The guideline does not apply to postoperative patients, post-myocardial infarction patients, those with class IV heart failure or valvular heart disease, or patients taking antiarrhythmic medications.
The objective of this guideline is to recommend pharmacologic management of newly diagnosed atrial fibrillation. The evidence category for this guideline is management. Outcomes considered are control of heart rate and stroke risk reduction. The committees used the Guyatt method of grading recommendations,1 a qualitative approach to the literature. These were revised to comply with the SORT taxonomy.2
Guideline relevance and limitations
Atrial fibrillation is common, affecting anywhere from 1% of the American population at age 60 to 8% at age 80. It is more common in men than women. Even if patients are asymptomatic, they are at increased risk of stroke (1.9%–18% per year).
A lengthy bibliography accompanies the guideline. Tables of supporting evidence are lacking, which makes it more difficult to analyze the final recommendations. The guideline is weakened by the lack of a cost-effectiveness analysis.
Grade A Recommendations
- Prescribe long-term warfarin at therapeutic levels unless stroke risk is low, as determined by risk factors: congestive heart failure, hypertension, age 75 years older, diabetes mellitus, or history of transient ischemic event/cerebrovascular accident. Warfarin should not be prescribed if there are contraindications of thrombocytopenia, recent trauma, surgery, or alcoholism.
- Atenolol (Tenormin), metoprolol (Lopressor, Toprol-XL), diltiazem, and verapamil are optimal choices for rate control during exercise and at rest. Digoxin (Lanoxin is a second-line agent and is only effective at rest.
Grade B Recommendations
- Rate control with anticoagulation is the primary goal of treatment. Consider rhythm control according to a patient’s symptoms.
- Cardioversion by electrical conversion and pharmacologic conversion are both appropriate.
- For patients who elect cardioversion, options include 1) early antiocoagulation and cardioversion (with transesophageal echocardiography confirming absence of mural thrombus), or 2) delayed cardioversion with pre- and post-anticoagulation.
- Most patients who convert to sinus rhythm do not need maintenance rhythm therapy. When quality of life is threatened, the best agents are amiodarone (Cordarone), disopyramide (Norpace), propafenone (Rythmol), and sotalol (Betapace).
Guideline development and evidence review
This guideline is based on background papers published by McNamara3 and the John Hopkins Evidence-Based Practice Center4 under contract with the Agency for Healthcare Research and Quality. There are 57 references.
The guideline group reviewed the evidence and made graded recommendations regarding rate control versus rhythm control, stroke prevention and anticoagulation, electrical conversion versus pharmacologic conversion, the role of transesophageal echocardiography in guiding therapy, and maintenance therapy.
Source for this guideline
Snow V, Weiss KB, LeFevre M, et al.Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2003; 139: 1009–1017.
Other guidelines on atrial fibrillation
ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation.
A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation).
This 2001 guideline is the work of an international panel. Algorithms are provided for pharma-cologic management of patients with newly diagnosed atrial fibrillation, pharmacologic management of patients with recurrent paroxysmal atrial fibrillation, antiarrythmic drug therapy to maintain sinus rhythm in patients with recurrent paroxysmal or persistent atrial fibrillation, and pharmacologic management of patients with recurrent persistent or permanent atrial fibrillation. Recent evidence regarding rate control versus rhythm control was not available at publication of this guideline.
Sources: American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2001; 38:1266i–lxx. (580 references) Fuster V, Ryden LE, Asinger RW, et al.ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology. Eur Heart J 2001; 22:1852–1923. (580 references)
Antithrombotic therapy in atrial fibrillation.
In: Sixth ACCP Consensus Conference on Antithrombotic Therapy.
This is an excellent review of a grading scheme for stroke risk and choice of anti-thrombotic agents.
Source: Albers GW, Dalen JE, Laupacis A, et al.Antithrombotic therapy in atrial fibrillation. Chest 2001; 119(1 Suppl):194S–206S. (103 references)
Atrial fibrillation: drug treatment and electric cardioversion.
This Finnish guideline makes recommendations regarding drug treatment, anticoagulation, and cardioversion for patients with atrial fibrillation and atrial flutter. The recommendations are not graded.
Source: Finnish Medical Society Duodecim. Atrial Fibrillation: Drug Treatment and Electric Cardioversion. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 Mar 4. Various pages.
Preventive health care, 2000 update.
Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke.
This guideline is from 2000 and found insufficient evidence to recommend for or against ambulatory electrocardiography to detect atrial fibrillation for patients after stroke or TIA.
Source: Bell C, Kapral M. Use of ambulatory electrocardiography for the detection of paroxysmal atrial fibrillation in patients with stroke. Canadian Task Force on Preventive Health Care. Can J Neurol Sc. 2000; 27:25–31. (78 references)
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.
1. Guyatt GH, Sackett DL, Sinclair JC, et al. User’s guide to the medical literature:IX.A method for grading health care recommendations. JAMA 1995;274:1800.-
2. Ebell M, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am Fam Physician 2004;69:548-556.
3. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Int Med 2003;139:1018-1033.
4. McNamara RL, Bass EB, Miller MR, et al. Evidence report on the management of new onset atrial fibrillation.Agency for Healthcare Research and Quality publication no. AHRQ 01-E026. Rockville, MD: Agency for Healthcare Research and Quality; January 2001.