User login
Malpractice Counsel: Allergic Reaction Versus Anaphylaxis
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
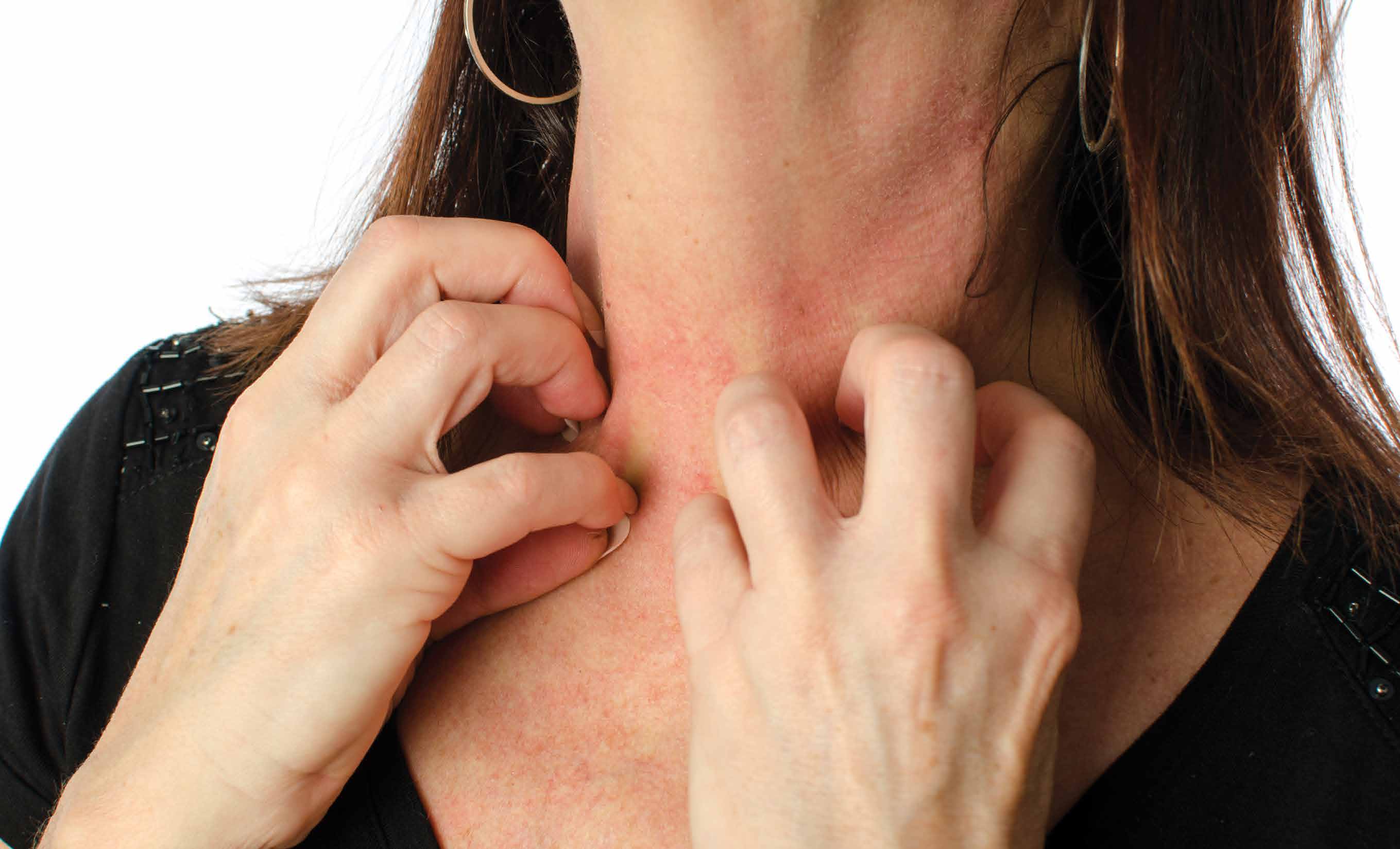
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
Allergic Reaction Versus Anaphylaxis
A 34-year-old woman presented to ED with complaints of an allergic reaction, the onset of which began approximately 1 hour prior. The patient did not know what might have caused her symptoms. She complained of hives and itching all over; she denied difficulty swallowing, wheezing, and shortness of breath. Her medical history was unremarkable. She was on no medications, and she denied any alcohol or tobacco use. She had no known medication or food allergies.
Physical examination revealed a woman in mild discomfort, secondary to generalized itching. Her vital signs, including pulse oximetry, were normal. There was no swelling of the face, lips, or oropharynx. The lungs were clear to auscultation bilaterally. The heart and abdominal examinations were normal. Examination of the skin revealed diffuse urticaria without petechiae or purpura.
The emergency physician (EP) ordered 125 mg of methylprednisolone sodium succinate and 25 mg of diphenhydramine intravenously (IV). After approximately 1 hour, the hives and itching decreased and the patient felt improved. She was diagnosed with an allergic reaction and discharged home with a prescription for diphenhydramine and a methylprednisolone dose pack.
The following day, the patient collapsed at home and emergency medical services was called. Unfortunately, the patient could not be resuscitated and was pronounced dead at the scene. An autopsy revealed the patient had died from anaphylaxis and laryngeal edema, with an extremely elevated tryptase level of 200 ng/mL (normal, <11.5 ng/mL).
The patient’s family sued the EP for failure to diagnose and treat anaphylaxis, failure to treat with epinephrine, and failure to admit the patient to the hospital. The defense claimed the patient did not present with anaphylaxis, but rather simply a worsening of the hives and angioedema, and that the treatment provided was appropriate. The jury found in favor of the defendants.
Discussion
It does not appear the patient presented with anaphylaxis on the first visit, but may have had it on the second visit. In 2004, the National Institutes of Allergy and Infectious Disease (NIAID) panel and the Food Allergy and Anaphylaxis Network (FAAN) developed criteria for the diagnosis of anaphylaxis.1 According to the criteria, anaphylaxis is likely when any one of the following three criteria are present: (1) acute onset of symptoms involving the skin or mucosa (eg, pruritus, hives, angioedema), and either respiratory compromise (eg, dyspnea, wheezing, stridor, hypoxia) or hypotension/end-organ dysfunction (eg, syncope, incontinence); (2) two or more symptoms (eg, respiratory compromise, hypotension/end-organ dysfunction, persistent gastrointestinal [GI] symptoms such as vomiting, diarrhea, or crampy abdominal pain) that occur rapidly after exposure involving the skin or mucosa; or (3) hypotension from a known allergen to the patient. The accuracy of these criteria has been retrospectively evaluated in an ED study, and found to have a 97% sensitivity and an 82% specificity.2 The negative predictive value was good at 98%, but the positive predictive value was only 69%.2
When a patient presents with minimal or subtle symptoms, anaphylaxis can be a very difficult diagnosis to make in the ED early on in the process. While no EP will miss the diagnosis in a patient with hives, hypotension, and wheezing, it can be easy to miss when the predominant symptoms are GI, such as nausea, vomiting, or diarrhea. In addition, the differential diagnosis for the presentation of anaphylaxis in the ED can be extremely broad and include vasovagal reaction, asthma attack, myocardial infarction, gastroenteritis, panic attack, or airway obstruction.
Due to the nature of emergency medicine, EPs must consider multiple etiologies before determining an evaluation and management plan. While recognizing there are limitations to the NIAID/FAAN criteria, EPs should be aware of them. We are very good at treating these types of symptoms with antihistamines and steroids; however, we frequently fail to give epinephrine when indicated. It is important to remember that epinephrine is the first-line treatment for anaphylaxis—not corticosteroids or antihistamines.3
Reasons for not administering epinephrine are multiple. First, as discussed above, if the diagnosis of anaphylaxis is not considered, the EP is not going to administer the drug of choice. Secondly, EPs have been taught to have a healthy respect for epinephrine and its effects, especially in older patients. Due to this cautious approach, epinephrine is frequently not given to patients with mild symptoms or to those who present early in the course of disease.
Emergency physicians have experience giving epinephrine subcutaneously, but not nearly as much with the intramuscular (IM) route. This is important, because an IM injection in the anterolateral thigh is the recommended location for the treatment of anaphylaxis. The dose should be weight based (0.01 mg/kg) to a maximum of 0.5 mg. This dose can be given every 5 to 15 minutes as necessary to control symptoms.3 The dosing is important to remember, since many EDs stock only autoinjectable epinephrine devices for use in anaphylaxis. These autoinjectors only contain 0.3 mg of epinephrine, so some patients may be underdosed if used.
In the management of allergic reactions and anaphylaxis, EPs frequently administer antihistamines and corticosteroids. While there is no direct evidence to support their use in the management of anaphylaxis, theoretical benefits do exist.3 This, combined with the excellent medication safety profile and lack of serious side effects, make these two medication classes appropriate for use in the ED.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
- Allergic Reaction Versus Anaphylaxis
- Manivannan V, Decker WW, Stead LG, Li JT, Campbell RL. National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2(1):3-5.
- Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol. 2012;129(3):748-752.
- Campbell RL, Li JT, Nicklas RA, Sadosty AT; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599-608.
White House launches new plan to combat multidrug-resistant TB
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
Key clinical point: A new action plan to combat the rise of multidrug-resistant tuberculosis focuses on strengthening the domestic capacity to treat the MDR-TB, improving international capacity and collaboration to combat the disease, and accelerating research and treatment developments.
Move to Allow Patients to Request 'Refund' Appealing and Risky
We’ve all seen hundreds of commercials from companies advertising products and services with a money-back guarantee. The Men’s Warehouse, for example, has been promising men across the globe for over a decade, “You’re going to like the way you look. I guarantee it!” But to date, no one has made such a “guarantee” in the healthcare industry. Buying a suit is not exactly like getting your gallbladder removed.
We know that medical diagnoses and treatments are filled with uncertainty in expected processes and outcomes, because the factors that are dependent on these processes and outcomes are endless. These include patient factors (overall health, functional status, comorbid conditions), procedural factors (emergency versus elective, time of day or night), and facility factors (having the optimal team with skills that match the patient need, having all the right products and equipment). Although we know that many medical procedures have a relatively predictable risk of complications, unpredictable complications still occur, so how can we ever offer a guarantee for the interventions we perform on patients?
First of Its Kind
David Feinberg, MD, MBA, president and CEO of Geisinger Health System, is doing just that. This healthcare system has developed an application, called the Geisinger ProvenExperience, which can be downloaded onto a smartphone. After a procedure, each patient is given a code for the condition that was treated. With that code, the patient can enter feedback on the services provided and can then request a refund if they are not fully satisfied.
Most remarkably, the request for a refund is based on the judgment of the recipient, not on that of the provider(s). At a recent public meeting, Dr. Feinberg said of the new program: “We’re going to do everything right. That’s our job, that’s our promise to you … and you’re the judge. If you don’t think so, we’re going to apologize, we’re going to try to fix it for the next guy, and, as a small token of appreciation, we’re going to give you some money back.”1
Although many are skeptical about whether or not the program will be successful, much less viable, Dr. Feinberg contends that early feedback on the program has shown that most patients don’t actually want their money back. Instead, if their needs have not been met, most have just wanted a sincere apology and a commitment to make things better for others. Dr. Feinberg also contests that even if this is not the best or only approach to improving healthcare (quickly), we should all feel compelled to do something about our repeated failures in meeting patient expectations in the quality and/or experience of their care; and because no other industry works this way, other than healthcare. Typically, when consumers get fed up with poor service in other industries, disruptive innovations (Uber, for example) are created to satisfy customers’ desires.
A New Paradigm?
In healthcare, patients certainly should be dissatisfied if they experience a preventable harm event. Some types of harm are considered “always preventable,” such as wrong-site surgery. These events are extremely rare and, thus, do not constitute most cases of harm in hospitals these days. Such “never events” are relatively well defined and have been adopted for nonpayment by Medicare and other insurers, which can serve to buffer a patient’s financial liability in the small number of these cases. For other, more common, types of preventable harm, some hospitals have instituted apology and disclosure policies, and some will also relieve the patient of the portion of the bill attributable to the preventable harm. But not all hospitals have adopted such policies, despite the fact that they are widely endorsed by influential agencies, including The Joint Commission, the American Medical Association, Leapfrog Group, the National Quality Forum, and the Agency for Healthcare Research and Quality.
And, even for hospitals that have adopted such “best practice” policies, there is not always clear consensus on what constitutes preventable harm. Generally, the “judgment call” about what constitutes preventable harm is made by healthcare systems and providers—not patients. In addition, many cases of harm that are not necessarily preventable can often result in great dissatisfaction for the patient. There are countless stories of patients who are unfortunately harmed in the course of medical procedures, but who were informed of the possible risks of the procedure and consented to have the procedure performed despite the risks. These situations, which are agonizingly difficult for the system, the providers, and the patients, have no good solutions. Systems cannot “own” all harm, such as those resulting from the disease process itself or from risky and invasive procedures intended to benefit the patient. And there is ongoing inconsistency in healthcare systems when it comes to their willingness and ability to consistently define preventable harm or to disclose, apologize, and forgive payments in such cases.
So, while this move to allow patients to ask for a “refund” seems both extremely appealing and extremely risky, it certainly seems as though it will greatly enhance the trust of patients and their families in the Geisinger Health System.
I, among others, will eagerly follow the results of this program; while getting a cholecystectomy is not the same as buying a men’s suit, I do hope that someday, I will be able to say to every patient entering my healthcare system that before they leave, “You’re going to like the way you feel. I guarantee it!” TH

References
1. Guydish M. Geisinger CEO: money-back guarantee for health care coming. November 6, 2015. Times Leader website. Available at: http://timesleader.com/news/492790/geisinger-ceo-money-back-guarantee-for-health-car-coming. Accessed December 5, 2015.
2. Luthra S. When something goes wrong at the hospital, who pays? November 11, 2015. Kaiser Health News. Available at: http://khn.org/news/when-something-goes-wrong-at-the-hospital-who-pays/?utm_source=Managed&utm_campaign=9e17712a95-Quality+%26+Patient+Safety+Update&utm_medium=email&utm_term=0_ebe1fa6178-9e17712a95-319388717. Accessed December 5, 2015.
We’ve all seen hundreds of commercials from companies advertising products and services with a money-back guarantee. The Men’s Warehouse, for example, has been promising men across the globe for over a decade, “You’re going to like the way you look. I guarantee it!” But to date, no one has made such a “guarantee” in the healthcare industry. Buying a suit is not exactly like getting your gallbladder removed.
We know that medical diagnoses and treatments are filled with uncertainty in expected processes and outcomes, because the factors that are dependent on these processes and outcomes are endless. These include patient factors (overall health, functional status, comorbid conditions), procedural factors (emergency versus elective, time of day or night), and facility factors (having the optimal team with skills that match the patient need, having all the right products and equipment). Although we know that many medical procedures have a relatively predictable risk of complications, unpredictable complications still occur, so how can we ever offer a guarantee for the interventions we perform on patients?
First of Its Kind
David Feinberg, MD, MBA, president and CEO of Geisinger Health System, is doing just that. This healthcare system has developed an application, called the Geisinger ProvenExperience, which can be downloaded onto a smartphone. After a procedure, each patient is given a code for the condition that was treated. With that code, the patient can enter feedback on the services provided and can then request a refund if they are not fully satisfied.
Most remarkably, the request for a refund is based on the judgment of the recipient, not on that of the provider(s). At a recent public meeting, Dr. Feinberg said of the new program: “We’re going to do everything right. That’s our job, that’s our promise to you … and you’re the judge. If you don’t think so, we’re going to apologize, we’re going to try to fix it for the next guy, and, as a small token of appreciation, we’re going to give you some money back.”1
Although many are skeptical about whether or not the program will be successful, much less viable, Dr. Feinberg contends that early feedback on the program has shown that most patients don’t actually want their money back. Instead, if their needs have not been met, most have just wanted a sincere apology and a commitment to make things better for others. Dr. Feinberg also contests that even if this is not the best or only approach to improving healthcare (quickly), we should all feel compelled to do something about our repeated failures in meeting patient expectations in the quality and/or experience of their care; and because no other industry works this way, other than healthcare. Typically, when consumers get fed up with poor service in other industries, disruptive innovations (Uber, for example) are created to satisfy customers’ desires.
A New Paradigm?
In healthcare, patients certainly should be dissatisfied if they experience a preventable harm event. Some types of harm are considered “always preventable,” such as wrong-site surgery. These events are extremely rare and, thus, do not constitute most cases of harm in hospitals these days. Such “never events” are relatively well defined and have been adopted for nonpayment by Medicare and other insurers, which can serve to buffer a patient’s financial liability in the small number of these cases. For other, more common, types of preventable harm, some hospitals have instituted apology and disclosure policies, and some will also relieve the patient of the portion of the bill attributable to the preventable harm. But not all hospitals have adopted such policies, despite the fact that they are widely endorsed by influential agencies, including The Joint Commission, the American Medical Association, Leapfrog Group, the National Quality Forum, and the Agency for Healthcare Research and Quality.
And, even for hospitals that have adopted such “best practice” policies, there is not always clear consensus on what constitutes preventable harm. Generally, the “judgment call” about what constitutes preventable harm is made by healthcare systems and providers—not patients. In addition, many cases of harm that are not necessarily preventable can often result in great dissatisfaction for the patient. There are countless stories of patients who are unfortunately harmed in the course of medical procedures, but who were informed of the possible risks of the procedure and consented to have the procedure performed despite the risks. These situations, which are agonizingly difficult for the system, the providers, and the patients, have no good solutions. Systems cannot “own” all harm, such as those resulting from the disease process itself or from risky and invasive procedures intended to benefit the patient. And there is ongoing inconsistency in healthcare systems when it comes to their willingness and ability to consistently define preventable harm or to disclose, apologize, and forgive payments in such cases.
So, while this move to allow patients to ask for a “refund” seems both extremely appealing and extremely risky, it certainly seems as though it will greatly enhance the trust of patients and their families in the Geisinger Health System.
I, among others, will eagerly follow the results of this program; while getting a cholecystectomy is not the same as buying a men’s suit, I do hope that someday, I will be able to say to every patient entering my healthcare system that before they leave, “You’re going to like the way you feel. I guarantee it!” TH

References
1. Guydish M. Geisinger CEO: money-back guarantee for health care coming. November 6, 2015. Times Leader website. Available at: http://timesleader.com/news/492790/geisinger-ceo-money-back-guarantee-for-health-car-coming. Accessed December 5, 2015.
2. Luthra S. When something goes wrong at the hospital, who pays? November 11, 2015. Kaiser Health News. Available at: http://khn.org/news/when-something-goes-wrong-at-the-hospital-who-pays/?utm_source=Managed&utm_campaign=9e17712a95-Quality+%26+Patient+Safety+Update&utm_medium=email&utm_term=0_ebe1fa6178-9e17712a95-319388717. Accessed December 5, 2015.
We’ve all seen hundreds of commercials from companies advertising products and services with a money-back guarantee. The Men’s Warehouse, for example, has been promising men across the globe for over a decade, “You’re going to like the way you look. I guarantee it!” But to date, no one has made such a “guarantee” in the healthcare industry. Buying a suit is not exactly like getting your gallbladder removed.
We know that medical diagnoses and treatments are filled with uncertainty in expected processes and outcomes, because the factors that are dependent on these processes and outcomes are endless. These include patient factors (overall health, functional status, comorbid conditions), procedural factors (emergency versus elective, time of day or night), and facility factors (having the optimal team with skills that match the patient need, having all the right products and equipment). Although we know that many medical procedures have a relatively predictable risk of complications, unpredictable complications still occur, so how can we ever offer a guarantee for the interventions we perform on patients?
First of Its Kind
David Feinberg, MD, MBA, president and CEO of Geisinger Health System, is doing just that. This healthcare system has developed an application, called the Geisinger ProvenExperience, which can be downloaded onto a smartphone. After a procedure, each patient is given a code for the condition that was treated. With that code, the patient can enter feedback on the services provided and can then request a refund if they are not fully satisfied.
Most remarkably, the request for a refund is based on the judgment of the recipient, not on that of the provider(s). At a recent public meeting, Dr. Feinberg said of the new program: “We’re going to do everything right. That’s our job, that’s our promise to you … and you’re the judge. If you don’t think so, we’re going to apologize, we’re going to try to fix it for the next guy, and, as a small token of appreciation, we’re going to give you some money back.”1
Although many are skeptical about whether or not the program will be successful, much less viable, Dr. Feinberg contends that early feedback on the program has shown that most patients don’t actually want their money back. Instead, if their needs have not been met, most have just wanted a sincere apology and a commitment to make things better for others. Dr. Feinberg also contests that even if this is not the best or only approach to improving healthcare (quickly), we should all feel compelled to do something about our repeated failures in meeting patient expectations in the quality and/or experience of their care; and because no other industry works this way, other than healthcare. Typically, when consumers get fed up with poor service in other industries, disruptive innovations (Uber, for example) are created to satisfy customers’ desires.
A New Paradigm?
In healthcare, patients certainly should be dissatisfied if they experience a preventable harm event. Some types of harm are considered “always preventable,” such as wrong-site surgery. These events are extremely rare and, thus, do not constitute most cases of harm in hospitals these days. Such “never events” are relatively well defined and have been adopted for nonpayment by Medicare and other insurers, which can serve to buffer a patient’s financial liability in the small number of these cases. For other, more common, types of preventable harm, some hospitals have instituted apology and disclosure policies, and some will also relieve the patient of the portion of the bill attributable to the preventable harm. But not all hospitals have adopted such policies, despite the fact that they are widely endorsed by influential agencies, including The Joint Commission, the American Medical Association, Leapfrog Group, the National Quality Forum, and the Agency for Healthcare Research and Quality.
And, even for hospitals that have adopted such “best practice” policies, there is not always clear consensus on what constitutes preventable harm. Generally, the “judgment call” about what constitutes preventable harm is made by healthcare systems and providers—not patients. In addition, many cases of harm that are not necessarily preventable can often result in great dissatisfaction for the patient. There are countless stories of patients who are unfortunately harmed in the course of medical procedures, but who were informed of the possible risks of the procedure and consented to have the procedure performed despite the risks. These situations, which are agonizingly difficult for the system, the providers, and the patients, have no good solutions. Systems cannot “own” all harm, such as those resulting from the disease process itself or from risky and invasive procedures intended to benefit the patient. And there is ongoing inconsistency in healthcare systems when it comes to their willingness and ability to consistently define preventable harm or to disclose, apologize, and forgive payments in such cases.
So, while this move to allow patients to ask for a “refund” seems both extremely appealing and extremely risky, it certainly seems as though it will greatly enhance the trust of patients and their families in the Geisinger Health System.
I, among others, will eagerly follow the results of this program; while getting a cholecystectomy is not the same as buying a men’s suit, I do hope that someday, I will be able to say to every patient entering my healthcare system that before they leave, “You’re going to like the way you feel. I guarantee it!” TH

References
1. Guydish M. Geisinger CEO: money-back guarantee for health care coming. November 6, 2015. Times Leader website. Available at: http://timesleader.com/news/492790/geisinger-ceo-money-back-guarantee-for-health-car-coming. Accessed December 5, 2015.
2. Luthra S. When something goes wrong at the hospital, who pays? November 11, 2015. Kaiser Health News. Available at: http://khn.org/news/when-something-goes-wrong-at-the-hospital-who-pays/?utm_source=Managed&utm_campaign=9e17712a95-Quality+%26+Patient+Safety+Update&utm_medium=email&utm_term=0_ebe1fa6178-9e17712a95-319388717. Accessed December 5, 2015.
Crowded hospital EDs not dealing effectively with patient flow
Even though most crowded U.S. hospital emergency departments were adopting measures to improve patient flow, they were not adopting the most effective interventions, according to a new study in Health Affairs.
Hospitals “have been slow to adopt interventions that require a change in protocols. This may reflect the fact that ED crowding is a low hospital-wide priority in many facilities, despite the fact that it continues to worsen,” Dr. Leah Honigman Warner, attending physician in the department of emergency medicine at Long Island Jewish Medical Center, New Hyde Park, N.Y., and colleagues wrote in the December 2015 issue of Health Affairs (2015 Dec.;34[12]:2151-2159. doi: 10.1377/hlthaff.2015.0603).
Researchers examined data collected from the Centers for Disease Control and Prevention’s National Hospital Ambulatory Medical Care Survey on emergency department crowding interventions from 2007 to 2010 and found that “while the average number of crowding interventions adopted by hospitals has increased in recent years, there is still a significant gap in the adoption of many of the strategies that can reduce ED crowding and make crowded EDs safer.”
For example, two interventions – the use of full capacity protocol and boarding in inpatient hallways – were not adopted by the majority of the most crowded quartile of hospitals. Sixty percent of hospitals have not adopted full capacity protocol, and 80% do not transfer admitted patients to wait in inpatient hallways when all beds are full, researchers noted.
To highlight the effectiveness of full capacity protocol, authors point to the Canadian province of Alberta, which adopted the measure and saw ED length-of-stay reduced by one-third and ED boarding reduced by half.
Hospitals during the study period were employing a number of other strategies, including physical space expansion, which has not been proven to help crowding, and technology-related interventions, such as the use of electronic dashboards and computer-assisted triage, both of which are growing and can help reduce length of stay.
“There are data to support the use of ED crowding interventions and proven best practices,” the researchers conclude. “Now is the time for a national campaign to develop the standards to reduce ED crowding and eliminate ED boarding, which will allow hospitals and EDs to provide the highest-quality acute care.”
The authors did not declare any conflicts of interest.
Even though most crowded U.S. hospital emergency departments were adopting measures to improve patient flow, they were not adopting the most effective interventions, according to a new study in Health Affairs.
Hospitals “have been slow to adopt interventions that require a change in protocols. This may reflect the fact that ED crowding is a low hospital-wide priority in many facilities, despite the fact that it continues to worsen,” Dr. Leah Honigman Warner, attending physician in the department of emergency medicine at Long Island Jewish Medical Center, New Hyde Park, N.Y., and colleagues wrote in the December 2015 issue of Health Affairs (2015 Dec.;34[12]:2151-2159. doi: 10.1377/hlthaff.2015.0603).
Researchers examined data collected from the Centers for Disease Control and Prevention’s National Hospital Ambulatory Medical Care Survey on emergency department crowding interventions from 2007 to 2010 and found that “while the average number of crowding interventions adopted by hospitals has increased in recent years, there is still a significant gap in the adoption of many of the strategies that can reduce ED crowding and make crowded EDs safer.”
For example, two interventions – the use of full capacity protocol and boarding in inpatient hallways – were not adopted by the majority of the most crowded quartile of hospitals. Sixty percent of hospitals have not adopted full capacity protocol, and 80% do not transfer admitted patients to wait in inpatient hallways when all beds are full, researchers noted.
To highlight the effectiveness of full capacity protocol, authors point to the Canadian province of Alberta, which adopted the measure and saw ED length-of-stay reduced by one-third and ED boarding reduced by half.
Hospitals during the study period were employing a number of other strategies, including physical space expansion, which has not been proven to help crowding, and technology-related interventions, such as the use of electronic dashboards and computer-assisted triage, both of which are growing and can help reduce length of stay.
“There are data to support the use of ED crowding interventions and proven best practices,” the researchers conclude. “Now is the time for a national campaign to develop the standards to reduce ED crowding and eliminate ED boarding, which will allow hospitals and EDs to provide the highest-quality acute care.”
The authors did not declare any conflicts of interest.
Even though most crowded U.S. hospital emergency departments were adopting measures to improve patient flow, they were not adopting the most effective interventions, according to a new study in Health Affairs.
Hospitals “have been slow to adopt interventions that require a change in protocols. This may reflect the fact that ED crowding is a low hospital-wide priority in many facilities, despite the fact that it continues to worsen,” Dr. Leah Honigman Warner, attending physician in the department of emergency medicine at Long Island Jewish Medical Center, New Hyde Park, N.Y., and colleagues wrote in the December 2015 issue of Health Affairs (2015 Dec.;34[12]:2151-2159. doi: 10.1377/hlthaff.2015.0603).
Researchers examined data collected from the Centers for Disease Control and Prevention’s National Hospital Ambulatory Medical Care Survey on emergency department crowding interventions from 2007 to 2010 and found that “while the average number of crowding interventions adopted by hospitals has increased in recent years, there is still a significant gap in the adoption of many of the strategies that can reduce ED crowding and make crowded EDs safer.”
For example, two interventions – the use of full capacity protocol and boarding in inpatient hallways – were not adopted by the majority of the most crowded quartile of hospitals. Sixty percent of hospitals have not adopted full capacity protocol, and 80% do not transfer admitted patients to wait in inpatient hallways when all beds are full, researchers noted.
To highlight the effectiveness of full capacity protocol, authors point to the Canadian province of Alberta, which adopted the measure and saw ED length-of-stay reduced by one-third and ED boarding reduced by half.
Hospitals during the study period were employing a number of other strategies, including physical space expansion, which has not been proven to help crowding, and technology-related interventions, such as the use of electronic dashboards and computer-assisted triage, both of which are growing and can help reduce length of stay.
“There are data to support the use of ED crowding interventions and proven best practices,” the researchers conclude. “Now is the time for a national campaign to develop the standards to reduce ED crowding and eliminate ED boarding, which will allow hospitals and EDs to provide the highest-quality acute care.”
The authors did not declare any conflicts of interest.
FROM HEALTH AFFAIRS
Concerns Grow as Top Clinicians Choose Nonclinical Roles
On a spring day a couple of years ago, I met with some internal medicine residents in a “Healthcare Systems Immersion” elective. I was to provide thoughts about the nonclinical portion of my work that I spend consulting with other hospitalist groups.
I asked for their thoughts about whether the ranks of doctors providing direct bedside care were losing too many of the most talented clinicians to nonclinical roles. The most vocal resident was confident that was not the case; these doctors would ultimately have a positive impact on the care of larger numbers of patients through administrative work than through direct patient care.
I wonder if she is right.
Numerous Hospitalists Opt for Nonnclinical Work
It seems like lots of hospitalists are transitioning to nonclinical work. My experience is that most who have administrative or other nonclinical roles continue—for part of their time—to provide direct patient care. But some leave clinical work behind altogether. Some of them are very prominent people in our field, like the top physician at CMS, the current U.S. Surgeon General, and this year’s most influential physician executive as judged by Modern Healthcare. I think it is pretty cool that these people come from our specialty.
I couldn’t find published survey data on the portion of hospitalists, or doctors in any specialty, who have entirely (or almost entirely) nonclinical roles. My impression is that this was a vanishingly small number across all specialties 30 or 40 years ago, but it seems to have increased pretty dramatically in the last 10 years. At the start of my career, few hospitals had a physician in an administrative position. Now it is common.
Physician leadership roles now include information technology (CMIO), quality (CQO), leader of the employed physician group, and hospital CEO (at least two hospitalists I know are in this role). And there are lots of nonclinical roles for doctors outside of hospitals.
Pros, Cons for Healthcare
I’ve had mixed feelings watching many people leave clinical practice. Most of them, like those mentioned above, continue to make important contributions to our healthcare system; they improve the services and care patients receive. Yet it seems like some of the best clinicians are taken from active practice and are difficult to replace.
At the start of my career, the few doctors who left clinical practice for nonclinical work tended to do so late in their careers. Now many make this choice very early in their careers. Of the six or seven residents I met with above, several planned to pursue entirely nonclinical work either immediately upon completing residency or after just a few years of clinical practice. They were at one of the top internal medicine programs in the country and will, presumably, provide direct clinical care to a really small number of patients over their careers.
It makes me wonder if there is a meaningful effect of more talented people having, and exercising, the option to leave clinical practice, resulting in a tilt toward somewhat-less-talented doctors left to treat patients. I hope there is no meaningful effect in this direction, but I’m not sure.
Reasons to Move
My experience is that most doctors who have left clinical work will wax eloquent about how they really loved it and weren’t fleeing it but did so because they wanted to “try something new” or contribute to healthcare in other ways. I’m suspicious that for many of them this isn’t entirely true. Some must have been fleeing it. They were burned out, tired of being on call, and so on, and were eager to find relief from clinical work more than they were “drawn to a new career challenge.” They just don’t want to admit it.
I sometimes think about what several nationally prominent hospitalist leaders have said to me over my career. Not long ago, one said, “Wow. You’re still seeing patients and making rounds? I can’t believe it. You need to find something better.”
This doctor seemed to equate an entire career spent in clinical practice as something done mostly by those who aren’t talented enough to have other options. What a change from 30 or 40 years ago.
Several years ago, in a very moving conversation, another nationally prominent hospitalist leader told me, “It’s all about the patient and how we care for them at the bedside. There’s no better way we can spend our time.”
The Best Career
Within a few years, he left clinical practice entirely, even though he was still mid-career.
I hold in highest esteem hospitalists and other doctors who spend a full career in direct patient care and do it well. At the top of that list is my own dad, who is up there with Osler when it comes to dedicated physicians.
Of course, those who spend most or all of their time in nonclinical work really can make important contributions that help the healthcare system better serve patients, in some cases clearly making a bigger difference for more patients than they could via direct clinical care. We need talented people in both roles, but we also need to always be looking for ways to minimize the numbers of doctors who feel the need to flee a clinical career.
Like many hospitalists, I think about these things a lot when making decisions about my own career. I hope we all have the wisdom to make the best choices for ourselves, and for the patients we set out to serve when we entered medical school. TH

On a spring day a couple of years ago, I met with some internal medicine residents in a “Healthcare Systems Immersion” elective. I was to provide thoughts about the nonclinical portion of my work that I spend consulting with other hospitalist groups.
I asked for their thoughts about whether the ranks of doctors providing direct bedside care were losing too many of the most talented clinicians to nonclinical roles. The most vocal resident was confident that was not the case; these doctors would ultimately have a positive impact on the care of larger numbers of patients through administrative work than through direct patient care.
I wonder if she is right.
Numerous Hospitalists Opt for Nonnclinical Work
It seems like lots of hospitalists are transitioning to nonclinical work. My experience is that most who have administrative or other nonclinical roles continue—for part of their time—to provide direct patient care. But some leave clinical work behind altogether. Some of them are very prominent people in our field, like the top physician at CMS, the current U.S. Surgeon General, and this year’s most influential physician executive as judged by Modern Healthcare. I think it is pretty cool that these people come from our specialty.
I couldn’t find published survey data on the portion of hospitalists, or doctors in any specialty, who have entirely (or almost entirely) nonclinical roles. My impression is that this was a vanishingly small number across all specialties 30 or 40 years ago, but it seems to have increased pretty dramatically in the last 10 years. At the start of my career, few hospitals had a physician in an administrative position. Now it is common.
Physician leadership roles now include information technology (CMIO), quality (CQO), leader of the employed physician group, and hospital CEO (at least two hospitalists I know are in this role). And there are lots of nonclinical roles for doctors outside of hospitals.
Pros, Cons for Healthcare
I’ve had mixed feelings watching many people leave clinical practice. Most of them, like those mentioned above, continue to make important contributions to our healthcare system; they improve the services and care patients receive. Yet it seems like some of the best clinicians are taken from active practice and are difficult to replace.
At the start of my career, the few doctors who left clinical practice for nonclinical work tended to do so late in their careers. Now many make this choice very early in their careers. Of the six or seven residents I met with above, several planned to pursue entirely nonclinical work either immediately upon completing residency or after just a few years of clinical practice. They were at one of the top internal medicine programs in the country and will, presumably, provide direct clinical care to a really small number of patients over their careers.
It makes me wonder if there is a meaningful effect of more talented people having, and exercising, the option to leave clinical practice, resulting in a tilt toward somewhat-less-talented doctors left to treat patients. I hope there is no meaningful effect in this direction, but I’m not sure.
Reasons to Move
My experience is that most doctors who have left clinical work will wax eloquent about how they really loved it and weren’t fleeing it but did so because they wanted to “try something new” or contribute to healthcare in other ways. I’m suspicious that for many of them this isn’t entirely true. Some must have been fleeing it. They were burned out, tired of being on call, and so on, and were eager to find relief from clinical work more than they were “drawn to a new career challenge.” They just don’t want to admit it.
I sometimes think about what several nationally prominent hospitalist leaders have said to me over my career. Not long ago, one said, “Wow. You’re still seeing patients and making rounds? I can’t believe it. You need to find something better.”
This doctor seemed to equate an entire career spent in clinical practice as something done mostly by those who aren’t talented enough to have other options. What a change from 30 or 40 years ago.
Several years ago, in a very moving conversation, another nationally prominent hospitalist leader told me, “It’s all about the patient and how we care for them at the bedside. There’s no better way we can spend our time.”
The Best Career
Within a few years, he left clinical practice entirely, even though he was still mid-career.
I hold in highest esteem hospitalists and other doctors who spend a full career in direct patient care and do it well. At the top of that list is my own dad, who is up there with Osler when it comes to dedicated physicians.
Of course, those who spend most or all of their time in nonclinical work really can make important contributions that help the healthcare system better serve patients, in some cases clearly making a bigger difference for more patients than they could via direct clinical care. We need talented people in both roles, but we also need to always be looking for ways to minimize the numbers of doctors who feel the need to flee a clinical career.
Like many hospitalists, I think about these things a lot when making decisions about my own career. I hope we all have the wisdom to make the best choices for ourselves, and for the patients we set out to serve when we entered medical school. TH

On a spring day a couple of years ago, I met with some internal medicine residents in a “Healthcare Systems Immersion” elective. I was to provide thoughts about the nonclinical portion of my work that I spend consulting with other hospitalist groups.
I asked for their thoughts about whether the ranks of doctors providing direct bedside care were losing too many of the most talented clinicians to nonclinical roles. The most vocal resident was confident that was not the case; these doctors would ultimately have a positive impact on the care of larger numbers of patients through administrative work than through direct patient care.
I wonder if she is right.
Numerous Hospitalists Opt for Nonnclinical Work
It seems like lots of hospitalists are transitioning to nonclinical work. My experience is that most who have administrative or other nonclinical roles continue—for part of their time—to provide direct patient care. But some leave clinical work behind altogether. Some of them are very prominent people in our field, like the top physician at CMS, the current U.S. Surgeon General, and this year’s most influential physician executive as judged by Modern Healthcare. I think it is pretty cool that these people come from our specialty.
I couldn’t find published survey data on the portion of hospitalists, or doctors in any specialty, who have entirely (or almost entirely) nonclinical roles. My impression is that this was a vanishingly small number across all specialties 30 or 40 years ago, but it seems to have increased pretty dramatically in the last 10 years. At the start of my career, few hospitals had a physician in an administrative position. Now it is common.
Physician leadership roles now include information technology (CMIO), quality (CQO), leader of the employed physician group, and hospital CEO (at least two hospitalists I know are in this role). And there are lots of nonclinical roles for doctors outside of hospitals.
Pros, Cons for Healthcare
I’ve had mixed feelings watching many people leave clinical practice. Most of them, like those mentioned above, continue to make important contributions to our healthcare system; they improve the services and care patients receive. Yet it seems like some of the best clinicians are taken from active practice and are difficult to replace.
At the start of my career, the few doctors who left clinical practice for nonclinical work tended to do so late in their careers. Now many make this choice very early in their careers. Of the six or seven residents I met with above, several planned to pursue entirely nonclinical work either immediately upon completing residency or after just a few years of clinical practice. They were at one of the top internal medicine programs in the country and will, presumably, provide direct clinical care to a really small number of patients over their careers.
It makes me wonder if there is a meaningful effect of more talented people having, and exercising, the option to leave clinical practice, resulting in a tilt toward somewhat-less-talented doctors left to treat patients. I hope there is no meaningful effect in this direction, but I’m not sure.
Reasons to Move
My experience is that most doctors who have left clinical work will wax eloquent about how they really loved it and weren’t fleeing it but did so because they wanted to “try something new” or contribute to healthcare in other ways. I’m suspicious that for many of them this isn’t entirely true. Some must have been fleeing it. They were burned out, tired of being on call, and so on, and were eager to find relief from clinical work more than they were “drawn to a new career challenge.” They just don’t want to admit it.
I sometimes think about what several nationally prominent hospitalist leaders have said to me over my career. Not long ago, one said, “Wow. You’re still seeing patients and making rounds? I can’t believe it. You need to find something better.”
This doctor seemed to equate an entire career spent in clinical practice as something done mostly by those who aren’t talented enough to have other options. What a change from 30 or 40 years ago.
Several years ago, in a very moving conversation, another nationally prominent hospitalist leader told me, “It’s all about the patient and how we care for them at the bedside. There’s no better way we can spend our time.”
The Best Career
Within a few years, he left clinical practice entirely, even though he was still mid-career.
I hold in highest esteem hospitalists and other doctors who spend a full career in direct patient care and do it well. At the top of that list is my own dad, who is up there with Osler when it comes to dedicated physicians.
Of course, those who spend most or all of their time in nonclinical work really can make important contributions that help the healthcare system better serve patients, in some cases clearly making a bigger difference for more patients than they could via direct clinical care. We need talented people in both roles, but we also need to always be looking for ways to minimize the numbers of doctors who feel the need to flee a clinical career.
Like many hospitalists, I think about these things a lot when making decisions about my own career. I hope we all have the wisdom to make the best choices for ourselves, and for the patients we set out to serve when we entered medical school. TH

Blanket hardship exemption for 2015 meaningful use authorized by Congress
For doctors unable to meet meaningful use requirements for 2015, Congress has approved a blanket process for those applying for a hardship exemption to avoid having a penalty applied to their Medicare payments in 2017.
By voice vote in the House on Dec. 18 and a unanimous consent vote later that day in the Senate, Congress passed the Patient Access and Medicare Protection Act (S. 2425), which allows CMS to grant hardship exemptions globally, rather than on a case-by-case basis.
There are a number of reasons a hardship exemption would be issued, including infrastructure-related problems, unforeseen circumstances, lack of face-to-face interactions, and lack of available certified electronic health records.
Doctors seeking the exemption will need to apply by March 15, 2016; hospitals must do so by April 1, 2016.
“Moving forward, this process will now allow doctors to avoid erroneous penalties that would have otherwise caused harm for patients seeking quality care,” Rep. Renee Ellmers (R-N.C.) said in a statement.
For doctors unable to meet meaningful use requirements for 2015, Congress has approved a blanket process for those applying for a hardship exemption to avoid having a penalty applied to their Medicare payments in 2017.
By voice vote in the House on Dec. 18 and a unanimous consent vote later that day in the Senate, Congress passed the Patient Access and Medicare Protection Act (S. 2425), which allows CMS to grant hardship exemptions globally, rather than on a case-by-case basis.
There are a number of reasons a hardship exemption would be issued, including infrastructure-related problems, unforeseen circumstances, lack of face-to-face interactions, and lack of available certified electronic health records.
Doctors seeking the exemption will need to apply by March 15, 2016; hospitals must do so by April 1, 2016.
“Moving forward, this process will now allow doctors to avoid erroneous penalties that would have otherwise caused harm for patients seeking quality care,” Rep. Renee Ellmers (R-N.C.) said in a statement.
For doctors unable to meet meaningful use requirements for 2015, Congress has approved a blanket process for those applying for a hardship exemption to avoid having a penalty applied to their Medicare payments in 2017.
By voice vote in the House on Dec. 18 and a unanimous consent vote later that day in the Senate, Congress passed the Patient Access and Medicare Protection Act (S. 2425), which allows CMS to grant hardship exemptions globally, rather than on a case-by-case basis.
There are a number of reasons a hardship exemption would be issued, including infrastructure-related problems, unforeseen circumstances, lack of face-to-face interactions, and lack of available certified electronic health records.
Doctors seeking the exemption will need to apply by March 15, 2016; hospitals must do so by April 1, 2016.
“Moving forward, this process will now allow doctors to avoid erroneous penalties that would have otherwise caused harm for patients seeking quality care,” Rep. Renee Ellmers (R-N.C.) said in a statement.
Budget deal with NIH, CDC funding boost clears Congress
A budget deal that includes increases in funding to both the National Institutes of Health and the Centers for Disease Control and Prevention quickly passed through Congress and is expected to be signed by President Obama.
The omnibus budget agreement, announced Dec. 15, cleared the House on Dec. 18 by a 316-113 vote, with five representatives not voting. The agreement was quickly passed the same day in the Senate by a 65-33 vote. If signed, the budget bill would fund the government through the end of fiscal year 2016.
The agreement would boost funding to the NIH by $2 billion, bringing the total budget to $32 billion. It would increase funding for the CDC by about $308 million, bringing the 2016 budget to $7.2 billion.
The bill would cut $30 million from the Agency for Healthcare Research and Quality budget and keep the budgets at the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology at their fiscal 2015 levels.
Absent from the bill are any changes to the Meaningful Use program, as well as any cuts to Planned Parenthood funding.
A budget deal that includes increases in funding to both the National Institutes of Health and the Centers for Disease Control and Prevention quickly passed through Congress and is expected to be signed by President Obama.
The omnibus budget agreement, announced Dec. 15, cleared the House on Dec. 18 by a 316-113 vote, with five representatives not voting. The agreement was quickly passed the same day in the Senate by a 65-33 vote. If signed, the budget bill would fund the government through the end of fiscal year 2016.
The agreement would boost funding to the NIH by $2 billion, bringing the total budget to $32 billion. It would increase funding for the CDC by about $308 million, bringing the 2016 budget to $7.2 billion.
The bill would cut $30 million from the Agency for Healthcare Research and Quality budget and keep the budgets at the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology at their fiscal 2015 levels.
Absent from the bill are any changes to the Meaningful Use program, as well as any cuts to Planned Parenthood funding.
A budget deal that includes increases in funding to both the National Institutes of Health and the Centers for Disease Control and Prevention quickly passed through Congress and is expected to be signed by President Obama.
The omnibus budget agreement, announced Dec. 15, cleared the House on Dec. 18 by a 316-113 vote, with five representatives not voting. The agreement was quickly passed the same day in the Senate by a 65-33 vote. If signed, the budget bill would fund the government through the end of fiscal year 2016.
The agreement would boost funding to the NIH by $2 billion, bringing the total budget to $32 billion. It would increase funding for the CDC by about $308 million, bringing the 2016 budget to $7.2 billion.
The bill would cut $30 million from the Agency for Healthcare Research and Quality budget and keep the budgets at the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology at their fiscal 2015 levels.
Absent from the bill are any changes to the Meaningful Use program, as well as any cuts to Planned Parenthood funding.
ACA marketplace sees late surge in new enrollees
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
Physician Compare: Expanded data cause concern
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med
Docs to CMS: Delay Meaningful Use Stage 3
The American Medical Association is asking the Centers for Medicare & Medicaid Services to revise the meaningful use program to better align with requirements of last year’s Medicare Access and CHIP Reauthorization Act (MACRA) and to allow for smoother transition to value-based payment models.
In a Dec. 15 letter to CMS, the AMA issued a list of recommendations for meaningful use Stage 3 that aim to address challenges with using electronic health records (EHRs) and to help move toward MACRA’s alternative payment models (APM) and Merit-Based Incentive Payment System (MIPS).
“Doctors want to spend their time with patients, not measuring the number of clicks,” Dr. Steven J. Stack, AMA president, said in a statement. “We want a successful transition to digital health records, and we also want the new Medicare law to succeed. It will take thoughtful changes in the regulations to support physicians as they treat patients through new models of care.”
The AMA’s recommendations come in response to CMS’ final rule for meaningful use Stage 3, effective Dec. 15. The final rule simplified Stage 3 and gave doctors 1 more year – until Jan. 1, 2018 – to comply.
The AMA requested that CMS immediately adopt the association’s revisions for meaningful use Stage 3, including that the agency provide more flexibility and allow for multiple methods/paths to achieve desired end goals; remove threshold requirements for measures outside physicians’ control; and eliminate its pass-fail program design. Scrapping a pass-fail approach is the only way the ... program can align and operate within MIPS and APMs, Dr. James L. Madara, AMA executive vice president and CEO wrote in the letter.
The AMA also criticized Stage 3 for taking a poor approach to interoperability. The current measures are too focused on the quantity of information moved and “not the relevance of exchanges or the underlying business case for transmitting data,” Dr. Madara wrote. The AMA wants the measures to be refocused to address specific instances of data exchange, such as closing the referral loop, team-based care, and notification of tests/admissions.
According to the AMA, CMS should:
• Re-orient measures away from process-based tasks to highlight goals that are useful to patients and physicians.
• Encourage new technology functions to be the focus of certification rather than placing requirements on physicians and patients that may not yet be feasible.
• Support the reuse of data to reduce the burden on documentation.
The AMA’s recommendations are in line with concerns by the American Academy of Family Physicians over Stage 3, according to Dr. Robert L. Wergin, AAFP board chair. In a Dec. 2 letter to CMS, the AAFP said the final rule fell short of expectations and, in fact, places further obstacles in the way of improved health, better health care, and lower cost.
Interoperability challenges remain a top concern with the program, Dr. Wergin said in an interview. He cited a 27% decrease in physician satisfaction with EHRs since the launch of the meaningful use program, according to a 2014 survey.
“The whole concept of electronic health records, as family physicians, we saw the potential to help us care for our patients and help us track their progress,” Dr. Wergin said. “I would call it a potential unrealized. It really hasn’t developed into what we thought it could do. There’s a lot of frustrations.”
The AAFP calls for CMS to hit the pause button on meaningful use until 2019 – long enough to allow:
• The health care industry time to focus on interoperability issues.
• Vendors, physicians, and other health care professionals time to focus on designing and implementing the functionality and work flows necessary to achieve value-based payment.
• Regulators time to modify meaningful use regulations and align them with pending MACRA rules.
Similar concerns were expressed in a Nov. 20 letter from the GOP Doctors Caucus to Speaker of the House Paul Ryan (R-Wisc.). The 18-member caucus requested Speaker Ryan’s help in pressing for a delay of Stage 3 and a blanket hardship waiver exception for Stage 2. Implementation of more-stringent criteria is likely to create “a chilling effect on further EMR adoption as physicians conclude that the cost of implementation is simply not worth the bureaucratic hassle,” according to the letter. “Members of our caucus, as well as numerous congressional health care leaders, have engaged CMS on these issues to warn them of the potential negative consequences of placing these new requirements on providers in order to meet an arbitrary deadline. CMS has ignored Congress. Congressional action is the only solution left for preserving patient access, choice and quality.”
But Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., does not believe that the AMA’s recommendations nor other changes to the meaningful use program will make it better.
“I think they’re going about this entirely the wrong way,” said Dr. Bilhartz who blogs at bilhartzmd.com. “Meaningful use should not be delayed, but frankly abandoned. I don’t necessarily believe that the Department of Health & Human Service set out to try to design a system that would impair a physician’s ability to care for patients. I do believe without a doubt, that is exactly what has happened.”
On Twitter @legal_med
The American Medical Association is asking the Centers for Medicare & Medicaid Services to revise the meaningful use program to better align with requirements of last year’s Medicare Access and CHIP Reauthorization Act (MACRA) and to allow for smoother transition to value-based payment models.
In a Dec. 15 letter to CMS, the AMA issued a list of recommendations for meaningful use Stage 3 that aim to address challenges with using electronic health records (EHRs) and to help move toward MACRA’s alternative payment models (APM) and Merit-Based Incentive Payment System (MIPS).
“Doctors want to spend their time with patients, not measuring the number of clicks,” Dr. Steven J. Stack, AMA president, said in a statement. “We want a successful transition to digital health records, and we also want the new Medicare law to succeed. It will take thoughtful changes in the regulations to support physicians as they treat patients through new models of care.”
The AMA’s recommendations come in response to CMS’ final rule for meaningful use Stage 3, effective Dec. 15. The final rule simplified Stage 3 and gave doctors 1 more year – until Jan. 1, 2018 – to comply.
The AMA requested that CMS immediately adopt the association’s revisions for meaningful use Stage 3, including that the agency provide more flexibility and allow for multiple methods/paths to achieve desired end goals; remove threshold requirements for measures outside physicians’ control; and eliminate its pass-fail program design. Scrapping a pass-fail approach is the only way the ... program can align and operate within MIPS and APMs, Dr. James L. Madara, AMA executive vice president and CEO wrote in the letter.
The AMA also criticized Stage 3 for taking a poor approach to interoperability. The current measures are too focused on the quantity of information moved and “not the relevance of exchanges or the underlying business case for transmitting data,” Dr. Madara wrote. The AMA wants the measures to be refocused to address specific instances of data exchange, such as closing the referral loop, team-based care, and notification of tests/admissions.
According to the AMA, CMS should:
• Re-orient measures away from process-based tasks to highlight goals that are useful to patients and physicians.
• Encourage new technology functions to be the focus of certification rather than placing requirements on physicians and patients that may not yet be feasible.
• Support the reuse of data to reduce the burden on documentation.
The AMA’s recommendations are in line with concerns by the American Academy of Family Physicians over Stage 3, according to Dr. Robert L. Wergin, AAFP board chair. In a Dec. 2 letter to CMS, the AAFP said the final rule fell short of expectations and, in fact, places further obstacles in the way of improved health, better health care, and lower cost.
Interoperability challenges remain a top concern with the program, Dr. Wergin said in an interview. He cited a 27% decrease in physician satisfaction with EHRs since the launch of the meaningful use program, according to a 2014 survey.
“The whole concept of electronic health records, as family physicians, we saw the potential to help us care for our patients and help us track their progress,” Dr. Wergin said. “I would call it a potential unrealized. It really hasn’t developed into what we thought it could do. There’s a lot of frustrations.”
The AAFP calls for CMS to hit the pause button on meaningful use until 2019 – long enough to allow:
• The health care industry time to focus on interoperability issues.
• Vendors, physicians, and other health care professionals time to focus on designing and implementing the functionality and work flows necessary to achieve value-based payment.
• Regulators time to modify meaningful use regulations and align them with pending MACRA rules.
Similar concerns were expressed in a Nov. 20 letter from the GOP Doctors Caucus to Speaker of the House Paul Ryan (R-Wisc.). The 18-member caucus requested Speaker Ryan’s help in pressing for a delay of Stage 3 and a blanket hardship waiver exception for Stage 2. Implementation of more-stringent criteria is likely to create “a chilling effect on further EMR adoption as physicians conclude that the cost of implementation is simply not worth the bureaucratic hassle,” according to the letter. “Members of our caucus, as well as numerous congressional health care leaders, have engaged CMS on these issues to warn them of the potential negative consequences of placing these new requirements on providers in order to meet an arbitrary deadline. CMS has ignored Congress. Congressional action is the only solution left for preserving patient access, choice and quality.”
But Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., does not believe that the AMA’s recommendations nor other changes to the meaningful use program will make it better.
“I think they’re going about this entirely the wrong way,” said Dr. Bilhartz who blogs at bilhartzmd.com. “Meaningful use should not be delayed, but frankly abandoned. I don’t necessarily believe that the Department of Health & Human Service set out to try to design a system that would impair a physician’s ability to care for patients. I do believe without a doubt, that is exactly what has happened.”
On Twitter @legal_med
The American Medical Association is asking the Centers for Medicare & Medicaid Services to revise the meaningful use program to better align with requirements of last year’s Medicare Access and CHIP Reauthorization Act (MACRA) and to allow for smoother transition to value-based payment models.
In a Dec. 15 letter to CMS, the AMA issued a list of recommendations for meaningful use Stage 3 that aim to address challenges with using electronic health records (EHRs) and to help move toward MACRA’s alternative payment models (APM) and Merit-Based Incentive Payment System (MIPS).
“Doctors want to spend their time with patients, not measuring the number of clicks,” Dr. Steven J. Stack, AMA president, said in a statement. “We want a successful transition to digital health records, and we also want the new Medicare law to succeed. It will take thoughtful changes in the regulations to support physicians as they treat patients through new models of care.”
The AMA’s recommendations come in response to CMS’ final rule for meaningful use Stage 3, effective Dec. 15. The final rule simplified Stage 3 and gave doctors 1 more year – until Jan. 1, 2018 – to comply.
The AMA requested that CMS immediately adopt the association’s revisions for meaningful use Stage 3, including that the agency provide more flexibility and allow for multiple methods/paths to achieve desired end goals; remove threshold requirements for measures outside physicians’ control; and eliminate its pass-fail program design. Scrapping a pass-fail approach is the only way the ... program can align and operate within MIPS and APMs, Dr. James L. Madara, AMA executive vice president and CEO wrote in the letter.
The AMA also criticized Stage 3 for taking a poor approach to interoperability. The current measures are too focused on the quantity of information moved and “not the relevance of exchanges or the underlying business case for transmitting data,” Dr. Madara wrote. The AMA wants the measures to be refocused to address specific instances of data exchange, such as closing the referral loop, team-based care, and notification of tests/admissions.
According to the AMA, CMS should:
• Re-orient measures away from process-based tasks to highlight goals that are useful to patients and physicians.
• Encourage new technology functions to be the focus of certification rather than placing requirements on physicians and patients that may not yet be feasible.
• Support the reuse of data to reduce the burden on documentation.
The AMA’s recommendations are in line with concerns by the American Academy of Family Physicians over Stage 3, according to Dr. Robert L. Wergin, AAFP board chair. In a Dec. 2 letter to CMS, the AAFP said the final rule fell short of expectations and, in fact, places further obstacles in the way of improved health, better health care, and lower cost.
Interoperability challenges remain a top concern with the program, Dr. Wergin said in an interview. He cited a 27% decrease in physician satisfaction with EHRs since the launch of the meaningful use program, according to a 2014 survey.
“The whole concept of electronic health records, as family physicians, we saw the potential to help us care for our patients and help us track their progress,” Dr. Wergin said. “I would call it a potential unrealized. It really hasn’t developed into what we thought it could do. There’s a lot of frustrations.”
The AAFP calls for CMS to hit the pause button on meaningful use until 2019 – long enough to allow:
• The health care industry time to focus on interoperability issues.
• Vendors, physicians, and other health care professionals time to focus on designing and implementing the functionality and work flows necessary to achieve value-based payment.
• Regulators time to modify meaningful use regulations and align them with pending MACRA rules.
Similar concerns were expressed in a Nov. 20 letter from the GOP Doctors Caucus to Speaker of the House Paul Ryan (R-Wisc.). The 18-member caucus requested Speaker Ryan’s help in pressing for a delay of Stage 3 and a blanket hardship waiver exception for Stage 2. Implementation of more-stringent criteria is likely to create “a chilling effect on further EMR adoption as physicians conclude that the cost of implementation is simply not worth the bureaucratic hassle,” according to the letter. “Members of our caucus, as well as numerous congressional health care leaders, have engaged CMS on these issues to warn them of the potential negative consequences of placing these new requirements on providers in order to meet an arbitrary deadline. CMS has ignored Congress. Congressional action is the only solution left for preserving patient access, choice and quality.”
But Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., does not believe that the AMA’s recommendations nor other changes to the meaningful use program will make it better.
“I think they’re going about this entirely the wrong way,” said Dr. Bilhartz who blogs at bilhartzmd.com. “Meaningful use should not be delayed, but frankly abandoned. I don’t necessarily believe that the Department of Health & Human Service set out to try to design a system that would impair a physician’s ability to care for patients. I do believe without a doubt, that is exactly what has happened.”
On Twitter @legal_med