User login
Unassigned, Undocumented Patients Take a Toll on Healthcare and Hospitalists
When a patient must remain in the acute care hospital setting—despite being well enough to transition to a lower level of care, costs continue to mount as the patient receives care at the most expensive level.
“But policymakers must understand that reducing support for essential hospitals might save dollars in the short term but ultimately threatens access to care and creates greater costs in the long run,” says Beth Feldpush, DrPH, senior vice president of policy and advocacy for America’s Essential Hospitals in Washington, D.C. The group represents more than 250 essential hospitals, which fill a safety net role and provide communitywide services, such as trauma, neonatal intensive care, and disaster response.
“Our hospitals, which already operate at a loss on average, cannot continue to sustain federal and state funding cuts,” Dr. Feldpush says. “Access to care for vulnerable patients and entire communities will suffer if we continue to chip away at crucial sources of support, such as Medicaid and Medicare disproportionate share hospital funding and payment for outpatient services.”
The Affordable Care Act (ACA) makes many changes to the healthcare system that are designed to improve the quality, value of, and access to healthcare services.
“While many are good in theory, they have faced challenges in practice,” Dr. Feldpush says.
For example, the law’s authors included deep cuts to Medicaid and Medicare disproportionate share hospital (DSH) payments, which support hospitals that provide a large volume of uncompensated care. They made these cuts with the assumption that Medicare expansion and the ACA health insurance marketplace would significantly increase coverage, lessening the need for DSH payments. The U.S. Supreme Court’s decision to give states the option of expanding Medicaid has resulted in expansion in only about half of the states, however.
“But the DSH cuts remain, meaning our hospitals are getting significantly less support for the same or more uncompensated care,” Dr. Feldpush says.
Likewise, the ACA put into place many quality incentive programs for Medicare, including those designed to reduce preventable readmissions and hospital-acquired conditions and to encourage more value-based purchasing.
“The goals are obviously good ones, but the quality measures used to calculate incentive payments or penalties fail to account for the sociodemographic challenges our patients face—and that our hospitals can’t control,” she says. “So, these programs disproportionately penalize our hospitals, which, in turn, creates a vicious circle that reduces the funding they need to make improvements.”
Access to equitable healthcare for low-income, uninsured, and other vulnerable patients is a national problem, Dr. Feldpush continues. But the severity of the problem can vary by community and region—in states that have chosen not to expand their Medicaid programs, for example, or in economically depressed areas. TH
When a patient must remain in the acute care hospital setting—despite being well enough to transition to a lower level of care, costs continue to mount as the patient receives care at the most expensive level.
“But policymakers must understand that reducing support for essential hospitals might save dollars in the short term but ultimately threatens access to care and creates greater costs in the long run,” says Beth Feldpush, DrPH, senior vice president of policy and advocacy for America’s Essential Hospitals in Washington, D.C. The group represents more than 250 essential hospitals, which fill a safety net role and provide communitywide services, such as trauma, neonatal intensive care, and disaster response.
“Our hospitals, which already operate at a loss on average, cannot continue to sustain federal and state funding cuts,” Dr. Feldpush says. “Access to care for vulnerable patients and entire communities will suffer if we continue to chip away at crucial sources of support, such as Medicaid and Medicare disproportionate share hospital funding and payment for outpatient services.”
The Affordable Care Act (ACA) makes many changes to the healthcare system that are designed to improve the quality, value of, and access to healthcare services.
“While many are good in theory, they have faced challenges in practice,” Dr. Feldpush says.
For example, the law’s authors included deep cuts to Medicaid and Medicare disproportionate share hospital (DSH) payments, which support hospitals that provide a large volume of uncompensated care. They made these cuts with the assumption that Medicare expansion and the ACA health insurance marketplace would significantly increase coverage, lessening the need for DSH payments. The U.S. Supreme Court’s decision to give states the option of expanding Medicaid has resulted in expansion in only about half of the states, however.
“But the DSH cuts remain, meaning our hospitals are getting significantly less support for the same or more uncompensated care,” Dr. Feldpush says.
Likewise, the ACA put into place many quality incentive programs for Medicare, including those designed to reduce preventable readmissions and hospital-acquired conditions and to encourage more value-based purchasing.
“The goals are obviously good ones, but the quality measures used to calculate incentive payments or penalties fail to account for the sociodemographic challenges our patients face—and that our hospitals can’t control,” she says. “So, these programs disproportionately penalize our hospitals, which, in turn, creates a vicious circle that reduces the funding they need to make improvements.”
Access to equitable healthcare for low-income, uninsured, and other vulnerable patients is a national problem, Dr. Feldpush continues. But the severity of the problem can vary by community and region—in states that have chosen not to expand their Medicaid programs, for example, or in economically depressed areas. TH
When a patient must remain in the acute care hospital setting—despite being well enough to transition to a lower level of care, costs continue to mount as the patient receives care at the most expensive level.
“But policymakers must understand that reducing support for essential hospitals might save dollars in the short term but ultimately threatens access to care and creates greater costs in the long run,” says Beth Feldpush, DrPH, senior vice president of policy and advocacy for America’s Essential Hospitals in Washington, D.C. The group represents more than 250 essential hospitals, which fill a safety net role and provide communitywide services, such as trauma, neonatal intensive care, and disaster response.
“Our hospitals, which already operate at a loss on average, cannot continue to sustain federal and state funding cuts,” Dr. Feldpush says. “Access to care for vulnerable patients and entire communities will suffer if we continue to chip away at crucial sources of support, such as Medicaid and Medicare disproportionate share hospital funding and payment for outpatient services.”
The Affordable Care Act (ACA) makes many changes to the healthcare system that are designed to improve the quality, value of, and access to healthcare services.
“While many are good in theory, they have faced challenges in practice,” Dr. Feldpush says.
For example, the law’s authors included deep cuts to Medicaid and Medicare disproportionate share hospital (DSH) payments, which support hospitals that provide a large volume of uncompensated care. They made these cuts with the assumption that Medicare expansion and the ACA health insurance marketplace would significantly increase coverage, lessening the need for DSH payments. The U.S. Supreme Court’s decision to give states the option of expanding Medicaid has resulted in expansion in only about half of the states, however.
“But the DSH cuts remain, meaning our hospitals are getting significantly less support for the same or more uncompensated care,” Dr. Feldpush says.
Likewise, the ACA put into place many quality incentive programs for Medicare, including those designed to reduce preventable readmissions and hospital-acquired conditions and to encourage more value-based purchasing.
“The goals are obviously good ones, but the quality measures used to calculate incentive payments or penalties fail to account for the sociodemographic challenges our patients face—and that our hospitals can’t control,” she says. “So, these programs disproportionately penalize our hospitals, which, in turn, creates a vicious circle that reduces the funding they need to make improvements.”
Access to equitable healthcare for low-income, uninsured, and other vulnerable patients is a national problem, Dr. Feldpush continues. But the severity of the problem can vary by community and region—in states that have chosen not to expand their Medicaid programs, for example, or in economically depressed areas. TH
Health spending growth soars after years of low growth
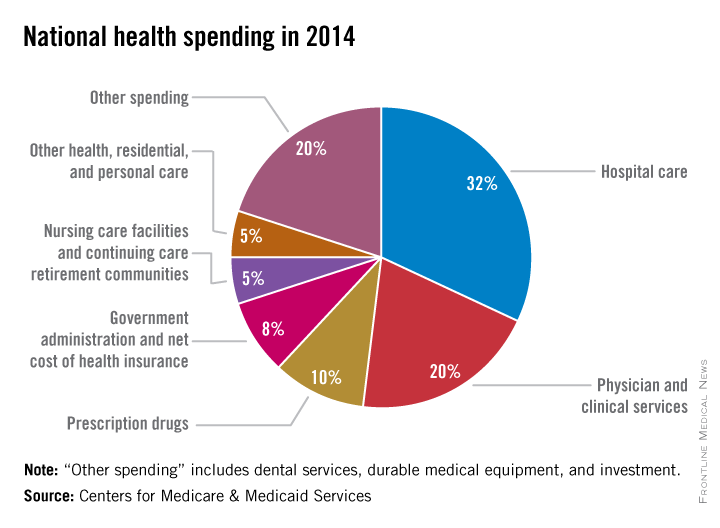
Five years of low growth in national health spending expenditures reversed substantially in 2014, driven by new Affordable Care Act coverage mandates and higher prescription drug spending, largely for new hepatitis C treatments.
U.S. health spending climbed to $3 trillion and grew by 5.3%, as compared with 2013 when growth was 2.9%. Per capita spending in 2014 was up 4.5% to $9,523 from $9,115 in 2013, according to Centers for Medicare & Medicaid Services Office of the Actuary (Health Affairs. 2015 Dec 2 doi: 10.1377/hlthaff.2015.1194).
Anne B. Martin, an economist with actuary’s office, said the 2014 numbers reversed several years of historically low spending growth that tracked with the sluggish economy. She attributed the uptick in growth to the ACA’s access mandates as well as prescription drug purchases, namely those for HCV, which added $11.3 billion in new spending.
“We can’t necessarily say that the [low-growth] cycle has been broken, but this 2014 phenomenon is driven primarily by the ACA expansion and the one-time impact of bringing the new hepatitis C drug into the 2014 mix,” Ms. Martin said.
Between 2013 and 2014, 8.7 million additional patients were enrolled in public and private health insurance, bringing the total insured share of the population from 86% to 88.8%, the highest coverage rate since 1987, according to Ms. Martin and her colleagues.
The growth rate for private health insurance spending went from 1.6% in 2013 to 4.4% in 2014. The $991 billion spent reflected the addition of 2.2 million newly insured patients, and higher rates of spending on medications, clinical services, and inpatient care, compared with 2013.
Federal government spending grew the fastest in 2014 at 11.7%, an 8.2% faster growth rate than in 2013.
In 2014, 28% of all health care purchases were made by the federal government, up from 26% in 2013.
Medicaid-specific spending totaled $495.8 billion, an 11% growth rate in 2014, up from 5.9% in 2013, reflecting the addition of 7.7 million Medicaid enrollees, various increases in prescription drug rebates, and updated provider fees.
Medicare spending jumped to 5.5%, up from 3.0% in 2013, largely due to prescription drugs, although Micah Hartman, a statistician in the Office of the Actuary, said that the per-enrollee spending rate was 2.4% in 2014, up from –0.2% in 2013, which was due to physician and clinical services, higher administrative costs, as well as the net cost of insurance, including fees and administrative costs.
Mr. Hartman singled out Medicare Advantage as a key contributor, noting that the 9.7% increase in growth for that program was from ACA-stipulated fees.
Overall, pharmaceutical spending was $297.7 billion in 2014, according to the report, attributable to novel HCV drugs, other new treatments, fewer-than-normal patent expirations and, in some cases, a doubling of the costs for certain brand-name drugs. The overall 2014 pharmaceutical expenditure growth rate was 12.2%, compared with 2.4% in 2013, the largest differential since 2002.
Physician and clinical services in 2014 went from a growth rate of 2.5% to 4.6%, with total spending at $603.7 billion. Hospital spending last year was $971.8 billion, with a spending growth rate of 4.1%, compared with 3.5% in 2013.
On Twitter @whitneymcknight
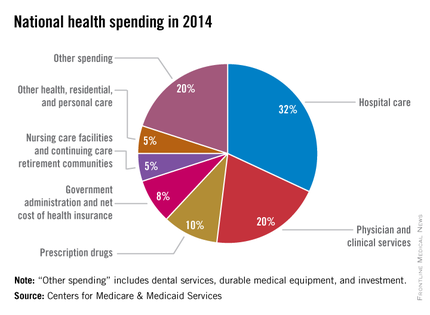
Five years of low growth in national health spending expenditures reversed substantially in 2014, driven by new Affordable Care Act coverage mandates and higher prescription drug spending, largely for new hepatitis C treatments.
U.S. health spending climbed to $3 trillion and grew by 5.3%, as compared with 2013 when growth was 2.9%. Per capita spending in 2014 was up 4.5% to $9,523 from $9,115 in 2013, according to Centers for Medicare & Medicaid Services Office of the Actuary (Health Affairs. 2015 Dec 2 doi: 10.1377/hlthaff.2015.1194).

Anne B. Martin, an economist with actuary’s office, said the 2014 numbers reversed several years of historically low spending growth that tracked with the sluggish economy. She attributed the uptick in growth to the ACA’s access mandates as well as prescription drug purchases, namely those for HCV, which added $11.3 billion in new spending.
“We can’t necessarily say that the [low-growth] cycle has been broken, but this 2014 phenomenon is driven primarily by the ACA expansion and the one-time impact of bringing the new hepatitis C drug into the 2014 mix,” Ms. Martin said.
Between 2013 and 2014, 8.7 million additional patients were enrolled in public and private health insurance, bringing the total insured share of the population from 86% to 88.8%, the highest coverage rate since 1987, according to Ms. Martin and her colleagues.
The growth rate for private health insurance spending went from 1.6% in 2013 to 4.4% in 2014. The $991 billion spent reflected the addition of 2.2 million newly insured patients, and higher rates of spending on medications, clinical services, and inpatient care, compared with 2013.
Federal government spending grew the fastest in 2014 at 11.7%, an 8.2% faster growth rate than in 2013.
In 2014, 28% of all health care purchases were made by the federal government, up from 26% in 2013.
Medicaid-specific spending totaled $495.8 billion, an 11% growth rate in 2014, up from 5.9% in 2013, reflecting the addition of 7.7 million Medicaid enrollees, various increases in prescription drug rebates, and updated provider fees.
Medicare spending jumped to 5.5%, up from 3.0% in 2013, largely due to prescription drugs, although Micah Hartman, a statistician in the Office of the Actuary, said that the per-enrollee spending rate was 2.4% in 2014, up from –0.2% in 2013, which was due to physician and clinical services, higher administrative costs, as well as the net cost of insurance, including fees and administrative costs.
Mr. Hartman singled out Medicare Advantage as a key contributor, noting that the 9.7% increase in growth for that program was from ACA-stipulated fees.
Overall, pharmaceutical spending was $297.7 billion in 2014, according to the report, attributable to novel HCV drugs, other new treatments, fewer-than-normal patent expirations and, in some cases, a doubling of the costs for certain brand-name drugs. The overall 2014 pharmaceutical expenditure growth rate was 12.2%, compared with 2.4% in 2013, the largest differential since 2002.
Physician and clinical services in 2014 went from a growth rate of 2.5% to 4.6%, with total spending at $603.7 billion. Hospital spending last year was $971.8 billion, with a spending growth rate of 4.1%, compared with 3.5% in 2013.
On Twitter @whitneymcknight
Five years of low growth in national health spending expenditures reversed substantially in 2014, driven by new Affordable Care Act coverage mandates and higher prescription drug spending, largely for new hepatitis C treatments.
U.S. health spending climbed to $3 trillion and grew by 5.3%, as compared with 2013 when growth was 2.9%. Per capita spending in 2014 was up 4.5% to $9,523 from $9,115 in 2013, according to Centers for Medicare & Medicaid Services Office of the Actuary (Health Affairs. 2015 Dec 2 doi: 10.1377/hlthaff.2015.1194).

Anne B. Martin, an economist with actuary’s office, said the 2014 numbers reversed several years of historically low spending growth that tracked with the sluggish economy. She attributed the uptick in growth to the ACA’s access mandates as well as prescription drug purchases, namely those for HCV, which added $11.3 billion in new spending.
“We can’t necessarily say that the [low-growth] cycle has been broken, but this 2014 phenomenon is driven primarily by the ACA expansion and the one-time impact of bringing the new hepatitis C drug into the 2014 mix,” Ms. Martin said.
Between 2013 and 2014, 8.7 million additional patients were enrolled in public and private health insurance, bringing the total insured share of the population from 86% to 88.8%, the highest coverage rate since 1987, according to Ms. Martin and her colleagues.
The growth rate for private health insurance spending went from 1.6% in 2013 to 4.4% in 2014. The $991 billion spent reflected the addition of 2.2 million newly insured patients, and higher rates of spending on medications, clinical services, and inpatient care, compared with 2013.
Federal government spending grew the fastest in 2014 at 11.7%, an 8.2% faster growth rate than in 2013.
In 2014, 28% of all health care purchases were made by the federal government, up from 26% in 2013.
Medicaid-specific spending totaled $495.8 billion, an 11% growth rate in 2014, up from 5.9% in 2013, reflecting the addition of 7.7 million Medicaid enrollees, various increases in prescription drug rebates, and updated provider fees.
Medicare spending jumped to 5.5%, up from 3.0% in 2013, largely due to prescription drugs, although Micah Hartman, a statistician in the Office of the Actuary, said that the per-enrollee spending rate was 2.4% in 2014, up from –0.2% in 2013, which was due to physician and clinical services, higher administrative costs, as well as the net cost of insurance, including fees and administrative costs.
Mr. Hartman singled out Medicare Advantage as a key contributor, noting that the 9.7% increase in growth for that program was from ACA-stipulated fees.
Overall, pharmaceutical spending was $297.7 billion in 2014, according to the report, attributable to novel HCV drugs, other new treatments, fewer-than-normal patent expirations and, in some cases, a doubling of the costs for certain brand-name drugs. The overall 2014 pharmaceutical expenditure growth rate was 12.2%, compared with 2.4% in 2013, the largest differential since 2002.
Physician and clinical services in 2014 went from a growth rate of 2.5% to 4.6%, with total spending at $603.7 billion. Hospital spending last year was $971.8 billion, with a spending growth rate of 4.1%, compared with 3.5% in 2013.
On Twitter @whitneymcknight
FROM THE JOURNAL HEALTH AFFAIRS
After 3 years of decline, hospital injury rates plateau, report finds
The rate of avoidable complications affecting patients in hospitals leveled off in 2014 after 3 years of declines, according to a federal report released Dec. 1.
Hospitals have averted many types of injuries where clear preventive steps have been identified, but they still struggle to avert complications with broader causes and less clear-cut solutions, government and hospital officials said.
There were at least 4 million infections and other potentially avoidable injuries in hospitals last year, the study estimated. That translates to about 12 of every 100 hospital stays. Among the most common complications that were measured – each occurring a quarter million times or more – were bed sores, falls, bad reactions to drugs used to treat diabetes, and kidney damage that develops after contrast dyes are injected through catheters to help radiologists take images of blood vessels.
The frequency of hospital complications last year was 17% lower than in 2010 but the same as in 2013, indicating that some patient safety improvements made by hospitals and the government are sticking. But the lack of improvement raised concerns that it is becoming harder for hospitals to further reduce the chances that a patient may be harmed during a visit.
“We are still trying to understand all the factors involved, but I think the improvements we saw from 2010 to 2013 were very likely the low-hanging fruit, the easy problems to solve,” said Dr. Richard Kronick, director of the U.S. Agency for Healthcare Research and Quality (AHRQ), which conducted the study.
The Obama administration has been focusing on lowering the rates of medical infections and injuries as it tracks a slew of patient safety initiatives created by the Affordable Care Act. Those include Medicare penalties for poor-performing hospitals, wider use of electronic records to help track patient care and prevent mistakes, and grants to collaborations of medical providers formed to improve the quality of patient care and identify the best ways of addressing each type of problem.
The AHRQ report calculated national rates for 27 specific complications by extrapolating from 30,000 medical cases that officials examined. Decreases in infections, medicine reactions, and other complications since 2010 have resulted in 2.1 million fewer incidents of harm, 87,000 fewer deaths, and $20 billion in health care savings, the report concluded.
“Those are real people that are not dying, getting infections or other adverse events in the hospital,” said Dr. Patrick Conway, chief medical officer for the Centers for Medicare & Medicaid Services.
Some of the most significant progress was made in lowering the number of infections from central lines inserted into veins – down 72% from 2010. Medical researchers have proven that these infections can be virtually eliminated if doctors and nurses follow a clear set of procedures.
Infections from urinary catheters decreased by 38% and surgical site infections dropped by 18%. In all three cases, the reductions exceeded the goals set by the administration. Dr. Conway noted that hospitals had a financial motivation to cut these infections as they are used to determine whether hospitals get Medicare bonuses and penalties each year.
However, hospitals have not made headway in trimming the numbers of falls or pneumonia cases in patients breathing through mechanical ventilators, the report found. And the rates of adverse drug reactions and complications during childbirth were higher than what the administration estimated they should be for 2014.
Dr. Conway said that complications are difficult to address because they involve tradeoffs that can cause other problems. For instance, he said, hospitals have to balance efforts to reduce falls with the need to help unstable patients improve their ability to walk. “We’ve got to work with providers to figure out what’s the sweet spot that can keep mobilization occurring but decrease the rate of falls,” he said.
Even though overall complication rates were flat, the report found that some types of injuries became less common in 2014. One was the number of blood clots that form after surgery and travel to the lung; those rates dropped by 30% in a year.
Maulik Joshi, an executive at the American Hospital Association, predicted that complications will become even rarer in future years. “Hospitals are working on projects that are just not reflected in these data points,” he said.
But a few conditions became more prevalent in 2014. Infections from methicillin-resistant Staphylococcus aureus increased by 55% to an estimated 17,000 incidents last year. The number of times a catheter punctured a femoral artery during an angiography increased by 25% to 74,000, the report estimated.
“We think we addressed a lot of the areas where there was a strong evidence base on how to improve patient safety,” Dr. Conway said. “We’ll now have to tackle that next wave that has multiple causes.”
The rate of avoidable complications affecting patients in hospitals leveled off in 2014 after 3 years of declines, according to a federal report released Dec. 1.
Hospitals have averted many types of injuries where clear preventive steps have been identified, but they still struggle to avert complications with broader causes and less clear-cut solutions, government and hospital officials said.
There were at least 4 million infections and other potentially avoidable injuries in hospitals last year, the study estimated. That translates to about 12 of every 100 hospital stays. Among the most common complications that were measured – each occurring a quarter million times or more – were bed sores, falls, bad reactions to drugs used to treat diabetes, and kidney damage that develops after contrast dyes are injected through catheters to help radiologists take images of blood vessels.
The frequency of hospital complications last year was 17% lower than in 2010 but the same as in 2013, indicating that some patient safety improvements made by hospitals and the government are sticking. But the lack of improvement raised concerns that it is becoming harder for hospitals to further reduce the chances that a patient may be harmed during a visit.
“We are still trying to understand all the factors involved, but I think the improvements we saw from 2010 to 2013 were very likely the low-hanging fruit, the easy problems to solve,” said Dr. Richard Kronick, director of the U.S. Agency for Healthcare Research and Quality (AHRQ), which conducted the study.
The Obama administration has been focusing on lowering the rates of medical infections and injuries as it tracks a slew of patient safety initiatives created by the Affordable Care Act. Those include Medicare penalties for poor-performing hospitals, wider use of electronic records to help track patient care and prevent mistakes, and grants to collaborations of medical providers formed to improve the quality of patient care and identify the best ways of addressing each type of problem.
The AHRQ report calculated national rates for 27 specific complications by extrapolating from 30,000 medical cases that officials examined. Decreases in infections, medicine reactions, and other complications since 2010 have resulted in 2.1 million fewer incidents of harm, 87,000 fewer deaths, and $20 billion in health care savings, the report concluded.
“Those are real people that are not dying, getting infections or other adverse events in the hospital,” said Dr. Patrick Conway, chief medical officer for the Centers for Medicare & Medicaid Services.
Some of the most significant progress was made in lowering the number of infections from central lines inserted into veins – down 72% from 2010. Medical researchers have proven that these infections can be virtually eliminated if doctors and nurses follow a clear set of procedures.
Infections from urinary catheters decreased by 38% and surgical site infections dropped by 18%. In all three cases, the reductions exceeded the goals set by the administration. Dr. Conway noted that hospitals had a financial motivation to cut these infections as they are used to determine whether hospitals get Medicare bonuses and penalties each year.
However, hospitals have not made headway in trimming the numbers of falls or pneumonia cases in patients breathing through mechanical ventilators, the report found. And the rates of adverse drug reactions and complications during childbirth were higher than what the administration estimated they should be for 2014.
Dr. Conway said that complications are difficult to address because they involve tradeoffs that can cause other problems. For instance, he said, hospitals have to balance efforts to reduce falls with the need to help unstable patients improve their ability to walk. “We’ve got to work with providers to figure out what’s the sweet spot that can keep mobilization occurring but decrease the rate of falls,” he said.
Even though overall complication rates were flat, the report found that some types of injuries became less common in 2014. One was the number of blood clots that form after surgery and travel to the lung; those rates dropped by 30% in a year.
Maulik Joshi, an executive at the American Hospital Association, predicted that complications will become even rarer in future years. “Hospitals are working on projects that are just not reflected in these data points,” he said.
But a few conditions became more prevalent in 2014. Infections from methicillin-resistant Staphylococcus aureus increased by 55% to an estimated 17,000 incidents last year. The number of times a catheter punctured a femoral artery during an angiography increased by 25% to 74,000, the report estimated.
“We think we addressed a lot of the areas where there was a strong evidence base on how to improve patient safety,” Dr. Conway said. “We’ll now have to tackle that next wave that has multiple causes.”
The rate of avoidable complications affecting patients in hospitals leveled off in 2014 after 3 years of declines, according to a federal report released Dec. 1.
Hospitals have averted many types of injuries where clear preventive steps have been identified, but they still struggle to avert complications with broader causes and less clear-cut solutions, government and hospital officials said.
There were at least 4 million infections and other potentially avoidable injuries in hospitals last year, the study estimated. That translates to about 12 of every 100 hospital stays. Among the most common complications that were measured – each occurring a quarter million times or more – were bed sores, falls, bad reactions to drugs used to treat diabetes, and kidney damage that develops after contrast dyes are injected through catheters to help radiologists take images of blood vessels.
The frequency of hospital complications last year was 17% lower than in 2010 but the same as in 2013, indicating that some patient safety improvements made by hospitals and the government are sticking. But the lack of improvement raised concerns that it is becoming harder for hospitals to further reduce the chances that a patient may be harmed during a visit.
“We are still trying to understand all the factors involved, but I think the improvements we saw from 2010 to 2013 were very likely the low-hanging fruit, the easy problems to solve,” said Dr. Richard Kronick, director of the U.S. Agency for Healthcare Research and Quality (AHRQ), which conducted the study.
The Obama administration has been focusing on lowering the rates of medical infections and injuries as it tracks a slew of patient safety initiatives created by the Affordable Care Act. Those include Medicare penalties for poor-performing hospitals, wider use of electronic records to help track patient care and prevent mistakes, and grants to collaborations of medical providers formed to improve the quality of patient care and identify the best ways of addressing each type of problem.
The AHRQ report calculated national rates for 27 specific complications by extrapolating from 30,000 medical cases that officials examined. Decreases in infections, medicine reactions, and other complications since 2010 have resulted in 2.1 million fewer incidents of harm, 87,000 fewer deaths, and $20 billion in health care savings, the report concluded.
“Those are real people that are not dying, getting infections or other adverse events in the hospital,” said Dr. Patrick Conway, chief medical officer for the Centers for Medicare & Medicaid Services.
Some of the most significant progress was made in lowering the number of infections from central lines inserted into veins – down 72% from 2010. Medical researchers have proven that these infections can be virtually eliminated if doctors and nurses follow a clear set of procedures.
Infections from urinary catheters decreased by 38% and surgical site infections dropped by 18%. In all three cases, the reductions exceeded the goals set by the administration. Dr. Conway noted that hospitals had a financial motivation to cut these infections as they are used to determine whether hospitals get Medicare bonuses and penalties each year.
However, hospitals have not made headway in trimming the numbers of falls or pneumonia cases in patients breathing through mechanical ventilators, the report found. And the rates of adverse drug reactions and complications during childbirth were higher than what the administration estimated they should be for 2014.
Dr. Conway said that complications are difficult to address because they involve tradeoffs that can cause other problems. For instance, he said, hospitals have to balance efforts to reduce falls with the need to help unstable patients improve their ability to walk. “We’ve got to work with providers to figure out what’s the sweet spot that can keep mobilization occurring but decrease the rate of falls,” he said.
Even though overall complication rates were flat, the report found that some types of injuries became less common in 2014. One was the number of blood clots that form after surgery and travel to the lung; those rates dropped by 30% in a year.
Maulik Joshi, an executive at the American Hospital Association, predicted that complications will become even rarer in future years. “Hospitals are working on projects that are just not reflected in these data points,” he said.
But a few conditions became more prevalent in 2014. Infections from methicillin-resistant Staphylococcus aureus increased by 55% to an estimated 17,000 incidents last year. The number of times a catheter punctured a femoral artery during an angiography increased by 25% to 74,000, the report estimated.
“We think we addressed a lot of the areas where there was a strong evidence base on how to improve patient safety,” Dr. Conway said. “We’ll now have to tackle that next wave that has multiple causes.”
ICD-10 Flexibility Helps Transition to New Coding Systems, Principles, Payer Policy Requirements
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Poor Continuity of Patient Care Increases Work for Hospitalist Groups
I think every hospitalist group should diligently try to maximize hospitalist-patient continuity, but many seem to adopt schedules and other operational practices that erode it. Let’s walk through the issue of continuity, starting with some history.
Inpatient Continuity in Old Healthcare System
Proudly carrying a pager nearly the size of a loaf of bread and wearing a white shirt and pants with Converse All Stars, I served as a hospital orderly in the 1970s. This position involved things like getting patients out of bed, placing Foley catheters, performing chest compressions during codes, and transporting the bodies of the deceased to the morgue. I really enjoyed the work, and the experience serves as one of my historical frames of reference for how hospital care has evolved since then.
The way I remember it, nearly everyone at the hospital worked a predictable schedule. RN staffing was the same each day; it didn’t vary based on census. Each full-time RN worked five shifts a week, eight hours each. Most or all would work alternate weekends and would have two compensatory days off during the following work week. This resulted in terrific continuity between nurse and patient, and the long length of stays meant patients and nurses got to know one another really well.
Continuity Takes a Hit
But things have changed. Nurse-patient continuity seems to have declined significantly as a result of two main forces: the hospital’s efforts to reduce staffing costs by varying nurse staffing to match daily patient volume, and nurses’ desire for a wide variety of work schedules. Asking a bedside nurse in today’s hospital whether the patient’s confusion, diarrhea, or appetite is meaningfully different today than yesterday typically yields the same reply. “This is my first day with the patient; I’ll have to look at the chart.”
I couldn’t find many research articles or editorials regarding hospital nurse-patient continuity from one day to the next. But several researchers seem to have begun studying this issue and have recently published a proposed framework for assessing it, and I found one study showing it wasn’t correlated with rates of pressure ulcers.1,2.
My anecdotal experience tells me continuity between the patient and caregivers of all stripes matters a lot. Research will be valuable in helping us to better understand its most significant costs and benefits, but I’m already convinced “Continuity is King” and should be one of the most important factors in the design of work schedules and patient allocation models for nurses and hospitalists alike.
While some might say we should wait for randomized trials of continuity to determine its importance, I’m inclined to see it like the authors of “Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials.” As a ding against those who insist on research data when common sense may be sufficient, they concluded “…that everyone might benefit if the most radical protagonists of evidence-based medicine organised and participated in a double-blind, randomised, placebo-controlled, crossover trial of the parachute.3
Continuity and Hospitalists
On top of what I see as erosion in nurse-patient continuity, the arrival of hospitalists disrupted doctor-patient continuity across the inpatient and outpatient setting. While there was significant concern about this when our field first took off in the 1990s, it seems to be getting a great deal less attention over the last few years. In many hospitalist groups I work with, it is one of the last factors considered when creating a work schedule. Factors that are examined include the following:
- Solely for provider convenience, a group might regularly schedule a provider for only two or three consecutive daytime shifts, or sometimes only single days;
- Groups that use unit-based hospital (a.k.a. “geographic”) staffing might have a patient transfer to a different attending hospitalist solely as a result of moving to a room in a different nursing unit; and
- As part of morning load leveling, some groups reassign existing patients to a new hospitalist.
I think all groups should work hard to avoid doing these things. And while I seem to be a real outlier on this one, I think the benefits of a separate daytime hospitalist admitter shift are not worth the cost of having different doctors always do the admission and first follow-up visit. Most groups should consider moving the admitter into an additional rounder position and allocating daytime admissions across all hospitalists.
One study found that hospitalist discontinuity was not associated with adverse events, and another found it was associated with higher length of stay for selected diagnoses.4,5 But there is too little research to draw hard conclusions. I’m convinced poor continuity increases the possibility of handoff-related errors, likely results in lower patient satisfaction, and increases the overall work of the hospitalist group, because more providers have to take the time to get to know the patient.
Although there will always be some tension between terrific continuity and a sustainable hospitalist lifestyle—a person can work only so many consecutive days before wearing out—every group should thoughtfully consider whether they are doing everything reasonable to maximize continuity. After all, continuity is king.

References
- Stifter J, Yao Y, Lopez KC, Khokhar A, Wilkie DJ, Keenan GM. Proposing a new conceptual model and an exemplar measure using health information technology to examine the impact of relational nurse continuity on hospital-acquired pressure ulcers. ANS Adv Nurs Sci. 2015;38(3):241-251.
- Stifter J, Yao Y, Lodhi MK, et al. Nurse continuity and hospital-acquired pressure ulcers: a comparative analysis using an electronic health record “big data” set. Nurs Res. 2015;64(5):361-371.
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459-1461.
- O’Leary KJ, Turner J, Christensen N, et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147-151.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
I think every hospitalist group should diligently try to maximize hospitalist-patient continuity, but many seem to adopt schedules and other operational practices that erode it. Let’s walk through the issue of continuity, starting with some history.
Inpatient Continuity in Old Healthcare System
Proudly carrying a pager nearly the size of a loaf of bread and wearing a white shirt and pants with Converse All Stars, I served as a hospital orderly in the 1970s. This position involved things like getting patients out of bed, placing Foley catheters, performing chest compressions during codes, and transporting the bodies of the deceased to the morgue. I really enjoyed the work, and the experience serves as one of my historical frames of reference for how hospital care has evolved since then.
The way I remember it, nearly everyone at the hospital worked a predictable schedule. RN staffing was the same each day; it didn’t vary based on census. Each full-time RN worked five shifts a week, eight hours each. Most or all would work alternate weekends and would have two compensatory days off during the following work week. This resulted in terrific continuity between nurse and patient, and the long length of stays meant patients and nurses got to know one another really well.
Continuity Takes a Hit
But things have changed. Nurse-patient continuity seems to have declined significantly as a result of two main forces: the hospital’s efforts to reduce staffing costs by varying nurse staffing to match daily patient volume, and nurses’ desire for a wide variety of work schedules. Asking a bedside nurse in today’s hospital whether the patient’s confusion, diarrhea, or appetite is meaningfully different today than yesterday typically yields the same reply. “This is my first day with the patient; I’ll have to look at the chart.”
I couldn’t find many research articles or editorials regarding hospital nurse-patient continuity from one day to the next. But several researchers seem to have begun studying this issue and have recently published a proposed framework for assessing it, and I found one study showing it wasn’t correlated with rates of pressure ulcers.1,2.
My anecdotal experience tells me continuity between the patient and caregivers of all stripes matters a lot. Research will be valuable in helping us to better understand its most significant costs and benefits, but I’m already convinced “Continuity is King” and should be one of the most important factors in the design of work schedules and patient allocation models for nurses and hospitalists alike.
While some might say we should wait for randomized trials of continuity to determine its importance, I’m inclined to see it like the authors of “Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials.” As a ding against those who insist on research data when common sense may be sufficient, they concluded “…that everyone might benefit if the most radical protagonists of evidence-based medicine organised and participated in a double-blind, randomised, placebo-controlled, crossover trial of the parachute.3
Continuity and Hospitalists
On top of what I see as erosion in nurse-patient continuity, the arrival of hospitalists disrupted doctor-patient continuity across the inpatient and outpatient setting. While there was significant concern about this when our field first took off in the 1990s, it seems to be getting a great deal less attention over the last few years. In many hospitalist groups I work with, it is one of the last factors considered when creating a work schedule. Factors that are examined include the following:
- Solely for provider convenience, a group might regularly schedule a provider for only two or three consecutive daytime shifts, or sometimes only single days;
- Groups that use unit-based hospital (a.k.a. “geographic”) staffing might have a patient transfer to a different attending hospitalist solely as a result of moving to a room in a different nursing unit; and
- As part of morning load leveling, some groups reassign existing patients to a new hospitalist.
I think all groups should work hard to avoid doing these things. And while I seem to be a real outlier on this one, I think the benefits of a separate daytime hospitalist admitter shift are not worth the cost of having different doctors always do the admission and first follow-up visit. Most groups should consider moving the admitter into an additional rounder position and allocating daytime admissions across all hospitalists.
One study found that hospitalist discontinuity was not associated with adverse events, and another found it was associated with higher length of stay for selected diagnoses.4,5 But there is too little research to draw hard conclusions. I’m convinced poor continuity increases the possibility of handoff-related errors, likely results in lower patient satisfaction, and increases the overall work of the hospitalist group, because more providers have to take the time to get to know the patient.
Although there will always be some tension between terrific continuity and a sustainable hospitalist lifestyle—a person can work only so many consecutive days before wearing out—every group should thoughtfully consider whether they are doing everything reasonable to maximize continuity. After all, continuity is king.

References
- Stifter J, Yao Y, Lopez KC, Khokhar A, Wilkie DJ, Keenan GM. Proposing a new conceptual model and an exemplar measure using health information technology to examine the impact of relational nurse continuity on hospital-acquired pressure ulcers. ANS Adv Nurs Sci. 2015;38(3):241-251.
- Stifter J, Yao Y, Lodhi MK, et al. Nurse continuity and hospital-acquired pressure ulcers: a comparative analysis using an electronic health record “big data” set. Nurs Res. 2015;64(5):361-371.
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459-1461.
- O’Leary KJ, Turner J, Christensen N, et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147-151.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
I think every hospitalist group should diligently try to maximize hospitalist-patient continuity, but many seem to adopt schedules and other operational practices that erode it. Let’s walk through the issue of continuity, starting with some history.
Inpatient Continuity in Old Healthcare System
Proudly carrying a pager nearly the size of a loaf of bread and wearing a white shirt and pants with Converse All Stars, I served as a hospital orderly in the 1970s. This position involved things like getting patients out of bed, placing Foley catheters, performing chest compressions during codes, and transporting the bodies of the deceased to the morgue. I really enjoyed the work, and the experience serves as one of my historical frames of reference for how hospital care has evolved since then.
The way I remember it, nearly everyone at the hospital worked a predictable schedule. RN staffing was the same each day; it didn’t vary based on census. Each full-time RN worked five shifts a week, eight hours each. Most or all would work alternate weekends and would have two compensatory days off during the following work week. This resulted in terrific continuity between nurse and patient, and the long length of stays meant patients and nurses got to know one another really well.
Continuity Takes a Hit
But things have changed. Nurse-patient continuity seems to have declined significantly as a result of two main forces: the hospital’s efforts to reduce staffing costs by varying nurse staffing to match daily patient volume, and nurses’ desire for a wide variety of work schedules. Asking a bedside nurse in today’s hospital whether the patient’s confusion, diarrhea, or appetite is meaningfully different today than yesterday typically yields the same reply. “This is my first day with the patient; I’ll have to look at the chart.”
I couldn’t find many research articles or editorials regarding hospital nurse-patient continuity from one day to the next. But several researchers seem to have begun studying this issue and have recently published a proposed framework for assessing it, and I found one study showing it wasn’t correlated with rates of pressure ulcers.1,2.
My anecdotal experience tells me continuity between the patient and caregivers of all stripes matters a lot. Research will be valuable in helping us to better understand its most significant costs and benefits, but I’m already convinced “Continuity is King” and should be one of the most important factors in the design of work schedules and patient allocation models for nurses and hospitalists alike.
While some might say we should wait for randomized trials of continuity to determine its importance, I’m inclined to see it like the authors of “Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials.” As a ding against those who insist on research data when common sense may be sufficient, they concluded “…that everyone might benefit if the most radical protagonists of evidence-based medicine organised and participated in a double-blind, randomised, placebo-controlled, crossover trial of the parachute.3
Continuity and Hospitalists
On top of what I see as erosion in nurse-patient continuity, the arrival of hospitalists disrupted doctor-patient continuity across the inpatient and outpatient setting. While there was significant concern about this when our field first took off in the 1990s, it seems to be getting a great deal less attention over the last few years. In many hospitalist groups I work with, it is one of the last factors considered when creating a work schedule. Factors that are examined include the following:
- Solely for provider convenience, a group might regularly schedule a provider for only two or three consecutive daytime shifts, or sometimes only single days;
- Groups that use unit-based hospital (a.k.a. “geographic”) staffing might have a patient transfer to a different attending hospitalist solely as a result of moving to a room in a different nursing unit; and
- As part of morning load leveling, some groups reassign existing patients to a new hospitalist.
I think all groups should work hard to avoid doing these things. And while I seem to be a real outlier on this one, I think the benefits of a separate daytime hospitalist admitter shift are not worth the cost of having different doctors always do the admission and first follow-up visit. Most groups should consider moving the admitter into an additional rounder position and allocating daytime admissions across all hospitalists.
One study found that hospitalist discontinuity was not associated with adverse events, and another found it was associated with higher length of stay for selected diagnoses.4,5 But there is too little research to draw hard conclusions. I’m convinced poor continuity increases the possibility of handoff-related errors, likely results in lower patient satisfaction, and increases the overall work of the hospitalist group, because more providers have to take the time to get to know the patient.
Although there will always be some tension between terrific continuity and a sustainable hospitalist lifestyle—a person can work only so many consecutive days before wearing out—every group should thoughtfully consider whether they are doing everything reasonable to maximize continuity. After all, continuity is king.

References
- Stifter J, Yao Y, Lopez KC, Khokhar A, Wilkie DJ, Keenan GM. Proposing a new conceptual model and an exemplar measure using health information technology to examine the impact of relational nurse continuity on hospital-acquired pressure ulcers. ANS Adv Nurs Sci. 2015;38(3):241-251.
- Stifter J, Yao Y, Lodhi MK, et al. Nurse continuity and hospital-acquired pressure ulcers: a comparative analysis using an electronic health record “big data” set. Nurs Res. 2015;64(5):361-371.
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327(7429):1459-1461.
- O’Leary KJ, Turner J, Christensen N, et al. The effect of hospitalist discontinuity on adverse events. J Hosp Med. 2015;10(3):147-151.
- Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
Stark Law: More flexibility starting in 2016
Medicare has relaxed some requirements of the Stark Law through its 2016 fee schedule and created new exceptions for compensation arrangements under the statute. The changes make it easier to recruit nonphysician employees, share rental space, and operate on expired contracts without fear of violating the law.
“These rules show the government was seeking to give some flexibility in the area of Stark procedure or technical issues,” said Julie E. Kass, a Washington area health law attorney who specializes in Stark and antikickback laws. “I think what [the Centers for Medicare & Medicaid Services] was seeing, and the kinds of issues being disclosed, weren’t things that were going to raise the risk of fraud or abuse to the program. Recognizing that, they wanted to make sure there weren’t unnecessary concerns about these, or unnecessary [efforts].”
What should you know about the Stark Law modifications? Ms. Kass and Philadelphia-based health law attorney Karl A. Thallner Jr. discussed the latest changes in a recent interview.
Nonphysician recruitment
Starting in 2016, hospitals can assist in the recruitment of nonphysician health professionals for physician practices. In the past, hospitals could not because remuneration could be considered a compensation relationship between the hospital and the practice.
In the fee schedule final rule, CMS expands its definition of nonphysician provider to mean physician assistant, nurse practitioner, clinical nurse specialist, certified nurse-midwife, clinical psychologist, or clinical social worker. Hospitals, rural health clinics, and federally qualified health centers can provide recruitment assistance and retention payments to physician practices to employ nonphysician providers. CMS also loosened its original proposal that said the nonphysician provider would have to be a bona fide employee of the physician practice. Instead, they can be independent contractors as long as they contract directly with the practice, according to the final rule. Third-party companies do not qualify.
While the change is primarily positive, it does have limitations, Mr. Thallner said. The subsidy amount from the hospital for example, can be only 50% of employment costs and can last just 2 years.
“At some point, the practice is going to have to assume the full risk of the person,” he said. “But one might envision some scenarios where this might be helpful to physician practice in a community where there’s some need for start-up support.”
Holdover extension
Physicians who have compensation arrangements that fall under a Stark Law exception no longer need to panic if their agreement expires and they neglect to redraft a new contract. The 2016 rule releases doctors from potential violations if such an agreement expires, but the arrangement continues under the same terms.
In the past, doctors had a 6-month grace period to renew an arrangement agreement once the contract expired. CMS noted it receives numerous disclosures of actual or potential violations relating to writing requirements of compensation exceptions through the self-referral disclosure protocol, which allows providers and suppliers to disclose actual or potential violations of the physician self-referral law to CMS and authorizes the Health & Human Services department to reduce the amount potentially owed for disclosed violations. However, arrangements that continue beyond the 6-month period do not necessarily pose a risk of program or patient abuse, provided that the arrangement continues to satisfy the specific requirements of the applicable exception, the agency stated.
The agency has eliminated the time limitation on contract holdovers if the agreements meet requirements related to fair market value and so long as the compensation does not take into account the volume or value of referrals or other business generated between the parties.
“This one [is] helpful if you find you have an agreement that has slipped through the cracks,” Mr. Thallner said.
Practices should still be monitoring agreements after they expire to ensure that compensation levels remain appropriate and take efforts to redraft if changes are identified, he stressed.
Timeshare reprieve
Timeshare arrangements for office space, equipment, personnel, supplies, and other services are allowed starting in 2016.
Previously, physicians who did not require traditional office spaces could only lease from sources who could pose a referral relationship on a part-time basis and those rentals had to me meet specific rental criteria. The renter was required to have exclusive use of the space and 1-year contract.
CMS now acknowledges that in some cases – such as in rural or underserved areas – there may be a community need for short-term specialty services in which exclusive use of an office is not necessary. Under a timeshare arrangement, a hospital or local practice may ask a specialist from a neighboring community to use space owned by the hospital or practice on a limited or as-needed basis. Often, the specialist does not establish an additional office, but instead creates a timeshare-like arrangement for the space, equipment, and services necessary to treat patients.
To timeshare, doctors must meet the following requirements:
• The arrangement is set out in writing, signed by the parties, and specifies the premises, equipment, personnel, items, supplies, and services covered by the arrangement.
• The arrangement is between a physician and a hospital or a physician organization of which the physician is not an owner, employee, or contractor.
• The arrangement is not conditioned on the licensee’s referral of patients to the licensor.
• The compensation over the term of the arrangement is set in advance, consistent with fair market value.
The timeshare exception reduces hassles and makes it easier for doctors to share work spaces for short durations of time, Ms. Kass said.
“Physicians are able to either license or be the licensor or licensee of a timeshare arrangement rather than having a lease on a part-time basis,” she said. “You can still create a lease on a part-time basis using the old rental of space rules, but if you’re leasing a whole office, complete with all of the equipment and personnel and the space, you are able to do that for a day, for a week, in periods of time. Now one single exception can help you with that.”
On Twitter @legal_med
Medicare has relaxed some requirements of the Stark Law through its 2016 fee schedule and created new exceptions for compensation arrangements under the statute. The changes make it easier to recruit nonphysician employees, share rental space, and operate on expired contracts without fear of violating the law.
“These rules show the government was seeking to give some flexibility in the area of Stark procedure or technical issues,” said Julie E. Kass, a Washington area health law attorney who specializes in Stark and antikickback laws. “I think what [the Centers for Medicare & Medicaid Services] was seeing, and the kinds of issues being disclosed, weren’t things that were going to raise the risk of fraud or abuse to the program. Recognizing that, they wanted to make sure there weren’t unnecessary concerns about these, or unnecessary [efforts].”
What should you know about the Stark Law modifications? Ms. Kass and Philadelphia-based health law attorney Karl A. Thallner Jr. discussed the latest changes in a recent interview.
Nonphysician recruitment
Starting in 2016, hospitals can assist in the recruitment of nonphysician health professionals for physician practices. In the past, hospitals could not because remuneration could be considered a compensation relationship between the hospital and the practice.
In the fee schedule final rule, CMS expands its definition of nonphysician provider to mean physician assistant, nurse practitioner, clinical nurse specialist, certified nurse-midwife, clinical psychologist, or clinical social worker. Hospitals, rural health clinics, and federally qualified health centers can provide recruitment assistance and retention payments to physician practices to employ nonphysician providers. CMS also loosened its original proposal that said the nonphysician provider would have to be a bona fide employee of the physician practice. Instead, they can be independent contractors as long as they contract directly with the practice, according to the final rule. Third-party companies do not qualify.
While the change is primarily positive, it does have limitations, Mr. Thallner said. The subsidy amount from the hospital for example, can be only 50% of employment costs and can last just 2 years.
“At some point, the practice is going to have to assume the full risk of the person,” he said. “But one might envision some scenarios where this might be helpful to physician practice in a community where there’s some need for start-up support.”
Holdover extension
Physicians who have compensation arrangements that fall under a Stark Law exception no longer need to panic if their agreement expires and they neglect to redraft a new contract. The 2016 rule releases doctors from potential violations if such an agreement expires, but the arrangement continues under the same terms.
In the past, doctors had a 6-month grace period to renew an arrangement agreement once the contract expired. CMS noted it receives numerous disclosures of actual or potential violations relating to writing requirements of compensation exceptions through the self-referral disclosure protocol, which allows providers and suppliers to disclose actual or potential violations of the physician self-referral law to CMS and authorizes the Health & Human Services department to reduce the amount potentially owed for disclosed violations. However, arrangements that continue beyond the 6-month period do not necessarily pose a risk of program or patient abuse, provided that the arrangement continues to satisfy the specific requirements of the applicable exception, the agency stated.
The agency has eliminated the time limitation on contract holdovers if the agreements meet requirements related to fair market value and so long as the compensation does not take into account the volume or value of referrals or other business generated between the parties.
“This one [is] helpful if you find you have an agreement that has slipped through the cracks,” Mr. Thallner said.
Practices should still be monitoring agreements after they expire to ensure that compensation levels remain appropriate and take efforts to redraft if changes are identified, he stressed.
Timeshare reprieve
Timeshare arrangements for office space, equipment, personnel, supplies, and other services are allowed starting in 2016.
Previously, physicians who did not require traditional office spaces could only lease from sources who could pose a referral relationship on a part-time basis and those rentals had to me meet specific rental criteria. The renter was required to have exclusive use of the space and 1-year contract.
CMS now acknowledges that in some cases – such as in rural or underserved areas – there may be a community need for short-term specialty services in which exclusive use of an office is not necessary. Under a timeshare arrangement, a hospital or local practice may ask a specialist from a neighboring community to use space owned by the hospital or practice on a limited or as-needed basis. Often, the specialist does not establish an additional office, but instead creates a timeshare-like arrangement for the space, equipment, and services necessary to treat patients.
To timeshare, doctors must meet the following requirements:
• The arrangement is set out in writing, signed by the parties, and specifies the premises, equipment, personnel, items, supplies, and services covered by the arrangement.
• The arrangement is between a physician and a hospital or a physician organization of which the physician is not an owner, employee, or contractor.
• The arrangement is not conditioned on the licensee’s referral of patients to the licensor.
• The compensation over the term of the arrangement is set in advance, consistent with fair market value.
The timeshare exception reduces hassles and makes it easier for doctors to share work spaces for short durations of time, Ms. Kass said.
“Physicians are able to either license or be the licensor or licensee of a timeshare arrangement rather than having a lease on a part-time basis,” she said. “You can still create a lease on a part-time basis using the old rental of space rules, but if you’re leasing a whole office, complete with all of the equipment and personnel and the space, you are able to do that for a day, for a week, in periods of time. Now one single exception can help you with that.”
On Twitter @legal_med
Medicare has relaxed some requirements of the Stark Law through its 2016 fee schedule and created new exceptions for compensation arrangements under the statute. The changes make it easier to recruit nonphysician employees, share rental space, and operate on expired contracts without fear of violating the law.
“These rules show the government was seeking to give some flexibility in the area of Stark procedure or technical issues,” said Julie E. Kass, a Washington area health law attorney who specializes in Stark and antikickback laws. “I think what [the Centers for Medicare & Medicaid Services] was seeing, and the kinds of issues being disclosed, weren’t things that were going to raise the risk of fraud or abuse to the program. Recognizing that, they wanted to make sure there weren’t unnecessary concerns about these, or unnecessary [efforts].”
What should you know about the Stark Law modifications? Ms. Kass and Philadelphia-based health law attorney Karl A. Thallner Jr. discussed the latest changes in a recent interview.
Nonphysician recruitment
Starting in 2016, hospitals can assist in the recruitment of nonphysician health professionals for physician practices. In the past, hospitals could not because remuneration could be considered a compensation relationship between the hospital and the practice.
In the fee schedule final rule, CMS expands its definition of nonphysician provider to mean physician assistant, nurse practitioner, clinical nurse specialist, certified nurse-midwife, clinical psychologist, or clinical social worker. Hospitals, rural health clinics, and federally qualified health centers can provide recruitment assistance and retention payments to physician practices to employ nonphysician providers. CMS also loosened its original proposal that said the nonphysician provider would have to be a bona fide employee of the physician practice. Instead, they can be independent contractors as long as they contract directly with the practice, according to the final rule. Third-party companies do not qualify.
While the change is primarily positive, it does have limitations, Mr. Thallner said. The subsidy amount from the hospital for example, can be only 50% of employment costs and can last just 2 years.
“At some point, the practice is going to have to assume the full risk of the person,” he said. “But one might envision some scenarios where this might be helpful to physician practice in a community where there’s some need for start-up support.”
Holdover extension
Physicians who have compensation arrangements that fall under a Stark Law exception no longer need to panic if their agreement expires and they neglect to redraft a new contract. The 2016 rule releases doctors from potential violations if such an agreement expires, but the arrangement continues under the same terms.
In the past, doctors had a 6-month grace period to renew an arrangement agreement once the contract expired. CMS noted it receives numerous disclosures of actual or potential violations relating to writing requirements of compensation exceptions through the self-referral disclosure protocol, which allows providers and suppliers to disclose actual or potential violations of the physician self-referral law to CMS and authorizes the Health & Human Services department to reduce the amount potentially owed for disclosed violations. However, arrangements that continue beyond the 6-month period do not necessarily pose a risk of program or patient abuse, provided that the arrangement continues to satisfy the specific requirements of the applicable exception, the agency stated.
The agency has eliminated the time limitation on contract holdovers if the agreements meet requirements related to fair market value and so long as the compensation does not take into account the volume or value of referrals or other business generated between the parties.
“This one [is] helpful if you find you have an agreement that has slipped through the cracks,” Mr. Thallner said.
Practices should still be monitoring agreements after they expire to ensure that compensation levels remain appropriate and take efforts to redraft if changes are identified, he stressed.
Timeshare reprieve
Timeshare arrangements for office space, equipment, personnel, supplies, and other services are allowed starting in 2016.
Previously, physicians who did not require traditional office spaces could only lease from sources who could pose a referral relationship on a part-time basis and those rentals had to me meet specific rental criteria. The renter was required to have exclusive use of the space and 1-year contract.
CMS now acknowledges that in some cases – such as in rural or underserved areas – there may be a community need for short-term specialty services in which exclusive use of an office is not necessary. Under a timeshare arrangement, a hospital or local practice may ask a specialist from a neighboring community to use space owned by the hospital or practice on a limited or as-needed basis. Often, the specialist does not establish an additional office, but instead creates a timeshare-like arrangement for the space, equipment, and services necessary to treat patients.
To timeshare, doctors must meet the following requirements:
• The arrangement is set out in writing, signed by the parties, and specifies the premises, equipment, personnel, items, supplies, and services covered by the arrangement.
• The arrangement is between a physician and a hospital or a physician organization of which the physician is not an owner, employee, or contractor.
• The arrangement is not conditioned on the licensee’s referral of patients to the licensor.
• The compensation over the term of the arrangement is set in advance, consistent with fair market value.
The timeshare exception reduces hassles and makes it easier for doctors to share work spaces for short durations of time, Ms. Kass said.
“Physicians are able to either license or be the licensor or licensee of a timeshare arrangement rather than having a lease on a part-time basis,” she said. “You can still create a lease on a part-time basis using the old rental of space rules, but if you’re leasing a whole office, complete with all of the equipment and personnel and the space, you are able to do that for a day, for a week, in periods of time. Now one single exception can help you with that.”
On Twitter @legal_med
UnitedHealth warns of marketplace exit: Start of a trend or push for White House action?
UnitedHealthGroup laid out a litany of reasons on Nov. 19 as to why it might stop selling individual health insurance through federal and state markets in 2017 – a move some see as an effort to compel the Obama administration to ease regulations and make good on promised payments.
Those problems, including low participation by healthy people, have led to financial losses, according to UnitedHealth. If not addressed, similar issues could affect other insurers, causing more to exit the market in the coming years, some Wall Street analysts and policy experts said.
Many said they anticipate the federal government will act to forestall widespread departures, particularly because continued withdrawals could be politically explosive during an election year.
A key piece of President Obama’s signature health care law, the online marketplaces, also called exchanges, opened in 2014 for people who buy their own insurance because they don’t get it through their jobs. Enrollment, while growing, has fallen short of capturing the share of the eligible uninsured that was anticipated. This year, the marketplaces saw enrollment of more than 9 million customers, although the law’s expansion of Medicaid enrollment in many states also has played a large role in reducing the overall number of uninsured.
Only a month ago, United sounded more optimistic about business on the exchanges. But on Nov. 19, the insurer said it would cut its earnings forecast and projected hundreds of millions in losses stemming from the policies it sells through the health law’s marketplaces.
The turnaround led some analysts to ask the insurer what had changed.
Stephen Hemsley, UnitedHealth chief executive officer, said too many healthy people dropped coverage and noted slower than expected enrollment. A major factor, he added, was far higher costs for those who signed up for 2015 coverage under special exemptions after the general open-enrollment period ended. Those exemptions included, for example, people who lost their insurance, moved, or suffered a hardship, such as an eviction or had their utilities turned off. United said it did not see a similar increase in costs for people who bought policies from private brokers or websites instead of the government marketplaces after open enrollment, suggesting the reason was partly that the company’s eligibility assessments were more thorough.
The firm did not say it would halt sales in 2017 but warned that it would strongly consider doing so based on what happens in the next few months.
“We cannot sustain these losses,” Mr. Hemsley said on a phone call with Wall Street analysts. “We can’t subsidize a marketplace that doesn’t appear at the moment to be sustaining itself.”
Although it’s the nation’s largest insurer, United captured only a small percentage of consumers who currently have coverage through the Affordable Care Act marketplace, in part because it sat out the first year of enrollment and really ramped up only for this year’s coverage.
While seen as a serious challenge to the health care law, United’s decision alone doesn’t mark the death knell for the exchanges. In remarks to analysts and press reports on Nov. 19, Aetna and Kaiser Permanente re-affirmed their commitment to selling through the marketplaces.
Ben Wakana, a spokesman for the Health and Human Services department, defended the government marketplaces, noting that 9 of 10 of policyholders re-enrolling have a choice of three or more insurers for next year. “The reality is we continue to see more people signing up for health insurance and more issuers entering the marketplaces, and at the end of January, we believe we’ll be looking at another successful open enrollment – just like the last two,” he said. “[Thursday’s] statement by one issuer is not indicative of the marketplace’s strength and viability.”
But insurers, including Humana, Aetna, and some of the large Blue Cross Blue Shield plans, were losing money or barely breaking even on their marketplace business, according to earnings reports.
“If there are no changes, all the large publicly traded companies will end up leaving,” said Ana Gupte, analyst with Leerink Partners. “But I would be very surprised if [the Department of Health & Human Services] doesn’t do something to accommodate their issues.”
Those options would be limited to what the agency could do without congressional action, many analysts said. Still, that could include relaxing some regulations or reconsidering some of the exemptions that allow people to sign up after the open-enrollment period.
Former insurance executive and consultant Robert Laszewski said the administration needs to relax the rules to give insurers more flexibility to design plans that would attract healthier people. He said the costs – including deductibles and premiums – were too high for many people, particularly those with few medical needs.
“Disproportionately, the sick are signing up and the healthy are dropping out,” said Mr. Laszewski, adding that alternative plans with fewer benefits but lower costs should be made available.
Economist Len Nichols cautioned, however, that most of the law’s benefit requirements – taken individually – add little to the cost of a plan. Removing the bigger-ticket requirements, such as coverage for maternity care, would leave consumers without adequate coverage, said Nichols, who directs the George Mason University Center for Health Research and Ethics in Fairfax, Va.
Mr. Nichols, Ms. Gupte, and other analysts agree with the industry’s trade lobby, which says one thing the administration could do is make good on a promise to pay insurers under a temporary program designed to redistribute profits from some insurers that did especially well to offset losses others experienced in the marketplace plans. That program, however, has paid only about 13 cents on the dollar of what was promised, mainly because fewer insurers than expected made money.
Earlier this month, HHS Secretary Sylvia Burwell said the administration is exploring ways it might be able to help make those payments, although such a move comes too late to save many of the dozen insurance cooperatives that have announced they will pull out of the market in January. The less-than-anticipated payments are often cited as a main factor in the co-ops demise.
UnitedHealthGroup laid out a litany of reasons on Nov. 19 as to why it might stop selling individual health insurance through federal and state markets in 2017 – a move some see as an effort to compel the Obama administration to ease regulations and make good on promised payments.
Those problems, including low participation by healthy people, have led to financial losses, according to UnitedHealth. If not addressed, similar issues could affect other insurers, causing more to exit the market in the coming years, some Wall Street analysts and policy experts said.
Many said they anticipate the federal government will act to forestall widespread departures, particularly because continued withdrawals could be politically explosive during an election year.
A key piece of President Obama’s signature health care law, the online marketplaces, also called exchanges, opened in 2014 for people who buy their own insurance because they don’t get it through their jobs. Enrollment, while growing, has fallen short of capturing the share of the eligible uninsured that was anticipated. This year, the marketplaces saw enrollment of more than 9 million customers, although the law’s expansion of Medicaid enrollment in many states also has played a large role in reducing the overall number of uninsured.
Only a month ago, United sounded more optimistic about business on the exchanges. But on Nov. 19, the insurer said it would cut its earnings forecast and projected hundreds of millions in losses stemming from the policies it sells through the health law’s marketplaces.
The turnaround led some analysts to ask the insurer what had changed.
Stephen Hemsley, UnitedHealth chief executive officer, said too many healthy people dropped coverage and noted slower than expected enrollment. A major factor, he added, was far higher costs for those who signed up for 2015 coverage under special exemptions after the general open-enrollment period ended. Those exemptions included, for example, people who lost their insurance, moved, or suffered a hardship, such as an eviction or had their utilities turned off. United said it did not see a similar increase in costs for people who bought policies from private brokers or websites instead of the government marketplaces after open enrollment, suggesting the reason was partly that the company’s eligibility assessments were more thorough.
The firm did not say it would halt sales in 2017 but warned that it would strongly consider doing so based on what happens in the next few months.
“We cannot sustain these losses,” Mr. Hemsley said on a phone call with Wall Street analysts. “We can’t subsidize a marketplace that doesn’t appear at the moment to be sustaining itself.”
Although it’s the nation’s largest insurer, United captured only a small percentage of consumers who currently have coverage through the Affordable Care Act marketplace, in part because it sat out the first year of enrollment and really ramped up only for this year’s coverage.
While seen as a serious challenge to the health care law, United’s decision alone doesn’t mark the death knell for the exchanges. In remarks to analysts and press reports on Nov. 19, Aetna and Kaiser Permanente re-affirmed their commitment to selling through the marketplaces.
Ben Wakana, a spokesman for the Health and Human Services department, defended the government marketplaces, noting that 9 of 10 of policyholders re-enrolling have a choice of three or more insurers for next year. “The reality is we continue to see more people signing up for health insurance and more issuers entering the marketplaces, and at the end of January, we believe we’ll be looking at another successful open enrollment – just like the last two,” he said. “[Thursday’s] statement by one issuer is not indicative of the marketplace’s strength and viability.”
But insurers, including Humana, Aetna, and some of the large Blue Cross Blue Shield plans, were losing money or barely breaking even on their marketplace business, according to earnings reports.
“If there are no changes, all the large publicly traded companies will end up leaving,” said Ana Gupte, analyst with Leerink Partners. “But I would be very surprised if [the Department of Health & Human Services] doesn’t do something to accommodate their issues.”
Those options would be limited to what the agency could do without congressional action, many analysts said. Still, that could include relaxing some regulations or reconsidering some of the exemptions that allow people to sign up after the open-enrollment period.
Former insurance executive and consultant Robert Laszewski said the administration needs to relax the rules to give insurers more flexibility to design plans that would attract healthier people. He said the costs – including deductibles and premiums – were too high for many people, particularly those with few medical needs.
“Disproportionately, the sick are signing up and the healthy are dropping out,” said Mr. Laszewski, adding that alternative plans with fewer benefits but lower costs should be made available.
Economist Len Nichols cautioned, however, that most of the law’s benefit requirements – taken individually – add little to the cost of a plan. Removing the bigger-ticket requirements, such as coverage for maternity care, would leave consumers without adequate coverage, said Nichols, who directs the George Mason University Center for Health Research and Ethics in Fairfax, Va.
Mr. Nichols, Ms. Gupte, and other analysts agree with the industry’s trade lobby, which says one thing the administration could do is make good on a promise to pay insurers under a temporary program designed to redistribute profits from some insurers that did especially well to offset losses others experienced in the marketplace plans. That program, however, has paid only about 13 cents on the dollar of what was promised, mainly because fewer insurers than expected made money.
Earlier this month, HHS Secretary Sylvia Burwell said the administration is exploring ways it might be able to help make those payments, although such a move comes too late to save many of the dozen insurance cooperatives that have announced they will pull out of the market in January. The less-than-anticipated payments are often cited as a main factor in the co-ops demise.
UnitedHealthGroup laid out a litany of reasons on Nov. 19 as to why it might stop selling individual health insurance through federal and state markets in 2017 – a move some see as an effort to compel the Obama administration to ease regulations and make good on promised payments.
Those problems, including low participation by healthy people, have led to financial losses, according to UnitedHealth. If not addressed, similar issues could affect other insurers, causing more to exit the market in the coming years, some Wall Street analysts and policy experts said.
Many said they anticipate the federal government will act to forestall widespread departures, particularly because continued withdrawals could be politically explosive during an election year.
A key piece of President Obama’s signature health care law, the online marketplaces, also called exchanges, opened in 2014 for people who buy their own insurance because they don’t get it through their jobs. Enrollment, while growing, has fallen short of capturing the share of the eligible uninsured that was anticipated. This year, the marketplaces saw enrollment of more than 9 million customers, although the law’s expansion of Medicaid enrollment in many states also has played a large role in reducing the overall number of uninsured.
Only a month ago, United sounded more optimistic about business on the exchanges. But on Nov. 19, the insurer said it would cut its earnings forecast and projected hundreds of millions in losses stemming from the policies it sells through the health law’s marketplaces.
The turnaround led some analysts to ask the insurer what had changed.
Stephen Hemsley, UnitedHealth chief executive officer, said too many healthy people dropped coverage and noted slower than expected enrollment. A major factor, he added, was far higher costs for those who signed up for 2015 coverage under special exemptions after the general open-enrollment period ended. Those exemptions included, for example, people who lost their insurance, moved, or suffered a hardship, such as an eviction or had their utilities turned off. United said it did not see a similar increase in costs for people who bought policies from private brokers or websites instead of the government marketplaces after open enrollment, suggesting the reason was partly that the company’s eligibility assessments were more thorough.
The firm did not say it would halt sales in 2017 but warned that it would strongly consider doing so based on what happens in the next few months.
“We cannot sustain these losses,” Mr. Hemsley said on a phone call with Wall Street analysts. “We can’t subsidize a marketplace that doesn’t appear at the moment to be sustaining itself.”
Although it’s the nation’s largest insurer, United captured only a small percentage of consumers who currently have coverage through the Affordable Care Act marketplace, in part because it sat out the first year of enrollment and really ramped up only for this year’s coverage.
While seen as a serious challenge to the health care law, United’s decision alone doesn’t mark the death knell for the exchanges. In remarks to analysts and press reports on Nov. 19, Aetna and Kaiser Permanente re-affirmed their commitment to selling through the marketplaces.
Ben Wakana, a spokesman for the Health and Human Services department, defended the government marketplaces, noting that 9 of 10 of policyholders re-enrolling have a choice of three or more insurers for next year. “The reality is we continue to see more people signing up for health insurance and more issuers entering the marketplaces, and at the end of January, we believe we’ll be looking at another successful open enrollment – just like the last two,” he said. “[Thursday’s] statement by one issuer is not indicative of the marketplace’s strength and viability.”
But insurers, including Humana, Aetna, and some of the large Blue Cross Blue Shield plans, were losing money or barely breaking even on their marketplace business, according to earnings reports.
“If there are no changes, all the large publicly traded companies will end up leaving,” said Ana Gupte, analyst with Leerink Partners. “But I would be very surprised if [the Department of Health & Human Services] doesn’t do something to accommodate their issues.”
Those options would be limited to what the agency could do without congressional action, many analysts said. Still, that could include relaxing some regulations or reconsidering some of the exemptions that allow people to sign up after the open-enrollment period.
Former insurance executive and consultant Robert Laszewski said the administration needs to relax the rules to give insurers more flexibility to design plans that would attract healthier people. He said the costs – including deductibles and premiums – were too high for many people, particularly those with few medical needs.
“Disproportionately, the sick are signing up and the healthy are dropping out,” said Mr. Laszewski, adding that alternative plans with fewer benefits but lower costs should be made available.
Economist Len Nichols cautioned, however, that most of the law’s benefit requirements – taken individually – add little to the cost of a plan. Removing the bigger-ticket requirements, such as coverage for maternity care, would leave consumers without adequate coverage, said Nichols, who directs the George Mason University Center for Health Research and Ethics in Fairfax, Va.
Mr. Nichols, Ms. Gupte, and other analysts agree with the industry’s trade lobby, which says one thing the administration could do is make good on a promise to pay insurers under a temporary program designed to redistribute profits from some insurers that did especially well to offset losses others experienced in the marketplace plans. That program, however, has paid only about 13 cents on the dollar of what was promised, mainly because fewer insurers than expected made money.
Earlier this month, HHS Secretary Sylvia Burwell said the administration is exploring ways it might be able to help make those payments, although such a move comes too late to save many of the dozen insurance cooperatives that have announced they will pull out of the market in January. The less-than-anticipated payments are often cited as a main factor in the co-ops demise.
ICD-10 Under ACP Scrutiny
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
Law & Medicine: To whom do doctors owe a duty?
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: A doctor may owe a duty of care in the setting of:
A. A cyber relationship.
B. A special relationship.
C. Both A and B.
D. Neither A nor B.
Answer: C. Ascertaining whether a defendant owes a duty to a claimant is the first inquiry in the tort of negligence. To say there is no duty owed is to deny liability altogether, however obvious the breach or horrendous the foreseeable injuries.
Thus, duty is used as a filter mechanism to reduce frivolous suits or otherwise control the tide of litigation, to prevent “liability in an indeterminate amount for an indeterminate time to an indeterminate class.”
Duty in the context of medical negligence is not usually in dispute, as it is plainly owed by a doctor to his or her patient. It arises out of the doctor-patient relationship. Whether a relationship has been formed in the first place is a threshold inquiry. Where a doctor accepts a patient who is seeking his or her services, the relationship is readily evident. Duty is also established when the doctor begins the evaluation process in a typical encounter.
However, a phone inquiry by a potential patient, without more, may be insufficient to create this relationship, although this may depend on the nature of the phone conversation and the doctor’s response.
Likewise, a “curbside” consultation sought by a colleague does not normally translate into a duty for the doctor offering the opinion. Presumably casual advice given freely and understood as such at social gatherings does not add up to a doctor-patient relationship, and courts will look to reasonableness as the touchstone in deciding whether such a relationship was ever formed.
Still, there are some medical situations where a legitimate question of duty can be raised. With the growth of electronic medical records and communication, medical encounters in cyberspace will emerge as an increasing source of litigation.
Internet liability can be far reaching. In addition to risks governing negligence, informed consent, and privacy/confidentiality, there are additional issues of product liability, cross-border jurisdictional conflicts, and others.
The threshold question when assessing cyberspace liability arising, for example, from the use of doctor-operated medical websites concerns duty, because its existence or denial will determine whether the case can go forward in the first place. Although not the typical office or hospital patient, a plaintiff may argue successfully that a doctor-patient relationship had nonetheless been formed in cyberspace.
It is possible that such a relationship will be found in some circumstances, relevant factors being knowledge of names of subscribers, frequency of interactions, specificity of queries, and so on. In particular, a subscription fee is likely to be construed as evidence of soliciting and accepting a more committed interaction, so it places the operator of the website at greater legal risk. A specific disclaimer is a standard precaution but may not be enough to definitively protect against a lawsuit.
Courts have ruled in favor of plaintiffs despite the absence of face-to-face interaction with a physician. In one case, a doctor speaking to a patient from the emergency department was deemed to have formed a doctor-patient relationship (O’Neill v. Montefiore Hospital, 11 A.D.2d 132 (N.Y.A.D. 1 Dept. 1960). In another, an on-call neurologist’s telephone advice to the treating doctor likewise raised the issue of legal duty (Lection v. Dyll, 65 S.W.3d 696 (Tex. App. Dallas 2001).
The state of Hawaii now permits telehealth services to be reimbursable, notwithstanding the absence of face-to-face contact (HI Rev Stat § 431:10A-116.3[a]). With this law, an online encounter will likely translate into a professional relationship – with corresponding legal duty of due care.
In the case of a Good Samaritan physician – i.e., one who offers gratuitous aid to a stranger in need of medical assistance – courts are unlikely to find a professional relationship, because there is no common law duty to help a stranger.
However, once treatment has begun, there is a duty not to make matters worse. So, all 50 states have enacted Good Samaritan statutes, which protect against liability arising out of negligent rescue. Note that statutory protection is generally excluded for Good Samaritan acts performed within a hospital setting, under the theory that doctors have an ongoing relationship with the hospital and are already obligated to provide emergency care within its walls.
Another category of legal duty concerns nonpatient third parties. The complaint may relate to a failure to warn family members of a patient’s contagious disease, or the transmissible condition may have been missed and an innocent third party was injured as a result.
Another situation where duty to a third party might arise is the learning of a credible threat of harm directed at a named individual. This is famously known as the Tarasoff doctrine, after a California case in which the court imposed a duty on a college psychologist to directly warn an intended victim of harm by his patient – even though that meant breaching confidentiality of a professional relationship, and the victim was a nonpatient third party (Tarasoff v. Regents of University of California, 551 P.2d 334 [Cal. 1976]).
A doctor may also incur liability for automobile injuries sustained by one other than his or her own patient. In a Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old bystander. The driver alleged that the prescription medication prazosin caused him to lose control of the car.
In ruling that the health care provider was liable to the injured bystander, the Hawaii Supreme Court held that physicians have a duty to warn their patients of potential adverse medication effects, and this responsibility should extend to third parties (McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 [Haw. 2002]).
A foreseeable and unreasonable risk of harm is an important factor, but not the only decisive factor, in construing the existence of a legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of “existing social values, customs, and considerations of policy.”
In a Massachusetts case, a family practitioner had failed to warn his patient of the risk of diabetic drugs when operating a vehicle. Just 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court used the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist (Arsenault v. McConarty, 21 Mass. L. Rptr. 500 [2006]).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Senate panel targets drug prices during FDA commissioner nomination hearing
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
AT THE SENATE HELP COMMITTEE HEARING