User login
Product News: 06 2017
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Avène Complexion Correcting Shield SPF 50+
Pierre Fabre Dermo-Cosmetique USA adds the Avène Complexion Correcting Shield SPF 50+ mineral sunscreen to its physician-dispensed sun care line. This tinted moisturizer, available in 3 shades, provides 24-hour hydration and an effective antioxidant defense against sun-induced free radicals. Avène Complexion Correcting Shield provides an instant blurring effect to camouflage skin imperfections such as large pores, uneven skin tone, redness, fine lines, and wrinkles. For more information, visit www.aveneusa.com.
Coppertone Clearly Sheer Whipped Sunscreen
Bayer introduces Coppertone Clearly Sheer Whipped Sunscreen, a rich and creamy formula available in sun protection factor 30 and 50. Coppertone Clearly Sheer Whipped Sunscreen absorbs quickly to leave skin feeling soft and smooth. It offers broad-spectrum UVA/UVB protection and is water resistant for up to 80 minutes.For more information, visit www.coppertone.com.
DerMend Mature Skin Solutions
Ferndale Healthcare launches DerMend Mature Skin Solutions, an over-the-counter line consisting of 3 products specifically designed for patients aged 50 years and older. The Fragile Skin Moisturizing Formula rejuvenates thin and fragile skin with hyaluronic acid, retinol, glycolic acid, niacinaminde, and 5 ceramides. The Moisturizing Anti-Itch Lotion is steroid free and contains
Jan Marini Sunscreens
Jan Marini Skin Research, Inc, introduces Antioxidant Daily Face Protectant SPF 33 and Marini Physical Protectant SPF 45, both providing broad-spectrum UVA/UVB protection. Antioxidant Daily Face Protectant provides oil control and advanced hydration for daily use to reduce and address damage caused by sun exposure. Marini Physical Protectant utilizes purely physical filters to decrease the risk of premature skin aging and features a universal tint with a sheer matte finish. For more information, visit www.janmarini.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product News: 05 2017
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
In-Office Diagnostic Needle Arthroscopy
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
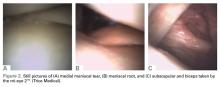
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
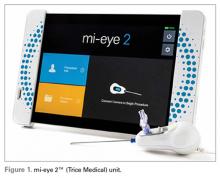
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
Product News: 04 2017
Cutanea Life Sciences, Inc, launches Aktipak (erythromycin 3% and benzoyl peroxide 5%) Gel, a prescription combination therapy indicated for acne vulgaris. Aktipak is packaged in a pocket-sized, dual-chamber pouch that contains erythromycin and benzoyl peroxide in separate chambers to enable convenient on-the-go use. Immediately prior to use, the patient cuts or twists open the pouch, squeezes the 2 gels into the palm of the hand, mixes the gels together, and applies the mix to the area affected by acne. Aktipak has an 18-month shelf life and does not require refrigeration. Results can be seen within 8 weeks. For more information, visit www.aktipak.com.
Glytone Acne BPO Clearing Cleanser
Pierre Fabre Group introduces the Glytone Acne BPO Clearing Cleanser (4.5% encapsulated benzoyl peroxide [BPO]) with time-released technology to control the delivery of BPO and enhance penetration. The targeted delivery system adheres to the skin and penetrates the lipid layer while releasing the encapsulated BPO once warmed by the skin, providing optimal efficacy to inhibit the growth of acne-causing bacteria with minimal irritation. Glytone Acne BPO Clearing Cleanser is dispensed by physicians and can be used with other products in the Glytone acne product line for optimal results. For more information, visit www.glytone-usa.com.
Juvéderm Vollure XC
Allergan announces US Food and Drug Administration approval of Juvéderm Vollure XC for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds in adults older than 21 years. It utilizes VYCROSS technology, which blends different weights of hyaluronic acid, contributing to the gel’s duration. Long-lasting results have been demonstrated up to 18 months. For more information, visit www.juvederm.com.
Neutrogena Light Therapy Acne Mask
Johnson & Johnson Consumer Inc presents the Neutrogena Light Therapy Acne Mask, an LED device utilizing red and blue light to treat acne at home. The mask contains 12 blue LED bulbs that kill Propionibacterium acnes bacteria and 9 red LED bulbs to penetrate deep into the skin to calm inflammation. The mask can be used for 10 minutes each night and shuts off automatically. Results have been seen in 1 week for mild to moderate acne. For more information, visit www.neutrogena.com.
3% Retinol Peel ProSystem
NeoStrata Company, Inc, introduces the 3% Retinol Peel ProSystem featuring Retinol Boosting Complex to exfoliate and improve the appearance of fine lines and winkles, help reduce acne, and improve skin laxity, while promoting a bright, even, and clear complexion. This physician-strength peel is applied in the office but is removed at home after 8 hours or overnight. This peel has demonstrated improvement in acne and skin texture as well as diminished pigmentation. For more information, visit www.neostrata.com.
Siliq
Valeant Pharmaceuticals International, Inc, announces US Food and Drug Administration approval of the Biologics License Application for Siliq (brodalumab) injection. Siliq, an IL-17 inhibitor, is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies. Siliq has a black box warning for patients with a history of suicidal thoughts or behavior and was approved with a Risk Evaluation and Mitigation Strategy involving a one-time enrollment for physicians and one-time informed consent for patients. Sales and marketing in the United States will begin in the second half of 2017. For more information, visit www.valeant.com.
Thermi
Thermi, an Almirall company, announces “The Art of Thermi” campaign focusing on 2 Thermi devices: ThermiRF and Thermi250. ThermiRF is temperature-controlled radiofrequency technology that uses heat to produce aesthetic outcomes for soft tissue applications. Thermi250 is a high-powered, temperature-controlled radiofrequency system emitting at 470 kHz designed with a user-friendly interface to offer versatility for targeting cellulite. For more information, visit www.thermi.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Cutanea Life Sciences, Inc, launches Aktipak (erythromycin 3% and benzoyl peroxide 5%) Gel, a prescription combination therapy indicated for acne vulgaris. Aktipak is packaged in a pocket-sized, dual-chamber pouch that contains erythromycin and benzoyl peroxide in separate chambers to enable convenient on-the-go use. Immediately prior to use, the patient cuts or twists open the pouch, squeezes the 2 gels into the palm of the hand, mixes the gels together, and applies the mix to the area affected by acne. Aktipak has an 18-month shelf life and does not require refrigeration. Results can be seen within 8 weeks. For more information, visit www.aktipak.com.
Glytone Acne BPO Clearing Cleanser
Pierre Fabre Group introduces the Glytone Acne BPO Clearing Cleanser (4.5% encapsulated benzoyl peroxide [BPO]) with time-released technology to control the delivery of BPO and enhance penetration. The targeted delivery system adheres to the skin and penetrates the lipid layer while releasing the encapsulated BPO once warmed by the skin, providing optimal efficacy to inhibit the growth of acne-causing bacteria with minimal irritation. Glytone Acne BPO Clearing Cleanser is dispensed by physicians and can be used with other products in the Glytone acne product line for optimal results. For more information, visit www.glytone-usa.com.
Juvéderm Vollure XC
Allergan announces US Food and Drug Administration approval of Juvéderm Vollure XC for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds in adults older than 21 years. It utilizes VYCROSS technology, which blends different weights of hyaluronic acid, contributing to the gel’s duration. Long-lasting results have been demonstrated up to 18 months. For more information, visit www.juvederm.com.
Neutrogena Light Therapy Acne Mask
Johnson & Johnson Consumer Inc presents the Neutrogena Light Therapy Acne Mask, an LED device utilizing red and blue light to treat acne at home. The mask contains 12 blue LED bulbs that kill Propionibacterium acnes bacteria and 9 red LED bulbs to penetrate deep into the skin to calm inflammation. The mask can be used for 10 minutes each night and shuts off automatically. Results have been seen in 1 week for mild to moderate acne. For more information, visit www.neutrogena.com.
3% Retinol Peel ProSystem
NeoStrata Company, Inc, introduces the 3% Retinol Peel ProSystem featuring Retinol Boosting Complex to exfoliate and improve the appearance of fine lines and winkles, help reduce acne, and improve skin laxity, while promoting a bright, even, and clear complexion. This physician-strength peel is applied in the office but is removed at home after 8 hours or overnight. This peel has demonstrated improvement in acne and skin texture as well as diminished pigmentation. For more information, visit www.neostrata.com.
Siliq
Valeant Pharmaceuticals International, Inc, announces US Food and Drug Administration approval of the Biologics License Application for Siliq (brodalumab) injection. Siliq, an IL-17 inhibitor, is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies. Siliq has a black box warning for patients with a history of suicidal thoughts or behavior and was approved with a Risk Evaluation and Mitigation Strategy involving a one-time enrollment for physicians and one-time informed consent for patients. Sales and marketing in the United States will begin in the second half of 2017. For more information, visit www.valeant.com.
Thermi
Thermi, an Almirall company, announces “The Art of Thermi” campaign focusing on 2 Thermi devices: ThermiRF and Thermi250. ThermiRF is temperature-controlled radiofrequency technology that uses heat to produce aesthetic outcomes for soft tissue applications. Thermi250 is a high-powered, temperature-controlled radiofrequency system emitting at 470 kHz designed with a user-friendly interface to offer versatility for targeting cellulite. For more information, visit www.thermi.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Cutanea Life Sciences, Inc, launches Aktipak (erythromycin 3% and benzoyl peroxide 5%) Gel, a prescription combination therapy indicated for acne vulgaris. Aktipak is packaged in a pocket-sized, dual-chamber pouch that contains erythromycin and benzoyl peroxide in separate chambers to enable convenient on-the-go use. Immediately prior to use, the patient cuts or twists open the pouch, squeezes the 2 gels into the palm of the hand, mixes the gels together, and applies the mix to the area affected by acne. Aktipak has an 18-month shelf life and does not require refrigeration. Results can be seen within 8 weeks. For more information, visit www.aktipak.com.
Glytone Acne BPO Clearing Cleanser
Pierre Fabre Group introduces the Glytone Acne BPO Clearing Cleanser (4.5% encapsulated benzoyl peroxide [BPO]) with time-released technology to control the delivery of BPO and enhance penetration. The targeted delivery system adheres to the skin and penetrates the lipid layer while releasing the encapsulated BPO once warmed by the skin, providing optimal efficacy to inhibit the growth of acne-causing bacteria with minimal irritation. Glytone Acne BPO Clearing Cleanser is dispensed by physicians and can be used with other products in the Glytone acne product line for optimal results. For more information, visit www.glytone-usa.com.
Juvéderm Vollure XC
Allergan announces US Food and Drug Administration approval of Juvéderm Vollure XC for correction of moderate to severe facial wrinkles and folds such as the nasolabial folds in adults older than 21 years. It utilizes VYCROSS technology, which blends different weights of hyaluronic acid, contributing to the gel’s duration. Long-lasting results have been demonstrated up to 18 months. For more information, visit www.juvederm.com.
Neutrogena Light Therapy Acne Mask
Johnson & Johnson Consumer Inc presents the Neutrogena Light Therapy Acne Mask, an LED device utilizing red and blue light to treat acne at home. The mask contains 12 blue LED bulbs that kill Propionibacterium acnes bacteria and 9 red LED bulbs to penetrate deep into the skin to calm inflammation. The mask can be used for 10 minutes each night and shuts off automatically. Results have been seen in 1 week for mild to moderate acne. For more information, visit www.neutrogena.com.
3% Retinol Peel ProSystem
NeoStrata Company, Inc, introduces the 3% Retinol Peel ProSystem featuring Retinol Boosting Complex to exfoliate and improve the appearance of fine lines and winkles, help reduce acne, and improve skin laxity, while promoting a bright, even, and clear complexion. This physician-strength peel is applied in the office but is removed at home after 8 hours or overnight. This peel has demonstrated improvement in acne and skin texture as well as diminished pigmentation. For more information, visit www.neostrata.com.
Siliq
Valeant Pharmaceuticals International, Inc, announces US Food and Drug Administration approval of the Biologics License Application for Siliq (brodalumab) injection. Siliq, an IL-17 inhibitor, is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy and have failed to respond or have lost response to other systemic therapies. Siliq has a black box warning for patients with a history of suicidal thoughts or behavior and was approved with a Risk Evaluation and Mitigation Strategy involving a one-time enrollment for physicians and one-time informed consent for patients. Sales and marketing in the United States will begin in the second half of 2017. For more information, visit www.valeant.com.
Thermi
Thermi, an Almirall company, announces “The Art of Thermi” campaign focusing on 2 Thermi devices: ThermiRF and Thermi250. ThermiRF is temperature-controlled radiofrequency technology that uses heat to produce aesthetic outcomes for soft tissue applications. Thermi250 is a high-powered, temperature-controlled radiofrequency system emitting at 470 kHz designed with a user-friendly interface to offer versatility for targeting cellulite. For more information, visit www.thermi.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
High-Resolution Wireless Ultrasound
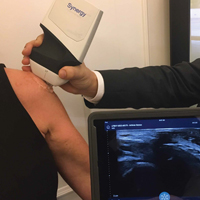
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
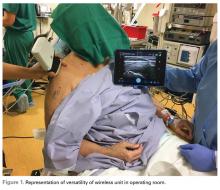
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
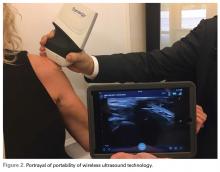
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
Arthrex Synergy MSK Ultrasound by Clarius(http://www.synergy-ultrasound.com/)
Three scanners are capable of targeting different tissue types and depths. We prefer the Synergy MSK Linear Ultrasound by Clarius, a linear transducer that can evaluate tissue to depths of 7 cm and use frequencies from 4 MHz to 13 MHz. Its battery holds a standby charge for 7 days and can be actively used for 45 minutes. The unit has a magnesium shell; with the battery removed, the unit can be completely immersed in liquid without being damaged, which allows for easy cleaning and, potentially, sterilization with a soak solution. Color Doppler (for blood-flow assessment) and proprietary advanced needle visualization technology will be available in June.
The app is simply controlled with typical smart-device gestures. Depth control requires a finger swipe, and zoom takes a pinch. Other controls, such as optimal gain and frequency settings, are automated. Images and videos can be stored on the device and uploaded either to the Clarius Cloud or to a PACS (picture archiving and communication system) device. New features will allow the device to use a Synergy arthroscopy tower (Arthrex) as its display for surgeons and anesthesiologists in the surgical suite.
This technology finally allows ultrasound to be used in the operating room without the hassles of cumbersome machines and the potential contamination by the sleeves covering the cord that connects the transducer and the base unit (Figure 1).
Recent studies have demonstrated new ultrasound-guided surgical techniques for biceps tenodesis,4 anterolateral ligament reconstruction,13 medial patellofemoral ligament repair or reconstruction,14 and medial collateral ligament internal bracing.4
This small device can also be easily used on sports fields, as it can be carried in a pocket with a smart phone or tablet. With its 10- to 15-second start-up, it is readily available and allows for immediate evaluation of a player. No longer does a player need to be taken off the field for a radiograph. The same advantage of portability means the unit is appropriate for emergency department physicians and staff.
Surgical pearl: Overall, ultrasound is an imaging technology that has improved the accuracy and efficacy of injections. Wireless capability, portability, and versatility with high-resolution images improve this modality further and extend our reach into surgical, office, hospital, and sports settings. The ease, convenience, and reasonable price of high-resolution wireless ultrasound make it an attractive tool for physicians, nursing staff, athletic trainers, and physical therapists.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
1. Eustace JA, Brophy DP, Gibney RP, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
2. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
3. Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
4. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
5. Kang MN, Rizio L, Prybicien M, Middlemas DA, Blacksin MF. The accuracy of subacromial corticosteroid injections: a comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 suppl):61S-66S.
6. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
7. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
8. Partington PF, Broome GH. Diagnostic injection around the shoulder: hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
9. Rutten MJ, Maresch BJ, Jager GJ, de Waal Malefijt MC. Injection of the subacromial-subdeltoid bursa: blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
10. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
11. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
12. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: an arthrographic evaluation. Arthroscopy. 2002;18(8):887-891.
13. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
14. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of the medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. In press.
Robotic-Assisted Total Knee Arthroplasty
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
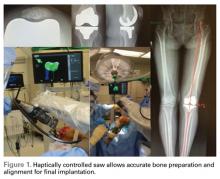
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
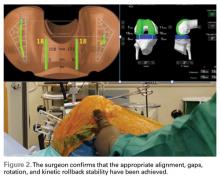
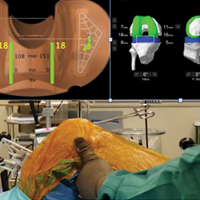
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
Stryker(http://www.stryker.com/en-us/products/Orthopaedics/MakoRobotic-ArmAssistedSurgery/index.htm)
Mako Robotic-Arm Assisted Surgery
The role of new technology in the treatment of knee arthritis is to enable accurate execution of the surgical plan for each individual’s arthritic presentation. A robotic-assisted approach allows a surgeon to perform a unicompartmental to a tricompartmental knee replacement in a consistent and reproducible manner.1
The desire is to address the technical inaccuracies (malalignment, malrotation, and soft tissue imbalance) that lead to early revisions and patient dissatisfaction.
Preoperative planning utilizing a computed tomography- based approach enables the evaluation of the entire limb pathology, and aids the surgeon in“patient-matching” the implant position based on anatomic references 3-dimensionally.
Intraoperative tracking informs the surgeon on pre-resection alignment, and flexion-extension gaps. The surgeon can define a fixed vs correctable deformity, and then adjust the implant position prior to cutting, if required, while defining the desired implant and limb alignment.
Haptically guiding the saw allows the surgeon to perform accurate bony cuts in 3 planes while protecting the soft tissues (Figure 1).
Trialing with integrated sensors allows me to evaluate the effects of the alignment and gaps on the soft tissue balance, and kinematic rollback with dynamic testing.2
The goal of robotic sensor-assisted surgery is to develop a patient specific preoperative plan, and then assist in accurate, dynamic modifications based on the patient’s limb alignment and soft tissue tension. The final implant position can be evaluated through a full range of motion (ROM), and stability defined. This information is then collected, and the effects of implant position and various limb alignment targets on soft tissue balance are evaluated as it relates to functional outcomes and patient satisfaction measurements.
Surgical pearl: Using the Mako Robotic-Arm Assisted Surgery, I performed the first robotic-assisted total knee replacement in June 2016, and have performed over 80 cases to date. Early results are showing improved accuracy, early ROM, and a decreased postoperative utilization of therapy and assistive devices. Multi-centered studies will enable the evaluation of robotic surgical approaches on short- and long-term outcomes.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
1. Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplasty. 2016;31(10):2353-2363.
2. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.