User login
Bariatric surgery + medical therapy: Effective Tx for T2DM?
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
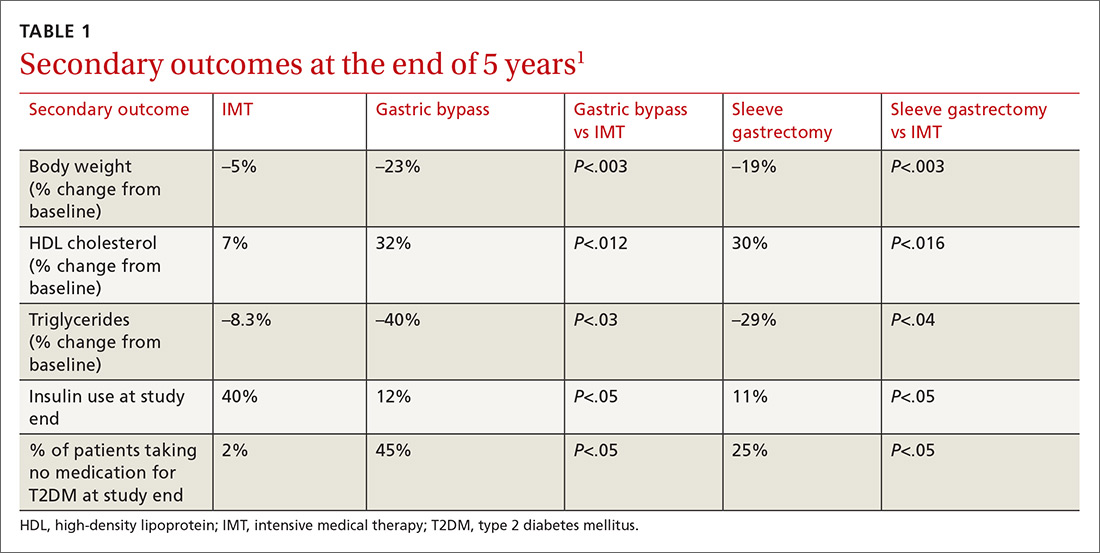
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.

WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.

WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
PRACTICE CHANGER
Consider bariatric surgery with medical therapy as a treatment option for adults with uncontrolled type 2 diabetes and a body mass index ≥27 kg/m2.1
STRENGTH OF RECOMMENDATION
B: Based on a nonblinded, single-center, randomized controlled trial.
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
How do these 3 diabetes agents compare in reducing mortality?
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old man with taype 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care hemoglobin A1c is 9.5%, and he is currently taking only metformin 1000 mg bid. You are considering adding an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and cardiovascular (CV) mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people, or 1 of every 11, now struggles to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of medications available that are aimed at lowering blood sugar and improving diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The “American Diabetes Association Standards of Medical Care in Diabetes” points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin, and the GLP-1 agonist liraglutide, as agents that should be added to metformin and lifestyle modification in patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists and the American College of Endocrinology guidelines do include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in strength of recommendations for these classes is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s are associated with better mortality outcomes than DPP-4s
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176,310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to reduce all-cause mortality and CV endpoints in patients with T2DM. The authors analyzed English-language RCTs that followed patients with T2DM for at least 12 weeks and compared SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors to one another, to placebo, or to no treatment.
A majority of the patients in both the intervention and control groups were taking additional diabetes medications, such as metformin, prior to enrollment and during the trials. About half of the patients analyzed were enrolled in trials that specifically evaluated patients at elevated CV risk, which is notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group, hazard ratio [HR]=0.80; 95% credible interval [CrI], 0.71-0.89; absolute risk difference [RD]= –1%; number needed to treat [NNT]=100; GLP-1 agonist group, HR=0.88; 95% CrI, 0.81-0.94; absolute RD= -0.6%; NNT=167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR=1.02; 95% CrI, 0.94-1.11; absolute RD=0.1%). Both the SGLT-2 inhibitor (HR=0.78; 95% CrI, 0.68-0.90; absolute RD= –0.9%; NNT=111) and GLP-1 agonist (HR=0.86; 95% CrI, 0.77-0.96; absolute RD= –0.5%; NNT=200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR=0.79; 95% Crl, 0.69-0.91; absolute RD= –0.8%; NNT=125) and GLP-1 agonist (HR=0.85; Crl, 95% 0.77-0.94; absolute RD= –0.5%; NNT=200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR=0.62; 95% CrI, 0.54-0.72; absolute RD= –1.1%; NNT=91) and MIs (HR=0.86; 95% CrI, 0.77–0.97; absolute RD= –0.6%; NNT=167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR=0.67; 95% CrI, 0.57 to 0.80; absolute RD= –0.9; NNT=111) or DPP-4 inhibitors (HR=0.55; 95% CrI, 0.46-0.67; absolute RD= –1.1%; NNT=91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups saw lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists, HR=2; 95% CrI, 1.70-2.37; absolute RD=4.7%; number needed to harm [NNH]=21; SGLT-2 inhibitors, HR=1.8; 95% CrI, 1.44-2.25; absolute RD=5.8%; NNH=17; and DPP-4 inhibitors, HR=1.93; 95% CrI, 1.59-2.35; absolute RD=3.1%; NNH=32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR]=4.19; 95% confidence interval [CI], 3.45-5.09; absolute RD=6%; NNH=16), but not of urinary tract infection or lower limb amputation, although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR=1.58; 95% CI, 1.04-2.39; absolute RD=0.1%; NNH=1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than is the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 classes of medication.
However, there was relatively low heterogeneity among the studies included (I2=12), which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation may represent challenges
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors, and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists, must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes–2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Published December 2015. Updated May 2017. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
PRACTICE CHANGER
Consider adding a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a glucagon-like peptide 1 (GLP-1) agonist to the treatment regimen of patients with poorly controlled type 2 diabetes—especially those with higher CV risk. Doing so can reduce all-cause and cardiovascular (CV) mortality 1
STRENGTH OF RECOMMENDATION
B: Based on a network meta-analysis of 236 randomized controlled trials.
Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
When to “Undiagnose” Asthma

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
Less is more when it comes to ketorolac for pain
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
PRACTICE CHANGER
Use a low dose (10 mg) of intravenous ketorolac for moderate to severe acute pain in adults because it is as effective as higher doses (15-30 mg).1
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality randomized controlled trial.
Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
What’s the best treatment setting for stable PE patients?
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.
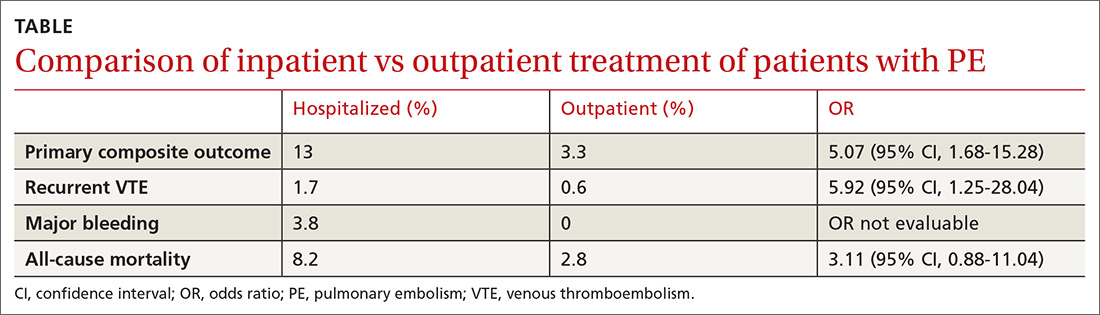
WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.

WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate is 90 bpm. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the Centers for Disease Control and Prevention, venous thromboembolism (VTE) affects approximately 900,000 people each year, and approximately 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attacks and strokes.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk of long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE disease recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, <70,000/mm3); expected to be compliant with treatment; and the patient feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized vs outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable patients with acute PE
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital vs outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via computed tomography scan or high-probability ventilation-perfusion scan and excluded if they were <19 years of age, diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 patient characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome was rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts was 63.2 years for the inpatient group and 63.6 years for the outpatient group. Overall, the cohorts were well matched.
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR] = 5.07; 95% confidence interval [CI], 1.68-15.28), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (TABLE). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR = 4.9; 95% CI, 2.62-9.17). The results remained similar for high-risk patients (Class III-V) based on their PESI score.

WHAT’S NEW
A higher rate of AEs in those treated as inpatients vs outpatients
This trial supports the CHEST guideline recommendations3 to manage hemodynamically stable patients with acute PE as outpatients. It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
A good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not a randomized controlled trial (RCT). This study defined outpatient management as patients discharged from the ED or hospitalized for <48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges, while the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low molecular weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain insurance carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. Centers for Disease Control and Prevention. Venous Thromboembolism Data & Statistics. February 5, 2018. https://www.cdc.gov/ncbddd/dvt/data.html. Accessed July 6, 2018.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
PRACTICE CHANGER
Manage patients with acute pulmonary embolism (PE) who are hemodynamically stable in the outpatient setting to decrease adverse events—regardless of their initial risk category.1
STRENGTH OF RECOMMENDATION
B: Based upon a good-quality retrospective cohort propensity score analysis.
Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.