User login
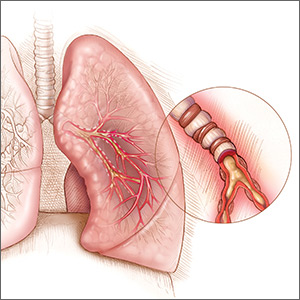
Should you reassess your patient’s asthma diagnosis?
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 45-year-old woman presents to your office for a yearly visit. Two years ago she was started on an inhaled corticosteroid (ICS) and a bronchodilator rescue inhaler after being diagnosed with asthma based on her history and physical exam findings. She has had no exacerbations since then. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 years have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance. And treatment entails significant costs and possible adverse effects. Without some sort of pulmonary function measurements or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication usage is cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications, and how they did in the subsequent year.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on people with a recent (<5 years) asthma diagnosis, so as to represent contemporary diagnostic practice and to make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers in Canada. Patients were excluded if they were using long-term oral steroids, pregnant or breastfeeding, unable to tolerate spirometry or methacholine challenges, or had a history of more than 10 pack-years of smoking.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in the first second of expiration (FEV1). If there was no improvement, the patient took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. If the patient did well with another methacholine challenge about 1 month later, maintenance medications were stopped, and the patient underwent a third methacholine challenge 3 weeks later.
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the adults with physician-diagnosed asthma, 33.1% (95% confidence interval [CI], 29.4%-36.8%) no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. The investigators also found 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma, including ischemic heart disease, subglottic stenosis, and bronchiectasis.
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well off all asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds are of no benefit for about one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the last 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, there appears to be no benefit to using asthma medications. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 While patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study, over 40% of patients who no longer had asthma were objectively proven to have had asthma at their original diagnosis.
CAVEATS
High level of rigor and the absence of a randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests, most including methacholine challenges, as well as oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. The results here are consistent with those of a study that looked at asthma disappearance in groups of patients with and without obesity. In that study, approximately 30% of both groups of patients no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only about 3% of patients who had their medications stopped reported worsening of symptoms.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a physician’s typical work, and it may take some time and effort to educate and monitor patients through the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma,* by state - National Health Interview Survey,† 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org. Accessed June 15, 2018.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
PRACTICE CHANGER
Consider tapering medications and retesting spirometry in adults with well-controlled asthma, as many may no longer have the disease.1
STRENGTH OF RECOMMENDATION
A: Based on a high-quality prospective cohort study and consistent findings in other studies.
Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
Time to Stop Glucosamine and Chondroitin for Knee OA?

A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member. Should you tell the patient to continue taking the medication?
Knee OA is a common condition in the United States, affecting an estimated 12% of adults ages 60 and older and 16% of those ages 70 and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that firstline treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical NSAIDs; the latter are also used if pain is unresponsive to acetaminophen.3,4 If initial therapy is inadequate to control pain, tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate are alternatives.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses ≥ 800 mg/d compared with placebo (level of evidence, low; risk for bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N > 200) with adequate methods of blinding and allocation concealment found no difference in pain.8 There was no statistically significant difference in adverse events for glucosamine plus chondroitin vs placebo, based on data from three studies included in the review.8
This RCT from Roman-Blas et al evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid), maker of the combination of chondroitin plus glucosamine used in the study.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Chondroitin + glucosamine not better than placebo
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in nine rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate (1,200 mg) plus glucosamine sulfate (1,500 mg) (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate-to-severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Knee pain severity was defined by a self-reported global pain score of 40 to 80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics; average age in the CS/GS group was 67 and in the placebo group, 65. Exclusion criteria included BMI ≥ 35, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary endpoint was mean reduction in global pain score on a 0- to 100-mm VAS at six months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P < .03.1
Results. In the intention-to-treat analysis at six months, patients in the placebo group had a greater reduction in pain than the CS/GC group (–20 mm vs –12 mm; P = .029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P = .01).
Continue to: In the CS/GS group...
In the CS/GS group, 31% did not complete the six-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P = .018).1
WHAT’S NEW
Pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone, or different dosing, would lead to the same outcome is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[9]:566-568).
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.

A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member. Should you tell the patient to continue taking the medication?
Knee OA is a common condition in the United States, affecting an estimated 12% of adults ages 60 and older and 16% of those ages 70 and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that firstline treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical NSAIDs; the latter are also used if pain is unresponsive to acetaminophen.3,4 If initial therapy is inadequate to control pain, tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate are alternatives.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses ≥ 800 mg/d compared with placebo (level of evidence, low; risk for bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N > 200) with adequate methods of blinding and allocation concealment found no difference in pain.8 There was no statistically significant difference in adverse events for glucosamine plus chondroitin vs placebo, based on data from three studies included in the review.8
This RCT from Roman-Blas et al evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid), maker of the combination of chondroitin plus glucosamine used in the study.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Chondroitin + glucosamine not better than placebo
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in nine rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate (1,200 mg) plus glucosamine sulfate (1,500 mg) (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate-to-severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Knee pain severity was defined by a self-reported global pain score of 40 to 80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics; average age in the CS/GS group was 67 and in the placebo group, 65. Exclusion criteria included BMI ≥ 35, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary endpoint was mean reduction in global pain score on a 0- to 100-mm VAS at six months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P < .03.1
Results. In the intention-to-treat analysis at six months, patients in the placebo group had a greater reduction in pain than the CS/GC group (–20 mm vs –12 mm; P = .029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P = .01).
Continue to: In the CS/GS group...
In the CS/GS group, 31% did not complete the six-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P = .018).1
WHAT’S NEW
Pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone, or different dosing, would lead to the same outcome is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[9]:566-568).

A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member. Should you tell the patient to continue taking the medication?
Knee OA is a common condition in the United States, affecting an estimated 12% of adults ages 60 and older and 16% of those ages 70 and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that firstline treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical NSAIDs; the latter are also used if pain is unresponsive to acetaminophen.3,4 If initial therapy is inadequate to control pain, tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate are alternatives.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses ≥ 800 mg/d compared with placebo (level of evidence, low; risk for bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N > 200) with adequate methods of blinding and allocation concealment found no difference in pain.8 There was no statistically significant difference in adverse events for glucosamine plus chondroitin vs placebo, based on data from three studies included in the review.8
This RCT from Roman-Blas et al evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid), maker of the combination of chondroitin plus glucosamine used in the study.1
Continue to: STUDY SUMMARY
STUDY SUMMARY
Chondroitin + glucosamine not better than placebo
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in nine rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate (1,200 mg) plus glucosamine sulfate (1,500 mg) (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate-to-severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Knee pain severity was defined by a self-reported global pain score of 40 to 80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics; average age in the CS/GS group was 67 and in the placebo group, 65. Exclusion criteria included BMI ≥ 35, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary endpoint was mean reduction in global pain score on a 0- to 100-mm VAS at six months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P < .03.1
Results. In the intention-to-treat analysis at six months, patients in the placebo group had a greater reduction in pain than the CS/GC group (–20 mm vs –12 mm; P = .029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P = .01).
Continue to: In the CS/GS group...
In the CS/GS group, 31% did not complete the six-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P = .018).1
WHAT’S NEW
Pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone, or different dosing, would lead to the same outcome is unknown.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[9]:566-568).
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
The (Sterile) Gloves Are Coming Off

Your practice manager, on a quest to reduce expenses, asks whether your practice could reduce the amount of money spent on gloves for procedures. How do you reply?
The effect of a small difference spread over a large number of events can be sizable. For example, the added cost of using sterile, as opposed to nonsterile, gloves for minor procedures is relatively modest and certainly worthwhile if the sterile gloves reduce the number of surgical site infections (SSIs). However, if there is no difference in SSIs, the extra cost becomes a large unnecessary expense, given the volume of minor procedures performed.
The decision to use sterile gloves often stems from habit, product availability, or perceived benefit of fewer SSIs.2 Providers’ choice of gloves varies widely, despite some evidence comparing sterile and nonsterile gloves.3-5
STUDY SUMMARY
Sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 RCTs and observational (prospective or retrospective) studies compared infection rates using sterile versus nonsterile gloves in 11,071 unique patients. The methods used in the review followed the Cochrane collaboration guidelines.6 Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. A total of 512 publications were reviewed for inclusion; 14 met the criteria but one study was removed due to incomplete data, leaving 13 trials with a total of 11,071 patients for the analysis. In the RCTs, 1,360 patients were randomly assigned to treatment with sterile gloves and 1,381 to treatment with nonsterile gloves as the intervention. In the prospective or retrospective observational trials, 4,680 patients were treated with sterile gloves, and 3,650 were treated with nonsterile gloves. Heterogeneity was low. Of note, the researchers performed a subgroup analysis on nine studies (4 RCTs and 5 observational studies) involving only cutaneous surgeries; these represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile and nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR], 1.06). There was also no difference in infection rate in the subgroup analysis (2.2% vs 2.2%, respectively; RR, 1.02) or an analysis limited to only RCTs.
WHAT’S NEW
Highest-quality evidence shows no difference
This systematic review found no difference in SSI rates when using sterile versus nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
Continue to: CAVEATS
CAVEATS
Risk for bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk for bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Providers may worry about potential medicolegal ramifications from this change.1 Lastly, some providers may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[8]:507-508).
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016; 152(9): 1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204(6):976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015(1);202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21(3):148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. http://methods.cochrane.org/. Accessed August 24, 2018.

Your practice manager, on a quest to reduce expenses, asks whether your practice could reduce the amount of money spent on gloves for procedures. How do you reply?
The effect of a small difference spread over a large number of events can be sizable. For example, the added cost of using sterile, as opposed to nonsterile, gloves for minor procedures is relatively modest and certainly worthwhile if the sterile gloves reduce the number of surgical site infections (SSIs). However, if there is no difference in SSIs, the extra cost becomes a large unnecessary expense, given the volume of minor procedures performed.
The decision to use sterile gloves often stems from habit, product availability, or perceived benefit of fewer SSIs.2 Providers’ choice of gloves varies widely, despite some evidence comparing sterile and nonsterile gloves.3-5
STUDY SUMMARY
Sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 RCTs and observational (prospective or retrospective) studies compared infection rates using sterile versus nonsterile gloves in 11,071 unique patients. The methods used in the review followed the Cochrane collaboration guidelines.6 Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. A total of 512 publications were reviewed for inclusion; 14 met the criteria but one study was removed due to incomplete data, leaving 13 trials with a total of 11,071 patients for the analysis. In the RCTs, 1,360 patients were randomly assigned to treatment with sterile gloves and 1,381 to treatment with nonsterile gloves as the intervention. In the prospective or retrospective observational trials, 4,680 patients were treated with sterile gloves, and 3,650 were treated with nonsterile gloves. Heterogeneity was low. Of note, the researchers performed a subgroup analysis on nine studies (4 RCTs and 5 observational studies) involving only cutaneous surgeries; these represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile and nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR], 1.06). There was also no difference in infection rate in the subgroup analysis (2.2% vs 2.2%, respectively; RR, 1.02) or an analysis limited to only RCTs.
WHAT’S NEW
Highest-quality evidence shows no difference
This systematic review found no difference in SSI rates when using sterile versus nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
Continue to: CAVEATS
CAVEATS
Risk for bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk for bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Providers may worry about potential medicolegal ramifications from this change.1 Lastly, some providers may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[8]:507-508).

Your practice manager, on a quest to reduce expenses, asks whether your practice could reduce the amount of money spent on gloves for procedures. How do you reply?
The effect of a small difference spread over a large number of events can be sizable. For example, the added cost of using sterile, as opposed to nonsterile, gloves for minor procedures is relatively modest and certainly worthwhile if the sterile gloves reduce the number of surgical site infections (SSIs). However, if there is no difference in SSIs, the extra cost becomes a large unnecessary expense, given the volume of minor procedures performed.
The decision to use sterile gloves often stems from habit, product availability, or perceived benefit of fewer SSIs.2 Providers’ choice of gloves varies widely, despite some evidence comparing sterile and nonsterile gloves.3-5
STUDY SUMMARY
Sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 RCTs and observational (prospective or retrospective) studies compared infection rates using sterile versus nonsterile gloves in 11,071 unique patients. The methods used in the review followed the Cochrane collaboration guidelines.6 Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. A total of 512 publications were reviewed for inclusion; 14 met the criteria but one study was removed due to incomplete data, leaving 13 trials with a total of 11,071 patients for the analysis. In the RCTs, 1,360 patients were randomly assigned to treatment with sterile gloves and 1,381 to treatment with nonsterile gloves as the intervention. In the prospective or retrospective observational trials, 4,680 patients were treated with sterile gloves, and 3,650 were treated with nonsterile gloves. Heterogeneity was low. Of note, the researchers performed a subgroup analysis on nine studies (4 RCTs and 5 observational studies) involving only cutaneous surgeries; these represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile and nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR], 1.06). There was also no difference in infection rate in the subgroup analysis (2.2% vs 2.2%, respectively; RR, 1.02) or an analysis limited to only RCTs.
WHAT’S NEW
Highest-quality evidence shows no difference
This systematic review found no difference in SSI rates when using sterile versus nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
Continue to: CAVEATS
CAVEATS
Risk for bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk for bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Providers may worry about potential medicolegal ramifications from this change.1 Lastly, some providers may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[8]:507-508).
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016; 152(9): 1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204(6):976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015(1);202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21(3):148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. http://methods.cochrane.org/. Accessed August 24, 2018.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016; 152(9): 1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204(6):976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015(1);202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21(3):148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. http://methods.cochrane.org/. Accessed August 24, 2018.
Time to stop glucosamine and chondroitin for knee OA?
ILLUSTRATIVE CASE
A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member.
Should you tell the patient it’s okay to continue the medication?
Knee OA in the United States is a common condition and affects an estimated 12% of adults 60 years and older and 16% of adults 70 years and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that first-line treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical nonsteroidal anti-inflammatory drugs (either initially or if unresponsive to acetaminophen).3,4 Alternative medication options for patients with an inadequate response to initial therapy include tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence of OA.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional recommendation).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses of ≥800 mg/d compared with placebo (level of evidence, low; risk of bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
[polldaddy:10097537]
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N>200) with adequate methods of blinding and allocation concealment found no difference in pain.8
Continue to: Three studies included...
Three studies included in the 2015 systematic review provided data on adverse events when comparing glucosamine plus chondroitin vs placebo, and found no statistically significant difference.8
This randomized controlled trial (RCT) from Roman-Blas et al1 evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid, Spain) maker of the combination of chondroitin plus glucosamine used in the study.
STUDY SUMMARY
Chondroitin + glucosamine was not better than placebo for pain
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in 9 rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate 1200 mg plus glucosamine sulfate 1500 mg (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate to severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Level of knee pain was defined by a self-reported global pain score of 40-80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics, and the average age in the CS/GS group was 67 years vs 65 years in the placebo group. Exclusion criteria included body mass index of ≥35 kg/m2, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary end point was mean reduction in global pain score on a 0- to 100-mm VAS at 6 months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Continue to: Baseline global pain scores were...
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P<.03.1
Results. In the intention-to-treat analysis at 6 months, patients in the placebo group had a greater reduction in pain than the CS/GC group (-20 mm vs -12 mm; P=.029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P=.01).
In the CS/GS group, 31% did not complete the 6-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P=.018).1
WHAT’S NEW
A pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings to CS or GS alone, or different dosages
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone or different dosing would lead to the same outcome is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
An all-too-common product presents challenges
CS/GC is available over the counter and advertised directly to consumers. With this medication so readily available, identifying patients who are taking the supplement and encouraging discontinuation can be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
ILLUSTRATIVE CASE
A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member.
Should you tell the patient it’s okay to continue the medication?
Knee OA in the United States is a common condition and affects an estimated 12% of adults 60 years and older and 16% of adults 70 years and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that first-line treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical nonsteroidal anti-inflammatory drugs (either initially or if unresponsive to acetaminophen).3,4 Alternative medication options for patients with an inadequate response to initial therapy include tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence of OA.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional recommendation).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses of ≥800 mg/d compared with placebo (level of evidence, low; risk of bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
[polldaddy:10097537]
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N>200) with adequate methods of blinding and allocation concealment found no difference in pain.8
Continue to: Three studies included...
Three studies included in the 2015 systematic review provided data on adverse events when comparing glucosamine plus chondroitin vs placebo, and found no statistically significant difference.8
This randomized controlled trial (RCT) from Roman-Blas et al1 evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid, Spain) maker of the combination of chondroitin plus glucosamine used in the study.
STUDY SUMMARY
Chondroitin + glucosamine was not better than placebo for pain
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in 9 rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate 1200 mg plus glucosamine sulfate 1500 mg (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate to severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Level of knee pain was defined by a self-reported global pain score of 40-80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics, and the average age in the CS/GS group was 67 years vs 65 years in the placebo group. Exclusion criteria included body mass index of ≥35 kg/m2, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary end point was mean reduction in global pain score on a 0- to 100-mm VAS at 6 months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Continue to: Baseline global pain scores were...
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P<.03.1
Results. In the intention-to-treat analysis at 6 months, patients in the placebo group had a greater reduction in pain than the CS/GC group (-20 mm vs -12 mm; P=.029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P=.01).
In the CS/GS group, 31% did not complete the 6-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P=.018).1
WHAT’S NEW
A pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings to CS or GS alone, or different dosages
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone or different dosing would lead to the same outcome is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
An all-too-common product presents challenges
CS/GC is available over the counter and advertised directly to consumers. With this medication so readily available, identifying patients who are taking the supplement and encouraging discontinuation can be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 65-year-old man with moderately severe osteoarthritis (OA) of the knee presents to your office for his annual exam. During the medication review, the patient mentions he is using glucosamine and chondroitin for his knee pain, which was recommended by a family member.
Should you tell the patient it’s okay to continue the medication?
Knee OA in the United States is a common condition and affects an estimated 12% of adults 60 years and older and 16% of adults 70 years and older.2 The primary goals of OA therapy are to minimize pain and improve function. The American Academy of Orthopedic Surgeons (AAOS) and the American College of Rheumatology (ACR) agree that first-line treatment recommendations include aerobic exercise, resistance training, and weight loss.
Initial pharmacologic therapies include full-strength acetaminophen or oral/topical nonsteroidal anti-inflammatory drugs (either initially or if unresponsive to acetaminophen).3,4 Alternative medication options for patients with an inadequate response to initial therapy include tramadol, other opioids, duloxetine, or intra-articular injections with corticosteroids or hyaluronate.3,4 Total knee replacement may be indicated in moderate or severe knee OA with radiographic evidence of OA.5 Vitamin D, lateral wedge insoles, and antioxidants are not currently recommended.6
Prior studies evaluating glucosamine and/or chondroitin have provided conflicting results regarding evidence on pain reduction, function, and quality of life. Therefore, guidelines on OA management do not recommend their use (AAOS, strong; ACR, conditional recommendation).3,4 However, consumption remains high, with 6.5 million US adults reporting use of glucosamine and/or chondroitin in the prior 30 days.7
A 2015 systematic review of 43 randomized trials evaluating oral chondroitin sulfate for OA of varying severity suggested there may be a significant decrease in short-term and long-term pain with doses of ≥800 mg/d compared with placebo (level of evidence, low; risk of bias, high).8 However, no significant difference was noted in short- or long-term function, and the trials were highly heterogeneous.
[polldaddy:10097537]
Studies included in the 2015 systematic review found that glucosamine plus chondroitin did not have a significant effect on short- or long-term pain or physical function compared with placebo. Although glucosamine plus chondroitin led to significantly decreased pain compared with other medication, sensitivity analyses conducted for larger studies (N>200) with adequate methods of blinding and allocation concealment found no difference in pain.8
Continue to: Three studies included...
Three studies included in the 2015 systematic review provided data on adverse events when comparing glucosamine plus chondroitin vs placebo, and found no statistically significant difference.8
This randomized controlled trial (RCT) from Roman-Blas et al1 evaluated chondroitin and glucosamine vs placebo in patients with more severe OA. The study was supported by Tedec-Meiji Farma (Madrid, Spain) maker of the combination of chondroitin plus glucosamine used in the study.
STUDY SUMMARY
Chondroitin + glucosamine was not better than placebo for pain
This multicenter, randomized, double-blind, placebo-controlled trial was conducted in 9 rheumatology referral centers and one orthopedic center in Spain. The trial evaluated the efficacy of chondroitin sulfate 1200 mg plus glucosamine sulfate 1500 mg (CS/GS) compared with placebo in 164 patients with Grade 2 or 3 knee OA and moderate to severe knee pain. OA grade was ascertained using the Kellgren-Lawrence scale, corresponding to osteophytes and either possible (Grade 2) or definite (Grade 3) joint space narrowing. Level of knee pain was defined by a self-reported global pain score of 40-80 mm on a 100-mm visual analog scale (VAS).
No significant difference was noted in group characteristics, and the average age in the CS/GS group was 67 years vs 65 years in the placebo group. Exclusion criteria included body mass index of ≥35 kg/m2, concurrent arthritic conditions, and any coexisting chronic disease that would prevent successful completion of the trial.1
The primary end point was mean reduction in global pain score on a 0- to 100-mm VAS at 6 months. Secondary outcomes included mean reduction in total and subscale scores in pain and function on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) index (0–100-mm VAS for each) and the use of rescue medication.
Continue to: Baseline global pain scores were...
Baseline global pain scores were 62 mm in both groups. Acetaminophen, up to 3 g/d, was the only allowed rescue medication. Clinic visits occurred at 4, 12, and 24 weeks. A statistically significant difference between groups was defined as P<.03.1
Results. In the intention-to-treat analysis at 6 months, patients in the placebo group had a greater reduction in pain than the CS/GC group (-20 mm vs -12 mm; P=.029). No other difference was noted between the placebo and CS/GS groups in the total or subscales of the WOMAC index, and no difference was noted in use of acetaminophen. More patients in the placebo group had at least a 50% improvement in pain or function compared with the CS/GS group (47.4% vs 27.5%; P=.01).
In the CS/GS group, 31% did not complete the 6-month treatment period, compared with 18% in the placebo group. More patients dropped out because of adverse effects (diarrhea, upper abdominal pain, and constipation) in the CS/GS group than the placebo group (33 vs 19; P=.018).1
WHAT’S NEW
A pharma-sponsored study finds treatment ineffective
The effectiveness of CS/GS for the treatment of knee OA has been in question for years, but this RCT is the first trial sponsored by a pharmaceutical company to evaluate CS/GS efficacy. This trial found evidence of a lack of efficacy. In patients with more severe OA of the knee, placebo was more effective than CS/GS, and CS/GS had significantly more adverse events. Therefore, it may be time to advise patients to stop taking their CS/GS supplement.
CAVEATS
Cannot generalize findings to CS or GS alone, or different dosages
The study compared only one medication dosing regimen using a combination of CS and GS. Whether either agent alone or different dosing would lead to the same outcome is unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
An all-too-common product presents challenges
CS/GC is available over the counter and advertised directly to consumers. With this medication so readily available, identifying patients who are taking the supplement and encouraging discontinuation can be a challenge.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
1. Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
2. Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33:2271-2279.
3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64:465-474.
4. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd ed. J Am Acad Orthop Surg. 2013;21:577-579.
5. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155.
6. Ebell MH. Osteoarthritis: rapid evidence review. Am Fam Physician. 2018;97:523-526.
7. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;(79):1-16.
8. Singh JA, Noorbaloochi S, MacDonald R, et al. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;(1):CD005614.
PRACTICE CHANGER
Tell patients with moderately severe osteoarthritis to stop taking their glucosamine and chondroitin as it is less effective than placebo.1
STRENGTH OF RECOMMENDATION
B: Based on single, good-quality randomized controlled trial.
Roman-Blas JA, Castañeda S, Sánchez-Pernaute O, et al. Combined treatment with chondroitin sulfate and glucosamine sulfate shows no superiority over placebo for reduction of joint pain and functional impairment in patients with knee osteoarthritis: a six-month multicenter, randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 2017;69:77-85.
Time to switch to nonsterile gloves for these procedures?
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Your practice manager comes to you to discuss ways that you can reduce expenses. He asks whether the practice could reduce the amount of money spent on gloves for procedures. How do you reply?
A decision involving a small difference, spread over a larger number of events, can have a sizable effect. An example is whether to use sterile vs nonsterile gloves for minor procedures. The cost difference between a box of sterile gloves and a box of nonsterile gloves is relatively small, and certainly worth the difference if the more expensive sterile gloves reduce the number of surgical site infections (SSIs).
However, if there is no difference in the number of SSIs, there may be no value to the extra cost, which, given the number of such procedures, becomes a large unnecessary expense. The choice to use sterile gloves often stems from habit, product availability, or the perceived benefit of fewer SSIs.2 While some evidence exists comparing glove choice, there is wide variability in physicians’ choice of gloves.3-5 This large systematic review compared rates of SSIs using sterile vs nonsterile gloves.
STUDY SUMMARY
RCTs/observational studies find sterile no better than nonsterile gloves
This systematic review and meta-analysis of 13 randomized controlled trials (RCTs) and observational (prospective or retrospective) studies compared infection rates using sterile vs nonsterile gloves in 11,071 unique patients undergoing cutaneous surgery, including Mohs microsurgery or outpatient dental procedures. The methods used in the review followed the Cochrane collaboration guidelines.6 The inclusion criteria were that the studies had to be either RCTs or observational studies. Patients included in each study underwent outpatient cutaneous or mucosal surgical procedures, including laceration repair, standard excisions, Mohs micrographic surgery, or tooth extractions. In addition to glove type, documentation of postoperative SSI was necessary for inclusion.
Methodology. The authors of the analysis reviewed a total of 512 publications for inclusion; of these, 14 met the inclusion criteria. One study was later removed due to incomplete data, leaving a total of 13 trials for the analysis. Of the 11,071 patients included in the final analysis, 1360 patients were randomly assigned to treatment with sterile gloves, while 1381 patients were assigned to treatment with nonsterile gloves as the intervention in a clinical trial. The remaining patients participated in either prospective or retrospective observational trials; 4680 patients were treated with sterile gloves, and 3650 patients were treated with nonsterile gloves. Heterogeneity was low for the included studies. Of note, the researchers performed a subgroup analysis on 9 total studies (4 RCTs and 5 observational studies) involving cutaneous surgeries only. These represented procedures most likely performed in the primary care setting.
The primary outcome of this review was postoperative wound infection. The results did not show any difference in SSIs between sterile vs nonsterile gloves in all trials (2% vs 2.1%; relative risk [RR]=1.06; 95% confidence interval [CI], 0.81-1.39). There was also no difference in infection rates in the subgroup analysis of 9 trials limited to cutaneous surgery (2.2% vs 2.2%, respectively; RR=1.02; 95% CI, 0.78-1.34) or when the analysis was limited to only RCTs.
[polldaddy:10063798]
Continue to: WHAT'S NEW
WHAT’S NEW
Highest-quality evidence shows no difference in SSIs
This systematic review found no difference in SSI rates when using sterile vs nonsterile gloves. Given that the analysis represents the highest-quality level of evidence (a systematic review of RCTs) and that sterile gloves are several times more expensive per pair than nonsterile gloves, the findings should impact future practice.
CAVEATS
A risk of bias and limited applicability
Not every trial in this meta-analysis was an RCT, and the inclusion of observational studies increases the risk of bias. However, the results of the observational studies were similar to those of the RCTs, somewhat alleviating this potential threat to validity.
It is worth noting that more extensive surgeries and more complicated repairs were not included in the trials, meaning that the findings are limited to oral surgery, Mohs micrographic surgery, standard incisions, and laceration repairs.
CHALLENGES TO IMPLEMENTATION
Inertia, medicolegal concerns, and personal preference
Clinical inertia may lead to slow adoption of these recommendations. Physicians may worry about potential medicolegal ramifications from this change.1 Lastly, some physicians may prefer the fit and feel of sterile gloves for their procedures.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
1. Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
2. Creamer J, Davis K, Rice W. Sterile gloves: do they make a difference? Am J Surg. 2012;204:976-979.
3. Heal C, Sriharan S, Buttner PG, et al. Comparing non-sterile to sterile gloves for minor surgery: a prospective randomised controlled non-inferiority trial. Med J Aust. 2015;202:27-31.
4. Ghafouri HB, Zoofaghari SJ, Kasnavieh MH, et al. A pilot study on the repair of contaminated traumatic wounds in the emergency department using sterile versus non-sterile gloves. Hong Kong J Emerg Med. 2014;21:148-152.
5. Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
6. Cochrane Methods. London, UK: The Cochrane Collaboration. 2018. Available at: http://methods.cochrane.org/. Accessed July 15, 2018.
PRACTICE CHANGER
Using nonsterile gloves for common primary care skin procedures causes no more infections than using sterile gloves.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 13 randomized controlled trials.
Brewer JD, Gonzalez AB, Baum CL, et al. Comparison of sterile vs nonsterile gloves in cutaneous surgery and common outpatient dental procedures: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:1008-1014.
Let Low-risk Moms Eat During Labor?
A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.

A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).

A 23-year-old nulliparous woman at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the past three hours and says she’s hungry. What type of diet should you order for this patient? Should you place any restrictions in the order?
Since the first reports of Mendelson syndrome (aspiration during general anesthesia) in the early 1940s, many health care providers managing laboring women restrict their diets to clear liquids or less, with little evidence to support the decision.2 In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a general-population study of 172,334 adults who underwent a total of 215,488 surgeries with general anesthesia, the risk for aspiration was 1:895 for emergency procedures and 1:3886 for elective procedures.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less-restrictive diet during labor.
STUDY SUMMARY
Not one case of aspiration
This meta-analysis of 10 RCTs, including 3,982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less-restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥ 37 weeks, two studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥ 30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Continue to: Results
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes. Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal ICU were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk, 1.00). None of the 3,982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
An outdated practice, per the data
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less-restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk for complications/surgery during labor are not justified based on current data.
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less-restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[6]:379-380).
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38(11):1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78(1):56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14(4):171-175.
6. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
First-time, mild diverticulitis: Antibiotics or watchful waiting?
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with over-the-counter medications. You suspect diverticulitis and obtain an abdominal computed tomography (CT) scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis.
How would you treat him?
Diverticulitis is common; about 200,000 people per year are admitted to the hospital because of diverticulitis in the United States.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized control trial (RCT; N=623) found that antibiotic treatment (compared with no antibiotic treatment) for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by a lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, non-uniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
RCT finds that watchful waiting is just as effective as antibiotic Tx
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adult patients in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomized to receive IV administration of amoxicillin-clavulanate 1200 mg 4 times daily for at least 48 hours followed by 625 mg PO 3 times daily for 10 total days of antibiotic treatment (n=266) or to be observed (n=262). Computerized randomization, with a random varying block size and stratified by Hinchey classification and center, was performed, and allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic treatment adverse effects; and all-cause mortality.
Continue to: Results
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 days vs 12 days; P=.15; hazard ratio [HR] for functional recovery=0.91; lower limit of 1-sided 95% confidence interval, 0.78). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up (complicated diverticulitis, 3.8% vs 2.6%, respectively; P=.377), recurrent diverticulitis (3.4% vs 3%; P=.494), readmission (17.6% vs 12%; P=.148), or adverse events (48.5% vs 54.5%; P=.221). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 days; P=.006). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
WHAT’S NEW
A study that looks at a true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate, or requirement for percutaneous drainage.7,8 This study is the first one to look at functional return to work (a true patient-oriented outcome). And it is the only study to look out to 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize findings to patients with worse forms of diverticulitis
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease), and is not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis maybe more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
Continuet to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018 Jan 11. doi: 10.1111/codi.14013.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
PRACTICE CHANGER
For mild, computed tomography-proven acute diverticulitis, consider observation only instead of antibiotic therapy.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.
Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.1