User login
Bariatric Surgery + Medical Therapy: Effective Tx for T2DM?

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
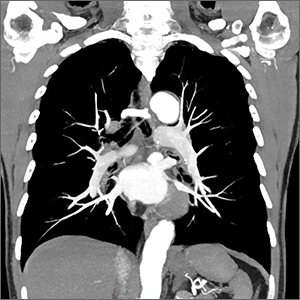
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
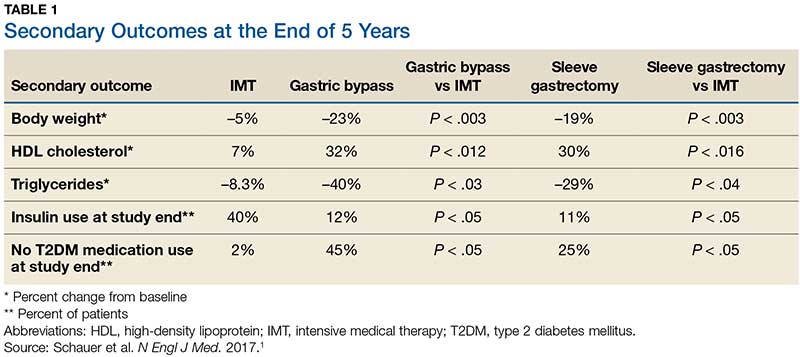
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1
WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1

WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).

A 46-year-old woman presents with a BMI of 28, a 4-year history of type 2 diabetes mellitus (T2DM), and an A1C of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 U/d, with minimal change in A1C. Should you recommend bariatric surgery?
One in 11 Americans has diabetes, and at least 95% of those have T2DM.2,3 The treatment of T2DM is generally multimodal to target the various mechanisms that cause hyperglycemia. Strategies may include making lifestyle modifications, decreasing insulin resistance, increasing insulin secretion, replacing insulin, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) recommends diet, exercise, and behavioral modifications as firstline therapy for diabetes management, but these methods are often inadequate.2 In addition to various pharmacotherapeutic strategies for some populations with T2DM, the ADA recommends bariatric surgery for those with a BMI ≥ 35 and uncontrolled hyperglycemia.2,4
However, this recommendation is based only on short-term studies. For example, in a single-center, nonblinded RCT of 60 patients with a BMI ≥ 35, the average baseline A1C levels of 8.65 ± 1.45% were reduced to 7.7 ± 0.6% in the IMT group and to 6.4 ± 1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35), gastric bypass yielded better outcomes than sleeve gastrectomy: 93% of patients in the former group and 47% of those in the latter group achieved remission of T2DM over a 12-month period.6
The current study by Schauer et al examined the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up: surgery + IMT works
This study was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were ages 20 to 60, had a BMI of 27 to 43, and had an A1C > 7%. Patients with a history of bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Patients were randomly placed in a 1:1:1 fashion into 3 groups: IMT (as defined by the ADA) only, IMT and gastric bypass, or IMT and sleeve gastrectomy. The primary outcome was the number of patients with an A1C ≤ 6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period, leaving 149. Of these, 134 completed the 5-year follow-up. Eight patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment, and 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an A1C of ≤ 6% than in the IMT group (14 of 49 gastric bypass patients, 11 of 47 sleeve gastrectomy patients, and 2 of 38 IMT patients). Compared with those in the IMT group, the patients in the 2 surgery groups showed greater reductions from baseline in body weight and triglyceride levels and greater increases from baseline in HDL cholesterol levels; they also required less antidiabetes medication for glycemic control (see Table).1

WHAT’S NEW?
Big benefits, minimal adverse effects
Prior studies evaluating the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates that bariatric surgery plus IMT has long-term benefits with minimal adverse events, compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM in patients with a BMI ≥ 27, which is below the starting BMI (35) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications—eg, gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis—in this patient population is significant.1 Other potential complications include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1C, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who underwent gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up, compared with patients who underwent sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to those of IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:102-104).
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Association. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S81-S89.
3. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: CDC, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 27, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011; 146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
A better approach to the diagnosis of PE
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
PRACTICE CHANGER
Do not order computed tomography pulmonary angiography when evaluating patients for suspected pulmonary embolism unless: (1) the patient has a
STRENGTH OF RECOMMENDATION
A: Based on a prospective, multicenter, cohort study of 3616 patients with clinically suspected pulmonary embolism.1
van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
Less Is More When It Comes to Ketorolac for Pain

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).

A 46-year-old man with no significant medical history presents to the emergency department (ED) with right flank pain and nausea. CT reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning to start him on IV ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective NSAID. As a nonopiate analgesic, it is often the first choice for the treatment of acute pain in the flank, abdomen, musculoskeletal system, or head.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries an FDA black-box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose” at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when the anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the ceiling dose of ketorolac as 10 mg across dosage forms—yet the majority of research and most health care providers in current practice use higher doses (20 to 60 mg).4,5 The FDA-approved labeling provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose in at least 97% of patients who received IV doses and at least 96% of those who received intramuscular (IM) doses in a US ED.6 If 10 mg of ketorolac is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of ketorolac in 240 adult patients (ages 18 to 65) presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset within the past 30 days.
Patients were randomly assigned to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, which were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0-to-10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine (0.1 mg/kg) was offered. The primary outcome was a numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue
The treatment groups were similar in terms of demographics and baseline vital signs. Mean age was 39 to 42. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had experienced pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, with no difference between the groups: mean pain scores postintervention were 5.1 for the 10- and 15-mg group and 4.8 for the 30-mg group. There was no difference between the groups at any other time intervals. There was also no difference between groups in the number of patients who needed rescue medication at 30 minutes (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose of IV ketorolac is just as effective as higher doses for acute pain control.
CAVEATS
2-hour limit; no look at long-term effects
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects such as bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
10-mg single-dose vial not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared (as it was in this study). However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses will. CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[1]:41-42).
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017; 70:177-184.
2. Buckley MM, Brogden RN. Ketorolac: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39: 86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Laboratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
Can vitamin D prevent acute respiratory infections?
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Ms. M is a 55-year-old woman who is generally healthy, but who was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D level of 8 ng/mL). She is being seen for her second episode of acute viral bronchitis in the past 6 months. She has no significant smoking or exposure history, no history of asthma, and takes no respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/week in bolus dosing, but is that your best option in this case?
Acute respiratory tract infections (ARTIs) include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common cause of ambulatory care visits, accounting for almost 120 million, or about 10% of all visits, per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D protects against ARTIs, but only in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N=10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk of bias. The Cochrane risk of bias tool was used to address threats to validity.
The review and meta-analysis included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or vitamin D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI, and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR] = 0.88; 95% confidence interval [CI], 0.81-0.96; number needed to treat [NNT] = 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR = 0.81; 95% CI, 0.72-0.91), but bolus dosing (≥ 30,000 IU) was not (AOR = 0.97; 95% CI, 0.86-1.10).
Continue to: In 2-step analysis...
In 2-step analysis, patients benefited who: had baseline circulating 25-hydroxyvitamin D concentrations < 10 ng/mL (AOR = 0.30; 95% CI, 0.17-0.53; NNT = 4); had baseline circulating 25-hydroxyvitamin D levels of 10 to 28 ng/mL (AOR = 0.75; 95% CI, 0.60-0.95; NNT = 15); were ages 1.1 to 15.9 years (AOR = 0.59; 95% CI, 0.45-0.79); were ages 16 to 65 years (AOR = 0.79; 95% CI, 0.63-0.99); or had a body mass index < 25 (AOR = 0.82; 95% CI, 0.71-0.95).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25-hydroxyvitamin D ≥ 30 ng/mL did not appear to provide benefit (AOR = 0.96; 95% CI, 0.78-1.18). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR = 0.98; 95% CI, 0.80-1.20).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25-hydroxyvitamin D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or vitamin D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect of vitamin D supplementation on the prevention of ARTIs was noted in those who were most severely vitamin D deficient (those with circulating 25-hydroxyvitamin levels < 10 ng/mL, NNT = 4; 10-28 ng/mL, NNT = 15). There was no demonstrable effect once circulating 25-hydroxyvitamin D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs, but the doses used were much lower than the commonly used 10,000 to 50,000 IU bolus doses, which were ineffective in reducing ARTIs in the current meta-analysis. Since bolus dosing is an ingrained practice for many providers, changing this may prove challenging.
Continue to: In addition...
In addition, the authors of the study suggest that one of the ways to provide this level of vitamin D is through food fortification, but food fortification is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health Care Surveys. http://www.cdc.gov/nchs/dhcs.htm. Accessed April 17, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blind trial in young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
PRACTICE CHANGER
Reduce acute respiratory tract infections in those with significant vitamin D deficiency (circulating 25-hydroxyvitamin D levels < 10 ng/mL) with daily or weekly vitamin D supplementation—not bolus vitamin D treatment.1
STRENGTH OF RECOMMENDATION
A: Based on a systematic review and meta-analysis of 25 trials.
Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
What’s the Best Treatment Setting for Stable Pulmonary Embolism?
A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute-onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate, 90 beats/min. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the CDC, venous thromboembolism (VTE) affects about 900,000 people each year, and about 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attack and stroke.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk for long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, < 70,000/mm3); expected to be compliant with treatment; and feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized versus outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable PE patients
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital versus outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via CT or high-probability ventilation-perfusion scan and excluded if they were younger than 19, were diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome included rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts wa
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR], 5.07), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (see Table). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR, 4.9). The results remained similar for high-risk patients (Class III-V) based on their PESI score.
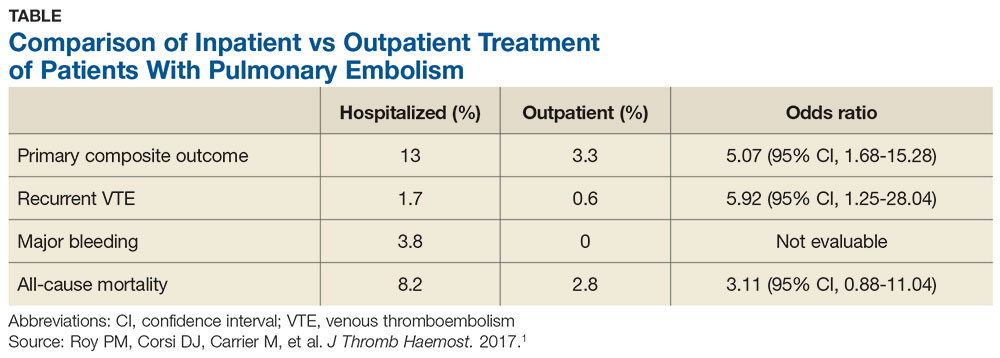
WHAT’S NEW
Higher rate of adverse events in inpatients
This trial supports the CHEST guideline recommendations to manage hemodynamically stable patients with acute PE as outpatients.3 It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
Good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not an RCT. This study defined outpatient management as patients discharged from the ED or hospitalized for < 48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges; the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low-molecular-weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[12]:777-779).
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. CDC. Venous thromboembolism data & statistics. www.cdc.gov/ncbddd/dvt/data.html. Accessed April 26, 2019.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.

A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute-onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate, 90 beats/min. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the CDC, venous thromboembolism (VTE) affects about 900,000 people each year, and about 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attack and stroke.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk for long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, < 70,000/mm3); expected to be compliant with treatment; and feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized versus outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable PE patients
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital versus outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via CT or high-probability ventilation-perfusion scan and excluded if they were younger than 19, were diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome included rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts wa
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR], 5.07), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (see Table). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR, 4.9). The results remained similar for high-risk patients (Class III-V) based on their PESI score.

WHAT’S NEW
Higher rate of adverse events in inpatients
This trial supports the CHEST guideline recommendations to manage hemodynamically stable patients with acute PE as outpatients.3 It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
Good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not an RCT. This study defined outpatient management as patients discharged from the ED or hospitalized for < 48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges; the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low-molecular-weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[12]:777-779).

A 63-year-old woman with a history of hypertension presents to the emergency department (ED) with acute-onset shortness of breath and pleuritic chest pain after traveling across the country for a work conference. She has no history of cancer, liver disease, or renal disease. Her blood pressure is 140/80 mm Hg, and her heart rate, 90 beats/min. You diagnose an acute PE in this patient and start anticoagulation. Should you admit her to the hospital to decrease morbidity and mortality?
According to the CDC, venous thromboembolism (VTE) affects about 900,000 people each year, and about 60,000 to 100,000 of these patients die annually.2 Pulmonary embolism is the third leading cause of death from cardiovascular disease, following heart attack and stroke.3 Prompt diagnosis and treatment with systemic anticoagulation improves patient outcomes and decreases the risk for long-term complications.
The 2016 American College of Chest Physicians (CHEST) guideline on antithrombotic therapy for VTE recommends home treatment or early discharge over standard discharge (after the first 5 days of treatment) for patients who meet the following clinical criteria: “clinically stable with good cardiopulmonary reserve; no contraindications such as recent bleeding, severe renal or liver disease, or severe thrombocytopenia (ie, < 70,000/mm3); expected to be compliant with treatment; and feels well enough to be treated at home.”3
The guideline states that various clinical decision tools, such as the Pulmonary Embolism Severity Index (PESI), can aid in identifying low-risk patients to be considered for treatment at home. The PESI uses age, gender, vital signs, mental status, and a history of cancer, lung, and cardiac disease to stratify patients by risk.4
A systematic review of 1 randomized controlled trial (RCT) and 7 observational studies found that in low-risk patients, outpatient treatment was as safe as inpatient treatment.5 This more recent study determines the net clinical benefit of hospitalized versus outpatient management in a wider range of patients with acute PE, regardless of initial risk.1
STUDY SUMMARY
Hospitalization confers no benefit to stable PE patients
This retrospective, propensity-matched cohort study compared rates of adverse events in 1127 patients with acute PE managed in the hospital versus outpatient setting.1 Patients were classified as outpatients if they were discharged from the ED or discharged from the hospital within 48 hours of admission. Patients were included if a symptomatic acute PE was diagnosed via CT or high-probability ventilation-perfusion scan and excluded if they were younger than 19, were diagnosed with a PE during hospitalization, had chronic PE, or were hemodynamically unstable, among other factors. The investigators calculated PESI scores for all patients.
Propensity scores matched patients on 28 characteristics and known risk factors for adverse events to ensure the groups were similar. The primary outcome was rate of adverse events, including recurrent VTE, major bleeding, or death at 14 days. The secondary outcome included rates of the above during the 3-month follow-up period.
Continue to: Of the 1127 eligible patients...
Of the 1127 eligible patients, 1081 were included in the matched cohort, with 576 (53%) treated as hospitalized patients and 505 (47%) treated as outpatients. The mean age of the matched cohorts wa
The 14-day rate of adverse events was higher in hospitalized patients than in outpatients (13% vs 3.3%; odds ratio [OR], 5.07), with each of the adverse events that made up the primary outcome occurring more frequently in the hospitalized group (see Table). The rate of adverse events at 3 months was also greater for hospitalized patients compared with outpatients (21.7% vs 6.9%; OR, 4.9). The results remained similar for high-risk patients (Class III-V) based on their PESI score.

WHAT’S NEW
Higher rate of adverse events in inpatients
This trial supports the CHEST guideline recommendations to manage hemodynamically stable patients with acute PE as outpatients.3 It adds to the conversation by demonstrating higher rates of adverse events with hospitalization, even in high-risk subgroups (PESI Class III-V).
CAVEATS
Good study, but it wasn’t an RCT
While this is a well-designed cohort study, it is not an RCT. This study defined outpatient management as patients discharged from the ED or hospitalized for < 48 hours. However, only 59 of the 544 patients in the outpatient group were early hospital discharges; the rest were never admitted. Finally, a specialized thrombosis clinic followed up with the patients within 24 hours of discharge, and patients had telephone access to specialized health care professionals; such organization of care contributed to the safe outpatient management of these PE patients.
CHALLENGES TO IMPLEMENTATION
Insurance coverage may present an issue
Medication coverage of direct oral anticoagulants and low-molecular-weight heparin may present a barrier to patients treated in the outpatient setting who have no insurance or are insured by certain carriers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[12]:777-779).
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. CDC. Venous thromboembolism data & statistics. www.cdc.gov/ncbddd/dvt/data.html. Accessed April 26, 2019.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
1. Roy PM, Corsi DJ, Carrier M, et al. Net clinical benefit of hospitalization versus outpatient management of patients with acute pulmonary embolism. J Thromb Haemost. 2017;15:685-694.
2. CDC. Venous thromboembolism data & statistics. www.cdc.gov/ncbddd/dvt/data.html. Accessed April 26, 2019.
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315-352.
4. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172:1041-1046.
5. Vinson DR, Zehtabchi S, Yealy DM. Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Ann Emerg Med. 2012;60:651-662.
How Do These 3 Diabetes Agents Compare in Reducing Mortality?
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.

A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).

A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
Should you switch the DAPT agent one month after ACS?
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 60-year-old man is seen in your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). He underwent percutaneous coronary intervention (PCI) with placement of one stent. He received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk of bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after myocardial infarction (MI).2 Current American Cardiology Association and European Society of Cardiology guidelines recommend patients with coronary artery disease who have had a recent MI continue DAPT with aspirin and a P2Y12 blocker (ie, clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACSto reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (ie, prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events when compared to clopidogrel.5-7 These data led to a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies evaluating the newer P2Y12 agents continue to show strong evidence for their use in the first month following PCI, while also demonstrating an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the current study, which tested switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior to unchanged DAPT
This open-label RCT (N = 646) examined changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, all patients received a loading dose of ticagrelor 180 mg or prasugrel 60 mg. Subsequently, all patients in the trial took aspirin (75 mg/d) and one of the newer P2Y12 inhibitors (prasugrel 10 mg/d or ticagrelor 90 mg BID) for 1 month. For those enrollees who had no adverse events after 30 days, half were randomly switched to aspirin and clopidogrel 75 mg/d and the other half remained on aspirin and their newer P2Y12 blocker in a 1:1 ratio. For the next year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (as defined by the Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1 year post-ACS).
The average age of the participants was 60 years; 40% had experienced a STEMI and 60% had a non–STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched DAPT group and 75% of the unchanged DAPT group were still taking their medication. At the 1-year follow-up, the composite outcome was lower in the switched group, compared with the unchanged group (13% vs 26%; hazard ratio [HR] = 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT] = 8).
All bleeding events (ranging from minimal to fatal) were lower in the switched group (9% vs 24%; HR = 0.39; 95% CI, 0.27-0.57; NNT = 7), and bleeding events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in the switched group (4% vs 15%; HR = 0.30, 95% CI, 0.18-0.50; NNT = 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR = 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Fewer bleeding events without an increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
This trial was an open-label, unblinded study. The investigators who adjudicated critical events were blinded to the treatment allocation, but some events, such as minor bleeding and medication discontinuation, could be self-reported by patients. In addition, the investigators used a less-than-ideal method (opaque envelopes) to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
Implementation may require changing a cardiologist’s prescription
Implementing this practice change is facilitated by the fact that clopidogrel is currently less expensive than the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. So the family physician (FP) may not be responsible for the DAPT switch initially. Further, switching may necessitate coordination with the cardiologist, as FPs may be hesitant to change cardiologists’ prescriptions. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082-1115.
3. Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2015;37:267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001-2015.
PRACTICE CHANGER
Switch to clopidogrel from one of the newer P2Y12 blockers 1 month after an acute coronary event, while continuing aspirin, to decrease bleeding events without increasing the risk of ischemic events.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT).
Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078.
First-time, Mild Diverticulitis: Antibiotics or Watchful Waiting?

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).

A 58-year-old man presents to your office with a 2-day history of moderate (6/10) left lower quadrant pain, mild fever (none currently), 2 episodes of vomiting, no diarrhea, and no relief with OTC medications. You suspect diverticulitis and obtain an abdominal CT scan, which shows mild, uncomplicated (Hinchey stage 1a) diverticulitis. How would you treat this patient?
Diverticulitis is common; each year, about 200,000 people in the United States are admitted to the hospital because of it.2,3 Health care providers typically treat diverticular disease with antibiotics and bowel rest.2,3 While severe forms of diverticulitis often require parenteral antibiotics and/or surgery, practitioners are increasingly managing the condition with oral antibiotics.4
One previous randomized controlled trial (RCT; N = 623) found that antibiotic treatment for acute uncomplicated diverticulitis did not speed recovery or prevent complications (perforation or abscess formation) or recurrence at 12 months.5 The study’s strengths included limiting enrollment to people with CT-proven diverticulitis, using a good randomization and concealment process, and employing intention-to-treat analysis. The study was limited by the lack of a standardized antibiotic regimen across centers, previous diverticulitis diagnoses in 40% of patients, nonuniform follow-up processes to confirm anatomic resolution, and the lack of assessment to confirm resolution.5
STUDY SUMMARY
Watchful waiting just as effective as antibiotics
This newer study was a single-blind RCT that compared treatment with antibiotics to observation among 528 adults in the Netherlands. Patients were enrolled if they had CT-proven, primary, left-sided, uncomplicated acute diverticulitis (Hinchey stage 1a and 1b).1 (The Hinchey classification is based on radiologic findings, with 0 for clinical diverticulitis only, 1a for confined pericolic inflammation or phlegmon, and 1b for pericolic or mesocolic abscess.6) Exclusion criteria included suspicion of colonic cancer by CT or ultrasound (US), previous CT/US-proven diverticulitis, sepsis, pregnancy, or antibiotic use in the previous 4 weeks.1
Observational vs antibiotic treatment. Enrolled patients were randomly assigned to receive amoxicillin-clavulanate (1,200 mg by IV qid for at least 48 hours, followed by 625 mg po tid, for 10 total days; n = 266) or to be observed (n = 262). Randomization was performed by computer, with a random varying block size and stratification by Hinchey classification and center; allocation was concealed. The investigators were masked to the allocation until all analyses were completed.1
The primary outcome was the time to functional recovery (resumption of pre-illness work activities) during a 6-month follow-up period. Secondary outcomes included hospital readmission rate; complicated, ongoing, and recurrent diverticulitis; sigmoid resection; other nonsurgical intervention; antibiotic adverse effects; and all-cause mortality.
Results. Median recovery time for observational treatment was not inferior to antibiotic treatment (14 d vs 12 d; hazard ratio for functional recovery, 0.91). Observation was not inferior to antibiotics for any of the secondary endpoints at 6 and 12 months of follow-up: complicated diverticulitis (3.8% vs 2.6%, respectively), recurrent diverticulitis (3.4% vs 3%), readmission (17.6% vs 12%), or adverse events (48.5% vs 54.5%). Initial hospitalization length of stay was shorter in the observation group (2 vs 3 d). The researchers conducted a 24-month telephone follow-up, but no differences from the 12-month follow-up were noted.1
Continue to: WHAT'S NEW
WHAT’S NEW
Study looked at true patient-oriented outcome
Previous studies of treatment options for acute uncomplicated diverticulitis looked at short-term outcomes, or at readmission, recurrence, and surgical intervention rate or requirement for percutaneous drainage.7,8 This study is the first to look at functional return to work (a true patient-oriented outcome). And it is the only study to follow up at 24 months to gauge long-term outcomes with observational treatment.
CAVEATS
Can’t generalize to worse cases
It is worth noting that the findings of this study apply only to the mildest form of CT-proven acute diverticulitis (those patients classified as having Hinchey 1a disease) and are not generalizable to patients with more severe forms. Not enough patients with Hinchey 1b acute diverticulitis were enrolled in the study to reach any conclusions about treatment.
Various guidelines issued outside the United States recommend antibiotics for uncomplicated diverticulitis; however, the American Gastroenterological Association (AGA) indicates that antibiotics should be used selectively.1,9,10 This recommendation was based on an emerging understanding that diverticulitis may be more inflammatory than infectious in nature. The AGA guideline authors acknowledge that their conclusion was based on low-quality evidence.9
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to implementing this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[7]:435-436,438).
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.
1. Daniels L, Ünlü Ç, de Korte N, et al, for the Dutch Diverticular Disease (3D) Collaborative Study Group. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104:52-61.
2. Wheat CL, Strate LL. Trends in hospitalization for diverticulitis and diverticular bleeding in the United States from 2000 to 2010. Clin Gastroenterol Hepatol. 2016;14:96-103.e1.
3. Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
4. Shabanzadeh DM, Wille-Jørgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database Syst Rev. 2012;11:CD009092.
5. Chabok A, Påhlman L, Hjern F, et al. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99:532-539.
6. Klarenbeek BR, de Korte N, van der Peet DL, et al. Review of current classifications for diverticular disease and a translation into clinical practice. Int J Colorectal Dis. 2012;27:207-214.
7. Tandon A, Fretwell VL, Nunes QM, et al. Antibiotics versus no antibiotics in the treatment of acute uncomplicated diverticulitis - a systematic review and meta-analysis. Colorectal Dis. 2018;20(3):179-188.
8. Feingold D, Steele SR, Lee S, et al. Practice parameters for the treatment of sigmoid diverticulitis. Dis Colon Rectum. 2014;57:284-294.
9. Stollman N, Smalley W, Hirano I; AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology. 2015;149:1944-1949.
10. Sartelli M, Viale P, Catena F, et al. 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2013;8:3.