User login
Yeast Infection in Pregnancy? Think Twice About Fluconazole

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.

A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although the latter are recommended as firstline therapy, the ease of oral therapy makes it an attractive option.3,4
However, the safety of oral fluconazole during pregnancy has recently come under scrutiny. Case reports have linked high-dose use with congenital malformation.5,6 These case reports led to epidemiologic studies in which no such association was found.7,8
A large cohort study involving 1,079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk for congenital malformation or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriage.10 However, the validity of both studies’ findings was limited by small numbers of participants.
The current study is the largest to date to evaluate whether use of fluconazole in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth, compared to topical azoles.
STUDY SUMMARY
Increased risk for miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole–exposed pregnancy was matched with up to four unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented by filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. Of the total cohort, 3,315 pregnancies were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortion occurred in 147 of these pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR], 1.48).
Rates of stillbirth. Of 5,382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR, 1.32). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were four times more likely than lower doses (150 mg) to be associated with stillbirth (HRs, 4.10 and 0.99, respectively).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk for spontaneous abortion, compared to topical azole use (130 of 2,823 pregnancies vs 118 of 2,823 pregnancies; HR, 1.62)—but not an increased risk for stillbirth (20 of 4,301 pregnancies vs 22 of 4,301 pregnancies; HR, 1.18).
WHAT'S NEW
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk for spontaneous abortion. By comparing treatments in a sensitivity analysis, the researchers were able to eliminate Candida infections causing spontaneous abortion as a confounding factor. In addition, this study challenges the balance between ease of use and safety.
CAVEATS
A skewed population?
This cohort study using a Danish hospital registry may not be generalizable to a larger, non-Scandinavian population. Those not seeking care through a hospital were likely missed; if those seeking care through the hospital had a higher risk for abortion, the results could be biased. However, this would not have affected the results of the comparison between the two active treatments.
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically.
In all, given the large sample size and the care taken to match each exposed pregnancy with up to four unexposed pregnancies, these limitations likely had little influence on the overall findings.
CHALLENGES TO IMPLEMENTATION
Balancing ease of use with safety
Given the ease of using oral fluconazole, compared with daily topical azole therapy, many clinicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(9):624-626.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al; Vaginal Infections and Prematurity Study Group. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidiasis. Practitioner. 1985;229:655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
Monitoring home BP readings just got easier
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
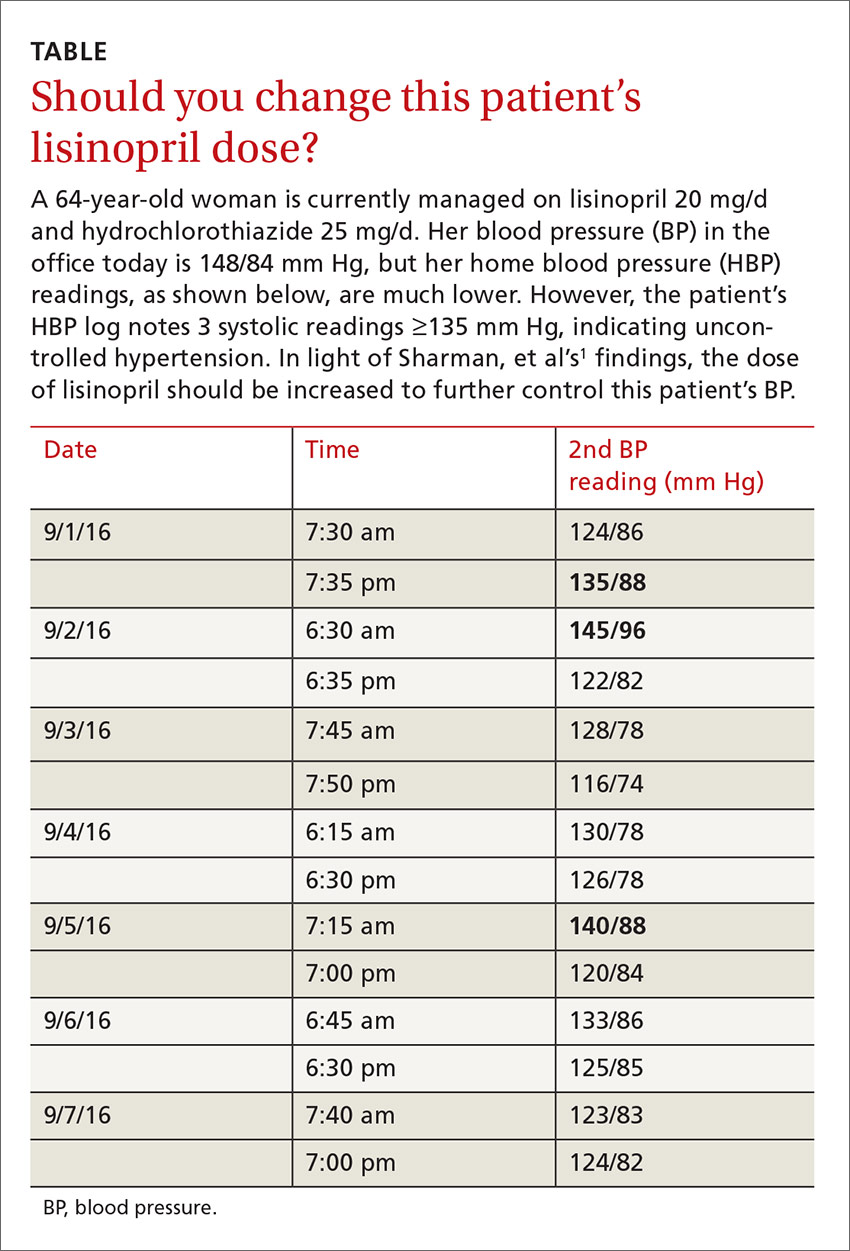
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Yeast infection in pregnancy? Think twice about fluconazole
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Avoid prescribing oral fluconazole in early pregnancy because it is associated with a higher rate of spontaneous abortion than is topical azole therapy.1
Strength of recommendation
B: Based on large cohort study performed in Denmark.
Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
Illustrative Case
A 25-year-old woman who is 16 weeks pregnant with her first child is experiencing increased vaginal discharge associated with vaginal itching. A microscopic examination of the discharge confirms your suspicions of vaginal candidiasis. Is oral fluconazole or a topical azole your treatment of choice?
Because of the increased production of sex hormones, vaginal candidiasis is common during pregnancy, affecting up to 10% of pregnant women in the United States.1,2 Treatment options include oral fluconazole and a variety of topical azoles. Although topical azoles are recommended as first-line therapy,3 the ease of oral therapy makes it an attractive treatment option.4 The safety of oral fluconazole during pregnancy, however, has recently come under scrutiny.
Case reports have linked high-dose fluconazole use during pregnancy with congenital malformations.5,6 These case reports led to epidemiological studies evaluating fluconazole’s safety, but, in these studies, no association with congenital malformations was found.7,8
A large cohort study involving 1079 fluconazole-exposed pregnancies and 170,453 unexposed pregnancies found no increased risk of congenital malformations or stillbirth; rates of spontaneous abortion and miscarriage were not evaluated.9 A prospective cohort study of 226 pregnant women found no association between fluconazole use during the first trimester and miscarriages.10 However, the validity of both studies’ findings was limited by small numbers of participants. The current study is the largest to date to evaluate whether use of fluconazole compared to that of topical azoles in early pregnancy is associated with increased rates of spontaneous abortion and stillbirth.
Study Summary
Fluconazole significantly increases risk of miscarriage, but not stillbirth
This nationwide cohort study, conducted using the Medical Birth Register in Denmark, evaluated more than 1.4 million pregnancies occurring from 1997 to 2013 for exposure to oral fluconazole between 7 and 22 weeks’ gestation. Each oral fluconazole-exposed pregnancy was matched with up to 4 unexposed pregnancies (based on propensity score, maternal age, calendar year, and gestational age) and to pregnancies exposed to intravaginal formulations of topical azoles. Exposure to fluconazole was documented based on filled prescriptions from the National Prescription Register. Primary outcomes were rates of spontaneous abortion (loss before 22 weeks) and stillbirth (loss after 23 weeks).
Rates of spontaneous abortion. From the total cohort of more than 1.4 million pregnancies, 3315 were exposed to oral fluconazole between 7 and 22 weeks’ gestation. Spontaneous abortions occurred in 147 of the 3315 fluconazole-exposed pregnancies and in 563 of 13,246 unexposed, matched pregnancies (hazard ratio [HR]=1.48; 95% confidence interval [CI], 1.23-1.77).
Rates of stillbirth. Of 5382 pregnancies exposed to fluconazole from week 7 to birth, 21 resulted in stillbirth; 77 stillbirths occurred in the 21,506 unexposed matched pregnancies (HR=1.32; 95% CI, 0.82-2.14). In a sensitivity analysis, however, higher doses of fluconazole (350 mg) were 4 times more likely to be associated with stillbirth (HR=4.10; 95% CI, 1.89-8.90) than lower doses (150 mg) (HR= 0.99; 95% CI, 0.56-1.74).
Oral fluconazole vs topical azole. Use of oral fluconazole in pregnancy was associated with an increased risk of spontaneous abortion when compared to topical azole use: 130 of 2823 pregnancies vs 118 of 2823 pregnancies, respectively (HR=1.62; 95% CI, 1.26-2.07), but not an increased risk of stillbirths: 20 of 4301 pregnancies vs 22 of 4301 pregnancies, respectively (HR=1.18; 95% CI, 0.64-2.16).
What’s New
A sizeable study with a treatment comparison
The authors found that exposure in early pregnancy to oral fluconazole, as compared to topical azoles, increases the risk of spontaneous abortion. By comparing treatments in a sensitivity analysis, the confounder of Candida infections causing spontaneous abortion was removed. In addition, when considering the ease of dosing of fluconazole as compared with topical imidazoles, this study challenges the balance of ease of use with safety.
Caveats
A skewed population and limited generalizability?
This large cohort study using the National Patient Register in Denmark may not be generalizable to a larger, non-Scandinavian population. Since a hospital registry was used, those not seeking care through the hospital were likely missed. If patients
In addition, the study focused on women exposed from 7 to 22 weeks’ gestation; the findings may not be generalizable to fluconazole exposure prior to 7 weeks. Likewise, the registry is unlikely to capture very early spontaneous abortions that are not recognized clinically. In all, given the large sample size and the care taken to match each exposed pregnancy with up to 4 unexposed pregnancies, these limitations are likely to have had little influence on the overall findings of the study.
Challenges to Implementation
Balancing ease of use with safety
Given the ease of using oral fluconazole vs daily topical azole therapy, many physicians and patients may still opt for oral treatment.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
1. Mølgaard-Nielsen D, Svanström H, Melbye M, et al. Association between use of oral fluconazole during pregnancy and risk of spontaneous abortion and stillbirth. JAMA. 2016;315:58-67.
2. Cotch MF, Hillier SL, Gibbs RS, et al. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178:374-380.
3. Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
4. Tooley PJ. Patient and doctor preferences in the treatment of vaginal candidosis. Practitioner. 1985;229: 655-660.
5. Aleck KA, Bartley DL. Multiple malformation syndrome following fluconazole use in pregnancy: report of an additional patient. Am J Med Genet. 1997;72:253-256.
6. Lee BE, Feinberg M, Abraham JJ, et al. Congenital malformations in an infant born to a woman treated with fluconazole. Pediatr Infect Dis J. 1992;11:1062-1064.
7. Jick SS. Pregnancy outcomes after maternal exposure to fluconazole. Pharmacotherapy. 1999;19:221-222.
8. Mølgaard-Nielsen D, Pasternak B, Hviid A. Use of oral fluconazole during pregnancy and the risk of birth defects. N Engl J Med. 2013;369:830-839.
9. Nørgaard M, Pedersen L, Gislum M, et al. Maternal use of fluconazole and risk of congenital malformations: a Danish population-based cohort study. J Antimicrob Chemother. 2008;62:172-176.
10. Mastroiacovo P, Mazzone T, Botto LD, et al. Prospective assessment of pregnancy outcomes after first-trimester exposure to fluconazole. Am J Obstet Gynecol. 1996;175:1645-1650.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
On-demand Pill Protocol Protects Against HIV

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.

In most high-income countries, including the US, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 In the absence of a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the CDC began recommending daily use of tenofovir, disoproxil, fumarate, and emtricitabine (TDF-FTC) in high-risk individuals as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT], 46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably due to the difficulty in getting patients to take the medication daily.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 This is because, until now, no studies had demonstrated the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays study—a double-blind, multicenter study conducted in France and Canada—assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis proposed that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk for HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection—defined as having a history of unprotected anal sex with at least two partners in the past six months. Other inclusion criteria included an age of at least 18 and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance < 60 mL/min, alanine aminotransferase level more than 2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. Those who withdrew consent, were lost to follow-up, or acquired HIV-1 infection were excluded, and the remaining study participants were randomized to take TDF-FTC (n = 199) or placebo (n = 201) before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. Participants were instructed to take a loading dose of two pills of TDF-FTC or placebo with food two to 24 hours prior to intercourse, a third pill 24 hours later, and a fourth pill 24 hours after the third.
If there were multiple consecutive days of sexual intercourse, participants were to take one pill on each day of intercourse, followed by the two postexposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the PrEP; otherwise, they were to begin again with two pills two to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants four and eight weeks after enrollment, then every eight weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count, drug levels in plasma, and with an at-home, computer-assisted interview completed by participants prior to each visit.
Participants received counseling from a peer community member and were offered preventive services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took their assigned pills correctly. Participants were followed for a median of 9.3 months. Overall, 72% of participants took the study drugs (TDF-FTC or placebo), but 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. After 20 months, the study was unblinded and is now continuing as an open-label study because of the discontinuation of another PrEP study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk for HIV without PrEP. The open-label part of the study (iPrex-OLE) completed enrollment and data gathering in November 2013. The data analysis and results are pending.9
Eighty-six percent experienced relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with three of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group and two of the new cases were in the TDF-FTC group. This translated to an 86% relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group (NNT, 17 over 9.3 months).
The two cases in the TDF-FTC group occurred in participants found to be nonadherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain, which were seen more commonly in the treatment group than in the placebo group (14% vs 5%; number needed to harm, 11). There were also mild increases in serum creatinine level, but only two participants had a transient decrease in creatinine clearance to < 60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction nearly doubles
This is the first study to look at on-demand PrEP with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the increased benefit of on-demand PrEP is likely due to improved compliance with medication use.
CAVEATS
Can adherence be maintained?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we need to wait for the results of the open-label study to fully assess the risk for adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of the medication; the retail price of TDF-FTC is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(8):556-558.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014. A clinical practice guideline. www.cdc.gov/hiv/pdf/prepguide lines2014.pdf. Accessed August 9, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. iPrEx open-label extension. www.iprexnews.com. Accessed August 9, 2016.
10. GoodRx. Truvada. www.goodrx.com/truvada. Accessed August 9, 2016.
Light Therapy For Nonseasonal Major Depressive Disorder?
PRACTICE CHANGER
Consider treatment with bright light therapy, alone or in combination with fluoxetine, for patients with nonseasonal major depressive disorder.1
Strength of Recommendation
B: Based on a single moderate-quality randomized controlled trial.1
A 38-year-old woman recently diagnosed with major depressive disorder (MDD) without a seasonal pattern presents to discuss treatment options. Her Hamilton Depression Rating Scale (HAM-D) score is 22, and she is not suicidal. Should you consider bright light therapy in addition to pharmacotherapy?
MDD is one of the most common psychiatric illnesses in the United States, affecting approximately one in five adults at some point in their lives.2 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are considered effective firstline pharmacotherapy options for MDD.2,3 Despite their effectiveness, however, studies have shown that only about 40% of patients with MDD achieve remission with firstline or secondline drugs.2 In addition, pharmacologic agents have a higher frequency of treatment-associated adverse effects than fluorescent light therapy.4
A Cochrane systematic review of 20 studies (N = 620) demonstrated the effectiveness of combined light therapy and pharmacotherapy in treating nonseasonal MDD but found no benefit to light used as monotherapy.5 However, the majority of the studies were of poor quality, occurred in the inpatient setting, and lasted less than four weeks.
In a five-week, controlled, double-blind trial not included in the Cochrane review, 102 patients with nonseasonal MDD were randomized to receive either active treatment (bright light therapy) plus sertraline (50 mg/d) or sham light treatment (using a dim red light) plus sertraline (50 mg/d). The investigators found a statistically significant reduction in depression score in the active treatment group compared to the sham light group, based on the HAM-D, the Hamilton 6-Item Subscale, the Melancholia Scale, and the seven atypical items from the Structured Interview Guide for the Seasonal Affective Disorder version of the HAM-D.6,7
Continue for the study summary >>
STUDY SUMMARY
Light therapy improves nonseasonal depression
This latest study was an eight-week, randomized, double-blind, placebo- and sham-controlled clinical trial evaluating the benefit of light therapy with and without pharmacotherapy for nonseasonal MDD.1 The investigators enrolled 122 adult patients (ages 19 to 60) from outpatient psychiatry clinics who had a diagnosis of MDD (diagnosed by a psychiatrist) and a HAM-D8 score of at least 20. Subjects had to be off psychotropic medication for at least two weeks prior to the first visit; they were subsequently monitored for one week to identify spontaneous responders and give patients time to better regulate their sleep-wake cycle (with the goal of sleeping only between 10 PM and 8 AM daily).
The investigators randomly assigned patients to one of four treatment groups:
• Active light monotherapy (10,000-lux fluorescent white light for 30 min/d early in the morning) plus a placebo pill
• Fluoxetine (20 mg/d) plus sham light therapy
• Placebo pills with sham light therapy; or
• Combined active light therapy with fluoxetine (20 mg/d).
Sham light therapy consisted of the use of an inactivated negative ion generator, used in the same fashion as a light box. All patients were analyzed based on modified intention to treat. Adherence was assessed through review of patients’ daily logs of device treatment times and through pill counts.
The primary outcome at eight weeks was the change from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item questionnaire with a worst score of 60.9 Secondary outcomes were treatment response (≥ 50% reduction in MADRS score) and remission (MADRS score ≤ 10) at the final eighth-week visit. MADRS scoring was used because of its sensitivity to treatment-induced changes and its high correlation with the HAM-D scale.
At the end of eight weeks, the mean changes in MADRS scores from baseline were: light monotherapy, 13.4; fluoxetine monotherapy, 8.8; combination therapy, 16.9; and placebo, 6.5. The improvement was significant in the light monotherapy treatment group and in the combination therapy group, compared with the placebo group, and in the combination group, compared with the fluoxetine treatment group; improvement was not significant for the fluoxetine treatment group compared with the placebo group, however.
The treatment response (≥ 50% MADRS improvement) rate was highest in the combination treatment group (75.9%), followed by light monotherapy (50%), placebo (33.3%), and fluoxetine monotherapy (29%). There was a significant response effect for the combination versus placebo treatment group.
Similarly, there was a higher remission rate in the combination treatment group (58.6%) than in the placebo, light monotherapy, or fluoxetine treatment groups (30%, 43.8%, and 19.4%, respectively). Combination therapy was superior to placebo in treatment response (≥ 50% reduction in the MADRS score) and remission (MADRS ≤ 10), with numbers needed to treat of 2.4 and 3.5, respectively.
By the end of the eight-week study period, 16 of 122 patients had dropped out. Two reported lack of efficacy, five reported adverse effects, and the remainder cited administrative reasons, were lost to follow-up, or withdrew consent.
WHAT’S NEW
New evidence on a not-so-new treatment
We now have evidence that bright light therapy, either alone or in combination with fluoxetine, is efficacious for increasing the remission rate of nonseasonal MDD.
Continue for caveats >>
CAVEATS
Variables may have affected results
Among the study’s limitations: use of a single SSRI (other, more potent SSRIs might work better); location (southern Canada; benefits may differ in regions farther south); and exclusion of pregnant and breastfeeding women from the study population.
Furthermore, the trial duration was relatively short, and the investigators did not attain their preplanned sample size for the study. This limited the power to detect clinically significant seasonal treatment effects and differences between the fluoxetine and placebo groups, regardless of whether they received active phototherapy.
CHALLENGES TO IMPLEMENTATION
Commercial insurance doesn’t usually cover light therapy
Bright light therapy is fairly safe, and some evidence exists supporting its use in the treatment of nonseasonal MDD; however, the data for its use in this area are limited.10 Since few studies have tested light therapy for nonseasonal MDD, uncertainty remains about patient selection, as well as optimal dose, timing, and duration in the management of nonseasonal MDD.11 Although the associated risks are minimal, bright light therapy can lead to mania or hypomania; clinicians need to monitor for such effects when initiating therapy.3
Lastly, commercial insurance does not usually cover light therapy. The average price of the bright light devices, which are available in medical supply stores and online, ranges from $118 to $237.4,11 However, such devices are reusable, making the amortized cost almost negligible and perhaps negating this concern.12
REFERENCES
1. Lam RW, Levitt AJ, Levitan RD, et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:56-63.
2. Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342:324-330.
3. Gelenberg AJ, Freeman CMP, Markowitz JC, et al. American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed July 5, 2016.
4. Lam RW, Tam EM. A Clinician’s Guide to Using Light Therapy. New York, NY: Cambridge University Press; 2009. www.ubcmood.ca/sad/SAD%20resources%20package%202009.pdf. Accessed July 5, 2016.
5. Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004;2:CD004050.
6. Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr Scand Suppl. 2004;425:7-28.
7. Martiny K, Lunde M, Unden M, et al. Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr Scand. 2005;112:117-125.
8. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
9. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
10. Oldham MA, Ciraulo DA. Use of bright light therapy among psychiatrists in Massachusetts: an e-mail survey. Prim Care Companion CNS Disord. 2014;16(3). Epub 2014 Jun 26.
11. Sloane PD, Figueiro M, Cohen L. Light as therapy for sleep disorders and depression in older adults. Clin Geriatr. 2008;16:25-31.
12. Kripke DF. A breakthrough treatment for major depression. J Clin Psychiatry. 2015;76:e660-e661.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(7):486-488.
PRACTICE CHANGER
Consider treatment with bright light therapy, alone or in combination with fluoxetine, for patients with nonseasonal major depressive disorder.1
Strength of Recommendation
B: Based on a single moderate-quality randomized controlled trial.1
A 38-year-old woman recently diagnosed with major depressive disorder (MDD) without a seasonal pattern presents to discuss treatment options. Her Hamilton Depression Rating Scale (HAM-D) score is 22, and she is not suicidal. Should you consider bright light therapy in addition to pharmacotherapy?
MDD is one of the most common psychiatric illnesses in the United States, affecting approximately one in five adults at some point in their lives.2 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are considered effective firstline pharmacotherapy options for MDD.2,3 Despite their effectiveness, however, studies have shown that only about 40% of patients with MDD achieve remission with firstline or secondline drugs.2 In addition, pharmacologic agents have a higher frequency of treatment-associated adverse effects than fluorescent light therapy.4
A Cochrane systematic review of 20 studies (N = 620) demonstrated the effectiveness of combined light therapy and pharmacotherapy in treating nonseasonal MDD but found no benefit to light used as monotherapy.5 However, the majority of the studies were of poor quality, occurred in the inpatient setting, and lasted less than four weeks.
In a five-week, controlled, double-blind trial not included in the Cochrane review, 102 patients with nonseasonal MDD were randomized to receive either active treatment (bright light therapy) plus sertraline (50 mg/d) or sham light treatment (using a dim red light) plus sertraline (50 mg/d). The investigators found a statistically significant reduction in depression score in the active treatment group compared to the sham light group, based on the HAM-D, the Hamilton 6-Item Subscale, the Melancholia Scale, and the seven atypical items from the Structured Interview Guide for the Seasonal Affective Disorder version of the HAM-D.6,7
Continue for the study summary >>
STUDY SUMMARY
Light therapy improves nonseasonal depression
This latest study was an eight-week, randomized, double-blind, placebo- and sham-controlled clinical trial evaluating the benefit of light therapy with and without pharmacotherapy for nonseasonal MDD.1 The investigators enrolled 122 adult patients (ages 19 to 60) from outpatient psychiatry clinics who had a diagnosis of MDD (diagnosed by a psychiatrist) and a HAM-D8 score of at least 20. Subjects had to be off psychotropic medication for at least two weeks prior to the first visit; they were subsequently monitored for one week to identify spontaneous responders and give patients time to better regulate their sleep-wake cycle (with the goal of sleeping only between 10 PM and 8 AM daily).
The investigators randomly assigned patients to one of four treatment groups:
• Active light monotherapy (10,000-lux fluorescent white light for 30 min/d early in the morning) plus a placebo pill
• Fluoxetine (20 mg/d) plus sham light therapy
• Placebo pills with sham light therapy; or
• Combined active light therapy with fluoxetine (20 mg/d).
Sham light therapy consisted of the use of an inactivated negative ion generator, used in the same fashion as a light box. All patients were analyzed based on modified intention to treat. Adherence was assessed through review of patients’ daily logs of device treatment times and through pill counts.
The primary outcome at eight weeks was the change from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item questionnaire with a worst score of 60.9 Secondary outcomes were treatment response (≥ 50% reduction in MADRS score) and remission (MADRS score ≤ 10) at the final eighth-week visit. MADRS scoring was used because of its sensitivity to treatment-induced changes and its high correlation with the HAM-D scale.
At the end of eight weeks, the mean changes in MADRS scores from baseline were: light monotherapy, 13.4; fluoxetine monotherapy, 8.8; combination therapy, 16.9; and placebo, 6.5. The improvement was significant in the light monotherapy treatment group and in the combination therapy group, compared with the placebo group, and in the combination group, compared with the fluoxetine treatment group; improvement was not significant for the fluoxetine treatment group compared with the placebo group, however.
The treatment response (≥ 50% MADRS improvement) rate was highest in the combination treatment group (75.9%), followed by light monotherapy (50%), placebo (33.3%), and fluoxetine monotherapy (29%). There was a significant response effect for the combination versus placebo treatment group.
Similarly, there was a higher remission rate in the combination treatment group (58.6%) than in the placebo, light monotherapy, or fluoxetine treatment groups (30%, 43.8%, and 19.4%, respectively). Combination therapy was superior to placebo in treatment response (≥ 50% reduction in the MADRS score) and remission (MADRS ≤ 10), with numbers needed to treat of 2.4 and 3.5, respectively.
By the end of the eight-week study period, 16 of 122 patients had dropped out. Two reported lack of efficacy, five reported adverse effects, and the remainder cited administrative reasons, were lost to follow-up, or withdrew consent.
WHAT’S NEW
New evidence on a not-so-new treatment
We now have evidence that bright light therapy, either alone or in combination with fluoxetine, is efficacious for increasing the remission rate of nonseasonal MDD.
Continue for caveats >>
CAVEATS
Variables may have affected results
Among the study’s limitations: use of a single SSRI (other, more potent SSRIs might work better); location (southern Canada; benefits may differ in regions farther south); and exclusion of pregnant and breastfeeding women from the study population.
Furthermore, the trial duration was relatively short, and the investigators did not attain their preplanned sample size for the study. This limited the power to detect clinically significant seasonal treatment effects and differences between the fluoxetine and placebo groups, regardless of whether they received active phototherapy.
CHALLENGES TO IMPLEMENTATION
Commercial insurance doesn’t usually cover light therapy
Bright light therapy is fairly safe, and some evidence exists supporting its use in the treatment of nonseasonal MDD; however, the data for its use in this area are limited.10 Since few studies have tested light therapy for nonseasonal MDD, uncertainty remains about patient selection, as well as optimal dose, timing, and duration in the management of nonseasonal MDD.11 Although the associated risks are minimal, bright light therapy can lead to mania or hypomania; clinicians need to monitor for such effects when initiating therapy.3
Lastly, commercial insurance does not usually cover light therapy. The average price of the bright light devices, which are available in medical supply stores and online, ranges from $118 to $237.4,11 However, such devices are reusable, making the amortized cost almost negligible and perhaps negating this concern.12
REFERENCES
1. Lam RW, Levitt AJ, Levitan RD, et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:56-63.
2. Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342:324-330.
3. Gelenberg AJ, Freeman CMP, Markowitz JC, et al. American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed July 5, 2016.
4. Lam RW, Tam EM. A Clinician’s Guide to Using Light Therapy. New York, NY: Cambridge University Press; 2009. www.ubcmood.ca/sad/SAD%20resources%20package%202009.pdf. Accessed July 5, 2016.
5. Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004;2:CD004050.
6. Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr Scand Suppl. 2004;425:7-28.
7. Martiny K, Lunde M, Unden M, et al. Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr Scand. 2005;112:117-125.
8. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
9. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
10. Oldham MA, Ciraulo DA. Use of bright light therapy among psychiatrists in Massachusetts: an e-mail survey. Prim Care Companion CNS Disord. 2014;16(3). Epub 2014 Jun 26.
11. Sloane PD, Figueiro M, Cohen L. Light as therapy for sleep disorders and depression in older adults. Clin Geriatr. 2008;16:25-31.
12. Kripke DF. A breakthrough treatment for major depression. J Clin Psychiatry. 2015;76:e660-e661.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(7):486-488.
PRACTICE CHANGER
Consider treatment with bright light therapy, alone or in combination with fluoxetine, for patients with nonseasonal major depressive disorder.1
Strength of Recommendation
B: Based on a single moderate-quality randomized controlled trial.1
A 38-year-old woman recently diagnosed with major depressive disorder (MDD) without a seasonal pattern presents to discuss treatment options. Her Hamilton Depression Rating Scale (HAM-D) score is 22, and she is not suicidal. Should you consider bright light therapy in addition to pharmacotherapy?
MDD is one of the most common psychiatric illnesses in the United States, affecting approximately one in five adults at some point in their lives.2 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are considered effective firstline pharmacotherapy options for MDD.2,3 Despite their effectiveness, however, studies have shown that only about 40% of patients with MDD achieve remission with firstline or secondline drugs.2 In addition, pharmacologic agents have a higher frequency of treatment-associated adverse effects than fluorescent light therapy.4
A Cochrane systematic review of 20 studies (N = 620) demonstrated the effectiveness of combined light therapy and pharmacotherapy in treating nonseasonal MDD but found no benefit to light used as monotherapy.5 However, the majority of the studies were of poor quality, occurred in the inpatient setting, and lasted less than four weeks.
In a five-week, controlled, double-blind trial not included in the Cochrane review, 102 patients with nonseasonal MDD were randomized to receive either active treatment (bright light therapy) plus sertraline (50 mg/d) or sham light treatment (using a dim red light) plus sertraline (50 mg/d). The investigators found a statistically significant reduction in depression score in the active treatment group compared to the sham light group, based on the HAM-D, the Hamilton 6-Item Subscale, the Melancholia Scale, and the seven atypical items from the Structured Interview Guide for the Seasonal Affective Disorder version of the HAM-D.6,7
Continue for the study summary >>
STUDY SUMMARY
Light therapy improves nonseasonal depression
This latest study was an eight-week, randomized, double-blind, placebo- and sham-controlled clinical trial evaluating the benefit of light therapy with and without pharmacotherapy for nonseasonal MDD.1 The investigators enrolled 122 adult patients (ages 19 to 60) from outpatient psychiatry clinics who had a diagnosis of MDD (diagnosed by a psychiatrist) and a HAM-D8 score of at least 20. Subjects had to be off psychotropic medication for at least two weeks prior to the first visit; they were subsequently monitored for one week to identify spontaneous responders and give patients time to better regulate their sleep-wake cycle (with the goal of sleeping only between 10 PM and 8 AM daily).
The investigators randomly assigned patients to one of four treatment groups:
• Active light monotherapy (10,000-lux fluorescent white light for 30 min/d early in the morning) plus a placebo pill
• Fluoxetine (20 mg/d) plus sham light therapy
• Placebo pills with sham light therapy; or
• Combined active light therapy with fluoxetine (20 mg/d).
Sham light therapy consisted of the use of an inactivated negative ion generator, used in the same fashion as a light box. All patients were analyzed based on modified intention to treat. Adherence was assessed through review of patients’ daily logs of device treatment times and through pill counts.
The primary outcome at eight weeks was the change from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item questionnaire with a worst score of 60.9 Secondary outcomes were treatment response (≥ 50% reduction in MADRS score) and remission (MADRS score ≤ 10) at the final eighth-week visit. MADRS scoring was used because of its sensitivity to treatment-induced changes and its high correlation with the HAM-D scale.
At the end of eight weeks, the mean changes in MADRS scores from baseline were: light monotherapy, 13.4; fluoxetine monotherapy, 8.8; combination therapy, 16.9; and placebo, 6.5. The improvement was significant in the light monotherapy treatment group and in the combination therapy group, compared with the placebo group, and in the combination group, compared with the fluoxetine treatment group; improvement was not significant for the fluoxetine treatment group compared with the placebo group, however.
The treatment response (≥ 50% MADRS improvement) rate was highest in the combination treatment group (75.9%), followed by light monotherapy (50%), placebo (33.3%), and fluoxetine monotherapy (29%). There was a significant response effect for the combination versus placebo treatment group.
Similarly, there was a higher remission rate in the combination treatment group (58.6%) than in the placebo, light monotherapy, or fluoxetine treatment groups (30%, 43.8%, and 19.4%, respectively). Combination therapy was superior to placebo in treatment response (≥ 50% reduction in the MADRS score) and remission (MADRS ≤ 10), with numbers needed to treat of 2.4 and 3.5, respectively.
By the end of the eight-week study period, 16 of 122 patients had dropped out. Two reported lack of efficacy, five reported adverse effects, and the remainder cited administrative reasons, were lost to follow-up, or withdrew consent.
WHAT’S NEW
New evidence on a not-so-new treatment
We now have evidence that bright light therapy, either alone or in combination with fluoxetine, is efficacious for increasing the remission rate of nonseasonal MDD.
Continue for caveats >>
CAVEATS
Variables may have affected results
Among the study’s limitations: use of a single SSRI (other, more potent SSRIs might work better); location (southern Canada; benefits may differ in regions farther south); and exclusion of pregnant and breastfeeding women from the study population.
Furthermore, the trial duration was relatively short, and the investigators did not attain their preplanned sample size for the study. This limited the power to detect clinically significant seasonal treatment effects and differences between the fluoxetine and placebo groups, regardless of whether they received active phototherapy.
CHALLENGES TO IMPLEMENTATION
Commercial insurance doesn’t usually cover light therapy
Bright light therapy is fairly safe, and some evidence exists supporting its use in the treatment of nonseasonal MDD; however, the data for its use in this area are limited.10 Since few studies have tested light therapy for nonseasonal MDD, uncertainty remains about patient selection, as well as optimal dose, timing, and duration in the management of nonseasonal MDD.11 Although the associated risks are minimal, bright light therapy can lead to mania or hypomania; clinicians need to monitor for such effects when initiating therapy.3
Lastly, commercial insurance does not usually cover light therapy. The average price of the bright light devices, which are available in medical supply stores and online, ranges from $118 to $237.4,11 However, such devices are reusable, making the amortized cost almost negligible and perhaps negating this concern.12
REFERENCES
1. Lam RW, Levitt AJ, Levitan RD, et al. Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:56-63.
2. Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342:324-330.
3. Gelenberg AJ, Freeman CMP, Markowitz JC, et al. American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. 3rd ed. 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed July 5, 2016.
4. Lam RW, Tam EM. A Clinician’s Guide to Using Light Therapy. New York, NY: Cambridge University Press; 2009. www.ubcmood.ca/sad/SAD%20resources%20package%202009.pdf. Accessed July 5, 2016.
5. Tuunainen A, Kripke DF, Endo T. Light therapy for non-seasonal depression. Cochrane Database Syst Rev. 2004;2:CD004050.
6. Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr Scand Suppl. 2004;425:7-28.
7. Martiny K, Lunde M, Unden M, et al. Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr Scand. 2005;112:117-125.
8. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
9. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
10. Oldham MA, Ciraulo DA. Use of bright light therapy among psychiatrists in Massachusetts: an e-mail survey. Prim Care Companion CNS Disord. 2014;16(3). Epub 2014 Jun 26.
11. Sloane PD, Figueiro M, Cohen L. Light as therapy for sleep disorders and depression in older adults. Clin Geriatr. 2008;16:25-31.
12. Kripke DF. A breakthrough treatment for major depression. J Clin Psychiatry. 2015;76:e660-e661.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(7):486-488.
On-demand pill protocol protects against HIV
Offer patients at high risk for human immunodeficiency virus (HIV), particularly men who have sex with men, preexposure prophylaxis (PrEP) with a combination pill of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) on an on-demand basis to decrease HIV-1 infection rates.
Strength of recommendation
B: Based on one good quality randomized control trial.1
Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
ILLUSTRATIVE CASE
Your patient is a 31-year-old man who has sex with men. He is sexually active with several different partners. He asks you if there is anything he can do to decrease his risk of becoming infected with human immunodeficiency virus (HIV). Besides recommending condom use, what should you offer him?
In most high-income countries, including the United States, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 Without a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the Centers for Disease Control and Prevention (CDC) began recommending daily use of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) in high-risk individuals, as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT]=46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably because of the difficulty in getting patients to take the medication on a daily basis.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 That is because, until now, there have been no studies demonstrating the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays (IPERGAY) study was a double-blind, multicenter study conducted in France and Canada that assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis was that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk of HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection. The investigators defined high risk as having a history of unprotected anal sex with at least 2 partners in the previous 6 months. Other inclusion criteria included age ≥18 years, and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance <60 mL/min, alanine aminotransferase level >2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. After excluding those who withdrew consent, were lost to follow-up, or who acquired HIV-1 infection, the study participants (199 in the TDF-FTC group and 201 in the placebo group) were randomized to take TDF-FTC or placebo before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. The participants were instructed to take a loading dose of 2 pills of TDF-FTC or placebo with food 2 to 24 hours prior to intercourse, followed by a third pill 24 hours after taking the first 2 pills, and a fourth pill 24 hours after the third pill. If there were multiple consecutive days with episodes of sexual intercourse, participants were to take one pill on each of the days of intercourse, and then the 2 post-exposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the preexposure prophylaxis; otherwise, they were to begin again with 2 pills 2 to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants 4 and 8 weeks after enrollment, and then every 8 weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count and drug levels in plasma, as well as with an at-home, computer-assisted interview completed by each participant prior to each visit.
Participants received counseling from a peer community member and were offered preventative services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took the pills correctly. The participants were followed for a median of 9.3 months. Overall, 72% of the participants took the study drugs (TDF-FTC or placebo), although 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. The study was unblinded after 20 months and is continuing as an open-label study because of the discontinuation of another preexposure prophylaxis study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk of contracting HIV without PrEP. The open-label part of the study, iPrex-OLE, completed enrollment and data gathering in November 2013, and the data analysis and results are presently pending.9
Eighty-six percent relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with 3 of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group (6.6 infections per 100 person-years) and 2 of the new cases were in the TDF-FTC group (incidence 0.91 per 100 person-years). This translated to a relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group of 86% (95% confidence interval, 40%-98%; P=.002; NNT=17 over 9.3 months).
The 2 study participants in the TDF-FTC group diagnosed with new HIV-1 were found to be non-adherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain and were seen at a greater rate (14% vs 5%, P=.002; number needed to harm=11) in the treatment group than in the placebo group. There were also mild increases in serum creatinine level (seen in 18% of the TDF-FTC group), but only 2 participants had a transient decrease in creatinine clearance to <60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction with on-demand use is nearly double that of daily use
This is the first study to look at on-demand preexposure prophylaxis with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the higher benefit of on-demand PrEP is likely due to increased compliance with medication use.
CAVEATS
Is fewer pills enough to maintain adherence over time?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we will need to wait for the results of the open-label study to fully assess the risks of adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of TDF-FTC, the retail price of which is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. A clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. Accessed June 4, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. IPrEx open-label extension. Available at: http://www.iprexnews.com. Accessed July 13, 2016.
10. GoodRx. Truvada. Available at: https://www.goodrx.com/truvada. Accessed June 4, 2016.
Offer patients at high risk for human immunodeficiency virus (HIV), particularly men who have sex with men, preexposure prophylaxis (PrEP) with a combination pill of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) on an on-demand basis to decrease HIV-1 infection rates.
Strength of recommendation
B: Based on one good quality randomized control trial.1
Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
ILLUSTRATIVE CASE
Your patient is a 31-year-old man who has sex with men. He is sexually active with several different partners. He asks you if there is anything he can do to decrease his risk of becoming infected with human immunodeficiency virus (HIV). Besides recommending condom use, what should you offer him?
In most high-income countries, including the United States, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 Without a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the Centers for Disease Control and Prevention (CDC) began recommending daily use of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) in high-risk individuals, as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT]=46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably because of the difficulty in getting patients to take the medication on a daily basis.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 That is because, until now, there have been no studies demonstrating the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays (IPERGAY) study was a double-blind, multicenter study conducted in France and Canada that assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis was that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk of HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection. The investigators defined high risk as having a history of unprotected anal sex with at least 2 partners in the previous 6 months. Other inclusion criteria included age ≥18 years, and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance <60 mL/min, alanine aminotransferase level >2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. After excluding those who withdrew consent, were lost to follow-up, or who acquired HIV-1 infection, the study participants (199 in the TDF-FTC group and 201 in the placebo group) were randomized to take TDF-FTC or placebo before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. The participants were instructed to take a loading dose of 2 pills of TDF-FTC or placebo with food 2 to 24 hours prior to intercourse, followed by a third pill 24 hours after taking the first 2 pills, and a fourth pill 24 hours after the third pill. If there were multiple consecutive days with episodes of sexual intercourse, participants were to take one pill on each of the days of intercourse, and then the 2 post-exposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the preexposure prophylaxis; otherwise, they were to begin again with 2 pills 2 to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants 4 and 8 weeks after enrollment, and then every 8 weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count and drug levels in plasma, as well as with an at-home, computer-assisted interview completed by each participant prior to each visit.
Participants received counseling from a peer community member and were offered preventative services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took the pills correctly. The participants were followed for a median of 9.3 months. Overall, 72% of the participants took the study drugs (TDF-FTC or placebo), although 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. The study was unblinded after 20 months and is continuing as an open-label study because of the discontinuation of another preexposure prophylaxis study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk of contracting HIV without PrEP. The open-label part of the study, iPrex-OLE, completed enrollment and data gathering in November 2013, and the data analysis and results are presently pending.9
Eighty-six percent relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with 3 of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group (6.6 infections per 100 person-years) and 2 of the new cases were in the TDF-FTC group (incidence 0.91 per 100 person-years). This translated to a relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group of 86% (95% confidence interval, 40%-98%; P=.002; NNT=17 over 9.3 months).
The 2 study participants in the TDF-FTC group diagnosed with new HIV-1 were found to be non-adherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain and were seen at a greater rate (14% vs 5%, P=.002; number needed to harm=11) in the treatment group than in the placebo group. There were also mild increases in serum creatinine level (seen in 18% of the TDF-FTC group), but only 2 participants had a transient decrease in creatinine clearance to <60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction with on-demand use is nearly double that of daily use
This is the first study to look at on-demand preexposure prophylaxis with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the higher benefit of on-demand PrEP is likely due to increased compliance with medication use.
CAVEATS
Is fewer pills enough to maintain adherence over time?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we will need to wait for the results of the open-label study to fully assess the risks of adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of TDF-FTC, the retail price of which is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Offer patients at high risk for human immunodeficiency virus (HIV), particularly men who have sex with men, preexposure prophylaxis (PrEP) with a combination pill of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) on an on-demand basis to decrease HIV-1 infection rates.
Strength of recommendation
B: Based on one good quality randomized control trial.1
Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
ILLUSTRATIVE CASE
Your patient is a 31-year-old man who has sex with men. He is sexually active with several different partners. He asks you if there is anything he can do to decrease his risk of becoming infected with human immunodeficiency virus (HIV). Besides recommending condom use, what should you offer him?
In most high-income countries, including the United States, HIV-1 infection continues to occur in high-risk groups, especially among men who have sex with men (MSM).2 Without a vaccine, condom use has served as the primary method of preventing infection.
In 2014, the Centers for Disease Control and Prevention (CDC) began recommending daily use of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) in high-risk individuals, as a form of preexposure prophylaxis (PrEP).3-5 This recommendation is based primarily on the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a relative reduction of 44% (number needed to treat [NNT]=46 over 1.2 years) in the incidence of new HIV-1 infection among men and transgender women who have sex with men when TDF-FTC was used on a daily basis.6 However, the effectiveness of this strategy in the real world has not been as high as hoped, presumably because of the difficulty in getting patients to take the medication on a daily basis.7,8
While it would likely improve adherence rates, the use of prophylaxis in an on-demand manner is not currently recommended.5 That is because, until now, there have been no studies demonstrating the effectiveness of PrEP used episodically and taken only around the time of potential exposure.
STUDY SUMMARY
Fewer pills improves adherence, reduces HIV infection rates
The Intervention Preventive de l’Exposition aux Risques avec et pour les Gays (IPERGAY) study was a double-blind, multicenter study conducted in France and Canada that assessed the efficacy and safety of prophylaxis with TDF-FTC used in an on-demand fashion by MSM.1 The study hypothesis was that adherence would be higher if chemoprophylaxis was taken only around the time of intercourse, rather than daily, and that this would further reduce the risk of HIV infection.
The study randomized 414 participants who were considered to be at high risk for acquiring HIV-1 infection. The investigators defined high risk as having a history of unprotected anal sex with at least 2 partners in the previous 6 months. Other inclusion criteria included age ≥18 years, and male or transgender female sex. Exclusion criteria included current HIV infection, hepatitis B or C infection, creatinine clearance <60 mL/min, alanine aminotransferase level >2.5 times the upper limit of normal, and significant glycosuria or proteinuria.
The pill and visit schedule. After excluding those who withdrew consent, were lost to follow-up, or who acquired HIV-1 infection, the study participants (199 in the TDF-FTC group and 201 in the placebo group) were randomized to take TDF-FTC or placebo before and after sexual activity. The dose of TDF-FTC was fixed at 300 mg of TDF and 200 mg of FTC per pill. The participants were instructed to take a loading dose of 2 pills of TDF-FTC or placebo with food 2 to 24 hours prior to intercourse, followed by a third pill 24 hours after taking the first 2 pills, and a fourth pill 24 hours after the third pill. If there were multiple consecutive days with episodes of sexual intercourse, participants were to take one pill on each of the days of intercourse, and then the 2 post-exposure pills. If sexual activity resumed within a week of the prior episode, participants were instructed to take only one pill when resuming the preexposure prophylaxis; otherwise, they were to begin again with 2 pills 2 to 24 hours prior to intercourse and repeat the protocol.
Study coordinators followed participants 4 and 8 weeks after enrollment, and then every 8 weeks subsequently. The investigators tested the participants for HIV-1 and HIV-2 at each visit and assessed adherence by pill count and drug levels in plasma, as well as with an at-home, computer-assisted interview completed by each participant prior to each visit.
Participants received counseling from a peer community member and were offered preventative services and testing for other sexually transmitted infections. They were given free condoms and gel at each visit, as well as enough pills (TDF-FTC or placebo) to cover daily use until their next visit.
Forty-three percent took the pills correctly. The participants were followed for a median of 9.3 months. Overall, 72% of the participants took the study drugs (TDF-FTC or placebo), although 29% took a suboptimal dose. There was no change in the sexual behavior of the participants during the study. The study was unblinded after 20 months and is continuing as an open-label study because of the discontinuation of another preexposure prophylaxis study in the United Kingdom, which showed an NNT of 13 to prevent one new HIV infection per year.3
An independent data and safety monitoring board recommended the unblinding because the placebo group was considered to be at significantly increased risk of contracting HIV without PrEP. The open-label part of the study, iPrex-OLE, completed enrollment and data gathering in November 2013, and the data analysis and results are presently pending.9
Eighty-six percent relative reduction in HIV. The primary end-point was the diagnosis of HIV-1 infection, and the results were based on an intention-to-treat analysis. HIV-1 infection was diagnosed in 19 study participants, with 3 of those new cases occurring between the time of randomization and enrollment. Fourteen of the cases were in the placebo group (6.6 infections per 100 person-years) and 2 of the new cases were in the TDF-FTC group (incidence 0.91 per 100 person-years). This translated to a relative reduction in the incidence of new HIV-1 seroconversion in the TDF-FTC group of 86% (95% confidence interval, 40%-98%; P=.002; NNT=17 over 9.3 months).
The 2 study participants in the TDF-FTC group diagnosed with new HIV-1 were found to be non-adherent to the prescribed prophylaxis, as they returned 58 and 60 of the 60 pills administered to them, and no study drugs were found in their plasma samples.
Adverse events included gastrointestinal symptoms of nausea, vomiting, diarrhea, and abdominal pain and were seen at a greater rate (14% vs 5%, P=.002; number needed to harm=11) in the treatment group than in the placebo group. There were also mild increases in serum creatinine level (seen in 18% of the TDF-FTC group), but only 2 participants had a transient decrease in creatinine clearance to <60 mL/min. None of the participants discontinued medications due to renal issues.
WHAT’S NEW
Risk reduction with on-demand use is nearly double that of daily use
This is the first study to look at on-demand preexposure prophylaxis with TDF-FTC to decrease the incidence of HIV-1 infection in high-risk MSM. The risk reduction in this study (86%) was much better than the 44% seen in the prior study that used daily PrEP in this population.6 We suspect the higher benefit of on-demand PrEP is likely due to increased compliance with medication use.
CAVEATS
Is fewer pills enough to maintain adherence over time?
The median length of follow-up in the study was 9.3 months. One concern is that adherence may wane over time, decreasing the efficacy of the prophylaxis. Continued efforts to improve compliance with this type of PrEP may be needed to ensure efficacy. Since the study was shortened and reported early, we will need to wait for the results of the open-label study to fully assess the risks of adverse events.
CHALLENGES TO IMPLEMENTATION
Efficacy and convenience come at a cost
The main challenge to implementation could be the cost of TDF-FTC, the retail price of which is about $50 per dose.10 Insurance coverage for the medication varies.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. A clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. Accessed June 4, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. IPrEx open-label extension. Available at: http://www.iprexnews.com. Accessed July 13, 2016.
10. GoodRx. Truvada. Available at: https://www.goodrx.com/truvada. Accessed June 4, 2016.
1. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237-2246.
2. Beyrer C, Sullivan P, Sanchez J, et al. The increase in global HIV epidemics in MSM. AIDS. 2013;27:2665-2678.
3. McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387:53-60.
4. Youle M, Wainberg MA. Could chemoprophylaxis be used as an HIV prevention strategy while we wait for an effective vaccine? AIDS. 2003;17:937-938.
5. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States – 2014. A clinical practice guideline. Available at: https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf. Accessed June 4, 2016.
6. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599.
7. Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509-518.
8. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411-422.
9. IPrEx open-label extension. Available at: http://www.iprexnews.com. Accessed July 13, 2016.
10. GoodRx. Truvada. Available at: https://www.goodrx.com/truvada. Accessed June 4, 2016.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.