User login
Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.
Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
When medical care is based on consistent, good-quality evidence, most physicians adopt it. However, not all care is well supported by the literature and may, in fact, be overused without offering benefit to patients. Choosing Wisely, at www.choosingwisely.org, is a health care initiative that highlights screening and testing recommendations from specialty societies in an effort to encourage patients and clinicians to talk about how to make high-value, effective health care decisions and avoid overuse. (See “Test and Tx overutilization: A bigger problem than you might think"1-3).
SIDEBAR
Test and Tx overutilization: A bigger problem than you might think
Care that isn’t backed up by the medical literature is adopted by some physicians and not adopted by others, leading to practice variations. Some variation is to be expected, since no 2 patients require exactly the same care, but substantial variations may be a clue to overuse.
A 2006 analysis of inpatient lab studies found that doctors ordered an average of 2.96 studies per patient per day, but only 29% of these tests (0.95 test/patient/day) contributed to management.1 A 2016 systematic review found more than 800 studies on overuse were published in a single year.2 One study of thyroid nodules followed almost 1000 patients with nodules as they underwent routine follow-up imaging. At the end of the study, 7 were found to have cancer, but of those, only 3 had enlarging or changing nodules that would have been detected with the follow-up imaging being studied. Three of the cancers were stable in size and 1 was found incidentally.3
Enabling physician and patient dialogue. The initiative began in 2010 when the American Board of Internal Medicine convened a panel of experts to identify low-value tests and therapies. Their list took the form of a “Top Five Things” that may not be high value in patient care, and it used language tailored to patients and physicians so that they could converse meaningfully. Physicians could use the evidence to make a clinical decision, and patients could feel empowered to ask informed questions about recommendations they received. The initiative has now expanded to include ways that health care systems can reduce low-value interventions.

Scope of participation. Since the first Choosing Wisely recommendations were published in 2013, more than 80 professional associations have contributed lists of their own. Professional societies participate voluntarily. The American Academy of Family Physicians (AAFP), Society of General Internal Medicine, and American Academy of Pediatrics (AAP) have contributed lists relevant to primary care. All Choosing Wisely recommendations can be searched or sorted by specialty organization. Recommendations are reviewed and revised regularly. If the evidence becomes conflicted or contradictory, recommendations are withdrawn.
Making meaningful improvements by Choosing Wisely
Several studies have shown that health care systems can implement Choosing Wisely recommendations to reduce overuse of unnecessary tests. A 2015 study examined the effect of applying a Choosing Wisely recommendation to reduce the use of continuous pulse oximetry in pediatric inpatients with asthma, wheezing, or bronchiolitis. The recommendation, from the Society of Hospital Medicine–Pediatric Hospital Medicine, advises against continuous pulse oximetry in children with acute respiratory illnesses unless the child is using supplemental oxygen.4 This study, done at the Cincinnati Children’s Hospital Medical Center, found that within 3 months of initiating a protocol on all general pediatrics floors, the average time on pulse oximetry after meeting clinical goals decreased from 10.7 hours to 3.1 hours. In addition, the percentage of patients who had their continuous pulse oximetry stopped within 2 hours of clinical stability (a goal time) increased from 25% to 46%.5
Patients are important drivers of health care utilization. A 2003 study showed that physicians are more likely to order referrals, tests, and prescriptions when patients ask for them, and that nearly 1 in 4 patients did so.6 A 2002 study found that physicians granted all but 3% of patient’s requests for orders or tests, and that fulfilling requests correlated with patient satisfaction in the specialty office studied (cardiology) but not in the primary care (internal medicine) office.7
From its inception, Choosing Wisely has considered patients as full partners in conversations about health care utilization. Choosing Wisely partners with Consumer Reports to create and disseminate plain-language summaries of recommendations. Community groups and physician organizations have also participated in implementation efforts. In 2018, Choosing Wisely secured a grant to expand outreach to diverse or underserved communities.
Choosing Wisely recommendations are not guidelines or mandates. They are intended to be evidence-based advice from a specialty society to its members and to patients about care that is often unnecessary. The goal is to create a conversation and not to eliminate these services from ever being offered or used.
Continue to: Improve your practice with these 10 primary care recommendations
Improve your practice with these 10 primary care recommendations
1 Avoid imaging studies in early acute low back pain without red flags.
Both the AAFP and the American Society of Anesthesiologists recommend against routine X-rays, magnetic resonance imaging, and computed tomography (CT) scans in the first 6 weeks of acute low back pain (LBP).8,9 The American College of Emergency Physicians (ACEP) recommends against routine lumbar spine imaging for emergency department (ED) patients.10 In all cases, imaging is indicated if the patient has any signs or symptoms of neurologic deficits or other indications, such as signs of spinal infection or fracture. However, as ACEP notes, diagnostic imaging does not typically help identify the cause of acute LBP, and when it does, it does not reduce the time to symptom improvement.10
2 Prescribe oral contraceptives on the basis of a medical history and a blood pressure measurement. No routine pelvic exam or other physical exam is necessary.
This AAFP recommendation11 is based on clinical practice guidelines from the American College of Obstetricians and Gynecologists (ACOG) and other research.12 The ACOG practice guideline supports provision of hormonal contraception without a pelvic exam, cervical cancer (Pap) testing, urine pregnancy testing, or testing for sexually transmitted infections. ACOG guidelines also support over-the-counter provision of hormonal contraceptives, including combined oral contraceptives.12
3 Stop recommending daily self-glucose monitoring for patients with diabetes who are not using insulin.
Both the AAFP and the Society for General Internal Medicine recommend against daily blood sugar checks for people who do not use insulin.13,14 A Cochrane review of 9 trials (3300 patients) found that after 6 months, hemoglobin A1C was reduced by 0.3% in people who checked their sugar daily compared with those who did not, but this difference was not significant after a year.15 Hypoglycemic episodes were more common in the “checking” group, and there were no differences in quality of life. A qualitative study found that blood sugar results had little impact on patients’ motivation to change behavior.16
4 Don’t screen for herpes simplex virus (HSV) infection in asymptomatic adults, even those who are pregnant.
This AAFP recommendation17 comes from a US Preventive Services Task Force (USPSTF) Grade D recommendation.18 Most people with positive HSV-2 serology have had an outbreak; even those who do not think they have had one will realize that they had the symptoms once they hear them described.18 With available tests, 1 in 2 positive results for HSV-2 among asymptomatic people will be a false-positive.18
There is no known cure, intervention, or reduction in transmission for infected patients who do not have symptoms.18 Also, serologically detected HSV-2 does not reliably predict genital herpes; and HSV-1 has been found to cause an increasing percentage of genital infection cases.18
Continue to: 5 Don't screen for testicular cancer in asymptomatic individuals
5 Don’t screen for testicular cancer in asymptomatic individuals.
This AAFP recommendation19 also comes from a USPSTF Grade D recommendation.20 A 2010 systematic review found no evidence to support screening of asymptomatic people with a physical exam or ultrasound. All available studies involved symptomatic patients.20
6 Stop recommending cough and cold medicines for children younger than 4 years.
The AAP recommends that clinicians discourage the use of any cough or cold medicine for children in this age-group.21 A 2008 study found that more than 7000 children annually presented to EDs for adverse events from cough and cold medicines.22 Previous studies found no benefit in reducing symptoms.23 In children older than 12 months, a Cochrane review found that honey has a modest benefit for cough in single-night trials.24
7 Avoid performing serum allergy panels.
The American Academy of Allergy, Asthma, and Immunology discourages the use of serum panel testing when patients present with allergy symptoms.25 A patient can have a strong positive immunoglobulin E (IgE) serum result to an allergen and have no clinical allergic symptoms or can have a weak positive serum result and a strong clinical reaction. Targeted skin or serum IgE testing—for example, testing for cashew allergy in a patient known to have had a reaction after eating one—is reasonable.26
8 Avoid routine electroencephalography (EEG), head CT, and carotid ultrasound as initial work-up for simple syncope in adults.
These recommendations, from the American Epilepsy Society,27 ACEP,28 American College of Physicians,29 and American Academy of Neurology (AAN),30 emphasize the low yield of routine work-ups for patients with simple syncope. The AAN notes that 40% of people will experience syncope during adulthood and most will not have carotid disease, which generally manifests with stroke-like symptoms rather than syncope. One study found that approximately 1 in 8 patients referred to an epilepsy clinic had neurocardiogenic syncope rather than epilepsy.31
EEGs have high false-negative and false-positive rates, and history-taking is a better tool with which to make a diagnosis. CT scans performed in the ED were found to contribute to the diagnosis of simple syncope in fewer than 2% of cases of syncope, compared with orthostatic blood pressure (25% of cases).32
Continue to: 9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age
9 Wait to refer children with umbilical hernias to pediatric surgery until they are 4 to 5 years of age.
The AAP Section on Surgery offers evidence that the risk-benefit analysis strongly favors waiting on intervention.33 About 1 in 4 children will have an umbilical hernia, and about 85% of cases will resolve by age 5. The strangulation rate with umbilical hernias is very low, and although the risk of infection with surgery is likewise low, the risk of recurrence following surgery before the age of 4 is as high as 2.4%.34 The AAP Section on Surgery recommends against strapping or restraining the hernia, as well.
10 Avoid using appetite stimulants, such as megesterol, and high-calorie nutritional supplements to treat anorexia and cachexia in older adults.
Instead, the American Geriatrics Society recommends that physicians encourage caregivers to serve appealing food, provide support with eating, and remove barriers to appetite and nutrition.35 A Cochrane review showed that high-calorie supplements, such as Boost or Ensure, are associated with very modest weight gain—about 2% of weight—but are not associated with an increased life expectancy or improved quality of life.36
Prescription appetite stimulants are associated with adverse effects and yield inconsistent benefits in older adults. Megesterol, for example, was associated with headache, gastrointestinal adverse effects, insomnia, weakness, and fatigue. Mirtazapine is associated with sedation and fatigue.37
CORRESPONDENCE
Kathleen Rowland, MD, MS, Rush Copley Family Medicine Residency, Rush Medical College, 600 South Paulina, Kidston House Room 605, Chicago IL 60612; [email protected].
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
1. Miyakis S, Karamanof G, Liontos M, et al. Factors contributing to inappropriate ordering of tests in an academic medical department and the effect of an educational feedback strategy. Postgrad Med J. 2006;82:823-829.
2. Morgan DJ, Dhruva SS, Wright SM, et al. Update on medical overuse: a systematic review. JAMA Intern Med. 2016;176:1687-1692.
3. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926-935.
4. Choosing Wisely. Society of Hospital Medicine—Pediatric hospital medicine. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. www.choosingwisely.org/clinician-lists/society-hospital-medicine-pediatric-continuous-pulse-oximetry-in-children-with-acute-respiratory-illness/. Accessed September 28, 2020.
5. Schondelmeyer AC, Simmons JM, Statile AM, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135:e1044-e1051.
6. Kravitz RL, Bell RA, Azari R, et al. Direct observation of requests for clinical services in office practice: what do patients want and do they get it? Arch Intern Med. 2003;163:1673-1681.
7. Kravitz RL, Bell RA, Franz CE, et al. Characterizing patient requests and physician responses in office practice. Health Serv Res. 2002;37:217-238.
8. Choosing Wisely. American Academy of Family Physicians. Don’t do imaging for low back pain within the first six weeks, unless red flags are present. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-imaging-low-back-pain/. Accessed September 28, 2020.
9. Choosing Wisely. American Society of Anesthesiologists–Pain Medicine. Avoid imaging studies (MRI, CT or X-rays) for acute low back pain without specific indications. www.choosingwisely.org/clinician-lists/american-society-anesthesiologists-imaging-studies-for-acute-low-back-pain/. Accessed September 28, 2020.
10. Choosing Wisely. American College of Emergency Physicians. Avoid lumbar spine imaging in the emergency department for adults with non-traumatic back pain unless the patient has severe or progressive neurologic deficits or is suspected of having a serious underlying condition (such as vertebral infection, cauda equina syndrome, or cancer with bony metastasis). www.choosingwisely.org/clinician-lists/acep-lumbar-spine-imaging-in-the-ed/. Accessed September 28, 2020.
11. Choosing Wisely. American Academy of Family Physicians. Don’t require a pelvic exam or other physical exam to prescribe oral contraceptive medications. www.choosingwisely.org/clinician-lists/american-academy-family-physicians-pelvic-or-physical-exams-to-prescribe-oral-contraceptives/. Accessed September 28, 2020.
12. Over-the-counter access to hormonal contraception. ACOG Committee Opinion, Number 788. Obstet Gynecol. 2019;134:e96-e105. https://journals.lww.com/greenjournal/Fulltext/2019/10000/Over_the_Counter_Access_to_Hormonal_Contraception_.46.aspx. Accessed September 28, 2020.
13. Choosing Wisely. American Academy of Family Physicians. Don’t routinely recommend daily home glucose monitoring for patients who have Type 2 diabetes mellitus and are not using insulin. www.choosingwisely.org/clinician-lists/aafp-daily-home-glucose-monitoring-for-patients-with-type-2-diabetes. Accessed September 28, 2020.
14. Choosing Wisely. Society of General Internal Medicine. Don’t recommend daily home finger glucose testing in patients with Type 2 diabetes mellitus not using insulin. www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed September 28, 2020.
15. Malanda UL, Welschen LM, Riphagen II, et al. Self‐monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012(1):CD005060.
16. Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335:493.
17. Choosing Wisely. American Academy of Family Physicians. Don’t screen for genital herpes simplex virus infection (HSV) in asymptomatic adults, including pregnant women. www.choosingwisely.org/clinician-lists/aafp-genital-herpes-screening-in-asymptomatic-adults/. Accessed September 28, 2020.
18. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530.
19. Choosing Wisely. American Academy of Family Physicians. Don’t screen for testicular cancer in asymptomatic adolescent and adult males. www.choosingwisely.org/clinician-lists/aafp-testicular-cancer-screening-in-asymptomatic-adolescent-and-adult-men/. Accessed September 28, 2020.
20. Lin K, Sharangpani R. Screening for testicular cancer: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2010;153:396-399.
21. Choosing Wisely. American Academy of Pediatrics. Cough and cold medicines should not be prescribed, recommended or used for respiratory illnesses in young children. www.choosingwisely.org/clinician-lists/american-academy-pediatrics-cough-and-cold-medicines-for-children-under-four/. Accessed September 28, 2020.
22. Schaefer MK, Shehab N, Cohen AL, et al. Adverse events from cough and cold medications in children. Pediatrics. 2008;121:783-787.
23. Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18:184-188.
24. Oduwole O, Udoh EE, Oyo‐Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev. 2018(4):CD007094.
25. Choosing Wisely. American Academy of Allergy, Asthma & Immunology. Don’t perform unproven diagnostic tests, such as immunoglobulin G(lgG) testing or an indiscriminate battery of immunoglobulin E(lgE) tests, in the evaluation of allergy. www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunology-diagnostic-tests-for-allergy-evaluation/. Accessed September 28, 2020.
26. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: report from the American College of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol. 2008;101:580-592.
27. Choosing Wisely. American Epilepsy Society. Do not routinely order electroencephalogram (EEG) as part of initial syncope work-up. www.choosingwisely.org/clinician-lists/aes-eeg-as-part-of-initial-syncope-work-up/. Accessed September 28, 2020.
28. Choosing Wisely. American College of Emergency Physicians. Avoid CT of the head in asymptomatic adult patients in the emergency department with syncope, insignificant trauma and a normal neurological evaluation. www.choosingwisely.org/clinician-lists/acep-avoid-head-ct-for-asymptomatic-adults-with-syncope/. Accessed September 28, 2020.
29. Choosing Wisely. American College of Physicians. In the evaluation of simple syncope and a normal neurological examination, don’t obtain brain imaging studies (CT or MRI). www.choosingwisely.org/clinician-lists/american-college-physicians-brain-imaging-to-evaluate-simple-syncope/. Accessed September 28, 2020.
30. Choosing Wisely. American Academy of Neurology. Don’t perform imaging of the carotid arteries for simple syncope without other neurologic symptoms. www.choosingwisely.org/clinician-lists/american-academy-neurology-carotid-artery-imaging-for-simple-syncope/. Accessed September 28, 2020.
31. Josephson CB, Rahey S, Sadler RM. Neurocardiogenic syncope: frequency and consequences of its misdiagnosis as epilepsy. Can J Neurol Sci. 2007;34:221-224.
32. Mendu ML, McAvay G, Lampert R, et al. Yield of diagnostic tests in evaluating syncopal episodes in older patients. Arch Intern Med. 2009;169:1299-1305.
33. Choosing Wisely. American Academy of Pediatrics–Section on Surgery. Avoid referring most children with umbilical hernias to a pediatric surgeon until around age 4-5 years. www.choosingwisely.org/clinician-lists/aap-sosu-avoid-surgery-referral-for-umbilical-hernias-until-age-4-5/. Accessed September 28, 2020.
34. Antonoff MB, Kreykes NS, Saltzman DA, et al. American Academy of Pediatrics Section on Surgery hernia survey revisited. J Pediatr Surg. 2005;40:1009-1014.
35. Choosing Wisely. American Geriatrics Society. Avoid using prescription appetite stimulants or high-calorie supplements for treatment of anorexia or cachexia in older adults; instead, optimize social supports, discontinue medications that may interfere with eating, provide appealing food and feeding assistance, and clarify patient goals and expectations. www.choosingwisely.org/clinician-lists/american-geriatrics-society-prescription-appetite-stimulants-to-treat-anorexia-cachexia-in-elderly/. Accessed September 28, 2020.
36. Milne AC, Potter J, Vivanti A, et al. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Sys Rev. 2009(2):CD003288.
37. Fox CB, Treadway AK, Blaszczyk AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383-397.
Fever, petechiae, and joint pain
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.
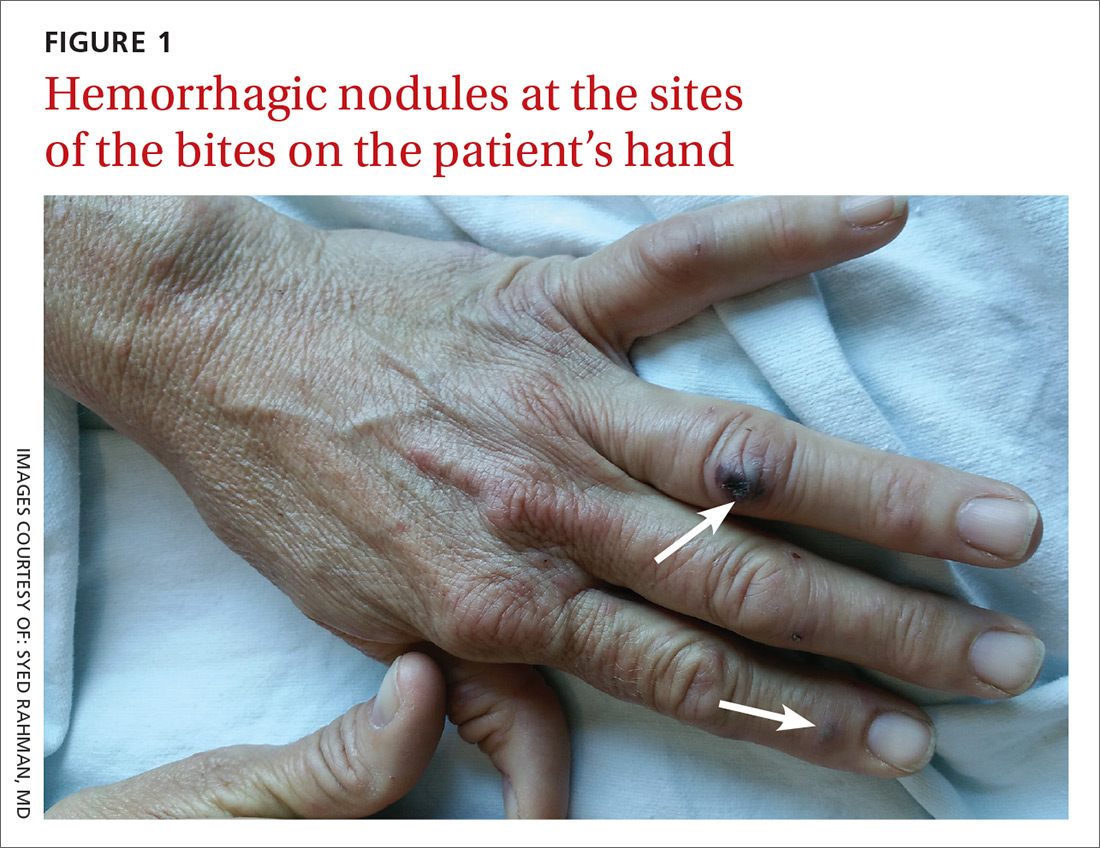
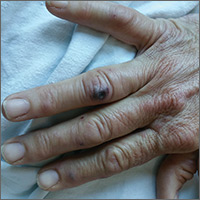
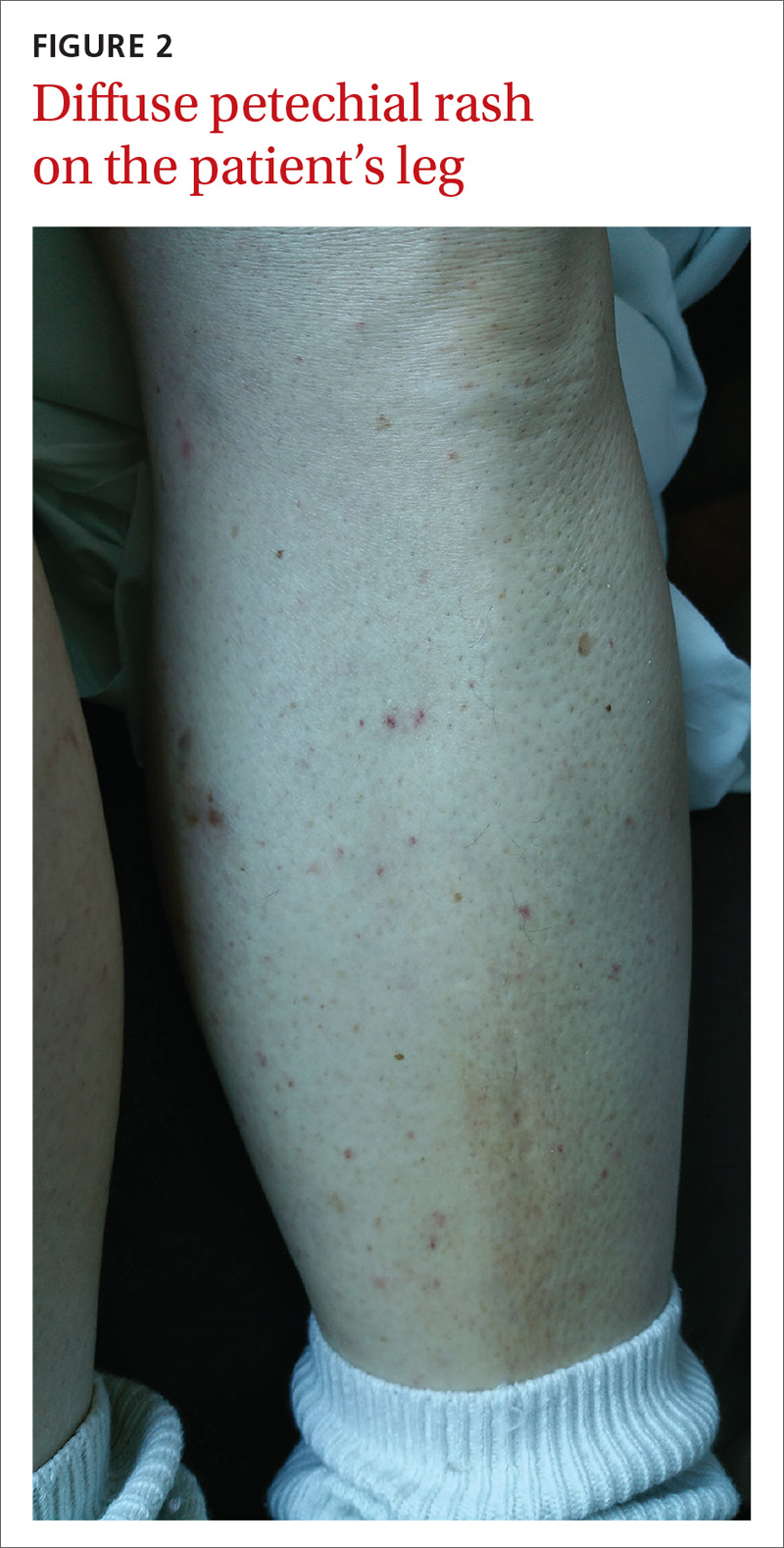
The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.
The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.

The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.

The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
A 59-year-old woman presented to our emergency department with a rash, severe acute pain in her left hip and lower back, and dyspnea on exertion. She denied having a headache and her mental status was at baseline. The woman reported exposure to rats and snakes one week prior to presentation, and mentioned getting bitten by a rat multiple times on the back of both of her hands while feeding it to her son’s pet snake. The patient had a history of a left hip replacement, with a revision and bone graft 5 years earlier.

The patient had a fever of 103° F during the physical examination. She had erythematous papules and central hemorrhagic eschars at the sites of the bites (FIGURE 1). She also had nonblanching petechiae on both of her lower legs (FIGURE 2) and on the dorsal and palmar aspects of her hands.

The patient’s lab work showed mild normocytic anemia with a hemoglobin level of 11.4 g/dL (normal, 12-16 g/dL) and a platelet count of 129,000/mcL (normal, 130,000-400,000/mcL). Her white blood cell count, chemistries, brain natriuretic peptide test, and chest x-ray were normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Rat bite fever
Based on the patient’s symptoms, history, and lab work, we concluded that this was a case of rat bite fever. RBF is a zoonotic systemic illness caused by infection from either the gram-negative bacillus Streptobacillus moniliformis, commonly found in the United States, or the gram-negative rod Spirillum minus, commonly seen in Asia. Anyone with exposure to rats is at risk for RBF, especially pet shop employees, lab workers, and people living in areas with rat infestations.1
The rash associated with RBF can be petechial, purpuric, or maculopapular, but the presence of hemorrhagic nodules and ulcers at the site of the bite is especially indicative of the illness. The rash often involves the hands and feet, including the palms and soles.
To make the diagnosis of RBF, a careful history and a high index of suspicion are important. Fever and rigor are often the first symptoms to appear, beginning 3 to 10 days after the bite. Three to 4 days after the onset of fever, up to 75% of patients will develop a rash.2 Joint and muscle aches are also common, as is a migrating pattern of arthritis.2,3
Rule out other infections related to animal exposure
The differential diagnosis for RBF includes other animal-related infections, such as those from snake bites, leptospirosis, rabies, and pasteurellosis.
Symptoms associated with snake bite injuries appear rapidly after the bite and vary with the type of snake toxin. Hemotoxic symptoms may include intense pain, edema, petechiae, and ecchymosis from coagulopathy. Neurotoxic symptoms may include ptosis, weakness, and paresthesias. All snake bites should be treated with supportive care, and antivenin is indicated when symptoms or history indicate a bite from a venomous snake. Venomous snakes are rarely intentionally kept as pets.2
Leptospirosis is a zoonotic bacterial infection that may be spread through the urine of rats, dogs, or other mammals. Symptoms may be mild and limited to conjunctivitis, vomiting, and fever; life-threatening symptoms include hemorrhage and kidney failure. A petechial rash is not typical.4 Beta-lactam antibiotics are the treatment of choice.
Rabies is a viral infection that occurs after exposure to infected animals (most commonly raccoons, bats, skunks, and foxes). Symptoms include fever and mental status changes that can lead to death; rash is not a typical symptom. Exposed patients should receive post-exposure prophylaxis with immune globulin or a rabies vaccine.5
Pasteurellosis may also cause hemorrhagic nodules at the site of the bite or scratch, but bites are typically caused by larger animals such as dogs and livestock. Other symptoms include fever, sepsis, and osteomyelitis. Treatment includes amoxicillin-clavulanate or a fluoroquinolone-clindamycin combination.6
In cases of high suspicion, special culture tubes may be needed
Blood cultures and cerebrospinal fluid cultures are often falsely negative. Special culture tubes without polyanethol sulfonate preservative, which inhibits the growth of S moniliformis, may be required in cases of high suspicion. S moniliformis polymerase chain reaction may be available in some specialized labs.7,8
Treatment options include 7 to 10 days of antibiotic therapy with oral penicillin 500 mg 4 times daily, amoxicillin-clavulanate 875/125 mg twice daily, or oral doxycycline 100 mg every 12 hours.9
RBF may be fatal if not treated.3 Complications may include bacteremia, septicemia, meningitis, and endocarditis.
Our patient received empiric intravenous ceftriaxone 1 g every 24 hours and her fever and joint pain resolved within 48 hours. On Day 3 she was discharged home to complete a 10-day course of oral amoxicillin-clavulanate 875/125 mg. Her primary care physician reported that the rash resolved and the patient made a full recovery.
CORRESPONDENCE
Kate Rowland, MD, MS, Rush-Copley Family Medicine Residency, 2020 Ogden Ave. Suite 325, Aurora, IL 60504; [email protected].
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
1. Centers for Disease Control and Prevention. Rat-bite fever (RBF). Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/rat-bite-fever/index.html. Accessed December 1, 2015.
2. Elliott SP. Rat bite fever and Streptobacillus moniliformis. Clin Microbiol Rev. 2007;20:13-22.
3. Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1374.
4. Rabinowitz PM, Gordon Z, Odofin L. Pet-related infections. Am Fam Physician. 2007;76:1314-1322.
5. Fishbein DB, Robinson LE. Rabies. N Engl J Med. 1993;329:1632-1638.
6. Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26:631-655.
7. Eng J. Effect of sodium polyanethol sulfonate in blood cultures. J Clin Microbiol. 1975;1:119-123.
8. Nakagomi D, Deguchi N, Yagasaki A, et al. Rat-bite fever identified by polymerase chain reaction detection of Streptobacillus moniliformis DNA. J Dermatol. 2008;35:667-670.
9. Bush LM, Perez MT. Rat-bite fever. In: The Merck Manual of Diagnosis and Therapy. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2011.
Monitoring Home BP Readings Just Got Easier

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5
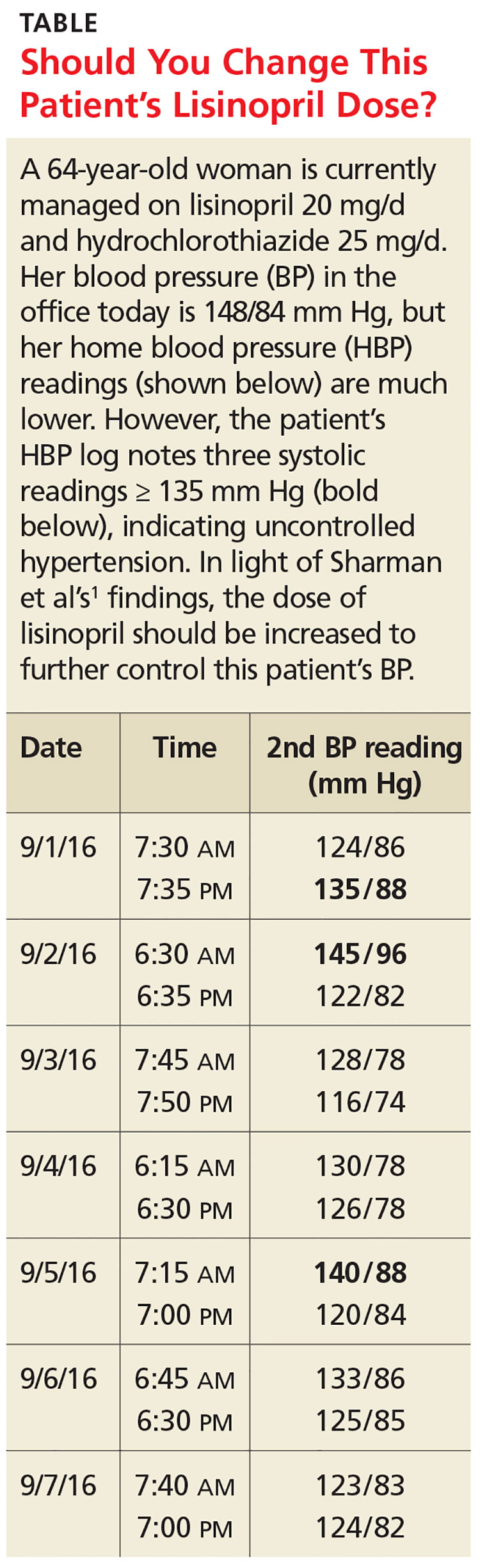
HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5

HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.

A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see Table). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory BP monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension, but ABPM is not always acceptable to patients.3-5

HBP monitoring for long-term follow-up
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values < 130/80 mm Hg may be considered normal, while a mean HBP ≥ 135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for three to seven days prior to a patient’s follow-up appointment, with two readings taken one to two minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care providers accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
3 of 10 readings = predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥ 135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, age 18 or older, and taking three or fewer antihypertensive medications. Patients were excluded if they had a significant abnormal left ventricular mass index (women > 59 g/m2; men > 64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP > 180/100 mm Hg.
Approximately half of the participants were women (53%). Average BMI was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. Medication compliance was verified by a study nurse at a clinic visit.
The patients were instructed to take two BP readings (one minute apart) at home three times daily, in the morning (between 6
The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least three of the last 10 HBP readings were elevated (≥ 135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥ 130 mm Hg). When patients had less than three HBP elevations out of 10 readings, their mean (± standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (± 11.1) mm Hg and their mean systolic HBP value was 120.4 (± 9.8) mm Hg. When patients had three or more HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (± 11.2) mm Hg and their mean systolic HBP value was 147.4 (± 10.5) mm Hg.
The positive and negative predictive values of three or more HBP elevations were 0.85 and 0.56, respectively, for a 24-hour systolic ABP of ≥ 130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of three or more elevations for mean 24-hour ABP systolic readings ≥ 130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥ 135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (three of the past 10 measurements ≥ 135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
BP goals are hazy, patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥ 130 mm Hg for overall 24-hour ABP and ≥ 135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that (1) The study focused only on systolic BP goals; (2) patients in the study adhered to precise instructions on BP monitoring; HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and (3) while end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost, sizing of cuffs
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people.
Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements. The British Hypertension Society maintains a list of validated BP devices on its website: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2016;65(10):719-722.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996; 1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertension Society. BP Monitors. http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Monitoring home BP readings just got easier
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5
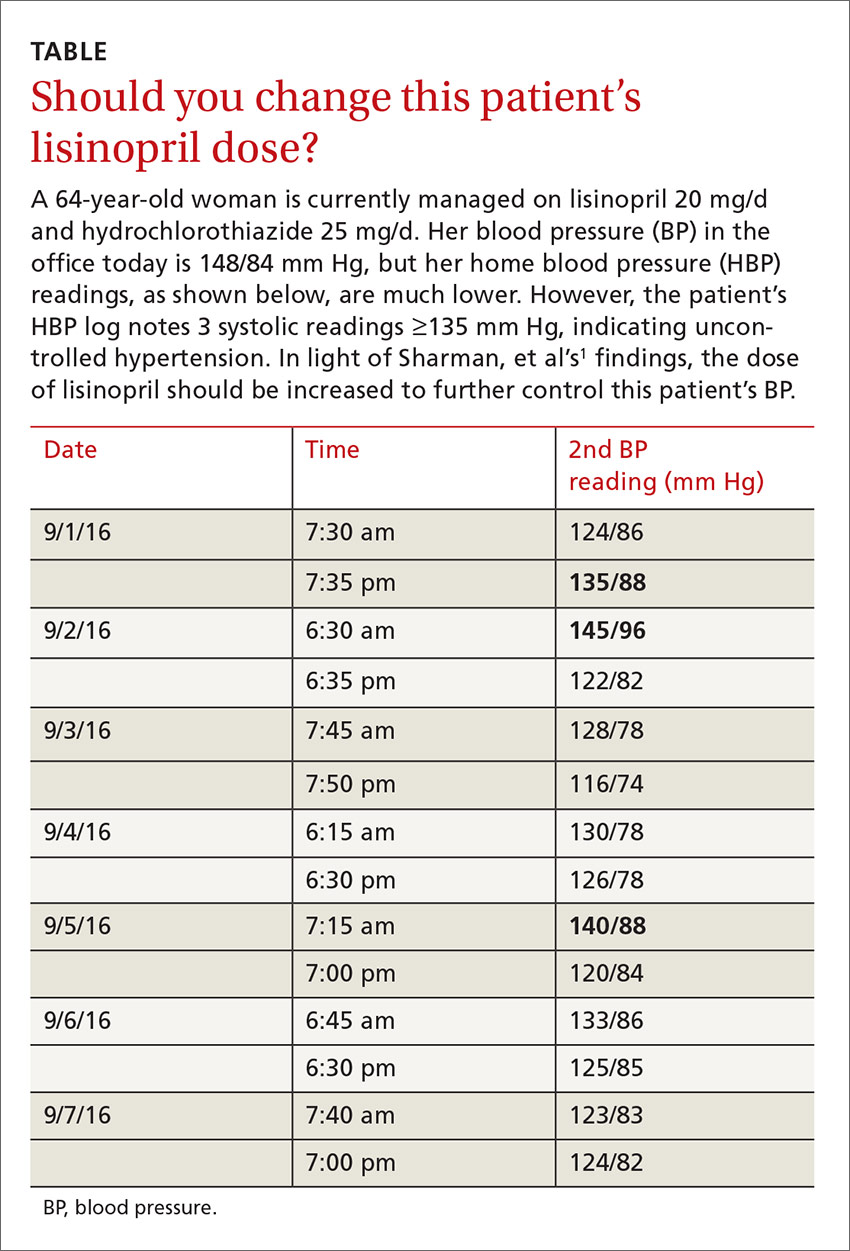
Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PRACTICE CHANGER
Use this easy “3 out of 10 rule” to quickly sift through home blood pressure readings and identify patients with uncontrolled hypertension who require pharmacologic management.1
Strength of recommendation
B: Based on a single, good quality, multicenter trial.
Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
ILLUSTRATIVE CASE
A 64-year-old woman presents to your office for a follow-up visit for her hypertension. She is currently managed on lisinopril 20 mg/d and hydrochlorothiazide 25 mg/d without any problems. The patient’s blood pressure (BP) in the office today is 148/84 mm Hg, but her home blood pressure (HBP) readings are much lower (see TABLE). Should you increase her lisinopril dose today?
Hypertension has been diagnosed on the basis of office readings of BP for almost a century, but the readings can be so inaccurate that they are not useful.2 The US Preventive Services Task Force recommends the use of ambulatory blood pressure monitoring (ABPM) to accurately diagnose hypertension in all patients, while The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends ABPM for patients suspected of having white-coat hypertension and any patient with resistant hypertension,3,4 but ABPM is not always acceptable to patients.5

Guidelines recommend HBP monitoring for long-term follow-up of hypertension
The European Society of Hypertension practice guideline on HBP monitoring suggests that HBP values <130/80 mm Hg may be considered normal, while a mean HBP ≥135/85 mm Hg is considered elevated.9 The guideline recommends HBP monitoring for 3 to 7 days prior to a patient’s follow-up appointment with 2 readings taken one to 2 minutes apart in the morning and evening.9 In a busy clinic, averaging all of these home values can be time-consuming.
So how can primary care physicians accurately and efficiently streamline the process? This study sought to answer that question.
STUDY SUMMARY
When 3 of 10 readings are elevated, it’s predictive
This multicenter trial compared HBP monitoring to 24-hour ABPM in 286 patients with uncomplicated essential hypertension to determine the optimal percentage of HBP readings needed to diagnose uncontrolled BP (HBP ≥135/85 mm Hg). Patients were included if they were diagnosed with uncomplicated hypertension, not pregnant, ≥18 years of age, and taking ≤3 antihypertensive medications. Medication compliance was verified by a study nurse at a clinic visit. Patients were excluded if they had a significant abnormal left ventricular mass index (women >59 g/m2; men >64 g/m2), coronary artery or renal disease, secondary hypertension, serum creatinine exceeding 1.6 mg/dL, aortic valve stenosis, upper limb obstructive atherosclerosis, or BP >180/100 mm Hg.
Approximately half of the participants were women (53%), average body mass index was 29.4 kg/m2, and the average number of hypertension medications being taken was 2.4. The patients were instructed to take 2 BP readings (one minute apart) at home 3 times daily, in the morning (between 6 am and 10 am), at noon, and in the evening (between 6 pm and 10 pm), and to record only the second reading for 7 days. Only the morning and evening readings were used for analysis in the study. The 24-hour ABP was measured every 30 minutes during the daytime hours and every 60 minutes overnight. The primary outcome was to determine the optimal number of systolic HBP readings above goal (135 mm Hg), from the last 10 recordings, that would best predict elevated 24-hour ABP. Secondary outcomes were various cardiovascular markers of target end-organ damage.
The researchers found that if at least 3 of the last 10 HBP readings were elevated (≥135 mm Hg systolic), the patient was likely to have hypertension on 24-hour ABPM (≥130 mm Hg). When patients had <3 HBP elevations out of 10 readings, their mean (±standard deviation [SD]) 24-hour ambulatory daytime systolic BP was 132.7 (±11.1) mm Hg and their mean systolic HBP value was 120.4 (±9.8) mm Hg. When patients had ≥3 HBP elevations, their mean 24-hour ambulatory daytime systolic BP was 143.4 (±11.2) mm Hg and their mean systolic HBP value was 147.4 (±10.5) mm Hg.
The positive and negative predictive values of ≥3 HBP elevations were 0.85 (95% confidence interval [CI], 0.78-0.91) and 0.56 (95% CI, 0.48-0.64), respectively, for a 24-hour systolic ABP of ≥130 mm Hg. Three elevations or more in HBP, out of the last 10 readings, was also an indicator for target organ disease assessed by aortic stiffness and increased left ventricular mass and decreased function.
The sensitivity and specificity of ≥3 elevations for mean 24-hour ABP systolic readings ≥130 mm Hg were 62% and 80%, respectively, and for 24-hour ABP daytime systolic readings ≥135 mm Hg were 65% and 77%, respectively.
WHAT’S NEW
Monitoring home BP can be simplified
The researchers found that HBP monitoring correlates well with ABPM and that their method provides clinicians with a simple way (3 of the past 10 measurements ≥135 mm Hg systolic) to use HBP readings to make clinical decisions regarding BP management.
CAVEATS
Ideal BP goals are hazy, and a lot of patient education is required
Conflicting information and opinions remain regarding the ideal intensive and standard BP goals in different populations.10,11 Systolic BP goals in this study (≥130 mm Hg for overall 24-hour ABP and ≥135 mm Hg for 24-hour ABP daytime readings) are recommended by some experts, but are not commonly recognized goals in the United States. This study found good correlation between HBP and ABPM at these goals, and it seems likely that this correlation could be extrapolated for similar BP goals.
Other limitations are that: 1) The study focused only on systolic BP goals; 2) Patients in the study adhered to precise instructions on BP monitoring. HBP monitoring requires significant patient education on the proper use of the equipment and the monitoring schedule; and 3) While end-organ complication outcomes showed numerical decreases in function, the clinical significance of these reductions for patients is unclear.
CHALLENGES TO IMPLEMENTATION
Cost of device and improper cuff sizes could be barriers
The cost of HBP monitors ($40-$60) has decreased significantly over time, but the devices are not always covered by insurance and may be unobtainable for some people. Additionally, patients should be counseled on how to determine the appropriate cuff size to ensure the accuracy of the measurements.
The British Hypertensive Society maintains a list of validated BP devices on their Web site: http://bhsoc.org/bp-monitors/bp-monitors.12
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
1. Sharman JE, Blizzard L, Kosmala W, et al. Pragmatic method using blood pressure diaries to assess blood pressure control. Ann Fam Med. 2016;14:63-69.
2. Sebo P, Pechère-Bertschi A, Herrmann FR, et al. Blood pressure measurements are unreliable to diagnose hypertension in primary care. J Hypertens. 2014;32:509-517.
3. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Int Med. 2015;163:778-786. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed June 16, 2016.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560-2572.
5. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1:197-203.
6. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self-measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919-1927.
7. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012;30:449-456.
8. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary. A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1-9.
9. Parati G, Stergiou GS, Asmar R, et al; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779-785.
10. The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717.
12. British Hypertensive Society. BP Monitors. Available at: http://bhsoc.org/bp-monitors/bp-monitors. Accessed June 27, 2016.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
Should You Bypass Anticoagulant “Bridging” Before and After Surgery?
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
PRACTICE CHANGER
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤ 4) who are receiving warfarin. The risks outweigh the benefits.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality randomized controlled trial.1
CASE A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped one to two days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a three- to seven-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk for operative bleeding, the warfarin must be stopped days prior to the procedure, but clinicians often worry that this will increase the risk for arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy five days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk for bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (see the table) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score, 0-2) should not be bridged, and patients at high risk (CHADS2 score, 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for patients considered to be at moderate risk (CHADS2 score, 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7Researchers found no difference in ATE events in eight studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR], 0.80).7 The group that received bridging had an increased risk for overall bleeding in 13 studies and of major bleeding in five studies.7This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk for bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding, 3.84).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
Continue for study summary >>
STUDY SUMMARY
When it comes to stroke/TIA, there’s no advantage to bridging
This double-blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1,884 patients with atrial fibrillation who were taking warfarin and whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke and had been taking it for at least 12 weeks, with a goal International Normalized Ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (six weeks prior). Patients undergoing cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥ 3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin five days before their procedure and began subcutaneous dalteparin (100 IU/kg) or an identical placebo three days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin > 2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (four events) in the no-bridging group vs 0.3% (three events) in the bridging group. Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH], 53; relative risk [RR], 0.41).
The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH, 11; 12% vs 20.9%). There were no differences between groups in other secondary outcomes.
Continue for what's new >>
WHAT’S NEW
High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score, 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk for ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score, 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS
Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤ 3. About 3% had a CHADS2 score ≥ 5. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADS2. This may reflect a limit of the predictive value of CHADS2 but should not have affected the rate of bleeding or ATE outcomes in this study.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Providers may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and clinicians, and they pose a greater risk for morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk for bleeding than stroke, which is in contrast to some providers’ experience and current recommendations.
A clinician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, clinicians may be reluctant to provide care in contrast to these recommendations.
REFERENCES
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015; 175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review.JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence.Circulation. 2006;114:119-125.
7. Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates.Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).Circulation. 2015;131:488-494.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(12):794-795, 800.
Should you bypass anticoagulant “bridging” before and after surgery?
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop using low molecular weight heparin (LMWH) for surgical procedures to “bridge” low- to moderate-risk patients with atrial fibrillation (CHADS2 score ≤4) who are receiving warfarin. The risks outweigh the benefits.1
Strength of recommendation
B: Based on a single good-quality randomized control trial.
Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
Illustrative case
A 75-year-old man comes to your office for surgical clearance before right knee replacement surgery. He has diabetes and high blood pressure, and is taking warfarin for atrial fibrillation. He is scheduled for surgery in a week. What is the safest way to manage his warfarin in the perioperative period?
More than 2 million people are being treated with oral anticoagulation in North America to prevent stroke, or to prevent or treat venous thromboembolism.2 Since 2010, several new oral anticoagulants have been approved, including dabigatran, apixaban, and rivaroxaban. These new medications have a shorter half-life than older anticoagulants, which enables them to be stopped 1 to 2 days before surgery.
On the other hand, warfarin—which remains a common choice for anticoagulation—has a 3- to 7-day onset and elimination.3,4 This long clinical half-life presents a special challenge during the perioperative period. To reduce the risk of operative bleeding, the warfarin must be stopped days prior to the procedure, but physicians often worry that this will increase the risk of arterial or venous thromboembolism, including stroke.
An estimated 250,000 patients need perioperative management of their anticoagulation each year.5 As the US population continues to age and the incidence of conditions requiring anticoagulation (particularly atrial fibrillation) increases, this number is only going to rise.6
Current guidelines on bridging. American College of Chest Physicians (ACCP) guidelines recommend transition to “a short-acting anticoagulant, consisting of subcutaneous low molecular weight heparin (LMWH) or intravenous unfractionated heparin, for a 10- to 12-day period during interruption of vitamin K antagonist (VKA) therapy.”5 Furthermore, for an appropriate bridging regimen, the ACCP guidelines recommend stopping VKA therapy 5 days prior to the procedure and utilizing LMWH from within 24 to 48 hours of stopping VKA therapy until up to 24 hours before surgery.5 Postoperatively, VKA or LMWH therapy should be reinitiated within 24 hours and 24 to 72 hours, respectively, depending on the patient’s risk of bleeding during surgery.5
These guidelines recommend using CHADS2 scoring (TABLE3) to determine arterial thromboembolism (ATE) risk in atrial fibrillation.3,5 Patients at low risk for ATE (CHADS2 score 0-2) should not be bridged, and patients at high risk (CHADS2 score of 5-6) should always be bridged.5 These guidelines are less clear about bridging recommendations for moderate-risk patients (CHADS2 score 3-4).
Previous evidence on bridging. A 2012 meta-analysis of 34 studies evaluated the safety and efficacy of perioperative bridging with heparin in patients receiving VKA.7 Researchers found no difference in ATE events in 8 studies that compared groups that received bridging vs groups that simply stopped anticoagulation (odds ratio [OR]=0.80; 95% confidence interval [CI], 0.42–1.54).7 The group that received bridging had an increased risk of overall bleeding in 13 studies, and of major bleeding in 5 studies.7 This meta-analysis was limited by poor study quality and variation in the indication for VKA therapy.
A 2015 subgroup analysis of a larger cohort study of patients receiving anticoagulants for atrial fibrillation found an increased risk of bleeding when their anticoagulation was interrupted for procedures (OR for major bleeding=3.84; 95% CI, 2.07-7.14; P<.0001).8
Douketis et al1 conducted a randomized trial to clarify the need for and safety of bridging anticoagulation for ATE in patients with atrial fibrillation who were receiving warfarin.
STUDY SUMMARY: When it comes to stroke/TIA, there’s no advantage to bridging
This double blind, placebo-controlled trial compared bridging with dalteparin, a form of LMWH, to placebo among 1884 patients with atrial fibrillation on warfarin whose anticoagulation therapy needed to be interrupted for an elective procedure. Patients were included if they were receiving warfarin to prevent stroke, and had been on warfarin for at least 12 weeks, with a goal international normalized ratio (INR) of 2.0 to 3.0. Exclusion criteria included having a mechanical heart valve or having a stroke/transient ischemic attack (TIA; 12 weeks prior) or major bleeding (6 weeks prior). Cardiac, intracranial, and intraspinal surgeries were also excluded from the study.
The patients’ mean CHADS2 score was 2.3; 38.3% of patients had a CHADS2 score ≥3, and 9.4% of patients had a history of stroke. Forty-four percent of patients underwent a gastrointestinal procedure, 17.2% underwent a cardiothoracic procedure, and 9.2% underwent an orthopedic procedure.
Patients stopped taking warfarin 5 days before their procedure, and began subcutaneous dalteparin, 100 IU/kg, or an identical placebo 3 days before the procedure. The dalteparin/placebo was stopped 24 hours before the procedure and restarted after the procedure, until the patient’s INR was in the therapeutic range. Warfarin was resumed on the evening of the procedure or the following day.
The primary efficacy outcome was ATE, including stroke, TIA, or systemic embolism. The primary safety endpoint was major bleeding (defined as bleeding at a critical anatomic site, symptomatic or clinically overt bleeding, or a decrease in hemoglobin >2 g/dL). Secondary efficacy and safety outcomes included minor bleeding, acute myocardial infarction, deep vein thrombosis, pulmonary embolism, and death. Outcomes were assessed within 37 days of the procedure.
The incidence of ATE was 0.4% (4 events) in the no-bridging group vs 0.3% (3 events) in the bridging group (95% CI, -0.6 to 0.8; P=.01 for non-inferiority; P=.73 for superiority). Major bleeding occurred in 1.3% of the no-bridging group (12 events) and in 3.2% of the bridging group (29 events), indicating that no bridging was superior in terms of the major bleeding outcome (number needed to harm [NNH]=53; relative risk [RR]=0.41; 95% CI, 0.20-0.78; P=.005). The no-bridging group also had significantly fewer minor bleeds in comparison to the bridging group (NNH=11; 12% vs 20.9%; P<.001). There were no differences between groups in other secondary outcomes.
WHAT'S NEW: High-quality evidence suggests it’s OK to stop warfarin before surgery
This is the largest good-quality study to evaluate perioperative bridging in patients with atrial fibrillation who were at low or moderate risk for ATE (CHADS2 score 0-4). Previous studies suggested bridging increased bleeding and offered limited benefit for reducing the risk of ATE. However, this is the first study to include a large group of moderate-risk patients (CHADS2 score 3-4). This trial provides high-quality evidence to support the practice of simply stopping warfarin in the perioperative period, rather than bridging with LMWH.
CAVEATS: Findings might not apply to patients at highest risk
Most patients in this study had a CHADS2 score ≤3. About 3% had a CHADS2 score ≥5 or higher. It’s not clear whether these findings apply to patients with a CHADS2 score of 5 or 6.
This trial categorized ATE risk using the CHADS2 score, rather than the CHA2DS2-VASc, which includes additional risk factors and may more accurately predict stroke risk. Both patients who received bridging therapy and those who did not had a lower rate of stroke than predicted by CHADs2. This may reflect a limit of the predictive value of CHADS2, but should not have affected the rate of bleeding or ATE outcomes in this study.
CHALLENGES TO IMPLEMENTATION: Physicians may hesitate to disregard current guidelines
Strokes are devastating events for patients, families, and physicians, and they pose a greater risk of morbidity and mortality compared to bleeding. However, this study suggests patients who receive bridging have a higher risk of bleeding than stroke, which is in contrast to some physicians’ experience and current recommendations.
A physician caring for a patient who’s had a stroke may be inclined to recommend bridging despite the lack of efficacy and evidence of bleeding risk. Additionally, until guidelines reflect the most current research, physicians may be reluctant to provide care in contrast to these recommendations.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
1. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823-833.
2. Guyatt GH, Akl EA, Crowther M, et al; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
3. Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175:1163-1168.
4. Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962.
5. Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e326S-e350S.
6. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125.
7. Siegal, D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin k antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630-1639.
8. Steinberg B, Peterson E, Kim S, et al; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488-494.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Helmets for Positional Skull Deformities: A Good Idea or Not?
PRACTICE CHANGER
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
STRENGTH OF RECOMMENDATION
B: Based on a single-blind, randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well-child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull and ask about using a helmet to help correct the deformity. How would you counsel them?
Approximately 45% of infants ages 7 to 12 weeks are estimated to have positional skull deformity (PSD), although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are two common forms of PSD: plagiocephaly and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity (eg, craniosynostosis). Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skull to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against use of helmets for infants with mild to moderate deformities but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly identified early (ie, when the child is 7 to 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to four to eight weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
Continue for study summary >>
STUDY SUMMARY
Helmets: No help, some harm
In this single-blind RCT of 84 infants (ages 5 or 6 months) with moderate or severe PSD, helmet therapy (n = 42) was compared to no intervention (allowing natural growth; n = 42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until age 1 year, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group received usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months, as measured by the oblique diameter difference index (ODDI; a unitless measurement of plagiocephaly calculated as the ratio of measures of two dimensions of cranial diameter) and the cranioproportional index (CPI; a similar measurement of brachycephaly). Infants were considered fully recovered if they achieved an ODDI score < 104% and a CPI score < 90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At study’s end, the reduction in ODDI and CPI scores was almost the same in both groups. Ten children in the helmet group (26%) and nine in the control group (23%) experienced complete resolution of their PSD.
Both groups were similar in secondary outcomes of infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20 to 80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group (–3.9).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
Continue for new evidence on helmets for PSD >>
WHAT’S NEW
Stronger evidence that helmets are not effective
Previously, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety.
It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1,935 vs $196, respectively).1
CAVEATS
Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe PSD or those with skull deformity due to secondary causes.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Parents may find this evidence hard to accept
To appropriately implement this recommendation, a clinician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another clinician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary and costly and causes adverse effects.
REFERENCES
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131: e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(1):44-46.
PRACTICE CHANGER
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
STRENGTH OF RECOMMENDATION
B: Based on a single-blind, randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well-child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull and ask about using a helmet to help correct the deformity. How would you counsel them?
Approximately 45% of infants ages 7 to 12 weeks are estimated to have positional skull deformity (PSD), although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are two common forms of PSD: plagiocephaly and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity (eg, craniosynostosis). Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skull to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against use of helmets for infants with mild to moderate deformities but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly identified early (ie, when the child is 7 to 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to four to eight weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
Continue for study summary >>
STUDY SUMMARY
Helmets: No help, some harm
In this single-blind RCT of 84 infants (ages 5 or 6 months) with moderate or severe PSD, helmet therapy (n = 42) was compared to no intervention (allowing natural growth; n = 42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until age 1 year, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group received usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months, as measured by the oblique diameter difference index (ODDI; a unitless measurement of plagiocephaly calculated as the ratio of measures of two dimensions of cranial diameter) and the cranioproportional index (CPI; a similar measurement of brachycephaly). Infants were considered fully recovered if they achieved an ODDI score < 104% and a CPI score < 90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At study’s end, the reduction in ODDI and CPI scores was almost the same in both groups. Ten children in the helmet group (26%) and nine in the control group (23%) experienced complete resolution of their PSD.
Both groups were similar in secondary outcomes of infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20 to 80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group (–3.9).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
Continue for new evidence on helmets for PSD >>
WHAT’S NEW
Stronger evidence that helmets are not effective
Previously, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety.
It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1,935 vs $196, respectively).1
CAVEATS
Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe PSD or those with skull deformity due to secondary causes.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Parents may find this evidence hard to accept
To appropriately implement this recommendation, a clinician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another clinician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary and costly and causes adverse effects.
REFERENCES
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131: e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(1):44-46.
PRACTICE CHANGER
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
STRENGTH OF RECOMMENDATION
B: Based on a single-blind, randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well-child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull and ask about using a helmet to help correct the deformity. How would you counsel them?
Approximately 45% of infants ages 7 to 12 weeks are estimated to have positional skull deformity (PSD), although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are two common forms of PSD: plagiocephaly and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity (eg, craniosynostosis). Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skull to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against use of helmets for infants with mild to moderate deformities but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly identified early (ie, when the child is 7 to 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to four to eight weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
Continue for study summary >>
STUDY SUMMARY
Helmets: No help, some harm
In this single-blind RCT of 84 infants (ages 5 or 6 months) with moderate or severe PSD, helmet therapy (n = 42) was compared to no intervention (allowing natural growth; n = 42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until age 1 year, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group received usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months, as measured by the oblique diameter difference index (ODDI; a unitless measurement of plagiocephaly calculated as the ratio of measures of two dimensions of cranial diameter) and the cranioproportional index (CPI; a similar measurement of brachycephaly). Infants were considered fully recovered if they achieved an ODDI score < 104% and a CPI score < 90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At study’s end, the reduction in ODDI and CPI scores was almost the same in both groups. Ten children in the helmet group (26%) and nine in the control group (23%) experienced complete resolution of their PSD.
Both groups were similar in secondary outcomes of infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20 to 80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group (–3.9).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
Continue for new evidence on helmets for PSD >>
WHAT’S NEW
Stronger evidence that helmets are not effective
Previously, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety.
It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1,935 vs $196, respectively).1
CAVEATS
Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe PSD or those with skull deformity due to secondary causes.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
Continue for challenges to implementation >>
CHALLENGES TO IMPLEMENTATION
Parents may find this evidence hard to accept
To appropriately implement this recommendation, a clinician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another clinician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary and costly and causes adverse effects.
REFERENCES
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131: e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(1):44-46.
Helmets for positional skull deformities: A good idea, or not?
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
Strength of recommendation
B: Based on a single-blind, randomized controlled trial (RCT).
van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
Illustrative case
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull, and ask about using a helmet to help correct the deformity. How would you counsel them?
Positional skull deformity (PSD) is a common problem of infancy. Approximately 45% of infants ages 7 to 12 weeks are estimated to have PSD, although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are 2 common forms of PSD: plagiocephaly, and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. Children with severe plagiocephaly have a misshapen, asymmetric skull, while children with brachycephaly have a flattened skull. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity, such as craniosynostosis. Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skill to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against using helmets for infants with mild to moderate deformities, but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly caught early (7 and 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to 4 to 8 weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
STUDY SUMMARY: Helmets for infants: No help and some harm
This single-blind RCT of 84 infants ages 5 or 6 months with moderate or severe PSD compared helmet therapy (n=42) to no intervention (allowing natural growth, n=42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until they were a year old, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group had usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months as measured by the oblique diameter difference index (ODDI), a unitless measurement of plagiocephaly calculated by taking the ratio of measures of 2 dimensions of cranial diameter, and the cranioproportional index (CPI), a similar measurement of brachycephaly. Infants were considered fully recovered if they achieved an ODDI score of <104% and a CPI score of <90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At the end of the study, the reduction in ODDI and CPI scores was almost the same in both the helmet therapy and the control groups. Ten children in the helmet group (26%) and 9 in the control group (23%) experienced complete resolution of their PSD (P=.74).
Secondary outcomes included infant motor development, infant quality of life, parental satisfaction with the shape of their infant’s head, and parental anxiety. Both groups were similar in infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20-80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group: (-3.9; 95% confidence interval, -7.5 to -0.2; P=.04).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
WHAT’S NEW: RCT provides stronger evidence that helmets are not effective
This is the first RCT that assessed helmet therapy for PSD in children.1 Before this, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety. It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1935 vs $196, respectively).1
CAVEATS: Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe cases of PSD or those with skull deformity due to secondary causes, such as craniosynostosis, who were excluded from this study.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
CHALLENGES TO IMPLEMENTATION: Parents may find this evidence hard to accept
To appropriately implement this recommendation, a family physician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another physician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary, costly, and causes adverse effects.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131:e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
Strength of recommendation
B: Based on a single-blind, randomized controlled trial (RCT).
van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
Illustrative case
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull, and ask about using a helmet to help correct the deformity. How would you counsel them?
Positional skull deformity (PSD) is a common problem of infancy. Approximately 45% of infants ages 7 to 12 weeks are estimated to have PSD, although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are 2 common forms of PSD: plagiocephaly, and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. Children with severe plagiocephaly have a misshapen, asymmetric skull, while children with brachycephaly have a flattened skull. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity, such as craniosynostosis. Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skill to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against using helmets for infants with mild to moderate deformities, but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly caught early (7 and 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to 4 to 8 weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
STUDY SUMMARY: Helmets for infants: No help and some harm
This single-blind RCT of 84 infants ages 5 or 6 months with moderate or severe PSD compared helmet therapy (n=42) to no intervention (allowing natural growth, n=42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until they were a year old, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group had usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months as measured by the oblique diameter difference index (ODDI), a unitless measurement of plagiocephaly calculated by taking the ratio of measures of 2 dimensions of cranial diameter, and the cranioproportional index (CPI), a similar measurement of brachycephaly. Infants were considered fully recovered if they achieved an ODDI score of <104% and a CPI score of <90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At the end of the study, the reduction in ODDI and CPI scores was almost the same in both the helmet therapy and the control groups. Ten children in the helmet group (26%) and 9 in the control group (23%) experienced complete resolution of their PSD (P=.74).
Secondary outcomes included infant motor development, infant quality of life, parental satisfaction with the shape of their infant’s head, and parental anxiety. Both groups were similar in infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20-80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group: (-3.9; 95% confidence interval, -7.5 to -0.2; P=.04).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
WHAT’S NEW: RCT provides stronger evidence that helmets are not effective
This is the first RCT that assessed helmet therapy for PSD in children.1 Before this, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety. It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1935 vs $196, respectively).1
CAVEATS: Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe cases of PSD or those with skull deformity due to secondary causes, such as craniosynostosis, who were excluded from this study.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
CHALLENGES TO IMPLEMENTATION: Parents may find this evidence hard to accept
To appropriately implement this recommendation, a family physician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another physician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary, costly, and causes adverse effects.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Do not recommend helmet therapy for positional skull deformity in infants and children. Wearing a helmet causes adverse effects but does not alter the natural course of head growth.1
Strength of recommendation
B: Based on a single-blind, randomized controlled trial (RCT).
van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
Illustrative case
The parents of a 6-month-old girl with moderate plagiocephaly bring their daughter in for a well child visit. Previously, you had recommended that the parents increase “tummy time” when the baby is awake, change her position in bed, and monitor the progression of the condition. They do not feel these interventions have made a difference in the shape of their daughter’s skull, and ask about using a helmet to help correct the deformity. How would you counsel them?
Positional skull deformity (PSD) is a common problem of infancy. Approximately 45% of infants ages 7 to 12 weeks are estimated to have PSD, although three-quarters of them have mild cases.2 The incidence of PSD began to increase in 1992 after the American Academy of Pediatrics (AAP) introduced its “Back to Sleep” campaign, which encouraged parents to place their infants on their back at bedtime to reduce sudden infant death syndrome.3
There are 2 common forms of PSD: plagiocephaly, and brachycephaly.1 Plagiocephaly is unilateral occipital flattening, which may be accompanied by ipsilateral forehead prominence and asymmetrical ears. Brachycephaly is symmetric flattening of the back of the head, which can lead to prominence of the temporal areas, making the head appear wide. Children with severe plagiocephaly have a misshapen, asymmetric skull, while children with brachycephaly have a flattened skull. The cranial sutures remain open in both kinds of PSD.
Evaluating infants for PSD is part of the routine physical exam, and when the condition is noted, the exam should also differentiate PSD from other causes of skull deformity, such as craniosynostosis. Infants and preschool-aged children with PSD may score lower on developmental testing than children without skull deformity.4 However, these differences are small and inconsistent (2-3 points on a 100-point scale).4 Skull deformity persists into adolescence in only 1% to 2% of patients.5
Neither the AAP nor the American Academy of Family Physicians has a guideline or consensus statement on PSD. Helmets are intended to correct PSD by fitting closely to an infant’s head but allowing room for the skill to grow at the flattened area.1 A 2011 clinical report by Laughlin et al6 recommended against using helmets for infants with mild to moderate deformities, but stated that there was little evidence of harm. Earlier studies have suggested that physical therapy might be effective for plagiocephaly caught early (7 and 8 weeks of age).7,8 Biggs9 suggested considering helmet therapy for infants whose cranial sutures remain open and who do not respond to 4 to 8 weeks of physical therapy for PSD. van Wijk et al1 conducted an RCT to explore the risks and benefits of helmet therapy for children with PSD.
STUDY SUMMARY: Helmets for infants: No help and some harm
This single-blind RCT of 84 infants ages 5 or 6 months with moderate or severe PSD compared helmet therapy (n=42) to no intervention (allowing natural growth, n=42). Infants were excluded if they had very severe PSD or skull deformity from another cause, such as torticollis or craniosynostosis.
Infants in the helmet therapy group received a custom-made helmet that they wore 23 hours a day until they were a year old, with regular evaluation by an orthotist and modification of the helmet as necessary to allow skull growth. The control group had usual care and no helmet.
The primary outcome was improvement in skull shape at age 24 months as measured by the oblique diameter difference index (ODDI), a unitless measurement of plagiocephaly calculated by taking the ratio of measures of 2 dimensions of cranial diameter, and the cranioproportional index (CPI), a similar measurement of brachycephaly. Infants were considered fully recovered if they achieved an ODDI score of <104% and a CPI score of <90%. These scores indicate a normal head shape; higher scores indicate worse PSD.
At the end of the study, the reduction in ODDI and CPI scores was almost the same in both the helmet therapy and the control groups. Ten children in the helmet group (26%) and 9 in the control group (23%) experienced complete resolution of their PSD (P=.74).
Secondary outcomes included infant motor development, infant quality of life, parental satisfaction with the shape of their infant’s head, and parental anxiety. Both groups were similar in infant motor development, infant quality of life, and parental satisfaction. Parental anxiety was assessed using the Spielberger State-Trait Anxiety Inventory (scores range from 20-80; a higher score indicates greater anxiety). There was less parental anxiety in the helmet therapy group: (-3.9; 95% confidence interval, -7.5 to -0.2; P=.04).
All parents of infants in the helmet therapy group reported at least one adverse effect from the intervention. These effects included skin irritation (96%), bad helmet odor (76%), pain associated with the helmet (33%), and feeling hindered from cuddling their child (77%).
WHAT’S NEW: RCT provides stronger evidence that helmets are not effective
This is the first RCT that assessed helmet therapy for PSD in children.1 Before this, the evidence on helmets for PSD had been obtained mainly from observational or poorly designed studies with significant flaws.6 This study by van Wijk et al1 included objective measurement of skull deformity, along with clinically meaningful outcomes of parental satisfaction, motor development, and parental anxiety. It also found that helmet therapy was significantly more expensive than care that focused on waiting for PSD to resolve on its own ($1935 vs $196, respectively).1
CAVEATS: Results may not apply to all infants with skull deformity
These findings do not apply to infants with very severe cases of PSD or those with skull deformity due to secondary causes, such as craniosynostosis, who were excluded from this study.1 In addition, this is the only RCT to date that has assessed helmet use in PSD, so it is possible that future studies will find helmets are effective.
CHALLENGES TO IMPLEMENTATION: Parents may find this evidence hard to accept
To appropriately implement this recommendation, a family physician must be comfortable making the assessment of mild, moderate, severe, or very severe PSD. Referral to physical therapy might be appropriate for infants with very severe PSD.
If another physician or physical therapist recommends helmet therapy—or if a parent requests it—explaining the findings of this study may be challenging. We believe that the reduction in parental anxiety in the helmet group likely occurred because the parents believed that the helmet would accelerate the normal reshaping of the skull shape that occurs spontaneously in almost all infants with PSD. Since this study shows that helmets don’t help correct skull deformities, parents can be assured that a helmet is unnecessary, costly, and causes adverse effects.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131:e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
1. van Wijk RM, van Vlimmeren LA, Groothuis-Oudshoorn CG, et al. Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ. 2014;348:g2741.
2. Mawji A, Vollman AR, Hatfield J, et al. The incidence of positional plagiocephaly: a cohort study. Pediatrics. 2013;132:298-304.
3. Peitsch WK, Keefer CH, LaBrie RA, et al. Incidence of cranial asymmetry in healthy newborns. Pediatrics. 2002;110:e72.
4. Collett BR, Gray KE, Starr JR, et al. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131:e109-e115.
5. Roby BB, Finkelstein M, Tibesar RJ, et al. Prevalence of positional plagiocephaly in teens born after the “Back to Sleep” campaign. Otolaryngol Head Neck Surg. 2012;146:823-828.
6. Laughlin J, Luerssen TG, Dias MS; Committee on Practice and Ambulatory Medicine, Section on Neurological Surgery. Prevention and management of positional skull deformities in infants. Pediatrics. 2011;128:1236-1241.
7. van Vlimmeren LA, van der Graaf Y, Boere-Boonekamp MM, et al. Effect of pediatric physical therapy on deformational plagiocephaly in children with positional preference: a randomized controlled trial. Arch Pediatr Adolesc Med. 2008;162:712-718.
8. Vargish, L, Mendoza MD, Ewigman, B. Use physical therapy to head off this deformity in infants. Consider early PT to prevent severe deformational plagiocephaly. J Fam Pract. 2009;58:E1-E3.
9. Biggs WS. Diagnosis and management of positional head deformity. Am Fam Physician. 2003;67:1953-1956.
Copyright © 2015 Family Physicians Inquiries Network. All rights reserved.
Probiotics for colic? A PURL update
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
In “Colicky baby? Here’s a surprising remedy” (J Fam Pract. 2011;60:34-36), we summarized a 2010 double-blind randomized controlled trial (RCT) that found the probiotic Lactobacillus reuteri DSM 17938 reduced daily crying time in colicky, exclusively breastfed infants.1
A recently published RCT of the same probiotic by Sung et al2 adds to the body of evidence and suggests that the jury may still be out as to the value of probiotics for colicky babies.
The newer study (which also measured colic using modified Wessel’s criteria) included babies who were formula-fed as well as those who were breastfed. When researchers looked at all babies as a single group, those who received probiotics fussed significantly more than those who received placebo at nearly all of the postintervention time points. However, when they delved deeper, the researchers noted that an increase in fussing occurred only among infants on formula. On the other hand, the time that breastfed infants spent crying or fussing did not vary significantly between those who received probiotics and those who received placebo.
Both the 2010 and 2014 studies used valid RCT methods with low risk for bias, so we’re not clear why the results (especially for breastfed infants) differed. The 2010 study was done in Italy and required breastfeeding moms to avoid cow’s milk, while the 2014 Sung et al2 study was conducted in Australia and did not have this requirement, so environmental factors may have played a role. The reporting method in the Sung et al2 study—a well-validated, detailed diary of infant behaviors—may have led to less parent recall error than the diary used in the 2010 study. All in all, we can only conclude that it is unclear whether probiotics work to reduce crying in colicky infants.
A safe bet may be to avoid recommending probiotics for colicky formula-fed infants, since no study of this population has shown probiotics are effective, and in the Sung et al2 study, they appeared to worsen symptoms. For breastfed babies, there is no evidence of harm, and mixed evidence on whether probiotics help.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
1. Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 2010;126:e526-e533.
2. Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial. BMJ. 2014;348:g2107.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Low-dose penicillin for recurrent cellulitis?
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.