User login
A pivot in training: My path to reproductive psychiatry
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
Ethics do not end at the bedside: A commentary about scientific authorship
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
Sound moral principles are essential in the development of all physicians. Given how heavily each clinical encounter is laden with ethical implications, this is taught early in medical school. The medical student and resident physician must be able to make ethical and moral decisions on a consistent basis.
Speaking as a psychiatrist in training, there is an intimate relationship between psychiatry and moral questions.1 Issues such as determining an individual’s ability to make decisions about their medical care, hospitalizing patients against their will, and involuntarily administering medication are an almost-daily occurrence.2 Physicians, especially those who practice psychiatric medicine, must be ethically grounded to properly make these difficult but common decisions. It is also imperative that residents are given proper guidance in ethical practice in structured didactics and hands-on training.
However, many residents may be unfamiliar with ethics in research, more specifically ethical authorship. While some trainees might have participated in scholarly activities before residency, residency is the time to discover one’s interests, and residents are encouraged to engage in research. Unfortunately, many of the considerations surrounding ethical authorship are not emphasized, and questionable practices are common.3 In this article, I summarize the different faces of unethical authorship, and call for a greater emphasis on ethical authorship in medical residency training programs.
What drives unethical authorship practices
One of the main drivers for the increase in unethical practices is the need to publish to advance one’s academic career. The academic principle of “publish or perish” pressures many faculty researchers.3 The impact of this expectation plays a significant role in potentially unethical authorship practices, and also has increased the number of publications of mediocre quality or fraudulent data.4 This mindset has also seeped into the clinical world because promotions and financial bonuses are incentives for attending physicians to perform scholarly work. Due to these incentives and pressures, a senior academician might compel a junior researcher to include them as a coauthor on the junior researcher’s paper, even when the senior’s contributions to the paper might be limited.5
Most journals have specific criteria for authorship. The International Committee of Medical Journal Editors (ICMJE) has 4 core criteria for authorship: 1) substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) providing final approval of the version to be published, and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.5,6 One survey found that in certain journals, approximately 15% of authors met full ICMJE authorship criteria, while one-half claimed there were substantial contributions but did not state anything more specific.7
There are several types of authorship abuse.5 Gift authorship is when authorship is awarded to a friend either out of respect or in hopes that friend will return the favor (quid pro quo). Ghost authorship occurs when a third party commissions an author to write or help write a paper (eg, when a pharmaceutical company hires writers to produce a paper about a medication they manufacture) or when legitimate authors are denied recognition on a paper. Honorary authorship occurs when authorship is granted with the hope that the reputation of the honorary author will increase the chances of the paper getting published and possibly boost citations.
While these forms of authorship abuse occur with unsettling frequency, they might not be common among physician trainees who do not engage in full-time research.5 Resident authors might be more likely to experience coercive authorship.
Continue to: Coercive authorship is when...
Coercive authorship is when an individual in a superior position (such as an attending physician) forces their name onto a paper of a junior individual (such as a resident). Kwok8 called this “The White Bull effect,” based on Greek mythology in which Zeus transformed himself into a white bull to seduce Europa. The White Bull represents the predatory nature of the senior individual who exploits ambiguous institutional research regulations to their benefit.8 They stretch out the ICMJE criteria, only superficially satisfying them to justify authorship. In this scenario, the attending physician with promotional incentives notices the work of a resident and demands authorship, given their role as the “supervising” physician (akin to general supervision of a research group). This is not justification for authorship per the ICMJE or any major medical journal criteria. However, a resident with limited research experience may agree to include the attending as a coauthor for a variety of reasons, including fear of a poor performance evaluation or professionalism complaints, or just to maintain a positive working relationship.
Serious implications
While there are countless reasons to be concerned about this behavior, the central issue is the attending physician’s role to train and/or mentor the resident. As previously stated, a physician—especially one practicing psychiatric medicine—must be of morally sound mind. A resident being taught unethical behaviors by their attending physician has dangerous implications. Academic dishonesty does not occur in vacuum. It is likely that dishonest and unethical behavior in research matters can cross over into the clinical arena. One study found that individuals who exhibit dishonest academic behavior are more likely to violate workplace policies.9 Also, these behaviors lead to increased moral disengagement in all areas.10,11 Imagining a morally disengaged attending psychiatrist practicing medicine and training the next generation of psychiatrists is unsettling.
My hope is that residency programs discourage this detrimental conduct in their departments and support those trying to uphold integrity.
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
1. Scher S, Kozlowska K. Teaching ethics in psychiatry: time to reset. Harv Rev Psychiatry. 2020;28(5):328-333. doi:10.1097/HRP.0000000000000258
2. Allen NG, Khan JS, Alzahri MS, et al. Ethical issues in emergency psychiatry. Emerg Med Clin North Am. 2015;33(4):863-874. doi:10.1016/j.emc.2015.07.012
3. Pfleegor AG, Katz M, Bowers MT. Publish, perish, or salami slice? Authorship ethics in an emerging field. Journal of Business Ethics. 2019;156(1):189-208.
4. Rivera H. Fake peer review and inappropriate authorship are real evils. J Korean Med Sci. 2018;34(2):e6. doi:10.3346/jkms.2019.34.e6
5. Strange K. Authorship: why not just toss a coin? Am J Physiol Cell Physiol. 2008;295(3):C567-C575. doi:10.1152/ajpcell.00208.2008
6. Ali MJ. ICMJE criteria for authorship: why the criticisms are not justified? Graefes Arch Clin Exp Ophthalmol. 2021;259(2):289-290. doi:10.1007/s00417-020-04825-2
7. Malički M, Jerončić A, Marušić M, et al. Why do you think you should be the author on this manuscript? Analysis of open-ended responses of authors in a general medical journal. BMC Med Res Methodol. 2012;12:189. doi:10.1186/1471-2288-12-189
8. Kwok LS. The White Bull effect: abusive coauthorship and publication parasitism. J Med Ethics. 2005;31(9):554-556. doi:10.1136/jme.2004.010553
9. Harding TS, Carpenter DD, Finelli CJ, et al. Does academic dishonesty relate to unethical behavior in professional practice? An exploratory study. Sci Eng Ethics. 2004;10(2):311-324. doi:10.1007/s11948-004-0027-3
10. Shu LL, Gino F. Sweeping dishonesty under the rug: how unethical actions lead to forgetting of moral rules. J Pers Soc Psychol. 2012;102(6):1164-1177. doi:10.1037/a0028381
11. Shu LL, Gino F, Bazerman MH. Dishonest deed, clear conscience: when cheating leads to moral disengagement and motivated forgetting. Pers Soc Psychol Bull. 2011;37(3):330-349. doi:10.1177/0146167211398138
The importance of diversity in psychiatry
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
In a sea of blonde hair and blue eyes, my black hair and brown eyes stood out. At the time, I was a medical student and one of the few people of color rotating through the inpatient child psychiatric unit. While I was aware I looked “different,” I discovered that my young patients had an unbridled curiosity about such differences. Common questions I received included “Where are you from? Why are your eyes so small? Is it because you eat rice?” Their questions were never of malicious intent, but rather due to my patient’s unfamiliarity with the Asian-American community and with Black, Indigenous, and people of color (BIPOC) communities in general.
Therefore, it came as no surprise that my BIPOC patients could keenly detect similarities. I could see their eyes widen, a spark of recognition, surprise, or even perhaps relief, when they saw my dark hair or the color of my skin. For members of minority racial/ethnic groups in a predominantly White society, there is a special kinship with other underrepresented BIPOC individuals. We are a community; our shared experiences of discrimination and disadvantages bind us together.
Perhaps it was because of our similarities that my BIPOC patients felt comfortable sharing their most intimate secrets: struggling with social anxiety due to language barriers in school, feeling anxious about balancing their familial expectations vs being “American,” or wishing they were dead due to the color of their skin. It hurt to hear this from my patients. My BIPOC patients’ narratives shared a common theme of fear. Fear that others wouldn’t understand their experiences. Fear that no one would understand their pain. When I reflect upon my own experiences with racism, from microaggressions to outright threats, I am reminded of my own fears, loneliness, and pain. It is these experiences that fuel every BIPOC medical student, resident, and physician to provide culturally sensitive care to patients and promote greater mental health for the BIPOC community.
Why diversity matters
Diversity is important in health care. Our patients come from various backgrounds and cultural experiences. A 2019 survey recruited participants who self-identified with >1 race or as a member of an interracial family relationship, to evaluate their preferences in clinicians.1 Through thematic evaluation of participants’ responses, researchers noted that participants expressed a preference for clinicians who identified as a person of color.1 Participants desired clinicians who were culturally sensitive, who could connect and empathize with their experiences as people of color.1 Ultimately, by having a diverse array of clinicians, health care systems ensure that medical professionals can make important connections with patients due to shared experiences.
I remember talking to a mother about her daughter’s suicide attempt. During our conversation, the mother began to shake her head. “She doesn’t have depression,” she exclaimed. “She needs to snap out of it.” As I listened to her, I was reminded of my own grandmother.
My grandmother struggled with depression throughout her life, yet she was adamant she was “fine.” For my grandmother, her insistence that she did not have depression was rooted in shame. In our community, depression was not viewed as a disease, but rather a moral failing. My patient’s mother shared a similar attitude towards depression, believing her daughter was struggling due to her lack of willpower.
As the only person of color on the treatment team, I understood the importance of helping others on the team to also understand the mother’s perspective—doing so changed the dynamics of the relationship between the team and the family. Rather than having an antagonistic view of the mother who seemed to be callous of her daughter’s needs, the team viewed her differently; she was now understood as a mother who was overwhelmed and lacked an understanding of the disease. This changed the treatment team’s focus. The first step was to educate the family about depression, before providing therapeutic and medication treatments.
To fully understand the patient, the physician must place the story in the correct context, recognizing how the intersectionality of race, sexuality, socioeconomic status, and culture impact mental health. I am now a resident, and as a physician, my primary goal is to be an advocate for patients. To improve patient care, we must continue to find ways to improve diversity in the field of psychiatry. One crucial way is for clinicians to share their stories and be vulnerable with our colleagues, as our patients are with us. Through sharing our personal narratives, we further honor and encourage greater diversity.
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
1. Snyder CR, Truitt AR. Exploring the provider preferences of multiracial patients. J Patient Exp. 2020;7(4):479-483. doi:10.1177/2374373519851694
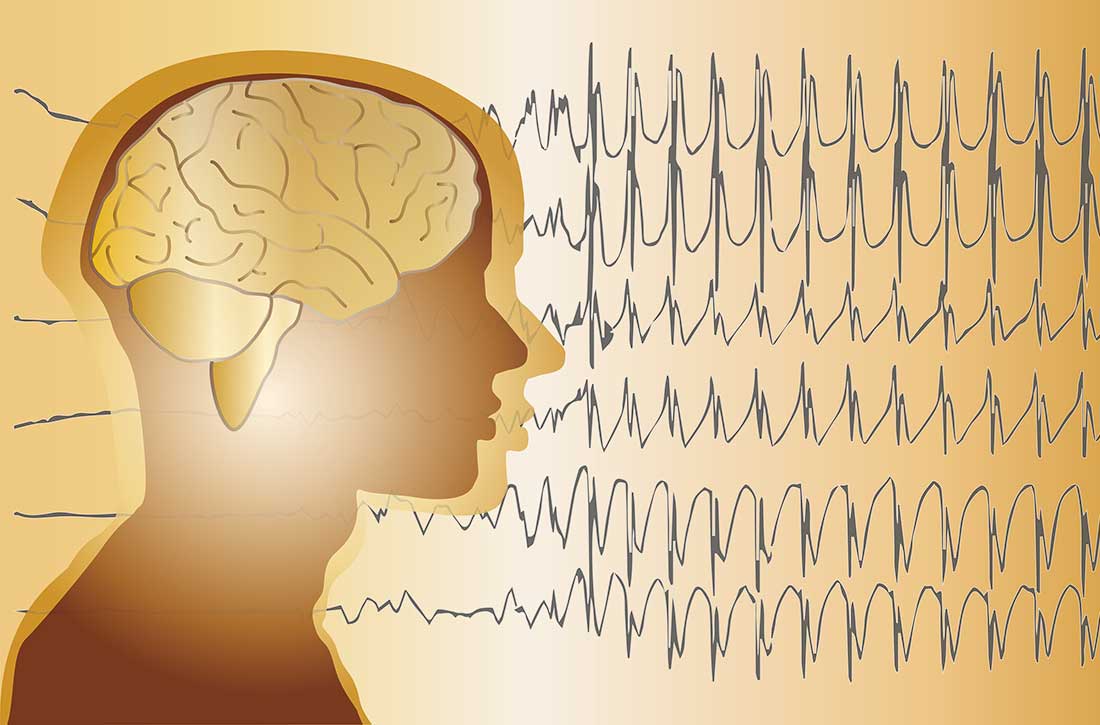
Co-occurring psychogenic nonepileptic seizures and possible true seizures
Psychogenic nonepileptic seizures (PNES) are a physical manifestation of a psychological disturbance. They are characterized by episodes of altered subjective experience and movements that can resemble epilepsy, syncope, or other paroxysmal disorders, but are not caused by neuronal hypersynchronization or other epileptic semiology.
Patients with PNES may present to multiple clinicians and hospitals for assessment. Access to outside hospital records can be limited, which can lead to redundant testing and increased health care costs and burden. Additionally, repeat presentations can increase stigmatization of the patient and delay or prevent appropriate therapeutic management, which might exacerbate a patient’s underlying psychiatric condition and could be dangerous in a patient with a co-occurring true seizure disorder. Though obtaining and reviewing external medical records can be cumbersome, doing so may prevent unnecessary testing, guide medical treatment, and strengthen the patient-doctor therapeutic alliance.
In this article, I discuss our treatment team’s management of a patient with PNES who, based on our careful review of records from previous hospitalizations, may have had a co-occurring true seizure disorder.
Case report
Ms. M, age 31, has a medical history of anxiety, depression, first-degree atrioventricular block, type 2 diabetes, and PNES. She presented to the ED with witnessed seizure activity at home.
According to collateral information, earlier that day Ms. M said she felt like she was seizing and began mumbling, but returned to baseline within a few minutes. Later, she demonstrated intermittent upper and lower extremity shaking for more than 1 hour. At one point, Ms. M appeared to be not breathing. However, upon initiation of chest compressions, she began gasping for air and immediately returned to baseline.
In the ED, Ms. M demonstrated multiple seizure-like episodes every 5 minutes, each lasting 5 to 10 seconds. These episodes were described as thrashing of the bilateral limbs and head crossing midline with eyes closed. No urinary incontinence or tongue biting was observed. Following each episode, Ms. M was unresponsive to verbal or tactile stimuli but intermittently opened her eyes. Laboratory test results were notable for an elevated serum lactate and positive for cannabinoids on urine drug screen.
Ms. M expressed frustration when told that her seizures were psychogenic. She was adamant that she had a true seizure disorder, demanded testing, and threatened to leave against medical advice without it. She said her brother had epilepsy, and thus she knew how seizures present. The interview was complicated by Ms. M’s mistrust and Cluster B personality disorder traits, such as splitting staff into “good and bad.” Ultimately, she was able to be reassured and did not leave the hospital.
Continue to: The treatment team...
The treatment team reviewed external records from 2 hospitals, Hospital A and Hospital B. These records showed well-documented inpatient and outpatient Psychiatry and Neurology diagnoses of PNES and other conversion disorders. Her medications included
Ms. M’s first lifetime documented seizure occurred in May 2020, when she woke up with tongue biting, extremity shaking (laterality was unclear), and urinary incontinence followed by fatigue. She did not go to the hospital after this first episode. In June 2020, she presented and was admitted to Hospital A after similar seizure-like activity. While admitted and monitored on continuous EEG (cEEG), she had numerous events consistent with a nonepileptic etiology without a postictal state. A brain MRI was unremarkable, and Ms. M was diagnosed with PNES.
She presented to Hospital B in October 2020 reporting seizure-like activity. Hospital B reviewed Hospital A’s brain MRI and found right temporal lobe cortical dysplasia that was not noted in Hospital A’s MRI read. Ms. M again underwent cEEG while at Hospital B and had 2 recorded nonepileptic events. Interestingly, the cEEG demonstrated
Ms. M documented 3 seizure-like events between October and December 2020. She documented activity with and without full-body convulsions, some with laterality, some with loss of consciousness, and some preceded by an aura of impending doom. Ms. M was referred to psychotherapy and instructed to continue topiramate 100 mg every 12 hours for seizure prophylaxis.
Ms. M presented to Hospital B again in March 2022 reporting seizure-like activity. A brain MRI found cortical dysplasia in the right temporal lobe, consistent with the MRI at Hospital A in June 2020. cEEG was also repeated at Hospital B and was unremarkable. Oxcarbazepine 300 mg every 12 hours was added to Ms. M’s medications.
Ultimately, based on an external record review, our team (at Hospital C) concluded Ms. M had a possible true seizure co-occurrence with PNES. To avoid redundant testing, we did not repeat imaging or cEEG. Instead, we increased the patient’s oxcarbazepine to 450 mg every 12 hours, for both its effectiveness in temporal seizures and its mood-stabilizing properties. Moreover, in collecting our own data to draw a conclusion by a thorough record review, we gained Ms. M’s trust and strengthened the therapeutic alliance. She was agreeable to forgo more testing and continue outpatient follow-up with our hospital’s Neurology team.
Take-home points
Although PNES and true seizure disorder may not frequently co-occur, this case highlights the importance of clinician due diligence when evaluating a potential psychogenic illness, both for patient safety and clinician liability. By trusting our patients and drawing our own data-based conclusions, we can cultivate a safer and more satisfactory patient-clinician experience in the context of psychosomatic disorders.
1. Bajestan SN, LaFrance WC Jr. Clinical approaches to psychogenic nonepileptic seizures. Focus (Am Psychiatr Publ). 2016;14(4):422-431. doi:10.1176/appi.focus.20160020
2. Dickson JM, Dudhill H, Shewan J, et al. Cross-sectional study of the hospital management of adult patients with a suspected seizure (EPIC2). BMJ Open. 2017;7(7):e015696. doi:10.1136/bmjopen-2016-015696
3. Kutlubaev MA, Xu Y, Hackett ML, et al. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav. 2018;89:70-78. doi:10.1016/j.yebeh.2018.10.010
Psychogenic nonepileptic seizures (PNES) are a physical manifestation of a psychological disturbance. They are characterized by episodes of altered subjective experience and movements that can resemble epilepsy, syncope, or other paroxysmal disorders, but are not caused by neuronal hypersynchronization or other epileptic semiology.
Patients with PNES may present to multiple clinicians and hospitals for assessment. Access to outside hospital records can be limited, which can lead to redundant testing and increased health care costs and burden. Additionally, repeat presentations can increase stigmatization of the patient and delay or prevent appropriate therapeutic management, which might exacerbate a patient’s underlying psychiatric condition and could be dangerous in a patient with a co-occurring true seizure disorder. Though obtaining and reviewing external medical records can be cumbersome, doing so may prevent unnecessary testing, guide medical treatment, and strengthen the patient-doctor therapeutic alliance.
In this article, I discuss our treatment team’s management of a patient with PNES who, based on our careful review of records from previous hospitalizations, may have had a co-occurring true seizure disorder.
Case report
Ms. M, age 31, has a medical history of anxiety, depression, first-degree atrioventricular block, type 2 diabetes, and PNES. She presented to the ED with witnessed seizure activity at home.
According to collateral information, earlier that day Ms. M said she felt like she was seizing and began mumbling, but returned to baseline within a few minutes. Later, she demonstrated intermittent upper and lower extremity shaking for more than 1 hour. At one point, Ms. M appeared to be not breathing. However, upon initiation of chest compressions, she began gasping for air and immediately returned to baseline.
In the ED, Ms. M demonstrated multiple seizure-like episodes every 5 minutes, each lasting 5 to 10 seconds. These episodes were described as thrashing of the bilateral limbs and head crossing midline with eyes closed. No urinary incontinence or tongue biting was observed. Following each episode, Ms. M was unresponsive to verbal or tactile stimuli but intermittently opened her eyes. Laboratory test results were notable for an elevated serum lactate and positive for cannabinoids on urine drug screen.
Ms. M expressed frustration when told that her seizures were psychogenic. She was adamant that she had a true seizure disorder, demanded testing, and threatened to leave against medical advice without it. She said her brother had epilepsy, and thus she knew how seizures present. The interview was complicated by Ms. M’s mistrust and Cluster B personality disorder traits, such as splitting staff into “good and bad.” Ultimately, she was able to be reassured and did not leave the hospital.
Continue to: The treatment team...
The treatment team reviewed external records from 2 hospitals, Hospital A and Hospital B. These records showed well-documented inpatient and outpatient Psychiatry and Neurology diagnoses of PNES and other conversion disorders. Her medications included
Ms. M’s first lifetime documented seizure occurred in May 2020, when she woke up with tongue biting, extremity shaking (laterality was unclear), and urinary incontinence followed by fatigue. She did not go to the hospital after this first episode. In June 2020, she presented and was admitted to Hospital A after similar seizure-like activity. While admitted and monitored on continuous EEG (cEEG), she had numerous events consistent with a nonepileptic etiology without a postictal state. A brain MRI was unremarkable, and Ms. M was diagnosed with PNES.
She presented to Hospital B in October 2020 reporting seizure-like activity. Hospital B reviewed Hospital A’s brain MRI and found right temporal lobe cortical dysplasia that was not noted in Hospital A’s MRI read. Ms. M again underwent cEEG while at Hospital B and had 2 recorded nonepileptic events. Interestingly, the cEEG demonstrated
Ms. M documented 3 seizure-like events between October and December 2020. She documented activity with and without full-body convulsions, some with laterality, some with loss of consciousness, and some preceded by an aura of impending doom. Ms. M was referred to psychotherapy and instructed to continue topiramate 100 mg every 12 hours for seizure prophylaxis.
Ms. M presented to Hospital B again in March 2022 reporting seizure-like activity. A brain MRI found cortical dysplasia in the right temporal lobe, consistent with the MRI at Hospital A in June 2020. cEEG was also repeated at Hospital B and was unremarkable. Oxcarbazepine 300 mg every 12 hours was added to Ms. M’s medications.
Ultimately, based on an external record review, our team (at Hospital C) concluded Ms. M had a possible true seizure co-occurrence with PNES. To avoid redundant testing, we did not repeat imaging or cEEG. Instead, we increased the patient’s oxcarbazepine to 450 mg every 12 hours, for both its effectiveness in temporal seizures and its mood-stabilizing properties. Moreover, in collecting our own data to draw a conclusion by a thorough record review, we gained Ms. M’s trust and strengthened the therapeutic alliance. She was agreeable to forgo more testing and continue outpatient follow-up with our hospital’s Neurology team.
Take-home points
Although PNES and true seizure disorder may not frequently co-occur, this case highlights the importance of clinician due diligence when evaluating a potential psychogenic illness, both for patient safety and clinician liability. By trusting our patients and drawing our own data-based conclusions, we can cultivate a safer and more satisfactory patient-clinician experience in the context of psychosomatic disorders.
Psychogenic nonepileptic seizures (PNES) are a physical manifestation of a psychological disturbance. They are characterized by episodes of altered subjective experience and movements that can resemble epilepsy, syncope, or other paroxysmal disorders, but are not caused by neuronal hypersynchronization or other epileptic semiology.
Patients with PNES may present to multiple clinicians and hospitals for assessment. Access to outside hospital records can be limited, which can lead to redundant testing and increased health care costs and burden. Additionally, repeat presentations can increase stigmatization of the patient and delay or prevent appropriate therapeutic management, which might exacerbate a patient’s underlying psychiatric condition and could be dangerous in a patient with a co-occurring true seizure disorder. Though obtaining and reviewing external medical records can be cumbersome, doing so may prevent unnecessary testing, guide medical treatment, and strengthen the patient-doctor therapeutic alliance.
In this article, I discuss our treatment team’s management of a patient with PNES who, based on our careful review of records from previous hospitalizations, may have had a co-occurring true seizure disorder.
Case report
Ms. M, age 31, has a medical history of anxiety, depression, first-degree atrioventricular block, type 2 diabetes, and PNES. She presented to the ED with witnessed seizure activity at home.
According to collateral information, earlier that day Ms. M said she felt like she was seizing and began mumbling, but returned to baseline within a few minutes. Later, she demonstrated intermittent upper and lower extremity shaking for more than 1 hour. At one point, Ms. M appeared to be not breathing. However, upon initiation of chest compressions, she began gasping for air and immediately returned to baseline.
In the ED, Ms. M demonstrated multiple seizure-like episodes every 5 minutes, each lasting 5 to 10 seconds. These episodes were described as thrashing of the bilateral limbs and head crossing midline with eyes closed. No urinary incontinence or tongue biting was observed. Following each episode, Ms. M was unresponsive to verbal or tactile stimuli but intermittently opened her eyes. Laboratory test results were notable for an elevated serum lactate and positive for cannabinoids on urine drug screen.
Ms. M expressed frustration when told that her seizures were psychogenic. She was adamant that she had a true seizure disorder, demanded testing, and threatened to leave against medical advice without it. She said her brother had epilepsy, and thus she knew how seizures present. The interview was complicated by Ms. M’s mistrust and Cluster B personality disorder traits, such as splitting staff into “good and bad.” Ultimately, she was able to be reassured and did not leave the hospital.
Continue to: The treatment team...
The treatment team reviewed external records from 2 hospitals, Hospital A and Hospital B. These records showed well-documented inpatient and outpatient Psychiatry and Neurology diagnoses of PNES and other conversion disorders. Her medications included
Ms. M’s first lifetime documented seizure occurred in May 2020, when she woke up with tongue biting, extremity shaking (laterality was unclear), and urinary incontinence followed by fatigue. She did not go to the hospital after this first episode. In June 2020, she presented and was admitted to Hospital A after similar seizure-like activity. While admitted and monitored on continuous EEG (cEEG), she had numerous events consistent with a nonepileptic etiology without a postictal state. A brain MRI was unremarkable, and Ms. M was diagnosed with PNES.
She presented to Hospital B in October 2020 reporting seizure-like activity. Hospital B reviewed Hospital A’s brain MRI and found right temporal lobe cortical dysplasia that was not noted in Hospital A’s MRI read. Ms. M again underwent cEEG while at Hospital B and had 2 recorded nonepileptic events. Interestingly, the cEEG demonstrated
Ms. M documented 3 seizure-like events between October and December 2020. She documented activity with and without full-body convulsions, some with laterality, some with loss of consciousness, and some preceded by an aura of impending doom. Ms. M was referred to psychotherapy and instructed to continue topiramate 100 mg every 12 hours for seizure prophylaxis.
Ms. M presented to Hospital B again in March 2022 reporting seizure-like activity. A brain MRI found cortical dysplasia in the right temporal lobe, consistent with the MRI at Hospital A in June 2020. cEEG was also repeated at Hospital B and was unremarkable. Oxcarbazepine 300 mg every 12 hours was added to Ms. M’s medications.
Ultimately, based on an external record review, our team (at Hospital C) concluded Ms. M had a possible true seizure co-occurrence with PNES. To avoid redundant testing, we did not repeat imaging or cEEG. Instead, we increased the patient’s oxcarbazepine to 450 mg every 12 hours, for both its effectiveness in temporal seizures and its mood-stabilizing properties. Moreover, in collecting our own data to draw a conclusion by a thorough record review, we gained Ms. M’s trust and strengthened the therapeutic alliance. She was agreeable to forgo more testing and continue outpatient follow-up with our hospital’s Neurology team.
Take-home points
Although PNES and true seizure disorder may not frequently co-occur, this case highlights the importance of clinician due diligence when evaluating a potential psychogenic illness, both for patient safety and clinician liability. By trusting our patients and drawing our own data-based conclusions, we can cultivate a safer and more satisfactory patient-clinician experience in the context of psychosomatic disorders.
1. Bajestan SN, LaFrance WC Jr. Clinical approaches to psychogenic nonepileptic seizures. Focus (Am Psychiatr Publ). 2016;14(4):422-431. doi:10.1176/appi.focus.20160020
2. Dickson JM, Dudhill H, Shewan J, et al. Cross-sectional study of the hospital management of adult patients with a suspected seizure (EPIC2). BMJ Open. 2017;7(7):e015696. doi:10.1136/bmjopen-2016-015696
3. Kutlubaev MA, Xu Y, Hackett ML, et al. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav. 2018;89:70-78. doi:10.1016/j.yebeh.2018.10.010
1. Bajestan SN, LaFrance WC Jr. Clinical approaches to psychogenic nonepileptic seizures. Focus (Am Psychiatr Publ). 2016;14(4):422-431. doi:10.1176/appi.focus.20160020
2. Dickson JM, Dudhill H, Shewan J, et al. Cross-sectional study of the hospital management of adult patients with a suspected seizure (EPIC2). BMJ Open. 2017;7(7):e015696. doi:10.1136/bmjopen-2016-015696
3. Kutlubaev MA, Xu Y, Hackett ML, et al. Dual diagnosis of epilepsy and psychogenic nonepileptic seizures: systematic review and meta-analysis of frequency, correlates, and outcomes. Epilepsy Behav. 2018;89:70-78. doi:10.1016/j.yebeh.2018.10.010
Disability in medicine: My experience
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
What does a doctor look like? Throughout history, this concept has shifted due to societal norms and increased access to medical education. Today, the idea of a physician has expanded to incorporate a myriad of people; however, stigma still exists in medicine regarding mental illness and disability. I would like to share my personal journey through high school, college, medical school, and now residency, and how my identity and struggles have shaped me into the physician I am today. There are few conversations around disability—especially disability and mental health—in medicine, and through my own advocacy, I have met many students with disability who feel that medical school is unattainable. Additionally, I have met many medical students, residents, and pre-health advisors who are happy for the experience to learn more about a marginalized group in medicine. My hope in sharing my story is to offer a space for conversation about intersectionality within medical communities and how physicians and physicians in training can facilitate that change, regardless of their position or specialty. Additionally, I hope to shed light on the unique mental health needs of patients with disabilities and how mental health clinicians can address those needs.
Perceived weaknesses turned into strengths
“Why do you walk like that?” “What is that brace on your leg?” The early years of my childhood were marked by these questions and others like them. I was the kid with the limp, the kid with a brace on his leg, and the kid who disappeared multiple times a week for doctor’s appointments or physical therapy. I learned to deflect these questions or give nebulous answers about an accident or injury. The reality is that I was born with cerebral palsy (CP). My CP manifested as hemiparesis on the left side of my body. I was in aggressive physical therapy throughout childhood, received Botox injections for muscle spasticity, and underwent corrective surgery on my left leg to straighten my foot. In childhood, the diagnosis meant nothing more than 2 words that sounded like they belonged to superheroes in comic books. Even with supportive parents and family, I kept my disability a secret, much like the powers and abilities of my favorite superheroes.
However, like all great origin stories, what I once thought were weaknesses turned out to be strengths that pushed me through college, medical school, and now psychiatry residency. Living with a disability has shaped how I see the world and relate to my patients. My experience has helped me connect to my patients in ways others might not. These properties are important in any physician but vital in psychiatry, where many patients feel neglected or stigmatized; this is another reason there should be more doctors with disabilities in medicine. Unfortunately, systemic barriers are still in place that disincentivize those with a disability from pursuing careers in medicine. Stories like mine are important to inspire a reexamination of what a physician should be and how medicine, patients, and communities benefit from this change.
My experience through medical school
My path to psychiatry and residency was shaped by my early experience with the medical field and treatment. From the early days of my diagnosis at age 4, I was told that my brain was “wired differently” and that, because of this disruption in circuitry, I would have difficulty with physical activity. I grew to appreciate the intricacies of the brain and pathology to understand my body. With greater understanding came the existential realization that I would live with a disability for the rest of my life. Rather than dream of a future where I would be “normal,” I focused on adapting my life to my normal. An unfortunate reality of this normal was that no doctor would be able to relate to me, and my health care would focus on limitations rather than possibilities.
I focused on school as a distraction and slowly warmed to the idea of pursuing medicine as a career. The seed was planted years prior by the numerous doctors’ visits and procedures, and was cultivated by a desire to understand pathologies and offer treatment to patients from the perspective of a patient. When I applied to medical school, I did not know how to address my CP. Living as a person with CP was a core reason for my decision to pursue medicine, but I was afraid that a disclosure of disability would preclude any admission to medical school. Research into programs offered little guidance because most institutions only listed vague “physical expectations” of each student. There were times I doubted if I would be accepted anywhere. Many programs I reached out to about my situation seemed unenthusiastic about the prospect of a student with CP, and when I brought up my CP in interviews, the reaction was often of surprise and an admission that they had forgotten about “that part” of my application. Fortunately, I was accepted to medical school, but still struggled with the fear that one day I would be found out and not allowed to continue. No one in my class or school was like me, and a meeting with an Americans with Disabilities Act coordinator who asked me to reexamine the physical competencies of the school before advancing to clinical clerkships only further reinforced this fear. I decided to fly under the radar and not say anything about my disability to my attendings. I slowly worked my way through clerkships by making do with adapted ways to perform procedures and exams with additional practice and maneuvering at home. I found myself drawn to psychiatry because of the similarities I saw in the patients and myself. I empathized with how the patients struggled with chronic conditions that left them feeling separated from society and how they felt that their diagnosis was something they needed to hide. When medical school ended and I decided to pursue psychiatry, I wanted to share my story to inspire others with a disability to consider medicine as a career given their unique experiences. My experience thus far has been uplifting as my journey has echoed so many others.
A need for greater representation
Disability representation in medicine is needed more than ever. According to the CDC, >60 million adults in the United States (1 in 4) live with a disability.1 Although the physical health disparities are often discussed, there is less conversation surrounding mental health for individuals with disabilities. A 2018 study by Cree et al2 found that approximately 17.4 million adults with disabilities experienced frequent mental distress, defined as reporting ≥14 mentally unhealthy days in the past 30 days. Furthermore, compared to individuals without a disability, those with a disability are statistically more likely to have suicidal ideation, suicidal planning, and suicide attempts.3 One way to address this disparity is to recruit medical students with disabilities to become physicians with disabilities. Evidence suggests that physicians who are members of groups that are underrepresented in medicine are more likely to deliver care to underrepresented patients.4 However, medical schools and institutions have been slow to address the disparity. A 2019 survey found an estimated 4.6% of medical students responded “yes” when asked if they had a disability, with most students reporting a psychological or attention/hyperactive disorder.5 Existing barriers include restrictive language surrounding technical standards influenced by long-standing vestiges of what a physician should be.6
An opportunity to connect with patients
I now do not see myself as having a secret identity to hide. Although my CP does not give me any superpowers, it has given me the opportunity to connect with my patients and serve as an example of why medical school recruitment and admissions should expand. Psychiatrists have been on the forefront of change in medicine and can shift the perception of a physician. In doing so, we not only enrich our field but also the lives of our patients who may need it most.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
1. Okoro CA, Hollis ND, Cyrus AC, et al. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(32):882-887.
2. Cree RA, Okoro CA, Zack MM, et al. Frequent mental distress among adults, by disability status, disability type, and selected characteristics—United States 2018. MMWR Morb Mortal Wkly Rep. 2020;69(36):1238-1243.
3. Marlow NM, Xie Z, Tanner R, et al. Association between disability and suicide-related outcomes among US adults. Am J Prev Med. 2021;61(6):852-862.
4. Thurmond VB, Kirch DG. Impact of minority physicians on health care. South Med J. 1998;91(11):1009-1013.
5. Meeks LM, Case B, Herzer K, et al. Change in prevalence of disabilities and accommodation practices among US medical schools, 2016 vs 2019. JAMA. 2019;322(20):2022-2024.
6. Stauffer C, Case B, Moreland CJ, et al. Technical standards from newly established medical schools: a review of disability inclusive practices. J Med Educ Curric Dev. 2022;9:23821205211072763.
Should residents be taught how to prescribe monoamine oxidase inhibitors?
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
What else can I offer this patient?
This thought passed through my mind as the patient’s desperation grew palpable. He had experienced intractable major depressive disorder (MDD) for years and had exhausted multiple classes of antidepressants, trying various combinations without any relief.
The previous resident had arranged for intranasal ketamine treatment, but the patient was unable to receive it due to lack of transportation. As I combed through the list of the dozens of medications the patient previously had been prescribed, I noticed the absence of a certain class of agents: monoamine oxidase inhibitors (MAOIs).
My knowledge of MAOIs stemmed from medical school, where the dietary restrictions, potential for hypertensive crisis, and capricious drug-drug interactions were heavily emphasized while their value was minimized. I did not have any practical experience with these medications, and even the attending physician disclosed he had not prescribed an MAOI in more than 30 years. Nonetheless, both the attending physician and patient agreed that the patient would try one.
Following a washout period, the patient began tranylcypromine. After taking tranylcypromine 40 mg/d for 3 months, he reported he felt like a weight had been lifted off his chest. He felt less irritable and depressed, more energetic, and more hopeful for the future. He also felt that his symptoms were improving for the first time in many years.
An older but still potentially helpful class of medications
MDD is one of the leading causes of disability in the United States, affecting millions of people. Its economic burden is estimated to be more than $200 billion, with a large contingent consisting of direct medical cost and suicide-related costs.1 MDD is often recurrent—60% of patients experience another episode within 5 years.2 Most of these patients are classified as having treatment-resistant depression (TRD), which typically is defined as the failure to respond to 2 different medications given at adequate doses for a sufficient duration.3 The Sequenced Treatment Alternatives to Relieve Depression trial suggested that after each medication failure, depression becomes increasingly difficult to treat, with many patients developing TRD.4 For some patients with TRD, MAOIs may be a powerful and beneficial option.5,6 Studies have shown that MAOIs (at adequate doses) can be effective in approximately one-half of patients with TRD. Patients with anxious, endogenous, or atypical depression may also respond to MAOIs.7
MAOIs were among the earliest antidepressants on the market, starting in the late 1950s with isocarboxazid, phenelzine, tranylcypromine, and selegiline. The use of MAOIs as a treatment for depression was serendipitously discovered when iproniazid, a tuberculosis drug, was observed to have mood-elevating adverse effects that were explained by its monoamine oxidase (MAO) inhibitory properties.8 This sparked the hypothesis that a deficiency in serotonin, norepinephrine, and dopamine played a central role in depressive disorders. MAOs encompass a class of enzymes that metabolize catecholamines, which include the previously mentioned neurotransmitters and the trace amine tyramine. The MAO isoenzymes also inhabit many tissues, including the central and peripheral nervous system, liver, and intestines.
There are 2 subtypes of MAOs: MAO-A and MAO-B. MAO-A inhibits tyramine, serotonin, norepinephrine, and dopamine. MAO-B is mainly responsible for the degradation of dopamine, which makes MAO-B inhibitors (ie, rasagiline) useful in treating Parkinson disease.9
Continue to: For most psychiatrists...
For most psychiatrists, MAOIs have fallen out of favor due to their discomfort with their potential adverse effects and drug-drug interactions, the dietary restrictions patients must face, and the perception that newer medications have fewer adverse effects.10 Prescribing an MAOI requires the clinician to remain vigilant of any new medication the patient is taking that may potentiate intrasynaptic serotonin, which may include certain antibiotics or analgesics, causing serotonin syndrome. Close monitoring of the patient’s diet also is necessary so the patient avoids foods rich in tyramine that may trigger a hypertensive crisis. This is because excess tyramine can precipitate an increase in catecholamine release, causing a dangerous increase in blood pressure. However, many foods have safe levels of tyramine (<6 mg/serving), although the perception of tyramine levels in modern foods remains overestimated.5
Residents need to know how to use MAOIs
Psychiatrists should weigh the risks and benefits prior to prescribing any new medication, and MAOIs should be no exception. A patient’s enduring pain is often overshadowed by the potential for adverse effects, which occasionally is overemphasized. Other treatments for severe psychiatric illnesses (such as lithium and clozapine) are also declining due to these agents’ requirement for cumbersome monitoring and potential for adverse effects despite evidence of their superior efficacy and antisuicidal properties.11,12
Fortunately, there are many novel therapies available that can be effective for patients with TRD, including transcranial magnetic stimulation, ketamine, and vagal nerve stimulation. However, as psychiatrists, especially during training, our armamentarium should be equipped with all modalities of psychopharmacology. Training and teaching residents to prescribe MAOIs safely and effectively may add a glimmer of hope for an otherwise hopeless patient.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653-665.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-191.
3. Gaynes BN, Lux L, Gartlehner G, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134-145.
4. Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
5. Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
6. Amsterdam JD, Shults J. MAOI efficacy and safety in advanced stage treatment-resistant depression--a retrospective study. J Affect Disord. 2005;89(1-3):183-188.
7. Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371-386.
8. Ramachandraih CT, Subramanyam N, Bar KJ, et al. Antidepressants: from MAOIs to SSRIs and more. Indian J Psychiatry. 2011;53(2):180-182.
9. Tipton KF. 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna). 2018;125(11):1519-1551.
10. Gillman PK, Feinberg SS, Fochtmann LJ. Revitalizing monoamine oxidase inhibitors: a call for action. CNS Spectr. 2020;25(4):452-454.
11. Kelly DL, Wehring HJ, Vyas G. Current status of clozapine in the United States. Shanghai Arch Psychiatry. 2012;24(2):110-113.
12. Tibrewal P, Ng T, Bastiampillai T, et al. Why is lithium use declining? Asian J Psychiatr. 2019;43:219-220.
The light at the end of the tunnel: Reflecting on a 7-year training journey
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
Throughout my training, a common refrain from more senior colleagues was that training “goes by quickly.” At the risk of sounding cliché, and even after a 7-year journey spanning psychiatry and preventive medicine residencies as well as a consultation-liaison psychiatry fellowship, I agree without reservations that it does indeed go quickly. In the waning days of my training, reflection and nostalgia have become commonplace, as one might expect after such a meaningful pursuit. In sharing my reflections, I hope others progressing through training will also reflect on elements that added meaning to their experience and how they might improve the journey for future trainees.
Residency is a team sport
One realization that quickly struck me was that residency is a team sport, and finding supportive communities is essential to survival. Other residents, colleagues, and mentors played integral roles in making my experience rewarding. Training might be considered a shared traumatic experience, but having peers to commiserate with at each step has been among its greatest rewards. Residency automatically provided a cohort of colleagues who shared and validated my experiences. Additionally, having mentors who have been through it themselves and find ways to improve the training experience made mine superlative. Mentors assisted me in tailoring my training and developing interests that I could integrate into my future practice. The interpersonal connections I made were critical in helping me survive and thrive during training.
See one, do one, teach one
Residency and fellowship programs might be considered “see one, do one, teach one”1 at large scale. Since their inception, these programs—designed to develop junior physicians—have been inherently educational in nature. The structure is elegant, allowing trainees to continue learning while incrementally gaining more autonomy and teaching responsibility.2 Naively, I did not understand that implicit within my education was an expectation to become an educator and hone my teaching skills. Initially, being a newly minted resident receiving brand-new 3rd-year medical students charged me with apprehension. Thoughts I internalized, such as “these students probably know more than me” or “how can I be responsible for patients and students simultaneously,” may have resulted from a paucity of instruction about teaching available during medical school.3,4 I quickly found, though, that teaching was among the most rewarding facets of training. Helping other learners grow became one of my passions and added to my experience.
Iron sharpens iron
Although my experience was enjoyable, I would be remiss without also considering accompanying trials and tribulations. Seemingly interminable night shifts, sleep deprivation, lack of autonomy, and system inefficiencies frustrated me. Eventually, these frustrations seemed less bothersome. These challenges likely had not vanished with time, but perhaps my capacity to tolerate distress improved—likely corresponding with increasing skill and confidence. These challenges allowed me to hone my clinical decision-making abilities while under duress. My struggles and frustrations were not unique but perhaps lessons themselves.
Residency is not meant to be easy. The crucible of residency taught me that I had resilience to draw upon during challenging times. “Iron sharpens iron,” as the adage goes, and I believe adversity ultimately helped me become a better psychiatrist.
Self-reflection is part of completing training
Reminders that my journey is at an end are everywhere. Seeing notes written by past residents or fellows reminds me that soon I too will merely be a name in the chart to future trainees. Perhaps this line of thought is unfair, reducing my training experience to notes I signed—whereas my training experience was defined by connections made with colleagues and mentors, opportunities to teach junior learners, and confidence gained by overcoming adversity.
While becoming an attending psychiatrist fills me with trepidation, fear need not be an inherent aspect of new beginnings. Reflection has been a powerful practice, allowing me to realize what made my experience so meaningful, and that training is meant to be process-oriented rather than outcome-oriented. My reflection has underscored the realization that challenges are inherent in training, although not without purpose. I believe these struggles were meant to allow me to build meaningful relationships with colleagues, discover joy in teaching, and build resiliency.
The purpose of residencies and fellowships should be to produce clinically excellent psychiatrists, but I feel the journey was as important as the destination. Psychiatrists likely understand this better than most, as we were trained to thoughtfully approach the process of termination with patients.5 While the conclusion of our training journeys may seem unceremonious or anticlimactic, the termination process should include self-reflection on meaningful facets of training. For me, this reflection has itself been invaluable, while also making me hopeful to contribute value to the training journeys of future psychiatrists.
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
1. Gorrindo T, Beresin EV. Is “See one, do one, teach one” dead? Implications for the professionalization of medical educators in the twenty-first century. Acad Psychiatry. 2015;39(6):613-614. doi:10.1007/s40596-015-0424-8
2. Wright Jr. JR, Schachar NS. Necessity is the mother of invention: William Stewart Halsted’s addiction and its influence on the development of residency training in North America. Can J Surg. 2020;63(1):E13-E19. doi:10.1503/cjs.003319
3. Dandavino M, Snell L, Wiseman J. Why medical students should learn how to teach. Med Teach. 2007;29(6):558-565. doi:10.1080/01421590701477449
4. Liu AC, Liu M, Dannaway J, et al. Are Australian medical students being taught to teach? Clin Teach. 2017;14(5):330-335. doi:10.1111/tct.12591
5. Vasquez MJ, Bingham RP, Barnett JE. Psychotherapy termination: clinical and ethical responsibilities. J Clin Psychol. 2008;64(5):653-665. doi:10.1002/jclp.20478
Postop analgesia in Saudi Arabia and the United States: A resident’s perspective
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
I had the opportunity to experience first-hand acute postoperative pain management in both the United States and Saudi Arabia. In this article, I discuss some of the differences in how postop pain is managed in each location, potential reasons for these differences, how they may impact patients over time, and the psychiatrist’s role in raising awareness about the hazards of overprescribing analgesic medications.
Vast differences in postop opioid prescribing
From personal observation and literature review, I was appalled by the amount of oxycodone tablets patients are typically discharged home with after a surgical procedure in the United States. Depending on the extent of the surgical procedure, opioid-naïve patients were routinely discharged with 40 to 120 tablets of oxycodone 5 mg. A ventral hernia repair or laparotomy was on the high end of how much oxycodone was provided, and a laparoscopic cholecystectomy or inguinal hernia repair was on the low end. At least one study has supported this observation, finding a wide variation and excessive doses of opioids prescribed postop.1 Notably, among opioids obtained by postsurgical patients, 42% to 71% of all tablets went unused.2 Nevertheless, prescribing in this manner became the standard for postop pain management—possibly in an effort to maximize patient satisfaction on surveys. Additionally, marketing and promotion by the pharmaceutical industry appears to have considerably amplified the prescription, sales, and availability of opioids.3
Signing those prescriptions always left a bad taste in my mouth out of concern for the potential for initiating chronic opioid use.4 Personally, I would prescribe the lowest reasonable number of narcotic tablets for my patients, along with acetaminophen and ibuprofen, knowing that nonsteroidal anti-inflammatory drugs are sufficient for treating postop pain and will decrease opioid requirements, therefore minimizing opiate-induced adverse events.5 Overtreatment of pain with narcotics as first-line therapy is particularly problematic when treating postop pain in children after minor procedures, such as an umbilical hernia repair.Allowing children to resort to a narcotic analgesic agent as a first-line therapy had the potential to develop into an opioid use disorder (OUD) later in life if environmental factors tipped the scales.6
In the hospital in Saudi Arabia where I initially trained, surgery residents were not permitted to prescribe narcotics. The standard of care was to discharge patients with acetaminophen and ibuprofen. In cases where there was an indication for pain treatment with narcotics, stringent regulations were in place. For example, in my experience, which is corroborated by one study,6 special “narcotic forms” are required in the Middle East. In most of these countries, access to these forms is restricted.7 Moreover, pharmacists would only accept this special form when attested to by the surgery consultant (the equivalent of an attending physician in the United States). These consultants would typically write a prescription for 9 to 15 oxycodone 5 mg tablets. Patients receiving such medications were closely watched and followed up in the surgery clinic 3 to 5 days after discharge. Patients were also required to fill out a form detailing their contact information, including their home address and national ID number, to be able to pick up their prescription. Furthermore, apart from 2 Middle East countries, opioids were only available from hospital pharmacies, which were independent of the general hospital pharmacy in location and staff training.8
The psychiatrist’s role
Adapting similar stringent practices for prescribing narcotics in the United States might reduce 1 risk factor for OUD in postop patients. Surgeons attempt to provide the best care by maximizing analgesia, but psychiatrists see firsthand the consequences of overprescribing, and play a direct role in managing patients’ OUDs. As psychiatrists, we have a duty to continue to raise awareness and alert other clinicians about the hazards of overprescribing narcotic analgesic agents.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
1. Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709-714.
2. Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066-1071.
3. Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health. 2009;99(2):221-227.
4. Sun EC, Darnall BD, Baker LC, et al. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-1293.
5. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. doi: 10.1007/s11916-016-0591-7
6. Pollini RA, Banta-Green CJ, Cuevas-Mota J, et al. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abuse Rehabil. 2011;2(1):173-180.
7. Cleary J, Silbermann M, Scholten W, et al. Formulary availability and regulatory barriers to accessibility of opioids for cancer pain in the Middle East: a report from the Global Opioid Policy Initiative (GOPI). Ann Oncol. 2013;24 Suppl 11:xi51-xi59. doi: 10.1093/annonc/mdt503
8. Lankenau SE, Teti M, Silva K, et al. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23(1):37-44.
Neurosurgical treatment of OCD: Patient selection, safety, and access
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
Obsessive-compulsive disorder (OCD) is typically a severe, chronic illness in which patients have recurrent, unwanted thoughts, urges, and compulsions.1 It causes significant morbidity and lost potential over time, and is the world’s 10th-most disabling disorder in terms of lost income and decreased quality of life, and the fifth-most disabling mental health condition.2 Patients with OCD (and their clinicians) are often desperate for an efficacious treatment, but we must ensure that those who are not helped by traditional psychotherapeutic and/or pharmacologic treatments are appropriate for safe neurosurgical intervention.
Pros and cons of neurosurgical therapies
Most patients with OCD are effectively treated with cognitive-behavioral therapy and pharmacotherapy in the form of selective serotonin reuptake inhibitors, clomipramine, or second-generation antipsychotics. However, up to 5% of individuals with OCD will have symptoms refractory to these traditional therapies.3 These cases require more aggressive forms of therapy, including radiofrequency ablation surgeries and deep brain stimulation (DBS). The efficacy of both therapies is similar at 40% to 60%.4,5 While these treatments can be life-changing for patients fortunate to receive them, they are not without issue.
Only a limited number of institutions offer these neurosurgical techniques, and for many patients, those locations may be inaccessible. Patients may not experience relief simply due to where they live, difficult logistics, and the high cost requisite to receive care. If fortunate enough to live near a participating institution or have the means to travel to one, the patient and clinician must then choose the best option based on the nuances of the patient’s situation.
Ablation techniques, such as gamma knife or magnetic resonance–guided ultrasound, are simpler and more cost-effective. A drawback of this approach, however, is that it is irreversible. Lesioned structures are irreparable, as are the adverse effects of the surgery, which, while rare, may include a persistent minimally conscious state or necrotic cysts.4 A benefit of this approach is that there is no need for lengthy follow-up as seen with DBS.
DBS is more complicated. In addition to having to undergo an open neurosurgical procedure, these patients require long-term follow-up and monitoring. A positive aspect is the device can be turned off or removed. However, the amount of follow-up and adjustments is significant. These patients need access to clinicians skilled in DBS device management.
Finally, we must consider the chronically ill patient’s perspective after successful treatment. While the patient’s symptoms may improve, their lives and identities likely developed around their symptoms. Bosanac et al6 describe this reality well in a case study in which a patient with OCD was “burdened with normality” after successful DBS treatment. He was finally able to work, build meaningful relationships, and approach previously unattainable social milestones. This was an overwhelming experience for him, and he and his family needed guidance into the world in which most of us find comfort.
As ablation techniques, DBS, and other cutting-edge therapies for OCD come to the forefront of modern care, clinicians must remember to keep patient safety first. Verify follow-up care before committing patients to invasive and irreversible treatments. While general access is currently poor, participating institutions should consider advertising and communicating that there is an accessible network available for these chronically ill individuals.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
1. Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63.
2. World Health Organization. The Global Burden of Disease: 2004 Update. World Health Organization; 2008.
3. Jenike MA, Rauch SL. Managing the patient with treatment-resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55 Suppl:11-17.
4. Rasmussen SA, Noren G, Greenberg BD, et al. Gamma ventral capsulotomy in intractable obsessive-compulsive disorder. Biol Psychiatry. 2018;84(5):355-364.
5. Kumar KK, Appelboom, G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473.
6. Bosanac P, Hamilton BE, Lucak J, et al. Identity challenges and ‘burden of normality’ after DBS for severe OCD: a narrative case study. BMC Psychiatry. 2018;18(1):186.
The impact of COVID-19 on adolescents’ mental health
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
While the COVID-19 pandemic has impacted the mental health of a wide range of individuals, its adverse effects have been particularly detrimental to adolescents. In this article, I discuss evidence that shows the effects of the pandemic on adolescent patients, potential reasons for this increased distress, and what types of coping mechanisms adolescents have used to counter these effects.
Increases in multiple measures of psychopathology
Multiple online surveys and other studies have documented the pandemic’s impact on younger individuals. In the United States, visits to emergency departments by pediatric patients increased in the months after the first lockdown period.1 Several studies found increased rates of anxiety and depression among adolescents during the COVID-19 pandemic.2,3 In an online survey of 359 children and 3,254 adolescents in China, 22% of respondents reported that they experienced depressive symptoms.3 In an online survey of 1,054 Canadian adolescents, 43% said they were “very concerned” about the pandemic.4 In an online survey of 7,353 adolescents in the United States, 37% reported suicidal ideation during the pandemic compared to 17% in 2017.5 A Chinese study found that smartphone and internet addiction was significantly associated with increased levels of depressive symptoms during the pandemic.3 In a survey in the Philippines, 16.3% of adolescents reported moderate-to-severe psychological impairment during the pandemic; the rates of COVID-19–related anxiety were higher among girls vs boys.6 Alcohol and cannabis use increased among Canadian adolescents during the pandemic, according to an online survey.7 Adolescents with anorexia nervosa reported a 70% increase in poor eating habits and more thoughts associated with eating disorders during the pandemic.8 A Danish study found that children and adolescents newly diagnosed with obsessive-compulsive disorder (OCD) or who had completed treatment exhibited worsening OCD, anxiety, and depressive symptoms during the pandemic.9 An online survey of 6,196 Chinese adolescents found that those with a higher number of pre-pandemic adverse childhood experiences, such as abuse and neglect, had elevated posttraumatic stress symptoms and anxiety during the onset of the pandemic.10
Underlying causes of pandemic-induced distress
Limited social connectedness during the pandemic is a major reason for distress among adolescents. A review of 80 studies found that social isolation and loneliness as a result of social distancing and quarantining were associated with an increased risk of depression, anxiety, suicidal ideation, and self-harm.11 Parents’ stress about the risks of COVID-19 was correlated with worsening mental health in their adolescent children.12 A Chinese study found that the amount of time students spent on smartphones and social media doubled during the pandemic.13 In an online survey of 7,890 Chinese adolescents, greater social media, internet, and smartphone use was associated with increased anxiety and depression.14 This may be in part the result of adolescents spending time reading COVID-related news.
Coping mechanisms to increase well-being
Researchers have identified several positive coping mechanisms adolescents employed during the pandemic. Although some data suggest that increased internet use raises the risk of COVID-related distress, for certain adolescents, using social media to stay connected with friends and relatives was a buffer for feelings of loneliness and might have increased mental well-being.15 Other common coping mechanisms include relying on faith, volunteering, and starting new hobbies.16 During the pandemic, there were higher rates of playing outside and increased physical activity, which correlated with positive mental health outcomes.16 An online survey of 1,040 adolescents found that those who looked to the future optimistically and confidently had a higher health-related quality of life.17
Continuing an emphasis on adolescent well-being
Although data are limited, adolescents can continue to use these coping mechanisms to maintain their well-being, even if COVID-related restrictions are lifted or reimplemented. During these difficult times, it is imperative for adolescents to get the mental health services they need, and for psychiatric clinicians to continue to find avenues to promote resilience and mental wellness among young patients.
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828
1. Leeb RT, Bitsko RH, Radhakrishnan L, et al. Mental health–related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1-October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1675-1680. doi:10.15585/mmwr.mm6945a3
2. Oosterhoff B, Palmer CA, Wilson J, et al. Adolescents’ motivations to engage in social distancing during the COVID-19 pandemic: associations with mental and social health. J Adolesc Health. 2020;67(2):179-185. doi:10.1016/j.jadohealth.2020.05.004
3. Duan L, Shao X, Wang Y, et al. An investigation of mental health status of children and adolescents in China during the outbreak of COVID-19. J Affect Disord. 2020;275:112-118. doi:10.1016/j.jad.2020.06.029
4. Ellis WE, Dumas TM, Forbes LM. Physically isolated but socially connected: psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Can J Behav Sci. 2020;52(3):177-187. doi:10.1037/cbs0000215
5. Murata S, Rezeppa T, Thoma B, et al. The psychiatric sequelae of the COVID-19 pandemic in adolescents, adults, and health care workers. Depress Anxiety. 2021;38(2):233-246. doi:10.1002/da.23120
6. Tee ML, Tee CA, Anlacan JP, et al. Psychological impact of COVID-19 pandemic in the Philippines. J Affect Disord. 2020;277:379-391. doi:10.1016/j.jad.2020.08.043
7. Dumas TM, Ellis W, Litt DM. What does adolescent substance use look like during the COVID-19 pandemic? Examining changes in frequency, social contexts, and pandemic-related predictors. J Adolesc Health. 2020;67(3):354-361. doi:10.1016/j.jadohealth.2020.06.018
8. Schlegl S, Maier J, Meule A, et al. Eating disorders in times of the COVID-19 pandemic—results from an online survey of patients with anorexia nervosa. Int J Eat Disord. 2020;53:1791-1800. doi:10.1002/eat.23374.
9. Nissen JB, Højgaard D, Thomsen PH. The immediate effect of COVID-19 pandemic on children and adolescents with obsessive compulsive disorder. BMC Psychiatry. 2020;20(1):511. doi:10.1186/s12888-020-02905-5
10. Guo J, Fu M, Liu D, et al. Is the psychological impact of exposure to COVID-19 stronger in adolescents with pre-pandemic maltreatment experiences? A survey of rural Chinese adolescents. Child Abuse Negl. 2020;110(Pt 2):104667. doi:10.1016/j.chiabu.2020.104667
11. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid Systematic Review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1218-1239.e3. doi:10.1016/j.jaac.2020.05.009
12. Spinelli M, Lionetti F, Setti A, et al. Parenting stress during the COVID-19 outbreak: socioeconomic and environmental risk factors and implications for children emotion regulation. Fam Process. 2021;60(2):639-653. doi:10.1111/famp.12601
13. Chen IH, Chen CY, Pakpour AH, et al. Internet-related behaviors and psychological distress among schoolchildren during COVID-19 school suspension. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1099-1102.e1. doi:10.1016/j.jaac.2020.06.007
14. Li W, Zhang Y, Wang J, et al. Association of home quarantine and mental health among teenagers in Wuhan, China, during the COVID-19 pandemic. JAMA Pediatr. 2021;175(3):313-316. doi:10.1001/jamapediatrics.2020.5499
15. Janssen, LHC, Kullberg, MJ, Verkuil B, et al. Does the COVID-19 pandemic impact parents’ and adolescents’ well-being? An EMA-study on daily affect and parenting. PLoS One. 2020;15(10):e0240962. doi:10.1371/journal.pone.0240962
16. Banati P, Jones N, Youssef S. Intersecting vulnerabilities: the impacts of COVID-19 on the psycho-emotional lives of young people in low- and middle-income countries. Eur J Dev Res. 2020;32(5):1613-1638. doi:10.1057/s41287-020-00325-5
17. Ravens-Sieberer U, Kaman A, Otto C, et al. Mental health and quality of life in children and adolescents during the COVID-19 pandemic—results of the COPSY study. Dtsch Arztebl Int. 2020;117(48):828-829. doi:10.3238/arztebl.2020.0828