User login
Hemodynamic principles key to managing right ventricular heart failure
PHOENIX – In the clinical opinion of Dr. Nevin M. Katz, caring for critical care patients after cardiothoracic surgery requires a multidisciplinary team.
“It used to be just the surgeons and the residents and anesthesiologists, but in this era, it’s a broad team,” Dr. Katz said at the annual meeting of the Society of Thoracic Surgeons. “Coordination of the team, which includes surgeons, anesthesiologists, physician assistants, bedside nurses, nurse practitioners, perfusionists, pharmacists, respiratory therapists, and nutritionists is very important, and it’s important that members of the team be on the same page.”
Dr. Katz, a cardiovascular surgeon/intensivist at Johns Hopkins University, Baltimore, went on to offer tips for managing right ventricular failure in cardiac surgical patients. He recommends that clinicians consider five basic parameters of hemodynamic management: the heart rate and rhythm; the preload; the afterload; contractility; and the surgical result, including the potential for an anatomic problem and the risk of cardiac tamponade. “One must also consider a cardiac assist device,” he said.
He recommended that the cardiac index goal for cardiovascular patients in the ICU be in the range of 2.2-4.4 L per min/m2. The recommended hemodynamic goals also included systemic blood pressure ranges with a systolic pressure of 90-140 mm Hg and a mean arterial pressure of 70-90 mm Hg; a left arterial pressure or pulmonary capillary wedge pressure of 5-18 mm Hg, a right arterial pressure or central venous pressure of 5-15 mm Hg, and a systemic vascular resistance of 800-1,200 dynes per sec/cm5. “When treating right ventricular failure or complex patients, I think advanced PA [pulmonary artery] catheters are valuable, although they’re not absolutely necessary,” he said.
Complementary technologies available in most ICUs can help clinicians manage these patients, particularly ultrasound. With ultrasound, “we can determine where the patient is on the ventricular function curve, regional versus global dysfunction, right ventricular versus left ventricular dysfunction, valve dysfunction, and cardiac tamponade,” said Dr. Katz, who also created the Foundation for the Advancement of Cardiothoracic Care, also known as FACTS-Care. “It’s a very important monitoring modality.”
An important goal in managing patients with right ventricular failure is to establish an optimal heart rate and rhythm. “We have modalities to treat bradycardia and heart block,” he said. “Loss of atrial contraction is very important. If we can avoid atrial fibrillation, that’s good. Ventricular arrhythmias can be a problem, and nowadays we can treat atrioventricular and ventricular dyssynchrony.”
Optimal preload requires a focus on volume responsiveness, Dr. Katz continued. “Where is the patient on that ventricular function curve?” he asked. “With the advanced PA catheters, there are ways to look at that. You would like to be on the ascending part of that curve.” Clinicians can also use pulsus paradoxus, a variation of systemic arterial pulse volume. “That will indicate that perhaps you’re low in volume, but you can use stroke volume variation with an advanced PA catheter,” he said. “If your stroke volume variation is greater than 15% you’re on the ascending part of the curve. But if your stroke volume variation is less than 15%, your volume is probably optimal and you’re not going to be volume responsive.”
Clinicians who lack the benefit of an advanced PA catheter can assess volume responsiveness with passive leg raising.
Low preload causes of RV failure include hypovolemia, bleeding, third-spacing, high urine output, and cardiac tamponade. High preload can be a problem, too, from excess fluid administration, tricuspid or pulmonary valve regurgitation, or from left to right shunting.
Overall, optimal management of RV failure depends on the coordination of the multidisciplinary critical care team.
Dr. Katz reported having no financial disclosures.
PHOENIX – In the clinical opinion of Dr. Nevin M. Katz, caring for critical care patients after cardiothoracic surgery requires a multidisciplinary team.
“It used to be just the surgeons and the residents and anesthesiologists, but in this era, it’s a broad team,” Dr. Katz said at the annual meeting of the Society of Thoracic Surgeons. “Coordination of the team, which includes surgeons, anesthesiologists, physician assistants, bedside nurses, nurse practitioners, perfusionists, pharmacists, respiratory therapists, and nutritionists is very important, and it’s important that members of the team be on the same page.”
Dr. Katz, a cardiovascular surgeon/intensivist at Johns Hopkins University, Baltimore, went on to offer tips for managing right ventricular failure in cardiac surgical patients. He recommends that clinicians consider five basic parameters of hemodynamic management: the heart rate and rhythm; the preload; the afterload; contractility; and the surgical result, including the potential for an anatomic problem and the risk of cardiac tamponade. “One must also consider a cardiac assist device,” he said.
He recommended that the cardiac index goal for cardiovascular patients in the ICU be in the range of 2.2-4.4 L per min/m2. The recommended hemodynamic goals also included systemic blood pressure ranges with a systolic pressure of 90-140 mm Hg and a mean arterial pressure of 70-90 mm Hg; a left arterial pressure or pulmonary capillary wedge pressure of 5-18 mm Hg, a right arterial pressure or central venous pressure of 5-15 mm Hg, and a systemic vascular resistance of 800-1,200 dynes per sec/cm5. “When treating right ventricular failure or complex patients, I think advanced PA [pulmonary artery] catheters are valuable, although they’re not absolutely necessary,” he said.
Complementary technologies available in most ICUs can help clinicians manage these patients, particularly ultrasound. With ultrasound, “we can determine where the patient is on the ventricular function curve, regional versus global dysfunction, right ventricular versus left ventricular dysfunction, valve dysfunction, and cardiac tamponade,” said Dr. Katz, who also created the Foundation for the Advancement of Cardiothoracic Care, also known as FACTS-Care. “It’s a very important monitoring modality.”
An important goal in managing patients with right ventricular failure is to establish an optimal heart rate and rhythm. “We have modalities to treat bradycardia and heart block,” he said. “Loss of atrial contraction is very important. If we can avoid atrial fibrillation, that’s good. Ventricular arrhythmias can be a problem, and nowadays we can treat atrioventricular and ventricular dyssynchrony.”
Optimal preload requires a focus on volume responsiveness, Dr. Katz continued. “Where is the patient on that ventricular function curve?” he asked. “With the advanced PA catheters, there are ways to look at that. You would like to be on the ascending part of that curve.” Clinicians can also use pulsus paradoxus, a variation of systemic arterial pulse volume. “That will indicate that perhaps you’re low in volume, but you can use stroke volume variation with an advanced PA catheter,” he said. “If your stroke volume variation is greater than 15% you’re on the ascending part of the curve. But if your stroke volume variation is less than 15%, your volume is probably optimal and you’re not going to be volume responsive.”
Clinicians who lack the benefit of an advanced PA catheter can assess volume responsiveness with passive leg raising.
Low preload causes of RV failure include hypovolemia, bleeding, third-spacing, high urine output, and cardiac tamponade. High preload can be a problem, too, from excess fluid administration, tricuspid or pulmonary valve regurgitation, or from left to right shunting.
Overall, optimal management of RV failure depends on the coordination of the multidisciplinary critical care team.
Dr. Katz reported having no financial disclosures.
PHOENIX – In the clinical opinion of Dr. Nevin M. Katz, caring for critical care patients after cardiothoracic surgery requires a multidisciplinary team.
“It used to be just the surgeons and the residents and anesthesiologists, but in this era, it’s a broad team,” Dr. Katz said at the annual meeting of the Society of Thoracic Surgeons. “Coordination of the team, which includes surgeons, anesthesiologists, physician assistants, bedside nurses, nurse practitioners, perfusionists, pharmacists, respiratory therapists, and nutritionists is very important, and it’s important that members of the team be on the same page.”
Dr. Katz, a cardiovascular surgeon/intensivist at Johns Hopkins University, Baltimore, went on to offer tips for managing right ventricular failure in cardiac surgical patients. He recommends that clinicians consider five basic parameters of hemodynamic management: the heart rate and rhythm; the preload; the afterload; contractility; and the surgical result, including the potential for an anatomic problem and the risk of cardiac tamponade. “One must also consider a cardiac assist device,” he said.
He recommended that the cardiac index goal for cardiovascular patients in the ICU be in the range of 2.2-4.4 L per min/m2. The recommended hemodynamic goals also included systemic blood pressure ranges with a systolic pressure of 90-140 mm Hg and a mean arterial pressure of 70-90 mm Hg; a left arterial pressure or pulmonary capillary wedge pressure of 5-18 mm Hg, a right arterial pressure or central venous pressure of 5-15 mm Hg, and a systemic vascular resistance of 800-1,200 dynes per sec/cm5. “When treating right ventricular failure or complex patients, I think advanced PA [pulmonary artery] catheters are valuable, although they’re not absolutely necessary,” he said.
Complementary technologies available in most ICUs can help clinicians manage these patients, particularly ultrasound. With ultrasound, “we can determine where the patient is on the ventricular function curve, regional versus global dysfunction, right ventricular versus left ventricular dysfunction, valve dysfunction, and cardiac tamponade,” said Dr. Katz, who also created the Foundation for the Advancement of Cardiothoracic Care, also known as FACTS-Care. “It’s a very important monitoring modality.”
An important goal in managing patients with right ventricular failure is to establish an optimal heart rate and rhythm. “We have modalities to treat bradycardia and heart block,” he said. “Loss of atrial contraction is very important. If we can avoid atrial fibrillation, that’s good. Ventricular arrhythmias can be a problem, and nowadays we can treat atrioventricular and ventricular dyssynchrony.”
Optimal preload requires a focus on volume responsiveness, Dr. Katz continued. “Where is the patient on that ventricular function curve?” he asked. “With the advanced PA catheters, there are ways to look at that. You would like to be on the ascending part of that curve.” Clinicians can also use pulsus paradoxus, a variation of systemic arterial pulse volume. “That will indicate that perhaps you’re low in volume, but you can use stroke volume variation with an advanced PA catheter,” he said. “If your stroke volume variation is greater than 15% you’re on the ascending part of the curve. But if your stroke volume variation is less than 15%, your volume is probably optimal and you’re not going to be volume responsive.”
Clinicians who lack the benefit of an advanced PA catheter can assess volume responsiveness with passive leg raising.
Low preload causes of RV failure include hypovolemia, bleeding, third-spacing, high urine output, and cardiac tamponade. High preload can be a problem, too, from excess fluid administration, tricuspid or pulmonary valve regurgitation, or from left to right shunting.
Overall, optimal management of RV failure depends on the coordination of the multidisciplinary critical care team.
Dr. Katz reported having no financial disclosures.
EXPERT ANALYSIS AT THE STS ANNUAL MEETING
Therapeutic hypothermia called biggest recent advance in cardiac arrest
SNOWMASS, COLO. – By far the most-important contributor to improved outcomes following out-of-hospital cardiac arrest during the past decade has been therapeutic hypothermia, Dr. N.A. Mark Estes III said at the Annual Cardiovascular Conference at Snowmass.
The No. 1 cause of in-hospital death in patients who arrive at the hospital with a perfusable rhythm following resuscitation from out-of-hospital cardiac arrest isn’t sepsis, hepatic or renal failure, or cardiogenic shock. It’s neurologic death caused by anoxic brain injury, which begins several hours after cardiac arrest and continues for about 48 hours. This is where therapeutic hypothermia has made a huge difference, said Dr. Estes, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
“One-half of out-of-hospital cardiac arrest survivors experience secondary anoxic brain damage of varying degrees. Until recently, there was no treatment with documented efficacy in preventing this damage. Despite multiple agents being looked at for neuroprevention, none really has worked. But therapeutic hypothermia has drastically improved outcomes. More than half of patients who arrive at the hospital with a perfusable rhythm and receive therapeutic hypothermia are discharged relatively neurologically intact. That’s a huge difference from what we used to see,” the electrophysiologist observed.
Indeed, the proportion of U.S. patients who experience out-of-hospital cardiac arrest and survive to hospital discharge neurologically intact is “dismal” at about 10%, he noted.
Virtually all specialized cardiac arrest centers now provide therapeutic hypothermia using various protocols. The demonstrated effectiveness of this postresuscitation therapy was an impetus for the American Heart Association policy statement calling for creation of regional cardiac resuscitation systems of care (Circulation. 2010 Feb 9;121[5]:709-29). To date, however, such organized systems exist in only a handful of states or portions of states.
Nonetheless, when an out-of-hospital cardiac arrest patient arrives at a community hospital that can’t provide emergency coronary angiography and therapeutic hypothermia, it’s appropriate to stabilize that patient in the emergency department and then transfer to a hospital that can, according to Dr. Estes.
The mechanism by which therapeutic hypothermia works has been well elucidated. The treatment curbs the process by which ischemia as a second blow triggers formation of oxygen free radicals, glutamate release, calcium shifts, and mitochondrial dysfunction, with resultant destruction of brain tissue.
Roughly 250,000 sudden cardiac deaths (SCDs) occur annually in this country. In addition to more widespread availability of therapeutic hypothermia and other forms of specialized postresuscitation care through creation of regional systems of care for out-of-hospital cardiac arrest, there are other opportunities for improving outcomes. These include earlier activation of the chain of survival that begins with a bystander dialing 911 as well as greater availability of public access defibrillation.
Dr. Estes emphasized that while these measures will further improve outcomes of cardiac arrest, they won’t actually reduce its frequency. By far the greatest opportunity in that realm lies in primordial prevention of coronary artery disease; that is, prevention of the risk factors for CAD. After all, he noted, 80% of all SCDs are associated with underlying ischemic heart disease. In 30% of SCDs, the fatal event is the first manifestation of previously unrecognized CAD. Another one-third of SCDs occur in patients with known CAD, but who weren’t considered at high risk for SCD because of their preserved left ventricular ejection fraction.
“There are a number of luminaries in the field who feel that if we’re really going to make an impact on sudden cardiac death, it’s going to be through primordial prevention of CAD,” the cardiologist said.
For this reason, he was thrilled to hear Dr. Robert A. Vogel elsewhere at the conference describe research by investigators at Affiris AG in Vienna who’ve created a peptide-based vaccine that inhibits PCSK9. Moreover, they showed it to be effective in sharply lowering LDL in mice (PLoS One. 2014 Dec 4;9[12]:e114469).
“I believe that in my lifetime, we will have an antiatherosclerotic vaccine that will lower LDL to an extent where this disease will not disappear but may get to a manageable extent, perhaps a 10% lifetime risk instead of the 55% lifetime risk of MI or stroke that we as Americans currently have,” predicted Dr. Vogel of the University of Colorado, Denver.
Dr. Vogel reported serving as a consultant to the National Football League and the Pritikin Longevity Center as well as acting as the national coordinator for the Sanofi-sponsored ODYSSEY Outcomes trial studying the PCSK9 inhibitor alirocumab (Praluent).
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
SNOWMASS, COLO. – By far the most-important contributor to improved outcomes following out-of-hospital cardiac arrest during the past decade has been therapeutic hypothermia, Dr. N.A. Mark Estes III said at the Annual Cardiovascular Conference at Snowmass.
The No. 1 cause of in-hospital death in patients who arrive at the hospital with a perfusable rhythm following resuscitation from out-of-hospital cardiac arrest isn’t sepsis, hepatic or renal failure, or cardiogenic shock. It’s neurologic death caused by anoxic brain injury, which begins several hours after cardiac arrest and continues for about 48 hours. This is where therapeutic hypothermia has made a huge difference, said Dr. Estes, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
“One-half of out-of-hospital cardiac arrest survivors experience secondary anoxic brain damage of varying degrees. Until recently, there was no treatment with documented efficacy in preventing this damage. Despite multiple agents being looked at for neuroprevention, none really has worked. But therapeutic hypothermia has drastically improved outcomes. More than half of patients who arrive at the hospital with a perfusable rhythm and receive therapeutic hypothermia are discharged relatively neurologically intact. That’s a huge difference from what we used to see,” the electrophysiologist observed.
Indeed, the proportion of U.S. patients who experience out-of-hospital cardiac arrest and survive to hospital discharge neurologically intact is “dismal” at about 10%, he noted.
Virtually all specialized cardiac arrest centers now provide therapeutic hypothermia using various protocols. The demonstrated effectiveness of this postresuscitation therapy was an impetus for the American Heart Association policy statement calling for creation of regional cardiac resuscitation systems of care (Circulation. 2010 Feb 9;121[5]:709-29). To date, however, such organized systems exist in only a handful of states or portions of states.
Nonetheless, when an out-of-hospital cardiac arrest patient arrives at a community hospital that can’t provide emergency coronary angiography and therapeutic hypothermia, it’s appropriate to stabilize that patient in the emergency department and then transfer to a hospital that can, according to Dr. Estes.
The mechanism by which therapeutic hypothermia works has been well elucidated. The treatment curbs the process by which ischemia as a second blow triggers formation of oxygen free radicals, glutamate release, calcium shifts, and mitochondrial dysfunction, with resultant destruction of brain tissue.
Roughly 250,000 sudden cardiac deaths (SCDs) occur annually in this country. In addition to more widespread availability of therapeutic hypothermia and other forms of specialized postresuscitation care through creation of regional systems of care for out-of-hospital cardiac arrest, there are other opportunities for improving outcomes. These include earlier activation of the chain of survival that begins with a bystander dialing 911 as well as greater availability of public access defibrillation.
Dr. Estes emphasized that while these measures will further improve outcomes of cardiac arrest, they won’t actually reduce its frequency. By far the greatest opportunity in that realm lies in primordial prevention of coronary artery disease; that is, prevention of the risk factors for CAD. After all, he noted, 80% of all SCDs are associated with underlying ischemic heart disease. In 30% of SCDs, the fatal event is the first manifestation of previously unrecognized CAD. Another one-third of SCDs occur in patients with known CAD, but who weren’t considered at high risk for SCD because of their preserved left ventricular ejection fraction.
“There are a number of luminaries in the field who feel that if we’re really going to make an impact on sudden cardiac death, it’s going to be through primordial prevention of CAD,” the cardiologist said.
For this reason, he was thrilled to hear Dr. Robert A. Vogel elsewhere at the conference describe research by investigators at Affiris AG in Vienna who’ve created a peptide-based vaccine that inhibits PCSK9. Moreover, they showed it to be effective in sharply lowering LDL in mice (PLoS One. 2014 Dec 4;9[12]:e114469).
“I believe that in my lifetime, we will have an antiatherosclerotic vaccine that will lower LDL to an extent where this disease will not disappear but may get to a manageable extent, perhaps a 10% lifetime risk instead of the 55% lifetime risk of MI or stroke that we as Americans currently have,” predicted Dr. Vogel of the University of Colorado, Denver.
Dr. Vogel reported serving as a consultant to the National Football League and the Pritikin Longevity Center as well as acting as the national coordinator for the Sanofi-sponsored ODYSSEY Outcomes trial studying the PCSK9 inhibitor alirocumab (Praluent).
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
SNOWMASS, COLO. – By far the most-important contributor to improved outcomes following out-of-hospital cardiac arrest during the past decade has been therapeutic hypothermia, Dr. N.A. Mark Estes III said at the Annual Cardiovascular Conference at Snowmass.
The No. 1 cause of in-hospital death in patients who arrive at the hospital with a perfusable rhythm following resuscitation from out-of-hospital cardiac arrest isn’t sepsis, hepatic or renal failure, or cardiogenic shock. It’s neurologic death caused by anoxic brain injury, which begins several hours after cardiac arrest and continues for about 48 hours. This is where therapeutic hypothermia has made a huge difference, said Dr. Estes, professor of medicine and director of cardiac arrhythmia services at Tufts University, Boston.
“One-half of out-of-hospital cardiac arrest survivors experience secondary anoxic brain damage of varying degrees. Until recently, there was no treatment with documented efficacy in preventing this damage. Despite multiple agents being looked at for neuroprevention, none really has worked. But therapeutic hypothermia has drastically improved outcomes. More than half of patients who arrive at the hospital with a perfusable rhythm and receive therapeutic hypothermia are discharged relatively neurologically intact. That’s a huge difference from what we used to see,” the electrophysiologist observed.
Indeed, the proportion of U.S. patients who experience out-of-hospital cardiac arrest and survive to hospital discharge neurologically intact is “dismal” at about 10%, he noted.
Virtually all specialized cardiac arrest centers now provide therapeutic hypothermia using various protocols. The demonstrated effectiveness of this postresuscitation therapy was an impetus for the American Heart Association policy statement calling for creation of regional cardiac resuscitation systems of care (Circulation. 2010 Feb 9;121[5]:709-29). To date, however, such organized systems exist in only a handful of states or portions of states.
Nonetheless, when an out-of-hospital cardiac arrest patient arrives at a community hospital that can’t provide emergency coronary angiography and therapeutic hypothermia, it’s appropriate to stabilize that patient in the emergency department and then transfer to a hospital that can, according to Dr. Estes.
The mechanism by which therapeutic hypothermia works has been well elucidated. The treatment curbs the process by which ischemia as a second blow triggers formation of oxygen free radicals, glutamate release, calcium shifts, and mitochondrial dysfunction, with resultant destruction of brain tissue.
Roughly 250,000 sudden cardiac deaths (SCDs) occur annually in this country. In addition to more widespread availability of therapeutic hypothermia and other forms of specialized postresuscitation care through creation of regional systems of care for out-of-hospital cardiac arrest, there are other opportunities for improving outcomes. These include earlier activation of the chain of survival that begins with a bystander dialing 911 as well as greater availability of public access defibrillation.
Dr. Estes emphasized that while these measures will further improve outcomes of cardiac arrest, they won’t actually reduce its frequency. By far the greatest opportunity in that realm lies in primordial prevention of coronary artery disease; that is, prevention of the risk factors for CAD. After all, he noted, 80% of all SCDs are associated with underlying ischemic heart disease. In 30% of SCDs, the fatal event is the first manifestation of previously unrecognized CAD. Another one-third of SCDs occur in patients with known CAD, but who weren’t considered at high risk for SCD because of their preserved left ventricular ejection fraction.
“There are a number of luminaries in the field who feel that if we’re really going to make an impact on sudden cardiac death, it’s going to be through primordial prevention of CAD,” the cardiologist said.
For this reason, he was thrilled to hear Dr. Robert A. Vogel elsewhere at the conference describe research by investigators at Affiris AG in Vienna who’ve created a peptide-based vaccine that inhibits PCSK9. Moreover, they showed it to be effective in sharply lowering LDL in mice (PLoS One. 2014 Dec 4;9[12]:e114469).
“I believe that in my lifetime, we will have an antiatherosclerotic vaccine that will lower LDL to an extent where this disease will not disappear but may get to a manageable extent, perhaps a 10% lifetime risk instead of the 55% lifetime risk of MI or stroke that we as Americans currently have,” predicted Dr. Vogel of the University of Colorado, Denver.
Dr. Vogel reported serving as a consultant to the National Football League and the Pritikin Longevity Center as well as acting as the national coordinator for the Sanofi-sponsored ODYSSEY Outcomes trial studying the PCSK9 inhibitor alirocumab (Praluent).
Dr. Estes reported serving as a consultant to Boston Scientific, Medtronic, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
How to use two new game-changing heart failure drugs
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
SNOWMASS, COLO. – Ivabradine and sacubitril/valsartan are paradigm-changing drugs approved last year for the treatment of heart failure with reduced ejection fraction – and it’s entirely reasonable to begin using them now in the appropriate patients, Dr. Akshay S. Desai said at the Annual Cardiovascular Conference at Snowmass.
The impressive positive results seen in the pivotal trials for these novel agents – the SHIFT trial for ivabradine (Corlanor) and PARADIGM-HF for sacubitril/valsartan (Entresto) – have rocked the heart failure world.
The studies showed that, in the right patients, these two medications improve heart failure morbidity and mortality significantly beyond what’s achievable with the current gold standard, guideline-directed medical therapy. That’s exciting because even though great therapeutic strides have been made during the past 15 years, symptomatic patients with heart failure with reduced ejection fraction (HFrEF) treated with optimal guideline-directed pharmacotherapy still have substantial residual risk for heart failure hospitalization and death, noted Dr. Desai, director of heart failure disease management at Brigham and Women’s Hospital in Boston.
The U.S. heart failure guidelines panel hasn’t yet addressed the use of either of these recently approved drugs, but Dr. Desai provided his best sense of the data and how he thinks physicians might start using them now.
Ivabradine and sacubitril/valsartan are first-in-class agents with novel mechanisms of action. Ivabradine’s demonstrated safety and efficacy in the SHIFT trial confirmed the hypothesis that elevated heart rate is a legitimate therapeutic target in HFrEF.
Sacubitril/valsartan, an angiotensin II receptor/neprilysin inhibitor formerly known as LCZ696, provides what is to date a unique ability to enhance the activity of endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P, adrenomedullin, and calcitonin gene–related peptide. These peptides are antifibrotic, antihypertrophic, and they promote vasodilation and diuresis, thus counteracting the adverse effects of neurohormonal activation. But in HFrEF, these vasoactive peptides are less active and patients are less sensitive to them.
Ivabradine
This selective sinus node inhibitor decreases heart rate and has essentially no other effects. The drug has been available for years in Europe, and the European Society of Cardiology (ESC) has had sufficient time to integrate ivabradine into its guidelines for pharmacotherapy in HFrEF.
The ESC treatment algorithm for HFrEF (Eur Heart J. 2012 Jul;33[14]:1787-847) is built upon a foundation of thiazide diuretics to relieve signs and symptoms of congestion along with a beta-blocker and an ACE inhibitor or angiotensin receptor blocker (ARB). In a patient who still has New York Heart Association class II-IV symptoms after those drugs are titrated to guideline-recommended target levels or maximally tolerated doses, a mineralocorticoid receptor antagonist – either spironolactone or eplerenone – is added. And, in a patient who still remains symptomatic, has a left ventricular ejection fraction of 35% or less, is in sinus rhythm, and has a heart rate of 70 beats per minute or more, it’s time to consider adding ivabradine.
“This is how our own guidelines may elect to incorporate ivabradine, but of course, we don’t know yet,” Dr. Desai observed.
In the randomized, double-blind SHIFT trial involving 6,558 HFrEF patients who fit the description of ivabradine candidates described in the ESC guidelines, those who received ivabradine titrated to a maximum of 7.5 mg twice daily experienced a 26% reduction in hospital admissions for worsening heart failure, compared with placebo, a 26% reduction in deaths from heart failure, and fewer adverse events than the control group (Lancet. 2010 Sep 11;376[9744]:875-85).
The important question is who should get ivabradine and who should just get a little more beta-blocker in order to slow the heart rate. The fact is, many heart failure patients simply can’t tolerate the guideline-recommended target dose of beta-blocker therapy, which is 12.5 mg twice daily of carvedilol or its equivalent. Indeed, only 26% of SHIFT participants were able to do so.
“My interpretation of the SHIFT trial is that the goal is to reduce heart rate by any means necessary; preferentially, with a beta-blocker, and with ivabradine as an adjunct in patients who can’t get to target doses,” the cardiologist said.
Sacubitril/valsartan
In the landmark double-blind, 8,442-patient PARADIGM-HF trial, the group randomized to sacubitril/valsartan had a 20% reduction in the primary endpoint of cardiovascular death or heart failure hospitalization over 27 months of follow-up, compared with controls on enalapril at the guideline-recommended dose of 10 mg twice a day. The number needed to treat (NNT) was 21. Moreover, all-cause mortality was reduced by 16% (N Engl J Med. 2014 Sep 11;37[11]:993-1004).
In a recent follow-up cause of death analysis, Dr. Desai and his coinvestigators reported that 81% of all deaths in PARADIGM-HF were cardiovascular in nature. The NNT for sacubitril/valsartan in order to prevent one cardiovascular death was 32. The risk of sudden cardiac death was reduced by 80%, while the risk of death due to worsening heart failure was decreased by 21% (Eur Heart J 2015 Aug 7;36[30]:1990-7).
In another secondary analysis from the PARADIGM-HF investigators, the use of the angiotensin receptor/neprilysin inhibitor was shown to prevent clinical progression of surviving patients with heart failure much more effectively than enalapril. The sacubitril/valsartan group was 34% less likely to have an emergency department visit for worsening heart failure, 18% less likely to require intensive care, and 22% less likely to receive an implantable heart failure device or undergo cardiac transplantation. The reduction in the rate of heart failure hospitalization became significant within the first 30 days (Circulation. 2015 Jan 6;131[1]:54-61).
Moreover, the absolute benefit of sacubitril/valsartan in PARADIGM-HF was consistent across the full spectrum of patient risk (J Am Coll Cardiol. 2015 Nov 10;66[19]:2059-71).
To put this into perspective, Dr. Desai continued, for every 1,000 HFrEF patients switched from an ACE inhibitor or ARB to sacubitril/valsartan, the absolute benefit over the course of 27 months includes 31 fewer cardiovascular deaths, 28 fewer hospitalizations for heart failure, and 37 fewer hospitalizations for any reason.
“This is potent therapy for patients with HFrEF who have the right phenotype,” he observed.
While substitution of sacubitril/valsartan for an ACE inhibitor or ARB may be appropriate in many patients with chronic HFrEF who continue to have NYHA Class II-IV symptoms on guideline-directed medical therapy, several caveats apply, according to Dr. Desai.
It’s important to be aware of the PARADIGM-HF eligibility criteria, because it’s only in patients who fit that profile that sacubitril/valsartan provides evidence-based therapy. There are as yet no data to support the drug’s use in patients with new-onset HFrEF, acute decompensated HFrEF, in patients who are immediately post-MI, or in those with advanced chronic kidney disease, he emphasized.
“I think you have to be mindful of eligibility because the label that’s applied to this drug is basically ‘patients with HFrEF who are treated with guideline-directed medical therapy.’ There’s no specific requirement that you follow the detailed eligibility criteria of the PARADIGM-HF trial, but you should realize that the drug is known to be effective only in patients who fit the PARADIGM-HF eligibility profile,” he said.
Dr. Desai gave a few clinical pearls for prescribing sacubitril/valsartan. For most patients, the initial recommended dose is 49/51 mg twice daily. In those with low baseline blood pressure and tenuous hemodynamics, it’s appropriate to initiate therapy at 24/26 mg BID. It’s important to halt ACE inhibitor therapy 36 hours prior to starting sacubitril/valsartan so as to avoid overlap and consequent increased risk of angioedema. And while serum n-terminal prohormone brain natriuretic peptide (NT-proBNP) remains a useful biomarker to monitor heart rate severity and response to treatment while a patient is on sacubitril/valsartan, BNP is not because serum levels of that biomarker rise with neprilysin inhibition.
Dr. Desai reported receiving research support from Novartis and St. Jude Medical and serving as a consultant to those companies as well as Merck and Relypsa.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
VIDEO: Preventing healthcare acquired infections after CT surgery
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
FDA approves marketing of sutureless aortic valve
LivaNova announced that the U.S. Food and Drug Administration has announced the approval of their Perceval Sutureless Heart Valve. The approval was issued Jan. 8 and is effective immediately, and LivaNova indicated that it will begin commercial distribution of the device in the United States over the coming quarter.
According to an FDA summary document, the Perceval Sutureless Heart Valve is a bioprosthetic valve designed to replace a diseased native or a malfunctioning prosthetic aortic valve via open heart surgery. The self-expanding stent frame consists of a tissue component made from bovine pericardium and a self-expandable nitinol stent, along with a dedicated delivery system that allows physicians to position and anchor the valve suturelessly.
The Perceval heart valve is supplied unmounted and must be loaded onto an accompanying holder by reducing the valve size using a supplied polycarbonate collapser. The holder is used for sternal approaches and includes a rigid shaft with an end section that houses the valve prosthesis during delivery. A separate holder, approximately 3 cm longer, is also available for minimally invasive procedures. After implantation, the physician uses the a post-dilation catheter to expand the valve in situ.
Perceval’s approval was based upon the results of a pivotal European study performed to assess the device, the CAVALIER (Safety and Effectiveness Study of Perceval S Valve for Extended CE Mark) trial. The prospective, multicenter, nonrandomized clinical study was conducted at 26 investigational sites in Austria, Belgium, England, France, Germany, and the Netherlands.
Patients were treated between Feb. 23, 2010, and Sept. 30, 2013, and the database for the PDA-assessed data collected through Nov. 5, 2014, and included 658 patients.
The differences between the New York Heart Association (NYHA) class at 12 months and the baseline were calculated. In total, 77.5% of patients showed a decrease of NYHA equal to at least one class, whereas 19.7% of patients remained stable over the time. Only 2.8% of patients showed a worsened clinical status.
Reduction in mean gradients and increase in effective orifice area were both observed at 1 year follow-up, according to the FDA report.
At the 1-, 2-, and 3-year follow-up time points, 75.1% or greater of the implanted patients with available data had improved by one to three classes and at the 4-year follow-up time point 72.6% of the patients had improved by one to three classes, according to the report. In addition, at the 1-, 2-, and 3-year follow-up time points, 92.9% or greater of the patients were in NYHA Class I and Class II, and at the 4-year follow-up time point, 86.3% of the patients were in NYHA Class I and Class II.
Although there was a predominance of women in the study population (64.4%), patients of both sexes demonstrated acceptable hemodynamic outcomes and significant improvement in functional status, according to the FDA summary.
Sutureless valves are considered to have a promising future according to a recent international consensus panel recommendationpublished online in the European Journal of Cardio-Thoracic Surgery (2015 Oct 29. doi: 10.1093/ejcts/ezv369). The report assessed various benefits of sutureless and rapid deployment technology, and concluded these devices “may represent a helpful tool in aortic valve replacement for patients requiring a biological valve.” However, further evidence will be needed to reaffirm the benefit of sutureless and rapid deployment valves, they concluded.
LivaNova announced that the U.S. Food and Drug Administration has announced the approval of their Perceval Sutureless Heart Valve. The approval was issued Jan. 8 and is effective immediately, and LivaNova indicated that it will begin commercial distribution of the device in the United States over the coming quarter.
According to an FDA summary document, the Perceval Sutureless Heart Valve is a bioprosthetic valve designed to replace a diseased native or a malfunctioning prosthetic aortic valve via open heart surgery. The self-expanding stent frame consists of a tissue component made from bovine pericardium and a self-expandable nitinol stent, along with a dedicated delivery system that allows physicians to position and anchor the valve suturelessly.
The Perceval heart valve is supplied unmounted and must be loaded onto an accompanying holder by reducing the valve size using a supplied polycarbonate collapser. The holder is used for sternal approaches and includes a rigid shaft with an end section that houses the valve prosthesis during delivery. A separate holder, approximately 3 cm longer, is also available for minimally invasive procedures. After implantation, the physician uses the a post-dilation catheter to expand the valve in situ.
Perceval’s approval was based upon the results of a pivotal European study performed to assess the device, the CAVALIER (Safety and Effectiveness Study of Perceval S Valve for Extended CE Mark) trial. The prospective, multicenter, nonrandomized clinical study was conducted at 26 investigational sites in Austria, Belgium, England, France, Germany, and the Netherlands.
Patients were treated between Feb. 23, 2010, and Sept. 30, 2013, and the database for the PDA-assessed data collected through Nov. 5, 2014, and included 658 patients.
The differences between the New York Heart Association (NYHA) class at 12 months and the baseline were calculated. In total, 77.5% of patients showed a decrease of NYHA equal to at least one class, whereas 19.7% of patients remained stable over the time. Only 2.8% of patients showed a worsened clinical status.
Reduction in mean gradients and increase in effective orifice area were both observed at 1 year follow-up, according to the FDA report.
At the 1-, 2-, and 3-year follow-up time points, 75.1% or greater of the implanted patients with available data had improved by one to three classes and at the 4-year follow-up time point 72.6% of the patients had improved by one to three classes, according to the report. In addition, at the 1-, 2-, and 3-year follow-up time points, 92.9% or greater of the patients were in NYHA Class I and Class II, and at the 4-year follow-up time point, 86.3% of the patients were in NYHA Class I and Class II.
Although there was a predominance of women in the study population (64.4%), patients of both sexes demonstrated acceptable hemodynamic outcomes and significant improvement in functional status, according to the FDA summary.
Sutureless valves are considered to have a promising future according to a recent international consensus panel recommendationpublished online in the European Journal of Cardio-Thoracic Surgery (2015 Oct 29. doi: 10.1093/ejcts/ezv369). The report assessed various benefits of sutureless and rapid deployment technology, and concluded these devices “may represent a helpful tool in aortic valve replacement for patients requiring a biological valve.” However, further evidence will be needed to reaffirm the benefit of sutureless and rapid deployment valves, they concluded.
LivaNova announced that the U.S. Food and Drug Administration has announced the approval of their Perceval Sutureless Heart Valve. The approval was issued Jan. 8 and is effective immediately, and LivaNova indicated that it will begin commercial distribution of the device in the United States over the coming quarter.
According to an FDA summary document, the Perceval Sutureless Heart Valve is a bioprosthetic valve designed to replace a diseased native or a malfunctioning prosthetic aortic valve via open heart surgery. The self-expanding stent frame consists of a tissue component made from bovine pericardium and a self-expandable nitinol stent, along with a dedicated delivery system that allows physicians to position and anchor the valve suturelessly.
The Perceval heart valve is supplied unmounted and must be loaded onto an accompanying holder by reducing the valve size using a supplied polycarbonate collapser. The holder is used for sternal approaches and includes a rigid shaft with an end section that houses the valve prosthesis during delivery. A separate holder, approximately 3 cm longer, is also available for minimally invasive procedures. After implantation, the physician uses the a post-dilation catheter to expand the valve in situ.
Perceval’s approval was based upon the results of a pivotal European study performed to assess the device, the CAVALIER (Safety and Effectiveness Study of Perceval S Valve for Extended CE Mark) trial. The prospective, multicenter, nonrandomized clinical study was conducted at 26 investigational sites in Austria, Belgium, England, France, Germany, and the Netherlands.
Patients were treated between Feb. 23, 2010, and Sept. 30, 2013, and the database for the PDA-assessed data collected through Nov. 5, 2014, and included 658 patients.
The differences between the New York Heart Association (NYHA) class at 12 months and the baseline were calculated. In total, 77.5% of patients showed a decrease of NYHA equal to at least one class, whereas 19.7% of patients remained stable over the time. Only 2.8% of patients showed a worsened clinical status.
Reduction in mean gradients and increase in effective orifice area were both observed at 1 year follow-up, according to the FDA report.
At the 1-, 2-, and 3-year follow-up time points, 75.1% or greater of the implanted patients with available data had improved by one to three classes and at the 4-year follow-up time point 72.6% of the patients had improved by one to three classes, according to the report. In addition, at the 1-, 2-, and 3-year follow-up time points, 92.9% or greater of the patients were in NYHA Class I and Class II, and at the 4-year follow-up time point, 86.3% of the patients were in NYHA Class I and Class II.
Although there was a predominance of women in the study population (64.4%), patients of both sexes demonstrated acceptable hemodynamic outcomes and significant improvement in functional status, according to the FDA summary.
Sutureless valves are considered to have a promising future according to a recent international consensus panel recommendationpublished online in the European Journal of Cardio-Thoracic Surgery (2015 Oct 29. doi: 10.1093/ejcts/ezv369). The report assessed various benefits of sutureless and rapid deployment technology, and concluded these devices “may represent a helpful tool in aortic valve replacement for patients requiring a biological valve.” However, further evidence will be needed to reaffirm the benefit of sutureless and rapid deployment valves, they concluded.
The palliative path: Talking with elderly patients facing emergency surgery
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
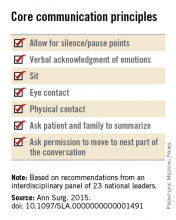
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
An expert panel has developed a communication framework to improve treatment of older, seriously ill patients who have surgical emergencies, which has been published online in Annals of Surgery.
A substantial portion of older patients who undergo emergency surgeries already have serious life-limiting illnesses such as cardiopulmonary disease, renal failure, liver failure, dementia, severe neurological impairment, or malignancy. The advisory panel based its work on the premise that surgery in these circumstances can lead to significant further morbidity, health care utilization, functional decline, prolonged hospital stay or institutionalization, and death, with attendant physical discomfort and psychological distress at the end of these patients’ lives.
Surgeons consulted in the emergency setting for these patients are hampered by patients unable to communicate well because they are in extremis, by surrogates who are unprepared for their role, and by time constraints, lack of familiarity with the patient, poor understanding of the illness by patients and families, prognostic uncertainty, and inadequate advance care planning. In addition, “many surgeons lack skills to engage in conversations about end-of-life care, or are too unfamiliar with palliative options to discuss them well,” or feel obligated to maintain postoperative life support despite the patient’s wishes, said Dr. Zara Cooper, of Ariadne Labs and the Center for Surgery and Public Health at Brigham and Women’s Hospital, both in Boston, and her associates.
To address these issues and assist surgeons in caring for such patients, an expert panel of 23 national leaders in acute care surgery, general surgery, surgical oncology, palliative medicine, critical care, emergency medicine, anesthesia, and health care innovation was convened at Harvard Medical School, Boston.
The focus of the panel’s recommendations was a structured communications framework prototype to facilitate shared decision-making in these difficult circumstances.
Among the panel’s recommendations for surgeons were the following priorities:
• Review the medical record and consult the treatment team to fully understand the patient’s current condition, comorbidities, expected illness trajectory, and preferences for end-of-life care.
• Assess functional performance as part of the routine history and physical to fully understand the patient’s fitness for surgery.
• Formulate a prognosis regarding the patient’s overall health both with and without surgery.
The panel offered a set of principles and specific elements for the meeting with the patient and family:
• The surgeon should begin by introducing himself or herself; according to reports in the literature, physicians fail to do this approximately half of the time.
• Pay attention to nonverbal communication, such as eye contact and physical contact, as this is critical to building rapport. Immediately address pain, anxiety, and other indicators of distress, to maximize the patients’ and the families’ engagement in subsequent medical discussions. “Although adequate analgesia may render a patient unable to make their own decisions, surrogates are more likely to make appropriate decisions when they feel their loved one is comfortable,” the panel noted.
• Allow pauses and silences to occur. Let the patient and the family process information and their own emotions.
• Elicit the patients’ or the surrogates’ understanding of the illness and their views of the patients’ likely trajectory, correcting any inaccuracies. This substantially influences their decisions regarding the aggressiveness of subsequent treatments.
• Inform the patient and family of the life-threatening nature of the patient’s acute condition and its potential impact on the rest of his or her life, including the possibility of prolonged life support, ICU stay, burdensome treatment, and loss of independence. Use accepted techniques for breaking bad news, and check to be sure the patient understands what was conveyed.
• At this point, the surgeon should synthesize and summarize the information from the patient, the family, and the medical record, then pause to give them time to process the information and to assess their emotional state. It is helpful to label and respond to the patient’s emotions at this juncture, and to build empathy with statements such as “I know this is difficult news, and I wish it were different.”
• Describe the benefits, burdens, and range of likely outcomes if surgery is undertaken and if it is not. The surgeon should use nonmedical language to describe symptoms, and should convey his or her expectations regarding length of hospitalization, need for and duration of life support, burdensome symptoms, discharge to an institution, and functional recovery.
• Surgeons should be able to communicate palliative options possible either in combination with surgery or instead of surgery. Palliative care can aid in managing advanced symptoms, providing psychosocial support for patients and caregivers, facilitating interdisciplinary communication, and facilitating medical decisions and care transitions.
• Avoid describing surgical procedures as “doing everything” and palliative care as “doing nothing.” This can make patients and families “feel abandoned, fearful, isolated, and angry, and fails to encompass palliative care’s practices of proactive communication, aggressive symptom management, and timely emotional support to alleviate suffering and affirm quality of life,” the panel said.
• Surgeons should explicitly support the patients’ medical decisions, whether or not they choose surgery.
The panel also cited a few factors that would assist surgeons in following these recommendations. First, surgeons must recognize the importance of communicating well with seriously ill older patients and acknowledge that this is a crucial clinical skill for them to cultivate. They must also recognize that palliative care is vital to delivering high-quality surgical care. Surgeons should consider discharging patients to hospice, which can improve pain and symptom management, improve patient and family satisfaction with care, and avoid unwanted hospitalization or cardiopulmonary resuscitation.
“There are a number of major barriers to introducing palliative care in these situations. One is an education problem - the perception on the part of patients and clinicians, and surgeons in particular, that palliative care is only limited to end-of-life care, which it is not. It is a misperception of what palliative care means in this equation - that palliative care and hospice are the same thing, which they absolutely are not,”said Dr. Cooper in an interview.
”The definition of palliative care has evolved over the past decade and the focus of palliative care is on quality of life and alleviating symptoms. End-of-life palliative care is part of that, and as patients get closer to the end of life, symptom management and quality of life become more focal than life-prolonging treatment... But for patients with chronic and serious illness, there has to be a role for palliative care because we know that when patients feel better, they tend to live longer. And when patients feel their emotional concerns and physical needs are being addressed, they tend to do better. Patients families have improved satisfaction when their loved one receives palliative care,” she noted.”
However, the number of palliative providers is completely inadequate to meet the needs of the number of seriously ill patients, she said. And a lot of hospital-based palliative care is by necessity limited to end-of-life care because of a lack of palliative resources.
Dr. Atul Gawande, a coauthor of the panel recommendations, wrote a best-selling book, Being Mortal (New York: Metropolitan Books, 2014) addressing the shortcomings and potential remaking of medical care in the context of age-related frailty, grave illness, and death. Dr. Cooper noted that there is a growing sentiment among the general public that they want to have their quality of life addressed in the type of medical care they receive. She said that Dr. Gawande’s book tapped into the perception of a lack of recognition of personhood of seriously ill patients.
“We often focus on diagnosis and we don’t have the ‘bandwidth’ to focus on the person carrying that diagnosis, and our patients and focus on the person carrying that diagnosis, but our patients and their families are demanding different types of care. So, ultimately, the patients will be the ones to push us to do better for them.”
The next steps to further developing a widely used and validated communication framework would be to create educational opportunities for clinicians to develop clinical skills in communication with seriously ill patients and palliative care, and to study the impact of these initiatives on improving outcomes most relevant to older patient. This work was supported by the Ariadne Labs, a Joint Center for Health System Innovation at Brigham and Women’s Hospital. Dr. Cooper and her associates reported having no relevant financial disclosures.
FROM ANNALS OF SURGERY
Meta-analysis backs SPRINT findings, argues for lower BP targets
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
FROM THE LANCET
Key clinical point: Blood pressure lowering is associated with significant reductions in vascular events in patients with a range of comorbidities and baseline blood pressures.
Major finding: Each 10–mm Hg reduction in systolic blood pressure is associated with a 20% reduction in major cardiovascular disease events.
Data source: A meta-analysis of 123 randomized controlled trials of blood pressure lowering treatment, involving a total of 613,815 participants.
Disclosures: Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
Aortic valve replacement: Transcatheter soars past surgical
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.
To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement (TAVR) since the procedure’s introduction in 2007 occurred in patients unsuited to a surgical approach because of their advanced age or elevated risk, according to an analysis published online Dec. 17 in the New England Journal of Medicine.
The surgical aortic valve replacement (SAVR) is still the standard of care, but many have questioned how the relatively new transcatheter approach has affected clinical practice overall, said Dr. Jochen Reinöhl of the Heart Center, University of Freiburg (Germany) and his associates.

To assess the evolution of treatment since TAVR was introduced, the investigators analyzed data from the Institute for the Hospital Remuneration System, which tracks all patient data regarding diagnoses, comorbidities, and procedures throughout the country. They focused on all 88,573 admissions for isolated surgical aortic valve replacements (55,992 procedures) and for isolated TAVR (32,581 procedures) performed in Germany during 2007-2013.
The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of SAVRs declined only slightly, from 8,622 to 7,048 per year. Patients aged 80 years and older accounted for almost all of the dramatic increase in transcatheter procedures, the investigators said (N Engl J Med. 2015 Dec 17;373:2438-47 [doi:10.1056/NEJMoa1500893]).
Overall in-hospital mortality was significantly higher with TAVR (6.5%) than with SAVR (2.8%), for an odds ratio of 2.41. This likely reflects the significantly greater risk of patients selected for TAVR, compared with those undergoing surgery, they said.
Mortality decreased over time in both patient groups, from 3.8% to 2.2% with surgery and from 13.2% to 5.4% with TAVR. In the case of TAVR, this decline is likely from a “learning curve” effect among clinicians, improvements in patient care, and advances in treatment devices. In the case of surgery, the mortality decline is probably due in part to the shift of high-risk patients from SAVR to the transcatheter approach, Dr. Reinöhl and his associates said.
Similarly, complications were significantly more common with TAVR. The need for permanent pacemaker implantation was the most frequently reported complication of TAVR, occurring in 17.7% of the transcatheter group but only 4.0% of the surgical group. Stroke rates (2.5% vs. 1.8%) and rates of acute kidney injury (5.5% vs. 3.0%) followed a similar pattern. In contrast, bleeding complications were more frequent with surgery (14.0% vs. 8.2%).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In Germany, almost all of the marked increase in the use of transcatheter aortic valve replacement occurred among patients unsuited for a surgical approach.
Major finding: The number of TAVR procedures increased markedly over time, from 144 to 9,147 per year, while the number of surgical procedures declined only slightly, from 8,622 to 7,048 per year.
Data source: A retrospective analysis of all 88,573 surgical and TAVR performed in Germany in 2007-2013.
Disclosures: This study was supported by the Heart Center at Freiburg University. Dr. Reinöhl and one of his associates reported receiving personal fees from Edwards Lifesciences and Direct Flow Medical.
Setting a new standard for aortic root repair?
Over the past 3 decades surgery for aortic root replacement has seen a dramatic decline in rates of death and complications, but there have been few studies comparing which technique would be best for specific patients, and those that have been done have been limited by selection bias or small patient numbers.
But a team of investigators from Weill Cornell Medical College in New York have analyzed results of three different aortic root replacement (ARR) procedures over a 17-year period and found that the rates of death during surgery and complications were less than 1% regardless of the technique. They published their results in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1120-9).

“In the current era, aortic root replacement can be performed with very low perioperative risk in high-volume aortic centers,” said Dr. Mario Gaudino and coauthors. “The type of operation performed does not affect early or late survival.”
They compared results of three different approaches to ARR performed in 890 consecutive patients in their institution from May 1997 to January 2014: mechanical composite valved graft (mCVG) in 289 patients; biologic composite valved graft (bCVG) in 421; and valve-sparing reconstruction (VSR) in 180. Then the researchers applied propensity matching to neutralize the differences in the baseline characteristics between the different procedures.
The overall rate of death from the operation was 0.2%, but the two patients who died did so in the first 5 years of the study. There were no deaths in the VSR group, and the incidence of complications after surgery was less than 0.5%. Three-year survival was 94.8% and 5-year survival was 89.4%, and reintervention rates at 5 years were 0% for the mCVG group, 2.4% for the bCVG group, and 7.3% for those who had VSR. “Although mCVG remains the gold standard for durability, bCVG and VSR are excellent options for those who either cannot take or wish to avoid long-term anticoagulation,” Dr. Gaudino and colleagues said.
At the time of surgery, 332 patients (37.3%) had at least one associated cardiac procedure, led by arch replacement (149 patients) and coronary artery bypass (81 patients). Eighty-four patients (9.4%) had two or more associated procedures. The bCVG and mCVG groups had the highest rates of associated cardiac procedures.
Before propensity matching, bCVG patients were older and had more comorbidities and worse functional class, while the mCVG group had higher rates of redo procedures and urgent or emergent operations. Connective tissue disorders were most common in the VSR group.
The results paralleled data from the Society of Thoracic Surgery’s Adult Cardiac Surgery Database, Dr. Gaudino and colleagues said, including a fivefold increase in the number of root replacements performed annually during the study period and a shift away from the traditional mCVG operation to widespread adoption of the bCVG and VSR procedures in the later years of the study.
“Surgeons with extensive experience in aortic surgery can tailor their choice of ARR to the procedure that best suits the individual patient based on their baseline characteristics,” Dr. Gaudino and coauthors said.
Dr. Gaudino and his coauthors had no disclosures.
The results of elective aortic root surgery that Dr. Mario Gaudino and his colleagues reported are “the most impressive … ever published and probably difficult, if not impossible, to reproduce,” Dr. Tirone David of the University of Toronto said in his invited commentary in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1130-1).
Dr. David cited the study’s low rates of deaths and complications. “They had only two deaths early on in their experience and no deaths among the most recent 804 patients operated on since 2002,” he said. “And there is more,” he said: only four strokes, one heart attack, one sternal infection, and only 11.2% of patients receiving blood transfusion. “And to make us ordinary surgeons even more envious, more than one-third of their patients had combined procedures,” Dr. David said.

|
Dr. Tirone David |
He said Dr. Gaudino and his colleagues have set a new standard for early outcomes of elective aortic root surgery. “These outcomes are difficult to emulate but we have to try,” Dr. David said. “To be an obsessive-compulsive surgeon who pays enormous attention to technical details is not enough because even patients who have perfectly executed operations may suffer serious and occasionally fatal postoperative complications.”
The results of elective aortic root surgery that Dr. Mario Gaudino and his colleagues reported are “the most impressive … ever published and probably difficult, if not impossible, to reproduce,” Dr. Tirone David of the University of Toronto said in his invited commentary in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1130-1).
Dr. David cited the study’s low rates of deaths and complications. “They had only two deaths early on in their experience and no deaths among the most recent 804 patients operated on since 2002,” he said. “And there is more,” he said: only four strokes, one heart attack, one sternal infection, and only 11.2% of patients receiving blood transfusion. “And to make us ordinary surgeons even more envious, more than one-third of their patients had combined procedures,” Dr. David said.

|
Dr. Tirone David |
He said Dr. Gaudino and his colleagues have set a new standard for early outcomes of elective aortic root surgery. “These outcomes are difficult to emulate but we have to try,” Dr. David said. “To be an obsessive-compulsive surgeon who pays enormous attention to technical details is not enough because even patients who have perfectly executed operations may suffer serious and occasionally fatal postoperative complications.”
The results of elective aortic root surgery that Dr. Mario Gaudino and his colleagues reported are “the most impressive … ever published and probably difficult, if not impossible, to reproduce,” Dr. Tirone David of the University of Toronto said in his invited commentary in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1130-1).
Dr. David cited the study’s low rates of deaths and complications. “They had only two deaths early on in their experience and no deaths among the most recent 804 patients operated on since 2002,” he said. “And there is more,” he said: only four strokes, one heart attack, one sternal infection, and only 11.2% of patients receiving blood transfusion. “And to make us ordinary surgeons even more envious, more than one-third of their patients had combined procedures,” Dr. David said.

|
Dr. Tirone David |
He said Dr. Gaudino and his colleagues have set a new standard for early outcomes of elective aortic root surgery. “These outcomes are difficult to emulate but we have to try,” Dr. David said. “To be an obsessive-compulsive surgeon who pays enormous attention to technical details is not enough because even patients who have perfectly executed operations may suffer serious and occasionally fatal postoperative complications.”
Over the past 3 decades surgery for aortic root replacement has seen a dramatic decline in rates of death and complications, but there have been few studies comparing which technique would be best for specific patients, and those that have been done have been limited by selection bias or small patient numbers.
But a team of investigators from Weill Cornell Medical College in New York have analyzed results of three different aortic root replacement (ARR) procedures over a 17-year period and found that the rates of death during surgery and complications were less than 1% regardless of the technique. They published their results in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1120-9).

“In the current era, aortic root replacement can be performed with very low perioperative risk in high-volume aortic centers,” said Dr. Mario Gaudino and coauthors. “The type of operation performed does not affect early or late survival.”
They compared results of three different approaches to ARR performed in 890 consecutive patients in their institution from May 1997 to January 2014: mechanical composite valved graft (mCVG) in 289 patients; biologic composite valved graft (bCVG) in 421; and valve-sparing reconstruction (VSR) in 180. Then the researchers applied propensity matching to neutralize the differences in the baseline characteristics between the different procedures.
The overall rate of death from the operation was 0.2%, but the two patients who died did so in the first 5 years of the study. There were no deaths in the VSR group, and the incidence of complications after surgery was less than 0.5%. Three-year survival was 94.8% and 5-year survival was 89.4%, and reintervention rates at 5 years were 0% for the mCVG group, 2.4% for the bCVG group, and 7.3% for those who had VSR. “Although mCVG remains the gold standard for durability, bCVG and VSR are excellent options for those who either cannot take or wish to avoid long-term anticoagulation,” Dr. Gaudino and colleagues said.
At the time of surgery, 332 patients (37.3%) had at least one associated cardiac procedure, led by arch replacement (149 patients) and coronary artery bypass (81 patients). Eighty-four patients (9.4%) had two or more associated procedures. The bCVG and mCVG groups had the highest rates of associated cardiac procedures.
Before propensity matching, bCVG patients were older and had more comorbidities and worse functional class, while the mCVG group had higher rates of redo procedures and urgent or emergent operations. Connective tissue disorders were most common in the VSR group.
The results paralleled data from the Society of Thoracic Surgery’s Adult Cardiac Surgery Database, Dr. Gaudino and colleagues said, including a fivefold increase in the number of root replacements performed annually during the study period and a shift away from the traditional mCVG operation to widespread adoption of the bCVG and VSR procedures in the later years of the study.
“Surgeons with extensive experience in aortic surgery can tailor their choice of ARR to the procedure that best suits the individual patient based on their baseline characteristics,” Dr. Gaudino and coauthors said.
Dr. Gaudino and his coauthors had no disclosures.
Over the past 3 decades surgery for aortic root replacement has seen a dramatic decline in rates of death and complications, but there have been few studies comparing which technique would be best for specific patients, and those that have been done have been limited by selection bias or small patient numbers.
But a team of investigators from Weill Cornell Medical College in New York have analyzed results of three different aortic root replacement (ARR) procedures over a 17-year period and found that the rates of death during surgery and complications were less than 1% regardless of the technique. They published their results in the Journal of Thoracic and Cardiovascular Surgery (2015;150:1120-9).

“In the current era, aortic root replacement can be performed with very low perioperative risk in high-volume aortic centers,” said Dr. Mario Gaudino and coauthors. “The type of operation performed does not affect early or late survival.”
They compared results of three different approaches to ARR performed in 890 consecutive patients in their institution from May 1997 to January 2014: mechanical composite valved graft (mCVG) in 289 patients; biologic composite valved graft (bCVG) in 421; and valve-sparing reconstruction (VSR) in 180. Then the researchers applied propensity matching to neutralize the differences in the baseline characteristics between the different procedures.
The overall rate of death from the operation was 0.2%, but the two patients who died did so in the first 5 years of the study. There were no deaths in the VSR group, and the incidence of complications after surgery was less than 0.5%. Three-year survival was 94.8% and 5-year survival was 89.4%, and reintervention rates at 5 years were 0% for the mCVG group, 2.4% for the bCVG group, and 7.3% for those who had VSR. “Although mCVG remains the gold standard for durability, bCVG and VSR are excellent options for those who either cannot take or wish to avoid long-term anticoagulation,” Dr. Gaudino and colleagues said.
At the time of surgery, 332 patients (37.3%) had at least one associated cardiac procedure, led by arch replacement (149 patients) and coronary artery bypass (81 patients). Eighty-four patients (9.4%) had two or more associated procedures. The bCVG and mCVG groups had the highest rates of associated cardiac procedures.
Before propensity matching, bCVG patients were older and had more comorbidities and worse functional class, while the mCVG group had higher rates of redo procedures and urgent or emergent operations. Connective tissue disorders were most common in the VSR group.
The results paralleled data from the Society of Thoracic Surgery’s Adult Cardiac Surgery Database, Dr. Gaudino and colleagues said, including a fivefold increase in the number of root replacements performed annually during the study period and a shift away from the traditional mCVG operation to widespread adoption of the bCVG and VSR procedures in the later years of the study.
“Surgeons with extensive experience in aortic surgery can tailor their choice of ARR to the procedure that best suits the individual patient based on their baseline characteristics,” Dr. Gaudino and coauthors said.
Dr. Gaudino and his coauthors had no disclosures.
Key clinical point: Aortic root replacement surgery can be performed with minimal risk of death and complications regardless of the approach taken.
Major finding: Overall rate of death was 0.2%, with none since 2002, and the overall rate of complications was below 0.5% in the study cohort.
Data source: Review of prospective data on 890 aortic root replacement operations performed over a 17-year period at a single center.
Disclosures: The study authors had no relationships to disclose.
ACC/AHA risk estimator underpredicts in HIV+ individuals
ORLANDO – The 2013 ACC/AHA atherosclerotic cardiovascular disease risk calculator isn’t reliably applicable to HIV-positive adults in its present form because it consistently underpredicts their MI risk, Dr. Michael J. Feinstein reported at the American Heart Association scientific sessions.
That’s the bad news. The good news is that “a simple, data-derived refit of the pooled cohort equations may improve the model’s performance in HIV-positive individuals,” said Dr. Feinstein of Northwestern University, Chicago.
Tweaking the risk calculator to enhance its accuracy in the HIV-positive population is particularly important because this population is growing in size and aging. And as Dr. Feinstein and coinvestigators showed in another study presented at the AHA meeting, the proportion of deaths due to cardiovascular disease in HIV-positive adults is shooting upward as they live longer because of treatment advances.
The investigators’ analysis of data from the Centers for Disease Control and Prevention national Wonder database showed that the proportion of deaths due to cardiovascular disease more than doubled between 1999 and 2013. Meanwhile, proportionate cardiovascular disease mortality declined by 22% in the general population and by 28% among individuals with inflammatory polyarthropathies.
That the ACC/AHA risk calculator in its present form doesn’t perform adequately in HIV-positive individuals hadn’t previously been shown, but it doesn’t really come as a surprise, according to Dr. Feinstein. After all, it’s known that this population is at 1.5- to 2-fold increased risk for MI and roughly 5-fold increased risk for sudden cardiac death, compared to the general population, where the risk calculator works best.
“Most data suggest that even in the setting of optimally treated HIV and undetectable viral load there’s still an underlying viral reservoir that appears to be driving inflammation and atherothrombotic and even nonatherothrombotic events in this population,” he said.
Dr. Feinstein and coworkers evaluated the 2013 ACC/AHA risk calculator in 11,901 HIV-positive black or white adults enrolled in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) database for whom 5-year follow-up was available; 52% of the subjects were aged 40 or older at baseline.
Running each of these nearly 12,000 subjects through the risk calculator, the predicted result was that 103 of them would have an acute MI during the 5-year follow-up period. In reality, 132 MIs were observed. The discrepancy between the risk calculator predictions and observed MI rates was greatest in the 63% of HIV-positive subjects deemed at low risk, with an estimated 10-year risk of atherosclerotic cardiovascular disease of less than 5%.
Among white men, the risk calculator was remarkably consistent in underpredicting MIs. Regardless of whether the risk calculator put their estimated 10-year risk at less than 5%, 5% to less than 7.5%, 7.5% to less than 10%, or at least 10%, the actual observed MI rates were 67%-68% greater across the board than predicted.
Dr. Feinstein and coworkers refit the ACC/AHA risk calculator on a trial basis by incorporating variables related to HIV-positivity into the risk equations. Then they reanalyzed the tool’s performance in the same nearly 12,000 HIV-infected subjects. They found the discrimination and calibration of the revised risk equations improved substantially and met the standard of “acceptable” by statisticians’ standards.
The next step in this ongoing CNICS project will be to validate the provisionally refit risk calculator’s performance in a separate database of HIV-infected adults with adjudicated MIs. If the results are again positive, it will be a relatively straightforward matter to introduce the revisions into the ACC/AHA risk calculator, particularly since the senior coinvestigator in this project is Dr. Donald M. Lloyd-Jones, also of Northwestern University, who played a central role in developing the 2013 risk calculator.
Dr. Feinstein reported having no financial conflicts regarding this study.
ORLANDO – The 2013 ACC/AHA atherosclerotic cardiovascular disease risk calculator isn’t reliably applicable to HIV-positive adults in its present form because it consistently underpredicts their MI risk, Dr. Michael J. Feinstein reported at the American Heart Association scientific sessions.
That’s the bad news. The good news is that “a simple, data-derived refit of the pooled cohort equations may improve the model’s performance in HIV-positive individuals,” said Dr. Feinstein of Northwestern University, Chicago.
Tweaking the risk calculator to enhance its accuracy in the HIV-positive population is particularly important because this population is growing in size and aging. And as Dr. Feinstein and coinvestigators showed in another study presented at the AHA meeting, the proportion of deaths due to cardiovascular disease in HIV-positive adults is shooting upward as they live longer because of treatment advances.
The investigators’ analysis of data from the Centers for Disease Control and Prevention national Wonder database showed that the proportion of deaths due to cardiovascular disease more than doubled between 1999 and 2013. Meanwhile, proportionate cardiovascular disease mortality declined by 22% in the general population and by 28% among individuals with inflammatory polyarthropathies.
That the ACC/AHA risk calculator in its present form doesn’t perform adequately in HIV-positive individuals hadn’t previously been shown, but it doesn’t really come as a surprise, according to Dr. Feinstein. After all, it’s known that this population is at 1.5- to 2-fold increased risk for MI and roughly 5-fold increased risk for sudden cardiac death, compared to the general population, where the risk calculator works best.
“Most data suggest that even in the setting of optimally treated HIV and undetectable viral load there’s still an underlying viral reservoir that appears to be driving inflammation and atherothrombotic and even nonatherothrombotic events in this population,” he said.
Dr. Feinstein and coworkers evaluated the 2013 ACC/AHA risk calculator in 11,901 HIV-positive black or white adults enrolled in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) database for whom 5-year follow-up was available; 52% of the subjects were aged 40 or older at baseline.
Running each of these nearly 12,000 subjects through the risk calculator, the predicted result was that 103 of them would have an acute MI during the 5-year follow-up period. In reality, 132 MIs were observed. The discrepancy between the risk calculator predictions and observed MI rates was greatest in the 63% of HIV-positive subjects deemed at low risk, with an estimated 10-year risk of atherosclerotic cardiovascular disease of less than 5%.
Among white men, the risk calculator was remarkably consistent in underpredicting MIs. Regardless of whether the risk calculator put their estimated 10-year risk at less than 5%, 5% to less than 7.5%, 7.5% to less than 10%, or at least 10%, the actual observed MI rates were 67%-68% greater across the board than predicted.
Dr. Feinstein and coworkers refit the ACC/AHA risk calculator on a trial basis by incorporating variables related to HIV-positivity into the risk equations. Then they reanalyzed the tool’s performance in the same nearly 12,000 HIV-infected subjects. They found the discrimination and calibration of the revised risk equations improved substantially and met the standard of “acceptable” by statisticians’ standards.
The next step in this ongoing CNICS project will be to validate the provisionally refit risk calculator’s performance in a separate database of HIV-infected adults with adjudicated MIs. If the results are again positive, it will be a relatively straightforward matter to introduce the revisions into the ACC/AHA risk calculator, particularly since the senior coinvestigator in this project is Dr. Donald M. Lloyd-Jones, also of Northwestern University, who played a central role in developing the 2013 risk calculator.
Dr. Feinstein reported having no financial conflicts regarding this study.
ORLANDO – The 2013 ACC/AHA atherosclerotic cardiovascular disease risk calculator isn’t reliably applicable to HIV-positive adults in its present form because it consistently underpredicts their MI risk, Dr. Michael J. Feinstein reported at the American Heart Association scientific sessions.
That’s the bad news. The good news is that “a simple, data-derived refit of the pooled cohort equations may improve the model’s performance in HIV-positive individuals,” said Dr. Feinstein of Northwestern University, Chicago.
Tweaking the risk calculator to enhance its accuracy in the HIV-positive population is particularly important because this population is growing in size and aging. And as Dr. Feinstein and coinvestigators showed in another study presented at the AHA meeting, the proportion of deaths due to cardiovascular disease in HIV-positive adults is shooting upward as they live longer because of treatment advances.
The investigators’ analysis of data from the Centers for Disease Control and Prevention national Wonder database showed that the proportion of deaths due to cardiovascular disease more than doubled between 1999 and 2013. Meanwhile, proportionate cardiovascular disease mortality declined by 22% in the general population and by 28% among individuals with inflammatory polyarthropathies.
That the ACC/AHA risk calculator in its present form doesn’t perform adequately in HIV-positive individuals hadn’t previously been shown, but it doesn’t really come as a surprise, according to Dr. Feinstein. After all, it’s known that this population is at 1.5- to 2-fold increased risk for MI and roughly 5-fold increased risk for sudden cardiac death, compared to the general population, where the risk calculator works best.
“Most data suggest that even in the setting of optimally treated HIV and undetectable viral load there’s still an underlying viral reservoir that appears to be driving inflammation and atherothrombotic and even nonatherothrombotic events in this population,” he said.
Dr. Feinstein and coworkers evaluated the 2013 ACC/AHA risk calculator in 11,901 HIV-positive black or white adults enrolled in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) database for whom 5-year follow-up was available; 52% of the subjects were aged 40 or older at baseline.
Running each of these nearly 12,000 subjects through the risk calculator, the predicted result was that 103 of them would have an acute MI during the 5-year follow-up period. In reality, 132 MIs were observed. The discrepancy between the risk calculator predictions and observed MI rates was greatest in the 63% of HIV-positive subjects deemed at low risk, with an estimated 10-year risk of atherosclerotic cardiovascular disease of less than 5%.
Among white men, the risk calculator was remarkably consistent in underpredicting MIs. Regardless of whether the risk calculator put their estimated 10-year risk at less than 5%, 5% to less than 7.5%, 7.5% to less than 10%, or at least 10%, the actual observed MI rates were 67%-68% greater across the board than predicted.
Dr. Feinstein and coworkers refit the ACC/AHA risk calculator on a trial basis by incorporating variables related to HIV-positivity into the risk equations. Then they reanalyzed the tool’s performance in the same nearly 12,000 HIV-infected subjects. They found the discrimination and calibration of the revised risk equations improved substantially and met the standard of “acceptable” by statisticians’ standards.
The next step in this ongoing CNICS project will be to validate the provisionally refit risk calculator’s performance in a separate database of HIV-infected adults with adjudicated MIs. If the results are again positive, it will be a relatively straightforward matter to introduce the revisions into the ACC/AHA risk calculator, particularly since the senior coinvestigator in this project is Dr. Donald M. Lloyd-Jones, also of Northwestern University, who played a central role in developing the 2013 risk calculator.
Dr. Feinstein reported having no financial conflicts regarding this study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The 2013 ACC/AHA risk estimator needs a tune-up before it can be reliably applied to HIV-infected adults.
Major finding: Observed rates of acute MI over time in HIV-positive white men were 67%-68% higher than predicted by the 2013 ACC/AHA atherosclerotic cardiovascular disease risk calculator regardless of whether the men were deemed at low, intermediate, or high baseline risk.
Data source: A study comparing the predicted number of acute MIs in a population of nearly 12,000 HIV-positive adults over a 5-year period as determined via the 2013 ACC/AHA atherosclerotic cardiovascular disease risk calculator with the actual observed number of adjudicated MIs.
Disclosures: The study was conducted free of commercial support. The presenter reported having no financial conflicts of interest.