User login
Methylphenidate Linked to Small Increase in CV Event Risk
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
TOPLINE:
Methylphenidate was associated with a small increased risk for cardiovascular events in individuals taking the drug for more than 6 months in a new cohort study.
METHODOLOGY:
- The retrospective, population-based cohort study was based on national Swedish registry data and included 26,710 patients with attention-deficit/hyperactivity disorder (ADHD) aged 12-60 years (median age 20) who had been prescribed methylphenidate between 2007 and 2012. They were each matched on birth date, sex, and county with up to 10 nonusers without ADHD (a total of 225,672 controls).
- Rates of cardiovascular events, including ischemic heart disease, venous thromboembolism, heart failure, or tachyarrhythmias 1 year before methylphenidate treatment and 6 months after treatment initiation were compared between individuals receiving methylphenidate and matched controls using a Bayesian within-individual design.
TAKEAWAY:
- The overall incidence of cardiovascular events was 1.51 per 10,000 person-weeks for individuals receiving methylphenidate and 0.77 for the matched controls.
- Individuals taking methylphenidate had a 70% posterior probability for a greater than 10% increased risk for cardiovascular events than controls and a 49% posterior probability for an increased risk larger than 20%.
- No difference was found in this risk between individuals with and without a history of cardiovascular disease.
IN PRACTICE:
The researchers concluded that these results support a small (10%) increased risk for cardiovascular events in individuals receiving methylphenidate compared with matched controls after 6 months of treatment. The probability of finding a difference in risk between users and nonusers decreased when considering risk for 20% or larger, with no evidence of differences between those with and without a history of cardiovascular disease. They said the findings suggest the decision to initiate methylphenidate should incorporate considerations of potential adverse cardiovascular effects among the broader benefits and risks for treatment for individual patients.
SOURCE:
The study, led by Miguel Garcia-Argibay, PhD, Örebro University, Örebro, Sweden, was published online in JAMA Network Open on March 6.
LIMITATIONS:
The data were observational, and thus, causality could not be inferred. Lack of information on methylphenidate dose meant that it was not possible to assess a dose effect. Compliance with the medication was also not known, and the association may therefore have been underestimated. The findings of this study were based on data collected from a Swedish population, which may not be representative of other populations.
DISCLOSURES:
The study received funding from the European Union’s Horizon 2020 research and innovation program and the Swedish Research Council for Health, Working Life, and Welfare.
A version of this article appeared on Medscape.com.
No End in Sight for National ADHD Drug Shortage
Nearly 18 months after the US Food and Drug Administration (FDA) first acknowledged a national shortage of Adderall, the most common drug used to treat attention-deficit/hyperactivity disorder (ADHD),
The first shortage of immediate release formulations of amphetamine mixed salts (Adderall, Adderall IR) was reported by the FDA in October 2022. Now, the list includes Focalin, Ritalin, and Vyvanse, among others.
Adding to the ongoing crisis, the FDA announced in early February that Azurity Pharmaceuticals was voluntarily withdrawing one lot of its Zenzedi (dextroamphetamine sulfate) 30 mg tablets because of contamination with the antihistamine, carbinoxamine.
For the roughly 10 million adults and 6 million children in the United States grappling with ADHD, getting a prescription filled with the exact medication ordered by a physician is dictated by geographic location, insurance formularies, and pharmacy supply chains. It’s particularly challenging for those who live in rural or underserved areas with limited access to nearby pharmacies.
“Not a day goes by when I don’t hear from a number of unfortunately struggling patients about this shortage,” said Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, Maryland.
The ADHD drug shortage is now well into its second year, and clinicians and advocates alike say there is no apparent end in sight.
How Did We Get Here?
Manufacturers and federal agencies blame the shortage on rising demand and each other, while clinicians say that insurers, drug distributors, and middlemen are also playing a role in keeping medications out of patients’ hands.
In August 2023, the Drug Enforcement Administration (DEA), which sets quotas for the production of controlled substances, and the FDA publicly blamed manufacturers for the shortages, claiming they were not using up their allocations.
At the time, the DEA said manufacturers made and sold only 70% of their quota, nearly 1 billion doses short of what they were allowed to produce and ship that year.
The agencies also noted a record-high number of prescriptions for stimulants from 2012 to 2021. Driven in part by telehealth, the demand intensified during the pandemic. One recent study reported a 14% increase in ADHD stimulant prescriptions between 2020 and 2022.
Insurers also play a role in the shortage, David Goodman, MD, an assistant professor of psychiatry and behavioral sciences also at Johns Hopkins University, told this news organization.
Stepped therapy — in which patients must try one, two, or three medications before they are authorized to receive a more expensive or newer drug — contributes to the problem, Dr. Goodman said. Demand for such medications is high and supply low. In addition, some insurers only provide coverage for in-network pharmacies, regardless of the ability of other providers outside such networks to fill prescriptions.
“If the insurance dictates where you get your pills, and that pharmacy doesn’t have the pills or that pharmacy chain in your area doesn’t have those pills, you’re out of luck,” Dr. Goodman said.
Patients as Detectives
To get prescriptions filled, patients must “turn into detectives,” Laurie Kulikosky, CEO of Children and Adults with Attention-Deficit/Hyperactivity Disorder, told this news organization. “It’s a huge stressor.”
Tracking which ADHD medications are available, on back order, or discontinued requires frequent checking of the FDA’s drug shortages website.
Some manufacturers of generic versions of mixed amphetamine salts are only fulfilling orders for existing contracts, while others say new product won’t be available until at least April or as late as September. All blame the delay on the shortage of active ingredients.
Teva, which makes both the brand and generic of Adderall, reported on the FDA’s site that its manufacturing and distribution is at record-high levels, but demand continues to rise.
The branded Concerta is available, but some makers of generic methylphenidate reported supplies won’t be available until July.
Lisdexamfetamine dimesylate in almost all dosages is either unavailable, available in restricted quantities, or on extended back order. However, the branded product Vyvanse is available.
Industry, Government Respond
In a November 2023 statement, the DEA reported that 17 of 18 drug manufacturers the agency contacted planned to use their full DEA quota and increase production for that year. The agency said it had made it easier for manufacturers to request changes in allocations and that periodically updating quotas was a possibility.
This news organization asked the DEA whether any manufacturers had not met their 2023 quotas, but an agency spokesperson said it would not comment.
An FDA spokesperson said it could help manufacturers ask for bigger quotas and to increase production, noting that in 2023, the DEA increased the quota for methylphenidate following an FDA request.
“The FDA is in frequent communication with the manufacturers of ADHD stimulant medications and the DEA, and we will continue to monitor supply,” the spokesperson said.
For 2024, the FDA told the DEA that it predicted a 3.1% increase in use of amphetamine, methylphenidate (including dexmethylphenidate), and lisdexamfetamine. The DEA took that into account when it issued its final quotas for 2024. Whether those amounts will be enough remains to be seen.
With many drugs — not just those for ADHD — in short supply, in February, the US Department of Health and Human Services (HHS) and the Federal Trade Commission opened an inquiry of sorts, seeking comments on how middlemen and others were influencing pricing and supply of generic drugs.
“When you’re prescribed an important medication by your doctor and you learn the drug is out of stock, your heart sinks,” HHS Secretary Xavier Becerra said in a statement. “This devastating reality is the case for too many Americans who need generic drugs for ADHD, cancer, and other conditions.”
On the comments site, which is open until April 15, many of the 4000-plus complaints filed to-date are from individuals with ADHD.
Dr. Pawar said clinicians can’t know what’s going on between the FDA, the DEA, and manufacturers, adding that, “they need to sit together and figure something out.”
Even Members of Congress have had trouble getting answers. In October, Rep. Abigail Spanberger (D-Virginia) and a dozen colleagues wrote to the FDA and DEA seeking information on how the agencies were responding to stimulant shortages. The DEA has still not replied.
Workarounds the Only Option?
In the past, physicians would prescribe the optimal medication for individual patients based on clinical factors. Now, one of the major factors in determining drug choice is the agent that has “the highest likelihood of benefit and the lowest likelihood of administrative demand or burden,” Dr. Goodman said.
With so many medications in short supply, clinicians have figured out workarounds to get prescriptions filled, but they don’t often pan out.
If a patient needs a 60-mg daily dose of a medication and the pharmacy doesn’t have any 60-mg pills, Dr. Goodman said he might write a prescription for a 30-mg pill to be taken twice a day. However, insurers often will cover only 30 pills for a month, which can thwart this strategy.
Dr. Pawar said he sometimes prescribes Journay PM in lieu of Concerta because it is often available. But insurers may deny coverage of Journay PM because it is a newer medication, he said. When prescribing ADHD medications, he also provides his patients with a list of potential substitutes so they can ask the pharmacist if any are in stock.
With no end to the shortage in sight, clinicians must often prescribe multiple medications until their patients are able to find one that’s available. In addition, patients are burdened with making calls and visits to multiple pharmacies until they find one that can fill their prescription.
Meanwhile, the ripple effects to the ADHD drug shortage continue to spread. Extended periods without treatment can lead to declining job performance or job loss, fractured relationships, and even financial distress, Dr. Goodman said.
“If you go without your pills for a month and you’re not performing, your work declines and you lose your job as a result, that’s not on you — that’s on the fact that you can’t get your treatment,” he noted. “The shortage is no longer an inconvenience.”
Dr. Goodman, Dr. Pawar, and Ms. Kulikosky reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Nearly 18 months after the US Food and Drug Administration (FDA) first acknowledged a national shortage of Adderall, the most common drug used to treat attention-deficit/hyperactivity disorder (ADHD),
The first shortage of immediate release formulations of amphetamine mixed salts (Adderall, Adderall IR) was reported by the FDA in October 2022. Now, the list includes Focalin, Ritalin, and Vyvanse, among others.
Adding to the ongoing crisis, the FDA announced in early February that Azurity Pharmaceuticals was voluntarily withdrawing one lot of its Zenzedi (dextroamphetamine sulfate) 30 mg tablets because of contamination with the antihistamine, carbinoxamine.
For the roughly 10 million adults and 6 million children in the United States grappling with ADHD, getting a prescription filled with the exact medication ordered by a physician is dictated by geographic location, insurance formularies, and pharmacy supply chains. It’s particularly challenging for those who live in rural or underserved areas with limited access to nearby pharmacies.
“Not a day goes by when I don’t hear from a number of unfortunately struggling patients about this shortage,” said Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, Maryland.
The ADHD drug shortage is now well into its second year, and clinicians and advocates alike say there is no apparent end in sight.
How Did We Get Here?
Manufacturers and federal agencies blame the shortage on rising demand and each other, while clinicians say that insurers, drug distributors, and middlemen are also playing a role in keeping medications out of patients’ hands.
In August 2023, the Drug Enforcement Administration (DEA), which sets quotas for the production of controlled substances, and the FDA publicly blamed manufacturers for the shortages, claiming they were not using up their allocations.
At the time, the DEA said manufacturers made and sold only 70% of their quota, nearly 1 billion doses short of what they were allowed to produce and ship that year.
The agencies also noted a record-high number of prescriptions for stimulants from 2012 to 2021. Driven in part by telehealth, the demand intensified during the pandemic. One recent study reported a 14% increase in ADHD stimulant prescriptions between 2020 and 2022.
Insurers also play a role in the shortage, David Goodman, MD, an assistant professor of psychiatry and behavioral sciences also at Johns Hopkins University, told this news organization.
Stepped therapy — in which patients must try one, two, or three medications before they are authorized to receive a more expensive or newer drug — contributes to the problem, Dr. Goodman said. Demand for such medications is high and supply low. In addition, some insurers only provide coverage for in-network pharmacies, regardless of the ability of other providers outside such networks to fill prescriptions.
“If the insurance dictates where you get your pills, and that pharmacy doesn’t have the pills or that pharmacy chain in your area doesn’t have those pills, you’re out of luck,” Dr. Goodman said.
Patients as Detectives
To get prescriptions filled, patients must “turn into detectives,” Laurie Kulikosky, CEO of Children and Adults with Attention-Deficit/Hyperactivity Disorder, told this news organization. “It’s a huge stressor.”
Tracking which ADHD medications are available, on back order, or discontinued requires frequent checking of the FDA’s drug shortages website.
Some manufacturers of generic versions of mixed amphetamine salts are only fulfilling orders for existing contracts, while others say new product won’t be available until at least April or as late as September. All blame the delay on the shortage of active ingredients.
Teva, which makes both the brand and generic of Adderall, reported on the FDA’s site that its manufacturing and distribution is at record-high levels, but demand continues to rise.
The branded Concerta is available, but some makers of generic methylphenidate reported supplies won’t be available until July.
Lisdexamfetamine dimesylate in almost all dosages is either unavailable, available in restricted quantities, or on extended back order. However, the branded product Vyvanse is available.
Industry, Government Respond
In a November 2023 statement, the DEA reported that 17 of 18 drug manufacturers the agency contacted planned to use their full DEA quota and increase production for that year. The agency said it had made it easier for manufacturers to request changes in allocations and that periodically updating quotas was a possibility.
This news organization asked the DEA whether any manufacturers had not met their 2023 quotas, but an agency spokesperson said it would not comment.
An FDA spokesperson said it could help manufacturers ask for bigger quotas and to increase production, noting that in 2023, the DEA increased the quota for methylphenidate following an FDA request.
“The FDA is in frequent communication with the manufacturers of ADHD stimulant medications and the DEA, and we will continue to monitor supply,” the spokesperson said.
For 2024, the FDA told the DEA that it predicted a 3.1% increase in use of amphetamine, methylphenidate (including dexmethylphenidate), and lisdexamfetamine. The DEA took that into account when it issued its final quotas for 2024. Whether those amounts will be enough remains to be seen.
With many drugs — not just those for ADHD — in short supply, in February, the US Department of Health and Human Services (HHS) and the Federal Trade Commission opened an inquiry of sorts, seeking comments on how middlemen and others were influencing pricing and supply of generic drugs.
“When you’re prescribed an important medication by your doctor and you learn the drug is out of stock, your heart sinks,” HHS Secretary Xavier Becerra said in a statement. “This devastating reality is the case for too many Americans who need generic drugs for ADHD, cancer, and other conditions.”
On the comments site, which is open until April 15, many of the 4000-plus complaints filed to-date are from individuals with ADHD.
Dr. Pawar said clinicians can’t know what’s going on between the FDA, the DEA, and manufacturers, adding that, “they need to sit together and figure something out.”
Even Members of Congress have had trouble getting answers. In October, Rep. Abigail Spanberger (D-Virginia) and a dozen colleagues wrote to the FDA and DEA seeking information on how the agencies were responding to stimulant shortages. The DEA has still not replied.
Workarounds the Only Option?
In the past, physicians would prescribe the optimal medication for individual patients based on clinical factors. Now, one of the major factors in determining drug choice is the agent that has “the highest likelihood of benefit and the lowest likelihood of administrative demand or burden,” Dr. Goodman said.
With so many medications in short supply, clinicians have figured out workarounds to get prescriptions filled, but they don’t often pan out.
If a patient needs a 60-mg daily dose of a medication and the pharmacy doesn’t have any 60-mg pills, Dr. Goodman said he might write a prescription for a 30-mg pill to be taken twice a day. However, insurers often will cover only 30 pills for a month, which can thwart this strategy.
Dr. Pawar said he sometimes prescribes Journay PM in lieu of Concerta because it is often available. But insurers may deny coverage of Journay PM because it is a newer medication, he said. When prescribing ADHD medications, he also provides his patients with a list of potential substitutes so they can ask the pharmacist if any are in stock.
With no end to the shortage in sight, clinicians must often prescribe multiple medications until their patients are able to find one that’s available. In addition, patients are burdened with making calls and visits to multiple pharmacies until they find one that can fill their prescription.
Meanwhile, the ripple effects to the ADHD drug shortage continue to spread. Extended periods without treatment can lead to declining job performance or job loss, fractured relationships, and even financial distress, Dr. Goodman said.
“If you go without your pills for a month and you’re not performing, your work declines and you lose your job as a result, that’s not on you — that’s on the fact that you can’t get your treatment,” he noted. “The shortage is no longer an inconvenience.”
Dr. Goodman, Dr. Pawar, and Ms. Kulikosky reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Nearly 18 months after the US Food and Drug Administration (FDA) first acknowledged a national shortage of Adderall, the most common drug used to treat attention-deficit/hyperactivity disorder (ADHD),
The first shortage of immediate release formulations of amphetamine mixed salts (Adderall, Adderall IR) was reported by the FDA in October 2022. Now, the list includes Focalin, Ritalin, and Vyvanse, among others.
Adding to the ongoing crisis, the FDA announced in early February that Azurity Pharmaceuticals was voluntarily withdrawing one lot of its Zenzedi (dextroamphetamine sulfate) 30 mg tablets because of contamination with the antihistamine, carbinoxamine.
For the roughly 10 million adults and 6 million children in the United States grappling with ADHD, getting a prescription filled with the exact medication ordered by a physician is dictated by geographic location, insurance formularies, and pharmacy supply chains. It’s particularly challenging for those who live in rural or underserved areas with limited access to nearby pharmacies.
“Not a day goes by when I don’t hear from a number of unfortunately struggling patients about this shortage,” said Aditya Pawar, MD, a child and adolescent psychiatrist with the Kennedy Krieger Institute and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins School of Medicine, Baltimore, Maryland.
The ADHD drug shortage is now well into its second year, and clinicians and advocates alike say there is no apparent end in sight.
How Did We Get Here?
Manufacturers and federal agencies blame the shortage on rising demand and each other, while clinicians say that insurers, drug distributors, and middlemen are also playing a role in keeping medications out of patients’ hands.
In August 2023, the Drug Enforcement Administration (DEA), which sets quotas for the production of controlled substances, and the FDA publicly blamed manufacturers for the shortages, claiming they were not using up their allocations.
At the time, the DEA said manufacturers made and sold only 70% of their quota, nearly 1 billion doses short of what they were allowed to produce and ship that year.
The agencies also noted a record-high number of prescriptions for stimulants from 2012 to 2021. Driven in part by telehealth, the demand intensified during the pandemic. One recent study reported a 14% increase in ADHD stimulant prescriptions between 2020 and 2022.
Insurers also play a role in the shortage, David Goodman, MD, an assistant professor of psychiatry and behavioral sciences also at Johns Hopkins University, told this news organization.
Stepped therapy — in which patients must try one, two, or three medications before they are authorized to receive a more expensive or newer drug — contributes to the problem, Dr. Goodman said. Demand for such medications is high and supply low. In addition, some insurers only provide coverage for in-network pharmacies, regardless of the ability of other providers outside such networks to fill prescriptions.
“If the insurance dictates where you get your pills, and that pharmacy doesn’t have the pills or that pharmacy chain in your area doesn’t have those pills, you’re out of luck,” Dr. Goodman said.
Patients as Detectives
To get prescriptions filled, patients must “turn into detectives,” Laurie Kulikosky, CEO of Children and Adults with Attention-Deficit/Hyperactivity Disorder, told this news organization. “It’s a huge stressor.”
Tracking which ADHD medications are available, on back order, or discontinued requires frequent checking of the FDA’s drug shortages website.
Some manufacturers of generic versions of mixed amphetamine salts are only fulfilling orders for existing contracts, while others say new product won’t be available until at least April or as late as September. All blame the delay on the shortage of active ingredients.
Teva, which makes both the brand and generic of Adderall, reported on the FDA’s site that its manufacturing and distribution is at record-high levels, but demand continues to rise.
The branded Concerta is available, but some makers of generic methylphenidate reported supplies won’t be available until July.
Lisdexamfetamine dimesylate in almost all dosages is either unavailable, available in restricted quantities, or on extended back order. However, the branded product Vyvanse is available.
Industry, Government Respond
In a November 2023 statement, the DEA reported that 17 of 18 drug manufacturers the agency contacted planned to use their full DEA quota and increase production for that year. The agency said it had made it easier for manufacturers to request changes in allocations and that periodically updating quotas was a possibility.
This news organization asked the DEA whether any manufacturers had not met their 2023 quotas, but an agency spokesperson said it would not comment.
An FDA spokesperson said it could help manufacturers ask for bigger quotas and to increase production, noting that in 2023, the DEA increased the quota for methylphenidate following an FDA request.
“The FDA is in frequent communication with the manufacturers of ADHD stimulant medications and the DEA, and we will continue to monitor supply,” the spokesperson said.
For 2024, the FDA told the DEA that it predicted a 3.1% increase in use of amphetamine, methylphenidate (including dexmethylphenidate), and lisdexamfetamine. The DEA took that into account when it issued its final quotas for 2024. Whether those amounts will be enough remains to be seen.
With many drugs — not just those for ADHD — in short supply, in February, the US Department of Health and Human Services (HHS) and the Federal Trade Commission opened an inquiry of sorts, seeking comments on how middlemen and others were influencing pricing and supply of generic drugs.
“When you’re prescribed an important medication by your doctor and you learn the drug is out of stock, your heart sinks,” HHS Secretary Xavier Becerra said in a statement. “This devastating reality is the case for too many Americans who need generic drugs for ADHD, cancer, and other conditions.”
On the comments site, which is open until April 15, many of the 4000-plus complaints filed to-date are from individuals with ADHD.
Dr. Pawar said clinicians can’t know what’s going on between the FDA, the DEA, and manufacturers, adding that, “they need to sit together and figure something out.”
Even Members of Congress have had trouble getting answers. In October, Rep. Abigail Spanberger (D-Virginia) and a dozen colleagues wrote to the FDA and DEA seeking information on how the agencies were responding to stimulant shortages. The DEA has still not replied.
Workarounds the Only Option?
In the past, physicians would prescribe the optimal medication for individual patients based on clinical factors. Now, one of the major factors in determining drug choice is the agent that has “the highest likelihood of benefit and the lowest likelihood of administrative demand or burden,” Dr. Goodman said.
With so many medications in short supply, clinicians have figured out workarounds to get prescriptions filled, but they don’t often pan out.
If a patient needs a 60-mg daily dose of a medication and the pharmacy doesn’t have any 60-mg pills, Dr. Goodman said he might write a prescription for a 30-mg pill to be taken twice a day. However, insurers often will cover only 30 pills for a month, which can thwart this strategy.
Dr. Pawar said he sometimes prescribes Journay PM in lieu of Concerta because it is often available. But insurers may deny coverage of Journay PM because it is a newer medication, he said. When prescribing ADHD medications, he also provides his patients with a list of potential substitutes so they can ask the pharmacist if any are in stock.
With no end to the shortage in sight, clinicians must often prescribe multiple medications until their patients are able to find one that’s available. In addition, patients are burdened with making calls and visits to multiple pharmacies until they find one that can fill their prescription.
Meanwhile, the ripple effects to the ADHD drug shortage continue to spread. Extended periods without treatment can lead to declining job performance or job loss, fractured relationships, and even financial distress, Dr. Goodman said.
“If you go without your pills for a month and you’re not performing, your work declines and you lose your job as a result, that’s not on you — that’s on the fact that you can’t get your treatment,” he noted. “The shortage is no longer an inconvenience.”
Dr. Goodman, Dr. Pawar, and Ms. Kulikosky reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Stimulants for ADHD Not Linked to Prescription Drug Misuse
TOPLINE:
The use of stimulant therapy by adolescents with attention-deficit/hyperactivity disorder (ADHD) was not associated with later prescription drug misuse (PDM), a new study showed. However, misuse of prescription stimulants during adolescence was associated with significantly higher odds of later PDM.
METHODOLOGY:
- Data came from 11,066 participants in the ongoing Monitoring the Future panel study (baseline cohort years 2005-2017), a multicohort US national longitudinal study of adolescents followed into adulthood, in which procedures and measures are kept consistent across time.
- Participants (ages 17 and 18 years, 51.7% female, 11.2% Black, 15.7% Hispanic, and 59.6% White) completed self-administered questionnaires, with biennial follow-up during young adulthood (ages 19-24 years).
- The questionnaires asked about the number of occasions (if any) in which respondents used a prescription drug (benzodiazepine, opioid, or stimulant) on their own, without a physician’s order.
- Baseline covariates included sex, race, ethnicity, grade point average during high school, parental education, past 2-week binge drinking, past-month cigarette use, and past-year marijuana use, as well as demographic factors.
TAKEAWAY:
- Overall, 9.9% of participants reported lifetime stimulant therapy for ADHD, and 18.6% reported lifetime prescription stimulant misuse at baseline.
- Adolescents who received stimulant therapy for ADHD were less likely to report past-year prescription stimulant misuse as young adults compared with their same-age peers who did not receive stimulant therapy (adjusted odds ratio, 0.71; 95% CI, 0.52-0.99).
- The researchers found no significant differences between adolescents with or without lifetime stimulants in later incidence or prevalence of past-year PDM during young adulthood.
- The most robust predictor of prescription stimulant misuse during young adulthood was prescription stimulant misuse during adolescence; similarly, the most robust predictors of prescription opioid and prescription benzodiazepine misuse during young adulthood were prescription opioid and prescription benzodiazepine misuse (respectively) during adolescence.
IN PRACTICE:
“These findings amplify accumulating evidence suggesting that careful monitoring and screening during adolescence could identify individuals who are at relatively greater risk for PDM and need more comprehensive substance use assessment,” the authors wrote.
SOURCE:
Sean Esteban McCabe, PhD, professor and director, Center for the Study of Drugs, Alcohol, Smoking and Health, University of Michigan School of Nursing, Ann Arbor, was the lead and corresponding author of the study. It was published online on February 7 in Psychiatric Sciences.
LIMITATIONS:
Some subpopulations with higher rates of substance use, including youths who left school before completion and institutionalized populations, were excluded from the study, which may have led to an underestimation of PDM. Moreover, some potential confounders (eg, comorbid psychiatric conditions) were not assessed.
DISCLOSURES:
This study was supported by a research award from the US Food and Drug Administration and research awards from the National Institute on Drug Abuse of the NIH. Dr. McCabe reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of stimulant therapy by adolescents with attention-deficit/hyperactivity disorder (ADHD) was not associated with later prescription drug misuse (PDM), a new study showed. However, misuse of prescription stimulants during adolescence was associated with significantly higher odds of later PDM.
METHODOLOGY:
- Data came from 11,066 participants in the ongoing Monitoring the Future panel study (baseline cohort years 2005-2017), a multicohort US national longitudinal study of adolescents followed into adulthood, in which procedures and measures are kept consistent across time.
- Participants (ages 17 and 18 years, 51.7% female, 11.2% Black, 15.7% Hispanic, and 59.6% White) completed self-administered questionnaires, with biennial follow-up during young adulthood (ages 19-24 years).
- The questionnaires asked about the number of occasions (if any) in which respondents used a prescription drug (benzodiazepine, opioid, or stimulant) on their own, without a physician’s order.
- Baseline covariates included sex, race, ethnicity, grade point average during high school, parental education, past 2-week binge drinking, past-month cigarette use, and past-year marijuana use, as well as demographic factors.
TAKEAWAY:
- Overall, 9.9% of participants reported lifetime stimulant therapy for ADHD, and 18.6% reported lifetime prescription stimulant misuse at baseline.
- Adolescents who received stimulant therapy for ADHD were less likely to report past-year prescription stimulant misuse as young adults compared with their same-age peers who did not receive stimulant therapy (adjusted odds ratio, 0.71; 95% CI, 0.52-0.99).
- The researchers found no significant differences between adolescents with or without lifetime stimulants in later incidence or prevalence of past-year PDM during young adulthood.
- The most robust predictor of prescription stimulant misuse during young adulthood was prescription stimulant misuse during adolescence; similarly, the most robust predictors of prescription opioid and prescription benzodiazepine misuse during young adulthood were prescription opioid and prescription benzodiazepine misuse (respectively) during adolescence.
IN PRACTICE:
“These findings amplify accumulating evidence suggesting that careful monitoring and screening during adolescence could identify individuals who are at relatively greater risk for PDM and need more comprehensive substance use assessment,” the authors wrote.
SOURCE:
Sean Esteban McCabe, PhD, professor and director, Center for the Study of Drugs, Alcohol, Smoking and Health, University of Michigan School of Nursing, Ann Arbor, was the lead and corresponding author of the study. It was published online on February 7 in Psychiatric Sciences.
LIMITATIONS:
Some subpopulations with higher rates of substance use, including youths who left school before completion and institutionalized populations, were excluded from the study, which may have led to an underestimation of PDM. Moreover, some potential confounders (eg, comorbid psychiatric conditions) were not assessed.
DISCLOSURES:
This study was supported by a research award from the US Food and Drug Administration and research awards from the National Institute on Drug Abuse of the NIH. Dr. McCabe reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of stimulant therapy by adolescents with attention-deficit/hyperactivity disorder (ADHD) was not associated with later prescription drug misuse (PDM), a new study showed. However, misuse of prescription stimulants during adolescence was associated with significantly higher odds of later PDM.
METHODOLOGY:
- Data came from 11,066 participants in the ongoing Monitoring the Future panel study (baseline cohort years 2005-2017), a multicohort US national longitudinal study of adolescents followed into adulthood, in which procedures and measures are kept consistent across time.
- Participants (ages 17 and 18 years, 51.7% female, 11.2% Black, 15.7% Hispanic, and 59.6% White) completed self-administered questionnaires, with biennial follow-up during young adulthood (ages 19-24 years).
- The questionnaires asked about the number of occasions (if any) in which respondents used a prescription drug (benzodiazepine, opioid, or stimulant) on their own, without a physician’s order.
- Baseline covariates included sex, race, ethnicity, grade point average during high school, parental education, past 2-week binge drinking, past-month cigarette use, and past-year marijuana use, as well as demographic factors.
TAKEAWAY:
- Overall, 9.9% of participants reported lifetime stimulant therapy for ADHD, and 18.6% reported lifetime prescription stimulant misuse at baseline.
- Adolescents who received stimulant therapy for ADHD were less likely to report past-year prescription stimulant misuse as young adults compared with their same-age peers who did not receive stimulant therapy (adjusted odds ratio, 0.71; 95% CI, 0.52-0.99).
- The researchers found no significant differences between adolescents with or without lifetime stimulants in later incidence or prevalence of past-year PDM during young adulthood.
- The most robust predictor of prescription stimulant misuse during young adulthood was prescription stimulant misuse during adolescence; similarly, the most robust predictors of prescription opioid and prescription benzodiazepine misuse during young adulthood were prescription opioid and prescription benzodiazepine misuse (respectively) during adolescence.
IN PRACTICE:
“These findings amplify accumulating evidence suggesting that careful monitoring and screening during adolescence could identify individuals who are at relatively greater risk for PDM and need more comprehensive substance use assessment,” the authors wrote.
SOURCE:
Sean Esteban McCabe, PhD, professor and director, Center for the Study of Drugs, Alcohol, Smoking and Health, University of Michigan School of Nursing, Ann Arbor, was the lead and corresponding author of the study. It was published online on February 7 in Psychiatric Sciences.
LIMITATIONS:
Some subpopulations with higher rates of substance use, including youths who left school before completion and institutionalized populations, were excluded from the study, which may have led to an underestimation of PDM. Moreover, some potential confounders (eg, comorbid psychiatric conditions) were not assessed.
DISCLOSURES:
This study was supported by a research award from the US Food and Drug Administration and research awards from the National Institute on Drug Abuse of the NIH. Dr. McCabe reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
Stimulant Medications for ADHD — the Good, the Bad, and the Ugly
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Children with attention-deficit/hyperactivity disorder (ADHD) are mainly cared for in primary care settings by us. Management of this chronic neurodevelopmental condition that affects 5+% of children worldwide should include proper diagnosis, assessment for contributing and comorbid conditions, behavioral intervention (the primary treatment for preschoolers), ensuring good sleep and nutrition, and usually medication.
Because stimulants are very effective for reducing ADHD symptoms, we may readily begin these first-line medications even on the initial visit when the diagnosis is determined. But are we really thoughtful about knowing and explaining the potential short- and long-term side effects of these medications that may then be used for many years? Considerable discussion with the child and parents may be needed to address their concerns, balanced with benefits, and to make a plan for their access and use of stimulants (and other medications for ADHD not the topic here).
Consider the Side Effects
In children older than 6 years, some form of either a methylphenidate (MPH) or a dextroamphetamine (DA) class of stimulant have been shown to be equally effective in reducing symptoms of ADHD in about 77% of cases, but side effects are common, mostly mild, and mostly in the first months of use. These include reduced appetite, abdominal pain, headache, weight loss, tics, jitteriness, and delays in falling asleep. About half of all children treated will have one of these adverse effects over 5 years, with reduced appetite the most common. There is no difference in effectiveness or side effects by presentation type, i.e. hyperactive, inattentive, or combined, but the DA forms are associated with more side effects than MPH (10% vs. 6%). Medicated preschoolers have more and different side effects which, in addition to those above, may include listlessness, social withdrawal, and repetitive movements. Fortunately, we can reassure families that side effects can usually be readily managed by slower ramp up of dose, spacing to ensure appetite for meals, extra snacks, attention to bowel patterns and bedtime routines, or change in medication class.
Rates of tics while on stimulants are low irrespective of whether DA or MPH is used, and are usually transient, but difficult cases may occur, sometimes as part of Tourette’s, although not a contraindication. Additional side effects of concern are anxiety, irritability, sadness, and overfocusing that may require a change in class of stimulant or to a nonstimulant. Keep in mind that these symptoms may represent comorbid conditions to ADHD, warranting counseling intervention rather than being a medication side effect. Both initial assessment for ADHD and monitoring should look for comorbidities whether medication is used or not.
Measuring height, weight, pulse, and blood pressure should be part of ADHD care. How concerned should you and the family be about variations? Growth rate declines are more common in preschool children; in the PATS study height varied by 20.3%, and weight by 55.2%, more in heavier children. Growth can be protected by providing favored food for school, encouraging eating when hungry, and an evening fourth meal. You can reassure families that, even with continual use of stimulant medicines for years and initial deficits of 2 cm and 2.7 kg compared to expected, no significant differences remain in adulthood.
This longitudinal growth data was collected when short-acting stimulants were the usual, rather than the now common long-acting stimulants given 7 days per week, however. Children on transdermal MPH with 12-hour release over 3 years showed a small but significant delay in growth with the mean deficit rates 1.3 kg/year mainly in the first year, and 0.68 cm/year in height in the second year. If we see growth not recovering as it is expected to after the first year of treatment, we can advise shorter-acting forms, and medication “holidays” on weekends or vacations, that reduce but do not end the deficits. When concerned, a nonstimulant can be selected.
Blood pressure and pulse rate are predictably slightly increased on stimulants (about 2-4 mm Hg and about 3-6 bpm) but not clinically significantly. Although ECGs are not routinely recommended, careful consideration and consultation is warranted before starting stimulants for any patient with structural cardiac abnormalities, unexplained syncope, exertional chest pain, or a family history of sudden death in children or young adults. Neither current nor former users of stimulants for ADHD were found to have greater rates of cardiac events than the general population, however.
Misuse and abuse
Misuse and diversion of stimulants is common (e.g. 26% diverted MPH in the past month; 14% of 12th graders divert DA), often undetected, and potentially dangerous. And the problem is not limited to just the kids. Sixteen percent of parents reported diversion of stimulant medication to another household member, mainly to themselves. Stimulant overdose can occur, especially taken parenterally, and presents with dilated pupils, tremor, agitation, hyperreflexia, combative behavior, confusion, hallucinations, delirium, anxiety, paranoia, movement disorders, and/or seizures. Fortunately, overdose of prescribed stimulants is rarely fatal if medically managed, but recent “fake” Adderall (not from pharmacies) has been circulating. These fake drugs may contain lethal amounts of fentanyl or methamphetamine. Point out to families that a peer-provided stimulant not prescribed for them may have underlying medical or psychiatric issues that increase adverse events. Selling stimulants can have serious legal implications, with punishments ranging from fines to incarceration. A record of arrest during adolescence increases the likelihood of high school dropout, lack of 4-year college education, and later employment barriers. Besides these serious outcomes, it is useful to remind patients that if they deviate from your recommended dosing that you, and others, will not prescribe for them in the future the medication that has been supporting their successful functioning.
You can be fooled about being able to tell if your patients are misusing or diverting the stimulants you prescribe. Most (59%) physicians suspect that more than one of their patients with ADHD has diverted or feigned symptoms (66%) to get a prescription. Women were less likely to suspect their patients than are men, though, so be vigilant! Child psychiatrists had the highest suspicion with their greater proportion of patients with ADHD plus conduct or substance use disorder, who account for 83% of misusers/diverters. We can use education about misuse, pill counts, contracts on dosing, or switching to long-acting or nonstimulants to curb this.
Additional concerns
With more ADHD diagnosis and stimulants used for many years should we worry about longer-term issues? There have been reports in rodent models and a few children of chromosomal changes with stimulant exposure, but reviewers do not interpret these as an individual cancer risk. Record review of patients who received stimulants showed lower numbers of cancer than expected. Nor is there evidence of reproductive effects of stimulants, although use during pregnancy is not cleared.
Stimulants carry a boxed warning as having high potential for abuse and psychological or physical dependence, which is unsurprising given their effects on brain reward pathways. However, neither past nor present use of stimulants for ADHD has been associated with greater substance use long term.
To top off these issues, recent shortages of stimulants complicate ADHD management. Most states require electronic prescribing, US rules only allowing one transfer of such e-prescriptions. With many pharmacies refusing to tell families about availability, we must make multiple calls to locate a source. Pharmacists could help us by looking up patient names of abusers on the registry and identifying sites with adequate supplies.
Dr. Howard is assistant professor of pediatrics at The Johns Hopkins University School of Medicine, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
ADHD Symptoms Linked With Physical Comorbidities
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Investigators from the French Health and Medical Research Institute (INSERM), University of Bordeaux, and Charles Perrens Hospital, alongside their Canadian, British, and Swedish counterparts, have shown that attention-deficit/hyperactivity disorder (ADHD) or attention-deficit disorder without hyperactivity is linked with physical health problems. Cédric Galéra, MD, PhD, child and adolescent psychiatrist and epidemiologist at the Bordeaux Population Health Research Center (INSERM/University of Bordeaux) and the Charles Perrens Hospital, explained these findings to this news organization.
A Bilateral Association
ADHD is a neurodevelopmental condition that develops in childhood and is characterized by high levels of inattention or agitation and impulsiveness. Some studies have revealed a link between ADHD and medical comorbidities, but these studies were carried out on small patient samples and were cross-sectional.
A new longitudinal study published in Lancet Child and Adolescent Health has shown a reciprocal link between ADHD and physical health problems. The researchers conducted statistical analyses to measure the links between ADHD symptoms and subsequent development of certain physical conditions and, conversely, between physical problems during childhood and subsequent development of ADHD symptoms.
Children From Quebec
The study was conducted by a team headed by Dr. Galéra in collaboration with teams from Britain, Sweden, and Canada. “We studied a Quebec-based cohort of 2000 children aged between 5 months and 17 years,” said Dr. Galéra.
“The researchers in Quebec sent interviewers to question parents at home. And once the children were able to answer for themselves, from adolescence, they were asked to answer the questions directly,” he added.
The children were assessed on the severity of their ADHD symptoms as well as their physical condition (general well-being, any conditions diagnosed, etc.).
Dental Caries, Excess Weight
“We were able to show links between ADHD in childhood and physical health problems in adolescence. There is a greater risk for dental caries, infections, injuries, wounds, sleep disorders, and excess weight.
“Accounting for socioeconomic status and mental health problems such as anxiety and depression or medical treatments, we observed that dental caries, wounds, excess weight, and restless legs syndrome were the conditions that cropped up time and time again,” said Dr. Galéra.
On the other hand, the researchers noted that certain physical health issues in childhood were linked with the onset of ADHD at a later stage. “We discovered that asthma in early childhood, injuries, sleep disturbances, epilepsy, and excess weight were associated with ADHD. Taking all above-referenced features into account, we were left with just wounds and injuries as well as restless legs syndrome as being linked to ADHD,” Dr. Galéra concluded.
For Dr. Galéra, the study illustrates the direction and timing of the links between physical problems and ADHD. “This reflects the link between physical and mental health. It’s important that all healthcare professionals be alert to this. Psychiatrists and mental health professionals must be vigilant about the physical health risks, and pediatricians and family physicians must be aware of the fact that children can present with physical conditions that will later be linked with ADHD. Each of them must be able to refer their young patients to their medical colleagues to ensure that these people receive the best care,” he emphasized.
The team will continue to study this cohort to see which problems emerge in adulthood. They also wish to study the Elfe cohort, a French longitudinal study of children.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
ADHD Plus Comorbidities Linked to Increased Schizophrenia Risk
TOPLINE:
Attention-deficit/hyperactivity disorder (ADHD) and comorbid psychiatric disorders are associated with a twofold increased risk for schizophrenia, new research shows.
METHODOLOGY:
- Investigators analyzed the data of 211,705 people aged 5-19 years (74% male; 54% aged 5-9 years) diagnosed with ADHD during 2010-2018 from the Health Insurance Review and Assessment Service database of South Korea.
- Participants with a diagnosis of schizophrenia or psychosis anytime in the 3 years prior to ADHD diagnosis were excluded.
- Investigators split participants into two groups — a group of those diagnosed with at least one psychiatric comorbidity within a year of ADHD diagnosis and another group comprising those with ADHD and no psychiatric comorbidities.
TAKEAWAY:
- 37% (77,890) of those with ADHD had at least one comorbid psychiatric disorder.
- Participants with one psychiatric comorbidity had a 2.1-fold increased risk for a schizophrenia diagnosis than participants with no comorbidity (adjusted hazard ratio [aHR], 2.14; 95% CI, 2.05-2.23).
- Schizophrenia risk increased with each additional comorbidity. There was a fourfold increased risk for schizophrenia in study participants with three or more psychiatric comorbidities (aHR, 4.26; 95% CI, 3.90-4.65) than those with no comorbidity.
- Psychiatric comorbidities included autism spectrum disorder, which had the strongest link to increased schizophrenia risk (aHR, 2.43; 95% CI, 2.26-2.62). Other comorbidities that showed strong associations were intellectual disability (aHR, 1.83; 95% CI, 1.72-1.95), tic disorder (aHR, 1.77; 95% CI, 1.66-1.88), depression (aHR,1.68; 95% CI, 1.60-1.77), and bipolar disorder (aHR, 1.67; 95% CI, 1.53-1.83).
IN PRACTICE:
“To our knowledge, this is the first study to investigate schizophrenia risk among children and adolescents with ADHD, with a particular focus on psychiatric comorbidities,” the researchers wrote. They also noted that although patients had no psychiatric comorbidities at the time of ADHD diagnosis, the occurrence of psychiatric comorbidities was frequently observed prior to schizophrenia diagnosis.
“These findings highlighted the significance of carefully monitoring psychiatric comorbidities in patients with ADHD to effectively mitigate the burden of schizophrenia,” they noted.
SOURCE:
Soo Min Jeon, PharmD, PhD, of Jeju National University in Jeju, South Korea, led the study, which was published online on November 30, 2023 in JAMA Network Open.
LIMITATIONS:
Since the diagnosis of ADHD, schizophrenia, and other psychiatric comorbidities were based on diagnostic codes, the possibility of underdiagnosis or overdiagnosis cannot be ruled out. Also, some patients with ADHD chose the general health consultation (International Classification of Diseases - Z code) due to the social stigma surrounding mental health problems.
DISCLOSURES:
The study was funded by the Basic Science Research Program through the Ministry of Education and the Health Insurance Review and Assessment Service. Author disclosures can be found in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
Attention-deficit/hyperactivity disorder (ADHD) and comorbid psychiatric disorders are associated with a twofold increased risk for schizophrenia, new research shows.
METHODOLOGY:
- Investigators analyzed the data of 211,705 people aged 5-19 years (74% male; 54% aged 5-9 years) diagnosed with ADHD during 2010-2018 from the Health Insurance Review and Assessment Service database of South Korea.
- Participants with a diagnosis of schizophrenia or psychosis anytime in the 3 years prior to ADHD diagnosis were excluded.
- Investigators split participants into two groups — a group of those diagnosed with at least one psychiatric comorbidity within a year of ADHD diagnosis and another group comprising those with ADHD and no psychiatric comorbidities.
TAKEAWAY:
- 37% (77,890) of those with ADHD had at least one comorbid psychiatric disorder.
- Participants with one psychiatric comorbidity had a 2.1-fold increased risk for a schizophrenia diagnosis than participants with no comorbidity (adjusted hazard ratio [aHR], 2.14; 95% CI, 2.05-2.23).
- Schizophrenia risk increased with each additional comorbidity. There was a fourfold increased risk for schizophrenia in study participants with three or more psychiatric comorbidities (aHR, 4.26; 95% CI, 3.90-4.65) than those with no comorbidity.
- Psychiatric comorbidities included autism spectrum disorder, which had the strongest link to increased schizophrenia risk (aHR, 2.43; 95% CI, 2.26-2.62). Other comorbidities that showed strong associations were intellectual disability (aHR, 1.83; 95% CI, 1.72-1.95), tic disorder (aHR, 1.77; 95% CI, 1.66-1.88), depression (aHR,1.68; 95% CI, 1.60-1.77), and bipolar disorder (aHR, 1.67; 95% CI, 1.53-1.83).
IN PRACTICE:
“To our knowledge, this is the first study to investigate schizophrenia risk among children and adolescents with ADHD, with a particular focus on psychiatric comorbidities,” the researchers wrote. They also noted that although patients had no psychiatric comorbidities at the time of ADHD diagnosis, the occurrence of psychiatric comorbidities was frequently observed prior to schizophrenia diagnosis.
“These findings highlighted the significance of carefully monitoring psychiatric comorbidities in patients with ADHD to effectively mitigate the burden of schizophrenia,” they noted.
SOURCE:
Soo Min Jeon, PharmD, PhD, of Jeju National University in Jeju, South Korea, led the study, which was published online on November 30, 2023 in JAMA Network Open.
LIMITATIONS:
Since the diagnosis of ADHD, schizophrenia, and other psychiatric comorbidities were based on diagnostic codes, the possibility of underdiagnosis or overdiagnosis cannot be ruled out. Also, some patients with ADHD chose the general health consultation (International Classification of Diseases - Z code) due to the social stigma surrounding mental health problems.
DISCLOSURES:
The study was funded by the Basic Science Research Program through the Ministry of Education and the Health Insurance Review and Assessment Service. Author disclosures can be found in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
Attention-deficit/hyperactivity disorder (ADHD) and comorbid psychiatric disorders are associated with a twofold increased risk for schizophrenia, new research shows.
METHODOLOGY:
- Investigators analyzed the data of 211,705 people aged 5-19 years (74% male; 54% aged 5-9 years) diagnosed with ADHD during 2010-2018 from the Health Insurance Review and Assessment Service database of South Korea.
- Participants with a diagnosis of schizophrenia or psychosis anytime in the 3 years prior to ADHD diagnosis were excluded.
- Investigators split participants into two groups — a group of those diagnosed with at least one psychiatric comorbidity within a year of ADHD diagnosis and another group comprising those with ADHD and no psychiatric comorbidities.
TAKEAWAY:
- 37% (77,890) of those with ADHD had at least one comorbid psychiatric disorder.
- Participants with one psychiatric comorbidity had a 2.1-fold increased risk for a schizophrenia diagnosis than participants with no comorbidity (adjusted hazard ratio [aHR], 2.14; 95% CI, 2.05-2.23).
- Schizophrenia risk increased with each additional comorbidity. There was a fourfold increased risk for schizophrenia in study participants with three or more psychiatric comorbidities (aHR, 4.26; 95% CI, 3.90-4.65) than those with no comorbidity.
- Psychiatric comorbidities included autism spectrum disorder, which had the strongest link to increased schizophrenia risk (aHR, 2.43; 95% CI, 2.26-2.62). Other comorbidities that showed strong associations were intellectual disability (aHR, 1.83; 95% CI, 1.72-1.95), tic disorder (aHR, 1.77; 95% CI, 1.66-1.88), depression (aHR,1.68; 95% CI, 1.60-1.77), and bipolar disorder (aHR, 1.67; 95% CI, 1.53-1.83).
IN PRACTICE:
“To our knowledge, this is the first study to investigate schizophrenia risk among children and adolescents with ADHD, with a particular focus on psychiatric comorbidities,” the researchers wrote. They also noted that although patients had no psychiatric comorbidities at the time of ADHD diagnosis, the occurrence of psychiatric comorbidities was frequently observed prior to schizophrenia diagnosis.
“These findings highlighted the significance of carefully monitoring psychiatric comorbidities in patients with ADHD to effectively mitigate the burden of schizophrenia,” they noted.
SOURCE:
Soo Min Jeon, PharmD, PhD, of Jeju National University in Jeju, South Korea, led the study, which was published online on November 30, 2023 in JAMA Network Open.
LIMITATIONS:
Since the diagnosis of ADHD, schizophrenia, and other psychiatric comorbidities were based on diagnostic codes, the possibility of underdiagnosis or overdiagnosis cannot be ruled out. Also, some patients with ADHD chose the general health consultation (International Classification of Diseases - Z code) due to the social stigma surrounding mental health problems.
DISCLOSURES:
The study was funded by the Basic Science Research Program through the Ministry of Education and the Health Insurance Review and Assessment Service. Author disclosures can be found in the original paper.
A version of this article appeared on Medscape.com.
Adult ADHD: Tips for an accurate diagnosis
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
Long-term use of ADHD meds and CVD risk: New data
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
results of a large Swedish nested case-control study suggest.
The increased risk was evident only for hypertension and arterial disease, was dose dependent, and was higher for stimulant than nonstimulant ADHD medications.
“Clinicians should be vigilant in monitoring signs and symptoms of cardiovascular diseases, particularly among those receiving higher doses,” Zheng Chang, PhD, principal researcher, department of medical epidemiology and biostatistics, Karolinska Institutet, Stockholm, said in an interview.
“Treatment decisions, as always, should be based on careful weighing of potential benefits and risks at individual patient level, rather than simple one-size-fits-all recommendations,” Dr. Chang added.
The study was published online in JAMA Psychiatry
Filling in the research gaps
The use of medications to treat ADHD has increased markedly over the past decades in both children and adults. The potential risk for CVD associated with long-term ADHD medication use remains unclear. Most “longitudinal” studies that have looked at the association have an average follow-up time of no more than 2 years, the authors note.
In contrast, the Swedish study assessed the association between cumulative use of ADHD medication in children and adults followed for up to 14 years and also looked at whether associations differ across types of medication and dosages, types of CVD, gender, and age.
Among 278,027 individuals aged 6-64 years diagnosed with ADHD or dispensed ADHD medication, 10,388 with CVD were identified and matched to 51,672 controls without CVD.
Longer cumulative duration of ADHD medication use was associated with a statistically significant increased risk for CVD, compared with no use.
When the risk for specific CVDs was examined, long-term use of ADHD medication (compared with no use) was associated with an increased risk for hypertension and arterial disease but not arrhythmias, heart failure, ischemic heart disease, thromboembolic disease, or cerebrovascular disease.
For hypertension, the adjusted odds ratio was 1.72 (95% confidence interval, 1.51-1.97) for 3 to ≤ 5 years and 1.80 (95% CI, 1.55-2.08) for > 5 years of medication use. For arterial disease, the AOR was 1.65 (95% CI, 1.11-2.45) for 3 to ≤ 5 years and 1.49 (95% CI, 0.96-2.32) for > 5 years of use.
Stimulants confer greatest risk
Across the 14-year follow-up period, each additional year of ADHD medication use was associated with an average 4% increased CVD risk, with a larger 8% increased risk in the first 3 years of cumulative use, followed by stable risk over the remaining follow-up.
Similar risks were observed in children and adults, as well as in females and males.
When focusing on specific ADHD medications, compared with no use, long-term use of the stimulant methylphenidate was associated with an increased risk for CVD (AOR, 1.20 [95% CI, 1.10-1.31] for 3 to ≤ 5 years and 1.19 [95% CI, 1.08-1.31] for > 5 years).
The same was true for long-term use of the stimulant lisdexamfetamine (AOR, 1.23 [95% CI, 1.05-1.44] for 2 to ≤ 3 years and 1.17 [95% CI, 0.98-1.40] for > 3 years).
In contrast, use of the nonstimulant atomoxetine was associated with elevated CVD risk only for the first year of use (AOR, 1.07; 95% CI, 1.01-1.13).
The increased risk for CVD occurred only above certain average daily doses: 45 mg for methylphenidate and lisdexamfetamine, 22.5 mg for amphetamines, and 120 mg for atomoxetine.
The authors note that, although they accounted for a wide range of potential confounding variables, considering the observational nature of the study and the possibility of residual confounding, they could not prove causality.
‘Tricky trade-offs’
The coauthors of an editorial in JAMA Psychiatry (2023 Nov 22. doi: 10.1001/jamapsychiatry.2023.4126) note that the study “should remind us that clinical decision-making is often based on tricky trade-offs that should be considered at the individual patient level.”
Given that hypertension is the leading cause of CV morbidity and mortality worldwide, the increased likelihood of hypertension with long-term use of ADHD medications “cannot be disregarded,” write Samuele Cortese, MD, PhD, and Cristiano Fava, MD, PhD, with University of Southampton (England).
“These findings are especially relevant given the reported association between ADHD and physical conditions, such as obesity, which further contribute to increased cardiovascular risk,” they add.
Dr. Cortese and Dr. Fava say that the increased CV risk – averaging 4% per year and stabilizing after 3 years of treatment – “should be carefully weighed against the established benefits, on a case-by-case basis.”
“Importantly,” they write, “large real-world self-controlled studies have shown that individuals with ADHD experience significantly fewer unintentional physical injuries, motor vehicle crashes, substance use disorders, and criminal acts, as well as improved academic functioning, during periods when they are taking, compared with periods when they are not taking, methylphenidate.”
The risk-benefit ratio, however, may be lower in people with preexisting heart conditions. However, more evidence and precise recommendations are needed in relation to the treatment of individuals with ADHD and preexisting CV conditions, the editorial writers say.
This study was supported by grants from the Swedish Research Council for Health, Working Life, and Welfare and the European Union’s Horizon 2020 research and innovation program. The authors and editorial writers have no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Adult ADHD: A sensible approach to diagnosis and treatment
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33
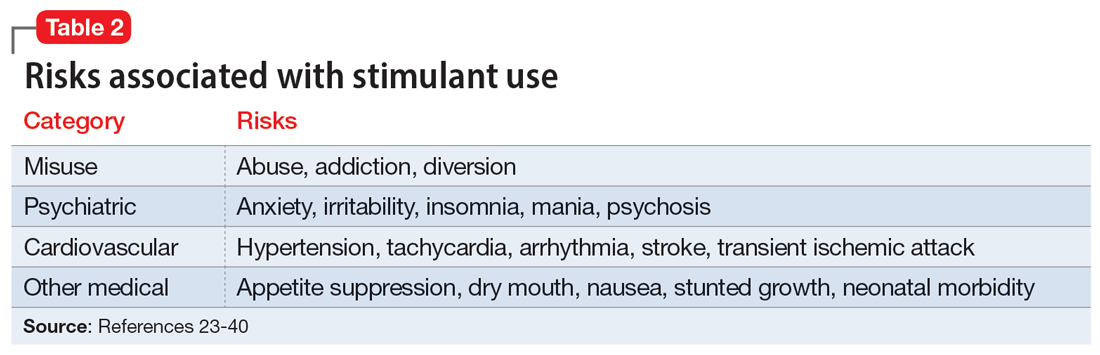
The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.
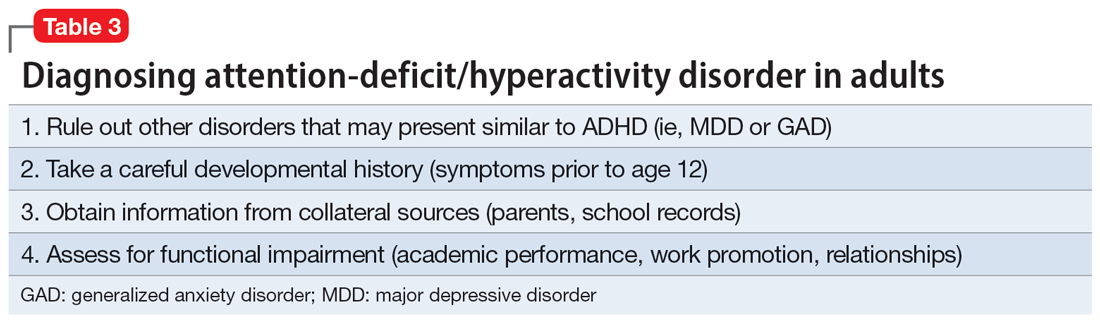
Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.
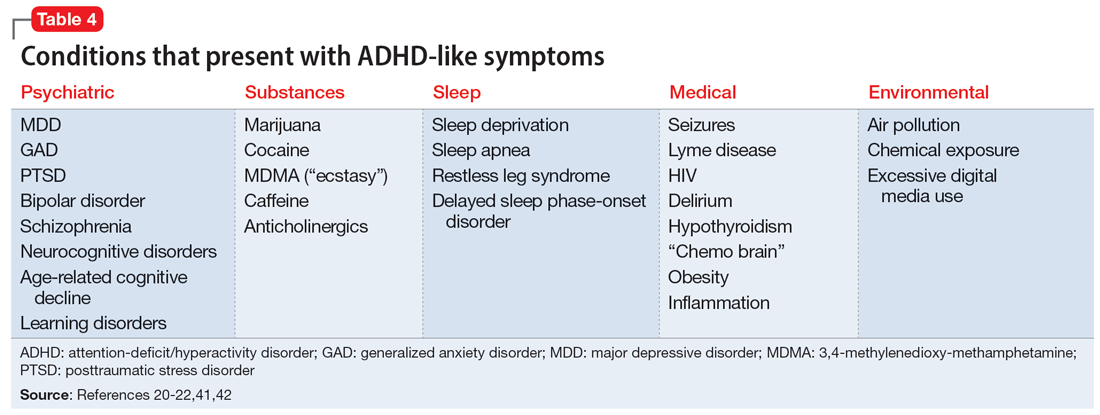
Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48
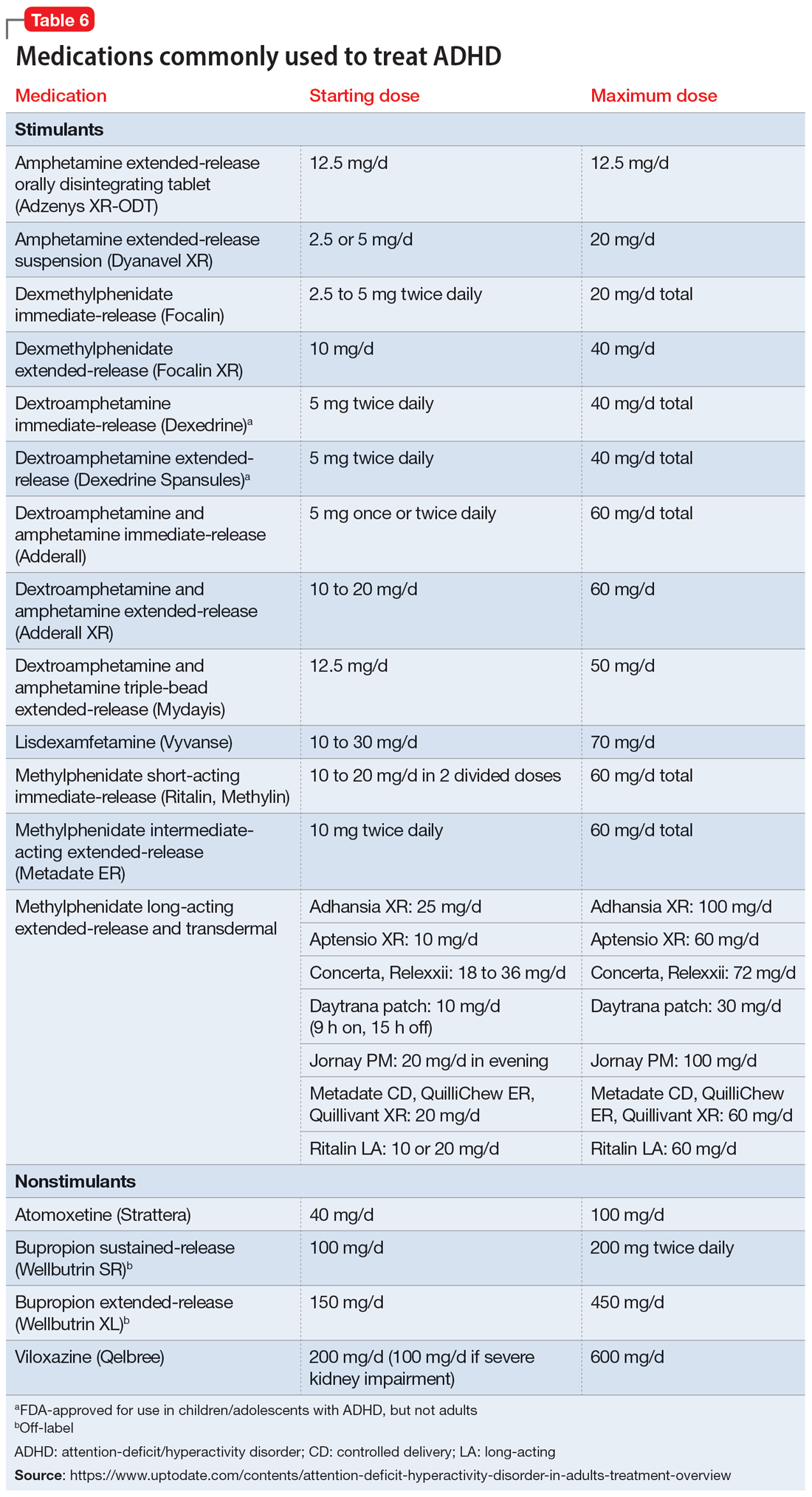
In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is common, with an estimated worldwide prevalence of 5.29% among children and adolescents and 2.5% among adults.1 DSM-5-TR classifies ADHD as a neurodevelopmental disorder, “a group of conditions with onset in the developmental period [that] typically manifest early in development, often before the child enters school.”2 Because of the expectation that ADHD symptoms emerge early in development, the diagnostic criteria specify that symptoms must have been present prior to age 12 to qualify as ADHD. However, recent years have shown a significant increase in the number of patients being diagnosed with ADHD for the first time in adulthood. One study found that the diagnosis of ADHD among adults in the United States doubled between 2007 and 2016.3
First-line treatment for ADHD is the stimulants methylphenidate and amphetamine/dextroamphetamine. In the United States, these medications are classified as Schedule II controlled substances, indicating a high risk for abuse. However, just as ADHD diagnoses among adults have increased, so have prescriptions for stimulants. For example, Olfson et al4 found that stimulant prescriptions among young adults increased by a factor of 10 between 1994 and 2009.
The increased prevalence of adult patients diagnosed with ADHD and taking stimulants frequently places clinicians in a position to consider the validity of existing diagnoses and evaluate new patients with ADHD-related concerns. In this article, we review some of the challenges associated with diagnosing ADHD in adults, discuss the risks of stimulant treatment, and present a practical approach to the diagnosis and treatment of ADHD in adults.
Challenges in diagnosis
DSM-5-TR diagnostic criteria for ADHD are summarized in Table 1. Establishing a diagnosis of adult ADHD can be challenging. As with many psychiatric conditions, symptoms of ADHD are highly subjective. Retrospectively diagnosing a developmental condition in adults is often biased by the patient’s current functioning.5 ADHD has a high heritability and adults may inquire about the diagnosis if their children are diagnosed with ADHD.6 Some experts have cautioned that clinicians must be careful in diagnosing ADHD in adults.7 Just as there are risks associated with underdiagnosing ADHD, there are risks associated with overdiagnosis. Overdiagnosis may medicalize normal variants in the population and lead to unnecessary treatment and a misappropriation of limited medical resources.8 Many false positive cases of late-onset ADHD may be attributable to nonimpairing cognitive fluctuations.9

Poor diagnostic practices can impede accuracy in establishing the presence or absence of ADHD. Unfortunately, methods of diagnosing adult ADHD have been shown to vary widely in terms of information sources, diagnostic instruments used, symptom threshold, and whether functional impairment is a requirement for diagnosis.10 A common practice in diagnosing adult ADHD involves asking patients to complete self-report questionnaires that list symptoms of ADHD, such as the Adult ADHD Self-Report Scale developed by the World Health Organization.11 However, self-reports of ADHD in adults are less reliable than informant reports, and some young adults without ADHD overreport symptoms.12,13 Symptom checklists are particularly susceptible to faking, which lessens their diagnostic value.14
The possibility of malingered symptoms of ADHD further increases the diagnostic difficulty. College students may be particularly susceptible to overreporting ADHD symptoms in order to obtain academic accommodations or stimulants in the hopes of improving school performance.15 One study found that 25% to 48% of college students self-referred for ADHD evaluations exaggerated their symptoms.16 In another study, 31% of adults failed the Word Memory Test, which suggests noncredible performance in their ADHD evaluation.17 College students can successfully feign ADHD symptoms in both self-reported symptoms and computer-based tests of attention.18 Harrison et al19 summarized many of these concerns in their 2007 study of ADHD malingering, noting the “almost perfect ability of the Faking group to choose items … that correspond to the DSM-IV symptoms, and to report these at levels even higher than persons with diagnosed ADHD.” They suggested “Clinicians should be suspicious of students or young adults presenting for a first-time diagnosis who rate themselves as being significantly symptomatic, yet have managed to achieve well in school and other life activities.”19
Another challenge in correctly diagnosing adult ADHD is identifying other conditions that may impair attention.20 Psychiatric conditions that may impair concentration include anxiety disorders, chronic stress, posttraumatic stress disorder, recent trauma, major depressive disorder (MDD), and bipolar disorder (BD). Undiagnosed learning disorders may present like ADHD. Focus can be negatively affected by sleep disorders such as sleep apnea, restless leg syndrome, or delayed sleep phase-onset disorder. Marijuana, cocaine, 3,4-methylenedioxy-methamphetamine (MDMA; “ecstasy”), caffeine, or prescription medications such as anticholinergics can also impair attention. Medical conditions that can present with attentional or executive functioning deficits include seizures, Lyme disease, HIV, encephalopathy, hypothyroidism, and “chemo brain.”21 Environmental factors such as age-related cognitive decline, sleep deprivation, inflammation, obesity, air pollution, chemical exposure, and excessive use of digital media may also produce symptoms similar to ADHD. Two studies of adult-onset ADHD concluded that 93% to 95% of cases were better explained by other conditions such as sleep disorders, substance use disorders, or another psychiatric disorder.22
Continue to: Risks associated with treatment
Risks associated with treatment
With or without an accurate ADHD diagnosis, prescribing stimulants presents certain risks (Table 223-40). One of the more well-known risks of stimulants is addiction or misuse.23 An estimated 5 million American adults misused prescription stimulants in 2016.24 Despite stimulants’ status as controlled substances, long-term concurrent use of stimulants with opioids is common among adults with ADHD.25 College students are particularly susceptible to misusing or diverting stimulants, often to improve their academic performance.26 At 1 university, 22% of students had misused stimulants in the past year.27 Prescribing short-acting stimulants (rather than extended-release formulations) increases the likelihood of misuse.28 Patients prescribed stimulants begin to receive requests to divert their medications to others as early as elementary school, and by college more than one-third of those taking stimulants have been asked to give, sell, or trade their medications.29 Diversion of stimulants by students with ADHD is prevalent, with 62% of patients engaging in diversion during their lifetime.15 Diverted stimulants can come from family members, black market sources, or deceived clinicians.30 Although students’ stimulant misuse/diversion often is academically motivated, nonmedical use of psychostimulants does not appear to have a statistically significant effect on improving grade point average.31 Despite a negligible impact on grades, most students who take stimulants identify their effect as strongly positive, producing a situation in which misusers of stimulants have little motivation to stop.32 While some patients might ask for a stimulant prescription with the rationale that liking the effects proves they have ADHD, this is inappropriate because most individuals like the effects of stimulant medications.33

The use of stimulants increases the risk for several adverse psychiatric outcomes. Stimulants increase the risk of anxiety, so exercise caution when prescribing to patients with a comorbid anxiety disorder.34 Stimulants can also worsen irritability and insomnia, 2 issues common among patients with ADHD.32 Use of stimulant medications can trigger manic episodes. Viktorin et al35 found a >6-fold increase in manic episodes among patients with BD receiving methylphenidate monotherapy compared to those receiving a combination of methylphenidate and a mood stabilizer.35 The use of methylphenidate and amphetamine can lead to new-onset psychosis (or exacerbation of pre-existing psychotic illness); amphetamine use is associated with a higher risk of psychosis than methylphenidate.36
General medical adverse effects are also possible with stimulant use. Stimulants’ adverse effect profiles include appetite suppression, dry mouth, and nausea. Long-term use poses a risk for stunting growth in children.1 Using stimulants during pregnancy is associated with higher risk for neonatal morbidity, including preterm birth, CNS-related disorders, and seizures.37 Stimulants can raise blood pressure and increase heart rate. Serious cardiovascular events associated with stimulant use include ventricular arrhythmias, strokes, and transient ischemic attacks.38
Nonstimulant ADHD treatments are less risky than stimulants but still require monitoring for common adverse effects. Atomoxetine has been associated with sedation, growth retardation (in children), and in severe cases, liver injury or suicidal ideation.39 Bupropion (commonly used off-label for ADHD) can lower the seizure threshold and cause irritability, anorexia, and insomnia.39 Viloxazine, a newer agent, can cause hypertension, increased heart rate, nausea, drowsiness, headache, and insomnia.40
Sensible diagnosing
Given the challenges in accurately diagnosing ADHD in adults, we present a sensible approach to making the diagnosis (Table 3). The first step is to rule out other conditions that might better explain the patient’s symptoms. A thorough clinical interview (including a psychiatric review of symptoms) is the cornerstone of an initial diagnostic assessment. The use of validated screening questionnaires such as the Patient Health Questionnaire-9 and General Anxiety Disorder-7 may also provide information regarding psychiatric conditions that require additional evaluation.

Continue to: Some of the most common conditions...
Some of the most common conditions we see mistaken for ADHD are MDD, generalized anxiety disorder (GAD), and BD. In DSM-5-TR, 1 of the diagnostic criteria for MDD is “diminished ability to think or concentrate, or indecisiveness, nearly every day (either by subjective account or as observed by others).”41 Similarly, criteria for GAD include “difficulty concentrating.”42 DSM-5-TR also includes distractibility as one of the criteria for mania/hypomania. Table 420-22,41,42 lists other psychiatric, substance-related, medical, and environmental conditions that can produce ADHD-like symptoms. Referring to some medical and environmental explanations for inattention, Aiken22 pointed out, “Patients who suffer from these problems might ask their doctor for a stimulant, but none of those syndromes require a psychopharmacologic approach.” ADHD can be comorbid with other psychiatric conditions, so the presence of another psychiatric illness does not automatically rule out ADHD. If alternative psychiatric diagnoses have been identified, these can be discussed with the patient and treatment offered that targets the specified condition.

Once alternative explanations have been ruled out, focus on the patient’s developmental history. DSM-5-TR conceptualizes ADHD as a neurodevelopmental disorder, meaning it is expected to emerge early in life. Whereas previous editions of DSM specified that ADHD symptoms must be present before age 7, DSM-5 modified this age threshold to before age 12.1 This necessitates taking a careful life history in order to understand the presence or absence of symptoms at earlier developmental stages.5 ADHD should be verified by symptoms apparent in childhood and present across the lifespan.15
While this retrospective history is necessary, histories that rely on self-report alone are often unreliable. Collateral sources of information are generally more reliable when assessing for ADHD symptoms.13 Third-party sources can help confirm that any impairment is best attributed to ADHD rather than to another condition.15 Unfortunately, the difficulty of obtaining collateral information means it is often neglected, even in the literature.10 A parent is the ideal informant for gathering collateral information regarding a patient’s functioning in childhood.5 Suggested best practices also include obtaining collateral information from interviews with significant others, behavioral questionnaires completed by parents (for current and childhood symptoms), review of school records, and consideration of intellectual and achievement testing.43 If psychological testing is pursued, include validity testing to detect feigned symptoms.18,44
When evaluating for ADHD, assess not only for the presence of symptoms, but also if these symptoms produce significant functional impairment.13,15 Impairments in daily functioning can include impaired school participation, social participation, quality of relationships, family conflict, family activities, family functioning, and emotional functioning.45 Some symptoms may affect functioning in an adult’s life differently than they did during childhood, from missed work appointments to being late picking up kids from school. Research has shown that the correlation between the number of symptoms and functional impairment is weak, which means someone could experience all of the symptoms of ADHD without experiencing functional impairment.45 To make an accurate diagnosis, it is therefore important to clearly establish both the number of symptoms the patient is experiencing and whether these symptoms are clearly linked to functional impairments.10
Sensible treatment
Once a diagnosis of ADHD has been clearly established, clinicians need to consider how best to treat the condition (Table 5). Stimulants are generally considered first-line treatment for ADHD. In randomized clinical trials, they showed significant efficacy; for example, one study of 146 adults with ADHD found a 76% improvement with methylphenidate compared to 19% for the placebo group.46 Before starting a stimulant, certain comorbidities should be ruled out. If a patient has glaucoma or pheochromocytoma, they may first need treatment from or clearance by other specialists. Stimulants should likely be held in patients with hypertension, angina, or cardiovascular defects until receiving medical clearance. The risks of stimulants need to be discussed with female patients of childbearing age, weighing the benefits of treatment against the risks of medication use should the patient get pregnant. Patients with comorbid psychosis or uncontrolled bipolar illness should not receive stimulants due to the risk of exacerbation. Patients with active substance use disorders (SUDs) are generally not good candidates for stimulants because of the risk of misusing or diverting stimulants and the possibility that substance abuse may be causing their inattentive symptoms. Patients whose SUDs are in remission may cautiously be considered as candidates for stimulants. If patients misuse their prescribed stimulants, they should be switched to a nonstimulant medication such as atomoxetine, bupropion, guanfacine, or clonidine.47

Continue to: Once a patient is deemed...
Once a patient is deemed to be a candidate for stimulants, clinicians need to choose between methylphenidate or amphetamine/dextroamphetamine formulations. Table 6 lists medications that are commonly prescribed to treat ADHD; unless otherwise noted, these are FDA-approved for this indication. As a general rule, for adults, long-acting stimulant formulations are preferred over short-acting formulations.28 Immediate-release stimulants are more prone to misuse or diversion compared to extended-release medications.29 Longer-acting formulations may also provide better full-day symptom control.48

In contrast to many other psychiatric medications, it may be beneficial to encourage periodically taking breaks or “medication holidays” from stimulants. Planned medication holidays for adults can involve intentionally not taking the medication over the weekend when the patient is not involved in work or school responsibilities. Such breaks have been shown to reduce adverse effects of stimulants (such as appetite suppression and insomnia) without significantly increasing ADHD symptoms.49 Short breaks can also help prevent medication tolerance and the subsequent need to increase doses.50 Medication holidays provide an opportunity to verify the ongoing benefits of the medication. It is advisable to periodically assess whether there is a continued need for stimulant treatment.51 If patients do not tolerate stimulants or have other contraindications, nonstimulants should be considered.
Lastly, no psychiatric patient should be treated with medication alone, and nonpharmacologic approaches should be incorporated as needed. Clear instructions, visual aids, nonverbal cues, frequent breaks to stand and stretch, schedules, normalizing failure as part of growth, and identifying triggers for emotional reactivity may help patients with ADHD.52 In a study of the academic performance of 92 college students taking medication for ADHD and 146 control students, treatment with stimulants alone did not eliminate the academic achievement deficit of those individuals with ADHD.53 Good study habits (even without stimulants) appeared more important in overcoming the achievement disparity of students with ADHD.53 Providing psychoeducation and training in concrete organization and planning skills have shown benefit.54 Practice of skills on a daily basis appears to be especially beneficial.55
Bottom Line
A sensible approach to diagnosing attention-deficit/hyperactivity disorder (ADHD) in adults includes ruling out other disorders that may present similar to ADHD, taking an appropriate developmental history, obtaining collateral information, and assessing for functional impairment. Sensible treatment involves ruling out comorbidities that stimulants could worsen, selecting extended-release stimulants, incorporating medication holidays, and using nonpharmacologic interventions.
Related Resources
- National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. https://www.nice.org.uk/guidance/ng87
- Substance Abuse and Mental Health Services Administration. Advisory: Prescription Stimulant Misuse Among Youth and Young Adults. https://store.samhsa.gov/product/prescription-stimulant-misuse-among-youth-young-adults/PEP21-06-01-003
Drug Brand Names
Amphetamine • Adzenys, Dyanavel, others
Atomoxetine • Strattera
Bupropion • Wellbutrin, Forfivo
Clonidine • Catapres, Kapvay
Dexmethylphenidate • Focalin
Dextroamphetamine • Dexedrine
Dextroamphetamine and amphetamine • Adderall, Mydayis
Guanfacine • Intuniv, Tenex
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin, others
Viloxazine • Qelbree
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
1. Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450-462.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:35.
3. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
4. Olfson M, Blanco C, Wang S, et al. Trends in office-based treatment of adults with stimulants in the United States. J Clin Psychiatry. 2013;74(1):43-50.
5. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956.
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24(4):562-575.
7. Solanto MV. Child vs adult onset of attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2017;74(4):421.
8. Jummani RR, Hirsch E, Hirsch GS. Are we overdiagnosing and overtreating ADHD? Psychiatric Times. Published May 31, 2017. Accessed March 17, 2023. https://www.psychiatrictimes.com/view/are-we-overdiagnosing-and-overtreating-adhd
9. Sibley MH, Rohde LA, Swanson JM, et al; Multimodal Treatment Study of Children with ADHD (MTA) Cooperative Group. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149.
10. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165.
11. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. 2017;74(5):520-527.
12. Faraone SV, Biederman J. Can attention-deficit/hyperactivity disorder onset occur in adulthood? JAMA Psychiatry. 2016;73(7):655-656.
13. Sibley MH, Pelham WE, Molina BSG, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052-1061.
14. Sollman MJ, Ranseen JD, Berry DT. Detection of feigned ADHD in college students. Psychol Assess. 2010;22(2):325-335.
15. Green AL, Rabiner DL. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9(3):559-568.
16. Sullivan BK, May K, Galbally L. Symptom exaggeration by college adults in attention-deficit hyperactivity disorder and learning disorder assessments. Appl Neuropsychol. 2007;14(3):189-207.
17. Suhr J, Hammers D, Dobbins-Buckland K, et al. The relationship of malingering test failure to self-reported symptoms and neuropsychological findings in adults referred for ADHD evaluation. Arch Clin Neuropsychol. 2008;23(5):521-530.
18. Lee Booksh R, Pella RD, Singh AN, et al. Ability of college students to simulate ADHD on objective measures of attention. J Atten Disord. 2010;13(4):325-338.
19. Harrison AG, Edwards MJ, Parker KC. Identifying students faking ADHD: preliminary findings and strategies for detection. Arch Clin Neuropsychol. 2007;22(5):577-588.
20. Lopez R, Micoulaud-Franchi JA, Galeria C, et al. Is adult-onset attention deficit/hyperactivity disorder frequent in clinical practice? Psychiatry Res. 2017;257:238-241.
21. Bhatia R. Rule out these causes of inattention before diagnosing ADHD. Current Psychiatry. 2016;15(10):32-33.
22. Aiken C. Adult-onset ADHD raises questions. Psychiatric Times. 2021;38(3):24.
23. Bjorn S, Weyandt LL. Issues pertaining to misuse of ADHD prescription medications. Psychiatric Times. 2018;35(9):17-19.
24. Compton WM, Han B, Blanco C, et al. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018;175(8):741-755.
25. Wei YJ, Zhu Y, Liu W, et al. Prevalence of and factors associated with long-term concurrent use of stimulants and opioids among adults with attention-deficit/hyperactivity disorder. JAMA Netw Open. 2018;1(4):e181152. doi:10.1001/jamanetworkopen.2018.1152
26. Benson K, Flory K, Humphreys KL, et al. Misuse of stimulant medication among college students: a comprehensive review and meta-analysis. Clin Child Fam Psychol Rev. 2015;18(1):50-76.
27. Benson K, Woodlief DT, Flory K, et al. Is ADHD, independent of ODD, associated with whether and why college students misuse stimulant medication? Exp Clin Psychopharmacol. 2018;26(5):476-487.
28. Froehlich TE. ADHD medication adherence in college students-- a call to action for clinicians and researchers: commentary on “transition to college and adherence to prescribed attention deficit hyperactivity disorder medication.” J Dev Behav Pediatr. 2018;39(1):77-78.
29. Wilens TE, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21-31.
30. Vrecko S. Everyday drug diversions: a qualitative study of the illicit exchange and non-medical use of prescription stimulants on a university campus. Soc Sci Med. 2015;131:297-304.
31. Munro BA, Weyandt LL, Marraccini ME, et al. The relationship between nonmedical use of prescription stimulants, executive functioning and academic outcomes. Addict Behav. 2017;65:250-257.
32. Rabiner DL, Anastopoulos AD, Costello EJ, et al. Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord. 2009;13(3)259-270.
33. Tayag Y. Adult ADHD is the wild west of psychiatry. The Atlantic. Published April 14, 2023. Accessed May 3, 2023. https://www.theatlantic.com/health/archive/2023/04/adult-adhd-diagnosis-treatment-adderall-shortage/673719/
34. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev. 2018;87:255-270.
35. Viktorin A, Rydén E, Thase ME, et al. The risk of treatment-emergent mania with methylphenidate in bipolar disorder. Am J Psychiatry. 2017;174(4):341-348.
36. Moran LV, Ongur D, Hsu J, et al. Psychosis with methylphenidate or amphetamine in patients with ADHD. N Engl J Med. 2019; 380(12):1128-1138.
37. Nörby U, Winbladh B, Källén K. Perinatal outcomes after treatment with ADHD medication during pregnancy. Pediatrics. 2017;140(6):e20170747. doi:10.1542/peds.2017-0747
38. Tadrous M, Shakeri A, Chu C, et al. Assessment of stimulant use and cardiovascular event risks among older adults. JAMA Netw Open. 2021;4(10):e2130795. doi:10.1001/jamanetworkopen.2021.30795
39. Daughton JM, Kratochvil CJ. Review of ADHD pharmacotherapies: advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48(3):240-248.
40. Qelbree [package insert]. Rockville, MD: Supernus Pharmaceuticals; 2021.
41. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:183.
42. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022:250.
43. DuPaul GJ, Weyandt LL, O’Dell SM, et al. College students with ADHD: current status and future directions. J Atten Disord. 2009;13(3):234-250.
44. Edmundson M, Berry DTR, Combs HL, et al. The effects of symptom information coaching on the feigning of adult ADHD. Psychol Assess. 2017;29(12):1429-1436.
45. Gordon M, Antshel K, Faraone S, et al. Symptoms versus impairment: the case for respecting DSM-IV’s criterion D. J Atten Disord. 2006;9(3):465-475.
46. Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456-463.
47. Osser D, Awidi B. Treating adults with ADHD requires special considerations. Psychiatric News. Published August 30, 2018. Accessed March 17, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.pp8a1
48. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown, RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
49. Martins S, Tramontina S, Polanczyk G, et al. Weekend holidays during methylphenidate use in ADHD children: a randomized clinical trial. J Child Adolesc Psychopharmacol. 2004;14(2):195-206.
50. Ibrahim K, Donyai P. Drug holidays from ADHD medication: international experience over the past four decades. J Atten Disord. 2015;19(7):551-568.
51. Matthijssen AM, Dietrich A, Bierens M, et al. Continued benefits of methylphenidate in ADHD after 2 years in clinical practice: a randomized placebo-controlled discontinuation study. Am J Psychiatry. 2019;176(9):754-762.
52. Mason EJ, Joshi KG. Nonpharmacologic strategies for helping children with ADHD. Current Psychiatry. 2018;7(1):42,46.
53. Advokat C, Lane SM, Luo C. College students with and without ADHD: comparison of self-report of medication usage, study habits, and academic achievement. J Atten Disord. 2011;15(8):656-666.
54. Knouse LE, Cooper-Vince C, Sprich S, et al. Recent developments in the psychosocial treatment of adult ADHD. Expert Rev Neurother. 2008;8(10):1537-1548.
55. Evans SW, Owens JS, Wymbs BT, et al. Evidence-based psychosocial treatments for children and adolescents with attention deficit/hyperactivity disorder. J Clin Child Adolesc Psychol. 2018;47(2):157-198.
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
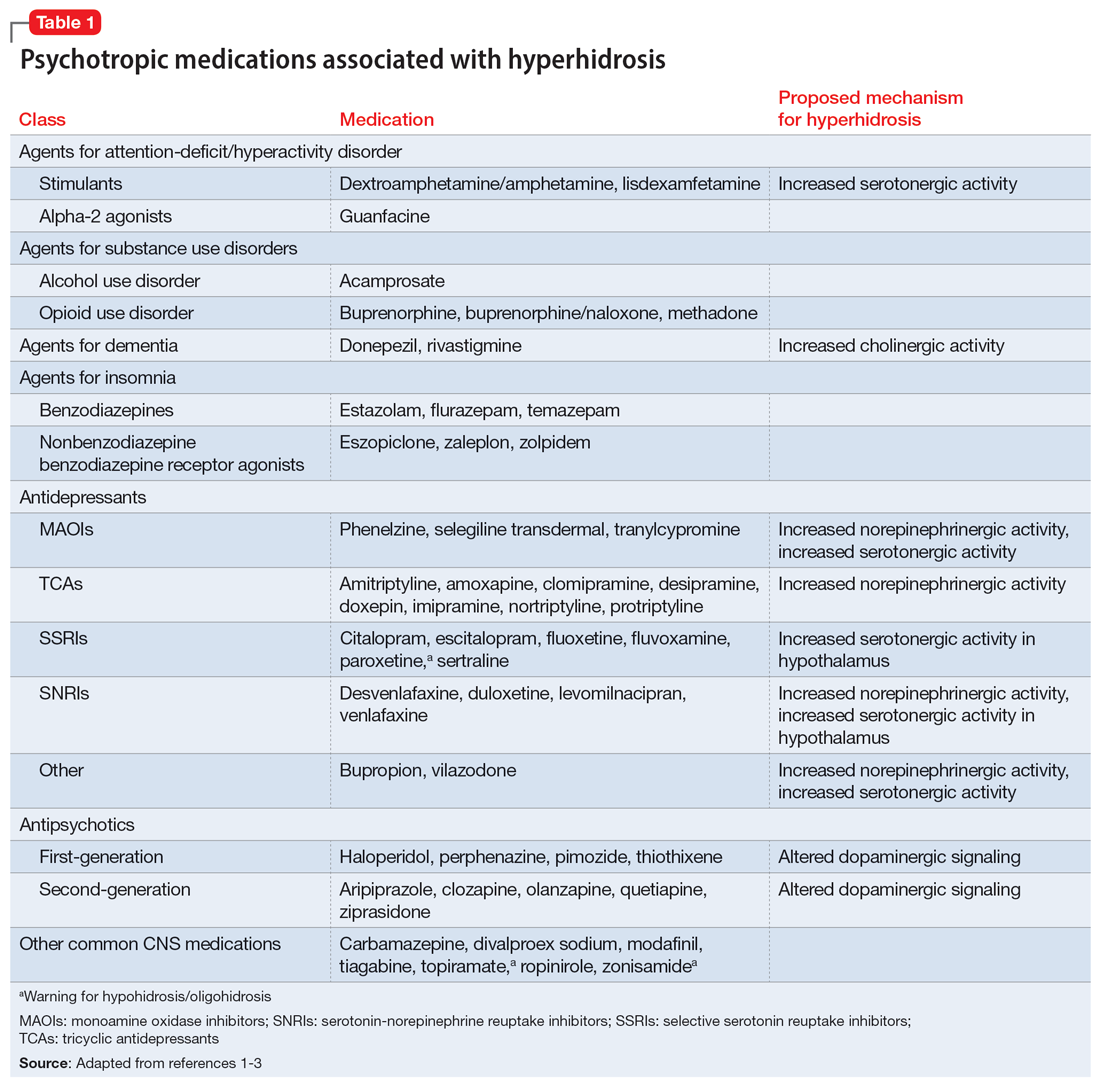
The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
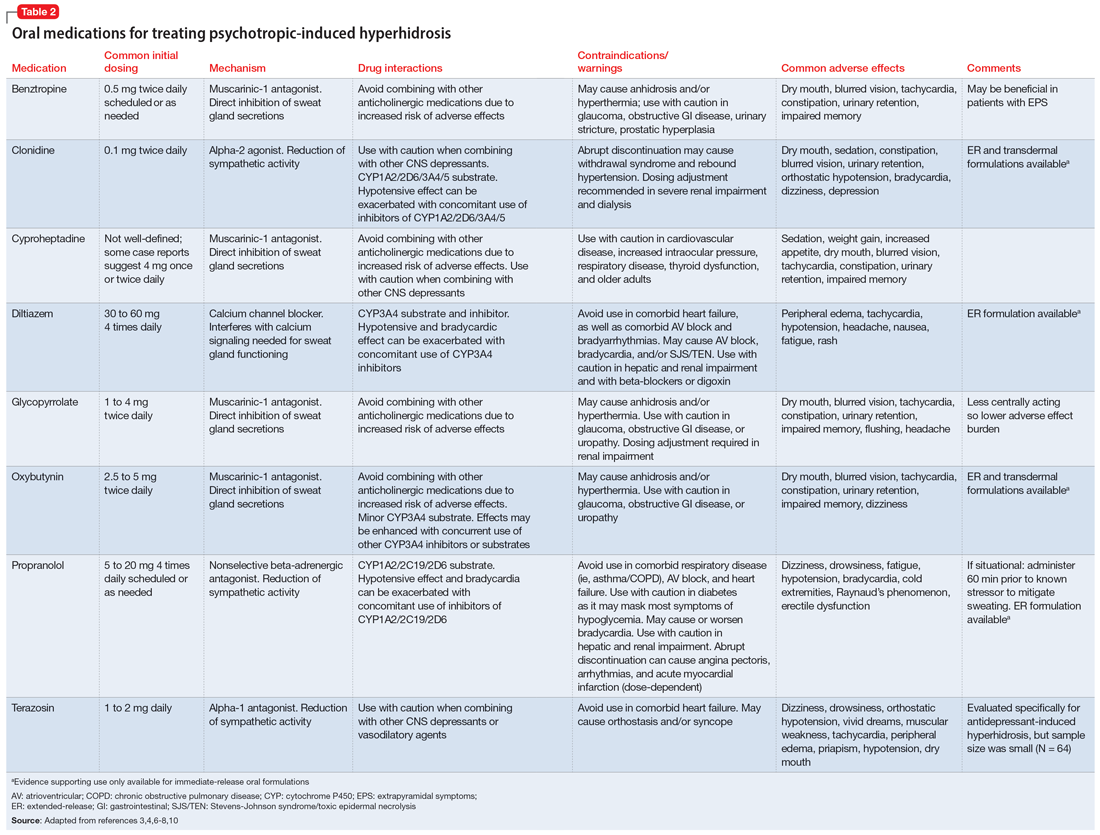
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449





