User login
Selinexor trials placed on partial hold
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
The US Food and Drug Administration (FDA) has placed a partial clinical hold on all trials of selinexor (KPT-330).
Selinexor is an inhibitor being evaluated in multiple trials of patients with relapsed and/or refractory hematologic and solid tumor malignancies.
While the partial clinical hold remains in effect, patients with stable disease or better may remain on selinexor.
However, no new patients may be enrolled in selinexor trials until the hold is lifted.
The FDA has indicated that the partial clinical hold is due to incomplete information in the existing version of the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, said it has amended the brochure, updated the informed consent documents accordingly, and submitted the documents to the FDA as requested.
As of March 10, Karyopharm had provided all requested materials to the FDA believed to be required to lift the partial clinical hold. By regulation, the FDA has 30 days from the receipt of Karyopharm’s submission to notify the company whether the partial clinical hold is lifted.
Karyopharm said it is working with the FDA to seek the release of the hold and resume enrollment in its selinexor trials as expeditiously as possible. The company believes its previously disclosed enrollment rates and timelines for its ongoing trials will remain materially unchanged.
About selinexor
Selinexor is a selective inhibitor of nuclear export (SINE) XPO1 antagonist. The drug binds with and inhibits XPO1, leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to induce apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor. The drug is currently being evaluated in several trials across multiple cancer indications.
One of these is the phase 2 SOPRA trial, in which selinexor is being compared to investigator’s choice of therapy (1 of 3 potential salvage therapies). The trial is enrolling patients 60 years of age or older with relapsed or refractory acute myeloid leukemia who are ineligible for standard intensive chemotherapy and/or transplant.
The SADAL study is a phase 2b trial comparing high and low doses of selinexor in patients with relapsed and/or refractory de novo diffuse large B-cell lymphoma who have no therapeutic options of demonstrated clinical benefit.
STORM is a phase 2b trial evaluating selinexor and low-dose dexamethasone in patients with heavily pretreated multiple myeloma (MM). And STOMP is a phase 1b/2 study evaluating selinexor in combination with existing therapies across the broader population in MM.
Karyopharm is also planning a randomized, phase 3 study known as BOSTON. In this trial, researchers will compare selinexor plus bortezomib and low-dose dexamethasone to bortezomib and low-dose dexamethasone in MM patients who have had 1 to 3 prior lines of therapy.
Additional phase 1, 2, and 3 studies are ongoing or currently planned.
Drug receives orphan designation for DLBCL
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
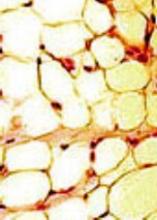
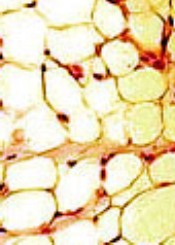
Study confirms increased adiposity in HSCT survivors
ORLANDO, FL—A recently conducted study confirms that survivors of hematopoietic stem cell transplant (HSCT) have increased body fat mass and lower lean mass compared to normal controls. And this is despite having a comparable body mass index (BMI).
Researchers say the abnormalities in adipokine levels—leptin and adiponectin—could provide insight into the mechanisms that contribute to the metabolic syndrome and cardiovascular complications that often develop in HSCT survivors.
Leptin and adiponectin are associated with obesity, insulin secretion, insulin resistance, endothelial function, vascular homeostasis, and atherosclerosis.
“So knowing that there is a dynamic interplay between obesity and insulin resistance and cytokine and adipokine profiles and, ultimately, insulin-resistance syndrome, we sought to evaluate, as part of a larger study, how treatment effects, including high-dose chemotherapy and radiation, alter cytokine profiles as well as obesity and body composition,” said Tyler G. Ketterl, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Ketterl presented the findings at the 2017 BMT Tandem Meetings as abstract 52.*
Study design
The research team compared 151 HSCT recipients who had survived more than 2 years after transplant with 92 sibling controls.
HSCT survivors were randomly recruited from 2 centers—Fred Hutchinson Cancer Research Center and University of Minnesota Masonic Children’s Hospital—and were younger than 21 years when diagnosed.
The researchers evaluated all participants for body composition, cardiovascular risk factors, and adipokines using anthropomorphic measurements, DXA scans for muscle and fat mass, and laboratory bloodwork.
The team stratified the HSCT survivors by the preparative regimen they had received—total body irradiation (TBI) alone, TBI plus cranial radiation (CRT), and chemotherapy alone.
Study population
Males comprised more than half the study population in each arm, 58% of HSCT survivors and 54% of siblings.
Nine percent and 8% in the HSCT and sibling arms, respectively, were non-white and/or Hispanic, and the mean current ages were 24.0 (range, 10-51) for HSCT survivors and 24.2 (range, 10-48) for siblings.
The survivors’ mean age at diagnosis was 9.1 years (range, 0.4–20.6), their mean age at transplant was 11.2 years (range, 0.6–32.6), and the mean time from transplant to study participation was 13.5 years (range, 2.6–32).
Most patients received a transplant for leukemia—54 (36%) for acute myeloid leukemia, 46 (31%) for acute lymphoblastic leukemia, and 15 (10%) for chronic myeloid leukemia. Thirteen (9%) received transplants for myelodysplastic syndromes, 12 (8%) for Hodgkin lymphoma, and 10 (6%) for non-Hodgkin lymphoma.
A little more than half had TBI (85, 56%) as the preparative regimen, 31 (21%) had TBI plus CRT, and 35 (23%) had chemotherapy only.
About three-quarters (116, 77%) had an allogeneic transplant, and 35 (23%) had an autologous transplant.
Results
Overall, HSCT survivors had significantly lower adiponectin levels than siblings (P<0.001).
Survivors who received TBI with or without CRT had significantly lower adiponectin levels than siblings (P<0.001), while survivors who received chemotherapy alone did not (P=0.42).
Adiponectin is involved in insulin sensitization, hepatoprotective action, antiatherogenic action, protection against the development of diabetes, and regulation of lipid metabolism.
Overall, survivors had significantly higher leptin levels than siblings (P<0.001).
This held true regardless of conditioning regimen, although levels for patients who received chemotherapy only were not as significantly high (P=0.02) as for survivors who received TBI (P<0.001).
Leptin helps increase energy expenditure, decrease appetite and food uptake, modify insulin sensitivity on muscles and liver, prevent ectopic lipid deposition, and regulate immune function.
BMI adjusted for age, sex, and Tanner stage was not significantly different between survivors and siblings, but percent fat mass was significantly higher across all conditioning regimens for survivors compared to siblings (P<0.001).
“And this goes along with previous data,” Dr Ketterl said, “that shows sarcopenic obesity is common amongst transplant survivors.”
The researchers believe these significant differences may provide insight into the underlying risk of developing metabolic syndrome and cardiovascular complications in transplant survivors. ![]()
*Some details in the abstract differ from the presentation.
ORLANDO, FL—A recently conducted study confirms that survivors of hematopoietic stem cell transplant (HSCT) have increased body fat mass and lower lean mass compared to normal controls. And this is despite having a comparable body mass index (BMI).
Researchers say the abnormalities in adipokine levels—leptin and adiponectin—could provide insight into the mechanisms that contribute to the metabolic syndrome and cardiovascular complications that often develop in HSCT survivors.
Leptin and adiponectin are associated with obesity, insulin secretion, insulin resistance, endothelial function, vascular homeostasis, and atherosclerosis.
“So knowing that there is a dynamic interplay between obesity and insulin resistance and cytokine and adipokine profiles and, ultimately, insulin-resistance syndrome, we sought to evaluate, as part of a larger study, how treatment effects, including high-dose chemotherapy and radiation, alter cytokine profiles as well as obesity and body composition,” said Tyler G. Ketterl, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Ketterl presented the findings at the 2017 BMT Tandem Meetings as abstract 52.*
Study design
The research team compared 151 HSCT recipients who had survived more than 2 years after transplant with 92 sibling controls.
HSCT survivors were randomly recruited from 2 centers—Fred Hutchinson Cancer Research Center and University of Minnesota Masonic Children’s Hospital—and were younger than 21 years when diagnosed.
The researchers evaluated all participants for body composition, cardiovascular risk factors, and adipokines using anthropomorphic measurements, DXA scans for muscle and fat mass, and laboratory bloodwork.
The team stratified the HSCT survivors by the preparative regimen they had received—total body irradiation (TBI) alone, TBI plus cranial radiation (CRT), and chemotherapy alone.
Study population
Males comprised more than half the study population in each arm, 58% of HSCT survivors and 54% of siblings.
Nine percent and 8% in the HSCT and sibling arms, respectively, were non-white and/or Hispanic, and the mean current ages were 24.0 (range, 10-51) for HSCT survivors and 24.2 (range, 10-48) for siblings.
The survivors’ mean age at diagnosis was 9.1 years (range, 0.4–20.6), their mean age at transplant was 11.2 years (range, 0.6–32.6), and the mean time from transplant to study participation was 13.5 years (range, 2.6–32).
Most patients received a transplant for leukemia—54 (36%) for acute myeloid leukemia, 46 (31%) for acute lymphoblastic leukemia, and 15 (10%) for chronic myeloid leukemia. Thirteen (9%) received transplants for myelodysplastic syndromes, 12 (8%) for Hodgkin lymphoma, and 10 (6%) for non-Hodgkin lymphoma.
A little more than half had TBI (85, 56%) as the preparative regimen, 31 (21%) had TBI plus CRT, and 35 (23%) had chemotherapy only.
About three-quarters (116, 77%) had an allogeneic transplant, and 35 (23%) had an autologous transplant.
Results
Overall, HSCT survivors had significantly lower adiponectin levels than siblings (P<0.001).
Survivors who received TBI with or without CRT had significantly lower adiponectin levels than siblings (P<0.001), while survivors who received chemotherapy alone did not (P=0.42).
Adiponectin is involved in insulin sensitization, hepatoprotective action, antiatherogenic action, protection against the development of diabetes, and regulation of lipid metabolism.
Overall, survivors had significantly higher leptin levels than siblings (P<0.001).
This held true regardless of conditioning regimen, although levels for patients who received chemotherapy only were not as significantly high (P=0.02) as for survivors who received TBI (P<0.001).
Leptin helps increase energy expenditure, decrease appetite and food uptake, modify insulin sensitivity on muscles and liver, prevent ectopic lipid deposition, and regulate immune function.
BMI adjusted for age, sex, and Tanner stage was not significantly different between survivors and siblings, but percent fat mass was significantly higher across all conditioning regimens for survivors compared to siblings (P<0.001).
“And this goes along with previous data,” Dr Ketterl said, “that shows sarcopenic obesity is common amongst transplant survivors.”
The researchers believe these significant differences may provide insight into the underlying risk of developing metabolic syndrome and cardiovascular complications in transplant survivors. ![]()
*Some details in the abstract differ from the presentation.
ORLANDO, FL—A recently conducted study confirms that survivors of hematopoietic stem cell transplant (HSCT) have increased body fat mass and lower lean mass compared to normal controls. And this is despite having a comparable body mass index (BMI).
Researchers say the abnormalities in adipokine levels—leptin and adiponectin—could provide insight into the mechanisms that contribute to the metabolic syndrome and cardiovascular complications that often develop in HSCT survivors.
Leptin and adiponectin are associated with obesity, insulin secretion, insulin resistance, endothelial function, vascular homeostasis, and atherosclerosis.
“So knowing that there is a dynamic interplay between obesity and insulin resistance and cytokine and adipokine profiles and, ultimately, insulin-resistance syndrome, we sought to evaluate, as part of a larger study, how treatment effects, including high-dose chemotherapy and radiation, alter cytokine profiles as well as obesity and body composition,” said Tyler G. Ketterl, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Ketterl presented the findings at the 2017 BMT Tandem Meetings as abstract 52.*
Study design
The research team compared 151 HSCT recipients who had survived more than 2 years after transplant with 92 sibling controls.
HSCT survivors were randomly recruited from 2 centers—Fred Hutchinson Cancer Research Center and University of Minnesota Masonic Children’s Hospital—and were younger than 21 years when diagnosed.
The researchers evaluated all participants for body composition, cardiovascular risk factors, and adipokines using anthropomorphic measurements, DXA scans for muscle and fat mass, and laboratory bloodwork.
The team stratified the HSCT survivors by the preparative regimen they had received—total body irradiation (TBI) alone, TBI plus cranial radiation (CRT), and chemotherapy alone.
Study population
Males comprised more than half the study population in each arm, 58% of HSCT survivors and 54% of siblings.
Nine percent and 8% in the HSCT and sibling arms, respectively, were non-white and/or Hispanic, and the mean current ages were 24.0 (range, 10-51) for HSCT survivors and 24.2 (range, 10-48) for siblings.
The survivors’ mean age at diagnosis was 9.1 years (range, 0.4–20.6), their mean age at transplant was 11.2 years (range, 0.6–32.6), and the mean time from transplant to study participation was 13.5 years (range, 2.6–32).
Most patients received a transplant for leukemia—54 (36%) for acute myeloid leukemia, 46 (31%) for acute lymphoblastic leukemia, and 15 (10%) for chronic myeloid leukemia. Thirteen (9%) received transplants for myelodysplastic syndromes, 12 (8%) for Hodgkin lymphoma, and 10 (6%) for non-Hodgkin lymphoma.
A little more than half had TBI (85, 56%) as the preparative regimen, 31 (21%) had TBI plus CRT, and 35 (23%) had chemotherapy only.
About three-quarters (116, 77%) had an allogeneic transplant, and 35 (23%) had an autologous transplant.
Results
Overall, HSCT survivors had significantly lower adiponectin levels than siblings (P<0.001).
Survivors who received TBI with or without CRT had significantly lower adiponectin levels than siblings (P<0.001), while survivors who received chemotherapy alone did not (P=0.42).
Adiponectin is involved in insulin sensitization, hepatoprotective action, antiatherogenic action, protection against the development of diabetes, and regulation of lipid metabolism.
Overall, survivors had significantly higher leptin levels than siblings (P<0.001).
This held true regardless of conditioning regimen, although levels for patients who received chemotherapy only were not as significantly high (P=0.02) as for survivors who received TBI (P<0.001).
Leptin helps increase energy expenditure, decrease appetite and food uptake, modify insulin sensitivity on muscles and liver, prevent ectopic lipid deposition, and regulate immune function.
BMI adjusted for age, sex, and Tanner stage was not significantly different between survivors and siblings, but percent fat mass was significantly higher across all conditioning regimens for survivors compared to siblings (P<0.001).
“And this goes along with previous data,” Dr Ketterl said, “that shows sarcopenic obesity is common amongst transplant survivors.”
The researchers believe these significant differences may provide insight into the underlying risk of developing metabolic syndrome and cardiovascular complications in transplant survivors. ![]()
*Some details in the abstract differ from the presentation.
Connective tissue diseases reported in patients receiving immune checkpoint inhibitors
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Key clinical point:
Major finding: In a cohort of 447 patients, 4 with metastatic cancer developed connective tissue disease following anti-PD-1/PDL-1 treatment.
Data source: A prospective, multicenter, academic registry was screened for reports of CTD among patients being treated with anti-PD1/PDL-1 agents.
Disclosures: One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Adding rituximab to reduced intensity conditioning boosts PFS
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
ORLANDO – Rituximab conferred a significant progression-free survival benefit in reduced intensity conditioning regimens for patients with B-cell non-Hodgkin lymphoma who underwent allogeneic hematopoietic cell transplantation, based on data from the Center for International Blood & Marrow Transplant Research.
Further, higher cumulative rituximab doses appeared to confer a benefit in overall survival.
Rituximab is frequently a component of reduced intensity conditioning (RIC) regimens in allogeneic hematopoietic cell transplantation (HCT), but there has been a “paucity of comparative data” for rituximab-containing (R-RIC) versus non–R-RIC conditioning regimens for allogeneic transplant patients, Narendranath Epperla, MD, of the Medical College of Wisconsin, Milwaukee, said during the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society of Blood and Marrow Transplantation.
Using data from the Center for International Blood & Marrow Transplant Research, Dr. Epperla and his colleagues identified 1,022 patients who received rituximab and 379 patients who did not with diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma. The patients received their first RIC or non-myeloablative allogeneic HCT between 2008 and 2014. The donors were matched, and either related or 8x8 allele-matched unrelated; the graft source could be bone marrow or peripheral blood. Graft versus host disease (GVHD) suppression was calcineurin inhibitor based.
Patients who had received myeloablative conditioning, or who had received radioimmunotherapy or alemtuzumab were excluded, as were those who received alternative donor allografts.
Dr. Epperla and his colleagues factored in patient and disease characteristics, as well as differences in transplant regimen, in determining the adjusted cumulative incidence of relapse or progression, as well as the incidence of nonrelapse mortality.
In the multivariable analysis, overall survival did not differ between the R-RIC and the non–R-RIC cohorts (relative risk [RR] of all-cause mortality, R-RIC = 0.83, 95% CI 0.67-1.03, P = .09).
Based on the cumulative dose of rituximab that patients had received, though, “we noted that patients who got higher doses of rituximab had lower risk of nonrelapse mortality,” Dr. Epperla said. “Higher cumulative doses of rituximab seem to confer overall survival benefit.” This was true even though the higher rituximab doses had no significant effect on the risk of therapy failure, nonrelapse mortality, or the risk of progression/relapse.
When the cumulative rituximab dose was 2,000 to 3,375 mg/m2, the hazard ratio for all-cause mortality fell to 0.43 compared to a cumulative rituximab dose of less than 1,000 mg/m2 (95% confidence interval [CI] 0.21-0.90, P = .02).
Among the R-RIC group, there was a nonsignificant trend toward reduced risk of progression or relapse (relative risk of progression/relapse, R-RIC = 0.79, 95% CI 0.63-1.01, P = .055). However, the R-RIC group fared significantly better in terms of progression-free survival (RR of PFS, R-RIC = 0.76, 95% CI 0.62-0.92, P = .006).
After transplant, patients in the R-RIC group were no more likely than those in the non–R-RIC group to experience chronic GVHD (RR of GVHD, R-RIC = 1.15, 95% CI 0.96-1.39, P = .13). There was no difference in the adjusted curves of nonrelapse mortality between the groups (RR of nonrelapse mortality, R-RIC = 0.90, 95% CI 0.67-1.22, P = .51).
Also, there were no fatal cytopenias in the R-RIC arm, although the literature warrants some concern for increased risk of infection with rituximab, Dr. Epperla said.
At baseline, there were no significant differences in demographic characteristics between the nonrituximab and rituximab arms of the study population. More than 90% of patients were white, and 65% were male; the median age was 57 years (range, 18-74).
Patients had been diagnosed about 3 years before receiving HCT; about 60% of patients had a baseline Karnofsky performance score greater than 90, and the HCT comorbidity index was 2. About 86% of patients were chemosensitive, and patients in both study arms had received a median of three prior lines of therapy.
There were some differences in conditioning regimens between the two groups. “There were a significantly higher number of patients in the nonrituximab group who received fludarabine/busulfan, while there were a significantly high number in the rituximab group who received a fludarabine/cyclophosphamide-based conditioning regimen,” Dr. Epperla said. Follicular lymphomas were more common in the R-RIC arm, while diffuse large B-cell lymphomas were seen more in the non–R-RIC arm.
Given the survival benefit and similar rates of chronic GVHD seen in the retrospective analysis, a prospective, randomized head-to-head trial of R-RIC versus non–R-RIC is warranted, Dr. Epperla concluded.
During the postpresentation discussion, Dr. Epperla acknowledged the variability of the lymphomas in the study, but that there was no significant statistical effect of specific histologies on the findings in a subgroup analysis. Dr. Epperla added that the chemosensitivity status at transplant was checked to account for patient exposure to rituximab before RIC, and that there was no effect of prior rituximab exposure on the outcomes examined.
Dr. Epperla reported no conflicts of interest.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: Patients with rituximab-containing RIC regimens had better progression-free survival (PFS; relative risk of PFS, non–R-RIC=1, R-RIC=076, 95% CI 0.62-092, P = .006).
Data source: Retrospective review of 1,022 allogeneic HCT B-cell non-Hodgkin lymphoma patients who received rituximab and 379 who did not.
Disclosures: The data were obtained from the Center for International Blood & Marrow Transplant Research. Dr. Epperla reported no disclosures.
Potential therapeutic strategy for BL, DLBCL
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
Preclinical research has revealed a potential strategy for treating Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL).
Investigators discovered that miR-28 inhibits the growth of B-cell lymphomas, but this microRNA is often lost in these lymphomas.
Re-expressing miR-28 in mouse models of BL and DLBCL inhibited tumor growth, which supports the potential of synthetic miR-28 analogs for the treatment of these lymphomas.
In fact, the investigators believe their work could lead to the development of the first miRNA analog therapy for the treatment of B-cell lymphoma and provide the basis for clinical trials.
Almudena Ramiro, PhD, of Centro Nacional de Investigaciones Cardiovasculares in Madrid, Spain, and her colleagues described the work in Blood.
The team characterized the function of miR-28 in the biology of mature B lymphocytes and in the development of lymphomas associated with this cell type.
The investigators found that miR-28 regulates the terminal differentiation of B lymphocytes, a fundamental process in the biology of these cells that generates memory B lymphocytes and highly specific plasma cells.
But the team found that miR-28 expression is lost in several germinal center-derived lymphoma subtypes, including BL, DLBCL, follicular lymphoma, and chronic lymphocytic leukemia.
In vitro experiments showed that miR-28 expression dampens B-cell receptor signaling and diminishes the proliferation and survival of primary B cells and lymphoma cells.
And in vivo experiments showed that re-establishing miR-28 expression slows tumor growth in DLBCL and BL.
The investigators re-expressed miR-28 in xenograft models of BL and DLBCL via the use of viral vectors or synthetic molecules and found that both methods blocked tumor growth. The same effect was observed in mice with established BL tumors.
Dr Ramiro and her colleagues said these results reveal the therapeutic potential of miR-28 and provide ample justification for the initiation of clinical trials of miR-28-based therapies to treat B-cell lymphomas. ![]()
CCSs’ subsequent cancer risk decreased from ’70s to ’90s
Childhood cancer survivors (CCSs) who were diagnosed in the 1990s have a lower risk of subsequent malignancies than CCSs diagnosed in the 1970s, according to research published in JAMA.
The data suggest this outcome is associated with a reduction in the overall use and median dose of therapeutic radiation over time.
Past research has shown an association between therapeutic radiation and the development of subsequent neoplasms in CCSs. Studies have also linked specific chemotherapeutic agents to subsequent neoplasms.
This information has been used to modify childhood cancer treatment over time, with the hope of reducing the risk of subsequent neoplasms while maintaining or improving 5-year survival.
To assess the effects of these treatment modifications, Gregory Armstrong, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted a study of CCSs.
The researchers evaluated 23,603 five-year CCSs (mean age at diagnosis, 7.7 years) treated at pediatric hospitals in the US and Canada from 1970 through 1999, with follow-up through December 2015.
The most common initial diagnoses were acute lymphoblastic leukemia (35.1%), Hodgkin lymphoma (11.1%), and astrocytoma (9.6%).
Subsequent neoplasms, malignancies
At a mean follow-up of 20.5 years, 1639 CCSs had experienced 3115 subsequent neoplasms, including 1026 malignancies, 233 benign meningiomas, and 1856 non-melanoma skin cancers. The most common neoplasms were breast and thyroid cancers.
The 15-year cumulative incidence of subsequent neoplasms decreased by decade of diagnosis. The incidence was 2.9% for patients diagnosed in the 1970s, 2.4% for those diagnosed in the ’80s, and 1.5% for those diagnosed in the ’90s. For the 1970s vs 1980s, the P value was 0.02. For the 1970s vs 1990s and for the 1980s vs 1990s, the P value was <0.001.
The 15-year cumulative incidence of subsequent malignancies also decreased by decade of diagnosis—2.1% for the ’70s, 1.7% for the ’80s, and 1.3% for the ’90s. The P value was <0.001 for the ’70s vs the ’90s and the ’80s vs the ’90s.
Risk factors
In multivariable analyses, female CCSs had a higher rate of subsequent neoplasms (including malignancies) than males.
In addition, high doses of alkylating agents and platinum agents were associated with increased rates of subsequent malignancies.
The researchers noted that, although there was a decrease in the median cumulative dose of alkylating agents over time, the proportion of CCSs receiving these agents increased. And both the median cumulative dose of platinum agents and the proportion of CCSs receiving these agents increased from the ’70s to the ’90s.
Finally, therapeutic radiation was associated with increased rates of subsequent malignant neoplasms, meningiomas, and non-melanoma skin cancers.
This corresponded with the researchers’ findings that the proportion of individuals receiving radiation and the median dose of radiation both decreased over time.
The proportion of individuals receiving any radiation therapy was 77.7% in the ’70s, 56.7% in the ’80s, and 36.8% in the ’90s. The median dose of radiation was 30.0 Gy in the ’70s, 24.0 Gy in the ’80s, and 26.0 Gy in the ’90s.
“The most ominous late effect of pediatric cancer treatment is a second malignancy,” Dr Armstrong said. “This study shows efforts to reduce the late effects of treatment are paying off. The risk of second cancers for survivors increases with age, so it is good to see the reduction emerging early in survivorship while survivors are still young.” ![]()
Childhood cancer survivors (CCSs) who were diagnosed in the 1990s have a lower risk of subsequent malignancies than CCSs diagnosed in the 1970s, according to research published in JAMA.
The data suggest this outcome is associated with a reduction in the overall use and median dose of therapeutic radiation over time.
Past research has shown an association between therapeutic radiation and the development of subsequent neoplasms in CCSs. Studies have also linked specific chemotherapeutic agents to subsequent neoplasms.
This information has been used to modify childhood cancer treatment over time, with the hope of reducing the risk of subsequent neoplasms while maintaining or improving 5-year survival.
To assess the effects of these treatment modifications, Gregory Armstrong, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted a study of CCSs.
The researchers evaluated 23,603 five-year CCSs (mean age at diagnosis, 7.7 years) treated at pediatric hospitals in the US and Canada from 1970 through 1999, with follow-up through December 2015.
The most common initial diagnoses were acute lymphoblastic leukemia (35.1%), Hodgkin lymphoma (11.1%), and astrocytoma (9.6%).
Subsequent neoplasms, malignancies
At a mean follow-up of 20.5 years, 1639 CCSs had experienced 3115 subsequent neoplasms, including 1026 malignancies, 233 benign meningiomas, and 1856 non-melanoma skin cancers. The most common neoplasms were breast and thyroid cancers.
The 15-year cumulative incidence of subsequent neoplasms decreased by decade of diagnosis. The incidence was 2.9% for patients diagnosed in the 1970s, 2.4% for those diagnosed in the ’80s, and 1.5% for those diagnosed in the ’90s. For the 1970s vs 1980s, the P value was 0.02. For the 1970s vs 1990s and for the 1980s vs 1990s, the P value was <0.001.
The 15-year cumulative incidence of subsequent malignancies also decreased by decade of diagnosis—2.1% for the ’70s, 1.7% for the ’80s, and 1.3% for the ’90s. The P value was <0.001 for the ’70s vs the ’90s and the ’80s vs the ’90s.
Risk factors
In multivariable analyses, female CCSs had a higher rate of subsequent neoplasms (including malignancies) than males.
In addition, high doses of alkylating agents and platinum agents were associated with increased rates of subsequent malignancies.
The researchers noted that, although there was a decrease in the median cumulative dose of alkylating agents over time, the proportion of CCSs receiving these agents increased. And both the median cumulative dose of platinum agents and the proportion of CCSs receiving these agents increased from the ’70s to the ’90s.
Finally, therapeutic radiation was associated with increased rates of subsequent malignant neoplasms, meningiomas, and non-melanoma skin cancers.
This corresponded with the researchers’ findings that the proportion of individuals receiving radiation and the median dose of radiation both decreased over time.
The proportion of individuals receiving any radiation therapy was 77.7% in the ’70s, 56.7% in the ’80s, and 36.8% in the ’90s. The median dose of radiation was 30.0 Gy in the ’70s, 24.0 Gy in the ’80s, and 26.0 Gy in the ’90s.
“The most ominous late effect of pediatric cancer treatment is a second malignancy,” Dr Armstrong said. “This study shows efforts to reduce the late effects of treatment are paying off. The risk of second cancers for survivors increases with age, so it is good to see the reduction emerging early in survivorship while survivors are still young.” ![]()
Childhood cancer survivors (CCSs) who were diagnosed in the 1990s have a lower risk of subsequent malignancies than CCSs diagnosed in the 1970s, according to research published in JAMA.
The data suggest this outcome is associated with a reduction in the overall use and median dose of therapeutic radiation over time.
Past research has shown an association between therapeutic radiation and the development of subsequent neoplasms in CCSs. Studies have also linked specific chemotherapeutic agents to subsequent neoplasms.
This information has been used to modify childhood cancer treatment over time, with the hope of reducing the risk of subsequent neoplasms while maintaining or improving 5-year survival.
To assess the effects of these treatment modifications, Gregory Armstrong, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues conducted a study of CCSs.
The researchers evaluated 23,603 five-year CCSs (mean age at diagnosis, 7.7 years) treated at pediatric hospitals in the US and Canada from 1970 through 1999, with follow-up through December 2015.
The most common initial diagnoses were acute lymphoblastic leukemia (35.1%), Hodgkin lymphoma (11.1%), and astrocytoma (9.6%).
Subsequent neoplasms, malignancies
At a mean follow-up of 20.5 years, 1639 CCSs had experienced 3115 subsequent neoplasms, including 1026 malignancies, 233 benign meningiomas, and 1856 non-melanoma skin cancers. The most common neoplasms were breast and thyroid cancers.
The 15-year cumulative incidence of subsequent neoplasms decreased by decade of diagnosis. The incidence was 2.9% for patients diagnosed in the 1970s, 2.4% for those diagnosed in the ’80s, and 1.5% for those diagnosed in the ’90s. For the 1970s vs 1980s, the P value was 0.02. For the 1970s vs 1990s and for the 1980s vs 1990s, the P value was <0.001.
The 15-year cumulative incidence of subsequent malignancies also decreased by decade of diagnosis—2.1% for the ’70s, 1.7% for the ’80s, and 1.3% for the ’90s. The P value was <0.001 for the ’70s vs the ’90s and the ’80s vs the ’90s.
Risk factors
In multivariable analyses, female CCSs had a higher rate of subsequent neoplasms (including malignancies) than males.
In addition, high doses of alkylating agents and platinum agents were associated with increased rates of subsequent malignancies.
The researchers noted that, although there was a decrease in the median cumulative dose of alkylating agents over time, the proportion of CCSs receiving these agents increased. And both the median cumulative dose of platinum agents and the proportion of CCSs receiving these agents increased from the ’70s to the ’90s.
Finally, therapeutic radiation was associated with increased rates of subsequent malignant neoplasms, meningiomas, and non-melanoma skin cancers.
This corresponded with the researchers’ findings that the proportion of individuals receiving radiation and the median dose of radiation both decreased over time.
The proportion of individuals receiving any radiation therapy was 77.7% in the ’70s, 56.7% in the ’80s, and 36.8% in the ’90s. The median dose of radiation was 30.0 Gy in the ’70s, 24.0 Gy in the ’80s, and 26.0 Gy in the ’90s.
“The most ominous late effect of pediatric cancer treatment is a second malignancy,” Dr Armstrong said. “This study shows efforts to reduce the late effects of treatment are paying off. The risk of second cancers for survivors increases with age, so it is good to see the reduction emerging early in survivorship while survivors are still young.” ![]()
Phase III trial: VZV protects auto-HCT patients
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Overall incidence of herpes zoster was 32.8 cases/1,000 patient-years vs. 91.8/1,000 patient-years in patients in the vaccine and placebo groups, respectively.
Data source: A randomized, placebo-controlled phase III trial involving 1,230 patients.
Disclosures: Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
Drug granted orphan status for follicular lymphoma
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma. ![]()
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma. ![]()
The US Food and Drug Administration (FDA) has granted orphan designation to G100 for the treatment of follicular lymphoma.
G100 is a synthetic small-molecule toll-like receptor-4 agonist, glucopyranosyl lipid A, formulated in a stable emulsion.
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved.
G100 is being developed by Immune Design. The company says G100 works by leveraging the activation of innate and adaptive immunity in the tumor microenvironment to create an immune response against the tumor’s pre-existing antigens.
According to Immune Design, clinical and preclinical data have demonstrated G100’s ability to activate tumor-infiltrating lymphocytes, macrophages, and dendritic cells, and promote antigen-presentation and the recruitment of T cells to the tumor.
The ensuing induction of local and systemic immune responses has been shown to result in local and abscopal tumor control in preclinical studies.
In fact, G100, when combined with local radiation, demonstrated efficacy against A20 lymphoma in mice. This research was presented in a poster at the 2016 ASH Annual Meeting (abstract 4166).
In this study, investigators evaluated the immune response and therapeutic effects of intratumoral G100 alone, local radiation alone, and concomitant G100 and local radiation in mice with A20 lymphoma.
The investigators said the combination therapy demonstrated:
- Synergistic antitumor effects in both injected as well as uninjected tumors (abscopal effects)
- Synergistic induction of pro-inflammatory cytokine and chemokine environment, as well as induction of genes governing antigen processing and presentation
- Increased infiltration of T cells, including CD4 and CD8 T cells, in treated tumors.
In contrast, tumors that received only radiation had significantly lower T-cell levels than untreated tumors.
“These findings highlight the potential beneficial effect that immunotherapy with G100 could provide when given with radiation by modulating the tumor microenvironment to generate a systemic, durable, T-cell anti-tumor response,” said study investigator Ramesh Rengan, MD, of the University of Washington in Seattle.
“As shown in this model, G100 may hold potential as a treatment for lymphoma patients.”
To test that theory, Immune Design is conducting a phase 1/2 trial of G100 given with local radiation or the anti-PD-1 agent pembrolizumab to patients with follicular lymphoma. ![]()
Factors linked to B-NHL in Palestinians, Israelis
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()
New research has revealed factors that may increase the risk of B-cell non-Hodgkin lymphoma (B-NHL) in Israelis and Palestinians.
This large-scale, epidemiological study indicated that each group had its own unique risk factors.
However, in both groups, recreational sun exposure, black hair-dye use, a history of hospitalization for infection, and having a first-degree relative with a hematopoietic malignancy were all associated with B-NHL.
A team of Palestinian and Israeli researchers reported these findings in PLOS ONE.
The researchers noted that Israelis and Palestinians share the same ecosystem but differ in terms of lifestyle, health behaviors, and medical systems. Yet both populations report high incidences of NHL.
To gain some insight into this phenomenon, the team conducted a study examining risk factors for B-NHL and its subtypes in these two populations.
The researchers looked at medical history, environmental factors, and lifestyle factors in 823 B-NHL patients and 808 healthy controls.
There were 516 Israeli Jews with B-NHL and 307 Palestinian Arabs with B-NHL. The mean age at diagnosis was 60 and 51, respectively.
The proportion of patients with diffuse large B-cell lymphoma was 71% of Palestinian Arabs and 41% of Israeli Jews. The proportion of patients with follicular lymphoma was 14% and 28%, respectively. And the proportion of patients with marginal zone lymphoma was 2% and 14%, respectively.
Using data from questionnaires, pathology review, serology, and genotyping, the researchers uncovered potential risk factors for B-NHL common to both populations and other factors unique to each population.
Results
The data showed that, in both Palestinian Arabs and Israeli Jews, B-NHL was associated with:
- Recreational sun exposure (odds ratio [OR]=1.4)
- Black hair-dye use (OR=1.70)
- A history of hospitalization for infection (OR=1.68)
- Having a first-degree relative with a hematopoietic malignancy (OR=1.69).
Smoking was associated with follicular lymphoma in both populations (OR=1.46). And greater-than-monthly indoor pesticide use was associated with diffuse large B-cell lymphoma in both populations (OR=2.01).
There was an inverse association between alcohol use and B-NHL for both populations (OR=0.46).
Among Palestinian Arabs only, risk factors for B-NHL included gardening (OR=1.93) and a history of herpes (OR=3.73), mononucleosis (OR=6.34), rubella (OR=2.86), and blood transfusion (OR=2.53).
Risk factors that applied to Israeli Jews only included growing fruits and vegetables (OR=1.87) and self-reported autoimmune diseases (OR=1.99).
The researchers said differences in risk factors by ethnicity could reflect differences in lifestyle, medical systems, and reporting patterns, while variations by B-NHL subtypes suggest specific causal factors for different types of disease. However, these findings require further investigation to reveal their mechanisms.
“Apart from the scientific contribution that this research provides in terms of understanding risk factors for NHL, the study entails an important research cooperation among many institutions,” said study author Ora Paltiel, of Hadassah-Hebrew University Medical Organization in Jerusalem, Israel.
“The study provided opportunities for training Palestinian and Israeli researchers and will provide for intellectual interaction for years to come. The data collected will also provide a research platform for the future study of lymphoma. Epidemiologic research has the potential to improve and preserve human health, and it can also serve as a bridge to dialogue among nations.” ![]()