User login
‘Troubling’ trend in narcotic prescribing for ankylosing spondylitis
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.
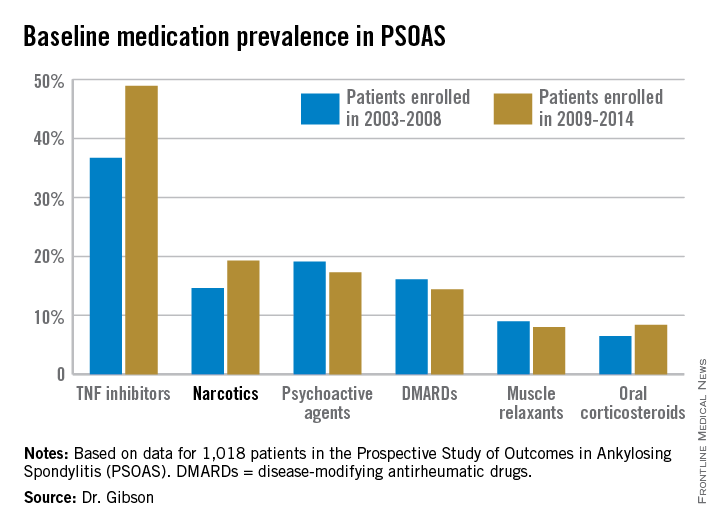
The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.

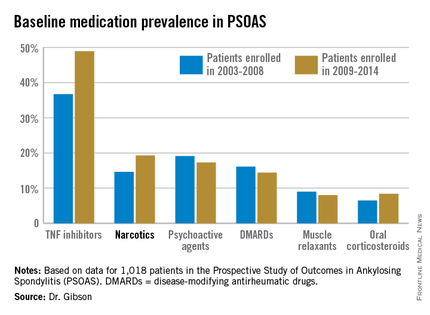
The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
DENVER – Even as prescribing of tumor necrosis factor inhibitors for patients with ankylosing spondylitis climbed in the last half-dozen years, patients’ use of prescription narcotics increased as well.
This was the unexpected finding of a comprehensive analysis of trends in medication use over a 12-year period among 1,018 ankylosing spondylitis patients participating in the ongoing multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS), Dr. Mary C. Gibson reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“Even with treatment with TNF inhibitors, patients are often requiring additional pain control with narcotic medications. This could reflect inadequate pain control from directed therapy or a trend toward change in pain control use in general practice,” observed Dr. Gibson of the University of Texas, Houston.

The patients enrolled in PSOAS during 2003-2014 are being followed longitudinally. They were recruited from community practice settings as well as academic centers. Dr. Gibson analyzed their medication use at the time of their baseline enrollment visit on a year-by-year basis, then stepped back to look at broader trends, comparing baseline medication use for patients enrolled in 2003-2008 to those enrolled during 2009-2014.
Not surprisingly, significantly more patients entered the study on a TNF inhibitor during the enrollment years 2009-2014 than during the first half of the study period: 49% compared with 37% during 2003-2008. What came as a surprise, however, was the time trend in narcotic use: 14.6% of ankylosing spondylitis patients in the 2003-2008 cohort were on narcotics, compared with 19.3% who enrolled since then.
“That’s a little bit troubling,” said Dr. John D. Reveille, professor and vice chair of rheumatology at the University of Texas, Houston, and head of PSOAS.
Oxycodone use nearly tripled between the first and second half of the study period: 1.1% were on the drug at enrollment in 2003-2008 compared with 3.1% in 2009-2014.
Of note, baseline use of oxycodone peaked in the enrollment class of 2012, 5.6% of whom were on the medication when they joined PSOAS. For narcotics overall, the peak year was 2008: over 27% of patients who enrolled in the study that year were on narcotic therapy at the time.
The use of NSAIDs – a guideline-recommended first-line therapy in ankylosing spondylitis – trended lower over time, albeit not significantly so. Among patients who enrolled in PSOAS during 2003-2008, over 70% were on NSAID therapy, compared with 66% of those who enrolled later.
There was no significant change over time in the use of DMARDs, psychoactive medications, muscle relaxants, or corticosteroids.
PSOAS is sponsored by the National Institutes of Health. Dr. Gibson reported having no financial conflicts.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: The use of prescription narcotics by patients with ankylosing spondylitis has gone up during the past 12 years, even as their use of tumor necrosis factor inhibitor therapy has grown.
Major finding: Among a large cohort of patients with ankylosing spondylitis, 14.6% were on prescription narcotic therapy during 2003-2008, compared with 19.3% in 2009-2014.
Data source: Analysis of 1,018 patients with ankylosing spondylitis, from PSOAS – a prospective, multicenter, longitudinal cohort study.
Disclosures: The ongoing PSOAS study is funded by the National Institutes of Health. The presenter reported having no conflicts of interest.
Exercise boosts medication’s benefits in ankylosing spondylitis
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
DENVER – Recreational exercise and tumor necrosis factor–inhibitor therapy provide synergistic improvement in the functional status of patients with ankylosing spondylitis, according to an analysis from the multicenter Prospective Study of Outcomes in Ankylosing Spondylitis (PSOAS).
Among 683 PSOAS participants prospectively followed for up to 12 years, those who consistently engaged in moderate recreational exercise, defined as at least 120 minutes of exercise per week, had significantly lower, more favorable Bath Ankylosing Spondylitis Functional Index (BASFI) scores during years 1-5 than did those who exercised less, even after controlling for baseline function and other potential confounders in a multivariate analysis, Dr. Lianne S. Gensler said at the annual meeting of the Spondyloarthritis Research and Treatment Network.
Additionally, a significant interaction was observed between exercise time and anti-TNF therapy. BASFI scores improved through 5 years of follow-up in TNF inhibitor users regardless of their exercise status, but the impact on functional status was “strikingly” greater in those patients on a TNF inhibitor who engaged in at least 120 minutes of exercise per week, according to Dr. Gensler of the University of California, San Francisco.
“This suggests that it’s not simply taking a TNF inhibitor that’s going to promote long-term function. These patients should also be exercising to synergize and get the best out of their therapy,” she emphasized.
The PSOAS cohort included averaged 43 years of age at baseline, with a mean 19-year disease duration and an average of 2.1 comorbid conditions. In a multivariate longitudinal analysis, several factors emerged as significant predictors of more pronounced functional impairment over time: being age 40 or older at baseline was associated with a 25% increased risk, opiate use was associated with a 12% increase in risk, a baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of at least 40 conferred a 19% increase in risk, as did a modified Stoke Ankylosing Spondylitis Spinal Score of 4 or greater.
Dr. Gensler drew a sharp distinction between the effects of moderate recreational exercise and occupational physical activity in ankylosing spondylitis patients. In an earlier landmark study involving 397 members of the PSOAS cohort with at least a 20-year history of ankylosing spondylitis, investigators mapped the patients’ work histories, the physical activities their work entailed, and the time they spent at each activity. Jobs that involved repeated bending, stretching, twisting, and vibration exposure were associated with worse BASFI scores and greater radiographic disease severity (Arthritis Rheum. 2008 Jun 15;59[6]:822-32).
Taking a step back to summarize what’s known today about what affects outcomes in ankylosing spondylitis patients, Dr. Gensler listed in the ‘bad’ column smoking, ongoing inflammation, baseline damage to the spine, certain occupational activities involving biomechanical stress, adiposity, and low socioeconomic status.
What’s good for patients with ankylosing spondylitis is physical therapy and back exercises – both will receive favorable marks in soon-to-be-published American College of Rheumatology guidelines on the management of ankylosing spondylitis that Dr. Gensler worked on – as well as TNF-inhibitor therapy, NSAIDs, and recreational exercise, which wasn’t addressed in the former guidelines.
Dr. Gensler reported receiving research funds from or serving as a consultant to AbbVie, Amgen, Celgene, Janssen, and UCB.
EXPERT ANALYSIS FROM THE 2015 SPARTAN ANNUAL MEETING
New era ahead in spondyloarthritis therapy
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
DENVER – The emerging new era in the treatment of spondyloarthritis is combined blockade of tumor necrosis factor–alpha (TNF-alpha) and interleukin-17 using a bispecific antibody, Dr. Siba P. Raychaudhuri predicted at the annual meeting of the Spondyloarthritis Research and Treatment Network.
“This is the next generation of antibody therapies for spondyloarthritis,” said Dr. Raychaudhuri, a rheumatologist at the University of California, Davis.
This novel approach has a sound theoretical basis. Moreover, studies in mouse models, including models of rheumatoid arthritis (Arthritis Rheumatol. 2015 Jan;67[1]:51-62), have been positive. And proprietary bispecific anti-TNF-alpha/IL-17 antibodies are now in phase I and phase II clinical trials for various forms of spondyloarthritis, he noted.
The impetus for development of this new therapy is widespread agreement that while TNF inhibition has been a step forward in the treatment of axial spondyloarthritis, the efficacy is less than hoped for with these potent and costly agents.
“Things have been sort of gray for almost the last 10 years. All the evidence suggests we’re not getting full efficacy with anti-TNF therapy. We’re getting stuck at about a 60% ASAS 20 response,” he observed.
The explanation for this merely middling success rate appears to be that TNF inhibition induces a counterregulatory uptick in the IL-17 pathway. And in animal models, IL-17 is a key mediator of joint inflammation and destruction.
Dr. Raychaudhuri noted that brodalumab, an investigational human monoclonal antibody directed against IL-17 receptor A, has been shown “reasonably effective” in a phase II study in patients with psoriatic arthritis, which is part of the spondyloarthritis family of autoimmune diseases, achieving an ACR 20 response rate of 39% at week 12 in the high-dose arm (N Engl J Med. 2014 Jun 12;370[24]:2295-306).
The expectation is that by inhibiting both TNF-alpha and IL-17 using a bispecific protein composed of fragments of two different monoclonal antibodies, the result will be reduced growth and differentiation of proinflammatory T cells, decreased T cell migration, and synergistic clinical efficacy, compared with what’s attainable by addressing either target alone.
Dr. Raychaudhuri reported receiving research grants from or serving as a consultant to Abbott, Amgen, Celgene, UCB, and Janssen Biotech.
EXPERT ANALYSIS FROM THE 2015 SPARTAN ANNUAL MEETING
New spondyloarthritis criteria in the works
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
DENVER – The Spondyloarthritis Research and Treatment Network has voted decisively to create new classification criteria for axial spondyloarthritis, including both ankylosing spondylitis and nonradiographic axial spondyloarthritis.
Following the vote, in which 64% of SPARTAN members attending the group’s annual meeting voted that “this is the right time to proceed,” comoderator Dr. Liron Caplan declared, “This is a very strong push. We’ll need to begin work on our proposal immediately.”
The detailed proposal will contain the justification for new classification criteria as well as outline how SPARTAN will go about creating the criteria in an evidence-based manner with input and financing from industry and patient support groups. The proposal needs to be filed by next March in order to be considered for roughly $135,000 in joint funding from the American College of Rheumatology and the European League Against Rheumatism.
A major impetus for the ambitious project was a frank conversation SPARTAN officers had in the Spring of 2014 with officials at the Food and Drug Administration. The rheumatologists sought an explanation for the FDA’s seemingly counterintuitive rejection of adalimumab (Humira) and certolizumab pegol (Cimzia) for treatment of nonradiographic axial spondyloarthritis (nr-axSpA) on the basis of the very same randomized, controlled trials that earlier resulted in approval for that indication by the European Medicines Agency.
The FDA representatives deemed the Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) used in those and other SpA trials to be overly broad. They felt the ASAS criteria lack sufficient sensitivity and specificity to ensure that large numbers of patients with fibromyalgia – a far more common disorder – wouldn’t be misclassified as having SpA and treated inappropriately with biologics, which aren’t effective in fibromyalgia, explained Dr. John D. Reveille, professor and vice-chair of rheumatology at the University of Texas, Houston, who attended the meeting.
“The only physicians who can prescribe an approved anti-TNF drug or other biologic in Europe are rheumatologists. The reason the FDA was nervous about approving drugs using the ASAS criteria is because any physician in the U.S. can prescribe those drugs once they’re approved. So the authorities made a regulatory decision about who the people are who are educated or not educated in the diagnosis and management of patients with SpA,” according to Dr. Michael H. Weisman, chair and director of rheumatology at Cedars-Sinai Medical Center in Los Angeles.
Dr. Caplan of the University of Colorado, Denver, observed that developing new and improved criteria for SpA will be a multiyear endeavor. A top goal is to shed light on the natural history of nr-axSpA, about which almost nothing is known. This will probably entail developing a sizable cohort of patients with nr-axSpA, following them for 5 years or so to see who develops ankylosing spondylitis, then looking back to identify reliable predictors.
The SPARTAN vote was preceded by a formal debate on the merits of replacing the ASAS criteria. Dr. Muhammad Asim Khan, professor of medicine at Case Western Reserve University, Cleveland, who 30 years ago was a pioneer in the concept of spondylitic disease without x-ray evidence of sacroiliitis, argued that the ASAS criteria aren’t adequately doing what disease classification criteria are supposed to do: provide a simple, clear means of identifying a homogeneous population for clinical trials. By letting in substantial numbers of patients with fibromyalgia, the criteria water down the clinical outcomes of investigational therapies for SpA.
The opposing argument was provided by ASAS representative Dr. Filip Van den Bosch. He conceded the ASAS classification criteria “aren’t perfect,” but over time they have become familiar to rheumatologists worldwide.
“Modifying the criteria will lead to years of uncertainty,” argued Dr. Van den Bosch, professor of rheumatology at Ghent (Belgium) University.
If, as some have proposed, SPARTAN were to tighten the ASAS criteria by insisting that a patient lacking x-ray evidence of sacroiliitis must possess three or four SpA features instead of the two now required, the result would be a drastic reduction in the sensitivity of the criteria, he continued.
“The challenge of making a correct diagnosis of axSpA cannot be solved by modifying the ASAS criteria. I cannot think of a new SpA feature that’s been identified in the last 5 years that could be added on top of what’s already in there,” the rheumatologist said.
What’s needed, he added, is a concerted effort to better educate physicians about axSpA, including interpretation of spinal x-rays and MRIs, the disease’s signs and symptoms, and how to differentiate SpA from other disorders that cause back pain.
While nearly two-thirds of the SPARTAN membership voted in favor of developing new classification criteria, some prominent voices were raised in dissent.
“I agree with Filip,” said Dr. Weisman. “The problem is not with the criteria, it’s who’s using them.”
Dr. Robert D. Inman, professor of medicine at the University of Toronto, predicted “it’s an exercise in futility” to try to come up with a single set of classification criteria that will provide the high degree of sensitivity that patients and clinicians want so that people who present with early axSpA are not missed while at the same time providing the maximum specificity that payers and regulatory officials seek.
Dr. Sindhu Johnson, who as cochair of the ACR Classification Criteria Subcommittee has shepherded the development of new criteria for a number of rheumatologic diseases, offered the SPARTAN group some practical tips. Because European studies tend to be comprised of monoethnic cohorts, the ACR will look favorably upon a successful SPARTAN effort to incorporate African Americans, Asians, Native Americans, and other minorities in their study cohorts. And SPARTAN should be open and receptive to critical feedback from stakeholders during the criteria development process.
“Get buy in. There’s no point in developing criteria that are extremely rigorous scientifically but that the global community feels are useless, that use tests they don’t have access to. This is one of the criticisms around the Sjögren’s criteria: they require ophthalmologic testing and salivary gland biopsy, and for rheumatologists – even academic rheumatologists at recruiting centers for clinical trials – they don’t have access to this, or it takes too much time. You need to come up with something pragmatic to get buy in,” advised Dr. Johnson of the University of Toronto.
By the way, she added, if the project wins ACR and EULAR funding it can only be named an ACR/EULAR product. “No other organization can be named in the headline,” according to Dr. Johnson.
AT THE 2015 SPARTAN ANNUAL MEETING
Study yields insight into nonradiographic axial spondyloarthritis progression
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
DENVER – Only one-quarter of patients newly identified as having nonradiographic axial spondyloarthritis will progress to ankylosing spondylitis within 15 years, according to findings from the Rochester (Minn.) Epidemiology Project.
Disease progression occurred far more frequently and rapidly in patients who qualified for nonradiographic axial spondyloarthritis (nr-AxSpA) on the basis of positive pelvic MRI findings than in those who met only the clinical criteria for nr-AxSpA, Dr. Runsheng Wang reported at the annual meeting of the Spondyloarthritis Research and Treatment Network.
The Rochester Epidemiology Project is a unique health care research resource. Essentially all residents in Olmsted County, Minn., the home of the Mayo Clinic, have signed off on participation in the project. Dr. Weng’s study focused on 16- to 45-year-old county residents who reported new-onset chronic back pain during 1985-2010, none of whom showed the radiographic evidence of sacroiliitis required for a diagnosis of ankylosing spondylitis.
The study began with 1,142 patients who for a variety of reasons underwent a pelvic MRI. Eighteen of them ultimately met ASAS (Assessment of Spondyloarthritis International Society) imaging criteria for nr-AxSpA. They formed the imaging arm of the study.
Another 1,009 patients in the target age group presented with chronic back pain but no MRI findings. Sixty-five of them were entered into the clinical nr-AxSpA arm of the study on the basis of being positive for HLA-B27, explained Dr. Wang, a clinical scholar in rheumatology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Subjects were followed for a mean of 10.7 years. The overall rate of progression from nr-AxSpA to ankylosing spondylitis was 6.4% at 5 years, 17.3% at 10 years, and 26.4% at 15 years.
These data help fill an unmet need regarding understanding of the prognosis of nr-AxSpA. The two previous studies by other investigators have limitations: one German study showed an 11.2% rate of conversion to ankylosing spondylitis but was limited to 2 years of follow-up (Ann Rheum Dis. 2011 Aug;70[8]:1369-1374), while a Norwegian study comprising 20 patients showed a 20% progression rate over 8 years, Dr. Wang noted.
In her Minnesota study, patients who met criteria for nr-AxSpA based upon MRI criteria were 3.5-fold more likely to progress to ankylosing spondylitis with evidence of sacroiliitis on X-ray, compared with those in the clinical arm. There was a hint of a gender difference: Men were 1.58 times more likely to progress to ankylosing spondylitis than were women. However, this difference didn’t reach statistical significance.
Audience members were particularly interested in whether the Minnesota data demonstrated that treatment with NSAIDs and other agents had an impact upon the progression rate. Dr. Wang replied that the study size was simply too small to allow for a reasonable multivariate analysis examining this key question.
The National Institutes of Health funded the study. Dr. Wang reported having no financial conflicts of interest.
AT THE 2015 SPARTAN ANNUAL MEETING
Key clinical point: Radiographic evidence of sacroiliitis is s-l-o-w to develop in patients with nonradiographic axial spondyloarthritis.
Major finding: Fifteen years after patients were identified as having nonradiographic axial spondyloarthritis based upon MRI or clinical findings, 26% of them had progressed to ankylosing spondylitis as defined by sacroiliitis on x-ray.
Data source: The Rochester (Minn.) Epidemiology Project, a unique medical records database incorporating all residents of Olmsted County, Minn.
Disclosures: The National Institutes of Health funded the study. The presenter reported having no relevant financial disclosures.
Counseling key to guiding rheumatic disease treatment during pregnancy
ROME – New therapies introduced over the past 15 years have vastly improved the lives of patients with rheumatic diseases but are also creating complex treatment scenarios as more female patients consider starting a family or become pregnant while taking medication. At the European Congress of Rheumatology, experts discussed the importance of contraceptive counseling, pregnancy planning, and how to treat pregnant women with rheumatic diseases.
“Nowadays we have such better treatments for our patients that sexuality and contraception is becoming more and more important,” said Dr. Eliza Chakravarty of the Oklahoma Medical Research Foundation in Oklahoma City. Yet contraception is “quite underused in our patients,” she observed.
Indeed, in one study looking at the risk of unintended pregnancy in women with lupus, over half (55%) of the 212 women surveyed admitted that they had unprotected sex at least once, with almost a quarter (23%) saying that this was usually in the preceding 3 months (Arthritis Care Res. 2008;59:863-6).
This underuse of contraception is not unique to lupus patients, and there are many reasons why women with rheumatic diseases who are sexually active may not always use adequate contraception. One of these reasons was the lack of time during consultations, which tend to focus on disease management rather than issues such as contraceptive counseling. Other reasons may be misconceptions on fertility or a lack of understanding of the teratogenicity of medications or the effect that hormones may have on disease activity. Women also may be reluctant to take additional medicines or feel uncomfortable discussing the topic with their rheumatologists.
“Patients see their rheumatologist more frequently than any other [health care] provider,” Dr. Chakravarty said, adding that continual follow-up appointments over time provide an opportunity to discuss fertility and changing contraception needs. “Contraception is a component of effective disease management for young women,” she said.
There are many contraceptive methods available, each with pros and cons and varying efficacy, so women have multiple options to choose from to suit their personal preferences and lifestyles. Dr. Chakravarty suggested that, of all the available methods, long-acting reversible contraceptive methods, such as the subdermal implant or intrauterine device, were perhaps the best choice for many women with rheumatic diseases since they are associated with a low rate of accidental pregnancy and after insertion there is little or nothing to do or remember until the devices needed replacing after 3-10 years.
Dr. Monika Østensen of the Norwegian National Advisory Unit on Pregnancy and Rheumatic Diseases at St. Olavs Hospital-Trondheim University Hospital, echoed the need for repeated family-planning advice. Of course, pregnancies occur even with the best planning, she said, and the question then is how to manage the rheumatic disease.
The need for treatment will depending on the type of disease, she said, noting that the need generally decreased during pregnancy in women with rheumatoid arthritis (RA) but increased in those with ankylosing spondylitis, most notably in the first and second trimesters (Ann. Rheum. Dis. 2004;63:1212-7). The ideal situation would be to adjust disease-directed therapy if needed before conception, although methotrexate is the only drug with proven teratogenicity that is used for RA and ankylosing spondylitis and that should definitely be stopped, she said.
There is a lot of evidence that methotrexate needs to be stopped before pregnancy. One recent prospective study (Arthritis Rheumatol. 2014;66:1101-10) looked at pregnancy outcomes and methotrexate use and found a high rate of spontaneous abortions if it was used within 10 weeks of conception (14.4%) or in the first trimester (42.5%). The rate of major birth defects was 6.6% if methotrexate was used in early pregnancy, versus 2.9% for women without rheumatic disease or 3.5% in women with rheumatic disease who were not taking the disease-modifying antirheumatic drug (DMARD).
Dr. Østensen advised that, when stopping methotrexate, women should be counseled to wait for one menstrual cycle before attempting to conceive and not to stop therapy without perhaps checking for fertility or switching to another pregnancy-compatible drug. She also stressed that patients who want to become pregnant or who do become pregnant should not immediately stop taking their medication without consulting a rheumatologist. Such an action could result in disease flares, and the aim of therapy should always be to keep the patient in remission or low disease activity and continue drugs that support remission while trying to minimize any likely effects on the fetus.
Leflunomide (Arava), rituximab (Rituxan), abatacept (Orencia), and newer biologics such as tocilizumab (Actemra), ustekinumab (Stelara), and tofacitinb (Xeljanz) are contraindicated because of lack of data on whether they are safe for the fetus. If a woman did become pregnant while taking any of these drugs or even methotrexate, then taking a careful history and performing fetal ultrasound and amniocentesis may be the best approach to determine what action to take and if pregnancy termination should be considered.
Tumor necrosis factor-alpha inhibitors (TNFi), the best-studied biologic DMARDs, can be given before conception and during the first and early second trimester; however, use in late pregnancy requires different considerations because transplacental passage varies based on differences related to their structure. Some TNFis, such as certolizumab pegol (Cimzia), have small affinity to the fetal Fc receptor or no Fc part and show low transplacental passage to the fetus. TNFis that possess an Fc part of immunoglobulin G1, however, allow high amounts of transfer and should be avoided in the third trimester whenever possible, Dr. Østensen said.
Data are sparse on human pregnancy exposure and fetal side effects and outcomes for most other biologics, so decisions to use biologics targeting B-cells, T-cell activation, or cytokines like interleukin (IL)-6, IL-23, IL-17, or IL-1beta must be based on the severity of maternal disease and reserved for cases in which no other safe options are available, Dr. Østensen cautioned.
So how can acute arthritis be treated during pregnancy? Options in RA include NSAIDs, short-term oral prednisone with rapid tapering or intramuscular injection of triamcinolone. Intra-articular steroid injection also might be considered, and TNFi in patients with severe polyarthritis. Spondyloarthritis might be treated with NSAIDs, intramuscular triamcinolone, or intra-articular steroid injection, with TNFi in severe cases.
Newer approaches to managing arthritis during pregnancy are to perhaps prescribe a TNFi with a low propensity for transplacental passage or to use a flexible regimen of TNFi by reducing the dose or prolonging the interval between administrations.
Continuing medications such as hydroxychloroquine, sulfasalazine, or azathioprine might be in some patients’ best interests to support remission and keep disease activity low, Dr. Østensen suggested. She noted that prednisone should be used at a low (5-7.5 mg) dose during pregnancy and that the aim should be to try to slowly reduce the use of pregnancy-compatible medications that are not necessary for continued remission, keeping a close eye on patients’ disease activity to ensure that no flares occur.
Dr. Chakravarty had no financial disclosures. Dr. Østensen reported receiving speaker fees and honoraria from UCB, Roche, AbbVie, MSD, Pfizer, and New Bridge.
ROME – New therapies introduced over the past 15 years have vastly improved the lives of patients with rheumatic diseases but are also creating complex treatment scenarios as more female patients consider starting a family or become pregnant while taking medication. At the European Congress of Rheumatology, experts discussed the importance of contraceptive counseling, pregnancy planning, and how to treat pregnant women with rheumatic diseases.
“Nowadays we have such better treatments for our patients that sexuality and contraception is becoming more and more important,” said Dr. Eliza Chakravarty of the Oklahoma Medical Research Foundation in Oklahoma City. Yet contraception is “quite underused in our patients,” she observed.
Indeed, in one study looking at the risk of unintended pregnancy in women with lupus, over half (55%) of the 212 women surveyed admitted that they had unprotected sex at least once, with almost a quarter (23%) saying that this was usually in the preceding 3 months (Arthritis Care Res. 2008;59:863-6).
This underuse of contraception is not unique to lupus patients, and there are many reasons why women with rheumatic diseases who are sexually active may not always use adequate contraception. One of these reasons was the lack of time during consultations, which tend to focus on disease management rather than issues such as contraceptive counseling. Other reasons may be misconceptions on fertility or a lack of understanding of the teratogenicity of medications or the effect that hormones may have on disease activity. Women also may be reluctant to take additional medicines or feel uncomfortable discussing the topic with their rheumatologists.
“Patients see their rheumatologist more frequently than any other [health care] provider,” Dr. Chakravarty said, adding that continual follow-up appointments over time provide an opportunity to discuss fertility and changing contraception needs. “Contraception is a component of effective disease management for young women,” she said.
There are many contraceptive methods available, each with pros and cons and varying efficacy, so women have multiple options to choose from to suit their personal preferences and lifestyles. Dr. Chakravarty suggested that, of all the available methods, long-acting reversible contraceptive methods, such as the subdermal implant or intrauterine device, were perhaps the best choice for many women with rheumatic diseases since they are associated with a low rate of accidental pregnancy and after insertion there is little or nothing to do or remember until the devices needed replacing after 3-10 years.
Dr. Monika Østensen of the Norwegian National Advisory Unit on Pregnancy and Rheumatic Diseases at St. Olavs Hospital-Trondheim University Hospital, echoed the need for repeated family-planning advice. Of course, pregnancies occur even with the best planning, she said, and the question then is how to manage the rheumatic disease.
The need for treatment will depending on the type of disease, she said, noting that the need generally decreased during pregnancy in women with rheumatoid arthritis (RA) but increased in those with ankylosing spondylitis, most notably in the first and second trimesters (Ann. Rheum. Dis. 2004;63:1212-7). The ideal situation would be to adjust disease-directed therapy if needed before conception, although methotrexate is the only drug with proven teratogenicity that is used for RA and ankylosing spondylitis and that should definitely be stopped, she said.
There is a lot of evidence that methotrexate needs to be stopped before pregnancy. One recent prospective study (Arthritis Rheumatol. 2014;66:1101-10) looked at pregnancy outcomes and methotrexate use and found a high rate of spontaneous abortions if it was used within 10 weeks of conception (14.4%) or in the first trimester (42.5%). The rate of major birth defects was 6.6% if methotrexate was used in early pregnancy, versus 2.9% for women without rheumatic disease or 3.5% in women with rheumatic disease who were not taking the disease-modifying antirheumatic drug (DMARD).
Dr. Østensen advised that, when stopping methotrexate, women should be counseled to wait for one menstrual cycle before attempting to conceive and not to stop therapy without perhaps checking for fertility or switching to another pregnancy-compatible drug. She also stressed that patients who want to become pregnant or who do become pregnant should not immediately stop taking their medication without consulting a rheumatologist. Such an action could result in disease flares, and the aim of therapy should always be to keep the patient in remission or low disease activity and continue drugs that support remission while trying to minimize any likely effects on the fetus.
Leflunomide (Arava), rituximab (Rituxan), abatacept (Orencia), and newer biologics such as tocilizumab (Actemra), ustekinumab (Stelara), and tofacitinb (Xeljanz) are contraindicated because of lack of data on whether they are safe for the fetus. If a woman did become pregnant while taking any of these drugs or even methotrexate, then taking a careful history and performing fetal ultrasound and amniocentesis may be the best approach to determine what action to take and if pregnancy termination should be considered.
Tumor necrosis factor-alpha inhibitors (TNFi), the best-studied biologic DMARDs, can be given before conception and during the first and early second trimester; however, use in late pregnancy requires different considerations because transplacental passage varies based on differences related to their structure. Some TNFis, such as certolizumab pegol (Cimzia), have small affinity to the fetal Fc receptor or no Fc part and show low transplacental passage to the fetus. TNFis that possess an Fc part of immunoglobulin G1, however, allow high amounts of transfer and should be avoided in the third trimester whenever possible, Dr. Østensen said.
Data are sparse on human pregnancy exposure and fetal side effects and outcomes for most other biologics, so decisions to use biologics targeting B-cells, T-cell activation, or cytokines like interleukin (IL)-6, IL-23, IL-17, or IL-1beta must be based on the severity of maternal disease and reserved for cases in which no other safe options are available, Dr. Østensen cautioned.
So how can acute arthritis be treated during pregnancy? Options in RA include NSAIDs, short-term oral prednisone with rapid tapering or intramuscular injection of triamcinolone. Intra-articular steroid injection also might be considered, and TNFi in patients with severe polyarthritis. Spondyloarthritis might be treated with NSAIDs, intramuscular triamcinolone, or intra-articular steroid injection, with TNFi in severe cases.
Newer approaches to managing arthritis during pregnancy are to perhaps prescribe a TNFi with a low propensity for transplacental passage or to use a flexible regimen of TNFi by reducing the dose or prolonging the interval between administrations.
Continuing medications such as hydroxychloroquine, sulfasalazine, or azathioprine might be in some patients’ best interests to support remission and keep disease activity low, Dr. Østensen suggested. She noted that prednisone should be used at a low (5-7.5 mg) dose during pregnancy and that the aim should be to try to slowly reduce the use of pregnancy-compatible medications that are not necessary for continued remission, keeping a close eye on patients’ disease activity to ensure that no flares occur.
Dr. Chakravarty had no financial disclosures. Dr. Østensen reported receiving speaker fees and honoraria from UCB, Roche, AbbVie, MSD, Pfizer, and New Bridge.
ROME – New therapies introduced over the past 15 years have vastly improved the lives of patients with rheumatic diseases but are also creating complex treatment scenarios as more female patients consider starting a family or become pregnant while taking medication. At the European Congress of Rheumatology, experts discussed the importance of contraceptive counseling, pregnancy planning, and how to treat pregnant women with rheumatic diseases.
“Nowadays we have such better treatments for our patients that sexuality and contraception is becoming more and more important,” said Dr. Eliza Chakravarty of the Oklahoma Medical Research Foundation in Oklahoma City. Yet contraception is “quite underused in our patients,” she observed.
Indeed, in one study looking at the risk of unintended pregnancy in women with lupus, over half (55%) of the 212 women surveyed admitted that they had unprotected sex at least once, with almost a quarter (23%) saying that this was usually in the preceding 3 months (Arthritis Care Res. 2008;59:863-6).
This underuse of contraception is not unique to lupus patients, and there are many reasons why women with rheumatic diseases who are sexually active may not always use adequate contraception. One of these reasons was the lack of time during consultations, which tend to focus on disease management rather than issues such as contraceptive counseling. Other reasons may be misconceptions on fertility or a lack of understanding of the teratogenicity of medications or the effect that hormones may have on disease activity. Women also may be reluctant to take additional medicines or feel uncomfortable discussing the topic with their rheumatologists.
“Patients see their rheumatologist more frequently than any other [health care] provider,” Dr. Chakravarty said, adding that continual follow-up appointments over time provide an opportunity to discuss fertility and changing contraception needs. “Contraception is a component of effective disease management for young women,” she said.
There are many contraceptive methods available, each with pros and cons and varying efficacy, so women have multiple options to choose from to suit their personal preferences and lifestyles. Dr. Chakravarty suggested that, of all the available methods, long-acting reversible contraceptive methods, such as the subdermal implant or intrauterine device, were perhaps the best choice for many women with rheumatic diseases since they are associated with a low rate of accidental pregnancy and after insertion there is little or nothing to do or remember until the devices needed replacing after 3-10 years.
Dr. Monika Østensen of the Norwegian National Advisory Unit on Pregnancy and Rheumatic Diseases at St. Olavs Hospital-Trondheim University Hospital, echoed the need for repeated family-planning advice. Of course, pregnancies occur even with the best planning, she said, and the question then is how to manage the rheumatic disease.
The need for treatment will depending on the type of disease, she said, noting that the need generally decreased during pregnancy in women with rheumatoid arthritis (RA) but increased in those with ankylosing spondylitis, most notably in the first and second trimesters (Ann. Rheum. Dis. 2004;63:1212-7). The ideal situation would be to adjust disease-directed therapy if needed before conception, although methotrexate is the only drug with proven teratogenicity that is used for RA and ankylosing spondylitis and that should definitely be stopped, she said.
There is a lot of evidence that methotrexate needs to be stopped before pregnancy. One recent prospective study (Arthritis Rheumatol. 2014;66:1101-10) looked at pregnancy outcomes and methotrexate use and found a high rate of spontaneous abortions if it was used within 10 weeks of conception (14.4%) or in the first trimester (42.5%). The rate of major birth defects was 6.6% if methotrexate was used in early pregnancy, versus 2.9% for women without rheumatic disease or 3.5% in women with rheumatic disease who were not taking the disease-modifying antirheumatic drug (DMARD).
Dr. Østensen advised that, when stopping methotrexate, women should be counseled to wait for one menstrual cycle before attempting to conceive and not to stop therapy without perhaps checking for fertility or switching to another pregnancy-compatible drug. She also stressed that patients who want to become pregnant or who do become pregnant should not immediately stop taking their medication without consulting a rheumatologist. Such an action could result in disease flares, and the aim of therapy should always be to keep the patient in remission or low disease activity and continue drugs that support remission while trying to minimize any likely effects on the fetus.
Leflunomide (Arava), rituximab (Rituxan), abatacept (Orencia), and newer biologics such as tocilizumab (Actemra), ustekinumab (Stelara), and tofacitinb (Xeljanz) are contraindicated because of lack of data on whether they are safe for the fetus. If a woman did become pregnant while taking any of these drugs or even methotrexate, then taking a careful history and performing fetal ultrasound and amniocentesis may be the best approach to determine what action to take and if pregnancy termination should be considered.
Tumor necrosis factor-alpha inhibitors (TNFi), the best-studied biologic DMARDs, can be given before conception and during the first and early second trimester; however, use in late pregnancy requires different considerations because transplacental passage varies based on differences related to their structure. Some TNFis, such as certolizumab pegol (Cimzia), have small affinity to the fetal Fc receptor or no Fc part and show low transplacental passage to the fetus. TNFis that possess an Fc part of immunoglobulin G1, however, allow high amounts of transfer and should be avoided in the third trimester whenever possible, Dr. Østensen said.
Data are sparse on human pregnancy exposure and fetal side effects and outcomes for most other biologics, so decisions to use biologics targeting B-cells, T-cell activation, or cytokines like interleukin (IL)-6, IL-23, IL-17, or IL-1beta must be based on the severity of maternal disease and reserved for cases in which no other safe options are available, Dr. Østensen cautioned.
So how can acute arthritis be treated during pregnancy? Options in RA include NSAIDs, short-term oral prednisone with rapid tapering or intramuscular injection of triamcinolone. Intra-articular steroid injection also might be considered, and TNFi in patients with severe polyarthritis. Spondyloarthritis might be treated with NSAIDs, intramuscular triamcinolone, or intra-articular steroid injection, with TNFi in severe cases.
Newer approaches to managing arthritis during pregnancy are to perhaps prescribe a TNFi with a low propensity for transplacental passage or to use a flexible regimen of TNFi by reducing the dose or prolonging the interval between administrations.
Continuing medications such as hydroxychloroquine, sulfasalazine, or azathioprine might be in some patients’ best interests to support remission and keep disease activity low, Dr. Østensen suggested. She noted that prednisone should be used at a low (5-7.5 mg) dose during pregnancy and that the aim should be to try to slowly reduce the use of pregnancy-compatible medications that are not necessary for continued remission, keeping a close eye on patients’ disease activity to ensure that no flares occur.
Dr. Chakravarty had no financial disclosures. Dr. Østensen reported receiving speaker fees and honoraria from UCB, Roche, AbbVie, MSD, Pfizer, and New Bridge.
EXPERT ANALYSIS FROM THE EULAR 2015 CONGRESS
Inspiratory muscle training boosts lung function in ankylosing spondylitis
ROME – A program of inspiratory muscle training combined with standard muscular rehabilitation exercise significantly improved lung function in patients with ankylosing spondylitis.
Compared to patients who engaged only in the rehabilitation exercise, the combination program resulted in significantly greater gains in both physical and physiologic measures of pulmonary function, Dr. Razvan Dragoi reported at the annual meeting of the European League Against Rheumatism.
“We assessed resting pulmonary function and ran cardiopulmonary exercise tests at the start and end of the study and saw significant improvements across all measures of lung function in the group undergoing inspiratory muscle training,” said Dr. Dragoi of the Victor Babes University of Medicine and Pharmacy, Romania. “When you compare these findings with the conventional exercise group – which saw small, nonsignificant improvements – it’s clear that adding inspiratory training to an exercise program has clear health benefits for patients.”
The study randomized 54 patients with ankylosing spondylitis to two exercise interventions, Dr. Dragoi said in an interview. “Both groups in our study performed a weekly group session for about 40 minutes per session, managed by a physiotherapist. They were then provided with simple, step-by-step written instructions with illustrations in order to practice these exercises at home,” 5 days each week, for 40 minutes at a time.
The program consisted of 20 exercises: motion and flexibility exercises of the cervical, thoracic, and lumbar spine; stretching of the hamstring muscles, erector spine muscle, and shoulder muscles; abdominal and diaphragm breathing exercises, and chest expansion exercises. The patients were required to achieve a level of perceived exertion of “somewhat hard.”
They also completed an exercise training diary in order to assess their compliance with the recommended program.
The investigational group, however, added another level of training. “In addition to the conventional exercise training, patients performed supervised inspiratory muscle training, three times a week, totaling 24 sessions. This used a real-time computer-assisted device (Trainair, Project Electronics Limited, United Kingdom).”
The training load was based on 80% of the patient’s sustained maximum inspiratory pressure. The patients started by performing six loaded inspiration with a 60-second rest period between each inspiration. This sequence of six exercises continued with 45-, 30-, and 15-, 10- and 5-second rest periods up to 36 loaded inspirations. The training session duration was about 30 minutes.
The study assessed a number of physical and physiologic endpoints, including chest expansion, forced vital capacity and expiratory volumes, and measures of oxygen and carbon dioxide exchange.
Only one outcome – chest expansion – improved significantly in the control arm, increasing from 69 cm at baseline to 72 cm by 8 weeks. In the intensified arm, however, every outcome improved significantly, including chest expansion (66 cm-94 cm), forced vital capacity (78.6%-82.7%), forced expiratory volume (71%-74.6%), and peak oxygen uptake (1.7-2 L/min). The measures of oxygen and carbon dioxide exchange also showed significant improvements.
Dr. Dragoi didn’t follow the patients to assess how long the exercise-related improvements lasted, but like all exercise, he said, the program would have to be repeated to maintain them. “We do have a follow-up in mind, and will be conducting that soon, but we do not know how many patients will be available for the follow-up.”
He had no financial disclosures.
On Twitter @Alz_Gal
ROME – A program of inspiratory muscle training combined with standard muscular rehabilitation exercise significantly improved lung function in patients with ankylosing spondylitis.
Compared to patients who engaged only in the rehabilitation exercise, the combination program resulted in significantly greater gains in both physical and physiologic measures of pulmonary function, Dr. Razvan Dragoi reported at the annual meeting of the European League Against Rheumatism.
“We assessed resting pulmonary function and ran cardiopulmonary exercise tests at the start and end of the study and saw significant improvements across all measures of lung function in the group undergoing inspiratory muscle training,” said Dr. Dragoi of the Victor Babes University of Medicine and Pharmacy, Romania. “When you compare these findings with the conventional exercise group – which saw small, nonsignificant improvements – it’s clear that adding inspiratory training to an exercise program has clear health benefits for patients.”
The study randomized 54 patients with ankylosing spondylitis to two exercise interventions, Dr. Dragoi said in an interview. “Both groups in our study performed a weekly group session for about 40 minutes per session, managed by a physiotherapist. They were then provided with simple, step-by-step written instructions with illustrations in order to practice these exercises at home,” 5 days each week, for 40 minutes at a time.
The program consisted of 20 exercises: motion and flexibility exercises of the cervical, thoracic, and lumbar spine; stretching of the hamstring muscles, erector spine muscle, and shoulder muscles; abdominal and diaphragm breathing exercises, and chest expansion exercises. The patients were required to achieve a level of perceived exertion of “somewhat hard.”
They also completed an exercise training diary in order to assess their compliance with the recommended program.
The investigational group, however, added another level of training. “In addition to the conventional exercise training, patients performed supervised inspiratory muscle training, three times a week, totaling 24 sessions. This used a real-time computer-assisted device (Trainair, Project Electronics Limited, United Kingdom).”
The training load was based on 80% of the patient’s sustained maximum inspiratory pressure. The patients started by performing six loaded inspiration with a 60-second rest period between each inspiration. This sequence of six exercises continued with 45-, 30-, and 15-, 10- and 5-second rest periods up to 36 loaded inspirations. The training session duration was about 30 minutes.
The study assessed a number of physical and physiologic endpoints, including chest expansion, forced vital capacity and expiratory volumes, and measures of oxygen and carbon dioxide exchange.
Only one outcome – chest expansion – improved significantly in the control arm, increasing from 69 cm at baseline to 72 cm by 8 weeks. In the intensified arm, however, every outcome improved significantly, including chest expansion (66 cm-94 cm), forced vital capacity (78.6%-82.7%), forced expiratory volume (71%-74.6%), and peak oxygen uptake (1.7-2 L/min). The measures of oxygen and carbon dioxide exchange also showed significant improvements.
Dr. Dragoi didn’t follow the patients to assess how long the exercise-related improvements lasted, but like all exercise, he said, the program would have to be repeated to maintain them. “We do have a follow-up in mind, and will be conducting that soon, but we do not know how many patients will be available for the follow-up.”
He had no financial disclosures.
On Twitter @Alz_Gal
ROME – A program of inspiratory muscle training combined with standard muscular rehabilitation exercise significantly improved lung function in patients with ankylosing spondylitis.
Compared to patients who engaged only in the rehabilitation exercise, the combination program resulted in significantly greater gains in both physical and physiologic measures of pulmonary function, Dr. Razvan Dragoi reported at the annual meeting of the European League Against Rheumatism.
“We assessed resting pulmonary function and ran cardiopulmonary exercise tests at the start and end of the study and saw significant improvements across all measures of lung function in the group undergoing inspiratory muscle training,” said Dr. Dragoi of the Victor Babes University of Medicine and Pharmacy, Romania. “When you compare these findings with the conventional exercise group – which saw small, nonsignificant improvements – it’s clear that adding inspiratory training to an exercise program has clear health benefits for patients.”
The study randomized 54 patients with ankylosing spondylitis to two exercise interventions, Dr. Dragoi said in an interview. “Both groups in our study performed a weekly group session for about 40 minutes per session, managed by a physiotherapist. They were then provided with simple, step-by-step written instructions with illustrations in order to practice these exercises at home,” 5 days each week, for 40 minutes at a time.
The program consisted of 20 exercises: motion and flexibility exercises of the cervical, thoracic, and lumbar spine; stretching of the hamstring muscles, erector spine muscle, and shoulder muscles; abdominal and diaphragm breathing exercises, and chest expansion exercises. The patients were required to achieve a level of perceived exertion of “somewhat hard.”
They also completed an exercise training diary in order to assess their compliance with the recommended program.
The investigational group, however, added another level of training. “In addition to the conventional exercise training, patients performed supervised inspiratory muscle training, three times a week, totaling 24 sessions. This used a real-time computer-assisted device (Trainair, Project Electronics Limited, United Kingdom).”
The training load was based on 80% of the patient’s sustained maximum inspiratory pressure. The patients started by performing six loaded inspiration with a 60-second rest period between each inspiration. This sequence of six exercises continued with 45-, 30-, and 15-, 10- and 5-second rest periods up to 36 loaded inspirations. The training session duration was about 30 minutes.
The study assessed a number of physical and physiologic endpoints, including chest expansion, forced vital capacity and expiratory volumes, and measures of oxygen and carbon dioxide exchange.
Only one outcome – chest expansion – improved significantly in the control arm, increasing from 69 cm at baseline to 72 cm by 8 weeks. In the intensified arm, however, every outcome improved significantly, including chest expansion (66 cm-94 cm), forced vital capacity (78.6%-82.7%), forced expiratory volume (71%-74.6%), and peak oxygen uptake (1.7-2 L/min). The measures of oxygen and carbon dioxide exchange also showed significant improvements.
Dr. Dragoi didn’t follow the patients to assess how long the exercise-related improvements lasted, but like all exercise, he said, the program would have to be repeated to maintain them. “We do have a follow-up in mind, and will be conducting that soon, but we do not know how many patients will be available for the follow-up.”
He had no financial disclosures.
On Twitter @Alz_Gal
AT EULAR 2015
Key clinical point: Inspiratory muscle training combined with conventional muscle rehabilitation boosted pulmonary function in patients with ankylosing spondylitis.
Major finding: The combination increased chest expansion by almost 30 cm as well as improving physiologic measures of lung function.
Data source: The trail randomized 54 patients to two exercise regimens.
Disclosures: Dr. Dragoi had no financial disclosures.
NSAID dosing frequency matters little in ankylosing spondylitis
ROME – Radiographic progression occurs at similar rates in the spines of ankylosing spondylitis patients treated over 2 years with either continuous or on-demand diclofenac, according to results from a randomized, prospective multicenter trial presented at the European Congress of Rheumatology.
In the ENRADAS (Effects of NSAIDs on Radiographic Damage in Ankylosing Spondylitis) trial, Dr. Joachim Sieper of Charite-Universitätsmedizin Berlin and his colleagues compared continuous treatment with at least 50% of the maximum 150-mg daily dose of the NSAID diclofenac and on-demand treatment with diclofenac. During the entire 2 years of the study, no patient received treatment with a tumor necrosis factor blocker or any other drug other than diclofenac.
The investigators measured radiographic spine progression using the modified Stoke Ankylosing Spondylitis Spinal Score(mSASSS). At baseline, patients randomized to continuous treatment who had complete radiographic follow-up had a mean mSASSS of 10.9, while those randomized to the on-demand arm had a mean mSASSS of 16.4. After 2 years on treatment, the average change in mSASSS from baseline was 1.28 for the 62 patients in the continuous-treatment group and 0.79 for the 60 patients in the on-demand group, a difference that was not statistically significant, Dr. Sieper said.
There also was no statistically significant between-group difference when the analysis focused on the subgroup of patients who were C-reactive protein positive at baseline, 55% and 58% of patients in the groups, respectively, who had their average mSASSS increase by 1.68, compared with 0.96. Similarly, when the analysis focused only on patients who had syndesmophytes at baseline, 53% and 62% of patients, respectively, the average increases in mSASSS were 2.11 and 0.95, a difference that was not statistically significant. Presence of C-reactive protein and syndesmophytes are both known risk factors for radiographic progression.
Patient characteristics were similar in the two groups. NSAID intake over the 2-year study period, measured using a 0-100 composite score based on treatment duration and NSAID doses and intervals, was a mean of 76 vs. 44 for the continuous and on-demand groups, respectively. At the study’s end, 77% of patients remained on diclofenac and had not switched to another NSAID.
Side effects were similar in both groups, with 19 serious adverse events in the continuous-treatment patients and 21 serious adverse events in the on-demand patients.
Previous studies have suggested that NSAIDs given continuously over 2 years reduce radiographic progression, compared with on-demand therapy, in ankylosing spondylitis patients. Similar effects were seen in a prospective cohort.
“In our study, continuous vs. on-demand treatment … did not prevent radiographic progression in [ankylosing spondylitis]. It is highly unlikely that the results would have been different with a higher number of patients, because we found a trend for less progression in the on-demand group,” Dr. Sieper said.
Additional study is needed to determine whether an NSAID other than diclofenac, specifically a COX-2 selective drug, would have a different effect on radiographic progression, he said.
Dr. Sieper reported receiving honorarium for consultancies, speaker’s bureaus, or grants from AbbVie, Janssen, Merck, Lily, Novartis, Pfizer, and UCB.
ROME – Radiographic progression occurs at similar rates in the spines of ankylosing spondylitis patients treated over 2 years with either continuous or on-demand diclofenac, according to results from a randomized, prospective multicenter trial presented at the European Congress of Rheumatology.
In the ENRADAS (Effects of NSAIDs on Radiographic Damage in Ankylosing Spondylitis) trial, Dr. Joachim Sieper of Charite-Universitätsmedizin Berlin and his colleagues compared continuous treatment with at least 50% of the maximum 150-mg daily dose of the NSAID diclofenac and on-demand treatment with diclofenac. During the entire 2 years of the study, no patient received treatment with a tumor necrosis factor blocker or any other drug other than diclofenac.
The investigators measured radiographic spine progression using the modified Stoke Ankylosing Spondylitis Spinal Score(mSASSS). At baseline, patients randomized to continuous treatment who had complete radiographic follow-up had a mean mSASSS of 10.9, while those randomized to the on-demand arm had a mean mSASSS of 16.4. After 2 years on treatment, the average change in mSASSS from baseline was 1.28 for the 62 patients in the continuous-treatment group and 0.79 for the 60 patients in the on-demand group, a difference that was not statistically significant, Dr. Sieper said.
There also was no statistically significant between-group difference when the analysis focused on the subgroup of patients who were C-reactive protein positive at baseline, 55% and 58% of patients in the groups, respectively, who had their average mSASSS increase by 1.68, compared with 0.96. Similarly, when the analysis focused only on patients who had syndesmophytes at baseline, 53% and 62% of patients, respectively, the average increases in mSASSS were 2.11 and 0.95, a difference that was not statistically significant. Presence of C-reactive protein and syndesmophytes are both known risk factors for radiographic progression.
Patient characteristics were similar in the two groups. NSAID intake over the 2-year study period, measured using a 0-100 composite score based on treatment duration and NSAID doses and intervals, was a mean of 76 vs. 44 for the continuous and on-demand groups, respectively. At the study’s end, 77% of patients remained on diclofenac and had not switched to another NSAID.
Side effects were similar in both groups, with 19 serious adverse events in the continuous-treatment patients and 21 serious adverse events in the on-demand patients.
Previous studies have suggested that NSAIDs given continuously over 2 years reduce radiographic progression, compared with on-demand therapy, in ankylosing spondylitis patients. Similar effects were seen in a prospective cohort.
“In our study, continuous vs. on-demand treatment … did not prevent radiographic progression in [ankylosing spondylitis]. It is highly unlikely that the results would have been different with a higher number of patients, because we found a trend for less progression in the on-demand group,” Dr. Sieper said.
Additional study is needed to determine whether an NSAID other than diclofenac, specifically a COX-2 selective drug, would have a different effect on radiographic progression, he said.
Dr. Sieper reported receiving honorarium for consultancies, speaker’s bureaus, or grants from AbbVie, Janssen, Merck, Lily, Novartis, Pfizer, and UCB.
ROME – Radiographic progression occurs at similar rates in the spines of ankylosing spondylitis patients treated over 2 years with either continuous or on-demand diclofenac, according to results from a randomized, prospective multicenter trial presented at the European Congress of Rheumatology.
In the ENRADAS (Effects of NSAIDs on Radiographic Damage in Ankylosing Spondylitis) trial, Dr. Joachim Sieper of Charite-Universitätsmedizin Berlin and his colleagues compared continuous treatment with at least 50% of the maximum 150-mg daily dose of the NSAID diclofenac and on-demand treatment with diclofenac. During the entire 2 years of the study, no patient received treatment with a tumor necrosis factor blocker or any other drug other than diclofenac.
The investigators measured radiographic spine progression using the modified Stoke Ankylosing Spondylitis Spinal Score(mSASSS). At baseline, patients randomized to continuous treatment who had complete radiographic follow-up had a mean mSASSS of 10.9, while those randomized to the on-demand arm had a mean mSASSS of 16.4. After 2 years on treatment, the average change in mSASSS from baseline was 1.28 for the 62 patients in the continuous-treatment group and 0.79 for the 60 patients in the on-demand group, a difference that was not statistically significant, Dr. Sieper said.
There also was no statistically significant between-group difference when the analysis focused on the subgroup of patients who were C-reactive protein positive at baseline, 55% and 58% of patients in the groups, respectively, who had their average mSASSS increase by 1.68, compared with 0.96. Similarly, when the analysis focused only on patients who had syndesmophytes at baseline, 53% and 62% of patients, respectively, the average increases in mSASSS were 2.11 and 0.95, a difference that was not statistically significant. Presence of C-reactive protein and syndesmophytes are both known risk factors for radiographic progression.
Patient characteristics were similar in the two groups. NSAID intake over the 2-year study period, measured using a 0-100 composite score based on treatment duration and NSAID doses and intervals, was a mean of 76 vs. 44 for the continuous and on-demand groups, respectively. At the study’s end, 77% of patients remained on diclofenac and had not switched to another NSAID.
Side effects were similar in both groups, with 19 serious adverse events in the continuous-treatment patients and 21 serious adverse events in the on-demand patients.
Previous studies have suggested that NSAIDs given continuously over 2 years reduce radiographic progression, compared with on-demand therapy, in ankylosing spondylitis patients. Similar effects were seen in a prospective cohort.
“In our study, continuous vs. on-demand treatment … did not prevent radiographic progression in [ankylosing spondylitis]. It is highly unlikely that the results would have been different with a higher number of patients, because we found a trend for less progression in the on-demand group,” Dr. Sieper said.
Additional study is needed to determine whether an NSAID other than diclofenac, specifically a COX-2 selective drug, would have a different effect on radiographic progression, he said.
Dr. Sieper reported receiving honorarium for consultancies, speaker’s bureaus, or grants from AbbVie, Janssen, Merck, Lily, Novartis, Pfizer, and UCB.
AT THE EULAR 2015 CONGRESS
Key clinical point: There are no differences with respect to radiographic progression in the spines of ankylosing spondylitis patients treated over 2 years with either continuous or on-demand diclofenac.
Major finding: The average 2-year change in mSASSS from baseline was 1.28 for the 62 patients in the continuous-treatment group and 0.79 for the 60 patients in the on-demand group, a difference that was not statistically significant.
Data source: A randomized, prospective multicenter trial of 122 patients with ankylosing spondylitis.
Disclosures: Dr. Sieper reported receiving honorarium for consultancies, speaker’s bureaus, or grants from AbbVie, Janssen, Merck, Lily, Novartis, Pfizer, and UCB.
VIDEO: EULAR updates cardiovascular-disease risk recommendations
ROME – The European League Against Rheumatism introduced an update to its 2009 recommendations on assessing and managing cardiovascular-disease risk in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis.
The update features an expanded evidence base for the recommendations, especially for psoriatic arthritis and ankylosing spondylitis, Dr. Michael T. Nurmohamed said in an interview at the European Congress of Rheumatology. One new element in the revision included a scaling down of the previously suggested annual assessment to a more flexible approach to the timing of serial assessments based on the risk level of individual patients. Another addition is the possible use of carotid ultrasound to measure atherosclerotic burden as a complement to more routinely-measured risk factors such as blood pressure and serum lipids, said Dr. Nurmohamed, convener of the current task force as well as the panel that formulated the first version (Ann. Rheum. Dis. 2010;69:325-31).
A second EULAR task force recently developed new recommendations on assessing and managing other comorbidities in patients with rheumatologic diseases, such as osteoporosis, cancer, peptic ulcers, and renal dysfunction. Chronic kidney disease is an important modifier of cardiovascular-disease risk, and hence the new comorbidity recommendations complement the new cardiovascular-disease statement, said Dr. Nurmohamed, professor and head of the rheumatology research department at VU University Medical Center in Amsterdam.
Dr. Nurmohamed had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The European League Against Rheumatism introduced an update to its 2009 recommendations on assessing and managing cardiovascular-disease risk in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis.
The update features an expanded evidence base for the recommendations, especially for psoriatic arthritis and ankylosing spondylitis, Dr. Michael T. Nurmohamed said in an interview at the European Congress of Rheumatology. One new element in the revision included a scaling down of the previously suggested annual assessment to a more flexible approach to the timing of serial assessments based on the risk level of individual patients. Another addition is the possible use of carotid ultrasound to measure atherosclerotic burden as a complement to more routinely-measured risk factors such as blood pressure and serum lipids, said Dr. Nurmohamed, convener of the current task force as well as the panel that formulated the first version (Ann. Rheum. Dis. 2010;69:325-31).
A second EULAR task force recently developed new recommendations on assessing and managing other comorbidities in patients with rheumatologic diseases, such as osteoporosis, cancer, peptic ulcers, and renal dysfunction. Chronic kidney disease is an important modifier of cardiovascular-disease risk, and hence the new comorbidity recommendations complement the new cardiovascular-disease statement, said Dr. Nurmohamed, professor and head of the rheumatology research department at VU University Medical Center in Amsterdam.
Dr. Nurmohamed had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
ROME – The European League Against Rheumatism introduced an update to its 2009 recommendations on assessing and managing cardiovascular-disease risk in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis.
The update features an expanded evidence base for the recommendations, especially for psoriatic arthritis and ankylosing spondylitis, Dr. Michael T. Nurmohamed said in an interview at the European Congress of Rheumatology. One new element in the revision included a scaling down of the previously suggested annual assessment to a more flexible approach to the timing of serial assessments based on the risk level of individual patients. Another addition is the possible use of carotid ultrasound to measure atherosclerotic burden as a complement to more routinely-measured risk factors such as blood pressure and serum lipids, said Dr. Nurmohamed, convener of the current task force as well as the panel that formulated the first version (Ann. Rheum. Dis. 2010;69:325-31).
A second EULAR task force recently developed new recommendations on assessing and managing other comorbidities in patients with rheumatologic diseases, such as osteoporosis, cancer, peptic ulcers, and renal dysfunction. Chronic kidney disease is an important modifier of cardiovascular-disease risk, and hence the new comorbidity recommendations complement the new cardiovascular-disease statement, said Dr. Nurmohamed, professor and head of the rheumatology research department at VU University Medical Center in Amsterdam.
Dr. Nurmohamed had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE EULAR 2015 CONGRESS
Rheumatologists support biosimilars, with caveats
Biosimilars are poised to flood the U.S. pharmaceutical market, bringing biologic therapies within reach of many patients who previously could not pay for them. In a position statement issued last month, the American College of Rheumatology voiced its support for the new agents, but only if they are carefully regulated and tracked – and if prescribing decisions are left up to physicians and patients, not pharmacists or payers.
“As more biologic drugs are used to treat rheumatic diseases, rheumatologists are increasingly concerned about their high cost and patients being unable to afford them,” ACR president William St.Clair said in a written statement. “We agree that less-expensive biologic therapies are needed, and recognize that biosimilars provide an opportunity to reduce treatment costs.”
The ACR position statement follows the U.S. Food and Drug Administration’s approval on March 6 of the first biosimilar, Sandoz’s Zarxio (filgrastim-sndz), which is used in cancer treatment and is based on Amgen’s Neupogen (filgrastim). Patents on many other biologics will expire by about 2020, clearing the way for biosimilars used in rheumatology, dermatology, and other fields. Among these, Celltrion’s infliximab biosimilar Remsima (CT-P13) was submitted for FDA approval last year for the treatment of rheumatoid arthritis, Crohn’s disease, and several other conditions. The FDA postponed a March 17 meeting to review Celltrion’s application, stating that it had requested more information from the company.
The ACR has echoed the FDA’s apparent caution regarding biosimilars. Although only the clinically inactive components of biosimilars are allowed to differ from their reference agents, today’s biologics are so molecularly complex that biosimilars need rigorous studies in humans to assess their safety and efficacy, the organization stated. “It is uncertain whether patients will respond to these drugs the same way they would to an original biologic, because biologics are very sensitive to manufacturing changes,” Dr. St.Clair said. “Even minor differences in a biosimilar’s molecular structure, purity, or other chemical properties could change the way a patient responds to the drug.”
For this reason, postmarketing studies in children and adults also will be essential, the ACR stated. Dr. Scott Zashin, a rheumatologist at Presbyterian Hospital, Dallas, and who is also with the University of Texas in Dallas, agreed. “Studies will need to be conducted to determine the benefit and risks,” he said in an interview. “In addition, registries would be needed to help assess long-term safety in larger numbers of patients than is feasible in a clinical trial.”
The ACR also raised concerns about switching patients back and forth between originator biologics and biosimilars, even if the biosimilar met stringent criteria demonstrating its interchangeability with the reference agent. “Experience outside the United States with biosimilar erythropoietin has indicated that a serious adverse reaction due to immunogenicity is a valid and very real concern for biosimilar products,” the ACR stated. “At this time, there are too many unknown variables to presume that repeated switching of biologic drugs would be safe practice.”
Because the FDA has not specified what would be needed to prove that a biologic and its biosimilar are truly interchangeable in the treatment of rheumatic conditions, pharmacists should not be allowed to substitute a biosimilar for a prescribed biologic without written approval from the physician, the ACR stated. But if pharmacists are legally allowed to substitute a biosimilar, they should be required to notify both physicians and patients so that patients can be monitored for adverse effects, the organization added.
Dr. Zashin agreed. “I would pretty much treat the biosimilars as generics, and leave it up to the FDA to decide if they are inferior,” he said. “But there are some generics on the market now (small molecules, not biologics) that I feel are inferior to the brand drug. This would be no different if biosimilars are approved.” For that reason, he would not accept a pharmacist substituting a biosimilar if he prescribed the branded drug, unless the patient preferred and specifically requested the less-expensive product, he said.
Along the same lines, the ACR believes that physicians should be able to designate a prescription as “brand only” if they are concerned about the safety of a biosimilar and that payers should not be able to force patients to switch to a biosimilar if they are already responding well to the biologic. But under current circumstances, “the insurance company would dictate what the patient could receive,” Dr. Zashin cautioned. If the patient refused the biosimilar, “I expect they would penalize the patient and make the brand quite costly.”
Finally, the ACR position statement recommended that biosimilars have distinctive names to easily distinguish them from their reference agents. Otherwise, name confusion could lead to prescribing errors and mistakes during surveillance for adverse events, the organization said.
“We will continue to follow the various issues surrounding the distribution, monitoring, and reimbursement of biosimilars very closely as state and federal policies are proposed that affect our patients and the rheumatologists who serve them,” Dr. St.Clair concluded. “Ensuring patients have easy access to affordable treatment options and rheumatology care continues to be a high priority for us.”
Biosimilars are poised to flood the U.S. pharmaceutical market, bringing biologic therapies within reach of many patients who previously could not pay for them. In a position statement issued last month, the American College of Rheumatology voiced its support for the new agents, but only if they are carefully regulated and tracked – and if prescribing decisions are left up to physicians and patients, not pharmacists or payers.
“As more biologic drugs are used to treat rheumatic diseases, rheumatologists are increasingly concerned about their high cost and patients being unable to afford them,” ACR president William St.Clair said in a written statement. “We agree that less-expensive biologic therapies are needed, and recognize that biosimilars provide an opportunity to reduce treatment costs.”
The ACR position statement follows the U.S. Food and Drug Administration’s approval on March 6 of the first biosimilar, Sandoz’s Zarxio (filgrastim-sndz), which is used in cancer treatment and is based on Amgen’s Neupogen (filgrastim). Patents on many other biologics will expire by about 2020, clearing the way for biosimilars used in rheumatology, dermatology, and other fields. Among these, Celltrion’s infliximab biosimilar Remsima (CT-P13) was submitted for FDA approval last year for the treatment of rheumatoid arthritis, Crohn’s disease, and several other conditions. The FDA postponed a March 17 meeting to review Celltrion’s application, stating that it had requested more information from the company.
The ACR has echoed the FDA’s apparent caution regarding biosimilars. Although only the clinically inactive components of biosimilars are allowed to differ from their reference agents, today’s biologics are so molecularly complex that biosimilars need rigorous studies in humans to assess their safety and efficacy, the organization stated. “It is uncertain whether patients will respond to these drugs the same way they would to an original biologic, because biologics are very sensitive to manufacturing changes,” Dr. St.Clair said. “Even minor differences in a biosimilar’s molecular structure, purity, or other chemical properties could change the way a patient responds to the drug.”
For this reason, postmarketing studies in children and adults also will be essential, the ACR stated. Dr. Scott Zashin, a rheumatologist at Presbyterian Hospital, Dallas, and who is also with the University of Texas in Dallas, agreed. “Studies will need to be conducted to determine the benefit and risks,” he said in an interview. “In addition, registries would be needed to help assess long-term safety in larger numbers of patients than is feasible in a clinical trial.”
The ACR also raised concerns about switching patients back and forth between originator biologics and biosimilars, even if the biosimilar met stringent criteria demonstrating its interchangeability with the reference agent. “Experience outside the United States with biosimilar erythropoietin has indicated that a serious adverse reaction due to immunogenicity is a valid and very real concern for biosimilar products,” the ACR stated. “At this time, there are too many unknown variables to presume that repeated switching of biologic drugs would be safe practice.”
Because the FDA has not specified what would be needed to prove that a biologic and its biosimilar are truly interchangeable in the treatment of rheumatic conditions, pharmacists should not be allowed to substitute a biosimilar for a prescribed biologic without written approval from the physician, the ACR stated. But if pharmacists are legally allowed to substitute a biosimilar, they should be required to notify both physicians and patients so that patients can be monitored for adverse effects, the organization added.
Dr. Zashin agreed. “I would pretty much treat the biosimilars as generics, and leave it up to the FDA to decide if they are inferior,” he said. “But there are some generics on the market now (small molecules, not biologics) that I feel are inferior to the brand drug. This would be no different if biosimilars are approved.” For that reason, he would not accept a pharmacist substituting a biosimilar if he prescribed the branded drug, unless the patient preferred and specifically requested the less-expensive product, he said.
Along the same lines, the ACR believes that physicians should be able to designate a prescription as “brand only” if they are concerned about the safety of a biosimilar and that payers should not be able to force patients to switch to a biosimilar if they are already responding well to the biologic. But under current circumstances, “the insurance company would dictate what the patient could receive,” Dr. Zashin cautioned. If the patient refused the biosimilar, “I expect they would penalize the patient and make the brand quite costly.”
Finally, the ACR position statement recommended that biosimilars have distinctive names to easily distinguish them from their reference agents. Otherwise, name confusion could lead to prescribing errors and mistakes during surveillance for adverse events, the organization said.
“We will continue to follow the various issues surrounding the distribution, monitoring, and reimbursement of biosimilars very closely as state and federal policies are proposed that affect our patients and the rheumatologists who serve them,” Dr. St.Clair concluded. “Ensuring patients have easy access to affordable treatment options and rheumatology care continues to be a high priority for us.”
Biosimilars are poised to flood the U.S. pharmaceutical market, bringing biologic therapies within reach of many patients who previously could not pay for them. In a position statement issued last month, the American College of Rheumatology voiced its support for the new agents, but only if they are carefully regulated and tracked – and if prescribing decisions are left up to physicians and patients, not pharmacists or payers.
“As more biologic drugs are used to treat rheumatic diseases, rheumatologists are increasingly concerned about their high cost and patients being unable to afford them,” ACR president William St.Clair said in a written statement. “We agree that less-expensive biologic therapies are needed, and recognize that biosimilars provide an opportunity to reduce treatment costs.”
The ACR position statement follows the U.S. Food and Drug Administration’s approval on March 6 of the first biosimilar, Sandoz’s Zarxio (filgrastim-sndz), which is used in cancer treatment and is based on Amgen’s Neupogen (filgrastim). Patents on many other biologics will expire by about 2020, clearing the way for biosimilars used in rheumatology, dermatology, and other fields. Among these, Celltrion’s infliximab biosimilar Remsima (CT-P13) was submitted for FDA approval last year for the treatment of rheumatoid arthritis, Crohn’s disease, and several other conditions. The FDA postponed a March 17 meeting to review Celltrion’s application, stating that it had requested more information from the company.
The ACR has echoed the FDA’s apparent caution regarding biosimilars. Although only the clinically inactive components of biosimilars are allowed to differ from their reference agents, today’s biologics are so molecularly complex that biosimilars need rigorous studies in humans to assess their safety and efficacy, the organization stated. “It is uncertain whether patients will respond to these drugs the same way they would to an original biologic, because biologics are very sensitive to manufacturing changes,” Dr. St.Clair said. “Even minor differences in a biosimilar’s molecular structure, purity, or other chemical properties could change the way a patient responds to the drug.”
For this reason, postmarketing studies in children and adults also will be essential, the ACR stated. Dr. Scott Zashin, a rheumatologist at Presbyterian Hospital, Dallas, and who is also with the University of Texas in Dallas, agreed. “Studies will need to be conducted to determine the benefit and risks,” he said in an interview. “In addition, registries would be needed to help assess long-term safety in larger numbers of patients than is feasible in a clinical trial.”
The ACR also raised concerns about switching patients back and forth between originator biologics and biosimilars, even if the biosimilar met stringent criteria demonstrating its interchangeability with the reference agent. “Experience outside the United States with biosimilar erythropoietin has indicated that a serious adverse reaction due to immunogenicity is a valid and very real concern for biosimilar products,” the ACR stated. “At this time, there are too many unknown variables to presume that repeated switching of biologic drugs would be safe practice.”
Because the FDA has not specified what would be needed to prove that a biologic and its biosimilar are truly interchangeable in the treatment of rheumatic conditions, pharmacists should not be allowed to substitute a biosimilar for a prescribed biologic without written approval from the physician, the ACR stated. But if pharmacists are legally allowed to substitute a biosimilar, they should be required to notify both physicians and patients so that patients can be monitored for adverse effects, the organization added.
Dr. Zashin agreed. “I would pretty much treat the biosimilars as generics, and leave it up to the FDA to decide if they are inferior,” he said. “But there are some generics on the market now (small molecules, not biologics) that I feel are inferior to the brand drug. This would be no different if biosimilars are approved.” For that reason, he would not accept a pharmacist substituting a biosimilar if he prescribed the branded drug, unless the patient preferred and specifically requested the less-expensive product, he said.
Along the same lines, the ACR believes that physicians should be able to designate a prescription as “brand only” if they are concerned about the safety of a biosimilar and that payers should not be able to force patients to switch to a biosimilar if they are already responding well to the biologic. But under current circumstances, “the insurance company would dictate what the patient could receive,” Dr. Zashin cautioned. If the patient refused the biosimilar, “I expect they would penalize the patient and make the brand quite costly.”
Finally, the ACR position statement recommended that biosimilars have distinctive names to easily distinguish them from their reference agents. Otherwise, name confusion could lead to prescribing errors and mistakes during surveillance for adverse events, the organization said.
“We will continue to follow the various issues surrounding the distribution, monitoring, and reimbursement of biosimilars very closely as state and federal policies are proposed that affect our patients and the rheumatologists who serve them,” Dr. St.Clair concluded. “Ensuring patients have easy access to affordable treatment options and rheumatology care continues to be a high priority for us.”