User login
State laws, regulatory concerns complicate biosimilars landscape
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
SAN FRANCISCO – A growing number of states are passing laws that set the parameters for how originator biologics may be replaced with biosimilars, and not all of them are helpful to physicians trying to maintain some knowledge and control over how prescriptions for biologics are dispensed, according to speakers at the annual meeting of the American College of Rheumatology.
These laws – now passed by a total of 19 states and Puerto Rico, including 12 in 2015 – address a requirement built into the Biologics Price Competition and Innovation Act of 2009 (part of the Affordable Care Act) that introduced the term “interchangeability” into the biosimilars approval pathway, stating that an approved biosimilar “may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.” The law also gives 1 year of exclusive marketing rights to the first biosimilar approved as being interchangeable with the reference product.
Although regulations on how the Food and Drug Administration will deem a biosimilar to be interchangeable have yet to be drafted, it’s important for physicians to play an active role in shaping the laws that address interchangeability, said Dr. J. Eugene Huffstutter, a rheumatologist in private practice in Hixson, Tenn., and clinical assistant professor of medicine at the University of Tennessee, Chattanooga.
Dr. Huffstutter encouraged rheumatologists and other physicians to work with their local state medical associations to contact their state legislators to explain to them the need for strict laws on how biosimilars can be dispensed. He suggested that good state legislation on biosimilars should contain provisions stating that:
• Only FDA-approved interchangeable biosimilars can be substituted.
• No substitution can be made with “dispense as written” prescription.
• The prescribing physician must be notified of substitution within 1-5 days by electronic record or fax.
• The pharmacy must maintain a record of the dispensed drug.
Organizations including the ACR, the Coalition of State Rheumatology Organizations, patient groups, electronic prescribers, state medical societies, and pharmaceutical companies (for the most part) support strong state laws on biosimilars, while the pharmacy lobby and insurance companies have opposed them, Dr. Huffstutter said.
The 19 states with biosimilars laws include California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Louisiana, Massachusetts, New Jersey, North Carolina, North Dakota, Oregon, Tennessee, Texas, Utah, Virginia, and Washington, along with Puerto Rico. However, the laws in Oregon and Virginia will sunset in 2016. Another seven states have 2015 or current session legislation on biosimilar substitution: Hawaii, Maryland, Michigan, Mississippi, Oklahoma, Pennsylvania, and Vermont. States with bills that failed or were not acted upon at adjournment include Arizona, Arkansas, Nevada, and Rhode Island, according to information provided to Dr. Huffstutter by the Coalition.
State laws may differ on substitution notification, he noted. In Tennessee, the law (H.B. 572) states that notification should occur in “a reasonable period of time” instead of a defined period. “What may be reasonable for one person may not be reasonable for another. I really think 5 days is the outside time, or 5 business days, because if you’re thinking about some of the biosimilars that are coming down the pike, they’ll be given on a weekly basis, so you’ll at least know before [patients] get their second shot what they’re receiving,” Dr. Huffstutter noted.
Idaho is unique in that it impaneled a board of pharmacy regulation on biosimilar substitution rather than a separate law. It states that “A pharmacist may substitute an interchangeable biosimilar product for a prescribed biological product if the biosimilar has been determined by the FDA to be interchangeable and published in the Purple Book; the prescriber does not indicate by any means that the prescribed biological product must be dispensed; and the name of the drug and the manufacturer or the NDC [National Drug Code] number is documented in the patient medical record.”
The situation may become trickier as more biosimilars are approved, noted Dr. Huffstutter, who is a member of the ACR’s Government Affairs Committee and is a liaison to the Committee on Rheumatologic Care. It could potentially be a problem when a patient is taking a biosimilar for a particular originator biologic and then other biosimilars for that originator join the marketplace. A patient could potentially be switched from one biosimilar to another when a prescription is filled if the company marketing the second biosimilar happens to win a competitive bid with an insurance company, he said.
The filgrastim biosimilar called Zarxio is the only biosimilar currently approved in the United States, but the FDA has received two applications for biosimilars for inflammatory diseases: one from Celltrion for infliximab biosimilar Remsima (August 2014) and one from Sandoz for an etanercept biosimilar (October 2015). In addition to two infliximab biosimilars that have been approved in other countries, one etanercept biosimilar that has been approved in South Korea, and one adalimumab biosimilar approved in India, there are many others in development to treat inflammatory diseases as of July 2015, including 12 for adalimumab, 9 for etanercept, 5 for infliximab, 2 for tocilizumab, and 7 for rituximab, according to Dr. Jonathan Kay, professor of medicine and director of clinical research in the division of rheumatology at the University of Massachusetts, Worcester.
Unresolved regulatory concerns
In separate presentations at the meeting, Dr. Kay described many of the nuances that define biosimilars, which go by different terminology in other countries, such as “follow-on biologic” in Japan, “subsequent-entry biological” in Canada, and “similar biological medicinal product” in the European Union. They are defined as a legitimate copy of an off-patent biopharmaceutical that has undergone rigorous analytical comparison and clinical assessment, in comparison to its reference product, and are approved by a regulatory agency according to a specific pathway for biosimilar evaluation. These differ from biomimetics, which are developed as a replica of a biopharmaceutical but have not been developed, assessed, or approved according to regulatory guidelines for biosimilars.
The FDA takes a “totality of the evidence” approach to establish biosimilarity. The biosimilar pathway for approval is different from the originator pathway by relying more on analytical and preclinical studies and less on the clinical pharmacology and clinical trial data to support biosimilarity.
However, the agency has not yet published final guidance for all steps in demonstrating biosimilarity, particularly for clinical pharmacology data. Draft guidance says the biosimilar must be analyzed and assigned to an assessment grade: not similar, similar, highly similar, and highly similar with fingerprintlike similarity.
During production, both originator and biosimilar biopharmaceuticals can have protein-folding variants, misfolding, aggregation, enzymatic cleavage, and degradation that can lead to inactivation of the protein or increased immunogenicity. Even so, most biosimilars are not identical to the originator because of post-translational modifications, but they do not matter as long as they are not clinically meaningful. Even originator products can drift over time because small changes in manufacturing processes can lead to gradual changes in the molecule, Dr. Kay said.
Extrapolation of indications from the originator to a biosimilar is another area of concern, Dr. Kay said. The FDA will consider the extrapolation of data from a clinical trial of a biosimilar conducted in one disease to support approval for additional indications for which the originator product is already licensed, but not across indications with different mechanisms of action, such as between rheumatoid arthritis and non-Hodgkin’s lymphoma for rituximab. But for which inflammatory diseases should a biosimilar be studied to provide adequate information for extrapolation of indications? Dr. Kay asked. This situation brings about questions of whether dermatologists would be comfortable using a biosimilar that has been studied for rheumatoid arthritis to treat psoriasis or gastroenterologists using a drug with data only from psoriatic arthritis to treat Crohn’s disease or ulcerative colitis.
Both the Coalition of State Rheumatology Organizations and the ACR oppose extrapolation of indications from the clinical trial data of a biosimilar in one disease to support approval of additional indications for which the originator product is already licensed, but Dr. Kay thought that “extrapolation of indications makes perfect sense” because it won’t be possible to review a biosimilar for indications not already approved for the originator and it’s natural to extrapolate to already approved indications once the protein is shown to function nearly identically to the originator.
Ideally, the nomenclature system for biosimilars should clearly identify each product to improve pharmacovigilance and to differentiate between products that have not been determined to be interchangeable, Dr. Kay said. In August, the FDA announced draft guidance proposing that all biopharmaceutical products (originator and biosimilars) would have a nonproprietary name that includes a suffix of four lowercase letters that is devoid of meaning. For example, all products that share a core name such as replicamab would be named replicamab-cznm, replicamab-hixf, and so on, Dr. Kay said. Some people have voiced concern that it would be better for names to have some meaning, such as using an abbreviated name for the developing company along with the nonproprietary name of the originator product, but others noted that method could be problematic when companies merge or are acquired by others.
Dr. Huffstutter disclosed relationships with Janssen, UCB, Lilly, Pfizer, Genentech, Bristol-Myers Squibb, and Celgene. Dr. Kay has received grant and research support from many pharmaceutical companies and has served as a consultant or on an advisory board for many pharmaceutical companies, including those developing biosimilars.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
ACR: Adalimumab best TNFi for anterior uveitis in ankylosing spondylitis
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
SAN FRANCISCO – Adalimumab was the clear winner over infliximab and etanercept for anterior uveitis in a Scandinavian database study of 1,365 patients with ankylosing spondylitis.
Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab (Humira). Flares per 100 patient-years dropped by almost 70%.
There was less improvement with infliximab (Remicade), which tended to be used in low doses in the study, less than 5 mg/kg. Office visits dropped from 50.4 to 27.3 per 100 patient-years for the 605 patients who started on it. Flares decreased by about 40%.
Etanercept (Enbrel), meanwhile, seemed to make uveitis worse, a concern that’s been raised previously. Office visits for uveitis increased from 38.1 to 56.5 per 100 patient-years for the 354 patients started on etanercept. Flares increased by about 20%.
The results were presented at the annual meeting of the American College of Rheumatology.
Among patients who were uveitis free in the 2 years prior to starting a tumor necrosis factor inhibitor (TNFi), the risk of flare was far greater with etanercept than with adalimumab (adjusted hazard ratio, 3.69; 95% confidence interval, 1.61-8.46). The risk also was higher for infliximab, though not significantly so (adjusted HR, 1.67; 95% CI, 0.69-4.04).
About 2% of adalimumab patients had their first bout of uveitis after starting it, versus 5.3% of infliximab and 13.7% of etanercept patients.
“A clear reduction in uveitis rates was observed for adalimumab, a slight reduction for infliximab, and a marked increase for etanercept, irrespective of the method for counting flares,” reported Dr. Elisabeth Lie, a rheumatology fellow at Gothenburg (Sweden) University.
In the adjusted analysis, “adalimumab and infliximab were associated with significantly lower hazard for first uveitis flare than etanercept,” noted Dr. Lie.
“Right now, we are still favoring infliximab because it comes as a biosimilar, so it’s inexpensive; I think we are giving it the benefit of the doubt. Still, these results are so favorable for adalimumab” that they make “a strong case to at least have a lower threshold to switch to it if you are not successful with your first choice,” Dr. Lie said in an interview.
The etanercept findings are “at least a reminder that if you have a patient with any history of uveitis, choose another option. I’ve seen a few” cases apparently triggered by etanercept. When that happens, “switch to something else,” she said.
The problem with etanercept may be related to its structure or immunologic effects, but no one really knows for sure, she added.
The study linked the first-time use of a TNFi by ankylosing spondylitis patients in the Swedish Biologics Register to their office visits for anterior uveitis as captured in the country’s National Patient Register. Dr. Lie and her coinvestigators analyzed the 2 years before and after the start of a TNFi. Adalimumab was more frequently used toward the end of the study period, which ran from 2003 through 2010.
Patients were in their early 40s, on average, and almost three-quarters were men. Infliximab patients most often used concomitant disease-modifying antirheumatic drugs and had the highest rate of comorbid inflammatory bowel disease, at 10.4%. Adalimumab patients had the lowest median baseline C-reactive protein at 10 mg/L, but highest pretreatment rate of uveitis, at 28%.
Dr. Lie is a speaker and consultant for AbbVie, the maker of adalimumab, and Pfizer, the maker of etanercept.
AT The ACR ANNUAL MEETING
Key clinical point: Pick adalimumab or perhaps infliximab for anterior uveitis in AS, not etanercept.
Major finding: Office visits for uveitis dropped from 42.6 to 13.4 per 100 patient-years for the 406 patients who started on adalimumab; office visits increased from 38.1 to 56.5 per 100 patient-years for the 354 patients who started on etanercept.
Data source: Database study of 1,365 ankylosing spondylitis patients with anterior uveitis.
Disclosures: The presenting investigator is a speaker and consultant for AbbVie, maker of adalimumab, and Pfizer, maker of etanercept.
ACR: Video capsule endoscopy finds Crohn’s disease best in spondyloarthropathy patients
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
AT THE ACR ANNUAL MEETING
Key clinical point:Video capsule endoscopy is better than ileocolonoscopy for detection of Crohn’s disease in patients with spondyloarthropathies.
Major finding: Video capsule endoscopy detected Crohn’s disease in 45% of patients, compared with 14% detected by ileocolonoscopy (P =.036).
Data source: Prospective study of 64 patients with SpA who underwent video capsule endoscopy followed by ileocolonoscopy.
Disclosures: AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
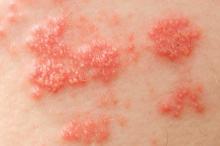
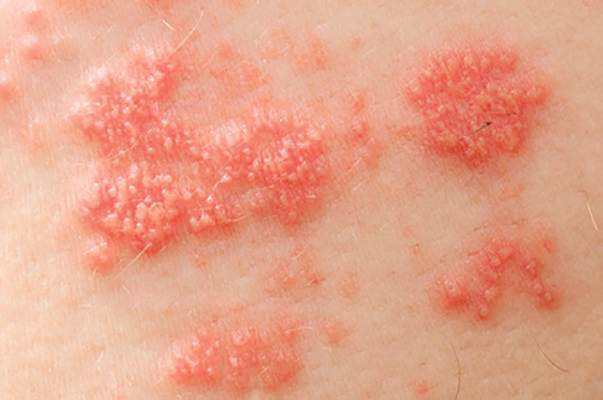
Stroke risk rose in autoimmune disease patients after herpes zoster
Stroke risk was 50% higher in the month after patients with autoimmune diseases developed herpes zoster, compared with the next 2-6 years, according to Dr. Leonard H. Calabrese.
“These data provide urgency for developing strategies to reduce the risk of varicella zoster virus in vulnerable immunosuppressed patients,” said Dr. Calabrese of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
Immunosuppressive therapies increase the frequency and complexity of herpes zoster, which is a known risk factor for stroke. To examine the temporal relationship between herpes zoster and stroke among immunosuppressed patients, Dr. Calabrese and his associates studied Medicare data for almost 51,000 patients with new-onset herpes zoster who also had physician-diagnosed ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis. The researchers excluded patients with a history of stroke.
In the multivariable analysis, stroke was 1.5 times more likely during the 6 months immediately after herpes zoster than in the 2-6 years after herpes zoster (95% confidence interval, 1.06-2.12). During this 6-month window, there were 9.8 strokes per 1,000 person-years, compared with 8.7 per 1,000 person-years in the 2-6 years after herpes zoster. Stroke risk also remained somewhat elevated during the entire year after herpes zoster (incidence rate ratio, 1.3; 95% CI, 1.05-1.61).
In general, stroke was more likely to occur among patients who were older, were receiving high-dose glucocorticoids, or had diabetes, hypertension, atrial fibrillation, or a history of transient ischemic attack, he said at the annual meeting of the American College of Rheumatology in San Francisco.
Dr. Calabrese disclosed relationships with Bristol-Myers Squibb, Crescendo, AbbVie, Genentech, Biogen, Pfizer, Sanofi-Aventis Pharmaceutical, and Johnson & Johnson. Two coauthors also disclosed relationships with several pharmaceutical companies. The other four coinvestigators had no disclosures.
Stroke risk was 50% higher in the month after patients with autoimmune diseases developed herpes zoster, compared with the next 2-6 years, according to Dr. Leonard H. Calabrese.
“These data provide urgency for developing strategies to reduce the risk of varicella zoster virus in vulnerable immunosuppressed patients,” said Dr. Calabrese of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
Immunosuppressive therapies increase the frequency and complexity of herpes zoster, which is a known risk factor for stroke. To examine the temporal relationship between herpes zoster and stroke among immunosuppressed patients, Dr. Calabrese and his associates studied Medicare data for almost 51,000 patients with new-onset herpes zoster who also had physician-diagnosed ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis. The researchers excluded patients with a history of stroke.
In the multivariable analysis, stroke was 1.5 times more likely during the 6 months immediately after herpes zoster than in the 2-6 years after herpes zoster (95% confidence interval, 1.06-2.12). During this 6-month window, there were 9.8 strokes per 1,000 person-years, compared with 8.7 per 1,000 person-years in the 2-6 years after herpes zoster. Stroke risk also remained somewhat elevated during the entire year after herpes zoster (incidence rate ratio, 1.3; 95% CI, 1.05-1.61).
In general, stroke was more likely to occur among patients who were older, were receiving high-dose glucocorticoids, or had diabetes, hypertension, atrial fibrillation, or a history of transient ischemic attack, he said at the annual meeting of the American College of Rheumatology in San Francisco.
Dr. Calabrese disclosed relationships with Bristol-Myers Squibb, Crescendo, AbbVie, Genentech, Biogen, Pfizer, Sanofi-Aventis Pharmaceutical, and Johnson & Johnson. Two coauthors also disclosed relationships with several pharmaceutical companies. The other four coinvestigators had no disclosures.
Stroke risk was 50% higher in the month after patients with autoimmune diseases developed herpes zoster, compared with the next 2-6 years, according to Dr. Leonard H. Calabrese.
“These data provide urgency for developing strategies to reduce the risk of varicella zoster virus in vulnerable immunosuppressed patients,” said Dr. Calabrese of the department of rheumatic and immunologic diseases at the Cleveland Clinic.
Immunosuppressive therapies increase the frequency and complexity of herpes zoster, which is a known risk factor for stroke. To examine the temporal relationship between herpes zoster and stroke among immunosuppressed patients, Dr. Calabrese and his associates studied Medicare data for almost 51,000 patients with new-onset herpes zoster who also had physician-diagnosed ankylosing spondylitis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or rheumatoid arthritis. The researchers excluded patients with a history of stroke.
In the multivariable analysis, stroke was 1.5 times more likely during the 6 months immediately after herpes zoster than in the 2-6 years after herpes zoster (95% confidence interval, 1.06-2.12). During this 6-month window, there were 9.8 strokes per 1,000 person-years, compared with 8.7 per 1,000 person-years in the 2-6 years after herpes zoster. Stroke risk also remained somewhat elevated during the entire year after herpes zoster (incidence rate ratio, 1.3; 95% CI, 1.05-1.61).
In general, stroke was more likely to occur among patients who were older, were receiving high-dose glucocorticoids, or had diabetes, hypertension, atrial fibrillation, or a history of transient ischemic attack, he said at the annual meeting of the American College of Rheumatology in San Francisco.
Dr. Calabrese disclosed relationships with Bristol-Myers Squibb, Crescendo, AbbVie, Genentech, Biogen, Pfizer, Sanofi-Aventis Pharmaceutical, and Johnson & Johnson. Two coauthors also disclosed relationships with several pharmaceutical companies. The other four coinvestigators had no disclosures.
FROM THE ACR ANNUAL MEETING
Key clinical point: Stroke risk increased by 50% in the month after patients with autoimmune diseases had an episode of herpes zoster.
Major finding: The risk of stroke was 50% higher in the 6 months immediately after incident herpes zoster than 2-6 years after herpes zoster (95% CI, 1.06- 2.12).
Data source: An analysis of Medicare data for 50,929 patients with autoimmune disease and incident herpes zoster between 2006 and 2012.
Disclosures: Dr. Calabrese disclosed relationships with Bristol-Myers Squibb, Crescendo, AbbVie, Genentech, Biogen, Pfizer, Sanofi-Aventis Pharmaceutical, and Johnson & Johnson. Two coauthors also disclosed relationships with several pharmaceutical companies. The other four coinvestigators had no disclosures.
Shingles vaccine protection lasted 5-6 years in autoimmune disease patients
Protection against shingles appeared to wane between the fifth and sixth years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients, reported Dr. Huifeng Yun, assistant professor of epidemiology at the University of Alabama, Birmingham. Based on the findings, clinicians could consider revaccinating patients with autoimmune diseases about 5 years after their first live herpes zoster vaccine, she said.*
The long-term Shingles Prevention Study recently showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals, but the duration of protection for patients with autoimmune diseases was unclear, the investigators said. Therefore, they retrospectively studied Medicare data for more than 130,000 such patients between 2006 and 2012. About one-third of patients were vaccinated against herpes zoster, and 47% had rheumatoid arthritis, 32% had psoriasis, 21% had inflammatory bowel disease, 5% had psoriatic arthritis, and 1% had ankylosing spondylitis.
Rates of herpes zoster among vaccinated patients rose from 0.75 per 100 person-years during the first year after vaccination to 1.36 during the sixth year. In the adjusted analysis, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52; 95% confidence interval, 0.45-0.61) and remained substantially less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92; 95% CI, 0.45-1.86). There was no overall change in risk among unvaccinated patients during the study period, the investigators noted.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
* Correction, 11/13/2015: The article previously misstated Dr. Yun's gender.
Protection against shingles appeared to wane between the fifth and sixth years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients, reported Dr. Huifeng Yun, assistant professor of epidemiology at the University of Alabama, Birmingham. Based on the findings, clinicians could consider revaccinating patients with autoimmune diseases about 5 years after their first live herpes zoster vaccine, she said.*
The long-term Shingles Prevention Study recently showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals, but the duration of protection for patients with autoimmune diseases was unclear, the investigators said. Therefore, they retrospectively studied Medicare data for more than 130,000 such patients between 2006 and 2012. About one-third of patients were vaccinated against herpes zoster, and 47% had rheumatoid arthritis, 32% had psoriasis, 21% had inflammatory bowel disease, 5% had psoriatic arthritis, and 1% had ankylosing spondylitis.
Rates of herpes zoster among vaccinated patients rose from 0.75 per 100 person-years during the first year after vaccination to 1.36 during the sixth year. In the adjusted analysis, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52; 95% confidence interval, 0.45-0.61) and remained substantially less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92; 95% CI, 0.45-1.86). There was no overall change in risk among unvaccinated patients during the study period, the investigators noted.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
* Correction, 11/13/2015: The article previously misstated Dr. Yun's gender.
Protection against shingles appeared to wane between the fifth and sixth years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients, reported Dr. Huifeng Yun, assistant professor of epidemiology at the University of Alabama, Birmingham. Based on the findings, clinicians could consider revaccinating patients with autoimmune diseases about 5 years after their first live herpes zoster vaccine, she said.*
The long-term Shingles Prevention Study recently showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals, but the duration of protection for patients with autoimmune diseases was unclear, the investigators said. Therefore, they retrospectively studied Medicare data for more than 130,000 such patients between 2006 and 2012. About one-third of patients were vaccinated against herpes zoster, and 47% had rheumatoid arthritis, 32% had psoriasis, 21% had inflammatory bowel disease, 5% had psoriatic arthritis, and 1% had ankylosing spondylitis.
Rates of herpes zoster among vaccinated patients rose from 0.75 per 100 person-years during the first year after vaccination to 1.36 during the sixth year. In the adjusted analysis, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52; 95% confidence interval, 0.45-0.61) and remained substantially less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92; 95% CI, 0.45-1.86). There was no overall change in risk among unvaccinated patients during the study period, the investigators noted.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
* Correction, 11/13/2015: The article previously misstated Dr. Yun's gender.
FROM THE ACR ANNUAL MEETING
Key clinical point: Protection against shingles appeared to wane between the fifth and sixth years after patients with autoimmune diseases received the live herpes zoster vaccine.
Major finding: The rate of herpes zoster rose from 0.75 per 100 person-years in the first year after vaccination to 1.36 in the sixth year, when it approached the rate among unvaccinated patients.
Data source: Retrospective analysis of 130,107 Medicare patients with autoimmune diseases between 2006 and 2012.
Disclosures: Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
No stone left unturned in ACR 2015 clinical sessions
If you haven’t dealt with a chikungunya patient yet, you probably will soon.
The mosquito-borne virus, long considered a tropical disease, is on the upswing in the United States, carried back especially by vacationers returning from the Caribbean. It causes an inflammatory arthritis that’s hard to recognize and treat.
“We’ve actually even had some here in Seattle,” said Dr. Gregory Gardner, a rheumatologist at the University of Washington, Seattle.
So a session this year at the annual meeting of the American College of Rheumatology, “Coming to a Joint Near You: Chikungunya” on Sunday, Nov. 8, at 8:30 a.m., is probably one that attendees won’t want to miss, said Dr. Gardner, one of the many planners of this year’s meeting.
The session will cover chikungunya immunopathology, clinical manifestations, and treatment – just about all you need to know to handle a case. The chikungunya session is just one example of the meeting’s timely, on-point clinical information. Another one that’s likely to be popular is “Maintenance Therapy in ANCA-Associated Vasculitis: Evaluation and Treatment of Patients in Remission,” on Monday, Nov. 9, at 7:30 a.m., and “The Great Debate: Long-Term, Low-Dose Corticosteroid Use in the Treatment of Rheumatoid Arthritis” on Sunday at 2:30 p.m. While some worry about the long-term side effects of steroids in rheumatoid arthritis, others think the approach is safe and possibly even disease modifying, Dr. Gardner said.
The clinical sessions will be complimented by a full slate of original science. “We’ve had more abstracts submitted this year than to any other previous meeting,” over 4,000. “There’s great research being presented,” said Dr. Victoria Shanmugam, a Washington, D.C., rheumatologist and also a planner of this year’s meeting.
Getting into the late-breaking abstract session on Tuesday, Nov. 10, at 4:30 p.m. “was incredibly competitive this year,” she said. Just a handful of the 70 submissions made it. Investigators who did will share their latest findings on tocilizumab for giant cell arteritis; baricitinib versus placebo or adalimumab for rheumatoid arthritis; adalimumab with methotrexate for uveitis in juvenile idiopathic arthritis; epratuzumab in moderate to severe systemic lupus; tofacitinib in ankylosing spondylitis; and interleukin-17a inhibition in active ankylosing spondylitis.
There’ll also be a strong show of original research in the plenary sessions at 11 a.m. Sunday, Monday, and Tuesday. Studies in Tuesday’s session, for instance, include investigations into the pharmacogenetics of allopurinol in gout and the role of anakinra in recurrent pericarditis.
Results from the Scleroderma Lung Study II, which pitted oral cyclophosphamide against mycophenolate mofetil for interstitial lung disease in systemic sclerosis, will be revealed Sunday at 4:30 p.m. in the “Systemic Sclerosis, Fibrosing Syndromes, and Raynaud’s – Clinical Aspects and Therapeutics I” session. Until now, there haven’t been strong prospective data to support the use of mycophenolate in scleroderma lung disease. “There will be a lot of interest in this. People have been waiting for these results to come out,” Dr. Shanmugam said.
Planners have done something new this year by bringing fertility and pregnancy research into its own dedicated abstract session, “Reproductive Issues in Rheumatic Disorders: Basic and Clinical Aspects,” on Monday at 4:30 p.m.
It’s recognition of the central role those issues play in rheumatology. Fertility and pregnancy are a “major clinical challenge we face in daily practice. Our diseases often present during pregnancy, and we tend to have diseases that affect young women. Patients have a lot of questions,” Dr. Shanmugam said.
The abstract session will be a mix of basic and clinical research across a range of rheumatic conditions. The hope is to break down the silos in rheumatology to foster collaboration and move the field forward, she said.
That session will be complemented by “Reproductive Issues in Rheumatology” on Wednesday at 11:00 a.m. – focusing on biologics in pregnancy and pregnancy outcomes in rheumatoid arthritis – as well as “Pregnancy and Infertility in Rheumatic Disease” on Monday at 2:30 p.m.
Macrophage activation is getting its share of attention, too, especially in adults, for instance during the “Nuts and Bolts of Macrophage Activation Syndrome” session on Monday at 8:30 a.m.
“Historically, it’s mainly been studied in children. We wanted to focus on bringing this into adult rheumatology. I think this is going to be a very good session,” Dr. Shanmugam said.
If you haven’t dealt with a chikungunya patient yet, you probably will soon.
The mosquito-borne virus, long considered a tropical disease, is on the upswing in the United States, carried back especially by vacationers returning from the Caribbean. It causes an inflammatory arthritis that’s hard to recognize and treat.
“We’ve actually even had some here in Seattle,” said Dr. Gregory Gardner, a rheumatologist at the University of Washington, Seattle.
So a session this year at the annual meeting of the American College of Rheumatology, “Coming to a Joint Near You: Chikungunya” on Sunday, Nov. 8, at 8:30 a.m., is probably one that attendees won’t want to miss, said Dr. Gardner, one of the many planners of this year’s meeting.
The session will cover chikungunya immunopathology, clinical manifestations, and treatment – just about all you need to know to handle a case. The chikungunya session is just one example of the meeting’s timely, on-point clinical information. Another one that’s likely to be popular is “Maintenance Therapy in ANCA-Associated Vasculitis: Evaluation and Treatment of Patients in Remission,” on Monday, Nov. 9, at 7:30 a.m., and “The Great Debate: Long-Term, Low-Dose Corticosteroid Use in the Treatment of Rheumatoid Arthritis” on Sunday at 2:30 p.m. While some worry about the long-term side effects of steroids in rheumatoid arthritis, others think the approach is safe and possibly even disease modifying, Dr. Gardner said.
The clinical sessions will be complimented by a full slate of original science. “We’ve had more abstracts submitted this year than to any other previous meeting,” over 4,000. “There’s great research being presented,” said Dr. Victoria Shanmugam, a Washington, D.C., rheumatologist and also a planner of this year’s meeting.
Getting into the late-breaking abstract session on Tuesday, Nov. 10, at 4:30 p.m. “was incredibly competitive this year,” she said. Just a handful of the 70 submissions made it. Investigators who did will share their latest findings on tocilizumab for giant cell arteritis; baricitinib versus placebo or adalimumab for rheumatoid arthritis; adalimumab with methotrexate for uveitis in juvenile idiopathic arthritis; epratuzumab in moderate to severe systemic lupus; tofacitinib in ankylosing spondylitis; and interleukin-17a inhibition in active ankylosing spondylitis.
There’ll also be a strong show of original research in the plenary sessions at 11 a.m. Sunday, Monday, and Tuesday. Studies in Tuesday’s session, for instance, include investigations into the pharmacogenetics of allopurinol in gout and the role of anakinra in recurrent pericarditis.
Results from the Scleroderma Lung Study II, which pitted oral cyclophosphamide against mycophenolate mofetil for interstitial lung disease in systemic sclerosis, will be revealed Sunday at 4:30 p.m. in the “Systemic Sclerosis, Fibrosing Syndromes, and Raynaud’s – Clinical Aspects and Therapeutics I” session. Until now, there haven’t been strong prospective data to support the use of mycophenolate in scleroderma lung disease. “There will be a lot of interest in this. People have been waiting for these results to come out,” Dr. Shanmugam said.
Planners have done something new this year by bringing fertility and pregnancy research into its own dedicated abstract session, “Reproductive Issues in Rheumatic Disorders: Basic and Clinical Aspects,” on Monday at 4:30 p.m.
It’s recognition of the central role those issues play in rheumatology. Fertility and pregnancy are a “major clinical challenge we face in daily practice. Our diseases often present during pregnancy, and we tend to have diseases that affect young women. Patients have a lot of questions,” Dr. Shanmugam said.
The abstract session will be a mix of basic and clinical research across a range of rheumatic conditions. The hope is to break down the silos in rheumatology to foster collaboration and move the field forward, she said.
That session will be complemented by “Reproductive Issues in Rheumatology” on Wednesday at 11:00 a.m. – focusing on biologics in pregnancy and pregnancy outcomes in rheumatoid arthritis – as well as “Pregnancy and Infertility in Rheumatic Disease” on Monday at 2:30 p.m.
Macrophage activation is getting its share of attention, too, especially in adults, for instance during the “Nuts and Bolts of Macrophage Activation Syndrome” session on Monday at 8:30 a.m.
“Historically, it’s mainly been studied in children. We wanted to focus on bringing this into adult rheumatology. I think this is going to be a very good session,” Dr. Shanmugam said.
If you haven’t dealt with a chikungunya patient yet, you probably will soon.
The mosquito-borne virus, long considered a tropical disease, is on the upswing in the United States, carried back especially by vacationers returning from the Caribbean. It causes an inflammatory arthritis that’s hard to recognize and treat.
“We’ve actually even had some here in Seattle,” said Dr. Gregory Gardner, a rheumatologist at the University of Washington, Seattle.
So a session this year at the annual meeting of the American College of Rheumatology, “Coming to a Joint Near You: Chikungunya” on Sunday, Nov. 8, at 8:30 a.m., is probably one that attendees won’t want to miss, said Dr. Gardner, one of the many planners of this year’s meeting.
The session will cover chikungunya immunopathology, clinical manifestations, and treatment – just about all you need to know to handle a case. The chikungunya session is just one example of the meeting’s timely, on-point clinical information. Another one that’s likely to be popular is “Maintenance Therapy in ANCA-Associated Vasculitis: Evaluation and Treatment of Patients in Remission,” on Monday, Nov. 9, at 7:30 a.m., and “The Great Debate: Long-Term, Low-Dose Corticosteroid Use in the Treatment of Rheumatoid Arthritis” on Sunday at 2:30 p.m. While some worry about the long-term side effects of steroids in rheumatoid arthritis, others think the approach is safe and possibly even disease modifying, Dr. Gardner said.
The clinical sessions will be complimented by a full slate of original science. “We’ve had more abstracts submitted this year than to any other previous meeting,” over 4,000. “There’s great research being presented,” said Dr. Victoria Shanmugam, a Washington, D.C., rheumatologist and also a planner of this year’s meeting.
Getting into the late-breaking abstract session on Tuesday, Nov. 10, at 4:30 p.m. “was incredibly competitive this year,” she said. Just a handful of the 70 submissions made it. Investigators who did will share their latest findings on tocilizumab for giant cell arteritis; baricitinib versus placebo or adalimumab for rheumatoid arthritis; adalimumab with methotrexate for uveitis in juvenile idiopathic arthritis; epratuzumab in moderate to severe systemic lupus; tofacitinib in ankylosing spondylitis; and interleukin-17a inhibition in active ankylosing spondylitis.
There’ll also be a strong show of original research in the plenary sessions at 11 a.m. Sunday, Monday, and Tuesday. Studies in Tuesday’s session, for instance, include investigations into the pharmacogenetics of allopurinol in gout and the role of anakinra in recurrent pericarditis.
Results from the Scleroderma Lung Study II, which pitted oral cyclophosphamide against mycophenolate mofetil for interstitial lung disease in systemic sclerosis, will be revealed Sunday at 4:30 p.m. in the “Systemic Sclerosis, Fibrosing Syndromes, and Raynaud’s – Clinical Aspects and Therapeutics I” session. Until now, there haven’t been strong prospective data to support the use of mycophenolate in scleroderma lung disease. “There will be a lot of interest in this. People have been waiting for these results to come out,” Dr. Shanmugam said.
Planners have done something new this year by bringing fertility and pregnancy research into its own dedicated abstract session, “Reproductive Issues in Rheumatic Disorders: Basic and Clinical Aspects,” on Monday at 4:30 p.m.
It’s recognition of the central role those issues play in rheumatology. Fertility and pregnancy are a “major clinical challenge we face in daily practice. Our diseases often present during pregnancy, and we tend to have diseases that affect young women. Patients have a lot of questions,” Dr. Shanmugam said.
The abstract session will be a mix of basic and clinical research across a range of rheumatic conditions. The hope is to break down the silos in rheumatology to foster collaboration and move the field forward, she said.
That session will be complemented by “Reproductive Issues in Rheumatology” on Wednesday at 11:00 a.m. – focusing on biologics in pregnancy and pregnancy outcomes in rheumatoid arthritis – as well as “Pregnancy and Infertility in Rheumatic Disease” on Monday at 2:30 p.m.
Macrophage activation is getting its share of attention, too, especially in adults, for instance during the “Nuts and Bolts of Macrophage Activation Syndrome” session on Monday at 8:30 a.m.
“Historically, it’s mainly been studied in children. We wanted to focus on bringing this into adult rheumatology. I think this is going to be a very good session,” Dr. Shanmugam said.
FROM THE ACR ANNUAL MEETING
Infections from endemic fungi, mycobacteria rare in patients on TNFIs
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The incidence of select mycobacterial and fungal infections in patients taking TNFIs is low.
Major finding: Only 0.51% of patients taking TNFIs developed the mycobacterial and fungal infections targeted in this study.
Data source: A case-control study of 30,772 patients taking TNFIs.
Disclosures: The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
EADV: Comorbid spondyloarthropathy common in hidradenitis suppurativa
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
COPENHAGEN – Back pain is surprisingly common in patients with hidradenitis suppurativa, and more than half of affected patients showed MRI evidence of axial spondyloarthropathy, Dr. Sylke Schneider-Burrus reported at the Annual Congress of the European Academy of Dermatology and Venereology.
“Our study demonstrates that back pain and spondyloarthropathy are very common among hidradenitis suppurativa patients and that neither history nor clinical parameters provide any hints for the presence of spondyloarthropathy. Therefore, we strongly suggest that hidradenitis suppurativa patients should be evaluated for spondyloarthropathy and affected patients should be treated systemically with TNF-alpha blockers in order to avoid chronic joint alterations,” said Dr. Schneider-Burrus, a dermatologist at Charite University Hospital in Berlin.
Hidradenitis suppurativa (HS) is a chronic, recurrent, scarring, inflammatory skin disease of the hair follicles. It causes painful, purulent, foul-smelling fistulating sinuses in the axillae, groin, and perianal region.
Because several other chronic inflammatory diseases affecting epithelial tissue have been associated with increased rates of axial spondyloarthropathy – notably, Crohn’s disease, ulcerative colitis, and psoriasis – Dr. Schneider-Burrus and coinvestigators wondered whether that might true of HS as well.
She presented a survey of 100 HS patients. To her surprise, fully 71% indicated they suffer from back pain, with lower back complaints predominating.
Forty-eight HS patients with back pain consented to undergo a pelvic MRI exam. Fifteen of the 48 (32%) showed clear MRI evidence of spondyloarthropathy, including sacroiliac erosions and subchondral sclerosis, while another 12 showed active sacroiliac synovitis and other acute inflammatory changes.
No significant differences were found between HS patients with and without axial spondyloarthropathy in terms of age at onset of HS, disease duration, HS severity as reflected in Sartorius score, age at MRI, body mass index, or smoking status.
Dr. Schneider-Burrus reported serving as a paid investigator for and consultant to Novartis and AbbVie.
AT THE EADV CONGRESS
Key clinical point: Axial spondyloarthropathy is extremely common in patients with hidradenitis suppurativa.
Major finding: Seventy-one percent of surveyed hidradenitis suppurativa patients reported suffering from back pain, and 56% of affected patients showed MRI evidence of axial spondyloarthropathy.
Data source: A back pain survey of 100 patients with hidradenitis suppurativa along with pelvic MRI exams in the 48 who reported back pain.
Disclosures: The presenter reported serving as a paid investigator for and consultant to Novartis and AbbVie.
New treatment recommendations issued for ankylosing spondylitis, nonradiographic axial SpA
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.
New recommendations for the treatment of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis largely echo each other in many respective clinical circumstances that are addressed for active and stable disease, including strongly advising the use of nonsteroidal anti-inflammatory drugs and physical therapy as initial therapy.
The recommendations, issued by the American College of Rheumatology (ACR), the Spondylitis Association of America, and the Spondyloarthritis Research and Treatment Network, are the same as those outlined at the 2014 ACR annual meeting in advance of their simultaneous publication Sept. 24 in Arthritis & Rheumatology (doi: 10.1002/art.39298) and Arthritis Care & Research (doi: 10.1002/acr.22708).
The main difference in recommendations for the treatment of active ankylosing spondylitis (AS) and nonradiographic axial spondyloarthritis (SpA) is that in patients with active AS despite treatment with NSAIDs, treatment with a tumor necrosis factor inhibitor (TNFi) is strongly advised, whereas for patients with nonradiographic axial SpA, a TNFi is conditionally recommended over no treatment with a TNFi. The authors noted that this treatment question “had the highest level of evidence among those for nonradiographic axial SpA.”
The authors also said that the recommendations are limited in that they “did not examine the full range of treatment alternatives for patients with active peripheral arthritis or enthesitis, advanced options for patients who do not respond to first- and second-level systemic treatments, use of analgesics, or the use of imaging in disease monitoring.” They also did not address the cost-effectiveness of treatment options.
Spondyloarthritides undergoing a renaissance, but challenges exist
Rheumatologists’ understanding of the spectrum of spondyloarthritis is undergoing an evolution that has revealed an underrecognition of the disease, particularly in women, but regulatory recognition of the spectrum of disease in the United States has yet to catch up with reality, according to Dr. Philip Mease.
More sophisticated imaging and laboratory techniques has meant that spondyloarthritis (SpA) is being discovered in up to twice as many people as originally thought, many of whom are women, said Dr. Mease, director of rheumatology research at Swedish Medical Center and clinical professor at the University of Washington, both in Seattle.
It’s not that SpA occurs as frequently in women as in men, but that it presents in a less-severe form with often little visible x-ray damage but with evidence of inflammation on MRI or in lab tests, he said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“These patients can be just as significantly impacted as patients with more ‘objective’ ankylosing spondylitis and can benefit as much from therapy such as anti-TNF [tumor necrosis factor] medications,” he said.
Evidence shows the prevalence of all spondyloarthritides in the United States is 0.346%-1.31%, compared with a rheumatoid arthritis prevalence of 0.6%-1.0%. Data from the German spondyloarthritis inception cohort has shown that the burden of disease in ankylosing spondylitis and nonradiographic axial SpA (nr-axSpA) is similar, Dr. Mease said.
The Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) have gone some way toward identifying patients earlier, he said, but the biggest controversy surrounding the management of nr-axSpA in the United States has been the Food and Drug Administration’s lack of recognition of the disease, making it difficult to treat patients early.
The FDA is concerned about the potential for misdiagnosis and inappropriate use of anti-TNFs for these noninflammatory conditions, he said. “They readily understand that patients who are MRI positive or CRP [C-reactive protein] elevated and x-ray negative exist and are significantly affected by their disease, but they are also aware that there can be similarity of symptomatology between these types of patients and patients with mechanical or degenerative spine disease and/or fibromyalgia.”
As such, there is a need for more information on the natural history of the disease from clinical registries and trials in order to gain more understanding on how to accurately diagnose patients, he said.
More education to U.S. physicians is also needed, he added, noting that this particular controversy is not present in other parts of the world, such as the European Union, where etanercept, adalimumab, and certolizumab pegol have been approved by the European Medicines Agency for the treatment of nr-axSpA.
“There is need for better understanding about this patient population and better education of health-care providers to get them to think about this condition and refer their patients to rheumatologists for evaluation and appropriate therapy,” he added.
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
Rheumatologists’ understanding of the spectrum of spondyloarthritis is undergoing an evolution that has revealed an underrecognition of the disease, particularly in women, but regulatory recognition of the spectrum of disease in the United States has yet to catch up with reality, according to Dr. Philip Mease.
More sophisticated imaging and laboratory techniques has meant that spondyloarthritis (SpA) is being discovered in up to twice as many people as originally thought, many of whom are women, said Dr. Mease, director of rheumatology research at Swedish Medical Center and clinical professor at the University of Washington, both in Seattle.
It’s not that SpA occurs as frequently in women as in men, but that it presents in a less-severe form with often little visible x-ray damage but with evidence of inflammation on MRI or in lab tests, he said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“These patients can be just as significantly impacted as patients with more ‘objective’ ankylosing spondylitis and can benefit as much from therapy such as anti-TNF [tumor necrosis factor] medications,” he said.
Evidence shows the prevalence of all spondyloarthritides in the United States is 0.346%-1.31%, compared with a rheumatoid arthritis prevalence of 0.6%-1.0%. Data from the German spondyloarthritis inception cohort has shown that the burden of disease in ankylosing spondylitis and nonradiographic axial SpA (nr-axSpA) is similar, Dr. Mease said.
The Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) have gone some way toward identifying patients earlier, he said, but the biggest controversy surrounding the management of nr-axSpA in the United States has been the Food and Drug Administration’s lack of recognition of the disease, making it difficult to treat patients early.
The FDA is concerned about the potential for misdiagnosis and inappropriate use of anti-TNFs for these noninflammatory conditions, he said. “They readily understand that patients who are MRI positive or CRP [C-reactive protein] elevated and x-ray negative exist and are significantly affected by their disease, but they are also aware that there can be similarity of symptomatology between these types of patients and patients with mechanical or degenerative spine disease and/or fibromyalgia.”
As such, there is a need for more information on the natural history of the disease from clinical registries and trials in order to gain more understanding on how to accurately diagnose patients, he said.
More education to U.S. physicians is also needed, he added, noting that this particular controversy is not present in other parts of the world, such as the European Union, where etanercept, adalimumab, and certolizumab pegol have been approved by the European Medicines Agency for the treatment of nr-axSpA.
“There is need for better understanding about this patient population and better education of health-care providers to get them to think about this condition and refer their patients to rheumatologists for evaluation and appropriate therapy,” he added.
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
Rheumatologists’ understanding of the spectrum of spondyloarthritis is undergoing an evolution that has revealed an underrecognition of the disease, particularly in women, but regulatory recognition of the spectrum of disease in the United States has yet to catch up with reality, according to Dr. Philip Mease.
More sophisticated imaging and laboratory techniques has meant that spondyloarthritis (SpA) is being discovered in up to twice as many people as originally thought, many of whom are women, said Dr. Mease, director of rheumatology research at Swedish Medical Center and clinical professor at the University of Washington, both in Seattle.
It’s not that SpA occurs as frequently in women as in men, but that it presents in a less-severe form with often little visible x-ray damage but with evidence of inflammation on MRI or in lab tests, he said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
“These patients can be just as significantly impacted as patients with more ‘objective’ ankylosing spondylitis and can benefit as much from therapy such as anti-TNF [tumor necrosis factor] medications,” he said.
Evidence shows the prevalence of all spondyloarthritides in the United States is 0.346%-1.31%, compared with a rheumatoid arthritis prevalence of 0.6%-1.0%. Data from the German spondyloarthritis inception cohort has shown that the burden of disease in ankylosing spondylitis and nonradiographic axial SpA (nr-axSpA) is similar, Dr. Mease said.
The Assessment of SpondyloArthritis Society (ASAS) classification criteria (Ann Rheum Dis. 2009;68:777-83) have gone some way toward identifying patients earlier, he said, but the biggest controversy surrounding the management of nr-axSpA in the United States has been the Food and Drug Administration’s lack of recognition of the disease, making it difficult to treat patients early.
The FDA is concerned about the potential for misdiagnosis and inappropriate use of anti-TNFs for these noninflammatory conditions, he said. “They readily understand that patients who are MRI positive or CRP [C-reactive protein] elevated and x-ray negative exist and are significantly affected by their disease, but they are also aware that there can be similarity of symptomatology between these types of patients and patients with mechanical or degenerative spine disease and/or fibromyalgia.”
As such, there is a need for more information on the natural history of the disease from clinical registries and trials in order to gain more understanding on how to accurately diagnose patients, he said.
More education to U.S. physicians is also needed, he added, noting that this particular controversy is not present in other parts of the world, such as the European Union, where etanercept, adalimumab, and certolizumab pegol have been approved by the European Medicines Agency for the treatment of nr-axSpA.
“There is need for better understanding about this patient population and better education of health-care providers to get them to think about this condition and refer their patients to rheumatologists for evaluation and appropriate therapy,” he added.
Dr. Mease disclosed that he has received research grants, consultation fees, and/or speaker honoraria from AbbVie, Amgen, Biogen, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, and UCB. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES