User login
FDA approves cefiderocol for multidrug-resistant, complicated urinary tract infections
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
The Food and Drug Administration announced that it has approved cefiderocol (Fetroja), an IV antibacterial drug to treat complicated urinary tract infections (cUTIs), including kidney infections, caused by multidrug-resistant gram-negative microorganisms in patients 18 years of age or older.
The safety and effectiveness of cefiderocol was demonstrated in a pivotal study of 448 patients with cUTIs. Published results indicated that 73% of patients had resolution of symptoms and eradication of the bacteria approximately 7 days after completing treatment, compared with 55% in patients who received an alternative antibiotic.
observed in comparison to patients treated with other antibiotics in a trial of critically ill patients having multidrug-resistant gram-negative bacterial infections (clinical trials. gov NCT02714595).
The cause of the increase in mortality has not been determined, according to the FDA. Some of the deaths in the study were attributable to worsening or complications of infection, or underlying comorbidities, in patients treated for hospital-acquired/ventilator-associated pneumonia (i.e., nosocomial pneumonia), bloodstream infections, or sepsis. Thus, safety and efficacy of cefiderocol has not been established for the treating these types of infections, according to the announcement.
Adverse reactions observed in patients treated with cefiderocol included diarrhea, constipation, nausea, vomiting, elevations in liver tests, rash, infusion-site reactions, and candidiasis. The FDA added that cefiderocol should not be used in persons known to have a severe hypersensitivity to beta-lactam antibacterial drugs.
“A key global challenge the FDA faces as a public health agency is addressing the threat of antimicrobial-resistant infections, like cUTIs. This approval represents another step forward in the FDA’s overall efforts to ensure safe and effective antimicrobial drugs are available to patients for treating infections,” John Farley, MD, acting director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research said in the FDA press statement.
Fetroja is a product of Shionogi.
FROM THE FDA
CDC releases update of 2013 Antibiotic Resistance Threats Report
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
“You and I are living in a time when some miracle drugs no longer perform miracles and families are being ripped apart by a microscopic enemy. The time for action is now and we can be part of the solution,” said Robert R. Redfield, MD, director of the Centers for Disease Control and Prevention in his foreword to the new CDC report on antibiotic resistance.
In this update of the previous 2013 report, The current report uses EHRs and other data sources obtained by the CDC for relevant infections extrapolated to develop national disease incidence. The report focuses on “the top 18 pathogens that require attention now,” advises specific steps be taken to address these pathogens, and puts into perspective the future of antibiotic development, their use and abuse, and the continuing threat of antibiotic resistance.
The CDC categorizes these 18 pathogens as either an urgent, serious, or concerning threat.
Urgent Threats
- Carbapenem-resistant Acinetobacter, which cause pneumonia and wound, bloodstream, and urinary tract infections; they tend to affect patients in ICUs. Of particular concern, some Acinetobacter are resistant to nearly all antibiotics, with few new drugs in development (8,500 hospital infections in 2017; 700 deaths).
- Candida auris, a drug-resistant fungus that was first identified in 2009 in Asia and has quickly become a cause of severe infections around the world; it is extremely difficult to eradicate from health care settings. It began spreading in the United States in 2015, with 323 cases reported in 2018 (90% resistant to at least one antifungal, and 30% resistant to at least two antifungals).
- Clostridioides difficile, which can cause life-threatening diarrhea, most often in people who have taken antibiotics for other conditions. It is the most common health care–associated infection, and although decreasing in the health care system, it has not decreased in community settings (223,900 hospital infections in 2017, and 12,800 estimated deaths).
- Carbapenem-resistant Enterobacteriaceae, which most frequently infect patients who require devices such as catheters and those taking long courses of some antibiotics. Of particular concern is the fact that these bacteria contain a transmissible plasmid that can transfer their drug resistance to other pathogens (13,100 hospital infections in 2017, and 1,100 estimated deaths).
- Drug-resistant Neisseria gonorrhoeae, which is a sexually transmitted disease that can result in life-threatening ectopic pregnancy, lead to infertility, and can increase the risk of getting and giving HIV; it can also cause cardiovascular and neurological problems. It is resistant to all but one class of antibiotics, and half of all infections are resistant to at least one antibiotic (550,000 drug-resistant infections yearly).
Serious Threats
- Drug-resistant Campylobacter.
- Drug-resistant Candida.
- Extended spectrum beta-lactamase–producing Enterobacteriaceae.
- Vancomycin-resistant Enterococci.
- Multidrug-resistant Pseudomonas aeruginosa.
- Drug-resistant nontyphoidal Salmonella.
- Drug-resistant Salmonella serotype Typhi.
- Drug-resistant Shigella.
- Methicillin-resistant Staphylococcus aureus (MRSA).
- Drug-resistant Streptococcus pneumoniae.
- Drug-resistant Tuberculosis.
Concerning Threats
These comprise erythromycin-resistant group A Streptococcus and clindamycin-resistant group B Streptococcus.
In addition, the CDC has established a Watch List of three pathogens to be wary of: azole-resistant Aspergillus fumigatus, drug-resistant Mycoplasma genitalium, and drug-resistant Bordetella pertussis.
Because antibiotic resistance is a global phenomenon caused by and affecting everyone, the CDC provided solutions to the problem of antibiotic resistance at every level of society. This “comprehensive and coordinated response implements the U.S. National Action Plan for Combating Antibiotic-Resistant Bacteria” and includes cooperation with the Department of Health and Human Services, Department of Veterans Affairs, Department of Defense, Department of State, and Department of Agriculture, according to the report.
The key components of this response include using data and new technologies to detect and track antibiotic resistance; infection prevention and containment, especially in terms of outbreak response; improving antibiotic use across populations (one successful example being a 16% decrease of outpatient antibiotic prescribing to children during 2011-2017); improvements in the identification and intervention in the environment including water and soil and in sanitation; and a significant investment in vaccines, diagnostics, and novel therapeutics (the CDC provided nearly $110 million to 96 institutions for work in these areas).
The report also details some hope in the development of new antibiotics. As of June 2019, there were 42 new antibiotics in development, including 4 with new drug applications submitted, 17 with the potential to treat serious gram negative bacteria, and 11 that could address the urgent threats of gonorrhea or C. difficile. Overall, a quarter of these new antibiotics represent a novel drug class or use a novel mechanism of action.
Furthermore, 84% of U.S. hospitals report a stewardship program meeting all seven of CDC’s Core Elements of Hospital Antibiotic Stewardship. Proper stewardship is at the core of preventing the development of new antibiotic resistant pathogen strains.
In addition, the CDC noted a 5% overall decline in antibiotic prescribing in outpatient settings during 2011-2016.
“The problem will get worse if we do not act now, but we can make a difference,” according to Dr. Redfield. “Simply, here’s what works. Preventing infections protects everyone. Improving antibiotic use in people and animals slows the threat and helps preserve today’s drugs and those yet to come. Detecting threats and implementing interventions to keep germs from becoming widespread saves lives.”
In response to the release of the report, the AMA issued a supporting statement and cited its collection of educational resources for physicians focused on antibiotic use, resistance, and stewardship.
Similarly, the Society for Healthcare Epidemiology of America (SHEA) stated that hospitals were “a bright spot” in the CDC report and offered tools and resources available to educate and inform health care professionals about best practices in infection prevention and control, as well as antibiotic stewardship.
SOURCE: CDC. Antibiotic Resistance Threats in the United States 2019.
Rifabutin-based triple therapy for H. pylori gets high marks
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
SAN ANTONIO – David Y. Graham, MD, asserted at the annual meeting of the American College of Gastroenterology.
The drug, recently approved as Talicia, is a rifabutin-based triple therapy. Each capsule contains 50 mg of rifabutin, 1,000 mg of amoxicillin, and 40 mg of omeprazole. As in the pivotal phase 3 trial led by Dr. Graham, the approved treatment regimen calls for adults to take four capsules every 8 hours for 14 days.
The impetus for developing the new therapy centers on the growing problem of resistance to long-standard agents for H. pylori eradication, including metronidazole and clarithromycin. The World Health Organization has declared H. pylori eradication to be a high priority for therapeutic development. Rifabutin resistance is rare: In one study, 413 of 414 strains of H. pylori were sensitive to the antibiotic, noted Dr. Graham, professor of medicine at Baylor College of Medicine, Houston.
He presented the results of the pivotal phase 3, double-blind, multicenter, active comparator trial, known as ERADICATE Hp2, in which 455 participants with confirmed H. pylori infection were randomized to a course of the all-in-one-capsule triple drug combo or to dual therapy with four capsules, each containing 1,000 mg of amoxicillin and 40 mg of omeprazole, every 8 hours for 14 days.
The primary endpoint was H. pylori eradication as documented by a negative urea breath test obtained 4-6 weeks after completing 14 days of treatment. The rate was 84% with the rifabutin-based combo, compared with 58% seen with the high-dose dual therapy. Moreover, in a prespecified secondary analysis restricted to the 391 participants who were confirmed to be actually taking their medication as evidenced by a positive blood level measured on day 13, the eradication rates rose to 90% and 65%, respectively.
The antimicrobial resistance rates documented in this study were eye opening: 17% of patients’ strains were resistant to clarithromycin, 44% to metronidazole, and 10.5% to both. Of concern, 6.4% of participants’ strains were amoxicillin resistant.
“For the first time we saw a low level – but a definite level – of amoxicillin resistance. That’s something we had not seen previously,” Dr. Graham said.
No rifabutin resistance was detected before or after treatment.
The side effect profiles of the two treatment regimens were similar. Diarrhea was reported by 9% of participants, headache by 7%, and nausea by 5%. No serious adverse events occurred in the 14-day study.
The efficacy of the rifabutin-based therapy wasn’t affected by metronidazole or clarithromycin resistance.
The ERADICATE Hp2 trial was sponsored by RedHill Biopharma of Tel Aviv. Dr. Graham reported having no financial conflicts.
REPORTING FROM ACG 2019
IDWeek examined hot topics in the clinical treatment of infectious diseases
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
WASHINGTON – The top existential threats to health today are climate change and overpopulation, but third in this list is antimicrobial resistance, according to Helen Boucher, MD, of Tufts Medical Center, Boston. In her talk at an annual scientific meeting on infectious diseases, however, she focused on the last, presenting the hottest developments in the clinical science of treating and identifying disease-causing agents.
In particular, she discussed two of the most important developments in the area of rapid diagnostics: cell-free microbial DNA in plasma and the use of next-generation gene sequencing for determining disease etiology.
Using a meta-genomics test, cell-free microbial DNA can be identified in plasma from more than 1,000 relevant bacteria, DNA viruses, fungi, and parasites. Though importantly, RNA viruses are not detectable using this technology, she added. Although current sampling is of plasma, this might expand to the ability to use urine in the future. She discussed its particular use in sepsis, as outlined in a paper in Nature Microbiology (2019;4[4]:663-74). The researchers examined 350 suspected sepsis patients and they found a 93% sensitivity, compared with reference standards, using this new test. The main issue with the test was a high incidence of false positives.
Another test Dr. Boucher discussed was the use of meta-genomic next-generation sequencing. She referred to a 2019 paper in the New England Journal of Medicine, which discussed the use of clinical meta-genomic next-generation sequencing of cerebrospinal fluid for the diagnosis of meningitis and encephalitis (2019;380[27]:2327-40). Next-generation sequencing identified 13% of patients positive who were missed using standard screening. However, a number of patients were not diagnosed using the new test, showing that this technique was an improvement over current methods, but not 100% successful.
Dr. Boucher stressed the need for “diagnostic stewardship” to identify the correct microbial agent causing disease, allowing for the use of appropriate treatment rather than shotgun approaches to prevent the development of antibiotic resistance. This practice requires collaboration between the clinical laboratory, pharmacists, and infectious disease specialists.
Dr. Boucher then switched to the area of therapeutics, focusing on the introduction of new antibiotics and other innovations in disease treatment methodologies, especially in the field of transplant ID.
“We have new drugs. That is the good news,” with the goals of the 10 x ’20 initiative to develop 10 new systemic antibiotics by 2020, having “been met and then some,” said Dr. Boucher.
“We now have 13 new drugs, systemically available antibiotics, available by August 2019,” she added, discussing several of the new drugs.
In addition, she pointed out several studies that have indicated that shorter courses of antibiotics are better than longer, and that, in many cases, oral therapy is better than intravenous.
In the burgeoning area of transplant ID studies, Dr. Boucher discussed new research showing that vaccinations in transplanted patients can be advised in several instances, though may require higher dosing, and how the use of hepatitis C virus–positive organs for transplant is showing good results and increasing the availability of organs for transplant.
Dr. Boucher has served on data review committees for Actelion and Medtronix and has served as a consultant/advisor for Cerexa, Durata Therapeutics, Merck (adjudication committee), Rib-X, and Wyeth/Pfizer (data safety monitoring committee).
EXPERT ANALYSIS FROM IDWEEK 2019
Oral beta-lactams provide noninferior postdischarge pyelonephritis treatment
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
WASHINGTON – Patients hospitalized for pyelonephritis and discharged after receiving intravenous antibiotic treatment who then received step-down treatment with an oral beta-lactam had 30-day outcomes that were noninferior to patients who received an oral fluoroquinolone or trimethoprim-sulfamethoxazole as their discharge regimen, in a retrospective study of 211 patients managed at either of two U.S. hospitals.
This was the largest comparison reported on oral beta-lactam drugs for postdischarge treatment of pyelonephritis relative to the standard oral agents, fluoroquinolones and trimethoprim-sulfamethoxazole (Bactrim), Athena Hobbs, PharmD, said at an annual scientific meeting on infectious diseases. The superiority of an oral fluoroquinolone or trimethoprim-sulfamethoxazole and inferiority of oral beta-lactam drugs were cited in 2010 guidelines for managing pyelonephritis from the Infectious Diseases Society of America (Clin Infect Dis. 2011 March 1;52 [5]: e103-20).
Although limited as a nonrandomized, retrospective comparison, the finding of at least similar efficacy by beta-lactam agents “opens new treatment options” that avoid issues with drug resistance and adverse effects from treatment with fluoroquinolones or trimethoprim-sulfamethoxazole, Dr. Hobbs said in a video interview. Beta-lactams have already been embraced for this indication by some hospitalists, demonstrated by their use of beta-lactam antibiotics for 122 (58%) of the 211 patients included in the study. Among the 89 patients discharged on a non–beta-lactam, 69 (78%) had fluoroquinolone treatment and the remaining 20 patients went home taking trimethoprim-sulfamethoxazole. The new finding “confirms that we are not doing harm to patients,” with this existing practice of mostly prescribing an oral beta-lactam drug, noted Dr. Hobbs, an infectious diseases pharmacy specialist at Baptist Memorial Hospital in Memphis.
The study included patients aged 18-89 years hospitalized during 2014-2017 for a primary diagnosis of pyelonephritis at Baptist or at a second Hospital in Austin, Tex. The study excluded patients in intensive care, with a urologic abnormality, pregnant women, and patients treated with an intravenous antibiotic other than a beta-lactam for more than 24 hours. The most commonly used intravenous drugs were cefazolin and ceftriaxone. The enrolled patients averaged just over 40 years old, and more than 90% were women.
The study’s primary outcome was the 30-day rate of either hospital readmission or an ED visit for pyelonephritis or a urinary tract infection. This occurred in 4.9% of the patients discharged on an oral course of a beta-lactam drug, and in 5.6% of those discharged on either a fluoroquinolone or trimethoprim-sulfamethoxazole, a difference that was not statistically significant and that met the prespecified criteria for noninferiority, Dr. Hobbs reported. The most commonly prescribed oral beta-lactam was cefuroxime in about half the patients, followed by cephalexin or cefadroxil in about a quarter of patients, and amoxicillin with clavulanate in 19%. The two arms of the study also showed no significant difference in infection recurrences during 90-day follow-up.
The study received no commercial funding. Dr. Hobbs had no relevant disclosures.
REPORTING FROM IDWEEK 2019
Hospital slashes S. aureus vancomycin resistance
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
LJUBLJANA, SLOVENIA – , Johannes Huebner, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
He presented a retrospective analysis of S. aureus isolates obtained from 540 patients at the Dr. von Hauner Children’s Hospital, Munich, from 2002 to 2017. All were either newly identified methicillin-resistant S. aureus (MRSA) or specimens from bacteremic children with invasive MRSA or methicillin-sensitive S. aureus (MSSA). The strains were tested for vancomycin resistance and minimum inhibitory concentration (MIC). The results from the 200 isolates obtained from 2002 to 2009 were then compared to the 340 specimens from 2010 to 2017, when antibiotic stewardship programs rose to the fore at the pediatric hospital.
All samples proved to be vancomycin sensitive. The further good news was there was absolutely no evidence of the worrisome vancomycin MIC creep that has been described at some centers. On the contrary, the MIC was significantly lower in the later samples, at 0.99 mcg/mL, compared with 1.11 mcg/mL in the earlier period. Moreover, the prevalence of heterogeneous glycopeptide-intermediate S. aureus (hGISA) – a phenotype that has been associated with increased rates of treatment failure – improved from 25% in the earlier period to 6% during the later period, reported Dr. Huebner, head of the division of pediatric infectious diseases at the children’s hospital, part of the University of Munich.
Vancomycin MICs weren’t significantly different between the MRSA and MSSA samples.
Based upon this favorable institutional experience, vancomycin remains the first-line treatment for suspected severe gram-positive cocci infections as well as proven infections involving MRSA at Dr. von Hauner Children’s Hospital.
These vancomycin MIC and hGISA data underscore the importance of periodically monitoring local S. aureus antimicrobial susceptibilities, which, as in this case, can differ from the broader global trends. The vancomycin MIC creep issue hadn’t been studied previously in German hospitals, according to Dr. Huebner.
He and his coworkers have published details of the elements of pediatric antibiotic stewardship programs they have found to be most effective (Infection. 2017 Aug;45[4]:493-504) as well as a systematic review of studies on the favorable economic impact of such programs (J Hosp Infect. 2019 Aug;102[4]:369-376).
Dr. Huebner reported having no financial conflicts regarding his study, which was conducted free of commercial support.
REPORTING FROM ESPID 2019
Key clinical point: Staphylococcus aureus vancomycin MIC creep is reversible through dedicated antimicrobial stewardship.
Major finding: The prevalence of hGISA in MRSA and MSSA specimens improved from 25% during 2002-2009 to 6% during 2010-2017 at one German tertiary children’s hospital.
Study details: This was a retrospective single-center analysis of vancomycin resistance trends over time in 540 S. aureus specimens gathered in 2002-2017.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
FDA approves Recarbrio for cUTI, cIAI treatment in adults
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
The Recarbrio is a three-drug combo injection containing imipenem/cilastatin, an antibiotic previously approved by the FDA, and relebactam, a beta-lactamase inhibitor.
The efficacy of Recarbrio was supported by data on the efficacy of imipenem/cilastatin in the treatment of cUTI and cIAI and by in vitro studies and animal models of infection with treatment by relebactam. The safety was assessed in a pair of clinical studies, one that assessed cUTI patients and another that assessed cIAI patients.
The most common adverse events reported were nausea, diarrhea, headache, fever, and increased liver enzymes. Treatment with Recarbrio is not recommended in patients taking ganciclovir, valproic acid, or divalproex sodium because there is an increased risk of seizures, according to the FDA.
“The FDA remains focused on facilitating the development of safe and effective new antibacterial drugs to give patients more options to fight serious infections. It is important that the use of Recarbrio be reserved for situations when there are limited or no alternative antibacterial drugs for treating a patient’s infection,” Ed Cox, MD, MPH, director for the Office of Antimicrobial Products in FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
Study finds increase in dermatologic prescriptions for postsurgical antibiotics
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
WASHINGTON – , according to an analysis presented at the annual meeting of the American Academy of Dermatology.
John Barbieri, MD, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, who presented the results, and colleagues examined data about antibiotic use in dermatology between 2008 and 2016. Overall, antibiotic use decreased during this period, primarily for chronic conditions such as acne and rosacea. On the other hand, antibiotic use associated with surgical visits increased by approximately 70%.
Using data from 2008 to 2016 in the Optum Clinformatics DataMart deidentified commercial claims database, the researchers performed a repeated cross-sectional analysis of oral antibiotic prescriptions associated with encounters for surgical procedures performed by dermatologists. They found that oral antibiotic prescriptions increased from 2.9% to 4.4% of visits for benign excisions, from 4.2% to 6.3% of visits for malignant excisions, and from 9.9% to 13.8% of visits for Mohs procedures during this time period. Oral antibiotic prescribing was more common for procedures that entailed a flap or graft and among patients with diabetes, female patients, and younger patients.
The investigators observed greater than twofold variation in antibiotic-prescribing rates across geographic census divisions. “If higher-prescribing divisions were to develop antibiotic prescribing rates similar to [those of] lower-prescribing divisions, antibiotic use could be decreased by over 50%,” Dr. Barbieri said. Before prescribing antibiotic prophylaxis, dermatologists should consider which patients benefit most from it, he added.
The investigators also examined the duration of antibiotic courses. The median course duration of postoperative antibiotics was 7 days. Randomized, controlled trials that collectively included more than 600 patients have failed to demonstrate a benefit of long postoperative courses of antibiotics, compared with perioperative antibiotics alone, said Dr. Barbieri. “While it may be hard to have true perioperative antibiotics available in a dermatology surgical clinic, there likely are opportunities to reduce these postoperative courses to a day or 3 days from this mean of 7 days that we observed in the study.” Reducing these courses would decrease the risk of antibiotic-associated complications such as nausea, diarrhea, and skin rashes, he added.
SOURCE: AAD 2019, Abstract 11356.
REPORTING FROM AAD 2019
Candida auris: Dangerous and here to stay
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.
This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
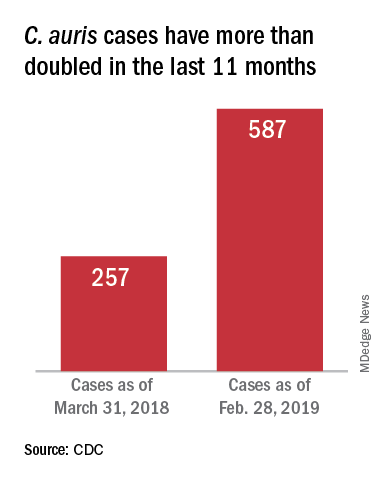
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”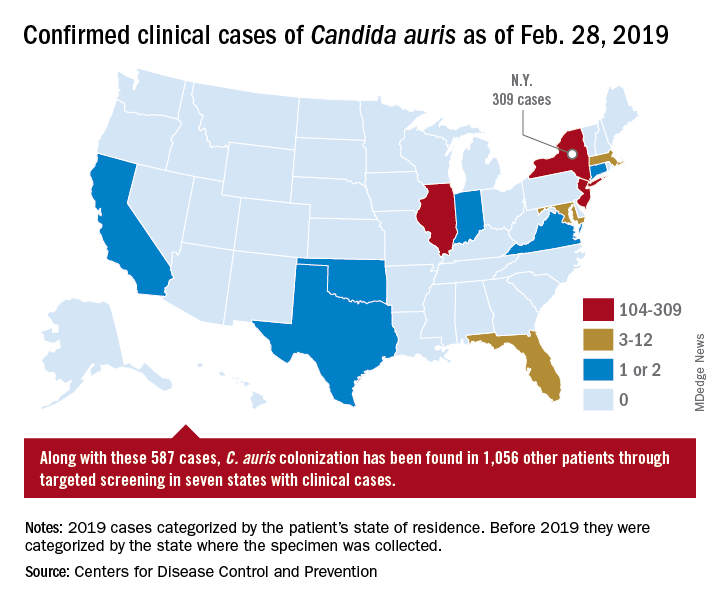
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.

This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Critical care units and long-term care facilities are on alert for cases of Candida auris, a novel fungal infection that is both dangerous to vulnerable patients and difficult to eradicate. The increased profile of C. auris is not a welcome development but is no surprise to critical care physicians.

This pathogen was first identified in 2009 and has since been found in increasing numbers of patients all over the world. As expected, cases of C. auris are on the rise in the United States.
The Centers for Disease Control and Prevention stated “Candida auris is an emerging fungus that presents a serious global health threat.” This is an opportunistic pathogen that hits critically ill patients and those with compromised immunity.
On March 29, 2019, CDC reported that confirmed clinical cases of C. auris in the United States have more than doubled over the past year, from 257 cases in 2018 to 587 cases with an additional 1,056 colonized patients identified as of February 2019. “Most C. auris cases in the United States have been detected in the New York City area, New Jersey, and the Chicago area. Strains of C. auris in the United States have been linked to other parts of the world. U.S. C. auris cases are a result of inadvertent introduction into the United States from a patient who recently received health care in a country where C. auris has been reported or a result of local spread after such an introduction.”
Case reports have found a mortality rate of up to 50% in patients with C. auris candidemia. The total number of cases is still small, but the trajectory is clear. The hunt is on in labs all over the world for optimal treatments and processes to handle outbreaks.
Jeniel Nett, MD, an infectious disease specialist, and a team of investigators at the University of Wisconsin, Madison, have focused their research on the characteristics of C. auris and its progression in patients and in medical facilities.
According to Dr. Nett, it’s not clear why this emerging threat has cropped up in multiple locations globally. “Candida auris was first recognized in 2009, in Japan, and relatively quickly we saw emergence of this species in relatively distant locations,” she said, adding that independent clades in these locations ruled out transmission as the source of the multiple outbreaks. Antifungal resistance is an epidemiologic area of concern and increased antifungal use may be a contributor, she said.

Once established, the organism is persistent: “It is found on mattresses, on bedsheets, IV poles, and a lot of reusable equipment,” said Dr. Nett in an interview. “It appears to persist in the environment for weeks – maybe longer.” In addition, “it seems to behave differently than a lot of the Candida species that we see; it readily colonizes the skin” to a much greater extent than does other Candida species, she said. “This allows it to be transmitted readily person to person, particularly in the hospitalized setting.” However, it can also colonize both the urinary and respiratory tracts, she said.
Which patients are susceptible to C. auris candidemia? “Many of these patients have undergone multiple procedures; they may have undergone mechanical ventilation as well as different surgical procedures,” said Dr. Nett. Affected patients often have received many rounds of antibiotic and antifungal treatment as well, she said, and may have an underlying illness like diabetes or malignancy.
Studies of C. auris outbreaks have begun to appear in the literature and give clinicians some perspective on the progression of an outbreak and potential strategies for containment. A prospective cohort study of a large outbreak of C. auris was conducted by Alba Ruiz-Gaitán, MD, and her colleagues at La Fe University and Polytechnic Hospital, Valencia, Spain (Expert Rev Anti Infect Ther. 2019 Apr;17[4]:295-305). The researchers followed 114 patients who were colonized with C. auris or had C. auris candidemia. The patients were compared with 114 case-matched controls within the hospital’s adult surgical and medical intensive care units over an 11-month period during the hospital’s protracted outbreak.
The investigators found a crude mortality rate of 58.5% at 30 days for patients with C. auris candidemia. All isolates in the study were completely resistant to fluconazole and had reduced susceptibility to voriconazole.
In critical care units at Hospital La Fe, investigators found C. auris on 25% of blood pressure cuffs, 10% of patient tables and keyboards, and 8% of infusion pumps.
Among the patients at Hospital La Fe, multivariable analysis revealed that those most likely to develop C. auris colonization or candidemia were individuals with polytrauma, cardiovascular disease, and cancer.
Patients receiving parenteral nutrition (odds ratio, 3.49), mechanical ventilation (OR, 2.43), and especially those having indwelling central venous catheters (OR, 13.48) were more likely to be colonized or have candidemia as well, according to Dr. Ruiz-Gaitán and her coauthors.
Once identified, how should C. auris be treated? “The majority of strains – upward of 90% – are resistant to fluconazole,” said Dr. Nett. “Moreover, 30%-50% of them are resistant to another antifungal, often amphotericin B. The isolates that we see in the United States are most often susceptible to an echinocandin, and echinocandins remain the choice for treatment of Candida auris pending susceptibility tests.”
However, in Valencia, “The susceptibility to echinocandins presented interesting features. These antifungals were not fungicidal against C. auris,” wrote Dr. Ruiz-Gaitán and her colleagues. They found that for caspofungin, “most isolates presented a clear paradoxical growth after 24 hours of incubation.” Additionally, fungal growth was inhibited at lower caspofungin concentrations, but rebounded at higher levels. Similar patterns were seen for anidulafungin and micafungin, they said.
These findings meant that Hospital La Fe patients received initial treatment with echinocandins, with the addition of liposomal amphotericin B or isavuconazole if candidemia persisted or clinical response was not seen, wrote the investigators.
Patient presentation is similar to other forms of candidiasis, said Dr. Nett. “Patients often have fever, chills, leukocytosis, and this persists despite antibacterial therapy… If Candida auris is suspected, the first course of action would be to place the patient in isolation, and laboratory staff should be alerted regarding the diagnosis.”
Most large clinical laboratories, she said, can now detect C. auris. Matrix-assisted laser desorption/ionization–time of flight is the identification technique of choice, provided that the databases are updated.
Smaller laboratories that use phenotypic tests may misidentify C. auris as another Candida species, or even as Saccharomyces cerevisiae – common beer yeast. Facilities without matrix-assisted laser desorption/ionization can find guidance for interpretation of phenotypic testing on the CDC website as well, said Dr. Nett.
After experiencing what they believe to be the largest C. auris outbreak at a single European hospital, Dr. Ruiz-Gaitán and her colleagues offered best-practice tips for treatment of patients with C. auris candidemia. These include removing mechanical devices as early as is safely practical; performing ophthalmologic examinations for endophthalmitis, a known C. auris complication; obtaining blood cultures every other day to track antimicrobial therapy to the point of sterilization; and searching for metastatic foci if blood cultures remain positive.
All instances of C. auris laboratory identification should be reported to the CDC at [email protected], and to local and state health agencies. The CDC recommends strict isolation and cleaning protocols, similar to those used for the spore-forming Clostridium difficile.
Dr. Nett reported funding support from the National Institutes of Health, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. She reported no conflicts of interest. Dr. Ruiz-Gaitán and her collaborators reported funding from Instituto de Salud Carlos III, Spain, and the Spanish Ministry of Science and University. They reported no conflicts of interest.
Anti-infective update addresses SSSI choices
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
ORLANDO – What’s new in infectious disease therapeutics for dermatologists? He ran through an array of updates at the Orlando Dermatology Aesthetic and Clinical Conference.
While naturally occurring smallpox was globally eradicated in 1980, small research stores are held in the United States and Russia, and effective antivirals are part of a strategy to combat bioweapons. Tecovirimat (TPOXX) is an antiviral that inhibits a major envelope protein that poxviruses need to produce extracellular virus. Approved by the Food and Drug Administration in mid-2018, it is currently the only antiviral for treating variola virus infection approved in the United States, noted Dr. Finch of the University of Connecticut, Farmington. He added that 2 million doses are currently held in the U.S. Strategic National Stockpile.
Another anti-infective agent that won’t be used by those practicing in the United States, but which promises to alleviate a significant source of suffering in the developing world, is moxidectin. The anthelmintic had previously been approved for veterinary uses, but in June 2018, the FDA approved moxidectin to treat onchocerciasis, also known as river blindness. The drug defeats the parasitic worm by binding to glutamate-gated chloride ion channels; it is licensed by the nonprofit Medicines Development for Global Health.
Another antiparasitic drug, benznidazole, was approved to treat children aged 2-12 years with Chagas disease in 2017, Dr. Finch said.
Also in 2017, a topical quinolone, ozenoxacin (Xepi) was approved to treat impetigo in adults and children aged at least 2 months. Formulated as a 1% cream, ozenoxacin is applied twice daily for 5 days. In clinical trials, ozenoxacin was shown to be noninferior to retapamulin, he said.
A new topical choice is important as mupirocin resistance climbs, Dr. Finch added. A recent Greek study showed that 20% (437) of 2,137 staph infections studied were mupirocin resistant. Of the 20%, all but one were skin and skin structure infections (SSSIs), with 88% of these being impetigo.
In the United States, mupirocin resistance has been seen in one in three outpatients in a Florida study and in 31% of patients in a New York City sample. Other studies have shown mupirocin resistance in Staphylococcus aureus isolates with resistance in the 10%-15% range among children with SSSIs, Dr. Finch said.
Two other new antibiotics to fight SSSIs can each be administered orally or intravenously. One, omadacycline (Nuzyra), is a novel tetracycline that maintains efficacy against bacteria that express tetracycline resistance through efflux and ribosomal protection. Approved in late 2018 for acute bacterial SSSIs, omadacycline treats not just methicillin-sensitive and methicillin-resistant S. aureus, but also Streptococcus species and gram-negative rods such as Enterobacter and Klebsiella pneumoniae, Dr. Finch noted.
Another new fluorinated quinolone, approved in 2017, delafloxacin (Baxdela) has broad spectrum activity against gram-negative and gram-positive bacteria.
Dr. Finch reported that he has no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2019






