User login
Will you have cardiac arrest? New tech may predict if and when
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Deaths from COVID-19 may have caught more attention lately, but heart disease remains the leading cause of death in the United States.
More than 300,000 Americans will die this year of sudden cardiac arrest (also called sudden cardiac death, or SCD), when the heart abruptly stops working.
These events happen suddenly and often without warning, making them nearly impossible to predict. But that may be changing, thanks to 3D imaging and artificial intelligence (AI) technology under study at Johns Hopkins University, Baltimore.
There, researchers are working to create more accurate and personalized models of the heart – and not just any heart, your heart, if you have heart disease.
“Right now, a clinician can only say whether a patient is at risk or not at risk for sudden death,” says Dan Popescu, PhD, a Johns Hopkins research scientist and first author of a new study on AI’s ability to predict sudden cardiac arrest. “With this new technology, you can have much more nuanced predictions of probability of an event over time.”
Put another way: With AI, clinicians may be able not only to predict if someone is at risk for sudden cardiac arrest, but also when it is most likely to happen. They can do this using a much clearer and more personalized look at the electrical “wiring” of your heart.
Your heart, the conductor
Your heart isn’t just a metronome responsible for keeping a steady stream of blood pumping to tissues with every beat. It’s also a conductor through which vital energy flows.
To make the heart beat, electrical impulses flow from the top to the bottom of the organ. Healthy heart cells relay this electricity seamlessly. But in a heart damaged by inflammation or a past heart attack, scar tissue will block the energy flow.
When an electrical impulse encounters a scarred area, the signal can become erratic, disrupting the set top-to-bottom path and causing irregular heartbeats (arrhythmias), which increase someone’s danger of sudden cardiac death.
Seeing the heart in 3D
Today’s tests offer some insights into the heart’s makeup. For example, MRI scans can reveal damaged areas. PET scans can show inflammation. And EKGs can record the heart’s electrical signals from beat to beat.
But all these technologies offer only a snapshot, showing heart health at a moment in time. They can’t predict the future. That’s why scientists at Johns Hopkins are going further to develop 3D digital replicas of a person’s heart, known as computational heart models.
Computational models are computer-simulated replicas that combine mathematics, physics, and computer science. These models have been around for a long time and are used in many fields, ranging from manufacturing to economics.
In heart medicine, these models are populated with digital “cells,” which imitate living cells and can be programmed with different electrical properties, depending on whether they are healthy or diseased.
“Currently available imaging and testing (MRIs, PETs, EKGs) give some representation of the scarring, but you cannot translate that to what is going to happen over time,” says Natalia Trayanova, PhD, of the Johns Hopkins department of biomedical engineering.
“With computational heart models, we create a dynamic digital image of the heart. We can then give the digital image an electrical stimulus and assess how the heart is able to respond. Then you can better predict what is going to happen.”
The computerized 3D models also mean better, more accurate treatment for heart conditions.
For example, a common treatment for a type of arrhythmia known as atrial fibrillation is ablation, or burning some heart tissue. Ablation stops the erratic electrical impulses causing the arrhythmia, but it can also damage otherwise healthy heart cells.
A personalized computational heart model could allow doctors to see more accurately what areas should and shouldn’t be treated for a specific patient.
Using deep learning AI to predict health outcomes
Dr. Trayanova’s colleague Dr. Popescu is applying deep learning and AI to do more with computerized heart models to predict the future.
In a recent paper in Nature Cardiovascular Research, the research team showed their algorithm assessed the health of 269 patients and was able to predict the chance of sudden cardiac arrest up to 10 years in advance.
“This is really the first time ever, as far as we know, where deep learning technology has been proven to analyze scarring of the heart in a successful way,” Dr. Popescu says.
Dr. Popescu and Dr. Trayanova say the AI algorithm gathers information from the 3D computational heart models with patient data like MRIs, ethnicity, age, lifestyle, and other clinical information. Analyzing all these data can produce accurate and consistent estimates about how long patients might live if they are at risk for sudden death.
“You can’t afford to be wrong. If you are wrong, you can actually impact a patient’s quality of life dramatically,” Dr. Popescu says. “Having clinicians use this technology in the decision-making process will provide confidence in a better diagnosis and prognosis.”
While the current study was specifically about patients with a particular type of heart disease, Dr. Popescu says his algorithm can also be trained to assess other health conditions.
So when might you see this being used outside of a research study? Dr. Trayanova predicts 3D imaging of heart models could be available in 2 years, but first the technique must be tested in more clinical trials – some of which are happening right now.
Adding AI to the heart models will require more studies and Food and Drug Administration approval, so the timeline is less clear. But perhaps the biggest hurdle is that after approval the technologies would need to be adopted and used by clinicians and caregivers.
“The much harder question to answer is, ‘When will doctors be perfectly comfortable with AI tools?’ And I don’t know the answer,” Dr. Popescu says. “How to use AI as an aid in the decision-making process is something that’s not currently taught.”
A version of this article first appeared on WebMD.com.
Study points to causal role for Lp(a) in atrial fibrillation
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
Although lipoprotein(a) is causally related to coronary artery disease and aortic valve stenosis – two known risk factors for atrial fibrillation (AFib) – evidence linking Lp(a) to a causal role in the development of AFib has been lukewarm at best.
A recent Mendelian randomization study showed only a nominally significant effect of Lp(a) on AFib, whereas an ARIC substudy showed high levels of Lp(a) to be associated with elevated ischemic stroke risk but not incident AFib.
A new study that adds the heft of Mendelian randomization to large observational and genetic analyses, however, implicates Lp(a) as a potential causal mediator of AFib, independent of its known effects on atherosclerotic cardiovascular disease (ASCVD).
“Why this is exciting is because it shows that Lp(a) has effects beyond the arteries and beyond the aortic valve, and that provides two things,” senior author Guillaume Paré, MD, MSc, Population Health Research Institute, Hamilton, Ontario, told this news organization.
“First, it provides a potential means to decrease the risk, because there are all these Lp(a) inhibitors in development,” he said. “But I think the other thing is that it just points to a new pathway that leads to atrial fibrillation development that could potentially be targeted with other drugs when it’s better understood. We don’t pretend that we understand the biology there, but it opens this possibility.”
The results were published in the Journal of the American College of Cardiology.
Using data from 435,579 participants in the UK Biobank, the researchers identified 20,432 cases of incident AFib over a median of 11 years of follow-up. They also constructed a genetic risk score for Lp(a) using genetic variants within 500 kb of the LPA gene.
After common AFib risk factors were controlled for, results showed a 3% increased risk for incident AFib per 50 nmol/L increase in Lp(a) at enrollment (hazard ratio, 1.03; 95% confidence interval, 1.02-1.05).
A Mendelian randomization analysis showed a similar association between genetically predicted Lp(a) and AFib (odds ratio, 1.03; 95% CI, 1.02-1.05).
To replicate the results, the investigators performed separate Mendelian randomization analyses using publicly available genome-wide association study (GWAS) statistics from the largest GWAS of AFib involving more than 1 million participants and from the FinnGen cohort involving more than 114,000 Finnish residents.
The analyses showed a 3% increase in risk for AFib in the genome-wide study (OR, 1.03; 95% CI, 1.02-1.05) and an 8% increase in risk in the Finnish study (OR, 1.08; 95% CI, 1.04-1.12) per 50 nmol/L increase in Lp(a).
There was no evidence that the effect of observed or genetically predicted Lp(a) was modified by prevalent ischemic heart disease or aortic stenosis.
Further, MR analyses revealed no risk effect of low-density-lipoprotein cholesterol or triglycerides on AFib.
Notably, only 39% of Lp(a) was mediated through ASCVD, suggesting that Lp(a) partly influences AFib independent of its known effect on ASCVD.
“To me, the eureka moment is when we repeated the same analysis for LDL cholesterol and it had absolutely no association with AFib,” Dr. Paré said. “Because up to that point, there was always this lingering doubt that, well, it’s because of coronary artery disease, and that’s logical. But the signal is completely flat with LDL, and we see this strong signal with Lp(a).”
Another ‘red flag’
Erin D. Michos, MD, MHS, senior author of the ARIC substudy and associate director of preventive cardiology at Johns Hopkins School of Medicine, Baltimore, said the findings are “another red flag that lipoprotein(a) is a marker we need to pay attention to and potentially needs treatment.”
“The fact that it was Mendelian randomization does suggest that there’s a causal role,” she said. “I think the relationship is relatively modest compared to its known risk for stroke, ASCVD, coronary disease, and aortic stenosis, ... which may be why we didn’t see it in the ARIC cohort with 12,000 participants. You needed to have a million participants and 60,000 cases to see an effect here.”
Dr. Michos said she hopes the findings encourage increased testing, particularly with multiple potential treatments currently in the pipeline. She pointed out that the researchers estimated that the experimental antisense agent pelacarsen, which lowers Lp(a) by about 80%, would translate into about an 8% reduction in AFib risk, or “the same effect as 2 kg of weight loss or a 5 mm Hg reduction in blood pressure, which we do think are meaningful.”
Adding to this point in an accompanying editorial, Daniel Seung Kim, MD, PhD, and Abha Khandelwal, MD, MS, Stanford University School of Medicine, California, say that “moreover, reduction of Lp(a) levels would have multifactorial effects on CAD, cerebrovascular/peripheral artery disease, and AS risk.
“Therefore, approaches to reduce Lp(a) should be prioritized to further reduce the morbidity and mortality of a rapidly aging population,” they write.
The editorialists also join the researchers in calling for inclusion of AFib as a secondary outcome in ongoing Lp(a) trials, in addition to cerebrovascular disease and peripheral vascular disease.
Unanswered questions
As to what’s driving the risk effect of Lp(a), first author Pedrum Mohammadi-Shemirani, PhD, also from the Population Health Research Institute, explained that in aortic stenosis, “mechanical stress increases endothelial permeability, allowing Lp(a) to infiltrate valvular tissue and induce gene expression that results in microcalcifications and cell death.”
“So, in theory, a similar sort of mechanism could be at play in atrial tissue that may lead to damage and the electrical remodeling that causes atrial fibrillation,” he told this news organization.
Dr. Mohammadi-Shemirani also noted that Lp(a) has proinflammatory properties, but added that any potential mechanisms are “speculative and require further research to disentangle.”
Dr. Paré and colleagues say follow-up studies are also warranted, noting that generalizability of the results may be limited because AFib cases were found using electronic health records in the population-scale cohorts and because few UK Biobank participants were of non-European ancestry and Lp(a) levels vary among ethnic groups.
Another limitation is that the number of kringle IV type 2 domain repeats within the LPA gene, the largest contributor to genetic variation in Lp(a), could not be directly measured. Still, 71.4% of the variation in Lp(a) was explained using the genetic risk score alone, they say.
Dr. Paré holds the Canada Research Chair in Genetic and Molecular Epidemiology and Cisco Systems Professorship in Integrated Health Biosystems. Dr. Mohammadi-Shemirani is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institute of Health Research. Dr. Michos reports consulting for Novartis and serving on advisory boards for Novartis, AstraZeneca, Bayer, and Boehringer Ingelheim. Dr. Kim reports grant support from the National Institutes of Health and the American Heart Association. Dr. Khandelwal serves on the advisory board of Amgen and has received funding from Novartis CTQJ and Akcea.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
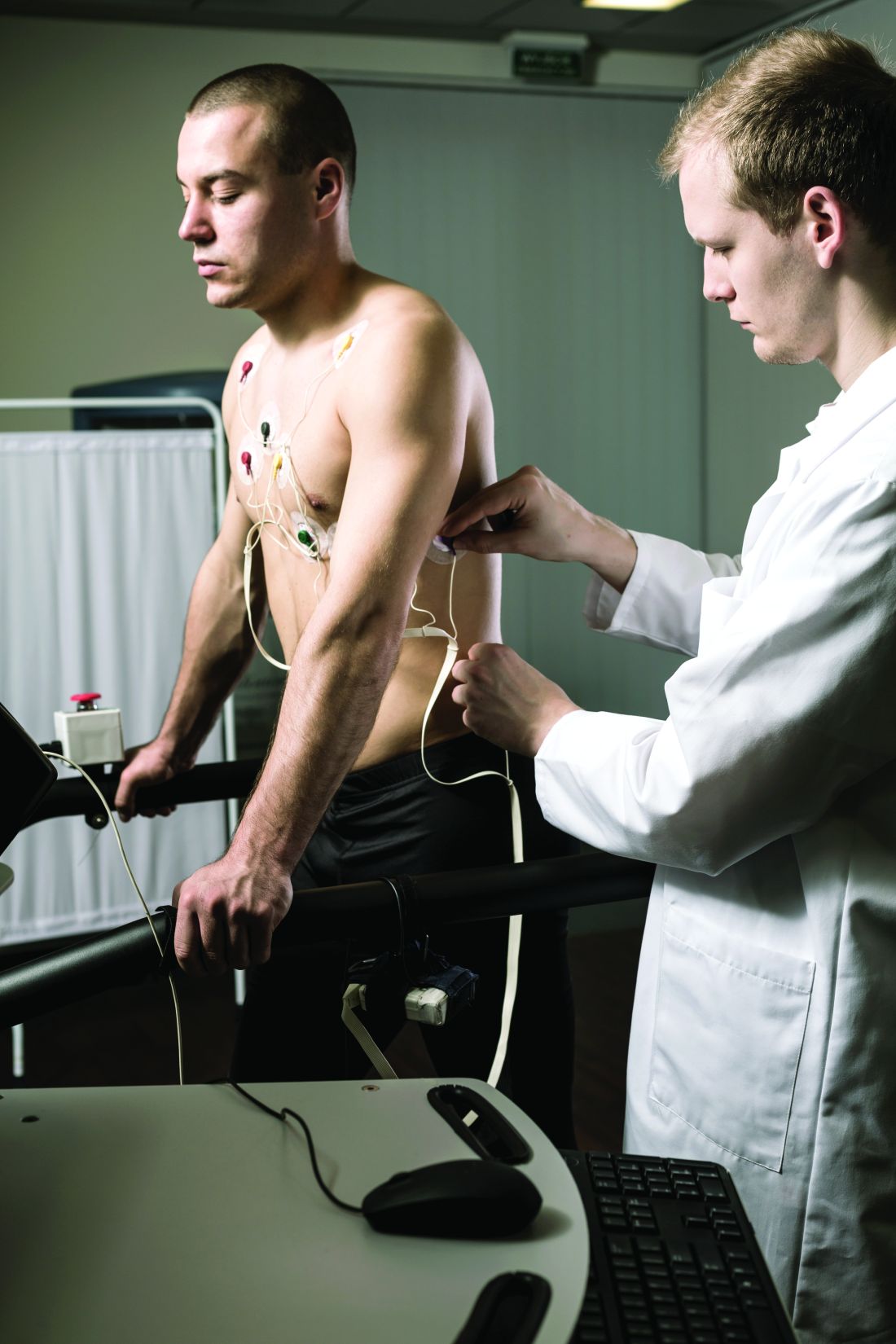
Young and older athletes show similar arrhythmia patterns with fQRS
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
The prevalence of exercise-induced arrhythmias in young athletes with fragmented QRS (fQRS) patterns in lead V1 was 27%, similar to that seen in adult athletes, based on data from nearly 700 individuals.
Recent data suggest that fQRS complex in lead V1 (fQRSV1) in healthy athletes may promote arrhythmias in the context of training-induced right ventricular remodeling, but the prevalence and significance in young athletes has not been well studied, Guilia Quinto, MD, of the University of Padova (Italy) said in a presentation at the annual congress of the European Association of Preventive Cardiology.
Dr. Quinto and colleagues assessed data from of young athletes on ventricular arrhythmias during exercise tests.
The study population included 684 young athletes with a mean age of 15 years; 64% were male. Baseline data collection included medical history, physical exam, resting ECG, standardized maximum exercise tolerance, and echocardiography evaluation.
The overall prevalence of fQRSV1 was 27%. Individuals with fQRSV1 were significantly less likely than those without fQRSV1 to be female (22% vs. 43%), and to present with a lower resting heart rate (66.98 beats per minute vs. 70.08 beats per minute).
Echocardiographic data showed that individuals with fQRSV1 had significantly different morphological and functional right ventricular characteristics.
Notably, right ventricular end-diastolic diameter was 20.42 mm/m2 among individuals with fQRSV1 and 19.81 mm/m2 in those without, a significant difference (P = .019), Dr. Quinto said. Tricuspid annulus plain systolic excursion also differed significantly; 24.33 mm and 23.75 mm for individuals with and without fQRSV1, respectively (P = .013).
However, the individuals with fQRSV1 showed no increased occurrence of any type of exercise-induced arrhythmias regardless of morphology or complexity, said Dr. Quinto.
The prevalence of common and uncommon arrhythmias among individuals with and without fQRSV1 was 31% versus 34% and 13% versus 11%, respectively; these differences were not significant.
The study findings were limited by the relatively small size, but were strengthened by the review of echocardiographic data by two independent physicians, she said.
The results show that the overall prevalence of fQRSV1 in young athletes is comparable with patterns seen in studies of adult athletes, and no differences in exercise-induced arrhythmias occurred despite differences in right ventricular characteristics, she concluded.
Expanded insight into evaluation
The ECG pattern identified in the current study is often encountered in the evaluation of athletes, but its importance was unknown, Matthew Martinez, MD, a sports cardiologist at the Atlantic Health System in Morristown, N.J., said in an interview.
“Studies of ECG findings in athletes continues to inform us about which findings are important to evaluate. This study furthers our understanding of how to proceed,” and will serve as a guide for additional testing to reduce athlete risk, he said.
Looking ahead, “this study should guide clinicians about additional testing and evaluation when fQRS is present in adolescent athletes compared to adults,” Dr. Martinez noted. However, additional research is needed to determine which is the next best test, and whether the patient requires ongoing surveillance, or whether a single evaluation is sufficient, he said. “Further study should focus on best practices after fQRS is identified and whether outcomes can be linked to this finding.”
The study received no outside funding. Dr. Quinto and Dr. Martinez had no financial conflicts to disclose.
FROM ESC PREVENTIVE CARDIOLOGY 2022
New smart device shows highly accurate AFib detection: mAFA II
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
Screening for heart rhythm disorders with a smartphone app and a wearable device had a high rate of correctly detecting atrial fibrillation (AFib) in a large new study.
The mAFA II study, conducted in a mass low-risk population in China, showed that more than 93% of possible AFib episodes detected by the smartphone app were confirmed to be AFib on further monitoring.
The study also used the app to screen for obstructive sleep apnea and found that sleep apnea was the most common risk factor associated with increased AFib susceptibility, and those identified as having the most severe sleep apnea were 1.5 times more likely to have AFib than those who did not have this condition.
This suggests that tools suitable for detecting both AFib and sleep apnea can work synergistically to further enhance health monitoring, said lead author, Yutao Guo, MD, professor of internal medicine at Chinese PLA General Hospital, Beijing.
Dr. Guo presented the mAFA II study at the American College of Cardiology (ACC) 2022 Scientific Session held in Washington, D.C., and online.
The trial, which involved more than 2.8 million participants, is the largest study to date to demonstrate how wearable consumer technologies can be used to screen for heart problems during everyday activities, Dr. Guo noted.
“Consumer-led screening with these technologies could increase early diagnosis of AFib and facilitate an integrated approach to fully implement clustered risk management to reduce AFib burden and its related complications,” she concluded.
Discussant of the study at the ACC session at which it was presented, Jodie Hurwitz, MD, Director of the Electrophysiology Lab at Medical City Hospital, Dallas, called this “a pretty impressive study. To get a 93.8% confirmation of AFib with these devices is great.”
But Dr. Hurwitz pointed out that the age of patients in the study was relatively young (average 37 years), and the group who really need to use such a device is much older than that.
“The take-home messages from this study are that AFib wearable detection algorithms have the ability to detect true AFib and that they might also be able to detect risk factors (such as sleep apnea) that predispose to AFib possibly even before AFib is present,” Dr. Hurwitz commented.
Moderator of the session, Edward Fry, MD, cardiologist at Ascension St. Vincent Heart Center, Indianapolis, and incoming president of the ACC, described the area of AFib screening with smart devices as “fascinating, especially with the perspective of the scalability of these types of studies.”
The mAFA II study tracked more than 2.8 million people who used a Huawei phone app together with Huawei and Honor smart devices incorporating photoplethysmography (PPG) technology, a light-based method to monitor blood flow and pulse. If an abnormal rhythm was detected, the wearer would be contacted by a clinician to set up an appointment for a clinical assessment.
Over the course of 4 years of the study, 12,244 (0.4%) of users received a notification of suspected AFib. Among 5,227 people who chose to follow up with a clinician, AFib was confirmed in 93.8% of patients using standard AFib diagnostic tools, including clinical evaluation, an electrocardiogram, and 24-hour Holter monitoring.
In this study, a subset of the individuals screened for AFib were also screened for signs of sleep apnea using the same PPG technology to detect physiological changes in parameters including oxygenation and respiratory rates. The app is also able to determine whether the individual is awake or asleep. Dr. Guo noted that the PPG algorithm for obstructive sleep apnea risk has been validated, compared with polysomnography or home sleep apnea tests.
Using measurements of apnea (signalled by a reduced respiratory rate) and hypopnea (when oxygenation would decrease), the apnea–hypopnea index (AHI) is calculated to determine the severity of the sleep apnea.
Of the 961,931 participants screened for sleep apnea, about 18,000 were notified they may have the condition.
Obstructive sleep apnea was the most reported common risk factor associated with increased AFib susceptibility, and those individuals with the highest risk sleep apnea (more than 80% monitoring measures with AHI greater than or equal to 30 during sleep) resulted in a 1.5-fold increase in prevalent AFib, Dr. Guo reported.
The mAFA II is the latest of several studies to show that AFib can be detected with various smartphone apps and wearable devices. Previous studies have included the Fitbit Heart Study and the Apple Heart Study.
Dr. Hurwitz told this news organization that the electrophysiologist community is enthusiastic about this new smart device technology.
“I sent my sister one so she could determine if she develops AFib: That’s a pretty good endorsement,” she commented, but added that there are still concerns about the rate of false-positive results.
Dr. Hurwitz said she suspected that there will probably be meaningful differences between the different apps and devices, but the algorithms are all proprietary, and the use of photoplethysmography seems to make a big difference.
She noted that the detection of sleep apnea in the current study was a novel approach. “This is important, as sleep apnea is felt to contribute to AFib, and treating it is felt to decrease the frequency of AFib. Perhaps if patients with sleep apnea were treated before they had documented AFib, the AFib burden could be reduced,” she said.
She added that further studies were needed to fine tune the algorithms and to try and identify other factors or heart rate variabilities that may predict future risk of AFib.
The study was funded by the National Natural Science Foundation of China. Dr. Guo reports no disclosures.
A version of this article first appeared on Medscape.com.
COVID-19 cardiovascular complications in children: AHA statement
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Empagliflozin rapidly improves acute heart failure symptoms in hospitalized patients
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
AT ACC 2022
FDA approves Fitbit’s AFib-detection software
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
A popular fitness tracker company has received approval from the Food and Drug Administration for a new software algorithm to detect atrial fibrillation (AFib), Fitbit announced on April 11.
The algorithm will be the basis of an upcoming Fitbit feature called Irregular Heart Rhythm Notifications, the company said in a press release.
The approval was based on data from the Fitbit Heart Study, which was conducted entirely virtually in more than 455,000 U.S. adults. Participants who had an irregular heart rhythm detected by the software algorithm were notified and invited to meet with a telehealth doctor. They then received a 1-week ECG patch monitor to wear along with the smartwatch or fitness tracker.
Results, presented at the annual scientific sessions of the American Heart Association in November 2021, showed that the positive predictive value of the Fitbit algorithm for detecting undiagnosed AFib with a range of wearable devices was 98%. Notably, irregular heart rhythm detection occurred in 1% of participants overall and 4% of those older than 65 years.
The algorithm works by using an optical measurement method called photoplethysmography (PPG), along with heart rate input from the Fitbit’s photodetector device.
It operates only when the user is still or at rest, so overnight use is important for detection, the company noted.
The upcoming Irregular Heart Rhythm Notifications feature will complement the existing ECG app, providing two ways to detect AFib. The ECG app provides a “spot-check approach” in which the users can screen themselves, and the PPG-based feature will allow for long-term heart rhythm assessment, the statement explained.
“Undiagnosed atrial fibrillation can lead to strokes, and early detection of atrial fibrillation may allow doctors to prescribe medications that are effective at preventing strokes,” said Steven A. Lubitz, MD, MPH, a cardiologist at Harvard University and Massachusetts General Hospital, both in Boston, at the AHA meeting.
TAVI device shows less deterioration than surgery 5 years out
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
Structural aortic valve deterioration (SVD) at 5 years is lower following repair with a contemporary transcatheter implantation (TAVI) device than with surgery, according to a pooled analysis of major trials.
For healthier patients with a relatively long life expectancy, this is important information for deciding whether to undergo TAVI or surgical aortic valve repair (SAVR), Michael J. Reardon, MD, said at the annual scientific sessions of the American College of Cardiology.
“Every week I get this question about which repair is more durable,” said Dr. Reardon, whose study was not only designed to compare device deterioration but to evaluate the effect of SVD on major outcomes.
In this analysis, the rates of SVD were compared for the self-expanding supra-annular CoreValve Evolut device and SAVR. The SVD curves separated within the first year. At 5 years, the differences were highly significant favoring TAVI (2.57% vs. 4.38%; P = .0095).
As part of this analysis, the impact of SVD was also assessed independent of type of repair. At 5 years, those with SVD relative to those without had an approximately twofold increase in all-cause mortality, cardiovascular mortality, and hospitalization of aortic valve worsening. These risks were elevated regardless of type of valve repair.
The data presented by Dr. Reardon can be considered device specific. The earlier PARTNER 2A study comparing older- and newer-generation TAVI devices with SAVR produced a different result. When a second-generation balloon-expandable SAPIEN XT device and a third-generation SAPIEN 3 device were compared with surgery, neither device achieved lower SVD rates relative to SAVR.
In PARTNER 2A, the SVD rate for the older device was nearly three times greater than SAVR (1.61 vs. 0.58 per 100 patient-years). The numerically higher SVD rates for the newer device (0.68 vs. 0.58 per 100 patient-years) was not statistically different, but the TAVI device was not superior.
More than 4,000 patients evaluated at 5 years
In the analysis presented by Dr. Reardon, data were pooled from the randomized CoreValve U.S. High-Risk Pivotal Trial and the SURTAVI Intermediate Risk Trial. Together, these studies randomized 971 patients to surgery and 1,128 patients to TAVI. Data on an additional 2,663 patients treated with the Evolut valve in two registries were added to the randomized trial data, providing data on 4,762 total patients with 5-year follow-up.
SVD was defined by two criteria. The first was a mean gradient increase of at least 10 mm Hg plus a mean overall gradient of at least 20 mm Hg as measured with echocardiography and assessed, when possible, by an independent core laboratory. The second was new-onset or increased intraprosthetic aortic regurgitation of at least moderate severity.
When graphed over time, the SVD curves separated in favor of TAVI after about 6 months of follow-up. The shape of the curves also differed. Unlike the steady rise in SVD observed in the surgery group, the SVD rate in the TAVI group remained below 1% for almost 4 years before beginning to climb.
There was greater relative benefit for the TAVI device in patients with annular diameters of 23 mm or less. Unlike the rise in SVD rates that began about 6 months after SAVR, the SVD rates in the TAVI patients remained at 0% for more than 2 years. At 5 years, the differences remained significant favoring TAVI (1.39% vs. 5.86%; P = .049).
In those with larger annular diameters, there was still a consistently lower SVD rate over time for TAVI relative to SAVR, but the trend for an advantage at 5 years fell just short of significance (2.48% vs. 3.96%; P = .067).
SVD linked to doubling of mortality
SVD worsened outcomes. When all data surgery and TAVI data were pooled, the hazard ratios corresponded with about a doubling of risk for major adverse outcomes, including all-cause mortality (HR, 1.98; P < .001), cardiovascular mortality (HR, 1.82; P = .008), and hospitalization for aortic valve disease or worsening heart failure (HR, 2.11; P = .01). The relative risks were similar in the two treatment groups, including the risk of all-cause mortality (HR, 2.24; P < .001 for TAVI vs. HR, 2.45; P = .002 for SAVR).
The predictors for SVD on multivariate analysis included female sex, increased body surface area, prior percutaneous coronary intervention, and a prior diagnosis of atrial fibrillation.
Design improvements in TAVI devices are likely to explain these results, said Dr. Reardon, chair of cardiovascular research at Houston Methodist Hospital.
“The CoreValve/Evolut supra-annular, self-expanding bioprosthesis is the first and only transcatheter bioprosthesis to demonstrate lower rates of SVD, compared with surgery,” Dr. Reardon said.
This analysis validated the risks posed by the definition of SVD applied in this study, which appears to be a practical tool for tracking valve function and patient risk. Dr. Reardon also said that the study confirms the value of serial Doppler transthoracic echocardiography as a tool for monitoring SVD.
Several experts agreed that this is important new information.
“This is a remarkable series of findings,” said James McClurken, MD, who is a cardiovascular surgeon affiliated with Temple University, Philadelphia, and practices in Doylestown, Penn. By both demonstrating the prognostic importance of SVD and showing differences between the study device and SAVR, this trial will yield practical data to inform patients about relative risks and benefits.
Athena Poppas, MD, the new president of the ACC and a professor of medicine at Brown University, Providence, R.I., called this study “practice changing” for the same reasons. She also thinks it has valuable data for guiding choice of intervention.
Overall, the data are likely to change thinking about the role of TAVI and surgery in younger, fit patients, according to Megan Coylewright, MD, chief of cardiology at Erlanger Cardiology, Chattanooga, Tenn.
“There are patients [in need of aortic valve repair] with a long life expectancy who have been told you have to have a surgical repair because we know they last longer,” she said. Although she said that relative outcomes after longer follow-up remain unknown, “I think this does throw that comment into question.”
Dr. Reardon has financial relationships with Abbott, Boston Scientific, Medtronic, and Gore Medical. Dr. Poppas and McClurken reported no potential financial conflicts of interest. Dr. Coylewright reported financial relationships with Abbott, Alleviant, Boston Scientific, Cardiosmart, Edwards Lifesciences, Medtronic, and Occlutech. The study received financial support from Medtronic.
FROM ACC 2022
Extraction of infected implanted cardiac devices rare, despite guidelines
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
FROM ACC 2022
FDA approves leadless, single-chamber pacemaker system
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.