User login
Traumatic Anterior Shoulder Instability: The US Military Experience
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
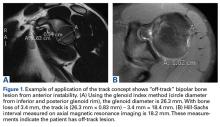
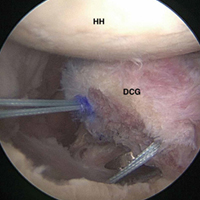
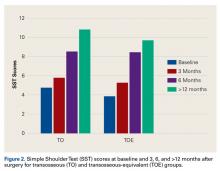
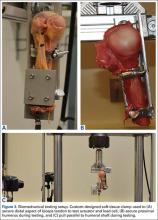
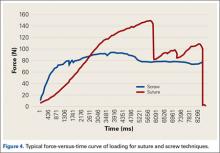
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Arthroscopic stabilization performed early results in better outcomes in patients with Bankart lesions.
- A subcritical level of bone loss of 13.5% has been shown to have a significant effect on outcomes, in addition to the established “critical amount”.
- Bone loss is a bipolar issue. Both sides must be considered in order to properly address shoulder instability.
- Off-track measurement has been shown to be even more positively predictive of outcomes than glenoid bone loss assessment.
- There are several bone loss management options including, the most common coracoid transfer, as well as distal tibial allograft and distal clavicular autograft.
Given its relatively young age, high activity level, and centralized medical care system, the US military population is ideal for studying traumatic anterior shoulder instability. There is a long history of military surgeons who have made significant contributions that have advanced our understanding of this pathology and its treatment and results. In this article, we describe the scope, treatment, and results of this pathology in the US military population.
Incidence and Pathology
At the United States Military Academy (USMA), Owens and colleagues1 studied the incidence of shoulder instability, including dislocation and subluxation, and found anterior instability events were far more common than in civilian populations. The incidence of shoulder instability was 0.08 per 1000 person-years in the general US population vs 1.69 per 1000 person-years in US military personnel. The factors associated with increased risk of shoulder instability injury in the military population were male sex, white race, junior enlisted rank, and age under 30 years. Owens and colleagues2 noted that subluxation accounted for almost 85% of the total anterior instability events. Owens and colleagues3 found the pathology in subluxation events was similar to that in full dislocations, with a soft-tissue anterior Bankart lesion and a Hill-Sachs lesion detected on magnetic resonance imaging in more than 90% of patients. In another study at the USMA, DeBerardino and colleagues4 noted that 97% of arthroscopically assessed shoulders in first-time dislocators involved complete detachment of the capsuloligamentous complex from the anterior glenoid rim and neck—a so-called Bankart lesion. Thus, in a military population, anterior instability resulting from subluxation or dislocation is a common finding that is often represented by a soft-tissue Bankart lesion and a Hill-Sachs defect.
Natural History of Traumatic Anterior Shoulder Instability in the Military
Several studies have evaluated the outcomes of nonoperative and operative treatment of shoulder instability. Although most have found better outcomes with operative intervention, Aronen and Regan5 reported good results (25% recurrence at nearly 3-year follow-up) with nonoperative treatment and adherence to a strict rehabilitation program. Most other comparative studies in this population have published contrary results. Wheeler and colleagues6 studied the natural history of anterior shoulder dislocations in a USMA cadet cohort and found recurrent instability after shoulder dislocation in 92% of cadets who had nonoperative treatment. Similarly, DeBerardino and colleagues4 found that, in the USMA, 90% of first-time traumatic anterior shoulder dislocations managed nonoperatively experienced recurrent instability. In a series of Army soldiers with shoulder instability, Bottoni and colleagues7 reported that 75% of nonoperatively managed patients had recurrent instability, and, of these, 67% progressed to surgical intervention. Nonoperative treatment for a first-time dislocation is still reasonable if a cadet or soldier needs to quickly return to functional duties. Athletes who develop shoulder instability during their playing season have been studied in a military population as well. In a multicenter study of service academy athletes with anterior instability, Dickens and colleagues8 found that, with conservative management and accelerated rehabilitation of in-season shoulder instability, 73% of athletes returned to sport by a mean of 5 days. However, the durability of this treatment should be questioned, as 64% later experienced recurrence.
Arthroscopic Stabilization of Acute Anterior Shoulder Dislocations
In an early series of cases of traumatic anterior shoulder instability in USMA cadets, Wheeler and colleagues6 found that, at 14 months, 78% of arthroscopically stabilized cases and 92% of nonoperatively treated cases were successful. Then, in the 1990s, DeBerardino and colleagues4 studied a series of young, active patients in the USMA and noted significantly better results with arthroscopic treatment, vs nonoperative treatment, at 2- to 5-year follow-up. Of the arthroscopically treated shoulders, 88% remained stable during the study and returned to preinjury activity levels, and 12% experienced recurrent instability (risk factors included 2+ sulcus sign, poor capsular labral tissue, and history of bilateral shoulder instability). In a long-term follow-up (mean, 11.7 years; range, 9.1-13.9 years) of the same cohort, Owens and colleagues9 found that 14% of patients available for follow-up had undergone revision stabilization surgery, and, of these, 21% reported experiencing subluxation events. The authors concluded that, in first-time dislocators in this active military population, acute arthroscopic Bankart repair resulted in excellent return to athletics and subjective function, and had acceptable recurrence and reoperation rates. Bottoni and colleagues,7 in a prospective, randomized evaluation of arthroscopic stabilization of acute, traumatic, first-time shoulder dislocations in the Army, noted an 89% success rate for arthroscopic treatment at an average follow-up of 36 months, with no recurrent instability. DeBerardino and colleagues10 compared West Point patients treated nonoperatively with those arthroscopically treated with staples, transglenoid sutures, or bioabsorbable anchors. Recurrence rates were 85% for nonoperative treatment, 22% for staples, 14% for transglenoid sutures, and 10% for bioabsorbable anchors.
Arthroscopic Versus Open Stabilization of Anterior Shoulder Instability
In a prospective, randomized clinical trial comparing open and arthroscopic shoulder stabilization for recurrent anterior instability in active-duty Army personnel, Bottoni and colleagues11 found comparable clinical outcomes. Stabilization surgery failed clinically in only 3 cases, 2 open and 1 arthroscopic. The authors concluded that arthroscopic stabilization can be safely performed for recurrent shoulder instability and that arthroscopic outcomes are similar to open outcomes. In a series of anterior shoulder subluxations in young athletes with Bankart lesions, Owens and colleagues12 found that open and arthroscopic stabilization performed early resulted in better outcomes, regardless of technique used. Recurrent subluxation occurred at a mean of 17 months in 3 of the 10 patients in the open group and 3 of the 9 patients in the arthroscopic group, for an overall recurrence rate of 31%. The authors concluded that, in this patient population with Bankart lesions caused by anterior subluxation events, surgery should be performed early.
Bone Lesions
Burkhart and De Beer13 first noted that bone loss has emerged as one of the most important considerations in the setting of shoulder instability in active patients. Other authors have found this to be true in military populations.14,15
The diagnosis of bone loss may include historical findings, such as increased number and ease of dislocations, as well as dislocation in lower positions of abduction. Physical examination findings may include apprehension in the midrange of motion. Advanced imaging, such as magnetic resonance arthrography, has since been validated as equivalent to 3-dimensional computed tomography (3-D CT) in determining glenoid bone loss.16 In 2007, Mologne and colleagues15 studied the amount of glenoid bone loss and the presence of fragmented bone or attritional bone loss and its effect on outcomes. They evaluated 21 patients who had arthroscopic treatment for anterior instability with anteroinferior glenoid bone loss between 20% and 30%. Average follow-up was 34 months. All patients received 3 or 4 anterior anchors. No patient with a bone fragment incorporated into the repair experienced recurrence or subluxation, whereas 30% of patients with attritional bone loss had recurrent instability.15
Classifying Bone Loss and Recognizing Its Effects
Burkhart and De Beer13 helped define the role and significance of bone loss in the setting of shoulder instability. They defined significant bone loss as an engaging Hill-Sachs lesion of the humerus in an abducted and externally rotated position or an “inverted pear” lesion of the glenoid. Overall analysis revealed recurrence in 4% of cases without significant bone loss and 65% of cases with significant bone loss. In a subanalysis of contact-sport athletes in the setting of bone loss, the failure rate increased to 89%, from 6.5%. Aiding in the quantitative assessment of glenoid bone loss, Itoi and colleagues17 showed that 21% glenoid bone loss resulted in instability that would not be corrected by a soft-tissue procedure alone. Bone loss of 20% to 25% has since been considered a “critical amount,” above which an arthroscopic Bankart has been questioned. More recently, several authors have shown that even less bone loss can have a significant effect on outcomes. Shaha and colleagues18 established that a subcritical level of bone loss (13.5%) on the anteroinferior glenoid resulted in clinical failure (as determined with the Western Ontario Shoulder Instability Index) even in cases in which frank recurrence or subluxation was avoided. It is thought that, in recurrent instability, glenoid bone loss incident rate is as high as 90%, and the corresponding percentage of patients with Hill-Sachs lesions is almost 100%.19,20 Thus, it is increasingly understood that bone loss is a bipolar issue and that both sides must be considered in order to properly address shoulder instability in this setting. In 2007, Yamamoto and colleagues21 introduced the glenoid track, a method for predicting whether a Hill-Sachs lesion will engage. Di Giacomo and colleagues22 refined the track concept to quantitatively determine which lesions will engage in the setting of both glenoid and humeral bone loss. Metzger and colleagues,23 confirming the track concept arthroscopically, found that manipulation with anesthesia and arthroscopic visualization was well predicted by preoperative track measurements, and thus these measurements can be a good guide for surgical management (Figures 1A, 1B).
Strategies for Addressing Bone Loss in Anterior Shoulder Instability
Several approaches for managing bone loss in shoulder instability have been described—the most common being coracoid transfer (Latarjet procedure). Waterman and colleagues25 recently studied the effects of coracoid transfer, distal tibial allograft, and iliac crest augmentation on anterior shoulder instability in US military patients treated between 2006 and 2012. Of 64 patients who underwent a bone block procedure, 16 (25%) had a complication during short-term follow-up. Complications included neurologic injury, pain, infection, hardware failure, and recurrent instability.
Conclusion
Traumatic anterior shoulder instability is a common pathology that continues to significantly challenge the readiness of the US military. Military surgeon-researchers have a long history of investigating approaches to the treatment of this pathology—applying good science to a large controlled population, using a single medical record, and demonstrating a commitment to return service members to the ready defense of the nation.
Am J Orthop. 2017;46(4):184-189. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
1. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791-796.
2. Owens BD, Duffey ML, Nelson BJ, DeBerardino TM, Taylor DC, Mountcastle SB. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
3. Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am. 2010;92(7):1605-1611.
4. DeBerardino TM, Arciero RA, Taylor DC, Uhorchak JM. Prospective evaluation of arthroscopic stabilization of acute, initial anterior shoulder dislocations in young athletes. Two- to five-year follow-up. Am J Sports Med. 2001;29(5):586-592.
5. Aronen JG, Regan K. Decreasing the incidence of recurrence of first time anterior shoulder dislocations with rehabilitation. Am J Sports Med. 1984;12(4):283-291.
6. Wheeler JH, Ryan JB, Arciero RA, Molinari RN. Arthroscopic versus nonoperative treatment of acute shoulder dislocations in young athletes. Arthroscopy. 1989;5(3):213-217.
7. Bottoni CR, Wilckens JH, DeBerardino TM, et al. A prospective, randomized evaluation of arthroscopic stabilization versus nonoperative treatment in patients with acute, traumatic, first-time shoulder dislocations. Am J Sports Med. 2002;30(4):576-580.
8. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42(12):2842-2850.
9. Owens BD, DeBerardino TM, Nelson BJ, et al. Long-term follow-up of acute arthroscopic Bankart repair for initial anterior shoulder dislocations in young athletes. Am J Sports Med. 2009;37(4):669-673.
10. DeBerardino TM, Arciero RA, Taylor DC. Arthroscopic stabilization of acute initial anterior shoulder dislocation: the West Point experience. J South Orthop Assoc. 1996;5(4):263-271.
11. Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. Am J Sports Med. 2006;34(11):1730-1737.
12. Owens BD, Cameron KL, Peck KY, et al. Arthroscopic versus open stabilization for anterior shoulder subluxations. Orthop J Sports Med. 2015;3(1):2325967115571084.
13. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy. 2000;16(7):677-694.14. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Clinical validation of the glenoid track concept in anterior glenohumeral instability. J Bone Joint Surg Am. 2016;98(22):1918-1923.
15. Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. Am J Sports Med. 2007;35(8):1276-1283.
16. Markenstein JE, Jaspars KC, van der Hulst VP, Willems WJ. The quantification of glenoid bone loss in anterior shoulder instability; MR-arthro compared to 3D-CT. Skeletal Radiol. 2014;43(4):475-483.
17. Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35-46.
18. Shaha JS, Cook JB, Song DJ, et al. Redefining “critical” bone loss in shoulder instability: functional outcomes worsen with “subcritical” bone loss. Am J Sports Med. 2015;43(7):1719-1725.
19. Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482-493.
20. Provencher MT, Frank RM, Leclere LE, et al. The Hill-Sachs lesion: diagnosis, classification, and management. J Am Acad Orthop Surg. 2012;20(4):242-252.
21. Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649-656.
22. Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy. 2014;30(1):90-98.
23. Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213.
24. Arciero RA, Parrino A, Bernhardson AS, et al. The effect of a combined glenoid and Hill-Sachs defect on glenohumeral stability: a biomechanical cadaveric study using 3-dimensional modeling of 142 patients. Am J Sports Med. 2015;43(6):1422-1429.
25. Waterman BR, Chandler PJ, Teague E, Provencher MT, Tokish JM, Pallis MP. Short-term outcomes of glenoid bone block augmentation for complex anterior shoulder instability in a high-risk population. Arthroscopy. 2016;32(9):1784-1790.
26. Schroder DT, Provencher MT, Mologne TS, Muldoon MP, Cox JS. The modified Bristow procedure for anterior shoulder instability: 26-year outcomes in Naval Academy midshipmen. Am J Sports Med. 2006;34(5):778-786.
27. Provencher MT, Frank RM, Golijanin P, et al. Distal tibia allograft glenoid reconstruction in recurrent anterior shoulder instability: clinical and radiographic outcomes. Arthroscopy. 2017;33(5):891-897.
28. Tokish JM, Fitzpatrick K, Cook JB, Mallon WJ. Arthroscopic distal clavicular autograft for treating shoulder instability with glenoid bone loss. Arthrosc Tech. 2014;3(4):e475-e481.
In-Office Diagnostic Needle Arthroscopy
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
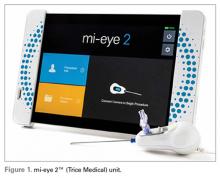
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
mi-eye 2™ (https://tricemedical.com/mi-eye/)
Over the past decade, magnetic resonance imaging (MRI) has been the gold standard for identification of intra-articular soft tissue pathology of the knee. Limitations, however, do exist for the use of MRI in diagnosing injuries. Various studies have reported MRI sensitivity and specificity to be 86% and 91% in diagnosis of knee pathology.1 These numbers can be lower in the setting of previous surgery. Furthermore, some patients cannot have MRIs, while for others, MRIs would be inconclusive. This includes patients who are morbidly obese, claustrophobic, renally impaired, have implanted medical devices, have metal within their bodies, or have had previous surgical intervention to the affected joint.
As an alternative to MRI, in-office needle arthroscopy offers a cost-effective, minimally invasive tool that can provide similar or greater diagnostic accuracy.2,3 The ability to provide real-time dynamic visualization of the patient’s anatomy allows for more accurate decision making by the physician and can potentially reduce the time from injury to diagnosis to recovery.4 It can be performed in a variety of joints, including the knee, shoulder, elbow, and ankle. Indications for use include patients with suspected meniscal tears, anterior cruciate ligament (ACL) tears, loose bodies, rotator cuff tears, and labral tears, as well as pre-arthroplasty evaluations and second-look evaluations of cartilage procedures.
The mi-eye 2™ (Trice Medical) is an in-office diagnostic needle arthroscope that can provide immediate diagnostic capabilities (Figure 1).
For billing purposes, the procedure is coded as a diagnostic arthroscopy of the affected joint. Should the diagnostic evaluation reveal pathology that requires surgical intervention, a modifier 58 code can be attached to allow for full reimbursement of both the in-office procedure and the surgical procedure.
Surgical pearl: It is important to properly position the patient in order to efficiently access the knee. For examination of the knee, we recommend positioning the patient’s knee flexed at either 45° with a bump beneath the knee, or at 90° with the knee off the end of the bed. I begin to anesthetize by placing 10 cc of 1% lidocaine into the joint. Additionally, I use 5 cc of 1% lidocaine to create a skin wheel around the anticipated portal of entry. I allow 5 to 7 minutes for anesthetization prior to performing the procedure. During this time I routinely move to another patient examination room to prevent a delay in patient flow.
When entering the knee joint I recommend placing the portal 1 cm above the joint line and 1 cm medial or lateral to the patellar tendon. This will aid in avoiding the fat pat upon entry. When entering the joint I aim toward the notch and use the ACL as my reference point before moving into the medial or lateral compartment. I typically enter through the side of suspected pathology, and then continue on with the remainder of the evaluation. For focused evaluation of the patellofemoral joint, a suprapatellar portal can be utilized. Dynamic evaluation can be performed by manipulating the leg. If a bloody field is encountered (acute ACL tears), the field of view can be cleared through irrigating the joint with 30 cc sterile saline flushes. I inject the fluid into the joint through the leer lock access and then withdraw it back into the same syringe. This fluid can be discarded and the steps repeated as necessary. At the conclusion of the procedure it is recommended to drain the joint of the injected saline. Through the leer lock, a steroid or platelet-rich plasma injection can be delivered if desired by the physician.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
1. Crawford R, Walley G, Bridgman S, Maffulli N. Magnetic resonance imaging versus arthroscopy in the diagnosis of knee pathology, concentrating on meniscal lesions and ACL tears: a systematic review. Br Med Bull. 2007;84:5-23.
2. Voigt JD, Mosier M, Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12(5):523-535.
3. Gramas DA, Antounian FS, Peterfy CG, Genant HK, Lane NE. Assessment of needle arthroscopy, standard arthroscopy, physical examination, and magnetic resonance imaging in knee pain: a pilot study. J Clin Rheumatol. 1995;1(1):26-34.
4. O’Donnell JF. Trice Medical Literature. #4-10-0032 Rev A.
Precision and Accuracy of Identification of Anatomical Surface Landmarks by 30 Expert Hip Arthroscopists
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
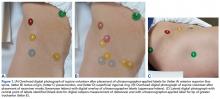
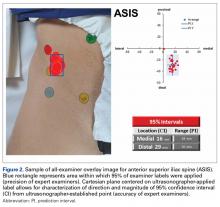
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
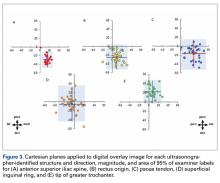
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
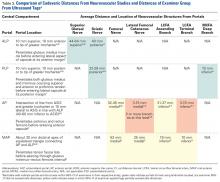
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Surface landmarks are routinely used for physical examination and surgical technique.
- Common surface landmarks used in establishing arthroscopic portals may be more difficult to accurately identify than previously thought.
- The greater trochanter was the surface landmark most precisely identified by expert examiners.
- Ultrasound examination identified landmarks varied from landmarks identified by palpation alone.
Anatomical surface landmarks about the hip and lower abdomen are often referenced when placing arthroscopic portals and office-based injections.1-3 However, the degree to which these landmarks can be reproducibly identified using only visual inspection and palpation is unknown.
Safe access to the hip joint and surrounding structures during hip arthroscopy has been a focus in the orthopedic literature. Authors have described anatomical relationships of recommended portals to neurovascular and other anatomical structures.4-6 This information has been reported in millimeters to centimeters of safety based on cadaver dissection studies.4-7We conducted a study to assess expert hip arthroscopists’ ability to identify, using only physical examination techniques, the anatomical structures used for reference when creating safe starting points for arthroscopic access. We hypothesized that variance in examiner-identified points would exceed safe distances from neurovascular structures for the most commonly used hip arthroscopic portals. The volunteer in this study provided written informed consent for print and electronic publication of this article.
Methods
In this study, we prospectively assessed 30 expert hip arthroscopic surgeons’ ability to identify commonly referenced surface landmarks on the adult male hip, using only inspection and manual palpation. Surgeons were defined as experts on the basis of their status as hip arthroscopy instructors at the Orthopaedic Learning Center (Rosemont, IL) for the Arthroscopy Association of North America and industry-sponsored hip arthroscopy education faculty (Arthrex). Five surface landmarks were selected for their relevance to publications on safe portal placement2-5: anterior superior iliac spine (ASIS), tip of greater trochanter (GT), rectus origin (RO), superficial inguinal ring (SIR), and psoas tendon (PT).
A healthy adult male volunteer was placed supine on an examination table and exposed distally from the mid abdomen, with the perineum and the genital area covered bikini-style. An expert musculoskeletal ultrasonographer used a handheld musculoskeletal ultrasound transducer (Sonosite) to identify the 5 landmarks. Short- and long-axis images of each structure were obtained. The examiner applied a round (1 cm in diameter), uniquely colored adhesive label to the skin over each location. A professional photographer using a Canon digital camera and fixed mounts made precise overhead and lateral images. The positional integrity and scale of these images were confirmed with referral to constant anatomical skin features. Images were archived for analysis (Figure 1A).
After the ultrasonographer’s labels were removed, each of the 30 expert hip arthroscopic surgeons identified the structures by static physical examination (inspection and palpation only) and applied the same colored labels to the skin.
Imaging software (Adobe Photoshop Creative Suite 5.1) was used to superimpose the digital images of the examiner labels on those of the ultrasound-verified anatomical labels (Figure 1C). Measurements were then taken with digital calipers to determine average distance from ultrasound label; accuracy within 10 mm of verified ultrasound label; true average location (TAL) determined by 95% confidence interval (CI); and interobserver variability calculated by 95% prediction interval, which determined the probability of where an additional examiner data point would lie.
In the second arm of the study, examiner data were compared with previously published data on arthroscopic portal safety.
Results
Average absolute distance from examiner labels to ultrasonographer labels was 31 mm for ASIS, 24 mm for GT, 26 mm for RO, 19 mm for SIR, and 35 mm for PT (Figure 2).
Of the 30 surgeons, 1 (3%) came within 10 mm of the ultrasound for ASIS, 1 (3%) for GT, 4 (13%) for RO, 5 (17%) for SIR, and 1 (3%) for PT (Table 1).
TAL as determined by CI was 16 mm medial and 29 mm inferior for ASIS; 8 mm anterior and 22 mm superior for GT; 10 mm medial and 25 mm inferior for RO; 5 mm lateral and 5 mm inferior for SIR; and 28 mm medial and 16 mm inferior for PT (Figure 3, Table 2). Interobserver variability determined by prediction interval had a range of 18 mm medial to lateral × 36 mm proximal to distal for ASIS; 33 mm anterior to posterior × 48 mm superior to inferior for GT; 41 mm medial to distal × 54 mm proximal to distal for RO; 51 mm medial to lateral × 74 mm proximal to distal for SIR; and 49 mm medial to distal × 61 mm proximal to distal for PT.
Given the difference between examiner data (direction and distance from ultrasound labels) and published data (distance to significant neurovascular structures), inaccurate identification of surface landmarks has the potential to lead to AP and MAP damage (Table 3). The examiner GT and ASIS surface landmarks used for AP overlapped directly with the safe distances for the lateral femoral cutaneous nerve and the terminal branch of the lateral circumflex femoral artery.
Discussion
Others have investigated examiners’ use of palpation, compared with ultrasound, to identify common shoulder and knee structures.8-10 In a 2011 systematic review, Gilliland and colleagues11 confirmed that accuracy was improved with use of ultrasound (vs palpation) for injections in the shoulder, hip, knee, wrist, and ankle. Given the scarcity of data in this setting, we conducted the present study to assess the precision and accuracy of expert arthroscopists in identifying common surface landmarks. We hypothesized that physical examination and ultrasound examination would differ significantly in precisely and accurately identifying these landmarks.
Working with a standard awake volunteer, our test group of examiners was consistently inaccurate when they accepted ultrasonographer-placed labels as the ideal. Precision within the group, however, trended toward close agreement; examiners consistently placed labels in the same direction and approximate magnitude away from ultrasonographer labels. This suggests that a discrepancy between the ultrasonographic surface structure definitions taught to ultrasonographers and the manually identified definitions taught to surgeons for arthroscopy (training bias) can generate differences in landmark identification.
Given reported low rates of complications in the creation of standard surface anatomy portals, more data is needed to correlate whether safe distance guidelines best apply to the points identified by hip experts or the points identified by ultrasonographers. In a 2013 systematic review, Harris and colleagues8 found a 7.5% overall complication rate, with temporary neuropraxia 1 of the 2 most common complications. Whether adding ultrasound to physical examination for the creation of some or all portals will reduce the incidence of these problems is unknown. Regardless of the anatomical area referenced by experts for portal creation, the tight grouping of examiner marks in our study supports a consensus regarding the location of the landmarks studied.
In our study of the use of surface anatomical landmarks for the creation of portals, we analyzed 4 previously described locations: ALP, AP, PLP, and MAP. ALP, AP, and PLP directly reference at least 1 surface anatomical structure; AP references 2 anatomical structures (ASIS, GT); and MAP indirectly references ASIS and GT and directly references ALP and AP. In cadaveric and radiographic studies, 7 neurovascular structures have been described in proximity to ALP, AP, MAP, and PLP: superior gluteal nerve, sciatic nerve, femoral nerve, lateral femoral cutaneous nerve, lateral circumflex femoral artery, and medial circumflex femoral artery.5,6 Our results showed that use of surface anatomy in AP and MAP creation most likely places structures at risk, given the overlap of examiner CIs and the previously published cadaveric5,6 and radiographic7 data.
Hua and colleagues12 confirmed the feasibility of using ultrasound for the creation of hip arthroscopy portals. More data is needed to assess how the standard palpation-and-fluoroscopy method described by Byrd3 compares with an ultrasound-guided technique in safety and cost. However, data from our study should not be used to justify a demand for ultrasound during arthroscopy portal establishment, as limitations do not permit such a recommendation.
With diagnostic injection remaining a mainstay of differential diagnosis and treatment about the hip,1 the data presented here suggest a potential for ultrasound in enhancing outcomes. There is evidence supporting the role of image guidance in improving palpation accuracy in the area of the biceps tendon in the forearm.10 Potentially, identification and treatment of specific extra-articular structures surrounding the hip could be made safer with more routine use of ultrasound.
Limitations
This study had several limitations. The surgeons were limited to palpation and static examination of a body in its natural state. Hip arthroscopic portals typically are created under traction and after a standard perineal post is placed for hip arthroscopy. In addition, in an awake injection setting, the clinician may receive patient feedback in the form of limb movement or speech. To what degree palpation or ultrasound will be affected in these scenarios is unknown.
Another limitation is the lack of serial examination by each examiner—intrarater variability could not be gauged. In addition, with only 1 ultrasonographic examination performed, there is the potential that adding ultrasonographic examinations, or having an examiner perform serial physical examinations, could better define the precision of each component. Given the practical limitations of our volunteer’s time and the schedules of 30 expert arthroscopists, we kept the chosen study design for its single setting.
Conclusion
Visual inspection and manual palpation are standard means of identifying common surface anatomical landmarks for the creation of arthroscopy portals and the placement of injections. Our study results showed variance in landmark identification between expert examiners and an ultrasonographer. The degree of variance exceeded established neurovascular safe zones, particularly for AP and MAP. This new evidence calls for further investigation into the best, safest means of performing hip arthroscopic techniques and injection-based interventions.
Am J Orthop. 2017;46(1):E65-E70. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
1. Byrd JW, Potts EA, Allison RK, Jones KS. Ultrasound-guided hip injections: a comparative study with fluoroscopy-guided injections. Arthroscopy. 2014;30(1):42-46.
2. Dienst M, Seil R, Kohn DM. Safe arthroscopic access to the central compartment of the hip. Arthroscopy. 2005;21(12):1510-1514.
3. Byrd JW. Hip arthroscopy, the supine approach: technique and anatomy of the intraarticular and peripheral compartments. Tech Orthop. 2005;20(1):17-31.
4. Bond JL, Knutson ZA, Ebert A, Guanche CA. The 23-point arthroscopic examination of the hip: basic setup, portal placement, and surgical technique. Arthroscopy. 2009;25(4):416-429.
5. Roberson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-1026.
6. Byrd JW, Pappas JN, Pedley MJ. Hip arthroscopy: an anatomic study of portal placement and relationship to the extra-articular structures. Arthroscopy. 1995;11(4):418-423.
7. Watson JN, Bohnenkamp F, El-Bitar Y, Moretti V, Domb BG. Variability in locations of hip neurovascular structures and their proximity to hip arthroscopic portals. Arthroscopy. 2014;30(4):462-467.
8. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
9. Jacobson JA, Bedi A, Sekiya JK, Blankenbaker DG. Evaluation of the painful athletic hip: imaging options and imaging-guided injections. AJR Am J Roentgenol. 2012;199(3):516-524.
10. Gazzillo GP, Finnoff JT, Hall MM, Sayeed YA, Smith J. Accuracy of palpating the long head of the biceps tendon: an ultrasonographic study. PM R. 2011;3(11):1035-1040.
11. Gilliland CA, Salazar LD, Borchers JR. Ultrasound versus anatomic guidance for intra-articular and periarticular injection: a systematic review. Phys Sportsmed. 2011;39(3):121-131.
12. Hua Y, Yang Y, Chen S, et al. Ultrasound-guided establishment of hip arthroscopy portals. Arthroscopy. 2009;25(12):1491-1495.
Poorer Arthroscopic Outcomes of Mild Dysplasia With Cam Femoroacetabular Impingement Versus Mixed Femoroacetabular Impingement in Absence of Capsular Repair
Take-Home Points
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
Take-Home Points
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
Complications and Risk Factors for Morbidity in Elective Hip Arthroscopy: A Review of 1325 Cases
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
Hip Arthroscopy
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Multicenter Outcomes After Hip Arthroscopy: Epidemiology (MASH Study Group). What Are We Seeing in the Office, and Who Are We Choosing to Treat?
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Arthroscopic Transosseous and Transosseous-Equivalent Rotator Cuff Repair: An Analysis of Cost, Operative Time, and Clinical Outcomes
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The rate of medical visits for rotator cuff pathology and the US incidence of arthroscopic rotator cuff repair (RCR) have increased over the past 10 years.1 The increased use of RCR has been justified with improved patient outcomes.2,3 Advances in surgical techniques and instrumentation have contributed to better outcomes for patients with rotator cuff pathology.3-5 Several studies have validated RCR with functional outcome measures, cost–benefit analysis, and health-related quality-of-life measurements.6-9
Healthcare reimbursement models are being changed to include capitated care, pay for performance, and penalties.10 Given the changing healthcare climate and the increasing incidence of RCR, it is becoming increasingly important for orthopedic surgeons to critically evaluate and modify their practice and procedures to decrease costs without compromising outcomes.11 RCR outcome studies have focused on comparing open/mini-open with arthroscopic techniques, and single-row with double-row techniques, among others.4,12-18 Furthermore, several studies on the cost-effectiveness of these surgical techniques have been conducted.19-21Arthroscopic anchorless (transosseous [TO]) RCR, which is increasingly popular,22 combines the minimal invasiveness of arthroscopic procedures with the biomechanical strength of open TO repair. In addition, this technique avoids the potential complications and costs associated with suture anchors, such as anchor pullout and greater tuberosity osteolysis.22,23 Several studies have documented the effectiveness of this technique.24-26 Biomechanical and clinical outcome data supporting arthroscopic TO-RCR have been published, but there are no reports of studies that have analyzed the cost savings associated with this technique.
In this study, we compared implant costs associated with arthroscopic TO-RCR and arthroscopic TO-equivalent (TOE) RCR. We also evaluated these techniques’ operative time and outcomes. Our hypothesis was that arthroscopic TO-RCR can be performed at lower cost and without increasing operative time or compromising outcomes.
Materials and Methods
Our Institutional Review Board approved this study. Between February 2013 and January 2014, participating surgeons performed 43 arthroscopic TO-RCRs that met the study’s inclusion criteria. Twenty-one of the 43 patients enrolled and became the study group. The control group of 21 patients, who underwent arthroscopic TOE-RCR the preceding year (between January 2012 and January 2013), was matched to the study group on tear size and concomitant procedures, including biceps treatment, labral treatment, acromioplasty, and distal clavicle excision (Table 1).
The primary outcome measure was implant cost (amount paid by institution). Cost was determined and reported by an independent third party using Cerner Surginet as the operating room documentation system and McKessen Pathways Materials Management System for item pricing.
All arthroscopic RCRs were performed by 1 of 3 orthopedic surgeons fellowship-trained in either sports medicine or shoulder and elbow surgery. Using the Cofield classification,27 the treating surgeon recorded the size of the rotator cuff tear: small (<1 cm), medium (1-3 cm), large (3-5 cm), massive (>5 cm). The surgeon also recorded the number of suture anchors used, repair technique, biceps treatment, execution of subacromial decompression, execution of distal clavicle excision, and intraoperative complications. TO repair surgical technique is described in the next section. TOE repair was double-row repair with suture anchors. The number of suture anchors varied by tear size: small (3 anchors), medium (2-5 anchors), large (4-6 anchors), massive (4-5 anchors).
Secondary outcome measures were operative time (time from cut to close) and scores on pain VAS (visual analog scale), SANE (Single Assessment Numeric Evaluation), and SST (Simple Shoulder Test). Demographic information was also obtained: age, sex, body mass index, smoking status (Table 1). All patients were asked to fill out questionnaires before surgery and 3, 6, and >12 months after surgery. Outcome surveys were scored by a single research coordinator, who recorded each patient’s outcome scores at the preoperative and postoperative intervals. Follow-up of >12 months was reached by 17 (81%) of the 21 TO patients and 14 (67%) of the 21 TOE patients. For >12 months, the overall rate of follow-up was 74%.
All patients followed the same postoperative rehabilitation protocol: sling immobilization with pendulums for 6 weeks starting at 2 weeks, passive range of motion starting at 6 weeks, and active range of motion starting at 8 weeks. At 3 months, they were allowed progressive resistant exercises with a 10-pound limit, and at 4.5 months they progressed to a 20-pound limit. At 6 months, they were cleared for discharge.
Surgical Technique: Arthroscopic Transosseous Repair
Surgery was performed with the patient in either the beach-chair position or the lateral decubitus position, based on surgeon preference. Our technique is similar to what has been described in the past.22,28 The glenohumeral joint is accessed through a standard posterior portal, followed by an anterior accessory portal through the rotator interval. Standard diagnostic arthroscopy is performed and intra-articular pathology addressed. Next, the scope is placed in the subacromial space through the posterior portal. A lateral subacromial portal is established and cannulated, and a bursectomy performed. The scope is then placed in a posterolateral portal for better visualization of the rotator cuff tear. The greater tuberosity is débrided with a curette to prepare the bed for repair. An ArthroTunneler (Tornier) is used to pass sutures through the greater tuberosity. For standard 2-tunnel repair, 3 sutures are placed through each tunnel. All 6 sutures are next passed (using a suture passer) through the rotator cuff. The second and fifth suture ends that are passed through the cuff are brought out through the cannula and tied together. They are then brought into the shoulder by pulling on the opposite ends and tied alongside the greater tuberosity to create a box stitch. The box stitch acts as a medial row fixation and as a rip stitch that strengthens the vertical mattress sutures against pullout. The other 4 sutures are tied in vertical mattress configuration.
Statistical Analysis
After obtaining the TO and TOE implant costs, we compared them using a generalized linear model with negative binomial distribution and an identity link function so returned parameters were in additive dollars. This comparison included evaluation of tear size and concomitant procedures. Operative times for TO and TOE were obtained and evaluated, and then compared using time-to-event analysis and the log-rank test. Outcome scores were obtained from patients at baseline and 3, 6, and >12 months after surgery and were compared using a linear mixed model that identified change in outcome scores over time, and difference in outcome scores between the TO and TOE groups.
Results
Table 1 lists patient demographics, including age, sex, body mass index, smoking status, and concomitant procedures. The TO and TOE groups had identical tear-size distributions. In addition, they had similar numbers of concomitant procedures, though our study was underpowered to confirm equivalence. Treatment techniques differed: more biceps tenodesis cases in the TO group (n = 12) than in the TOE group (n = 2) and more biceps tenotomy cases in the TOE group (n = 8) than in the TO group (n = 1).
TO implant cost was significantly lower than TOE implant cost for all tear sizes and independent of concomitant procedures (Figure 1).
Operative time was not significantly different between the TO and TOE groups. Mean (SD) operative time was 82.38 (24.09) minutes for the TO group and 81.71 (17.27) minutes for the TOE group. With all other factors controlled, mean operative time was 5.96 minutes shorter for the TOE group, but the difference was not significant (P = .549).
There was no significant difference in preoperative pain VAS (P = .93), SANE (P = .35), or SST (P = .36) scores between the TO and TOE groups.
Discussion
RCR is one of the most common orthopedic surgical procedures, and its use has increased over the past decade.9,21 This increase coincides with the emergence of new repair techniques and implants. These advancements come at a cost. Given the increasingly cost-conscious healthcare environment and its changing reimbursement models, now surgeons must evaluate the economics of their surgical procedures in an attempt to decrease costs without compromising outcomes. We hypothesized that arthroscopic TO-RCR can be performed at lower cost relative to arthroscopic TOE-RCR and without increasing operative time or compromising short-term outcomes.
Studies on the cost-effectiveness of different RCR techniques have been conducted.19-21 Adla and colleagues19 found that open RCR was more cost-effective than arthroscopic RCR, with most of the difference attributable to disposables and suture anchors. Genuario and colleagues21 found that double-row RCR was not as cost-effective as single-row RCR in treating tears of any size. They attributed the difference to 2 more anchors and about 15 more minutes in the operating room.
The increased interest in healthcare costs and the understanding that a substantial part of the cost of arthroscopic RCR is attributable to implants (suture anchors, specifically) led to recent efforts to eliminate the need for anchors. Newly available instrumentation was designed to assist in arthroscopic anchorless repair constructs using the concepts of traditional TO repair.22 Although still considered to be the RCR gold standard, TO fixation has been used less often in recent years, owing to the shift from open to arthroscopic surgery.24 Arthroscopic TO-RCR allows for all the benefits of arthroscopic surgery, plus the biological and mechanical benefits of traditional open or mini-open TO repair. In addition, this technique eliminates the cost of anchors. Kummer and colleagues25 confirmed with biomechanical testing that arthroscopic TO repair and double-row TOE repair are similar in strength, with a trend of less tendon displacement in the TO group.
Our study results support the hypothesis that arthroscopic TO repair provides significant cost savings over tear size–matched arthroscopic TOE repair. Implant cost was substantially higher for TOE repair than for TO repair. Mean (SD) total savings of $946.91 ($100.70) (P < .0001) can be realized performing TO rather than TOE repair. In the United States, where about 250,000 RCRs are performed each year, the use of TO repair would result in an annual savings of almost $250 million.6Operative time was analyzed as well. Running an operating room in the United States costs an estimated $62 per minute (range, $22-$133 per minute).29 Much of this cost is indirect, unrelated to the surgery (eg, capital investment, personnel, insurance), and is being paid even when the operating room is not in use. Therefore, for the hospital’s bottom line, operative time savings are less important than direct cost savings (supplies, implants). However, operative time has more of an effect on the surgeon’s bottom line, and longer procedures reduce the number of surgeries that can be performed and billed. We found no significant difference in operative time between TO and TOE repairs. Critical evaluation revealed that operative time was 5.96 minutes shorter for TOE repairs, but this difference was not significant (P = .677).
Our study results showed no significant difference in clinical outcomes between TO and TOE repair patients. Both groups’ outcome scores improved. At all follow-ups, both groups’ VAS, SANE, and SST scores were significantly improved. Overall, this is the first study to validate the proposed cost benefit of arthroscopic TO repair and confirm no compromise in patient outcomes.
This study had limitations. First, it enrolled relatively few patients, particularly those with small tears. In addition, despite the fact that patients were matched on tear size and concomitant procedures, the groups differed in their biceps pathology treatments. Of the 13 TO patients who had biceps treatment, 12 underwent tenodesis (1 had tenotomy); in contrast, of the 10 TOE patients who had biceps treatment, only 2 underwent tenodesis (8 had tenotomy). The difference is explained by the consecutive course of this study and the increasing popularity of tenodesis over tenotomy. The TOE group underwent surgery before the TO group did, at a time when the involved surgeons were routinely performing tenotomy more than tenodesis. We did not include the costs of implants related to biceps treatment in our analysis, as our focus was on the implant cost of RCR. As for operative time, biceps tenodesis would be expected to extend surgery and potentially affect the comparison of operative times between the TO and TOE groups. However, despite the fact that 12 of the 13 TO patients underwent biceps tenodesis, there was no significant difference in overall operative time. Last, regarding the effect of biceps treatment on clinical outcomes, there are no data showing improved outcomes with tenodesis over tenotomy in the setting of RCR.
A final limitation is lack of data from longer term (>12 months) follow-up for all patients. Our analysis included cost and operative time data for all 42 enrolled patients, but our clinical outcome data represent only 74% of the patients enrolled. Eleven of the 42 patients were lost to follow-up at >12 months, and outcome scores could not be obtained, despite multiple attempts at contact (phone, mail, email). The study design and primary outcome variable focused on cost analysis rather than clinical outcomes. Nevertheless, our data support our hypothesis that there is no difference in clinical outcomes between TO and TOE repairs.
Conclusion
Arthroscopic TO-RCR provides significant cost savings over arthroscopic TOE-RCR without increasing operative time or compromising outcomes. Arthroscopic TO-RCR may have an important role in the evolving healthcare environment and its changing reimbursement models.
Am J Orthop. 2016;45(7):E415-E420. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
1. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
2. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
3. Wolf BR, Dunn WR, Wright RW. Indications for repair of full-thickness rotator cuff tears. Am J Sports Med. 2007;35(6):1007-1016.
4. Yamaguchi K, Ball CM, Galatz LM. Arthroscopic rotator cuff repair: transition from mini-open to all-arthroscopic. Clin Orthop Relat Res. 2001;(390):83-94.
5. Yamaguchi K, Levine WN, Marra G, Galatz LM, Klepps S, Flatow EL. Transitioning to arthroscopic rotator cuff repair: the pros and cons. Instr Course Lect. 2003;52:81-92.
6. Mather RC 3rd, Koenig L, Acevedo D, et al. The societal and economic value of rotator cuff repair. J Bone Joint Surg Am. 2013;95(22):1993-2000.
7. Milne JC, Gartsman GM. Cost of shoulder surgery. J Shoulder Elbow Surg. 1994;3(5):295-298.
8. Savoie FH 3rd, Field LD, Jenkins RN. Costs analysis of successful rotator cuff repair surgery: an outcome study. Comparison of gatekeeper system in surgical patients. Arthroscopy. 1995;11(6):672-676.
9. Vitale MA, Vitale MG, Zivin JG, Braman JP, Bigliani LU, Flatow EL. Rotator cuff repair: an analysis of utility scores and cost-effectiveness. J Shoulder Elbow Surg. 2007;16(2):181-187.
10. Ihejirika RC, Sathiyakumar V, Thakore RV, et al. Healthcare reimbursement models and orthopaedic trauma: will there be change in patient management? A survey of orthopaedic surgeons. J Orthop Trauma. 2015;29(2):e79-e84.
11. Black EM, Higgins LD, Warner JJ. Value-based shoulder surgery: practicing outcomes-driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1000-1009.
12. Barber FA, Hapa O, Bynum JA. Comparative testing by cyclic loading of rotator cuff suture anchors containing multiple high-strength sutures. Arthroscopy. 2010;26(9 suppl):S134-S141.
13. Barros RM, Matos MA, Ferreira Neto AA, et al. Biomechanical evaluation on tendon reinsertion by comparing trans-osseous suture and suture anchor at different stages of healing: experimental study on rabbits. J Shoulder Elbow Surg. 2010;19(6):878-883.
14. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662-669.
15. Ghodadra NS, Provencher MT, Verma NN, Wilk KE, Romeo AA. Open, mini-open, and all-arthroscopic rotator cuff repair surgery: indications and implications for rehabilitation. J Orthop Sports Phys Ther. 2009;39(2):81-89.
16. Pietschmann MF, Fröhlich V, Ficklscherer A, et al. Pullout strength of suture anchors in comparison with transosseous sutures for rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2008;16(5):504-510.
17. van der Zwaal P, Thomassen BJ, Nieuwenhuijse MJ, Lindenburg R, Swen JW, van Arkel ER. Clinical outcome in all-arthroscopic versus mini-open rotator cuff repair in small to medium-sized tears: a randomized controlled trial in 100 patients with 1-year follow-up. Arthroscopy. 2013;29(2):266-273.
18. Wang VM, Wang FC, McNickle AG, et al. Medial versus lateral supraspinatus tendon properties: implications for double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2456-2463.
19. Adla DN, Rowsell M, Pandey R. Cost-effectiveness of open versus arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2010;19(2):258-261.
20. Churchill RS, Ghorai JK. Total cost and operating room time comparison of rotator cuff repair techniques at low, intermediate, and high volume centers: mini-open versus all-arthroscopic. J Shoulder Elbow Surg. 2010;19(5):716-721.
21. Genuario JW, Donegan RP, Hamman D, et al. The cost-effectiveness of single-row compared with double-row arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(15):1369-1377.
22. Garofalo R, Castagna A, Borroni M, Krishnan SG. Arthroscopic transosseous (anchorless) rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1031-1035.
23. Benson EC, MacDermid JC, Drosdowech DS, Athwal GS. The incidence of early metallic suture anchor pullout after arthroscopic rotator cuff repair. Arthroscopy. 2010;26(3):310-315.
24. Baudi P, Rasia Dani E, Campochiaro G, Rebuzzi M, Serafini F, Catani F. The rotator cuff tear repair with a new arthroscopic transosseous system: the Sharc-FT®. Musculoskelet Surg. 2013;97(suppl 1):57-61.
25. Kummer FJ, Hahn M, Day M, Meislin RJ, Jazrawi LM. A laboratory comparison of a new arthroscopic transosseous rotator cuff repair to a double row transosseous equivalent rotator cuff repair using suture anchors. Bull Hosp Joint Dis. 2013;71(2):128-131.
26. Kuroda S, Ishige N, Mikasa M. Advantages of arthroscopic transosseous suture repair of the rotator cuff without the use of anchors. Clin Orthop Relat Res. 2013;471(11):3514-3522.
27. Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet. 1982;154(5):667-672.
28. Paxton ES, Lazarus MD. Arthroscopic transosseous rotator cuff repair. Orthop Knowledge Online J. 2014;12(2). http://orthoportal.aaos.org/oko/article.aspx?article=OKO_SHO052#article. Accessed October 4, 2016.
29. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
Biomechanical Evaluation of Two Arthroscopic Biceps Tenodesis Techniques: Proximal Interference Screw and Modified Percutaneous Intra-Articular Transtendon
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
Quality and Quantity of the Elbow Arthroscopy Literature: A Systematic Review and Meta-Analysis
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
Although elbow arthroscopy was first described in the 1930s, it has become increasingly popular in the last 30 years.1 While initially considered as a tool for diagnosis and loose body removal, indications have expanded to include treatment of osteochondritis dissecans (OCD), treatment of lateral epicondylitis, fixation of fractures, and others.2-5 Miyake and colleagues6 found a significant improvement in range of motion, both flexion and extension, and outcome scores when elbow arthroscopy was used to remove impinging osteophytes. Babaqi and colleagues7 found significant improvement in pain, satisfaction, and outcome scores in 31 patients who underwent elbow arthroscopy for lateral epicondylitis refractory to nonsurgical management. The technical difficulty of the procedure, lower frequency of pathology amenable to arthroscopic intervention, and potential neurovascular complications make the elbow less frequently evaluated with the arthroscope vs other joints, such as the knee and shoulder.2,8,9
Geographic distribution of subjects undergoing elbow arthroscopy, the indications used, surgical techniques being performed, and their associated clinical outcomes have received little to no recognition in the peer-reviewed literature.10 Differences in the elbow arthroscopy literature include characteristics related to the patient (age, gender, hand dominance, duration of symptoms), study (level of evidence, number of subjects, number of participating centers, design), indication (lateral epicondylitis, loose bodies, olecranon osteophytes, OCD), surgical technique, and outcome. Evidence-based medicine and clinical practice guidelines direct surgeons in clinical decision-making. Payers investigate the cost of surgical interventions and the value that surgery may provide, while following trends in different surgical techniques. Regulatory agencies and associations emphasize subjective patient-reported outcomes as the primary outcome measured in high-quality trials. Thus, in discussion of complex surgical interventions such as elbow arthroscopy, it is important to characterize the studies, subjects, and surgeries across the world to understand the geographic similarities and differences to optimize care in this clinical situation.
The goal of this study was to perform a systematic review and meta-analysis of elbow arthroscopy literature to identify and compare the characteristics of the studies published, the subjects analyzed, and surgical techniques performed across continents and countries to answer these questions: “Across the world, what demographic of patients are undergoing elbow arthroscopy, what are the most common indications for elbow arthroscopy, and how good is the evidence?” The authors hypothesized that patients who undergo elbow arthroscopy will be largely age <40 years, the most common indication for elbow arthroscopy will be a release/débridement, and the evidence for elbow arthroscopy will be poor. Also, no significant differences will exist in elbow arthroscopy publications, subjects, outcomes, and techniques based on continent/country of publication.
Methods
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a PRISMA checklist.11 Systematic review registration was performed using the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; registration number, CRD42014010580; registration date, July 15, 2014).12 Two study authors independently conducted the search on June 23, 2014 using the following databases: Medline, Cochrane Central Register of Controlled Trials, SportDiscus, and CINAHL. The electronic search citation algorithm used was: (elbow) AND arthroscopy) NOT shoulder) NOT knee) NOT ankle) NOT wrist) NOT hip) NOT dog) NOT cadaver). English language Level I-IV evidence (2012 update by the Oxford Centre for Evidence-Based Medicine13) clinical studies were eligible for inclusion into this study. Abstracts were ineligible for inclusion. All references in selected studies were cross-referenced for inclusion if they were missed during the initial search. Duplicate subject publications within separate unique studies were not reported twice. The study with longer duration follow-up, higher level of evidence, greater number of subjects, or more detailed subject, surgical technique, or outcome reporting was retained for inclusion. Level V evidence reviews, expert opinion articles, letters to the editor, basic science, biomechanical studies, open elbow surgery, imaging, surgical technique, and classification studies were excluded.
All included patients underwent elbow arthroscopy for either intra- or extra-articular elbow pathology (ulnotrochlear osteoarthritis, lateral epicondylitis, rheumatoid arthritis, post-traumatic contracture, osteonecrosis of the capitellum or radial head, osteoid osteoma, and others). There was no minimum follow-up duration or rehabilitation requirement. The study and subject demographic parameters that we analyzed included year of publication, years of subject enrollment, presence of study financial conflict of interest, number of subjects and elbows, elbow dominance, gender, age, body mass index, diagnoses treated, type of anesthesia (block or general), and surgical positioning. Postoperative splint application and pain management, and whether a continuous passive motion machine was used and whether a drain was placed were recorded. Clinical outcome scores were DASH (Disability of the Arm, Shoulder, and Hand), Morrey score, MEPS (Mayo Elbow Performance Score), Andrews-Carson score, Timmerman-Andrews score, LES (Liverpool Elbow Score), Tegner score, HSS (Hospital for Special Surgery Score), VAS (Visual Analog Scale), EFA (Elbow Functional Assessment), Short Form-12 (SF-12), Short Form-36 (SF-36), Kerlan-Jobe Orthopaedic Clinic (KJOC) Shoulder and Elbow Questionnaire, and MAESS (Modified Andrews Elbow Scoring System). Radiographs, computed tomography (CT), computed tomography arthrography (CTA), magnetic resonance imaging (MRI), and magnetic resonance arthrography (MRA) data were extracted when available. Range of motion (flexion, extension, supination, and pronation) and grip strength data, both preoperative and postoperative, were extracted when available. Study methodological quality was evaluated using the Modified Coleman Methodology Score (MCMS).14
Statistical Analysis
Study descriptive statistics were calculated. Continuous variable data were reported as weighted means ± weighted standard deviations. Categorical variable data were reported as frequencies with percentages. For all statistical analysis either measured and calculated from study data extraction or directly reported from the individual studies, P < .05 was considered statistically significant. Study, subject, and surgical outcomes data were compared using 1-way analysis of variance (ANOVA) tests. Where applicable, study, subject, and surgical outcomes data were also compared using 2-sample and 2-proportion Z-test calculators with α .05 because of the difference in sample sizes between compared groups. To examine trends over time, Pearson’s correlation coefficients were calculated. For the purposes of analysis, the indications of “osteoarthritis,” “arthrofibrosis,” “loose body removal,” “ulnotrochlear osteoarthritis causing stiffness,” “post-traumatic contracture/stiffness,” and “post-operative elbow contracture” were combined into the indication “release and débridement.” For the 3 most common indications for arthroscopy (OCD, lateral epicondylitis, and release and débridement) data were combined into 5-year increments to overcome the smaller sample size within each of these categories, and Pearson’s correlation coefficients were calculated to determine if number of reported cases covaried with year period. Within these 3 diagnoses, ANOVA analyses were performed to determine whether the number of cases differed between continents and countries.
Results
A total of 353 studies were located, and, after implementation of the exclusion criteria, 112 studies were included in the final analysis (Figure 1; 3093 subjects; 3168 elbows; 64% male; mean age, 34.9 ± 14.68 years). There was a mean of 33.4 ± 26.02 months of follow-up, and 75% of surgeries involved the dominant elbow (Table 1). Most studies were level IV evidence (94.6%), had a low MCMS (mean 28.1 ± 8.06; poor rating), and were single-center investigations (94.6%). Most studies did not report financial conflicts of interest (56.3%) (Tables 1 and 2). From 1985 through 2014, the number of publications significantly increased with time (P = .004) among all continents. The MCMS was unchanged over time (P = .247) (Figure 2A), as was the level of evidence (P = .094) (Figure 2B). Conflicts of interest significantly increased with time (P = .025) (Figure 3).
Among continents, North America published the largest number of studies (54), and had the largest number of patients (1395) and elbow surgeries (1425) (Table 1). The United States published the largest number of studies (43%). There were no significant differences between age (P = .331), length of follow-up (P = .403), MCMS (P = .123), and level of evidence (P = .288) between continents. Of the 32 studies that reported the use of preoperative MRI, studies from Asia reported significantly more MRI scans than those from other continents (P = .040); there were no other significant differences between continents in reference to preoperative imaging studies or other demographic information.
The most common surgical indications were OCD (Figure 4), lateral epicondylitis (Figure 5), and release and débridement (Figure 6, Table 3; all studies listed indications). The number of reported cases for these 3 indications significantly increased over time (OCD P = .005, lateral epicondylitis P = .044, release and débridement P = .042) but did not significantly differ between regions (P > .05 in all cases).
Thirty-two (28.6%) studies reported the use of outcome measures (16 different outcome scores were used by the included studies). Asia reported outcome measures in 9 of 23 studies (39%), Europe in 12 of 35 studies (34%) and North America in 11 of 54 (20%) of studies. The MEPS was the most frequently used outcome score in 9.8% of studies, followed by VAS for pain in 5.3% of cases. North American studies reported a significantly higher increase in extension after elbow arthroscopy than Asia (P = .0432) (Figure 7), with no differences in flexion (P = .699), pronation (P = .376), or supination (P = .408). No significant differences were observed between continents in the type of anesthesia chosen (general anesthesia [P = .94] or regional anesthesia [P = .85]). Asia and Europe performed elbow arthroscopy most frequently in the lateral decubitus position, while North American studies most often used the supine position (Table 4).
Twenty (17.9%) studies reported the use of a postoperative splint, 12 (10.7%) studies reported use of a drain, 2 (1.79%) studies reported use of a hinged elbow brace, 9 (8.03%) studies reported use of a continuous passive motion machine postoperatively, and 3 (2.68%) studies reported use of an indwelling axillary catheter for postoperative pain management. Of 130 reported surgical complications (4.1%), the most frequent complication was transient sensory ulnar nerve palsy (1.5%), followed by persistent wound drainage (.76%), and transient sensory radial nerve palsy (.38%). Other reported complications included infection (.22%), transient sensory palsy of the median nerve (.19%), heterotopic ossification (.13%), complete transection of the ulnar nerve (.10%), loose body formation (.06%), hematoma formation (.06%), transient sensory palsy of the posterior interosseous (.06%), or anterior interosseous nerve (.03%), and complete transection of the radial (.03%), or median nerve (.03%).
Discussion
Elbow arthroscopy is an evolving surgical procedure that is used to treat intra- and extra-articular pathologies of the elbow. Outcomes of elbow arthroscopy for certain conditions have generally been reported as good, with improvements seen in pain, functional scores, and range of motion.6,15-17 The authors’ hypotheses were mostly confirmed in that the average age of patients undergoing elbow arthroscopy was <40 years, release/débridement was one of the most common indications (along with lateral epicondylitis and OCD), and the general evidence for elbow arthroscopy was poor. Also, there were almost no differences between continents/countries related to patient indications, preoperative imaging, anesthesia choice, indications, postoperative protocols, and outcomes (although the number of studies that reported outcomes was low and could have skewed the results), with the exception of a higher number of preoperative MRI scans in Asia. Some of the notable findings of this study included: 1) the number of studies published on elbow arthroscopy is significantly increasing with time, despite a lack of improvement in the level of evidence; 2) the majority of studies on elbow arthroscopy do not report a surgical outcome score; and 3) the number of reported cases for the 3 most common indications significantly increased over time (OCD, P = .005; lateral epicondylitis, P = .044; release and débridement, P = .042) but did not differ between regions (P > .05 in all cases).
The indications for elbow arthroscopy have grown dramatically in the past 2 decades to include both intra- and extra-articular pathologies.18 Despite this increase in the number of indications for elbow arthroscopy, the study did not find a significant difference between countries/continents in the indications each used for elbow arthroscopy patients. There was a trend towards an increase in OCD cases in all continents, especially Asia (Figure 4), with time. Interestingly, while not statistically significant, there was variation among countries for surgical indications. In North America, removal of loose bodies accounts for 18% of patients, while in Europe this accounted for only 9% and in Asia for 1%. Post-traumatic stiffness was the indication for elbow arthroscopy in Europe in 19% of patients vs 7% in North America and 10% in Asia. In Asia, OCD accounts for 40% of arthroscopies, 7% in Europe, and 14% in North America (Figure 4) (Table 3).
This study demonstrated that the mean increase in elbow extension gained after surgery in North America was significantly greater when compared with studies from Asia, but the gain in flexion, pronation, and supination was similar across continents. The underlying cause of this difference in improvement in elbow extension between nations is unclear, although differences in diagnosis could account for some variation. This study did not examine differences in rehabilitation protocols, and certainly, it is plausible that protocol variations by country could account for some discrepancy. Furthermore, differences in functional needs may vary by continent and could have driven this result.
This study found no routine reporting of outcome scores by elbow arthroscopy studies from any continent, and that when outcome scores are reported, there is substantial inconsistency with regard to the actual scoring system used. No continent reported outcome scores in more than 40% of the studies published from that area, and the variation of outcome scores used, even from a single region, was large. This makes comparing clinical outcomes between studies difficult, even when performing identical procedures for identical indications, because there is no standardized method of reporting outcomes. To allow comparison of studies and generalizability of the results to different populations, a more standardized approach to outcome reporting needs to be instituted in the elbow arthroscopy literature. To date, there is no standardized score that has been validated for reporting clinical outcomes after elbow arthroscopy.19 Hence, it is not surprising that there were 16 different outcome scores reported throughout the 112 studies analyzed in this review, with the most frequent score, the MEPS, reported in a total of 10 studies. As medicine moves towards pay scales that are based on patient outcomes, it will become more important to define a clear outcome score that can be used to assess these patients, and reliably report scores. This will allow comparison of patients across nations to determine the best surgical treatment for different clinical problems. A validation study comparing these outcome scores to determine which score best summarizes the patient’s level of pain and function after surgery would be beneficial, because this could identify 1 score that could be standardized to allow comparison among reported outcomes.
Limitations
This study had several limitations. Despite having 2 authors search independently for studies, some studies could have been missed during the search process, introducing possible selection bias. Including only published studies could have introduced publication bias. Numerous studies did not report all the variables the authors examined. This could have skewed some results, and had additional variables been reported, could have altered the data to show significant differences in some measured variables. Because this study did not compare outcome measures for varying pathologies, conclusions cannot be drawn on the best treatment options for different indications. Case reports could have lowered the MCMS score and the average in studies reporting outcomes. Furthermore, the poor quality of the underlying data used in this study could limit the validity/generalizability of the results because this is a systematic review, and its level of evidence is only as high as the studies it includes. Because the primary aim was to report on demographics, this study did not examine concomitant pathology at the time of surgery or rehabilitation protocols.
Conclusion
The quantity, but not the quality, of arthroscopic elbow publications has significantly increased over time. Most patients undergo elbow arthroscopy for lateral epicondylitis, OCD, and release and débridement. Pathology and indications do not appear to differ geographically with more men undergoing elbow arthroscopy than women.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.
1. Khanchandani P. Elbow arthroscopy: review of the literature and case reports. Case Rep Orthop. 2012;2012:478214.
2. Dodson CC, Nho SJ, Williams RJ 3rd, Altchek DW. Elbow arthroscopy. J Am Acad Orthop Surg. 2008;16(10):574-585.
3. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 1):47-62.
4. Kelly EW, Morrey BF, O’Driscoll SW. Complications of elbow arthroscopy. J Bone Joint Surg Am. 2001;83-A(1):25-34.
5. Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37-40.
6. Miyake J, Shimada K, Oka K, et al. Arthroscopic debridement in the treatment of patients with osteoarthritis of the elbow, based on computer simulation. Bone Joint J. 2014;96-B(2):237-241.
7. Babaqi AA, Kotb MM, Said HG, AbdelHamid MM, ElKady HA, ElAssal MA. Short-term evaluation of arthroscopic management of tennis elbow; including resection of radio-capitellar capsular complex. J Orthop. 2014;11(2):82-86.
8. Gay DM, Raphael BS, Weiland AJ. Revision arthroscopic contracture release in the elbow resulting in an ulnar nerve transection: a case report. J Bone Joint Surg Am. 2010;92(5):1246-1249.
9. Haapaniemi T, Berggren M, Adolfsson L. Complete transection of the median and radial nerves during arthroscopic release of post-traumatic elbow contracture. Arthroscopy. 1999;15(7):784-787.
10. Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28(2):272-282.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ. 2009;339:b2700.
12. PROSPERO. International Prospective Register of Ongoing Systematic Reviews. The University of York CfRaDP-Iprosr-v. 2013 [cited 2014]. http://www.crd.york.ac.uk/PROSPERO/. Accessed March 17, 2016.
13. Oxford Centre for Evidence-Based Medicine - levels of evidence (March 2009). Centre for Evidence-Based Medicine Web site. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed July 6, 2016.
14. Cowan J, Lozano-Calderόn S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
15. Jones GS, Savoie FH 3rd. Arthroscopic capsular release of flexion contractures (arthrofibrosis) of the elbow. Arthroscopy. 1993;9(3):277-283.
16. O’Brien MJ, Lee Murphy R, Savoie FH 3rd. A preliminary report of acute and subacute arthroscopic repair of the radial ulnohumeral ligament after elbow dislocation in the high-demand patient. Arthroscopy. 2014;30(6):679-687.
17. Rhyou IH, Kim KW. Is posterior synovial plica excision necessary for refractory lateral epicondylitis of the elbow? Clin Orthop Relat Res. 2013;471(1):284-290.
18. Jerosch J, Schunck J. Arthroscopic treatment of lateral epicondylitis: indication, technique and early results. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):379-382.
19. Tijssen M, van Cingel R, van Melick N, de Visser E. Patient-Reported Outcome questionnaires for hip arthroscopy: a systematic review of the psychometric evidence. BMC Musculoskelet Disord. 2011;12:117.