User login
A probiotic reduces disease severity in children and adolescents with atopic dermatitis
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Key clinical point: Coadjuvant treatment with a specific probiotic preparation reduced disease severity in children and adolescents with atopic dermatitis (AD), as evidenced by a decrease in Scoring of Atopic Dermatitis (SCORAD) and Investigator’s Global Assessment (IGA) scores.
Major finding: At 12 weeks, patients receiving the probiotic preparation vs placebo had a significantly higher rate of achieving at least a 1-point improvement in IGA score (90.5% vs 56.7%; P < .002) and lower SCORAD score (13.52 vs 18.96; P = .041).
Study details: This study included 70 patients aged 4-17 years with AD who were randomly assigned to receive the probiotic preparation (containing Bifidobacterium lactis, Bifidobacterium longum, and Lactobacillus casei; n = 35) or placebo (n = 35) daily for 12 weeks.
Disclosures: This study was funded by Biopolis SL. The authors declared no conflicts of interest.
Source: Feíto-Rodríguez M et al. Randomised double blind placebo controlled clinical trial to evaluate the effect of a mixture of probiotic strains on symptom severity and the use of corticosteroids in children and adolescents with atopic dermatitis. Clin Exp Dermatol. 2023 (Jan 13). Doi: 10.1093/ced/llad007
Atopic dermatitis with hand eczema: Upadacitinib is safe and effective in daily practice
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Key clinical point: Upadacitinib can serve as an effective treatment option for atopic dermatitis (AD) and concomitant hand eczema (HE) in daily practice.
Major finding: At week 16, the mean Eczema Area and Severity Index (EASI) score decreased significantly from 17.2 (at baseline) to 4.8 (P < .001) and 50.0%, 40.6%, 59.3%, and 74.1% of patients achieved EASI-75, an Investigator’s Global Assessment score of (almost) clear, Hand Eczema Severity Index-75, and a score of (almost) clear on the photographic guide, respectively. Adverse events were generally mild in severity.
Study details: This multicenter prospective observational study included 38 patients with AD from the BioDay registry who received once-daily upadacitinib over 16 weeks, of which 32 patients had concomitant HE.
Disclosures: The BioDay registry is funded by Sanofi/Regeneron and others. Some authors declared serving as advisors, speakers, or consultants for or receiving consulting fees from various sources, including the registry funders.
Source: Kamphuis E, Loman L, et al. Experiences from daily practice of upadacitinib treatment on atopic dermatitis with a focus on hand eczema: Results from the BioDay registry. Contact Dermatitis. 2023 (Jan 9). Doi: 10.1111/cod.14276
Dupilumab a favorable treatment option for moderate-to-severe atopic dermatitis
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Key clinical point: Dupilumab provides rapid improvement in atopic dermatitis (AD) signs and symptoms and is well tolerated in patients with moderate-to-severe AD in a real-world setting.
Major finding: At week 12, the percentages of patients who achieved ≥75% improvement in the Eczema Area and Severity Index, an Investigator’s Global Assessment score of 0/1 with a ≥2-point reduction from baseline, and a ≥4-point decrease in itch-numerical rating scale score were 59.4%, 33.0%, and 57.0%, respectively. Adverse event rates were lower than those reported in previous phase 3 trials.
Study details: Findings are from a 12-week analysis of the multicenter prospective real-life study PROLEAD including 828 dupilumab-naive adult patients with moderate-to-severe AD who received dupilumab.
Disclosures: This study was funded by Sanofi. Some authors reported ties with various organizations, including Sanofi. Three authors declared being employees of or holding stock or stock options in Sanofi.
Source: Augustin M et al. Dupilumab demonstrates rapid onset of action in improving signs, symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb). 2023 (Feb 4). Doi: 10.1007/s13555-023-00894-3
Tralokinumab counters difficult-to-treat moderate-to-severe atopic dermatitis
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Tralokinumab counters difficult-to-treat moderate-to-severe atopic dermatitis
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Key clinical point: Tralokinumab was effective and safe in a real-world cohort of patients with moderate-to-severe atopic dermatitis (AD) who had failed prior systemic therapy.
Major finding: At last review (24 weeks of maximum follow-up), 59% of patients were still on tralokinumab therapy and showed a decrease in the median Numeric Rating Scale Peak Pruritus Score over the past 7 days (from 5 at baseline to 2) but without any change in the median Investigator Global Assessment score. Treatment-related adverse events were mostly mild in severity.
Study details: Findings are from an observational, prospective cohort study including 37 patients aged ≥15 years with moderate-to-severe AD who had failed prior therapy with immunosuppressants, biologics, or a Janus kinase inhibitor and received subcutaneous tralokinumab.
Disclosures: This study did not receive any funding. DJ Hijnen declared serving as an investigator for and receiving consulting fees from various sources.
Source: Schlösser AR et al. Tralokinumab for moderate-to-severe atopic dermatitis patients: First daily practice results. Clin Exp Dermatol. 2023 (Jan 26). Doi: 10.1093/ced/llad038
Risk for atopic dermatitis in children alters with the mode of delivery
Key clinical point: Children born by cesarean section or instrumental vaginal delivery are at a greater risk of developing atopic dermatitis (AD) compared with those born by uncomplicated vaginal delivery.
Major finding: Children aged <1 year born by instrumental vaginal delivery (adjusted hazard ratio [aHR] 1.10; 95% CI 1.07-1.13), emergency cesarean section (aHR 1.12; 95% CI 1.10-1.15), and elective caesarean section (aHR 1.13; 95% CI 1.10-1.16) were at a higher risk for AD compared with those born by uncomplicated vaginal delivery, with the risk being similar in children aged ≥1 year.
Study details: This prospective population-based study included 1,399,406 children aged ≤5 years with available information on the mode of delivery and mother's identity.
Disclosures: This study was supported by the Swedish Research Council, Swedish Heart-Lung Foundation, and Stiftelsen Frimurare Barnhuset i Stockholm. The authors declared no conflicts of interest.
Source: Mubanga M et al. Mode of delivery and offspring atopic dermatitis in a Swedish nationwide study. Pediatr Allergy Immunol. 2023;34(1):e13904 (Jan 11). Doi: 10.1111/pai.13904
Key clinical point: Children born by cesarean section or instrumental vaginal delivery are at a greater risk of developing atopic dermatitis (AD) compared with those born by uncomplicated vaginal delivery.
Major finding: Children aged <1 year born by instrumental vaginal delivery (adjusted hazard ratio [aHR] 1.10; 95% CI 1.07-1.13), emergency cesarean section (aHR 1.12; 95% CI 1.10-1.15), and elective caesarean section (aHR 1.13; 95% CI 1.10-1.16) were at a higher risk for AD compared with those born by uncomplicated vaginal delivery, with the risk being similar in children aged ≥1 year.
Study details: This prospective population-based study included 1,399,406 children aged ≤5 years with available information on the mode of delivery and mother's identity.
Disclosures: This study was supported by the Swedish Research Council, Swedish Heart-Lung Foundation, and Stiftelsen Frimurare Barnhuset i Stockholm. The authors declared no conflicts of interest.
Source: Mubanga M et al. Mode of delivery and offspring atopic dermatitis in a Swedish nationwide study. Pediatr Allergy Immunol. 2023;34(1):e13904 (Jan 11). Doi: 10.1111/pai.13904
Key clinical point: Children born by cesarean section or instrumental vaginal delivery are at a greater risk of developing atopic dermatitis (AD) compared with those born by uncomplicated vaginal delivery.
Major finding: Children aged <1 year born by instrumental vaginal delivery (adjusted hazard ratio [aHR] 1.10; 95% CI 1.07-1.13), emergency cesarean section (aHR 1.12; 95% CI 1.10-1.15), and elective caesarean section (aHR 1.13; 95% CI 1.10-1.16) were at a higher risk for AD compared with those born by uncomplicated vaginal delivery, with the risk being similar in children aged ≥1 year.
Study details: This prospective population-based study included 1,399,406 children aged ≤5 years with available information on the mode of delivery and mother's identity.
Disclosures: This study was supported by the Swedish Research Council, Swedish Heart-Lung Foundation, and Stiftelsen Frimurare Barnhuset i Stockholm. The authors declared no conflicts of interest.
Source: Mubanga M et al. Mode of delivery and offspring atopic dermatitis in a Swedish nationwide study. Pediatr Allergy Immunol. 2023;34(1):e13904 (Jan 11). Doi: 10.1111/pai.13904
Dupilumab shows rapid and sustained efficacy and favorable safety in erythrodermic atopic dermatitis
Key clinical point: Dupilumab with or without concomitant topical corticosteroids (TCS) was safe and provided rapid and sustained improvements in atopic dermatitis (AD) signs and symptoms in patients with erythrodermic AD.
Major finding: At week 16, dupilumab without and with concomitant TCS vs placebo provided significant improvements in the percentage of AD-affected body surface area (P = .02 and P < .001, respectively), Eczema Area and Severity Index score (P = .002 and P < .001, respectively), and Peak Pruritus Numerical Rating Scale score (P < .001 and P = .002, respectively), with improvements observed from week 1. Most treatment-emergent adverse events were mild or moderate in severity.
Study details: This post hoc analysis of six multicenter randomized trials included 209 patients with erythrodermic AD who were randomly assigned to receive dupilumab or placebo (both with or without concomitant TCS).
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported ties with various organizations, including Sanofi/Regeneron. Four authors declared being employees of or holding stock or stock options in Sanofi/Regeneron.
Source: Paller AS et al. Efficacy and safety of dupilumab in patients with erythrodermic atopic dermatitis: A post hoc analysis of 6 randomized clinical trials. JAMA Dermatol. 2023 (Feb 1). Doi: 10.1001/jamadermatol.2022.6192
Key clinical point: Dupilumab with or without concomitant topical corticosteroids (TCS) was safe and provided rapid and sustained improvements in atopic dermatitis (AD) signs and symptoms in patients with erythrodermic AD.
Major finding: At week 16, dupilumab without and with concomitant TCS vs placebo provided significant improvements in the percentage of AD-affected body surface area (P = .02 and P < .001, respectively), Eczema Area and Severity Index score (P = .002 and P < .001, respectively), and Peak Pruritus Numerical Rating Scale score (P < .001 and P = .002, respectively), with improvements observed from week 1. Most treatment-emergent adverse events were mild or moderate in severity.
Study details: This post hoc analysis of six multicenter randomized trials included 209 patients with erythrodermic AD who were randomly assigned to receive dupilumab or placebo (both with or without concomitant TCS).
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported ties with various organizations, including Sanofi/Regeneron. Four authors declared being employees of or holding stock or stock options in Sanofi/Regeneron.
Source: Paller AS et al. Efficacy and safety of dupilumab in patients with erythrodermic atopic dermatitis: A post hoc analysis of 6 randomized clinical trials. JAMA Dermatol. 2023 (Feb 1). Doi: 10.1001/jamadermatol.2022.6192
Key clinical point: Dupilumab with or without concomitant topical corticosteroids (TCS) was safe and provided rapid and sustained improvements in atopic dermatitis (AD) signs and symptoms in patients with erythrodermic AD.
Major finding: At week 16, dupilumab without and with concomitant TCS vs placebo provided significant improvements in the percentage of AD-affected body surface area (P = .02 and P < .001, respectively), Eczema Area and Severity Index score (P = .002 and P < .001, respectively), and Peak Pruritus Numerical Rating Scale score (P < .001 and P = .002, respectively), with improvements observed from week 1. Most treatment-emergent adverse events were mild or moderate in severity.
Study details: This post hoc analysis of six multicenter randomized trials included 209 patients with erythrodermic AD who were randomly assigned to receive dupilumab or placebo (both with or without concomitant TCS).
Disclosures: This study was funded by Sanofi-Regeneron Pharmaceuticals Inc. Some authors reported ties with various organizations, including Sanofi/Regeneron. Four authors declared being employees of or holding stock or stock options in Sanofi/Regeneron.
Source: Paller AS et al. Efficacy and safety of dupilumab in patients with erythrodermic atopic dermatitis: A post hoc analysis of 6 randomized clinical trials. JAMA Dermatol. 2023 (Feb 1). Doi: 10.1001/jamadermatol.2022.6192
FDA expands oral JAK abrocitinib to adolescents with AD
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
Abrocitinib, taken once daily, previously was approved only for treating adults aged 18 and older.
It joins upadacitinib (Rinvoq), previously the only oral JAK inhibitor to be approved for use by adolescents aged 12 through 17 with refractory moderate to severe AD.
The indication has been expanded for teens whose disease is not adequately controlled with other systemic drugs, including biologics, or those for whom use of those drugs is not advised.
Prescribing information was updated to reflect data from JADE TEEN, a phase 3, randomized, placebo-controlled trial that supported the indication for adolescents. That trial evaluated both the 100-mg and 200-mg doses of abrocitinib in comparison with placebo in 285 adolescents aged 12-18 who had moderate to severe AD and who were also receiving background therapy with topical medications.
The most common toxicities that were reported in at least 1% of patients treated with abrocitinib for up to 16 weeks included nasopharyngitis, nausea, and headache.
Efficacy measures included improvements in itch, skin clearance, and disease severity using the Investigator Global Assessment (IGA), the Peak Pruritus Numerical Rating Scale (PP-NRS), and the Eczema Area and Severity Index (EASI), according to the Pfizer statement announcing the expanded approval.
Select JADE TEEN findings include the following:
- IGA response rate of 0 or 1 at week 12: 39% with abrocitinib 100 mg; 46% with abrocitinib 200 mg; and 24% with placebo.
- EASI-75 response rate at week 12: 64%, 71%, and 41%, respectively.
- Proportion of participants achieving PP-NRS with at least a 4-point decrease from baseline at week 2: 13%, 25%, and 8%, respectively.
Data included in the prescribing information now encompass five randomized, placebo-controlled clinical trials and a long-term extension study with more than 1,600 patients treated with abrocitinib, according to the statement from Pfizer.
In a 2021 story, when JADE TEEN trial results were presented, Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego, told this news organization that he welcomed oral JAKs as a weapon against atopic dermatitis.
He noted that moderate to severe AD can have a tremendous impact on adolescents. “Traditionally, we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control,” he said.
Abrocitinib is not recommended for use with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
AD, one of the most common inflammatory skin diseases, affects approximately 5%-10% of adults in the United States and approximately 11% of children. About one in three adults and one in three children and adolescents aged 17 and younger with AD have moderate to severe disease.
JAK inhibition is thought to modulate multiple cytokines involved in AD, including interleukin (IL)–4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin.
Prescribing information includes a warning that use of abrocitinib should be avoided by patients with an active, serious infection, including localized infections. A boxed warning is included in the labels of JAK inhibitors regarding the risk of serious infections, mortality, major cardiovascular events, and thrombosis.
Treatment risks and benefits should be carefully considered for patients with chronic or recurrent infections or those who have lived in or traveled in areas of endemic tuberculosis or endemic mycoses, the information states.
A version of this article first appeared on Medscape.com.
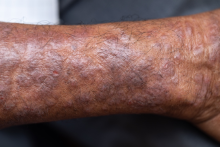
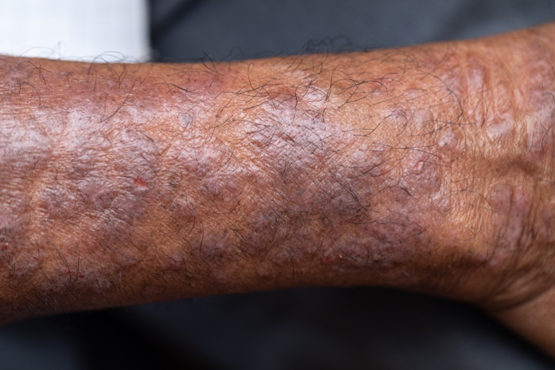
Pruritic rash on arms and legs
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Atopic dermatitis (AD) is one of the most common chronic, inflammatory skin diseases encountered by dermatologists. AD is characterized by pruritus and a chronic course of exacerbations and remissions. AD is thought to involve the interplay of genetic predisposition, immune dysregulation, and environmental factors. It is also associated with other allergic conditions, including asthma.
Although AD typically presents with pruritus as the hallmark symptom in all patients, the appearance of skin lesions may vary among different skin types. In individuals with light-colored skin, AD often appears as erythematous patches and plaques. It also more commonly affects the flexor surfaces of the skin. In individuals with darker skin tones, AD may more often result in follicularly centered papules, lichenification, and pigmentary changes. Lesions may also present on extensor surfaces rather than the typical flexure surfaces. Erythema in darker skin types may appear reddish-brown, have a violaceous hue, or be an ashen gray or darker brown color rather than bright red. Because erythema is more difficult to detect in darker skin types, clinicians may mistakenly minimize the severity of AD.
Clinical severity may also differ between ethnicities. Black patients have an increased tendency toward hyperlinearity of the palms, periorbital dark circles, Dennie-Morgan lines, and diffuse xerosis. Compared with White patients, Black patients with AD are also more likely to develop prurigo nodularis and lichenification. In contrast, Asian patients with AD often experience psoriasiform features, with lesions having more well-defined borders and increased scaling and lichenification.
Beyond differences in clinical appearance, AD may appear molecularly and histologically distinct in ethnic skin. One study suggests that Black patients with AD may have decreased Th1 and Th17 but share similar upregulation of Th2 and Th22 as seen in White patients. Another study showed that Asian patients may have higher Th17 and Th22 and lower Th1/interferon compared with White patients.
Regardless of skin type, treatment goals remain the same. Treatment goals aim to repair and improve the function of the skin barrier while preventing and managing flares. Clinical studies have shown that skincare regimens incorporating ceramide-containing moisturizers may improve AD by increasing the lipid content in the skin. This may offer clinical benefit in patients with skin of color. However, some treatments often used for AD may lead to other skin issues in skin in color. For example, long-term use of topical steroids may worsen hypopigmentation in darker skin types. Management strategies should take into account the unique clinical and genetic features of AD among different patient demographic groups.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 27-year-old student presents with a pruritic rash on his hands and in the bends of his arms and legs. He recently started clinical rotations in a nursing facility and has been using hand sanitizer multiple times per day, which has exacerbated the rash on his hands, causing them to ooze and sting. He describes the rash as itchy, especially at night. At times he reports that the itching causes difficulty sleeping. In addition, his skin has little cracks that frequently bleed. He notes that he has experienced similar symptoms in the past, which resolved with moisturizers and topical cream from the drugstore. He has tried over-the-counter hydrocortisone during this episode, with minimal improvement in symptoms. He denies any change in laundry detergents or use of new household products.
Physical examination reveals large erythematous plaques on the hands and flexure surfaces of his neck, antecubital fossa, and behind the knees with scattered excoriations. Erythematous, slightly lichenified coalescing papules are noted on the proximal arms. His face is clear. General skin pigmentation is brown and free of masses and lumps.