User login
FDA panel leans toward more robust breast implant surveillance
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
SILVER SPRING, MD. – A mandatory, comprehensive approach to collecting adverse event data from breast implant recipients was favored during a March 25 hearing by a Food and Drug Administration advisory panel that oversees surgical devices.
This additional data could offer more complete information during the informed consent process for breast implants and potentially validate a new, autoimmune-like syndrome – breast implant illness (BII).
On the first day of a scheduled 2-day hearing, the advisory panel held no votes and took no formal actions. After a day of expert presentations and comments from more than 40 members of the public – mostly personal stories from affected patients and from plastic surgeons who place breast implants, panel members discussed a handful of questions from the FDA about relevant data to collect to better define the risks posed to breast implant recipients from breast-implant associated anaplastic large cell lymphoma (BIA-ALCL) and BII.
The advisory panel meeting took place as reports recently appeared documenting the scope of BIA-ALCL (Plast Reconstr Surg. 2019 March;143[3S]:65S-73S) and how to diagnose and manage BIA-ALCL (Aesthetic Surg J. 2019 March;39[S1}:S3-S13), and the existence of BII (Plast Reconstr Surg. 2019 March;143[3S]:74S-81S).
During the day’s two public comment periods, the panel heard from several women who gave brief accounts of developing and dealing with BIA-ALCL or BII.
“We think it’s important that all breast implant patients be aware of the risk for BIA-ALCL,” said Binita Ashar, MD, director of the FDAs Division of Surgical Devices. The FDA “is asking the panel what further steps need to be taken to understand the BIA-ALCL risk,” said Dr. Ashar as she opened the meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee.
While the agency, as well as the plastic surgery community, have acknowledged the existence of BIA-ALCL since 2011, only recently have good data emerged on the scope of the complication. During the hearing, Mark W. Clemens, MD, a plastic surgeon at MD Anderson Cancer Center in Houston, reported on his analysis of 457 unique cases of BIA-ALCL reported to the FDA since 2011. He found that the vast majority of cases had occurred in women who had received textured implants while a relatively small minority were linked with the placement of smooth implants.
Further scrutiny of the reported details of each case showed that none of the lymphomas were linked with a confirmed instance of “pure” smooth implant exposure. He also estimated the U.S. incidence of BIA-ALCL as roughly one case for every 20,000 implants. Complete, en bloc removal of the implant seems to be the most effective way to treat the cancer; most explanted patients have a good prognosis, he said.
Despite the apparent link between textured implants specifically and onset of BIA-ALCL, some panel members did not see a ban on textured implants as the answer.
Texturing the implant helps to stabilize the implant in position. Without texturing “we would need to use something else to stabilize the implant, or there would be a tsunami of reoperations,” said panel member Mary H. McGrath, MD, professor of surgery at the University of California, San Francisco. The main alternative to texturing for stabilizing implants is to wrap them in place using surgical mesh, but that approach may also cause problems.
“Instead of just taking textured implants off the market, we need to also look at their advantages. A critical issue is informed consent,” said panel member Marc E. Lippman, MD, a professor of medicine at Georgetown University, Washington. Banning smooth implants based on what’s known so far “would be an extraordinary over reaction,” he said during the first day’s session.
Current U.S. anecdotal experience suggests that a ban may not even be necessary because “plastic surgeons are more and more walking away from textured implants” because of the apparent link to BIA-ALCL, Dr. McGrath said.
BII has been a more recent and more controversial complication of breast implants. As recently as September 2018, Dr. Ashar said in a written statement that “the agency continues to believe that the weight of the currently available scientific evidence does not conclusively demonstrate an association between breast implants and connective tissue diseases,” the types of symptoms that characterize BII.
While the panel heard no new, conclusive evidence of a causal link between breast implants and the range of symptoms that some implant recipients report and is now collectively known as BII, several participants seemed convinced that the syndrome was real and needed better surveillance and study.
“It’s in the same family as chronic fatigue syndrome and fibromyalgia. It’s not a diagnosis, but a set of symptoms.” said Benjamin O. Anderson, MD, a surgical oncologist and professor of surgery at the University of Washington in Seattle and a panel member. “It’s a giant challenge. BII is a constellation of difficult symptoms. We need to think about how we ask patients, what are your symptoms?”
Frank R. Lewis Jr., MD, committee chair, said a more standardized measure of the most common BII symptoms is needed. “That may be exceedingly difficult, with as many as a hundred reported symptoms,” said Dr. Lewis, executive director, emeritus, of the American Board of Surgery in Philadelphia.
The hearing featured results from some of the most research projects aimed at fleshing out an understanding of BII.
Diana Zuckerman, PhD, president of the National Center for Health Research, reported data she and her associates collected in an online survey completed in late 2018 and early 2019 by 449 women who had approached the Center for help in getting health insurance coverage for medically-necessary explantation of their breast implants.
Their most common symptoms included joint, muscle or back pain, weakness or stiffness; fatigue; “brain fog;” and anxiety and depression. More than two-thirds of the respondents had a family history and 3% had a personal history of an autoimmune disease, and 61% said their symptoms improved after their implants were removed, Dr. Zuckerman reported during her presentation to the panel.
During the discussion, panel members seemed intent on expanding mandatory, routine surveillance to all breast implants placed in U.S. practice.
Andrea L. Pusic, MD, president of the Plastic Surgery Foundation, summarized the recent launch of the National Breast Implant Registry by the Foundation and its parent organization, the American Society of Plastic Surgeons. These organizations, and plastic surgeons in general, would be amenable to collecting the data the FDA deemed necessary to better track BIA-ALCL and BII, said Dr. Pusic, professor of surgery at Harvard Medical School and chief of plastic and reconstructive surgery at Brigham and Women’s Hospital in Boston.
“Plastic surgeons are willing to enter these data because we know they are important,” she told the FDA panel.
Dr. Ashar, Dr. Clemens, Dr. McGrath, Dr. Lippman, Dr. Anderson, Dr. Lewis, Dr. Zuckerman, and Dr. Pusic reported having no relevant commercial disclosures.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
Socioeconomic status affects scleroderma severity in African Americans
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
“Not only do patients who manifest the diffuse cutaneous subset of disease experience a more severe course, but so do affected persons of African American race,” Nadia D. Morgan, MBBS, and Allan C. Gelber, MD, wrote in an accompanying editorial. The effects of socioeconomic status should not be overlooked based on the current study, in which the inclusion of socioeconomic factors eliminated the significance of association between race and mortality among scleroderma patients, they wrote.
“Overall, and in the context of these published reports which underscore the disproportionate and adverse impact of scleroderma among African Americans, and in light of the ongoing efforts of the GRASP study, the current paper by Moore et al. emphasizes the importance of socioeconomic status, and of socioeconomic determinants of health, to account for differences in clinically relevant outcomes,” they wrote.
Dr. Gelber is affiliated with the division of rheumatology at Johns Hopkins University, Baltimore. Dr. Morgan, who was also with Johns Hopkins, died before publication of the editorial. They made no conflict of interest disclosures.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
according to findings from an analysis of single-center cohort data over a 10-year period.
Indeed, among patients in the cohort of 402 scleroderma patients at MedStar Georgetown University Hospital in Washington, lower household income was predictive of higher mortality during follow-up, independent of race, according to first author Duncan F. Moore, MD, and his colleagues at the hospital.
Previous studies have demonstrated increased risk for scleroderma in African American patients, who also are more likely than non–African Americans to be diagnosed at a younger age and to have conditions including more diffuse cutaneous disease, more severe restrictive lung disease, more cardiac and renal involvement, and increased mortality, the authors wrote in Arthritis Care & Research.
“We did clearly show that African Americans have worse outcomes and severe pulmonary involvement, but I was surprised that there still was a major contribution of socioeconomic status affecting outcomes for all patients, even though only 10% of our patients were indigent and on medical assistance,” Virginia Steen, MD, senior author of the study and professor of rheumatology at Georgetown University, said in an interview. “I still feel strongly that there are likely genetic issues as to why African Americans have such severe disease. We are eager to learn more from the GRASP [Genome Research in African American Scleroderma Patients] study, which is specifically looking at the genetic issues in African American scleroderma patients,” she said.
Of the 402 scleroderma patients at MedStar Georgetown who were seen during 2006-2016, 202 were African American. A total of 186 African American and 184 non–African American patients in the study met the 2013 American College of Rheumatology/European League Against Rheumatism criteria for systemic sclerosis (SSc). Demographics including gender (87% female) and age (mean of 48 years) were similar between the groups.
Overall, the African American patients showed more severe lung disease, more pulmonary hypertension, and more severe cardiac involvement than did non–African American patients, and autoantibodies were significantly different between the groups.
During follow-up, mortality proved much higher among African Americans at 21%, compared with 11% in non–African Americans (P = .005). However, the unadjusted hazard ratio for death declined from 2.061 (P = .006) to a nonsignificant 1.256 after adjustment for socioeconomic variables.
All socioeconomic measures showed significant differences between the groups. African Americans were more likely to be single and disabled at the initial study visit and to have Medicaid, but they were less likely to be a homemaker, have private insurance, or have a college degree. African Americans’ $74,000 median household income (based on ZIP code) was also a statistically significant $23,000 less than non–African American patients. But the researchers noted that “for every additional $10,000 of household income, independent of race, the hazard of death during follow-up declined by 15.5%.”
Notable differences in antibodies appeared between the groups, with more African American patients having isolated nucleolar ANA, anti-U1RNP antibody, or other positive antinuclear antibodies without SSc-specific antibodies. African American patients also were less likely to have anticentromere or anti-RNA polymerase III antibodies.
The study findings were limited by several factors, including possible bias in the matching process and the use of only index values for socioeconomic variables, the researchers noted.
Regardless of relative socioeconomic and genetic influences, “it is clear that African Americans with scleroderma merit more intensive efforts to facilitate timely diagnosis and access to continued evaluation and suppressive treatment, particularly with respect to cardiopulmonary involvement,” they wrote.
Next steps for research, according to Dr. Steen, include studying clinical subsets of African American patients to try to identify factors to predict outcomes, including the nucleolar pattern ANA, overlap with lupus, history of hypertension, and the relationship with renal crisis.
“We are also looking at whether the African American patients are less responsive to mycophenolate than the non–African American patients. We definitely need to find ways to be more aggressive at identifying and treating African American patients early in their disease,” she added.
The researchers had no financial conflicts to disclose. Dr. Steen serves on the MDedge Rheumatology Editorial Advisory Board.
SOURCE: Moore DF et al. Arthritis Care Res. 2019 March 1. doi: 10.1002/acr.23861.
FROM ARTHRITIS CARE & RESEARCH
Resistant hypertension hits SLE patients hard
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
at a tertiary care center.
A patient with resistant hypertension either has blood pressure remaining above 140/90 mm Hg while taking three antihypertensive medications or requires the use of four or more antihypertensives to attain blood pressure control. Resistant hypertension, which was more likely to occur among blacks and patients with lower renal function, hypercholesterolemia, and increased inflammatory markers, increased the risk of death nearly threefold (hazard ratio, 2.91; P = .0005) when compared with those who didn’t have this condition.
The results of this analysis were published March 15 in Arthritis Care & Research (doi: 10.1002/acr.23880). We covered this study at the 2018 annual meeting of the American College of Rheumatology in Chicago before it was published in the journal. Read our previous story at the link above.
FROM ARTHRITIS CARE & RESEARCH
BTK inhibitor calms pemphigus vulgaris with low-dose steroids
WASHINGTON – An investigational molecule that blocks the downstream proinflammatory effects of B cells controlled disease activity and induced clinical remission in patients with pemphigus by 12 weeks.
At the end of a 24-week, open-label trial, a key driver of the sometimes-fatal blistering disease, Deedee Murrell, MD, said at the annual meeting of the American Academy of Dermatology.
The clinical efficacy plus a favorable safety profile supports the further development of the molecule, designed and manufactured by Principia Biopharma in San Francisco. The company is currently recruiting for a pivotal phase 3 trial of PRN1008 in 120 patients with moderate to severe pemphigus vulgaris.
Despite the recent approval of rituximab (Rituxan) for moderate to severe pemphigus, there remains an unmet need for a quick-acting, steroid-sparing, anti-inflammatory treatment, said Dr. Murrell, professor and head of the department of dermatology at the University of New South Wales, Sydney.
“We need something to use instead of high-dose steroids while we are waiting for rituximab to kick in, which can take 3 months,” and rituximab, which depletes B cells, puts patients at risk for infection, she said. “We need something that has rapid onset, is steroid sparing, safe for chronic administration, avoids B-cell depletion, and is convenient.”
Blocking the BTK receptor on B cells puts the brakes on the B-cell mediated inflammatory pathway, preventing activation of monocytes, macrophages, mast cells, basophils, and neutrophils. At the same time, however, it does not deplete the B-cell population, said Dr. Murrell, the lead investigator.
The BELIEVE study comprised 27 patients with mild to severe pemphigus of an average 6 years’ duration. Most (18) had relapsing disease; the remainder had newly diagnosed pemphigus. A majority (16) had severe disease, as measured by a score of 15 or more on the Pemphigus Disease Activity Index (PDAI). Almost all (23) were positive for antidesmoglein antibodies. Only one patient was negative for antibodies.
The mean corticosteroid dose at baseline was 14 mg/day, although that ranged from no steroids to 30 mg/day.
The study consisted of a 12-week treatment phase and a 12-week follow-up phase. During treatment, patients could take no more than 0.5 mg/kg of prednisone daily, although with 400 mg PRN1008 twice a day. They were allowed to undertake rescue immunosuppression if they experienced a disease flare.
The primary endpoint was disease control by day 29 as evidenced by no new lesions. Secondary endpoints were complete remission, minimization of prednisone, quality of life, antibody levels, and clinician measures including the PDAI and the Autoimmune Bullous Skin Disorder Intensity Score.
By the end of week 4, 54% of patients had achieved the primary endpoint. The benefit continued to expand, with 73% reaching that response by the end of week 12. During this period, the mean prednisone dose was 12 mg/day.
Among the 24 patients who completed the study, complete remission occurred in 17% by week 12. However, patients continued to respond through the follow-up period, even after the study medication was stopped. By week 24, 25% of these patients experienced a complete remission. At the point of remission, the mean steroid dose was 8 mg/day. The median duration of remission was 2 months after stopping PRN1008.
The PDAI fell by a median of 70% by week 12 and was maintained at that level by the end of week 24. The median level of antidesmoglein autoantibodies fell by up to 65%. Again, the improvement continued throughout the off-drug follow-up period. In subgroup analyses, PRN1008 was more effective in patients with moderate to severe disease than those with mild disease (80% response vs. 64%). It was equally effective in those with newly diagnosed disease (75% vs. 72%) and regardless of antibody level at baseline.
The adverse event profile was relatively benign. Most side effects were mild and transient, and included upper abdominal pain, headache, and nausea. There were two mild infections and one serious infection, which presented in a patient with a long-standing localized cellulitis that activated and was associated a high fever. It was culture negative and PRN1008 was restarted without issue.
There was also one serious adverse event and one death, both unrelated to the study drug. One patient developed a pancreatic cyst that was discovered on day 29. The patient dropped out of the study to have elective surgery. The death occurred in a patient who developed acute respiratory failure on day 8 of treatment, caused by an undiagnosed congenital pulmonary sequestration. The patient died of a brain embolism shortly after lung surgery.
Dr. Murrell designed the study and was an investigator. She reported a financial relationship with Principia, as well as with numerous other pharmaceutical companies.
SOURCE: Murrell D et al. AAD 2019, Session S034.
WASHINGTON – An investigational molecule that blocks the downstream proinflammatory effects of B cells controlled disease activity and induced clinical remission in patients with pemphigus by 12 weeks.
At the end of a 24-week, open-label trial, a key driver of the sometimes-fatal blistering disease, Deedee Murrell, MD, said at the annual meeting of the American Academy of Dermatology.
The clinical efficacy plus a favorable safety profile supports the further development of the molecule, designed and manufactured by Principia Biopharma in San Francisco. The company is currently recruiting for a pivotal phase 3 trial of PRN1008 in 120 patients with moderate to severe pemphigus vulgaris.
Despite the recent approval of rituximab (Rituxan) for moderate to severe pemphigus, there remains an unmet need for a quick-acting, steroid-sparing, anti-inflammatory treatment, said Dr. Murrell, professor and head of the department of dermatology at the University of New South Wales, Sydney.
“We need something to use instead of high-dose steroids while we are waiting for rituximab to kick in, which can take 3 months,” and rituximab, which depletes B cells, puts patients at risk for infection, she said. “We need something that has rapid onset, is steroid sparing, safe for chronic administration, avoids B-cell depletion, and is convenient.”
Blocking the BTK receptor on B cells puts the brakes on the B-cell mediated inflammatory pathway, preventing activation of monocytes, macrophages, mast cells, basophils, and neutrophils. At the same time, however, it does not deplete the B-cell population, said Dr. Murrell, the lead investigator.
The BELIEVE study comprised 27 patients with mild to severe pemphigus of an average 6 years’ duration. Most (18) had relapsing disease; the remainder had newly diagnosed pemphigus. A majority (16) had severe disease, as measured by a score of 15 or more on the Pemphigus Disease Activity Index (PDAI). Almost all (23) were positive for antidesmoglein antibodies. Only one patient was negative for antibodies.
The mean corticosteroid dose at baseline was 14 mg/day, although that ranged from no steroids to 30 mg/day.
The study consisted of a 12-week treatment phase and a 12-week follow-up phase. During treatment, patients could take no more than 0.5 mg/kg of prednisone daily, although with 400 mg PRN1008 twice a day. They were allowed to undertake rescue immunosuppression if they experienced a disease flare.
The primary endpoint was disease control by day 29 as evidenced by no new lesions. Secondary endpoints were complete remission, minimization of prednisone, quality of life, antibody levels, and clinician measures including the PDAI and the Autoimmune Bullous Skin Disorder Intensity Score.
By the end of week 4, 54% of patients had achieved the primary endpoint. The benefit continued to expand, with 73% reaching that response by the end of week 12. During this period, the mean prednisone dose was 12 mg/day.
Among the 24 patients who completed the study, complete remission occurred in 17% by week 12. However, patients continued to respond through the follow-up period, even after the study medication was stopped. By week 24, 25% of these patients experienced a complete remission. At the point of remission, the mean steroid dose was 8 mg/day. The median duration of remission was 2 months after stopping PRN1008.
The PDAI fell by a median of 70% by week 12 and was maintained at that level by the end of week 24. The median level of antidesmoglein autoantibodies fell by up to 65%. Again, the improvement continued throughout the off-drug follow-up period. In subgroup analyses, PRN1008 was more effective in patients with moderate to severe disease than those with mild disease (80% response vs. 64%). It was equally effective in those with newly diagnosed disease (75% vs. 72%) and regardless of antibody level at baseline.
The adverse event profile was relatively benign. Most side effects were mild and transient, and included upper abdominal pain, headache, and nausea. There were two mild infections and one serious infection, which presented in a patient with a long-standing localized cellulitis that activated and was associated a high fever. It was culture negative and PRN1008 was restarted without issue.
There was also one serious adverse event and one death, both unrelated to the study drug. One patient developed a pancreatic cyst that was discovered on day 29. The patient dropped out of the study to have elective surgery. The death occurred in a patient who developed acute respiratory failure on day 8 of treatment, caused by an undiagnosed congenital pulmonary sequestration. The patient died of a brain embolism shortly after lung surgery.
Dr. Murrell designed the study and was an investigator. She reported a financial relationship with Principia, as well as with numerous other pharmaceutical companies.
SOURCE: Murrell D et al. AAD 2019, Session S034.
WASHINGTON – An investigational molecule that blocks the downstream proinflammatory effects of B cells controlled disease activity and induced clinical remission in patients with pemphigus by 12 weeks.
At the end of a 24-week, open-label trial, a key driver of the sometimes-fatal blistering disease, Deedee Murrell, MD, said at the annual meeting of the American Academy of Dermatology.
The clinical efficacy plus a favorable safety profile supports the further development of the molecule, designed and manufactured by Principia Biopharma in San Francisco. The company is currently recruiting for a pivotal phase 3 trial of PRN1008 in 120 patients with moderate to severe pemphigus vulgaris.
Despite the recent approval of rituximab (Rituxan) for moderate to severe pemphigus, there remains an unmet need for a quick-acting, steroid-sparing, anti-inflammatory treatment, said Dr. Murrell, professor and head of the department of dermatology at the University of New South Wales, Sydney.
“We need something to use instead of high-dose steroids while we are waiting for rituximab to kick in, which can take 3 months,” and rituximab, which depletes B cells, puts patients at risk for infection, she said. “We need something that has rapid onset, is steroid sparing, safe for chronic administration, avoids B-cell depletion, and is convenient.”
Blocking the BTK receptor on B cells puts the brakes on the B-cell mediated inflammatory pathway, preventing activation of monocytes, macrophages, mast cells, basophils, and neutrophils. At the same time, however, it does not deplete the B-cell population, said Dr. Murrell, the lead investigator.
The BELIEVE study comprised 27 patients with mild to severe pemphigus of an average 6 years’ duration. Most (18) had relapsing disease; the remainder had newly diagnosed pemphigus. A majority (16) had severe disease, as measured by a score of 15 or more on the Pemphigus Disease Activity Index (PDAI). Almost all (23) were positive for antidesmoglein antibodies. Only one patient was negative for antibodies.
The mean corticosteroid dose at baseline was 14 mg/day, although that ranged from no steroids to 30 mg/day.
The study consisted of a 12-week treatment phase and a 12-week follow-up phase. During treatment, patients could take no more than 0.5 mg/kg of prednisone daily, although with 400 mg PRN1008 twice a day. They were allowed to undertake rescue immunosuppression if they experienced a disease flare.
The primary endpoint was disease control by day 29 as evidenced by no new lesions. Secondary endpoints were complete remission, minimization of prednisone, quality of life, antibody levels, and clinician measures including the PDAI and the Autoimmune Bullous Skin Disorder Intensity Score.
By the end of week 4, 54% of patients had achieved the primary endpoint. The benefit continued to expand, with 73% reaching that response by the end of week 12. During this period, the mean prednisone dose was 12 mg/day.
Among the 24 patients who completed the study, complete remission occurred in 17% by week 12. However, patients continued to respond through the follow-up period, even after the study medication was stopped. By week 24, 25% of these patients experienced a complete remission. At the point of remission, the mean steroid dose was 8 mg/day. The median duration of remission was 2 months after stopping PRN1008.
The PDAI fell by a median of 70% by week 12 and was maintained at that level by the end of week 24. The median level of antidesmoglein autoantibodies fell by up to 65%. Again, the improvement continued throughout the off-drug follow-up period. In subgroup analyses, PRN1008 was more effective in patients with moderate to severe disease than those with mild disease (80% response vs. 64%). It was equally effective in those with newly diagnosed disease (75% vs. 72%) and regardless of antibody level at baseline.
The adverse event profile was relatively benign. Most side effects were mild and transient, and included upper abdominal pain, headache, and nausea. There were two mild infections and one serious infection, which presented in a patient with a long-standing localized cellulitis that activated and was associated a high fever. It was culture negative and PRN1008 was restarted without issue.
There was also one serious adverse event and one death, both unrelated to the study drug. One patient developed a pancreatic cyst that was discovered on day 29. The patient dropped out of the study to have elective surgery. The death occurred in a patient who developed acute respiratory failure on day 8 of treatment, caused by an undiagnosed congenital pulmonary sequestration. The patient died of a brain embolism shortly after lung surgery.
Dr. Murrell designed the study and was an investigator. She reported a financial relationship with Principia, as well as with numerous other pharmaceutical companies.
SOURCE: Murrell D et al. AAD 2019, Session S034.
REPORTING FROM AAD 2019
Concurrent Keratoacanthomas and Nonsarcoidal Granulomatous Reactions in New and Preexisting Tattoos
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
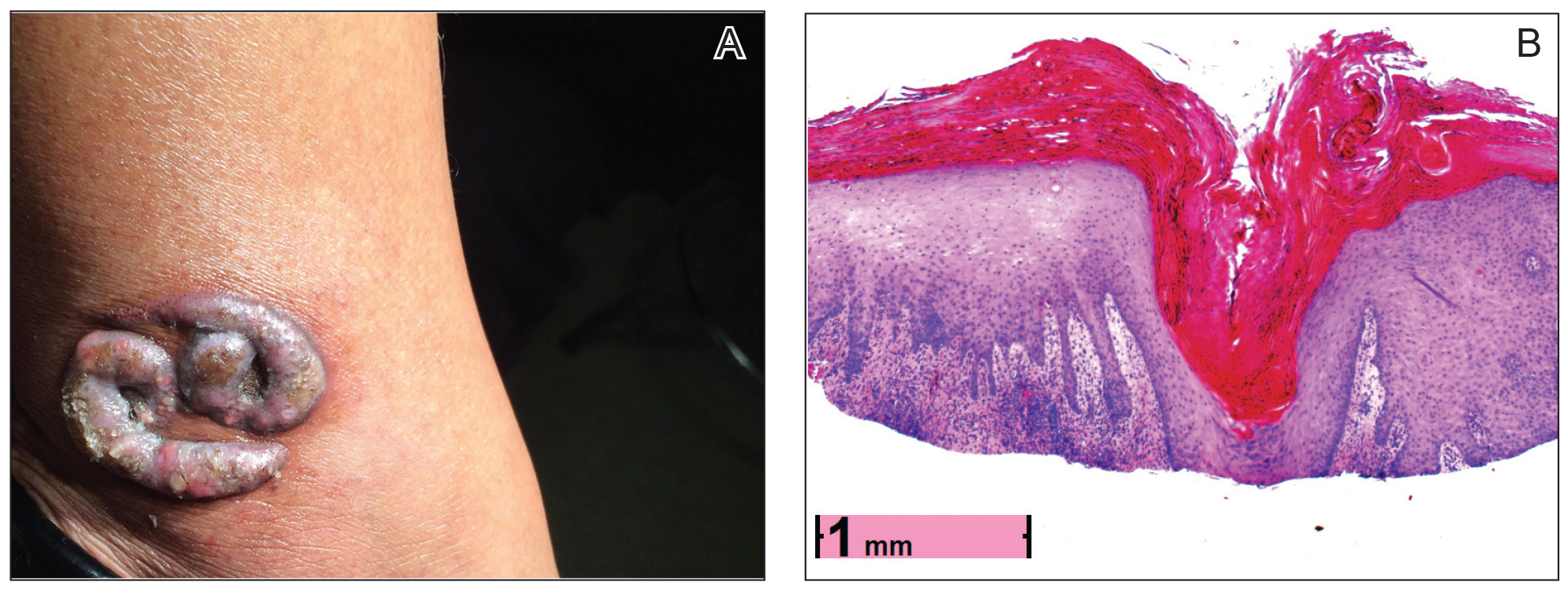
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.

contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
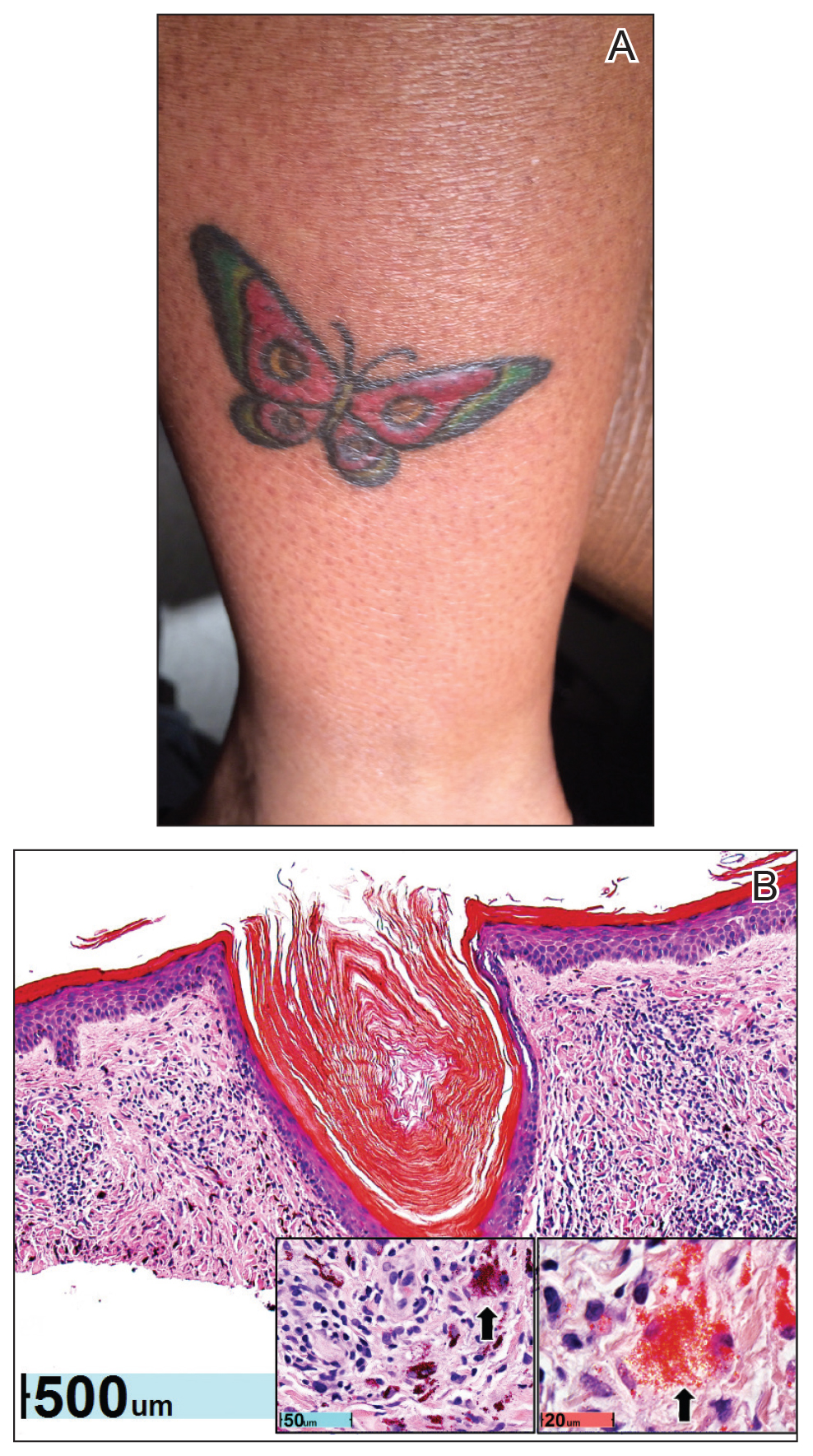
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
Practice Points
- Keratoacanthomas (KAs) are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution.
- The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, scarring disorders, and trauma (including tattoos).
- Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Our case adds additional weight to the idea that some KAs are primarily reactive phenomena sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
List of medications linked to drug-induced lupus expands
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
This new and updated list of possible lupus-inducing drugs includes a growing range of treatment categories, chemical structures, and pharmacologic actions. Yet it is still unclear what the common denominator is that links them.
Drug-induced lupus (DIL) is a peculiar adverse drug reaction that appears to be unrelated to any known property of the inducing agent, although cytokine modulating biologics are a possible exception. Nevertheless, the in vivo metabolism of dissimilar drugs to products with a common, reactive property may go some way to explaining how compounds with different pharmacologic and chemical structures could induce similar adverse reactions.
The findings by Arnaud et al. need better documentation than just positive pharmacovigilance signals. For example, a drug with a relatively high signal does not necessarily translate to a high propensity for causing lupus-like symptoms. It may be a reflection of high drug usage or an awareness of the report contributors for detecting new-onset systemic lupus erythematosus.
Regardless, this research serves to help and inform the medical community to increase the vigilance of previously unreported DIL and perhaps motivate the publication of novel, convincing case reports.
Robert L. Rubin, PhD, is with the University of New Mexico, Albuquerque. His comments are adapted from an editorial accompanying the report by Arnaud et al. (Ann Rheum Dis. 2019 Feb 13. doi: annrheumdis-2018-214785). He reported having no relevant disclosures.
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
leaving the overall number now standing at 118.
Among the 118 suspected drugs found in VigiBase, the WHO’s global deduplicated individual case safety reports (ICSR) database, 42 had not been previously reported in association with drug-induced lupus (DIL) and 76 had been previously reported in association with DIL in Medline. DIL was reported as a serious adverse event in 55.4% of cases, according to French researchers led by Laurent Arnaud, MD, PhD, of the department of rheumatology at Hôpitaux Universitaires de Strasbourg and Centre National de Références des Maladies Systémiques Rares, Strasbourg, France.
Dr. Arnaud and his colleagues conducted a case-noncase analysis for each drug associated with DIL in order to compare the proportion of specific adverse drug reactions (ADRs) reported for a single drug with the proportion of the same ADR for all other treatments in VigiBase, which receives reports from more than 130 country members of the WHO Programme for International Drug Monitoring and contains over 16 million deduplicated ICSRs recorded by pharmacovigilance centers since 1967. They searched for cases classified as systemic lupus erythematosus (SLE) and identified 12,166 ICSRs of DIL; from these they found 118 suspected drugs with significant pharmacovigilance signal from 8,163 ICSRs that mostly originated from the Americas (65%) and Europe (23%).
In line with what the study authors expected, the drugs associated with the highest number of DIL cases were the antitumor necrosis factor agents infliximab, adalimumab, and etanercept, and the drugs associated with the highest disproportional reporting of DIL were procainamide and hydralazine.
“This is an important finding because these are the two drugs associated with the highest risk of DIL in the literature, therefore confirming the reliability of our approach using a large pharmacovigilance database,” the researchers wrote in Annals of the Rheumatic Diseases.
Overall, DIL was considered definite for 9 drugs (procainamide, hydralazine, minocycline, quinidine, isoniazid, terbinafine, methyldopa, dihydralazine, and chlorpromazine), probable for 19 drugs, and possible for 45 drugs.
The median age of DIL onset was 49 years, which the authors noted was about 2 decades older than that of spontaneous SLE.
They also observed a marked predominance in females (female to male sex ratio, 4.3), a finding that contrasted with previous studies reporting a female to male sex ratio closer to 1:1.
Dr. Arnaud and his colleagues stated that their finding of a median delay between the reported start of suspected treatment and DIL occurrence of 172 days (interquartile range, 35-610 days) suggested that DIL mostly appears after a few months and usually within the first 2 years of treatment with the suspected drug.
“The analysis of the median reporting years for each suspected drug shows a clear evolution of suspected drugs during the past decades. This further underlines that the constantly changing spectrum of DIL should be monitored continuously, and further validates the interest of our approach using the WHO international pharmacovigilance database, the biggest database of this kind with over 16 million deduplicated ICSRs,” they wrote.
The researchers added that distinguishing DIL from SLE is important because its prognosis is usually good when the drug is withdrawn, but the spectrum of DIL is constantly evolving, with drugs once described as strongly linked to DIL now prescribed less frequently.
“The first case of DIL was reported in 1945 with sulfadiazine, while hydralazine DIL was first reported in 1953. Since then, pharmacopoeia has strongly evolved, and one could hypothesize that so has the spectrum of drugs that can induce DIL,” they wrote.
“The detailed list of suspected drugs may prove useful to physicians when confronted with potential DIL cases. Altogether, these findings may help in improving the identification of this constantly evolving disease,” they concluded.
The current study was limited by the lack of a uniform set of criteria for the diagnosis of DIL and by the level of reported details available in VigiBase.
The authors had no outside funding for the study and reported having no conflicts of interest.
SOURCE: Arnaud L et al. Ann Rheum Dis. 2019 Feb 4. doi: 10.1136/annrheumdis-2018-214598.
FROM ANNALS OF THE RHEUMATIC DISEASES
New findings raise questions about the role of ANAs in SLE
Antinuclear antibodies (ANAs) have long been considered an important marker in rheumatologic conditions, particularly for the diagnosis and classification of patients with systemic lupus erythematosus, but recent findings are raising new questions about their role.
“We’ve measured ANAs for a long time – it’s a very important test in rheumatology,” David S. Pisetsky, MD, PhD, explained in an interview.
However, even though this test has been around for decades, “some interesting things have developed around it that have made a lot of people, including me, take a second look,” said Dr. Pisetsky, professor of medicine and immunology at Duke University, Durham, N.C.
He elaborated on those recent findings, which relate to the findings of ANA negativity in patients with an established diagnosis of systemic lupus erythematosus (SLE) and to variability among ANA test kit findings, during a presentation at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Screening of patients during clinical trials for new treatments of SLE suggest that a significant number of people with lupus – 20%-30%, in fact – are ANA negative despite disease activity at the time the test is done,” he said.
For example, unpublished (but recently submitted) data from a phase 2 trial looking at the efficacy and safety of an interleukin-6 monoclonal antibody for the treatment of SLE showed that 23.8% of baseline samples from 183 SLE patients with positive historical ANA and clinically active lupus prior to randomization were ANA negative.
A particular concern with respect to such findings is that ANA positivity is typically a criterion for entry into clinical trials of therapies for lupus and prescription of medications approved for active lupus, Dr. Pisetsky said.
“On the other hand, about 20% of otherwise healthy people – especially women – can be ANA positive, so it’s always been problematic as a screening test due to these false positives, but these new findings suggest that in lupus a real concern is false negatives,” he said. “It’s quite a surprise.”
The findings raise questions about whether ANA negativity in SLE reflects the natural history of the disease, an effect of treatments, or a problem with the assays.
It appears an important problem relates to test kit variability, he said.
“There are lots of different ANA test kits. Their performance characteristics are very different. The performance of ANA tests is much more variable than people realize,” he said, citing data from an analysis that he and his colleagues conducted using 103 samples from a cohort of patients with established SLE.
In that 2017 study, an ANA enzyme-linked immunosorbent assay showed an ANA-negativity rate of 11.7% with zero indeterminate tests, whereas three different test kits showed ANA-negativity rates of 22.3% (with 8.7% of samples reported as indeterminate), 9.7% (with another 9.7% indeterminate), and 4.9% (with another 1.9% indeterminate), respectively. Multiplex testing showed a 13.6% ANA-negativity rate and an indeterminate rate of 7.8% (Ann Rheum Dis. 2018;77:911-3).
Only one sample tested negative for ANA on all three test kits, and disagreement about ANA negativity occurred in one-third of the samples, he said.
Anti–double-stranded DNA assays
Recent findings also raise questions about the use of assays that specifically assess for anti–double-stranded DNA (anti-dsDNA) antibodies, which are highly associated with SLE and have been used as a biomarker for the disease, Dr. Pisetsky said.
For example, a comparison of two anti-dsDNA assays showed discordant results with respect to negativity for anti-dsDNA antibodies in 64 of 181 samples from SLE patients. One assay showed a 70.7% rate of anti-dsDNA negativity and the other showed a 37.6% rate.
The concern regarding test variability relates to the issue of ANA positivity and eligibility for study enrollment and certain treatments; test variability can affect the diagnosis of patients with SLE because ANA positivity is an important finding in routine clinical care, and for anti-dsDNA, test variability can affect assessment of disease activity, he explained.
Tests may differ in a number of ways, such as in their specificity, sensitivity, avidity, and range of epitopes detected. Unfortunately, not enough is known at this point to make specific recommendations regarding best test kits, and while there are alternative technologies that could be useful for ANA testing, none has been validated for particular use in the assessment of trial eligibility, Dr. Pisetsky said.
Nonetheless, awareness of the test variability is important, especially when it comes to assessing patients for trial eligibility and prescribing medications, he added. “For practical, real-world utilization, people need to know about this.”
Dr. Pisetsky reported receiving ANA-related research support from Pfizer, conducting collaborative research with Bio-Rad and EuroImmun, and serving as an adviser to ImmunArray.
Antinuclear antibodies (ANAs) have long been considered an important marker in rheumatologic conditions, particularly for the diagnosis and classification of patients with systemic lupus erythematosus, but recent findings are raising new questions about their role.
“We’ve measured ANAs for a long time – it’s a very important test in rheumatology,” David S. Pisetsky, MD, PhD, explained in an interview.
However, even though this test has been around for decades, “some interesting things have developed around it that have made a lot of people, including me, take a second look,” said Dr. Pisetsky, professor of medicine and immunology at Duke University, Durham, N.C.
He elaborated on those recent findings, which relate to the findings of ANA negativity in patients with an established diagnosis of systemic lupus erythematosus (SLE) and to variability among ANA test kit findings, during a presentation at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Screening of patients during clinical trials for new treatments of SLE suggest that a significant number of people with lupus – 20%-30%, in fact – are ANA negative despite disease activity at the time the test is done,” he said.
For example, unpublished (but recently submitted) data from a phase 2 trial looking at the efficacy and safety of an interleukin-6 monoclonal antibody for the treatment of SLE showed that 23.8% of baseline samples from 183 SLE patients with positive historical ANA and clinically active lupus prior to randomization were ANA negative.
A particular concern with respect to such findings is that ANA positivity is typically a criterion for entry into clinical trials of therapies for lupus and prescription of medications approved for active lupus, Dr. Pisetsky said.
“On the other hand, about 20% of otherwise healthy people – especially women – can be ANA positive, so it’s always been problematic as a screening test due to these false positives, but these new findings suggest that in lupus a real concern is false negatives,” he said. “It’s quite a surprise.”
The findings raise questions about whether ANA negativity in SLE reflects the natural history of the disease, an effect of treatments, or a problem with the assays.
It appears an important problem relates to test kit variability, he said.
“There are lots of different ANA test kits. Their performance characteristics are very different. The performance of ANA tests is much more variable than people realize,” he said, citing data from an analysis that he and his colleagues conducted using 103 samples from a cohort of patients with established SLE.
In that 2017 study, an ANA enzyme-linked immunosorbent assay showed an ANA-negativity rate of 11.7% with zero indeterminate tests, whereas three different test kits showed ANA-negativity rates of 22.3% (with 8.7% of samples reported as indeterminate), 9.7% (with another 9.7% indeterminate), and 4.9% (with another 1.9% indeterminate), respectively. Multiplex testing showed a 13.6% ANA-negativity rate and an indeterminate rate of 7.8% (Ann Rheum Dis. 2018;77:911-3).
Only one sample tested negative for ANA on all three test kits, and disagreement about ANA negativity occurred in one-third of the samples, he said.
Anti–double-stranded DNA assays
Recent findings also raise questions about the use of assays that specifically assess for anti–double-stranded DNA (anti-dsDNA) antibodies, which are highly associated with SLE and have been used as a biomarker for the disease, Dr. Pisetsky said.
For example, a comparison of two anti-dsDNA assays showed discordant results with respect to negativity for anti-dsDNA antibodies in 64 of 181 samples from SLE patients. One assay showed a 70.7% rate of anti-dsDNA negativity and the other showed a 37.6% rate.
The concern regarding test variability relates to the issue of ANA positivity and eligibility for study enrollment and certain treatments; test variability can affect the diagnosis of patients with SLE because ANA positivity is an important finding in routine clinical care, and for anti-dsDNA, test variability can affect assessment of disease activity, he explained.
Tests may differ in a number of ways, such as in their specificity, sensitivity, avidity, and range of epitopes detected. Unfortunately, not enough is known at this point to make specific recommendations regarding best test kits, and while there are alternative technologies that could be useful for ANA testing, none has been validated for particular use in the assessment of trial eligibility, Dr. Pisetsky said.
Nonetheless, awareness of the test variability is important, especially when it comes to assessing patients for trial eligibility and prescribing medications, he added. “For practical, real-world utilization, people need to know about this.”
Dr. Pisetsky reported receiving ANA-related research support from Pfizer, conducting collaborative research with Bio-Rad and EuroImmun, and serving as an adviser to ImmunArray.
Antinuclear antibodies (ANAs) have long been considered an important marker in rheumatologic conditions, particularly for the diagnosis and classification of patients with systemic lupus erythematosus, but recent findings are raising new questions about their role.
“We’ve measured ANAs for a long time – it’s a very important test in rheumatology,” David S. Pisetsky, MD, PhD, explained in an interview.
However, even though this test has been around for decades, “some interesting things have developed around it that have made a lot of people, including me, take a second look,” said Dr. Pisetsky, professor of medicine and immunology at Duke University, Durham, N.C.
He elaborated on those recent findings, which relate to the findings of ANA negativity in patients with an established diagnosis of systemic lupus erythematosus (SLE) and to variability among ANA test kit findings, during a presentation at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“Screening of patients during clinical trials for new treatments of SLE suggest that a significant number of people with lupus – 20%-30%, in fact – are ANA negative despite disease activity at the time the test is done,” he said.
For example, unpublished (but recently submitted) data from a phase 2 trial looking at the efficacy and safety of an interleukin-6 monoclonal antibody for the treatment of SLE showed that 23.8% of baseline samples from 183 SLE patients with positive historical ANA and clinically active lupus prior to randomization were ANA negative.
A particular concern with respect to such findings is that ANA positivity is typically a criterion for entry into clinical trials of therapies for lupus and prescription of medications approved for active lupus, Dr. Pisetsky said.
“On the other hand, about 20% of otherwise healthy people – especially women – can be ANA positive, so it’s always been problematic as a screening test due to these false positives, but these new findings suggest that in lupus a real concern is false negatives,” he said. “It’s quite a surprise.”
The findings raise questions about whether ANA negativity in SLE reflects the natural history of the disease, an effect of treatments, or a problem with the assays.
It appears an important problem relates to test kit variability, he said.
“There are lots of different ANA test kits. Their performance characteristics are very different. The performance of ANA tests is much more variable than people realize,” he said, citing data from an analysis that he and his colleagues conducted using 103 samples from a cohort of patients with established SLE.
In that 2017 study, an ANA enzyme-linked immunosorbent assay showed an ANA-negativity rate of 11.7% with zero indeterminate tests, whereas three different test kits showed ANA-negativity rates of 22.3% (with 8.7% of samples reported as indeterminate), 9.7% (with another 9.7% indeterminate), and 4.9% (with another 1.9% indeterminate), respectively. Multiplex testing showed a 13.6% ANA-negativity rate and an indeterminate rate of 7.8% (Ann Rheum Dis. 2018;77:911-3).
Only one sample tested negative for ANA on all three test kits, and disagreement about ANA negativity occurred in one-third of the samples, he said.
Anti–double-stranded DNA assays
Recent findings also raise questions about the use of assays that specifically assess for anti–double-stranded DNA (anti-dsDNA) antibodies, which are highly associated with SLE and have been used as a biomarker for the disease, Dr. Pisetsky said.
For example, a comparison of two anti-dsDNA assays showed discordant results with respect to negativity for anti-dsDNA antibodies in 64 of 181 samples from SLE patients. One assay showed a 70.7% rate of anti-dsDNA negativity and the other showed a 37.6% rate.
The concern regarding test variability relates to the issue of ANA positivity and eligibility for study enrollment and certain treatments; test variability can affect the diagnosis of patients with SLE because ANA positivity is an important finding in routine clinical care, and for anti-dsDNA, test variability can affect assessment of disease activity, he explained.
Tests may differ in a number of ways, such as in their specificity, sensitivity, avidity, and range of epitopes detected. Unfortunately, not enough is known at this point to make specific recommendations regarding best test kits, and while there are alternative technologies that could be useful for ANA testing, none has been validated for particular use in the assessment of trial eligibility, Dr. Pisetsky said.
Nonetheless, awareness of the test variability is important, especially when it comes to assessing patients for trial eligibility and prescribing medications, he added. “For practical, real-world utilization, people need to know about this.”
Dr. Pisetsky reported receiving ANA-related research support from Pfizer, conducting collaborative research with Bio-Rad and EuroImmun, and serving as an adviser to ImmunArray.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Novel SSc classification scheme aims to improve risk stratification
CHICAGO – A simple new classification scheme that combines autoantibody specificity and extent of skin involvement could improve risk stratification of patients with systemic sclerosis, according to researchers at University College London.
“The Le Roy et al. classification of SSc [systemic sclerosis] into limited and diffuse cutaneous subtype remains the most commonly used classification system for systemic sclerosis, but autoantibodies are much better predictors of organ involvement, and while more sophisticated approaches exist, this proposed simple classification using antibodies and skin subset is relevant to clinical practice and could help risk stratification,” Svetlana I. Nihtyanova, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Nihtyanova, a clinical research fellow at University College London, reported how she and her colleagues at UCL divided 1,025 SSc patients into 12 subgroups based on skin subset and autoantibodies and then conducted Kaplan-Meier estimates of survival and cumulative incidence of organ complications to rank these 12 subgroups by endpoint estimates. They merged subgroups with similar ranking in multiple endpoints, ending up with seven groups in the final classification.
Group 1 comprised anti–centromere antibody–positive limited cutaneous SSc (lcSSc) patients and accounted for 29% of patients.
“This was the subgroup with the highest survival (72%) and the lowest incidence of pulmonary fibrosis (13%) and scleroderma renal crisis (no cases) at 20 years from onset,” she said, noting that the incidence of pulmonary hypertension in this group was similar to the average for the whole cohort.
Group 2 comprised all anti–RNA polymerase antibody–positive subjects and accounted for 11% of patients. This group had the highest incidence of scleroderma renal crisis (SRC; 32% at 20 years), but other organ complications and survival were similar to the cohort average.
Group 3 comprised Scl-70–positive lcSSc patients, and accounted for 11% of patients.
“Although incidence of pulmonary fibrosis in this group was the second highest (69% at 20 years), other complications were rare,” Dr. Nihtyanova said, adding that this group had the lowest incidence of pulmonary hypertension (6%) and the second lowest incidence of SRC (3%) at 20 years.
Group 4, conversely, included Scl-70–positive dcSSc patients and accounted for 11% of patients, who had a very poor prognosis; they had the highest incidence of pulmonary fibrosis (91%) and cardiac scleroderma (14%), and the worst survival (41%) at 20 years, she said.
Group 5 included all U3 RNP–positive patients, accounting for 5% of patients.
“Although survival in this group was not bad (70% at 20 years), the group had the highest pulmonary hypertension incidence (40%) and the second highest incidence of cardiac SSc (11%) at 20 years,” she noted.
Groups 6 and 7 (comprising 22% and 11% of study subjects, respectively) included lcSSc and diffuse cutaneous SSc (dcSSc) patients with other antibody specificities. Group 6 had low overall SRC and cardiac SSc risk, while other outcomes were similar to the cohort average. Group 7, however, had poor prognosis, with the second lowest survival (42% at 20 years) and above average rates of organ disease, particularly pulmonary fibrosis and SRC, she said.
Overall, estimated survival for the entire cohort was 60% at 20 years from onset, and in that time frame 44% developed significant pulmonary fibrosis, 25% pulmonary hypertension, 7% SRC, and 6% cardiac SSc. The patients had a mean age of 47 years at disease onset, and 16% were men. Diffuse cutaneous SSc was diagnosed in 35% of the subjects, she noted.
Dr. Nihtyanova reported having no disclosures.
SOURCE: Nihtyanova S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2935.
CHICAGO – A simple new classification scheme that combines autoantibody specificity and extent of skin involvement could improve risk stratification of patients with systemic sclerosis, according to researchers at University College London.
“The Le Roy et al. classification of SSc [systemic sclerosis] into limited and diffuse cutaneous subtype remains the most commonly used classification system for systemic sclerosis, but autoantibodies are much better predictors of organ involvement, and while more sophisticated approaches exist, this proposed simple classification using antibodies and skin subset is relevant to clinical practice and could help risk stratification,” Svetlana I. Nihtyanova, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Nihtyanova, a clinical research fellow at University College London, reported how she and her colleagues at UCL divided 1,025 SSc patients into 12 subgroups based on skin subset and autoantibodies and then conducted Kaplan-Meier estimates of survival and cumulative incidence of organ complications to rank these 12 subgroups by endpoint estimates. They merged subgroups with similar ranking in multiple endpoints, ending up with seven groups in the final classification.
Group 1 comprised anti–centromere antibody–positive limited cutaneous SSc (lcSSc) patients and accounted for 29% of patients.
“This was the subgroup with the highest survival (72%) and the lowest incidence of pulmonary fibrosis (13%) and scleroderma renal crisis (no cases) at 20 years from onset,” she said, noting that the incidence of pulmonary hypertension in this group was similar to the average for the whole cohort.
Group 2 comprised all anti–RNA polymerase antibody–positive subjects and accounted for 11% of patients. This group had the highest incidence of scleroderma renal crisis (SRC; 32% at 20 years), but other organ complications and survival were similar to the cohort average.
Group 3 comprised Scl-70–positive lcSSc patients, and accounted for 11% of patients.
“Although incidence of pulmonary fibrosis in this group was the second highest (69% at 20 years), other complications were rare,” Dr. Nihtyanova said, adding that this group had the lowest incidence of pulmonary hypertension (6%) and the second lowest incidence of SRC (3%) at 20 years.
Group 4, conversely, included Scl-70–positive dcSSc patients and accounted for 11% of patients, who had a very poor prognosis; they had the highest incidence of pulmonary fibrosis (91%) and cardiac scleroderma (14%), and the worst survival (41%) at 20 years, she said.
Group 5 included all U3 RNP–positive patients, accounting for 5% of patients.
“Although survival in this group was not bad (70% at 20 years), the group had the highest pulmonary hypertension incidence (40%) and the second highest incidence of cardiac SSc (11%) at 20 years,” she noted.
Groups 6 and 7 (comprising 22% and 11% of study subjects, respectively) included lcSSc and diffuse cutaneous SSc (dcSSc) patients with other antibody specificities. Group 6 had low overall SRC and cardiac SSc risk, while other outcomes were similar to the cohort average. Group 7, however, had poor prognosis, with the second lowest survival (42% at 20 years) and above average rates of organ disease, particularly pulmonary fibrosis and SRC, she said.
Overall, estimated survival for the entire cohort was 60% at 20 years from onset, and in that time frame 44% developed significant pulmonary fibrosis, 25% pulmonary hypertension, 7% SRC, and 6% cardiac SSc. The patients had a mean age of 47 years at disease onset, and 16% were men. Diffuse cutaneous SSc was diagnosed in 35% of the subjects, she noted.
Dr. Nihtyanova reported having no disclosures.
SOURCE: Nihtyanova S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2935.
CHICAGO – A simple new classification scheme that combines autoantibody specificity and extent of skin involvement could improve risk stratification of patients with systemic sclerosis, according to researchers at University College London.
“The Le Roy et al. classification of SSc [systemic sclerosis] into limited and diffuse cutaneous subtype remains the most commonly used classification system for systemic sclerosis, but autoantibodies are much better predictors of organ involvement, and while more sophisticated approaches exist, this proposed simple classification using antibodies and skin subset is relevant to clinical practice and could help risk stratification,” Svetlana I. Nihtyanova, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Nihtyanova, a clinical research fellow at University College London, reported how she and her colleagues at UCL divided 1,025 SSc patients into 12 subgroups based on skin subset and autoantibodies and then conducted Kaplan-Meier estimates of survival and cumulative incidence of organ complications to rank these 12 subgroups by endpoint estimates. They merged subgroups with similar ranking in multiple endpoints, ending up with seven groups in the final classification.
Group 1 comprised anti–centromere antibody–positive limited cutaneous SSc (lcSSc) patients and accounted for 29% of patients.
“This was the subgroup with the highest survival (72%) and the lowest incidence of pulmonary fibrosis (13%) and scleroderma renal crisis (no cases) at 20 years from onset,” she said, noting that the incidence of pulmonary hypertension in this group was similar to the average for the whole cohort.
Group 2 comprised all anti–RNA polymerase antibody–positive subjects and accounted for 11% of patients. This group had the highest incidence of scleroderma renal crisis (SRC; 32% at 20 years), but other organ complications and survival were similar to the cohort average.
Group 3 comprised Scl-70–positive lcSSc patients, and accounted for 11% of patients.
“Although incidence of pulmonary fibrosis in this group was the second highest (69% at 20 years), other complications were rare,” Dr. Nihtyanova said, adding that this group had the lowest incidence of pulmonary hypertension (6%) and the second lowest incidence of SRC (3%) at 20 years.
Group 4, conversely, included Scl-70–positive dcSSc patients and accounted for 11% of patients, who had a very poor prognosis; they had the highest incidence of pulmonary fibrosis (91%) and cardiac scleroderma (14%), and the worst survival (41%) at 20 years, she said.
Group 5 included all U3 RNP–positive patients, accounting for 5% of patients.
“Although survival in this group was not bad (70% at 20 years), the group had the highest pulmonary hypertension incidence (40%) and the second highest incidence of cardiac SSc (11%) at 20 years,” she noted.
Groups 6 and 7 (comprising 22% and 11% of study subjects, respectively) included lcSSc and diffuse cutaneous SSc (dcSSc) patients with other antibody specificities. Group 6 had low overall SRC and cardiac SSc risk, while other outcomes were similar to the cohort average. Group 7, however, had poor prognosis, with the second lowest survival (42% at 20 years) and above average rates of organ disease, particularly pulmonary fibrosis and SRC, she said.
Overall, estimated survival for the entire cohort was 60% at 20 years from onset, and in that time frame 44% developed significant pulmonary fibrosis, 25% pulmonary hypertension, 7% SRC, and 6% cardiac SSc. The patients had a mean age of 47 years at disease onset, and 16% were men. Diffuse cutaneous SSc was diagnosed in 35% of the subjects, she noted.
Dr. Nihtyanova reported having no disclosures.
SOURCE: Nihtyanova S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 2935.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The classification scheme for SSc risk stratification identified seven distinct SSc subgroups.
Study details: Development and testing of a novel risk classification scheme in 1,025 SSc patients.
Disclosures: Dr. Nihtyanova reported having no disclosures.
Source: Nihtyanova S et al. Arthritis Rheumatol. 2018;70(Suppl 10):Abstract 2935.
Increased cancer risk in dermatomyositis has temporal limits
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
The increased risk of cancer associated with anti-TIF1-Ab-positive dermatomyositis is limited almost exclusively to 3 years on either side of the onset of dermatomyositis, new research suggests.
Idiopathic inflammatory myopathy have been associated with malignancy, in particular dermatomyositis (DM) and the DM-specific antitranscriptional intermediary factor 1 antibody (anti-TIF1-Ab).
Around one-fifth of the 236 patients diagnosed with DM in the current study, published online Dec. 7 in Rheumatology, were anti-TIF1-Ab positive, and these patients had a more than threefold higher risk of developing cancer comapared with patients who were anti-TIF1-Ab negative (hazard ratio = 3.4, 95% confidence interval, 2.2-5.4; P less than .01).
Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
However, all the cancers in the anti-TIF1-Ab-positive group occurred within the 3 years before the onset of DM or within 2.5 years after onset. No anti-TIF1-Ab-positive patients developed cancers after this time, but some patients in the anti-TIF1-Ab-negative group did.
“This finding is not likely to be due to a disparity in follow-up time between anti-TIF1-Ab-positive and -negative cases, as the median follow-up times were similar for both groups: 10 years and 12 years, respectively,” wrote Alexander Oldroyd, MBChB, a clinical research fellow in the Centre for Musculoskeletal Research at the University of Manchester (England), and his coauthors. “Further, this finding is unlikely to be due to differences in cancer detection methods, as both cohorts’ cancer diagnoses were identified through HSCIC [U.K. Health and Social Care Information Centre] data, ensuring capture of all incident cancers during the follow-up period.”
Anti-TIF1-Ab-positive patients were more likely to develop cancer if they were older. None of the 15 anti-TIF1-Ab-positive patients who were aged under 39 when they developed DM went on to develop cancer. But cancer developed in around half of the anti-TIF1-Ab-positive patients who were aged 39 years or older when their DM began.
The anti-TIF1 antibody is commonly found in juvenile DM, but previous research has not found an association with an increased risk of cancer in this younger patient population.
“Our findings add strength to the hypothesis that there exists a subset of young adult anti-TIF1-Ab-positive cases who do not have a discernible increased risk of cancer, similar to that observed in TIF1-Ab-positive juvenile DM,” the authors wrote. They suggested that given the increased risk of malignancy in older patients who were anti-TIF1-Ab positive, this group should be subject to more detailed cancer screening.
Breast cancer was the most common malignancy among both anti-TIF1-Ab-positive and anti-TIF1-Ab-negative patients (33% and 25%, respectively). However, ovarian cancer was significantly more common among the anti-TIF1-Ab-positive patients than among the anti-TIF1-Ab-negative patients (19% vs. 2%; P less than .05); four of the five ovarian cancers in the entire cohort occurred in the anti-TIF1-Ab-positive group.
The authors noted that this confirmed the finding of a number of previous studies suggesting an increased risk of ovarian cancer with DM.
“However, this is the first large study to identify that ovarian cancer is overrepresented in anti-TIF1-Ab-positive individuals, suggesting that the true association between DM and ovarian cancer may be through possession of anti-TIF1-Abs,” they noted.
The authors wrote that they had aimed to inform cancer screening strategies among patients with DM.
“It may be that a focus on screening for cancer within the first 3 years after DM onset and particularly screening for ovarian cancer in anti-TIF1-Ab-positive female patients may be required,” they wrote. “Our findings also strengthen the hypothesis that inflammatory myopathies represent a paraneoplastic reaction initiated by attempted immune-mediated clearance of a cancer.”
The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
SOURCE: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
FROM RHEUMATOLOGY
Key clinical point: Patients with dermatomyositis are at increased risk of cancer only in the 3-year periods before and after the onset of dermatomyositis.
Major finding: Overall, 38% of patients in the anti-TIF1-Ab-positive group developed cancer during the 10-year follow-up, compared with 15% of patients with anti-TIF1-Ab-negative DM.
Study details: Cohort study of 236 people with dermatomyositis.
Disclosures: The study was supported by Arthritis Research UK, Myositis UK, the European Science Foundation for EuMyoNet, Association Francaise Contre Les Myopathies, the Medical Research Council, and the Manchester Academic Health Science Centre. No conflicts of interest were declared.
Source: Oldroyd A et al. Rheumatology. 2018 Dec 7. doi: 10.1093/rheumatology/key357.
Combination immunotherapy ups survival in ILD patients with anti-MDA5–positive dermatomyositis
CHICAGO – Early treatment with combined high-dose glucocorticoids, tacrolimus, and intravenous cyclophosphamide therapy significantly improves survival vs. step-up therapy in interstitial lung disease patients with anti–melanoma differentiation–associated gene 5 (anti-MDA5)–positive dermatomyositis, according to findings from a prospective, multicenter study.
However, the combination therapy was associated with a high risk of cytomegalovirus reactivation and other opportunistic infections that warrants careful monitoring of treated patients, Hideaki Tsuji, MD, reported at the annual meeting of the American College of Rheumatology.
ILD accompanied by anti-MDA5–positive dermatomyositis (DM) is often intractable and associated with high mortality in Japanese patients. Case reports have suggested improved outcomes with combined immunosuppressive therapy, but a standard treatment has not been established, said Dr. Tsuji of Kyoto University.
“Therefore, we evaluated the efficacy and safety of combined immunosuppressive therapy for anti-MDA5–positive DM with ILD in a prospective single-arm study,” he said, adding that early administration, a short interval of intravenous cyclophosphamide, use of plasmapheresis as an additional therapy, and control of opportunistic infections may contribute to the improved outcomes seen with the regimen in this study.
The primary endpoint of 6-month survival was reached by 24 (89%) of 27 patients treated with the combination regimen for 52 weeks, compared with 5 (33%) of 15 historical controls who received high-dose glucocorticoids followed by step-wise addition of immunosuppressants. At 12 months, the survival rates were 85% and 33%, respectively, Dr. Tsuji said.
Additionally, anti-MDA5 titer, serum ferritin level, C-reactive protein level, lactate dehydrogenase, and KL-6 level gradually decreased over the 52 months, and percent vital capacity increased with combination vs. step-up therapy, he noted.
Cytomegalovirus reactivation occurred in 90% of combination regimen patients vs. 33% of controls over the 52-week study period, he said, adding that pneumocystic pneumonia and sepsis also occurred in combination regimen group patients, and were associated with death in four patients.
When the 23 surviving patients in the combination regimen group were compared with the 4 in the group who died, it was noted that the deceased patients were significantly more likely to have cutaneous ulcers (75% vs. 13%), higher mean C-reactive protein level (2.7 vs. 0.77 mg/dL), and higher creatine kinase level (644.3 vs. 219.3 IU/L), respectively, before treatment, he said.
Study subjects were Japanese adults with new-onset anti-MDA5–positive dermatomyositis with interstitial lung disease (ILD) who were enrolled between July 2014 and September 2017.
They were treated with 1 mg/kg/day of prednisolone for 4 weeks with reduced doses thereafter, 500-1,000 mg/m2 of IV cyclophosphamide every 2 weeks for six cycles then every 4 weeks for up to a total of 10-15 treatments, and 10-12 ng/mL of tacrolimus (12-hour trough). Plasmapheresis was allowed in patients who progressed and needed oxygenation after the regimen was initiated, and it was administered in nine patients (31%) in the combination regimen group vs. one (7%) of the historical controls.
Given the different frequencies of rapidly progressive ILD in Asian vs. Western countries (39%-71% vs. 22%-57%, respectively), it is unclear whether the results seen in this study can be extrapolated to patients from the United States and Europe. Therefore, it is necessary to analyze the efficacy of the regimen in those patient populations, Dr. Tsuji said, also noting that future studies should evaluate risk-based modifications of the regimen to identify the optimal treatment for individuals based on factors such as age, respiratory dysfunction, hyperferritinemia, and treatment delay.
Dr. Tsuji reported having no disclosures.
SOURCE: Tsuji H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 838.
CHICAGO – Early treatment with combined high-dose glucocorticoids, tacrolimus, and intravenous cyclophosphamide therapy significantly improves survival vs. step-up therapy in interstitial lung disease patients with anti–melanoma differentiation–associated gene 5 (anti-MDA5)–positive dermatomyositis, according to findings from a prospective, multicenter study.
However, the combination therapy was associated with a high risk of cytomegalovirus reactivation and other opportunistic infections that warrants careful monitoring of treated patients, Hideaki Tsuji, MD, reported at the annual meeting of the American College of Rheumatology.
ILD accompanied by anti-MDA5–positive dermatomyositis (DM) is often intractable and associated with high mortality in Japanese patients. Case reports have suggested improved outcomes with combined immunosuppressive therapy, but a standard treatment has not been established, said Dr. Tsuji of Kyoto University.
“Therefore, we evaluated the efficacy and safety of combined immunosuppressive therapy for anti-MDA5–positive DM with ILD in a prospective single-arm study,” he said, adding that early administration, a short interval of intravenous cyclophosphamide, use of plasmapheresis as an additional therapy, and control of opportunistic infections may contribute to the improved outcomes seen with the regimen in this study.
The primary endpoint of 6-month survival was reached by 24 (89%) of 27 patients treated with the combination regimen for 52 weeks, compared with 5 (33%) of 15 historical controls who received high-dose glucocorticoids followed by step-wise addition of immunosuppressants. At 12 months, the survival rates were 85% and 33%, respectively, Dr. Tsuji said.
Additionally, anti-MDA5 titer, serum ferritin level, C-reactive protein level, lactate dehydrogenase, and KL-6 level gradually decreased over the 52 months, and percent vital capacity increased with combination vs. step-up therapy, he noted.
Cytomegalovirus reactivation occurred in 90% of combination regimen patients vs. 33% of controls over the 52-week study period, he said, adding that pneumocystic pneumonia and sepsis also occurred in combination regimen group patients, and were associated with death in four patients.
When the 23 surviving patients in the combination regimen group were compared with the 4 in the group who died, it was noted that the deceased patients were significantly more likely to have cutaneous ulcers (75% vs. 13%), higher mean C-reactive protein level (2.7 vs. 0.77 mg/dL), and higher creatine kinase level (644.3 vs. 219.3 IU/L), respectively, before treatment, he said.
Study subjects were Japanese adults with new-onset anti-MDA5–positive dermatomyositis with interstitial lung disease (ILD) who were enrolled between July 2014 and September 2017.
They were treated with 1 mg/kg/day of prednisolone for 4 weeks with reduced doses thereafter, 500-1,000 mg/m2 of IV cyclophosphamide every 2 weeks for six cycles then every 4 weeks for up to a total of 10-15 treatments, and 10-12 ng/mL of tacrolimus (12-hour trough). Plasmapheresis was allowed in patients who progressed and needed oxygenation after the regimen was initiated, and it was administered in nine patients (31%) in the combination regimen group vs. one (7%) of the historical controls.
Given the different frequencies of rapidly progressive ILD in Asian vs. Western countries (39%-71% vs. 22%-57%, respectively), it is unclear whether the results seen in this study can be extrapolated to patients from the United States and Europe. Therefore, it is necessary to analyze the efficacy of the regimen in those patient populations, Dr. Tsuji said, also noting that future studies should evaluate risk-based modifications of the regimen to identify the optimal treatment for individuals based on factors such as age, respiratory dysfunction, hyperferritinemia, and treatment delay.
Dr. Tsuji reported having no disclosures.
SOURCE: Tsuji H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 838.
CHICAGO – Early treatment with combined high-dose glucocorticoids, tacrolimus, and intravenous cyclophosphamide therapy significantly improves survival vs. step-up therapy in interstitial lung disease patients with anti–melanoma differentiation–associated gene 5 (anti-MDA5)–positive dermatomyositis, according to findings from a prospective, multicenter study.
However, the combination therapy was associated with a high risk of cytomegalovirus reactivation and other opportunistic infections that warrants careful monitoring of treated patients, Hideaki Tsuji, MD, reported at the annual meeting of the American College of Rheumatology.
ILD accompanied by anti-MDA5–positive dermatomyositis (DM) is often intractable and associated with high mortality in Japanese patients. Case reports have suggested improved outcomes with combined immunosuppressive therapy, but a standard treatment has not been established, said Dr. Tsuji of Kyoto University.
“Therefore, we evaluated the efficacy and safety of combined immunosuppressive therapy for anti-MDA5–positive DM with ILD in a prospective single-arm study,” he said, adding that early administration, a short interval of intravenous cyclophosphamide, use of plasmapheresis as an additional therapy, and control of opportunistic infections may contribute to the improved outcomes seen with the regimen in this study.
The primary endpoint of 6-month survival was reached by 24 (89%) of 27 patients treated with the combination regimen for 52 weeks, compared with 5 (33%) of 15 historical controls who received high-dose glucocorticoids followed by step-wise addition of immunosuppressants. At 12 months, the survival rates were 85% and 33%, respectively, Dr. Tsuji said.
Additionally, anti-MDA5 titer, serum ferritin level, C-reactive protein level, lactate dehydrogenase, and KL-6 level gradually decreased over the 52 months, and percent vital capacity increased with combination vs. step-up therapy, he noted.
Cytomegalovirus reactivation occurred in 90% of combination regimen patients vs. 33% of controls over the 52-week study period, he said, adding that pneumocystic pneumonia and sepsis also occurred in combination regimen group patients, and were associated with death in four patients.
When the 23 surviving patients in the combination regimen group were compared with the 4 in the group who died, it was noted that the deceased patients were significantly more likely to have cutaneous ulcers (75% vs. 13%), higher mean C-reactive protein level (2.7 vs. 0.77 mg/dL), and higher creatine kinase level (644.3 vs. 219.3 IU/L), respectively, before treatment, he said.
Study subjects were Japanese adults with new-onset anti-MDA5–positive dermatomyositis with interstitial lung disease (ILD) who were enrolled between July 2014 and September 2017.
They were treated with 1 mg/kg/day of prednisolone for 4 weeks with reduced doses thereafter, 500-1,000 mg/m2 of IV cyclophosphamide every 2 weeks for six cycles then every 4 weeks for up to a total of 10-15 treatments, and 10-12 ng/mL of tacrolimus (12-hour trough). Plasmapheresis was allowed in patients who progressed and needed oxygenation after the regimen was initiated, and it was administered in nine patients (31%) in the combination regimen group vs. one (7%) of the historical controls.
Given the different frequencies of rapidly progressive ILD in Asian vs. Western countries (39%-71% vs. 22%-57%, respectively), it is unclear whether the results seen in this study can be extrapolated to patients from the United States and Europe. Therefore, it is necessary to analyze the efficacy of the regimen in those patient populations, Dr. Tsuji said, also noting that future studies should evaluate risk-based modifications of the regimen to identify the optimal treatment for individuals based on factors such as age, respiratory dysfunction, hyperferritinemia, and treatment delay.
Dr. Tsuji reported having no disclosures.
SOURCE: Tsuji H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 838.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: 6-month survival was 89% vs. 33% with combination immunotherapy vs. step-up therapy.
Study details: A prospective, multicenter study of 27 patients and 15 historical controls.
Disclosures: Dr. Tsuji reported having no disclosures.
Source: Tsuji H et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 838.