User login
RELIEF: In Behçet’s, apremilast improves oral ulcers for up to 28 weeks
CHICAGO – Apremilast was effective and well tolerated for up to 28 weeks for the treatment of oral ulcers in patients with active Behçet’s disease, based on findings from the randomized, placebo-controlled, phase 3 RELIEF trial.
At baseline, mean oral ulcer counts were 4.2 in 104 patients randomized to receive the oral phosphodiesterase-4 inhibitor and 3.9 in 103 patients in the placebo group. Mean visual analog scale (VAS) pain scores were 61.2 and 60.8 in the two groups, respectively.
The primary study endpoint of area under the curve for total number of oral ulcers over a 12-week period (AUCWk0-12) – a measure that reflects the number of oral ulcers that occur over time and also accounts for the recurring-remitting course of oral ulcers – was achieved. AUCWk0-12 was significantly lower in the apremilast group than in the placebo group (129.54 vs. 222.14, respectively; P less than .0001), Gulen Hatemi, MD, reported at the annual meeting of the American College of Rheumatology.
From baseline to week 12, apremilast treatment also resulted in a significantly lower number of oral ulcers (mean of 1.1 vs. 2.0 for placebo at 12 weeks) and significantly reduced pain from oral ulcers at every visit from week 1 through week 12 of the study, compared with placebo (mean VAS score change from baseline, –40.7 vs. –15.9), said Dr. Hatemi, a professor of medicine at Istanbul University.
“The [12-week] complete response rate ... was 53% in the apremilast group and 22.3% in the placebo group. The [12-week] partial response rate ...was 76% in the apremilast group and 48% in the placebo group,” she said, adding that the efficacy of apremilast was sustained with continued treatment through 28 weeks.
Study participants were adults (mean age, 40 years) with active Behçet’s disease and three or more oral ulcers at randomization or two or more at screening and at randomization. All had been previously treated with at least one nonbiologic medication for oral ulcers and were allowed to have received previous biologic therapies for other disease manifestations. Those with active major organ involvement were excluded.
Treatment included a 30-mg dose of apremilast twice daily for 12 weeks or placebo. After 12 weeks, all patients received apremilast through at least 28 weeks of the 64-week study.
At the 28-week analysis, patients who were initially randomized to placebo and who switched to apremilast after week 12 had benefits comparable with those seen in those randomized to apremilast at the start of the study. A complete response was seen in 59% and 62% of patients in the groups, respectively, and a partial response was seen in 90% and 85%, respectively. Additionally, the mean change in the VAS score for oral ulcer pain in the groups at that time was –40.6 and –41.9, Dr. Hatemi said.
Apremilast was well tolerated in this study; the incidence of adverse events was comparable in the treatment and placebo groups during the 12-week placebo-controlled phase of the study – 78.8% and 71.8%, respectively. The most common events were diarrhea, nausea, headache, and upper respiratory tract infection, she said.
“These were generally mild to moderate, and only two patients had to discontinue the study due to gastrointestinal adverse events,” she said, noting that no new safety signals were observed.
Behçet’s disease is a chronic, relapsing, multisystem inflammatory disorder characterized by recurrent oral ulcers that can be disabling and have a substantial effect on quality of life. These findings, which include efficacy data up to 28 weeks and safety data for at least 100 patients exposed to apremilast for at least 1 year, demonstrate the efficacy of apremilast for the treatment oral ulcers in patients with Behçet’s disease, she said, noting that “the safety findings were consistent with the known safety profile of apremilast.”
The RELIEF study was supported by Celgene. Dr. Hatemi reported receiving grant/research support from Celgene and serving as a speaker for AbbVie, Mustafa Nevzet Pharmaceuticals, and UCB.
SOURCE: Hatemi G et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2789.
CHICAGO – Apremilast was effective and well tolerated for up to 28 weeks for the treatment of oral ulcers in patients with active Behçet’s disease, based on findings from the randomized, placebo-controlled, phase 3 RELIEF trial.
At baseline, mean oral ulcer counts were 4.2 in 104 patients randomized to receive the oral phosphodiesterase-4 inhibitor and 3.9 in 103 patients in the placebo group. Mean visual analog scale (VAS) pain scores were 61.2 and 60.8 in the two groups, respectively.
The primary study endpoint of area under the curve for total number of oral ulcers over a 12-week period (AUCWk0-12) – a measure that reflects the number of oral ulcers that occur over time and also accounts for the recurring-remitting course of oral ulcers – was achieved. AUCWk0-12 was significantly lower in the apremilast group than in the placebo group (129.54 vs. 222.14, respectively; P less than .0001), Gulen Hatemi, MD, reported at the annual meeting of the American College of Rheumatology.
From baseline to week 12, apremilast treatment also resulted in a significantly lower number of oral ulcers (mean of 1.1 vs. 2.0 for placebo at 12 weeks) and significantly reduced pain from oral ulcers at every visit from week 1 through week 12 of the study, compared with placebo (mean VAS score change from baseline, –40.7 vs. –15.9), said Dr. Hatemi, a professor of medicine at Istanbul University.
“The [12-week] complete response rate ... was 53% in the apremilast group and 22.3% in the placebo group. The [12-week] partial response rate ...was 76% in the apremilast group and 48% in the placebo group,” she said, adding that the efficacy of apremilast was sustained with continued treatment through 28 weeks.
Study participants were adults (mean age, 40 years) with active Behçet’s disease and three or more oral ulcers at randomization or two or more at screening and at randomization. All had been previously treated with at least one nonbiologic medication for oral ulcers and were allowed to have received previous biologic therapies for other disease manifestations. Those with active major organ involvement were excluded.
Treatment included a 30-mg dose of apremilast twice daily for 12 weeks or placebo. After 12 weeks, all patients received apremilast through at least 28 weeks of the 64-week study.
At the 28-week analysis, patients who were initially randomized to placebo and who switched to apremilast after week 12 had benefits comparable with those seen in those randomized to apremilast at the start of the study. A complete response was seen in 59% and 62% of patients in the groups, respectively, and a partial response was seen in 90% and 85%, respectively. Additionally, the mean change in the VAS score for oral ulcer pain in the groups at that time was –40.6 and –41.9, Dr. Hatemi said.
Apremilast was well tolerated in this study; the incidence of adverse events was comparable in the treatment and placebo groups during the 12-week placebo-controlled phase of the study – 78.8% and 71.8%, respectively. The most common events were diarrhea, nausea, headache, and upper respiratory tract infection, she said.
“These were generally mild to moderate, and only two patients had to discontinue the study due to gastrointestinal adverse events,” she said, noting that no new safety signals were observed.
Behçet’s disease is a chronic, relapsing, multisystem inflammatory disorder characterized by recurrent oral ulcers that can be disabling and have a substantial effect on quality of life. These findings, which include efficacy data up to 28 weeks and safety data for at least 100 patients exposed to apremilast for at least 1 year, demonstrate the efficacy of apremilast for the treatment oral ulcers in patients with Behçet’s disease, she said, noting that “the safety findings were consistent with the known safety profile of apremilast.”
The RELIEF study was supported by Celgene. Dr. Hatemi reported receiving grant/research support from Celgene and serving as a speaker for AbbVie, Mustafa Nevzet Pharmaceuticals, and UCB.
SOURCE: Hatemi G et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2789.
CHICAGO – Apremilast was effective and well tolerated for up to 28 weeks for the treatment of oral ulcers in patients with active Behçet’s disease, based on findings from the randomized, placebo-controlled, phase 3 RELIEF trial.
At baseline, mean oral ulcer counts were 4.2 in 104 patients randomized to receive the oral phosphodiesterase-4 inhibitor and 3.9 in 103 patients in the placebo group. Mean visual analog scale (VAS) pain scores were 61.2 and 60.8 in the two groups, respectively.
The primary study endpoint of area under the curve for total number of oral ulcers over a 12-week period (AUCWk0-12) – a measure that reflects the number of oral ulcers that occur over time and also accounts for the recurring-remitting course of oral ulcers – was achieved. AUCWk0-12 was significantly lower in the apremilast group than in the placebo group (129.54 vs. 222.14, respectively; P less than .0001), Gulen Hatemi, MD, reported at the annual meeting of the American College of Rheumatology.
From baseline to week 12, apremilast treatment also resulted in a significantly lower number of oral ulcers (mean of 1.1 vs. 2.0 for placebo at 12 weeks) and significantly reduced pain from oral ulcers at every visit from week 1 through week 12 of the study, compared with placebo (mean VAS score change from baseline, –40.7 vs. –15.9), said Dr. Hatemi, a professor of medicine at Istanbul University.
“The [12-week] complete response rate ... was 53% in the apremilast group and 22.3% in the placebo group. The [12-week] partial response rate ...was 76% in the apremilast group and 48% in the placebo group,” she said, adding that the efficacy of apremilast was sustained with continued treatment through 28 weeks.
Study participants were adults (mean age, 40 years) with active Behçet’s disease and three or more oral ulcers at randomization or two or more at screening and at randomization. All had been previously treated with at least one nonbiologic medication for oral ulcers and were allowed to have received previous biologic therapies for other disease manifestations. Those with active major organ involvement were excluded.
Treatment included a 30-mg dose of apremilast twice daily for 12 weeks or placebo. After 12 weeks, all patients received apremilast through at least 28 weeks of the 64-week study.
At the 28-week analysis, patients who were initially randomized to placebo and who switched to apremilast after week 12 had benefits comparable with those seen in those randomized to apremilast at the start of the study. A complete response was seen in 59% and 62% of patients in the groups, respectively, and a partial response was seen in 90% and 85%, respectively. Additionally, the mean change in the VAS score for oral ulcer pain in the groups at that time was –40.6 and –41.9, Dr. Hatemi said.
Apremilast was well tolerated in this study; the incidence of adverse events was comparable in the treatment and placebo groups during the 12-week placebo-controlled phase of the study – 78.8% and 71.8%, respectively. The most common events were diarrhea, nausea, headache, and upper respiratory tract infection, she said.
“These were generally mild to moderate, and only two patients had to discontinue the study due to gastrointestinal adverse events,” she said, noting that no new safety signals were observed.
Behçet’s disease is a chronic, relapsing, multisystem inflammatory disorder characterized by recurrent oral ulcers that can be disabling and have a substantial effect on quality of life. These findings, which include efficacy data up to 28 weeks and safety data for at least 100 patients exposed to apremilast for at least 1 year, demonstrate the efficacy of apremilast for the treatment oral ulcers in patients with Behçet’s disease, she said, noting that “the safety findings were consistent with the known safety profile of apremilast.”
The RELIEF study was supported by Celgene. Dr. Hatemi reported receiving grant/research support from Celgene and serving as a speaker for AbbVie, Mustafa Nevzet Pharmaceuticals, and UCB.
SOURCE: Hatemi G et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2789.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Apremilast is safe and effective for treating oral ulcers in patients with Behçet’s disease.
Major finding: The AUCWk0-12 was significantly lower with apremilast (129.54) versus placebo (222.14).
Study details: A randomized, placebo-controlled, phase 3 study of 207 patients.
Disclosures: The RELIEF study was supported by Celgene. Dr. Hatemi reported receiving grant/research support from Celgene and serving as a speaker for AbbVie, Mustafa Nevzet Pharmaceuticals, and UCB.
Source: Hatemi G et al. Arthritis Rheumatol. 2018;70(Suppl 10), Abstract 2789
Three drugs disappoint in SSc trials, but show some promise
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
CHICAGO – Recent randomized, placebo-controlled, phase 3 trials of tocilizumab, abatacept, and riociguat for the treatment of systemic sclerosis each failed to reach its primary endpoint of change from baseline in modified Rodnan Skin Score (mRSS).
Still, findings with respect to secondary endpoints and certain exploratory outcomes suggest each of the agents holds some promise in the systemic sclerosis (SSc) arena, according to the data presented at the annual meeting of the American College of Rheumatology.
Tocilizumab (Actemra)
In the double-blind portion of the phase 3 focuSSced trial of 212 patients with SSc, numerical improvement was observed for the primary endpoint of mean change in mRSS from baseline to week 48 with tocilizumab versus placebo (–6.14 vs. –4.41 points, respectively). The change in the treatment group was comparable with what was seen in the phase 2 faSScinate trial, but the decline in mRSS in the placebo group was much greater in phase 3 than in phase 2, and so the difference between the groups in the current study failed to reach statistical significance (P = .098), reported Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor.
The interleukin-6 (IL-6) receptor–alpha antibody was previously shown in the faSScinate trial to lead to numeric improvements in skin thickening as measured by the mRSS, as well as to clinically meaningful lung function preservation as measured by percent predicted forced vital capacity (FVC).
In the current phase 3 study, key secondary end points also appeared to favor tocilizumab, but since the primary endpoint for mRSS was not met, all other P values cannot be considered statistically significant despite the strength of the evidence and were reported for informational purposes only, he noted.
The median cumulative distribution of change from baseline to week 48 in percent predicted FVC with tocilizumab versus placebo was –0.6 vs. –3.9, respectively (descriptive P = .0015), and the mean change from baseline in FVC at week 48 was –24 mL vs. –190 mL (difference of 167 mL in favor of tocilizumab; descriptive P = .0001).
Time to treatment failure also favored tocilizumab, he said (hazard ratio, 0.63; descriptive P = .082), he said.
Patients were randomly assigned to receive either weekly 162-mg injections of subcutaneous tocilizumab or placebo for 48 weeks. Escape therapy was allowed beginning at week 16 if patients experienced declines in FVC or beginning at week 24 if they experienced worsened mRSS or worsened SSc complications, Dr. Khanna said.
“The key part is that no immunotherapy was allowed. ... So it’s a true randomized, placebo-controlled trial,” he said.
Most (81%) of the patients were women, and they had a mean age of 48 years, mean SSc duration of 23 months, mean mRSS of 20.4 units on a 0-51 scale, and a normal mean percent predicted FVC of 82.1%.
“HAQ-DI showed moderate disability of 1.2,” he noted.
Safety in the study was consistent with that seen in prior tocilizumab studies; no new safety signals were identified. Serious adverse events occurred in 13% and 17% of tocilizumab and placebo group patients , respectively, and serious infections were reported by 7% and 2%.
Although clinically meaningful and consistent differences in FVC favoring tocilizumab were shown in this study, the primary endpoint was not met, Dr. Khanna said.
“There were no statistically significant differences, largely driven by unexpected improvement in the placebo group, which was different than what we found in [the faSScinate] trial,” he said, noting, however, that the FVC findings in the current study were clinically meaningful.
Also, in a separate presentation at the meeting, he explained that the differences favoring tocilizumab were statistically significant when patient-level data from the trial were analyzed based on the ACR Composite Response Index in Systemic Sclerosis (CRISS). Those findings provide validation of the novel outcomes measure, he said.
Abatacept (Orencia)
Dr. Khanna also reported results of the 12-month, double-blind, randomized, placebo-controlled phase 2 ASSET trial of abatacept, which showed no significant difference in mRSS in patients with early diffuse cutaneous SSc (dfSSc) who were treated with 125 mg of the recombinant fusion protein weekly and those who received placebo. However, certain secondary outcomes favored abatacept. No concomitant immunotherapy was allowed.
The adjusted mean decrease in the mRSS among patients who completed the 12-month treatment period was –6.24 vs. –4.49 in 34 patients in the abatacept group and 35 in the placebo group, respectively (P = .28).
The secondary outcome measures of mean change in Health Assessment Questionnaire Disability Index (HAQ-DI), patients global assessment, physician global assessment, and ACR CRISS scores were statistically significant or showed numerical results favoring abatacept over placebo: mean decrease in HAQ-DI, –0.17 vs. –0.11 (P = .05), respectively; mean change in physician global assessment scores, –1.30 vs. –0.35 (P = .03); median ACR CRISS index, 0.68 vs. 0.01 (P = .03), decline in percent predicted FVC of 4.13% and 1.34% (P = .11).
Escape therapy was allowed at 6 months for worsening SSc, but it did not change the outcomes trajectory, he said. A larger proportion of placebo vs. abatacept subjects required escape immunosuppressive therapy (36% vs. 16%; P = .03).
Patients were enrolled between 2014 and 2018 at 27 U.S., Canadian, and U.K. sites. At baseline, participants had a mean age of 49 years, 75% were women, and mean disease duration was very short at 1.59 years, with 60% having disease duration of 18 months or less. The mean baseline mRSS was 22.4, mean percent predicted FVC was 85.3%, and mean HAQ-DI was 1.0.
Compliance with both treatments was greater than 98%. Abatacept was well tolerated with comparable adverse events (AEs), serious AEs, and AEs of special interest such as infections and malignancies between treatments, Dr. Khanna said, noting that two deaths occurred in the abatacept group (caused by scleroderma renal crisis in both cases at days 11 and 46) and one occurred in a placebo group patient who experienced sudden cardiac arrest at day 310.
Of note, mRSS showed large variability, despite recruiting an early dcSSc population, Dr. Khanna said.
The finding with respect to the primary outcome is consistent with other recent trials because of improvement in mRSS that’s part of the natural history of the disease, including the tocilizumab findings that he reported at the meeting. The findings with respect to secondary endpoints and safety show promise.
“Stay tuned for robust ongoing work on the relationship between clinical changes and ongoing mechanistic work,” he said.
Riociguat (Adempas)
Similarly, in the randomized, placebo-controlled phase 2b RISE-SSc study comparing riociguat and placebo for early dcSSc, the primary efficacy endpoint of mean change in mRSS did not reach statistical significance, but exploratory data suggested that the soluble guanylate cyclase stimulator prevented disease progression in patients with early dcSSc, reported Oliver Distler, MD, head of the connective tissue diseases program at University Hospital Zurich (Switzerland).
The mean mRSS at baseline was comparable in 60 patients randomized to receive riociguat and 61 in the placebo group (16.8 and 16.71, respectively). These mean values at week 52 dropped to 14.63 vs. 15.73, respectively (P = .08).
“So it was close, but it didn’t reach significance,” he said.
The difference in the mRSS progression rate, however, suggested significant effects favoring riociguat (descriptive P = .02), he said.
Further, mean change from baseline to week 52 in percent predicted FVC was not different overall between the groups, but a large difference favoring riociguat was seen among patients with scleroderma interstitial lung disease at baseline (mean change of –2.7 vs. –8.9), he said.
No differences were seen between the groups in HAQ-DI or patient and physician global assessment. The proportion of patients with probability of improvement at 52 weeks as measured using ACR CRISS was also the same at 18% in both treatment arms, he noted, ”but the CRISS is designed more for assessing disease regression than for assessing prevention of progression.”
Treatment was, however, well tolerated. At week 52, fewer serious adverse events occurred with riociguat group than in the placebo group (15% vs. 25%, respectively), and no new safety signals were observed, he said.
Riociguat has previously shown antifibrotic effects in animal models and efficacy in patients with pulmonary arterial hypertension associated with connective tissue disease, so it was hypothesized that patients with dcSSc might benefit from riociguat therapy, Dr. Distler explained.
Study subjects had very early dcSSc (duration of 18 months or less; mean of 9 months), mRSS of 10-22 units, FVC of 45% predicted or greater, and diffusion capacity of the lung for carbon monoxide of at least 40% of predicted at screening.
Riociguat was given at an individually adjusted dose between 0.5 mg and 2.5 mg three times daily.
The findings demonstrate a numeric decrease in mRSS over time with riociguat versus placebo and a prevention of progression with riociguat; the failure to reach the primary endpoint may be related to the small study size and the higher than expected regression rate in the placebo group, Dr. Distler said.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an National Institutes of Health/National Institute of Allergy and Infectious Diseases Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb. Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. In addition, he has a patent on mir-29 for the treatment of systemic sclerosis.
SOURCES: Khanna D et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 898 and Abstract 900; Distler O et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 903.
REPORTING FROM THE ACR ANNUAL MEETING
ACR CRISS: A way forward for scleroderma treatment trials?
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
CHICAGO – At least three phase 3 randomized scleroderma treatment trials presented at the annual meeting of the American College of Rheumatology failed to meet modified Rodnan Skin Score–based primary endpoints, but a different story emerged when data from the trials were analyzed using the novel ACR Composite Response Index in Systemic Sclerosis (CRISS).
The differences highlight the limitations of individual outcome measures like the modified Rodnan Skin Score (mRSS) and underscore the need for new measures that capture the complexity of systemic sclerosis (SSc) and are more sensitive to changes in disease severity, according to Robert Spiera, MD, director of the vasculitis and scleroderma program at the Hospital for Special Surgery in New York.
Such measures are needed to better assess the effects of treatment interventions in scleroderma trials, Dr. Spiera said in an interview.
The ACR CRISS
Development of the ACR CRISS was led by Dinesh Khanna, MBBS, a professor of medicine and director of the scleroderma program at the University of Michigan, Ann Arbor. He and his colleagues described the measure in 2016 (Arthritis Rheumatol. 2016 Feb;68[2]:299-311. doi: 10.1002/art.39501).
Its use involves a two-step process of identifying any significant disease worsening or new end-organ damage, and then calculating the probability of patient improvement after 1 year of treatment on a 0- to 1-point scale based on changes from baseline in five variables: the mRSS, percent predicted forced vital capacity (FVC), patient and physician global assessments, and the Health Assessment Questionnaire Disability Index (HAQ-DI).
A CRISS score of 0.6 or higher indicates likelihood that a patient improved on treatment. Of note, subjects with significant worsening of renal or cardiopulmonary involvement are classified as not improved (score of 0), regardless of improvements in other core items.
To devise the CRISS, the investigators compiled 150 patient profiles with standardized clinical outcome elements using patients with diffuse cutaneous systemic sclerosis (dcSSc). The profiles were assessed by 40 scleroderma experts who rated patient improvement or lack thereof over 12 months.
Using the 79% of profiles for which a consensus was reached, the investigators “fit logistic regression models in which the binary outcome referred to whether the patient was improved or not, and the changes in the core set items from baseline to follow-up were entered as covariates,” they explained.
This led to the selection of the five measures included in the final version, which was found to have sensitivity of 0.982 and specificity of 0.931. When evaluated in a previously completed 1-year randomized controlled trial, the index differentiated the effect of methotrexate from the effect of placebo (P = .02), they reported.
Based on these findings, the ACR board of directors granted “provisional” endorsement of the CRISS for use in SSc clinical studies, signifying that it had been quantitatively validated using patient data, but had not undergone validation using an external data set.
New data presented at the 2018 ACR meeting will likely lead to full approval of the measure once the studies validating the measure are published, according to Dr. Spiera.
The focuSSced study of tocilizumab
For example, in the phase 3 focuSSced study comparing the interleukin-6 (IL-6) receptor–alpha antibody tocilizumab (Actemra) with placebo in patients with SSc, the primary endpoint of mean change from baseline in mRSS was not met (–6.14 vs. –4.41 points with tocilizumab vs. placebo, respectively; P = .098), Dr. Khanna said during a report of findings from the double-blind portion of the study.
However, when the CRISS was applied and its performance prospectively assessed as an exploratory outcome at week 48 in the focuSSced trial, the differences between the groups were statistically significant. Dr. Khanna reported those findings in a separate presentation at the meeting, noting that they marked the first prospective evaluation of the ACR CRISS in a phase 3 trial in SSc.
The CRISS was applied – using a blinded review of adverse events and serious adverse events to complete step 1 – in all patients who received study treatment, stratified by baseline IL-6 levels.
Of 104 patients in the tocilizumab arm and 106 in the placebo arm, 6 and 13 patients, respectively, had cardio-pulmonary-renal involvement and therefore received a score of 0.
Using the ACR CRISS as a continuous measure, scores favored tocilizumab over placebo at week 48 with a median increase of 0.89 vs. 0.25 points (P = .023), and using the binary form of CRISS, 51% vs. 37% of patients in the treatment and placebo arms achieved the score cutoff of 0.60 or higher at week 48 (P = .035), he said.
“The focuSSced study validates the ACR CRISS endpoint for the first time in an independent prospective clinical trial and highlights the importance of step 1 as an indicator of reduced organ progression during 48 weeks of treatment,” Dr. Khanna concluded.
The ASSET trial of abatacept
Dr. Khanna also reported results from the 12-month phase 2 ASSET trial comparing abatacept (Orencia) and placebo in early dcSSc.
Again, the change from baseline in mRSS – the primary endpoint of the study – did not differ significantly with treatment vs. placebo (–6.24 vs. –4.49; P = .28).
And again, as presented separately in a poster session at ACR, application of CRISS at 12 months – a secondary outcome measure in the trial – showed a significant difference between the groups.
The investigators prospectively performed step 1 of the CRISS using a case report form. Of 63 patients with complete data available for relevant outcomes at 12 months, 10 (5 in each group) had cardio-pulmonary-renal involvement and thus were given a score of 0.
Overall, there was evidence of significantly improved CRISS scores in the abatacept group vs. the placebo group (P = .03), he said.
Most of the individual CRISS variables, except HAQ-DI and patient global assessment, had statistically significant correlations with the CRISS, he noted, adding that “although the degree of correlation is high between the mRSS and CRISS, there is evidence that CRISS may be more sensitive to clinically meaningful treatment changes than the standard skin score endpoint.”
“This suggests further validation of CRISS as an independent primary endpoint for scleroderma clinical trials,” he concluded.
The phase 2b RISE-SSc study of riociguat
As in the focuSSced and ASSET studies, the primary efficacy endpoint of mean change from baseline in mRSS was not met in the randomized, double-blind, placebo-controlled RISE-SSc study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator riociguat (Adempas) vs. placebo in 121 patients with early dcSSc, reported Oliver Distler, MD, a professor at University Hospital Zurich.
The mean change in mRSS at 52 weeks was 2.25 vs. 0.97 with riociguat vs. placebo, respectively (P = .08), although the difference in mRSS progression rate showed significant effects favoring riociguat (P = .02), he noted.
In this study, however, the proportion of patients with ACR CRISS probability of improvement (score of 0.60 or higher) at week 52 – a secondary study outcome – was the same at 18% in both arms, Dr. Distler said.
In a way, it’s helpful that the CRISS findings as well as the mRSS outcome in the RISE-SSc study were negative because it shows that “not everything comes up positive using the CRISS,” Dr. Spiera said.
An open-label extension trial of lenabasum
In the open-label extension of a phase 2 trial of lenabasum, Dr. Spiera and his colleagues also found the CRISS useful for assessing response in 36 patients. Lenabasum is a synthetic, nonimunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses.
The agent continued to demonstrate acceptable safety and tolerability in dcSSc with no severe or serious adverse events or study discontinuations related to treatment during 12 months of open-label extension dosing, both from baseline and from the start of the extension, they reported in a poster at the ACR meeting. These assessments were based on ACR CRISS score, mRSS, physician global assessment, and multiple patient-reported outcomes.
The median CRISS score was 92% at week 52, and mRSS declined by a mean of 9.4 points (41.3% from baseline). More than a third of patients (35%) achieved a low mRSS of 10 or less.
The investigators noted, however, that definitive attribution of the findings to lenabasum is limited by the use of background therapy, the potential for spontaneous improvement in patients, and open-label dosing.
Evaluating immunosuppressive therapy in SSc
In another study presented at the ACR meeting, Boyang Zheng, MD, a second-year rheumatology fellow at McGill University in Montreal and his colleagues evaluated the effect of current immunosuppressive therapy on the ACR CRISS in 301 adult dcSSc patients without prior immunosuppression who were part of the Canadian Scleroderma Research Group (CSRG) registry.
Patients newly treated with methotrexate, azathioprine, mycophenolate and/or cyclophosphamide for at least 2 years (47 patients) were considered “exposed patients,” and untreated patients with at least the same follow-up duration were considered control subjects (254 patients).
Inverse probability of treatment weighting (IPTW) was performed to balance potential confounders in the two groups, including age, sex, disease duration, and CRISS variables, in an effort “to create a statistical cohort that would resemble a randomized, controlled trial,” Dr Zheng explained.
Prior to IPTW, treated patients trended towards more improvement after 1 year, but with “unimpressive absolute values.”
“But [after IPTW], when you look at overall CRISS at 1 year, more of the treated patients had actually improved – 23% vs. 11.8% in the untreated patients. After adjusting for age, sex, and disease duration, immunosuppression was associated with an almost twofold higher likelihood of improvement, although this was not statistically significant,” he said.
“Most importantly, after our balancing in the statistical cohort – so after balancing and adjusting for covariates ... immunosuppression use was still associated with a higher likelihood of improving [with an] odds ratio of 1.85 and P-value of 0.018,” he said.
The treated patients were sicker, and the CRISS was still able to capture individual patient improvement, he added.
Though limited by the observational design and the fact that “IPTW balancing cannot correct for all possible confounders,” the findings “provide novel evidence to support the use of immunosuppression in diffuse systemic sclerosis, and this reassures us in our current practice; it provides evidence that the CRISS seems to have better sensitivity to change than the mean of individual disease measures,” he said.
However, it also shows that “we have a long way to go to have better treatment for our patients,” he added, noting that only a minority (23%) of the patients in this study improved on the CRISS.
Moving forward in systemic sclerosis
Indeed, in order to move forward in developing better treatment for SSc, it is important to have the best possible means for assessing the effects of the potential new treatments, Dr. Spiera said.
“The mRSS is a good, reliable outcome measure in terms of intra- and inter-rater reliability, and we know it has meaning in the real world; in a patient with rapidly progressive skin thickening and a skin score that is getting higher and higher, we know they have worse prognosis in terms of function and even survival,” he said. “On the other hand, it’s just one piece of this very complicated story when you’re dealing with patients with systemic sclerosis, some of whom can have important internal organ involvement and not even have skin involvement.”
The main challenge with the skin score is how it performs in clinical trials when it comes to demonstrating whether a drug works, he added.
“This is particularly relevant in an era where our better understanding of the disease has led to candidate therapeutic agents that we have reason to think might work, but where clinical trials haven’t shown a significant difference between the groups using the skin score.”
Conversely, there have been studies in which skin scores improve, but the patient is doing terribly, he noted.
“That patient would not be [shown to be] doing well using the CRISS,” he said, explaining that the complex formula used to determine the CRISS score, which is “heavily weighted toward skin score,” allows for an overall score that is “greater than the sum of its parts.” That is, if a patient is improving in multiple domains indicating that the patient is responding to therapy, more credit is given for each of those parts.
“So my sense, and I think it’s the consensus in the community of clinical investigators, is that the skin score is not adequately sensitive to change in the context of a trial and doesn’t capture the disease holistically enough to be the optimal measure for scleroderma clinical trials,” he said. “We think the CRISS score works better in terms of capturing improvement in the course of a trial, and also in capturing other outcomes that are really, really important to patients and to their physicians, like disability or lung function.”
The hope is that, given the evidence, regulatory agencies will look favorably on the ACR CRISS as an outcome measure in clinical trials moving forward, and that understanding of its use and value will increase across the rheumatology community, he said.
Dr. Spiera has received research grants, consulting fees, and/or other payments from many companies involved in SSc treatments, including Roche/Genentech, which markets tocilizumab, and Corbus Pharmaceuticals, which is developing lenabasum.
Dr. Khanna is a consultant to Roche/Genentech and Bayer, which markets riociguat, and other companies. He has received research grants from Bayer, Bristol-Myers Squibb (which markets abatacept), and Pfizer. The ASSET trial he presented was sponsored by an NIH/NIAID Clinical ACE grant and an investigator-initiated grant by Bristol-Myers Squibb.
Dr. Distler has a consultancy relationship and/or has received research funding from Bayer, Roche/Genentech, and other companies. He also has a patent for a treatment for systemic sclerosis.
Dr. Zheng reported having no disclosures.
REPORTING FROM THE ACR ANNUAL MEETING
Short-term lung function better predicts mortality risk in SSc
In addition to known risk factors, early reductions in lung function and diffusing capacity increased mortality risk in patients with systemic sclerosis and comorbid interstitial lung disease over 2 years in an analysis of data from the Scleroderma Lung Studies I and II.
First author Elizabeth R. Volkmann, MD, of the University of California, Los Angeles, and her colleagues also reported that although early treatment with cyclophosphamide or mycophenolate mofetil in these studies led to shorter-term improvement in surrogate measures of outcomes for systemic sclerosis and comorbid interstitial lung disease (SSc-ILD), either medication might not improve long-term outcomes. The findings were reported in the Annals of the Rheumatic Diseases.
The investigators identified specific factors linked to an increased risk of death over a median follow-up of 8 years in 158 systemic sclerosis-interstitial lung disease patients in the Scleroderma Lung Study I and median follow-up of 3.6 years in 142 patients in Scleroderma Lung Study II, both of which were randomized, controlled trials.
After follow-up was complete, 42% of participants in Scleroderma Lung Study I and 21% of participants in Scleroderma Lung Study II died, with the most common cause of death being complications related to systemic sclerosis. In addition, no statistical difference was seen in time to death between the intervention arms of both trials.
In building a risk prediction model based on findings from both trials, the investigators confirmed several known mortality risk predictors in systemic sclerosis, including increased age and baseline skin score, but also found that changes in forced vital capacity and diffusing capacity for carbon monoxide over 2 years were separately linked with a greater risk of death.
“These findings suggest that short-term changes in surrogate measures of systemic sclerosis-interstitial lung disease progression may have important effects on long-term outcomes,” the researchers wrote.
The researchers acknowledged the results emphasize the need to determine optimal length of treatment in patients with systemic sclerosis and comorbid interstitial lung disease.
“Systemic sclerosis providers should closely monitor lung function when interstitial lung disease is present to accurately identify declines in lung function and promptly intervene to improve patient outcomes,” the researchers concluded.
The study was funded by the National Institutes of Health and the Scleroderma Foundation. The authors reported no conflicts of interest.
SOURCE: Volkmann ER et al. Ann Rheum Dis. 2018 Nov 8. doi: 10.1136/annrheumdis-2018-213708.
In addition to known risk factors, early reductions in lung function and diffusing capacity increased mortality risk in patients with systemic sclerosis and comorbid interstitial lung disease over 2 years in an analysis of data from the Scleroderma Lung Studies I and II.
First author Elizabeth R. Volkmann, MD, of the University of California, Los Angeles, and her colleagues also reported that although early treatment with cyclophosphamide or mycophenolate mofetil in these studies led to shorter-term improvement in surrogate measures of outcomes for systemic sclerosis and comorbid interstitial lung disease (SSc-ILD), either medication might not improve long-term outcomes. The findings were reported in the Annals of the Rheumatic Diseases.
The investigators identified specific factors linked to an increased risk of death over a median follow-up of 8 years in 158 systemic sclerosis-interstitial lung disease patients in the Scleroderma Lung Study I and median follow-up of 3.6 years in 142 patients in Scleroderma Lung Study II, both of which were randomized, controlled trials.
After follow-up was complete, 42% of participants in Scleroderma Lung Study I and 21% of participants in Scleroderma Lung Study II died, with the most common cause of death being complications related to systemic sclerosis. In addition, no statistical difference was seen in time to death between the intervention arms of both trials.
In building a risk prediction model based on findings from both trials, the investigators confirmed several known mortality risk predictors in systemic sclerosis, including increased age and baseline skin score, but also found that changes in forced vital capacity and diffusing capacity for carbon monoxide over 2 years were separately linked with a greater risk of death.
“These findings suggest that short-term changes in surrogate measures of systemic sclerosis-interstitial lung disease progression may have important effects on long-term outcomes,” the researchers wrote.
The researchers acknowledged the results emphasize the need to determine optimal length of treatment in patients with systemic sclerosis and comorbid interstitial lung disease.
“Systemic sclerosis providers should closely monitor lung function when interstitial lung disease is present to accurately identify declines in lung function and promptly intervene to improve patient outcomes,” the researchers concluded.
The study was funded by the National Institutes of Health and the Scleroderma Foundation. The authors reported no conflicts of interest.
SOURCE: Volkmann ER et al. Ann Rheum Dis. 2018 Nov 8. doi: 10.1136/annrheumdis-2018-213708.
In addition to known risk factors, early reductions in lung function and diffusing capacity increased mortality risk in patients with systemic sclerosis and comorbid interstitial lung disease over 2 years in an analysis of data from the Scleroderma Lung Studies I and II.
First author Elizabeth R. Volkmann, MD, of the University of California, Los Angeles, and her colleagues also reported that although early treatment with cyclophosphamide or mycophenolate mofetil in these studies led to shorter-term improvement in surrogate measures of outcomes for systemic sclerosis and comorbid interstitial lung disease (SSc-ILD), either medication might not improve long-term outcomes. The findings were reported in the Annals of the Rheumatic Diseases.
The investigators identified specific factors linked to an increased risk of death over a median follow-up of 8 years in 158 systemic sclerosis-interstitial lung disease patients in the Scleroderma Lung Study I and median follow-up of 3.6 years in 142 patients in Scleroderma Lung Study II, both of which were randomized, controlled trials.
After follow-up was complete, 42% of participants in Scleroderma Lung Study I and 21% of participants in Scleroderma Lung Study II died, with the most common cause of death being complications related to systemic sclerosis. In addition, no statistical difference was seen in time to death between the intervention arms of both trials.
In building a risk prediction model based on findings from both trials, the investigators confirmed several known mortality risk predictors in systemic sclerosis, including increased age and baseline skin score, but also found that changes in forced vital capacity and diffusing capacity for carbon monoxide over 2 years were separately linked with a greater risk of death.
“These findings suggest that short-term changes in surrogate measures of systemic sclerosis-interstitial lung disease progression may have important effects on long-term outcomes,” the researchers wrote.
The researchers acknowledged the results emphasize the need to determine optimal length of treatment in patients with systemic sclerosis and comorbid interstitial lung disease.
“Systemic sclerosis providers should closely monitor lung function when interstitial lung disease is present to accurately identify declines in lung function and promptly intervene to improve patient outcomes,” the researchers concluded.
The study was funded by the National Institutes of Health and the Scleroderma Foundation. The authors reported no conflicts of interest.
SOURCE: Volkmann ER et al. Ann Rheum Dis. 2018 Nov 8. doi: 10.1136/annrheumdis-2018-213708.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Declines in forced vital capacity and diffusing capacity for carbon monoxide were significant, independent predictors of death over the study duration.
Study details: A long-term follow-up analysis of two independent randomized, controlled trials, which consisted of 158 and 142 adults, respectively.
Disclosures: The study was funded by the National Institutes of Health and the Scleroderma Foundation. The authors reported no conflicts of interest.
Source: Volkmann ER et al. Ann Rheum Dis. 2018 Nov 8. doi: 10.1136/annrheumdis-2018-213708.
Development of reversible B-cell inhibitor XmAb5871 for SLE moves forward
CHICAGO – The novel reversible B-cell inhibitor XmAb5871 showed promise for delaying loss of improvement after steroid therapy in patients with systemic lupus erythematosus in a randomized, placebo-controlled, phase 2 study.
Patients with moderate to severe nonorgan-threatening systemic lupus erythematosus (SLE) who underwent biweekly treatment with XmAb5871 after intramuscular glucocorticoid therapy more often achieved the trial’s primary endpoint of no loss of improvement through day 225 than did patients who took placebo. Overall, 21 (42%) of 50 patients who were randomized to receive active treatment reached this outcome, compared with 12 (29%) of 42 who received placebo based on the “efficacy-evaluable” patient population, defined as those who completed day 225, had a loss of improvement, or discontinued because of a drug-related adverse even. The difference between the treatment and placebo groups with respect to loss of improvement through day 225 reflected a positive trend, but did not reach statistical significance (P = .18), Debra J. Zack, MD, of Xencor in Monrovia, Calif., and her colleagues reported in a poster at the annual meeting of the American College of Rheumatology.
Of the patients who didn’t achieve the primary endpoint, 23 (46%) in the XmAb5871 arm versus 30 (71%) in the placebo arm experienced loss of improvement at any visit, the investigators noted. Six patients in the treatment arm discontinued for toxicity and were also considered nonresponders.
Treatment with XmAb5871 also led to a longer median time to loss of improvement of 230 days in comparison with the 130 days observed in placebo-treated patients. The difference was statistically significant in the efficacy-evaluable population (hazard ratio, 0.53; P = .025) and nearly statistically significant in the intent-to-treat (ITT) population of 52 patients in each group (HR, 0.59; P = .06), they noted.
Maintenance of improvement, which was another secondary endpoint, did not differ significantly between the groups in the efficacy-evaluable population (58.0% vs. 40.5%, respectively; P = .11), but did differ significantly with treatment versus placebo at both day 225 (response rates of 44.0% vs. 23.1%, respectively; P = .06) and at day 169 (57.7% vs. 34.6%; P = .02) in the ITT population, they said.
Of note, patients in the treatment group stayed in the study longer (median of 6.9 vs. 3.6 months) and received more infusions (median of 15.0 vs. 8.5).
“Antigen-activated B cells are down-regulated by engagement of immune complexes with the inhibitory Fcy receptor FcyRIIB on the B cell surface. XmAb5871 is an anti-CD19 monoclonal antibody engineered to enhance binding to FcyRIIB,” they explained, adding that the study was designed using unique methodology to minimize background medication and placebo responses to allow for better interpretation of the results in patients with complex, heterogeneous disease.
Participants were adults with a median age of 44.5 years; most (99 of 104) were women. All received an intramuscular glucocorticoid injection at the start of the screening period as background immunosuppressants were stopped, and those who experienced at least a 4-point decrease on the SLE disease activity index or at least a 1-grade decrease in at least one British Isles Lupus Activity Group A or B score were eligible for enrollment. Background immunosuppressants had to be stopped by the time of enrollment, but patients were allowed to remain on hydroxychloroquine and prednisone 10 mg or less daily or the equivalent.
Those enrolled were randomized to receive intravenous XmAb5871 at a dose of 5 mg/kg or placebo every 14 days for up to 16 doses until day 225 or loss of improvement (at which time patients could receive standard of care and withdraw from the study); all received intramuscular glucocorticoids (Depo-Medrol 80 mg [methylprednisolone acetate]) on days 1 and 15.
XmAb5871 was well tolerated in this study and had a safety profile consistent with that seen in previous SLE studies, with the exception of six withdrawals caused by infusion reactions, the investigators said. They noted that a subcutaneous formulation showed no infusion reactions or gastrointestinal-related infusion reactions in a bioavailability study of 40 healthy subjects, and so a formulation for subcutaneous injection will be used in future studies.
The vast majority of the 149 treatment-related adverse events that occurred in 41 patients in the current study (including 26% in placebo group patients) were mild or moderate in severity. A total of 13 serious adverse events (SAEs) occurred in 11 patients, and included fever, SLE flare (lung), atrial fibrillation, worsening hypertension, iron deficiency anemia, pneumonia (occurring 36 days, or 10 half-lives, after the last dose), infusion-related reaction, and vertigo in the XmAb5871 patients, and anemia SLE flare, SLE flare (enteritis), angioedema, and migraine headache in the placebo group.
“All SAEs were considered not or unlikely related except the infusion-related reaction. There were no deaths and no opportunistic infections,” they wrote.
Although the primary endpoint of loss of improvement by day 225 was not significantly better in the treatment group in this study, a positive trend was noted, the median time to loss of improvement was extended by 76%, and the risk of increased SLE disease activity was reduced by 47% in those who received XmAb5871, they said, concluding that the findings support further evaluation of the agent in patients with SLE.
The study was supported by Xencor.
SOURCE: Zack DJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L14.
CHICAGO – The novel reversible B-cell inhibitor XmAb5871 showed promise for delaying loss of improvement after steroid therapy in patients with systemic lupus erythematosus in a randomized, placebo-controlled, phase 2 study.
Patients with moderate to severe nonorgan-threatening systemic lupus erythematosus (SLE) who underwent biweekly treatment with XmAb5871 after intramuscular glucocorticoid therapy more often achieved the trial’s primary endpoint of no loss of improvement through day 225 than did patients who took placebo. Overall, 21 (42%) of 50 patients who were randomized to receive active treatment reached this outcome, compared with 12 (29%) of 42 who received placebo based on the “efficacy-evaluable” patient population, defined as those who completed day 225, had a loss of improvement, or discontinued because of a drug-related adverse even. The difference between the treatment and placebo groups with respect to loss of improvement through day 225 reflected a positive trend, but did not reach statistical significance (P = .18), Debra J. Zack, MD, of Xencor in Monrovia, Calif., and her colleagues reported in a poster at the annual meeting of the American College of Rheumatology.
Of the patients who didn’t achieve the primary endpoint, 23 (46%) in the XmAb5871 arm versus 30 (71%) in the placebo arm experienced loss of improvement at any visit, the investigators noted. Six patients in the treatment arm discontinued for toxicity and were also considered nonresponders.
Treatment with XmAb5871 also led to a longer median time to loss of improvement of 230 days in comparison with the 130 days observed in placebo-treated patients. The difference was statistically significant in the efficacy-evaluable population (hazard ratio, 0.53; P = .025) and nearly statistically significant in the intent-to-treat (ITT) population of 52 patients in each group (HR, 0.59; P = .06), they noted.
Maintenance of improvement, which was another secondary endpoint, did not differ significantly between the groups in the efficacy-evaluable population (58.0% vs. 40.5%, respectively; P = .11), but did differ significantly with treatment versus placebo at both day 225 (response rates of 44.0% vs. 23.1%, respectively; P = .06) and at day 169 (57.7% vs. 34.6%; P = .02) in the ITT population, they said.
Of note, patients in the treatment group stayed in the study longer (median of 6.9 vs. 3.6 months) and received more infusions (median of 15.0 vs. 8.5).
“Antigen-activated B cells are down-regulated by engagement of immune complexes with the inhibitory Fcy receptor FcyRIIB on the B cell surface. XmAb5871 is an anti-CD19 monoclonal antibody engineered to enhance binding to FcyRIIB,” they explained, adding that the study was designed using unique methodology to minimize background medication and placebo responses to allow for better interpretation of the results in patients with complex, heterogeneous disease.
Participants were adults with a median age of 44.5 years; most (99 of 104) were women. All received an intramuscular glucocorticoid injection at the start of the screening period as background immunosuppressants were stopped, and those who experienced at least a 4-point decrease on the SLE disease activity index or at least a 1-grade decrease in at least one British Isles Lupus Activity Group A or B score were eligible for enrollment. Background immunosuppressants had to be stopped by the time of enrollment, but patients were allowed to remain on hydroxychloroquine and prednisone 10 mg or less daily or the equivalent.
Those enrolled were randomized to receive intravenous XmAb5871 at a dose of 5 mg/kg or placebo every 14 days for up to 16 doses until day 225 or loss of improvement (at which time patients could receive standard of care and withdraw from the study); all received intramuscular glucocorticoids (Depo-Medrol 80 mg [methylprednisolone acetate]) on days 1 and 15.
XmAb5871 was well tolerated in this study and had a safety profile consistent with that seen in previous SLE studies, with the exception of six withdrawals caused by infusion reactions, the investigators said. They noted that a subcutaneous formulation showed no infusion reactions or gastrointestinal-related infusion reactions in a bioavailability study of 40 healthy subjects, and so a formulation for subcutaneous injection will be used in future studies.
The vast majority of the 149 treatment-related adverse events that occurred in 41 patients in the current study (including 26% in placebo group patients) were mild or moderate in severity. A total of 13 serious adverse events (SAEs) occurred in 11 patients, and included fever, SLE flare (lung), atrial fibrillation, worsening hypertension, iron deficiency anemia, pneumonia (occurring 36 days, or 10 half-lives, after the last dose), infusion-related reaction, and vertigo in the XmAb5871 patients, and anemia SLE flare, SLE flare (enteritis), angioedema, and migraine headache in the placebo group.
“All SAEs were considered not or unlikely related except the infusion-related reaction. There were no deaths and no opportunistic infections,” they wrote.
Although the primary endpoint of loss of improvement by day 225 was not significantly better in the treatment group in this study, a positive trend was noted, the median time to loss of improvement was extended by 76%, and the risk of increased SLE disease activity was reduced by 47% in those who received XmAb5871, they said, concluding that the findings support further evaluation of the agent in patients with SLE.
The study was supported by Xencor.
SOURCE: Zack DJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L14.
CHICAGO – The novel reversible B-cell inhibitor XmAb5871 showed promise for delaying loss of improvement after steroid therapy in patients with systemic lupus erythematosus in a randomized, placebo-controlled, phase 2 study.
Patients with moderate to severe nonorgan-threatening systemic lupus erythematosus (SLE) who underwent biweekly treatment with XmAb5871 after intramuscular glucocorticoid therapy more often achieved the trial’s primary endpoint of no loss of improvement through day 225 than did patients who took placebo. Overall, 21 (42%) of 50 patients who were randomized to receive active treatment reached this outcome, compared with 12 (29%) of 42 who received placebo based on the “efficacy-evaluable” patient population, defined as those who completed day 225, had a loss of improvement, or discontinued because of a drug-related adverse even. The difference between the treatment and placebo groups with respect to loss of improvement through day 225 reflected a positive trend, but did not reach statistical significance (P = .18), Debra J. Zack, MD, of Xencor in Monrovia, Calif., and her colleagues reported in a poster at the annual meeting of the American College of Rheumatology.
Of the patients who didn’t achieve the primary endpoint, 23 (46%) in the XmAb5871 arm versus 30 (71%) in the placebo arm experienced loss of improvement at any visit, the investigators noted. Six patients in the treatment arm discontinued for toxicity and were also considered nonresponders.
Treatment with XmAb5871 also led to a longer median time to loss of improvement of 230 days in comparison with the 130 days observed in placebo-treated patients. The difference was statistically significant in the efficacy-evaluable population (hazard ratio, 0.53; P = .025) and nearly statistically significant in the intent-to-treat (ITT) population of 52 patients in each group (HR, 0.59; P = .06), they noted.
Maintenance of improvement, which was another secondary endpoint, did not differ significantly between the groups in the efficacy-evaluable population (58.0% vs. 40.5%, respectively; P = .11), but did differ significantly with treatment versus placebo at both day 225 (response rates of 44.0% vs. 23.1%, respectively; P = .06) and at day 169 (57.7% vs. 34.6%; P = .02) in the ITT population, they said.
Of note, patients in the treatment group stayed in the study longer (median of 6.9 vs. 3.6 months) and received more infusions (median of 15.0 vs. 8.5).
“Antigen-activated B cells are down-regulated by engagement of immune complexes with the inhibitory Fcy receptor FcyRIIB on the B cell surface. XmAb5871 is an anti-CD19 monoclonal antibody engineered to enhance binding to FcyRIIB,” they explained, adding that the study was designed using unique methodology to minimize background medication and placebo responses to allow for better interpretation of the results in patients with complex, heterogeneous disease.
Participants were adults with a median age of 44.5 years; most (99 of 104) were women. All received an intramuscular glucocorticoid injection at the start of the screening period as background immunosuppressants were stopped, and those who experienced at least a 4-point decrease on the SLE disease activity index or at least a 1-grade decrease in at least one British Isles Lupus Activity Group A or B score were eligible for enrollment. Background immunosuppressants had to be stopped by the time of enrollment, but patients were allowed to remain on hydroxychloroquine and prednisone 10 mg or less daily or the equivalent.
Those enrolled were randomized to receive intravenous XmAb5871 at a dose of 5 mg/kg or placebo every 14 days for up to 16 doses until day 225 or loss of improvement (at which time patients could receive standard of care and withdraw from the study); all received intramuscular glucocorticoids (Depo-Medrol 80 mg [methylprednisolone acetate]) on days 1 and 15.
XmAb5871 was well tolerated in this study and had a safety profile consistent with that seen in previous SLE studies, with the exception of six withdrawals caused by infusion reactions, the investigators said. They noted that a subcutaneous formulation showed no infusion reactions or gastrointestinal-related infusion reactions in a bioavailability study of 40 healthy subjects, and so a formulation for subcutaneous injection will be used in future studies.
The vast majority of the 149 treatment-related adverse events that occurred in 41 patients in the current study (including 26% in placebo group patients) were mild or moderate in severity. A total of 13 serious adverse events (SAEs) occurred in 11 patients, and included fever, SLE flare (lung), atrial fibrillation, worsening hypertension, iron deficiency anemia, pneumonia (occurring 36 days, or 10 half-lives, after the last dose), infusion-related reaction, and vertigo in the XmAb5871 patients, and anemia SLE flare, SLE flare (enteritis), angioedema, and migraine headache in the placebo group.
“All SAEs were considered not or unlikely related except the infusion-related reaction. There were no deaths and no opportunistic infections,” they wrote.
Although the primary endpoint of loss of improvement by day 225 was not significantly better in the treatment group in this study, a positive trend was noted, the median time to loss of improvement was extended by 76%, and the risk of increased SLE disease activity was reduced by 47% in those who received XmAb5871, they said, concluding that the findings support further evaluation of the agent in patients with SLE.
The study was supported by Xencor.
SOURCE: Zack DJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L14.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: XmAb5871 shows promise in systemic lupus erythematosus.
Major finding: There was no loss of improvement through day 225 in 42% of patients treated with XmAb5871 versus 29% with placebo.
Study details: A randomized, placebo-controlled, phase 2 study.
Disclosures: The study was supported by Xencor. Dr. Zack is an employee of Xencor.
Source: Zack DJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L14.
Fear of blindness hobbles hydroxychloroquine treatment of SLE
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
CHICAGO – The dosage of hydroxychloroquine (HCQ) for treating patients with systemic lupus erythematosus (SLE) is often overly influenced by fear that the drug could cause blindness, Michelle A. Petri, MD, said at the annual meeting of the American College of Rheumatology.
HCQ “is the most important drug we have to treat lupus. It’s the only treatment shown to improve survival of lupus patients. There is no reason to make patients afraid of this very important drug. I am very concerned that fear of blindness is causing our patients to be less adherent” or making them receive an inadequate dosage, said Dr. Petri, a professor of medicine and the director of the Lupus Center at Johns Hopkins University in Baltimore. “I have had no patients who went blind on HCQ. A few patients developed retinopathy, but none went blind.”
Dr. Petri spoke about experiences with some of her SLE patients who were frightened by what an ophthalmologist told them about the retinal effects of HCQ and had their dosage of the drug unilaterally cut by the ophthalmologist.
“The message we should give patients is that retinopathy is a real complication that can happen, but usually not until after 16 years of treatment with HCQ, and we will work together to make sure you are regularly screened so that, if retinopathy developed, we would pick it up early and you’ll remain asymptomatic,” she said. ”We need to put the risk into perspective for our patients.”
Reports differ on the incidence of retinopathy in SLE patients on long-term HCQ treatment. During the session in which Dr. Petri spoke, James T. Rosenbaum, MD, cited a seminal report from 2014 that tracked 2,361 U.S. patients treated with HCQ daily for at least 5 years with regular retinal follow-up. The results showed a steady, cumulative increase in patients who developed retinopathy, an increase that was also dose dependent. For example, patients who received daily dosage of 5-5.9 mg/kg and took the drug for 20 or more years had a cumulative retinopathy incidence of 30% (JAMA Opthalmol. 2014 Dec;132[12]:1453-60). For patients on higher dosages, the cumulative risk at 20 years or longer jumped above 50%.
The findings from this study led directly to the most recent recommendations from the American Academy of Ophthalmology for retinopathy screening for patients on chronic HCQ treatment, said Dr. Rosenbaum, a professor of medicine and opthalmology at Oregon Health & Science University in Portland. The recommendations called for a dosage cap of less than 5 mg/kg real weight, a baseline retinal examination, and then at least annual follow-up examinations starting after 5 years of daily HCQ use, ideally using both automated visual fields and spectral-domain optical coherence tomography (Ophthalmology. 2016 June;123[6]:1386-94). The recommendations also noted that the presence of retinopathy risk factors, including renal disease or concomitant tamoxifen use, warrant starting screening at 5 years or sooner on HCQ.
Clinicians have often exceeded recommended dosage guidelines. Dr. Rosenbaum cited a 2017 report from one U.S. health care system that reviewed the treatment of 554 patients on HCQ and found that roughly half were overdosed in their starting regimen based on prevailing treatment recommendations (Opthalmology. 2017 May;124[5]:604-8).
But Dr. Petri contended that concerns about excessive HCQ dosages are overblown. At the meeting, she reported data showing a different perspective on the retinopathy risk of patients on chronic HCQ treatment. In a prospective series of 477 SLE patients on chronic HCQ treatment and followed at Johns Hopkins, the incidence of retinopathy was 10% among patients on treatment for 16 or more years, she reported in a talk at the meeting (Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2897).
A 10% retinopathy rate after 16 or more years on treatment “sounds a lot safer to patients” than rates as high as 30% or 50% that were reported in the JAMA Opthalmology report from 2014, Dr. Petri said. “There is a danger relying on one retrospective study,” she warned. In addition, the 10% risk for retinopathy is “manageable” when patients receive regular screening that produces early detection of retinal damage.
“There has been acceptance of suboptimal dosing of HCQ when discussion has only been about safety, not about efficacy. We need to put the risks into perspective and stop scaring patients.”
Dr. Petri presented her recommendations for treating SLE patients with HCQ: Treat with dosages as high as 6.5 mg/kg but without exceeding 400 mg/day, and cut the dosage for patients with renal insufficiency or failure, those with liver disease, or the elderly. In addition, Dr. Petri endorsed monitoring blood levels of HCQ. Data she reported at the meeting showed a roughly fourfold higher rate of retinopathy among patients who had a maximum HCQ blood level of 1,733 ng/mL or higher when compared with patients whose maximum level remained at 1,194 ng/mL or lower. Clinicians could use blood levels of HCQ to better focus screening and its intensity, she said. “We should embrace monitoring HCQ blood levels.”
Dr. Petri has been a consultant to Amgen, Exagen, GlaxoSmithKline, Inova Diagnostics, Janssen, Lilly, Merck, Novartis, Quintiles, and EMD Serono, and she has received research funding from AstraZeneca and Exagen. Dr. Rosenbaum has been a consultant to AbbVie, Eyevensys, Gilead, Janssen, Novartis, Regeneron, and Roche, and has received research funding from Pfizer.
REPORTING FROM THE ACR ANNUAL MEETING
Tofacitinib impresses in first trial for dermatomyositis
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
CHICAGO – Oral tofacitinib demonstrated strong clinical efficacy and good safety in the first-ever formal study of a Janus kinase inhibitor in patients with active, treatment-resistant dermatomyositis, Julie J. Paik, MD, reported at the annual meeting of the American College of Rheumatology.
Based upon the encouraging results of this small, open-label, proof-of-concept study, a larger randomized controlled trial is being planned, according to Dr. Paik, a rheumatologist at Johns Hopkins University in Baltimore.
The study included 10 dermatomyositis patients enrolled at the Johns Hopkins Myositis Clinic. All had previously failed at least two steroid-sparing drugs and/or high-dose steroids. After first going off all steroid-sparing agents and being limited to a maximum of 20 mg/day of prednisone, the participants were placed on 11 mg of open-label, extended-release tofacitinib (Xeljanz XR) once daily. Dr. Paik only reported results in 9 patients because the 10th hadn’t yet reached the 12-week mark.
The primary outcome was the rate of achievement of significant improvement at week 12 as defined using the validated International Myositis Assessment and Clinical Studies (IMACS) criteria, which require at least a 20% improvement in three of six core set measures coupled with no more than two measures worsening by 25% or more. By this standard, all nine patients met the primary endpoint. Five were rated as showing moderate improvement, and the other four demonstrated minimal improvement, based on the Total Improvement Score of the Myositis Response criteria. The median Total Improvement Score was 40, indicative of at least moderate improvement.
The mean CDASI (Cutaneous Dermatomyositis Disease Area and Severity Index) activity score – a key secondary endpoint – improved from 28 at baseline to 9.5 at week 12, which translates to a shift from moderate or severe disease to mild disease.
Levels of the alpha-chemokines CXCL9 and CXCL10, which are expressed in muscle affected by idiopathic inflammatory myopathies such as dermatomyositis, declined strongly over the course of 12 weeks of treatment to an extent that was just shy of statistical significance.
Myositis antibody titers didn’t change in response to tofacitinib therapy. Of note, six patients were positive for antitranscriptional intermediary factor 1-gamma, and five of the six were moderate responders to JAK inhibitor therapy.
Four patients were on 20 mg/day of prednisone at baseline; three of the four were able to go off steroids altogether over the course of 12 weeks.
The treatment was well tolerated, with no serious adverse events.
Asked if she thought 11 mg/day of tofacitinib was the right dose for this population of refractory dermatomyositis patients, Dr. Paik replied, “We’re not sure we have the right dose. We had one patient who didn’t have as robust a response, and I really wonder, if we gave her a higher dose, it would have made a difference.”
Extended-release tofacitinib at 11 mg/day is the approved dose for rheumatoid arthritis and psoriatic arthritis. However, 20 mg/day is approved for patients with ulcerative colitis, and Dr. Paik is lobbying for inclusion of a higher-dose arm in the randomized, controlled trial.
Prior to this formal proof-of-concept study, which was funded by Pfizer, the experience with tofacitinib in refractory dermatomyositis was limited to anecdotal case reports.
Dr. Paik reported having no financial conflicts.
SOURCE: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Among patients with refractory dermatomyositis, the mean Cutaneous Dermatomyositis Disease Area and Severity Index activity score improved from 28 at baseline to 9.5 after 12 weeks on oral tofacitinib.
Study details: This first-in-class, open-label, 12-week study included 10 patients with refractory dermatomyositis placed on extended-release tofacitinib at 11 mg once daily.
Disclosures: The presenter reported having no financial conflicts regarding the study, sponsored by Pfizer.
Source: Paik JJ et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L02
Scleroderma SCOT trial findings hold similar in lung disease
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Lung CT scores remain stable or improve following hematopoietic stem cell transplantation in patients with scleroderma-related interstitial lung disease when compared against monthly cyclophosphamide treatments.
Major finding: Quantitative interstitial lung disease scores decreased by 7% at 54 months in hematopoietic stem cell transplant patients versus no change in those who received cyclophosphamide (P = .024).
Study details: A study of 75 patients from the SCOT trial.
Disclosures: The investigators reported having no relevant disclosures.
Source: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
Genetic profile flags scleroderma patients with best HSCT responses
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: About 90% of fibroproliferative scleroderma patients had prolonged event-free survival after stem cell transplant, compared with about 35% of controls.
Study details: The study used data collected in the SCOT trial of 75 patients with severe scleroderma.
Disclosures: Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
Source: Franks J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1876.
Dueling SLE classification criteria: And the winner is...
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.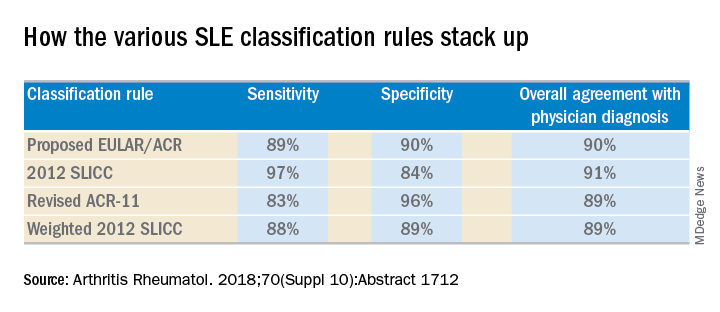
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: The 2012 SLICC SLE classification criteria are best suited for clinical practice.
Major finding:
Study details: This study compared the sensitivity, specificity, and overall agreement with physician diagnosis of four different sets of SLE classification criteria.
Disclosures: The study was supported by the National Institutes of Health. The presenter reported having no financial conflicts.
Source: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.



















