User login
Inflammatory markers may start in later stages of bipolar disorder
Interleukin-6, IL-1 receptor antagonist (IL-1RA), and tumor necrosis factor–alpha (TNF-alpha) activity was associated with inflammation and neurodegeneration in patients with chronic bipolar disorder, according to Sercan Karabulut, MD, of Kepez State Hospital in Antalya, Turkey, and associates.
In a study published in the Turkish Journal of Psychiatry, the investigators collected enzyme-linked immunosorbent assays from 30 patients with early-stage bipolar disorder, 77 with chronic disease, and 30 healthy controls. Early-stage disease patients were significantly younger than chronic patients (25.3 years vs. 37.8 years). reported Dr. Karabulut and associates.
Patients with chronic bipolar disorder had significantly increased levels of all measured markers, compared with those with early-stage disease and the healthy controls. IL-6 and IL-1RA levels correlated with neuron-specific enolase and S100B, biomarkers that are associated with glial alterations and neuronal damage. TNF-alpha correlated with scores on the Clinical Global Impressions Scale and other measures.
“The present findings support in part the hypothesis that inflammation starts at later stages of [bipolar disorder], presumably as an associated effect of gliosis and neuronal loss, which appear to be particularly associated with IL-1RA and IL-6 activity,” the investigators wrote. “TNF-alpha ... might be a useful prognostic marker in patients with [bipolar disorder].”
No conflicts of interest were reported.
SOURCE: Karabulut S et al. Turk Psikiyatri Derg. 2019 Winter;30(2):75-81.
Interleukin-6, IL-1 receptor antagonist (IL-1RA), and tumor necrosis factor–alpha (TNF-alpha) activity was associated with inflammation and neurodegeneration in patients with chronic bipolar disorder, according to Sercan Karabulut, MD, of Kepez State Hospital in Antalya, Turkey, and associates.
In a study published in the Turkish Journal of Psychiatry, the investigators collected enzyme-linked immunosorbent assays from 30 patients with early-stage bipolar disorder, 77 with chronic disease, and 30 healthy controls. Early-stage disease patients were significantly younger than chronic patients (25.3 years vs. 37.8 years). reported Dr. Karabulut and associates.
Patients with chronic bipolar disorder had significantly increased levels of all measured markers, compared with those with early-stage disease and the healthy controls. IL-6 and IL-1RA levels correlated with neuron-specific enolase and S100B, biomarkers that are associated with glial alterations and neuronal damage. TNF-alpha correlated with scores on the Clinical Global Impressions Scale and other measures.
“The present findings support in part the hypothesis that inflammation starts at later stages of [bipolar disorder], presumably as an associated effect of gliosis and neuronal loss, which appear to be particularly associated with IL-1RA and IL-6 activity,” the investigators wrote. “TNF-alpha ... might be a useful prognostic marker in patients with [bipolar disorder].”
No conflicts of interest were reported.
SOURCE: Karabulut S et al. Turk Psikiyatri Derg. 2019 Winter;30(2):75-81.
Interleukin-6, IL-1 receptor antagonist (IL-1RA), and tumor necrosis factor–alpha (TNF-alpha) activity was associated with inflammation and neurodegeneration in patients with chronic bipolar disorder, according to Sercan Karabulut, MD, of Kepez State Hospital in Antalya, Turkey, and associates.
In a study published in the Turkish Journal of Psychiatry, the investigators collected enzyme-linked immunosorbent assays from 30 patients with early-stage bipolar disorder, 77 with chronic disease, and 30 healthy controls. Early-stage disease patients were significantly younger than chronic patients (25.3 years vs. 37.8 years). reported Dr. Karabulut and associates.
Patients with chronic bipolar disorder had significantly increased levels of all measured markers, compared with those with early-stage disease and the healthy controls. IL-6 and IL-1RA levels correlated with neuron-specific enolase and S100B, biomarkers that are associated with glial alterations and neuronal damage. TNF-alpha correlated with scores on the Clinical Global Impressions Scale and other measures.
“The present findings support in part the hypothesis that inflammation starts at later stages of [bipolar disorder], presumably as an associated effect of gliosis and neuronal loss, which appear to be particularly associated with IL-1RA and IL-6 activity,” the investigators wrote. “TNF-alpha ... might be a useful prognostic marker in patients with [bipolar disorder].”
No conflicts of interest were reported.
SOURCE: Karabulut S et al. Turk Psikiyatri Derg. 2019 Winter;30(2):75-81.
FROM THE TURKISH JOURNAL OF PSYCHIATRY
FGF21 could be tied to psychopathology of bipolar mania
Patients’ fibroblast growth factor–21 levels dropped after 4 weeks of taking antipsychotics
Fibroblast growth factor–21 (FGF21), a protein that regulates carbohydrate and lipid metabolism, could be a biomarker in patients with bipolar mania, a new study suggests.
“In addition, our data indicates that FGF21 may monitor and/or prevent metabolic abnormalities induced by psychotropic drugs,” wrote Qing Hu of Xiamen City Xianyue Hospital, in Fujian, China, and associates. The study was published in Psychiatry Research.
To investigate how the expression of FGF21 changes in response to psychotropics taken by patients with bipolar mania, the researchers recruited 99 inpatients with bipolar mania with or without psychosis and 99 healthy controls. Eighty-two of the patients received psychotropics only, and 17 received psychotropics and lipid-lowering or hypotensive agents. Those in the smaller group were later excluded from follow-up.
At baseline, no significant differences were found between the patients and controls on several metabolic measures, such as cholesterol and apolipoprotein. The patients with bipolar mania had higher uric acid and triglyceride levels, although the latter was not statistically significant. However, compared with the FGF21 serum levels of the controls.
After 4 weeks of taking the antipsychotics, the patients experienced increases in several metabolic measures, such as BMI (23.68 kg/m2 vs. 24.02 kg/m2), LDL cholesterol (2.61 mg/dL vs. 2.98 mg/dL), and glucose (4.74 mg/dL vs. 4.88 mg/dL). However, their FGF21 levels declined, from 279.45 pg/mL to 215.12 pg/mL.
“In light of these findings, our future research will focus on investigating whether ... the change in FGF21 expression is a causal factor or a consequence of bipolar disorder,” the investigators wrote.
They cited several limitations. One is that psychotropic dosages were not discussed, and another is that evaluation data from the Young Mania Rating Scale were missing.
The researchers reported no conflicts of interest.
SOURCE: Hu Q et al. Psychiatry Res. 2019;272:643-8.
Patients’ fibroblast growth factor–21 levels dropped after 4 weeks of taking antipsychotics
Patients’ fibroblast growth factor–21 levels dropped after 4 weeks of taking antipsychotics
Fibroblast growth factor–21 (FGF21), a protein that regulates carbohydrate and lipid metabolism, could be a biomarker in patients with bipolar mania, a new study suggests.
“In addition, our data indicates that FGF21 may monitor and/or prevent metabolic abnormalities induced by psychotropic drugs,” wrote Qing Hu of Xiamen City Xianyue Hospital, in Fujian, China, and associates. The study was published in Psychiatry Research.
To investigate how the expression of FGF21 changes in response to psychotropics taken by patients with bipolar mania, the researchers recruited 99 inpatients with bipolar mania with or without psychosis and 99 healthy controls. Eighty-two of the patients received psychotropics only, and 17 received psychotropics and lipid-lowering or hypotensive agents. Those in the smaller group were later excluded from follow-up.
At baseline, no significant differences were found between the patients and controls on several metabolic measures, such as cholesterol and apolipoprotein. The patients with bipolar mania had higher uric acid and triglyceride levels, although the latter was not statistically significant. However, compared with the FGF21 serum levels of the controls.
After 4 weeks of taking the antipsychotics, the patients experienced increases in several metabolic measures, such as BMI (23.68 kg/m2 vs. 24.02 kg/m2), LDL cholesterol (2.61 mg/dL vs. 2.98 mg/dL), and glucose (4.74 mg/dL vs. 4.88 mg/dL). However, their FGF21 levels declined, from 279.45 pg/mL to 215.12 pg/mL.
“In light of these findings, our future research will focus on investigating whether ... the change in FGF21 expression is a causal factor or a consequence of bipolar disorder,” the investigators wrote.
They cited several limitations. One is that psychotropic dosages were not discussed, and another is that evaluation data from the Young Mania Rating Scale were missing.
The researchers reported no conflicts of interest.
SOURCE: Hu Q et al. Psychiatry Res. 2019;272:643-8.
Fibroblast growth factor–21 (FGF21), a protein that regulates carbohydrate and lipid metabolism, could be a biomarker in patients with bipolar mania, a new study suggests.
“In addition, our data indicates that FGF21 may monitor and/or prevent metabolic abnormalities induced by psychotropic drugs,” wrote Qing Hu of Xiamen City Xianyue Hospital, in Fujian, China, and associates. The study was published in Psychiatry Research.
To investigate how the expression of FGF21 changes in response to psychotropics taken by patients with bipolar mania, the researchers recruited 99 inpatients with bipolar mania with or without psychosis and 99 healthy controls. Eighty-two of the patients received psychotropics only, and 17 received psychotropics and lipid-lowering or hypotensive agents. Those in the smaller group were later excluded from follow-up.
At baseline, no significant differences were found between the patients and controls on several metabolic measures, such as cholesterol and apolipoprotein. The patients with bipolar mania had higher uric acid and triglyceride levels, although the latter was not statistically significant. However, compared with the FGF21 serum levels of the controls.
After 4 weeks of taking the antipsychotics, the patients experienced increases in several metabolic measures, such as BMI (23.68 kg/m2 vs. 24.02 kg/m2), LDL cholesterol (2.61 mg/dL vs. 2.98 mg/dL), and glucose (4.74 mg/dL vs. 4.88 mg/dL). However, their FGF21 levels declined, from 279.45 pg/mL to 215.12 pg/mL.
“In light of these findings, our future research will focus on investigating whether ... the change in FGF21 expression is a causal factor or a consequence of bipolar disorder,” the investigators wrote.
They cited several limitations. One is that psychotropic dosages were not discussed, and another is that evaluation data from the Young Mania Rating Scale were missing.
The researchers reported no conflicts of interest.
SOURCE: Hu Q et al. Psychiatry Res. 2019;272:643-8.
FROM PSYCHIATRY RESEARCH
Smartphone-based system rivals clinical assessments of anxiety in bipolar disorder
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
In patients with bipolar disorder currently in partial or full remission, self-reporting of anxiety to a smartphone-based system matched clinical evaluations of anxiety, according to Maria Faurholt-Jepsen, MD, and her associates.
A total of 84 patients with bipolar disorder who participated in the randomized, controlled, single-blind, parallel-group MONARCA II trial were included in the study, reported Dr. Faurholt-Jepsen of Rigshospitalet in Copenhagen, and her associates. All patients reported their anxiety to the smartphone-based system every day for a 9-month period; all patients underwent clinical evaluations of anxiety, functioning, patient-reported stress, and quality of life at five fixed time points over the study period. The study was published online by the Journal of Affective Disorders.
Self-reported anxiety was mild, with 19.3% of patients reporting anxiety during the study period, and 2.6% reporting severe anxiety. No association was seen between gender and anxiety days, or between bipolar disorder type and anxiety days. Patients reported depressed mood on 43.2% of the days when anxiety was also reported, and reported mania on 48.0% of the days when anxiety was reported.
Self-reported anxiety scores were positively associated with the anxiety subitems on a key rating scale (P = .0001). In addition, self-reported anxiety was associated with perceived stress, quality of life, and functioning (P = .0001 for all three).
“ Frequent fine-grained monitoring in clinical, high-risk and epidemiological populations provides an opportunity to gain a better understanding of the nature, correlates, and clinical implications of [bipolar disorder],” the investigators wrote.
Three coauthors reported consulting with Eli Lilly, Astra Zeneca, Servier, Bristol-Myers Squibb, Lundbeck, Sunovion, and Medilink. Two coauthors are cofounders and shareholders in Monsenso.
SOURCE: Faurholt-Jepsen M et al. J Affect Disord. 2019. doi: 10.1016/j.jad.2019.07.029.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
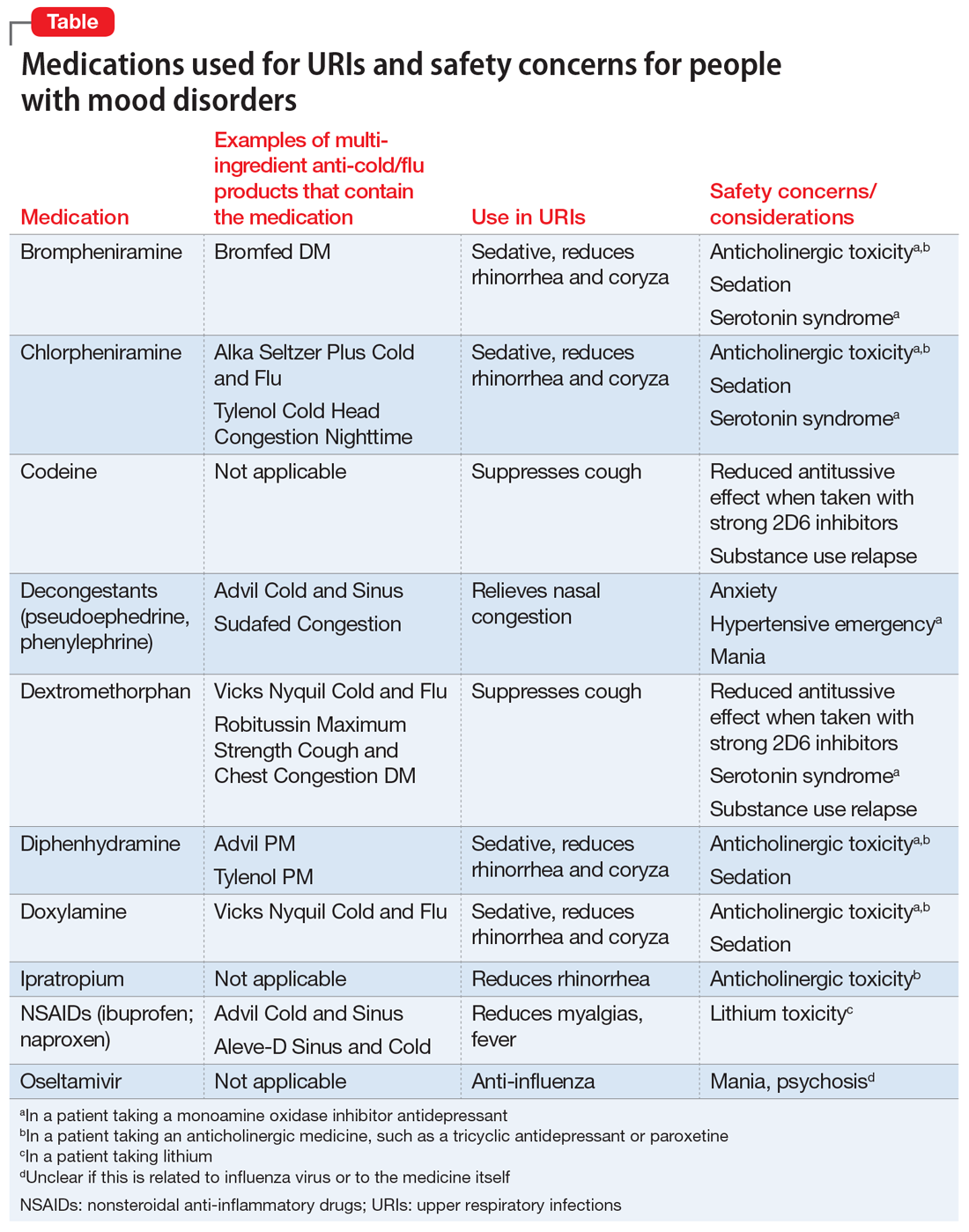
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.

Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Siblings of bipolar disorder patients at higher cardiometabolic disease risk
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
The siblings of patients with bipolar disorder have a higher prevalence of dyslipidemia and higher rates of ischemic stroke than do controls, results of a longitudinal cohort study suggest.
, wrote Wen-Yen Tsao, MD, of the department of psychiatry at Taipei Veterans General Hospital in Taiwan, and associates. Previous research has identified several overlapping genes between cardiometabolic diseases and mood disorders. In addition, polymorphisms of several genes tied to obesity have been associated with bipolar disorder.
In the current study, Dr. Tsao and associates analyzed the Taiwan National Health Insurance Research Database, which includes health care data from more than 99% of the Taiwanese population (J Affect Disord. 2019 Jun 15. doi: 10.1016/j.jad.2019.04.094). Adults born before 1990 who had no psychiatric disorders, a sibling with bipolar disorder, and a metabolic disorder were enrolled as the study cohort. A control group was identified randomly. By way of ICD-9-CM codes, people with type 2 diabetes, hypertension, dyslipidemia, and obesity were identified in both cohorts. The investigators followed the metabolic status of 7,225 unaffected siblings of bipolar disorder patients and 28,900 controls from 1996 to 2011.
Dr. Tsao and associates found that the family members who had siblings with bipolar disorder had a higher prevalence of dyslipidemia (5.4% vs. 4.5%; P = .001), compared with controls. The group with siblings with bipolar disorder also were diagnosed with type 2 diabetes at a younger age (34.81 vs. 37.22; P = .024), and had a higher prevalence of any stroke (1.5 vs. 1.1%; P = .007) and ischemic stroke (0.7% vs. 0.4%, P = .001), compared with controls.
A subanalysis showed that the higher risk of any stroke (odds ratio, 1.38; 95% confidence interval, 1.02-1.85) and ischemic stroke (OR, 2.43; 95% CI, 1.60-3.70) pertained only to male siblings. That gender-specific finding might be attributed to differences in plasma triglyceride clearance between men and women, the researchers wrote.
The findings might not be generalizable to other populations, the investigators noted. In addition, they said, the prevalence of cardiometabolic disease in the groups studied might be underestimated.
“Our results may motivate additional studies to evaluate genetic factors, psychosocial factors, and other pathophysiology of bipolar disorder,” they wrote.
The study was funded by Taiwan’s Ministry of Science and Technology, and Taipei Veterans General Hospital. The researchers cited no conflicts of interest.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Increased awareness needed of bipolar disorder in primary care
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
FROM GENERAL HOSPITAL PSYCHIATRY
Consider iatrogenesis in patients with new psychiatric symptoms
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
REPORTING FROM FOCUS ON NEUROPSYCHIATRY 2019
Patients with mood disorders may have altered microbiome
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
Discuss dietary interventions, such as probiotics, as ‘supplemental therapeutic options’
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Individuals with mood disorders might have an altered microbiome, but more information is needed to understand how the microorganisms that make up the microbiome affect patients’ health, an expert said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
“An increased understanding of the neurobiology of the microbiome is required so that the benefit that these microorganisms serve to human health can be fully harnessed,” said Emily G. Severance, PhD, assistant professor of pediatrics at John Hopkins University, Baltimore.
Diseases that involve the microbiome include those with a single identifiable infectious agent that produces persistent inflammation, central nervous system diseases with mucosal surface involvement, and diseases with “variable response to antibiotic and anti-inflammatory agents.”
“It’s becoming clear that [the microbiome is] integral for the modulation of the central nervous system,” which occurs through neurotransmitter production, Dr. Severance said at the meeting presented by Global Academy for Medical Education.
“We have an extensive enteric nervous system that has the very same receptors that the brain does,” she said. “If you have those receptors activated in the gut or [are] having the neurotransmitters produced in the gut, and if there’s a way for those neurotransmitters to reach the brain, that’s a very powerful mechanism to illustrate the gut-brain axis.”
In addition to neuropsychiatric diseases, the microbiome also can be involved in inflammatory gastrointestinal, systemic rheumatoid and autoimmune, chronic inflammatory lung, and periodontal diseases, as well as immune-mediated skin disorders. Mood disorders in particular have evidence for dysbiosis in low-level inflammation and leaky gut pathology, which is present in patients with depression, Dr. Severance said. “All these data suggest that We can do that because gut bacteria are easily accessed and can be altered through probiotics, prebiotics, diet, and fecal transplant, and in patients, Lactobacillus and Bifidobacterium combinations may improve mood, reduce anxiety, and enhance cognitive function.”
In addition, epidemiological studies show that antibiotic exposure can be a risk factor for developing mood disorders. One recent study found that anti-infective agents, particularly antibiotics, increased the risk of schizophrenia (hazard rate ratio, 2.05; 95% confidence interval, 1.77-2.38) and affective disorders (HRR, 2.59; 95% CI, 2.31-2.89), which the researchers attributed to brain inflammation, the microbiome, and environmental factors (Acta Psychiatr Scand. 2016 Nov 21. doi: 10.1111/acps.12671). In mice, other researchers found that those that received a fecal transplant with a “depression microbiota” showed symptoms of major depressive disorder, compared with mice that received a “healthy microbiota.” Those results suggest that change in microbiota can induce mood disorders (Mol Psychiatry. 2016 Apr 12. doi: 10.1038/mp.2016.44).
The evidence for probiotics is mixed, primarily because the study population in trials are so heterogeneous, but there is evidence for its efficacy in patients with mood disorders, Dr. Severance said. Probiotics have been shown to prevent rehospitalization for patients in mania. For example, one study showed reduced rehospitalization in patients with mania (8 of 33 patients) who received probiotics, compared with placebo (24 of 33 patients). Also, probiotic use was associated with fewer days of rehospitalization (Bipolar Disord. 2018 Apr 25. doi: 10. 1111/bdi.12652).
Meanwhile, a pilot study analyzing patients with irritable bowel syndrome and mild to moderate anxiety and/or depression found use of B. longum in this population reduced depression scores, but not anxiety or irritable bowel syndrome symptoms, compared with placebo (Gastroenterology. 2017 May 5. doi: 10.1053/j.gastro.2017.05.003).
Probiotic efficacy can be variable for patients with mood disorders, but the intervention is a “relatively low-risk, potentially high reward” option for these patients, Dr. Severance said. “Clinicians should inquire about patient GI conditions and overall GI health. Dietary interventions and the use of probiotics and their limitations should be discussed as supplemental therapeutic options.”
Dr. Severance reported no relevant financial disclosures.
Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM FOCUS ON NEUROPSYCHIATRY 2019
Unipolar vs bipolar depression: A clinician’s perspective
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
Mrs. W, age 36, who is married, has a history of military service, and is currently employed as a paralegal, is referred to our practice by her family physician. She complains of severe depression that impairs her ability to function at work. She had seen several other psychiatrists in both military and civilian settings, and had been treated with multiple antidepressants, including fluoxetine, sertraline, bupropion, and paroxetine.
At the time of her initial psychiatric evaluation, she is taking duloxetine, 90 mg/d, but still is experiencing depressive symptoms. She is tearful, sad, lacks energy, spends too much time in bed, and is experiencing thoughts of hopelessness, despair, and escape, verging on thoughts of suicide. As a result, she needs to scale back her work schedule to part-time. When asked about how long she had been suffering from depression, she responds “I’ve been depressed all my life.” She had been briefly hospitalized at age 16, when she made a suicide attempt by overdose. There had been no subsequent suicide attempts or psychiatric hospitalizations, although she acknowledges having intermittent suicidal thoughts.
Mrs. W’s clinical presentation is similar to that of many patients entering our practice—patients who have recurrent depression that began in early life and a history of failure to respond to multiple antidepressants. She and other patients with similar presentations are not suffering from treatment-resistant depression and in need of a trial of electroconvulsive therapy, transcranial magnetic stimulation, direct current stimulation, vagus nerve stimulation, or intranasal esketamine. She has bipolar disorder, and had been repeatedly misdiagnosed and treated inappropriately with antidepressant monotherapy.
In a previous article1 (“Controversies in bipolar disorder: Trust evidence or experience?,”
In this article, based on our more than 25 years of experience in diagnosing and treating psychiatric disorders in patients of all ages, we expand on those observations.
Misdiagnosis is common
Bipolar depression is frequently misdiagnosed as unipolar depression in outpatient2-8 and inpatient9 settings, and in children and adolescents.10 Mrs. W is typical of patients who have what we consider a bipolar spectrum disorder and receive an inaccurate diagnosis and treatment that is ineffective or may worsen the course of their illness.
Reliance on DSM-511 and its predecessor, DSM-IV, is a part of the problem of misdiagnosis because the diagnostic criteria for bipolar disorder fail to capture the clinical features of many patients with “softer” (less obvious manic and hypomanic) variants of the disorder.12,13 For example, DSM-5 criteria for a hypomanic episode (the mild high experienced by patients with a soft bipolar disorder) require that the episode lasts “at least 4 consecutive days” and is “present most of the day, nearly every day.” In our experience, the majority of hypomanic episodes are shorter—ranging from a half-day to 2 days, averaging perhaps 1.5 days.
Continue to: DSM-5 also requires...
DSM-5 also requires severity criteria for hypomania that patients with unequivocal hypomanic episodes often do not meet. For example, they may fail to experience flight of ideas or racing thoughts, or engage in activities such as “unrestrained buying sprees, sexual indiscretions, or foolish business investments.” These patients usually describe these mild highs as feeling normal and report a happier mood, more smiles and laughter, increased energy, less sleepiness, increased talkativeness, increased socialization, and improved motivation to complete tasks left undone and projects left unfinished because of the previous depressive episode. These softer (subthreshold) hypomanic episodes are authentic and, if clinicians do not identify them, may lead to misdiagnosis and inappropriate treatment.
Patients who present with depression often fail to report these brief, subthreshold hypomanic episodes or consider them to be irrelevant to their diagnosis and treatment.12,13 Probing questions can often elicit these unreported highs. For example, a patient with depression should be asked, “Have you had a single good day during the last month?” and “Where were you and what did you do during that day?” Eliciting a history of brief periods of improved mood is the key to differentiating between unipolar and bipolar depression. Screening instruments such as the Mood Disorders Questionnaire14 and the Bipolar Spectrum Diagnostic Scale15 may be helpful in distinguishing between unipolar and bipolar depression. However, we offer our thoughts on making that crucial distinction.
Distinguishing between these 2 types of depression
Although it may be difficult to distinguish between unipolar and bipolar depression, especially in the absence of a history of distinct manic or hypomanic episodes, we find the following criteria to be useful in making that determination.
Age of onset. Bipolar spectrum disorders typically begin earlier in life than unipolar depression.10,16-19 A typical presentation of bipolar disorder in children and adolescents is depression or agitated mixed states with features of both mania and depression, often accompanied by rapid mood cycling.20,21 Unipolar depression usually begins later in life, and patients do not have a history of significant depressive episodes or mood swings in childhood or adolescence. An important question to ask a patient with a chief complaint of depression is, “How old were you when you first experienced an episode of depression?”
Gender differences. Bipolar spectrum disorders with more subtle (softer) presentations, such as subthreshold highs, occur more often in women than men.22 However, overall rates of bipolar disorder may be slightly higher in men than in women.23 Unipolar melancholic depression occurs at approximately the same frequency in men and women.24
Continue to: Rapidity of onset
Rapidity of onset. Bipolar depressive episodes develop more rapidly than unipolar episodes. It is common for a patient with a bipolar spectrum disorder to transition from normal to very depressed virtually overnight, whereas in our clinical experience, unipolar episodes progress more slowly, often over several months.
Deliberate self-harm. Adolescents and young adults with a bipolar spectrum disorder frequently engage in self-injurious behavior, usually cutting with a knife, razor, or even sharp fingernails.25 Although these patients may also have thoughts of suicide and make suicide attempts, the individual usually perceives cutting as a means of gaining relief from tension and distress. These behaviors are often associated with a diagnosis of a personality disorder; in our opinion, however, they are hallmarks of a bipolar spectrum disorder.
ADHD. Bipolar disorder frequently co-occurs with attention-deficit/hyperactivity disorder (ADHD).26,27 Adults with bipolar disorder often have ADHD symptoms, which can complicate their treatment and cause functional impairment even after their mood disorder has been stabilized.28
Substance use disorders. Excessive use of alcohol and drugs is common among people with a wide range of psychiatric disorders, but patients with bipolar disorder have an unusually high rate of co-occurring substance use disorders—40% to 50%.29,30
Appetite and weight differences. Patients with unipolar depression usually experience loss of appetite and weight loss, whereas in our clinical experience, patients with bipolar depression often overeat, crave carbohydrates, and gain weight.
Continue to: Sleep problems
Sleep problems. Patients with bipolar depression have an increased need for sleep (the opposite of what they experience during highs), are sleepy during the day regardless of how many hours they sleep, and have difficulty getting up in the morning. Patients with unipolar depression also have a sleep disturbance: they may fall asleep easily, sleep for a few hours, and then awaken but are unable to fall back to sleep.31 Yet these patients usually do not complain of sleepiness during the day.
Diurnal variation of mood. Patients with unipolar depression often report that their depressive symptoms fluctuate in a circadian manner. For example, they may report that their depression is worse in the morning but improves toward evening.31 This regular alteration of circadian rhythm usually is not evident in patients with bipolar depression, whose mood may vary unpredictably or in response to stressors. Some patients with bipolar disorder, however, exhibit ultradian (ultra-rapid) mood cycling, which may be confused with the diurnal mood variation seen in patients with unipolar depression.
Tendency to recur. Although both unipolar and bipolar depressive episodes recur, a pattern of multiple recurring episodes beginning in early life is characteristic of bipolar spectrum disorders.
Behavioral history. Patients with bipolar depression are more likely than patients with unipolar depression to have a history of multiple marriages, multiple romantic relationships, episodes of promiscuity, legal problems, or financial extravagance.
Response to antidepressants. Patients with bipolar depression exhibit atypical responses to antidepressant monotherapy, such as worsening of depressive symptoms, initial improvement of mood with subsequent loss of effectiveness, premature response to an antidepressant (eg, improvement of mood within 1 to 2 days of beginning the antidepressant), fluctuation of depressive symptoms (mood cycling), or precipitation of a hypomanic or manic episode. We believe that a history of multiple failed antidepressant trials is compelling evidence of misdiagnosis of a bipolar spectrum disorder as unipolar depression.
Continue to: Genetics
Genetics. Bipolar disorder is one of the most heritable of illnesses.32 Family history is important, but affected relatives may have been misdiagnosed with unipolar depression or schizophrenia, or said to have experienced “nervous breakdowns.”
Consequences of misdiagnosis
Misdiagnosis of patients with bipolar disorder is not benign. We see patients who have suffered needlessly for years with severe depression and mood instability. After trying antidepressant after antidepressant without benefit, they begin to feel hopeless, believing they have tried everything and that nothing works for them. Often, these patients have dropped out of high school or college, or lost jobs, friends, and spouses due to their disabling but misdiagnosed psychiatric disorder. Patients with misdiagnosed bipolar disorder have an increased risk of suicide attempts and psychiatric hospitalization.5,8
Misdiagnosis of patients with bipolar disorder is not limited to nonpsychiatric physicians. The majority of patients with bipolar spectrum disorders are misdiagnosed by outpatient psychiatrists as having unipolar depression.2-7 At least 45% of patients hospitalized for depression have bipolar disorder—and most of these patients are treated inappropriately with antidepressants.9 The STAR*D study,33,34 a large randomized clinical trial of antidepressants, concluded that more than one-third of patients had not remitted from their depression after treatment with 3 different antidepressants. In our opinion, many of the nonresponding patients may have undiagnosed bipolar depression, which predictably leads to a failure to respond adequately to antidepressants. We believe that the customary inclusion and exclusion criteria used to select participants for these research studies miss subtle (subthreshold) hypomanic episodes that fall short of meeting DSM criteria for duration and severity. This phenomenon may account for the results of studies that conclude that antidepressants are, at best, minimally more effective than placebo.35
When a patient with a bipolar spectrum disorder is misdiagnosed and treated with an antidepressant, the usual result is mood destabilization. Reports of mood swings, increased crying, and suicidal thoughts and suicidal gestures in children, adolescents, and young adults treated with antidepressants led the FDA to issue a “black-box” warning.36 Because bipolar depression typically begins in youth,10,18,19 the behaviors cited in the warning may reflect misdiagnosis of bipolar depression as unipolar depression, and consequent mood destabilization as a result of treatment with an antidepressant in the absence of a mood stabilizer.
Depression and life stressors
Since many patients who are depressed present with a history of significant stressors, clinicians often face the problem of distinguishing between clinical depression and stress-induced depression. We believe that one typical symptom of depression—increased sensitivity to stressors—may help in making that distinction. A patient who is depressed will often attribute depression to stressors such as marital conflict, divorce, problems with a teenage child, work pressures, financial pressures, or the illness or death of a family member or pet. If clinical depression (unipolar or bipolar) is present, the symptoms are persistent, sometimes antedate the stressor by days or weeks, often outlast the stressor, increase in severity over time, and are disproportional to the stressor. Clinical depression can also cause the patient to become obsessed with traumatic events or losses that occurred many years earlier.
Continue to: Our approach to treatment
Our approach to treatment
Patients with mood disorders often benefit from a combination of pharmacologic management and psychotherapy. Psychotherapy is particularly important in addressing the functional impairment, diminished self-worth, and interpersonal conflicts that often accompany clinical depression. Several styles or systems of psychotherapy have been developed to benefit patients with mood disorders. Their effectiveness may depend on the patient’s ability to gain insight,37 but in our opinion, the most important attribute of helpful psychotherapy is the rapport established between the patient and the therapist, and the therapist’s ability to empathize with the patient and instill in the patient a sense of optimism and hope. We often recommend that patients attend meetings of the Depression and Bipolar Support Alliance (DBSA), a national support group with chapters throughout the country. Patients often find that attending these meetings is both educational and emotionally rewarding.
The foundational pharmacologic treatment for bipolar disorder is a mood stabilizer. The medications we consider to be effective mood stabilizers (some with an FDA indication for bipolar maintenance, some without) are lithium carbonate, divalproex sodium, carbamazepine, oxcarbazepine, and lamotrigine.
Each of these mood stabilizers has its advantages, disadvantages, risks, and adverse effects. For example, although divalproex is a reliable mood stabilizer, it has a significant risk of causing birth defects if taken during pregnancy and can cause increased appetite and weight gain. Carbamazepine has significant drug interactions and the potential to cause neurologic adverse effects, while oxcarbazepine, a derivative of carbamazepine, has fewer drug interactions but is more likely to cause hyponatremia. Lamotrigine must be titrated very slowly to reduce the risk of a potentially fatal skin rash (ie, Stevens-Johnson syndrome or toxic epidermal necrolysis). Lithium is effective but has a significant adverse-effect burden: impairment of renal function with long-term use, nephrogenic diabetes insipidus, hypothyroidism, hyperparathyroidism, acne, and weight gain. Lithium also has potential interactions with multiple commonly prescribed medications, including antihypertensives and diuretics, as well as over-the-counter pain relievers such as ibuprofen and naproxen.
Second-generation antipsychotics (SGAs) have mood stabilizing, antidepressant, and anti-manic properties and are often useful in managing bipolar disorder. In our experience, for patients with bipolar disorder, SGAs are best used in combination with a mood stabilizer. Although virtually all SGAs have demonstrated effectiveness in the treatment of psychosis and some phases of bipolar disorder, the newer agents (aripiprazole, brexpiprazole, lurasidone, and cariprazine) are relatively free of metabolic adverse effects such as weight gain, abnormal cholesterol levels, increased prolactin levels, insulin resistance, and increased risk of diabetes.
Antidepressants may be effective in treating unipolar depression, but when treating bipolar depression, they should be used cautiously and only in combination with a mood stabilizer.
Continue to: As we observed...
As we observed in our previous article,1 thyroid laboratory monitoring and supplementation are critical components of managing mood disorders (Box 138-41).
Box 1
Conventional laboratory reference ranges often indicate that thyroid-stimulating hormone (TSH) levels as high as 4.0, 4.5, or 5.0 mU/L are normal. A recent meta- analysis determined that treatment of subclinical hypothyroidism (elevated TSH with normal free thyroxine) does not benefit patients’ quality of life.38 Patients with mood disorders, however, often fail to respond to mood stabilizers and other psychiatric medications unless their TSH is <3.0 or even <2.5 mU/L.39,40 We typically augment with liothyronine because, unlike levothyroxine, it works quickly, does not require deiodination to be activated, and, contrary to some reports, its elimination and biologic half-life are sufficient for single daily dosing.41
Moving towards better diagnoses
The emergence of a criteria-based psychiatric system in 1980 with the publication of DSM-III, and its subsequent revisions and updates, constituted a major advance in psychiatric diagnosis. As we learn more about the pathophysiology, genetics, and epigenetics of psychiatric symptoms and syndromes, future diagnostic systems will improve problems of validity that have yet to be resolved. While we believe that, for the most part, DSM-5 was an advance over the previous diagnostic iteration, we have 2 issues with DSM-5 in terms of the diagnosis of bipolar disorder (Box 210,12,13,18,19,42).
Box 2
Based on our clinical experience treating thousands of patients over 25 years, we have 2 issues with DSM-5 regarding bipolar disorder:
1. The DSM-5 criteria for hypomania fail to reflect the features of clinical presentations commonly seen in our practice. The majority of patients with authentic bipolar syndromes do not have hypomanias that last for at least 4 days or reach the level of severity required for a DSM-5 diagnosis of hypomania. This results in misdiagnosis of patients with bipolar depression as suffering from unipolar depression, which leads to inappropriate treatment with antidepressant monotherapy.
2. Bipolar disorder frequently makes its first appearance in childhood and adolescence,10,18,19 and increasing numbers of young patients have been receiving this diagnosis.42 In our opinion, this increase reflects clinicians’ improved diagnostic skills. Perhaps alarmed by the increase in young people receiving a diagnosis of bipolar disorder, the authors of DSM-5 created a new diagnosis for children: disruptive mood dysregulation disorder. This diagnostic addition is based on the finding that children with these mood symptoms may not subsequently exhibit classic DSM-5 manic or hypomanic episodes. But the lack of such episodes does not preclude a diagnosis of bipolar disorder, because many adults with unequivocal bipolar spectrum disorders have subthreshold hypomanias and thus fail to exhibit classic manic or hypomanic episodes.12,13
A rose by any other name would smell as sweet. Children who exhibit symptoms of disruptive mood dysregulation disorder— chronic irritability and protracted temper outbursts—usually suffer from depression and mood instability. In our opinion, it is irrational and confusing to clinicians to separate out with a new diagnosis an arbitrarily defined group of children who exhibit substantially the same symptoms as those who receive a diagnosis of bipolar disorder.
Patients with a chief complaint of depression are often given a diagnosis of “major depression, rule out bipolar disorder.” We believe that this formula should be turned on its head. In our opinion, based on our clinical experience, we think that most patients who present to a clinician’s office or psychiatric hospital with depression have bipolar depression, not unipolar depression. We hope that our experience and observations derived from treating thousands of patients over more than 25 years may be helpful to clinicians who sometimes struggle to bring relief to their patients with mood disorders.
CASE CONTINUED
Return to work
Mrs. W is now doing well. She is taking a lower dosage of duloxetine, 60 mg/d, in combination with the mood stabilizer lamotrigine, 200 mg/d. She returns to work full-time as a paralegal and no longer is experiencing depressive episodes.
Bottom Line
Patients with bipolar depression are often misdiagnosed with unipolar depression and treated inappropriately with antidepressant monotherapy, which often results in mood destabilization. Based on our clinical experience, a careful assessment of select criteria, including age of onset, rapidity of onset, comorbidities, diurnal mood variations, and more, can be useful for distinguishing between unipolar and bipolar depression.
Related Resources
- Nasrallah HA. Misdiagnosing bipolar depression as major depressive disorder. Current Psychiatry. 2013;12(10):20-21,A.
- Ghaemi SN. Bipolar spectrum: a review of the concept and a vision for the future. Psychiatry Investig. 2013;10(3):218-224.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Carbamazepine • Tegretol, Equetro
Cariprazine • Vraylar
Divalproex • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Lamotrigine • Lamictal
Levothyroxine • Synthroid, Levoxyl
Liothyronine • Cytomel
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oxcarbazepine • Oxtellar XR, Trileptal
Paroxetine • Paxil
Sertraline • Zoloft
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
1. Miller GE, Noel RL. Controversies in bipolar disorder: trust evidence or experience? Current Psychiatry. 2009;8(2):27-28,31-33,39.
2. Glick ID. Undiagnosed bipolar disorder: new syndromes and new treatments. Prim Care Companion J Clin Psychiatry. 2004;6(1):27-33.
3. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52(1-3):135-144.
4. Blanco C, Laje G, Olfson M, et al. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry. 2002;159(6):1005-1010.
5. Shi L, Thiebaud P, McCombs JS. The impact of unrecognized bipolar disorders for patients treated with antidepressants in the fee-for-services California Medicaid (Medi-Cal) program. J Affect Disord. 2004;82(3):373-383.
6. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
7. Hughes T, Cardno A, West R, et al. Unrecognized bipolar disorder among UK primary care patients prescribed antidepressants: an observational study. Br J Gen Pract. 2016;66(643):e71-e77.
8. Keck PE Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatric Pract. 2008;14(Suppl 2):31-38.
9. Goldberg JF, Harrow M, Whiteside JF. Risk for bipolar illness in inpatients initially hospitalized for unipolar depression. Am J Psychiatry. 2001:158(8):1265-1270.
10. Chilakamarri JK, Filkowski MM, Ghaemi SN. Misdiagnosis of bipolar disorder in children and adolescents: a comparison with ADHD and major depressive disorder. Ann Clin Psychiatry. 2011;23(1):25-29.
11. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
12. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84(2-3):117-125.
13. Baldassano C. Distinctions between bipolar I and bipolar II depression. Current Psychiatry. 2017;16(8):S7-S16.
14. Hirschfeld MA, Williams JB, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: The Mood Disorder Questionnaire. Am J Psychiatry. 2000;157(11):1873-1875.
15. Ghaemi SN, Miller CJ, Berv DA, et al. Sensitivity and specificity a new bipolar spectrum diagnostic scale. J Affect Disorder. 2005;84(2-3):273-277
16. Suppes T, Leverich G, Keck P, et al. The Stanley Foundation Continuing Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disorder. 2001;67(1-3):45-49.
17. Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD). Biol Psychiatry. 2004;55(9):875-881.
18. Baldessarini RJ, Bolzani L, Kruz N, et al. Onset age of bipolar disorders at six international sites. J Affect Disord. 2010;121(1-2):143-146.
19. Post RM, Altshuler LL, Kupka R, et al. More childhood onset bipolar disorder in the United States than Canada or Europe: implications for treatment and prevention. Neurosci Biobehav Rev. 2017;74(Pt A):204-213.
20. Geller B, Luby J. Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1168-1176.
21. Findling RL, Gracious BL, McNamara NK, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
22. Arnold LM. Gender differences in bipolar disorder. Psychiatr Clin North Am. 2003;26(3):595-620.
23. Deflorio A, Jones I. Is sex important? Gender differences in bipolar disorder. Int Rev Psychiatry. 2010;22(5):437-452.
24. Bogren M, Brådvik L, Holmstrand C, et al. Gender differences in subtypes of depression by first incidence and age of onset: a follow-up of the Lunby population. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):179-189.
25. Singhal A, Ross J, Seminog O, et al. Risks of self-harm and suicide in people with specific psychiatric and physical disorders: comparisons between disorders using English national record linkage. J R Soc Med. 2014;107(5):194-204.
26. Joshi G, Wilens T. Comorbidity in pediatric bipolar disorder. Child Adolesc Psychiatr Clin N Amer. 2009;18(2):291-319.
27. Youngtrom EA, Arnold LE, Frazier TW. Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clin Psychol (New York). 2010;17(4):350-359.
28. McIntyre RS, Kennedy SH, Soczynska JK, et al. Attention-deficit/hyperactivity disorder in adults with bipolar disorder or major depressive disorder: results from the International Mood Disorders Collaborative Project. Prime Care Companion J Clin Psychiatry. 2010;12(3). doi:10.4088/PCC.09m00861gry.
29. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiological Catchment Area (ECA) Study. JAMA. 1990;264(19):2511-2518.
30. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349.
31. Agargun MY, Besiroglu L, Cilli AS, et al. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J Affect Disord. 2007;98(3):267-270.
32. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66(3):287-296.
33. Rush AJ, Trivedi MH, Wisniewski SR, et al. Buproprion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
34. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
35. Kirsch I. Antidepressants and the placebo effect. Z Psychol. 2014;222(3):128-134.
36. U.S. Food and Drug Administration. Suicidality in children and adolescents being treated with antidepressant medications. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm161679.htm. Published February 5, 2018. Accessed May 10, 2019.
37. Jennissen S, Huber J, Ehrenthal JC, et al. Association between insight and outcome of psychotherapy; systematic review and meta-analysis. Am J Psychiatry. 2018;175(10):961-969.
38. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism. JAMA. 2018;320(13):1349-1359.
39. Cole DP, Thase ME, Mallinger AG, et al. Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry. 2002;159(1):116-121.
40. Parmentier T, Sienaert P. The use of triiodothyronine (T3) in the treatment of bipolar depression: a review of the literature. J Affect Disord. 2018;229:410-414.
41. Koda-Kimbe MA, Alldredge BK. Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs (10th ed). Baltimore, MD: Walters Klower Health/Lippincott Williams & Wilkins; 2012.
42. Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64(9):1032-1039.
Reappraisal in emotion regulation unrelated to working memory in bipolar disorder
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
Working memory may not function properly during the reappraisal process of emotion regulation in patients with bipolar disorder, according to Dong Hun Oh of the department of psychiatry at Yonsei University, Seoul, South Korea, and associates.
For the study, published in the Journal of Affective Disorders, 43 patients with euthymic bipolar I disorder were recruited from a psychiatric outpatient clinic in Seoul and compared with 48 healthy controls. The Korean versions of the operation span task (OSPAN), emotion regulation questionnaire (K-ERQ), ruminative response scale (K-RRS), and Difficulties in Emotion Regulation Scale (K-DERS) were administered to all the patients.
In a between-group comparison of scores on the four scales measured, patients with bipolar disorder had significantly lower scores on OSPAN (P = .031), on the brooding section of the K-RRS (P =.016), and on the nonacceptance section of the K-DERS (P = .039). In a regression analysis of the interaction between working memory and the four components of emotion regulation (reappraisal, expressive suppression, brooding, and reflective pondering), a significant interaction was found in OSPAN scores for reappraisal between healthy controls and the BD group (P = .007). Brooding scores were significantly lower in the control group, but the interaction was not significant.
A simple slope analysis showed that, while working memory was worse in patients with bipolar disorder, a relationship between cognitive capacity and the efficacy of reappraisal was found only in healthy controls, the investigators noted.
“The absence of interaction between [working memory capacity] and reappraisal in euthymic [bipolar disorder] patients that we report may indicate that the positive effects of cognitive remediation or [working memory] training previously reported for healthy people, may not effectively improve [emotion regulation] for patients with [bipolar disorder],” they wrote.
Furthermore, if this is the case, cognitive interventions aimed at improving emotion regulation in patients with bipolar disorder might not work.
The investigators cited several limitations. One limitation was the small sample size; another was the use of self-administered questionnaires.
The study was supported by a faculty research grant from Yonsei University. The authors did not report any conflicts of interest.
SOURCE: Oh DH et al. J Affect Disord. 2019 Apr 8. doi: 10.1016/j.jad.2019.04.042.
FROM THE JOURNAL OF AFFECTIVE DISORDERS





