User login
Bipolar disorder or borderline personality disorder?
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
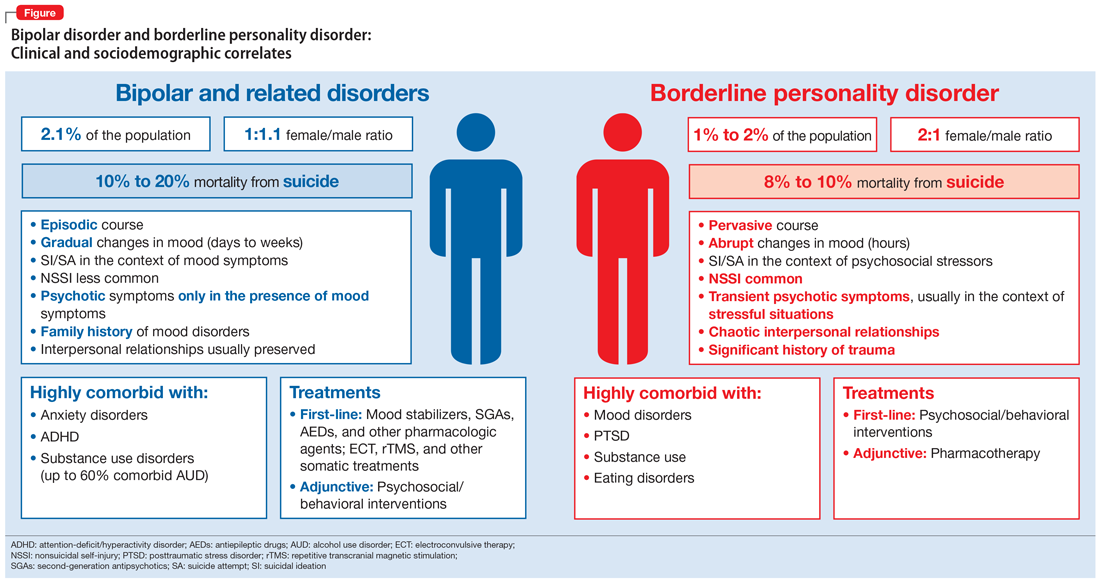
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9
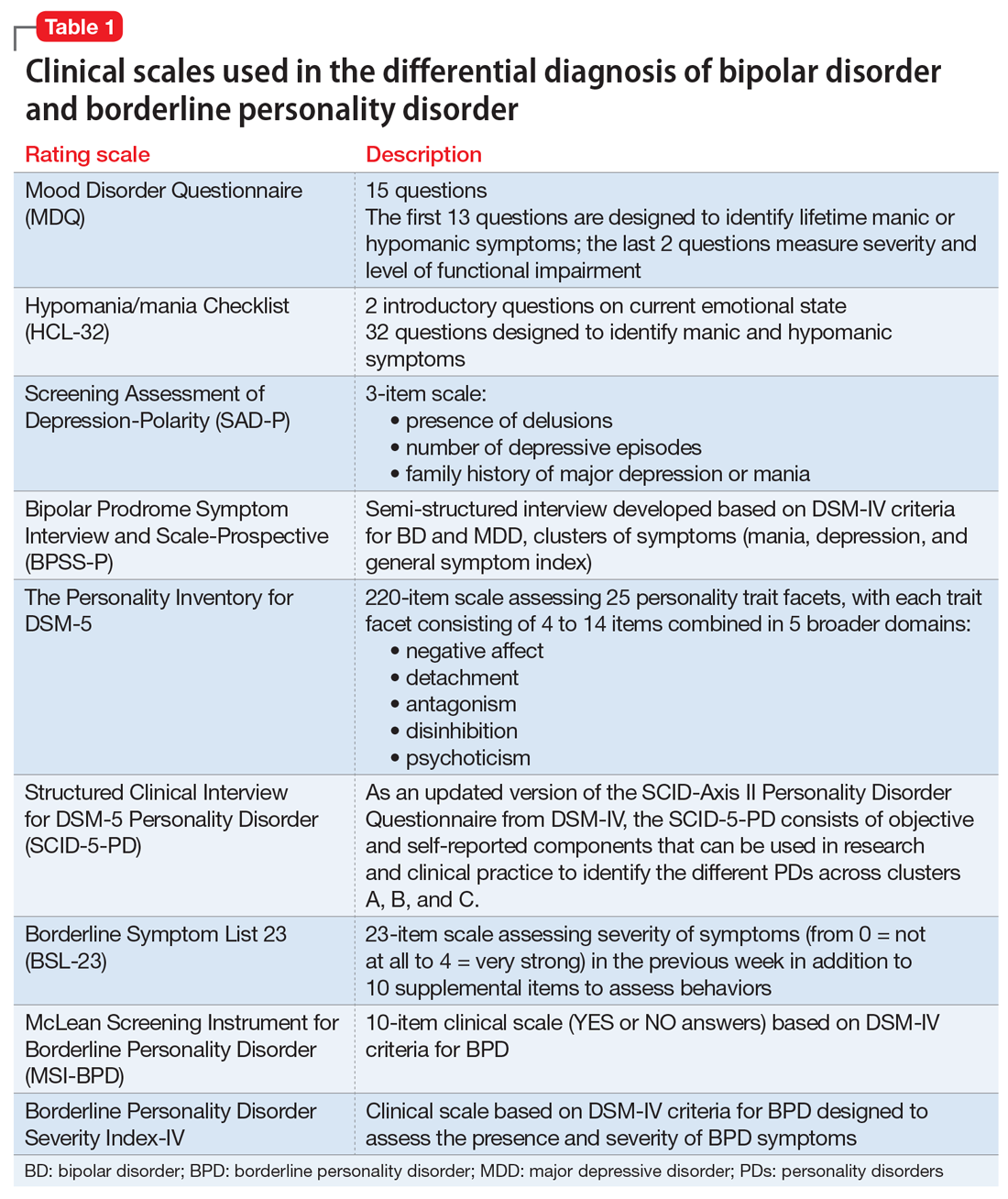
Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.
Continue to: Core features of BPD...
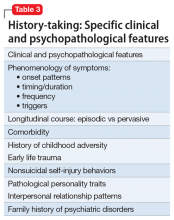
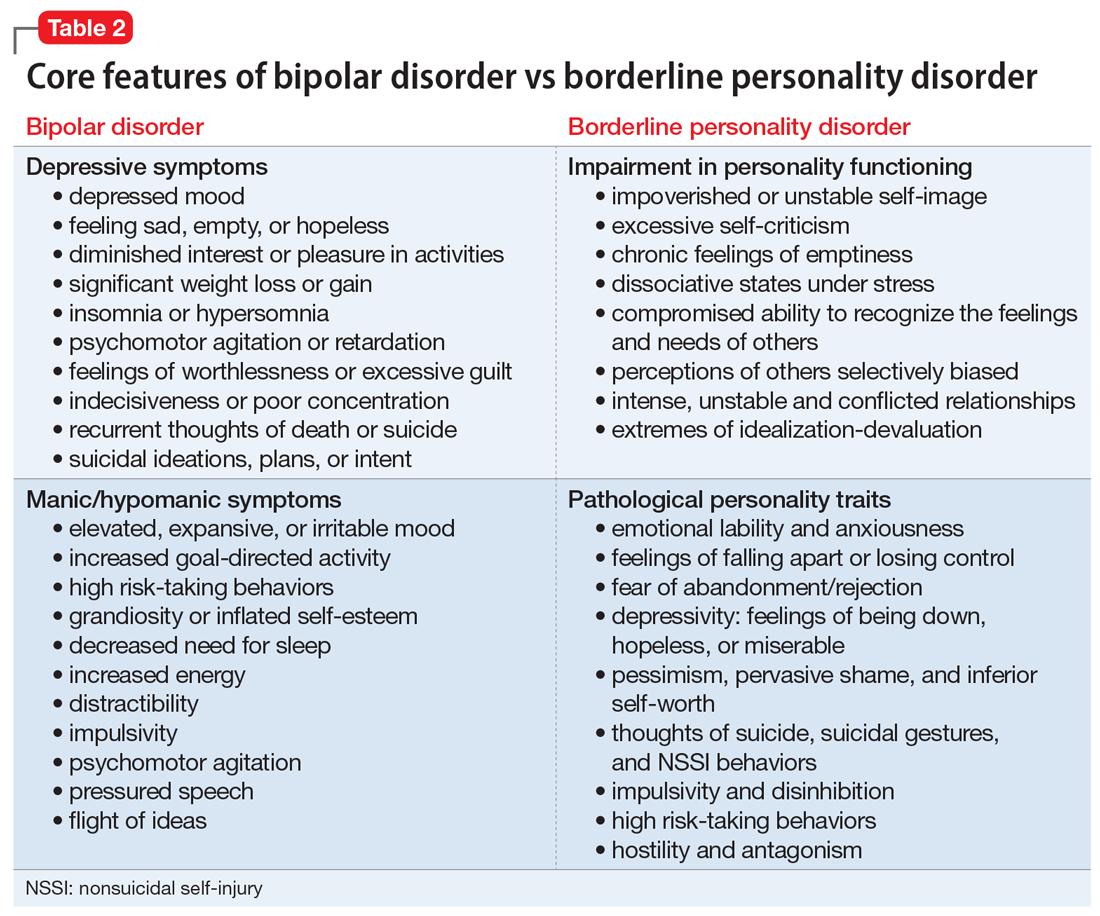
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).
Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9

Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.

Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).

Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
Although evidence suggests that bipolar disorder (BD) and borderline personality disorder (BPD) are distinct entities, their differential diagnosis is often challenging as a result of considerable overlap of phenotypical features. Moreover, BD and BPD frequently co-occur, which makes it even more difficult to differentiate these 2 conditions. Strategies for improving diagnostic accuracy are critical to optimizing patients’ clinical outcomes and long-term prognosis. Misdiagnosing these 2 conditions can be particularly deleterious, and failure to recognize their co-occurrence can result in additional burden to typically complex and severe clinical presentations.
This article describes key aspects of the differential diagnosis between BD and BPD, emphasizing core features and major dissimilarities between these 2 conditions, and discusses the implications of misdiagnosis. The goal is to highlight the clinical and psychopathological aspects of BD and BPD to help clinicians properly distinguish these 2 disorders.
Psychopathological and sociodemographic correlates
Bipolar disorder is a chronic and severe mental illness that is classified based on clusters of symptoms—manic, hypomanic, and depressive.1 It is among the 10 leading causes of disability worldwide, with significant morbidity arising from acute affective episodes and subacute states.2 Data suggest the lifetime prevalence of BPD is 2.1%, and subthreshold forms may affect an additional 2.4% of the US population.3 The onset of symptoms typically occurs during late adolescence or early adulthood, and mood lability and cyclothymic temperament are the most common prodromal features.4
In contrast, personality disorders, such as BPD, are characteristically pervasive and maladaptive patterns of emotional responses that usually deviate from an individual’s stage of development and cultural background.1 These disorders tend to cause significant impairment, particularly in personal, occupational, and social domains. Environmental factors, such as early childhood trauma, seem to play an important role in the genesis of personality disorders, which may be particularly relevant in BPD, a disorder characterized by marked impulsivity and a pattern of instability in personal relationships, self-image, and affect.1,5,6 Similarly to BD, BPD is also chronic and highly disabling.
According to the National Survey on Alcohol and Related Conditions (NESARC), approximately 15% of US adults were found to have at least one type of personality disorder, and 6% met criteria for a cluster B personality disorder (antisocial, borderline, narcissistic, and histrionic).7 The lifetime prevalence of BPD is nearly 2%, with higher estimates observed in psychiatric settings.7,8
As a result of the phenotypical resemblance between BD and BPD (Figure), the differential diagnosis is often difficult. Recent studies suggest that co-occurrence of BD and BPD is common, with rates of comorbid BPD as high as 29% in BD I and 24% in BD II.8,9 On the other hand, nearly 20% of individuals with BPD seem to have comorbid BD.8,9 Several studies suggest that comorbid personality disorders represent a negative prognostic factor in the course of mood disorders, and the presence of BPD in patients with BD seems to be associated with more severe clinical presentations, greater treatment complexity, a higher number of depressive episodes, poor inter-episode functioning, and higher rates of other comorbidities, such as substance use disorders (SUDs).8-11 The effect of BD on the course of BPD is unclear and fairly unexplored, although it has been suggested that better control of mood symptoms may lead to more stable psychosocial functioning in BPD.9

Whether BD and BPD are part of the same spectrum is a matter for debate.12-14 Multidimensional approaches have been proposed to better characterize these disorders in at-risk populations, based on structured interviews, self-administered and clinician-rated clinical scales (Table 1), neuroimaging studies, biological markers, and machine-learning models.15,16 Compelling evidence suggests that BD and BPD have distinct underlying neurobiological and psychopathological mechanisms12,13; however, the differential diagnosis still relies on phenotypical features, since the search for biological markers has not yet identified specific biomarkers that can be used in clinical practice.

Continue to: Core features of BPD...
Core features of BPD, such as mood lability, impulsivity, and risk-taking behaviors, are also part of the diagnostic criteria for BD (Table 2).1 Similarly, depressive symptoms prevail in the course of BD.17,18 This adds complexity to the differential because “depressivity” is also part of the diagnostic criteria for BPD.1 Therefore, comprehensive psychiatric assessments and longitudinal observations are critical to diagnostic accuracy and treatment planning. Further characterization of symptoms, such as onset patterns, clinical course, phenomenology of symptoms (eg, timing, frequency, duration, triggers), and personality traits, will provide information to properly distinguish these 2 syndromes when, for example, it is unclear if the “mood swings” and impulsivity are part of a mood or a personality disorder (Table 3).

Clinical features: A closer look
Borderline personality disorder. Affect dysregulation, emotional instability, impoverished and unstable self-image, and chronic feelings of emptiness are core features of BPD.1,5,19 These characteristics, when combined with a fear of abandonment or rejection, a compromised ability to recognize the feelings and needs of others, and extremes of idealization-devaluation, tend to culminate in problematic and chaotic relationships.6,19 Individuals with BPD may become suspicious or paranoid under stressful situations. Under these circumstances, individuals with BPD may also experience depersonalization and other dissociative symptoms.6,20 The mood lability and emotional instability observed in patients with BPD usually are in response to environmental factors, and although generally intense and out of proportion, they tend to be ephemeral and short-lived, typically lasting a few hours.1,5 The anxiety and depressive symptoms reported by patients with BPD frequently are associated with feelings of “falling apart” or “losing control,” pessimism, shame, and low self-esteem. Coping strategies tend to be poorly developed and/or maladaptive, and individuals with BPD usually display a hostile and antagonistic demeanor and engage in suicidal or nonsuicidal self-injury (NSSI) behaviors as means to alleviate overwhelming emotional distress. Impulsivity, disinhibition, poor tolerance to frustration, and risk-taking behaviors are also characteristic of BPD.1,5 As a result, BPD is usually associated with significant impairment in functioning, multiple hospitalizations, and high rates of comorbid mood disorders, posttraumatic stress disorder (PTSD), SUDs, and death by suicide.
Bipolar disorder. Conversely, the fluctuations in mood and affect observed in patients with BD are usually episodic rather than pervasive, and tend to last longer (typically days to weeks) compared with the transient mood shifts observed in patients with BPD.4,17,18 The impulsivity, psychomotor agitation, and increased goal-directed activity reported by patients with BD are usually seen in the context of an acute affective episode, and are far less common during periods of stability or euthymic affect.4,17,18 Grandiosity and inflated self-esteem—hallmarks of a manic or hypomanic state—seem to oppose the unstable self-image observed in BPD, although indecisiveness and low self-worth may be observed in individuals with BD during depressive episodes. Antidepressant-induced mania or hypomania, atypical depressive episodes, and disruptions in sleep and circadian rhythms may be predictors of BD.4,21 Furthermore, although psychosocial stressors may be associated with acute affective episodes in early stages of bipolar illness, over time minimal stressors are necessary to ignite new affective episodes.22,23 Although BD is associated with high rates of suicide, suicide attempts are usually seen in the context of an acute depressive episode, and NSSI behaviors are less common among patients with BD.24
Lastly, other biographical data, such as a history of early life trauma, comorbidity, and a family history of psychiatric illnesses, can be particularly helpful in establishing the differential diagnosis between BD and BPD.25 For instance, evidence suggests that the heritability of BD may be as high as 70%, which usually translates into an extensive family history of bipolar and related disorders.26 In addition, studies suggest a high co-occurrence of anxiety disorders, attention-deficit/hyperactivity disorder, and SUDs in patients with BD, whereas PTSD, SUDs, and eating disorders tend to be highly comorbid with BPD.27 Childhood adversity (ie, a history of physical, sexual, or emotional abuse, or neglect) seems to be pivotal in the genesis of BPD and may predispose these individuals to psychotic and dissociative symptoms, particularly those with a history of sexual abuse, while playing a more secondary role in BD.28-31
Implications of misdiagnosis
In the view of the limitations of the existing models, multidimensional approaches are necessary to improve diagnostic accuracy. Presently, the differential diagnosis of BD and BPD continues to rely on clinical findings and syndromic classifications. Misdiagnosing BD and BPD has adverse therapeutic and prognostic implications.32 For instance, while psychotropic medications and neuromodulatory therapies (eg, electroconvulsive therapy, repetitive transcranial magnetic stimulation) are considered first-line treatments for patients with BD, psychosocial interventions tend to be adjunctive treatments in BD.33 Conversely, although pharmacotherapy might be helpful for patients with BPD, psychosocial and behavioral interventions are the mainstay treatment for this disorder, with the strongest evidence supporting cognitive-behavioral therapy, dialectical behavioral therapy, mentalization-based therapy, and transference-focused therapy.34-36 Thus, misdiagnosing BD as BPD with comorbid depression may result in the use of antidepressants, which can be detrimental in BD. Antidepressant treatment of BD, particularly as monotherapy, has been associated with manic or hypomanic switch, mixed states, and frequent cycling.21 Moreover, delays in diagnosis and proper treatment of BD may result in protracted mood symptoms, prolonged affective episodes, higher rates of disability, functional impairment, and overall worse clinical outcomes.24 In addition, because behavioral and psychosocial interventions are usually adjunctive therapies rather than first-line interventions for patients with BD, misdiagnosing BPD as BD may ultimately prevent these individuals from receiving proper treatment, likely resulting in more severe functional impairment, multiple hospitalizations, self-inflicted injuries, and suicide attempts, since psychotropic medications are not particularly effective for improving self-efficacy and coping strategies, nor for correcting cognitive distortions, particularly in self-image, and pathological personality traits, all of which are critical aspects of BPD treatment.
Continue to: Several factors might...
Several factors might make clinicians reluctant to diagnose BPD, or bias them to diagnose BD more frequently. These include a lack of familiarity with the diagnostic criteria for BPD, the phenotypical resemblance between BP and BPD, or even concerns about the stigma and negative implications that are associated with a BPD diagnosis.32,37,38
Whereas BD is currently perceived as a condition with a strong biological basis, there are considerable misconceptions regarding BPD and its nature.4-6,22,26 As a consequence, individuals with BPD tend to be perceived as “difficult-to-treat,” “uncooperative,” or “attention-seeking.” These misconceptions may result in poor clinician-patient relationships, unmet clinical and psychiatric needs, and frustration for both clinicians and patients.37
Through advances in biological psychiatry, precision medicine may someday be a part of psychiatric practice. Biological “signatures” may eventually help clinicians in diagnosing and treating psychiatric disorders. Presently, however, rigorous history-taking and comprehensive clinical assessments are still the most powerful tools a clinician can use to accomplish these goals. Finally, destigmatizing psychiatric disorders and educating patients and clinicians are also critical to improving clinical outcomes and promoting mental health in a compassionate and empathetic fashion.
Bottom Line
Despite the phenotypical resemblance between bipolar disorder (BP) and borderline personality disorder (BPD), the 2 are independent conditions with distinct neurobiological and psychopathological underpinnings. Clinicians can use a rigorous assessment of pathological personality traits and characterization of symptoms, such as onset patterns, clinical course, and phenomenology, to properly distinguish between BP and BPD.
Related Resources
- Fiedorowicz JG, Black DW. Borderline, bipolar, or both? Frame your diagnosis on the patient history. Current Psychiatry. 2010; 9(1):21-24,29-32.
- Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
- National Institute of Mental Health. Overview on bipolar disorder. www.nimh.nih.gov/health/publications/bipolar-disorder/index.shtml. Revised October 2018.
- National Institute of Mental Health. Overview on borderline personality disorder. www.nimh.nih.gov/health/publications/borderline-personality-disorder-fact-sheet/index.shtml.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586.
3. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543-552.
4. Malhi GS, Bargh DM, Coulston CM, et al. Predicting bipolar disorder on the basis of phenomenology: implications for prevention and early intervention. Bipolar Disord. 2014;16(5):455-470.
5. Skodol AE, Gunderson JG, Pfohl B, et al. The borderline diagnosis I: psychopathology. Biol Psychiatry. 2002;51(12):936-950.
6. Skodol AE, Siever LJ, Livesley WJ, et al. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51(12):951-963.
7. Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609-1640.
8. McDermid J, Sareen J, El-Gabalawy R, et al. Co-morbidity of bipolar disorder and borderline personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2015;58:18-28.
9. Gunderson JG, Weinberg I, Daversa MT, et al. Descriptive and longitudinal observations on the relationship of borderline personality disorder and bipolar disorder. Am J Psychiatry. 2006;163(7):1173-1178.
10. Swartz HA, Pilkonis PA, Frank E, et al. Acute treatment outcomes in patients with bipolar I disorder and co-morbid borderline personality disorder receiving medication and psychotherapy. Bipolar Disord. 2005;7(2):192-197.
11. Riemann G, Weisscher N, Post RM, et al. The relationship between self-reported borderline personality features and prospective illness course in bipolar disorder. Int J Bipolar Disord. 2017;5(1):31.
12. de la Rosa I, Oquendo MA, García G, et al. Determining if borderline personality disorder and bipolar disorder are alternative expressions of the same disorder. J Clin Psychiatry. 2017;778(8):e994-e999. doi: 10.4088/JCP.16m11190.
13. di Giacomo E, Aspesi F, Fotiadou M, et al. Unblending borderline personality and bipolar disorders. J Psychiatr Res. 2017;91:90-97.
14. Parker G, Bayes A, McClure G, et al. Clinical status of comorbid bipolar disorder and borderline personality disorder. Br J Psychiatry. 2016;209(3):209-215.
15. Perez Arribas I, Goodwin GM, Geddes JR, et al. A signature-based machine learning model for distinguishing bipolar disorder and borderline personality disorder. Transl Psychiatry. 2018;8(1):274.
16. Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
17. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
18. Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
19. Oldham JM, Skodol AE, Bender DS. A current integrative perspective on personality disorders. American Psychiatric Publishing, Inc. 2005.
20. Herzog JI, Schmahl C. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front Psychiatry. 2018;9:420.
21. Barbuti M, Pacchiarotti I, Vieta E, et al. Antidepressant-induced hypomania/mania in patients with major depression: evidence from the BRIDGE-II-MIX study. J Affect Disord. 2017;219:187-192.
22. Post RM. Mechanisms of illness progression in the recurrent affective disorders. Neurotox Res. 2010;18(3-4):256-271.
23. da Costa SC, Passos IC, Lowri C, et al. Refractory bipolar disorder and neuroprogression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;70:103-110.
24. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931-939.
25. Zimmerman M, Martinez JH, Morgan TA, et al. Distinguishing bipolar II depression from major depressive disorder with comorbid borderline personality disorder: demographic, clinical, and family history differences. J Clin Psychiatry. 2013;74(9):880-886.
26. Hasler G, Drevets WC, Gould TD, et al. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93-105.
27. Brieger P, Ehrt U, Marneros A. Frequency of comorbid personality disorders in bipolar and unipolar affective disorders. Compr Psychiatry. 2003;44(1):28-34.
28. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288-297.
29. Leverich GS, Post RM. Course of bipolar illness after history of childhood trauma. Lancet. 2006;367(9516):1040-1042.
30. Golier JA, Yehuda R, Bierer LM, et al. The relationship of borderline personality disorder to posttraumatic stress disorder and traumatic events. Am J Psychiatry. 2003;160(11):2018-2024.
31. Nicol K, Pope M, Romaniuk L, et al. Childhood trauma, midbrain activation and psychotic symptoms in borderline personality disorder. Transl Psychiatry. 2015;5:e559. doi:10.1038/tp.2015.53.
32. Ruggero CJ, Zimmerman M, Chelminski I, et al. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. 2010;44(6):405-408.
33. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672-1682.
34. McMain S, Korman LM, Dimeff L. Dialectical behavior therapy and the treatment of emotion dysregulation. J Clin Psychol. 2001;57(2):183-196.
35. Cristea IA, Gentili C, Cotet CD, et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(4):319-328.
36. Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder. JAMA Psychiatry. 2015;72(75);475-482.
37. LeQuesne ER, Hersh RG. Disclosure of a diagnosis of borderline personality disorder. J Psychiatr Pract. 2004:10(3):170-176.
38. Young AH. Bipolar disorder: diagnostic conundrums and associated comorbidities. J Clin Psychiatry. 2009;70(8):e26. doi:10.4088/jcp.7067br6c.
Increased Parkinson’s disease risk seen with bipolar disorder
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
Patients with bipolar disorder may be at increased risk of Parkinson’s disease in later life, according to a systematic review and meta-analysis published in JAMA Neurology.
Patrícia R. Faustino, MD, from the faculty of medicine at the University of Lisboa (Portgual), and coauthors reviewed and analyzed seven articles – four cohort studies and three cross-sectional studies – that reported data on idiopathic Parkinson’s disease in patients with bipolar disorder, compared with those without. The meta-analysis found that individuals with a previous diagnosis of bipolar disorder had a 235% higher risk of being later diagnosed with Parkinson’s disease. Even after removing studies with a high risk of bias, the risk was still 3.21 times higher in those with bipolar disorder, compared with those without.
“The pathophysiological rationale between bipolar disorder and Parkinson’s disease might be explained by the dopamine dysregulation hypothesis, which states that the cyclical process of bipolar disorder in manic states leads to a down-regulation of dopamine receptor sensitivity (depression phase), which is later compensated by up-regulation (manic state),” the authors wrote. “Over time, this phenomenon may lead to an overall reduction of dopaminergic activity, the prototypical Parkinson’s disease state.”
Subgroup analysis revealed that subgroups with shorter follow-up periods – less than 9 years – had a greater increase in the risk of a later Parkinson’s disease diagnosis. The authors noted that this could represent misdiagnosis of parkinsonism – possibly drug induced – as Parkinson’s disease. The researchers also raised the possibility that the increased risk of Parkinson’s disease in patients with bipolar disorder could relate to long-term lithium use, rather than being a causal relationship. “However, treatment with lithium is foundational in bipolar disorder, and so to separate the causal effect from a potential confounder would be particularly difficult,” they wrote.
One of the studies included did explore the use of lithium, and found that lithium monotherapy was associated with a significant increase in the risk of being diagnosed with Parkinson’s disease or taking antiparkinsonism medication, compared with antidepressant therapy. However the authors commented that the diagnostic code may not differentiate Parkinson’s disease from other causes of parkinsonism.
Given their findings, the authors suggested that, if patients with bipolar disorder present with parkinsonism features, it may not necessarily be drug induced. In these patients, they recommended an investigation for Parkinson’s disease, perhaps using functional neuroimaging “as Parkinson’s disease classically presents with nigrostriatal degeneration while drug-induced parkinsonism does not.”
Two authors declared grants and personal fees from the pharmaceutical sector. No other conflicts of interest were reported.
SOURCE: Faustino PR et al. JAMA Neurol. 2019 Oct 14. doi: 10.1001/jamaneurol.2019.3446.
FROM JAMA NEUROLOGY
#MomsNeedToKnow mental health awareness campaign set to launch
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
One goal is to use social media to encourage women to let go of stigma
One goal is to use social media to encourage women to let go of stigma
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Pregnancy-related mental health conditions are the most common complication of pregnancy, yet half of all women suffering will not be treated.
I wanted to address the stigma associated with these conditions as well as the rampant misinformation online. So, I reached out to Jen Schwartz, patient advocate and founder of Motherhood Understand, an online community for moms impacted by maternal mental health conditions. Together, we conceived the idea for the #MomsNeedToKnow maternal mental health awareness campaign, which will run from Oct. 14 to 25. This is an evidence-based campaign, complete with references and citations, that speaks to patients where they are at, i.e., social media.
With my clinical expertise and Jen’s reach, we felt like it was a natural partnership, as well as an innovative approach to empowering women to take control of their mental health during the perinatal period. We teamed up with Jamina Bone, an illustrator, and developed 2 weeks of Instagram posts, focused on the themes of lesser-known diagnoses, maternal mental health myths, and treatment options. This campaign is designed to help women understand risk factors for perinatal mood and anxiety disorders, as well as the signs of these conditions. It will cover lesser-known diagnoses like postpartum obsessive-compulsive disorder and posttraumatic stress disorder, and will address topics such as the impact of infertility on mental health and clarify the roles of different clinicians who can help.
Moreover, the campaign aims to address stigma and myths around psychiatric treatment during pregnancy – and also provides resources.
Dr. Lakshmin, a perinatal psychiatrist, is clinical assistant professor of psychiatry at George Washington University in Washington.
This article was updated 10/12/19.
Regional brain activation lower in bipolar disorder patients after multiple manic episodes
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
Patients with bipolar disorder who had undergone multiple manic episodes had significantly lower regional activation in the prefrontal‐striatal‐amygdala networks than single-episode patients, according to Logan Borgelt of the department of psychiatry and behavioral neuroscience at the University of Cincinnati and associates.
For the study, the investigators collected functional MRI data from 57 first‐episode manic patients (mean age, 19 years; mean Young Mania Rating Scale [YMRS] score, 26) and 50 multiepisode patients (mean age, 32 years; mean YMRS score, 21) who performed a continuous task with emotional distractions, as well as MR spectroscopy from 52 first-episode patients (mean age, 19 years; mean YMRS score, 26) and 54 multiepisode patients (mean age, 32 years; mean YMRS, 22). The study was published in Bipolar Disorders.
The investigation found that activation of the bilateral ventrolateral prefrontal cortex (P = .0122 for left; P = .0007 for right), anterior cingulate cortex (P less than .0001), orbitofrontal cortex (P = .0133), putamen (P = .0032), caudate (P = .0008), and amygdala (P = .0215) was significantly lower in multiepisode patients than in single-episode patients. Glutamate and N‐acetylaspartate concentration in the anterior cingulate cortex was also lower in multiepisode patients.
The age of multiepisode patients was, in general, not heavily associated with worse activation; only the right putamen (r = 0.30) and right thalamus (r = 0.30) reached a moderate effect size.
“Our findings are consistent with a hypothesized vicious cycle in which progressive atrophic changes in the prefrontal cortex are associated with functional decrements in affective networks, which in turn contribute to both further neuronal loss and clinical observations of accelerating symptomatic recurrence. ,” the investigators wrote.
Mr. Borgelt reported no conflicts. Three coauthors reported consulting with, receiving support and honoraria from, or serving on the speaker’s bureaus for numerous sources. The remaining coauthors did not report any conflicts of interest.
SOURCE: Borgelt L et al. Bipolar Disord. 2019 Apr 26. doi: 10.1111/bdi.12782.
FROM BIPOLAR DISORDERS
Lithium drug interactions not quite as bad as imagined
SAN DIEGO – You don’t have to stop prescribing lithium when patients go on ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure treatment.
Some might opt to do that, but there’s no need to worry. In fact, the reason both classes are known to protect the kidneys is that they were tested with lithium; it was used to measure the drug’s effects on renal clearance, according to Stephen R. Saklad, PharmD, director of psychiatric pharmacy and clinical professor at the University of Texas at Austin.
There is interaction, but “lithium is not toxic in the presence of an ACE or an RB. You just have to adjust the dose,” he said at the annual Psych Congress.
Dr. Saklad shared lithium drug interaction pearls during a video interview at the meeting, including also using lithium with diuretics and NSAIDs. “The worst offender is probably my favorite of the NSAIDs, which is ibuprofen,” he said.
Most of the time with lithium, all that’s needed is a dose adjustment up or down of either it or the coadministered medication.
The overall guiding principle, he said, is that “lithium follows sodium. Anything that alters sodium in the body is going to alter sodium.”
SAN DIEGO – You don’t have to stop prescribing lithium when patients go on ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure treatment.
Some might opt to do that, but there’s no need to worry. In fact, the reason both classes are known to protect the kidneys is that they were tested with lithium; it was used to measure the drug’s effects on renal clearance, according to Stephen R. Saklad, PharmD, director of psychiatric pharmacy and clinical professor at the University of Texas at Austin.
There is interaction, but “lithium is not toxic in the presence of an ACE or an RB. You just have to adjust the dose,” he said at the annual Psych Congress.
Dr. Saklad shared lithium drug interaction pearls during a video interview at the meeting, including also using lithium with diuretics and NSAIDs. “The worst offender is probably my favorite of the NSAIDs, which is ibuprofen,” he said.
Most of the time with lithium, all that’s needed is a dose adjustment up or down of either it or the coadministered medication.
The overall guiding principle, he said, is that “lithium follows sodium. Anything that alters sodium in the body is going to alter sodium.”
SAN DIEGO – You don’t have to stop prescribing lithium when patients go on ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure treatment.
Some might opt to do that, but there’s no need to worry. In fact, the reason both classes are known to protect the kidneys is that they were tested with lithium; it was used to measure the drug’s effects on renal clearance, according to Stephen R. Saklad, PharmD, director of psychiatric pharmacy and clinical professor at the University of Texas at Austin.
There is interaction, but “lithium is not toxic in the presence of an ACE or an RB. You just have to adjust the dose,” he said at the annual Psych Congress.
Dr. Saklad shared lithium drug interaction pearls during a video interview at the meeting, including also using lithium with diuretics and NSAIDs. “The worst offender is probably my favorite of the NSAIDs, which is ibuprofen,” he said.
Most of the time with lithium, all that’s needed is a dose adjustment up or down of either it or the coadministered medication.
The overall guiding principle, he said, is that “lithium follows sodium. Anything that alters sodium in the body is going to alter sodium.”
REPORTING FROM PSYCH CONGRESS 2019
The challenges of caring for a physician with a mental illness
A physician’s mental health is important for the delivery of quality health care to his/her patients. Early identification and treatment of physicians with mental illnesses is challenging because physicians may neglect their own mental health due to the associated stigma, time constraints, or uncertainty regarding where to seek help. Physicians often worry about whom to confide in and harbor a fear that others will doubt his/her competence after recovery.1 Physicians have higher rates of suicide than the general population.2 According to data from the National Violent Death Reporting System, a diagnosed mental illness or a job problem significantly contribute to suicide among physicians.3 Additionally, physicians also have high rates of substance use and affective disorders.1,4
Here, we present the case of a physician we treated on an inpatient psychiatry unit who stirred profound emotions in us as trainees, and discuss how we managed this complicated scenario.
CASE REPORT
Dr. P, a 35-year-old male endocrinologist, was admitted to our inpatient psychiatry unit with a diagnosis of bipolar disorder, manic, severe, with psychotic features. Earlier that day, Dr. P had walked out of his private outpatient practice where he still had several appointments. After he had been missing for several hours, he was picked up by the police. Dr. P had 2 prior psychiatric admissions; the last one had occurred >10 years ago. A few weeks before this admission, he stopped taking lithium, while continuing escitalopram. He had not been keeping his appointments with his outpatient psychiatrist.
At admission, Dr. P had pressured speech, grandiose delusions, an expansive affect, and aggressive behavior. He was responding to internal stimuli with no insight into his illness. He was evasive when asked about hallucinations. Dr. P believed he was superior in intelligence and physical prowess to everyone in the emergency department (ED), and for that reason, the ED staff was persecuting him. His urine toxicology was negative.
On the inpatient unit, because Dr. P exhibited posturing, mutism, and negativism, catatonia associated with bipolar disorder was added to his diagnosis. For the first 2 days, his catatonia was managed with oral lorazepam, 2 mg twice daily. Dr. P was also observed giving medical advice to other patients on the unit, and was told to stop. Throughout his hospitalization, he dictated his own treatment and would frequently debate with his treatment team on the pharmacologic basis for treatment decisions, asserting his expertise as a physician and claiming to have a general clinical knowledge of the acute management of bipolar disorder.
Dr. P was eventually stabilized on oral lithium, 450 mg twice daily, and aripiprazole, 10 mg/d. He also received oral clonazepam, as needed for acute agitation, which was eventually tapered and discontinued. He gradually responded to treatment, and demonstrated improved insight. The treatment team met with Dr. P’s parents, who also were physicians, to discuss treatment goals, management considerations, and an aftercare plan. After spending 8 days in the hospital, Dr. P was discharged home to the care of his immediate family, and instructed to follow up with his outpatient psychiatrist. We do not know if he resumed clinical duties.
Managing an extremely knowledgeable patient
During his hospitalization, Dr. P frequently challenged our clinical knowledge; he would repeatedly remind us that he was a physician and that we were still trainees, which caused us to second-guess ourselves. Eventually, the attending physician on our team was able to impress upon Dr. P the clearly established roles of the treatment team and the patient. It was also important to maintain open communication channels with Dr. P and his family, and to address his anxiety by discussing the treatment plan in detail.5
Continue to: Although his queries on medication...
Although his queries on medication pharmacodynamics and pharmacokinetics were daunting, we empathized with him, recognizing that his knowledge invariably contributed to his anxiety. We engaged with Dr. P and his parents and elaborated on the rationale behind treatment decisions. This earned his trust and tremendously facilitated his recovery.
We were also cautious about using benzodiazepines to treat Dr. P’s catatonia because we were concerned that his knowledge could aid him in feigning symptoms to obtain these medications. Physicians have a high rate of prescription medication abuse, mainly opiates and benzodiazepines.2 The abuse of prescription medications by physicians is related to several psychological and psychiatric factors, including anxiety, depression, stress at work, personality problems, loss of loved ones, and pain. While treating physician patients, treatment decisions that include the use of opiates and benzodiazepines should be carefully considered.
A complicated scenario
Managing a physician patient can be a rewarding experience; however, there are several factors that can impact the experience, including:
- The treating physicians’ anxiety and countertransference/transference dynamics. We repeatedly imagined ourselves in Dr. P’s position and thought long and hard about how this scenario could happen to anyone in the medical profession; these thoughts induced significant anxiety in each of us. Further, interacting with Dr. P was reminiscent of our training under senior residents and attendings. Dr. P viewed us—his treatment team—as his trainees and challenged our clinical knowledge and actions.
- The physician-patient’s emotional responses, which may include anxiety, despair, denial, and an inability to accept role reversal.
Our medical culture needs a paradigm shift. We need a model designed to encourage early self-disclosure and treatment-seeking among physicians with mental illness. This will reduce the stigma towards mental illness in our profession.
1. Bianchi EF, Bhattacharyya MR, Meakin R. Exploring senior doctors’ beliefs and attitudes regarding mental illness within the medical profession: a qualitative study. BMJ Open. 2016;6(9):e012598. doi: 10.1136/bmjopen-2016 012598.
2. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
3. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
4. Schneck SA. “Doctoring” doctors and their families. JAMA. 1998;280(23):2039-2042.
5. Marshall EJ. Doctors’ health and fitness to practise: treating addicted doctors. Occup Med (Lond). 2008;58(5):334-340.
A physician’s mental health is important for the delivery of quality health care to his/her patients. Early identification and treatment of physicians with mental illnesses is challenging because physicians may neglect their own mental health due to the associated stigma, time constraints, or uncertainty regarding where to seek help. Physicians often worry about whom to confide in and harbor a fear that others will doubt his/her competence after recovery.1 Physicians have higher rates of suicide than the general population.2 According to data from the National Violent Death Reporting System, a diagnosed mental illness or a job problem significantly contribute to suicide among physicians.3 Additionally, physicians also have high rates of substance use and affective disorders.1,4
Here, we present the case of a physician we treated on an inpatient psychiatry unit who stirred profound emotions in us as trainees, and discuss how we managed this complicated scenario.
CASE REPORT
Dr. P, a 35-year-old male endocrinologist, was admitted to our inpatient psychiatry unit with a diagnosis of bipolar disorder, manic, severe, with psychotic features. Earlier that day, Dr. P had walked out of his private outpatient practice where he still had several appointments. After he had been missing for several hours, he was picked up by the police. Dr. P had 2 prior psychiatric admissions; the last one had occurred >10 years ago. A few weeks before this admission, he stopped taking lithium, while continuing escitalopram. He had not been keeping his appointments with his outpatient psychiatrist.
At admission, Dr. P had pressured speech, grandiose delusions, an expansive affect, and aggressive behavior. He was responding to internal stimuli with no insight into his illness. He was evasive when asked about hallucinations. Dr. P believed he was superior in intelligence and physical prowess to everyone in the emergency department (ED), and for that reason, the ED staff was persecuting him. His urine toxicology was negative.
On the inpatient unit, because Dr. P exhibited posturing, mutism, and negativism, catatonia associated with bipolar disorder was added to his diagnosis. For the first 2 days, his catatonia was managed with oral lorazepam, 2 mg twice daily. Dr. P was also observed giving medical advice to other patients on the unit, and was told to stop. Throughout his hospitalization, he dictated his own treatment and would frequently debate with his treatment team on the pharmacologic basis for treatment decisions, asserting his expertise as a physician and claiming to have a general clinical knowledge of the acute management of bipolar disorder.
Dr. P was eventually stabilized on oral lithium, 450 mg twice daily, and aripiprazole, 10 mg/d. He also received oral clonazepam, as needed for acute agitation, which was eventually tapered and discontinued. He gradually responded to treatment, and demonstrated improved insight. The treatment team met with Dr. P’s parents, who also were physicians, to discuss treatment goals, management considerations, and an aftercare plan. After spending 8 days in the hospital, Dr. P was discharged home to the care of his immediate family, and instructed to follow up with his outpatient psychiatrist. We do not know if he resumed clinical duties.
Managing an extremely knowledgeable patient
During his hospitalization, Dr. P frequently challenged our clinical knowledge; he would repeatedly remind us that he was a physician and that we were still trainees, which caused us to second-guess ourselves. Eventually, the attending physician on our team was able to impress upon Dr. P the clearly established roles of the treatment team and the patient. It was also important to maintain open communication channels with Dr. P and his family, and to address his anxiety by discussing the treatment plan in detail.5
Continue to: Although his queries on medication...
Although his queries on medication pharmacodynamics and pharmacokinetics were daunting, we empathized with him, recognizing that his knowledge invariably contributed to his anxiety. We engaged with Dr. P and his parents and elaborated on the rationale behind treatment decisions. This earned his trust and tremendously facilitated his recovery.
We were also cautious about using benzodiazepines to treat Dr. P’s catatonia because we were concerned that his knowledge could aid him in feigning symptoms to obtain these medications. Physicians have a high rate of prescription medication abuse, mainly opiates and benzodiazepines.2 The abuse of prescription medications by physicians is related to several psychological and psychiatric factors, including anxiety, depression, stress at work, personality problems, loss of loved ones, and pain. While treating physician patients, treatment decisions that include the use of opiates and benzodiazepines should be carefully considered.
A complicated scenario
Managing a physician patient can be a rewarding experience; however, there are several factors that can impact the experience, including:
- The treating physicians’ anxiety and countertransference/transference dynamics. We repeatedly imagined ourselves in Dr. P’s position and thought long and hard about how this scenario could happen to anyone in the medical profession; these thoughts induced significant anxiety in each of us. Further, interacting with Dr. P was reminiscent of our training under senior residents and attendings. Dr. P viewed us—his treatment team—as his trainees and challenged our clinical knowledge and actions.
- The physician-patient’s emotional responses, which may include anxiety, despair, denial, and an inability to accept role reversal.
Our medical culture needs a paradigm shift. We need a model designed to encourage early self-disclosure and treatment-seeking among physicians with mental illness. This will reduce the stigma towards mental illness in our profession.
A physician’s mental health is important for the delivery of quality health care to his/her patients. Early identification and treatment of physicians with mental illnesses is challenging because physicians may neglect their own mental health due to the associated stigma, time constraints, or uncertainty regarding where to seek help. Physicians often worry about whom to confide in and harbor a fear that others will doubt his/her competence after recovery.1 Physicians have higher rates of suicide than the general population.2 According to data from the National Violent Death Reporting System, a diagnosed mental illness or a job problem significantly contribute to suicide among physicians.3 Additionally, physicians also have high rates of substance use and affective disorders.1,4
Here, we present the case of a physician we treated on an inpatient psychiatry unit who stirred profound emotions in us as trainees, and discuss how we managed this complicated scenario.
CASE REPORT
Dr. P, a 35-year-old male endocrinologist, was admitted to our inpatient psychiatry unit with a diagnosis of bipolar disorder, manic, severe, with psychotic features. Earlier that day, Dr. P had walked out of his private outpatient practice where he still had several appointments. After he had been missing for several hours, he was picked up by the police. Dr. P had 2 prior psychiatric admissions; the last one had occurred >10 years ago. A few weeks before this admission, he stopped taking lithium, while continuing escitalopram. He had not been keeping his appointments with his outpatient psychiatrist.
At admission, Dr. P had pressured speech, grandiose delusions, an expansive affect, and aggressive behavior. He was responding to internal stimuli with no insight into his illness. He was evasive when asked about hallucinations. Dr. P believed he was superior in intelligence and physical prowess to everyone in the emergency department (ED), and for that reason, the ED staff was persecuting him. His urine toxicology was negative.
On the inpatient unit, because Dr. P exhibited posturing, mutism, and negativism, catatonia associated with bipolar disorder was added to his diagnosis. For the first 2 days, his catatonia was managed with oral lorazepam, 2 mg twice daily. Dr. P was also observed giving medical advice to other patients on the unit, and was told to stop. Throughout his hospitalization, he dictated his own treatment and would frequently debate with his treatment team on the pharmacologic basis for treatment decisions, asserting his expertise as a physician and claiming to have a general clinical knowledge of the acute management of bipolar disorder.
Dr. P was eventually stabilized on oral lithium, 450 mg twice daily, and aripiprazole, 10 mg/d. He also received oral clonazepam, as needed for acute agitation, which was eventually tapered and discontinued. He gradually responded to treatment, and demonstrated improved insight. The treatment team met with Dr. P’s parents, who also were physicians, to discuss treatment goals, management considerations, and an aftercare plan. After spending 8 days in the hospital, Dr. P was discharged home to the care of his immediate family, and instructed to follow up with his outpatient psychiatrist. We do not know if he resumed clinical duties.
Managing an extremely knowledgeable patient
During his hospitalization, Dr. P frequently challenged our clinical knowledge; he would repeatedly remind us that he was a physician and that we were still trainees, which caused us to second-guess ourselves. Eventually, the attending physician on our team was able to impress upon Dr. P the clearly established roles of the treatment team and the patient. It was also important to maintain open communication channels with Dr. P and his family, and to address his anxiety by discussing the treatment plan in detail.5
Continue to: Although his queries on medication...
Although his queries on medication pharmacodynamics and pharmacokinetics were daunting, we empathized with him, recognizing that his knowledge invariably contributed to his anxiety. We engaged with Dr. P and his parents and elaborated on the rationale behind treatment decisions. This earned his trust and tremendously facilitated his recovery.
We were also cautious about using benzodiazepines to treat Dr. P’s catatonia because we were concerned that his knowledge could aid him in feigning symptoms to obtain these medications. Physicians have a high rate of prescription medication abuse, mainly opiates and benzodiazepines.2 The abuse of prescription medications by physicians is related to several psychological and psychiatric factors, including anxiety, depression, stress at work, personality problems, loss of loved ones, and pain. While treating physician patients, treatment decisions that include the use of opiates and benzodiazepines should be carefully considered.
A complicated scenario
Managing a physician patient can be a rewarding experience; however, there are several factors that can impact the experience, including:
- The treating physicians’ anxiety and countertransference/transference dynamics. We repeatedly imagined ourselves in Dr. P’s position and thought long and hard about how this scenario could happen to anyone in the medical profession; these thoughts induced significant anxiety in each of us. Further, interacting with Dr. P was reminiscent of our training under senior residents and attendings. Dr. P viewed us—his treatment team—as his trainees and challenged our clinical knowledge and actions.
- The physician-patient’s emotional responses, which may include anxiety, despair, denial, and an inability to accept role reversal.
Our medical culture needs a paradigm shift. We need a model designed to encourage early self-disclosure and treatment-seeking among physicians with mental illness. This will reduce the stigma towards mental illness in our profession.
1. Bianchi EF, Bhattacharyya MR, Meakin R. Exploring senior doctors’ beliefs and attitudes regarding mental illness within the medical profession: a qualitative study. BMJ Open. 2016;6(9):e012598. doi: 10.1136/bmjopen-2016 012598.
2. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
3. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
4. Schneck SA. “Doctoring” doctors and their families. JAMA. 1998;280(23):2039-2042.
5. Marshall EJ. Doctors’ health and fitness to practise: treating addicted doctors. Occup Med (Lond). 2008;58(5):334-340.
1. Bianchi EF, Bhattacharyya MR, Meakin R. Exploring senior doctors’ beliefs and attitudes regarding mental illness within the medical profession: a qualitative study. BMJ Open. 2016;6(9):e012598. doi: 10.1136/bmjopen-2016 012598.
2. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
3. Gold KJ, Sen A, Schwenk TL. Details on suicide among US physicians: data from the National Violent Death Reporting System. Gen Hosp Psychiatry. 2013;35(1):45-49.
4. Schneck SA. “Doctoring” doctors and their families. JAMA. 1998;280(23):2039-2042.
5. Marshall EJ. Doctors’ health and fitness to practise: treating addicted doctors. Occup Med (Lond). 2008;58(5):334-340.
Premature mortality across most psychiatric disorders
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The 84-year-old state boxing champ: Bipolar disorder, or something else?
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
CASE Agitated, uncooperative, and irritable
Mr. X, age 84, presents to the emergency department with agitation, mania-like symptoms, and mood-congruent psychotic symptoms that started 2 weeks ago. Mr. X, who is accompanied by his wife, has no psychiatric history.
On examination, Mr. X is easily agitated and uncooperative. His speech is fast, but not pressured, with increased volume and tone. He states, “My mood is fantastic” with mood-congruent affect. His thought process reveals circumstantiality and loose association. Mr. X’s thought content includes flight of ideas and delusions of grandeur; he claims to be a state boxing champion and a psychologist. He also claims that he will run for Congress in the near future. He reports that he’s started knocking on his neighbors’ doors, pitched the idea to buy their house, and convinced them to vote for him as their congressman. He denies any suicidal or homicidal ideations. There is no evidence of perceptual disturbance. Mr. X undergoes a Mini-Mental State Examination (MMSE) and scores 26/30, which suggests no cognitive impairment. However, his insight and judgment are poor.
Mr. X’s physical examination is unremarkable. His laboratory workup includes a complete blood count, comprehensive metabolic panel, urinalysis, thyroid function test, vitamin B12 and folate levels, urine drug screen, and blood alcohol level. All results are within normal limits. He has no history of alcohol or recreational drug use as evident by the laboratory results and collateral information from his wife. Further, a non-contrast CT scan of his head shows no abnormality.
Approximately 1 month ago, Mr. X was diagnosed with restless leg syndrome (RLS). Mr. X’s medication regimen consists of gabapentin, 300 mg 3 times daily, prescribed years ago by his neurologist for neuropathic pain; and ropinirole, 3 mg/d, for RLS. His neurologist had prescribed him ropinirole, which was started at 1 mg/d and titrated to 3 mg/d within a 1-week span. Two weeks after Mr. X started this medication regimen, his wife reports that she noticed changes in his behavior, including severe agitation, irritability, delusions of grandeur, decreased need for sleep, and racing of thoughts.
[polldaddy:10417490]
The authors’ observations
Mr. X was diagnosed with medication (ropinirole)-induced bipolar and related disorder with mood-congruent psychotic features.
To determine this diagnosis, we initially considered Mr. X’s age and medical conditions, including stroke and space-occupying lesions of the brain. However, the laboratory and neuroimaging studies, which included a CT scan of the head and MRI of the brain, were negative. Next, because Mr. X had sudden onset manic symptoms after ropinirole was initiated, we considered the possibility of a substance/medication-induced bipolar and related disorder. Further, ropinirole is capable of producing the symptoms in criterion A of DSM-5 criteria for substance/medication-induced bipolar and related disorder. Mr. X met all DSM-5 criteria for substance/medication-induced bipolar and related disorder (Table1).

[polldaddy:10417494]
TREATMENT Medication adjustments and improvement
The admitting clinician discontinues ropinirole and initiates divalproex sodium, 500 mg twice a day. By Day 4, Mr. X shows significant improvement, including no irritable mood and regression of delusions of grandeur, and his sleep cycle returns to normal. At this time, the divalproex sodium is also discontinued.
Continue to: The authors' observations
The authors’ observations
Dopamine agonist agents are a standard treatment in the management of Parkinson’s disease and RLS.2-5 Ropinirole, a dopamine receptor agonist, has a high affinity for dopamine D2 and D3 receptor subtypes.4 Published reports have linked dopamine agonists to mania with psychotic features.6,7 In a study by Stoner et al,8 of 95 patients treated with ropinirole, 13 patients developed psychotic features that necessitated the use of antipsychotic medications or a lower dose of ropinirole.
The recommended starting dose for ropinirole is 0.25 mg/d. The dose can be increased to 0.5 mg in the next 2 days, and to 1 mg/d at the end of the first week.9 The mean effective daily dose is 2 mg/d, and maximum recommended dose is 4 mg/d.9 For Mr. X, ropinirole was quickly titrated to 3 mg/d over 1 week, which resulted in mania and psychosis. We suggest that when treating geriatric patients, clinicians should consider prescribing the lowest effective dose of psychotropic medications, such as ropinirole, to prevent adverse effects. Higher doses of dopamine agonists, especially in geriatric patients, increase the risk of common adverse effects, such as nausea (25% to 50%), headache (7% to 22%), fatigue (1% to 19%), dizziness (6% to 18%), and vomiting (5% to 11%).10 When prescribing dopamine agonists, clinicians should educate patients and their caregivers about the rare but potential risk of medication-induced mania and psychosis.
Mr. X’s case emphasizes the importance of a comprehensive psychiatric evaluation and medical workup to rule out a wide differential diagnosis when approaching new-onset mania and psychosis in geriatric patients.11 Our case contributes to the evidence that dopamine agonist medications are associated with mania and psychotic symptoms.
OUTCOME A return to baseline
On Day 12, Mr. X is discharged home in a stable condition. Two weeks later, at an outpatient follow-up visit, Mr. X is asymptomatic and has returned to his baseline functioning.
Bottom Line
When approaching new-onset mania and psychosis in geriatric patients, a comprehensive psychiatric evaluation and medical workup are necessary to rule out a wide differential diagnosis. Ropinirole use can lead to mania and psychotic symptoms, especially in geriatric patients. As should be done with all other dopaminergic agents, increase the dose of ropinirole with caution, and be vigilant for the emergence of signs of mania and/or psychosis.
Continue to: Related Resources
Related Resources
- Adabie A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29.
- Chen P, Dols A, Rej S, et al. Update on the epidemiology, diagnosis, and treatment of mania in older-age bipolar disorder. Curr Psychiatry Rep. 2017;19(8):46.
Drug Brand Names
Divalproex sodium • Depakote
Gabapentin • Neurontin
Ropinirole • Requip
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Singh A, Althoff R, Martineau RJ, et al. Pramipexole, ropinirole, and mania in Parkinson’s disease. Am J Psychiatry. 2005;162(4):814-815.
3. Weiss HD, Pontone GM. Dopamine receptor agonist drugs and impulse control disorders. JAMA Intern Med. 2014;174(12):1935-1937.
4 Shill HA, Stacy M. Update on ropinirole in the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:33-36.
5. Borovac JA. Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med. 2016;89(1):37-47.
6. Yüksel RN, Elyas Kaya Z, Dilbaz N, et al. Cabergoline-induced manic episode: case report. Ther Adv Psychopharmacol. 2016;6(3):229-231.
7. Perea E, Robbins BV, Hutto B. Psychosis related to ropinirole. Am J Psychiatry. 2006;163(3):547-548.
8. Stoner SC, Dahmen MM, Makos M, et al. An exploratory retrospective evaluation of ropinirole-associated psychotic symptoms in an outpatient population treated for restless legs syndrome or Parkinson’s disease. Ann Pharmacother. 2009;43(9):1426-1432.
9. Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
10. Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675-684.
11. Dols A, Beekman A. Older age bipolar disorder. Psychiatr Clin North Am. 2018;41(1):95-110.
Distinct mood, apathy profiles found in bipolar disorder patients
Neuroimaging
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
Neuroimaging
Neuroimaging
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
FROM NEUROIMAGE: CLINICAL
Virtual dark therapy tames manic episodes
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
COPENHAGEN – Bright light therapy is a well-established, guideline-recommended treatment for seasonal affective disorder, and many people prone to depression keep a light box at home. But are you ready to embrace the dark side – that is, dark therapy for bipolar mania, or its vastly more patient-friendly offshoot, virtual dark therapy?
And it’s a lot easier on patients than the massive sensory deprivation imposed by the original form of dark therapy, which entails keeping a patient with mania in a completely dark room for 14 hours per night, Tone E.G. Henriksen, MD, observed at the annual congress of the European College of Neuropsychopharmacology.
She was lead author of a pioneering randomized controlled trial demonstrating that bipolar patients who wore blue-blocking, orange-tinted glasses for 14 hours per evening while hospitalized for a manic episode experienced a significant improvement in scores on the Young Mania Rating Scale (YMRS), compared with patients randomized to wearing clear lenses. Moreover, the between-group difference achieved strong significance in just 3 days.
That’s a remarkable result, because bipolar mania is such a challenge to treat pharmacologically. The standard medications – mood stabilizers and antipsychotic agents – are slow in onset of effect, observed Dr. Henriksen, a psychiatrist at the University of Bergen (Norway).
Backing up, she noted there is strong evidence of seasonality to bipolar disorder, as highlighted in a systematic review of 51 publications (J Affect Disord. 2014 Oct;168:210-23). This recognition has prompted numerous researchers to focus attention on the abnormal circadian rhythms prevalent in patients with bipolar disorder, for which the light/dark cycle is a powerful synchronizing signal to the hypothalamic suprachiasmatic nucleus, the master clock of circadian rhythms. This understanding led to a landmark case control pilot study by Italian investigators who exposed 16 bipolar inpatients experiencing a manic episode to 14 hours of complete darkness from 6 p.m. to 8 a.m. for 3 consecutive nights. The outcome was a dramatic reduction in YMRS scores in the dark therapy group, compared with 16 matched control inpatients, with all participants on pharmacologic treatment as usual (Bipolar Disord. 2005 Feb;7[1]:98-101).
“This was really something,” Dr. Henriksen recalled.
She and her colleagues were impressed by other investigators’ discovery of specialized retinal ganglion cells, known as intrinsically photosensitive retinal ganglion cells, which are responsible for conveying the daylight signal to the brain. These specialized cells contain melanopsin, which is blue light sensitive. The Norwegian investigators reasoned that it might not be necessary to expose patients with mania to prolonged utter darkness to achieve rapid symptomatic improvement, as the Italian psychiatrists did. Instead, they hypothesized, it might be sufficient just to block the blue light, low-wavelength end of the spectrum. And that turned out to be the case.
Their randomized, single-blind, multicenter study included 23 patients with bipolar disorder who were hospitalized for manic symptoms. All remained on their standard background psychiatric medications while being randomized to wear orange-tinted, blue light–blocking glasses, which allowed passage of almost all light above 530 nm, or clear glasses. Participants were instructed to wear their glasses from 6 p.m. to 8 a.m. for 7 consecutive nights. They took their glasses off when they switched off the lights at bedtime, but they had to put them back on if they turned on a light before 8 a.m. The patients also wore an activity monitor.
The results were dramatic: The blue-blocking glasses group had a mean 14.1-point drop in their YMRS score from a baseline of about 25, compared with a mere 1.7-point decline in the control group. Moreover, Dr. Henriksen said, this result might actually underrepresent the true clinical effect of blocking blue light to the brain, since two patients in the blue-blocking glasses group experienced such rapid symptomatic improvement that they were moved from an acute psychiatric ward to a local hospital midstudy, a sudden change that triggered transient worsening of manic symptoms in both patients.
The investigators documented improved sleep efficiency in the blue-blocking group. Another noteworthy finding was that, in the blue-blocking group, the elements of the YMRS related to increased activation declined before the measures of distorted thoughts and perceptions. So did motor activity as recorded by actigraph. Meanwhile, nighttime activity worsened in the control group; they received substantially more sedatives, hypnotics, anxiolytic agents, and antipsychotic medications (Bipolar Disord. 2016 May;18[3]:221-32).
The mechanism underlying the improvement in sleep regularity and manic symptoms achieved by blocking blue light is not understood. Dr. Henriksen finds “very compelling” a theory put forth by prominent chronobiologist Daniel Kripke, MD, of the University of California, San Diego. He has shown in animal studies that a change in light exposure can trigger bifurcation in the circadian rhythms of the suprachiasmatic nucleus. The resultant suppression of melatonin secretion results in excess production of hypothalamic triiodothyronine, which in turn affects production of other key hormones. In patients with bipolar disorder, this could trigger mania, according to Dr. Kripke (F1000Res. 2015 May 6;4:107.
Dr. Henriksen reported having no financial conflicts regarding her study, which was conducted free of commercial support. She serves as a consultant to Chrono Chrome AS.
REPORTING FROM ECNP 2019