User login
Product granted fast track designation for aTTP
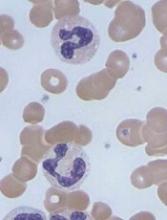
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()
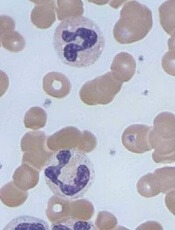
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to caplacizumab, an anti-von Willebrand factor (vWF) nanobody being developed for the treatment of acquired thrombotic thrombocytopenic purpura (aTTP).
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About caplacizumab
Caplacizumab is a bivalent anti-vWF nanobody being developed by Ablynx. Caplacizumab works by blocking the interaction of ultra-large vWF multimers with platelets, having an immediate effect on platelet aggregation and the ensuing formation and accumulation of the micro-clots that cause the severe thrombocytopenia, tissue ischemia, and organ dysfunction that occurs in patients with aTTP.
Researchers evaluated the efficacy and safety of caplacizumab, given with standard care for aTTP, in the phase 2 TITAN trial.
The trial enrolled 75 patients with aTTP. They all received the standard of care—daily plasma exchange and immunosuppressive therapy. Thirty-six patients were randomized to receive caplacizumab as well, and 39 were randomized to placebo.
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. Relapse was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively. Exacerbation was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required re-initiation of daily exchange.
The rate of adverse events thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of events that were possibly related was 54% and 8%, respectively.
A lower proportion of subjects in the caplacizumab arm experienced one or more major thromboembolic events or died, compared to the placebo arm—11.4% and 43.2%, respectively.
In addition, fewer caplacizumab-treated patients were refractory to treatment—5.7% vs 21.6%.
There were 2 deaths in the placebo arm, and both of those patients were refractory to treatment. There were no deaths reported in the caplacizumab arm.
Now, researchers are evaluating caplacizumab in the phase 3 HERCULES trial (NCT02553317). Results from this study are anticipated in the second half of 2017 are expected to support a planned biologics license application filing in the US in 2018. ![]()
Allele-matching in cord blood transplant yields better survival
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
Matching down to the allele level in umbilical cord blood transplantation between unrelated donors results in greater overall survival for those with nonmalignant diseases, such as aplastic anemia, researchers found in a retrospective study published in the Lancet Haematology.
The review (Lancet Haematol. 2017 Jul;4[7]:e325-33), the largest published on the topic, indicates that clinicians should change practice from the current standard of antigen-level matching, said Mary Eapen, MD, director of the Center for International Blood and Marrow Transplant Research (CIBMTR) in Wauwatosa, Wisconsin.
“Our findings,” Dr. Eapen wrote, “support a change in clinical practice to prioritization of units on allele-level HLA matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1.”
Data were pulled from cases reported to the Center for International Blood and Marrow Transplant Research or the European Group for Blood and Marrow Transplant. Researchers looked at 1,199 donor-recipient matches of cord blood transplantation for diseases, such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, and Fanconi anemia. Recipients could be as old as age 16, but most were age 5 or younger.
After adjustment for factors, including cytomegalovirus serostatus, the intensity of the conditioning regimen, and the total nucleated cell dose, the 5-year overall survival was 79% for transplants that were matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1. These results compare with 76% after transplants with one mismatch, 70% with two mismatches, 62% with three mismatches, and 49% with 4 or more mismatches.
Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched. There was no difference statistically between full matches and one mismatch, but the findings suggest that the mortality risk might prove significant with a larger sample size.
Researchers cautioned that, because most patients were age 5 or younger, the results might not be generalizable to older children.
Although HLA typing is available at CIBMTR for most blood cord transplants for nonmalignant diseases, full allele matches or just one mismatch are not the norm, Dr. Eapen wrote. Researchers said that they suspect this is because of difficulties finding matches or because a high total nucleated cell count is prioritized above HLA matching. They suggest clinicians change their decision making in this regard.
FROM THE LANCET HAEMATOLOGY
Key clinical point: HLA matching at the allele-level produces better survival in umbilical cord blood transplantation for nonmalignant diseases.
Major finding: Mortality risks were significantly higher for patients who received transplants with two (P = .018), three (P = .0001), and four or more mismatches (P less than .0001), compared with those whose transplants were fully matched at all eight alleles at HLA-A, HLA-B, HLA-C and HLA-DRB1.
Data source: A retrospective review of 1,199 cases reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) or the European Group for Blood and Marrow Transplant (EGBMT).
Disclosures: The authors reported no conflicts of interest.
Vaccination does not eliminate risk for meningococcal disease in eculizumab recipients
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
[email protected]
On Twitter @eaztweets
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
[email protected]
On Twitter @eaztweets
Patients taking eculizumab are at a significant risk for meningococcal disease even if they have received the quadrivalent meningococcal conjugate (MenACWY) and serogroup B (MenB) meningococcal vaccines, according to the Centers for Disease Control and Prevention’s Morbidity and Mortality Weekly Report, released July 7.
Between 2008 and 2016, 16 cases of meningococcal disease were reported in eculizumab users in 10 jurisdictions within the United States. Of those infected, 14 had received MenACWY and MenB vaccines as recommended by the Advisory Committee on Immunization Practices, according to the CDC report.
Required vaccination plus antimicrobial prophylaxis for the duration of eculizumab treatment might reduce the risk for meningococcal disease in these patients, but the addition of antibiotic prophylaxis is no guarantee that all cases of meningococcal disease would be prevented, wrote Lucy A. McNamara, PhD, of the division of bacterial diseases, National Center for Immunization and Respiratory Diseases, CDC, and her colleagues.
They advised physician and patient vigilance regarding meningococcal disease symptoms and urged that patients be advised to seek immediate care and be rapidly treated, regardless of meningococcal vaccination or antimicrobial prophylaxis status.
Health organizations in Europe, including France and the United Kingdom, are recommending eculizumab users receive penicillin during eculizumab treatment. A recent study of invasive meningococcal isolates in the United States found most were susceptible to penicillin, according to the report.
In the 16 U.S. cases reported, nongroupable Neisseria meningitidis caused meningococcal disease in 11 of the patients, serogroup Y was the cause in 4 patients, and the cause was not identified in 1 patient.
Ten patients had meningococcemia without meningitis, the researchers noted. “Initial symptoms of meningococcemia are often relatively mild and nonspecific and might include fever, chills, fatigue, vomiting, diarrhea, and aches or pains in the muscles, joints, chest, or abdomen; however, these symptoms can progress to severe illness and death within hours.”
Eculizumab (Soliris, Alexion Pharmaceuticals) is licensed in the United States for treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome, two diseases that are rare and can be fatal.
Eculizumab is associated with a 1,000-fold to 2,000-fold increased incidence of meningococcal disease among persons receiving the drug. The Food and Drug Administration–approved prescribing information includes a boxed warning regarding increased risk for meningococcal disease.
The CDC is collecting reports from state health departments for further analysis of the risk among eculizumab recipients.
The researchers reported having no conflicts of interest.
[email protected]
On Twitter @eaztweets
FROM MMWR
Monthly fitusiran showed promise in small phase I hemophilia trial
and increased thrombin production, according to the results of a small phase I dose-escalation study.
Compared with baseline, levels of antithrombin dropped by 70%-89%, K. John Pasi, MB, ChB, PhD, of the Royal London Haemophilia Centre and the London School of Medicine and Dentistry and his associates reported at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine. There were no thromboembolic events during the study, and the most common adverse events were mild injection-site reactions, they added.
Patients with hemophilia typically need frequent infusions of clotting factors, creating an urgent need for new therapies. Fitusiran is an investigational RNA interference (RNAi) therapy that targets antithrombin. This multicenter, open-label, phase I study included four healthy volunteers and 25 patients with moderate or severe hemophilia A or B without inhibitors. The healthy participants received a single subcutaneous injection of fitusiran (0.03 mg/kg) or placebo. The hemophilia patients received three injections of fitusiran either once weekly (0.015, 0.045, or 0.075 mg/kg) or once monthly (0.225, 0.45, 0.9, or 1.8 mg/kg, or a fixed dose of 80 mg (N Engl J Med. 2017 July 10. doi: 10.1056/NEJMoa1616569).
The single fitusiran dose and the weekly treatment regimens both produced consistent, sustained drops in plasma antithrombin levels, which supported a longer interval between doses, the researchers said. A monthly dose of 0.225 mg/kg cut antithrombin levels by about 70%, compared with baseline (standard deviation, ±9%), while a monthly dose of 1.8 mg/kg produced about an 89% (standard deviation, ±1%) drop in antithrombin levels. The fixed 80-mg dose consistently lowered antithrombin levels by about 87%, compared with baseline. The decreases in antithrombin varied little during the weeks between doses and were associated with increased thrombin generation, regardless of whether patients had hemophilia A or B, Dr. Pasi and his associates said.
One patient stopped treatment because of severe chest pain, but the investigators ruled out thrombosis as a cause. Fitusiran targets the liver, and 36% of patients developed transient increases in liver aminotransferase levels, they noted. Most of these patients had a history of hepatitis C virus infection. An extension study (NCT02554773) is ongoing to assess longer-term safety and efficacy findings. The investigators also are studying fitusiran in patients with inhibitory alloantibodies.
Alnylam Pharmaceuticals provided funding. Dr. Pasi disclosed research funding, advisory board fees, and travel grants from Alnylam and Genzyme. Four coinvestigators also disclosed receiving support from Alnylam during the conduct of the study.
and increased thrombin production, according to the results of a small phase I dose-escalation study.
Compared with baseline, levels of antithrombin dropped by 70%-89%, K. John Pasi, MB, ChB, PhD, of the Royal London Haemophilia Centre and the London School of Medicine and Dentistry and his associates reported at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine. There were no thromboembolic events during the study, and the most common adverse events were mild injection-site reactions, they added.
Patients with hemophilia typically need frequent infusions of clotting factors, creating an urgent need for new therapies. Fitusiran is an investigational RNA interference (RNAi) therapy that targets antithrombin. This multicenter, open-label, phase I study included four healthy volunteers and 25 patients with moderate or severe hemophilia A or B without inhibitors. The healthy participants received a single subcutaneous injection of fitusiran (0.03 mg/kg) or placebo. The hemophilia patients received three injections of fitusiran either once weekly (0.015, 0.045, or 0.075 mg/kg) or once monthly (0.225, 0.45, 0.9, or 1.8 mg/kg, or a fixed dose of 80 mg (N Engl J Med. 2017 July 10. doi: 10.1056/NEJMoa1616569).
The single fitusiran dose and the weekly treatment regimens both produced consistent, sustained drops in plasma antithrombin levels, which supported a longer interval between doses, the researchers said. A monthly dose of 0.225 mg/kg cut antithrombin levels by about 70%, compared with baseline (standard deviation, ±9%), while a monthly dose of 1.8 mg/kg produced about an 89% (standard deviation, ±1%) drop in antithrombin levels. The fixed 80-mg dose consistently lowered antithrombin levels by about 87%, compared with baseline. The decreases in antithrombin varied little during the weeks between doses and were associated with increased thrombin generation, regardless of whether patients had hemophilia A or B, Dr. Pasi and his associates said.
One patient stopped treatment because of severe chest pain, but the investigators ruled out thrombosis as a cause. Fitusiran targets the liver, and 36% of patients developed transient increases in liver aminotransferase levels, they noted. Most of these patients had a history of hepatitis C virus infection. An extension study (NCT02554773) is ongoing to assess longer-term safety and efficacy findings. The investigators also are studying fitusiran in patients with inhibitory alloantibodies.
Alnylam Pharmaceuticals provided funding. Dr. Pasi disclosed research funding, advisory board fees, and travel grants from Alnylam and Genzyme. Four coinvestigators also disclosed receiving support from Alnylam during the conduct of the study.
and increased thrombin production, according to the results of a small phase I dose-escalation study.
Compared with baseline, levels of antithrombin dropped by 70%-89%, K. John Pasi, MB, ChB, PhD, of the Royal London Haemophilia Centre and the London School of Medicine and Dentistry and his associates reported at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine. There were no thromboembolic events during the study, and the most common adverse events were mild injection-site reactions, they added.
Patients with hemophilia typically need frequent infusions of clotting factors, creating an urgent need for new therapies. Fitusiran is an investigational RNA interference (RNAi) therapy that targets antithrombin. This multicenter, open-label, phase I study included four healthy volunteers and 25 patients with moderate or severe hemophilia A or B without inhibitors. The healthy participants received a single subcutaneous injection of fitusiran (0.03 mg/kg) or placebo. The hemophilia patients received three injections of fitusiran either once weekly (0.015, 0.045, or 0.075 mg/kg) or once monthly (0.225, 0.45, 0.9, or 1.8 mg/kg, or a fixed dose of 80 mg (N Engl J Med. 2017 July 10. doi: 10.1056/NEJMoa1616569).
The single fitusiran dose and the weekly treatment regimens both produced consistent, sustained drops in plasma antithrombin levels, which supported a longer interval between doses, the researchers said. A monthly dose of 0.225 mg/kg cut antithrombin levels by about 70%, compared with baseline (standard deviation, ±9%), while a monthly dose of 1.8 mg/kg produced about an 89% (standard deviation, ±1%) drop in antithrombin levels. The fixed 80-mg dose consistently lowered antithrombin levels by about 87%, compared with baseline. The decreases in antithrombin varied little during the weeks between doses and were associated with increased thrombin generation, regardless of whether patients had hemophilia A or B, Dr. Pasi and his associates said.
One patient stopped treatment because of severe chest pain, but the investigators ruled out thrombosis as a cause. Fitusiran targets the liver, and 36% of patients developed transient increases in liver aminotransferase levels, they noted. Most of these patients had a history of hepatitis C virus infection. An extension study (NCT02554773) is ongoing to assess longer-term safety and efficacy findings. The investigators also are studying fitusiran in patients with inhibitory alloantibodies.
Alnylam Pharmaceuticals provided funding. Dr. Pasi disclosed research funding, advisory board fees, and travel grants from Alnylam and Genzyme. Four coinvestigators also disclosed receiving support from Alnylam during the conduct of the study.
FROM THE 2017 ISTH CONGRESS
Key clinical point: Once-monthly subcutaneous treatment with fitusiran led to dose-dependent lowering of antithrombin levels and increased thrombin production among patients with hemophilia A or B without inhibitors.
Major finding: Compared with baseline, levels of antithrombin dropped by 70%-89%. There were no episodes of thrombosis, and the most common adverse events were injection-site reactions.
Data source: A phase I dose-escalation study.
Disclosures: Alnylam Pharmaceuticals provided funding. Dr. Pasi disclosed research funding, advisory board fees, and travel grants from Alnylam and Genzyme. Four coinvestigators also disclosed receiving support from Alnylam during the conduct of the study.
Prophylactic emicizumab cut bleeds by 87% in hemophilia A with inhibitors
Once-weekly prophylactic therapy with emicizumab (ACE910) was associated an 87% lower rate of treated bleeding than no prophylaxis among patients with hemophilia A with inhibitors in the open-label, phase III HAVEN 1 trial.
After a median 24 weeks of treatment (range, 3-48 weeks), the 35 patients who were randomly assigned to prophylactic emicizumab (3.0 mg per kg for 4 weeks, followed by 1.5 mg per kg) had an annualized bleeding rate of 2.9 events, compared with an annualized rate of 23.3 events among the 18 patients who received no prophylaxis. The difference was statistically significant, reported Johannes Oldenburg, MD, PhD, and his associates at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine (N Engl J Med. 2017 Jul 10. doi: 10.1056/NEJMoa1703068).
In addition, 63% of patients who received prophylactic emicizumab had no bleeding events, compared with only 6% of the comparison group, said Dr. Oldenburg of Universitaetsklinikum Bonn, Germany. Emicizumab also was associated with a 79% lower rate of treated bleeds, compared with the historic rate among 24 patients who previously had been on prophylactic bypassing agents (P less than .001). No patient developed detectable antidrug antibodies.
The most common adverse events were injection site reactions (15% of patients), followed by headache, upper respiratory tract infection, arthralgia, and fatigue. Four patients developed thrombotic microangiopathy or cavernous sinus thrombosis and skin necrosis, all of whom had experienced breakthrough bleeding for which they had received multiple infusions of activated prothrombin complex concentrate at doses averaging more than 100 U per kg per day.
Patients with severe hemophilia A typically require prophylactic factor VIII infusions two to three times a week, but 30% develop neutralizing antibodies against factor VIII (inhibitors), which increases care burden, costs, and complication rates.
Emicizumab is a recombinant humanized bispecific monoclonal antibody that restores the function of missing active factor VIII by bridging activated factor IX and factor X, the investigators noted. A phase I study linked once-weekly emicizumab prophylaxis to markedly lower bleeding rates without dose-limiting toxicities (N Engl J Med 2016;374:2044-53). Subsequently, the phase III HAVEN 1 study (NCT02622321) compared once-weekly subcutaneous prophylactic emicizumab with on-demand (episodic) and prophylactic use of bypassing agents (BPAs) in 109 adults and adolescents (12 years and older) with hemophilia A with inhibitors. The primary endpoint was the difference in rates of treated bleeds between the groups. Patients had a median age of 28 years, all were male, and most had severe hemophilia at baseline. They typically had more than nine bleeding events in the 6 months before enrollment, with bleeding in more than one target joint and factor VIII titers of 180 Bethesda units per mL.
HAVEN 1 was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. Dr. Oldenburg and 13 coinvestigators disclosed ties to Chugai, Roche, and other pharmaceutical companies. The remaining coinvestigator had no disclosures.
The results of this phase III trial are of great importance to patients with for hemophilia A, offering “an elegant new solution for treatment.” Although it is not clear how best to treat the infrequent events of breakthrough bleeding when administering emicizumab, it is evident that repeated high doses of activated prothrombin complex concentrate should be avoided. Also, how to integrate emicizumab prophylaxis with current schedules for the induction of immune tolerance to factor VIII remains to be seen.
Meanwhile, weekly subcutaneous emicizumab prophylaxis offer great reductions in bleeding rates and improve quality of life “for this very challenging patient group.” Additional studies already are underway to ascertain the benefit of emicizumab prophylaxis in children with hemophilia with inhibitors, and a study for patients with hemophilia A without inhibitors is planned.
“These are extraordinary times for innovation in hemophilia therapy, and the introduction of emicizumab represents a major contribution toward achieving an enhanced standard of care for this lifelong bleeding disorder.”
David Lillicrap, MD, is with the department of pathology and molecular medicine at Queen’s University, Kingston, Ontario. He reported having no relevant conflicts of interest. These comments are from his accompanying editorial (N Engl J Med. 2017 June 10. doi: 10.1056/NEJMe1707802).
The results of this phase III trial are of great importance to patients with for hemophilia A, offering “an elegant new solution for treatment.” Although it is not clear how best to treat the infrequent events of breakthrough bleeding when administering emicizumab, it is evident that repeated high doses of activated prothrombin complex concentrate should be avoided. Also, how to integrate emicizumab prophylaxis with current schedules for the induction of immune tolerance to factor VIII remains to be seen.
Meanwhile, weekly subcutaneous emicizumab prophylaxis offer great reductions in bleeding rates and improve quality of life “for this very challenging patient group.” Additional studies already are underway to ascertain the benefit of emicizumab prophylaxis in children with hemophilia with inhibitors, and a study for patients with hemophilia A without inhibitors is planned.
“These are extraordinary times for innovation in hemophilia therapy, and the introduction of emicizumab represents a major contribution toward achieving an enhanced standard of care for this lifelong bleeding disorder.”
David Lillicrap, MD, is with the department of pathology and molecular medicine at Queen’s University, Kingston, Ontario. He reported having no relevant conflicts of interest. These comments are from his accompanying editorial (N Engl J Med. 2017 June 10. doi: 10.1056/NEJMe1707802).
The results of this phase III trial are of great importance to patients with for hemophilia A, offering “an elegant new solution for treatment.” Although it is not clear how best to treat the infrequent events of breakthrough bleeding when administering emicizumab, it is evident that repeated high doses of activated prothrombin complex concentrate should be avoided. Also, how to integrate emicizumab prophylaxis with current schedules for the induction of immune tolerance to factor VIII remains to be seen.
Meanwhile, weekly subcutaneous emicizumab prophylaxis offer great reductions in bleeding rates and improve quality of life “for this very challenging patient group.” Additional studies already are underway to ascertain the benefit of emicizumab prophylaxis in children with hemophilia with inhibitors, and a study for patients with hemophilia A without inhibitors is planned.
“These are extraordinary times for innovation in hemophilia therapy, and the introduction of emicizumab represents a major contribution toward achieving an enhanced standard of care for this lifelong bleeding disorder.”
David Lillicrap, MD, is with the department of pathology and molecular medicine at Queen’s University, Kingston, Ontario. He reported having no relevant conflicts of interest. These comments are from his accompanying editorial (N Engl J Med. 2017 June 10. doi: 10.1056/NEJMe1707802).
Once-weekly prophylactic therapy with emicizumab (ACE910) was associated an 87% lower rate of treated bleeding than no prophylaxis among patients with hemophilia A with inhibitors in the open-label, phase III HAVEN 1 trial.
After a median 24 weeks of treatment (range, 3-48 weeks), the 35 patients who were randomly assigned to prophylactic emicizumab (3.0 mg per kg for 4 weeks, followed by 1.5 mg per kg) had an annualized bleeding rate of 2.9 events, compared with an annualized rate of 23.3 events among the 18 patients who received no prophylaxis. The difference was statistically significant, reported Johannes Oldenburg, MD, PhD, and his associates at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine (N Engl J Med. 2017 Jul 10. doi: 10.1056/NEJMoa1703068).
In addition, 63% of patients who received prophylactic emicizumab had no bleeding events, compared with only 6% of the comparison group, said Dr. Oldenburg of Universitaetsklinikum Bonn, Germany. Emicizumab also was associated with a 79% lower rate of treated bleeds, compared with the historic rate among 24 patients who previously had been on prophylactic bypassing agents (P less than .001). No patient developed detectable antidrug antibodies.
The most common adverse events were injection site reactions (15% of patients), followed by headache, upper respiratory tract infection, arthralgia, and fatigue. Four patients developed thrombotic microangiopathy or cavernous sinus thrombosis and skin necrosis, all of whom had experienced breakthrough bleeding for which they had received multiple infusions of activated prothrombin complex concentrate at doses averaging more than 100 U per kg per day.
Patients with severe hemophilia A typically require prophylactic factor VIII infusions two to three times a week, but 30% develop neutralizing antibodies against factor VIII (inhibitors), which increases care burden, costs, and complication rates.
Emicizumab is a recombinant humanized bispecific monoclonal antibody that restores the function of missing active factor VIII by bridging activated factor IX and factor X, the investigators noted. A phase I study linked once-weekly emicizumab prophylaxis to markedly lower bleeding rates without dose-limiting toxicities (N Engl J Med 2016;374:2044-53). Subsequently, the phase III HAVEN 1 study (NCT02622321) compared once-weekly subcutaneous prophylactic emicizumab with on-demand (episodic) and prophylactic use of bypassing agents (BPAs) in 109 adults and adolescents (12 years and older) with hemophilia A with inhibitors. The primary endpoint was the difference in rates of treated bleeds between the groups. Patients had a median age of 28 years, all were male, and most had severe hemophilia at baseline. They typically had more than nine bleeding events in the 6 months before enrollment, with bleeding in more than one target joint and factor VIII titers of 180 Bethesda units per mL.
HAVEN 1 was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. Dr. Oldenburg and 13 coinvestigators disclosed ties to Chugai, Roche, and other pharmaceutical companies. The remaining coinvestigator had no disclosures.
Once-weekly prophylactic therapy with emicizumab (ACE910) was associated an 87% lower rate of treated bleeding than no prophylaxis among patients with hemophilia A with inhibitors in the open-label, phase III HAVEN 1 trial.
After a median 24 weeks of treatment (range, 3-48 weeks), the 35 patients who were randomly assigned to prophylactic emicizumab (3.0 mg per kg for 4 weeks, followed by 1.5 mg per kg) had an annualized bleeding rate of 2.9 events, compared with an annualized rate of 23.3 events among the 18 patients who received no prophylaxis. The difference was statistically significant, reported Johannes Oldenburg, MD, PhD, and his associates at the International Society on Thrombosis and Haemostasis congress and simultaneously in the New England Journal of Medicine (N Engl J Med. 2017 Jul 10. doi: 10.1056/NEJMoa1703068).
In addition, 63% of patients who received prophylactic emicizumab had no bleeding events, compared with only 6% of the comparison group, said Dr. Oldenburg of Universitaetsklinikum Bonn, Germany. Emicizumab also was associated with a 79% lower rate of treated bleeds, compared with the historic rate among 24 patients who previously had been on prophylactic bypassing agents (P less than .001). No patient developed detectable antidrug antibodies.
The most common adverse events were injection site reactions (15% of patients), followed by headache, upper respiratory tract infection, arthralgia, and fatigue. Four patients developed thrombotic microangiopathy or cavernous sinus thrombosis and skin necrosis, all of whom had experienced breakthrough bleeding for which they had received multiple infusions of activated prothrombin complex concentrate at doses averaging more than 100 U per kg per day.
Patients with severe hemophilia A typically require prophylactic factor VIII infusions two to three times a week, but 30% develop neutralizing antibodies against factor VIII (inhibitors), which increases care burden, costs, and complication rates.
Emicizumab is a recombinant humanized bispecific monoclonal antibody that restores the function of missing active factor VIII by bridging activated factor IX and factor X, the investigators noted. A phase I study linked once-weekly emicizumab prophylaxis to markedly lower bleeding rates without dose-limiting toxicities (N Engl J Med 2016;374:2044-53). Subsequently, the phase III HAVEN 1 study (NCT02622321) compared once-weekly subcutaneous prophylactic emicizumab with on-demand (episodic) and prophylactic use of bypassing agents (BPAs) in 109 adults and adolescents (12 years and older) with hemophilia A with inhibitors. The primary endpoint was the difference in rates of treated bleeds between the groups. Patients had a median age of 28 years, all were male, and most had severe hemophilia at baseline. They typically had more than nine bleeding events in the 6 months before enrollment, with bleeding in more than one target joint and factor VIII titers of 180 Bethesda units per mL.
HAVEN 1 was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. Dr. Oldenburg and 13 coinvestigators disclosed ties to Chugai, Roche, and other pharmaceutical companies. The remaining coinvestigator had no disclosures.
From 2017 ISTH CONGRESS
Key clinical point: .
Major finding: After a median 24 weeks of treatment, the intervention group averaged 2.9 bleeds per year vs. 23.3 events in the control group.
Data source: A phase III randomized multicenter open-label trial of 109 male adolescents and adults with hemophilia A and factor VIII inhibitors.
Disclosures: HAVEN 1 was funded by F. Hoffmann-La Roche and Chugai Pharmaceutical. Dr. Oldenburg and 13 coinvestigators disclosed ties to Chugai, Roche, and other pharmaceutical companies. The remaining coinvestigator had no disclosures.
Fostamatinib under review by FDA for chronic ITP
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission." ![]()
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission." ![]()
The US Food and Drug Administration (FDA) has accepted a new drug application (NDA) for the use of fostamatinib disodium (Tavalisse™) in the treatment of patients with chronic/persistent immune thrombocytopenia (ITP).
Fostamatinib is an oral spleen tyrosine kinase (SYK) inhibitor that investigators believe may address an underlying autoimmune cause of ITP by impeding platelet destruction.
Rigel Pharmaceuticals, the drug’s developer, anticipates an action date by the FDA of April 17, 2018.
The FDA previously granted fostamatinib orphan drug designation.
Data from 3 clinical studies support the new drug application. Study 047 and 048 were randomized placebo-controlled trials, and study 049 was an open-label extension study.
Together with an initial proof of concept study, the application included data on 163 ITP patients. Fostamatinib has been evaluated in over 4,600 individuals across all indications.
The patients enrolled in studies 047 and 048 had been diagnosed with persistent or chronic ITP, had failed at least 1 prior therapy for ITP, and had platelet counts consistently below 30,000/uL.
In both studies, patients were randomized in a 2:1 ratio to receive either fostamatinib or placebo orally twice a day for up to 24 weeks.
Study 047
In this study, 51 patients were randomized to fostamatinib and 25 to placebo.
The median platelet counts at baseline were 15,000/uL and 16,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 57 in both treatment arms. The duration of ITP was 7.5 years (range, 0.6-53) in the fostamatinib arm and 5.5 years (range, 0.4-45) in the placebo arm.
The primary efficacy endpoint in both study 047 and 048 was a stable platelet response, defined as achieving platelet counts greater than 50,000/uL for at least 4 of the 6 scheduled clinic visits between weeks 14 and 24 of treatment.
In study 047, the rate of stable platelet response was significantly higher in the fostamatinib arm than the placebo arm—18% (n=9) and 0%, respectively (P=0.026).
Study 048
Fifty patients were randomized to fostamatinib and 24 to placebo.
Patients’ median platelet counts at baseline were 16,000/uL and 21,000/uL in the fostamatinib and placebo arms, respectively.
The median age was 50 in both treatment arms. The duration of ITP was 8.8 years (range, 0.3-50) in the fostamatinib arm and 10.8 years (range, 0.9-29) in the placebo arm.
In this study, however, the difference in stable platelet response between the 2 arms was not significant—18% (n=9) and 4% (n=1), respectively (P=0.152).
However, when the data from study 047 and 048 were combined, the response rate was significantly higher in the fostamatinib arm than the placebo arm—18% (18/101) and 2% (1/49), respectively (P=0.007).
"The FDA acceptance for filing of our NDA is an exciting milestone for Rigel," Raul Rodriguez, Rigel's president and chief executive officer, said.
The approval of fostamatinib “will provide a new treatment option for patients with chronic or persistent ITP,” he affirmed. “We look forward to working closely with the FDA as they review our submission." ![]()
FDA approves Rebinyn for hemophilia B treatment
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.
FDA advisory committee supports L-glutamine for SCD
The available data on the use of L-glutamine powder for treating sickle cell disease is favorable in terms of the agent’s overall benefit-risk profile, a majority of the Food and Drug Administration’s Oncologic Drugs Advisory Committee agreed during a meeting May 24.
L-glutamine powder, which is used as an oral solution for treating sickle cell disease, showed moderate benefit in a phase III study and a smaller phase II study, and if approved by the FDA – which usually follows the recommendations of its advisory committees – would be only the second treatment approved for the debilitating and sometimes deadly disease. The first, hydroxyurea, was approved for use in adults in 1998.
The committee voted 10-3 in favor of L-glutamate, after hearing from representatives of the new drug marketing applicant, Emmaus Medical, about the efficacy and safety data, as well as from FDA representatives who analyzed the data, physicians who treat sickle cell patients, patient advocates, and patients and their family members who gave emotional testimony in favor of approving this treatment.
“This is clearly a bad disease. It’s worse than cancer in many ways. I think probably mostly from a stigma standpoint it has a desperate need [for treatments],” said acting committee chair Brian I. Rini, MD, who voted in favor of the agent.
While there were some concerns about the data, including questions about methodologies, differential dropout rates between study arms, baseline characteristics that may have affected outcomes, and discrepancies between the Emmaus Medical data and the FDA’s analyses of the data, “all seemed to come down in favor of the agent,” Dr. Rini said, citing its “modest but consistent benefit” and low risk.
“I think one thing that’s strikingly clear is that even any sort of modest benefit in this community, given the sequelae of crises, is significant; it doesn’t take much to produce a clinical impact, and that should be motivation to study more drugs in this disease,” he said.
Another focus among those who voted “yes” was on the overwhelming need for treatment for sickle cell patients, who spend “a hugely disproportionate part of their life in the health care system, and who have had a tremendous burden imposed on them by their disease,” according to Harold J. Burstein, MD, PhD, of Dana-Farber Cancer Institute in Boston, who said he was swayed by the low risk of toxicity and the corroborating evidence in the phase II and III trials.
“What I took away was that one fewer hospital visit per year was a clinically compelling benefit for any individual or family or hospital that might be caring for patients with sickle cell disease,” he said, referencing a finding that treated patients had three visits, compared with four visits among patients in the placebo group, in the phase III study.
The data presented to the committee during the meeting by Yutaka Niihara, MD, of Emmaus Medical, included findings from a phase III randomized, placebo-controlled multicenter study (GLUSCC09-01) involving patients aged 5 years and older with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises within the 12 months prior to screening. A total of 152 patients were randomized to receive oral L-glutamine at a dose of 0.3 mg/kg per day for 48 weeks followed by a 3-week tapering period, and 78 received placebo.
The Emmaus Medical analysis demonstrated a significant decrease in crisis events (median of 3 vs. 4) among treatment vs. placebo group patients, and the time to second crisis was delayed by 79 days in the treatment group (hazard ratio 0.68). The analysis, however, was complicated by the differential dropout rates (36% vs. 24% in the treatment and placebo arms, respectively), necessitating the use of imputation methods. Various methods were used to handle the missing data, and the findings with each of them favored L-glutamine, but each had important limitations, and while the FDA’s analysis of the various approaches showed that each favored L-glutamine over placebo with a range of reduction in the rates of crises from 0.4 to 0.9, this contributed to the decision by some panel member to vote against the agent.
The phase II study (Study 10478), which had a similar design, failed to meet its specified significance level for primary efficacy analysis, but showed a trend in favor of L-glutamine vs. placebo, Dr. Niihara said.
As for safety, Dr. Niihara reported that a safety population of 187 patients treated with L-glutamine and 111 treated with placebo in the phase II and III studies showed that most patients experienced a treatment-emergent adverse event – most often sickle cell anemia with crisis (66% and 72% in the groups, respectively) and acute chest syndrome (7% and 19%, respectively). Treatment-emergent adverse events led to withdrawal in 2.7% and 0.9% of patients, respectively. The most common adverse reactions were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
Bernard F. Cole, PhD, who was among the “no” votes, said he had concerns about “the limitations resulting from differential dropout” rates, which may have artificially shifted the risk profile.
“As a result of those limitations, it’s not clear whether patients at higher risk of a [sickle cell crisis] event might have disproportionately dropped out of the L-glutamine arm,” he explained. “My hope is that the sponsors can more thoroughly address the limitations of this pivotal trial with the FDA,” said Dr. Cole, a professor in the department of mathematics and statistics at the University of Vermont, Burlington.
Dr. Rini, despite his “yes” vote, agreed with the need for more data, noting that additional data analysis could help when it comes to clinical application of L-glutamine.
Specifically, more data regarding duration of therapy and quality data collection that “borrows from the cancer world ... in terms of rigor or data collection,” is needed, he said.
The committee members had no relevant conflicts of interests to disclose.
The available data on the use of L-glutamine powder for treating sickle cell disease is favorable in terms of the agent’s overall benefit-risk profile, a majority of the Food and Drug Administration’s Oncologic Drugs Advisory Committee agreed during a meeting May 24.
L-glutamine powder, which is used as an oral solution for treating sickle cell disease, showed moderate benefit in a phase III study and a smaller phase II study, and if approved by the FDA – which usually follows the recommendations of its advisory committees – would be only the second treatment approved for the debilitating and sometimes deadly disease. The first, hydroxyurea, was approved for use in adults in 1998.
The committee voted 10-3 in favor of L-glutamate, after hearing from representatives of the new drug marketing applicant, Emmaus Medical, about the efficacy and safety data, as well as from FDA representatives who analyzed the data, physicians who treat sickle cell patients, patient advocates, and patients and their family members who gave emotional testimony in favor of approving this treatment.
“This is clearly a bad disease. It’s worse than cancer in many ways. I think probably mostly from a stigma standpoint it has a desperate need [for treatments],” said acting committee chair Brian I. Rini, MD, who voted in favor of the agent.
While there were some concerns about the data, including questions about methodologies, differential dropout rates between study arms, baseline characteristics that may have affected outcomes, and discrepancies between the Emmaus Medical data and the FDA’s analyses of the data, “all seemed to come down in favor of the agent,” Dr. Rini said, citing its “modest but consistent benefit” and low risk.
“I think one thing that’s strikingly clear is that even any sort of modest benefit in this community, given the sequelae of crises, is significant; it doesn’t take much to produce a clinical impact, and that should be motivation to study more drugs in this disease,” he said.
Another focus among those who voted “yes” was on the overwhelming need for treatment for sickle cell patients, who spend “a hugely disproportionate part of their life in the health care system, and who have had a tremendous burden imposed on them by their disease,” according to Harold J. Burstein, MD, PhD, of Dana-Farber Cancer Institute in Boston, who said he was swayed by the low risk of toxicity and the corroborating evidence in the phase II and III trials.
“What I took away was that one fewer hospital visit per year was a clinically compelling benefit for any individual or family or hospital that might be caring for patients with sickle cell disease,” he said, referencing a finding that treated patients had three visits, compared with four visits among patients in the placebo group, in the phase III study.
The data presented to the committee during the meeting by Yutaka Niihara, MD, of Emmaus Medical, included findings from a phase III randomized, placebo-controlled multicenter study (GLUSCC09-01) involving patients aged 5 years and older with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises within the 12 months prior to screening. A total of 152 patients were randomized to receive oral L-glutamine at a dose of 0.3 mg/kg per day for 48 weeks followed by a 3-week tapering period, and 78 received placebo.
The Emmaus Medical analysis demonstrated a significant decrease in crisis events (median of 3 vs. 4) among treatment vs. placebo group patients, and the time to second crisis was delayed by 79 days in the treatment group (hazard ratio 0.68). The analysis, however, was complicated by the differential dropout rates (36% vs. 24% in the treatment and placebo arms, respectively), necessitating the use of imputation methods. Various methods were used to handle the missing data, and the findings with each of them favored L-glutamine, but each had important limitations, and while the FDA’s analysis of the various approaches showed that each favored L-glutamine over placebo with a range of reduction in the rates of crises from 0.4 to 0.9, this contributed to the decision by some panel member to vote against the agent.
The phase II study (Study 10478), which had a similar design, failed to meet its specified significance level for primary efficacy analysis, but showed a trend in favor of L-glutamine vs. placebo, Dr. Niihara said.
As for safety, Dr. Niihara reported that a safety population of 187 patients treated with L-glutamine and 111 treated with placebo in the phase II and III studies showed that most patients experienced a treatment-emergent adverse event – most often sickle cell anemia with crisis (66% and 72% in the groups, respectively) and acute chest syndrome (7% and 19%, respectively). Treatment-emergent adverse events led to withdrawal in 2.7% and 0.9% of patients, respectively. The most common adverse reactions were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
Bernard F. Cole, PhD, who was among the “no” votes, said he had concerns about “the limitations resulting from differential dropout” rates, which may have artificially shifted the risk profile.
“As a result of those limitations, it’s not clear whether patients at higher risk of a [sickle cell crisis] event might have disproportionately dropped out of the L-glutamine arm,” he explained. “My hope is that the sponsors can more thoroughly address the limitations of this pivotal trial with the FDA,” said Dr. Cole, a professor in the department of mathematics and statistics at the University of Vermont, Burlington.
Dr. Rini, despite his “yes” vote, agreed with the need for more data, noting that additional data analysis could help when it comes to clinical application of L-glutamine.
Specifically, more data regarding duration of therapy and quality data collection that “borrows from the cancer world ... in terms of rigor or data collection,” is needed, he said.
The committee members had no relevant conflicts of interests to disclose.
The available data on the use of L-glutamine powder for treating sickle cell disease is favorable in terms of the agent’s overall benefit-risk profile, a majority of the Food and Drug Administration’s Oncologic Drugs Advisory Committee agreed during a meeting May 24.
L-glutamine powder, which is used as an oral solution for treating sickle cell disease, showed moderate benefit in a phase III study and a smaller phase II study, and if approved by the FDA – which usually follows the recommendations of its advisory committees – would be only the second treatment approved for the debilitating and sometimes deadly disease. The first, hydroxyurea, was approved for use in adults in 1998.
The committee voted 10-3 in favor of L-glutamate, after hearing from representatives of the new drug marketing applicant, Emmaus Medical, about the efficacy and safety data, as well as from FDA representatives who analyzed the data, physicians who treat sickle cell patients, patient advocates, and patients and their family members who gave emotional testimony in favor of approving this treatment.
“This is clearly a bad disease. It’s worse than cancer in many ways. I think probably mostly from a stigma standpoint it has a desperate need [for treatments],” said acting committee chair Brian I. Rini, MD, who voted in favor of the agent.
While there were some concerns about the data, including questions about methodologies, differential dropout rates between study arms, baseline characteristics that may have affected outcomes, and discrepancies between the Emmaus Medical data and the FDA’s analyses of the data, “all seemed to come down in favor of the agent,” Dr. Rini said, citing its “modest but consistent benefit” and low risk.
“I think one thing that’s strikingly clear is that even any sort of modest benefit in this community, given the sequelae of crises, is significant; it doesn’t take much to produce a clinical impact, and that should be motivation to study more drugs in this disease,” he said.
Another focus among those who voted “yes” was on the overwhelming need for treatment for sickle cell patients, who spend “a hugely disproportionate part of their life in the health care system, and who have had a tremendous burden imposed on them by their disease,” according to Harold J. Burstein, MD, PhD, of Dana-Farber Cancer Institute in Boston, who said he was swayed by the low risk of toxicity and the corroborating evidence in the phase II and III trials.
“What I took away was that one fewer hospital visit per year was a clinically compelling benefit for any individual or family or hospital that might be caring for patients with sickle cell disease,” he said, referencing a finding that treated patients had three visits, compared with four visits among patients in the placebo group, in the phase III study.
The data presented to the committee during the meeting by Yutaka Niihara, MD, of Emmaus Medical, included findings from a phase III randomized, placebo-controlled multicenter study (GLUSCC09-01) involving patients aged 5 years and older with sickle cell disease or beta-0 thalassemia who had at least two episodes of painful crises within the 12 months prior to screening. A total of 152 patients were randomized to receive oral L-glutamine at a dose of 0.3 mg/kg per day for 48 weeks followed by a 3-week tapering period, and 78 received placebo.
The Emmaus Medical analysis demonstrated a significant decrease in crisis events (median of 3 vs. 4) among treatment vs. placebo group patients, and the time to second crisis was delayed by 79 days in the treatment group (hazard ratio 0.68). The analysis, however, was complicated by the differential dropout rates (36% vs. 24% in the treatment and placebo arms, respectively), necessitating the use of imputation methods. Various methods were used to handle the missing data, and the findings with each of them favored L-glutamine, but each had important limitations, and while the FDA’s analysis of the various approaches showed that each favored L-glutamine over placebo with a range of reduction in the rates of crises from 0.4 to 0.9, this contributed to the decision by some panel member to vote against the agent.
The phase II study (Study 10478), which had a similar design, failed to meet its specified significance level for primary efficacy analysis, but showed a trend in favor of L-glutamine vs. placebo, Dr. Niihara said.
As for safety, Dr. Niihara reported that a safety population of 187 patients treated with L-glutamine and 111 treated with placebo in the phase II and III studies showed that most patients experienced a treatment-emergent adverse event – most often sickle cell anemia with crisis (66% and 72% in the groups, respectively) and acute chest syndrome (7% and 19%, respectively). Treatment-emergent adverse events led to withdrawal in 2.7% and 0.9% of patients, respectively. The most common adverse reactions were constipation, nausea, headache, cough, pain in the extremities, back pain, chest pain, and abdominal pain.
Bernard F. Cole, PhD, who was among the “no” votes, said he had concerns about “the limitations resulting from differential dropout” rates, which may have artificially shifted the risk profile.
“As a result of those limitations, it’s not clear whether patients at higher risk of a [sickle cell crisis] event might have disproportionately dropped out of the L-glutamine arm,” he explained. “My hope is that the sponsors can more thoroughly address the limitations of this pivotal trial with the FDA,” said Dr. Cole, a professor in the department of mathematics and statistics at the University of Vermont, Burlington.
Dr. Rini, despite his “yes” vote, agreed with the need for more data, noting that additional data analysis could help when it comes to clinical application of L-glutamine.
Specifically, more data regarding duration of therapy and quality data collection that “borrows from the cancer world ... in terms of rigor or data collection,” is needed, he said.
The committee members had no relevant conflicts of interests to disclose.
Key clinical point:
Major finding: In a phase III study, there was a significant decrease in crisis events (median of 3 vs. 4) among treatment vs. placebo group patients.
Data source: A total of 152 patients were randomized to receive oral L-glutamine at a dose of 0.3 mg/kg per day for 48 weeks followed by a 3-week tapering period, and 78 received placebo.
Disclosures: The committee members had no relevant conflicts of interests to disclose.
Consider switch to clopidogrel for DAPT early post ACS
PARIS – A strategy of switching from prasugrel or ticagrelor to clopidogrel 1 month after percutaneous coronary intervention for acute coronary syndrome is superior to the guideline-recommended full 12 months of dual-antiplatelet therapy with either of the newer P2Y12 inhibitors, according to Thomas Cuisset, MD.
In the randomized TOPIC (Timing of Platelet Inhibition After Acute Coronary Syndrome) trial, this switch strategy resulted in a marked reduction in bleeding without an increased risk of ischemic events, compared with a full 12 months of standard dual-antiplatelet therapy (DAPT) using prasugrel (Effient) or ticagrelor (Brilinta).
He added that the cost savings of this switch strategy would be enormous, since generic clopidogrel is vastly less expensive than prasugrel or ticagrelor.
Twelve months of DAPT with aspirin plus either prasugrel or ticagrelor is the guideline-recommended DAPT regimen following PCI for ACS on the strength of the TRITON and PLATO trials, respectively, which showed that those agents were more effective than clopidogrel for the prevention of thrombotic events. But Dr. Cuisset and his coinvestigators noted that the risk of ischemic events was highest in the first month or so following ACS, while the risk of DAPT-related serious bleeding increased after the first month and continued for the duration.
“We need to use the new drugs, and we need to go for 1 year with DAPT. But does that mean we need to go for 1 year with the new drugs?” he asked.
This question was the impetus for TOPIC, an open-label, single-center randomized trial that included 646 ACS patients who were free of major adverse cardiovascular events during their first month on DAPT with prasugrel or ticagrelor. At that point they were randomized to remain on their standard regimen or switch to aspirin at 75 mg/day plus clopidogrel at 75 mg/day for months 2-12. The switch strategy is similar to the way pulmonary embolism is managed: an early phase of high-intensity therapy followed by a backing off to a less intensive regimen, said Dr. Cuisset, a cardiologist at Aix-Marseille University, Provence, France.
The primary endpoint in TOPIC was the cumulative 1-year rate of a composite of all-cause mortality, stroke, urgent revascularization, or clinically significant bleeding as reflected in a Bleeding Academic Research Consortium (BARC) grade 2 or greater bleeding. The primary endpoint occurred in 13.4% of the switch group, a 52% relative risk reduction, compared with the 26.3% cumulative incidence with standard DAPT.
This difference wasn’t due to any between-group disparity in ischemic events, but rather to a 70% reduction in the risk of BARC grade 2 or greater bleeding in the switch group: 4.0% vs. 14.9%.
Some physicians have already been switching to clopidogrel for DAPT after ACS, either because of safety or cost concerns. Now their practice is evidence based, Dr. Cuisset noted.
Asked why TOPIC didn’t use the more stringent bleeding endpoint of BARC grade 3-5 bleeding, the cardiologist replied that it would have required a larger trial to show a significant difference. Besides, he added, BARC grade 2 bleeding is clinically important because it has a negative impact on quality of life and can cause patients to discontinue DAPT, thereby increasing their risk of thrombosis.
The TOPIC protocol didn’t utilize a loading dose of clopidogrel when making the switch. Investigators started clopidogrel the day after stopping prasugrel and at least 12 hours after the final dose of ticagrelor.
Ideally, the novel TOPIC findings should be confirmed in a much larger, randomized, double-blind clinical trial capable of detecting any small differences in stent thrombosis or MI rates before physicians adopt a change in practice, but discussant Chaim Lotan, MD, director of the Heart Institute at Hadassah Medical Center in Jerusalem, dismissed that prospect as unlikely.
“I tried myself to do a similar study and found I got a lot of opposition from the pharma companies as well as from physicians who said, ‘How can you go against the guidelines?’ ” he said.
“I want to congratulate your team because I think this is a groundbreaking study that is going to dictate a changing of the guidelines,” he told Dr. Cuisset.
Dr. Cuisset reported having no financial conflicts regarding this investigator-driven study funded without commercial support.
Simultaneous with his presentation in Paris, the TOPIC findings were published online (Eur Heart J. 2017 May 16. doi: 10.1093/eurheartj/ehx175).
Dr. Cuisset reported no financial conflicts regarding this investigator-driven study funded without commercial support.
[email protected]
PARIS – A strategy of switching from prasugrel or ticagrelor to clopidogrel 1 month after percutaneous coronary intervention for acute coronary syndrome is superior to the guideline-recommended full 12 months of dual-antiplatelet therapy with either of the newer P2Y12 inhibitors, according to Thomas Cuisset, MD.
In the randomized TOPIC (Timing of Platelet Inhibition After Acute Coronary Syndrome) trial, this switch strategy resulted in a marked reduction in bleeding without an increased risk of ischemic events, compared with a full 12 months of standard dual-antiplatelet therapy (DAPT) using prasugrel (Effient) or ticagrelor (Brilinta).
He added that the cost savings of this switch strategy would be enormous, since generic clopidogrel is vastly less expensive than prasugrel or ticagrelor.
Twelve months of DAPT with aspirin plus either prasugrel or ticagrelor is the guideline-recommended DAPT regimen following PCI for ACS on the strength of the TRITON and PLATO trials, respectively, which showed that those agents were more effective than clopidogrel for the prevention of thrombotic events. But Dr. Cuisset and his coinvestigators noted that the risk of ischemic events was highest in the first month or so following ACS, while the risk of DAPT-related serious bleeding increased after the first month and continued for the duration.
“We need to use the new drugs, and we need to go for 1 year with DAPT. But does that mean we need to go for 1 year with the new drugs?” he asked.
This question was the impetus for TOPIC, an open-label, single-center randomized trial that included 646 ACS patients who were free of major adverse cardiovascular events during their first month on DAPT with prasugrel or ticagrelor. At that point they were randomized to remain on their standard regimen or switch to aspirin at 75 mg/day plus clopidogrel at 75 mg/day for months 2-12. The switch strategy is similar to the way pulmonary embolism is managed: an early phase of high-intensity therapy followed by a backing off to a less intensive regimen, said Dr. Cuisset, a cardiologist at Aix-Marseille University, Provence, France.
The primary endpoint in TOPIC was the cumulative 1-year rate of a composite of all-cause mortality, stroke, urgent revascularization, or clinically significant bleeding as reflected in a Bleeding Academic Research Consortium (BARC) grade 2 or greater bleeding. The primary endpoint occurred in 13.4% of the switch group, a 52% relative risk reduction, compared with the 26.3% cumulative incidence with standard DAPT.
This difference wasn’t due to any between-group disparity in ischemic events, but rather to a 70% reduction in the risk of BARC grade 2 or greater bleeding in the switch group: 4.0% vs. 14.9%.
Some physicians have already been switching to clopidogrel for DAPT after ACS, either because of safety or cost concerns. Now their practice is evidence based, Dr. Cuisset noted.
Asked why TOPIC didn’t use the more stringent bleeding endpoint of BARC grade 3-5 bleeding, the cardiologist replied that it would have required a larger trial to show a significant difference. Besides, he added, BARC grade 2 bleeding is clinically important because it has a negative impact on quality of life and can cause patients to discontinue DAPT, thereby increasing their risk of thrombosis.
The TOPIC protocol didn’t utilize a loading dose of clopidogrel when making the switch. Investigators started clopidogrel the day after stopping prasugrel and at least 12 hours after the final dose of ticagrelor.
Ideally, the novel TOPIC findings should be confirmed in a much larger, randomized, double-blind clinical trial capable of detecting any small differences in stent thrombosis or MI rates before physicians adopt a change in practice, but discussant Chaim Lotan, MD, director of the Heart Institute at Hadassah Medical Center in Jerusalem, dismissed that prospect as unlikely.
“I tried myself to do a similar study and found I got a lot of opposition from the pharma companies as well as from physicians who said, ‘How can you go against the guidelines?’ ” he said.
“I want to congratulate your team because I think this is a groundbreaking study that is going to dictate a changing of the guidelines,” he told Dr. Cuisset.
Dr. Cuisset reported having no financial conflicts regarding this investigator-driven study funded without commercial support.
Simultaneous with his presentation in Paris, the TOPIC findings were published online (Eur Heart J. 2017 May 16. doi: 10.1093/eurheartj/ehx175).
Dr. Cuisset reported no financial conflicts regarding this investigator-driven study funded without commercial support.
[email protected]
PARIS – A strategy of switching from prasugrel or ticagrelor to clopidogrel 1 month after percutaneous coronary intervention for acute coronary syndrome is superior to the guideline-recommended full 12 months of dual-antiplatelet therapy with either of the newer P2Y12 inhibitors, according to Thomas Cuisset, MD.
In the randomized TOPIC (Timing of Platelet Inhibition After Acute Coronary Syndrome) trial, this switch strategy resulted in a marked reduction in bleeding without an increased risk of ischemic events, compared with a full 12 months of standard dual-antiplatelet therapy (DAPT) using prasugrel (Effient) or ticagrelor (Brilinta).
He added that the cost savings of this switch strategy would be enormous, since generic clopidogrel is vastly less expensive than prasugrel or ticagrelor.
Twelve months of DAPT with aspirin plus either prasugrel or ticagrelor is the guideline-recommended DAPT regimen following PCI for ACS on the strength of the TRITON and PLATO trials, respectively, which showed that those agents were more effective than clopidogrel for the prevention of thrombotic events. But Dr. Cuisset and his coinvestigators noted that the risk of ischemic events was highest in the first month or so following ACS, while the risk of DAPT-related serious bleeding increased after the first month and continued for the duration.
“We need to use the new drugs, and we need to go for 1 year with DAPT. But does that mean we need to go for 1 year with the new drugs?” he asked.
This question was the impetus for TOPIC, an open-label, single-center randomized trial that included 646 ACS patients who were free of major adverse cardiovascular events during their first month on DAPT with prasugrel or ticagrelor. At that point they were randomized to remain on their standard regimen or switch to aspirin at 75 mg/day plus clopidogrel at 75 mg/day for months 2-12. The switch strategy is similar to the way pulmonary embolism is managed: an early phase of high-intensity therapy followed by a backing off to a less intensive regimen, said Dr. Cuisset, a cardiologist at Aix-Marseille University, Provence, France.
The primary endpoint in TOPIC was the cumulative 1-year rate of a composite of all-cause mortality, stroke, urgent revascularization, or clinically significant bleeding as reflected in a Bleeding Academic Research Consortium (BARC) grade 2 or greater bleeding. The primary endpoint occurred in 13.4% of the switch group, a 52% relative risk reduction, compared with the 26.3% cumulative incidence with standard DAPT.
This difference wasn’t due to any between-group disparity in ischemic events, but rather to a 70% reduction in the risk of BARC grade 2 or greater bleeding in the switch group: 4.0% vs. 14.9%.
Some physicians have already been switching to clopidogrel for DAPT after ACS, either because of safety or cost concerns. Now their practice is evidence based, Dr. Cuisset noted.
Asked why TOPIC didn’t use the more stringent bleeding endpoint of BARC grade 3-5 bleeding, the cardiologist replied that it would have required a larger trial to show a significant difference. Besides, he added, BARC grade 2 bleeding is clinically important because it has a negative impact on quality of life and can cause patients to discontinue DAPT, thereby increasing their risk of thrombosis.
The TOPIC protocol didn’t utilize a loading dose of clopidogrel when making the switch. Investigators started clopidogrel the day after stopping prasugrel and at least 12 hours after the final dose of ticagrelor.
Ideally, the novel TOPIC findings should be confirmed in a much larger, randomized, double-blind clinical trial capable of detecting any small differences in stent thrombosis or MI rates before physicians adopt a change in practice, but discussant Chaim Lotan, MD, director of the Heart Institute at Hadassah Medical Center in Jerusalem, dismissed that prospect as unlikely.
“I tried myself to do a similar study and found I got a lot of opposition from the pharma companies as well as from physicians who said, ‘How can you go against the guidelines?’ ” he said.
“I want to congratulate your team because I think this is a groundbreaking study that is going to dictate a changing of the guidelines,” he told Dr. Cuisset.
Dr. Cuisset reported having no financial conflicts regarding this investigator-driven study funded without commercial support.
Simultaneous with his presentation in Paris, the TOPIC findings were published online (Eur Heart J. 2017 May 16. doi: 10.1093/eurheartj/ehx175).
Dr. Cuisset reported no financial conflicts regarding this investigator-driven study funded without commercial support.
[email protected]
AT EuroPCR
Key clinical point:
Major finding: The cumulative 1-year incidence of all-cause mortality, stroke, urgent revascularization, or clinically significant bleeding was 13.4% in acute coronary syndrome patients who switched to clopidogrel after 1 month on prasugrel or ticagrelor for dual-antiplatelet therapy, compared with 26.3% in those who didn’t switch.
Data source: An open-label, single-center, randomized trial including 646 ACS patients.
Disclosures: The presenter reported no financial conflicts regarding this investigator-driven study funded without commercial support.
Intensive BP lowering may reduce larger hematoma expansion in ICH
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
BOSTON – Intensive systolic blood pressure reduction did not significantly reduce hematoma expansion, compared with standard systolic blood pressure reduction in the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II trial, but, in a post hoc analysis, it did show a strong trend toward reducing hematoma expansion in those with a larger initial hematoma volume.
In 450 patients randomized to receive intensive treatment and 426 randomized to receive standard treatment in the large, international, phase III randomized trial, the proportion of patients with any hematoma expansion was 46.4% and 52.3% , respectively (relative risk, 0.89; 95% confidence interval, 0.73-1.07). The proportion with hematoma expansion with an increase of greater than 33% was 18.9% and 24.4%, respectively (RR, 0.77; 95% CI, 0.58-1.03), Joshua N. Goldstein, MD, reported at the annual meeting of the American Academy of Neurology.
The confidence intervals suggested only a trend toward improvement, as the difference between the groups did not reach statistical significance, he said.
To determine if intensive systolic blood pressure reduction might have more of an impact among higher-risk patients, the investigators conducted a post hoc, secondary analysis in those with relatively larger hematomas. Among patients with an initial hematoma volume of at least 10 mL (at least half of the study population had smaller hematomas), the proportion with any hematoma expansion was 53.8% in the intensive treatment group and 61.3% in the standard treatment group (RR, 0.88; 95% CI, 0.67-1.13), and the proportion of patients with hematoma expansion of greater than 33% was 18% and 27.6%, respectively (RR, 0.67; 95% CI, 0.45-1.00), said Dr. Goldstein of Massachusetts General Hospital, Boston.
In those with an expansion greater than 33% and a hematoma volume of at least 6 cc, the finding was similar.
“The trend looks a little bit more aggressive,” he said, but the 95% confidence interval in those with larger hematomas reached 1.
Study subjects had elevated blood pressure at arrival, a Glasgow coma scale score of at least 5, and a hematoma volume of less than 60 cc. They were enrolled and randomized within 4.5 hours of symptom onset. Those in the intensive treatment group were treated with a goal of achieving between 110 and 139 mm Hg within 24 hours, and those in the standard treatment group were treated with a goal of achieving between 140 mm Hg and 179 mm Hg within 24 hours. They underwent baseline and 24-hour computed tomography scans, which were analyzed centrally by blinded investigators who recorded any increase of 0.5 mL or more, an increase of more than 33% , an increase of more than 33% or more than 6 mL, and an intraventricular hemorrhage volume greater than 2 mL.
These measures were correlated with death and disability, and hematoma enlargement was shown to be significantly associated with those outcomes at 3 months after randomization (RR, 1.59; 95% CI, 1.25-2.02), Dr. Goldstein said.
Previous studies have suggested that intensive lowering of systolic BP in patients with intracerebral hemorrhage can reduce the rate of hematoma expansion. As such, the hypothesis of the current study was that the intensive treatment of elevated blood pressures – arriving systolic blood pressure of greater than 180 mm Hg – would reduce the likelihood of death and disability at 3 months, Dr. Goldstein said,
The question is whether the biomarker – hematoma expansion – is really linked to clinical outcome, he said, “because a lot of our attempts to treat hematoma expansion are based on the assumption that, if we reduce hematoma expansion, we’re going to improve clinical outcomes.”
“In this trial ... the expanders had more death and disability than nonexpanders, and this was statistically significant, so hematoma expansion does seem to be a statistically significant predictor of poor outcome,” he said.
Thus, the strong trend toward a reduced risk of hematoma expansion with intensive blood pressure lowering in patients with larger hematoma volumes at baseline in this analysis is noteworthy, he said.
“It appears that the treatment is affecting the biomarker, that the treatment is affecting hematoma expansion ... and hematoma expansion was a significant predictor of death and disability ... so why didn’t we get the result we wanted from the trial?” he asked.
It may be that a greater magnitude of reduction in the risk of hematoma expansion was necessary, he said.
“In other words, even if our treatment is having an effect, it’s just not having a big enough effect to change outcomes,” he said, adding that future trials probably need to involve a much bigger impact on the risk of expansion to translate to a change in clinical outcomes.”
This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.
Key clinical point:
Major finding: Among patients with larger initial hematoma volume, expansion greater than 33% occurred in 18% vs. 28% in those with intense vs. standard blood pressure lowering, respectively (RR, 0.67).
Data source: The randomized phase III ATACH II trial involving 876 patients.
Disclosures: This study was sponsored by the National Institute of Neurological Disorders and Stroke. Dr. Goldstein reported having no disclosures.