User login
Lp(a) tied to more early CV events than familial hypercholesterolemia
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
Many more people are at risk for early cardiovascular events because of raised lipoprotein(a) levels than from having familial hypercholesterolemia (FH), a new study suggests.
The Danish study set out to try and establish a level of Lp(a) that would be associated with a cardiovascular risk similar to that seen with FH. As there are many different definitions of FH, results showed a large range of Lp(a) values that corresponded to risk levels of the different FH definitions.
However, if considering one of the broadest FH definitions (from MEDPED – Make Early Diagnoses, Prevent Early Deaths), which is the one most commonly used in the United States, results showed that the level of cardiovascular risk in patients with this definition of FH is similar to that associated with Lp(a) levels of around 70 mg/dL (0.7 g/L).
“While FH is fairly unusual, occurring in less than 1% of the population, levels of Lp(a) of 70 mg/dL or above are much more common, occurring in around 10% of the White population,” Børge Nordestgaard, MD, Copenhagen University Hospital, said in an interview. Around 20% of the Black population have such high levels, while levels in Hispanics are in between.
“Our results suggest that there will be many more individuals at risk of premature MI or cardiovascular death because of raised Lp(a) levels than because of FH,” added Dr. Nordestgaard, the senior author of the current study.
Dr. Nordestgaard explained that FH is well established to be a serious condition. “We consider FH to be the genetic disease that causes the most cases of early heart disease and early death worldwide.”
“But we know now that raised levels of Lp(a), which is also genetically determined, can also lead to an increased risk of cardiovascular events relatively early in life, and when you look into the numbers, it seems like high levels of Lp(a) could be more common than FH. We wanted to try and find the levels of Lp(a) that corresponded to similar cardiovascular risk as FH.”
The Danish study was published in the Journal of the American College of Cardiology.
The authors note that the 2019 joint European Society of Cardiology and European Atherosclerosis Society guidelines suggested that an Lp(a) level greater than 180 mg/dL (0.8 g/L) may confer a lifetime risk for heart disease equivalent to the risk associated with heterozygous FH, but they point out that this value was speculative and not based on a direct comparison of risk associated with the two conditions in the same population.
For their study, Dr. Nordestgaard and colleagues analyzed information from a large database of the Danish population, the Copenhagen General Population Study, including 69,644 individuals for whom data on FH and Lp(a) levels were available. As these conditions are genetically determined, and the study held records on individuals going back several decades, the researchers were able to analyze event rates over a median follow up time of 42 years. During this time, there were 4,166 cases of myocardial infarction and 11,464 cases of atherosclerotic cardiovascular disease (ASCVD).
Results showed that Lp(a) levels associated with MI risk equivalent to that of clinical FH ranged from 67 to 402 mg/dL depending on the definition used for FH. The Lp(a) level corresponding to the MI risk of genetically determined FH was 180 mg/dL.
In terms of risk of ASCVD events, the levels of Lp(a) corresponding to the risk associated with clinical FH ranged from 130 to 391 mg/dL, and the Lp(a) level corresponding to the ASCVD risk of genetically determined FH was 175 mg/dL.
“All these different definitions of FH may cause some confusion, but basically we are saying that if an individual is found to have an Lp(a) above 70 mg/dL, then they have a similar level of cardiovascular risk as that associated with the broadest definition of FH, and they should be taken as seriously as a patient diagnosed with FH,” Dr. Nordestgaard said.
He estimated that these individuals have approximately a doubling of cardiovascular risk, compared with the general population, and risk increases further with rising Lp(a) levels.
The researchers also found that if an individual has both FH and raised Lp(a) they are at very high risk, as these two conditions are independent of each other.
Although a specific treatment for lowering Lp(a) levels is not yet available, Dr. Nordestgaard stresses that it is still worth identifying individuals with raised Lp(a) as efforts can be made to address other cardiovascular risk factors.
“We know raised Lp(a) increases cardiovascular risk, but there are also many other factors that likewise increase this risk, and they are all additive. So, it is very important that individuals with raised Lp(a) levels address these other risk factors,” he said. “These include stopping smoking, being at healthy weight, exercising regularly, eating a heart-healthy diet, and aggressive treatment of raised LDL, hypertension, and diabetes. All these things will lower their overall risk of cardiovascular disease.”
And there is the promise of new drugs to lower Lp(a) on the horizon, with several such products now in clinical development.
Dr. Nordestgaard also points out that as Lp(a) is genetically determined, cascade screening of close relatives of the individual with raised Lp(a) should also take place to detect others who may be at risk.
Although a level of Lp(a) of around 70 mg/dL confers similar cardiovascular risk than some definitions of FH, Dr. Nordestgaard says lower levels than this should also be a signal for concern.
“We usually say Lp(a) levels of 50 mg/dL are when we need to start to take this seriously. And it’s estimated that about 20% of the White population will have levels of 50 mg/dL or over and even more in the Black population,” he added.
‘Screen for both conditions’
In an accompanying editorial, Pamela Morris, MD, Medical University of South Carolina, Charleston; Jagat Narula, MD, Icahn School of Medicine, New York; and Sotirios Tsimikas, MD, University of California, San Diego, say “the weight of evidence strongly supports that both genetic lipid disorders, elevated Lp(a) levels and FH, are causally associated with an increased risk of premature ASCVD and should be carefully considered in risk assessment and management for ASCVD risk reduction.”
Dr. Morris told this news organization that the current study found a very large range of Lp(a) levels that conferred a similar cardiovascular risk to FH, because of the many different definitions of FH in use.
“But this should not take away the importance of screening for raised Lp(a) levels,” she stressed.
“We know that increased Lp(a) levels signal a high risk of cardiovascular disease. A diagnosis of FH is also a high-risk condition,” she said. “Both are important, and we need to screen for both, but it is difficult to directly compare the two conditions because the different definitions of FH get in the way.”
Dr. Morris agrees with Dr. Nordestgaard that raised levels of Lp(a) may actually be more important for the population risk of cardiovascular disease than FH, as the prevalence of increased Lp(a) levels is higher.
“Because raised Lp(a) levels are more prevalent than confirmed FH, the risk to the population is greater,” she said.
Dr. Morris points out that cardiovascular risk starts to increase at Lp(a) levels of 30 mg/dL (75 nmol/L).
The editorialists recommend that “in addition to performing a lipid panel periodically according to evidence-based guidelines, measurement of Lp(a) levels should also be performed at least once in an individual’s lifetime for ASCVD risk assessment.”
They conclude that “it is vital to continue to raise awareness among clinicians and patients of these high-risk genetic lipid disorders. Our understanding of both disorders is rapidly expanding, and promising novel therapeutics may offer hope for prevention of cardiovascular disease in patients with elevated Lp(a) levels in the future.”
This work was supported by Copenhagen University Hospital – Herlev Gentofte, Denmark, and the Danish Beckett-Foundation. The Copenhagen General Population Study is supported by the Copenhagen County Foundation and Copenhagen University Hospital – Herlev Gentofte. Dr. Nordestgaard has been a consultant and a speaker for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, Abbott, and Esperion.
A version of this article first appeared on Medscape.com.
New studies change beliefs about cardiovascular disease
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to review a few of these.
The first is the TIME study. The TIME study looked at whether it matters if you give antihypertensive agents in the morning or the evening. This was a prospective, pragmatic, parallel-group study that was performed in the U.K. and published in The Lancet.
Their question was whether evening dosing of antihypertensives has benefit in cardiovascular outcomes in adults. They enrolled over 21,000 people with hypertension who were taking at least one antihypertensive medication. Patients were randomized to morning or evening dosing.
The primary outcome was death or hospitalization due to myocardial infarction or stroke. There was no difference. It doesn’t matter if you take your antihypertensive agent in the morning or the evening. I think this is important because, clinically, the simpler the regimen for the patient, the greater the adherence, leading to better outcomes.
I know I can safely ask a patient when they would rather take their medicine. For many people, that may be the morning because they’re brushing their teeth and they remember. If they want to take it in the evening, that’s fine, too. We’re no longer slave to telling a patient to take their antihypertensive medications in the evening.
At the meeting of the American Society of Nephrology, results from a study on the use of renin-angiotensin system (RAS) inhibitors in advanced CKD was presented, called the STOP ACEi trial. Again, another interesting trial asking a simple question. This was a randomized controlled trial (RCT) in patients who had an estimated glomerular filtration rate (eGFR) less than 30, and they were randomized to stop or continue therapy with their RAS inhibitors.
The primary outcome was the eGFR at 3 years. They enrolled 411 patients with a median baseline eGFR of 18. At 3 years, there was no difference in the eGFR between the groups. In the discontinuation group, the eGFR was 12.6 versus 13.3 in the continuation group. There were no differences in complications or anything else. Their conclusion was that among patients with advanced and progressive CKD, the discontinuation of a RAS inhibitor was not associated with a significant difference in the long-term rate of decrease in eGFR.
I think this is important because it changes our paradigm a bit. You can stop the RAS inhibitor; reduce the need for excessive medication in these patients; and, hopefully, focus on some newer medications that have been shown to prevent the decline in eGFR that are now available.
Next is from a letter published in JAMA, which asks the following question: Is diabetes itself an equivalent cardiovascular risk factor to those who have had a prior cardiovascular event?
We used to put having diabetes in that same high-risk category as people who’d already had a cardiovascular disease event. Well, have we made that any different? These authors are from Canada, and they did a retrospective population-based study looking at administrative health claims from Ontario, Canada, to assess the association of diabetes and prior cardiovascular disease with cardiovascular events from 1994 to 2014.
What I think is kind of cool, because I’m a diabetologist, is that over time the magnitude of the association between diabetes and cardiovascular event rates decreased. In somebody with diabetes, they don’t have the same high risk that a person who’s already had a cardiovascular event rate does. Diabetes is less of a risk factor for cardiovascular disease than having established cardiovascular disease, which means we’re treating diabetes better and reducing the risk for cardiovascular disease.
If you look at people with diabetes and a prior cardiovascular event, that’s still the very highest risk. The risk of people having another event who have established cardiovascular disease is pretty flat. Those people didn’t get better and the people with preexisting diabetes and cardiovascular events at baseline didn’t get much better, but those who had diabetes alone did improve in terms of looking at cardiovascular event rates.
I think this is good news because diabetes itself isn’t as high a cardiovascular risk factor as we once thought. It doesn’t mean that it isn’t a cardiovascular risk factor, but I think we’ve done better at mitigating the risk.
Finally, there is a relatively small study that was presented at the American Heart Association and published in the Journal of the American College of Cardiology, which asks whether supplements that are often used to lower LDL cholesterol are equivalent to a statin.
They compared six supplements with a placebo and with rosuvastatin, and looked to see what happened. This is not an outcome study, but a very short study, at 28 days, that used a placebo. They included 190 people with no history of cardiovascular disease but an increased 10-year risk for sclerotic cardiovascular disease.
The agents studied were rosuvastatin, placebo, fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice. Well, not surprisingly, rosuvastatin worked. It showed a 35% reduction in LDL cholesterol, and there was no significant impact on cholesterol levels with any of the other agents. The supplements yielded a similar response, as did the placebo. Side effects were similar, but they were most common with plant sterols and red yeast rice.
Clearly, a statin is better if you want to lower cholesterol levels. My approach, when patients want to take supplements, is to tell them what I know factually, which basically is that they don’t really cause much in the way of LDL cholesterol lowering. If I think the supplement isn’t going to hurt someone, I don’t tell them not to use it. I certainly tell them that they need to use agents that we know can actually reduce cardiovascular risk.
I think these studies really go through the gamut of asking questions. When can we stop an agent? What time of day do we need to give an agent? What, really, is the risk for type 2 diabetes with regard to cardiovascular events? What’s the value of supplements?
I think this is interesting, because I really encourage researchers to ask and answer these kinds of questions because it helps us clinically decide what’s best for treating our patients.
Thank you.
Dr. Peters is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She reported conflicts of interest with numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Persistent asthma linked to higher carotid plaque burden
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).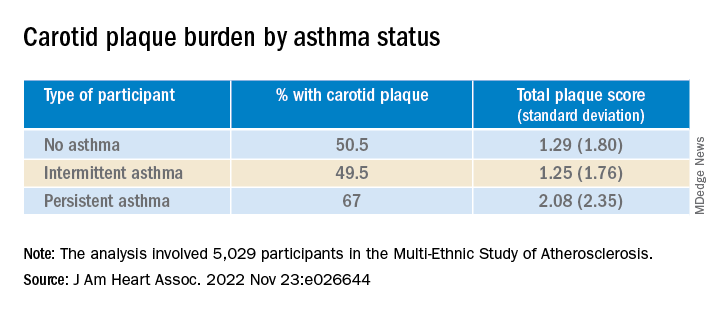
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.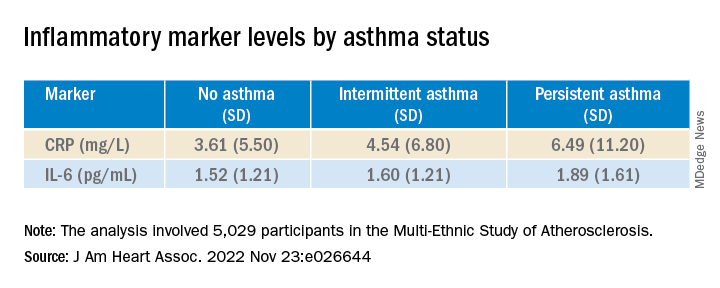
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D fails to stave off statin-related muscle symptoms
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not prevent muscle symptoms in new statin users or affect the likelihood of discontinuing a statin due to muscle pain and discomfort, a substudy of the VITAL trial indicates.
Among more than 2,000 randomized participants, statin-associated muscle symptoms (SAMS) were reported by 31% assigned to vitamin D and 31% assigned to placebo.
The two groups were equally likely to stop taking a statin due to muscle symptoms, at 13%.
No significant difference was observed in SAMS (odds ratio [OR], 0.97; 95% confidence interval [CI], 0.80-1.18) or statin discontinuations (OR, 1.04; 95% CI, 0.80-1.35) after adjustment for baseline variables and other characteristics, namely age, sex, and African-American race, previously found to be associated with SAMS in VITAL.
“We actually thought when we started out that maybe we were going to show something, that maybe it was going to be that the people who got the vitamin D were least likely to have a problem with a statin than all those who didn’t get vitamin D, but that is not what we showed,” senior author Neil J. Stone, MD, Northwestern University, Chicago, told this news organization.
He noted that patients in the clinic with low levels of vitamin D often have muscle pain and discomfort and that previous unblinded studies suggested vitamin D might benefit patients with SAMS and reduce statin intolerance.
As previously reported, the double-blind VITAL trial showed no difference in the primary prevention of cardiovascular disease or cancer at 5 years among 25,871 middle-aged adults randomized to vitamin D3 at 2000 IU/d or placebo, regardless of their baseline vitamin D level.
Unlike previous studies showing a benefit with vitamin D on SAMS, importantly, VITAL participants were unaware of whether they were taking vitamin D or placebo and were not expecting any help with their muscle symptoms, first author Mark A. Hlatky, MD, Stanford (Calif.) University, pointed out in an interview.
As to how many statin users turn to the popular supplement for SAMS, he said that number couldn’t be pinned down, despite a lengthy search. “But I think it’s very common, because up to half of people stop taking their statins within a year and many of these do so because of statin-associated muscle symptoms, and we found it in about 30% of people who have them. I have them myself and was motivated to study it because I thought this was an interesting question.”
The results were published online in JAMA Cardiology.
SAMS by baseline 25-OHD
The substudy included 2,083 patients who initiated statin therapy after randomization and were surveyed in early 2016 about their statin use and muscle symptoms.
Two-thirds, or 1,397 patients, had 25-hydroxy vitamin D (25-OHD) measured at baseline, with 47% having levels < 30 ng/mL and 13% levels < 20 ng/mL.
Serum 25-OHD levels were virtually identical in the two treatment groups (mean, 30.4 ng/mL; median, 30.0 ng/mL). The frequency of SAMS did not differ between those assigned to vitamin D or placebo (28% vs. 31%).
The odds ratios for the association with vitamin D on SAMS were:
- 0.86 in all respondents with 25-OHD measured (95% CI, 0.69-1.09).
- 0.87 in those with levels ≥ 30 ng/mL (95% CI, 0.64-1.19).
- 0.85 with levels of 20-30 ng/mL (95% CI, 0.56-1.28).
- 0.93 with levels < 20 ng/mL (95% CI, 0.50-1.74).
The test for treatment effect modification by baseline serum 25-OHD level was not significant (P for interaction = .83).
In addition, the rate of muscle symptoms was similar between participants randomized to vitamin D and placebo when researchers used a cutpoint to define low 25-OHD of < 30 ng/mL (27% vs. 30%) or < 20 ng/mL (33% vs. 35%).
“We didn’t find any evidence at all that the people who came into the study with low levels of vitamin D did better with the supplement in this case,” Dr. Hlatky said. “So that wasn’t the reason we didn’t see anything.”
Critics may suggest the trial didn’t use a high enough dose of vitamin D, but both Dr. Hlatky and Dr. Stone say that’s unlikely to be a factor in the results because 2,000 IU/d is a substantial dose and well above the recommended adult daily dose of 600-800 IU.
They caution that the substudy wasn’t prespecified, was smaller than the parent trial, and did not have a protocol in place to detail SAMS. They also can’t rule out the possibility that vitamin D may have an effect in patients who have confirmed intolerance to multiple statins, especially after adjustment for the statin type and dose.
“If you’re taking vitamin D to keep from having statin-associated muscle symptoms, this very carefully done substudy with the various caveats doesn’t support that and that’s not something I would give my patients,” Dr. Stone said.
“The most important thing from a negative study is that it allows you to focus your attention on things that may be much more productive rather than assuming that just giving everybody vitamin D will take care of the statin issue,” he added. “Maybe the answer is going to be somewhere else, and there’ll be a lot of people I’m sure who will offer their advice as what the answer is but, I would argue, we want to see more studies to pin it down. So people can get some science behind what they do to try to reduce statin-associated muscle symptoms.”
Paul D. Thompson, MD, chief of cardiology emeritus at Hartford (Conn.) Hospital, and a SAMS expert who was not involved with the research, said, “This is a useful publication, and it’s smart in that it took advantage of a study that was already done.”
He acknowledged being skeptical of a beneficial effect of vitamin D supplementation on SAMS, because some previous data have been retracted, but said that potential treatments are best tested in patients with confirmed statin myalgia, as was the case in his team’s negative trial of CoQ10 supplementation.
That said, the present “study was able to at least give some of the best evidence so far that vitamin D doesn’t do anything to improve symptoms,” Dr. Thompson said. “So maybe it will cut down on so many vitamin D levels [being measured] and use of vitamin D when you don’t really need it.”
The study was sponsored by the Hyperlipidemia Research Fund at Northwestern University. The VITAL trial was supported by grants from the National Institutes of Health, and Quest Diagnostics performed the laboratory measurements at no additional costs. Dr. Hlatky reports no relevant financial relationships. Dr. Stone reports a grant from the Hyperlipidemia Research Fund at Northwestern and honorarium for educational activity for Knowledge to Practice. Dr. Thompson is on the executive committee for a study examining bempedoic acid in patients with statin-associated muscle symptoms.
A version of this article first appeared on Medscape.com.
HDL cholesterol not linked to CHD risk in Blacks: REGARDS
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
IRONMAN galvanizes case for IV iron repletion in heart failure
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.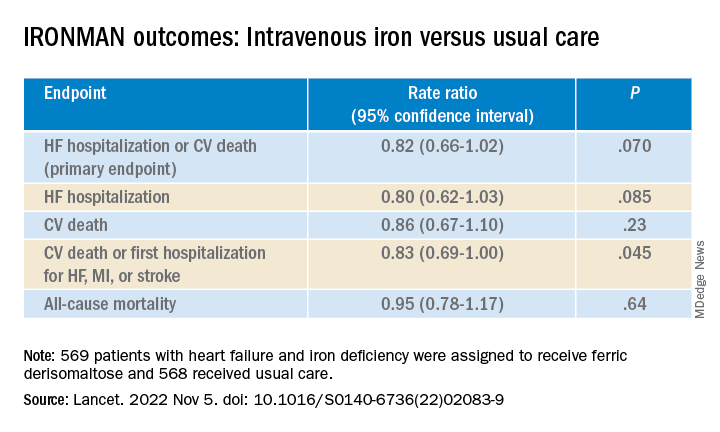
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
CHICAGO – Another major study appears to back the use of intravenous iron repletion in patients with heart failure (HF) and iron deficiency, strengthening largely consistent evidence, researchers say, that the treatment may improve symptoms and prevent some HF-related hospital admissions.
To be sure, the IRONMAN trial, which compared intravenous iron versus usual care in such patients – most with reduced ejection fraction and not hospitalized – failed to show a benefit for its primary endpoint. The 18% reduction in risk for HF hospitalization or cardiovascular (CV) death seen in the trial, however encouraging, can only be called a trend (P = .07).
But the intervention showed signs of benefit for some secondary endpoints, including quality of life scores, and hinted at such an effect on HF hospitalization. Risk for the latter endpoint dropped 20% (P = .085) over a median follow-up of 2.7 years.
The findings “build upon the other data we have that correcting iron deficiency can help improve well-being, and particularly reduce the risk of hospitalization, in a broad range of [HF] patients,” said Paul Kalra, MD, of the University of Glasgow and Portsmouth (England) Hospitals University NHS Trust.
The tested regimen “was well tolerated with no safety concerns” and offers “reassurance about the long-term safety” of the intravenous iron it used, ferric derisomaltose (MonoFerric), in patients with HF, Dr. Kalra said at a media briefing on the trial.
The remarks preceded his formal presentation of IRONMAN at the American Heart Association scientific sessions. Dr. Kalra is also lead author on the trial’s publication in The Lancet.
IRONMAN strengthens the base of evidence supporting intravenous iron in HF with iron deficiency, especially chronic HF in outpatients, Dr. Kalra and others said. It also supports efficacy for a form of intravenous iron not previously tested in a major HF trial.
Still, “the totality of data are now supporting intravenous iron per se,” regardless of the iron agent used, said Dr. Kalra. But ferric derisomaltose may have dosing advantages, he observed, “and we’ve now got these long-term safety data.”
The strongest prior support for intravenous iron in HF came from hospitalized patients who received it as ferric carboxymaltose (Ferinject) and were followed only 12 months. That was in the AFFIRM-AHF trial, published 2 years ago, which also missed its primary endpoint – the same one used in IRONMAN. Some outcomes in the two trials were similar.
The risk for HF hospitalization or CV death for intravenous iron therapy, compared with usual care, in AFFIRM-AHF fell 21% (P = .059), missing significance but apparently driven by a 26% drop in risk for HF readmissions (P = .013). But neither that trial nor IRONMAN suggested a benefit for CV mortality on its own.
The COVID effect
In IRONMAN, Dr. Kalra said, usual care could include oral iron supplementation, which 17% of patients in the control group received. That could potentially have kept the intravenous iron group from making a better showing for the primary endpoint, he proposed.
And some iron doses and other treatments were missed by a substantial number of patients in both groups who entered the trial after the United Kingdom’s national lockdown in response to the COVID-19 pandemic, he observed. “Patients were not able to come into hospitals for research visits, or in fact when they were able, may not have wanted to.”
So, the group conducted a “prespecified” sensitivity analysis that excluded the 9% of patients enrolled by the end of March 2020, about the time of the first lockdown, and followed the remainder for another 6 months.
In that analysis, risk for HF hospitalization or CV death declined 24% in the intravenous iron group, a marginal but significant result (P = .047) that was dominated by an improvement in HF hospitalizations.
Effects on guidelines
The intravenous iron recommendations in the European HF guidelines refer only to ferric carboxymaltose without mentioning other forms, such as ferric derisomaltose, “but this is now a class effect given the similarities between AFFIRM-AHF and IRONMAN,” said Gregory D. Lewis, MD, Mass General Brigham, Boston, invited discussant for Dr. Kalra’s presentation at the AHA session.
“In the United States, we relegate IV iron to improvement in functional capacity as a comorbidity of heart failure. Perhaps this role will expand,” added Dr. Lewis, who is medical director of his center’s heart transplant program.
He also wondered aloud whether the purported clinical benefits of intravenous iron in HF patients with iron deficiency, not as yet supported by a significant primary-endpoint showing in one of the major trials, currently justify expansion of its use in practice.
“With the benefits of IV iron on exercise capacity and quality of life, and the safety of administering high doses of IV iron,” potentially reducing HF polypharmacy, he noted, “should we be considering IV iron more commonly for utilization in our patients even if we find that heart failure hospitalizations and mortality are only modestly improved?”
IRONMAN “asked whether there’s benefit to IV iron in the longer term,” Kiran Musunuru, MD, PhD, MPH, University of Pennsylvania,Philadelphia, observed at the media briefing. As the trial was reported, “that does in fact, seem to be the case,” said Dr. Musunuru, who was not involved in IRONMAN.
Therefore, he said, “this study reinforces the message that we should be routinely monitoring our heart failure patients for iron deficiency and supplementing them as needed.”
A commentary linked to the IRONMAN publication agreed. The trial “increases the evidence base for the treatment of iron deficiency with intravenous iron supplementation,” wrote the editorialists, led by Theresa A. McDonagh, MD, King’s College Hospital and School of Cardiovascular Sciences, London.
Patients with acute or chronic HF, iron deficiency, and reduced or mildly reduced ejection fractions “should be offered treatment with intravenous iron to reduce their risk of hospital admission for heart failure,” they concluded.
Mostly reduced-EF outpatients
The open-label, blinded-endpoint IRONMAN trial, conducted at 70 centers in the United Kingdom, entered adults with HF, ejection fractions 45% or lower within the previous 2 years, and iron deficiency defined as transferrin saturation less than 20% or serum ferritin levels below 100 mcg/L, the report states. They were either hospitalized for HF, had such a hospitalization within the past 6 months, or were outpatients with elevated natriuretic peptide levels; the third category accounted for two thirds of the trial population.
Of the 1,137 randomized patients, 569 were assigned to receive intravenous ferric derisomaltose at weight- and hemoglobin-adjusted dosages; 568 went to the usual-care group.
Those receiving intravenous iron visited the trial clinic 4 weeks later and then every 4 months. At those visits, they received a round of ferric derisomaltose if their ferritin levels were below 100 mcg/L, or 400 mcg/L or lower if transferrin saturation was below 25%, the published report states.
Mean scores on the Minnesota Living with Heart Failure Questionnaire improved by a marginally significant 3.33 points (P = .050) at 4 months in the intravenous iron group. The gain receded to a nonsignificant 2.57 points by 20 months (P = .23).
In COVID-related sensitivity analysis, the intravenous iron group showed a significant benefit for the primary endpoint and a trend for improved HF hospitalizations.
- HF hospitalization or CV death: RR, 0.76 (95% confidence interval, 0.58-1.00; P = .047)
- HF hospitalization: RR 0.76 (95% CI, 0.56-1.03; P = .077)
Fewer patients in the intravenous iron group experienced serious cardiac adverse events, 36% compared with 43% in for those on usual care, P = .016.
The recently updated European Society of Cardiology guidelines for HF made it a class 1 recommendation to assess iron status in every patient, Kalra observed. “It doesn›t specify how frequently, but I think we should be thinking about every 4-6 months.”
Dr. Kalra disclosed receiving research grants from Pharmacosmos; and consulting or lecturing for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Pfizer, Pharmacosmos, Servier, and Vifor Pharma. Dr. Musunuru disclosed significant ownership interest in Verve Therapeutics and Variant Bio. Dr. Lewis disclosed relationships with NXT, American Regent, and RIVUS; and receiving research grants from Cytokinetics and Amgen.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Medical school culinary medicine programs grow despite limited funding
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
The way he sees it, the stakes couldn’t be higher. He believes doctors need to see food as medicine to be able to stem the tide of chronic disease.
About 6 in 10 adults in the United States live with chronic diseases, according to the Centers for Disease Control and Prevention, costing $4.1 trillion in annual health care costs. Adult obesity rates are rising, as are obesity-related conditions such as heart disease, stroke, type 2 diabetes, and certain types of cancer.
To turn the tide, Dr. Marvasti created a culinary medicine program in 2020 in collaboration with the University of Arizona Cooperative Extension and local chefs.
Dr. Marvasti, who is board certified in family medicine, graduated from the University of Arizona, Phoenix, where he serves as the director of the medical school’s Culinary Medicine Program.
The program offers an elective course for third- and fourth-year medical students, which introduces the evidence-based field of culinary medicine. Dr Marvasti’s goal is for the course to teach students how to use this science and the joy of cooking to improve long-term health outcomes for their patients.
As part of Dr. Marvasti’s program, students learn cooking fundamentals through chef demonstrations and hands-on practice – to teach students how food can be used to prevent and treat many chronic diseases.
One of the dishes students learn to make includes a quinoa salad made with cucumber, onion, bell peppers, corn, cherry tomatoes, beans, garlic, olive oil, and lemon juice. Another recipe includes a healthier take on dessert: Dark chocolate mousse made with three large, ripe avocados, dark chocolate powder, three tablespoons of agave or maple, coconut cream, nondairy milk, salt, and vanilla. Dr. Marvasti and his team are set to build out the existing program to develop additional resources for medically underserved and rural communities in Arizona, according to a statement from the university. These plans will be funded by a $750,000 grant from Novo Nordisk.
“We’re going to develop an open education curriculum to share, so it’s open access to everyone,” said Dr. Marvasti, who is also director of Public Health, Prevention and Health Promotion and an associate professor at the university. “It can be adaptable at the undergraduate, graduate, and postgraduate level.”
Dr. Marvasti and his colleagues at the University of Arizona aren’t alone. In fact, culinary medicine programs are sprouting some serious legs.
Culinary medicine programs catch on
Jaclyn Albin, MD, CCMS, an associate professor in the departments of internal medicine and pediatrics at UT Southwestern Medical Center, Dallas, conducted a scoping review of the literature on culinary medicine programs for medical students.* Her purpose was to learn how the programs were structured and how they assessed student knowledge and attitudes regarding nutrition counseling for patients.
Dr. Albin and her colleagues performed an initial literature search between June 1 and Aug. 1, 2020, of papers published between Jan. 1, 2012, and Aug. 1, 2020 – excluding some newer programs such as the one at the University of Arizona. The results of their research were published in Academic Medicine.
Ultimately, the authors identified and examined 34 programs offering medical student–focused culinary medicine courses.
Program instructors typically included a team of physicians, dietitians, chefs, and other professionals, the study found.
Most program participants exclusively taught medical students, though the training years of participants varied among programs, and they included first-, second-, third-, and fourth-year students. Some programs allowed students from outside their respective medical school to participate in the trainings.
As for the formats of the program, most included cohorts of 10-20 students attending multiple 2- to 3-hour sessions over the course of several months. The University of Alabama at Birmingham offers one of the longest courses, which spans 4-5 months, according to the paper. In contrast, the University of Rochester (N.Y.) program offers only a 1-day lab divided into four sessions, with each session lasting about 2 hours.
The culinary medicine programs’ course sessions tended to include a 10- to 30-minute didactic session involving videos, research articles, culinary theories, and other lectures, a 60- to 90-minute hands-on cooking session, and a 30-minute discussion around nutrition, culture, and patient care.
Most programs used pre- and post-program surveys to evaluate outcomes, though results varied between programs, according to the study. While each program evaluation had different metrics, the surveys generally revealed students felt more confident discussing dietary interventions with patients and in their own cooking skills following completion.
Course correction
Most of those programs are unfunded or minimally funded, Dr. Albin said.
Her own program, which is immensely popular with medical students, is one she teaches on a volunteer basis.
“I do this for free, in the evenings, because I believe in it,” she said.
Medical school education real estate is limited, so convincing medical schools to add something to the curriculum is difficult, Dr. Albin noted.
But it’s worth it, she said, because nutrition is the underpinning of so many diseases.
“Food is the top risk factor for early death in the U.S.,” Dr. Albin said. “I like to say that five times in a row. People have not digested it.”
During her culinary medicine courses, she also asks her medical students: “Who is comfortable in the kitchen?” Some sheepishly raise their hands, she said. Some don’t. Many don’t know anything about cooking.
Then she teaches students about healthy food and how to make it. As part of her program, medical students are given a pantry starter kit with olive oil and a variety of spices to take home and use.
Some recipes Dr. Albin teaches includes mango chili shrimp salad with lime vinaigrette, eggplant sliders, yellow vegetable curry, and strawberry banana chia pudding.
“If you figure out how to do it for your own busy, everyday life, you are now empowered to tell someone else about it,” she said.
A dietitian’s involvement
Milette Siler, RD, LD, CCMS, works with Dr. Albin to educate medical students and patients about food as medicine. A significant chunk of her job involves teaching future doctors what dietitians do.
When the class starts, many students don’t know two of the five basic things dietitians do, Ms. Siler said. By the end of the class, all students know what a dietitian does.
That’s important as students go on to become doctors.
“For us to remove barriers to care, we have to acknowledge most patients’ entry into health care is their physician,” she said. “The dietitian is often a referral. Doctors need to know enough to do no harm.”
Clinicians are often siloed, she said, and the key to better serving patients is partnership, transparency, and relationships. “I think everybody is at a point where everyone is saying what we’re doing isn’t working,” she said. “The American public deserves better, physicians deserve better, and clinicians deserve better.”
Popular with students
While the old guard has been slow to embrace the shift, her students have helped drive the growth of the culinary medicine field, Dr. Albin said.
“They are not settling for the inadequacy that somehow the rest of us did,” she continued. “I’m so hopeful for the future of the health system. We have a generation of people who will not stand for neglecting the most vital elements.”

Lyndon Bui, a second-year medical student at the University of Arizona, Phoenix, is an example of one of these people.
As a member of a culinary medicine interest group on campus, he said, he has learned a lot about the importance of diet for long-term health. This has given him confidence to talk about food and nutrition.
His group does cooking demos at the Phoenix Farmers Market using food from various local vendors. They usually make a salad from local greens and cook seasonal veggies in a stir fry, he said.
They’ve previously made salad with microgreens – young seedlings of edible vegetables and herbs – and pomegranate seeds with a honey mustard vinaigrette, eggplant or cucumber, and hummus on pita bread, as well as almond butter and honey sandwiches, according to the university.
The group also talks with people in the community, answers questions, and learns about community needs.
Mr. Bui’s participation in this group has helped him cultivate a passion for community outreach that he wants to incorporate into his career.
“I feel like I have the knowledge to provide better advice to patients,” he said. “Knowing all these things about food, I feel more comfortable talking about it and more inclined to refer to a dietitian when maybe I wouldn’t have before.”
Family physician applauds culinary medicine programs
When Angie Neison, MD, CCMS, went to medical school, she was surprised there wasn’t more education on nutrition.
In fact, on average, physicians receive less than 20 hours of nutrition education, according to the University of Arizona.
Now 15 years into her career as a family physician, Dr. Neison says nutrition is a huge part of her practice. She spends time working to bust myths about nutrition for her patients – including that healthy food is boring and bland, that making it is time consuming, and that healthy food is expensive. She also spends time teaching aspects of culinary medicine to her colleagues – many of whom are well into their careers – so they can better serve their patients.
It’s worth it to spend time learning about nutrition, she said, whether that’s as a medical student in a culinary medicine program or a practicing physician taking additional courses.
Nutrition education in medical school hasn’t been a priority, she said, maybe because there is so much to learn, or maybe because there is no money to be made in prevention.
“If doctors learn it, they are able to better guide patients,” she said.
Correction, 11/29/22: An earlier version of this article misstated Dr. Albin's institution.
FROM ACADEMIC MEDICINE
Statins boost glycemia slightly, but CVD benefits prevail
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
CHICAGO – A new, expanded meta-analysis confirmed the long-known effect that statin treatment has on raising blood glucose levels and causing incident diabetes, but it also documented that these effects are small and any risk they pose to statin users is dwarfed by the cholesterol-lowering effect of statins and their ability to reduce risk for atherosclerotic cardiovascular disease (ASCVD).
This meta-analysis of 23 trials with a total of more than 150,000 participants showed that statin therapy significantly increased the risk for new-onset diabetes and worsening glycemia, driven by a “very small but generalized increase in glucose,” with a greater effect from high-intensity statin regimens and a similar but somewhat more muted effect from low- and moderate-intensity statin treatment, David Preiss, MBChB, PhD, reported at the American Heart Association scientific sessions.
Dr. Preiss also stressed that despite this, “the cardiovascular benefits of statin therapy remain substantial and profound” in people regardless of whether they have diabetes, prediabetes, or normoglycemia when they start statin treatment, noting that the impact of even high-intensity statin treatment is “absolutely tiny” increases in hemoglobin A1c and blood glucose.
“This does not detract from the substantial benefit of statin treatment,” declared Dr. Preiss, a metabolic medicine specialist and endocrinologist at Oxford (England) University.
Small glycemia increases ‘nudge’ some into diabetes
The data Dr. Preiss reported showed that high-intensity statin treatment (atorvastatin at a daily dose of at least 40 mg, or rosuvastatin at a daily dose of at least 20 mg) led to an average increase in A1c levels of 0.08 percentage points among people without diabetes when their treatment began and 0.24 percentage points among people already diagnosed with diabetes. Blood glucose levels rose by an average of 0.04 mmol/L (less than 1 mg/d) in those without diabetes, and by an average 0.22 mmol/L (about 4 mg/dL) in those with diabetes. People who received low- or moderate-intensity statin regimens had significant but smaller increases.
“We’re not talking about people going from no diabetes to frank diabetes. We’re talking about [statins] nudging a very small number of people across a diabetes threshold,” an A1c of 6.5% that is set somewhat arbitrarily based on an increased risk for developing retinopathy, Dr. Preiss said. ”A person just needs to lose a [daily] can of Coke’s worth of weight to eliminate any apparent diabetes risk,” he noted.
Benefit outweighs risks by three- to sevenfold
Dr. Preiss presented two other examples of what his findings showed to illustrate the relatively small risk posed by statin therapy compared with its potential benefits. Treating 10,000 people for 5 years with a high-intensity statin regimen in those with established ASCVD (secondary prevention) would result in an increment of 150 extra people developing diabetes because of the hyperglycemic effect of statins, compared with an expected prevention of 1,000 ASCVD events. Among 10,000 people at high ASCVD risk and taking a high-intensity statin regimen for primary prevention 5 years of treatment would result in roughly 130 extra cases of incident diabetes while preventing about 500 ASCVD events.
In addition, applying the new risk estimates to the people included in the UK Biobank database, whose median A1c is 5.5%, showed that a high-intensity statin regimen could be expected to raise the prevalence of those with an A1c of 6.5% or greater from 4.5% to 5.7%.
Several preventive cardiologists who heard the report and were not involved with the analysis agreed with Dr. Preiss that the benefits of statin treatment substantially offset this confirmed hyperglycemic effect.
Risk ‘more than counterbalanced by benefit’
“He clearly showed that the small hyperglycemia risk posed by statin use is more than counterbalanced by its benefit for reducing ASCVD events,” commented Neil J. Stone, MD, a cardiologist and professor of medicine at Northwestern University, Chicago. “I agree that, for those with prediabetes who are on the road to diabetes with or without a statin, the small increase in glucose with a statin should not dissuade statin usage because the benefit is so large. Rather, it should focus efforts to improve diet, increase physical activity, and keep weight controlled.”
Dr. Stone also noted in an interview that in the JUPITER trial, which examined the effects of a daily 20-mg dose of rosuvastatin (Crestor), a high-intensity regimen, study participants with diabetes risk factors who were assigned to rosuvastatin had an onset of diabetes that was earlier than people assigned to placebo by only about 5.4 weeks, yet this group had evidence of significant benefit.
“I agree with Dr. Preiss that the benefits of statins in reducing heart attack, stroke, and cardiovascular death far outweigh their modest effects on glycemia,” commented Brendan M. Everett, MD, a cardiologist and preventive medicine specialist at Brigham and Women’s Hospital in Boston. “This is particularly true for those with preexisting prediabetes or diabetes, who have an elevated risk of atherosclerotic events and thus stand to derive more significant benefit from statins. The benefits of lowering LDL cholesterol with a statin for preventing seriously morbid, and potentially fatal, cardiovascular events far outweigh the extremely modest, or even negligible, increases in the risk of diabetes that could be seen with the extremely small increases in A1c,” Dr. Everett said in an interview.
The new findings “reaffirm that there is a increased risk [from statins] but the most important point is that it is a very, very tiny difference in A1c,” commented Marc S. Sabatine, MD, a cardiologist and professor at Harvard Medical School, Boston. “These data have been known for quite some time, but this analysis was done in a more rigorous way.” The finding of “a small increase in risk for diabetes is really because diabetes has a biochemical threshold and statin treatment nudges some people a little past a line that is semi-arbitrary. It’s important to be cognizant of this, but it in no way dissuades me from treating patients aggressively with statins to reduce their ASCVD risk. I would monitor their A1c levels, and if they go higher and can’t be controlled with lifestyle we have plenty of medications that can control it,” he said in an interview.
No difference by statin type
The meta-analysis used data from 13 placebo-controlled statin trials that together involved 123,940 participants and had an average 4.3 years of follow-up, and four trials that compared one statin with another and collectively involved 30,734 participants with an average 4.9 years of follow-up.
The analyses showed that high-intensity statin treatment increased the rate of incident diabetes by a significant 36% relative to controls and increased the rate of worsening glycemia by a significant 24% compared with controls. Low- or moderate-intensity statin regimens increased incident diabetes by a significant 10% and raised the incidence of worsening glycemia by a significant 10% compared with controls, Dr. Preiss reported.
These effects did not significantly differ by type of statin (the study included people treated with atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin), nor across a variety of subgroups based on age, sex, race, body mass index, diabetes risk, renal function, cholesterol levels, or cardiovascular disease. The effect was also consistent regardless of the duration of treatment.
Dr. Preiss also downplayed the magnitude of the apparent difference in risk posed by high-intensity and less intense statin regimens. “I suspect the apparent heterogeneity is true, but not quite as big as what we see,” he said.
The mechanisms by which statins have this effect remain unclear, but evidence suggests that it may be a direct effect of the main action of statins, inhibition of the HMG-CoA reductase enzyme.
The study received no commercial funding. Dr. Preiss and Dr. Stone had no disclosures. Dr. Everett has been a consultant to Eli Lilly, Gilead, Ipsen, Janssen, and Provention. Dr. Sabatine has been a consultant to Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol-Myers Squibb, DalCor, Dr Reddy’s Laboratories, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics.
AT AHA 2022
Dietary supplements hyped as LDL cholesterol lowering are a bust: SPORT
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a randomized trial of adults without cardiovascular disease but at increased cardiovascular risk.
In contrast, those who took the low dose of a high-potency statin in the eight-arm comparative study showed a significant 38% drop in LDL cholesterol levels over 28 days, a performance that blew away the six supplements containing fish oil, cinnamon, garlic, turmeric, plant sterols, or red yeast rice.
The supplements showed little or no effect on any measured lipid biomarkers, which also included total cholesterol and triglycerides, or C-reactive protein (CRP), which reflects systemic inflammation.
The findings undercut the widespread heart-health marketing claims for such supplements and could potentially restore faith in statins for the many patients looking for alternatives, researchers say.
“We all see patients that have their medication lists littered with dietary supplements,” observed Luke J. Laffin, MD, of the Cleveland Clinic Foundation. And it’s more than just heart patients who use them.
Almost $50 billion is spent on dietary supplements annually in the United States, and recent data suggest that more than three-fourths of the population use them, 18% of those based on specious heart-health claims, Dr. Laffin said in a Nov. 6 presentation at the American Heart Association scientific sessions.
The findings of the Supplements, Placebo, or Rosuvastatin Study (SPORT) and how they are framed for the public “are important for public health,” he said.
“As cardiologists, primary care doctors, and others, we really should use these results to have evidence-based discussions with patients” regarding the value of even low-dose statins and the supplements’ “lack of benefit,” said Dr. Laffin, lead author on the SPORT publication, which was published the same day in the Journal of the American College of Cardiology.
Patients assigned to low-dose rosuvastatin showed a mean 24.4% drop in total cholesterol levels over 28 days, the study’s primary endpoint. That differed from the placebo group and those for each supplement at P < .001.
They also averaged a 19.2% decrease in serum triglycerides, P < .05 for all group comparisons. None of the six supplements was significantly different from placebo for change in levels of either total cholesterol or triglycerides.
Nor were there significant differences in adverse events across the groups; there were no adverse changes in liver or kidney function tests or glucose levels; and there were no signs of musculoskeletal symptoms, the published report notes.
How to message the results
The SPORT trial is valuable for “addressing the void of data on supplements and cardiovascular health,” Chiadi E. Ndumele, MD, PhD, Johns Hopkins University, Baltimore, said as the invited discussant following Dr. Laffin’s presentation.
But they also send a reassuring message about statins, he noted. In a recent study of statin-nonadherent patients, 80% “were worried about statin side effects as the primary reason for not taking their statin, and 72% preferred using natural supplements instead of taking their prescription therapy,” Dr. Ndumele said. “The reason for this is clearly mistrust, misinformation, and a lack of evidence.”
The next step, he proposed, should be to get the study’s positive message about statins to the public, and especially patients “who are hesitant about statin use.” The current study “underscores the fact that using a low dose of a high-potency statin is associated with a very, very low risk of side effects.”
At a media briefing on SPORT, Amit Khera, MD, agreed the randomized trial provides some needed evidence that can be discussed with patients. “If someone’s coming to see me for cholesterol, we can say definitively now, at least there is data that these [supplements] don’t help your cholesterol and statins do.” Dr. Khera directs the preventive cardiology program at University of Texas Southwestern Medical Center, Dallas.
“I think for those who are there very specifically to lower their cholesterol, hopefully this will resonate,” he said.
“I personally didn’t see a lot of harms in using these supplements. But I also didn’t see any benefits,” Dr. Khera told this news organization.
“Now, if you’re taking them for other reasons, so be it. But if you need to lower your cholesterol for cardiovascular health reasons,” he said, “you need to know that they are minimally to not effective at all.”
But such supplements still “are not without harm,” Dr. Laffin proposed at the press conference. For example, they have potential for drug-drug interactions, “not only with cardiovascular medicines, but those taken for other reasons,” he said. “There are 90,000 supplements on the market in the United States today, and there are all kinds of potential safety issues associated with them.”
In patient discussions, Dr. Laffin said, “I do not think it’s good enough to say, you can waste your money [on supplements] as long as you’re taking your statin. These can actually be harmful in certain situations.”
SPORT, described as a single-center study, randomly assigned 199 participants from “throughout the Cleveland Clinic Health System in northeast Ohio” to one of the eight treatment groups. The investigators were blinded to treatment assignments, Dr. Laffin reported.
High adherence
Entry criteria included age 40 to 75 years with no history of cardiovascular disease, LDL-cholesterol from 70 to 189 mg/dL, and a 5%-20% 10-year risk of atherosclerotic cardiovascular disease by the pooled cohort equations. The predominantly White cohort averaged 64.4 years in age and 59% were women.
They were assigned to receive rosuvastatin 5 mg daily, placebo, or daily doses of supplements, with 25 patients per group, except the fish-oil group, which comprised 24 patients.
The daily supplement dosages were 2,400 mg for fish oil (Nature Made); 2,400 mg for cinnamon (NutriFlair), 5,000 mcg allicin for the garlic (Garlique), 4,500 mg for turmeric curcumin (BioSchwartz), 1,600 mg plant sterols (CholestOff Plus, Nature Made), and 2,400 mg red yeast rice (Arazo Nutrition).
Adherence to the assigned regimens was high, Dr. Laffin said, given that only four participants took less than 70% of their assigned doses.
Levels of LDL cholesterol in the statin group fell by 37.9% in 28 days, and by 35.2% relative to the placebo group (P < .001 for both differences), whereas any changes in LDL cholesterol among patients taking the most supplements were not significantly different from the placebo group. Of note, LDL cholesterol levels rose 7.8% (P = .01) compared with placebo among the group assigned to the garlic supplement.
Rosuvastatin had no apparent effect on HDL cholesterol levels, nor did most of the supplements; but such levels in patients taking the plant sterol supplement decreased by 7.1% (P = .02) compared to placebo and by 4% (P = .01) compared to the statin group.
None of the noncontrol groups, including those assigned to rosuvastatin, showed significant changes in high-sensitivity CRP levels compared with the placebo group. The lack of rosuvastatin effect on the inflammatory biomarker, the researchers speculated, is probably explained by the statins’ low dose as well as the limited size of the trial population.
There were two serious adverse events, including one deep venous thrombosis in the placebo group and a liver adenocarcinoma in a patient assigned to fish oil who “had not yet taken any of the study drug at the time of the serious adverse event,” the published report notes.
It remains open whether any of the assigned regimens could show different results over the long term, Dr. Laffin said. The SPORT trial’s 28-day duration, he said, “may not have fully captured the impact of supplements on lipid and inflammatory biomarkers.”
Nor is it known whether the supplements can potentially affect clinical outcomes. But “you could make an argument that it would be unethical” to randomize similar patients to a placebo-controlled, cardiovascular outcomes trial comparing the same six supplements and a statin.
Dr. Laffin has disclosed consulting or serving on a steering committee for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; receiving research funding from AstraZeneca; and having ownership interest in LucidAct Health and Gordy Health. Dr. Ndumele and Dr. Khera have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AHA 2022
New trial suggests CV benefit with EPA: RESPECT-EPA
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
FROM AHA 2022