User login
Triglyceride-lowering fails to show CV benefit in large fibrate trial
Twenty-five percent reduction has no effect
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
Twenty-five percent reduction has no effect
Twenty-five percent reduction has no effect
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
CHICAGO – Despite a 25% reduction in triglycerides (TGs) along with similar reductions in very-low-density lipoprotein (VLDL), and remnant cholesterol, a novel agent failed to provide any protection in a multinational trial against a composite endpoint of major adverse cardiovascular events (MACE) in patients with type 2 diabetes.
“Our data further highlight the complexity of lipid mediators of residual risk among patients with insulin resistance who are receiving statin therapy,” reported Aruna Das Pradhan, MD, of Harvard Medical School, Boston, and Queen Mary University, London.
It is the most recent in a series of trials that have failed to associate a meaningful reduction in TGs with protection from a composite MACE endpoint. This is a pattern that dates back 20 years, even though earlier trials did suggest that hypertriglyceridemia was a targetable risk factor.
No benefit from fibrates seen in statin era
“We have not seen a significant cardiovascular event reduction with a fibrate in the statin era,” according to Karol Watson, MD, PhD, director of the UCLA Women’s Cardiovascular Health Center, Los Angeles.
In the statin era, which began soon after the Helsinki Heart Study was published in 1987, Dr. Watson counted at least five studies with fibrates that had a null result.
In the setting of good control of LDL cholesterol, “fibrates have not been shown to further lower CV risk,” said Dr. Watson, who was invited by the AHA to discuss the PROMINENT trial.
In PROMINENT, 10,497 patients with type 2 diabetes were randomized to pemafibrate, a peroxisome proliferator-activated receptor a (PPAR-a) agonist, or placebo. Pemafibrate is not currently available in North America or Europe, but it is licensed in Japan for the treatment of hypertriglyceridemia.
The primary efficacy endpoint of the double-blind trial was a composite endpoint of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death.
The patients were eligible if they had TG levels from 200 to 400 mg/dL and HDL cholesterol levels of 40 mg/dL or below. Pemafibrate in a dose of 0.2 mg or placebo were taken twice daily. About two-thirds had a prior history of coronary heart disease. The goal was primary prevention in the remainder.
After a median follow-up of 3.4 years when the study was stopped for futility, the proportion of patients reaching a primary endpoint was slightly greater in the experimental arm (3.60 vs. 3.51 events per 100 patient-years). The hazard ratio, although not significant, was nominally in favor of placebo (hazard ratio, 1.03; P = .67).
When events within the composite endpoint were assessed individually, there was no signal of benefit for any outcome. The rates of death from any cause, although numerically higher in the pemafibrate group (2.44 vs. 2.34 per 100 patient years), were also comparable.
Lipid profile improved as predicted
Yet, in regard to an improvement in the lipid profile, pemafibrate performed as predicted. When compared to placebo 4 months into the trial, pemafibrate was associated with median reductions of 26.2% in TGs, 25.8% in VLDL, and 25.6% in remnant cholesterol, which is cholesterol transported in TG-rich lipoproteins after lipolysis and lipoprotein remodeling.
Furthermore, pemafibrate was associated with a median 27.6% reduction relative to placebo in apolipoprotein C-III and a median 4.8% reduction in apolipoprotein E, all of which would be expected to reduce CV risk.
The findings of PROMINENT were published online in the New England Journal of Medicine immediately after their presentation.
The findings of this study do not eliminate any hope for lowering residual CV risk with TG reductions, but they do suggest the relationship with other lipid subfractions is complex, according to Salim S. Virani, MD, PhD, a professor of cardiology at Baylor College of Medicine, Houston.
“I think that the lack of efficacy despite TG lowering may be largely due to a lack of an overall decrease in the apolipoprotein B level,” speculated Dr. Virani, who wrote an editorial that accompanied publication of the PROMINENT results.
He noted that pemafibrate is implicated in converting remnant cholesterol to LDL cholesterol, which might be one reason for a counterproductive effect on CV risk.
“In order for therapies that lower TG levels to be effective, they probably have to have mechanisms to increase clearance of TG-rich remnant lipoprotein cholesterol particles rather than just converting remnant lipoproteins to LDL,” Dr. Virani explained in an attempt to unravel the interplay of these variables.
Although this study enrolled patients “who would be predicted to have the most benefit from a TG-lowering strategy,” Dr. Watson agreed that these results do not necessarily extend to other means of lowering TG. However, it might draw into question the value of pemafibrate and perhaps other drugs in this class for treatment of hypertriglyceridemia. In addition to a lack of CV benefit, treatment was not without risks, including a higher rate of thromboembolism and adverse renal events.
Dr. Das Pradhan reported financial relationships with Denka, Medtelligence, Optum, Novo Nordisk, and Kowa, which provided funding for this trial. Dr. Watson reported financial relationships with Amarin, Amgen, Boehringer-Ingelheim, and Esperion.
AT AHA 2022
ACC/AHA issues updated guidance on aortic disease
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
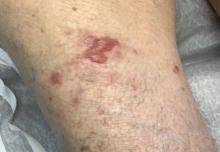
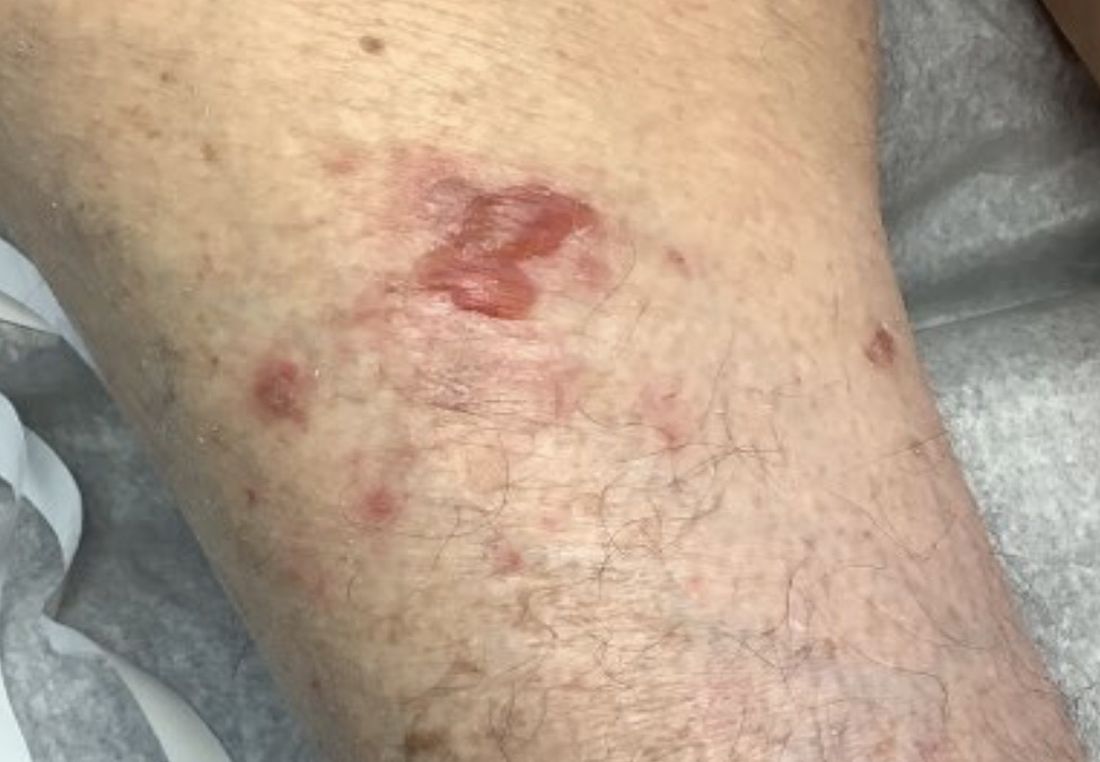
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
Combo thrombolytic approach fails to reduce ICH in stroke
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
A study evaluating a new approach using a combination of two thrombolytics designed to reduce bleeding risk in patients with acute ischemic stroke has not shown any benefit on the primary outcome of all intracranial hemorrhage (ICH).
However, there were some encouraging findings including a trend towards a reduction in symptomatic ICH, researchers report, and the combination approach did not show any depletion of fibrinogen levels, which suggests a potential lower bleeding risk.
“Although the main results of this study are neutral, we are encouraged that the combination approach with a low dose of alteplase followed by the new mutant pro-urokinase product looked as effective as full-dose alteplase alone, and there were some promising signs signaling a potential lower bleeding risk,” senior investigator, Diederik Dippel, MD, Erasmus University Medical Center, Rotterdam, the Netherlands, told this news organization.
The DUMAS study (Dual Thrombolytic Therapy With Mutant Pro-Urokinase and Low Dose Alteplase for Ischemic Stroke) was presented at the World Stroke Congress in Singapore by study coauthor Nadinda van der Ende, MD, also from Erasmus University Medical Center.
She pointed out that thrombolysis with intravenous alteplase increases the likelihood of a good outcome in acute ischemic stroke but can cause symptomatic intracranial hemorrhage, which can be associated with death and major disability.
Mutant pro-urokinase is a new thrombolytic agent, in development by Thrombolytic Science, Cambridge, Mass., formed by changing one amino acid in pro-urokinase to make it more stable. It is more fibrin specific than alteplase and therefore believed to have a lower risk of intracranial hemorrhage.
Fibrin is formed as the last step in the clotting process, and the precursor of fibrin in the blood is fibrinogen, Dr. van der Ende noted. Alteplase depletes fibrinogen, contributing to its increased bleeding risk, but mutant pro-urokinase is not believed to affect fibrinogen.
“Mutant pro-urokinase does not bind to intact fibrin. It only binds to fibrin that has already been primed by alteplase,” she explained.
The hypothesis behind the current study is that giving a small dose of alteplase will break down fibrin in the clot enough to expose the binding sites for mutant pro-urokinase, which can then be given to continue to lyse the clot.
As alteplase has a short half-life, it disappears quickly, and new fibrin is not affected. As mutant pro-urokinase can only lyse fibrin that is primed with alteplase, new hemostatic clots should stay intact. Animal studies have shown less bleeding from distant sites with this approach, Dr. van der Ende said.
The primary analysis of the phase 2 DUMAS study included 238 patients with mild ischemic stroke (median National Institutes of Health Stroke Scale [NIHSS] score 3) who met the standard criteria for IV alteplase.
They were randomized to alteplase alone at the regular dose of 0.9 mg/kg (max 90 mg) with a 10% bolus and the remaining given over 60 minutes; or to a combination of a 5-mg bolus of IV alteplase followed by mutant pro-urokinase at a dose of 40 mg given over 60 minutes.
The primary outcome was the rate of all intracranial hemorrhage (symptomatic and asymptomatic) detected by neuroimaging.
This occurred in 14% of patients in the full-dose alteplase group vs. 13% of patients in the combined alteplase/mutant pro-urokinase group, a nonsignificant difference: adjusted odds ratio, 0.99 (95% confidence interval, 0.46-2.14).
Secondary outcomes showed no significant differences in NIHSS scores at 24 hours or 5-7 days; functional outcome as measured by a shift analysis of the Modified Rankin Scale (mRS); final infarct volume; or perfusion deficit.
However, blood fibrinogen levels were not depleted and significantly higher in the alteplase/mutant pro-urokinase group than in the full-dose alteplase alone group.
In terms of safety, symptomatic ICH occurred in three patients in the alteplase group (3%) and in none (0%) in the combined alteplase/mutant pro-urokinase group; death occurred in 4% vs. 2% patients respectively; and major extracranial hemorrhage occurred in 1% in both groups.
Dr. Van der Ende concluded that the study showed an overall low rate of ICH; a combination of alteplase and mutant pro-urokinase was not superior to alteplase alone in reducing ICH rates in this population of patients with minor stroke; and mutant pro-urokinase appeared to be safe and, unlike alteplase, did not show any reduction in fibrinogen levels.
“We think the lack of an effect on fibrinogen with this new combination of a small alteplase bolus followed by mutant pro-urokinase infusion is promising,” Dr. Dippel commented. “The fact that there was no symptomatic ICH with the combination treatment is also encouraging. Although the primary endpoint of this trial was neutral, we still believe this is a very interesting approach, with the potential for reduced bleeding, compared with alteplase alone, but we need larger numbers to see an effect on outcomes.”
Dr. Dippel also pointed out that the study included only patients with minor stroke who were not eligible for endovascular therapy, and these patients have a low risk of a poor outcome and a low bleeding risk.
They are hoping to do another study in patients with more severe stroke, who have a higher bleeding risk and would have more to gain from this combination approach.
Because many patients with severe stroke now have immediate thrombectomy if they present to a comprehensive stroke center, a trial in severe stroke patients would have to be done in primary stroke centers, so if the patents are referred to thrombectomy, the thrombolytic would have a chance to work, Dr. Dippel added.
Commenting on the study for this news organization, Stefan Kiechl, MD, Medical University of Innsbruck (Austria), who is cochair of the World Stroke Congress scientific committee, said, “Alteplase is not fibrin specific, and also causes a degeneration of fibrinogen, which results in ‘fibrinogen depletion coagulopathy.’ It is assumed that 20%-40% of intracerebral bleeding after thrombolysis with alteplase is caused by this problem. DUMAS tests the combination of a substantially reduced alteplase [5 mg] dose plus mutant pro-urokinase to avoid this problem.”
The new thrombolysis protocol, however, did not result in a lower bleeding risk, compared to the comparator alteplase,” he added. “The main limitation of this study is that mainly patients with minor strokes were included. Patients with moderate and severe strokes, who have a substantial risk of bleeding, were not adequately addressed.”
The DUMAS trial was funded by an unrestricted grant from Thrombolytic Science, paid to the institution. Dr. Van der Ende and Dr. Dippel report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM WSC 2022
Uptake of high-sensitivity troponin assays lags in U.S. hospitals
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
Most hospitals in the United States have yet to transition from conventional to high-sensitivity cardiac troponin (hs-cTn) assays, despite their greater sensitivity for myocardial injury, a new National Cardiovascular Data Registry (NCDR) registry study indicates.
hs-cTn assays have been used in routine clinical practice in Europe, Canada, and Australia since 2010, but the first such assay did not gain approval in the United States until 2017. Although single-center studies have examined their efficacy and potential downstream consequences, few data exist on hs-cTn implementation nationally, explained study author Cian McCarthy, MB, BCh, BAO, Massachusetts General Hospital, Boston.
The results were published online in the Journal of the American College of Cardiology and will be presented Nov. 5 at the American Heart Association scientific sessions.
For the study, Dr. McCarthy and colleagues examined 550 hospitals participating in the NCDR Chest Pain-MI registry from January 2019 through September 2021.
Of the 251,000 patients included in the analysis (mean age, 64 years; 41.5% female), 155,049 had a non–ST-segment myocardial infarction (NSTEMI), 15,989 had unstable angina, and 79,962 had low-risk chest pain.
The hs-cTn assays included Roche Diagnostic’s Elecsys Gen5 STAT troponin T assay (23%); Abbott’s ARCHITECT STAT (17%); Beckman Coulter’s ACCESS (21%); and Siemens’ Atellica IM (18%), Dimension VISTA (14%), Dimension EXL (4%), and ADVIA Centaur (2%) troponin I assays.
During the study period, 11.5% of patients were evaluated with hs-cTn assays and the remainder were evaluated with conventional troponin assays. These patients were slightly older (65.0 vs. 64.0 years), more commonly White (83.1% vs. 79.9%), less likely to be of Hispanic or Latino ethnicity (8.9% vs. 10.0%), and less likely to be uninsured (6.8% vs. 8.3%; P for all < .001).
A slightly higher proportion of patients evaluated with hs-cTn assays were diagnosed with unstable angina (7.1% vs. 6.3%), a lower proportion with NSTEMI (61.1% vs. 61.9%), and a similar proportion with low-risk chest pain (31.8% vs. 31.9%) compared with those evaluated by conventional troponin assays.
Implementation, defined as at least 25% of patients evaluated by hs-cTn in each quarter, increased from 3.3% in the first quarter of 2019 to 32.6% in the third quarter of 2021 (P trend < .001).
Using higher implementation thresholds of at least 50% and 75% of patients evaluated by hs-cTn, the prevalence in 2021 was 28.9% and 24.7%, respectively.
“So still, the majority of the hospitals by the end of the third quarter 2021 were not using these assays,” Dr. McCarthy said.
Potential explanations for the slow uptake are that prospective comparative effectiveness trials of These assays have predominantly been in international populations and real-world data on U.S. implementation have been limited to integrated health networks at academic institutions.
Approval of several assays was also delayed and the study data cut off just before the October publication of the 2021 AHA/ACC Chest Pain guideline. “So, whether the chest pain guideline with the new class 1 recommendation for hs-cTn will lead to further uptake is something that will need to be looked at in the future,” he said.
Downstream testing
In adjusted analyses, hs-cTn use was associated with more echocardiography among patients with non-ST elevation–acute coronary syndrome (NSTE-ACS) (82.4% vs. 75.0%; odds ratio [OR], 1.43; 95% confidence interval [CI], 1.19-1.73), but not among those with low-risk chest pain (19.7% vs. 19.4%; OR, 0.93; 95% CI, 0.71-1.22) compared with conventional cTn assays.
Importantly, hs-cTn was not associated with a difference in stress testing or CT coronary angiography utilization.
Use of hs-cTn was associated with lower use of invasive coronary angiography among patients with low-risk chest pain (3.7% vs. 4.5%; OR, 0.73, 95% CI, 0.58-0.92) but similar use for NSTE-ACS (96.3% vs. 95.8%; OR, 0.99, 95% CI, 0.82-1.19).
Among patients with NSTE-ACS, there also was no difference in revascularization with percutaneous coronary intervention (PCI) (52.7% vs. 52.3%; OR, 0.99; 95% CI, 0.94-1.04) or coronary bypass graft surgery (9.4% vs. 9.1%; OR, 1.06; 95% CI, 0.94-1.18).
PCI (0.1% vs. 0.2%; P = .05) and bypass graft surgery (both 0.1%) were uncommon among patients with low-risk chest pain.
In-hospital mortality was similar among patients with low-risk chest pain evaluated using hs-cTn assays vs. conventional troponin assays (0% vs. 0.02%; P = .16) and among patients with NSTE-ACS (2.8% vs. 3.2%; OR, 0.98, 95% CI, 0.87-1.11).
Length of stay was slightly shorter with hs-cTn use for patients with low-risk chest pain (median, 5.8 vs. 6.2 hours; P < .001) and patients with NSTE-ACS (66.9 vs. 67.8 hours; P = .01).
“There was always a concern that maybe high-sensitivity cardiac troponin would dramatically increase testing and could even increase length of stay, but I think these data are reassuring, in that this study suggests high-sensitivity cardiac troponin is associated with a small reduction in length of stay and possibly more appropriate use of testing with echocardiography in STEMI and a reduction in invasive angiography in low-risk patients,” Dr. McCarthy said. “But the majority of hospitals haven’t implemented the assay.”
The authors pointed out that because registry entry of patients with low-risk chest pain and unstable angina is optional for participating sites, the percentage of patients with NSTEMI is higher than in typical chest pain analyses. This higher pretest probability for MI may thus affect post-test accuracy for a true positive result. “That stated, this is the exact scenario where higher sensitivity might be associated with favorable impact on utilization.”
Among other limitations: There was the potential for unmeasured confounders, the accuracy of diagnoses could not be confirmed, patients with type 2 MI were excluded from the registry, and post-discharge safety was not assessed.
“These data indicate further opportunities to more widely and effectively implement hs-cTn in the U.S. hospitals persist that could optimize care for patients with possible or definitive ACS,” Dr. McCarthy and colleagues concluded.
The study was funded by the American College of Cardiology’s National Cardiovascular Data Registry. Dr. McCarthy is supported by the National Heart, Lung, and Blood Institute and has received consulting income from Abbott Laboratories.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
Marital stress tied to worse outcome in young MI patients
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Severe marital stress was associated with worse recovery after myocardial infarction in a large U.S. cohort of married/partnered patients aged 55 years or younger.
Compared with patients who reported no or mild marital stress a month after their MI, patients who reported severe marital stress had worse physical and mental health, worse generic and cardiovascular quality of life, more frequent angina symptoms, and a greater likelihood of having a hospital readmission a year later.
These findings held true after adjusting for gender, age, race/ethnicity, and baseline health status (model 1) and after further adjusting for education and income levels and employment and insurance status (model 2).
A greater percentage of women than men reported having severe marital stress (39% vs. 30%; P = .001).
Cenjing Zhu, MPhil, a PhD candidate at Yale University, New Haven, Conn., and colleagues will present this study at the American Heart Association scientific sessions.
The results show that “both patients and care providers should be aware that stress experienced in one’s everyday life, such as marital stress, can affect AMI [acute MI] recovery,” Ms. Zhu said in an email.
Health care providers should consider incorporating screening for everyday stress during follow-up patient visits to better spot people at high risk of a poor recovery and further hospitalizations, she added. When possible, they could guide patients to resources to help them manage and reduce their stress levels.
According to Ms. Zhu, the findings suggest that “managing personal stress may be as important as managing other clinical risk factors during the recovery process.”
This study in younger patients with MI “shows that high levels of marital stress impair heart attack recovery, and women have greater impairment in their heart attack recovery compared to men,” AHA spokesperson Nieca Goldberg, MD, who was not involved with this research, told this news organization.
The study shows that “clinicians have to incorporate mental health as part of their assessment of all patients,” said Dr. Goldberg, a clinical associate professor of medicine at New York University and medical director of Atria New York City.
“Our mental health impacts our physical health,” she noted. “Questions about marital stress should be included as part of an overall assessment of mental health. This means assessing all patients for stress, anxiety, and depression.”
Patients who are experiencing marital stress should share the information with their doctor and discuss ways to be referred to therapists and cardiac rehabilitation providers, she said. “My final thought is, women have often been told that their cardiac symptoms are due to stress by doctors. Now we know stress impacts physical health and [is] no longer an excuse but a contributing factor to our physical health.”
Does marital stress affect young MI recovery?
Previous literature has linked psychological stress with worse cardiovascular outcomes, Ms. Zhu noted.
However, little is known about the prognostic impact of marital stress on 1-year health outcomes for younger people who survive an MI.
To investigate this, the researchers analyzed data from participants in the Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study.
The current study comprised 1,593 adults, including 1,020 female participants (64%), who were treated for MI at 103 hospitals in 30 U.S. states.
VIRGO enrolled participants in a 2:1 female-to-male ratio so as to enrich the inclusion of women, Ms. Zhu explained.
In the study, “partnered” participants were individuals who self-reported as “living as married/living with a partner.” There were 126 such patients (8%) in the current study.
The mean age of the patients was 47, and about 90% were 40-55 years old. Three quarters were White, 13% were Black, and 7% were Hispanic.
Marital stress was assessed on the basis of patients’ replies to 17 questions in the Stockholm Marital Stress Scale regarding the quality of their emotional and sexual relationships with their spouses/partners.
The researchers divided patients into three groups on the basis of their marital stress: mild or absent (lowest quartile), moderate (second quartile), and severe (upper two quartiles).
At 1 year after their MI, patients replied to questionnaires that assessed their health, quality of life, and depressive and angina symptoms. Hospital readmissions were determined on the basis of self-reports and medical records.
Compared to participants who reported no or mild marital stress, those who reported severe mental stress had significantly worse scores for physical and mental health and generic and cardiovascular quality of life, after adjusting for baseline health and demographics. They had worse scores for mental health and quality of life, after further adjusting for socioeconomic status.
In the fully adjusted model, patients who reported severe marital stress were significantly more likely to report more frequent chest pain/angina (odds ratio, 1.49; 95% confidence interval, 1.06-2.10; P = .023) and to have been readmitted to hospital for any cause (OR, 1.45; 95% CI, 1.04-2.00; P = .006), compared with the patients who reported no or mild marital stress.
Study limitations include the fact that the findings are based on self-reported questionnaire replies; they may not be generalizable to patients in other countries; and they do not extend beyond a period of 1 year.
The researchers call for further research “to understand this complex relationship and potential causal pathway associated with these findings.”
“Additional stressors beyond marital stress, such as financial strain or work stress, may also play a role in young adults’ recovery, and the interaction between these factors require further research,” Ms. Zhu noted in a press release from the AHA.
The study was funded by Canadian Institutes of Health Research. The VIRGO study was funded by the National Heart, Lung, and Blood Institute. Ms. Zhu and Dr. Goldberg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
USPSTF holds firm on postmenopausal hormone recommendations
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force moved forward their recommendations for using hormone therapy to prevent chronic conditions in postmenopausal women by keeping them the same.
The central message of the new recommendations, released on Nov. 1 as a statement published in JAMA, remains unchanged from the last update in 2017.
The message also remains simple: Don’t use hormone therapy for preventing chronic conditions, such as cardiovascular disease, cancer, and osteoporosis, or bone fracture.
The USPSTF summarized its recommendations in two brief statements: the group “recommends against the use of combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal persons” and “recommends against the use of estrogen alone for the primary prevention of chronic conditions in postmenopausal persons who have had a hysterectomy.”
This wording is identical to that used in the 2017 guidance (except it now refers to postmenopausal persons instead of specifically women). The recommendation against use of estrogen and progestin for prevention of chronic conditions in postmenopausal women was first made by the USPSTF in 2002.
An editorial accompanying the 2022 revision notes that the evidence cited by the USPSTF includes “only two additional, modest-sized trials” (that focused on the effects of hormone therapy on cognition and brain structure) compared with 2017, “as well as ancillary analyses of previous trials.”
A standard 5-year update
The 2022 revision and revisiting of the evidence base by the Task Force regarding the benefits and risks of postmenopausal hormone therapy occurred “as part of the Task Force’s standard approach, which includes updating each recommendation approximately every 5 years,” explained Carol M. Mangione, MD, who is USPSTF chair and chief of the division of general internal medicine and health services research at the University of California, Los Angeles.
“In our review we again found that while hormone therapy may reduce the risk of some conditions, it can also lead to serious harms such as an increase in the risk of blood clots and stroke,” Dr. Mangione said in an interview. “The harms cancel out any potential benefits overall.”
This new statement only applies to using menopausal hormone treatment for preventing chronic conditions in asymptomatic people but does not speak to using this treatment in managing people with perimenopausal symptoms such as hot flashes or vaginal dryness or treating people with premature or surgical menopause, Dr. Mangione highlighted.
No review for treating menopausal symptoms
“The Task Force encourages people who are experiencing symptoms of menopause to talk with their health care professional about the best treatment for them,” explained Dr. Mangione. “The Task Force did not review the evidence on the use of hormone therapy to treat symptoms of menopause.”
Osteoporosis and increased risk for bone fracture were among the conditions that accompany menopause reviewed by the USPSTF. The Task Force concluded that while “hormone therapy was associated with decreased risk of fractures,” after weighing the benefits and harms for preventing this condition, “there is no net benefit at the population level.”
This conclusion seems to contrast with the 2022 hormone therapy position statement of the North American Menopause Society (NAMS), released in July, which states: “For women aged younger than 60 years or who are within 10 years of menopause onset and have no contraindications, the benefit-risk ratio is favorable for treatment of bothersome vasomotor symptoms and prevention of bone loss.”
USPSTF, NAMS are ‘completely consistent’
However, Stephanie S. Faubion, MD, medical director of NAMS and director of the women’s health clinic at Mayo Clinic, Rochester, Minn., said the new USPSTF recommendations “are completely consistent” with the recent NAMS statement.
“We are entirely aligned with the recommendation to use hormone therapy for management of menopausal symptoms and not for chronic disease prevention or as an anti-aging strategy,” Dr. Faubion commented in an interview.
Dr. Faubion also stressed that “menopausal hormone therapy remains the most effective treatment for menopausal symptoms,” and that “women should not be reflexively directed to other pharmacologic therapies for management of menopausal symptoms.”
The distinction the USPSTF makes between its recommendations against using hormone therapy to prevent chronic conditions and its deferral of comment on use of the same treatment to manage perimenopausal symptoms is often forgotten, note Alison J. Huang, MD, and Deborah Grady, MD, in their editorial.
A problem of conflation
“Many patients and clinicians conflate these two different indications,” they write.
The notion that the net harms of menopausal hormone therapy outweigh the benefits “is now widely adopted as a rationale for foregoing menopausal hormone therapy for symptomatic treatment,” even though “nonhormonal treatments that are as effective as menopausal hormone therapy have not yet been identified,” say Dr. Huang and Dr. Grady, both physicians at the University of California, San Francisco.
In addition, alternative, nonhormonal options for treating perimenopausal symptoms have not received the same level of scrutiny as hormonal treatment, they say.
“It is arguably problematic to avoid menopausal hormone therapy and favor potentially less effective treatments, when the longer-term implications of those treatments for health have not been evaluated,” Dr. Huang and Dr. Grady write in their editorial.
In short, during menopause, people are at risk of being “frightened away from considering using menopausal hormone therapy for distressing symptoms,” they say.
“We can’t speak to whether or how often clinicians might be conflating the role of hormone therapy in treating symptoms and preventing chronic conditions,” answered Dr. Mangione.
“We hope to ensure that health professionals know that hormone therapy is not a beneficial way to reduce the risk of chronic conditions such as heart disease, cancer, and strokes,” she added. The new recommendations are an effort to “raise awareness about the value of considering other safe and effective ways for people to reduce their risk of chronic health problems as they age.”
The issue of timing
Another critique offered by Dr. Huang and Dr. Grady in their editorial is that “the scientific and medical community should let go of the past,” and should no longer invest additional resources in “trying to parse out subsets of menopausal patients who may derive some preventive benefit from menopausal hormone therapy for a limited amount of time.”
But Dr. Mangione disagreed.
The USPSTF “calls for more research that can help us understand whether health outcomes – both benefits and harms – differ depending on a person’s age or when they started hormone therapy related to when they went through menopause,” she said.
Dr. Mangione also highlighted the need for additional research on whether the benefits and risks of menopausal hormone therapy vary across racial and ethnic groups.
USPSTF receives no commercial funding. Dr. Mangione, Dr. Huang, and Dr. Grady have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
AHA 2022 to recapture in-person vibe but preserve global reach
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
Multiple menopause symptoms linked to increased cardiovascular risk
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
Up to 10 different menopausal symptoms were linked to an increased risk of cardiovascular disease when they were moderate to severe in women who initially had no evidence of cardiovascular disease, according to research presented at the North American Menopause Society annual meeting in Atlanta.
“The take-home message is that severe menopausal symptoms may increase the risk of cardiovascular disease,” Matthew Nudy, MD, an assistant professor of medicine at the Heart and Vascular Institute at Penn State University, Hershey, said in an interview about his findings. “Physicians and patients should be aware of this association. Women with severe symptoms may be more likely to see their physician, and this would be an ideal time to have their cardiovascular risk assessed.”
Margaret Nachtigall, MD, a clinical associate professor of obstetrics and gynecology at New York University and at NYU Langone Health, noted that these findings lined up with other studies showing an increased risk of cardiovascular disease in patients who have more symptoms, especially hot flashes.
“Other recent studies showed that an increase in severity of hot flush is associated with worse blood vessel function, leading to heart disease,” Dr. Nachtigall, who was not involved with the study, said in an interview. “The next step that makes sense is to try to eliminate these symptoms and hope that, in turn, would lower cardiovascular disease and improve survival.”
The researchers compared menopausal symptoms with cardiovascular outcomes and all-cause mortality in an observational cohort of 80,278 postmenopausal women for a median 8.2 years of follow-up. None of the women, all enrolled in the Women’s Health Initiative, had known cardiovascular disease at baseline. They had an average age of 63 years and average body mass index (BMI) of 25.9 at baseline. Most participants were White (86.7%), with 7% being Black and 4.1% Hispanic. Cardiovascular disease was a composite outcome that included hospitalized myocardial infarction, definite silent myocardial infarction, coronary death, stroke, congestive heart failure, angina, peripheral vascular disease, carotid artery disease, and coronary revascularization.
The researchers used a four-item Likert scale (0-3) to assess the severity of 15 symptoms experienced within the past 4 weeks at baseline: “night sweats, hot flashes, waking up several times at night, joint pain or stiffness, headaches or migraines, vaginal or genital dryness, heart racing or skipping beats, breast tenderness, dizziness, tremors (shakes), feeling tired, forgetfulness, mood swings, [feeling] restless or fidgety, and difficulty concentrating.”
The associations were adjusted for the following covariates: race/ethnicity, blood pressure, education, smoking status, bilateral oophorectomy, menopausal hormone therapy use (never/past/current), sleep duration, statin use, history of high cholesterol, aspirin use, use of antihypertensives, treated diabetes, and family history of heart attack. Continuous variables included age, age at menopause, BMI, blood pressure, and physical activity levels. Because of the high number of multiple comparisons, the researchers also used a Bonferroni correction to reduce the risk of spurious statistical significance.
The researchers found some clustering of symptoms. Among women who had at least two moderate or severe menopausal symptoms, more than half frequently woke up at night, had joint pain, or felt tired, the researchers reported. Those symptoms were also the most commonly reported ones overall. Younger women, between ages 50 and 59, were more likely than older women (60-79 years old) to experience vasomotor symptoms and all cognitive affective symptoms except forgetfulness.
The researchers identified 10 symptoms whose severity was significantly associated with cardiovascular disease. Compared to having no symptoms at all, the following moderate or severe symptoms were associated with an increased risk of a cardiovascular event after adjustment for covariates and corrected for multiple comparisons: night sweats – a 19% increased risk (P = .03), waking up several times at night – 11% increased risk (P = .05), joint pain or stiffness – 27% increased risk (P < .001), heart racing or skipping beats – 55% increased risk (P < .001), dizziness – 34% increased risk (P < .001), feeling tired – 35% increased risk (P < .001), forgetfulness – 25% increased risk (P < .001), mood swings – 21% increased risk (P = .02), feeling restless or fidgety – 29% increased risk (P < .001), and difficulty concentrating – 31% increased risk (P < .001)
In addition, all-cause mortality was associated with these symptoms when they were moderate or severe: heart racing or skipping beats (32% increased risk of all-cause mortality; hazard ratio, 1.32; P =.006), dizziness (HR, 1.58; P < .001), tremors (HR, 1.44; P < .001), feeling tired (HR, 1.26; P < .001), forgetfulness (HR, 1.29; P = .01), mood swings (HR, 1.35; P = .02), feeling restless or fidgety (HR, 1.35; P < .001), and difficulty concentrating (HR, 1.47; P < .001).
The symptom with the greatest association with all-cause mortality was dizziness, which was associated with an increased risk of 58% when rated moderate or severe. Any dizziness at all was linked to a 12% increased risk of cardiovascular disease, compared with no dizziness. Machine learning with the LASSO method determined that the symptoms most predictive of cardiovascular disease were dizziness, heart racing, feeling tired, and joint pain. The symptoms most associated with all-cause mortality, based on the machine learning algorithm, were dizziness, tremors, and feeling tired.
Dr. Nudy said that their study did not look at mitigation strategies. “Women should discuss with their physician the best methods for cardiovascular risk reduction,” he said. He also cautioned that severe menopausal symptoms can also indicate other health conditions that may require investigation.
“It is certainly possible some symptoms may represent other medical conditions we were unable to control for and may not be directly related to menopause,” such as autoimmune diseases, endocrine abnormalities, or subclinical cardiovascular disease, he said. Additional limitations of the study included an older cohort and retrospective assessment of menopausal symptoms only at baseline. In addition, ”we did not assess the cardiovascular risk among women whose symptoms persisted versus resolved during the study period,” Dr. Nudy said.
Dr. Nachtigall said a key message is that people who are experiencing these symptoms should try to get treatment for them and attempt to alleviate them, hopefully reducing the risk of heart disease and death.
”Estrogen treatment is one excellent option for some individuals and should be considered in the appropriate person,” Dr. Nachtigall said. “If estrogen treatment is to be considered, it should be given closer to menopause, within the first 10 years after menopause and in younger individuals (under 59) at start.”
Dr. Nachtigall referred to the NAMS 2022 position statement concluding that, for healthy women within 10 years of menopause who have bothersome menopause symptoms, “the benefits of hormone therapy outweigh its risks, with fewer cardiovascular events in younger versus older women.”
”Menopause and having menopausal symptoms is an opportunity for clinicians and patients to have a conversation about appropriate individualized management options,” Dr. Nachtigall said.
Women may also be able to mitigate their cardiovascular risk with regular exercise, eating a healthy diet, not smoking, and getting adequate sleep, Dr. Nachtigall said. But these healthy behaviors may not adequately treat moderate or severe menopausal symptoms.
“Some health care providers have said that because menopause happens naturally, individuals should just accept the symptoms and try to wait it out and not get treatment, but this study, as well as others, makes it clear that it actually may be beneficial to treat the symptoms,” Dr. Nachtigall said.
The research used no external funding. Dr. Nudy and Dr. Nachtigall had no disclosures.
FROM NAMS 2022
Best anticoagulant for minimizing bleeding risk identified
A commonly prescribed direct oral anticoagulant (DOAC) has the lowest risk of bleeding, say researchers. Used to prevent strokes in those with atrial fibrillation (AFib), DOACs have recently become more common than warfarin, the previous standard treatment, as they do not require as much follow-up monitoring – which was “particularly valuable” during the COVID-19 pandemic – and have “less risk” of side effects, highlighted the authors of a new study, published in Annals of Internal Medicine.
However, the authors explained that, although current guidelines recommend using DOACs over warfarin in patients with AFib, “head-to-head trial data do not exist to guide the choice of DOAC.” So, they set out to try and fill this evidence gap by doing a large-scale comparison between all DOACs – apixaban, dabigatran, edoxaban, and rivaroxaban – in routine clinical practice.
Wallis Lau, PhD, University College London, and co–lead author, said: “Direct oral anticoagulants have been prescribed with increasing frequency worldwide in recent years, but evidence comparing them directly has been limited.”
One drug stood out
For the multinational population-based cohort study the researchers compared the efficacy and risk of side effects for the four most common DOACs. They reviewed data – from five standardized electronic health care databases that covered 221 million people in the United Kingdom, France, Germany, and the United States – of 527,226 patients who had been newly diagnosed with AFib between 2010 and 2019, and who had received a new DOAC prescription. The study included 281,320 apixaban users, 61,008 dabigatran users, 12,722 edoxaban users, and 172,176 rivaroxaban users.
Database-specific hazard ratios of ischemic stroke or systemic embolism, intracranial hemorrhage, gastrointestinal bleeding, and all-cause mortality between DOACs were estimated using a Cox regression model stratified by propensity score and pooled using a random-effects model.
In total, 9,530 ischemic stroke or systemic embolism events, 841 intercranial hemorrhage events, 8,319 gastrointestinal bleeding events, and 1,476 deaths were identified over the study follow-up. The researchers found that all four drugs were comparable on outcomes for ischemic stroke, intercranial hemorrhage, and all-cause mortality.
However, they identified a difference in the risk of gastrointestinal bleeding, which they highlighted “is one of the most common and concerning side effects of DOACs.”
“Apixaban stood out as having lower risk of gastrointestinal bleeding,” said the authors, with a 19%-28% lower risk when compared directly with each of the other three DOACs. Specifically, apixaban use was associated with lower risk for gastrointestinal bleeding than use of dabigatran (HR, 0.81; 95% confidence interval, 0.70-0.94), edoxaban (HR, 0.77; 95% CI, 0.66-0.91), or rivaroxaban (HR, 0.72; 95% CI, 0.66-0.79).
The researchers also highlighted that their findings held true when looking at data only from those aged over 80, and those with chronic kidney disease, two groups that are “often underrepresented” in clinical trials.
Apixaban may be preferable
The researchers concluded that, compared with dabigatran, edoxaban, and rivaroxaban.
“Our results indicate that apixaban may be preferable to other blood thinners because of the lower rate of gastrointestinal bleeding and similar rates of stroke, a finding that we hope will be supported by randomized controlled trials,” said Dr. Lau.
However, he emphasized that, “as with all medications, potential risks and benefits can differ between people, so considering the full spectrum of outcomes and side effects will still be necessary for each individual patient.”
The authors all declared no conflicting interests.
A version of this article first appeared on Medscape UK.
A commonly prescribed direct oral anticoagulant (DOAC) has the lowest risk of bleeding, say researchers. Used to prevent strokes in those with atrial fibrillation (AFib), DOACs have recently become more common than warfarin, the previous standard treatment, as they do not require as much follow-up monitoring – which was “particularly valuable” during the COVID-19 pandemic – and have “less risk” of side effects, highlighted the authors of a new study, published in Annals of Internal Medicine.
However, the authors explained that, although current guidelines recommend using DOACs over warfarin in patients with AFib, “head-to-head trial data do not exist to guide the choice of DOAC.” So, they set out to try and fill this evidence gap by doing a large-scale comparison between all DOACs – apixaban, dabigatran, edoxaban, and rivaroxaban – in routine clinical practice.
Wallis Lau, PhD, University College London, and co–lead author, said: “Direct oral anticoagulants have been prescribed with increasing frequency worldwide in recent years, but evidence comparing them directly has been limited.”
One drug stood out
For the multinational population-based cohort study the researchers compared the efficacy and risk of side effects for the four most common DOACs. They reviewed data – from five standardized electronic health care databases that covered 221 million people in the United Kingdom, France, Germany, and the United States – of 527,226 patients who had been newly diagnosed with AFib between 2010 and 2019, and who had received a new DOAC prescription. The study included 281,320 apixaban users, 61,008 dabigatran users, 12,722 edoxaban users, and 172,176 rivaroxaban users.
Database-specific hazard ratios of ischemic stroke or systemic embolism, intracranial hemorrhage, gastrointestinal bleeding, and all-cause mortality between DOACs were estimated using a Cox regression model stratified by propensity score and pooled using a random-effects model.
In total, 9,530 ischemic stroke or systemic embolism events, 841 intercranial hemorrhage events, 8,319 gastrointestinal bleeding events, and 1,476 deaths were identified over the study follow-up. The researchers found that all four drugs were comparable on outcomes for ischemic stroke, intercranial hemorrhage, and all-cause mortality.
However, they identified a difference in the risk of gastrointestinal bleeding, which they highlighted “is one of the most common and concerning side effects of DOACs.”
“Apixaban stood out as having lower risk of gastrointestinal bleeding,” said the authors, with a 19%-28% lower risk when compared directly with each of the other three DOACs. Specifically, apixaban use was associated with lower risk for gastrointestinal bleeding than use of dabigatran (HR, 0.81; 95% confidence interval, 0.70-0.94), edoxaban (HR, 0.77; 95% CI, 0.66-0.91), or rivaroxaban (HR, 0.72; 95% CI, 0.66-0.79).
The researchers also highlighted that their findings held true when looking at data only from those aged over 80, and those with chronic kidney disease, two groups that are “often underrepresented” in clinical trials.
Apixaban may be preferable
The researchers concluded that, compared with dabigatran, edoxaban, and rivaroxaban.
“Our results indicate that apixaban may be preferable to other blood thinners because of the lower rate of gastrointestinal bleeding and similar rates of stroke, a finding that we hope will be supported by randomized controlled trials,” said Dr. Lau.
However, he emphasized that, “as with all medications, potential risks and benefits can differ between people, so considering the full spectrum of outcomes and side effects will still be necessary for each individual patient.”
The authors all declared no conflicting interests.
A version of this article first appeared on Medscape UK.
A commonly prescribed direct oral anticoagulant (DOAC) has the lowest risk of bleeding, say researchers. Used to prevent strokes in those with atrial fibrillation (AFib), DOACs have recently become more common than warfarin, the previous standard treatment, as they do not require as much follow-up monitoring – which was “particularly valuable” during the COVID-19 pandemic – and have “less risk” of side effects, highlighted the authors of a new study, published in Annals of Internal Medicine.
However, the authors explained that, although current guidelines recommend using DOACs over warfarin in patients with AFib, “head-to-head trial data do not exist to guide the choice of DOAC.” So, they set out to try and fill this evidence gap by doing a large-scale comparison between all DOACs – apixaban, dabigatran, edoxaban, and rivaroxaban – in routine clinical practice.
Wallis Lau, PhD, University College London, and co–lead author, said: “Direct oral anticoagulants have been prescribed with increasing frequency worldwide in recent years, but evidence comparing them directly has been limited.”
One drug stood out
For the multinational population-based cohort study the researchers compared the efficacy and risk of side effects for the four most common DOACs. They reviewed data – from five standardized electronic health care databases that covered 221 million people in the United Kingdom, France, Germany, and the United States – of 527,226 patients who had been newly diagnosed with AFib between 2010 and 2019, and who had received a new DOAC prescription. The study included 281,320 apixaban users, 61,008 dabigatran users, 12,722 edoxaban users, and 172,176 rivaroxaban users.
Database-specific hazard ratios of ischemic stroke or systemic embolism, intracranial hemorrhage, gastrointestinal bleeding, and all-cause mortality between DOACs were estimated using a Cox regression model stratified by propensity score and pooled using a random-effects model.
In total, 9,530 ischemic stroke or systemic embolism events, 841 intercranial hemorrhage events, 8,319 gastrointestinal bleeding events, and 1,476 deaths were identified over the study follow-up. The researchers found that all four drugs were comparable on outcomes for ischemic stroke, intercranial hemorrhage, and all-cause mortality.
However, they identified a difference in the risk of gastrointestinal bleeding, which they highlighted “is one of the most common and concerning side effects of DOACs.”
“Apixaban stood out as having lower risk of gastrointestinal bleeding,” said the authors, with a 19%-28% lower risk when compared directly with each of the other three DOACs. Specifically, apixaban use was associated with lower risk for gastrointestinal bleeding than use of dabigatran (HR, 0.81; 95% confidence interval, 0.70-0.94), edoxaban (HR, 0.77; 95% CI, 0.66-0.91), or rivaroxaban (HR, 0.72; 95% CI, 0.66-0.79).
The researchers also highlighted that their findings held true when looking at data only from those aged over 80, and those with chronic kidney disease, two groups that are “often underrepresented” in clinical trials.
Apixaban may be preferable
The researchers concluded that, compared with dabigatran, edoxaban, and rivaroxaban.
“Our results indicate that apixaban may be preferable to other blood thinners because of the lower rate of gastrointestinal bleeding and similar rates of stroke, a finding that we hope will be supported by randomized controlled trials,” said Dr. Lau.
However, he emphasized that, “as with all medications, potential risks and benefits can differ between people, so considering the full spectrum of outcomes and side effects will still be necessary for each individual patient.”
The authors all declared no conflicting interests.
A version of this article first appeared on Medscape UK.
FROM ANNALS OF INTERNAL MEDICINE