User login
U.S. vs. Europe: Costs of cardiac implant devices compared
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.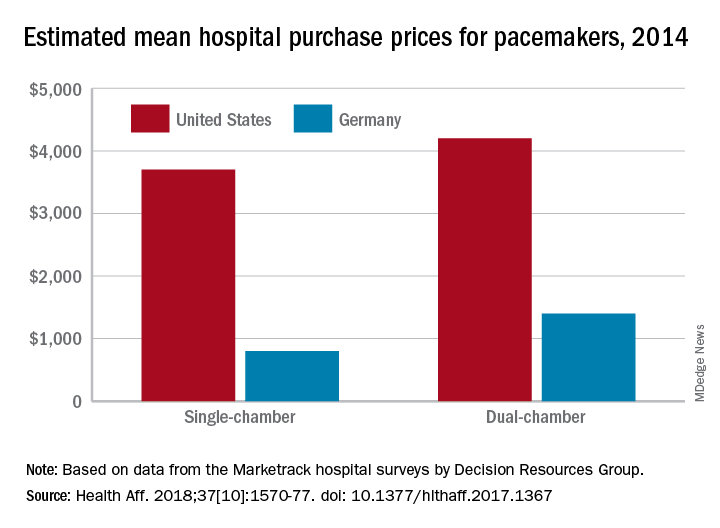
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
Prices that hospitals pay for cardiac implant devices are two to six times higher in the United States than in Europe, according to analysis of a large hospital panel survey.
U.S. hospitals had an estimated mean cost of $670 for a bare-metal stent in 2014, compared with $120 in Germany, and the mean costs for dual-chamber pacemakers that year were $4,200 in the United States and $1,400 in Germany, which had lower costs for cardiac devices than the other three European countries – United Kingdom, France, and Italy – included in the study, Martin Wenzl, MSc, and Elias Mossialos, MD, PhD, reported in Health Affairs.
France generally had the highest costs among the European countries, with Italy next and then the United Kingdom. The estimated cost of bare-metal stents was actually higher for French hospitals ($750) than for those in the United States, and Italy had mean prices similar to the United Sates for dual-chamber implantable cardioverter-defibrillators. The prices of implantable cardioverter-defibrillators and cardiac resynchronization devices with defibrillating function were the other exceptions, with the United Kingdom similar to or higher than the United States, said Mr. Wenzl and Dr. Mossialos, both of the London School of Economics and Political Science.
The analysis of data from Decision Resources Group’s Marketrack hospital surveys also showed significant variation between the hospitals in each country, with the exception of France, where payments are based on the specific device rather than the procedure and the system “creates weak incentives for hospitals to negotiate lower prices,” they said. In most of the device categories, “variation between hospitals in each country was similar to variation between countries,” they wrote, adding that prices in general “were only weakly correlated with volumes purchased by hospitals.”
The study was supported by a grant from the Commonwealth Fund. The investigators did not disclose any possible conflicts of interest.
SOURCE: Wenzi M, Mossialos E. Health Aff. 2018;37[10]:1570-77. doi: 10.1377/hlthaff.2017.1367.
FROM HEALTH AFFAIRS
Drug-coated balloons shown noninferior to DES in thin coronaries
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
Treating thin coronary arteries is a problem because they have a higher risk for in-stent restenosis, although usually we will put a stent in arteries that are at least 2.5 mm wide and sometimes in coronaries as narrow as 2.25 mm. That’s using the narrowest stent we have available. Sometimes in vessels this size, if the result from initial balloon angioplasty looks good on angiography, we accept that outcome and do not place a stent.
Steen Dalby Kristensen, MD , is a professor of cardiology at Aarhus University in Skejby, Denmark. He had no relevant disclosures. He made these comments in a video interview.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
MUNICH – for preventing the clinical consequences of restenosis during 12 months following coronary intervention, according to results from a prospective, randomized, multicenter trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Drug-coated balloons are already used to treat in-stent coronary restenosis. The findings of the current study establish the tested DCB as noninferior to a DES for treating coronary stenoses in narrow arteries less than 3 mm in diameter, Raban V. Jeger, MD, said at the annual congress of the European Society of Cardiology. The DCB approach avoids placing a metal stent in a narrow coronary and thus has no long-term risk for in-stent thrombosis, said Dr. Jeger, a professor of cardiology at Basel (Switzerland) University Hospital. Dr. Jeger acknowledged that the tested DCB is more expensive than the second-generation DES used as the comparator in most of the control patients, “but I think the benefit to patients is worth” the added cost, he said when discussing his report.
The BASKET-SMALL 2 (NCT01574534) study enrolled 758 patients at 14 centers in Switzerland, Germany, and Austria. The trial limited enrollment to patients who were scheduled to undergo percutaneous coronary intervention for stenosis in a coronary artery that was at least 2.0 mm and less than 3.0 mm in diameter and had first undergone successful predilatation without any flow-limiting dissections or residual stenosis, a step in the DCB procedure that adds to the procedure’s cost.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study randomized patients to treatment with either a balloon coated with paclitaxel/iopromide (SeQuent Please) or a DES. The first quarter of patients randomized into the DES arm received a first-generation, paclitaxel-eluting DES (Taxus Element); the remaining patients in the comparator arm received a second-generation everolimus-eluting DES (Xience). The DCB tested is not approved for U.S. marketing.
The primary endpoint was the combined rate of cardiac death, nonfatal MI, or target vessel revascularization during 12 months of follow-up. In the intention-to-treat analysis, this occurred in 7.33% of the DCB patients and in 7.45% of the DES patients, a difference that was not statistically significant and that met the prespecified criterion for noninferiority of the DCB. Concurrently with Dr. Jeger’s report at the congress, the results also appeared in an article published in The Lancet (Lancet. 2018 Sep 8;392[10190]:849-56).
One limitation of the study was that the first 25% of patients enrolled into the DES arm received a first-generation DES, while the remaining 75% received a second-generation device. Analysis of the primary endpoint by DES type showed that events occurred more than twice as often in the patients who received a first-generation DES, and their inclusion may have affected the comparator group’s results.
Coronary arteries that need percutaneous intervention and are less than 3 mm in diameter constitute about a third of all target vessels, and they are especially common among women and in patients with diabetes, Dr. Jeger said. Despite this, women made up about a quarter of the study enrollment, and about a third had diabetes. He also noted that a key aspect of adopting the DCB approach into routine practice is that operators would need to have the “courage” to accept some amount of recoil and “minor” dissections after DCB treatment and not feel compelled to correct these with a stent.
Other features of the BASKET-SMALL 2 trial also have raised concerns about the immediate clinical implications of the results, said Roxana Mehran, MD, a professor of medicine at Icahn School of Medicine at Mount Sinai, New York, and the congress’s designated discussant for the report.
The study began in 2012, which means it took more than 5 years to enroll and suggests that the study may have a selection bias. Dr. Mehran also questioned whether it was really a small vessel study, with an enrollment criterion of less than 3 mm in diameter. A future study should be done in “truly” small vessels, those thinner than 2.5 mm, she said.
Dr. Mehran agreed it’s attractive to speculate that, by using a DCB and avoiding stent placement, fewer patients will eventually have very-late adverse events, but this must be proven with longer follow-up and in larger numbers of patients, she said.
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: Drug-coated balloon treatment worked as well as drug-eluting stents in thin coronaries.
Major finding: Twelve-month MACE occurred in 7.33% of balloon-treated patients and in 7.45% of stent-treated patients.
Study details: BASKET-SMALL 2, an international, multicenter randomized trial with 758 patients.
Disclosures: The investigator-initiated study received partial funding from B. Braun, the company that markets the drug-coated balloon (SeQuent Please) tested in the study. Dr. Jeger has received research funding from B. Braun. Dr. Mehran has been a consultant to Abbott, Bayer, BSC, and CSL Behring and has received research funding from Abbott, Astra Zeneca, Bayer, BCC, DSI, and Janssen.
Prosthesis-patient mismatch post TAVR ups death risk 19%
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
SAN DIEGO – Severe prosthesis-patient mismatch (PPM) after transcatheter aortic valve replacement (TAVR) increases risk of adverse outcomes and may be preventable in some cases with careful preprocedural planning, suggests a registry-based retrospective cohort study of 62,125 patients treated in the contemporary era.
The study – the largest to date of this patient population – determined that about one in every eight patients undergoing TAVR ultimately had a severe mismatch between the hemodynamics of the valve prosthesis and the requirements for cardiac output. Compared with counterparts that have moderate or no PPM, these patients with severe PPM had a 12% higher adjusted risk of heart failure rehospitalization and a 19% higher adjusted risk of death, according to results reported at the Transcatheter Cardiovascular Therapeutics annual meeting and simultaneously published online (J Am Coll Cardiol. 2018 Sep 23. doi: 10.1016/j.jacc.2018.09.001).
Notably, some of the predictors of severe PPM, such as use of smaller-diameter valves and performance of a valve-in-valve procedure, were potentially modifiable.
“Our findings suggest that efforts should be made to identify this problem and limit the risk for PPM after TAVR,” concluded lead investigator Howard C. Herrmann, MD, a professor at the University of Pennsylvania and director of the cardiac catheterization laboratories, Hospital of the University of Pennsylvania, both in Philadelphia. “Awareness is really the first step in trying to fix it.”
“We spend a lot of time in the heart-team meetings looking at the CT scans for annular dimensions and the vascular access, but we don’t really talk too much about severe PPM or the risk of that,” he elaborated. “This [study] allows us to start to predict it, based on patient factors and what prosthesis we might be choosing for a patient, and it allows us to have that conversation and think about alternatives.
“There are alternatives to try to avoid PPM, everything from which prosthesis we choose to the size of the prosthesis, to whether we fracture a patient’s valve if we are doing a valve-in-valve procedure. In the future, in some situations, we might even choose a low-risk or low-intermediate-risk patient for surgery with an enlargement operation in order to get a larger effective orifice area. So there are choices that we can make, and we should start thinking about that in the heart-team approach.”
Findings in context
The new study reinforces the message “that hemodynamics matter,” he said. “To the extent that we can get larger valves in and get better results from those valves, it will reduce the frequency of PPM. That’s something as operators we don’t spend as much time focusing on, and this will refocus our attention in trying to prevent PPM by being more diligent in terms of prosthesis choice and some operator characteristics, to try to reduce the gradients and improve the effective orifice areas as much as we can.”
Panelist Jeffrey J. Popma, MD, an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston, noted that he and his colleagues have observed similar trends in their smaller studies but had difficulty teasing out contributors. “It really goes back to the preprocedural planning about what valve you can get in, and larger orifice area is certainly better,” he concurred. “So I do think that this is a phenomenal addition.”
Study details
For the study, Dr. Herrmann and his colleagues analyzed 2014-2107 data from the STS/ACC Transcatheter Valve Therapy Registry, a national surveillance and quality improvement system. They identified enrollees aged 65 years or older at the time of their TAVR procedure who had fee-for-service Medicare and linked them to Centers for Medicare & Medicaid Services claims data to assess outcomes.
Overall, 12.1% of patients had severe PPM, defined as an effective valve orifice area indexed to body surface area of less than 0.65 cm2/m2 on discharge echocardiography, and another 24.6% had moderate PPM, Dr. Herrmann reported at the meeting, sponsored by the Cardiovascular Research Foundation.
The strongest multivariate predictors of severe PPM were small prosthetic valve size (up to 23 mm in diameter) (odds ratio, 2.77), a valve-in-valve procedure (OR, 2.78), larger body surface area (OR, 1.71 per 0.2-U increase), and female sex (OR, 1.46). Odds also increased with decreasing age and were elevated for patients of nonwhite/Hispanic race and those having a lower ejection fraction, atrial fibrillation or flutter, or severe mitral or tricuspid regurgitation.
It was not possible to assess specific valves as predictors of mismatch because the registry prohibits comparisons across manufacturers, according to Dr. Herrmann.
One-year mortality, the study’s primary endpoint, was 17.2% in patients with severe PPM, compared with 15.8% in patients with moderate or no PPM (adjusted hazard ratio, 1.19; P less than .001). Findings were similar across subgroups.
The 1-year rate of heart failure rehospitalization was 14.7% in patients with severe PPM, compared with 12.2% in patients with moderate or no PPM (AHR, 1.12; P = .017).
“I would point out that these [outcome] curves are divergent at 1 year,” Dr. Herrmann noted. “So if we look at low-intermediate-risk and low-risk patients and younger patients, who may be more active and who see the effects of PPM more commonly and who are going to be living more than 1 year, we are going to have to consider this going forward in a more important way.”
Severe PPM did not significantly influence the rate of stroke (which stood at about 4% in each group) or worsen quality of life score at 1 year.
Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
REPORTING FROM TCT 2018
Key clinical point:
Major finding: Patients with severe PPM after TAVR had elevated risks of heart failure rehospitalization (adjusted hazard ratio, 1.12) and death (AHR, 1.19).
Study details: A retrospective cohort study of 62,125 patients aged 65 years or older who underwent TAVR and were captured in the national STS/ACC Transcatheter Valve Therapy Registry.
Disclosures: Dr. Herrmann disclosed receiving institutional grant/research support from Abbott Vascular, Bayer, Boston Scientific, Corvia Medical, Edwards Lifesciences, Medtronic, and St. Jude Medical, as well as consulting fees/honoraria from Edwards, Medtronic, and Siemens Healthineers.
Novel device improves mitral regurgitation 30% in REDUCE-FMR
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
SAN DIEGO – In patients with heart failure and functional mitral regurgitation, implantation of an investigational device led to reduced MR and improved left ventricular remodeling at 1 year, compared with patients who received sham treatment in the REDUCE-FMR trial.
The device showed promise in this trial, despite a small sample size, and its nature makes it possible to follow up with other procedures if the disease progresses. “The advantage of this technique is that all other options are still open,” Horst Sievert, MD, director of the CardioVascular Center in Frankfurt, said during a press conference at the Transcatheter Cardiovascular Therapeutics annual meeting.
The Carillon Mitral Counter System includes two anchors, one in the great cardiac vein and one in the coronary sinus, connected by a shaping ribbon. The tension of the ribbon bolsters the mitral annulus, which in turn reduces mitral regurgitation.
REDUCE-FMR recruited 120 patients from centers in eight countries and randomized 87 to the Carillon device (73 implanted) and 33 to a sham procedure. Sham patients were sedated and received a coronary sinus angiogram. Patients were included if they had dilated ischemic or nonischemic cardiomyopathy and moderate to severe functional MR, among other requirements. Exclusion criteria included existing coronary artery stents in the implant target zone, severe mitral annular calcification, and significant organic mitral valve pathology.
The primary endpoint was the mean reduction of regurgitant volume at 1 year. The treated patients had a 22% reduction of 7.1 mL, while the sham group on average had an 8% increase of 3.3 mL (P = .03). In the as-treated subpopulation, which comprised 45 patients in the treatment group and 13 controls, the values were –7.5 mL and +3.3 mL (P = .02). A per-protocol analysis, which excluded patients who did not meet protocol criteria, led to an amplification of the effect when the study design was adhered to (–12.5 mL vs. +1.3 mL), though this result did not achieve statistical significance owing to the small sample size.
For the safety endpoints, the researchers examined the frequency of major adverse events (MAE), including death, myocardial infarction, cardiac perforation, device embolism, and surgery or percutaneous coronary intervention related to the device at 1 year. In the treatment group, 16.1% experienced a MAE, compared with 18.2% of control patients, a statistically nonsignificant difference.
A secondary efficacy endpoint of change in left ventricular end-diastolic volume showed improvements in the treatment group at 6 months (–12.4 mL) and 12 months (–8.6 mL), compared with increases in the sham group at 6 months (+5.4 mL) and 12 months (+6.5 mL). A similar trend occurred in left ventricular end-systolic volume (–7.8 mL and –4.8 mL; +3.4 mL and +6.1 mL, respectively).
The study was conducted in a patient population similar to that of the COAPT trial, which examined implantation of Abbott’s MitraClip. That study, presented here at TCT 2018 and simultaneously published in the New England Journal of Medicine, also examined patients with heart failure and secondary MR.
However, in REDUCE-FMR, many of the patients had milder heart failure than the researchers had expected: 44.8% in the treatment group had NYHA class II, as did 48.5% in the sham group. That surprise may help identify an appropriate patient population. “I think this device may have a nice spot in between medical therapy and MitraClip implantation, because we have, by chance, a patient population with mild heart insufficiency and mild MR,” said Dr. Sievert.
The two devices also showed different physiologic effects, Michael Mack, MD, said at a press conference. “One subtle difference is that, in this trial, the difference is due to both positive left ventricular remodeling in the treatment arm and continued progression in the sham control. In COAPT, the difference in improvement that we saw was totally due to prevention of progression of disease. We just stabilized the disease to where it was at. So that’s an intriguing difference here, that you actually were able to demonstrate positive left ventricular remodeling,” noted Dr. Mack, medical director for cardiovascular surgery at Baylor Scott & White Medical Center, Plano, Tex. He was a coinvestigator in the COAPT trial.
REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
REPORTING FROM TCT 2018
Key clinical point: The device led to improvement in mitral regurgitation as well as ventricular remodeling.
Major finding:
Study details: REDUCE-FMR, a randomized, sham controlled trial of 120 patients from 8 countries.
Disclosures: REDUCE-FMR was funded by Cardiac Dimensions. Dr. Sievert has received consulting fees, travel expenses, and study honoraria from Cardiac Dimensions, and 35 other companies. Dr. Mack has received grant support or had a research contract with Abbott Vascular, Medtronic, and Edwards Lifesciences.
COAPT: MitraClip prolongs life in selected HF patients
SAN DIEGO – Among a carefully selected subset of heart failure patients and severe secondary mitral regurgitation, transcatheter mitral valve repair with the MitraClip reduced hospitalizations for heart failure by 47%, and death from any cause by 38% over 24 months, compared with maximal medical therapy alone.
That’s according to a randomized, open-label trial presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
The number needed to treat to prevent one heart failure (HF) hospitalization within 2 years was three; the number needed to treat to save one life was six. Only about 3% of patients had a device complication within 12 months of placement in the study, dubbed COAPT (the Heart Failure Patients with Functional Mitral Regurgitation Trial).
COAPT patients had grade 3+ or 4+ secondary mitral regurgitation, with a mean effective regurgitant orifice area (EROA) of 41 mm2. Their left ventricles were dilated, but not huge, with a mean left ventricular end-diastolic volume of 101 mL/m2. “We estimate that’s about 10% of heart failure patients,” said lead investigator and interventional cardiologist Gregg W. Stone, MD, a professor of medicine at Columbia University, New York.
MitraClip placement was performed in high-volume centers by experienced operators, and patients were on maximally tolerated doses of guideline-directed medical therapy, as per the 2013 American College of Cardiology/American Heart Association heart failure management guidelines. There was very little variation in treatment regimens during the 2-year trial (J Am Coll Cardiol. 2017;70:776-803).
Those parameters matter. Among HF patients who did not fit them in the recent Mitra-FR trial in France, MitraClip did not reduce rates of death or unplanned hospitalization (N Engl J Med. 2018 Aug 27. doi: 10.1056/NEJMoa1805374).
COAPT and Mitra-FR investigators said at the meeting that the studies are complimentary, not conflicting, because together, they define secondary mitral regurgitation (MR) patients who will and will not benefit from the device.
MR was less severe in Mitra-FR, with a mean EROA of 31 mm2, but left ventricles were more dilated, with a mean left ventricular end-diastolic volume of 135 mL/m2. Patients were on more real-world drug regimens that varied over the course of the trial. Also, the lower implantation rates and higher complication rates in Mitra-FR “suggests perhaps greater experience of the COAPT operators,” said Dr. Stone, who also is the director of cardiovascular research and education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center.
In short, “they were a different patient population than were enrolled in COAPT,” he said at the meeting, sponsored by the Cardiovascular Research Foundation, which Dr. Stone also codirects.
There was great excitement at TCT about COAPT because there was a startling benefit for patients who previously had few options. But many speakers worried that the hype surrounding the trial will drown out the critically important message about patient selection and that the clip will be used in HF patients who don’t fit the COAPT profile.
They also said that the emerging picture of benefit in patients with less ventricular dilation but more mitral regurgitation needs to be fleshed out and better quantified.
COAPT randomized 302 patients to MitraClip on a background of guideline-directed therapy and 312 to guideline-directed therapy alone. Participants who had mitral regurgitation caused by left ventricular dysfunction, were not surgical candidates, and remained symptomatic despite optimal treatment.
The annualized rate of all hospitalizations for HF within 24 months was 35.8% per patient-year in the device group, as compared with 67.9% per patient-year in the control group, for a relative reduction of 47% (P less than .001).
Death from any cause within 24 months occurred in 29.1% of the patients in the device group and 46.1% in the control group, yielding a reduction of 38% (P less than .001).
“We didn’t cure patients by fixing their MR. They still had 29% 2-year mortality, but we did markedly improve their quality of life. The only subgroup that didn’t benefit were patients that had an EORA of less than 30 mm2 and end diastolic volume greater than the median” of 96 mL/m2, which was “fascinating,” Dr. Stone said, and fit the emerging picture.
Mitral regurgitation grade fell to 1+ or lower in 82% of patients after clip placement and remained there in the majority of survivors at 2 years.
For a long time, “HF experts thought MR was just a marker of severe left ventricular dysfunction. What I think we see here is that secondary MR is not just a bystander. It contributes to the abnormal pathophysiology of these patients,” he said.
The trial was sponsored by MitraClip’s maker, Abbott. The company participated in site selection, management, and data analysis. Dr. Stone disclosed that his employer, Columbia University, receives royalties from Abbott for sale of the clip. Several fellow investigators disclosed grants, fees, and other financial ties to the company.
Simultaneously with the COAPT presentation, the results were published online (N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640).
Mitra-FR was funded by the French Ministry of Health and Research and Abbott.
This is really a blockbuster trial, because you see a statistically significant reduction in cardiovascular endpoints, which is something we almost never see in device-based trials. I think this is going to change clinical practice, but the question of generalizability is tricky. This was such a well-conducted trial; it may be difficult to generalize this to the practicing public. I was impressed by the MitraClip performance: the reduction in MR [mitral regurgitation], the lack of recurrence, and the small number of complications. Perhaps more than anything else, the difference between Mitra-FR and COAPT was the quality of the operators.
Martin B. Leon, MD , is the director of the Center for Interventional Vascular Therapy at Columbia University, N.Y., and the Cardiovascular Research Foundation’s founder and codirector of medical research and education. He was not involved in COAPT, and made his comments after the study presentation.
This is really a blockbuster trial, because you see a statistically significant reduction in cardiovascular endpoints, which is something we almost never see in device-based trials. I think this is going to change clinical practice, but the question of generalizability is tricky. This was such a well-conducted trial; it may be difficult to generalize this to the practicing public. I was impressed by the MitraClip performance: the reduction in MR [mitral regurgitation], the lack of recurrence, and the small number of complications. Perhaps more than anything else, the difference between Mitra-FR and COAPT was the quality of the operators.
Martin B. Leon, MD , is the director of the Center for Interventional Vascular Therapy at Columbia University, N.Y., and the Cardiovascular Research Foundation’s founder and codirector of medical research and education. He was not involved in COAPT, and made his comments after the study presentation.
This is really a blockbuster trial, because you see a statistically significant reduction in cardiovascular endpoints, which is something we almost never see in device-based trials. I think this is going to change clinical practice, but the question of generalizability is tricky. This was such a well-conducted trial; it may be difficult to generalize this to the practicing public. I was impressed by the MitraClip performance: the reduction in MR [mitral regurgitation], the lack of recurrence, and the small number of complications. Perhaps more than anything else, the difference between Mitra-FR and COAPT was the quality of the operators.
Martin B. Leon, MD , is the director of the Center for Interventional Vascular Therapy at Columbia University, N.Y., and the Cardiovascular Research Foundation’s founder and codirector of medical research and education. He was not involved in COAPT, and made his comments after the study presentation.
SAN DIEGO – Among a carefully selected subset of heart failure patients and severe secondary mitral regurgitation, transcatheter mitral valve repair with the MitraClip reduced hospitalizations for heart failure by 47%, and death from any cause by 38% over 24 months, compared with maximal medical therapy alone.
That’s according to a randomized, open-label trial presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
The number needed to treat to prevent one heart failure (HF) hospitalization within 2 years was three; the number needed to treat to save one life was six. Only about 3% of patients had a device complication within 12 months of placement in the study, dubbed COAPT (the Heart Failure Patients with Functional Mitral Regurgitation Trial).
COAPT patients had grade 3+ or 4+ secondary mitral regurgitation, with a mean effective regurgitant orifice area (EROA) of 41 mm2. Their left ventricles were dilated, but not huge, with a mean left ventricular end-diastolic volume of 101 mL/m2. “We estimate that’s about 10% of heart failure patients,” said lead investigator and interventional cardiologist Gregg W. Stone, MD, a professor of medicine at Columbia University, New York.
MitraClip placement was performed in high-volume centers by experienced operators, and patients were on maximally tolerated doses of guideline-directed medical therapy, as per the 2013 American College of Cardiology/American Heart Association heart failure management guidelines. There was very little variation in treatment regimens during the 2-year trial (J Am Coll Cardiol. 2017;70:776-803).
Those parameters matter. Among HF patients who did not fit them in the recent Mitra-FR trial in France, MitraClip did not reduce rates of death or unplanned hospitalization (N Engl J Med. 2018 Aug 27. doi: 10.1056/NEJMoa1805374).
COAPT and Mitra-FR investigators said at the meeting that the studies are complimentary, not conflicting, because together, they define secondary mitral regurgitation (MR) patients who will and will not benefit from the device.
MR was less severe in Mitra-FR, with a mean EROA of 31 mm2, but left ventricles were more dilated, with a mean left ventricular end-diastolic volume of 135 mL/m2. Patients were on more real-world drug regimens that varied over the course of the trial. Also, the lower implantation rates and higher complication rates in Mitra-FR “suggests perhaps greater experience of the COAPT operators,” said Dr. Stone, who also is the director of cardiovascular research and education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center.
In short, “they were a different patient population than were enrolled in COAPT,” he said at the meeting, sponsored by the Cardiovascular Research Foundation, which Dr. Stone also codirects.
There was great excitement at TCT about COAPT because there was a startling benefit for patients who previously had few options. But many speakers worried that the hype surrounding the trial will drown out the critically important message about patient selection and that the clip will be used in HF patients who don’t fit the COAPT profile.
They also said that the emerging picture of benefit in patients with less ventricular dilation but more mitral regurgitation needs to be fleshed out and better quantified.
COAPT randomized 302 patients to MitraClip on a background of guideline-directed therapy and 312 to guideline-directed therapy alone. Participants who had mitral regurgitation caused by left ventricular dysfunction, were not surgical candidates, and remained symptomatic despite optimal treatment.
The annualized rate of all hospitalizations for HF within 24 months was 35.8% per patient-year in the device group, as compared with 67.9% per patient-year in the control group, for a relative reduction of 47% (P less than .001).
Death from any cause within 24 months occurred in 29.1% of the patients in the device group and 46.1% in the control group, yielding a reduction of 38% (P less than .001).
“We didn’t cure patients by fixing their MR. They still had 29% 2-year mortality, but we did markedly improve their quality of life. The only subgroup that didn’t benefit were patients that had an EORA of less than 30 mm2 and end diastolic volume greater than the median” of 96 mL/m2, which was “fascinating,” Dr. Stone said, and fit the emerging picture.
Mitral regurgitation grade fell to 1+ or lower in 82% of patients after clip placement and remained there in the majority of survivors at 2 years.
For a long time, “HF experts thought MR was just a marker of severe left ventricular dysfunction. What I think we see here is that secondary MR is not just a bystander. It contributes to the abnormal pathophysiology of these patients,” he said.
The trial was sponsored by MitraClip’s maker, Abbott. The company participated in site selection, management, and data analysis. Dr. Stone disclosed that his employer, Columbia University, receives royalties from Abbott for sale of the clip. Several fellow investigators disclosed grants, fees, and other financial ties to the company.
Simultaneously with the COAPT presentation, the results were published online (N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640).
Mitra-FR was funded by the French Ministry of Health and Research and Abbott.
SAN DIEGO – Among a carefully selected subset of heart failure patients and severe secondary mitral regurgitation, transcatheter mitral valve repair with the MitraClip reduced hospitalizations for heart failure by 47%, and death from any cause by 38% over 24 months, compared with maximal medical therapy alone.
That’s according to a randomized, open-label trial presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
The number needed to treat to prevent one heart failure (HF) hospitalization within 2 years was three; the number needed to treat to save one life was six. Only about 3% of patients had a device complication within 12 months of placement in the study, dubbed COAPT (the Heart Failure Patients with Functional Mitral Regurgitation Trial).
COAPT patients had grade 3+ or 4+ secondary mitral regurgitation, with a mean effective regurgitant orifice area (EROA) of 41 mm2. Their left ventricles were dilated, but not huge, with a mean left ventricular end-diastolic volume of 101 mL/m2. “We estimate that’s about 10% of heart failure patients,” said lead investigator and interventional cardiologist Gregg W. Stone, MD, a professor of medicine at Columbia University, New York.
MitraClip placement was performed in high-volume centers by experienced operators, and patients were on maximally tolerated doses of guideline-directed medical therapy, as per the 2013 American College of Cardiology/American Heart Association heart failure management guidelines. There was very little variation in treatment regimens during the 2-year trial (J Am Coll Cardiol. 2017;70:776-803).
Those parameters matter. Among HF patients who did not fit them in the recent Mitra-FR trial in France, MitraClip did not reduce rates of death or unplanned hospitalization (N Engl J Med. 2018 Aug 27. doi: 10.1056/NEJMoa1805374).
COAPT and Mitra-FR investigators said at the meeting that the studies are complimentary, not conflicting, because together, they define secondary mitral regurgitation (MR) patients who will and will not benefit from the device.
MR was less severe in Mitra-FR, with a mean EROA of 31 mm2, but left ventricles were more dilated, with a mean left ventricular end-diastolic volume of 135 mL/m2. Patients were on more real-world drug regimens that varied over the course of the trial. Also, the lower implantation rates and higher complication rates in Mitra-FR “suggests perhaps greater experience of the COAPT operators,” said Dr. Stone, who also is the director of cardiovascular research and education at the Center for Interventional Vascular Therapy at New York-Presbyterian Hospital/Columbia University Medical Center.
In short, “they were a different patient population than were enrolled in COAPT,” he said at the meeting, sponsored by the Cardiovascular Research Foundation, which Dr. Stone also codirects.
There was great excitement at TCT about COAPT because there was a startling benefit for patients who previously had few options. But many speakers worried that the hype surrounding the trial will drown out the critically important message about patient selection and that the clip will be used in HF patients who don’t fit the COAPT profile.
They also said that the emerging picture of benefit in patients with less ventricular dilation but more mitral regurgitation needs to be fleshed out and better quantified.
COAPT randomized 302 patients to MitraClip on a background of guideline-directed therapy and 312 to guideline-directed therapy alone. Participants who had mitral regurgitation caused by left ventricular dysfunction, were not surgical candidates, and remained symptomatic despite optimal treatment.
The annualized rate of all hospitalizations for HF within 24 months was 35.8% per patient-year in the device group, as compared with 67.9% per patient-year in the control group, for a relative reduction of 47% (P less than .001).
Death from any cause within 24 months occurred in 29.1% of the patients in the device group and 46.1% in the control group, yielding a reduction of 38% (P less than .001).
“We didn’t cure patients by fixing their MR. They still had 29% 2-year mortality, but we did markedly improve their quality of life. The only subgroup that didn’t benefit were patients that had an EORA of less than 30 mm2 and end diastolic volume greater than the median” of 96 mL/m2, which was “fascinating,” Dr. Stone said, and fit the emerging picture.
Mitral regurgitation grade fell to 1+ or lower in 82% of patients after clip placement and remained there in the majority of survivors at 2 years.
For a long time, “HF experts thought MR was just a marker of severe left ventricular dysfunction. What I think we see here is that secondary MR is not just a bystander. It contributes to the abnormal pathophysiology of these patients,” he said.
The trial was sponsored by MitraClip’s maker, Abbott. The company participated in site selection, management, and data analysis. Dr. Stone disclosed that his employer, Columbia University, receives royalties from Abbott for sale of the clip. Several fellow investigators disclosed grants, fees, and other financial ties to the company.
Simultaneously with the COAPT presentation, the results were published online (N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640).
Mitra-FR was funded by the French Ministry of Health and Research and Abbott.
REPORTING FROM TCT 2018
Key clinical point: MitraClip reduced death and heart failure hospitalizations in certain patients, but
Major finding: Among a subset of heart failure patients with moderately dilated left ventricles and severe secondary mitral regurgitation, transcatheter mitral valve repair with the MitraClip reduced hospitalizations for heart failure within 24 months by 47%, and death from any cause within 24 months by 38%, compared with maximal medical therapy alone.
Study details: COAPT, a randomized, open-label trial with 614 subjects
Disclosures: The work was funded by MitraClip maker, Abbott. The company participated in site selection, management, and data analysis. The lead and several other investigators disclosed financial ties to the company.
DIVA results similar for drug-eluting, bare-metal stents
Drug-eluting stents (DESs) and less-expensive bare-metal stents (BMSs) performed equally well in patients with failed saphenous vein grafts after coronary artery bypass graft surgery, based on an analysis of patients in the DIVA trial.
The findings run counter to those of previous clinical trials, which had found drug-eluting stents perform better than bare-metal stents in these situations. “The study results have important economic implications in countries with high DES prices, such as the USA, because they suggest that the lower-cost BMS can be used in SVG [saphenous vein graft] lesions without compromising either safety or efficacy,” lead author Emmanouil S. Brilakis, MD, PhD, of Minneapolis Heart Institute and his coauthors said in reporting the results for the DIVA trial investigators in the Lancet.
The DIVA trial was a randomized, double-blind, controlled trial done at 25 U.S. Department of Veterans Affairs centers. Researchers randomly assigned 599 patients who had previous coronary artery bypass surgery to either the DES or BMS groups, and the study reported data from 597 patients. The combined endpoint comprised cardiac death, target vessel MI, or target vessel revascularization at 12 months and then over the entire length of follow-up, which ranged from 2 to 7 years. Operators used the DES or BMS of their choice.
While BMSs are presumed to be less expensive than DESs, the study authors did not provide prices or price ranges for the stents. Dr. Brilakis and his coauthors acknowledged that the financial implications depend on local stent pricing practices.
The cost-effectiveness of using DESs vs. BMSs has been controversial, with many studies reporting that BMS are cost-effective over the long-term because of the lower incidence of revascularization and later hospitalization. These studies did not differentiate between SVG and native vessels, however. Multiple studies have reported that the overall costs, including the cost for reintervention, are lower for DESs than for BMSs in native vessels. A Wake Forest study reported the average per procedure cost was $1,846 higher for a DES but the cost was offset after 3 years by lower revascularization rates (Circ Cardiovasc Qual Outcomes. 2011. doi: 10.1161/CIRCOUTCOMES.110.960187)
A recent Korean study found the total cost of DESs was about 5% higher (Yonsei Med J. 2014 Nov;55[6]:1533-41). A French study reported BMSs resulted in a cost reduction $217 per case (Open Heart. 2016 Aug 25;3[2]:e000445). But few, if any, studies have directly compared prices hospitals pay for DESs and BMSs.
Pricing aside, Dr. Brilakis and his coauthors reported no statistical differences in terms of outcomes between the DES and BMS groups. Baseline characteristics of both groups were similar, and the vessel failure rates were 17% in the DES group and 19% in the BMS group after 12 months of follow-up. After 2-7 years, “target vessel failure occurred in approximately one in three patients, with no difference between the bare-metal and drug-eluting stents,” Dr. Brilakis and his coauthors said.
There was no significant difference in cardiac death rates – 5% for DES patients and 4% for BMS patients – or in rates of target lesion revascularization, at 9% and 8%, respectively. Postprocedure medication rates were also similar between the two groups. For example, the rates of patients on P2Y12 inhibitors were 89% for both groups at 12 months and, among those who had follow-up at 36 months, 48% for DES and 44% for BMS.
Among the limitations of the study that Dr. Brilakis and his coauthors noted was the high proportion of men in the VA population – only two women, both in the DES group, participated in the study – and the interventionists doing the index SVG intervention were not masked to the type of stent used.
Dr. Brilakis disclosed relationships with Abbott Vascular, Amgen, Asahi, Boston Scientific, Cardinal Health, CSI, Elsevier, GE Healthcare, Medicure, Medtronic, Nitiloop, InfraRedx, and Osprey.
SOURCE: Brilakis ES et al. Lancet. 2018 May 19;391(10134);1997-2007.
The predominant use of second-generation drug-eluting stents in the DIVA study may explain why the researchers found no difference in outcomes for bare metal and drug-eluting stents.
Most patients in previous trials were treated with first-generation drug-eluting stents, but second-generation drug-eluting stents perform better than their first-generation counterparts in native coronary artery disease. One might think that this finding should also apply to saphenous vein bypass graft lesions in which atherosclerosis is more aggressive and the progress of the disease much faster, yet this was not the case in DIVA, and the study authors did not provide an explanation for this finding.
One possible reason for the comparability of outcomes in the drug-eluting stents and bare metal stents groups may be that saphenous vein bypass graft lesions may be more favorably disposed to paclitaxel, commonly used in first-generation drug-eluting stents, than the drugs found in the second-generation stents. The DIVA findings may indicate that the second-generation drug-eluting stents performed worse, not that the bare metal stents performed better.
Studies of only first-generation paclitaxel-eluting stents showed a sustained benefit. Any notion that the pathophysiology of saphenous vein grafts might make them more amenable to a bare metal stent while a drug-eluting stent is better suited for native vessels is purely speculative. Further research comparing the effect of different stent types in saphenous vein bypass graft failure is warranted.
Raban V. Jeger, MD, and Sven Möbius-Winkler, MD, made these remarks in an invited commentary. Dr. Jeger is with the University Hospital Basel (Switzerland), and Dr. Möbius-Winkler is with University Hospital Jena (Germany). Dr. Jeger disclosed he is the principal investigator of the BASKET-SAVAGE trial, which received funding from Boston Scientific Germany. Dr. Möbius-Winkler had no financial relationships to disclose.
The predominant use of second-generation drug-eluting stents in the DIVA study may explain why the researchers found no difference in outcomes for bare metal and drug-eluting stents.
Most patients in previous trials were treated with first-generation drug-eluting stents, but second-generation drug-eluting stents perform better than their first-generation counterparts in native coronary artery disease. One might think that this finding should also apply to saphenous vein bypass graft lesions in which atherosclerosis is more aggressive and the progress of the disease much faster, yet this was not the case in DIVA, and the study authors did not provide an explanation for this finding.
One possible reason for the comparability of outcomes in the drug-eluting stents and bare metal stents groups may be that saphenous vein bypass graft lesions may be more favorably disposed to paclitaxel, commonly used in first-generation drug-eluting stents, than the drugs found in the second-generation stents. The DIVA findings may indicate that the second-generation drug-eluting stents performed worse, not that the bare metal stents performed better.
Studies of only first-generation paclitaxel-eluting stents showed a sustained benefit. Any notion that the pathophysiology of saphenous vein grafts might make them more amenable to a bare metal stent while a drug-eluting stent is better suited for native vessels is purely speculative. Further research comparing the effect of different stent types in saphenous vein bypass graft failure is warranted.
Raban V. Jeger, MD, and Sven Möbius-Winkler, MD, made these remarks in an invited commentary. Dr. Jeger is with the University Hospital Basel (Switzerland), and Dr. Möbius-Winkler is with University Hospital Jena (Germany). Dr. Jeger disclosed he is the principal investigator of the BASKET-SAVAGE trial, which received funding from Boston Scientific Germany. Dr. Möbius-Winkler had no financial relationships to disclose.
The predominant use of second-generation drug-eluting stents in the DIVA study may explain why the researchers found no difference in outcomes for bare metal and drug-eluting stents.
Most patients in previous trials were treated with first-generation drug-eluting stents, but second-generation drug-eluting stents perform better than their first-generation counterparts in native coronary artery disease. One might think that this finding should also apply to saphenous vein bypass graft lesions in which atherosclerosis is more aggressive and the progress of the disease much faster, yet this was not the case in DIVA, and the study authors did not provide an explanation for this finding.
One possible reason for the comparability of outcomes in the drug-eluting stents and bare metal stents groups may be that saphenous vein bypass graft lesions may be more favorably disposed to paclitaxel, commonly used in first-generation drug-eluting stents, than the drugs found in the second-generation stents. The DIVA findings may indicate that the second-generation drug-eluting stents performed worse, not that the bare metal stents performed better.
Studies of only first-generation paclitaxel-eluting stents showed a sustained benefit. Any notion that the pathophysiology of saphenous vein grafts might make them more amenable to a bare metal stent while a drug-eluting stent is better suited for native vessels is purely speculative. Further research comparing the effect of different stent types in saphenous vein bypass graft failure is warranted.
Raban V. Jeger, MD, and Sven Möbius-Winkler, MD, made these remarks in an invited commentary. Dr. Jeger is with the University Hospital Basel (Switzerland), and Dr. Möbius-Winkler is with University Hospital Jena (Germany). Dr. Jeger disclosed he is the principal investigator of the BASKET-SAVAGE trial, which received funding from Boston Scientific Germany. Dr. Möbius-Winkler had no financial relationships to disclose.
Drug-eluting stents (DESs) and less-expensive bare-metal stents (BMSs) performed equally well in patients with failed saphenous vein grafts after coronary artery bypass graft surgery, based on an analysis of patients in the DIVA trial.
The findings run counter to those of previous clinical trials, which had found drug-eluting stents perform better than bare-metal stents in these situations. “The study results have important economic implications in countries with high DES prices, such as the USA, because they suggest that the lower-cost BMS can be used in SVG [saphenous vein graft] lesions without compromising either safety or efficacy,” lead author Emmanouil S. Brilakis, MD, PhD, of Minneapolis Heart Institute and his coauthors said in reporting the results for the DIVA trial investigators in the Lancet.
The DIVA trial was a randomized, double-blind, controlled trial done at 25 U.S. Department of Veterans Affairs centers. Researchers randomly assigned 599 patients who had previous coronary artery bypass surgery to either the DES or BMS groups, and the study reported data from 597 patients. The combined endpoint comprised cardiac death, target vessel MI, or target vessel revascularization at 12 months and then over the entire length of follow-up, which ranged from 2 to 7 years. Operators used the DES or BMS of their choice.
While BMSs are presumed to be less expensive than DESs, the study authors did not provide prices or price ranges for the stents. Dr. Brilakis and his coauthors acknowledged that the financial implications depend on local stent pricing practices.
The cost-effectiveness of using DESs vs. BMSs has been controversial, with many studies reporting that BMS are cost-effective over the long-term because of the lower incidence of revascularization and later hospitalization. These studies did not differentiate between SVG and native vessels, however. Multiple studies have reported that the overall costs, including the cost for reintervention, are lower for DESs than for BMSs in native vessels. A Wake Forest study reported the average per procedure cost was $1,846 higher for a DES but the cost was offset after 3 years by lower revascularization rates (Circ Cardiovasc Qual Outcomes. 2011. doi: 10.1161/CIRCOUTCOMES.110.960187)
A recent Korean study found the total cost of DESs was about 5% higher (Yonsei Med J. 2014 Nov;55[6]:1533-41). A French study reported BMSs resulted in a cost reduction $217 per case (Open Heart. 2016 Aug 25;3[2]:e000445). But few, if any, studies have directly compared prices hospitals pay for DESs and BMSs.
Pricing aside, Dr. Brilakis and his coauthors reported no statistical differences in terms of outcomes between the DES and BMS groups. Baseline characteristics of both groups were similar, and the vessel failure rates were 17% in the DES group and 19% in the BMS group after 12 months of follow-up. After 2-7 years, “target vessel failure occurred in approximately one in three patients, with no difference between the bare-metal and drug-eluting stents,” Dr. Brilakis and his coauthors said.
There was no significant difference in cardiac death rates – 5% for DES patients and 4% for BMS patients – or in rates of target lesion revascularization, at 9% and 8%, respectively. Postprocedure medication rates were also similar between the two groups. For example, the rates of patients on P2Y12 inhibitors were 89% for both groups at 12 months and, among those who had follow-up at 36 months, 48% for DES and 44% for BMS.
Among the limitations of the study that Dr. Brilakis and his coauthors noted was the high proportion of men in the VA population – only two women, both in the DES group, participated in the study – and the interventionists doing the index SVG intervention were not masked to the type of stent used.
Dr. Brilakis disclosed relationships with Abbott Vascular, Amgen, Asahi, Boston Scientific, Cardinal Health, CSI, Elsevier, GE Healthcare, Medicure, Medtronic, Nitiloop, InfraRedx, and Osprey.
SOURCE: Brilakis ES et al. Lancet. 2018 May 19;391(10134);1997-2007.
Drug-eluting stents (DESs) and less-expensive bare-metal stents (BMSs) performed equally well in patients with failed saphenous vein grafts after coronary artery bypass graft surgery, based on an analysis of patients in the DIVA trial.
The findings run counter to those of previous clinical trials, which had found drug-eluting stents perform better than bare-metal stents in these situations. “The study results have important economic implications in countries with high DES prices, such as the USA, because they suggest that the lower-cost BMS can be used in SVG [saphenous vein graft] lesions without compromising either safety or efficacy,” lead author Emmanouil S. Brilakis, MD, PhD, of Minneapolis Heart Institute and his coauthors said in reporting the results for the DIVA trial investigators in the Lancet.
The DIVA trial was a randomized, double-blind, controlled trial done at 25 U.S. Department of Veterans Affairs centers. Researchers randomly assigned 599 patients who had previous coronary artery bypass surgery to either the DES or BMS groups, and the study reported data from 597 patients. The combined endpoint comprised cardiac death, target vessel MI, or target vessel revascularization at 12 months and then over the entire length of follow-up, which ranged from 2 to 7 years. Operators used the DES or BMS of their choice.
While BMSs are presumed to be less expensive than DESs, the study authors did not provide prices or price ranges for the stents. Dr. Brilakis and his coauthors acknowledged that the financial implications depend on local stent pricing practices.
The cost-effectiveness of using DESs vs. BMSs has been controversial, with many studies reporting that BMS are cost-effective over the long-term because of the lower incidence of revascularization and later hospitalization. These studies did not differentiate between SVG and native vessels, however. Multiple studies have reported that the overall costs, including the cost for reintervention, are lower for DESs than for BMSs in native vessels. A Wake Forest study reported the average per procedure cost was $1,846 higher for a DES but the cost was offset after 3 years by lower revascularization rates (Circ Cardiovasc Qual Outcomes. 2011. doi: 10.1161/CIRCOUTCOMES.110.960187)
A recent Korean study found the total cost of DESs was about 5% higher (Yonsei Med J. 2014 Nov;55[6]:1533-41). A French study reported BMSs resulted in a cost reduction $217 per case (Open Heart. 2016 Aug 25;3[2]:e000445). But few, if any, studies have directly compared prices hospitals pay for DESs and BMSs.
Pricing aside, Dr. Brilakis and his coauthors reported no statistical differences in terms of outcomes between the DES and BMS groups. Baseline characteristics of both groups were similar, and the vessel failure rates were 17% in the DES group and 19% in the BMS group after 12 months of follow-up. After 2-7 years, “target vessel failure occurred in approximately one in three patients, with no difference between the bare-metal and drug-eluting stents,” Dr. Brilakis and his coauthors said.
There was no significant difference in cardiac death rates – 5% for DES patients and 4% for BMS patients – or in rates of target lesion revascularization, at 9% and 8%, respectively. Postprocedure medication rates were also similar between the two groups. For example, the rates of patients on P2Y12 inhibitors were 89% for both groups at 12 months and, among those who had follow-up at 36 months, 48% for DES and 44% for BMS.
Among the limitations of the study that Dr. Brilakis and his coauthors noted was the high proportion of men in the VA population – only two women, both in the DES group, participated in the study – and the interventionists doing the index SVG intervention were not masked to the type of stent used.
Dr. Brilakis disclosed relationships with Abbott Vascular, Amgen, Asahi, Boston Scientific, Cardinal Health, CSI, Elsevier, GE Healthcare, Medicure, Medtronic, Nitiloop, InfraRedx, and Osprey.
SOURCE: Brilakis ES et al. Lancet. 2018 May 19;391(10134);1997-2007.
FROM THE LANCET
Key clinical point: Drug-eluting and bare-metal stents had similar outcomes for saphenous vein bypass lesions.
Major finding: Target vessel failure was 17% for drug-eluting stents and 19% for bare metal stents.
Study details: The DIVA trial randomly assigned 599 patients with post-CABG saphenous vein bypass graft failure to drug-eluting or bare metal stents between Jan. 1, 2012, and Dec. 31, 2015.
Disclosures: Dr. Brilakis disclosed relationships with Abbott Vascular, Amgen, Asahi, Boston Scientific, Cardinal Health, CSI, Elsevier, GE Healthcare, Medicure, Medtronic, Nitiloop, InfraRedx, and Osprey.
Source: Brilakis ES et al. Lancet. 2018 May 19;391(10134);1997-2007.
Restrictive transfusions do not increase long-term CV surgery risk
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
MUNICH – in a large, randomized trial presented at the annual congress of the European Society of Cardiology, and published simultaneously in the New England Journal of Medicine.
The investigators previously reported that 28 day outcomes were non-inferior with the restrictive approach, but they wanted to look into 6 month results to rule out latent problems, such as sequelae from perioperative organ hypoxia.
The new outcomes from the study, dubbed the Transfusion Requirements in Cardiac Surgery (TRICS) III trial, offer some reassurance at a time when cardiac surgeons are shifting towards more restrictive transfusion policies (N Engl J Med. 2018 Aug 26.doi: 10.1056/NEJMoa1808561).
The trial randomized 2,317 patients to red cell transfusions if their hemoglobin concentrations fell below 7.5 g/dL intraoperatively or postoperatively. Another 2,347 were randomized to the liberal approach, with transfusions below 9.5 g/dL in the operating room and ICU, and below 8.5 g/dL outside of the ICU. The arms were well matched, with a mean score of 8 in both groups on the 47-point European System for Cardiac Operative Risk Evaluation I score.
At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive threshold); 6.2% of patients in the restrictive-threshold group, versus 6.4% in the liberal-arm, had died at that point, a statistically insignificant difference.
Also at 6 months, 43.8% of patients in the restrictive-threshold group, versus 42.8% in the liberal arm, met the study’s secondary composite outcome, which included the components of the primary outcome plus hospital readmissions, ED visits, and coronary revascularization. The difference was again statistically insignificant.
The restrictive strategy saved a lot of blood. Just over half of the patients, versus almost three-quarters in the liberal arm, were transfused during their index admissions. When transfused, patients in the restrictive arm received a median of 2 units of red cells; liberal-arm patients received a median of 3 units.
Unexpectedly, patients 75 years and older had a lower risk of poor outcomes with the restrictive strategy, while the liberal strategy was associated with lower risk in younger subjects. The findings “appear to contradict” current practice, “in which a liberal transfusion strategy is used in older patients undergoing cardiac or noncardiac surgery,” said investigators led by C. David Mazer, MD, a professor in the department of anesthesia at the University of Toronto.
“One could hypothesize that older patients may have unacceptable adverse effects related to transfusion (e.g., volume overload and inflammatory and infectious complications) or that there may be age-related differences in the adverse-effect profile of transfusion or anemia. ... Whether transfusion thresholds should differ according to age” needs to be determined, they said.
The participants were a mean of 72 years old, and 35% were women. The majority in both arms underwent coronary artery bypass surgery, valve surgery, or both. Heart transplants were excluded. The trial was conducted in 19 countries, including China and India; results were consistent across study sites.
The work was funded by the Canadian Institutes of Health Research, among others. Dr. Mazer had no relevant disclosures.
SOURCE: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
REPORTING FROM THE ESC CONGRESS 2018
Key clinical point: A restrictive transfusion strategy during cardiovascular surgery, versus a more traditional liberal approach, did not increase the risk of poor outcomes at 6 months.
Major finding: At 6 months, 17.4% of patients in the restrictive arm, versus 17.1% in the liberal-threshold group, met the primary composite outcome of death from any cause, myocardial infarction, stroke, or new onset renal failure with dialysis (P = .006 for noninferiority with the restrictive approach).
Study details: Randomized, multicenter trial with over 5,000 surgery patients
Disclosures: The work was funded by the Canadian Institutes of Health Research, among others. The lead investigator had no relevant disclosures.
Source: Mazer CD et al. N Engl J Med. 2018 Aug 26. doi:10.1056/NEJMoa1808561
Lung volume reduction procedures on the rise since 2011
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
AT ATS 2018
Key clinical point: Lung volume–reduction surgery remains an evidence-based therapy for a cohort of severe COPD patients.
Major finding: Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
Study details: A retrospective cohort analysis of 2,805 patients who underwent LVRS.
Disclosures: The researchers reported having no financial disclosures.
Source: Attaway A et al. Abstract 4436, ATS 2018.
Customized airway stents show promise in feasibility trial
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
SAN DIEGO – for whom conventional stents were not suitable or failed, results from a small study demonstrated.
“Anatomically complex airway stenosis remains a challenging situation,” lead study author Nicolas Guibert, MD, said at an international conference of the American Thoracic Society. “Conventional devices are either not suited or may result in a significant complication rate, including poor clinical tolerance, migration, or granulation tissue reaction due to lack of congruence.”
Dr. Guibert reported results from eight patients. Of these, three had posttransplant complex airway stenoses involving the bronchus intermedius. Each improved after placement of the customized stents. For example, one patient with vanishing bronchus intermedius syndrome experienced improvements in NYHA dyspnea score from 3 to 1, the VQ11 score from 22 to 11/55, and forced expiratory volume in 1 second (FEV1) from 70% to 107%. The stent was removed after 3 months. Meanwhile, a patient with localized malacia and stenosis of the right main bronchus experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 score from 27 to 15/55, and FEV1 from 70% to 102%. That person’s stent is still in place with no complications. Another patient with localized malacia and stenosis of the bronchus intermedius experienced improvements in FEV1 from 84% to 100%. That person’s device was removed after 3 months, with no residual stenosis.
A fourth patient underwent stent placement for localized malacia (cartilage ring rupture). That person experienced improvements in NYHA dyspnea score from 3 to 1, VQ11 from 23 to 15/55, and FEV1 from 66% to 92%, and peak flow from 49% to 82%. The device is still in place with no complications. A fifth patient received stent placement for extensive tracheobronchomalacia, but it had imperfect congruence and was removed after 3 months because it caused intense cough.
One patient with post-tracheotomy stenosis experienced improvements in NYHA dyspnea score from 3 to 0, VQ11 from 29 to 12/55, and peak flow from 45% to 81%. That person’s device is still in place, Dr. Guibert said. Two other patients treated for post-tracheotomy experienced stent migration (conventional stents also migrated in these two cases), despite good bronchoscopic congruence after placement.
“Tracheal diseases result in suboptimal congruence, probably due to higher respiratory variation,” Dr. Guibert said. “These devices need to be studied in less selected populations and the technology has to be improved.” He reported having no financial disclosures.
SOURCE: Guibert N et al. ATS 2018, Abstract 4433.
REPORTING FROM ATS 2018
Key clinical point: Customized, 3-D airway stents have the potential for improving tolerance and decreasing the complication rate.
Major finding: Congruence and outcomes tended to be better in stenoses involving the bronchial level (three of three, no complications).
Study details: A feasibility study of eight patients with nonmalignant, anatomically complex, and symptomatic stenosis for which conventional stents were not suitable.
Disclosures: Dr. Guibert reported having no financial disclosures.
Source: Guibert N et al. ATS 2018, Abstract 4433.
TAVR safe in low-risk aortic stenosis, early data indicate
WASHINGTON – , according to results of the first U.S. study to evaluate mortality in a low-risk population.
Other complications were also low relative to those seen in previously published studies with higher-risk populations. The preliminary results are “reassuring,” Ronald Waksman, MD, associate director of the division of cardiology, Medstar Health Institute, Washington, DC, reported at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
In this study, a low-risk eligibility criterion is a Society of Thoracic Surgeons score of 3% or less. The mean score in the first 125 patients was 1.9%.
At 30 days, there were no myocardial infarctions, no acute kidney injuries of stage II or greater, and no transient ischemic attacks. There was one nondisabling stroke, one paravalvular leak, and one coronary obstruction that required a coronary artery bypass graft. The rates of new onset atrial fibrillation, major vascular complications, and life-threatening or major bleeding events were lower than 5%.
Surgical atrial valve replacement (SAVR) currently has a IB recommendation for the treatment of symptomatic low-risk aortic stenosis, but TAVR is not currently indicated in this population, according to 2017 American Heart Association/American College of Cardiology guidelines. There is, however, substantial interest in extending TAVR to a low-risk population, according to Dr. Waksman.
“We know that there are potential benefits for TAVR versus SAVR that include reduced ICU and hospital length of stay, more rapid quality of life recovery, lower risk of bleeding, and lower risk of postprocedure AF [atrial fibrillation],” said Dr. Waksman in explaining the rationale for this and other ongoing studies testing TAVR in low-risk patients.
In this investigator-initiated study, which was conducted without industry support, 11 sites participated. Most were relatively low-volume centers with fewer than 150 TAVR procedures performed annually, according to Dr. Waksman. The choice of TAVR device was left to the discretion of the operator.
Reflecting a lower-risk population, the mean age of 74.6 years is younger than that of participants in previous TAVR trials. The mean left ventricular ejection fraction was 62.9%. Only 4.8% had a prior MI and 19.2% had a prior coronary artery bypass graft. Most procedures were performed under conscious sedation with fewer than 20% receiving general anesthesia. The mean fluoroscopy time was 16 minutes.
The degree of improvement in hemodynamics following TAVR in this population was called “excellent,” but Dr. Waksman did report a 12.5% incidence of hypo-attenuating leaflet thickening (HALT), an 11% incidence of reduced leaflet motion, and a 9.3% incidence of hypo-attenuation affecting motion. All were subclinical effects.
In a closer analysis of HALT, the rate was 14.4% in the 80.8% of patients who received antiplatelet therapy versus 4.8% in the 17.5% who received oral anticoagulation (some received neither). Dr. Waksman called the greater association of HALT with antiplatelet therapy “a potential signal” that deserves further study.
Full results from all 200 patients are expected in the fall of 2018.
Jeffrey Popma, MD, director of the interventional cardiology clinical service, Beth Israel Deaconess Hospital, Boston, and moderator of the session where the data were presented, called for a direct comparison with SAVR in low-risk patients to place the relative role of these options into context.
Neil Moat, a consultant cardiac surgeon at Royal Brompton & Harefield Hospital, London, agreed. Although he is also encouraged by the evidence of safety in low-risk patients, he labeled the rate of HALT in this study “a concern.”
Dr. Waksman reported financial relationships with Abbott Vascular, Amgen, AstraZeneca, Life Technologies, Med Alliance, Medtronic Vascular, and Symetis, among other companies.
WASHINGTON – , according to results of the first U.S. study to evaluate mortality in a low-risk population.
Other complications were also low relative to those seen in previously published studies with higher-risk populations. The preliminary results are “reassuring,” Ronald Waksman, MD, associate director of the division of cardiology, Medstar Health Institute, Washington, DC, reported at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
In this study, a low-risk eligibility criterion is a Society of Thoracic Surgeons score of 3% or less. The mean score in the first 125 patients was 1.9%.
At 30 days, there were no myocardial infarctions, no acute kidney injuries of stage II or greater, and no transient ischemic attacks. There was one nondisabling stroke, one paravalvular leak, and one coronary obstruction that required a coronary artery bypass graft. The rates of new onset atrial fibrillation, major vascular complications, and life-threatening or major bleeding events were lower than 5%.
Surgical atrial valve replacement (SAVR) currently has a IB recommendation for the treatment of symptomatic low-risk aortic stenosis, but TAVR is not currently indicated in this population, according to 2017 American Heart Association/American College of Cardiology guidelines. There is, however, substantial interest in extending TAVR to a low-risk population, according to Dr. Waksman.
“We know that there are potential benefits for TAVR versus SAVR that include reduced ICU and hospital length of stay, more rapid quality of life recovery, lower risk of bleeding, and lower risk of postprocedure AF [atrial fibrillation],” said Dr. Waksman in explaining the rationale for this and other ongoing studies testing TAVR in low-risk patients.
In this investigator-initiated study, which was conducted without industry support, 11 sites participated. Most were relatively low-volume centers with fewer than 150 TAVR procedures performed annually, according to Dr. Waksman. The choice of TAVR device was left to the discretion of the operator.
Reflecting a lower-risk population, the mean age of 74.6 years is younger than that of participants in previous TAVR trials. The mean left ventricular ejection fraction was 62.9%. Only 4.8% had a prior MI and 19.2% had a prior coronary artery bypass graft. Most procedures were performed under conscious sedation with fewer than 20% receiving general anesthesia. The mean fluoroscopy time was 16 minutes.
The degree of improvement in hemodynamics following TAVR in this population was called “excellent,” but Dr. Waksman did report a 12.5% incidence of hypo-attenuating leaflet thickening (HALT), an 11% incidence of reduced leaflet motion, and a 9.3% incidence of hypo-attenuation affecting motion. All were subclinical effects.
In a closer analysis of HALT, the rate was 14.4% in the 80.8% of patients who received antiplatelet therapy versus 4.8% in the 17.5% who received oral anticoagulation (some received neither). Dr. Waksman called the greater association of HALT with antiplatelet therapy “a potential signal” that deserves further study.
Full results from all 200 patients are expected in the fall of 2018.
Jeffrey Popma, MD, director of the interventional cardiology clinical service, Beth Israel Deaconess Hospital, Boston, and moderator of the session where the data were presented, called for a direct comparison with SAVR in low-risk patients to place the relative role of these options into context.
Neil Moat, a consultant cardiac surgeon at Royal Brompton & Harefield Hospital, London, agreed. Although he is also encouraged by the evidence of safety in low-risk patients, he labeled the rate of HALT in this study “a concern.”
Dr. Waksman reported financial relationships with Abbott Vascular, Amgen, AstraZeneca, Life Technologies, Med Alliance, Medtronic Vascular, and Symetis, among other companies.
WASHINGTON – , according to results of the first U.S. study to evaluate mortality in a low-risk population.
Other complications were also low relative to those seen in previously published studies with higher-risk populations. The preliminary results are “reassuring,” Ronald Waksman, MD, associate director of the division of cardiology, Medstar Health Institute, Washington, DC, reported at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
In this study, a low-risk eligibility criterion is a Society of Thoracic Surgeons score of 3% or less. The mean score in the first 125 patients was 1.9%.
At 30 days, there were no myocardial infarctions, no acute kidney injuries of stage II or greater, and no transient ischemic attacks. There was one nondisabling stroke, one paravalvular leak, and one coronary obstruction that required a coronary artery bypass graft. The rates of new onset atrial fibrillation, major vascular complications, and life-threatening or major bleeding events were lower than 5%.
Surgical atrial valve replacement (SAVR) currently has a IB recommendation for the treatment of symptomatic low-risk aortic stenosis, but TAVR is not currently indicated in this population, according to 2017 American Heart Association/American College of Cardiology guidelines. There is, however, substantial interest in extending TAVR to a low-risk population, according to Dr. Waksman.
“We know that there are potential benefits for TAVR versus SAVR that include reduced ICU and hospital length of stay, more rapid quality of life recovery, lower risk of bleeding, and lower risk of postprocedure AF [atrial fibrillation],” said Dr. Waksman in explaining the rationale for this and other ongoing studies testing TAVR in low-risk patients.
In this investigator-initiated study, which was conducted without industry support, 11 sites participated. Most were relatively low-volume centers with fewer than 150 TAVR procedures performed annually, according to Dr. Waksman. The choice of TAVR device was left to the discretion of the operator.
Reflecting a lower-risk population, the mean age of 74.6 years is younger than that of participants in previous TAVR trials. The mean left ventricular ejection fraction was 62.9%. Only 4.8% had a prior MI and 19.2% had a prior coronary artery bypass graft. Most procedures were performed under conscious sedation with fewer than 20% receiving general anesthesia. The mean fluoroscopy time was 16 minutes.
The degree of improvement in hemodynamics following TAVR in this population was called “excellent,” but Dr. Waksman did report a 12.5% incidence of hypo-attenuating leaflet thickening (HALT), an 11% incidence of reduced leaflet motion, and a 9.3% incidence of hypo-attenuation affecting motion. All were subclinical effects.
In a closer analysis of HALT, the rate was 14.4% in the 80.8% of patients who received antiplatelet therapy versus 4.8% in the 17.5% who received oral anticoagulation (some received neither). Dr. Waksman called the greater association of HALT with antiplatelet therapy “a potential signal” that deserves further study.
Full results from all 200 patients are expected in the fall of 2018.
Jeffrey Popma, MD, director of the interventional cardiology clinical service, Beth Israel Deaconess Hospital, Boston, and moderator of the session where the data were presented, called for a direct comparison with SAVR in low-risk patients to place the relative role of these options into context.
Neil Moat, a consultant cardiac surgeon at Royal Brompton & Harefield Hospital, London, agreed. Although he is also encouraged by the evidence of safety in low-risk patients, he labeled the rate of HALT in this study “a concern.”
Dr. Waksman reported financial relationships with Abbott Vascular, Amgen, AstraZeneca, Life Technologies, Med Alliance, Medtronic Vascular, and Symetis, among other companies.
REPORTING FROM CRT 2018
Key clinical point: In an interim analysis, transcatheter aortic valve replacement was found safe in low-risk patients with symptomatic aortic stenosis.
Major finding: Through 30 days of follow-up, the mortality rate was 0% or lower than observed in studies conducted in higher-risk populations.
Study details: Interim analysis of 125 patients in a prospective registry study.
Disclosures: Dr. Waksman reported financial relationships with Abbott Vascular, Amgen, AstraZeneca, Life Technologies, Med Alliance, Medtronic Vascular, and Symetis, among other companies.




















