User login
CAR T-cell therapy found safe, effective for HIV-associated lymphoma
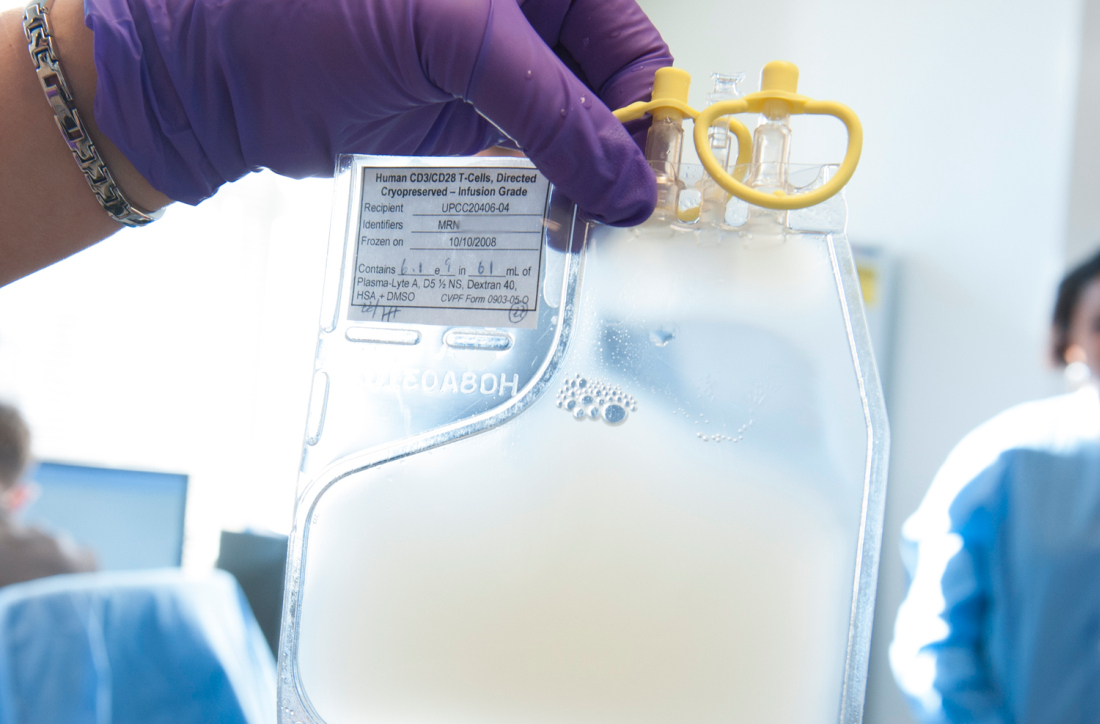
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
HIV positivity does not preclude chimeric antigen receptor (CAR) T-cell therapy for patients with aggressive lymphoma, a report of two cases suggests. Both of the HIV-positive patients, one of whom had long-term psychiatric comorbidity, achieved durable remission on axicabtagene ciloleucel (Yescarta) without undue toxicity.
“To our knowledge, these are the first reported cases of CAR T-cell therapy administered to HIV-infected patients with lymphoma,” Jeremy S. Abramson, MD, of Massachusetts General Hospital, Boston and his colleagues wrote in Cancer. “Patients with HIV and AIDS, as well as those with preexisting mental illness, should not be considered disqualified from CAR T-cell therapy and deserve ongoing studies to optimize efficacy and safety in this population.”
The Food and Drug Administration has approved two CAR T-cell products that target the B-cell antigen CD19 for the treatment of refractory lymphoma. But their efficacy and safety in HIV-positive patients are unknown because this group has been excluded from pivotal clinical trials.
Dr. Abramson and coauthors detail the two cases of successful anti-CD19 CAR T-cell therapy with axicabtagene ciloleucel in patients with HIV-associated, refractory, high-grade B-cell lymphoma.
The first patient was an HIV-positive man with diffuse large B-cell lymphoma (DLBCL) of germinal center B-cell subtype who was intermittently adherent to antiretroviral therapy. His comorbidities included posttraumatic stress disorder and schizoaffective disorder.
Previous treatments for DLBCL included dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), and rituximab, ifosfamide, carboplatin, and etoposide (RICE). A recurrence precluded high-dose chemotherapy with autologous stem cell support.
With close multidisciplinary management, including psychiatric consultation, the patient became a candidate for CAR T-cell therapy and received axicabtagene ciloleucel. He experienced grade 2 cytokine release syndrome and grade 3 neurologic toxicity, both of which resolved with treatment. Imaging showed complete remission at approximately 3 months that was sustained at 1 year. Additionally, he had an undetectable HIV viral load and was psychiatrically stable.
The second patient was a man with AIDS-associated, non–germinal center B-cell, Epstein-Barr virus–positive DLBCL who was adherent to antiretroviral therapy. His lymphoma had recurred rapidly after initially responding to dose-adjusted EPOCH-R and then was refractory to combination rituximab and lenalidomide. He previously had hepatitis B virus, cytomegalovirus, and Mycobacterium avium complex infections.
Because of prolonged cytopenias and infectious complications after the previous lymphoma treatments, the patient was considered a poor candidate for high-dose chemotherapy. He underwent CAR T-cell therapy with axicabtagene ciloleucel and had a complete remission on day 28. Additionally, his HIV infection remained well controlled.
“Although much remains to be learned regarding CAR T-cell therapy in patients with refractory hematologic malignancies, with or without HIV infection, the cases presented herein demonstrate that patients with chemotherapy-refractory, high-grade B-cell lymphoma can successfully undergo autologous CAR T-cell manufacturing, and subsequently can safely tolerate CAR T-cell therapy and achieve a durable complete remission,” the researchers wrote. “These cases have further demonstrated the proactive, multidisciplinary care required to navigate a patient with high-risk lymphoma through CAR T-cell therapy with attention to significant medical and psychiatric comorbidities.”
Dr. Abramson reported that he has acted as a paid member of the scientific advisory board and as a paid consultant for Kite Pharma, which markets Yescarta, and several other companies.
SOURCE: Abramson JS et al. Cancer. 2019 Sep 10. doi: 10.1002/cncr.32411.
FROM CANCER
CT103A elicits responses after prior CAR T-cell relapse
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
BOSTON – CT103A, a chimeric antigen receptor (CAR) T-cell therapy, is “active and effective” in patients with relapsed/refractory multiple myeloma, according to a speaker at the International Myeloma Workshop, held by the International Myeloma Society.
The anti–B-cell maturation antigen (BCMA) CAR T-cell therapy produced a 100% response rate in patients with heavily pretreated multiple myeloma, and three of four patients who had failed a prior CAR T-cell therapy achieved a stringent complete response after CT103A.
Chunrui Li, MD, PhD, of Tongji Hospital and Tongji Medical College, Huazhong University of Science, Wuhan, China, presented these results at the workshop.
Dr. Li noted that anti-BCMA CAR T-cell therapy has produced responses in myeloma patients, but approximately half of patients typically relapse in about a year. CAR T-cell infusions after relapse have not been effective in these patients.
In an effort to change that, Dr. Li and his colleagues developed CT103A, a lentiviral vector containing a CAR structure with a fully human single-chain fragment variant; CD8a hinger; and transmembrane, 4-1BB co-stimulatory, and CD3z activation domains.
Dr. Li and his colleagues evaluated CT103A in a phase 0 trial (ChiCTR1800018137) of 18 patients who had received at least three prior lines of therapy and had disease refractory to a proteasome inhibitor and an immunomodulatory agent.
The patients’ median age was 53.3 years (range, 38-66 years), and their median time since diagnosis was 32 months (range, 8-92 months). They had received a median of 4 (range, 3-6) prior therapies. All had received prior bortezomib and lenalidomide, seven had undergone a transplant, and four had been treated on a trial of murine anti-BCMA CAR T-cell therapy.
For the current trial, patients received lymphodepletion with cyclophosphamide and fludarabine, followed by CT103A at 1x106, 3x106, or 6x106 CAR T cells/kg.
There was one dose-limiting toxicity at the highest dose level – grade 4 cytokine release syndrome (CRS) in a patient who died at day 19 after CT103A infusion.
In all, 17 patients developed CRS, four with grade 1, eight with grade 2, four with grade 3, and one with grade 4 CRS. None of the patients developed neurologic toxicity.
Serious adverse events related to lymphodepletion and/or CT103A included prolonged cytopenia (n = 3), pulmonary infection (n = 2), herpes zoster (n = 1), pleuritis (n = 1), and hypoxemia (n = 1).
There were 17 patients evaluable for efficacy, and all of them achieved a response at some point. In eight patients, responses have lasted more than 200 days.
At the data cutoff, there were 10 stringent complete responses, two complete responses, and three very good partial responses. One patient progressed after achieving a very good partial response, and one patient achieved a partial response but ultimately died (likely of respiratory failure attributable to a lung infection).
Of the four patients who had previously received murine CAR T-cell therapy, one progressed, and three achieved a stringent complete response.
This study was funded by Nanjing Iaso Biotherapeutics. Dr. Li did not disclose any conflicts of interest.
SOURCE: Li C et al. IMW 2019, Abstract OAB-033.
REPORTING FROM IMW 2019
CAR T-cell therapy less effective in transformed follicular lymphoma
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
FROM BLOOD
Low intensity bridging may be best path to CAR T in adult ALL
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
FROM ASCO 2019
FDA approves first drug for steroid-refractory acute GVHD
The Food and Drug Administration has approved Jafaki (ruxolitinib) for treatment of steroid-refractory acute graft-versus-host disease (GVHD) in adult and pediatric patients 12 years and older.
Ruxolitinib will be made available to appropriate patients immediately, according to a statement from Incyte, which markets the drug. The company noted that ruxolitinib is the first FDA-approved treatment for this indication.
The approval is based on data from the open-label, single-arm, multicenter REACH1 trial, which studied ruxolitinib in combination with corticosteroids. The 71 patients in the trial had grade 2-4 acute GVHD after allogeneic hematopoietic stem cell transplant; of these patients, 49 were refractory to steroids alone, 12 had received at least two prior therapies for GVHD, and 10 did not otherwise meet the FDA definition of steroid refractory.
The trial’s primary endpoints were day-28 overall response rate and response duration. Among the 49 patients with steroid only–refractory GVHD, the overall response rate was 100% for grade 2 GVHD, 40.7% for grade 3, and 44.4% for grade 4. Median response duration was 16 days. For all 49 of these patients, the overall response rate was 57%, and the complete response rate was 31%.
Among all 71 participants, the most frequently reported adverse reactions were infections (55%) and edema (51%); anemia (71%), thrombocytopenia (75%), and neutropenia (58%) were the most common laboratory abnormalities.
The Food and Drug Administration has approved Jafaki (ruxolitinib) for treatment of steroid-refractory acute graft-versus-host disease (GVHD) in adult and pediatric patients 12 years and older.
Ruxolitinib will be made available to appropriate patients immediately, according to a statement from Incyte, which markets the drug. The company noted that ruxolitinib is the first FDA-approved treatment for this indication.
The approval is based on data from the open-label, single-arm, multicenter REACH1 trial, which studied ruxolitinib in combination with corticosteroids. The 71 patients in the trial had grade 2-4 acute GVHD after allogeneic hematopoietic stem cell transplant; of these patients, 49 were refractory to steroids alone, 12 had received at least two prior therapies for GVHD, and 10 did not otherwise meet the FDA definition of steroid refractory.
The trial’s primary endpoints were day-28 overall response rate and response duration. Among the 49 patients with steroid only–refractory GVHD, the overall response rate was 100% for grade 2 GVHD, 40.7% for grade 3, and 44.4% for grade 4. Median response duration was 16 days. For all 49 of these patients, the overall response rate was 57%, and the complete response rate was 31%.
Among all 71 participants, the most frequently reported adverse reactions were infections (55%) and edema (51%); anemia (71%), thrombocytopenia (75%), and neutropenia (58%) were the most common laboratory abnormalities.
The Food and Drug Administration has approved Jafaki (ruxolitinib) for treatment of steroid-refractory acute graft-versus-host disease (GVHD) in adult and pediatric patients 12 years and older.
Ruxolitinib will be made available to appropriate patients immediately, according to a statement from Incyte, which markets the drug. The company noted that ruxolitinib is the first FDA-approved treatment for this indication.
The approval is based on data from the open-label, single-arm, multicenter REACH1 trial, which studied ruxolitinib in combination with corticosteroids. The 71 patients in the trial had grade 2-4 acute GVHD after allogeneic hematopoietic stem cell transplant; of these patients, 49 were refractory to steroids alone, 12 had received at least two prior therapies for GVHD, and 10 did not otherwise meet the FDA definition of steroid refractory.
The trial’s primary endpoints were day-28 overall response rate and response duration. Among the 49 patients with steroid only–refractory GVHD, the overall response rate was 100% for grade 2 GVHD, 40.7% for grade 3, and 44.4% for grade 4. Median response duration was 16 days. For all 49 of these patients, the overall response rate was 57%, and the complete response rate was 31%.
Among all 71 participants, the most frequently reported adverse reactions were infections (55%) and edema (51%); anemia (71%), thrombocytopenia (75%), and neutropenia (58%) were the most common laboratory abnormalities.
CMS proposes payment increase for administering CAR T in the hospital
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.
ICYMI: NIH renames, streamlines gene therapy committee
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
Monitoring, early intervention key to CAR T safety
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
GLASGOW – Constant patient monitoring and early intervention with tocilizumab and steroids are essential to the safe delivery of chimeric antigen receptor (CAR) T-cell therapy in patients with non-Hodgkin lymphoma (NHL), according to a leading expert.
As a clinical researcher at MD Anderson Cancer Center in Houston, Loretta Nastoupil, MD has played an active role in the evolution of CAR T-cell therapy, from early trials to ongoing development of treatment protocols. During a presentation at the annual meeting of the British Society for Haematology, Dr. Nastoupil discussed leading topics in CAR T-cell therapy, with an emphasis on safe delivery.
“[Toxicity] is something we don’t talk about as much as we should, partly because this therapy works and it’s really exciting,” Dr. Nastoupil said. “But the toxicity is not something that I minimize, and it’s very challenging. It’s led us to restructure our inpatient services. It’s led to a lot of sleepless nights. These patients can do very, very well, or they can do very, very poorly in terms of toxicity and I think the most important strategy is recognition and early intervention.”
Monitoring
Early recognition depends on close monitoring, Dr. Nastoupil said, which is carried out by highly trained nursing staff who follow therapy-specific decision algorithms.
“We have nurses that are on the front line,” Dr. Nastoupil said. “They’re the most important group. We have staff that round on [patients] daily, but the nurses are there 24 hours a day. We have a flow sheet where they grade cytokine release syndrome and neurotoxicity every 8 hours, or if there is an acute change in symptoms or toxicity, they’ll do it in real time.”
Dr. Nastoupil said that if these toxicities are detected, intervention is occurring sooner than it did with some of the first patients to receive CAR-T cell therapy.
“Initially there was a lot of fear surrounding anything that would abort the CAR-T cell therapy,” Dr. Nastoupil said. “There was concern that if you were trying to mitigate some of the toxicity you might have a negative impact on efficacy ... [W]ith the first iteration of studies, generally we were waiting until grade 3 or higher cytokine release syndrome before initiating either tocilizumab and/or steroids. As the studies evolved, it started to move into grade 2 toxicity that we started using therapy, mostly because we started to see that those patients were still responding.”
At MD Anderson, these earlier interventions have decreased severity of adverse events.
“It’s rare nowadays to have grade 3 or 4 cytokine release syndrome because we are generally introducing abortive therapy at grade 2,” Dr. Nastoupil said, citing increased use of steroids and tocilizumab.
Currently, no consensus exists for managing these events, partly because clinicians are still learning about best management practices.
“There will be a consensus on management,” Dr. Nastoupil said. “I think that’s needed. The problem is, it will probably evolve as we get more experience with managing these patients. I think there’s been a little hesitation to put something out on paper knowing that a year from now that might change.”
Grading toxicity
In contrast, Dr. Nastoupil said that a consensus has been reached for grading acute toxicity. Of note, fever is now considered an essential element of cytokine release syndrome.
“The first thing we see [with cytokine release syndrome] is fever, generally speaking,” Dr. Nastoupil said. “That will prompt a workup for infection because these patients are going to be neutropenic. And we initiate broad spectrum antimicrobials.”
She said that some patients treated with CAR T-cell therapy have had disseminated fungal infections, so clinicians need to be on the lookout for septic shock.
To assess neurotoxicity, the team at MD Anderson uses an objective scoring system, called “CARTOX.” This helps maintain consistency when facing broadly different neurological presentations.
“There’s such a wide ranging spectrum of patients that are undergoing neurotoxicity you can’t expect someone, even myself, to be consistent when you are trying to tease out how serious it is,” Dr. Nastoupil said.
With CARTOX, nurses can easily score patients and call clinicians with results. Still, this doesn’t eliminate difficulties inherent to managing neurotoxicity, particularly when it is severe.
“I’d say one of the areas that is still very challenging is when [patients with neurotoxicity] are no longer responding,” Dr. Nastoupil said. “You have to be very mindful of seizure activity. We’ve had a couple of patients with status [epilepticus]. You don’t see seizure activity physically, but when you do an EEG, you pick it up.”
Dr. Nastoupil added that most centers are now giving patients prophylactic levetiracetam (Keppra) to lower seizure risk.
Choosing therapy
When selecting between the two therapies currently approved by the Food and Drug Administration – tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) – based on safety, Dr. Nastoupil said that rates of cytokine release syndrome appear similar, but neurotoxicity rates may differ.
“Cytokine release syndrome in my opinion is probably more similar than different in terms of grade 3 or higher because tocilizumab and steroids work quite well in aborting those toxicities,” Dr. Nastoupil said. “But neurotoxicity still sticks out in my mind as the most striking difference, where with axicabtagene you see more grade 3 or higher neurotoxicity, though very, very few deaths as a result of this. But it’s very challenging in terms of management.”
According to Dr. Nastoupil, comparisons between CAR T-cell therapies have been complicated by differences in clinical trial methodologies. However, she offered a general conclusion regarding efficacy.
“[W]hat I’ll tell you, at the end of the day, is [that existing CAR T-cell therapies] all seem to sort of settle out around 30%-40% in terms of durable responses,” Dr. Nastoupil said.
Dr. Nastoupil concluded her presentation with an overview and look to the future.
“I do think [CAR T-cell therapy] is transformative, particularly for our chemo refractory patients,” she said. “There is nothing else like it. The problem right now is that it is only durable in 40% of patients. So can we be better at selecting out patients that are more likely to respond? Does introducing this in earlier lines of therapy increase that fraction of patients that are potentially cured?”
Considering these questions, she said: “We need more patients. We need more data. We need longer follow-up to understand the nuances of this therapy.”
Dr. Nastoupil previously reported financial relationships with Celgene, Genentech, Gilead, Merck, Novartis, Spectrum, and TG Therapeutics.
EXPERT ANALYSIS FROM BSH 2019
Gene therapy restored immunity in newly diagnosed SCID-X1
For infants with newly diagnosed X-linked severe combined immunodeficiency (SCID-X1), lentiviral gene therapy and targeted busulfan conditioning successfully induced multilineage engraftment of transduced cells, researchers reported.
By 3-4 months after infusion, seven of eight patients had normal numbers of CD3+, CD4+, and naive CD4+ T cells; normal counts of natural killer (NK) cells; and vector marking of T cells, B cells, NK cells, myeloid cells, and bone marrow progenitors, Ewelina Mamcarz, MD, of St. Jude Children’s Research Hospital in Memphis, and her associates reported in the New England Journal of Medicine.
The eighth infant at first lacked a sufficient T-cell response but responded to a boost of gene-corrected cells without busulfan conditioning.
By 6-12 months after infusion, IgM levels also had normalized in seven of the eight infants and showed polyclonal patterns without clonal dominance, according to the investigators. Among four infants who were able to stop intravenous immunoglobulin therapy, three responded to vaccinations with tetanus, diphtheria, pertussis, polio, and pneumococcal polysaccharide. Such restoration of humoral immunity “has not been achieved in previously reported trials of gene therapy for infants with newly diagnosed SCID-X1,” wrote the investigators of this dual-center, phase 1/2 study.
X-linked severe combined immunodeficiency – “bubble boy disease” – is characterized by a lack of T cells, NK cells, and B cells, and is caused by mutations in IL2RG. Some 80% of affected infants have no matched sibling donor for hematopoietic stem cell transplantation, and transplantation from other donors can produce an inadequate response and graft-versus-host disease. Prior attempts at gene therapy with gamma-retroviral vectors had led to vector-induced leukemia or had failed to induce humoral immunity or normal NK cell production.
“Our new lentiviral vector gene therapy combined with nonmyeloablative busulfan conditioning has been successful in restoring immunity in five patients 7-23 years of age in whom a previous allogeneic hematopoietic stem cell transplantation for SCID-X1 had failed,” the investigators wrote. “We hypothesized that the combination of this lentiviral vector and low-exposure busulfan administered by means of pharmacokinetic dose targeting would be safe and effective as the primary treatment in infants with newly diagnosed SCID-X1.”
Their protocol included one to two daily intravenous doses of busulfan, targeting a cumulative area under the curve of 22 mg per hr/L. They calculated the first dose by weight and age using a population-based pharmacokinetic model and adjusted the second dose based on first-dose pharmacokinetics.
After a median of 16.4 months, all infants continued to grow normally and cleared previous infections, and there were no unanticipated side effects from bone marrow harvest, busulfan conditioning, or cell infusion.
“It is hoped that durable, complete adaptive immunity will be achieved in the majority of the patients over time,” the researchers wrote.
They continue to follow the patients to assess therapeutic safety, immune durability, and persistence of the transferred gene in hematopoietic and immune cells.
Study funders included the American Lebanese Syrian Associated Charities, the National Institutes of Health, the California Institute of Regenerative Medicine, and the Assisi Foundation of Memphis. St. Jude Children’s Research Hospital has licensed the gene therapy and partnered with Mustang Bio to develop and commercialize it. Dr. Mamcarz reported receiving grant support from the study funders.
SOURCE: Mamcarz E et al. N Engl J Med. 2019; 380:1525-34.
For infants with newly diagnosed X-linked severe combined immunodeficiency (SCID-X1), lentiviral gene therapy and targeted busulfan conditioning successfully induced multilineage engraftment of transduced cells, researchers reported.
By 3-4 months after infusion, seven of eight patients had normal numbers of CD3+, CD4+, and naive CD4+ T cells; normal counts of natural killer (NK) cells; and vector marking of T cells, B cells, NK cells, myeloid cells, and bone marrow progenitors, Ewelina Mamcarz, MD, of St. Jude Children’s Research Hospital in Memphis, and her associates reported in the New England Journal of Medicine.
The eighth infant at first lacked a sufficient T-cell response but responded to a boost of gene-corrected cells without busulfan conditioning.
By 6-12 months after infusion, IgM levels also had normalized in seven of the eight infants and showed polyclonal patterns without clonal dominance, according to the investigators. Among four infants who were able to stop intravenous immunoglobulin therapy, three responded to vaccinations with tetanus, diphtheria, pertussis, polio, and pneumococcal polysaccharide. Such restoration of humoral immunity “has not been achieved in previously reported trials of gene therapy for infants with newly diagnosed SCID-X1,” wrote the investigators of this dual-center, phase 1/2 study.
X-linked severe combined immunodeficiency – “bubble boy disease” – is characterized by a lack of T cells, NK cells, and B cells, and is caused by mutations in IL2RG. Some 80% of affected infants have no matched sibling donor for hematopoietic stem cell transplantation, and transplantation from other donors can produce an inadequate response and graft-versus-host disease. Prior attempts at gene therapy with gamma-retroviral vectors had led to vector-induced leukemia or had failed to induce humoral immunity or normal NK cell production.
“Our new lentiviral vector gene therapy combined with nonmyeloablative busulfan conditioning has been successful in restoring immunity in five patients 7-23 years of age in whom a previous allogeneic hematopoietic stem cell transplantation for SCID-X1 had failed,” the investigators wrote. “We hypothesized that the combination of this lentiviral vector and low-exposure busulfan administered by means of pharmacokinetic dose targeting would be safe and effective as the primary treatment in infants with newly diagnosed SCID-X1.”
Their protocol included one to two daily intravenous doses of busulfan, targeting a cumulative area under the curve of 22 mg per hr/L. They calculated the first dose by weight and age using a population-based pharmacokinetic model and adjusted the second dose based on first-dose pharmacokinetics.
After a median of 16.4 months, all infants continued to grow normally and cleared previous infections, and there were no unanticipated side effects from bone marrow harvest, busulfan conditioning, or cell infusion.
“It is hoped that durable, complete adaptive immunity will be achieved in the majority of the patients over time,” the researchers wrote.
They continue to follow the patients to assess therapeutic safety, immune durability, and persistence of the transferred gene in hematopoietic and immune cells.
Study funders included the American Lebanese Syrian Associated Charities, the National Institutes of Health, the California Institute of Regenerative Medicine, and the Assisi Foundation of Memphis. St. Jude Children’s Research Hospital has licensed the gene therapy and partnered with Mustang Bio to develop and commercialize it. Dr. Mamcarz reported receiving grant support from the study funders.
SOURCE: Mamcarz E et al. N Engl J Med. 2019; 380:1525-34.
For infants with newly diagnosed X-linked severe combined immunodeficiency (SCID-X1), lentiviral gene therapy and targeted busulfan conditioning successfully induced multilineage engraftment of transduced cells, researchers reported.
By 3-4 months after infusion, seven of eight patients had normal numbers of CD3+, CD4+, and naive CD4+ T cells; normal counts of natural killer (NK) cells; and vector marking of T cells, B cells, NK cells, myeloid cells, and bone marrow progenitors, Ewelina Mamcarz, MD, of St. Jude Children’s Research Hospital in Memphis, and her associates reported in the New England Journal of Medicine.
The eighth infant at first lacked a sufficient T-cell response but responded to a boost of gene-corrected cells without busulfan conditioning.
By 6-12 months after infusion, IgM levels also had normalized in seven of the eight infants and showed polyclonal patterns without clonal dominance, according to the investigators. Among four infants who were able to stop intravenous immunoglobulin therapy, three responded to vaccinations with tetanus, diphtheria, pertussis, polio, and pneumococcal polysaccharide. Such restoration of humoral immunity “has not been achieved in previously reported trials of gene therapy for infants with newly diagnosed SCID-X1,” wrote the investigators of this dual-center, phase 1/2 study.
X-linked severe combined immunodeficiency – “bubble boy disease” – is characterized by a lack of T cells, NK cells, and B cells, and is caused by mutations in IL2RG. Some 80% of affected infants have no matched sibling donor for hematopoietic stem cell transplantation, and transplantation from other donors can produce an inadequate response and graft-versus-host disease. Prior attempts at gene therapy with gamma-retroviral vectors had led to vector-induced leukemia or had failed to induce humoral immunity or normal NK cell production.
“Our new lentiviral vector gene therapy combined with nonmyeloablative busulfan conditioning has been successful in restoring immunity in five patients 7-23 years of age in whom a previous allogeneic hematopoietic stem cell transplantation for SCID-X1 had failed,” the investigators wrote. “We hypothesized that the combination of this lentiviral vector and low-exposure busulfan administered by means of pharmacokinetic dose targeting would be safe and effective as the primary treatment in infants with newly diagnosed SCID-X1.”
Their protocol included one to two daily intravenous doses of busulfan, targeting a cumulative area under the curve of 22 mg per hr/L. They calculated the first dose by weight and age using a population-based pharmacokinetic model and adjusted the second dose based on first-dose pharmacokinetics.
After a median of 16.4 months, all infants continued to grow normally and cleared previous infections, and there were no unanticipated side effects from bone marrow harvest, busulfan conditioning, or cell infusion.
“It is hoped that durable, complete adaptive immunity will be achieved in the majority of the patients over time,” the researchers wrote.
They continue to follow the patients to assess therapeutic safety, immune durability, and persistence of the transferred gene in hematopoietic and immune cells.
Study funders included the American Lebanese Syrian Associated Charities, the National Institutes of Health, the California Institute of Regenerative Medicine, and the Assisi Foundation of Memphis. St. Jude Children’s Research Hospital has licensed the gene therapy and partnered with Mustang Bio to develop and commercialize it. Dr. Mamcarz reported receiving grant support from the study funders.
SOURCE: Mamcarz E et al. N Engl J Med. 2019; 380:1525-34.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Factors emerge for mitigating CD19 CAR T toxicity
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
REPORTING FROM TCT 2019