User login
Adding checkpoint inhibitors to radiotherapy requires particular caution in this one scenario
Among scenarios where immune checkpoint inhibitors (ICIs) might be combined, particular caution is needed in the setting of brain metastases, according to authors of a recent clinical review.
While evidence to date is mixed, some studies do suggest that adding ICIs to high-dose stereotactic intracranial radiotherapy for brain metastases might increase the risk of treatment-related brain necrosis, the authors said.
By contrast, the balance of evidence suggests ICIs can be safely combined with palliative radiotherapy without site-specific increases in adverse events, they added.
Likewise, in patients with non–small-cell lung cancer, ICIs do not appear to increase incidence of grade 3 or greater pneumonitis when given after definitive chemoradiotherapy, in both retrospective and prospective investigations.
Nevertheless, the addition of ICIs to radiotherapy requires careful further study because of the potential for increased type or severity of toxicities, including the immune-related adverse events associated with ICIs, wrote corresponding author Jay S. Loeffler, MD, of Massachusetts General Hospital, Boston, and his colleagues.
“Caution is warranted when combining radiotherapy and ICI, especially with intracranial radiotherapy,” the researchers wrote. Their report is in Nature Reviews Clinical Oncology.
Some studies have indicated a higher rate of treatment-associated brain necrosis when ICIs are combined with intracranial radiotherapy, while others have shown no such trend, the authors said.
In one single-institution experience involving 180 patients with brain metastases undergoing stereotactic radiotherapy, incidence of treatment-associated brain necrosis was significantly higher in patients receiving an ICI, with an odds ratio of 2.4 (95% confidence interval, 1.06-5.44; P = .03).
Similarly, a retrospective single institution 480-patient study showed an incidence of treatment-associated brain necrosis of 20% for ICIs plus stereotactic radiotherapy versus 7% for radiotherapy alone (P less than .001), but substantial differences in baseline characteristics between groups limited the strength of the study’s conclusions, according to the researchers.
Increased risk is primarily in the form of asymptomatic or minimally symptomatic episodes in some series, the authors noted. A retrospective, 54-patient report showed a rate of treatment-associated brain necrosis of 30% when ICIs were combined with stereotactic radiotherapy, versus 21% for radiotherapy alone (P = .08), but the incidence of symptomatic cases was 15% in both groups, they noted.
“Intriguingly, the findings of several studies have demonstrated an association between [treatment-associated brain necrosis] and improved survival outcomes in patients with melanoma brain metastases that is similar to the independent observations of an analogous relationship between risk of [immune-related adverse events] in general and responsiveness to ICI,” the researchers wrote.
Most of the Food and Drug Administration–approved indications for ICIs are in the metastatic setting, where palliative radiotherapy is frequently important, the authors noted.
In two retrospective studies of patients with metastatic cancers receiving palliative radiotherapy with ICIs, there was a lack of clear association between the irradiated site and specific immune-related adverse events; that lack of association suggests that any toxicities arising from interactions between palliative radiotherapy and ICIs are mainly systemic, rather than local, the authors wrote.
Several retrospective series in advanced-stage melanoma patients have suggested that palliative radiotherapy plus ICIs is safe and does not significantly increase incidence of immune-related adverse events. However, findings from one series showed a correlation between both the ICI and radiotherapy dose given and the incidence of immune-related adverse events.
Prospective studies will be essential to optimize the balance between disease control and risk of morbidity associated with ICIs and radiotherapy combinations, the authors concluded.
The researchers declared no competing interests related to their review article.
SOURCE: Hwang WL, et al. Nat Rev Clin Oncol. 2018 Aug;15(8):477-494.
Among scenarios where immune checkpoint inhibitors (ICIs) might be combined, particular caution is needed in the setting of brain metastases, according to authors of a recent clinical review.
While evidence to date is mixed, some studies do suggest that adding ICIs to high-dose stereotactic intracranial radiotherapy for brain metastases might increase the risk of treatment-related brain necrosis, the authors said.
By contrast, the balance of evidence suggests ICIs can be safely combined with palliative radiotherapy without site-specific increases in adverse events, they added.
Likewise, in patients with non–small-cell lung cancer, ICIs do not appear to increase incidence of grade 3 or greater pneumonitis when given after definitive chemoradiotherapy, in both retrospective and prospective investigations.
Nevertheless, the addition of ICIs to radiotherapy requires careful further study because of the potential for increased type or severity of toxicities, including the immune-related adverse events associated with ICIs, wrote corresponding author Jay S. Loeffler, MD, of Massachusetts General Hospital, Boston, and his colleagues.
“Caution is warranted when combining radiotherapy and ICI, especially with intracranial radiotherapy,” the researchers wrote. Their report is in Nature Reviews Clinical Oncology.
Some studies have indicated a higher rate of treatment-associated brain necrosis when ICIs are combined with intracranial radiotherapy, while others have shown no such trend, the authors said.
In one single-institution experience involving 180 patients with brain metastases undergoing stereotactic radiotherapy, incidence of treatment-associated brain necrosis was significantly higher in patients receiving an ICI, with an odds ratio of 2.4 (95% confidence interval, 1.06-5.44; P = .03).
Similarly, a retrospective single institution 480-patient study showed an incidence of treatment-associated brain necrosis of 20% for ICIs plus stereotactic radiotherapy versus 7% for radiotherapy alone (P less than .001), but substantial differences in baseline characteristics between groups limited the strength of the study’s conclusions, according to the researchers.
Increased risk is primarily in the form of asymptomatic or minimally symptomatic episodes in some series, the authors noted. A retrospective, 54-patient report showed a rate of treatment-associated brain necrosis of 30% when ICIs were combined with stereotactic radiotherapy, versus 21% for radiotherapy alone (P = .08), but the incidence of symptomatic cases was 15% in both groups, they noted.
“Intriguingly, the findings of several studies have demonstrated an association between [treatment-associated brain necrosis] and improved survival outcomes in patients with melanoma brain metastases that is similar to the independent observations of an analogous relationship between risk of [immune-related adverse events] in general and responsiveness to ICI,” the researchers wrote.
Most of the Food and Drug Administration–approved indications for ICIs are in the metastatic setting, where palliative radiotherapy is frequently important, the authors noted.
In two retrospective studies of patients with metastatic cancers receiving palliative radiotherapy with ICIs, there was a lack of clear association between the irradiated site and specific immune-related adverse events; that lack of association suggests that any toxicities arising from interactions between palliative radiotherapy and ICIs are mainly systemic, rather than local, the authors wrote.
Several retrospective series in advanced-stage melanoma patients have suggested that palliative radiotherapy plus ICIs is safe and does not significantly increase incidence of immune-related adverse events. However, findings from one series showed a correlation between both the ICI and radiotherapy dose given and the incidence of immune-related adverse events.
Prospective studies will be essential to optimize the balance between disease control and risk of morbidity associated with ICIs and radiotherapy combinations, the authors concluded.
The researchers declared no competing interests related to their review article.
SOURCE: Hwang WL, et al. Nat Rev Clin Oncol. 2018 Aug;15(8):477-494.
Among scenarios where immune checkpoint inhibitors (ICIs) might be combined, particular caution is needed in the setting of brain metastases, according to authors of a recent clinical review.
While evidence to date is mixed, some studies do suggest that adding ICIs to high-dose stereotactic intracranial radiotherapy for brain metastases might increase the risk of treatment-related brain necrosis, the authors said.
By contrast, the balance of evidence suggests ICIs can be safely combined with palliative radiotherapy without site-specific increases in adverse events, they added.
Likewise, in patients with non–small-cell lung cancer, ICIs do not appear to increase incidence of grade 3 or greater pneumonitis when given after definitive chemoradiotherapy, in both retrospective and prospective investigations.
Nevertheless, the addition of ICIs to radiotherapy requires careful further study because of the potential for increased type or severity of toxicities, including the immune-related adverse events associated with ICIs, wrote corresponding author Jay S. Loeffler, MD, of Massachusetts General Hospital, Boston, and his colleagues.
“Caution is warranted when combining radiotherapy and ICI, especially with intracranial radiotherapy,” the researchers wrote. Their report is in Nature Reviews Clinical Oncology.
Some studies have indicated a higher rate of treatment-associated brain necrosis when ICIs are combined with intracranial radiotherapy, while others have shown no such trend, the authors said.
In one single-institution experience involving 180 patients with brain metastases undergoing stereotactic radiotherapy, incidence of treatment-associated brain necrosis was significantly higher in patients receiving an ICI, with an odds ratio of 2.4 (95% confidence interval, 1.06-5.44; P = .03).
Similarly, a retrospective single institution 480-patient study showed an incidence of treatment-associated brain necrosis of 20% for ICIs plus stereotactic radiotherapy versus 7% for radiotherapy alone (P less than .001), but substantial differences in baseline characteristics between groups limited the strength of the study’s conclusions, according to the researchers.
Increased risk is primarily in the form of asymptomatic or minimally symptomatic episodes in some series, the authors noted. A retrospective, 54-patient report showed a rate of treatment-associated brain necrosis of 30% when ICIs were combined with stereotactic radiotherapy, versus 21% for radiotherapy alone (P = .08), but the incidence of symptomatic cases was 15% in both groups, they noted.
“Intriguingly, the findings of several studies have demonstrated an association between [treatment-associated brain necrosis] and improved survival outcomes in patients with melanoma brain metastases that is similar to the independent observations of an analogous relationship between risk of [immune-related adverse events] in general and responsiveness to ICI,” the researchers wrote.
Most of the Food and Drug Administration–approved indications for ICIs are in the metastatic setting, where palliative radiotherapy is frequently important, the authors noted.
In two retrospective studies of patients with metastatic cancers receiving palliative radiotherapy with ICIs, there was a lack of clear association between the irradiated site and specific immune-related adverse events; that lack of association suggests that any toxicities arising from interactions between palliative radiotherapy and ICIs are mainly systemic, rather than local, the authors wrote.
Several retrospective series in advanced-stage melanoma patients have suggested that palliative radiotherapy plus ICIs is safe and does not significantly increase incidence of immune-related adverse events. However, findings from one series showed a correlation between both the ICI and radiotherapy dose given and the incidence of immune-related adverse events.
Prospective studies will be essential to optimize the balance between disease control and risk of morbidity associated with ICIs and radiotherapy combinations, the authors concluded.
The researchers declared no competing interests related to their review article.
SOURCE: Hwang WL, et al. Nat Rev Clin Oncol. 2018 Aug;15(8):477-494.
FROM NATURE REVIEWS CLINICAL ONCOLOGY
Key clinical point: Some studies suggest that adding ICIs to high-dose stereotactic intracranial radiotherapy for brain metastases might increase the risk of treatment-related brain necrosis.
Major finding: The balance of evidence suggests ICIs can be safely combined with palliative radiotherapy.
Study details: A literature review.
Disclosures: The researchers declared no competing interests related to their review article.
Source: Hwang WL et al. Nat Rev Clin Oncol. 2018 Aug;15(8):477-94.
Immunotherapies extend survival for melanoma patients with brain metastases
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
Since the Food and Drug Administration first approved checkpoint blockade immunotherapy (CBI) and BRAFV600-targeted therapy in 2011, survival times for patients with melanoma brain metastases (MBMs) have significantly improved, with a 91% increase in 4-year overall survival (OS) from 7.4% to 14.1%.
“The management of advanced melanoma has traditionally been tempered by limited responses to conventional therapies, resulting in a median overall survival (OS) of less than 1 year,” wrote J. Bryan Iorgulescu, MD, of Brigham and Women’s Hospital in Boston, and his colleagues. The report was published in Cancer Immunology Research. “The landscape of advanced melanoma treatment was revolutionized” by the approval of immunotherapy agents, beginning in 2011.
The current, retrospective study involved 2,753 patients with stage IV melanoma. Patient data were drawn from the National Cancer Database, with diagnoses made between 2010 and 2015. Patient management, overall survival, and disease characteristics were evaluated.
During initial review, the researchers found that 35.8% of patients with stage IV melanoma had brain involvement. These patients were further categorized by those with MBM only (39.7%) versus those with extracranial metastatic disease (60.3%), which included involvement of lung (82.9%), liver (8.1%), bone (6.0%), and lymph nodes or distant subcutaneous skin (3%). MBM-only disease was independently predicted by both younger age and geographic location.
Patients receiving first-line CBI therapy demonstrated improved 4-year OS (28.1% vs. 11.1%; P less than.001) and median OS (12.4 months vs. 5.2 months; P less than .001).
Improvements with CBI were most dramatic in patients with MBM-only disease. In these cases, 4-year OS improved from 16.9% to 51.5% (P less than .001), while median OS jumped from 7.7 months to 56.4 months (P less than .001).
Improved OS was also associated with fewer comorbidities, younger age, management at an academic cancer center, single-fraction stereotactic radiosurgery, and resection of the MBM.
“Our findings help bridge the gaps in early clinical trials of CBIs that largely excluded stage IV melanoma patients with MBMs, with checkpoint immunotherapy demonstrating a more than doubling of the median and 4-year OS of MBMs,” the authors concluded.
SOURCE: Iorgulescu et al. Cancer Immunol Res. 2018 July 12 doi: 10.1158/2326-6066.CIR-18-0067.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Checkpoint blockade immunotherapy and BRAFV600-targeted therapy improve survival for patients with melanoma brain metastases.
Major finding: Patients with melanoma brain metastases receiving first-line checkpoint blockade immunotherapy had an improved 4-year overall survival (28.1% vs. 11.1%; P less than .001) and median overall survival (12.4 months vs. 5.2 months; P less than .001).
Study details: A retrospective study of 2,753 patients with stage IV melanoma and brain metastases, from the National Cancer Database, between 2010 and 2015.
Disclosures: The study was supported by the National Institute of Health, Abbvie, Bristol-Myers Squibb, Merck, and others. No conflicts of interest were reported.
Source: Iorgulescu et al. Cancer Immunol Res. 2018 July 12. doi: 10.1158/2326-6066.CIR-18-0067.
Recombinant poliovirus appears safe, active as recurrent glioblastoma treatment
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
The potentially useful anticancer properties of viruses are just starting to be recognized and exploited, Dan L. Longo, MD, and Lindsey R. Baden, MD, both with the Dana-Farber Cancer Institute at Brigham and Women’s Hospital, Boston, said in an editorial.
One approach is the development of oncolytic viruses that can not only directly kill tumor cells, but can also prompt an immune response against viable tumor cells, they wrote. The study by Dr. Desjardins and her colleagues describes clinical experience with PVSRIPO, a recombinant, nonpathogenic polio-rhinovirus chimera. This engineered virus targets glioblastoma by gaining cell entry through the CD155 receptor, which is expressed on solid tumors.
The survival data showed a plateau, with a 36-month survival rate of 21%, compared with 4% for a historical control cohort of patients, Dr. Longo and Dr. Baden noted.
In this study, PVSRIPO was delivered into intracranial tumors using an indwelling catheter. One of the outstanding questions with viral approaches to cancer treatment, according to the editorialists, is how local administration impacts systemic immunity in terms of recognition and elimination of remote lesions.
“Much more needs to be learned, but the clinical results to date encourage further exploration of this new treatment approach,” Dr. Longo and Dr. Baden wrote.
This summary is based on an editorial written by Dr. Longo and Dr. Baden that appeared in the New England Journal of Medicine. Dr. Baden and Longo both reported employment by the New England Journal of Medicine as deputy editor. Dr. Baden reported grant support from the Ragon Institute, the National Institutes of Health and the National Institute of Allergy and Infectious Disease, and the Gates Foundation outside the submitted work and also reported involvement in HIV vaccine trials done in collaboration with NIH, HIV Vaccine Trials Network, and others.
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
Treatment with the recombinant poliovirus vaccine PVSRIPO in patients with recurrent glioblastoma can be delivered at a safe dose with efficacy that compares favorably with historical data, recently reported results of a phase 1, nonrandomized study suggest.
The survival rate at 36 months after intratumoral infusion of PVSRIPO was 21%, versus 4% in a control group of patients who would have met the study’s eligibility criteria, investigators wrote in the New England Journal of Medicine.
There was no evidence of virus shedding or viral neuropathogenicity in the study, which included 61 patients with recurrent World Health Organization grade IV malignant glioma. “Further investigations are warranted,” wrote Annick Desjardins, MD, of Duke University, Durham, N.C., and her coauthors.
The prognosis of WHO grade IV malignant glioma remains dismal despite aggressive therapy and decades of research focused on advanced surgery, radiation, chemotherapy, and targeted agents, Dr. Desjardins and her colleagues said.
Accordingly, they sought to evaluate the potential of PVSRIPO, a live-attenuated poliovirus type 1 vaccine with its viral internal ribosome entry site replaced by one of human rhinovirus type. The engineered virus gains entry via the CD155 receptor, which is upregulated in solid tumors such as glioblastomas and expressed in antigen-presenting cells.
“Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity,” the investigators wrote.
With a median follow-up of 27.6 months, the median overall survival for PVSRIPO-treated patients was 12.5 months, longer than the 11.3 months seen in the historical control group. It was also longer than the 6.6 months found in a second comparison group of patients who underwent therapy with tumor-treating fields, which involves application of alternating electrical current to the head.
Survival hit a “plateau” in the PVSRIPO-treated patients, investigators said, with an overall survival rate of 21% at both 24 and 36 months. That stood in contrast to a decline in the historical control group from 14% at 24 months to 4% at 36 months, and a decline from 8% to 3% in the tumor-treating-fields group.
The phase 1 study had a dose-escalation phase including 9 patients and a dose-expansion phase with 52 patients. In the dose-expansion phase, 19% of patients had grade 3 or greater adverse events attributable to PVSRIPO, according to the report.
Of all 61 patients, 69% had a vaccine-related grade 1 or 2 event as their most severe adverse event.
One patient death caused by complications from an intracranial hemorrhage was attributed to bevacizumab. As part of a study protocol amendment, bevacizumab at half the standard dose was allowed to control symptoms of locoregional inflammation, investigators said.
In an ongoing, phase 2, randomized trial, PVSRIPO is being evaluated alone or with lomustine in patients with recurrent WHO grade IV malignant glioma. The Food and Drug Administration granted breakthrough therapy designation to PVSRIPO in May 2016.
Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
SOURCE: Desjardins A et al .N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Delivery of PVSRIPO was safe, with efficacy comparing favorably with historical data.
Major finding: Overall survival reached 21% at 24 months and remained at 21% at 36 months.
Study details: A phase 1 study including 61 patients with recurrent World Health Organization grade IV glioma.
Disclosures: Seven study authors reported equity in Istari Oncology, a biotechnology company that is developing PVSRIPO. Study authors also reported disclosures related to Genentech/Roche, Celgene, Celldex, and Eli Lilly, among other entities. The study was supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, the National Institutes of Health, and others.
Source: Desjardins A et al. N Engl J Med. 2018 Jun 26. doi: 10.1056/NEJMoa1716435.
Screening for brain mets could improve quality of life for some with breast cancer
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
FROM JAMA ONCOLOGY
Key clinical point: Breast cancer patients presented with larger and more numerous brain metastases compared with non–small-cell lung cancer patients, but after brain-directed therapy, there were no differences in outcomes between groups.
Major finding: Median survival was 1.45 years for breast cancer patients and 1.09 for NSCLC patients.
Study details: A retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Disclosures: The authors reported no conflicts of interest.
Source: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
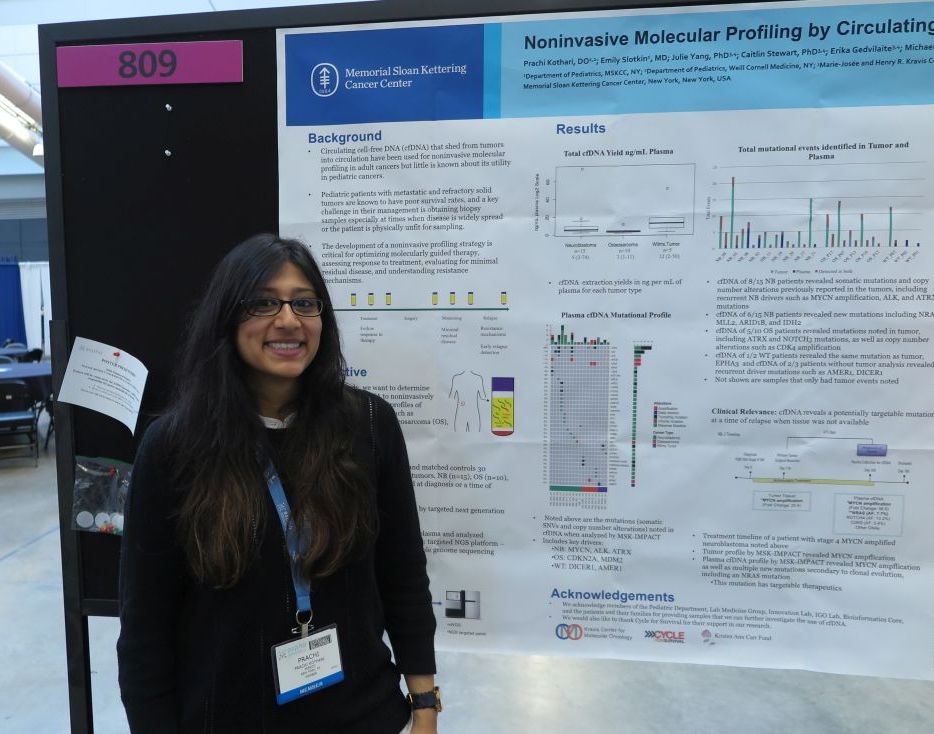
cfDNA reveals targetable mutations in pediatric neuroblastoma, sarcoma
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
PITTSBURGH – Genetic analysis of circulating free DNA (cfDNA) from pediatric solid tumors can noninvasively identify somatic mutations and copy number alterations that could be used to identify therapeutic targets, investigators reported.
An analysis of tumor specimens and plasma samples from children with neuroblastoma, osteosarcoma, and Wilms tumor revealed in cfDNA both somatic mutations and copy number alterations that had already been detected in the solid tumors, and new, potentially targetable mutations, reported Prachi Kothari, DO, and her colleagues from Memorial Sloan Kettering Cancer Center in New York.
“Circulating free DNA is much less invasive than a tumor biopsy, and you can do it throughout the patient’s entire timeline of treatment, so you get real-time information or after they relapse to see what’s going on if you’re not able to get a tumor biopsy,” Dr. Kothari said at annual meeting of the American Society of Pediatric Hematology/Oncology.
So-called “liquid biopsy” using cfDNA has been used for molecular profiling of adults malignancies, but there are few data on its use in pediatric tumors, Dr. Kothari said.
To see whether the technique could provide useful clinical information for the management of pediatric tumors, the investigators examined tumor samples taken at diagnosis or at the time of disease progression from 15 patients with neuroblastoma, 10 with osteosarcoma, and 5 with Wilms tumor. They analyzed the tumor samples using targeted next-generation sequencing (NGS), and cfDNA using three different genomic analysis techniques, including NGS, MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), and shallow whole genome sequencing.
For each of the tumor types studies, cfDNA analysis with the MSK-IMPACT platform identified key drivers of malignancy, including MYCN, ALK, and ATRX in neuroblastoma; CDKN2A and MDM2 in osteosarcoma; and DICER1 and AMER1 in Wilms tumor.
The cfDNA samples also revealed somatic mutations and copy number alterations previously reported in the tumors of 8 of the 15 patients with neuroblastoma, as well as potentially targetable new mutations in 6 of the 15 patients, including NRAS, MLL2, ARID1B, and IDH2.
For example, in one patient with stage 4 MYCN-amplified neuroblastoma, both tumor analysis and cfDNA revealed MYCN amplification, but cfDNA also show multiple new mutations, including a targetable NRAS mutation, secondary to clonal mutation.
In 5 of the 10 patients with osteosarcoma, cfDNA detected mutations that had been seen in the tumor samples, including mutations in ATRX and NOTCH3, and copy number alterations such as CDK4 amplification,
Of the five patients with Wilms tumors, cfDNA analysis was performed on two samples, one of which showed the same mutation as the tumor. Additionally, for the three patients without tumor analysis, cfDNA showed recurrent driver mutations such as AMER1 and DICER1.
The investigators have used the data from this study to create a genome-wide z score derived from shallow whole genome sequencing profiles and cfDNA, and found that a high genomewide z score, compared with a low score was significantly associated a more than four-fold greater risk for worse survival (hazard ratio, 4.42; P = .049).
“Establishing a platform using cfDNA to identify molecular profiles of these tumors can serve as a powerful tool for guiding treatment and monitoring response to treatment,” the investigators concluded.
The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
SOURCE: Kothari P et al. ASPHO 2018. Abstract #809.
REPORTING FROM ASPHO 2018
Key clinical point: Circulating free DNA analysis is a noninvasive method for detecting potential therapeutic targets.
Major finding: cfDNA revealed potentially targetable new mutations in 6 of 15 patients with neuroblastoma.
Study details: Retrospective analysis of tumor and plasma samples in 30 patients with neuroblastoma, osteosarcoma, or Wilms tumor.
Disclosures: The study was supported by Cycle for Survival and the Kristen Ann Carr Fund. The investigators reported having no conflicts of interest.
Source: Kothari P et al. ASPHO 2018. Abstract #809.
Surgery indicates higher survival with adrenal cortical carcinoma
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
SOURCE: Tella SH et al. Endo 2018.
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
SOURCE: Tella SH et al. Endo 2018.
CHICAGO –
These findings, uncovered using the largest cancer registry in the United States, will help give physicians insight into what the best course of action is for treating their patients, according to presenters.
“Surgical resection of the primary tumor improved survival in all stages of disease, whereas adjuvant therapy with chemotherapy or radiation improved overall survival only in stage IVpatients,” said Sri Harsha Tella, MD, endocrinologist at the University of South Carolina, Columbia. “These results may help the prognostication of patients in treatment decision making.”
Investigators conducted a retrospective study of 3,185 pathologically confirmed cases of ACC, registered into the National Cancer Database between 2004 and 2015.
Patients were mostly women with an average age of 55 years old and private insurance, with a nearly even split of patients with stage I-III (26%) and stage IV ACC (24%). Nearly three-quarters of those studied chose to have surgery, of which 31% chose open resection.
Patients with stage I-III ACC had a significant median survival rate of 63 months, compared with those who did not have surgery who had an average survival of 8 months.
In patients with stage IV ACC, surgery lengthened overall survival to 19 months, compared with 6 months for those without surgery, according to Dr. Tella and fellow investigators.
While surgery did have a greater positive effect on patients’ live spans across all stages, the impact of chemotherapy and radiation was significant only among stage IV patients who had complete surgery.
Those in the stage IV group who were given post-surgery adjuvant chemotherapy were likely to live an average of nearly 9 more months than did those who did not have chemotherapy after radiation (22 vs. 13), while those given radiation therapy saw an increase in survival by 19 months (29 vs. 10). These increases did not affect stage I-III patients, who had a similar rate of survival regardless of additional therapies after their surgery (24 vs. 25 months).
One possible explanation for why additional therapy made little difference in survival for stage I-III patients is that, given that the tumors did not spread as widely, the surgical procedures were likely to be more effective at removing most of the disease, according to Dr. Tella.
“One of the possibilities is that surgeons were able to get the whole mass out,” Dr. Tella hypothesized in response to a question from attendees. “On the other hand, patients with stage IV ACC may be more likely to have more presence of metastases and so would benefit more greatly from the removal of the primary tumor and then also additional therapy.”
Investigators noted that because of the structure of the registry, they were unable to determine the initiation and duration of chemotherapy, as well as doses of radiation therapy received by the patient.
A more robust database and future stage-specific, prospective clinical trials are needed in order to better understand these findings, Dr. Tella said.
The investigators reported no relevant financial disclosures.
SOURCE: Tella SH et al. Endo 2018.
REPORTING FROM ENDO 2018
Key clinical point: Patients who undergo surgical resection at all stages are more likely to survive.
Major finding: Patients stage I-III who underwent surgery survived over nearly 8 times longer than non-surgery patients (63 vs. 8 months [P less than .001]).
Study details: Retrospective study of 3,185 adrenal cortical carcinoma cases entered into the National Cancer Database between 2004 and 2015.
Disclosures: The presenter reported no relevant financial disclosures.
Source: Tella SH et al. Endo 2018.
Study: Test for PD-L1 amplification in solid tumors
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
SAN FRANCISCO – Amplification of programmed death-ligand 1 (PD-L1), also known as cluster of differentiation 274 (CD274), is rare in most solid tumors, but findings from an analysis in which a majority of patients with the alteration experienced durable responses to PD-1/PD-L1 blockade suggest that testing for it may be warranted.
Of 117,344 deidentified cancer patient samples from a large database, only 0.7% had PD-L1 amplification, which was defined as 6 or more copy number alterations (CNAs). The CNAs were found across more than 100 tumor histologies, Aaron Goodman, MD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
Of a subset of 2,039 clinically annotated patients from the database, who were seen at the University of California, San Diego (UCSD) Center for Personalized Cancer Therapy, 13 (0.6%) had PD-L1 CNAs, and 9 were treated with immune checkpoint blockade, either alone or in combination with another immunotherapeutic or targeted therapy, after a median of four prior systemic therapies.
The PD-1/PD-L1 blockade response rate in those nine patients was 67%, and median progression-free survival was 15.2 months; three objective responses were ongoing for at least 15 months, said Dr. Goodman of UCSD.
The findings are notable, because in unselected patients, the rates of response to immune checkpoint blockade range from 10% to 20%.
Lessons from cHL and solid tumors
“Over the past few years, investigators have identified numerous biomarkers that can select subgroups of patients with increased likelihoods of responding to PD-1 blockade,” he said, adding that biomarkers include PD-L1 expression by immunohistochemistry, microsatellite instability – with microsatellite instability–high tumors responding extremely well to immunotherapy, tumor mutational burden measured by whole exome sequencing and next generation sequencing, and possibly PD-L1 amplification.
Of note, response rates are high in patients with classical Hodgkin lymphoma (cHL). In general, cHL patients respond well to treatment, with the majority being cured by way of multiagent chemotherapy and radiation.
“But for the subpopulation that fails to respond to chemotherapy or relapses, outcomes still remain suboptimal. Remarkably, in the relapsed/refractory population of Hodgkin lymphoma ... response rates to single agent nivolumab and pembrolizumab were 65% to 87% [in recent studies],” he said. “Long-term follow-up demonstrates that the majority of these responses were durable and lasted over a year.”
The question is why relapsed/refractory cHL patients treated with immune checkpoint blockade have such a higher response rate than is typically seen in patients with solid tumors.
One answer might lie in the recent finding that nearly 100% of cHL tumors harbor amplification of 9p24.1; the 9p24.1 amplicon encodes the genes PD-L1, PD-L2, and JAK2, (and thus is also known as the PDJ amplicon), he explained, adding that “through gene dose-dependent increased expression of PD-L1 ligand on the Hodgkin lymphoma Reed-Sternberg cells, there is also JAK-STAT mediation of further expression of PD-L1 on the Reed-Sternberg cells.
An encounter with a patient with metastatic basal cell carcinoma – a “relatively unusual situation, as the majority of patients are cured with local therapy”– led to interest in looking at 9p24.1 alterations in solid tumors.
The patient had extensive metastatic disease, and had progressed through multiple therapies. Given his limited treatment options, next generation sequencing was performed on a biopsy from his tumor, and it revealed the PTCH1 alteration typical in basal cell carcinoma, as well as amplification of 9p24.1 with PD-L1, PD-L2, and JAK2 amplification. Nivolumab monotherapy was initiated.
“Within 2 months, he had an excellent partial response to therapy, and I’m pleased to say that he’s in an ongoing complete response 2 years later,” Dr. Goodman said.
It was that case that sparked the idea for the current study.
9p24.1 alterations and checkpoint blockade
“With my interest in hematologic malignancies, I was unaware that [9p24.1] amplification could occur in solid tumors, so the first aim was to determine the prevalence of chromosome 9p24.1 alterations in solid tumors. The next was to determine if patients with solid tumors and chromosome 9p24.1 alterations respond to PD-1/PD-L1 checkpoint blockade.
“What is astounding is [that PD-L1 amplification] was found in over 100 unique tumor histologies, although rare in most histologies,” Dr. Goodman said, noting that histologies with a statistically increased prevalence of PD-L1 amplification included breast cancer, head and neck squamous cell carcinoma, lung squamous cell carcinoma, and soft tissue sarcoma.
There also were some rare histologies with increased prevalence of PD-L1 amplification, including nasopharyngeal carcinoma, renal sarcomatoid carcinoma, bladder squamous cell carcinoma, and liver mixed hepatocellular cholangiocarcinoma, he said.
Tumors with a paucity of PD-L1 amplification included colorectal cancer, pancreatic cancer, and cutaneous melanoma, although even these still harbored a few patients with amplification, he said.
A closer look at the mutational burden in amplified vs. unamplified tumors showed a median of 7.4 vs. 3.6 mut/mb, but in the PD-L1 amplified group, 85% still had a low-to intermediate mutational burden of 1-20 mut/mb.
“Microsatellite instability and PD-L1 amplification were not mutually exclusive, but a rare event. Five of the 821 cases with PD-L1 amplification were microsatellite high; these included three carcinomas of unknown origin and two cases of gastrointestinal cancer,” he noted.
Treatment outcomes
In the 13 UCSD patients with PD-L1 amplification, nine different malignancies were identified, and all patients had advanced or metastatic disease and were heavily pretreated. Of the nine treated patients, five received anti-PD-1 monotherapy, one received anti-CTLA4/anti-PD-1 combination therapy, and three received a PD-1/PD-L1 inhibitor plus an investigational agent, which was immunotherapeutic, Dr. Goodman said.
The 67% overall response rate was similar to that seen in Hodgkin lymphoma, and many of the responses were durable; median overall survival was not reached.
Of note, genomic analysis in the 13 UCSD patients found to have PD-L1 amplification showed there were 143 total alterations in 70 different genes. All but one patient had amplification of PD-L1, PD-L2, and JAK2, and that one had amplification of PD-L1 and PD-L2.
Of six tumors with tissue available to test for PD-L1 expression by immunohistochemistry, four (67%) tested positive. None were microsatellite high, and tumor-infiltrating lymphocytes were present in five cases.
The tumors that tested negative for PD-L1 expression were from the patient with the rare basal cell cancer, and another with glioblastoma. Both responded to anti-PD1/PD-L1 therapy.
The glioblastoma patient was a 40-year-old man with progressive disease, who underwent standard surgical debulking followed by concurrent radiation therapy plus temozolomide. He progressed soon after completing the concurrent chemoradiation therapy, and genomic profiling revealed 12 alterations, including 9p24.1 amplification, Dr. Goodman said, adding that nivolumab therapy was initiated.
“By week 12, much of the tumor mass had started to resolve, and by week 26 it continued to decrease further. He continues to be in an ongoing partial response at 5.2 months,” he said.
Recommendations
The findings of this study demonstrate that PD-Ll amplification is rare in solid tumors.
“However, PD-L1 amplification appears to be tissue agnostic, as we have seen in over 100 tumor histologies. We also noted that PD-L1 amplification was enriched in many rare tumors with limited treatment options, including anaplastic thyroid cancer, sarcomatoid carcinoma, and some sarcomas. We believe testing for PD-L1 amplification may be warranted given the frequent responses that were durable and seemed to be independent of mutational burden,” he concluded.
Ravindra Uppaluri, MD, session chair and discussant for Dr. Goodman’s presentation, said that Dr. Goodman’s findings should be considered in the context of “the complex biology [of PD-L1/PD-L2] that has evolved over the last few years.”
He specifically mentioned the two patients without PD-L1 expression despite amplification, but with response to immune checkpoint blockade, and noted that “there are several things going on here ... and we really want to look at all these things.”
The PDJ amplicon, especially given “the ability to look at this with the targeted gene panels that many patients are getting,” is clearly contributing to biomarker stratification, said Dr. Uppaluri of Dana-Farber Cancer Institute and Brigham and Women’s Hospital, Boston.
However, it should be assessed as part of a “global biomarker” that includes tumor-infiltrating lymphocytes and tumor mutational burden, he said.
Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
SOURCE: Goodman A et al. ASCO-SITC, Abstract 47
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: Solid tumor patients with PD-L1 amplification had durable responses to PD-1/PD-L1 blockade.
Major finding: The overall response rate was 67% in nine patients treated with PD-1/PD-L1 blockade.
Study details: An analysis of more than 117,000 patient samples.
Disclosures: Dr. Goodman reported having no disclosures. Dr. Uppaluri has received grant/research support from NIH/NIDCR, Merck, and V Foundation, and has received honoraria from Merck.
Source: Goodman A et al. ASCO-SITC, Abstract 47.
FDA: Gadolinium retention prompts new GBCA class warning, safety measures
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
Gadolinium-based contrast agents (GBCAs) used for MRI will now carry a warning regarding their potential retention in the bodies and brains of treated patients, according to the Food and Drug Administration.
The FDA is requiring the new class warning, along with other safety measures, based on evidence showing that trace amounts of gadolinium can be retained in the body for months to years after treatment.
Specifically, the agency will require that patients receiving GBCAs first receive a Medication Guide and that GBCA manufacturers conduct human and animal studies to further assess GBCA safety. At this time, the only known adverse health effect of gadolinium retention is nephrogenic systemic fibrosis, which affects a small subgroup of patients with pre-existing kidney failure. No causal association has been established between gadolinium retention and reported adverse events in those with normal kidney function.
The FDA recommended that health care professionals consider the retention characteristics of GBCAs for patients who may be at higher risk for retention, including those requiring multiple lifetime doses, pregnant women, children, and patients with inflammatory conditions, but stressed that, although repeated GBCA imaging studies should be minimized when possible, they should not be avoided or deferred when they are necessary. In the safety alert, the FDA noted that administration of the GBCAs Dotarem (gadoterate meglumine), Gadavist (gadobutrol), and ProHance (gadoteridol) produce the lowest gadolinium levels in the body, and the three agents leave similar gadolinium levels in the body.
The agency encourages reports of adverse events or side effects related to the use of GBCAs to its MedWatch Safety information and Adverse Event Reporting Program. Reports can be submitted online at www.fda.gov/MedWatch/report or by calling 1-800-332-1088 to request a preaddressed form that can be mailed or faxed to 1-800-FDA-0178.
FDA approves biosimilar to bevacizumab
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
Glioblastoma: Prognosis is poor, but new therapies are emerging
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.
The questioning of former FBI director James B. Comey by Sen. John McCain (R-Ariz.) during a June 8 Senate Intelligence Committee hearing raised more than a few eyebrows; Sen. McCain seemed confused and disoriented, at one point referring to Mr. Comey as “President Comey,” but a possible medical explanation emerged soon after.
On July 14, Sen. McCain, 80, underwent surgery to remove a 5-cm blood clot that had been discovered above his left eye during a physical, and on July 19, the Mayo Clinic in Phoenix, where he had undergone the procedure, announced at his request that, “subsequent tissue pathology revealed that a primary brain tumor known as a glioblastoma was associated with the blood clot.”
Glioblastoma features
While Sen. McCain’s symptoms can’t necessarily be attributed to the glioblastoma, it is not unusual for glioblastoma patients to present with some sort of neurologic deficit, such as speech issues, unilateral weakness, or confusion, according to Eudocia Quant Lee, MD, a neuro-oncologist at Dana-Farber Cancer Institute, Boston.
Neuro-oncologist Manmeet Singh Ahluwalia, MD, of the Cleveland Clinic said seizures, persistent headaches, double or blurred vision, and changes in ability to think and learn can also be presenting symptoms.
Glioblastoma is the most common malignant primary brain tumor diagnosed in adults, with an estimated 12,000-13,000 new cases occurring each year in the United States. It is more common among older adults but can occur in younger patients. It arises in the brain and generally stays within the central nervous system, Dr. Lee explained, noting that it is much less common than lung cancer, breast cancer, and melanoma.
This is particularly true for older patients.
Prognosis and age
“We know, in general – as with most cancers – that the older you’re diagnosed with your cancer, the poorer your prognosis is,” she said, adding that other health issues and the ability to tolerate treatment can affect outcomes.
Outcomes also can be affected by type of surgery, functional status, extent of treatment, and molecular subtypes of the glioblastoma, Dr. Ahluwalia said.
Survival generally ranges about 14-18 months, although about 10% of patients live 5 years or longer.
The study, presented in a poster by Michael Weller, MD, of University Hospital and University of Zürich and his colleagues, also showed that, compared with the 398 older patients who survived less than 2 years (median, 6.2 months), those who survived longer had “more intensive up-front treatment and a trend toward higher initial Karnofsky performance scores as distinguishing clinical factors.”
In addition, molecular analyses showed more frequent O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in those with longer survival, while isocitrate dehydrogenase (IDH) mutations were restricted to single patients.
“Collectively, our findings confirm that LTS is rare in elderly patients with glioblastoma and that clinical and tumor-associated molecular factors linked to LTS resemble those in standard-age patients, except for less common IDH mutation,” the investigators wrote.
Another abstract published online in conjunction with the ASCO annual meeting looked at outcomes, based on age and MGMT analysis, and similarly found that aggressive treatment with chemoradiation is associated with better outcomes in both younger and older patients.
In that study by Suryanarayan Mohapatra, MD, of Cleveland Clinic–Fairview Hospital, and his colleagues – including Dr. Ahluwalia – 567 of 1,165 patients were aged 65 years or older. The benefits of chemoradiation therapy, which was associated with a significantly lower risk of death vs. radiation therapy alone in the study, were more pronounced among the older patients (hazard ratio, 0.45 vs. 0.61 for those under age 65 years), but the difference did not reach statistical significance.
Dr. Mohapatra and his colleagues also showed that more aggressive therapy resulted in better overall and progression-free survival regardless of MGMT methylation status, but that there was no difference between the age groups on this measure. Overall and progression-free survival also were significantly better with gross-total resection and subtotal resection vs. biopsy only, and with diagnosis during 2009 and later vs. during 2007-2008. However, a difference between the two age groups was seen with respect to overall survival only among those diagnosed during 2009 or later, with a more prominent impact among the younger group, the investigators reported.
“Older individuals often get less aggressive treatment. However, based on the research, active and functional older patients should get aggressive treatment,” Dr. Ahluwalia said. “We advocate tailor-made treatment that takes into account patient condition, location of tumor, functional status, etc., in addition to patient age.”
Standard treatment approaches
The first step in the treatment of glioblastomas is maximal safe therapy, Dr. Lee said.
“You want to achieve as much of a resection as possible without leaving the patient with some sort of permanent neurologic deficit that could severely compromise the quality of their life,” she explained.
Sen. McCain’s tumor was “completely resected by imaging criteria,” according to the Mayo Clinic statement, which also noted that treatment options might include a combination of chemotherapy and radiation.
Indeed, the standard of care for glioblastomas after surgery is combined chemotherapy and radiation – typically given as approximately 6 weeks of radiation combined with oral temozolomide chemotherapy – followed by 6 monthly cycles of temozolomide, she explained.
Radiation is sometimes given for only 3 weeks, but this option is mainly reserved for elderly patients, she said, adding that trials in patients aged 65-70 years have shown that this shorter course of radiation can be equally effective but potentially less toxic.
Emerging treatment approaches
Another treatment that has shown promise involves the use of tumor-treating fields (TTFields) – a locoregionally delivered antimitotic treatment that disrupts cell division and organelle assembly.
A 2015 phase 3 trial showed that adding TTFields to maintenance temozolomide significantly prolonged progression-free and overall survival, Dr. Ahluwalia said.
Other studies, including two presented during poster sessions at the ASCO annual meeting, have shown a progression-free survival benefit with the addition of bevacizumab to the treatment regimen. One open-label phase 2 study showed that hypofractionated radiotherapy in combination with IV bevacizumab every 2 weeks vs. radiotherapy alone in newly diagnosed patients over age 65 years improved progression-free survival (median, 7.6 vs. 4.8 months), but not overall survival (median, 12.1 vs. 12.2 months).
Another phase 2 study presented by Phioanh (Leia) Nghiemphu, MD, of the University of California, Los Angeles, showed that in newly diagnosed patients aged 70 years and older, upfront treatment with bevacizumab and temozolomide was associated with promising survival benefits (overall survival, 12.3 months; progression-free survival, 5.1 months) and tolerable side effects. The best survival in multivariate analysis was in patients who received radiotherapy at progression; it was unclear whether the addition of bevacizumab led to a survival advantage, but it may have allowed delay of radiotherapy treatment, she noted.
“Although we have no cure for glioblastoma, treatments can control tumor growth for a period of time, and there are additional promising therapies emerging every day to treat this deadly cancer, she said in an interview. “There has been increasing interest in developing better therapies for the older patients with glioblastoma with less toxicity and still-robust survival, such as the addition of bevacizumab or a short course of radiotherapy with temozolomide chemotherapy.”
Dr. Ahluwalia encourages clinical trial participation for patients diagnosed with glioblastoma and noted that he is particularly excited about immunotherapy and targeted therapy trials.
Dr. Lee has served as consultant to Eli Lilly. Dr. Ahluwalia disclosed a financial relationship with multiple companies, including Novocure, which markets a TTFields device. Dr. Weller disclosed a financial relationships with multiple companies, including Novocure; Merck Sharp & Dohme, which markets temozolomide; and Roche, which markets bevacizumab. Dr. Nghiemphu has received research funding from Genentech/Roche and Novartis. Dr. Mohapatra reporting having no disclosures.