User login
Pandemic-stressed youths call runaway hotline
The calls kept coming into the National Runaway Safeline during the pandemic: the desperate kids who wanted to bike away from home in the middle of the night, the isolated youths who felt suicidal, the teens whose parents had forced them out of the house.
To the surprise of experts who help runaway youths, the pandemic didn’t appear to produce a big rise or fall in the numbers of children and teens who had left home. Still, the crisis hit hard. As schools closed and households sheltered in place, youths reached out to the National Runaway Safeline to report heightened family conflicts and worsening mental health.
The Safeline, based in Chicago, is the country’s 24/7, federally designated communications system for runaway and homeless youths. Each year, it makes about 125,000 connections with young people and their family members through its hotline and other services.
In a typical year, teens aged 15-17 years are the main group that gets in touch by phone, live chat, email, or an online crisis forum, according to Jeff Stern, chief engagement officer at the Safeline.
But in the past 2 years, “contacts have skewed younger,” including many more children under age 12.
“I think this is showing what a hit this is taking on young children,” he said.
Without school, sports, and other activities, younger children might be reaching out because they’ve lost trusted sources of support. Callers have been as young as 9.
“Those ones stand out,” said a crisis center supervisor who asked to go by Michael, which is not his real name, to protect the privacy of his clients.
In November 2020, a child posted in the crisis forum: “I’m 11 and my parents treat me poorly. They have told me many times to ‘kill myself’ and I didn’t let that settle well with me. ... I have tried to run away one time from my house, but they found out, so they took my phone away and put screws on my windows so I couldn’t leave.”
Increasing numbers of children told Safeline counselors that their parents were emotionally or verbally abusive, while others reported physical abuse. Some said they experienced neglect, while others had been thrown out.
“We absolutely have had youths who have either been physically kicked out of the house or just verbally told to leave,” Michael said, “and then the kid does.”
Heightened family conflicts
The Safeline partners with the National Center for Missing and Exploited Children, which, despite widespread public perception, doesn’t work mainly with child abduction cases. Each year, the center assists with 29,000-31,000 cases, and 92% involve “endangered runaways,” said John Bischoff, vice president of the Missing Children Division. These children could be running away from home or foster care.
During the pandemic, the center didn’t spot major changes in its missing child numbers, “which honestly was shocking,” Mr. Bischoff said. “We figured we were either going to see an extreme rise or a decrease.
“But the reasons for the run were changing,” he said.
Many youths were fleeing out of frustration with quarantine restrictions, Mr. Bischoff said, as well as frustration with the unknown and their own lack of control over many situations.
At the runaway hotline, calls have been longer and more intense, with family problems topping the list of concerns. In 2019, about 57% of all contacts mentioned family dynamics. In 2020, that number jumped to 88%, according to Mr. Stern.
Some kids sought support for family problems that involved school. In October 2020, one 13-year-old wrote in the Safeline forum: “My mom constantly yells at me for no reason. I want to leave, but I don’t know how. I have also been really stressed about school because they haven’t been giving me the grades I would normally receive during actual school. She thinks I’m lying and that I don’t care. I just need somebody to help me.”
Many adults are under tremendous strain, too, Michael said.
“Parents might have gotten COVID last month and haven’t been able to work for 2 weeks, and they’re missing a paycheck now. Money is tight, there might not be food, everyone’s angry at everything.”
During the pandemic, the National Runaway Safeline found a 16% increase in contacts citing financial challenges.
Some children have felt confined in unsafe homes or have endured violence, as one 15-year-old reported in the forum: “I am the scapegoat out of four kids. Unfortunately, my mom has always been a toxic person. ... I’m the only kid she still hits really hard. She’s left bruises and scratches recently. ... I just have no solution to this.”
Worsening mental health
Besides family dynamics, mental health emerged as a top concern that youths reported in 2020. “This is something notable. It increased by 30% just in 1 year,” Mr. Stern said.
In November 2020, a 16-year-old wrote: “I can’t ever go outside. I’ve been stuck in the house for a very long time now since quarantine started. I’m scared. ... My mother has been taking her anger out on me emotionally. ... I have severe depression and I need help. Please, if there’s any way I can get out of here, let me know.”
The Safeline also has seen a rise in suicide-related contacts. Among children and teens who had cited a mental health concern, 18% said they were suicidal, Stern said. Most were between ages 12 and 16, but some were younger than 12.
When children couldn’t hang out with peers, they felt even more isolated if parents confiscated their phones, a common punishment, Michael said.
During the winter of 2020-21, “It felt like almost every digital contact was a youth reaching out on their Chromebook because they had gotten their phone taken away and they were either suicidal or considering running away,” he said. “That’s kind of their entire social sphere getting taken away.”
Reality check
Roughly 7 in 10 youths report still being at home when they reach out to the Safeline. Among those who do leave, Michael said, “They’re going sometimes to friends’ houses, oftentimes to a significant other’s house, sometimes to extended family members’ houses. Often, they don’t have a place that they’re planning to go. They just left, and that’s why they’re calling us.”
While some youths have been afraid of catching COVID-19 in general, the coronavirus threat hasn’t deterred those who have decided to run away, Michael said. “Usually, they’re more worried about being returned home.”
Many can’t comprehend the risks of setting off on their own.
In October 2021, a 15-year-old boy posted on the forum that his verbally abusive parents had called him a mistake and said they couldn’t wait for him to move out.
“So I’m going to make their dreams come true,” he wrote. “I’m going to go live in California with my friend who is a young YouTuber. I need help getting money to either fly or get a bus ticket, even though I’m all right with trying to ride a bike or fixing my dirt bike and getting the wagon to pull my stuff. But I’m looking for apartments in Los Angeles so I’m not living on the streets and I’m looking for a job. Please help me. My friend can’t send me money because I don’t have a bank account.”
“Often,” Michael said, “we’re reality-checking kids who want to hitchhike 5 hours away to either a friend’s or the closest shelter that we could find them. Or walk for 5 hours at 3 a.m. or bike, so we try to safety-check that.”
Another concern: online enticement by predators. During the pandemic, the National Center for Missing and Exploited Children saw cases in which children ran away from home “to go meet with someone who may not be who they thought they were talking to online,” Mr. Bischoff said. “It’s certainly something we’re keeping a close eye on.”
Fewer resources in the pandemic
The National Runaway Safeline provides information and referrals to other hotlines and services, including suicide prevention and mental health organizations. When youths have already run away and have no place to go, Michael said, the Safeline tries to find shelter options or seek out a relative who can provide a safe place to stay.
But finding shelters became tougher during the pandemic, when many had no room or shelter supply was limited. Some had to shut down for COVID-19–related deep cleanings, Michael said. Helping youths find transportation, especially with public transportation shutdowns, also was tough.
The Huckleberry House, a six-bed youth shelter in San Francisco, has stayed open throughout the pandemic with limited staffing, said Douglas Styles, PsyD. He’s the executive director of the Huckleberry Youth Programs, which runs the house.
The shelter, which serves Bay Area runaway and homeless youths ages 12-17, hasn’t seen an overall spike in demand, Dr. Styles said. But “what’s expanded is undocumented [youths] and young people who don’t have any family connections in the area, so they’re unaccompanied as well. We’ve seen that here and there throughout the years, but during the pandemic, that population has actually increased quite a bit.”
The Huckleberry House has sheltered children and teens who have run away from all kinds of homes, including affluent ones, Dr. Styles said.
Once children leave home, the lack of adult supervision leaves them vulnerable. They face multiple dangers, including child sex trafficking and exploitation, substance abuse, gang involvement, and violence. “As an organization, that scares us,” Mr. Bischoff said. “What’s happening at home, we’ll sort that out. The biggest thing we as an organization are trying to do is locate them and ensure their safety.”
To help runaways and their families get in touch, the National Runaway Safeline provides a message service and conference calling. “We can play the middleman, really acting on behalf of the young person – not because they’re right or wrong, but to ensure that their voice is really heard,” Mr. Stern said.
Through its national Home Free program, the Safeline partners with Greyhound to bring children back home or into an alternative, safe living environment by providing a free bus ticket.
These days, technology can expose children to harm online, but it can also speed their return home.
“When I was growing up, if you weren’t home by 5 o’clock, Mom would start to worry, but she really didn’t have any way of reaching you,” Mr. Bischoff said. “More children today have cellphones. More children are easily reachable. That’s a benefit.”
A version of this article first appeared on WebMD.com.
The calls kept coming into the National Runaway Safeline during the pandemic: the desperate kids who wanted to bike away from home in the middle of the night, the isolated youths who felt suicidal, the teens whose parents had forced them out of the house.
To the surprise of experts who help runaway youths, the pandemic didn’t appear to produce a big rise or fall in the numbers of children and teens who had left home. Still, the crisis hit hard. As schools closed and households sheltered in place, youths reached out to the National Runaway Safeline to report heightened family conflicts and worsening mental health.
The Safeline, based in Chicago, is the country’s 24/7, federally designated communications system for runaway and homeless youths. Each year, it makes about 125,000 connections with young people and their family members through its hotline and other services.
In a typical year, teens aged 15-17 years are the main group that gets in touch by phone, live chat, email, or an online crisis forum, according to Jeff Stern, chief engagement officer at the Safeline.
But in the past 2 years, “contacts have skewed younger,” including many more children under age 12.
“I think this is showing what a hit this is taking on young children,” he said.
Without school, sports, and other activities, younger children might be reaching out because they’ve lost trusted sources of support. Callers have been as young as 9.
“Those ones stand out,” said a crisis center supervisor who asked to go by Michael, which is not his real name, to protect the privacy of his clients.
In November 2020, a child posted in the crisis forum: “I’m 11 and my parents treat me poorly. They have told me many times to ‘kill myself’ and I didn’t let that settle well with me. ... I have tried to run away one time from my house, but they found out, so they took my phone away and put screws on my windows so I couldn’t leave.”
Increasing numbers of children told Safeline counselors that their parents were emotionally or verbally abusive, while others reported physical abuse. Some said they experienced neglect, while others had been thrown out.
“We absolutely have had youths who have either been physically kicked out of the house or just verbally told to leave,” Michael said, “and then the kid does.”
Heightened family conflicts
The Safeline partners with the National Center for Missing and Exploited Children, which, despite widespread public perception, doesn’t work mainly with child abduction cases. Each year, the center assists with 29,000-31,000 cases, and 92% involve “endangered runaways,” said John Bischoff, vice president of the Missing Children Division. These children could be running away from home or foster care.
During the pandemic, the center didn’t spot major changes in its missing child numbers, “which honestly was shocking,” Mr. Bischoff said. “We figured we were either going to see an extreme rise or a decrease.
“But the reasons for the run were changing,” he said.
Many youths were fleeing out of frustration with quarantine restrictions, Mr. Bischoff said, as well as frustration with the unknown and their own lack of control over many situations.
At the runaway hotline, calls have been longer and more intense, with family problems topping the list of concerns. In 2019, about 57% of all contacts mentioned family dynamics. In 2020, that number jumped to 88%, according to Mr. Stern.
Some kids sought support for family problems that involved school. In October 2020, one 13-year-old wrote in the Safeline forum: “My mom constantly yells at me for no reason. I want to leave, but I don’t know how. I have also been really stressed about school because they haven’t been giving me the grades I would normally receive during actual school. She thinks I’m lying and that I don’t care. I just need somebody to help me.”
Many adults are under tremendous strain, too, Michael said.
“Parents might have gotten COVID last month and haven’t been able to work for 2 weeks, and they’re missing a paycheck now. Money is tight, there might not be food, everyone’s angry at everything.”
During the pandemic, the National Runaway Safeline found a 16% increase in contacts citing financial challenges.
Some children have felt confined in unsafe homes or have endured violence, as one 15-year-old reported in the forum: “I am the scapegoat out of four kids. Unfortunately, my mom has always been a toxic person. ... I’m the only kid she still hits really hard. She’s left bruises and scratches recently. ... I just have no solution to this.”
Worsening mental health
Besides family dynamics, mental health emerged as a top concern that youths reported in 2020. “This is something notable. It increased by 30% just in 1 year,” Mr. Stern said.
In November 2020, a 16-year-old wrote: “I can’t ever go outside. I’ve been stuck in the house for a very long time now since quarantine started. I’m scared. ... My mother has been taking her anger out on me emotionally. ... I have severe depression and I need help. Please, if there’s any way I can get out of here, let me know.”
The Safeline also has seen a rise in suicide-related contacts. Among children and teens who had cited a mental health concern, 18% said they were suicidal, Stern said. Most were between ages 12 and 16, but some were younger than 12.
When children couldn’t hang out with peers, they felt even more isolated if parents confiscated their phones, a common punishment, Michael said.
During the winter of 2020-21, “It felt like almost every digital contact was a youth reaching out on their Chromebook because they had gotten their phone taken away and they were either suicidal or considering running away,” he said. “That’s kind of their entire social sphere getting taken away.”
Reality check
Roughly 7 in 10 youths report still being at home when they reach out to the Safeline. Among those who do leave, Michael said, “They’re going sometimes to friends’ houses, oftentimes to a significant other’s house, sometimes to extended family members’ houses. Often, they don’t have a place that they’re planning to go. They just left, and that’s why they’re calling us.”
While some youths have been afraid of catching COVID-19 in general, the coronavirus threat hasn’t deterred those who have decided to run away, Michael said. “Usually, they’re more worried about being returned home.”
Many can’t comprehend the risks of setting off on their own.
In October 2021, a 15-year-old boy posted on the forum that his verbally abusive parents had called him a mistake and said they couldn’t wait for him to move out.
“So I’m going to make their dreams come true,” he wrote. “I’m going to go live in California with my friend who is a young YouTuber. I need help getting money to either fly or get a bus ticket, even though I’m all right with trying to ride a bike or fixing my dirt bike and getting the wagon to pull my stuff. But I’m looking for apartments in Los Angeles so I’m not living on the streets and I’m looking for a job. Please help me. My friend can’t send me money because I don’t have a bank account.”
“Often,” Michael said, “we’re reality-checking kids who want to hitchhike 5 hours away to either a friend’s or the closest shelter that we could find them. Or walk for 5 hours at 3 a.m. or bike, so we try to safety-check that.”
Another concern: online enticement by predators. During the pandemic, the National Center for Missing and Exploited Children saw cases in which children ran away from home “to go meet with someone who may not be who they thought they were talking to online,” Mr. Bischoff said. “It’s certainly something we’re keeping a close eye on.”
Fewer resources in the pandemic
The National Runaway Safeline provides information and referrals to other hotlines and services, including suicide prevention and mental health organizations. When youths have already run away and have no place to go, Michael said, the Safeline tries to find shelter options or seek out a relative who can provide a safe place to stay.
But finding shelters became tougher during the pandemic, when many had no room or shelter supply was limited. Some had to shut down for COVID-19–related deep cleanings, Michael said. Helping youths find transportation, especially with public transportation shutdowns, also was tough.
The Huckleberry House, a six-bed youth shelter in San Francisco, has stayed open throughout the pandemic with limited staffing, said Douglas Styles, PsyD. He’s the executive director of the Huckleberry Youth Programs, which runs the house.
The shelter, which serves Bay Area runaway and homeless youths ages 12-17, hasn’t seen an overall spike in demand, Dr. Styles said. But “what’s expanded is undocumented [youths] and young people who don’t have any family connections in the area, so they’re unaccompanied as well. We’ve seen that here and there throughout the years, but during the pandemic, that population has actually increased quite a bit.”
The Huckleberry House has sheltered children and teens who have run away from all kinds of homes, including affluent ones, Dr. Styles said.
Once children leave home, the lack of adult supervision leaves them vulnerable. They face multiple dangers, including child sex trafficking and exploitation, substance abuse, gang involvement, and violence. “As an organization, that scares us,” Mr. Bischoff said. “What’s happening at home, we’ll sort that out. The biggest thing we as an organization are trying to do is locate them and ensure their safety.”
To help runaways and their families get in touch, the National Runaway Safeline provides a message service and conference calling. “We can play the middleman, really acting on behalf of the young person – not because they’re right or wrong, but to ensure that their voice is really heard,” Mr. Stern said.
Through its national Home Free program, the Safeline partners with Greyhound to bring children back home or into an alternative, safe living environment by providing a free bus ticket.
These days, technology can expose children to harm online, but it can also speed their return home.
“When I was growing up, if you weren’t home by 5 o’clock, Mom would start to worry, but she really didn’t have any way of reaching you,” Mr. Bischoff said. “More children today have cellphones. More children are easily reachable. That’s a benefit.”
A version of this article first appeared on WebMD.com.
The calls kept coming into the National Runaway Safeline during the pandemic: the desperate kids who wanted to bike away from home in the middle of the night, the isolated youths who felt suicidal, the teens whose parents had forced them out of the house.
To the surprise of experts who help runaway youths, the pandemic didn’t appear to produce a big rise or fall in the numbers of children and teens who had left home. Still, the crisis hit hard. As schools closed and households sheltered in place, youths reached out to the National Runaway Safeline to report heightened family conflicts and worsening mental health.
The Safeline, based in Chicago, is the country’s 24/7, federally designated communications system for runaway and homeless youths. Each year, it makes about 125,000 connections with young people and their family members through its hotline and other services.
In a typical year, teens aged 15-17 years are the main group that gets in touch by phone, live chat, email, or an online crisis forum, according to Jeff Stern, chief engagement officer at the Safeline.
But in the past 2 years, “contacts have skewed younger,” including many more children under age 12.
“I think this is showing what a hit this is taking on young children,” he said.
Without school, sports, and other activities, younger children might be reaching out because they’ve lost trusted sources of support. Callers have been as young as 9.
“Those ones stand out,” said a crisis center supervisor who asked to go by Michael, which is not his real name, to protect the privacy of his clients.
In November 2020, a child posted in the crisis forum: “I’m 11 and my parents treat me poorly. They have told me many times to ‘kill myself’ and I didn’t let that settle well with me. ... I have tried to run away one time from my house, but they found out, so they took my phone away and put screws on my windows so I couldn’t leave.”
Increasing numbers of children told Safeline counselors that their parents were emotionally or verbally abusive, while others reported physical abuse. Some said they experienced neglect, while others had been thrown out.
“We absolutely have had youths who have either been physically kicked out of the house or just verbally told to leave,” Michael said, “and then the kid does.”
Heightened family conflicts
The Safeline partners with the National Center for Missing and Exploited Children, which, despite widespread public perception, doesn’t work mainly with child abduction cases. Each year, the center assists with 29,000-31,000 cases, and 92% involve “endangered runaways,” said John Bischoff, vice president of the Missing Children Division. These children could be running away from home or foster care.
During the pandemic, the center didn’t spot major changes in its missing child numbers, “which honestly was shocking,” Mr. Bischoff said. “We figured we were either going to see an extreme rise or a decrease.
“But the reasons for the run were changing,” he said.
Many youths were fleeing out of frustration with quarantine restrictions, Mr. Bischoff said, as well as frustration with the unknown and their own lack of control over many situations.
At the runaway hotline, calls have been longer and more intense, with family problems topping the list of concerns. In 2019, about 57% of all contacts mentioned family dynamics. In 2020, that number jumped to 88%, according to Mr. Stern.
Some kids sought support for family problems that involved school. In October 2020, one 13-year-old wrote in the Safeline forum: “My mom constantly yells at me for no reason. I want to leave, but I don’t know how. I have also been really stressed about school because they haven’t been giving me the grades I would normally receive during actual school. She thinks I’m lying and that I don’t care. I just need somebody to help me.”
Many adults are under tremendous strain, too, Michael said.
“Parents might have gotten COVID last month and haven’t been able to work for 2 weeks, and they’re missing a paycheck now. Money is tight, there might not be food, everyone’s angry at everything.”
During the pandemic, the National Runaway Safeline found a 16% increase in contacts citing financial challenges.
Some children have felt confined in unsafe homes or have endured violence, as one 15-year-old reported in the forum: “I am the scapegoat out of four kids. Unfortunately, my mom has always been a toxic person. ... I’m the only kid she still hits really hard. She’s left bruises and scratches recently. ... I just have no solution to this.”
Worsening mental health
Besides family dynamics, mental health emerged as a top concern that youths reported in 2020. “This is something notable. It increased by 30% just in 1 year,” Mr. Stern said.
In November 2020, a 16-year-old wrote: “I can’t ever go outside. I’ve been stuck in the house for a very long time now since quarantine started. I’m scared. ... My mother has been taking her anger out on me emotionally. ... I have severe depression and I need help. Please, if there’s any way I can get out of here, let me know.”
The Safeline also has seen a rise in suicide-related contacts. Among children and teens who had cited a mental health concern, 18% said they were suicidal, Stern said. Most were between ages 12 and 16, but some were younger than 12.
When children couldn’t hang out with peers, they felt even more isolated if parents confiscated their phones, a common punishment, Michael said.
During the winter of 2020-21, “It felt like almost every digital contact was a youth reaching out on their Chromebook because they had gotten their phone taken away and they were either suicidal or considering running away,” he said. “That’s kind of their entire social sphere getting taken away.”
Reality check
Roughly 7 in 10 youths report still being at home when they reach out to the Safeline. Among those who do leave, Michael said, “They’re going sometimes to friends’ houses, oftentimes to a significant other’s house, sometimes to extended family members’ houses. Often, they don’t have a place that they’re planning to go. They just left, and that’s why they’re calling us.”
While some youths have been afraid of catching COVID-19 in general, the coronavirus threat hasn’t deterred those who have decided to run away, Michael said. “Usually, they’re more worried about being returned home.”
Many can’t comprehend the risks of setting off on their own.
In October 2021, a 15-year-old boy posted on the forum that his verbally abusive parents had called him a mistake and said they couldn’t wait for him to move out.
“So I’m going to make their dreams come true,” he wrote. “I’m going to go live in California with my friend who is a young YouTuber. I need help getting money to either fly or get a bus ticket, even though I’m all right with trying to ride a bike or fixing my dirt bike and getting the wagon to pull my stuff. But I’m looking for apartments in Los Angeles so I’m not living on the streets and I’m looking for a job. Please help me. My friend can’t send me money because I don’t have a bank account.”
“Often,” Michael said, “we’re reality-checking kids who want to hitchhike 5 hours away to either a friend’s or the closest shelter that we could find them. Or walk for 5 hours at 3 a.m. or bike, so we try to safety-check that.”
Another concern: online enticement by predators. During the pandemic, the National Center for Missing and Exploited Children saw cases in which children ran away from home “to go meet with someone who may not be who they thought they were talking to online,” Mr. Bischoff said. “It’s certainly something we’re keeping a close eye on.”
Fewer resources in the pandemic
The National Runaway Safeline provides information and referrals to other hotlines and services, including suicide prevention and mental health organizations. When youths have already run away and have no place to go, Michael said, the Safeline tries to find shelter options or seek out a relative who can provide a safe place to stay.
But finding shelters became tougher during the pandemic, when many had no room or shelter supply was limited. Some had to shut down for COVID-19–related deep cleanings, Michael said. Helping youths find transportation, especially with public transportation shutdowns, also was tough.
The Huckleberry House, a six-bed youth shelter in San Francisco, has stayed open throughout the pandemic with limited staffing, said Douglas Styles, PsyD. He’s the executive director of the Huckleberry Youth Programs, which runs the house.
The shelter, which serves Bay Area runaway and homeless youths ages 12-17, hasn’t seen an overall spike in demand, Dr. Styles said. But “what’s expanded is undocumented [youths] and young people who don’t have any family connections in the area, so they’re unaccompanied as well. We’ve seen that here and there throughout the years, but during the pandemic, that population has actually increased quite a bit.”
The Huckleberry House has sheltered children and teens who have run away from all kinds of homes, including affluent ones, Dr. Styles said.
Once children leave home, the lack of adult supervision leaves them vulnerable. They face multiple dangers, including child sex trafficking and exploitation, substance abuse, gang involvement, and violence. “As an organization, that scares us,” Mr. Bischoff said. “What’s happening at home, we’ll sort that out. The biggest thing we as an organization are trying to do is locate them and ensure their safety.”
To help runaways and their families get in touch, the National Runaway Safeline provides a message service and conference calling. “We can play the middleman, really acting on behalf of the young person – not because they’re right or wrong, but to ensure that their voice is really heard,” Mr. Stern said.
Through its national Home Free program, the Safeline partners with Greyhound to bring children back home or into an alternative, safe living environment by providing a free bus ticket.
These days, technology can expose children to harm online, but it can also speed their return home.
“When I was growing up, if you weren’t home by 5 o’clock, Mom would start to worry, but she really didn’t have any way of reaching you,” Mr. Bischoff said. “More children today have cellphones. More children are easily reachable. That’s a benefit.”
A version of this article first appeared on WebMD.com.
Children and COVID: The Omicron surge has become a retreat
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 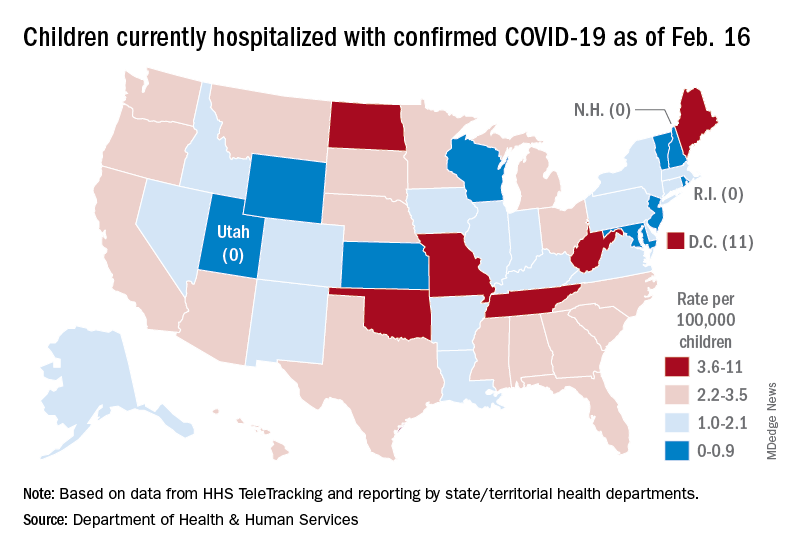
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
The Omicron decline continued for a fourth consecutive week as new cases of COVID-19 in children fell by 42% from the week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That 42% represents a drop from the 299,000 new cases reported for Feb. 4-10 down to 174,000 for the most recent week, Feb. 11-17. 
The overall count of COVID-19 cases in children is 12.5 million over the course of the pandemic, and that represents 19% of cases reported among all ages, the AAP and CHA said based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Hospital admissions also continued to fall, with the rate for children aged 0-17 at 0.43 per 100,000 population as of Feb. 20, down by almost 66% from the peak of 1.25 per 100,000 reached on Jan. 16, the Centers for Disease Control and Prevention reported.
A snapshot of the hospitalization situation shows that 1,687 children were occupying inpatient beds on Feb. 16, compared with 4,070 on Jan. 19, which appears to be the peak of the Omicron surge, according to data from the Department of Health & Human Services.
The state with the highest rate – 5.6 per 100,000 children – on Feb. 16 was North Dakota, although the District of Columbia came in at 11.0 per 100,000. They were followed by Oklahoma (5.3), Missouri (5.2), and West Virginia (4.1). There were three states – New Hampshire, Rhode Island, and Utah – with no children in the hospital on that date, the HHS said.
New vaccinations in children aged 5-11 years, which declined in mid- and late January, even as Omicron surged, continued to decline, as did vaccine completions. Vaccinations also fell among children aged 12-17 for the latest reporting week, Feb. 10-16, the AAP said in a separate report.
As more states and school districts drop mask mandates, data from the CDC indicate that 32.5% of 5- to 11-year olds and 67.4% of 12- to 17-year-olds have gotten at least one dose of the COVID-19 vaccine and that 25.1% and 57.3%, respectively, are fully vaccinated. Meanwhile, 20.5% of those fully vaccinated 12- to 17-year-olds have gotten a booster dose, the CDC said.
New MIS-C guidance addresses diagnostic challenges, cardiac care
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Updated guidance for health care providers on multisystem inflammatory syndrome in children (MIS-C) recognizes the evolving nature of the disease and offers strategies for pediatric rheumatologists, who also may be asked to recommend treatment for hyperinflammation in children with acute COVID-19.
Guidance is needed for many reasons, including the variable case definitions for MIS-C, the presence of MIS-C features in other infections and childhood rheumatic diseases, the extrapolation of treatment strategies from other conditions with similar presentations, and the issue of myocardial dysfunction, wrote Lauren A. Henderson, MD, MMSC, of Boston Children’s Hospital, and members of the American College of Rheumatology MIS-C and COVID-19–Related Hyperinflammation Task Force.
However, “modifications to treatment plans, particularly in patients with complex conditions, are highly disease, patient, geography, and time specific, and therefore must be individualized as part of a shared decision-making process,” the authors said. The updated guidance was published in Arthritis & Rheumatology.
Update needed in wake of Omicron
“We continue to see cases of MIS-C across the United States due to the spike in SARS-CoV-2 infections from the Omicron variant,” and therefore updated guidance is important at this time, Dr. Henderson told this news organization.
“MIS-C remains a serious complication of COVID-19 in children and the ACR wanted to continue to provide pediatricians with up-to-date recommendations for the management of MIS-C,” she said.
“Children began to present with MIS-C in April 2020. At that time, little was known about this entity. Most of the recommendations in the first version of the MIS-C guidance were based on expert opinion,” she explained. However, “over the last 2 years, pediatricians have worked very hard to conduct high-quality research studies to better understand MIS-C, so we now have more scientific evidence to guide our recommendations.
“In version three of the MIS-C guidance, there are new recommendations on treatment. Previously, it was unclear what medications should be used for first-line treatment in patients with MIS-C. Some children were given intravenous immunoglobulin while others were given IVIg and steroids together. Several new studies show that children with MIS-C who are treated with a combination of IVIg and steroids have better outcomes. Accordingly, the MIS-C guidance now recommends dual therapy with IVIg and steroids in children with MIS-C.”
Diagnostic evaluation
The guidance calls for maintaining a broad differential diagnosis of MIS-C, given that the condition remains rare, and that most children with COVID-19 present with mild symptoms and have excellent outcomes, the authors noted. The range of clinical features associated with MIS-C include fever, mucocutaneous findings, myocardial dysfunction, cardiac conduction abnormalities, shock, gastrointestinal symptoms, and lymphadenopathy.
Some patients also experience neurologic involvement in the form of severe headache, altered mental status, seizures, cranial nerve palsies, meningismus, cerebral edema, and ischemic or hemorrhagic stroke. Given the nonspecific nature of these symptoms, “it is imperative that a diagnostic evaluation for MIS-C include investigation for other possible causes, as deemed appropriate by the treating provider,” the authors emphasized. Other diagnostic considerations include the prevalence and chronology of COVID-19 in the community, which may change over time.
MIS-C and Kawasaki disease phenotypes
Earlier in the pandemic, when MIS-C first emerged, it was compared with Kawasaki disease (KD). “However, a closer examination of the literature shows that only about one-quarter to half of patients with a reported diagnosis of MIS-C meet the full diagnostic criteria for KD,” the authors wrote. Key features that separate MIS-C from KD include the greater incidence of KD among children in Japan and East Asia versus the higher incidence of MIS-C among non-Hispanic Black children. In addition, children with MIS-C have shown a wider age range, more prominent gastrointestinal and neurologic symptoms, and more frequent cardiac dysfunction, compared with those with KD.
Cardiac management
Close follow-up with cardiology is essential for children with MIS-C, according to the authors. The recommendations call for repeat echocardiograms for all children with MIS-C at a minimum of 7-14 days, then again at 4-6 weeks after the initial presentation. The authors also recommended additional echocardiograms for children with left ventricular dysfunction and cardiac aortic aneurysms.
MIS-C treatment
Current treatment recommendations emphasize that patients under investigation for MIS-C with life-threatening manifestations may need immunomodulatory therapy before a full diagnostic evaluation is complete, the authors said. However, patients without life-threatening manifestations should be evaluated before starting immunomodulatory treatment to avoid potentially harmful therapies for pediatric patients who don’t need them.
When MIS-C is refractory to initial immunomodulatory treatment, a second dose of IVIg is not recommended, but intensification therapy is advised with either high-dose (10-30 mg/kg per day) glucocorticoids, anakinra, or infliximab. However, there is little evidence available for selecting a specific agent for intensification therapy.
The task force also advises giving low-dose aspirin (3-5 mg/kg per day, up to 81 mg once daily) to all MIS-C patients without active bleeding or significant bleeding risk until normalization of the platelet count and confirmed normal coronary arteries at least 4 weeks after diagnosis.
COVID-19 and hyperinflammation
The task force also noted a distinction between MIS-C and severe COVID-19 in children. Although many children with MIS-C are previously healthy, most children who develop severe COVID-19 during an initial infection have complex conditions or comorbidities such as developmental delay or genetic anomaly, or chronic conditions such as congenital heart disease, type 1 diabetes, or asthma, the authors said. They recommend that “hospitalized children with COVID-19 requiring supplemental oxygen or respiratory support should be considered for immunomodulatory therapy in addition to supportive care and antiviral medications.”
The authors acknowledged the limitations and evolving nature of the recommendations, which will continue to change and do not replace clinical judgment for the management of individual patients. In the meantime, the ACR will support the task force in reviewing new evidence and providing revised versions of the current document.
Many questions about MIS-C remain, Dr. Henderson said in an interview. “It can be very hard to diagnose children with MIS-C because many of the symptoms are similar to those seen in other febrile illness of childhood. We need to identify better biomarkers to help us make the diagnosis of MIS-C. In addition, we need studies to provide information about what treatments should be used if children fail to respond to IVIg and steroids. Finally, it appears that vaccination [against SARS-CoV-2] protects against severe forms of MIS-C, and studies are needed to see how vaccination protects children from MIS-C.”
The development of the guidance was supported by the American College of Rheumatology. Dr. Henderson disclosed relationships with companies including Sobi, Pfizer, and Adaptive Biotechnologies (less than $10,000) and research support from the Childhood Arthritis and Rheumatology Research Alliance and research grant support from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS AND RHEUMATOLOGY
Subvariant may be more dangerous than original Omicron strain
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
, a lab study from Japan says.
“Our multiscale investigations suggest that the risk of BA.2 for global health is potentially higher than that of BA.1,” the researchers said in the study published on the preprint server bioRxiv. The study has not been peer-reviewed.
The researchers infected hamsters with BA.1 and BA.2. The hamsters infected with BA.2 got sicker, with more lung damage and loss of body weight. Results were similar when mice were infected with BA.1 and BA.2.
“Infection experiments using hamsters show that BA.2 is more pathogenic than BA.1,” the study said.
BA.1 and BA.2 both appear to evade immunity created by COVID-19 vaccines, the study said. But a booster shot makes illness after infection 74% less likely, CNN said.
What’s more, therapeutic monoclonal antibodies used to treat people infected with COVID didn’t have much effect on BA.2.
BA.2 was “almost completely resistant” to casirivimab and imdevimab and was 35 times more resistant to sotrovimab, compared to the original B.1.1 virus, the researchers wrote.
“In summary, our data suggest the possibility that BA.2 would be the most concerned variant to global health,” the researchers wrote. “Currently, both BA.2 and BA.1 are recognised together as Omicron and these are almost undistinguishable. Based on our findings, we propose that BA.2 should be recognised as a unique variant of concern, and this SARS-CoV-2 variant should be monitored in depth.”
If the World Health Organization recognized BA.2 as a “unique variant of concern,” it would be given its own Greek letter.
But some scientists noted that findings in the lab don’t always reflect what’s happening in the real world of people.
“I think it’s always hard to translate differences in animal and cell culture models to what’s going on with regards to human disease,” Jeremy Kamil, PhD, an associate professor of microbiology and immunology at Louisiana State University Health Shreveport, told Newsweek. “That said, the differences do look real.”
“It might be, from a human’s perspective, a worse virus than BA.1 and might be able to transmit better and cause worse disease,” Daniel Rhoads, MD, section head of microbiology at the Cleveland Clinic in Ohio, told CNN. He reviewed the Japanese study but was not involved in it.
Another scientist who reviewed the study but was not involved in the research noted that human immune systems are evolving along with the COVID variants.
“One of the caveats that we have to think about, as we get new variants that might seem more dangerous, is the fact that there’s two sides to the story,” Deborah Fuller, PhD, a virologist at the University of Washington School of Medicine, told CNN. “Our immune system is evolving as well. And so that’s pushing back on things.”
Scientists have already established that BA.2 is more transmissible than BA.1. The Omicron subvariant has been detected in 74 countries and 47 U.S. states, according to CNN. About 4% of Americans with COVID were infected with BA.2, the outlet reported, citing the CDC, but it’s now the dominant strain in other nations.
It’s not clear yet if BA.2 causes more severe illness in people. While BA.2 spreads faster than BA.1, there’s no evidence the subvariant makes people any sicker, an official with the World Health Organization said, according to CNBC.
A version of this article first appeared on WebMD.com.
Two factors linked to higher risk of long COVID in IBD
Two features are significantly associated with a higher risk for developing long COVID symptoms among people with inflammatory bowel disease (IBD), according to a large Danish population study.
People with Crohn’s disease (CD) who experienced adverse acute COVID-19, defined as requiring hospitalization, were nearly three times more likely to report persistent symptoms 12 weeks after acute infection.
“Long-term, persisting symptoms following COVID-19 is a frequently occurring problem, which is probably underappreciated. IBD specialists should therefore be aware of any of these symptoms and actively ask patients whether they have these problems,” lead author Mohamed Attauabi, MD, PhD, said in an interview.
Dr. Attauabi and colleagues also found that people with ulcerative colitis (UC) who discontinued immunosuppressive agents because of COVID-19 were 1.5 times more likely to experience long COVID symptoms, a result that surprised the researchers.
“This has not been shown before and remains to be confirmed,” said Dr. Attauabi, a fellow in the department of gastroenterology at Herlev Hospital at the University of Copenhagen.
Attauabi presented the results as a digital oral presentation at the 17th congress of the European Crohn’s and Colitis Organisation.
A closer look at IBD and COVID-19
Large, hospital-based studies of symptoms consistent with long COVID reveal a high prevalence of fatigue, sleep difficulties, and anxiety at 12 weeks or more post acute infection. However, these were not specific to people with CD or UC, Dr. Attauabi said.
“In patients with IBD, the risk of long-term sequelae of COVID-19 remains to be investigated,” he said.
Dr. Attauabi and colleagues studied 197 people with CD and 319 with UC, all of whom had polymerase chain reaction–confirmed COVID-19. Participants were prospectively enrolled in the population-based Danish IBD-COVID registry from January 28, 2020 to April 1, 2021. At a median of 5.1 months, a subset of 85 people with CD and 137 with UC agreed to report any post-COVID symptoms.
Older age, smoking, IBD disease activity, and presence of comorbidities were not associated with a significantly elevated risk of long COVID.
In a multivariate analysis, hospitalization for COVID-19 among people with CD was significantly associated with long COVID (odds ratio, 2.76; 95% confidence interval, 1.05-3.90; P = .04).
Furthermore, people with UC who stopped taking immunosuppressive agents also had a significantly higher risk (OR, 1.50; 95% CI, 1.07-10.22; P = .01).
“However, IBD medications such as systemic steroids were not associated with this outcome,” Dr. Attauabi said.
Fatigue most common long COVID symptom
Fatigue was the most common long COVID symptom, reported by 37% of patients with CD and 36% with UC.
Anosmia and ageusia were also common, reported by 29% and 28% of patients with CD, and 27% and 19% of those with UC, respectively.
“In our cohort of patients with UC or CD who developed COVID-19, the long-term health effects of COVID-19 did not appear to differ among patients with UC or CD nor according to IBD medications,” Dr. Attauabi said.
That is a “great study,” said session cochair Torsten Kucharzik, MD, PhD, head of internal medicine and gastroenterology at Lueneburg (Germany) Hospital.
When Dr. Kucharzik asked about smoking, Dr. Attauabi responded that they collected information on current and previous smoking, but they chose not to include the data because it was not statistically significant.
Dr. Attauabi has reported no relevant financial relationships. Dr. Kucharzik has reported receiving grants from Takeda and personal fees from companies including MSD/Essex, AbbVie, Falk Foundation, Biogen, Bristol-Myers Squibb, Arena, Celgene, Celltrion, Ferring, Janssen, Galapagos, Olympus, Mundipharma, Takeda, Amgen, Pfizer, Roche, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Two features are significantly associated with a higher risk for developing long COVID symptoms among people with inflammatory bowel disease (IBD), according to a large Danish population study.
People with Crohn’s disease (CD) who experienced adverse acute COVID-19, defined as requiring hospitalization, were nearly three times more likely to report persistent symptoms 12 weeks after acute infection.
“Long-term, persisting symptoms following COVID-19 is a frequently occurring problem, which is probably underappreciated. IBD specialists should therefore be aware of any of these symptoms and actively ask patients whether they have these problems,” lead author Mohamed Attauabi, MD, PhD, said in an interview.
Dr. Attauabi and colleagues also found that people with ulcerative colitis (UC) who discontinued immunosuppressive agents because of COVID-19 were 1.5 times more likely to experience long COVID symptoms, a result that surprised the researchers.
“This has not been shown before and remains to be confirmed,” said Dr. Attauabi, a fellow in the department of gastroenterology at Herlev Hospital at the University of Copenhagen.
Attauabi presented the results as a digital oral presentation at the 17th congress of the European Crohn’s and Colitis Organisation.
A closer look at IBD and COVID-19
Large, hospital-based studies of symptoms consistent with long COVID reveal a high prevalence of fatigue, sleep difficulties, and anxiety at 12 weeks or more post acute infection. However, these were not specific to people with CD or UC, Dr. Attauabi said.
“In patients with IBD, the risk of long-term sequelae of COVID-19 remains to be investigated,” he said.
Dr. Attauabi and colleagues studied 197 people with CD and 319 with UC, all of whom had polymerase chain reaction–confirmed COVID-19. Participants were prospectively enrolled in the population-based Danish IBD-COVID registry from January 28, 2020 to April 1, 2021. At a median of 5.1 months, a subset of 85 people with CD and 137 with UC agreed to report any post-COVID symptoms.
Older age, smoking, IBD disease activity, and presence of comorbidities were not associated with a significantly elevated risk of long COVID.
In a multivariate analysis, hospitalization for COVID-19 among people with CD was significantly associated with long COVID (odds ratio, 2.76; 95% confidence interval, 1.05-3.90; P = .04).
Furthermore, people with UC who stopped taking immunosuppressive agents also had a significantly higher risk (OR, 1.50; 95% CI, 1.07-10.22; P = .01).
“However, IBD medications such as systemic steroids were not associated with this outcome,” Dr. Attauabi said.
Fatigue most common long COVID symptom
Fatigue was the most common long COVID symptom, reported by 37% of patients with CD and 36% with UC.
Anosmia and ageusia were also common, reported by 29% and 28% of patients with CD, and 27% and 19% of those with UC, respectively.
“In our cohort of patients with UC or CD who developed COVID-19, the long-term health effects of COVID-19 did not appear to differ among patients with UC or CD nor according to IBD medications,” Dr. Attauabi said.
That is a “great study,” said session cochair Torsten Kucharzik, MD, PhD, head of internal medicine and gastroenterology at Lueneburg (Germany) Hospital.
When Dr. Kucharzik asked about smoking, Dr. Attauabi responded that they collected information on current and previous smoking, but they chose not to include the data because it was not statistically significant.
Dr. Attauabi has reported no relevant financial relationships. Dr. Kucharzik has reported receiving grants from Takeda and personal fees from companies including MSD/Essex, AbbVie, Falk Foundation, Biogen, Bristol-Myers Squibb, Arena, Celgene, Celltrion, Ferring, Janssen, Galapagos, Olympus, Mundipharma, Takeda, Amgen, Pfizer, Roche, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Two features are significantly associated with a higher risk for developing long COVID symptoms among people with inflammatory bowel disease (IBD), according to a large Danish population study.
People with Crohn’s disease (CD) who experienced adverse acute COVID-19, defined as requiring hospitalization, were nearly three times more likely to report persistent symptoms 12 weeks after acute infection.
“Long-term, persisting symptoms following COVID-19 is a frequently occurring problem, which is probably underappreciated. IBD specialists should therefore be aware of any of these symptoms and actively ask patients whether they have these problems,” lead author Mohamed Attauabi, MD, PhD, said in an interview.
Dr. Attauabi and colleagues also found that people with ulcerative colitis (UC) who discontinued immunosuppressive agents because of COVID-19 were 1.5 times more likely to experience long COVID symptoms, a result that surprised the researchers.
“This has not been shown before and remains to be confirmed,” said Dr. Attauabi, a fellow in the department of gastroenterology at Herlev Hospital at the University of Copenhagen.
Attauabi presented the results as a digital oral presentation at the 17th congress of the European Crohn’s and Colitis Organisation.
A closer look at IBD and COVID-19
Large, hospital-based studies of symptoms consistent with long COVID reveal a high prevalence of fatigue, sleep difficulties, and anxiety at 12 weeks or more post acute infection. However, these were not specific to people with CD or UC, Dr. Attauabi said.
“In patients with IBD, the risk of long-term sequelae of COVID-19 remains to be investigated,” he said.
Dr. Attauabi and colleagues studied 197 people with CD and 319 with UC, all of whom had polymerase chain reaction–confirmed COVID-19. Participants were prospectively enrolled in the population-based Danish IBD-COVID registry from January 28, 2020 to April 1, 2021. At a median of 5.1 months, a subset of 85 people with CD and 137 with UC agreed to report any post-COVID symptoms.
Older age, smoking, IBD disease activity, and presence of comorbidities were not associated with a significantly elevated risk of long COVID.
In a multivariate analysis, hospitalization for COVID-19 among people with CD was significantly associated with long COVID (odds ratio, 2.76; 95% confidence interval, 1.05-3.90; P = .04).
Furthermore, people with UC who stopped taking immunosuppressive agents also had a significantly higher risk (OR, 1.50; 95% CI, 1.07-10.22; P = .01).
“However, IBD medications such as systemic steroids were not associated with this outcome,” Dr. Attauabi said.
Fatigue most common long COVID symptom
Fatigue was the most common long COVID symptom, reported by 37% of patients with CD and 36% with UC.
Anosmia and ageusia were also common, reported by 29% and 28% of patients with CD, and 27% and 19% of those with UC, respectively.
“In our cohort of patients with UC or CD who developed COVID-19, the long-term health effects of COVID-19 did not appear to differ among patients with UC or CD nor according to IBD medications,” Dr. Attauabi said.
That is a “great study,” said session cochair Torsten Kucharzik, MD, PhD, head of internal medicine and gastroenterology at Lueneburg (Germany) Hospital.
When Dr. Kucharzik asked about smoking, Dr. Attauabi responded that they collected information on current and previous smoking, but they chose not to include the data because it was not statistically significant.
Dr. Attauabi has reported no relevant financial relationships. Dr. Kucharzik has reported receiving grants from Takeda and personal fees from companies including MSD/Essex, AbbVie, Falk Foundation, Biogen, Bristol-Myers Squibb, Arena, Celgene, Celltrion, Ferring, Janssen, Galapagos, Olympus, Mundipharma, Takeda, Amgen, Pfizer, Roche, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
FROM ECCO 2022
Ivermectin does not stop progression to severe COVID: randomized trial
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICNE
New ivermectin, HCQ scripts highest in GOP-dominated counties
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
New prescriptions of hydroxychloroquine (HCQ) and ivermectin increased in 2020, driven particularly by rates in counties with the highest proportion of Republican votes in the 2020 U.S. presidential election, according to a cross-sectional study published in JAMA Internal Medicine.
“Our findings are consistent with the hypothesis that U.S. prescribing of hydroxychloroquine and ivermectin during the COVID-19 pandemic may have been influenced by political affiliation,” wrote Michael L. Barnett, MD, of the Harvard T.H. Chan School of Public Health in Boston and colleagues.
The researchers used data from the OptumLabs Data Warehouse to analyze commercial and Medicare Advantage medical claims from January 2019 through December 2020 for more than 18.5 million adults living in counties with at least 50 enrollees.
Using U.S. Census data and 2020 presidential election results, the researchers classified counties according to their proportion of Republican voters and then examined whether those proportions were associated with that county’s rates of new prescriptions for HCQ, ivermectin, methotrexate sodium, and albendazole. Methotrexate is prescribed for similar conditions and indications as HCQ, and albendazole is prescribed for similar reasons as ivermectin, although neither of the comparison drugs has been considered for COVID-19 treatment.
The Food and Drug Administration issued an emergency use authorization (EUA) for HCQ as a COVID-19 treatment on March 28, 2020, but the agency revoked the EUA 3 months later on June 15. Ivermectin never received an EUA for COVID treatment, but an in vitro study published April 3, 2020 claimed it had an antiviral effect.
The National Institutes of Health recommended against using ivermectin as a COVID-19 treatment on Aug. 1, 2020, but a few months later, on Nov. 13, a flawed clinical trial – later retracted – claimed ivermectin was 90% effective in treating COVID-19. Despite the lack of evidence for ivermectin’s efficacy, a Senate committee meeting on Dec. 8, 2020, included testimony from a physician who promoted its use.
In comparing ivermectin and HCQ prescription rates with counties’ political composition, the researchers adjusted their findings to account for differences in the counties’ racial composition and COVID-19 incidence as well as enrollees’ age, sex, insurance type, income, comorbidity burden, and home in a rural or urban area.
The results showed an average of 20 new HCQ prescriptions per 100,000 enrollees in 2019, but 2020 saw a sharp increase and drop in new HCQ prescriptions in March-April 2020, independent of counties’ breakdown of political affiliation.
“However, after June 2020, coinciding with the revocation of the U.S. Food and Drug Administration’s emergency use authorization for hydroxychloroquine, prescribing volume was significantly higher in the highest vs. lowest Republican vote share counties,” the authors report. The gradual increase from June through December 2020 averaged to 42 new prescriptions per 100,000, a 146% increase over 2019 rates that was driven largely by the 25% of counties with the highest proportion of Republican voters.
Similarly, rates of new ivermectin prescriptions in December 2020 were more than nine times higher in counties with the highest Republican vote share, compared with new prescriptions throughout 2019. The researchers found no differences in new prescriptions for methotrexate or albendazole in 2020 based on counties’ proportion of Republican votes.
Since the study is an ecological, observational one, it cannot show causation or shed light on what role patients, physicians, or other factors might have played in prescribing patterns. Nevertheless, the authors noted the potentially negative implications of their findings.
“Because political affiliation should not be a factor in clinical treatment decisions, our findings raise concerns for public trust in a nonpartisan health care system,” the authors write.
Coauthor Ateev Mehrotra, MD, MPH, reported personal fees from Sanofi-Aventis, and coauthor Anupam B. Jena, MD, PhD, reported personal fees from Bioverativ, Merck, Janssen, Edwards Lifesciences, Novartis, Amgen, Eisai, Otsuka, Vertex, Celgene, Sanofi-Aventis, Precision Health Economics (now PRECISIONheor), Analysis Group, and Doubleday and hosting the podcast Freakonomics, M.D. The other coauthors have disclosed no relevant financial relationships. No external funding source was noted.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
Long COVID is real and consists of these conditions – or does it?
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
About 73% of U.S. estimated to be immune to Omicron variant
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
, a university health institute says.
About half of eligible Americans have received booster shots, and about 80 million confirmed COVID-19 infections have been reported. Many more infections have occurred but haven’t been officially recorded, The Associated Press reported.
The high percentage of immunity from vaccination and previous infection tends to prevent or shorten new illnesses and reduce the amount of virus circulating overall. Health experts are now discussing whether the number is high enough to stop new waves or reduce the burden on hospitals.
“I am optimistic even if we have a surge in summer, cases will go up, but hospitalizations and deaths will not,” Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington in Seattle, told the AP.
Dr. Mokdad works on COVID-19 forecasting for the university’s Institute for Health Metrics and Evaluation, which has been a reliable model during the pandemic. Dr. Mokdad calculated the 73% number for the AP.
“We have changed,” he said. “We have been exposed to this virus and we know how to deal with it.”
The United States is now reporting about 125,000 new cases per day, according to the data tracker from the New York Times, marking a 68% decrease from the past 2 weeks. Hospitalizations are also down 39%, and about 2,300 new deaths are being reported daily, marking a 13% decline.
There will be more outbreaks as new variants emerge, immunity wanes, and some people remain unvaccinated, Dr. Mokdad said. But the coronavirus is no longer new, and the entire population is no longer “immunologically naive.” Scientists are now trying to understand how long booster protection will last against Omicron and how many people have been infected who had mild or no symptoms that were never reported.
By the end of the Omicron surge, about three out of four people in the United States will have been infected, Shaun Truelove, PhD, an epidemiologist and disease modeler at Johns Hopkins University, told the AP.
“We know it’s a huge proportion of the population,” he said. “This varies a lot by location, and in some areas, we expect the number infected to be closer to one in two.”
That means different regions and groups of people have different levels of protection and risk. In Virginia, for instance, disease modelers estimate that about 45% of residents have the highest level of immunity by being vaccinated and boosted or vaccinated with a recent Omicron infection. Another 47% have immunity that has waned somewhat.
“That’s going to be a nice shield of armor for our population as a whole,” Bryan Lewis, PhD, an epidemiologist who leads the University of Virginia’s COVID-19 modeling team, told the outlet. “If we do get to very low case rates, we certainly can ease back on some of these restrictions.”
About 7% of Virginians are considered the most vulnerable because they were never vaccinated or infected, he noted. Nationwide, about 80 million Americans are still vulnerable, the AP reported.
“The 26% who could still get Omicron right now have to be very careful,” Dr. Mokdad said.
The percentages will continue to change as immunity wanes and new variants circulate in the country. For now, the Institute for Health Metrics and Evaluation model estimates that about 63% to 81% of Americans are protected.
“We’ve reached a much better position for the coming months, but with waning immunity, we shouldn’t take it for granted,” Dr. Mokdad said.
A version of this article first appeared on WebMD.com.
Post–COVID vaccine AHA cases raise eyebrows in Italy
“The overall number of cases observed does not allow ... any definitive conclusion over a possible causal relationship between SARS-CoV-2 vaccination and AHA, which would need more epidemiological and pharmacovigilance data about suspected vaccine-related adverse events,” Maria Cristina Leone, MD, of Azienda USL-IRCCS di Reggio Emilia (Italy), and colleagues reported online on Jan. 19, 2022, in a letter to the editors of Thrombosis Research.
The cases, observed in Reggio Emilia during the first 8 months of the vaccination campaign, occurred following receipt of mRNA BNT162b2 (Pfizer-BioNTech) vaccine. The AHA patients included two men and two women who ranged in age from 67 to 86 years.
During this time frame, 235,597 people received at least one dose of BNT162b2 vaccine, the authors noted.
In the 5 years prior, from January 2016 to December 2020, only zero to two cases of AHA were observed each year, totaling five cases, or 1.9 cases per million people/year. These numbers are in line with the estimated incidence of the disease, the researchers noted, adding that “it should nonetheless be underlined that vaccination benefits exceed potential side effects and play a central role in individual and public health to effectively protect people from COVID-19 and stop the pandemic.”
However, they also wrote that the “unusual observation of four cases of a rare disease during the first months of the vaccination campaign in our province could be of interest and could sensitize health care personnel toward a possible complication of SARS-CoV-2 immunization.”
AHA is a rare autoimmune disease caused by neutralizing autoantibodies against coagulation factor VIII. It is mainly associated with malignancy, autoimmune diseases, certain medications, and postnatal status.
“Sporadic AHA cases have been reported in association with infectious diseases or vaccinations,” the author noted, adding that associations between the BNT162b2 vaccine immune complications, including AHA, have also been reported by other authors.
Three of the four case patients in Reggio Emilia had “at least one common clinical association of AHA,” they found, suggesting that these associations could “reflect susceptibility to autoimmunity potentially triggered by vaccination.”
“Case four died due to complications from sepsis after being treated with steroid and rituximab, whereas the first three cases underwent clinical and laboratory remission after immunosuppressive therapy, and no relapse has been observed during follow-up, as in the other two cases reported: This could suggest a more favorable prognosis in respect to other non–vaccine-associated cases, but longer-term data are definitely needed,” they concluded.
The authors reported having no disclosures.
“The overall number of cases observed does not allow ... any definitive conclusion over a possible causal relationship between SARS-CoV-2 vaccination and AHA, which would need more epidemiological and pharmacovigilance data about suspected vaccine-related adverse events,” Maria Cristina Leone, MD, of Azienda USL-IRCCS di Reggio Emilia (Italy), and colleagues reported online on Jan. 19, 2022, in a letter to the editors of Thrombosis Research.
The cases, observed in Reggio Emilia during the first 8 months of the vaccination campaign, occurred following receipt of mRNA BNT162b2 (Pfizer-BioNTech) vaccine. The AHA patients included two men and two women who ranged in age from 67 to 86 years.
During this time frame, 235,597 people received at least one dose of BNT162b2 vaccine, the authors noted.
In the 5 years prior, from January 2016 to December 2020, only zero to two cases of AHA were observed each year, totaling five cases, or 1.9 cases per million people/year. These numbers are in line with the estimated incidence of the disease, the researchers noted, adding that “it should nonetheless be underlined that vaccination benefits exceed potential side effects and play a central role in individual and public health to effectively protect people from COVID-19 and stop the pandemic.”
However, they also wrote that the “unusual observation of four cases of a rare disease during the first months of the vaccination campaign in our province could be of interest and could sensitize health care personnel toward a possible complication of SARS-CoV-2 immunization.”
AHA is a rare autoimmune disease caused by neutralizing autoantibodies against coagulation factor VIII. It is mainly associated with malignancy, autoimmune diseases, certain medications, and postnatal status.
“Sporadic AHA cases have been reported in association with infectious diseases or vaccinations,” the author noted, adding that associations between the BNT162b2 vaccine immune complications, including AHA, have also been reported by other authors.
Three of the four case patients in Reggio Emilia had “at least one common clinical association of AHA,” they found, suggesting that these associations could “reflect susceptibility to autoimmunity potentially triggered by vaccination.”
“Case four died due to complications from sepsis after being treated with steroid and rituximab, whereas the first three cases underwent clinical and laboratory remission after immunosuppressive therapy, and no relapse has been observed during follow-up, as in the other two cases reported: This could suggest a more favorable prognosis in respect to other non–vaccine-associated cases, but longer-term data are definitely needed,” they concluded.
The authors reported having no disclosures.
“The overall number of cases observed does not allow ... any definitive conclusion over a possible causal relationship between SARS-CoV-2 vaccination and AHA, which would need more epidemiological and pharmacovigilance data about suspected vaccine-related adverse events,” Maria Cristina Leone, MD, of Azienda USL-IRCCS di Reggio Emilia (Italy), and colleagues reported online on Jan. 19, 2022, in a letter to the editors of Thrombosis Research.
The cases, observed in Reggio Emilia during the first 8 months of the vaccination campaign, occurred following receipt of mRNA BNT162b2 (Pfizer-BioNTech) vaccine. The AHA patients included two men and two women who ranged in age from 67 to 86 years.
During this time frame, 235,597 people received at least one dose of BNT162b2 vaccine, the authors noted.
In the 5 years prior, from January 2016 to December 2020, only zero to two cases of AHA were observed each year, totaling five cases, or 1.9 cases per million people/year. These numbers are in line with the estimated incidence of the disease, the researchers noted, adding that “it should nonetheless be underlined that vaccination benefits exceed potential side effects and play a central role in individual and public health to effectively protect people from COVID-19 and stop the pandemic.”
However, they also wrote that the “unusual observation of four cases of a rare disease during the first months of the vaccination campaign in our province could be of interest and could sensitize health care personnel toward a possible complication of SARS-CoV-2 immunization.”
AHA is a rare autoimmune disease caused by neutralizing autoantibodies against coagulation factor VIII. It is mainly associated with malignancy, autoimmune diseases, certain medications, and postnatal status.
“Sporadic AHA cases have been reported in association with infectious diseases or vaccinations,” the author noted, adding that associations between the BNT162b2 vaccine immune complications, including AHA, have also been reported by other authors.
Three of the four case patients in Reggio Emilia had “at least one common clinical association of AHA,” they found, suggesting that these associations could “reflect susceptibility to autoimmunity potentially triggered by vaccination.”
“Case four died due to complications from sepsis after being treated with steroid and rituximab, whereas the first three cases underwent clinical and laboratory remission after immunosuppressive therapy, and no relapse has been observed during follow-up, as in the other two cases reported: This could suggest a more favorable prognosis in respect to other non–vaccine-associated cases, but longer-term data are definitely needed,” they concluded.
The authors reported having no disclosures.
FROM THROMBOSIS RESEARCH