User login
Novel tool accurately predicts suicide after self-harm
Investigators have developed and validated a new risk calculator to help predict death by suicide in the 6-12 months after an episode of nonfatal self-harm, new research shows.
A study led by Seena Fazel, MBChB, MD, University of Oxford, England, suggests the Oxford Suicide Assessment Tool for Self-harm (OxSATS) may help guide treatment decisions and target resources to those most in need, the researchers note.
“Many tools use only simple high/low categories, whereas OxSATS includes probability scores, which align more closely with risk calculators in cardiovascular medicine, such as the Framingham Risk Score, and prognostic models in cancer medicine, which provide 5-year survival probabilities. This potentially allows OxSATS to inform clinical decision-making more directly,” Dr. Fazel told this news organization.
The findings were published online in BMJ Mental Health.
Targeted tool
Self-harm is associated with a 1-year risk of suicide that is 20 times higher than that of the general population. Given that about 16 million people self-harm annually, the impact at a population level is potentially quite large, the researchers note.
Current structured approaches to gauge suicide risk among those who have engaged in self-harm are based on tools developed for other purposes and symptom checklists. “Their poor to moderate performance is therefore not unexpected,” Dr. Fazel told this news organization.
In contrast, OxSATS was specifically developed to predict suicide mortality after self-harm.
Dr. Fazel’s group evaluated data on 53,172 Swedish individuals aged 10 years and older who sought emergency medical care after episodes of self-harm.
The development cohort included 37,523 individuals. Of these, 391 died by suicide within 12 months. The validation cohort included 15,649 individuals; of these people, 178 died by suicide within 12 months.
The final OxSATS model includes 11 predictors related to age and sex, as well as variables related to substance misuse, mental health, and treatment and history of self-harm.
“The performance of the model in external validation was good, with c-index at 6 and 12 months of 0.77,” the researchers note.
Using a cutoff threshold of 1%, the OxSATS correctly identified 68% of those who died by suicide within 6 months, while 71% of those who didn’t die were correctly classified as being at low risk. The figures for risk prediction at 12 months were 82% and 54%, respectively.
The OxSATS has been made into a simple online tool with probability scores for suicide at 6 and 12 months after an episode of self-harm, but without linkage to interventions. A tool on its own is unlikely to improve outcomes, said Dr. Fazel.
“However,” he added, “it can improve consistency in the assessment process, especially in busy clinical settings where people from different professional backgrounds and experience undertake such assessments. It can also highlight the role of modifiable risk factors and provide an opportunity to transparently discuss risk with patients and their carers.”
Valuable work
Reached for comment, Igor Galynker, MD, PhD, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, said that this is a “very solid study with a very large sample size and solid statistical analysis.”
Another strength of the research is the outcome of suicide death versus suicide attempt or suicidal ideation. “In that respect, it is a valuable paper,” Dr. Galynker, who directs the Mount Sinai Beth Israel Suicide Research Laboratory, told this news organization.
He noted that there are no new risk factors in the model. Rather, the model contains the typical risk factors for suicide, which include male sex, substance misuse, past suicide attempt, and psychiatric diagnosis.
“The strongest risk factor in the model is self-harm by hanging, strangulation, or suffocation, which has been shown before and is therefore unsurprising,” said Dr. Galynker.
In general, the risk factors included in the model are often part of administrative tools for suicide risk assessment, said Dr. Galynker, but the OxSATS “seems easier to use because it has 11 items only.”
Broadly speaking, individuals with mental illness and past suicide attempt, past self-harm, alcohol use, and other risk factors “should be treated proactively with suicide prevention measures,” he told this news organization.
As previously reported, Dr. Galynker and colleagues have developed the Abbreviated Suicide Crisis Syndrome Checklist (A-SCS-C), a novel tool to help identify which suicidal patients who present to the emergency department should be admitted to hospital and which patients can be safely discharged.
Funding for the study was provided by Wellcome Trust and the Swedish Research Council. Dr. Fazel and Dr. Galynker have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators have developed and validated a new risk calculator to help predict death by suicide in the 6-12 months after an episode of nonfatal self-harm, new research shows.
A study led by Seena Fazel, MBChB, MD, University of Oxford, England, suggests the Oxford Suicide Assessment Tool for Self-harm (OxSATS) may help guide treatment decisions and target resources to those most in need, the researchers note.
“Many tools use only simple high/low categories, whereas OxSATS includes probability scores, which align more closely with risk calculators in cardiovascular medicine, such as the Framingham Risk Score, and prognostic models in cancer medicine, which provide 5-year survival probabilities. This potentially allows OxSATS to inform clinical decision-making more directly,” Dr. Fazel told this news organization.
The findings were published online in BMJ Mental Health.
Targeted tool
Self-harm is associated with a 1-year risk of suicide that is 20 times higher than that of the general population. Given that about 16 million people self-harm annually, the impact at a population level is potentially quite large, the researchers note.
Current structured approaches to gauge suicide risk among those who have engaged in self-harm are based on tools developed for other purposes and symptom checklists. “Their poor to moderate performance is therefore not unexpected,” Dr. Fazel told this news organization.
In contrast, OxSATS was specifically developed to predict suicide mortality after self-harm.
Dr. Fazel’s group evaluated data on 53,172 Swedish individuals aged 10 years and older who sought emergency medical care after episodes of self-harm.
The development cohort included 37,523 individuals. Of these, 391 died by suicide within 12 months. The validation cohort included 15,649 individuals; of these people, 178 died by suicide within 12 months.
The final OxSATS model includes 11 predictors related to age and sex, as well as variables related to substance misuse, mental health, and treatment and history of self-harm.
“The performance of the model in external validation was good, with c-index at 6 and 12 months of 0.77,” the researchers note.
Using a cutoff threshold of 1%, the OxSATS correctly identified 68% of those who died by suicide within 6 months, while 71% of those who didn’t die were correctly classified as being at low risk. The figures for risk prediction at 12 months were 82% and 54%, respectively.
The OxSATS has been made into a simple online tool with probability scores for suicide at 6 and 12 months after an episode of self-harm, but without linkage to interventions. A tool on its own is unlikely to improve outcomes, said Dr. Fazel.
“However,” he added, “it can improve consistency in the assessment process, especially in busy clinical settings where people from different professional backgrounds and experience undertake such assessments. It can also highlight the role of modifiable risk factors and provide an opportunity to transparently discuss risk with patients and their carers.”
Valuable work
Reached for comment, Igor Galynker, MD, PhD, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, said that this is a “very solid study with a very large sample size and solid statistical analysis.”
Another strength of the research is the outcome of suicide death versus suicide attempt or suicidal ideation. “In that respect, it is a valuable paper,” Dr. Galynker, who directs the Mount Sinai Beth Israel Suicide Research Laboratory, told this news organization.
He noted that there are no new risk factors in the model. Rather, the model contains the typical risk factors for suicide, which include male sex, substance misuse, past suicide attempt, and psychiatric diagnosis.
“The strongest risk factor in the model is self-harm by hanging, strangulation, or suffocation, which has been shown before and is therefore unsurprising,” said Dr. Galynker.
In general, the risk factors included in the model are often part of administrative tools for suicide risk assessment, said Dr. Galynker, but the OxSATS “seems easier to use because it has 11 items only.”
Broadly speaking, individuals with mental illness and past suicide attempt, past self-harm, alcohol use, and other risk factors “should be treated proactively with suicide prevention measures,” he told this news organization.
As previously reported, Dr. Galynker and colleagues have developed the Abbreviated Suicide Crisis Syndrome Checklist (A-SCS-C), a novel tool to help identify which suicidal patients who present to the emergency department should be admitted to hospital and which patients can be safely discharged.
Funding for the study was provided by Wellcome Trust and the Swedish Research Council. Dr. Fazel and Dr. Galynker have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators have developed and validated a new risk calculator to help predict death by suicide in the 6-12 months after an episode of nonfatal self-harm, new research shows.
A study led by Seena Fazel, MBChB, MD, University of Oxford, England, suggests the Oxford Suicide Assessment Tool for Self-harm (OxSATS) may help guide treatment decisions and target resources to those most in need, the researchers note.
“Many tools use only simple high/low categories, whereas OxSATS includes probability scores, which align more closely with risk calculators in cardiovascular medicine, such as the Framingham Risk Score, and prognostic models in cancer medicine, which provide 5-year survival probabilities. This potentially allows OxSATS to inform clinical decision-making more directly,” Dr. Fazel told this news organization.
The findings were published online in BMJ Mental Health.
Targeted tool
Self-harm is associated with a 1-year risk of suicide that is 20 times higher than that of the general population. Given that about 16 million people self-harm annually, the impact at a population level is potentially quite large, the researchers note.
Current structured approaches to gauge suicide risk among those who have engaged in self-harm are based on tools developed for other purposes and symptom checklists. “Their poor to moderate performance is therefore not unexpected,” Dr. Fazel told this news organization.
In contrast, OxSATS was specifically developed to predict suicide mortality after self-harm.
Dr. Fazel’s group evaluated data on 53,172 Swedish individuals aged 10 years and older who sought emergency medical care after episodes of self-harm.
The development cohort included 37,523 individuals. Of these, 391 died by suicide within 12 months. The validation cohort included 15,649 individuals; of these people, 178 died by suicide within 12 months.
The final OxSATS model includes 11 predictors related to age and sex, as well as variables related to substance misuse, mental health, and treatment and history of self-harm.
“The performance of the model in external validation was good, with c-index at 6 and 12 months of 0.77,” the researchers note.
Using a cutoff threshold of 1%, the OxSATS correctly identified 68% of those who died by suicide within 6 months, while 71% of those who didn’t die were correctly classified as being at low risk. The figures for risk prediction at 12 months were 82% and 54%, respectively.
The OxSATS has been made into a simple online tool with probability scores for suicide at 6 and 12 months after an episode of self-harm, but without linkage to interventions. A tool on its own is unlikely to improve outcomes, said Dr. Fazel.
“However,” he added, “it can improve consistency in the assessment process, especially in busy clinical settings where people from different professional backgrounds and experience undertake such assessments. It can also highlight the role of modifiable risk factors and provide an opportunity to transparently discuss risk with patients and their carers.”
Valuable work
Reached for comment, Igor Galynker, MD, PhD, professor of psychiatry at the Icahn School of Medicine at Mount Sinai, New York, said that this is a “very solid study with a very large sample size and solid statistical analysis.”
Another strength of the research is the outcome of suicide death versus suicide attempt or suicidal ideation. “In that respect, it is a valuable paper,” Dr. Galynker, who directs the Mount Sinai Beth Israel Suicide Research Laboratory, told this news organization.
He noted that there are no new risk factors in the model. Rather, the model contains the typical risk factors for suicide, which include male sex, substance misuse, past suicide attempt, and psychiatric diagnosis.
“The strongest risk factor in the model is self-harm by hanging, strangulation, or suffocation, which has been shown before and is therefore unsurprising,” said Dr. Galynker.
In general, the risk factors included in the model are often part of administrative tools for suicide risk assessment, said Dr. Galynker, but the OxSATS “seems easier to use because it has 11 items only.”
Broadly speaking, individuals with mental illness and past suicide attempt, past self-harm, alcohol use, and other risk factors “should be treated proactively with suicide prevention measures,” he told this news organization.
As previously reported, Dr. Galynker and colleagues have developed the Abbreviated Suicide Crisis Syndrome Checklist (A-SCS-C), a novel tool to help identify which suicidal patients who present to the emergency department should be admitted to hospital and which patients can be safely discharged.
Funding for the study was provided by Wellcome Trust and the Swedish Research Council. Dr. Fazel and Dr. Galynker have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Do oral contraceptives increase depression risk?
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPIDEMIOLOGY AND PSYCHIATRIC SCIENCES
Risk Evaluation and Mitigation Strategy programs: How they can be improved
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).
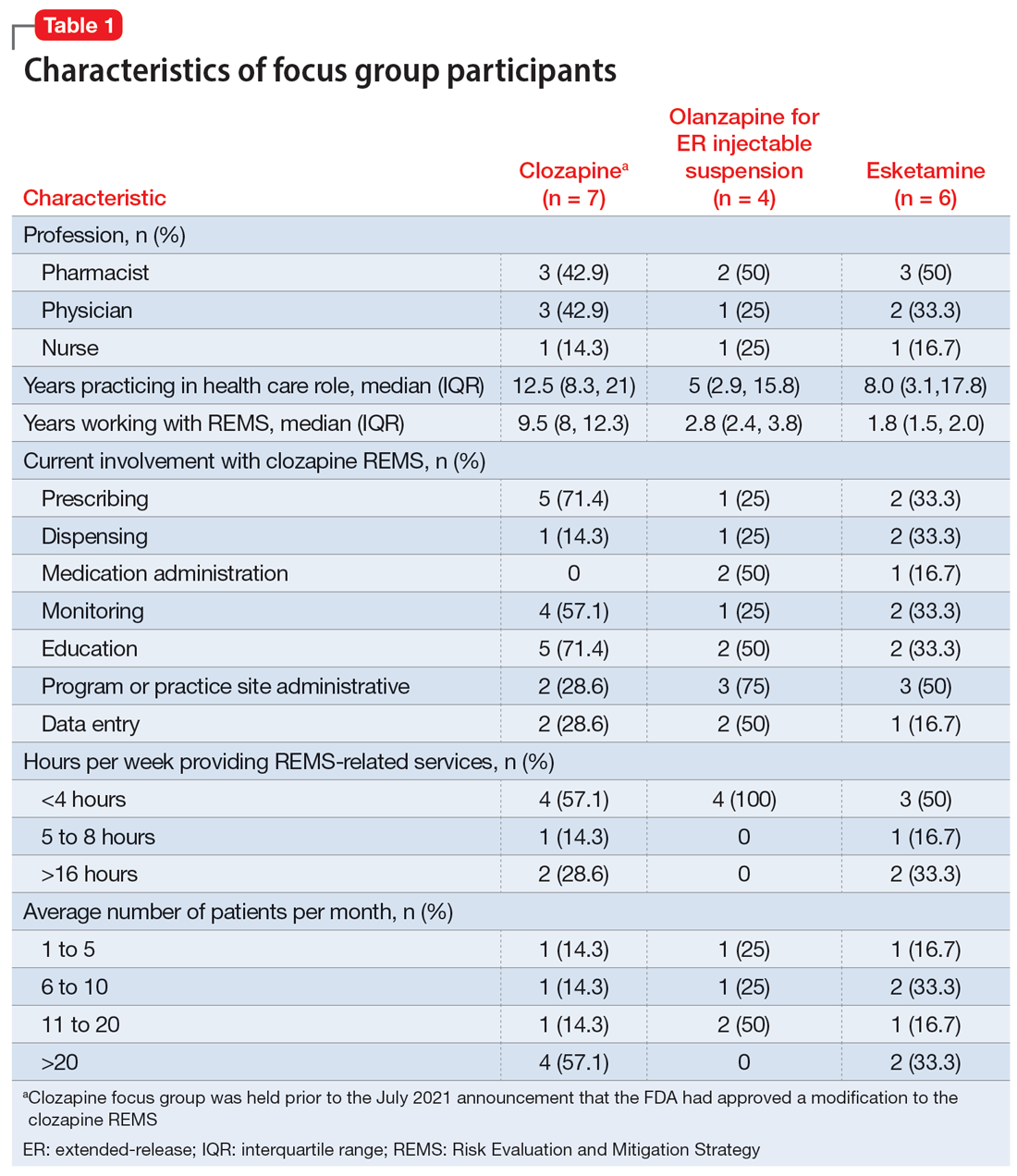
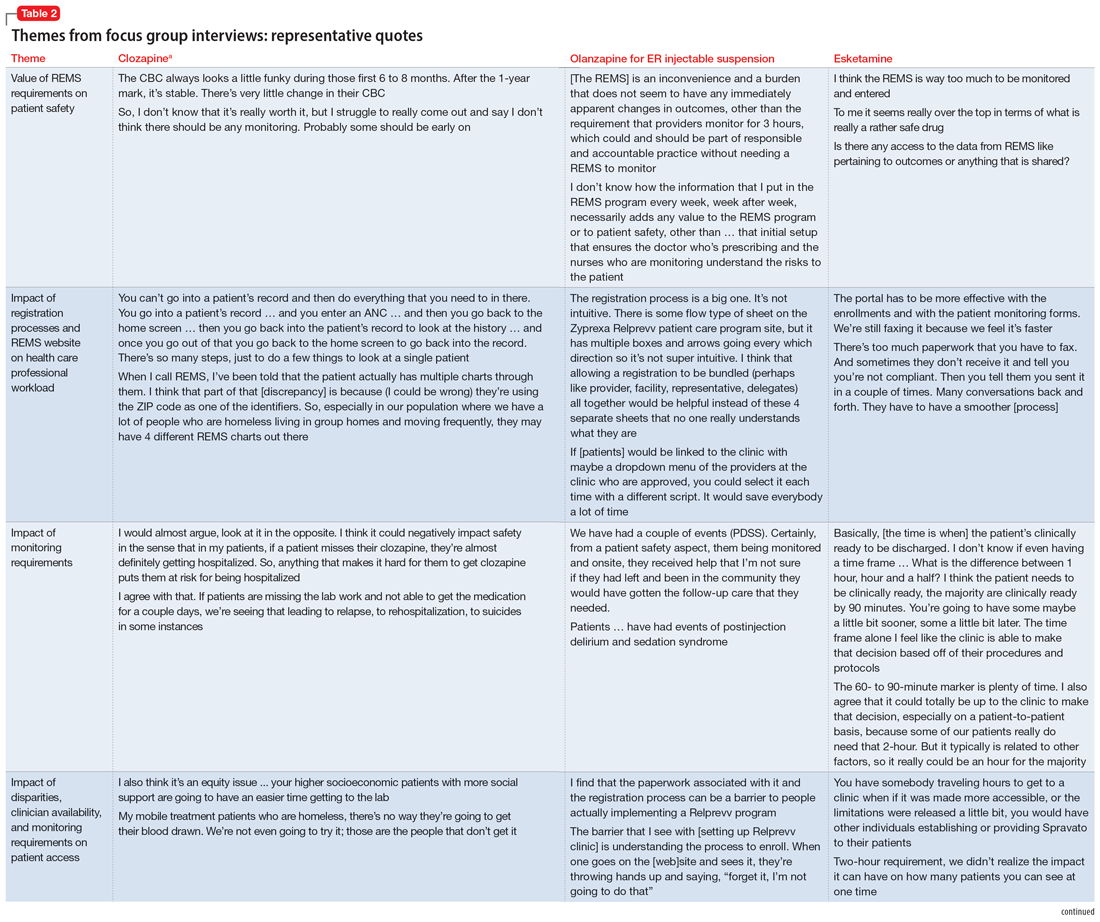
Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.

Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.
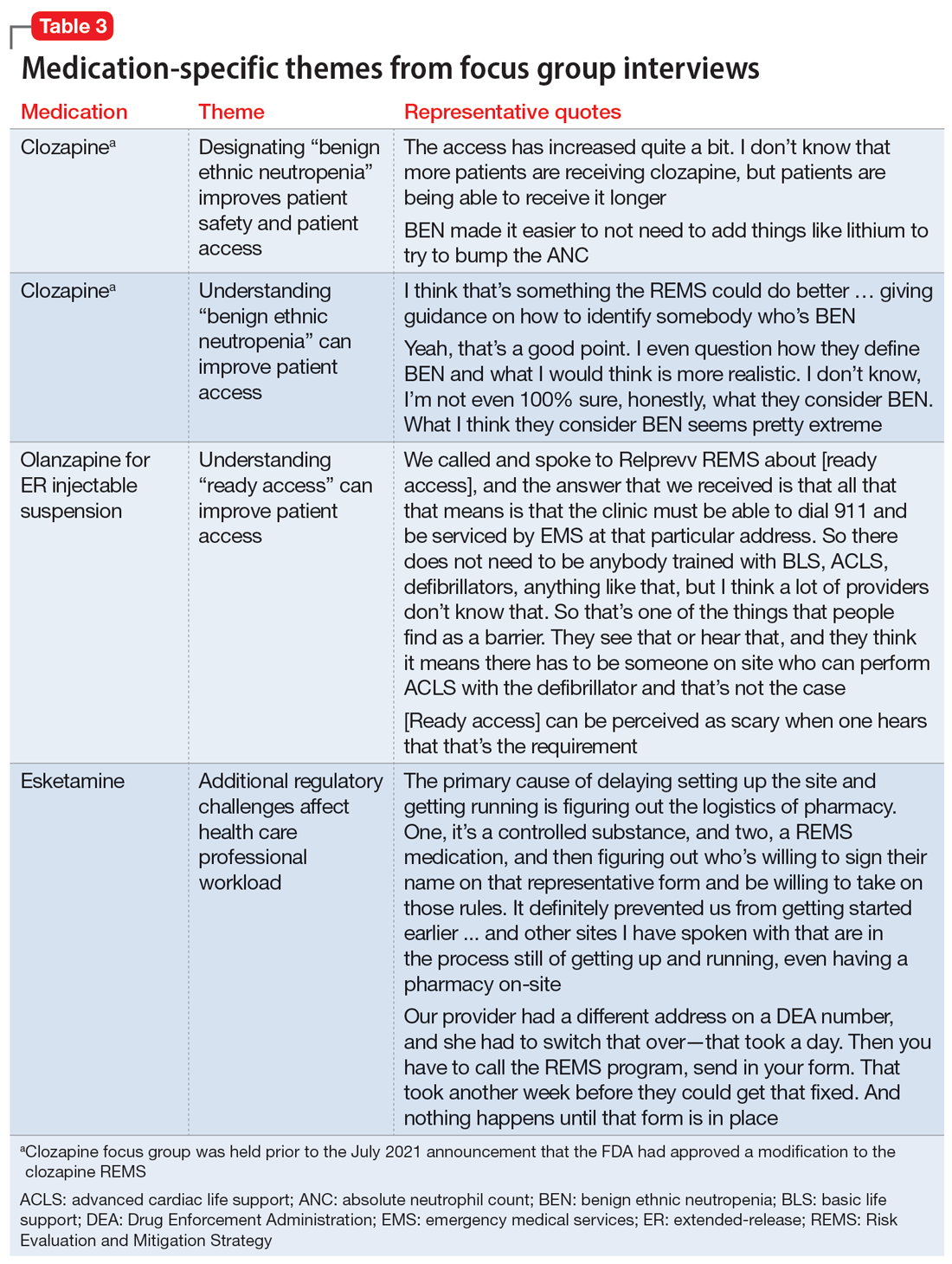
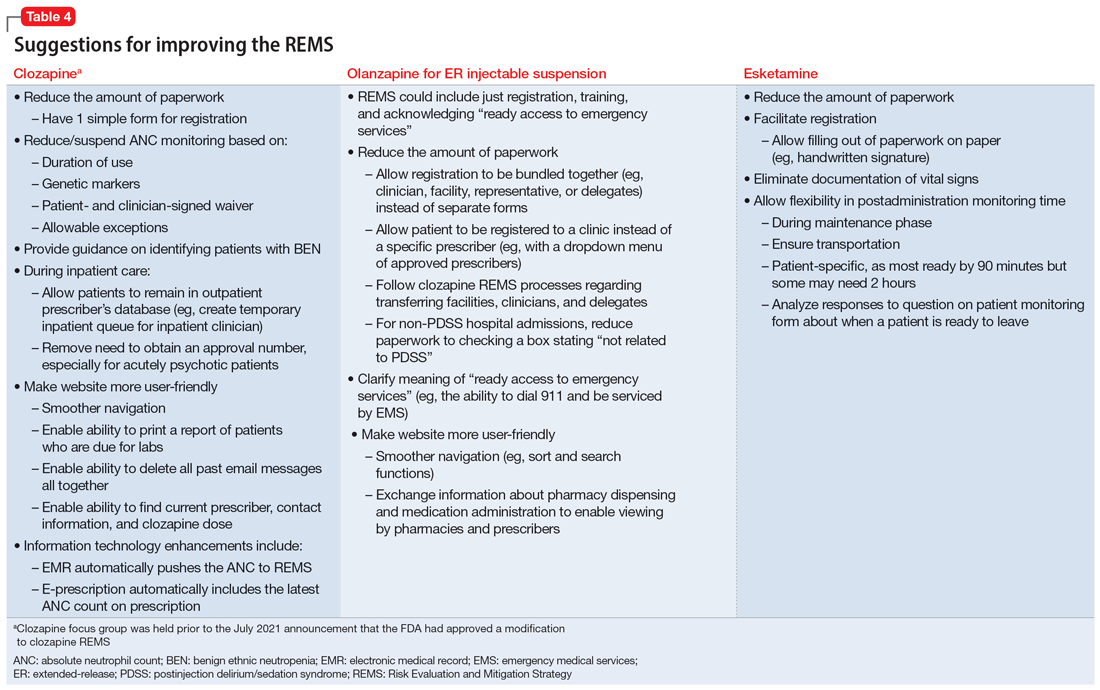
Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.
Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
Interventional psychiatry (Part 2)
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.
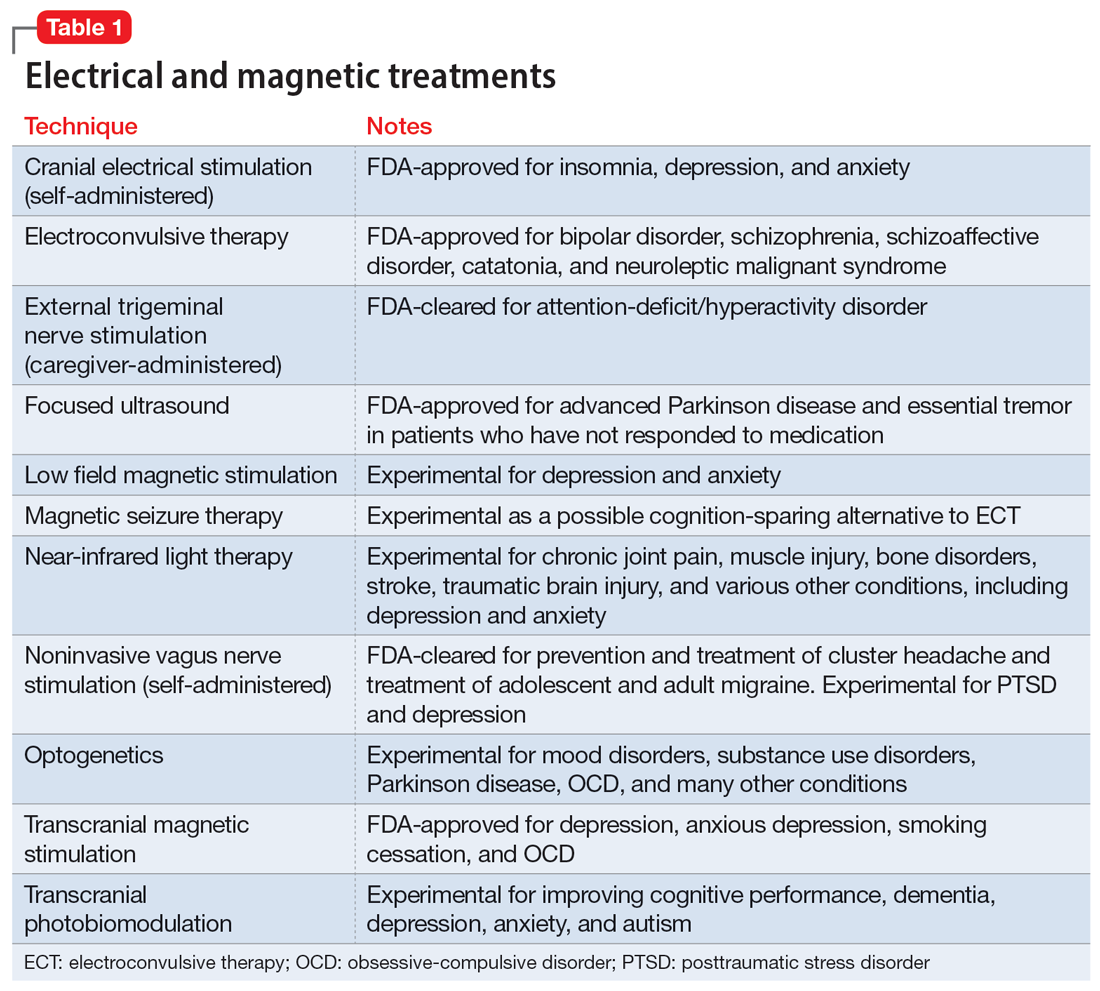

Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
Interventional psychiatry: What are the next steps?
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
Using apps in clinical practice: 8 studies
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
From smiling to smizing: Assessing the affect of a patient wearing a mask
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Physician suicide roundtable: 8 important initiatives that can help
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.
More than 30 experts question validity of serotonin/depression study
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
The authors of the article, however, stand by their conclusion.
“The methodology doesn’t conform to a conventional umbrella review,” said the commentary’s lead author, Sameer Jauhar, MD, PhD, first author of the commentary criticizing the review, which was published online in Molecular Psychiatry.
In addition, preeminent psychiatrist David J. Nutt, MD, PhD, Edmond J. Safra Professor of Neuropsychopharmacology, Imperial College London, is calling for the review to be retracted. In an interview with The Daily Mail, he said the article is “full of flaws and it should never have been published in the first place. Yet it has been frequently cited and people believe it is true. It’s essentially misinformation. That’s why I’m calling on the journal to retract it.” Dr. Nutt is one of the authors of the published commentary.
‘No convincing evidence’
Led by Joanna Moncrieff, MD, professor of clinical and social psychiatry, University College London, the authors analyzed systematic reviews and meta-analyses to determine whether low serotonin levels are, in fact, associated with depression.
Of 361 potential studies, 17 were selected for the review, including meta-analyses, systematic reviews, and a genetic association study.
The review included examinations of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in “body fluids,” 5HT1A receptor and serotonin transporter protein (SERT) availability in imaging and postmortem studies, investigations of SERT gene polymorphisms, interactions between SERT and stress in depression, and effects of tryptophan depletion on mood.
The tryptophan hypothesis suggests depression occurs through tryptophan depletion, which lowers available serotonin. According to the review, two crossover studies of patients with depression who were currently receiving or had recently received antidepressant treatment did not show substantial effects of depletion, and data from studies involving volunteers largely showed no effect.
Ultimately, Dr. Moncrieff and colleagues concluded that “there is no convincing evidence that depression is associated with, or caused by, lower serotonin concentrations or activity.”
‘Unconventional, odd’ methodology
However, Dr. Jauhar and the commentary’s coauthors disagree with the study’s conclusion. The researchers claim that “we don’t see depression symptoms in healthy volunteers when given tryptophan depletion; everyone knows that and agrees with that; it’s only in people vulnerable to depression who will have it.”
Furthermore, he said, the study’s conclusion does not consider that experimental medicine studies of tryptophan depletion are difficult to conduct. “You’re not going to have huge sample sizes as you would in a genetic study or big epidemiological studies.
Dr. Jauhar said he found it “unconventional” and “odd” that the review included individual tryptophan depletion studies that were not in the prespecified protocol.
For studies involving molecular imaging, Dr. Jauhar said the review’s inferences were “simplistic” and the review authors are “basically shaping the argument” to fit their desired narrative.
He also noted factual errors in the review. “They make a mistake when they talk about serotonin transporter imaging; they say there are no consistent findings across studies when, in fact, there are.”
With both tryptophan depletion and molecular imaging studies, the review “glosses over findings” from the original studies, said Dr. Jauhar.
For tryptophan depletion, “a more accurate, constructive conclusion would be that acute tryptophan depletion and decreased plasma tryptophan in depression indicate a role for 5-HT in those vulnerable to or suffering from depression, and that molecular imaging suggests the system is perturbed,” the commentators wrote.
“The proven efficacy of SSRIs in a proportion of people with depression lends credibility to this position,” they added.
Dr. Jauhar also took issue with criteria for certainty of finding of these and other studies used in the review. “If you’re setting the criteria yourself, it’s arbitrary.”
No new data
An umbrella review is supposed to be of the highest quality and should entail “taking out the studies and analysing them yourself,” but here, “all they have done is put a synthesis forward of other people’s reviews, so essentially there’s no new data there,” said Dr. Jauhar.
And sometimes the review’s findings differ from the original research. “When you have people who haven’t conducted original research themselves quoting someone else’s work and ignoring what those people say, we’re all in trouble,” said Dr. Jauhar.
In an additional commentary also published in Molecular Psychiatry, Jacob Pade Ramsøe Jacobsen, Evecxia Therapeutics, Durham, N.C., also criticized the review by Dr. Moncrieff and colleagues.
Its authors appear unfamiliar with serotonin biology and pharmacology, Dr. Jacobsen wrote.
“The review contains factual errors, makes conclusions serotonin neurobiology may not support, and quotes the cited literature in a selective manner,” he added.
“Most troubling, they misinterpret some data reviewed and intimate that serotonin reuptake inhibitor antidepressants, e.g., SSRIs, may decrease, rather than increase, serotonin function.”
If accepted by general practitioners and the public, the review’s conclusions “could lead to reduced use of antidepressants among patients in need and increased morbidity related to depression.”
Dr. Moncrieff pushes back
Responding to the torrent of criticism of her study, Dr. Moncrieff told this news organization via email that they stand by the review, adding that Dr. Jauhar and others “don’t want to let the cat out of the bag” that there’s no good evidence to support the hypothesis that low serotonin causes depression because it challenges antidepressant use.
“The idea that antidepressants work by correcting an underlying chemical imbalance or serotonin abnormality has led research up a blind alley and meant scientists have not taken the harmful effects of these drugs seriously enough.”
These critics, she added, “want business as usual – which means people will continue to be misinformed and exposed to harmful effects of drugs that have minimal and uncertain benefits.”
In a letter to the editor of Molecular Psychiatry, Dr. Moncrieff and her fellow authors maintain that they used approved and well-accepted methods for the umbrella review, including preregistering the protocol and using recommended search methods and quality assessments, and that they did not miss certain studies, as has been claimed.
In her blog, Dr. Moncrieff wrote that the “marginal differences between antidepressants and placebo that are apparent in clinical trials are likely to be produced by alternative, more plausible mechanisms like the emotional blunting effects of the drugs or by amplified placebo effects, rather than by targeting underlying biological mechanisms (since these have not been demonstrated).”
It also highlights “how we don’t know what antidepressants do to the brain exactly, which is a cause for concern,” she adds.
Dr. Jauhar has received honoraria for nonpromotional educational talks on antipsychotics from Janssen, Sunovion, and Lundbeck and on causes of schizophrenia for Boehringer-Ingelheim. He has also received honoraria for consulting on antipsychotics for LB Pharmaceuticals. He sits on Council for the British Association for Psychopharmacology and was a recent panel member for the Wellcome Trust.
A version of this article originally appeared on Medscape.com.
Agency issues advisory on mental health symptoms of long COVID
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.






