User login
Could new therapy for food ‘cues’ improve weight loss?
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
An intensive 1-year behavior therapy program aimed at changing a person’s response to food “cues” might help people with obesity lose a modest amount of weight, a randomized clinical trial suggests.
“Patients who are food-cue sensitive often feel out of control with their eating; they cannot resist food and/or cannot stop thinking about food,” said lead author Kerri N. Boutelle, PhD.
“Behavioral weight loss skills are not sufficient for these individuals,” so they designed this new approach, Dr. Boutelle, of the University of California, San Diego, explained in a press release.
The regulation of cues (ROC) intervention trains individuals to respond to their hunger and to resist eating highly craved foods (internal management), in contrast to behavioral weight loss programs that focus on counting calories (external management), Dr. Boutelle explained in an email.
The results of the Providing Adult Collaborative Interventions for Ideal Changes (PACIFIC) clinical trial, including follow-up out to 2 years, were published in JAMA Network Open.
Patients in the behavioral weight loss therapy group or the combined ROC and behavioral weight loss therapy group lost more weight at 6 months than patients in the ROC group – but then they slowly regained weight (whereas patients in the ROC group did not).
At 24 months, the three groups had a similar modest weight loss, compared with a control group that did not lose weight.
“We believe these internal management strategies are more durable over time,” said Dr. Boutelle.
However, two obesity experts, who helped develop the Canadian Adult Obesity Clinical Practice Guidelines, cautioned in emails that the intervention is very labor-intensive with less than 5% weight loss.
Four interventions
The trial was conducted at the Center for Healthy Eating and Activity Research at the University of California, San Diego, from December 2015 to December 2019.
Researchers randomized 271 adults with a mean BMI of 35 kg/m2 to one of four interventions:
- Regulation of cues: Patients were not given a prescribed diet but instead were given skills to tolerate cravings and respond better to hunger or satiety cues.
- Behavioral weight loss therapy: Patients were advised to follow a balanced, calorie-deficit diet based on their weight and given related skills.
- Combined regulation of cues plus behavioral weight loss therapy.
- Control: Patients received information about nutrition and stress management plus mindfulness training and were encouraged to find social support.
Therapy was given as 26 group sessions, 90 minutes each, over 12 months, with 16 weekly sessions, four biweekly sessions, and six monthly booster sessions.
Participants were asked to take part in 150 minutes of moderate to high intensity exercise each week and aim for 10,000 steps per day. All patients except those in the control group received a pedometer.
The patients were a mean age of 46 years, 82% were women and 62% were White.
At the end of the 12-month intervention, mean BMI had dropped by –1.18 kg/m2 in the ROC group and by –1.58 kg/m2 and –1.56 kg/m2 in the other two groups, compared with the control group, where BMI was virtually unchanged.
At 24 months follow-up, mean BMI was similar (roughly 33.5 kg/m2) in the ROC, the behavioral weight loss therapy, and the ROC plus behavioral weight loss therapy groups.
There was weight regain from 12 months in the latter two groups but not in the ROC group.
‘Nice study, but not practical’
“This is a nice study, but in no way is it practical,” Sean Wharton, MD, summarized.
“I think it may have difficulty finding its way into everyday practice,” said Dr. Wharton, adjunct professor at McMaster University, Hamilton, Ontario.
Also, “it does not compare ROC to pharmacotherapy,” he added, which is “quickly becoming the gold standard for obesity management. We have learned that adding intensive behavioral therapy – more visits and possibly a liquid diet as part of the weight management and some light group counseling – to pharmacotherapy does not add much.”
However, Dr. Wharton conceded that if an individual did not want, or could not take, pharmacotherapy and had access to ROC sessions, this might be a good option.
“The challenge will be offering this labor-intensive tool to 40% of Americans living with obesity,” he said.
The ROC intervention “is very different than a GP’s office that may see a patient two to three times/year max, with limited supports,” Dr. Wharton pointed out.
“It is labor-intensive, not reproducible in most places, and cannot be sustained forever. There is no evidence that the learning remains past the treatment interval. For example, 2 to 3 years later, are patients still adhering to ROC? Is weight still decreased or do they need to come to classes every month forever?”
‘Modest weight loss, doubtful long-term benefits’
Similarly, Arya M. Sharma, MD, said: “While this [ROC] approach may be helpful for some individuals, given the rather modest weight loss achieved (despite considerable efforts and a cash incentive), the long-term clinical benefits remain doubtful.”
The weight loss of less than 5% over 24 months is “in the ballpark of other behavioral weight-loss interventions,” said Dr. Sharma, of the University of Edmonton, Alberta, and past scientific director of Obesity Canada.
“I’m not convinced” about less weight regain, he added. “The difference between the groups is minimal. While this approach may well help individuals better deal with food cues, it does not change the underlying biology of weight regain.”
“This approach at best may help prevent future weight gain in susceptible individuals,” he speculated. “I would consider this more as a weight-stabilization than a weight-loss strategy.”
Next steps
Insurance doesn’t always cover weight loss with a mental health professional, Dr. Boutelle agreed. “However, there are eating disorder categories that also apply to many of our food-cue-sensitive patients, including binge eating,” she noted.
“We believe that ROC is an alternative model for weight loss that could be offered to patients who are interested or for whom behavioral weight loss has not been successful ... who are highly food-cue-responsive.”
The group is writing a manual about the ROC program to disseminate to other behavior therapists. They are also studying ROC in another clinical trial, Solutions for Hunger and Regulating Eating (SHARE). The ROC program is being offered at the UC San Diego Center for Healthy Eating and Activity Research, of which Dr. Boutelle is director.
The study was supported by grants from the National Institutes of Health. The researchers have reported no relevant financial relationships. Dr. Wharton has reported receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly, and Janssen. He is the medical director of a medical clinic specializing in weight management and diabetes. Dr. Sharma has reported receiving speakers bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Tirzepatide (Mounjaro) approved for type 2 diabetes
The “twincretin” era for treating patients with type 2 diabetes has begun, with the Food and Drug Administration’s approval of tirzepatide for this indication on May 13, making it the first approved agent that works as a dual agonist for the two principal human incretins.
Tirzepatide represents “an important advance in the treatment of type 2 diabetes,” the FDA’s Patrick Archdeacon, MD, associate director of the division of diabetes, lipid disorders, and obesity, said in a statement released by the agency.
That advance is based on tirzepatide’s engineering, which gives it agonist properties for both the glucagonlike peptide–1 (GLP-1) receptor, as well as the glucose-dependent insulinotropic polypeptide (GIP). Several agents are already approved for U.S. use from the class with single-agonist activity on the GLP-1 receptor, including semaglutide (Ozempic for treating patients with type 2 diabetes; Wegovy for weight loss).
The FDA’s approved label includes all three dosages of tirzepatide that underwent testing in the pivotal trials: 5 mg, 10 mg, and 15 mg, each delivered by subcutaneous injection once a week. Also approved was the 2.5-mg/week dose used when starting a patient on the agent. Gradual up-titration appears to minimize possible gastrointestinal adverse effects during initial tirzepatide use.
Tirzepatide, which will be marketed by Lilly as Mounjaro, will hit the U.S. market with much anticipation, based on results from five pivotal trials, all reported during the past year or so, that established the drug’s unprecedented efficacy for reducing hemoglobin A1c levels as well as triggering significant weight loss in most patients with a generally benign safety profile.
‘Impressive’ effects
The effects from tirzepatide on A1c and weight seen in these studies was “impressive, and will likely drive use of this agent,” commented Carol H. Wysham, MD, an endocrinologist at the MultiCare Rockwood Clinic in Spokane, Wash.
Tirzepatide received good notices in several editorials that accompanied the published reports of the pivotal trials. The first of these, a commentary from two U.K.-based endocrinologists, said that “tirzepatide appears to represent an advancement over current GLP-1 analogues, providing enhanced glycemic and weight benefits without an added penalty in terms of gastrointestinal adverse effects.”
The pivotal trials included head-to-head comparisons between tirzepatide and a 1.0-mg/week dose of semaglutide, as well as comparisons with each of two long-acting insulin analogs, insulin glargine (Lantus) and insulin degludec (Tresiba).
“These are the most important comparators,” Dr. Wysham said.
“Tirzepatide was appropriately compared with the best-in-class and most effective glucose-lowering agents currently available,” said Ildiko Lingvay, MD, an endocrinologist and professor at the University of Texas Southwestern Medical Center in Dallas.
“Given its outstanding efficacy at both lowering glucose and weight, I expect tirzepatide to have quick uptake among patients with diabetes,” Dr. Lingvay said. “The only limiting factor will be cost,” she added in an interview, highlighting the major stumbling block that could limit tirzepatide’s uptake.
“As with any new medication, access will be the biggest barrier to uptake,” agreed Alice Y.Y. Cheng, MD, an endocrinologist at the University of Toronto.
Lingering uncertainties
The timing of the comparison with semaglutide leaves some unanswered questions. The SURPASS-2 trial compared the three primary tirzepatide regimens (5 mg, 10 mg, and 15 mg/week) with a 1.0-mg/week dose of semaglutide, which was at the time the only approved dosage of semaglutide for patients with type 2 diabetes. Since then, a 2.0-mg/week dosage of semaglutide (Ozempic) received U.S. approval for treating patients with type 2 diabetes, and a 2.4-mg/week dosage (Wegovy) received an FDA nod for treating people with obesity.
The lack of head-to-head data for tirzepatide against the 2.0-mg/week dose of semaglutide “leaves a clinical gap,” said Dr. Cheng. Tirzepatide “represents an advance over semaglutide at the 1-mg/week dose, but we do not know for sure compared to the higher dose.”
Another important limitation for tirzepatide right now is that the agent’s obligatory cardiovascular outcome trial, SURPASS CVOT, with about 12,500 enrolled patients, will not have findings out until about 2025, leaving uncertainty until then about tirzepatide’s cardiovascular effects.
“We are missing the cardiovascular outcome data – very important data will come” from that trial, noted Dr. Wysham. “There will be some reluctance to use the agent in high-risk patients until we see the results.”
Given tirzepatide’s proven efficacy so far, the missing cardiovascular results “are not a limitation for most patients, but for patients with preexisting cardiovascular disease I will continue to use agents with proven benefits until the SURPASS CVOT results come out,” Dr. Lingvay said.
And then there is the cost issue, something that Lilly had not yet publicly addressed at the time that the FDA announced its decision.
An analysis of cost effectiveness published by the U.S. Institute for Clinical and Economic Review in February 2022 concluded that tirzepatide had a better impact on patient quality of life, compared with 1.0 mg/week semaglutide for treating patients with type 2 diabetes, which gave it a modest pricing cushion, compared with semaglutide of about $5,500 per quality-adjusted life-year gained. But the researchers who prepared the report admitted that tirzepatide’s cost-effectiveness was hard to estimate without knowing the drug’s actual price.
Dr. Wysham has financial ties to AstraZeneca, Abbott, Boehringer Ingelheim, Intercept, Janssen, Mylan, Novo Nordisk, and Sanofi. Dr. Lingvay has dies to Lilly, Novo Nordisk, Sanofi, Boehringer Ingelheim, Merck, Pfizer, and Mylan, Intarcia, MannKind, Valeritas, and several other drug and device makers.
A version of this article first appeared on Medscape.com.
The “twincretin” era for treating patients with type 2 diabetes has begun, with the Food and Drug Administration’s approval of tirzepatide for this indication on May 13, making it the first approved agent that works as a dual agonist for the two principal human incretins.
Tirzepatide represents “an important advance in the treatment of type 2 diabetes,” the FDA’s Patrick Archdeacon, MD, associate director of the division of diabetes, lipid disorders, and obesity, said in a statement released by the agency.
That advance is based on tirzepatide’s engineering, which gives it agonist properties for both the glucagonlike peptide–1 (GLP-1) receptor, as well as the glucose-dependent insulinotropic polypeptide (GIP). Several agents are already approved for U.S. use from the class with single-agonist activity on the GLP-1 receptor, including semaglutide (Ozempic for treating patients with type 2 diabetes; Wegovy for weight loss).
The FDA’s approved label includes all three dosages of tirzepatide that underwent testing in the pivotal trials: 5 mg, 10 mg, and 15 mg, each delivered by subcutaneous injection once a week. Also approved was the 2.5-mg/week dose used when starting a patient on the agent. Gradual up-titration appears to minimize possible gastrointestinal adverse effects during initial tirzepatide use.
Tirzepatide, which will be marketed by Lilly as Mounjaro, will hit the U.S. market with much anticipation, based on results from five pivotal trials, all reported during the past year or so, that established the drug’s unprecedented efficacy for reducing hemoglobin A1c levels as well as triggering significant weight loss in most patients with a generally benign safety profile.
‘Impressive’ effects
The effects from tirzepatide on A1c and weight seen in these studies was “impressive, and will likely drive use of this agent,” commented Carol H. Wysham, MD, an endocrinologist at the MultiCare Rockwood Clinic in Spokane, Wash.
Tirzepatide received good notices in several editorials that accompanied the published reports of the pivotal trials. The first of these, a commentary from two U.K.-based endocrinologists, said that “tirzepatide appears to represent an advancement over current GLP-1 analogues, providing enhanced glycemic and weight benefits without an added penalty in terms of gastrointestinal adverse effects.”
The pivotal trials included head-to-head comparisons between tirzepatide and a 1.0-mg/week dose of semaglutide, as well as comparisons with each of two long-acting insulin analogs, insulin glargine (Lantus) and insulin degludec (Tresiba).
“These are the most important comparators,” Dr. Wysham said.
“Tirzepatide was appropriately compared with the best-in-class and most effective glucose-lowering agents currently available,” said Ildiko Lingvay, MD, an endocrinologist and professor at the University of Texas Southwestern Medical Center in Dallas.
“Given its outstanding efficacy at both lowering glucose and weight, I expect tirzepatide to have quick uptake among patients with diabetes,” Dr. Lingvay said. “The only limiting factor will be cost,” she added in an interview, highlighting the major stumbling block that could limit tirzepatide’s uptake.
“As with any new medication, access will be the biggest barrier to uptake,” agreed Alice Y.Y. Cheng, MD, an endocrinologist at the University of Toronto.
Lingering uncertainties
The timing of the comparison with semaglutide leaves some unanswered questions. The SURPASS-2 trial compared the three primary tirzepatide regimens (5 mg, 10 mg, and 15 mg/week) with a 1.0-mg/week dose of semaglutide, which was at the time the only approved dosage of semaglutide for patients with type 2 diabetes. Since then, a 2.0-mg/week dosage of semaglutide (Ozempic) received U.S. approval for treating patients with type 2 diabetes, and a 2.4-mg/week dosage (Wegovy) received an FDA nod for treating people with obesity.
The lack of head-to-head data for tirzepatide against the 2.0-mg/week dose of semaglutide “leaves a clinical gap,” said Dr. Cheng. Tirzepatide “represents an advance over semaglutide at the 1-mg/week dose, but we do not know for sure compared to the higher dose.”
Another important limitation for tirzepatide right now is that the agent’s obligatory cardiovascular outcome trial, SURPASS CVOT, with about 12,500 enrolled patients, will not have findings out until about 2025, leaving uncertainty until then about tirzepatide’s cardiovascular effects.
“We are missing the cardiovascular outcome data – very important data will come” from that trial, noted Dr. Wysham. “There will be some reluctance to use the agent in high-risk patients until we see the results.”
Given tirzepatide’s proven efficacy so far, the missing cardiovascular results “are not a limitation for most patients, but for patients with preexisting cardiovascular disease I will continue to use agents with proven benefits until the SURPASS CVOT results come out,” Dr. Lingvay said.
And then there is the cost issue, something that Lilly had not yet publicly addressed at the time that the FDA announced its decision.
An analysis of cost effectiveness published by the U.S. Institute for Clinical and Economic Review in February 2022 concluded that tirzepatide had a better impact on patient quality of life, compared with 1.0 mg/week semaglutide for treating patients with type 2 diabetes, which gave it a modest pricing cushion, compared with semaglutide of about $5,500 per quality-adjusted life-year gained. But the researchers who prepared the report admitted that tirzepatide’s cost-effectiveness was hard to estimate without knowing the drug’s actual price.
Dr. Wysham has financial ties to AstraZeneca, Abbott, Boehringer Ingelheim, Intercept, Janssen, Mylan, Novo Nordisk, and Sanofi. Dr. Lingvay has dies to Lilly, Novo Nordisk, Sanofi, Boehringer Ingelheim, Merck, Pfizer, and Mylan, Intarcia, MannKind, Valeritas, and several other drug and device makers.
A version of this article first appeared on Medscape.com.
The “twincretin” era for treating patients with type 2 diabetes has begun, with the Food and Drug Administration’s approval of tirzepatide for this indication on May 13, making it the first approved agent that works as a dual agonist for the two principal human incretins.
Tirzepatide represents “an important advance in the treatment of type 2 diabetes,” the FDA’s Patrick Archdeacon, MD, associate director of the division of diabetes, lipid disorders, and obesity, said in a statement released by the agency.
That advance is based on tirzepatide’s engineering, which gives it agonist properties for both the glucagonlike peptide–1 (GLP-1) receptor, as well as the glucose-dependent insulinotropic polypeptide (GIP). Several agents are already approved for U.S. use from the class with single-agonist activity on the GLP-1 receptor, including semaglutide (Ozempic for treating patients with type 2 diabetes; Wegovy for weight loss).
The FDA’s approved label includes all three dosages of tirzepatide that underwent testing in the pivotal trials: 5 mg, 10 mg, and 15 mg, each delivered by subcutaneous injection once a week. Also approved was the 2.5-mg/week dose used when starting a patient on the agent. Gradual up-titration appears to minimize possible gastrointestinal adverse effects during initial tirzepatide use.
Tirzepatide, which will be marketed by Lilly as Mounjaro, will hit the U.S. market with much anticipation, based on results from five pivotal trials, all reported during the past year or so, that established the drug’s unprecedented efficacy for reducing hemoglobin A1c levels as well as triggering significant weight loss in most patients with a generally benign safety profile.
‘Impressive’ effects
The effects from tirzepatide on A1c and weight seen in these studies was “impressive, and will likely drive use of this agent,” commented Carol H. Wysham, MD, an endocrinologist at the MultiCare Rockwood Clinic in Spokane, Wash.
Tirzepatide received good notices in several editorials that accompanied the published reports of the pivotal trials. The first of these, a commentary from two U.K.-based endocrinologists, said that “tirzepatide appears to represent an advancement over current GLP-1 analogues, providing enhanced glycemic and weight benefits without an added penalty in terms of gastrointestinal adverse effects.”
The pivotal trials included head-to-head comparisons between tirzepatide and a 1.0-mg/week dose of semaglutide, as well as comparisons with each of two long-acting insulin analogs, insulin glargine (Lantus) and insulin degludec (Tresiba).
“These are the most important comparators,” Dr. Wysham said.
“Tirzepatide was appropriately compared with the best-in-class and most effective glucose-lowering agents currently available,” said Ildiko Lingvay, MD, an endocrinologist and professor at the University of Texas Southwestern Medical Center in Dallas.
“Given its outstanding efficacy at both lowering glucose and weight, I expect tirzepatide to have quick uptake among patients with diabetes,” Dr. Lingvay said. “The only limiting factor will be cost,” she added in an interview, highlighting the major stumbling block that could limit tirzepatide’s uptake.
“As with any new medication, access will be the biggest barrier to uptake,” agreed Alice Y.Y. Cheng, MD, an endocrinologist at the University of Toronto.
Lingering uncertainties
The timing of the comparison with semaglutide leaves some unanswered questions. The SURPASS-2 trial compared the three primary tirzepatide regimens (5 mg, 10 mg, and 15 mg/week) with a 1.0-mg/week dose of semaglutide, which was at the time the only approved dosage of semaglutide for patients with type 2 diabetes. Since then, a 2.0-mg/week dosage of semaglutide (Ozempic) received U.S. approval for treating patients with type 2 diabetes, and a 2.4-mg/week dosage (Wegovy) received an FDA nod for treating people with obesity.
The lack of head-to-head data for tirzepatide against the 2.0-mg/week dose of semaglutide “leaves a clinical gap,” said Dr. Cheng. Tirzepatide “represents an advance over semaglutide at the 1-mg/week dose, but we do not know for sure compared to the higher dose.”
Another important limitation for tirzepatide right now is that the agent’s obligatory cardiovascular outcome trial, SURPASS CVOT, with about 12,500 enrolled patients, will not have findings out until about 2025, leaving uncertainty until then about tirzepatide’s cardiovascular effects.
“We are missing the cardiovascular outcome data – very important data will come” from that trial, noted Dr. Wysham. “There will be some reluctance to use the agent in high-risk patients until we see the results.”
Given tirzepatide’s proven efficacy so far, the missing cardiovascular results “are not a limitation for most patients, but for patients with preexisting cardiovascular disease I will continue to use agents with proven benefits until the SURPASS CVOT results come out,” Dr. Lingvay said.
And then there is the cost issue, something that Lilly had not yet publicly addressed at the time that the FDA announced its decision.
An analysis of cost effectiveness published by the U.S. Institute for Clinical and Economic Review in February 2022 concluded that tirzepatide had a better impact on patient quality of life, compared with 1.0 mg/week semaglutide for treating patients with type 2 diabetes, which gave it a modest pricing cushion, compared with semaglutide of about $5,500 per quality-adjusted life-year gained. But the researchers who prepared the report admitted that tirzepatide’s cost-effectiveness was hard to estimate without knowing the drug’s actual price.
Dr. Wysham has financial ties to AstraZeneca, Abbott, Boehringer Ingelheim, Intercept, Janssen, Mylan, Novo Nordisk, and Sanofi. Dr. Lingvay has dies to Lilly, Novo Nordisk, Sanofi, Boehringer Ingelheim, Merck, Pfizer, and Mylan, Intarcia, MannKind, Valeritas, and several other drug and device makers.
A version of this article first appeared on Medscape.com.
NAFLD vs. MAFLD: What’s in a name?
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
Non-alcoholic fatty liver disease (NAFLD) and metabolic associated fatty liver disease (MAFLD) demonstrate highly similar clinical courses and mortality rates, and a name change may not be clinically beneficial, based on data from more than 17,000 patients.
Instead, etiologic subcategorization of fatty liver disease (FLD) should be considered, reported lead author Zobair M. Younossi, MD, of Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church, Va., and colleagues.
“There is debate about whether NAFLD is an appropriate name as the term ‘non-alcoholic’ overemphasizes the absence of alcohol use and underemphasizes the importance of the metabolic risk factors which are the main drivers of disease progression,” the investigators wrote in Hepatology. “It has been suggested that MAFLD may better reflect these risk factors. However, such a recommendation is made despite a lack of a general consensus on the definition of ‘metabolic health’ and disagreements in endocrinology circles about the term ‘metabolic syndrome.’ Nevertheless, a few investigators have suggested that MAFLD but not NAFLD is associated with increased fibrosis and mortality.”
To look for clinical differences between the two disease entities, Dr. Younossi and colleagues turned to the National Health and Nutrition Examination Survey (NHANES). Specifically, the NHANES III and NHANES 2017-2018 cohorts were employed, including 12,878 and 4,328 participants, respectively.
MAFLD was defined as FLD with overweight/obesity, evidence of metabolic dysregulation, or type 2 diabetes mellitus. NAFLD was defined as FLD without excessive alcohol consumption or other causes of chronic liver disease. Patients were sorted into four groups: NAFLD, MAFLD, both disease types, or neither disease type. Since the categories were not mutually exclusive, the investigators compared clinical characteristics based on 95% confidence intervals. If no overlap was found, then differences were deemed statistically significant.
Diagnoses of NAFLD and MAFLD were highly concordant (kappa coefficient = 0.83-0.94). After a median of 22.8 years follow-up, no significant differences were found between groups for cause-specific mortality, all-cause mortality, or major clinical characteristics except those inherent to the disease definitions (for example, lack of alcohol use in NAFLD). Greatest risk factors for advanced fibrosis in both groups were obesity, high-risk fibrosis, and type 2 diabetes mellitus.
As anticipated, by definition, alcoholic liver disease and excess alcohol use were documented in approximately 15% of patients with MAFLD, but in no patients with NAFLD. As such, alcoholic liver disease predicted liver-specific mortality for MAFLD (hazard ratio, 4.50; 95% confidence interval, 1.89-10.75) but not NAFLD. Conversely, insulin resistance predicted liver-specific mortality in NAFLD (HR, 3.57; 95% CI, 1.35-9.42) but not MAFLD (HR, 0.84; 95% CI, 0.36-1.95).
“These data do not support the notion that a name change from NAFLD to MAFLD will better capture the risk for long-term outcomes of these patients or better define metabolically at-risk patients who present with FLD,” the investigators concluded. “On the other hand, enlarging the definition to FLD with subcategories of ‘alcoholic,’ ‘non-alcoholic,’ ‘drug-induced,’ etc. has merit and needs to be further considered. In this context, a true international consensus group of experts supported by liver and non-liver scientific societies must undertake an evidence-based and comprehensive approach to this issue and assess both the benefits and risks of changing the name.”
According to Rohit Loomba, MD, director of the NAFLD research center and professor of medicine in the division of gastroenterology and hepatology at University of California, San Diego, the study offers a preview of the consequences if NAFLD were changed to MAFLD, most notably by making alcohol a key driver of outcomes.
“If we change the name of a disease entity ... how does that impact natural history?” Dr. Loomba asked in an interview. “This paper gives you an idea. If you start calling it MAFLD, then people are dying from alcohol use, and they’re not dying from what we are currently seeing patients with NAFLD die of.”
He also noted that the name change could disrupt drug development and outcome measures since most drugs currently in development are directed at nonalcoholic steatohepatitis (NASH).
“Is it worth the headache?” Dr. Loomba asked. “How are we going to define NASH-related fibrosis? That probably will remain the same because the therapies that we will use to address that will remain consistent with what we are currently pursuing. ... It’s probably premature to change the nomenclature before assessing the impact on finding new treatment.”
Dr. Younossi disclosed relationships with BMS, Novartis, Gilead, and others. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals, and Viking Therapeutics.
FROM HEPATOLOGY
First fatty liver guidelines for endocrinology, primary care
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
New clinical practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease (NAFLD) are the first to be targeted specifically to primary care and endocrinology clinical settings.
They include 34 evidence-based clinical practice recommendations for screening, diagnosis, management, and referral, presented in a table and an algorithm flow chart as well as detailed text.
The new guidelines are by the American Association of Clinical Endocrinology and cosponsored by the American Association for the Study of Liver Diseases. They were presented at the annual scientific & clinical congress of the AACE and simultaneously published in Endocrine Practice.
These are “the first of this type for this field of medicine. The vast majority of patients with NAFLD are being seen in the primary care and endocrinology settings. Only when they get to the more advanced disease are they being referred to the liver specialists. So, we need to be the ones who are diagnosing and managing these patients because there just aren’t enough liver specialists to do that,” Scott Isaacs, MD, cochair of the writing panel for the guidelines, said in an interview.
80 million Americans have NAFLD, but very few are aware
The spectrum of NAFLD ranges from nonprogressive steatosis to the progressive conditions nonalcoholic steatohepatitis, fibrotic NASH, and end-stage NASH cirrhosis. And NASH, in turn, is a major cause of liver cancer. NAFLD is also strongly associated with insulin resistance, type 2 diabetes, atherogenesis, and myocardial dysfunction.
The global prevalence of NAFLD is about 25% and NASH, about 12%-14%. However, a recent study found that, among patients in endocrine and primary care clinics, more than 70% of patients with type 2 diabetes and more than 90% with type 2 diabetes who had a body mass index above 35 kg/m2 also had NAFLD, and more than 20% of those patients had significant liver fibrosis.
Problematically, very few people are aware they have either. “It’s so common. At least 80 million Americans have this but only about 6% know they have it. We talk about it a lot, but it’s not talked about enough,” said Dr. Isaacs, an endocrinologist who practices in Atlanta.
In fact, most cases of NAFLD are diagnosed incidentally when people undergo an ultrasound or a CT scan for another reason. And, in about 70% of cases the liver enzymes are normal, and those patients rarely undergo liver workups, Dr. Isaacs noted.
In an accompanying editorial, Suthat Liangpunsakul, MD, wrote: “In my perspective, as a hepatologist, this AACE guideline is very practical and easy to incorporate into routine practice in primary care and endocrinology settings. ... Early identification and risk stratification of patients with NAFLD, especially the degree of hepatic fibrosis, are required to reduce downstream health care costs and triage unwarranted specialty care referrals.”
And “an effective screening strategy may also identify those in primary care and endocrinology settings who may benefit from an appropriate referral to hepatologists before the development of portal hypertension complications, decompensated liver disease, and hepatocellular carcinoma,” added Dr. Liangpunsakul, professor of medicine in the division of gastroenterology and hepatology at Indiana University, Indianapolis.
Screening advised using new FIB-4 test
The guideline calls for screening all patients at high risk for NAFLD, including those with prediabetes, type 2 diabetes, obesity, and/or two or more cardiometabolic risk factors, or those with hepatic steatosis found on imaging, and/or persistently elevated plasma aminotransferase levels (that is, for more than 6 months).
The recommended screening test is the Fibrosis-4 (FIB-4) index, calculated using the patient’s age, AST level, platelet count, and ALT level: FIB-4 score = age (years) x AST (U/L)/PLT (109/L) x ALT ½ (U/L).
Recently approved by the Food and Drug Administration, the FIB-4 has been demonstrated to help identify liver disease in primary care settings.
“We really want to encourage clinicians to do the screening. The first step is the FIB-4 test. It’s a mathematical calculation using blood tests that we do anyway,” Dr. Isaacs said in an interview.
The FIB-4 stratifies patients as being low, intermediate, or high risk for liver fibrosis. Those at low risk can be managed in primary care or endocrinology settings with a focus on obesity management and cardiovascular disease prevention. “Those at low risk on FIB-4 still have a high cardiovascular disease risk. They still need to be managed,” Dr. Isaacs observed.
For those at intermediate risk, a second noninvasive test – either a liver stiffness measurement by elastography or an enhanced liver fibrosis test – is advised. If the patient is found to be at high risk or is still indeterminant after two noninvasive tests, referral to a liver specialist for further testing, including possible biopsy, is advised.
Those found to be at high risk with the FIB-4 should also be referred to hepatology. In both the intermediate- and high-risk groups, management should be multidisciplinary, including a hepatologist, endocrinologist, and other professionals to prevent both cardiovascular disease and progression to cirrhosis, the guidelines say.
“The diagnosis isn’t about diagnosing liver fat. It’s about diagnosing fibrosis, or the risk for clinically significant fibrosis. That’s really where the challenge lies,” Dr. Isaacs commented.
NAFLD treatment in endocrinology and primary care: CVD prevention
During the presentation at the AACE meeting, guideline panel cochair Kenneth Cusi, MD, chief of endocrinology, diabetes, and metabolism at the University of Florida, Gainesville, summarized current and future treatments for NAFLD.
Lifestyle intervention, cardiovascular risk reduction, and weight loss for those who are overweight or obese are recommended for all patients with NAFLD, including structured weight-loss programs, antiobesity medications, and bariatric surgery if indicated.
There are currently no FDA-approved medications specifically for NASH, but pioglitazone, approved for type 2 diabetes, and glucagonlike peptide–1 agonists, approved for type 2 diabetes and weight loss, have been shown to be effective in treating the condition and preventing progression. Other treatments are in development, Dr. Cusi said.
The guideline also includes a section on diagnosis and management of NAFLD in children and adolescents. Here, the FIB-4 is not recommended because it isn’t accurate due to the age part of the equation, so liver enzyme tests are used in pediatric patients considered at high risk because of clinical factors. Management is similar to adults, except not all medications used in adults are approved for use in children.
In the editorial, Dr. Liangpunsakul cautioned that “the level of uptake and usage of the guideline may be an obstacle.”
To remedy that, he advised that “the next effort should gear toward distributing this guideline to the targeted providers and developing the ‘feedback platforms’ on its execution in the real-world. ... The successful implementation of this AACE guideline by the primary care providers and endocrinologists, hopefully, will deescalate the future burden of NAFLD-related morbidity and mortality.”
Dr. Isaacs and Dr. Liangpunsakul have reported no relevant financial relationships. Dr. Cusi has reported receiving research support towards the University of Florida as principal investigator from the National Institute of Health, Echosens, Inventiva, Nordic Bioscience, Novo Nordisk, Poxel, Labcorp, and Zydus, and is a consultant for Altimmune, Akero, Arrowhead, AstraZeneca, 89Bio, Bristol-Myers Squibb, Coherus, Intercept, Lilly, Madrigal, Merck, Novo Nordisk, Quest, Sagimet, Sonic Incytes, Terns, and Thera Technologies.
A version of this article first appeared on Medscape.com.
FROM AACE 2022
Exenatide linked to less hyperglycemia after stroke
Treatment with the diabetes drug exenatide was associated with a significant decrease in hyperglycemia in acute stroke patients, a new study shows.
The research could offer clinicians an alternative to insulin therapy to treat hyperglycemia and reduce glucose levels, which are elevated in up to 60% of stroke patients and associated with worse outcomes after stroke.
“Use of these diabetes drugs to control glucose in acute stroke has enormous potential,” said lead researcher Christopher Bladin, PhD, professor of neurology at Monash University and Eastern Health Clinical School, Australia.
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022 annual meeting in Lyon, France.
A better fix than insulin?
Hyperglycemia is common in stroke patients, including those who have no prior history of diabetes. Among stroke patients with normal blood glucose upon admission, about 30% will develop hyperglycemia within 48 hours of stroke onset.
Previous research suggests that hyperglycemia is a poor prognostic factor in patients with stroke and may reduce the efficacy of reperfusion therapies such as thrombolysis and mechanical thrombectomy.
“We’ve been looking for different ways of treating hyperglycemia for quite some time, and one of the obvious ways is to use insulin therapy,” Dr. Bladin said. “But as we’ve seen from multiple studies, insulin therapy is difficult.”
Insulin treatment is resource-heavy, significantly increases the risk for hypoglycemia, and some studies suggest the therapy isn’t associated with better outcomes.
An advantage to a GLP-1 agonist-like exenatide, Dr. Bladin added, is that it’s glucose-dependent. As the glucose level falls, the drug’s efficacy diminishes. It is delivered via an autoinjector and easy to administer.
A case for more study
To study exenatide’s efficacy in reducing hyperglycemia and improving neurologic outcomes, researchers developed the phase 2, international, multicenter, randomized controlled TEXAIS trial.
The study enrolled 350 patients following an ischemic stroke. Within 9 hours of stroke onset, patients received either standard care or a subcutaneous injection of 5 mg of exenatide twice daily for 5 days.
On admission, 42% of patients had hyperglycemia, defined as blood glucose > 7.0 mmol/L.
The study’s primary outcome was at least an 8-point improvement in National Institutes of Health Stroke Scale (NIHSS) score by 7 days after treatment with exenatide. Although there was a trend toward better scores with exenatide, the score was not significantly different between groups (56.7% with standard care versus 61.2% with exenatide; adjusted odds ratio, 1.22; P = .38).
However, when the researchers examined hyperglycemia frequency, they found significantly lower incidence in patients treated with exenatide (P = .002).
There were no cases of hypoglycemia in either group, and only 4% of the study group reported nausea or vomiting.
“Clearly exenatide is having some benefit in terms of keeping glucose under control, reducing hyperglycemia,” Dr. Bladin said. “It certainly lends itself to a larger phase 3 study which can look at this more completely.”
Value to clinicians
Commenting on the findings, Yvonne Chun, PhD, honorary senior clinical lecturer at University of Edinburgh, noted that, even though the study didn’t find a significant association with improved neurological outcomes, the reduced risk for hypoglycemia makes exenatide an attractive alternative to insulin therapy in stroke patients.
“The results are of value to clinicians, as exenatide could potentially be a safer medication to administer than an insulin infusion in acute stroke patients with hyperglycemia,” Dr. Chun said. “There is less risk of hypoglycemia with exenatide compared to standard care.”
However, Dr. Chun noted that more study is needed before exenatide can replace standard care. Dr. Bladin agrees and would like to pursue a phase 3 trial with a modified design to answer questions raised by Dr. Chun and others.
“The next phase could consider changing the primary outcome to an ordinal shift analysis on modified Rankin Scale – a very commonly used primary outcome in stroke clinical trials to assess improvement in disability,” Dr. Chun said. “The primary outcome used in the presented trial – an 8-point improvement on NIHSS – seemed too ambitious and does not inform disability of the patient post stroke.”
Dr. Bladin said he would also like to see the next phase enroll more patients, examine a higher dose of exenatide, and include better stratification of patients with a history of diabetes. Such a trial could yield findings demonstrating the drug’s effectiveness at reducing hyperglycemia and improving outcomes after stroke, he said.
“I can see the day patients will come in with acute stroke, and as they’re coming into the emergency department, they’ll simply get their shot of exenatide because we know it’s safe to use, and it doesn’t cause hypoglycemia,” Dr. Bladin said. “And from the moment that patient arrives the glucose control is underway.”
Dr. Bladin and Dr. Chun reported no relevant financial relationships. Study funding was not disclosed.
A version of this article first appeared on Medscape.com.
Treatment with the diabetes drug exenatide was associated with a significant decrease in hyperglycemia in acute stroke patients, a new study shows.
The research could offer clinicians an alternative to insulin therapy to treat hyperglycemia and reduce glucose levels, which are elevated in up to 60% of stroke patients and associated with worse outcomes after stroke.
“Use of these diabetes drugs to control glucose in acute stroke has enormous potential,” said lead researcher Christopher Bladin, PhD, professor of neurology at Monash University and Eastern Health Clinical School, Australia.
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022 annual meeting in Lyon, France.
A better fix than insulin?
Hyperglycemia is common in stroke patients, including those who have no prior history of diabetes. Among stroke patients with normal blood glucose upon admission, about 30% will develop hyperglycemia within 48 hours of stroke onset.
Previous research suggests that hyperglycemia is a poor prognostic factor in patients with stroke and may reduce the efficacy of reperfusion therapies such as thrombolysis and mechanical thrombectomy.
“We’ve been looking for different ways of treating hyperglycemia for quite some time, and one of the obvious ways is to use insulin therapy,” Dr. Bladin said. “But as we’ve seen from multiple studies, insulin therapy is difficult.”
Insulin treatment is resource-heavy, significantly increases the risk for hypoglycemia, and some studies suggest the therapy isn’t associated with better outcomes.
An advantage to a GLP-1 agonist-like exenatide, Dr. Bladin added, is that it’s glucose-dependent. As the glucose level falls, the drug’s efficacy diminishes. It is delivered via an autoinjector and easy to administer.
A case for more study
To study exenatide’s efficacy in reducing hyperglycemia and improving neurologic outcomes, researchers developed the phase 2, international, multicenter, randomized controlled TEXAIS trial.
The study enrolled 350 patients following an ischemic stroke. Within 9 hours of stroke onset, patients received either standard care or a subcutaneous injection of 5 mg of exenatide twice daily for 5 days.
On admission, 42% of patients had hyperglycemia, defined as blood glucose > 7.0 mmol/L.
The study’s primary outcome was at least an 8-point improvement in National Institutes of Health Stroke Scale (NIHSS) score by 7 days after treatment with exenatide. Although there was a trend toward better scores with exenatide, the score was not significantly different between groups (56.7% with standard care versus 61.2% with exenatide; adjusted odds ratio, 1.22; P = .38).
However, when the researchers examined hyperglycemia frequency, they found significantly lower incidence in patients treated with exenatide (P = .002).
There were no cases of hypoglycemia in either group, and only 4% of the study group reported nausea or vomiting.
“Clearly exenatide is having some benefit in terms of keeping glucose under control, reducing hyperglycemia,” Dr. Bladin said. “It certainly lends itself to a larger phase 3 study which can look at this more completely.”
Value to clinicians
Commenting on the findings, Yvonne Chun, PhD, honorary senior clinical lecturer at University of Edinburgh, noted that, even though the study didn’t find a significant association with improved neurological outcomes, the reduced risk for hypoglycemia makes exenatide an attractive alternative to insulin therapy in stroke patients.
“The results are of value to clinicians, as exenatide could potentially be a safer medication to administer than an insulin infusion in acute stroke patients with hyperglycemia,” Dr. Chun said. “There is less risk of hypoglycemia with exenatide compared to standard care.”
However, Dr. Chun noted that more study is needed before exenatide can replace standard care. Dr. Bladin agrees and would like to pursue a phase 3 trial with a modified design to answer questions raised by Dr. Chun and others.
“The next phase could consider changing the primary outcome to an ordinal shift analysis on modified Rankin Scale – a very commonly used primary outcome in stroke clinical trials to assess improvement in disability,” Dr. Chun said. “The primary outcome used in the presented trial – an 8-point improvement on NIHSS – seemed too ambitious and does not inform disability of the patient post stroke.”
Dr. Bladin said he would also like to see the next phase enroll more patients, examine a higher dose of exenatide, and include better stratification of patients with a history of diabetes. Such a trial could yield findings demonstrating the drug’s effectiveness at reducing hyperglycemia and improving outcomes after stroke, he said.
“I can see the day patients will come in with acute stroke, and as they’re coming into the emergency department, they’ll simply get their shot of exenatide because we know it’s safe to use, and it doesn’t cause hypoglycemia,” Dr. Bladin said. “And from the moment that patient arrives the glucose control is underway.”
Dr. Bladin and Dr. Chun reported no relevant financial relationships. Study funding was not disclosed.
A version of this article first appeared on Medscape.com.
Treatment with the diabetes drug exenatide was associated with a significant decrease in hyperglycemia in acute stroke patients, a new study shows.
The research could offer clinicians an alternative to insulin therapy to treat hyperglycemia and reduce glucose levels, which are elevated in up to 60% of stroke patients and associated with worse outcomes after stroke.
“Use of these diabetes drugs to control glucose in acute stroke has enormous potential,” said lead researcher Christopher Bladin, PhD, professor of neurology at Monash University and Eastern Health Clinical School, Australia.
The findings were presented at the European Stroke Organisation Conference (ESOC) 2022 annual meeting in Lyon, France.
A better fix than insulin?
Hyperglycemia is common in stroke patients, including those who have no prior history of diabetes. Among stroke patients with normal blood glucose upon admission, about 30% will develop hyperglycemia within 48 hours of stroke onset.
Previous research suggests that hyperglycemia is a poor prognostic factor in patients with stroke and may reduce the efficacy of reperfusion therapies such as thrombolysis and mechanical thrombectomy.
“We’ve been looking for different ways of treating hyperglycemia for quite some time, and one of the obvious ways is to use insulin therapy,” Dr. Bladin said. “But as we’ve seen from multiple studies, insulin therapy is difficult.”
Insulin treatment is resource-heavy, significantly increases the risk for hypoglycemia, and some studies suggest the therapy isn’t associated with better outcomes.
An advantage to a GLP-1 agonist-like exenatide, Dr. Bladin added, is that it’s glucose-dependent. As the glucose level falls, the drug’s efficacy diminishes. It is delivered via an autoinjector and easy to administer.
A case for more study
To study exenatide’s efficacy in reducing hyperglycemia and improving neurologic outcomes, researchers developed the phase 2, international, multicenter, randomized controlled TEXAIS trial.
The study enrolled 350 patients following an ischemic stroke. Within 9 hours of stroke onset, patients received either standard care or a subcutaneous injection of 5 mg of exenatide twice daily for 5 days.
On admission, 42% of patients had hyperglycemia, defined as blood glucose > 7.0 mmol/L.
The study’s primary outcome was at least an 8-point improvement in National Institutes of Health Stroke Scale (NIHSS) score by 7 days after treatment with exenatide. Although there was a trend toward better scores with exenatide, the score was not significantly different between groups (56.7% with standard care versus 61.2% with exenatide; adjusted odds ratio, 1.22; P = .38).
However, when the researchers examined hyperglycemia frequency, they found significantly lower incidence in patients treated with exenatide (P = .002).
There were no cases of hypoglycemia in either group, and only 4% of the study group reported nausea or vomiting.
“Clearly exenatide is having some benefit in terms of keeping glucose under control, reducing hyperglycemia,” Dr. Bladin said. “It certainly lends itself to a larger phase 3 study which can look at this more completely.”
Value to clinicians
Commenting on the findings, Yvonne Chun, PhD, honorary senior clinical lecturer at University of Edinburgh, noted that, even though the study didn’t find a significant association with improved neurological outcomes, the reduced risk for hypoglycemia makes exenatide an attractive alternative to insulin therapy in stroke patients.
“The results are of value to clinicians, as exenatide could potentially be a safer medication to administer than an insulin infusion in acute stroke patients with hyperglycemia,” Dr. Chun said. “There is less risk of hypoglycemia with exenatide compared to standard care.”
However, Dr. Chun noted that more study is needed before exenatide can replace standard care. Dr. Bladin agrees and would like to pursue a phase 3 trial with a modified design to answer questions raised by Dr. Chun and others.
“The next phase could consider changing the primary outcome to an ordinal shift analysis on modified Rankin Scale – a very commonly used primary outcome in stroke clinical trials to assess improvement in disability,” Dr. Chun said. “The primary outcome used in the presented trial – an 8-point improvement on NIHSS – seemed too ambitious and does not inform disability of the patient post stroke.”
Dr. Bladin said he would also like to see the next phase enroll more patients, examine a higher dose of exenatide, and include better stratification of patients with a history of diabetes. Such a trial could yield findings demonstrating the drug’s effectiveness at reducing hyperglycemia and improving outcomes after stroke, he said.
“I can see the day patients will come in with acute stroke, and as they’re coming into the emergency department, they’ll simply get their shot of exenatide because we know it’s safe to use, and it doesn’t cause hypoglycemia,” Dr. Bladin said. “And from the moment that patient arrives the glucose control is underway.”
Dr. Bladin and Dr. Chun reported no relevant financial relationships. Study funding was not disclosed.
A version of this article first appeared on Medscape.com.
FROM ESOC 2022
What is the glycemic risk index and why do we need it?
I want to talk about a new continuous glucose monitoring (CGM) metric known as glycemic risk index, or GRI. You may ask why we need another metric. We currently have multiple CGM metrics, including time in range, time below range, time above range, mean glucose, glucose management indicator (GMI), and coefficient of variation, and it seems like an overwhelming number of ways to look at the same data.
The problem is that no single metric tells you exactly what is happening with the patient. For instance, a patient could be at a target time in range of 70%, but that could mean that 30% of that patient’s time is spent too low or even very low, which is a very serious problem, versus if 30% of their time was spent in a somewhat but not very high range, which requires less immediate attention.
Dr. David Klonoff and colleagues, including me, decided to see if one number could be used to identify which patients needed more immediate attention and which needed less. He asked 330 clinicians to evaluate 225 CGM tracings and rank their clinical status in terms of these metrics: very low glucose and low glucose hypoglycemia, very high glucose and high glucose hyperglycemia, time in range, mean glucose, and coefficient of variation.
Then he took all the data and analyzed it in complex ways that I barely understood and came up with one number, the GRI, that captures what the clinicians considered important. The analysis showed that the clinician rankings depended primarily on two components: One related to hypoglycemia, which gives more weight to very low glucose than to low glucose hypoglycemia; and the other related to hyperglycemia, which gives greater weight to very high glucose than to high glucose.
These two components were combined into a single glycemic risk index, the GRI, that corresponds closely to the clinician rankings of the overall quality of glycemia. In terms of numbers, the best GRI is in the zero to 20th percentile and the worst in the 81st to 100th percentile. The GRI grid that is provided in the paper enables users to track sequential changes within an individual over time and compare groups of individuals.
As I said initially, at first I wasn’t sure of the utility of adding yet another number to the mix, but I realized that for triaging what I hope will be increasing amounts of CGM data in a health care system, this could help identify those patients who need the most urgent assistance. It can also help providers have an overall sense of how a patient is doing and whether or not they are improving.
The GRI is not yet in general use and needs to be studied to see if it is actually helpful in clinical practice; however, I like the concept. Given the need to increase provider understanding of CGM metrics overall, I think it is a good way for providers to identify which patients need further analysis of their CGM data for potential treatment modifications.
Thank you.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies.
A version of this article first appeared on Medscape.com.
I want to talk about a new continuous glucose monitoring (CGM) metric known as glycemic risk index, or GRI. You may ask why we need another metric. We currently have multiple CGM metrics, including time in range, time below range, time above range, mean glucose, glucose management indicator (GMI), and coefficient of variation, and it seems like an overwhelming number of ways to look at the same data.
The problem is that no single metric tells you exactly what is happening with the patient. For instance, a patient could be at a target time in range of 70%, but that could mean that 30% of that patient’s time is spent too low or even very low, which is a very serious problem, versus if 30% of their time was spent in a somewhat but not very high range, which requires less immediate attention.
Dr. David Klonoff and colleagues, including me, decided to see if one number could be used to identify which patients needed more immediate attention and which needed less. He asked 330 clinicians to evaluate 225 CGM tracings and rank their clinical status in terms of these metrics: very low glucose and low glucose hypoglycemia, very high glucose and high glucose hyperglycemia, time in range, mean glucose, and coefficient of variation.
Then he took all the data and analyzed it in complex ways that I barely understood and came up with one number, the GRI, that captures what the clinicians considered important. The analysis showed that the clinician rankings depended primarily on two components: One related to hypoglycemia, which gives more weight to very low glucose than to low glucose hypoglycemia; and the other related to hyperglycemia, which gives greater weight to very high glucose than to high glucose.
These two components were combined into a single glycemic risk index, the GRI, that corresponds closely to the clinician rankings of the overall quality of glycemia. In terms of numbers, the best GRI is in the zero to 20th percentile and the worst in the 81st to 100th percentile. The GRI grid that is provided in the paper enables users to track sequential changes within an individual over time and compare groups of individuals.
As I said initially, at first I wasn’t sure of the utility of adding yet another number to the mix, but I realized that for triaging what I hope will be increasing amounts of CGM data in a health care system, this could help identify those patients who need the most urgent assistance. It can also help providers have an overall sense of how a patient is doing and whether or not they are improving.
The GRI is not yet in general use and needs to be studied to see if it is actually helpful in clinical practice; however, I like the concept. Given the need to increase provider understanding of CGM metrics overall, I think it is a good way for providers to identify which patients need further analysis of their CGM data for potential treatment modifications.
Thank you.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies.
A version of this article first appeared on Medscape.com.
I want to talk about a new continuous glucose monitoring (CGM) metric known as glycemic risk index, or GRI. You may ask why we need another metric. We currently have multiple CGM metrics, including time in range, time below range, time above range, mean glucose, glucose management indicator (GMI), and coefficient of variation, and it seems like an overwhelming number of ways to look at the same data.
The problem is that no single metric tells you exactly what is happening with the patient. For instance, a patient could be at a target time in range of 70%, but that could mean that 30% of that patient’s time is spent too low or even very low, which is a very serious problem, versus if 30% of their time was spent in a somewhat but not very high range, which requires less immediate attention.
Dr. David Klonoff and colleagues, including me, decided to see if one number could be used to identify which patients needed more immediate attention and which needed less. He asked 330 clinicians to evaluate 225 CGM tracings and rank their clinical status in terms of these metrics: very low glucose and low glucose hypoglycemia, very high glucose and high glucose hyperglycemia, time in range, mean glucose, and coefficient of variation.
Then he took all the data and analyzed it in complex ways that I barely understood and came up with one number, the GRI, that captures what the clinicians considered important. The analysis showed that the clinician rankings depended primarily on two components: One related to hypoglycemia, which gives more weight to very low glucose than to low glucose hypoglycemia; and the other related to hyperglycemia, which gives greater weight to very high glucose than to high glucose.
These two components were combined into a single glycemic risk index, the GRI, that corresponds closely to the clinician rankings of the overall quality of glycemia. In terms of numbers, the best GRI is in the zero to 20th percentile and the worst in the 81st to 100th percentile. The GRI grid that is provided in the paper enables users to track sequential changes within an individual over time and compare groups of individuals.
As I said initially, at first I wasn’t sure of the utility of adding yet another number to the mix, but I realized that for triaging what I hope will be increasing amounts of CGM data in a health care system, this could help identify those patients who need the most urgent assistance. It can also help providers have an overall sense of how a patient is doing and whether or not they are improving.
The GRI is not yet in general use and needs to be studied to see if it is actually helpful in clinical practice; however, I like the concept. Given the need to increase provider understanding of CGM metrics overall, I think it is a good way for providers to identify which patients need further analysis of their CGM data for potential treatment modifications.
Thank you.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts, and three books, on diabetes, and has been an investigator for more than 40 research studies.
A version of this article first appeared on Medscape.com.
Grit your teeth for a lesser-known complication of diabetes
Type 2 diabetes was associated with a 20% increased risk of tooth loss after adjusting for multiple other risk factors in a meta-analysis of 22 recent observational studies from around the world.
The risk of tooth loss with type 2 diabetes (versus no diabetes) ranged from 15% higher in cross-sectional studies to 29% higher in cohort studies to five times higher in case-control studies.
“For diabetes, there are various known complications that are considered in [patient] treatment and management, including neuropathy, nephropathy, cardiovascular [disease] and hypertension, and kidney disease,” senior author Abdolhalim Rajabi, PhD, told this news organization in an email.
“However, a chronic complication of this disease, which may be less noticeable and less tangible, is missing teeth, which can also exacerbate other complications in patients with diabetes,” Dr. Rajabi, a biostatistician at Golestan University of Medical Sciences, Gorgan, Iran, continued.
The meta-analysis showed that “physicians should pay attention to [dental health] in the management and control of diabetic patients,” he summarized.
The analysis by Amir Reza Ahmadian, DDS, dean of the Faculty of Dentistry, Golestan University of Medical Sciences, and colleagues was recently published in BMC Endocrine Disorders.
“Our study is the first comprehensive meta-analysis about the association between [type 2 diabetes] and tooth loss,” Dr. Ahmadian and colleagues write. It summarizes articles in dentistry and medicine about “an important question:” the relationship between type 2 diabetes and tooth loss.
Nevertheless, “large-scale prospective studies are needed to validate the current results in the future,” they conclude.
Oral complications of diabetes
Diabetes increases the risk of oral disease directly by a gingival inflammatory response and indirectly by decreased saliva production due to antidiabetic medications.
Oral complications arising from this include dry mouth, tooth decay, and periodontal disease (gum disease). The latter ranges from gingivitis (gum inflammation) to severe periodontal disease (periodontitis) that can lead to tooth loss, the authors explain.
About a third of people with diabetes have severe periodontal disease, and the American Diabetes Association estimates that one in five cases of tooth loss in adults is related to diabetes.
Tooth loss has decreased over the past decades but is still a major health problem and is associated with poorer quality of life as well as risk of cardiovascular disease, hypertension, stroke, and cancer.
Previous studies and meta-analyses of the relationship between type 2 diabetes and tooth loss have reported inconsistent findings, and they did not include several more recent studies.
Therefore, Dr. Ahmadian and colleagues performed a meta-analysis of 13 cross-sectional, six cohort, and three case-control studies that investigated the link between type 2 diabetes and tooth loss published from 2007 to 2021.
Eleven studies were from North and South America: Brazil (2), Columbia (1), Mexico (2), and the United States (6). Seven studies were from Europe: Belgium (1), Finland (2), France (1), Germany (2), and Portugal (1). Four studies were from the Middle East and Asia: Saudi Arabia (1), South Korea (1), Thailand (1), and Yemen (1).
Diabetes was diagnosed based on glucose or A1c levels in half the studies and based on self-report in the other studies. Most studies investigated any tooth loss (16 studies) and the rest only considered loss of five or more teeth.
The meta-analysis included 677,532 patients, ranging from 60 to 379,021 patients per study. Most studies (77%) were judged to be of moderate or high quality.
The studies adjusted for confounders, including age, sex, place of residence, education, lifestyle factors (smoking, alcohol consumption, physical activity), use of medications and vitamin supplements, and health insurance.
Overall, after adjusting for confounders, participants with type 2 diabetes had a significantly (20%) greater risk of tooth loss than participants without diabetes (adjusted odds ratio, 1.20; P < 0.001).
The association persisted in the different study types. The risk of tooth loss was highest in the case-control studies (OR, 5.10), but was also significantly higher in the cohort (OR, 1.29) and cross-sectional studies (OR, 1.15).
The association “was also present in other subgroups, including ... method of diagnosing type 2 diabetes, continent, study quality, and number of tooth loss,” the researchers write.
“This event seems to be in line with what has been reported in other epidemiologic studies, as several cases have supported the link between diabetes, periodontal disease, and tooth decay,” which “are two common reasons for the endpoint of the tooth loss parameter,” they note.
The researchers did not find any publication bias. However, most of the studies were cross-sectional, so they cannot determine a causal relationship between diabetes and tooth loss.
The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Type 2 diabetes was associated with a 20% increased risk of tooth loss after adjusting for multiple other risk factors in a meta-analysis of 22 recent observational studies from around the world.
The risk of tooth loss with type 2 diabetes (versus no diabetes) ranged from 15% higher in cross-sectional studies to 29% higher in cohort studies to five times higher in case-control studies.
“For diabetes, there are various known complications that are considered in [patient] treatment and management, including neuropathy, nephropathy, cardiovascular [disease] and hypertension, and kidney disease,” senior author Abdolhalim Rajabi, PhD, told this news organization in an email.
“However, a chronic complication of this disease, which may be less noticeable and less tangible, is missing teeth, which can also exacerbate other complications in patients with diabetes,” Dr. Rajabi, a biostatistician at Golestan University of Medical Sciences, Gorgan, Iran, continued.
The meta-analysis showed that “physicians should pay attention to [dental health] in the management and control of diabetic patients,” he summarized.
The analysis by Amir Reza Ahmadian, DDS, dean of the Faculty of Dentistry, Golestan University of Medical Sciences, and colleagues was recently published in BMC Endocrine Disorders.
“Our study is the first comprehensive meta-analysis about the association between [type 2 diabetes] and tooth loss,” Dr. Ahmadian and colleagues write. It summarizes articles in dentistry and medicine about “an important question:” the relationship between type 2 diabetes and tooth loss.
Nevertheless, “large-scale prospective studies are needed to validate the current results in the future,” they conclude.
Oral complications of diabetes
Diabetes increases the risk of oral disease directly by a gingival inflammatory response and indirectly by decreased saliva production due to antidiabetic medications.
Oral complications arising from this include dry mouth, tooth decay, and periodontal disease (gum disease). The latter ranges from gingivitis (gum inflammation) to severe periodontal disease (periodontitis) that can lead to tooth loss, the authors explain.
About a third of people with diabetes have severe periodontal disease, and the American Diabetes Association estimates that one in five cases of tooth loss in adults is related to diabetes.
Tooth loss has decreased over the past decades but is still a major health problem and is associated with poorer quality of life as well as risk of cardiovascular disease, hypertension, stroke, and cancer.
Previous studies and meta-analyses of the relationship between type 2 diabetes and tooth loss have reported inconsistent findings, and they did not include several more recent studies.
Therefore, Dr. Ahmadian and colleagues performed a meta-analysis of 13 cross-sectional, six cohort, and three case-control studies that investigated the link between type 2 diabetes and tooth loss published from 2007 to 2021.
Eleven studies were from North and South America: Brazil (2), Columbia (1), Mexico (2), and the United States (6). Seven studies were from Europe: Belgium (1), Finland (2), France (1), Germany (2), and Portugal (1). Four studies were from the Middle East and Asia: Saudi Arabia (1), South Korea (1), Thailand (1), and Yemen (1).
Diabetes was diagnosed based on glucose or A1c levels in half the studies and based on self-report in the other studies. Most studies investigated any tooth loss (16 studies) and the rest only considered loss of five or more teeth.
The meta-analysis included 677,532 patients, ranging from 60 to 379,021 patients per study. Most studies (77%) were judged to be of moderate or high quality.
The studies adjusted for confounders, including age, sex, place of residence, education, lifestyle factors (smoking, alcohol consumption, physical activity), use of medications and vitamin supplements, and health insurance.
Overall, after adjusting for confounders, participants with type 2 diabetes had a significantly (20%) greater risk of tooth loss than participants without diabetes (adjusted odds ratio, 1.20; P < 0.001).
The association persisted in the different study types. The risk of tooth loss was highest in the case-control studies (OR, 5.10), but was also significantly higher in the cohort (OR, 1.29) and cross-sectional studies (OR, 1.15).
The association “was also present in other subgroups, including ... method of diagnosing type 2 diabetes, continent, study quality, and number of tooth loss,” the researchers write.
“This event seems to be in line with what has been reported in other epidemiologic studies, as several cases have supported the link between diabetes, periodontal disease, and tooth decay,” which “are two common reasons for the endpoint of the tooth loss parameter,” they note.
The researchers did not find any publication bias. However, most of the studies were cross-sectional, so they cannot determine a causal relationship between diabetes and tooth loss.
The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Type 2 diabetes was associated with a 20% increased risk of tooth loss after adjusting for multiple other risk factors in a meta-analysis of 22 recent observational studies from around the world.
The risk of tooth loss with type 2 diabetes (versus no diabetes) ranged from 15% higher in cross-sectional studies to 29% higher in cohort studies to five times higher in case-control studies.
“For diabetes, there are various known complications that are considered in [patient] treatment and management, including neuropathy, nephropathy, cardiovascular [disease] and hypertension, and kidney disease,” senior author Abdolhalim Rajabi, PhD, told this news organization in an email.
“However, a chronic complication of this disease, which may be less noticeable and less tangible, is missing teeth, which can also exacerbate other complications in patients with diabetes,” Dr. Rajabi, a biostatistician at Golestan University of Medical Sciences, Gorgan, Iran, continued.
The meta-analysis showed that “physicians should pay attention to [dental health] in the management and control of diabetic patients,” he summarized.
The analysis by Amir Reza Ahmadian, DDS, dean of the Faculty of Dentistry, Golestan University of Medical Sciences, and colleagues was recently published in BMC Endocrine Disorders.
“Our study is the first comprehensive meta-analysis about the association between [type 2 diabetes] and tooth loss,” Dr. Ahmadian and colleagues write. It summarizes articles in dentistry and medicine about “an important question:” the relationship between type 2 diabetes and tooth loss.
Nevertheless, “large-scale prospective studies are needed to validate the current results in the future,” they conclude.
Oral complications of diabetes
Diabetes increases the risk of oral disease directly by a gingival inflammatory response and indirectly by decreased saliva production due to antidiabetic medications.
Oral complications arising from this include dry mouth, tooth decay, and periodontal disease (gum disease). The latter ranges from gingivitis (gum inflammation) to severe periodontal disease (periodontitis) that can lead to tooth loss, the authors explain.
About a third of people with diabetes have severe periodontal disease, and the American Diabetes Association estimates that one in five cases of tooth loss in adults is related to diabetes.
Tooth loss has decreased over the past decades but is still a major health problem and is associated with poorer quality of life as well as risk of cardiovascular disease, hypertension, stroke, and cancer.
Previous studies and meta-analyses of the relationship between type 2 diabetes and tooth loss have reported inconsistent findings, and they did not include several more recent studies.
Therefore, Dr. Ahmadian and colleagues performed a meta-analysis of 13 cross-sectional, six cohort, and three case-control studies that investigated the link between type 2 diabetes and tooth loss published from 2007 to 2021.
Eleven studies were from North and South America: Brazil (2), Columbia (1), Mexico (2), and the United States (6). Seven studies were from Europe: Belgium (1), Finland (2), France (1), Germany (2), and Portugal (1). Four studies were from the Middle East and Asia: Saudi Arabia (1), South Korea (1), Thailand (1), and Yemen (1).
Diabetes was diagnosed based on glucose or A1c levels in half the studies and based on self-report in the other studies. Most studies investigated any tooth loss (16 studies) and the rest only considered loss of five or more teeth.
The meta-analysis included 677,532 patients, ranging from 60 to 379,021 patients per study. Most studies (77%) were judged to be of moderate or high quality.
The studies adjusted for confounders, including age, sex, place of residence, education, lifestyle factors (smoking, alcohol consumption, physical activity), use of medications and vitamin supplements, and health insurance.
Overall, after adjusting for confounders, participants with type 2 diabetes had a significantly (20%) greater risk of tooth loss than participants without diabetes (adjusted odds ratio, 1.20; P < 0.001).
The association persisted in the different study types. The risk of tooth loss was highest in the case-control studies (OR, 5.10), but was also significantly higher in the cohort (OR, 1.29) and cross-sectional studies (OR, 1.15).
The association “was also present in other subgroups, including ... method of diagnosing type 2 diabetes, continent, study quality, and number of tooth loss,” the researchers write.
“This event seems to be in line with what has been reported in other epidemiologic studies, as several cases have supported the link between diabetes, periodontal disease, and tooth decay,” which “are two common reasons for the endpoint of the tooth loss parameter,” they note.
The researchers did not find any publication bias. However, most of the studies were cross-sectional, so they cannot determine a causal relationship between diabetes and tooth loss.
The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM BMJ ENDOCRINE DISORDERS
Recommendations for improving federal diabetes programs: How primary care clinicians can help with implementation
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Screening for diabetes at normal BMIs could cut racial disparities
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
USPSTF recommendation roundup
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2
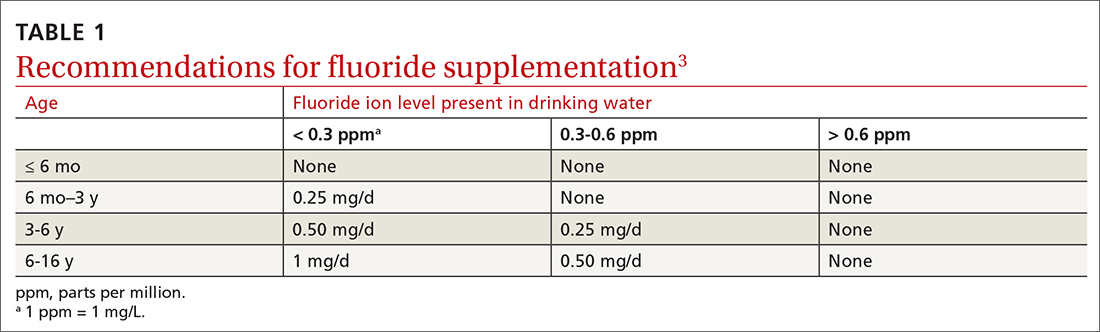
In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.
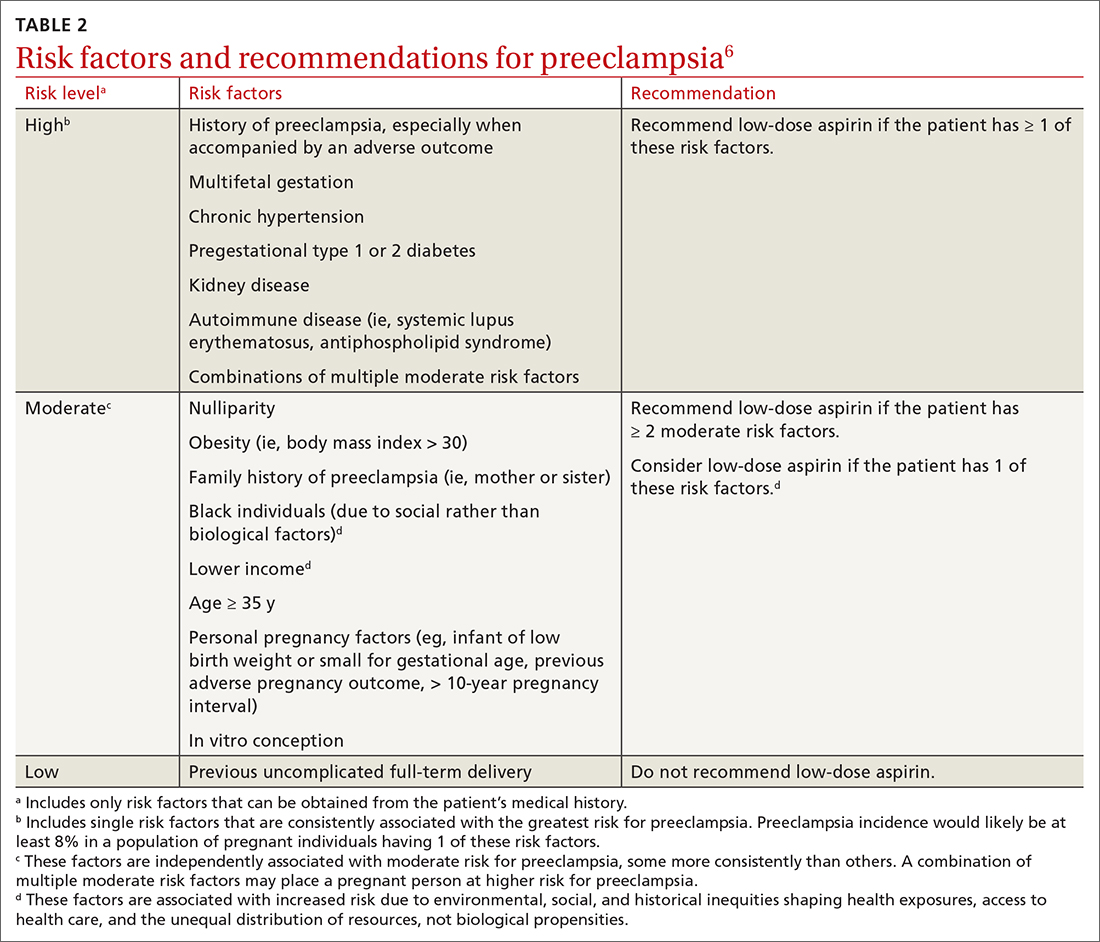
Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11
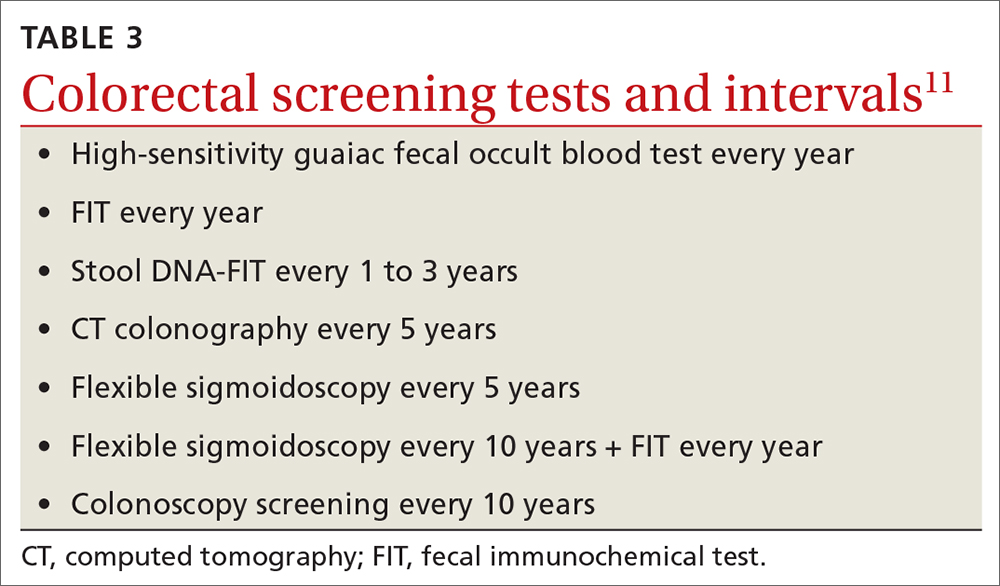
For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1
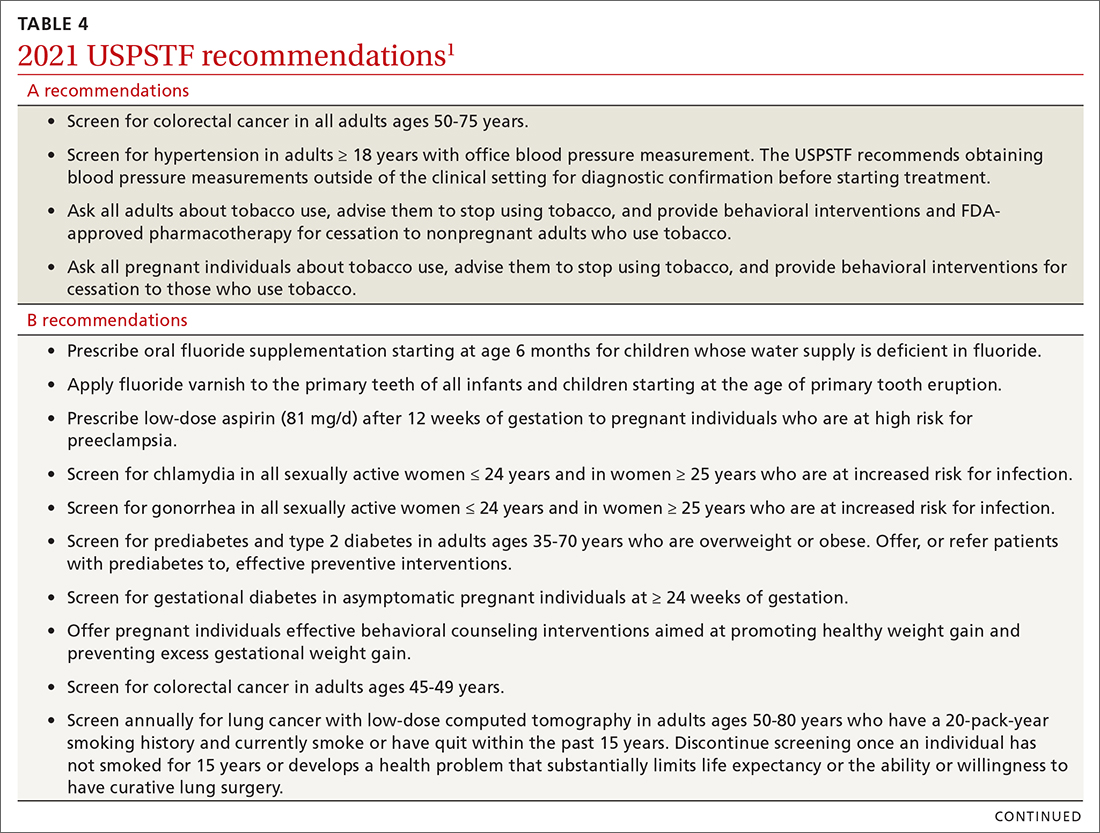
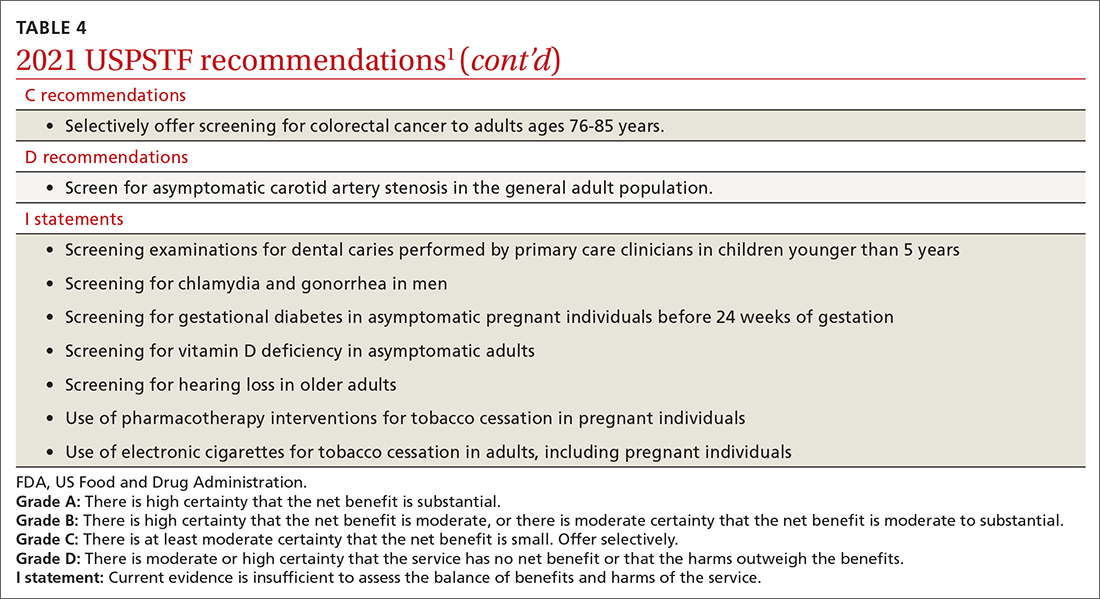
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


In 2021, the US Preventive Services Task Force (USPSTF) considered 13 topics and made a total of 23 recommendations. They reviewed only 1 new topic. The other 12 were updates of topics previously addressed; no changes were made in 9 of them. In 3, the recommended age of screening or the criteria for screening were expanded. This Practice Alert will review the recommendations made and highlight new recommendations and any changes to previous ones. All complete recommendation statements, rationales, clinical considerations, and evidence reports can be found on the USPSTF website at https://uspreventiveservicestaskforce.org/uspstf/home.1
Dental caries in children
Dental caries affect about 23% of children between the ages of 2 and 5 years and are associated with multiple adverse social outcomes and medical conditions.2 The best way to prevent tooth decay, other than regular brushing with fluoride toothpaste, is to drink water with recommended amounts of fluoride (≥ 0.6 parts fluoride per million parts water).2 The USPSTF reaffirmed its recommendation from 2014 that stated when a local water supply lacks sufficient fluoride, primary care clinicians should prescribe oral supplementation for infants and children in the form of fluoride drops starting at age 6 months. The dosage of fluoride depends on patient age and fluoride concentration in the local water (TABLE 13). The USPSTF also recommends applying topical fluoride as 5% sodium fluoride varnish, every 6 months, starting when the primary teeth erupt.2

In addition to fluoride supplements and topical varnish, should clinicians perform screening examinations looking for dental caries? The USPSTF feels there is not enough evidence to assess this practice and gives it an “I” rating (insufficient evidence).
Preventive interventions in pregnancy
In 2021, the USPSTF assessed 3 topics related to pregnancy and prenatal care.
Screening for gestational diabetes. The USPSTF gave a “B” recommendation for screening at 24 weeks of pregnancy or after, but an “I” statement for screening prior to 24 weeks.4 Screening can involve a 1-step or 2-step protocol.
The 2-step protocol is most commonly used in the United States. It involves first measuring serum glucose after a nonfasting 50-g oral glucose challenge; if the resulting level is high, the second step is a 75- or 100-g oral glucose tolerance test lasting 3 hours. The 1-step protocol involves measuring a fasting glucose level, followed by a 75-g oral glucose challenge with glucose levels measured at 1 and 2 hours.
Healthy weight gain in pregnancy. This was the only new topic the USPSTF assessed last year. The resulting recommendation is to offer pregnant women behavioral counseling to promote healthy weight gain and to prevent excessive weight gain in pregnancy. The recommended weight gain depends on the mother’s prepregnancy weight status: 28 to 40 lbs if the mother is underweight; 25 to 35 lbs if she is not under- or overweight; 15 to 25 lbs if she is overweight; and 11 to 20 lbs if she is obese.5 Healthy weight gain contributes to preventing gestational diabetes, emergency cesarean sections, and infant macrosomia.
Continue to: Low-dose aspirin
Low-dose aspirin. Reaffirming a recommendation from 2014, the USPSTF advises low-dose aspirin (81 mg/d) starting after 12 weeks’ gestation for all pregnant women who are at high risk for preeclampsia. TABLE 26 lists high- and moderate-risk conditions for preeclampsia and the recommendation for the use of low-dose aspirin.

Sexually transmitted infections
Screening for both chlamydia and gonorrhea in sexually active females through age 24 years was given a “B” recommendation, reaffirming the 2014 recommendation.7 Screening for these 2 sexually transmitted infections (STIs) is also recommended for women 25 years and older who are at increased risk of STIs. Risk is defined as having a new sex partner, more than 1 sex partner, a sex partner who has other sex partners, or a sex partner who has an STI; not using condoms consistently; having a previous STI; exchanging sex for money or drugs; or having a history of incarceration.
Screen for both infections simultaneously using a nucleic acid amplification test, testing all sites of sexual exposure. Urine testing can replace cervical, vaginal, and urethral testing. Those found to be positive for either STI should be treated according to the most recent treatment guidelines from the Centers for Disease Control and Prevention (CDC). And sexual partners should be advised to undergo testing.8,9
The USPSTF could not find evidence for the benefits and harms of screening for STIs in men. Remember that screening applies to those who are asymptomatic. Male sex partners of those found to be infected should be tested, as should those who show any signs or symptoms of an STI. A recent Practice Alert described the most current CDC guidance for diagnosing and treating STIs.9
Type 2 diabetes and prediabetes
Screening for type 2 diabetes (T2D) and prediabetes is now recommended for adults ages 35 to 70 years who are overweight or obese.10 The age to start screening has been lowered to 35 years from the previous recommendation in 2015, which recommended starting at age 40. In addition, the recommendation states that patients with prediabetes should be referred for preventive interventions. It is important that referral is included in the statement because the Affordable Care Act mandates that USPSTF “A” and “B” recommendations must be covered by commercial health insurance with no copay or deductible.
Continue to: Screening can be conducted...
Screening can be conducted using a fasting plasma glucose or A1C level, or with an oral glucose tolerance test. Interventions that can prevent or delay the onset of T2D in those with prediabetes include lifestyle interventions that focus on diet and physical activity, and the use of metformin (although metformin has not been approved for this by the US Food and Drug Administration).
Changes to cancer screening recommendations
In 2021, the USPSTF reviewed and modified its recommendations on screening for 2 types of cancer: colorectal and lung.
For colorectal cancer, the age at which to start screening was lowered from 50 years to 45 years.11 Screening at this earlier age is a “B” recommendation, because, while there is benefit from screening, it is less than for older age groups. Screening individuals ages 50 to 75 years remains an “A” recommendation, and for those ages 76 to 85 years it remains a “C” recommendation. A “C” recommendation means that the overall benefits are small but some individuals might benefit based on their overall health and prior screening results. In its clinical considerations, the USPSTF recommends against screening in those ages 85 and older but, curiously, does not list it as a “D” recommendation. The screening methods and recommended screening intervals for each appear in TABLE 3.11

For lung cancer, annual screening using low-dose computed tomography (CT) was first recommended by the USPSTF in 2013 for adults ages 55 to 80 years with a 30-pack-year smoking history. Screening could stop once 15 years had passed since smoking cessation. In 2021, the USPSTF lowered the age to initiate screening to 50 years, and the smoking history threshold to 20 pack-years.12 If these recommendations are followed, a current smoker who does not quit smoking could possibly receive 30 annual CT scans. The recommendation does state that screening should stop once a person develops a health condition that significantly affects life expectancy or ability to have lung surgery.
For primary prevention of lung cancer and other chronic diseases through smoking cessation, the USPSTF also reassessed its 2015 recommendations. It reaffirmed the “A” recommendation to ask adults about tobacco use and, for tobacco users, to recommend cessation and provide behavioral therapy and approved pharmacotherapy.13 The recommendation differed for pregnant adults in that the USPSTF is unsure about the potential harms of pharmacotherapy in pregnancy and gives that an “I” statement.13 An additional “I” statement was made about the use of electronic cigarettes for smoking cessation; the USPSTF recommends using behavioral and pharmacotherapy interventions with proven effectiveness and safety instead.
Continue to: 4 additional recommendation updates with no changes
4 additional recommendation updates with no changes
Screening for high blood pressure in adults ages 18 years and older continues to receive an “A” recommendation.14 Importantly, the recommendation states that confirmation of high blood pressure should be made in an out-of-office setting before initiating treatment. Screening for vitamin D deficiency in adults and hearing loss in older adults both continue with “I” statements,15,16 and screening for asymptomatic carotid artery stenosis continues to receive a “D” recommendation.17 The implications of the vitamin D “I” statement were discussed in a previous Practice Alert.18
Continuing value of the USPSTF
The USPSTF continues to set the gold standard for assessment of preventive interventions, and its decisions affect first-dollar coverage by commercial health insurance. The reaffirmation of past recommendations demonstrates the value of adhering to rigorous evidence-based methods (if they are done correctly, they rarely must be markedly changed). And the updating of screening criteria shows the need to constantly review the evolving evidence for current recommendations. Once again, however, funding and staffing limitations allowed the USPSTF to assess only 1 new topic. A listing of all the 2021 recommendations is in TABLE 4.1


1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.
1. USPSTF. Recommendation topics. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation-topics
2. USPSTF. Prevention of dental caries in children younger than 5 years: screening and interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-dental-caries-in-children-younger-than-age-5-years-screening-and-interventions1#bootstrap-panel—4
3. ADA. Dietary fluoride supplements: evidence-based clinical recommendations. Accessed April 14, 2022. www.ada.org/-/media/project/ada-organization/ada/ada-org/files/resources/research/ada_evidence-based_fluoride_supplement_chairside_guide.pdf?rev=60850dca0dcc41038efda83d42b1c2e0&hash=FEC2BBEA0C892FB12C098E33344E48B4
4. USPSTF. Gestational diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/gestational-diabetes-screening
5. USPSTF. Healthy weight and weight gain in pregnancy: behavioral counseling interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-weight-and-weight-gain-during-pregnancy-behavioral-counseling-interventions
6. USPSTF. Aspirin use to prevent preeclampsia and related morbidity and mortality: preventive medication. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication
7. USPSTF. Chlamydia and gonorrhea: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/chlamydia-and-gonorrhea-screening
8. Workowski KA, Bauchman LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
9. Campos-Outcalt D. CDC guidelines on sexually transmitted infections. J Fam Pract. 2021;70:506-509.
10. USPSTF. Prediabetes and type 2 diabetes: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-for-prediabetes-and-type-2-diabetes
11. USPSTF. Colorectal cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
12. USPSTF. Lung cancer: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
13. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
14. USPSTF. Hypertension in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hypertension-in-adults-screening
15. USPSTF. Vitamin D deficiency in adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-deficiency-screening
16. USPSTF. Hearing loss in older adults: screening. Accessed April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hearing-loss-in-older-adults-screening
17. USPSTF. Asymptomatic carotid artery stenosis: screening. Access April 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
18. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292.