User login
2021 in Review: Key Trials in Type 2 Diabetes (T2D)
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Full-press therapy rare in diabetes with ASCVD
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
FROM JAMA OPEN NETWORK
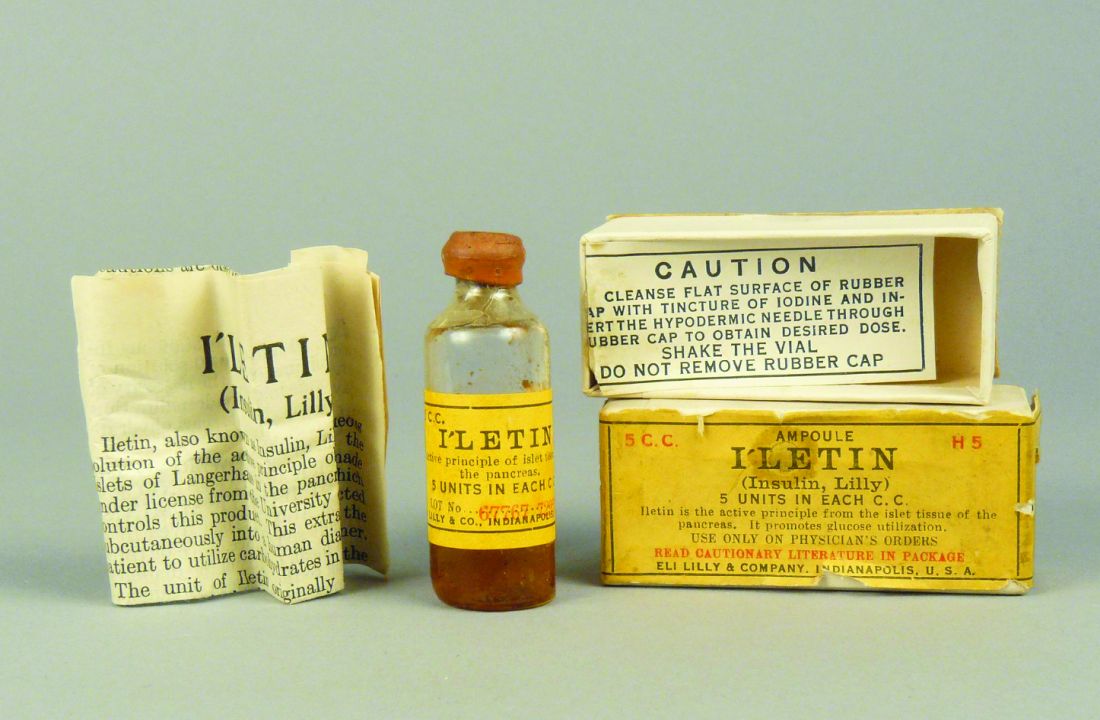
The battle of egos behind the life-saving discovery of insulin
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
PCOS common in adolescent girls with type 2 diabetes
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
Polycystic ovary syndrome is common in girls with type 2 diabetes, findings of a new study suggest, and authors say screening for PCOS is critical in this group.
In a systematic review and meta-analysis involving 470 girls (average age 12.9-16.1 years) with type 2 diabetes in six studies, the prevalence of PCOS was nearly 1 in 5 (19.58%; 95% confidence interval, 12.02%-27.14%; P = .002), substantially higher than that of PCOS in the general adolescent population.
PCOS, a complex endocrine disorder, occurs in 1.14%-11.04% of adolescent girls globally, according to the paper published online in JAMA Network Open.
The secondary outcome studied links to prevalence of PCOS with race and obesity.
Insulin resistance and compensatory hyperinsulinemia are present in 44%-70% of women with PCOS, suggesting that they are more likely to develop type 2 diabetes, according to the researchers led by Milena Cioana, BHSc, with the department of pediatrics, McMaster University, Hamilton, Ont.
Kelly A. Curran, MD, an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center in Oklahoma City, where she practices adolescent medicine, said in an interview that it has been known that women with PCOS have higher rates of diabetes and many in the field have suspected the relationship is bidirectional.
“In my clinical practice, I’ve seen a high percentage of women with type 2 diabetes present with irregular menses, some of whom have gone on to be diagnosed with PCOS,” said Dr. Curran, who was not involved with the study.
However, she said, she was surprised the prevalence of PCOS reported in this paper – nearly one in five – was so high. Early diagnosis is important for PCOS to prevent complications such as hypertension, hyperglycemia, and dyslipidemia.
Psychiatric conditions are also prevalent in patients with PCOS, including anxiety (18%), depression (16%), and ADHD (9%).
Dr. Curran agreed there is a need to screen for PCOS and to evaluate for other causes of irregular periods in patients with type 2 diabetes.
“Menstrual irregularities are often overlooked in young women without further work-up, especially in patients who have chronic illnesses,” she noted.
Results come with a caveat
However, the authors said, results should be viewed with caution because “studies including the larger numbers of girls did not report the criteria used to diagnose PCOS, which is a challenge during adolescence.”
Diagnostic criteria for PCOS during adolescence include the combination of menstrual irregularities according to time since their first period and clinical or biochemical hyperandrogenism after excluding other potential causes.
Dr. Curran explained that PCOS symptoms include irregular periods and acne which can overlap with normal changes in puberty. In her experience, PCOS is often diagnosed without patients meeting full criteria. She agreed further research with standardized criteria is urgently needed.
The European Society of Human Reproduction and Embryology/American Society of Reproductive Medicine, the Pediatric Endocrine Society, and the International Consortium of Paediatric Endocrinology guidelines suggest that using ultrasound to check the size of ovaries could help diagnose PCOS, but other guidelines are more conservative, the authors noted.
They added that “there is a need for a consensus to establish the pediatric criteria for diagnosing PCOS in adolescents to ensure accurate diagnosis and lower the misclassification rates.”
Assessing links to obesity and race
Still unclear, the authors wrote, is whether and how obesity and race affect prevalence of PCOS among girls with type 2 diabetes.
The authors wrote: “Although earlier studies suggested that obesity-related insulin resistance and hyperinsulinemia can contribute to PCOS pathogenesis, insulin resistance in patients with PCOS may be present independently of [body mass index]. Obesity seems to increase the risk of PCOS only slightly and might represent a referral bias for PCOS.”
Few studies included in the meta-analysis had race-specific data, so the authors were limited in assessing associations between race and PCOS prevalence.
“However,” they wrote, “our data demonstrate that Indian girls had the highest prevalence, followed by White girls, and then Indigenous girls in Canada.”
Further studies are needed to help define at-risk subgroups and evaluate treatment strategies, the authors noted.
They reported having no relevant financial relationships. Dr. Curran had no conflicts of interest.
FROM JAMA NETWORK OPEN
Fewer diabetes complications with NOACs in patients with AFib
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
The new research, which was published in Annals of Internal Medicine, found that taking non–vitamin K oral anticoagulants was associated with reduced diabetes complications and lower mortality vs. taking warfarin in the group examined.
In their paper, the researchers present the outcomes of a retrospective cohort study involving 30,209 patients with atrial fibrillation and diabetes. Of these, 19,909 were treated with non–vitamin K oral anticoagulants (NOACs) – dabigatran, rivaroxaban, apixaban, or edoxaban – and 10,300 were treated with warfarin.
Dr. Huei-Kai Huang from the Hualien (Taiwan) Tzu Chi Hospital and coauthors wrote that, while diabetes mellitus is an important risk factor for stroke, there’s not yet a good understanding of the effect of different oral anticoagulants on the risk for diabetes-related complications in patients with atrial fibrillation and diabetes.
“Recent evidence has suggested that NOAC and warfarin may have different effects on glycemic control through the vitamin K–related mechanisms,” coauthor Yu-Kang Tu, PhD, from the College of Public Health at the National Taiwan University in Taipei said in an interview. “It was therefore natural to further evaluate whether NOAC could help decrease various diabetes-related complications, compared with warfarin.”
Hazards with NOACS vs. warfarin
The researchers found that patients treated with NOACs had a 16% lower hazard of macrovascular complications – a composite of coronary artery disease, stroke, and peripheral vascular disease (95% confidence interval, 0.78-0.91; P < .001) – and a 21% lower hazard of microvascular complications including dialysis and lower-extremity amputations (95% CI, 0.73-0.85; P < .001).
NOAC therapy was also associated a 22% lower hazard of death (95% CI, 0.75-0.82; P < .001) and a 9% lower hazard for glycemic emergency (95% CI, 0.83-0.99; P = .043), which the authors defined as a composite of diabetic ketoacidosis, hyperosmolar hyperglycemic state, and hypoglycemia.
In particular, patients treated with NOACs showed significantly lower hazards for coronary artery disease, stroke, dialysis, amputation of lower extremities, and death from cardiovascular and noncardiovascular causes, compared with warfarin users.
The study also found that patients on higher volumes of NOAC medication had greater reductions in mortality and diabetes complications.
“Although our main findings can be explained by the potential differences in underlying mechanisms of action between NOAC and warfarin, we were still surprised with the significantly lower risks of retinopathy, neuropathy, and hypoglycemia in patients taking NOAC with high medication possession ratio,” Dr. Tu said.
Study provides more diabetes-specific outcomes data
Commenting on the findings, Dr. Peter Rossing, head of complications research at the Steno Diabetes Center in Copenhagen said there has long been discussion about whether the newer and more expensive NOACs offer greater benefits to patient with diabetes – beyond stroke prevention – compared with the older and cheaper warfarin. As such, this study was important in providing more diabetes-specific outcomes data and in a large population.
“The effect size they find is certainly meaningful and relevant and should support decision-making,” Dr. Rossing noted in an interview. The finding of reduced risk of amputation and mortality “fits in line with theory that maybe if you block vitamin K, you get calcification, you get vascular damage that leads to failure of the kidney and leads to limb amputations, and that is potentially prevented or not developed when you give the NOACs.”
Dr. John Camm, professor of clinical cardiology at St George’s University of London, said the findings of the benefits of NOACs in this patient group ,were confirmation of earlier, smaller studies, and were important not just for patients with atrial fibrillation and diabetes, but also those prone to diabetes.
“We know from previous studies from the same database, and also from Korea, [for example], that patients who are treated with NOACs as opposed to warfarin develop less diabetes,” he explained.
Dr. Camm said many guidelines around the world now suggest NOACs, and, in some cases, even advise against using vitamin K antagonists as a first option, except in certain situations, such as when patients have rheumatic heart disease, mild to moderate mitral stenosis in rheumatic disease, or prosthetic heart valves.
The researchers applied two methods to account for covariates that may have influenced whether patients received one class of treatment or the other. These achieved ‘appropriate balance’ of baseline characteristics such as comorbidities and baseline medication use for diabetes and other conditions, Dr. Tu and colleagues wrote.
The benefits of NOACs were less evident in younger patients, and the reductions in mortality and diabetes complications associated with NOACs did not reach statistical significance in those aged under 65 years. Regarding this, Dr. Camm noted that there was a debate as to whether patients under 65 years with atrial fibrillation and diabetes should be put on an anticoagulant.
The study was funded by Hualien Tzu Chi Hospital. No conflicts of interest were declared.
FROM ANNALS OF INTERNAL MEDICINE
Sports experts on T2D: Boost activity, cut sedentary time
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
The American College of Sports Medicine (ACSM) has issued new recommendations for exercise/physical activity in people with type 2 diabetes, which update a 2010 joint ACSM/American Diabetes Association position statement.
The guidance has been published in the February issue of Medicine & Science in Sports & Exercise.
“This consensus statement provides a brief summary of the current evidence and extends and updates the prior recommendations,” the authors explain.
In the past decade, there has been a “considerable amount” of research about exercise in people with type 2 diabetes, they add, while the prevalence of diabetes has steadily increased.
The updated recommendations have been “expanded to include physical activity – a broader, more comprehensive definition of human movement than planned exercise – and reducing sedentary time,” the authors note.
“The latest guidelines are applicable to most individuals with diabetes, including youth, with a few exceptions and modifications,” lead author Jill A. Kanaley, PhD, said in a press release from the ACSM.
The key takeaway is that “all individuals [with type 2 diabetes] should engage in regular physical activity, reduce sedentary time, and break up sitting time with frequent activity breaks,” said Dr. Kanaley, a professor in the department of nutrition and exercise physiology, University of Missouri, Columbia.
“Exercise can play an important role in managing type 2 diabetes, and workouts can be modified to fit the abilities of most people,” she stressed.
And those with type 2 diabetes who want to lose weight “should consider workouts of moderately high volume for 4 to 5 days per week,” she added.
Six key tips for physical activity in adults with type 2 diabetes
The consensus statement gives six key tips for physical activity in adults with type 2 diabetes, as follows.
- Regular aerobic exercise improves glycemic management; meta-analyses have reported fewer daily hyperglycemic episodes and reductions in A1c of 0.5%-0.7%.
- High-intensity resistance exercise, when performed safely, is better than low-to-moderate intensity resistance exercise for glucose management and attenuation of insulin levels. Resistance exercise typically results in improvements of 10% to 15% in strength, bone mineral density, blood pressure, lipid profile, skeletal muscle mass, and insulin sensitivity.
- Exercise after meals, such as taking a walk after dinner at one’s own pace, takes advantage of the blood glucose-stabilizing effects of exercise.
- Reduce sedentary time by taking regular breaks for small “doses” of physical activity, which can modestly attenuate postprandial glucose and insulin levels, particularly in individuals with insulin resistance and a higher body mass index.
- To prevent hypoglycemia during or after exercise, people taking insulin or insulin secretagogues should increase carbohydrate intake, or if possible, reduce insulin.
- People who are taking beta blockers should not rely on a heart monitor to measure workout intensity. They could ask a certified exercise professional about using ratings of perceived exertion to track how a workout feels.
Other recommendations
The consensus statement also summarizes precautions that people with complications of type 2 diabetes (such as neuropathy, retinopathy, kidney disease, and hypertension) should take.
Low impact exercises for flexibility can help introduce sedentary people to physical activity, the consensus group writes. Balance exercises can be helpful for older adults.
Weight loss greater than 5% can benefit A1c, blood lipid, and blood pressure levels. Moderate exercise 4 to 5 days a week can reduce visceral fat.
In studies of youth with type 2 diabetes, intensive lifestyle interventions plus metformin were not superior to metformin alone for managing glycemia. Physical activity goals are the same for youth with or without diabetes.
Pregnant women with diabetes should participate in at least 20 to 30 minutes of moderate-intensity exercise most days of the week.
Participating in an exercise program before and after bariatric surgery may enhance surgical outcomes.
Dr. Kanaley has reported receiving a grant from the National Institutes of Health. Disclosures for the other authors are listed in the article.
A version of this article first appeared on Medscape.com.
FDA okays 6-month implanted Eversense CGM for diabetes
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a new second-generation version of the implanted continuous glucose monitoring (CGM) system Eversense (Senseonics) that lasts for 6 months.
The Eversense E3 CGM system doubles the wear time from 3 months with the previous Eversense device approved in the United States in 2018. As before, the new system is approved for adults with diabetes aged 18 years and older.
This means that it will be the longest lasting CGM system available in the United States, with essentially two sensor insertion and removal procedures per year, the company said.
Data from the pivotal PROMISE trial of the 6-month version were presented at the American Diabetes Association Scientific Sessions in 2021, as reported by this news organization.
An older 6-month wear time version (Eversense XL) has been available in Europe since 2017. The new second-generation 6-month system is currently under regulatory review there.
The PROMISE trial included 181 participants with diabetes, about two-thirds with type 1 and one-third with type 2 diabetes, at eight clinical research sites.
“We repeatedly hear from our patients with diabetes that what they desire is a long-lasting sensor that is also highly accurate ... The next generation Eversense E3 System delivers on both,” said PROMISE study principal investigator Satish Garg, MD, professor of medicine and director of the adult diabetes program at the Barbara Davis Center, University of Colorado, Aurora, in a company press release.
The Eversense E3 consists of a fluorescence-based sensor, a transmitter, and a smartphone app that displays glucose values, trends, and alerts. The sensor is inserted subcutaneously into the upper arm by a certified health care professional in a brief office procedure. The transmitter is placed on the skin on top of the sensor. Glucose data are sent to the app automatically every 5 minutes.
The system includes an on-body vibratory alert as well as alerts on the app for high and low blood glucose values. Eversense readings may be used for treatment decisions, but users still must perform fingerstick glucose checks for calibration.
The regulatory review for the Eversense E3 was delayed for a year due to the COVID-19 pandemic. It will be distributed in the United States through a partnership with Ascensia Diabetes Care beginning in the second quarter of 2022, according to a Senseonics statement.
In addition, “the company expects the majority of its expenses for 2022 to be for research and development for ongoing feasibility and pivotal clinical trials for additional products in its product pipeline, including the start of its 365-day pivotal trial.”
Health care providers who want to offer the Eversense CGM System to their patients can sign up here or call 844-SENSE4U (844-736-7348).
Patients interested in getting started on Eversense can sign up here and will be among the first to know when Eversense E3 is commercially available.
A version of this article first appeared on Medscape.com.
Do latest SURPASS findings with twincretin in diabetes impress?
, new research shows.
The novel once-weekly injectable agent is nicknamed a twincretin because it combines two different gut-hormone activities. It works both as a glucagonlike peptide-1 (GLP-1) receptor agonist and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
Findings from the randomized phase 3 SURPASS-5 clinical trial were published online Feb. 8 in JAMA.
This is the latest in a series of SURPASS trials of tirzepatide in individuals with type 2 diabetes for which results have been presented at various conferences, announced by the company, and/or published since late 2020.
SURPASS-5 specifically investigated the effect on glycemic control of adding three different doses of once-weekly subcutaneous tirzepatide compared with placebo in 475 adults who hadn’t achieved target A1c levels using insulin glargine with or without metformin. Statistically significant reductions in A1c were found at 40 weeks for all three doses.
Moreover, authors Dominik Dahl, MD, group practice for internal medicine and diabetology, Hamburg, Germany, and colleagues note that the improvements in the tirzepatide groups “were associated with significantly lower insulin glargine use and significant bodyweight reduction compared with placebo.”
“Despite the differences in glycemic control between the tirzepatide and placebo groups, the rate of clinically significant or severe hypoglycemia was below one event per patient-year in all treatment groups,” they add.
However, concerns about the study protocol and generalizability were raised in an accompanying editorial by Stuart R. Chipkin, MD, of the School of Public Health & Health Sciences, University of Massachusetts Amherst.
“Importantly, the study did not compare tirzepatide with other treatments that could have been used to target the postprandial glycemic pattern of the study population,” he writes.
And ultimately, he says: “Even though the results of this investigation are important for demonstrating the potential clinical benefit of [tirzepatide], and may help to advance the goal of achieving U.S. Food and Drug Administration approval, the study may leave clinicians uncertain about when and how to best use tirzepatide to improve clinical outcomes for patients with type 2 diabetes.”
Significant A1c, weight reductions when added to insulin glargine
The randomized, phase 3 SURPASS-5 trial was conducted at 45 centers in eight countries between August 2019 and January 2021. The 475 adult participants had type 2 diabetes inadequately controlled (baseline A1c, 7.0%-10.5%) with once-daily insulin glargine, with or without metformin. They were randomized to receive once-weekly subcutaneous injections of tirzepatide in doses of 5 mg, 10 mg, or 15 mg, or volume-matched placebo injections over 40 weeks.
The mean changes from baseline in A1c at week 40, the primary study endpoint, were –2.11, –2.40, and –2.34 percentage points for the 5 mg, 10 mg, and 15 mg doses of tirzepatide, respectively (P < .001), versus a nonsignificant change of –0.86 percentage points with placebo. The differences from placebo at week 40 were also significant for the 10-mg and 15-mg doses (both P < .001).
Significantly higher proportions of patients receiving 5 mg, 10 mg, and 15 mg tirzepatide met the A1c target of less than 7% at week 40, compared with placebo (85%-90% vs. 34%; P < .001). Significantly higher proportions of patients in the 10-mg and 15-mg dose groups also achieved A1c less than 5.7% (42% and 50%, respectively, vs. 3%).
Mean fasting glucose was also reduced significantly with all doses of tirzepatide by 58.2 mg/dL, 64.0 mg/dL, and 62.6 mg/dL, respectively, versus 39.2 mg/dL with placebo (all P <0.001 vs. placebo).
At week 40, mean body weight reductions from baseline were 5.4 kg (11.9 lbs), 7.5 kg, and 8.8 kg versus just 1.6 kg with placebo (all P <0.001 vs. placebo).
All three tirzepatide doses were also associated with significant improvements from baseline in total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and triglycerides.
Gastrointestinal adverse events, hypoglycemia seen in minority
The most common treatment-emergent adverse events in the tirzepatide groups versus placebo were gastrointestinal, including diarrhea (12%-21% vs. 10%), nausea (13%-18% vs. 2.5%), vomiting (7%-13% vs. 2.5%), and decreased appetite (7%-14% vs. 1.7%). Most of these adverse events were mild to moderate in intensity and decreased over time in the tirzepatide groups.
There were no deaths in the study. Serious adverse events were reported by 8%-11% in the tirzepatide groups, compared with 8% in the placebo group. Drug discontinuation due to adverse events occurred in 6.0%, 8.4%, and 10.8% of the 5-mg, 10-mg, and 15-mg dose groups, respectively, versus 2.5% in the placebo group.
Rates of hypoglycemia (less than or equal to 70 mg/dL) ranged from 14.2% to 19.3% with tirzepatide versus 12.5% with placebo. There were three episodes of severe hypoglycemia (less than 54 mg/dL), two with 10 mg tirzepatide and one with 15 mg tirzepatide.
Editorial raises questions
In his editorial, Dr. Chipkin writes that the study “demonstrated that use of tirzepatide was associated with significant reductions in A1c and weight in a fairly homogeneous cohort of patients with type 2 diabetes who were receiving insulin glargine with or without metformin.”
“The protocol answered questions about efficacy but left open questions about generalizability and effectiveness in different populations, especially patients with certain complications or comorbid chronic diseases.” He also notes that younger adults and Black patients were not well-represented.
And the study didn’t allow for dividing up the glargine dose or for adding short-acting insulin before meals or any other pre-meal medications and “thus may represent a departure from usual care” in the setting of rising glucose levels.
The authors themselves acknowledge that “the postprandial glucose excursions observed in the placebo group suggest an additional prandial intervention was likely needed in some patients, despite the strict inclusion criteria and the treat-to-target-approach used in the study.”
Dr. Chipkin concludes that “although patients are likely to embrace a medication with weight loss outcomes, the protocol also leaves unanswered questions about reducing insulin and evaluating the comparative risk of adverse effects.”
The study was sponsored by Eli Lilly. Dr. Dahl has reported receiving personal fees from Eli Lilly during the conduct of the study and personal fees from Afimmune, Novo Nordisk, and Novartis outside the submitted work. Dr. Chipkin has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
The novel once-weekly injectable agent is nicknamed a twincretin because it combines two different gut-hormone activities. It works both as a glucagonlike peptide-1 (GLP-1) receptor agonist and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
Findings from the randomized phase 3 SURPASS-5 clinical trial were published online Feb. 8 in JAMA.
This is the latest in a series of SURPASS trials of tirzepatide in individuals with type 2 diabetes for which results have been presented at various conferences, announced by the company, and/or published since late 2020.
SURPASS-5 specifically investigated the effect on glycemic control of adding three different doses of once-weekly subcutaneous tirzepatide compared with placebo in 475 adults who hadn’t achieved target A1c levels using insulin glargine with or without metformin. Statistically significant reductions in A1c were found at 40 weeks for all three doses.
Moreover, authors Dominik Dahl, MD, group practice for internal medicine and diabetology, Hamburg, Germany, and colleagues note that the improvements in the tirzepatide groups “were associated with significantly lower insulin glargine use and significant bodyweight reduction compared with placebo.”
“Despite the differences in glycemic control between the tirzepatide and placebo groups, the rate of clinically significant or severe hypoglycemia was below one event per patient-year in all treatment groups,” they add.
However, concerns about the study protocol and generalizability were raised in an accompanying editorial by Stuart R. Chipkin, MD, of the School of Public Health & Health Sciences, University of Massachusetts Amherst.
“Importantly, the study did not compare tirzepatide with other treatments that could have been used to target the postprandial glycemic pattern of the study population,” he writes.
And ultimately, he says: “Even though the results of this investigation are important for demonstrating the potential clinical benefit of [tirzepatide], and may help to advance the goal of achieving U.S. Food and Drug Administration approval, the study may leave clinicians uncertain about when and how to best use tirzepatide to improve clinical outcomes for patients with type 2 diabetes.”
Significant A1c, weight reductions when added to insulin glargine
The randomized, phase 3 SURPASS-5 trial was conducted at 45 centers in eight countries between August 2019 and January 2021. The 475 adult participants had type 2 diabetes inadequately controlled (baseline A1c, 7.0%-10.5%) with once-daily insulin glargine, with or without metformin. They were randomized to receive once-weekly subcutaneous injections of tirzepatide in doses of 5 mg, 10 mg, or 15 mg, or volume-matched placebo injections over 40 weeks.
The mean changes from baseline in A1c at week 40, the primary study endpoint, were –2.11, –2.40, and –2.34 percentage points for the 5 mg, 10 mg, and 15 mg doses of tirzepatide, respectively (P < .001), versus a nonsignificant change of –0.86 percentage points with placebo. The differences from placebo at week 40 were also significant for the 10-mg and 15-mg doses (both P < .001).
Significantly higher proportions of patients receiving 5 mg, 10 mg, and 15 mg tirzepatide met the A1c target of less than 7% at week 40, compared with placebo (85%-90% vs. 34%; P < .001). Significantly higher proportions of patients in the 10-mg and 15-mg dose groups also achieved A1c less than 5.7% (42% and 50%, respectively, vs. 3%).
Mean fasting glucose was also reduced significantly with all doses of tirzepatide by 58.2 mg/dL, 64.0 mg/dL, and 62.6 mg/dL, respectively, versus 39.2 mg/dL with placebo (all P <0.001 vs. placebo).
At week 40, mean body weight reductions from baseline were 5.4 kg (11.9 lbs), 7.5 kg, and 8.8 kg versus just 1.6 kg with placebo (all P <0.001 vs. placebo).
All three tirzepatide doses were also associated with significant improvements from baseline in total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and triglycerides.
Gastrointestinal adverse events, hypoglycemia seen in minority
The most common treatment-emergent adverse events in the tirzepatide groups versus placebo were gastrointestinal, including diarrhea (12%-21% vs. 10%), nausea (13%-18% vs. 2.5%), vomiting (7%-13% vs. 2.5%), and decreased appetite (7%-14% vs. 1.7%). Most of these adverse events were mild to moderate in intensity and decreased over time in the tirzepatide groups.
There were no deaths in the study. Serious adverse events were reported by 8%-11% in the tirzepatide groups, compared with 8% in the placebo group. Drug discontinuation due to adverse events occurred in 6.0%, 8.4%, and 10.8% of the 5-mg, 10-mg, and 15-mg dose groups, respectively, versus 2.5% in the placebo group.
Rates of hypoglycemia (less than or equal to 70 mg/dL) ranged from 14.2% to 19.3% with tirzepatide versus 12.5% with placebo. There were three episodes of severe hypoglycemia (less than 54 mg/dL), two with 10 mg tirzepatide and one with 15 mg tirzepatide.
Editorial raises questions
In his editorial, Dr. Chipkin writes that the study “demonstrated that use of tirzepatide was associated with significant reductions in A1c and weight in a fairly homogeneous cohort of patients with type 2 diabetes who were receiving insulin glargine with or without metformin.”
“The protocol answered questions about efficacy but left open questions about generalizability and effectiveness in different populations, especially patients with certain complications or comorbid chronic diseases.” He also notes that younger adults and Black patients were not well-represented.
And the study didn’t allow for dividing up the glargine dose or for adding short-acting insulin before meals or any other pre-meal medications and “thus may represent a departure from usual care” in the setting of rising glucose levels.
The authors themselves acknowledge that “the postprandial glucose excursions observed in the placebo group suggest an additional prandial intervention was likely needed in some patients, despite the strict inclusion criteria and the treat-to-target-approach used in the study.”
Dr. Chipkin concludes that “although patients are likely to embrace a medication with weight loss outcomes, the protocol also leaves unanswered questions about reducing insulin and evaluating the comparative risk of adverse effects.”
The study was sponsored by Eli Lilly. Dr. Dahl has reported receiving personal fees from Eli Lilly during the conduct of the study and personal fees from Afimmune, Novo Nordisk, and Novartis outside the submitted work. Dr. Chipkin has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
The novel once-weekly injectable agent is nicknamed a twincretin because it combines two different gut-hormone activities. It works both as a glucagonlike peptide-1 (GLP-1) receptor agonist and as an agent that mimics the glucose-dependent insulinotropic polypeptide (GIP).
Findings from the randomized phase 3 SURPASS-5 clinical trial were published online Feb. 8 in JAMA.
This is the latest in a series of SURPASS trials of tirzepatide in individuals with type 2 diabetes for which results have been presented at various conferences, announced by the company, and/or published since late 2020.
SURPASS-5 specifically investigated the effect on glycemic control of adding three different doses of once-weekly subcutaneous tirzepatide compared with placebo in 475 adults who hadn’t achieved target A1c levels using insulin glargine with or without metformin. Statistically significant reductions in A1c were found at 40 weeks for all three doses.
Moreover, authors Dominik Dahl, MD, group practice for internal medicine and diabetology, Hamburg, Germany, and colleagues note that the improvements in the tirzepatide groups “were associated with significantly lower insulin glargine use and significant bodyweight reduction compared with placebo.”
“Despite the differences in glycemic control between the tirzepatide and placebo groups, the rate of clinically significant or severe hypoglycemia was below one event per patient-year in all treatment groups,” they add.
However, concerns about the study protocol and generalizability were raised in an accompanying editorial by Stuart R. Chipkin, MD, of the School of Public Health & Health Sciences, University of Massachusetts Amherst.
“Importantly, the study did not compare tirzepatide with other treatments that could have been used to target the postprandial glycemic pattern of the study population,” he writes.
And ultimately, he says: “Even though the results of this investigation are important for demonstrating the potential clinical benefit of [tirzepatide], and may help to advance the goal of achieving U.S. Food and Drug Administration approval, the study may leave clinicians uncertain about when and how to best use tirzepatide to improve clinical outcomes for patients with type 2 diabetes.”
Significant A1c, weight reductions when added to insulin glargine
The randomized, phase 3 SURPASS-5 trial was conducted at 45 centers in eight countries between August 2019 and January 2021. The 475 adult participants had type 2 diabetes inadequately controlled (baseline A1c, 7.0%-10.5%) with once-daily insulin glargine, with or without metformin. They were randomized to receive once-weekly subcutaneous injections of tirzepatide in doses of 5 mg, 10 mg, or 15 mg, or volume-matched placebo injections over 40 weeks.
The mean changes from baseline in A1c at week 40, the primary study endpoint, were –2.11, –2.40, and –2.34 percentage points for the 5 mg, 10 mg, and 15 mg doses of tirzepatide, respectively (P < .001), versus a nonsignificant change of –0.86 percentage points with placebo. The differences from placebo at week 40 were also significant for the 10-mg and 15-mg doses (both P < .001).
Significantly higher proportions of patients receiving 5 mg, 10 mg, and 15 mg tirzepatide met the A1c target of less than 7% at week 40, compared with placebo (85%-90% vs. 34%; P < .001). Significantly higher proportions of patients in the 10-mg and 15-mg dose groups also achieved A1c less than 5.7% (42% and 50%, respectively, vs. 3%).
Mean fasting glucose was also reduced significantly with all doses of tirzepatide by 58.2 mg/dL, 64.0 mg/dL, and 62.6 mg/dL, respectively, versus 39.2 mg/dL with placebo (all P <0.001 vs. placebo).
At week 40, mean body weight reductions from baseline were 5.4 kg (11.9 lbs), 7.5 kg, and 8.8 kg versus just 1.6 kg with placebo (all P <0.001 vs. placebo).
All three tirzepatide doses were also associated with significant improvements from baseline in total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and triglycerides.
Gastrointestinal adverse events, hypoglycemia seen in minority
The most common treatment-emergent adverse events in the tirzepatide groups versus placebo were gastrointestinal, including diarrhea (12%-21% vs. 10%), nausea (13%-18% vs. 2.5%), vomiting (7%-13% vs. 2.5%), and decreased appetite (7%-14% vs. 1.7%). Most of these adverse events were mild to moderate in intensity and decreased over time in the tirzepatide groups.
There were no deaths in the study. Serious adverse events were reported by 8%-11% in the tirzepatide groups, compared with 8% in the placebo group. Drug discontinuation due to adverse events occurred in 6.0%, 8.4%, and 10.8% of the 5-mg, 10-mg, and 15-mg dose groups, respectively, versus 2.5% in the placebo group.
Rates of hypoglycemia (less than or equal to 70 mg/dL) ranged from 14.2% to 19.3% with tirzepatide versus 12.5% with placebo. There were three episodes of severe hypoglycemia (less than 54 mg/dL), two with 10 mg tirzepatide and one with 15 mg tirzepatide.
Editorial raises questions
In his editorial, Dr. Chipkin writes that the study “demonstrated that use of tirzepatide was associated with significant reductions in A1c and weight in a fairly homogeneous cohort of patients with type 2 diabetes who were receiving insulin glargine with or without metformin.”
“The protocol answered questions about efficacy but left open questions about generalizability and effectiveness in different populations, especially patients with certain complications or comorbid chronic diseases.” He also notes that younger adults and Black patients were not well-represented.
And the study didn’t allow for dividing up the glargine dose or for adding short-acting insulin before meals or any other pre-meal medications and “thus may represent a departure from usual care” in the setting of rising glucose levels.
The authors themselves acknowledge that “the postprandial glucose excursions observed in the placebo group suggest an additional prandial intervention was likely needed in some patients, despite the strict inclusion criteria and the treat-to-target-approach used in the study.”
Dr. Chipkin concludes that “although patients are likely to embrace a medication with weight loss outcomes, the protocol also leaves unanswered questions about reducing insulin and evaluating the comparative risk of adverse effects.”
The study was sponsored by Eli Lilly. Dr. Dahl has reported receiving personal fees from Eli Lilly during the conduct of the study and personal fees from Afimmune, Novo Nordisk, and Novartis outside the submitted work. Dr. Chipkin has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Biomarkers predict cardiovascular risk in chronic kidney disease patients
Chronic kidney disease (CKD) patients may be at increased risk for atherosclerotic cardiovascular disease, but no ASCVD risk prediction models are currently in place to inform clinical care and prevention strategies, Joshua Bundy, PhD, of Tulane University, New Orleans, and colleagues wrote in their paper, published in the Journal of the American Society of Nephrology.
To improve the accuracy of ASCVD risk prediction, the researchers developed several models using data from the Chronic Renal Insufficiency Cohort (CRIC) study. This longitudinal cohort study included more than 2,500 adult CKD patients. The participants’ ages ranged from 21-74 years, with the mean age having been 55.8 years, and 52.0% of the cohort was male.
Kidney function was defined using the glomerular filtration rate; the mean estimated glomerular filtration rate (eGFR) of the study participants was 56.0 mL/min per 1.73m2. The primary endpoint for the prediction models was incident ASCVD, defined as a composite of incident fatal or nonfatal stroke or MI.
A total of 252 incident ASCVD events occurred during the first 10 years of follow-up from baseline (1.9 events per 1,000 person-years). Patients with ASCVD events were more likely to be older, Black, and current smokers. They also were more likely than those who did not experience ASCVD events to have less than a college level education, to have a history of diabetes, and to use blood pressure–lowering medications.
“In our study, we created two new prediction tools for patients with CKD: the first is a simple model that includes factors routinely measured by health care providers and the second is an expanded model with additional variables particularly important to patients with CKD, including measures of long-term blood sugar, inflammation, and kidney and heart injury,” he explained. “We found that the new models are better able to classify patients who will or will not have a stroke or heart attack within 10 years, compared with the standard models. The new tools may better assist health care providers and patients with CKD in shared decision-making for prevention of heart disease.”
Results
The area under the curve for a prediction model using coefficients estimated within the CRIC sample was 0.736. This represented an accuracy higher than the American College of Cardiology/American Heart Association Pooled Cohort Equations (PCE), which have shown an AUC of 0.730 (P = .03). The PCE were developed by the ACC and the AHA in 2013 to estimate ASCVD risk in the primary prevention population.
The second CRIC model that was developed using clinically available variables had an AUC of 0.760. However, the third CRIC biomarker-enriched model was even more effective, with an AUC of 0.771 – significantly higher than the clinical model (P = .001).
Model 1 included the ACC/AHA PCE variables with coefficients recalculated in the CRIC study sample. Model 2 (the CRIC Clinical Model) included age, HDL cholesterol, systolic BP, current smoking, urinary albumin-to-creatinine ratio (ACR), hemoglobin A1c, and hemoglobin. Model 3 (the CRIC Enriched Model) included age, total cholesterol, HDL cholesterol, current smoking, urinary ACR, A1c, apolipoprotein B, high-sensitivity C-reactive protein (hsCRP), troponin T, and N-terminal of the prohormone brain natriuretic peptide (NT-proBNP).
Both the clinical and biomarker models improved reclassification of non-ASCVD events, compared with the PCEs (6.6% and 10.0%, respectively).
Several factors not included in prior prediction models were important for atherosclerotic CVD prediction among patients with CKD, the researchers noted. These included variables routinely measured in clinical practice as well as biomarkers: measures of long-term glycemia (A1c), inflammation (hsCRP), kidney injury (urinary ACR), and cardiac injury (troponin T and NT-proBNP).
Patients who had an ASCVD event had higher levels of A1c, systolic and diastolic BP, urinary ACR, troponin T, and NT-proBNP; these patients also had lower levels of HDL cholesterol, eGFR, and hemoglobin, compared with those who did not have an event.
The study findings were limited by several factors including the selection of study participants based on a single assessment of kidney function, who had an above average baseline ASCVD risk, the researchers noted. Other limitations included the inability to include imaging variables in the models, and the overestimated risk in the highest predicted probability groups in the CRIC study.
However, the models significantly improve prediction beyond the ACC/AHA PCE in patients with CKD, they concluded.
Models may inform shared decision-making
The development of new prediction models is important, because cardiovascular disease is the leading cause of death among U.S. adults and preventing CVD is a major public health challenge, lead author Dr. Bundy said in an interview.
“In an effort to prevent CVD, risk prediction equations can help identify patients who are at high risk for developing CVD and who may benefit from initiation or intensification of preventive and/or therapeutic measures. Simultaneously, chronic kidney disease is prevalent and those with CKD are often considered at high risk for CVD,” he said.
“However, common risk prediction tools were developed for the general population and may not work as well in patients with CKD, who may have different risk factors. Improving risk prediction in patients with CKD may help identify those among this growing population who are truly at high risk, as well as identify those who are at low risk and less likely to benefit from invasive procedures,” Dr. Bundy explained.
Glomerular filtration rate was not a strong predictor of atherosclerotic CVD
“One of the surprising findings was that estimated glomerular filtration rate was not a strong predictor and was not included in our final models,” Dr. Bundy said.
“We know that eGFR is a very important measurement in this population, but our results suggest that, at least in our sample, urinary albumin-to-creatinine ratio and cardiac biomarkers like troponin T and NT-proBNP are stronger predictors of atherosclerotic CVD in a population with reduced kidney function,” he said.
“Patient characteristics like age, blood pressure, and cholesterol are used by health care providers to predict whether a person will have a heart attack or stroke. However, most currently available prediction tools were not made for use in patients with CKD, which is a condition that is becoming more common and is likely to be seen by more health care providers in family practice,” said Dr. Bundy. “These people with CKD may have different risk factors for heart disease.”
Models are useful for clinical practice
“We are seeing rising numbers of patients with CKD in the population because of increasing age, rising rates of diabetes, and hypertension,” Noel Deep, MD, said in an interview. “The current practice of medicine does not have CKD-specific prediction models for ASCVD development, and current risks are calculated based on prediction models developed for the general population.”
“Having a prediction model that incorporates criteria/variables associated with CKD improves our ability to accurately identify and address the risk of ASCVD in this particular patient population,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
“We always knew that CKD does place the individual at higher risk for developing ASCVD, but I was impressed by the significant improvement in the prediction models using CKD specific tools, such as cardiac biomarkers (NT-proBNP), intensity of diabetes control (A1c), tobacco use, urinary albuminuria, in addition to advancing age,” he said. “Many of the laboratory tests listed in this study are commonly available and can be easily incorporated into our evaluation for and management of ASCVD in our patients with CKD.”
“As a practicing primary care physician, I would say that this study emphasizes the importance of identifying and working toward mitigating the associated health risks that our patients with CKD might have coexisting and that significantly contribute to progression of CKD,” said Dr. Deep, who is also assistant clinical professor at the Medical College of Wisconsin, Wausau. “By addressing these risk factors, we can positively impact the health of our patients with CKD and decrease the morbidity and mortality, and health care costs. These predictive models can hopefully help us more accurately identify the risk of ASCVD thereby decreasing unnecessary diagnostic procedures and interventions which carry their own risks and morbidity.”
Looking ahead, “these predictive models should be assessed and validated in large studies in diverse populations and those with different risk factors for ASCVD because CKD can be caused by several different medical conditions each with potential to contribute to ASCVD,” Dr. Deep added.
Limitations and next steps
“Although we externally validated our models in two population-based cohort studies, the individuals in these datasets were selected based on only one assessment of kidney function,” Dr. Bundy noted. “Furthermore, the best practices for implementing risk prediction models in the clinic remain to be determined, especially as new models are developed.
“While our models show promising performance for predicting 10-year risk of atherosclerotic CVD, more clinical trials are needed to test implementation of these models for improving patient care and disease prevention.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support came from the University of Pennsylvania Clinical and Translational Science Award, Johns Hopkins University, the University of Maryland, Clinical and Translational Science Collaborative of Cleveland, the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research, University of Illinois at Chicago, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases, Kaiser Permanente, and the University of New Mexico. Lead author Dr. Bundy was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose.
This article was updated on 2/17/2021.
Chronic kidney disease (CKD) patients may be at increased risk for atherosclerotic cardiovascular disease, but no ASCVD risk prediction models are currently in place to inform clinical care and prevention strategies, Joshua Bundy, PhD, of Tulane University, New Orleans, and colleagues wrote in their paper, published in the Journal of the American Society of Nephrology.
To improve the accuracy of ASCVD risk prediction, the researchers developed several models using data from the Chronic Renal Insufficiency Cohort (CRIC) study. This longitudinal cohort study included more than 2,500 adult CKD patients. The participants’ ages ranged from 21-74 years, with the mean age having been 55.8 years, and 52.0% of the cohort was male.
Kidney function was defined using the glomerular filtration rate; the mean estimated glomerular filtration rate (eGFR) of the study participants was 56.0 mL/min per 1.73m2. The primary endpoint for the prediction models was incident ASCVD, defined as a composite of incident fatal or nonfatal stroke or MI.
A total of 252 incident ASCVD events occurred during the first 10 years of follow-up from baseline (1.9 events per 1,000 person-years). Patients with ASCVD events were more likely to be older, Black, and current smokers. They also were more likely than those who did not experience ASCVD events to have less than a college level education, to have a history of diabetes, and to use blood pressure–lowering medications.
“In our study, we created two new prediction tools for patients with CKD: the first is a simple model that includes factors routinely measured by health care providers and the second is an expanded model with additional variables particularly important to patients with CKD, including measures of long-term blood sugar, inflammation, and kidney and heart injury,” he explained. “We found that the new models are better able to classify patients who will or will not have a stroke or heart attack within 10 years, compared with the standard models. The new tools may better assist health care providers and patients with CKD in shared decision-making for prevention of heart disease.”
Results
The area under the curve for a prediction model using coefficients estimated within the CRIC sample was 0.736. This represented an accuracy higher than the American College of Cardiology/American Heart Association Pooled Cohort Equations (PCE), which have shown an AUC of 0.730 (P = .03). The PCE were developed by the ACC and the AHA in 2013 to estimate ASCVD risk in the primary prevention population.
The second CRIC model that was developed using clinically available variables had an AUC of 0.760. However, the third CRIC biomarker-enriched model was even more effective, with an AUC of 0.771 – significantly higher than the clinical model (P = .001).
Model 1 included the ACC/AHA PCE variables with coefficients recalculated in the CRIC study sample. Model 2 (the CRIC Clinical Model) included age, HDL cholesterol, systolic BP, current smoking, urinary albumin-to-creatinine ratio (ACR), hemoglobin A1c, and hemoglobin. Model 3 (the CRIC Enriched Model) included age, total cholesterol, HDL cholesterol, current smoking, urinary ACR, A1c, apolipoprotein B, high-sensitivity C-reactive protein (hsCRP), troponin T, and N-terminal of the prohormone brain natriuretic peptide (NT-proBNP).
Both the clinical and biomarker models improved reclassification of non-ASCVD events, compared with the PCEs (6.6% and 10.0%, respectively).
Several factors not included in prior prediction models were important for atherosclerotic CVD prediction among patients with CKD, the researchers noted. These included variables routinely measured in clinical practice as well as biomarkers: measures of long-term glycemia (A1c), inflammation (hsCRP), kidney injury (urinary ACR), and cardiac injury (troponin T and NT-proBNP).
Patients who had an ASCVD event had higher levels of A1c, systolic and diastolic BP, urinary ACR, troponin T, and NT-proBNP; these patients also had lower levels of HDL cholesterol, eGFR, and hemoglobin, compared with those who did not have an event.
The study findings were limited by several factors including the selection of study participants based on a single assessment of kidney function, who had an above average baseline ASCVD risk, the researchers noted. Other limitations included the inability to include imaging variables in the models, and the overestimated risk in the highest predicted probability groups in the CRIC study.
However, the models significantly improve prediction beyond the ACC/AHA PCE in patients with CKD, they concluded.
Models may inform shared decision-making
The development of new prediction models is important, because cardiovascular disease is the leading cause of death among U.S. adults and preventing CVD is a major public health challenge, lead author Dr. Bundy said in an interview.
“In an effort to prevent CVD, risk prediction equations can help identify patients who are at high risk for developing CVD and who may benefit from initiation or intensification of preventive and/or therapeutic measures. Simultaneously, chronic kidney disease is prevalent and those with CKD are often considered at high risk for CVD,” he said.
“However, common risk prediction tools were developed for the general population and may not work as well in patients with CKD, who may have different risk factors. Improving risk prediction in patients with CKD may help identify those among this growing population who are truly at high risk, as well as identify those who are at low risk and less likely to benefit from invasive procedures,” Dr. Bundy explained.
Glomerular filtration rate was not a strong predictor of atherosclerotic CVD
“One of the surprising findings was that estimated glomerular filtration rate was not a strong predictor and was not included in our final models,” Dr. Bundy said.
“We know that eGFR is a very important measurement in this population, but our results suggest that, at least in our sample, urinary albumin-to-creatinine ratio and cardiac biomarkers like troponin T and NT-proBNP are stronger predictors of atherosclerotic CVD in a population with reduced kidney function,” he said.
“Patient characteristics like age, blood pressure, and cholesterol are used by health care providers to predict whether a person will have a heart attack or stroke. However, most currently available prediction tools were not made for use in patients with CKD, which is a condition that is becoming more common and is likely to be seen by more health care providers in family practice,” said Dr. Bundy. “These people with CKD may have different risk factors for heart disease.”
Models are useful for clinical practice
“We are seeing rising numbers of patients with CKD in the population because of increasing age, rising rates of diabetes, and hypertension,” Noel Deep, MD, said in an interview. “The current practice of medicine does not have CKD-specific prediction models for ASCVD development, and current risks are calculated based on prediction models developed for the general population.”
“Having a prediction model that incorporates criteria/variables associated with CKD improves our ability to accurately identify and address the risk of ASCVD in this particular patient population,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
“We always knew that CKD does place the individual at higher risk for developing ASCVD, but I was impressed by the significant improvement in the prediction models using CKD specific tools, such as cardiac biomarkers (NT-proBNP), intensity of diabetes control (A1c), tobacco use, urinary albuminuria, in addition to advancing age,” he said. “Many of the laboratory tests listed in this study are commonly available and can be easily incorporated into our evaluation for and management of ASCVD in our patients with CKD.”
“As a practicing primary care physician, I would say that this study emphasizes the importance of identifying and working toward mitigating the associated health risks that our patients with CKD might have coexisting and that significantly contribute to progression of CKD,” said Dr. Deep, who is also assistant clinical professor at the Medical College of Wisconsin, Wausau. “By addressing these risk factors, we can positively impact the health of our patients with CKD and decrease the morbidity and mortality, and health care costs. These predictive models can hopefully help us more accurately identify the risk of ASCVD thereby decreasing unnecessary diagnostic procedures and interventions which carry their own risks and morbidity.”
Looking ahead, “these predictive models should be assessed and validated in large studies in diverse populations and those with different risk factors for ASCVD because CKD can be caused by several different medical conditions each with potential to contribute to ASCVD,” Dr. Deep added.
Limitations and next steps
“Although we externally validated our models in two population-based cohort studies, the individuals in these datasets were selected based on only one assessment of kidney function,” Dr. Bundy noted. “Furthermore, the best practices for implementing risk prediction models in the clinic remain to be determined, especially as new models are developed.
“While our models show promising performance for predicting 10-year risk of atherosclerotic CVD, more clinical trials are needed to test implementation of these models for improving patient care and disease prevention.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support came from the University of Pennsylvania Clinical and Translational Science Award, Johns Hopkins University, the University of Maryland, Clinical and Translational Science Collaborative of Cleveland, the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research, University of Illinois at Chicago, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases, Kaiser Permanente, and the University of New Mexico. Lead author Dr. Bundy was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose.
This article was updated on 2/17/2021.
Chronic kidney disease (CKD) patients may be at increased risk for atherosclerotic cardiovascular disease, but no ASCVD risk prediction models are currently in place to inform clinical care and prevention strategies, Joshua Bundy, PhD, of Tulane University, New Orleans, and colleagues wrote in their paper, published in the Journal of the American Society of Nephrology.
To improve the accuracy of ASCVD risk prediction, the researchers developed several models using data from the Chronic Renal Insufficiency Cohort (CRIC) study. This longitudinal cohort study included more than 2,500 adult CKD patients. The participants’ ages ranged from 21-74 years, with the mean age having been 55.8 years, and 52.0% of the cohort was male.
Kidney function was defined using the glomerular filtration rate; the mean estimated glomerular filtration rate (eGFR) of the study participants was 56.0 mL/min per 1.73m2. The primary endpoint for the prediction models was incident ASCVD, defined as a composite of incident fatal or nonfatal stroke or MI.
A total of 252 incident ASCVD events occurred during the first 10 years of follow-up from baseline (1.9 events per 1,000 person-years). Patients with ASCVD events were more likely to be older, Black, and current smokers. They also were more likely than those who did not experience ASCVD events to have less than a college level education, to have a history of diabetes, and to use blood pressure–lowering medications.
“In our study, we created two new prediction tools for patients with CKD: the first is a simple model that includes factors routinely measured by health care providers and the second is an expanded model with additional variables particularly important to patients with CKD, including measures of long-term blood sugar, inflammation, and kidney and heart injury,” he explained. “We found that the new models are better able to classify patients who will or will not have a stroke or heart attack within 10 years, compared with the standard models. The new tools may better assist health care providers and patients with CKD in shared decision-making for prevention of heart disease.”
Results
The area under the curve for a prediction model using coefficients estimated within the CRIC sample was 0.736. This represented an accuracy higher than the American College of Cardiology/American Heart Association Pooled Cohort Equations (PCE), which have shown an AUC of 0.730 (P = .03). The PCE were developed by the ACC and the AHA in 2013 to estimate ASCVD risk in the primary prevention population.
The second CRIC model that was developed using clinically available variables had an AUC of 0.760. However, the third CRIC biomarker-enriched model was even more effective, with an AUC of 0.771 – significantly higher than the clinical model (P = .001).
Model 1 included the ACC/AHA PCE variables with coefficients recalculated in the CRIC study sample. Model 2 (the CRIC Clinical Model) included age, HDL cholesterol, systolic BP, current smoking, urinary albumin-to-creatinine ratio (ACR), hemoglobin A1c, and hemoglobin. Model 3 (the CRIC Enriched Model) included age, total cholesterol, HDL cholesterol, current smoking, urinary ACR, A1c, apolipoprotein B, high-sensitivity C-reactive protein (hsCRP), troponin T, and N-terminal of the prohormone brain natriuretic peptide (NT-proBNP).
Both the clinical and biomarker models improved reclassification of non-ASCVD events, compared with the PCEs (6.6% and 10.0%, respectively).
Several factors not included in prior prediction models were important for atherosclerotic CVD prediction among patients with CKD, the researchers noted. These included variables routinely measured in clinical practice as well as biomarkers: measures of long-term glycemia (A1c), inflammation (hsCRP), kidney injury (urinary ACR), and cardiac injury (troponin T and NT-proBNP).
Patients who had an ASCVD event had higher levels of A1c, systolic and diastolic BP, urinary ACR, troponin T, and NT-proBNP; these patients also had lower levels of HDL cholesterol, eGFR, and hemoglobin, compared with those who did not have an event.
The study findings were limited by several factors including the selection of study participants based on a single assessment of kidney function, who had an above average baseline ASCVD risk, the researchers noted. Other limitations included the inability to include imaging variables in the models, and the overestimated risk in the highest predicted probability groups in the CRIC study.
However, the models significantly improve prediction beyond the ACC/AHA PCE in patients with CKD, they concluded.
Models may inform shared decision-making
The development of new prediction models is important, because cardiovascular disease is the leading cause of death among U.S. adults and preventing CVD is a major public health challenge, lead author Dr. Bundy said in an interview.
“In an effort to prevent CVD, risk prediction equations can help identify patients who are at high risk for developing CVD and who may benefit from initiation or intensification of preventive and/or therapeutic measures. Simultaneously, chronic kidney disease is prevalent and those with CKD are often considered at high risk for CVD,” he said.
“However, common risk prediction tools were developed for the general population and may not work as well in patients with CKD, who may have different risk factors. Improving risk prediction in patients with CKD may help identify those among this growing population who are truly at high risk, as well as identify those who are at low risk and less likely to benefit from invasive procedures,” Dr. Bundy explained.
Glomerular filtration rate was not a strong predictor of atherosclerotic CVD
“One of the surprising findings was that estimated glomerular filtration rate was not a strong predictor and was not included in our final models,” Dr. Bundy said.
“We know that eGFR is a very important measurement in this population, but our results suggest that, at least in our sample, urinary albumin-to-creatinine ratio and cardiac biomarkers like troponin T and NT-proBNP are stronger predictors of atherosclerotic CVD in a population with reduced kidney function,” he said.
“Patient characteristics like age, blood pressure, and cholesterol are used by health care providers to predict whether a person will have a heart attack or stroke. However, most currently available prediction tools were not made for use in patients with CKD, which is a condition that is becoming more common and is likely to be seen by more health care providers in family practice,” said Dr. Bundy. “These people with CKD may have different risk factors for heart disease.”
Models are useful for clinical practice
“We are seeing rising numbers of patients with CKD in the population because of increasing age, rising rates of diabetes, and hypertension,” Noel Deep, MD, said in an interview. “The current practice of medicine does not have CKD-specific prediction models for ASCVD development, and current risks are calculated based on prediction models developed for the general population.”
“Having a prediction model that incorporates criteria/variables associated with CKD improves our ability to accurately identify and address the risk of ASCVD in this particular patient population,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
“We always knew that CKD does place the individual at higher risk for developing ASCVD, but I was impressed by the significant improvement in the prediction models using CKD specific tools, such as cardiac biomarkers (NT-proBNP), intensity of diabetes control (A1c), tobacco use, urinary albuminuria, in addition to advancing age,” he said. “Many of the laboratory tests listed in this study are commonly available and can be easily incorporated into our evaluation for and management of ASCVD in our patients with CKD.”
“As a practicing primary care physician, I would say that this study emphasizes the importance of identifying and working toward mitigating the associated health risks that our patients with CKD might have coexisting and that significantly contribute to progression of CKD,” said Dr. Deep, who is also assistant clinical professor at the Medical College of Wisconsin, Wausau. “By addressing these risk factors, we can positively impact the health of our patients with CKD and decrease the morbidity and mortality, and health care costs. These predictive models can hopefully help us more accurately identify the risk of ASCVD thereby decreasing unnecessary diagnostic procedures and interventions which carry their own risks and morbidity.”
Looking ahead, “these predictive models should be assessed and validated in large studies in diverse populations and those with different risk factors for ASCVD because CKD can be caused by several different medical conditions each with potential to contribute to ASCVD,” Dr. Deep added.
Limitations and next steps
“Although we externally validated our models in two population-based cohort studies, the individuals in these datasets were selected based on only one assessment of kidney function,” Dr. Bundy noted. “Furthermore, the best practices for implementing risk prediction models in the clinic remain to be determined, especially as new models are developed.
“While our models show promising performance for predicting 10-year risk of atherosclerotic CVD, more clinical trials are needed to test implementation of these models for improving patient care and disease prevention.”
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support came from the University of Pennsylvania Clinical and Translational Science Award, Johns Hopkins University, the University of Maryland, Clinical and Translational Science Collaborative of Cleveland, the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research, University of Illinois at Chicago, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases, Kaiser Permanente, and the University of New Mexico. Lead author Dr. Bundy was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose.
This article was updated on 2/17/2021.
FROM THE JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY
Heavy cannabis use tied to less diabetes in women
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Women who used marijuana (cannabis) at least four times in the previous month (heavy users) were less likely to have type 2 diabetes than women who were light users or nonusers, in a nationally representative U.S. observational study.
In contrast, there were no differences in the prevalence of type 2 diabetes in men who were light or heavy cannabis users versus nonusers.
These findings are based on data from the 2013-2018 National Health and Nutrition Examination Survey (NHANES), whereby participants self-reported their cannabis use.
The study by Ayobami S. Ogunsola, MD, MPH, a graduate student at Texas A&M University, College Station, and colleagues was recently published in Cannabis and Cannabinoid Research.
What do the findings mean?
Although overall findings linking cannabis use and diabetes have been inconsistent, the gender differences in the current study are consistent with animal studies and some clinical studies, senior author Ibraheem M. Karaye, MD, MPH, said in an interview.
However, these gender differences need to be confirmed, and “we strongly recommend that more biological or biochemical studies be conducted that could actually tell us the mechanisms,” said Dr. Karaye, an assistant professor in the department of population health, Hofstra University, Hempstead, N.Y.
“It’s indisputable that medical marijuana has some medical benefits,” he added. “Women [who use cannabis] have been shown to lose more weight than men, for example.”
“If women [cannabis users] are less likely to develop diabetes or more likely to express improvement of symptoms of diabetes,” he noted, “this means that hyperglycemic medications that are being prescribed should be watched scrupulously. Otherwise, there is a risk that [women] may overrespond.”
That is, Dr. Karaye continued, women “may be at risk of developing hypoglycemia because the cannabis is acting synergistically with the regular drug that is being used to treat the diabetes.”
U.S. clinicians, especially in states with legalized medical marijuana, need to be aware of the potential synergy.
“One would have to consider the patient as a whole,” he stressed. “For example, a woman that uses medical marijuana may actually respond differently to hyperglycemic medication.”
Conflicting reports explained by sex differences?
Evidence on whether cannabis use is linked with type 2 diabetes is limited and conflicting, the researchers wrote. They hypothesized that these conflicting findings might be explained by sex differences.
To “help inform current diabetes prevention and mitigation efforts,” they investigated sex differences in cannabis use and prevalence of type 2 diabetes in 15,602 men and women in the 2013-2014, 2015-2016, and 2017-2018 NHANES surveys.
Participants were classified as having type 2 diabetes if they had a physician’s diagnosis; a 2-hour plasma glucose of at least 200 mg/dL (in a glucose tolerance test); fasting blood glucose of at least 126 mg/dL; or A1c of at least 6.5%.
About half of respondents were women (52%) and close to half (44%) were age 18-39.
More than a third (38%) had a body mass index (BMI) of at least 30 kg/m2, indicating obesity.
Roughly 1 in 10 had a diagnosis of type 2 diabetes (13.5%) or A1c of at least 6.5% (9.8%).
Close to a fifth smoked cigarettes (16%). Similarly, 14.5% used cannabis at least four times a week, 3.3% used it less often, and the rest did not use it. Half of participants were not physically active (49%).
Just over half had at least a college education (55%).
Heavy cannabis users were more likely to be younger than age 40 (57% of men, 57% of women), college graduates (54% of men, 63% of women), cigarette smokers (79% of men, 83% of women), and physically inactive (39% of men, 49% of women).
Among women, heavy cannabis users were 49% less likely to have type 2 diabetes than nonusers, after adjusting for age, sex, race/ethnicity, educational level, physical activity, tobacco use, alcohol use, marital status, difficulty walking, employment status, income, and BMI (adjusted odds ratio, 0.51; 95% confidence interval, 0.31-0.84).
There were no significant differences between light cannabis users versus nonusers and diabetes prevalence in women, or between light or heavy cannabis users versus nonusers and diabetes prevalence in men.
Limitations, yet biologically plausible
The researchers acknowledged several study limitations.
They do not know how long participants had used marijuana. The men and women may have underreported their cannabis use, especially in states where medical marijuana was not legal, and the NHANES data did not specify whether the cannabis was recreational or medicinal.
The study may have been underpowered to detect a smaller difference in men who used versus did not use marijuana.
And importantly, this was an observational study (a snapshot at one point in time), so it cannot say whether the heavy cannabis use in women caused a decreased likelihood of diabetes.
Nevertheless, the inverse association between cannabis use and presence of type 2 diabetes is biologically plausible, Dr. Ogunsola and colleagues wrote.
The two major cannabis compounds, cannabidiol and delta-9-tetrahydrocannabinol, stimulate CBD1 and CBD2 receptors in the central and peripheral nervous systems, respectively. And “activation of the CBD1 receptor increases insulin secretion, glucagon, and somatostatin, and activates metabolic processes in fat and skeletal muscles – mechanisms that improve glucose disposal,” they explained.
The researchers speculated that the sex differences they found for this association may be caused by differences in sex hormones, or the endocannabinoid system, or fat deposits.
Therefore, “additional studies are needed to investigate the sex-based heterogeneity reported in this study and to elucidate potential mechanisms for the observation,” they concluded.
The study did not receive any funding and the researchers have no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM CANNABIS AND CANNABINOID RESEARCH