User login
Food insecurity linked to metabolic syndrome in Hispanic/Latino youth
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
FROM PEDIATRICS
Inside insulin (Part 2): Approaching a cure for type 1 diabetes?
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate enough to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell–derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” said Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Ore.
Type 1 diabetes: The past 100 years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” noted Dr. Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes ... over [the age of] 20,” explained Dr. Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the Centers for Disease Control and Prevention. This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” said Dr. Stephens. “Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease.”
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19–related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, said Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for National Health Service England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Dr. Kar emphasized.
CGMs and automated insulin delivery: A ‘godsend’
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the NHS from April 2022, Dr. Kar pointed out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and U.K.-based medical historian who recently wrote a book, “Insulin, the Crooked Timber” (Oxford, England: Oxford University Press, 2022) uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Dr. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he said. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing insulin at scale
As described by Dr. Hall in his book, the journey from treating Mr. Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process – where pH guides bitterness – to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8,000 lb (approximately 3,600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Dr. Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in E. coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Dr. Hall explained.
Today, a whole range of insulins are available, including ultra–rapid-acting, short-acting, intermediate-acting, long-acting, ultra–long-acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Dr. Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Dr. Stephens explained.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than nonanalog human insulin. In the United Kingdom through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the United Kingdom and $12.00 in Canada.
Several U.S. states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act – which would cap copayments for insulin at $35 – currently hangs in the balance.
Alongside these moves, in 2020 the Food and Drug Administration approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stressed Dr. Kar. “For lifesaving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How near to a cure in the coming decades?
Looking ahead to the coming years, if not the next 100, Dr. Stephens highlighted two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Dr. Stephens added.
The second advance of interest is the development and transplantation of cells that produce insulin.
Dr. Stephens explained that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient – who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia – with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his hemoglobin A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem cell–derived islets, “it’s a whole new life,” he recently told the New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, said Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago.”
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the FDA in July 2021, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes specialist nurses/educators keep it human
Dr. Hall said he concurs with the late eminent U.K. diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Dr. Tattersall’s book, “Diabetes: A Biography,” Dr. Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses ... these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, diabetes specialist nurses were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist.
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concluded Dr. Hall, again quoting Dr. Tattersall.
A version of this article first appeared on Medscape.com.
CPAP has only small effect on metabolic syndrome
Continuous positive airway pressure (CPAP) may be only modestly effective for ameliorating metabolic syndrome in patients with moderate to severe obstructive sleep apnea (OSA).
That conclusion comes from investigators in a randomized controlled, trial, who found that, among 100 patients with OSA and a recent diagnosis of metabolic syndrome (MS), 18% of those assigned to use CPAP at night had a reversal of MS at 6 months of follow-up, compared with 4% of controls who were assigned to use nasal strips at night (P = .04).
The majority of patients assigned to CPAP still retained their MS diagnoses at 6 months, and CPAP did not significantly reduce individual components of the syndrome. Use of CPAP was, however, associated with small reductions in visceral fat and improvement in endothelial function, reported Sara Q.C. Giampa, PhD, from the University of São Paulo, and colleagues.
“Despite a significant rate of MS reversibility after CPAP therapy, most of the patients maintained the MS diagnosis. The modest effects of CPAP on MS reversibility underscore the need for combined therapy with CPAP, aiming to maximize metabolic syndrome recovery in parallel with improvements in OSA severity and related symptoms,” according to their study, reported in the journal CHEST®.
Asked whether he still recommends CPAP to patients with OSA and the metabolic syndrome, given the findings, corresponding author Luciano F. Drager, MD, PhD, replied “yes, definitely.”
“Despite the modest rate in reversing metabolic syndrome after CPAP, the rate was 5-fold higher than non-effective treatment (18% vs. 4%),” he said in an interview.
Dr. Drager noted that studies of other single interventions such as physical exercise to reverse MS in patients with OSA also had modest results.
A researcher who studies the relationship between sleep, circadian rhythms, and metabolism commented that, although the patients in the CPAP group were compliant with the assigned equipment and had both reductions in apneic events and improvement in oxygen saturation, the effect of CPAP on the metabolic syndrome was rather small.
“The CPAP was doing what we thought it was supposed to do, but it didn’t have the magnitude of effect on the metabolic syndrome as I expected or I think as the authors expected,” said Deanna Arble, PhD, assistant professor of biological science at Marquette University, Milwaukee.
She noted that the study also failed to detect a significant improvement in the blood pressure component of metabolic syndrome.
“In my experience and my review of the literature, blood pressure tends to be the one that’s improved most dramatically with CPAP,” she said.
Dr. Arble was not involved in the study.
Study details
In the trial, titled TREATOSA-MS, the investigators enrolled 100 patients with a recent diagnosis of metabolic syndrome and moderate to severe OSA, defined as 15 or more apnea-hypopnea index events per hour. The patients were stratified by body mass index and then randomized to undergo therapeutic CPAP or to use nasal strips for 6 months.
At baseline and at the end of each intervention investigators measured anthropometric variables, blood pressure, glucose, and lipid profiles. They also leptin and adiponectin, body composition, food intake, physical activity, subcutaneous and abdominal fat (visceral and hepatic), and endothelial function to control for potential confounders.
As noted previously, they found that after 6 months “most patients with OSA randomized to CPAP retained the MS diagnosis, but the rate of MS reversibility was higher than observed in the placebo group.” The difference in metabolic syndrome reversal, 18% with CPAP versus 4% with nasal strips, translated into a hazard ratio favoring CPAP of 5.27 (P = .04).
Also as noted, in analyses adjusted for baseline values, CPAP did not significantly improve either weight, liver fat, lip profiles, or the adiposity biomarkers leptin and adiponectin, but did have “very modest” influence on reducing visceral fat and improving endothelial function.
Rigorous study
Dr. Arble said that most studies of the association between OSA and metabolic syndrome have focused on only one or two of the parameters that were included in the TREATOSA-MS study, giving the findings additional weight.
“This could potentially be a very good, carefully controlled first insight into how obstructive sleep apnea is related to the metabolic syndrome,” she said.
The study was funded by grants Fundação de Amparo Q22 à Pesquisa do Estado de São Paulo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The authors and Dr. Arble reported having no conflicts of interest to disclose.
Continuous positive airway pressure (CPAP) may be only modestly effective for ameliorating metabolic syndrome in patients with moderate to severe obstructive sleep apnea (OSA).
That conclusion comes from investigators in a randomized controlled, trial, who found that, among 100 patients with OSA and a recent diagnosis of metabolic syndrome (MS), 18% of those assigned to use CPAP at night had a reversal of MS at 6 months of follow-up, compared with 4% of controls who were assigned to use nasal strips at night (P = .04).
The majority of patients assigned to CPAP still retained their MS diagnoses at 6 months, and CPAP did not significantly reduce individual components of the syndrome. Use of CPAP was, however, associated with small reductions in visceral fat and improvement in endothelial function, reported Sara Q.C. Giampa, PhD, from the University of São Paulo, and colleagues.
“Despite a significant rate of MS reversibility after CPAP therapy, most of the patients maintained the MS diagnosis. The modest effects of CPAP on MS reversibility underscore the need for combined therapy with CPAP, aiming to maximize metabolic syndrome recovery in parallel with improvements in OSA severity and related symptoms,” according to their study, reported in the journal CHEST®.
Asked whether he still recommends CPAP to patients with OSA and the metabolic syndrome, given the findings, corresponding author Luciano F. Drager, MD, PhD, replied “yes, definitely.”
“Despite the modest rate in reversing metabolic syndrome after CPAP, the rate was 5-fold higher than non-effective treatment (18% vs. 4%),” he said in an interview.
Dr. Drager noted that studies of other single interventions such as physical exercise to reverse MS in patients with OSA also had modest results.
A researcher who studies the relationship between sleep, circadian rhythms, and metabolism commented that, although the patients in the CPAP group were compliant with the assigned equipment and had both reductions in apneic events and improvement in oxygen saturation, the effect of CPAP on the metabolic syndrome was rather small.
“The CPAP was doing what we thought it was supposed to do, but it didn’t have the magnitude of effect on the metabolic syndrome as I expected or I think as the authors expected,” said Deanna Arble, PhD, assistant professor of biological science at Marquette University, Milwaukee.
She noted that the study also failed to detect a significant improvement in the blood pressure component of metabolic syndrome.
“In my experience and my review of the literature, blood pressure tends to be the one that’s improved most dramatically with CPAP,” she said.
Dr. Arble was not involved in the study.
Study details
In the trial, titled TREATOSA-MS, the investigators enrolled 100 patients with a recent diagnosis of metabolic syndrome and moderate to severe OSA, defined as 15 or more apnea-hypopnea index events per hour. The patients were stratified by body mass index and then randomized to undergo therapeutic CPAP or to use nasal strips for 6 months.
At baseline and at the end of each intervention investigators measured anthropometric variables, blood pressure, glucose, and lipid profiles. They also leptin and adiponectin, body composition, food intake, physical activity, subcutaneous and abdominal fat (visceral and hepatic), and endothelial function to control for potential confounders.
As noted previously, they found that after 6 months “most patients with OSA randomized to CPAP retained the MS diagnosis, but the rate of MS reversibility was higher than observed in the placebo group.” The difference in metabolic syndrome reversal, 18% with CPAP versus 4% with nasal strips, translated into a hazard ratio favoring CPAP of 5.27 (P = .04).
Also as noted, in analyses adjusted for baseline values, CPAP did not significantly improve either weight, liver fat, lip profiles, or the adiposity biomarkers leptin and adiponectin, but did have “very modest” influence on reducing visceral fat and improving endothelial function.
Rigorous study
Dr. Arble said that most studies of the association between OSA and metabolic syndrome have focused on only one or two of the parameters that were included in the TREATOSA-MS study, giving the findings additional weight.
“This could potentially be a very good, carefully controlled first insight into how obstructive sleep apnea is related to the metabolic syndrome,” she said.
The study was funded by grants Fundação de Amparo Q22 à Pesquisa do Estado de São Paulo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The authors and Dr. Arble reported having no conflicts of interest to disclose.
Continuous positive airway pressure (CPAP) may be only modestly effective for ameliorating metabolic syndrome in patients with moderate to severe obstructive sleep apnea (OSA).
That conclusion comes from investigators in a randomized controlled, trial, who found that, among 100 patients with OSA and a recent diagnosis of metabolic syndrome (MS), 18% of those assigned to use CPAP at night had a reversal of MS at 6 months of follow-up, compared with 4% of controls who were assigned to use nasal strips at night (P = .04).
The majority of patients assigned to CPAP still retained their MS diagnoses at 6 months, and CPAP did not significantly reduce individual components of the syndrome. Use of CPAP was, however, associated with small reductions in visceral fat and improvement in endothelial function, reported Sara Q.C. Giampa, PhD, from the University of São Paulo, and colleagues.
“Despite a significant rate of MS reversibility after CPAP therapy, most of the patients maintained the MS diagnosis. The modest effects of CPAP on MS reversibility underscore the need for combined therapy with CPAP, aiming to maximize metabolic syndrome recovery in parallel with improvements in OSA severity and related symptoms,” according to their study, reported in the journal CHEST®.
Asked whether he still recommends CPAP to patients with OSA and the metabolic syndrome, given the findings, corresponding author Luciano F. Drager, MD, PhD, replied “yes, definitely.”
“Despite the modest rate in reversing metabolic syndrome after CPAP, the rate was 5-fold higher than non-effective treatment (18% vs. 4%),” he said in an interview.
Dr. Drager noted that studies of other single interventions such as physical exercise to reverse MS in patients with OSA also had modest results.
A researcher who studies the relationship between sleep, circadian rhythms, and metabolism commented that, although the patients in the CPAP group were compliant with the assigned equipment and had both reductions in apneic events and improvement in oxygen saturation, the effect of CPAP on the metabolic syndrome was rather small.
“The CPAP was doing what we thought it was supposed to do, but it didn’t have the magnitude of effect on the metabolic syndrome as I expected or I think as the authors expected,” said Deanna Arble, PhD, assistant professor of biological science at Marquette University, Milwaukee.
She noted that the study also failed to detect a significant improvement in the blood pressure component of metabolic syndrome.
“In my experience and my review of the literature, blood pressure tends to be the one that’s improved most dramatically with CPAP,” she said.
Dr. Arble was not involved in the study.
Study details
In the trial, titled TREATOSA-MS, the investigators enrolled 100 patients with a recent diagnosis of metabolic syndrome and moderate to severe OSA, defined as 15 or more apnea-hypopnea index events per hour. The patients were stratified by body mass index and then randomized to undergo therapeutic CPAP or to use nasal strips for 6 months.
At baseline and at the end of each intervention investigators measured anthropometric variables, blood pressure, glucose, and lipid profiles. They also leptin and adiponectin, body composition, food intake, physical activity, subcutaneous and abdominal fat (visceral and hepatic), and endothelial function to control for potential confounders.
As noted previously, they found that after 6 months “most patients with OSA randomized to CPAP retained the MS diagnosis, but the rate of MS reversibility was higher than observed in the placebo group.” The difference in metabolic syndrome reversal, 18% with CPAP versus 4% with nasal strips, translated into a hazard ratio favoring CPAP of 5.27 (P = .04).
Also as noted, in analyses adjusted for baseline values, CPAP did not significantly improve either weight, liver fat, lip profiles, or the adiposity biomarkers leptin and adiponectin, but did have “very modest” influence on reducing visceral fat and improving endothelial function.
Rigorous study
Dr. Arble said that most studies of the association between OSA and metabolic syndrome have focused on only one or two of the parameters that were included in the TREATOSA-MS study, giving the findings additional weight.
“This could potentially be a very good, carefully controlled first insight into how obstructive sleep apnea is related to the metabolic syndrome,” she said.
The study was funded by grants Fundação de Amparo Q22 à Pesquisa do Estado de São Paulo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The authors and Dr. Arble reported having no conflicts of interest to disclose.
FROM CHEST
Ukrainian diabetes care, insulin access ‘severely disrupted’
with shortages resulting more from distribution problems than supply itself, according to multiple sources.
In 2021, there were about 2.3 million people with diabetes in Ukraine, roughly 7% of the total population. Of those, about 120,000 have type 1 diabetes and depend on insulin to live, while a similar number have insulin-treated type 2 diabetes.
Donations of insulin, other medications, and supplies have been pouring in since late February from sources including the Ukrainian diaspora, nongovernmental organizations, other European governments, universities, and product manufacturers. “The main problem now is logistic,” Boris Mankovsky, MD, president of the Ukrainian Diabetology Association, said in an interview.
Insulin manufacturer Novo Nordisk’s warehouse continues to operate, although deliveries have been curtailed because of shortages in delivery staff. The company is working to get medications to patients either through pharmacies or humanitarian organizations and has funded refugee support efforts, they said in a March 8 statement.
But even if the supplies reach the pharmacies, they may not reach patients for a variety of logistical reasons, noted Dr. Mankovsky, who is head of the department of diabetology at the P.L. Shupyk National Medical Academy for Postgraduate Education in Kyiv. “So, there are a lot of problems. I don’t know exactly where the main bottleneck is, but there are shortages, definitely.”
Insulin supplies have also been distributed very unequally by region and type, with various shipments containing long-acting, short-acting, analog, or human insulins. “We’re very grateful for all of it. But it’s not centrally coordinated, which of course is understandable, but it means that a lot of donations go to one place and no supply goes to another,” Dr. Mankovsky said.
Most of the donated supplies have been going to western Ukraine, where the capital Kyiv is located. “But the main problem now is the eastern part of Ukraine. It’s difficult and dangerous to deliver any supplies there, especially [with] the terrible situation in Mariupol. Eastern Ukraine now suffers the most, at least at this minute,” he said.
Diabetes specialists continue to work, at least for now
Ivan Smirnov, MD, PhD, head of the endocrinology department at Kharkiv Regional Hospital, in the northeastern part of the country, said in an email to this news organization: “I continue to stay in Kharkiv, in spite of the situation. A lot of people are killed, many people are wounded. My hospital is full of wounded civilians ... a lot of buildings are destroyed partly and some completely.”
Dr. Smirnov said that he and his colleagues “find the way to overcome the fear ... in constant work. Part of the work is online consulting assistance for routine patients. ... But the main time now is dedicated to providing the diabetes patients with insulin. This is a heavy job to do indeed.”
Dr. Mankovsky, who practices adult diabetology and endocrinology in Kyiv, continues to manage patients, but mostly remotely. “Practice is severely disrupted. I’m willing to see patients but it’s extremely difficult and dangerous for them and probably not possible to travel to see me. So that’s why all our communications now is distant, through phone or internet. ... We can communicate and I’m able to provide some recommendations for changes in treatment or some corrections in insulin therapy.”
Despite the Russians closing in on Kyiv, Dr. Mankovsky said, “I’ve decided to stay as long as possible. Then, nobody knows of course but I think I have to. ... We hear explosions every day. ... I’m in the center of the city and the streets are empty. It’s heartbreaking.”
Supplies are reaching refugees
Dr. Mankovsky said: “Now we have huge movement of refugees. Among them are a lot of people with diabetes who moved out of their place and nobody knows where they are. It’s really a huge disruption.”
According to the type 1 diabetes advocacy organization JDRF, many men with diabetes aged 18-60 are remaining in Ukraine to fight, despite the increased risk with the disease. But an estimated 15,000 children with type 1 diabetes and their families are attempting to escape the conflict by moving to the western regions of the country or over the borders.
“Those who make it to Hungary, Moldova, Poland, or Romania are being received with wonderful generosity. We have heard stories ranging from governments making it possible to pick up insulin free without a script to individuals emptying their cupboards of insulin for those whose need is urgent,” JDRF said in a statement on March 2.
For its part, Novo Nordisk has donated 55 million Danish kroner (about 7.3 million Euros, or $8.2 million U.S. dollars) to support international relief organizations in assisting refugees.
Ivan Tkac, MD, PhD, professor of medicine at Safárik University in Kosice, Slovakia, is assisting refugees, including those with diabetes. Slovakia is predominantly a transit country for refugees from Ukraine, he said in an interview.
“However, in solidarity with Ukraine, we are providing emergency medical care for both Ukrainians and refugees from third countries leaving Ukraine,” he said, noting that those individuals are primarily foreign students who had been studying there.
“Asylum seekers receive full medical insurance paid by the government of the Slovak Republic. As part of this care, the refugees are provided with the necessary amount of insulin and other antidiabetic drugs, as well as medical devices needed for the treatment of diabetes. The European Commission has pledged to supply Slovakia with the necessary quantities of medicines for the treatment of diabetes in the coming weeks as part of its assistance to the countries bordering Ukraine. In addition, some humanitarian organizations are organizing supplies of insulin and other medicines for soldiers fighting in the Ukrainian army,” Dr. Tkac said.
How you can help
A number of organizations are providing assistance specifically to people with diabetes, as well as broader medical assistance to people remaining in Ukraine and to refugees.
A collaboration between the Ministry of Health of Ukraine, the humanitarian agency Direct Relief, and the International Diabetes Federation is working to determine where supplies are short, to secure donations within Europe, and to open up “green corridors” within Ukraine to deliver them quickly to where they’re needed. They have asked those who wish to help to donate to Direct Relief and direct donations to the “Ukraine crisis.”
Another effort organized by IDF Europe is Connect Solidarity, a program that “aims to facilitate support from IDF Europe member associations across Europe wishing to help other national diabetes associations in Ukraine’s neighboring countries, provide advice, medicines, and supplies to Ukrainian refugees.”
IDF President Andrew Boulton, MD, told this news organization that he has been in almost daily contact with senior colleagues working in diabetes in Ukraine, and that he is working with the organization’s affiliated charity Life for a Child in addition to the other charitable agencies. “We will continue to do our utmost best to help those with diabetes living in Ukraine. However, this is, of course, very challenging, and we hope that we are doing the best we can in such a difficult situation. We all hope and pray that this situation is soon resolved.”
The European Association for the Study of Diabetes is taking a somewhat different approach, by encouraging its members to “support people with ill health, including diabetes, with donations through established [nongovernmental organizations] that have the capacity to help on site, such as United Nations High Commissioner for Refugees or International Committee of the Red Cross.”
Dr. Mankovsky told this news organization that he is very grateful for all the support from around the world: “Just thanks. I’ve got so much support, so many phone calls, so many letters ... not just me, all of us. People wise and friendship wise we feel support. It’s really important, emotionally and with insulin supply and other medications. Without that, it would be much more difficult.”
Pandemic-prompted changes enable wartime diabetes care
Dramatic changes in diabetes care delivery in Ukraine necessitated by the COVID-19 pandemic have proved indispensable during the Russian invasion.
In a piece published May 29, 2020, in the Journal of Diabetes Science and Technology, Dr. Mankovsky described how the pandemic hit just as Ukraine’s health system was pivoting from government controlled to insurance based.
Prior to the pandemic, patients with both type 1 and type 2 diabetes were regularly admitted to hospital for routine checkups, insulin dose management, and other treatments, a “remnant of the Soviet-era medical practice, which emphasized heavily on hospital admissions,” Dr. Mankovsky said in an interview.
This was the case, he wrote in the article, “despite the common understanding that such a system was a waste of resources ... this policy was changing much slower than we wanted.”
But the COVID-19 pandemic changed that practice “abruptly and dramatically,” so that all hospitalizations for patients with diabetes were stopped unless there was a real metabolic emergency.
Subsequently, Dr. Mankovsky wrote, “almost every health professional recognizes the particular importance of the new ways of communications with patients and with other colleagues.”
Indeed, in his email to this news organization, Dr. Smirnov mentioned that the routine diabetes management work he is still able to do remotely despite the extreme disruption in his region “is easy because of long-term COVID-period experience.”
Also because of the pandemic, insulin prescriptions were switched from traditional paper to electronic transfer, so that patients could easily pick them up at the pharmacy. “This new ... system proved to be not just very convenient for all parties involved, but in the current situation, it allowed us to prevent so many medically unnecessary visits to the clinics, which otherwise would have presented the real threat to the patients’ health and risk to get them infected,” Dr. Mankovsky wrote in 2020.
Now with the new danger, he said, “the inability to see patients is probably the least of our problems.”
A version of this article first appeared on Medscape.com.
with shortages resulting more from distribution problems than supply itself, according to multiple sources.
In 2021, there were about 2.3 million people with diabetes in Ukraine, roughly 7% of the total population. Of those, about 120,000 have type 1 diabetes and depend on insulin to live, while a similar number have insulin-treated type 2 diabetes.
Donations of insulin, other medications, and supplies have been pouring in since late February from sources including the Ukrainian diaspora, nongovernmental organizations, other European governments, universities, and product manufacturers. “The main problem now is logistic,” Boris Mankovsky, MD, president of the Ukrainian Diabetology Association, said in an interview.
Insulin manufacturer Novo Nordisk’s warehouse continues to operate, although deliveries have been curtailed because of shortages in delivery staff. The company is working to get medications to patients either through pharmacies or humanitarian organizations and has funded refugee support efforts, they said in a March 8 statement.
But even if the supplies reach the pharmacies, they may not reach patients for a variety of logistical reasons, noted Dr. Mankovsky, who is head of the department of diabetology at the P.L. Shupyk National Medical Academy for Postgraduate Education in Kyiv. “So, there are a lot of problems. I don’t know exactly where the main bottleneck is, but there are shortages, definitely.”
Insulin supplies have also been distributed very unequally by region and type, with various shipments containing long-acting, short-acting, analog, or human insulins. “We’re very grateful for all of it. But it’s not centrally coordinated, which of course is understandable, but it means that a lot of donations go to one place and no supply goes to another,” Dr. Mankovsky said.
Most of the donated supplies have been going to western Ukraine, where the capital Kyiv is located. “But the main problem now is the eastern part of Ukraine. It’s difficult and dangerous to deliver any supplies there, especially [with] the terrible situation in Mariupol. Eastern Ukraine now suffers the most, at least at this minute,” he said.
Diabetes specialists continue to work, at least for now
Ivan Smirnov, MD, PhD, head of the endocrinology department at Kharkiv Regional Hospital, in the northeastern part of the country, said in an email to this news organization: “I continue to stay in Kharkiv, in spite of the situation. A lot of people are killed, many people are wounded. My hospital is full of wounded civilians ... a lot of buildings are destroyed partly and some completely.”
Dr. Smirnov said that he and his colleagues “find the way to overcome the fear ... in constant work. Part of the work is online consulting assistance for routine patients. ... But the main time now is dedicated to providing the diabetes patients with insulin. This is a heavy job to do indeed.”
Dr. Mankovsky, who practices adult diabetology and endocrinology in Kyiv, continues to manage patients, but mostly remotely. “Practice is severely disrupted. I’m willing to see patients but it’s extremely difficult and dangerous for them and probably not possible to travel to see me. So that’s why all our communications now is distant, through phone or internet. ... We can communicate and I’m able to provide some recommendations for changes in treatment or some corrections in insulin therapy.”
Despite the Russians closing in on Kyiv, Dr. Mankovsky said, “I’ve decided to stay as long as possible. Then, nobody knows of course but I think I have to. ... We hear explosions every day. ... I’m in the center of the city and the streets are empty. It’s heartbreaking.”
Supplies are reaching refugees
Dr. Mankovsky said: “Now we have huge movement of refugees. Among them are a lot of people with diabetes who moved out of their place and nobody knows where they are. It’s really a huge disruption.”
According to the type 1 diabetes advocacy organization JDRF, many men with diabetes aged 18-60 are remaining in Ukraine to fight, despite the increased risk with the disease. But an estimated 15,000 children with type 1 diabetes and their families are attempting to escape the conflict by moving to the western regions of the country or over the borders.
“Those who make it to Hungary, Moldova, Poland, or Romania are being received with wonderful generosity. We have heard stories ranging from governments making it possible to pick up insulin free without a script to individuals emptying their cupboards of insulin for those whose need is urgent,” JDRF said in a statement on March 2.
For its part, Novo Nordisk has donated 55 million Danish kroner (about 7.3 million Euros, or $8.2 million U.S. dollars) to support international relief organizations in assisting refugees.
Ivan Tkac, MD, PhD, professor of medicine at Safárik University in Kosice, Slovakia, is assisting refugees, including those with diabetes. Slovakia is predominantly a transit country for refugees from Ukraine, he said in an interview.
“However, in solidarity with Ukraine, we are providing emergency medical care for both Ukrainians and refugees from third countries leaving Ukraine,” he said, noting that those individuals are primarily foreign students who had been studying there.
“Asylum seekers receive full medical insurance paid by the government of the Slovak Republic. As part of this care, the refugees are provided with the necessary amount of insulin and other antidiabetic drugs, as well as medical devices needed for the treatment of diabetes. The European Commission has pledged to supply Slovakia with the necessary quantities of medicines for the treatment of diabetes in the coming weeks as part of its assistance to the countries bordering Ukraine. In addition, some humanitarian organizations are organizing supplies of insulin and other medicines for soldiers fighting in the Ukrainian army,” Dr. Tkac said.
How you can help
A number of organizations are providing assistance specifically to people with diabetes, as well as broader medical assistance to people remaining in Ukraine and to refugees.
A collaboration between the Ministry of Health of Ukraine, the humanitarian agency Direct Relief, and the International Diabetes Federation is working to determine where supplies are short, to secure donations within Europe, and to open up “green corridors” within Ukraine to deliver them quickly to where they’re needed. They have asked those who wish to help to donate to Direct Relief and direct donations to the “Ukraine crisis.”
Another effort organized by IDF Europe is Connect Solidarity, a program that “aims to facilitate support from IDF Europe member associations across Europe wishing to help other national diabetes associations in Ukraine’s neighboring countries, provide advice, medicines, and supplies to Ukrainian refugees.”
IDF President Andrew Boulton, MD, told this news organization that he has been in almost daily contact with senior colleagues working in diabetes in Ukraine, and that he is working with the organization’s affiliated charity Life for a Child in addition to the other charitable agencies. “We will continue to do our utmost best to help those with diabetes living in Ukraine. However, this is, of course, very challenging, and we hope that we are doing the best we can in such a difficult situation. We all hope and pray that this situation is soon resolved.”
The European Association for the Study of Diabetes is taking a somewhat different approach, by encouraging its members to “support people with ill health, including diabetes, with donations through established [nongovernmental organizations] that have the capacity to help on site, such as United Nations High Commissioner for Refugees or International Committee of the Red Cross.”
Dr. Mankovsky told this news organization that he is very grateful for all the support from around the world: “Just thanks. I’ve got so much support, so many phone calls, so many letters ... not just me, all of us. People wise and friendship wise we feel support. It’s really important, emotionally and with insulin supply and other medications. Without that, it would be much more difficult.”
Pandemic-prompted changes enable wartime diabetes care
Dramatic changes in diabetes care delivery in Ukraine necessitated by the COVID-19 pandemic have proved indispensable during the Russian invasion.
In a piece published May 29, 2020, in the Journal of Diabetes Science and Technology, Dr. Mankovsky described how the pandemic hit just as Ukraine’s health system was pivoting from government controlled to insurance based.
Prior to the pandemic, patients with both type 1 and type 2 diabetes were regularly admitted to hospital for routine checkups, insulin dose management, and other treatments, a “remnant of the Soviet-era medical practice, which emphasized heavily on hospital admissions,” Dr. Mankovsky said in an interview.
This was the case, he wrote in the article, “despite the common understanding that such a system was a waste of resources ... this policy was changing much slower than we wanted.”
But the COVID-19 pandemic changed that practice “abruptly and dramatically,” so that all hospitalizations for patients with diabetes were stopped unless there was a real metabolic emergency.
Subsequently, Dr. Mankovsky wrote, “almost every health professional recognizes the particular importance of the new ways of communications with patients and with other colleagues.”
Indeed, in his email to this news organization, Dr. Smirnov mentioned that the routine diabetes management work he is still able to do remotely despite the extreme disruption in his region “is easy because of long-term COVID-period experience.”
Also because of the pandemic, insulin prescriptions were switched from traditional paper to electronic transfer, so that patients could easily pick them up at the pharmacy. “This new ... system proved to be not just very convenient for all parties involved, but in the current situation, it allowed us to prevent so many medically unnecessary visits to the clinics, which otherwise would have presented the real threat to the patients’ health and risk to get them infected,” Dr. Mankovsky wrote in 2020.
Now with the new danger, he said, “the inability to see patients is probably the least of our problems.”
A version of this article first appeared on Medscape.com.
with shortages resulting more from distribution problems than supply itself, according to multiple sources.
In 2021, there were about 2.3 million people with diabetes in Ukraine, roughly 7% of the total population. Of those, about 120,000 have type 1 diabetes and depend on insulin to live, while a similar number have insulin-treated type 2 diabetes.
Donations of insulin, other medications, and supplies have been pouring in since late February from sources including the Ukrainian diaspora, nongovernmental organizations, other European governments, universities, and product manufacturers. “The main problem now is logistic,” Boris Mankovsky, MD, president of the Ukrainian Diabetology Association, said in an interview.
Insulin manufacturer Novo Nordisk’s warehouse continues to operate, although deliveries have been curtailed because of shortages in delivery staff. The company is working to get medications to patients either through pharmacies or humanitarian organizations and has funded refugee support efforts, they said in a March 8 statement.
But even if the supplies reach the pharmacies, they may not reach patients for a variety of logistical reasons, noted Dr. Mankovsky, who is head of the department of diabetology at the P.L. Shupyk National Medical Academy for Postgraduate Education in Kyiv. “So, there are a lot of problems. I don’t know exactly where the main bottleneck is, but there are shortages, definitely.”
Insulin supplies have also been distributed very unequally by region and type, with various shipments containing long-acting, short-acting, analog, or human insulins. “We’re very grateful for all of it. But it’s not centrally coordinated, which of course is understandable, but it means that a lot of donations go to one place and no supply goes to another,” Dr. Mankovsky said.
Most of the donated supplies have been going to western Ukraine, where the capital Kyiv is located. “But the main problem now is the eastern part of Ukraine. It’s difficult and dangerous to deliver any supplies there, especially [with] the terrible situation in Mariupol. Eastern Ukraine now suffers the most, at least at this minute,” he said.
Diabetes specialists continue to work, at least for now
Ivan Smirnov, MD, PhD, head of the endocrinology department at Kharkiv Regional Hospital, in the northeastern part of the country, said in an email to this news organization: “I continue to stay in Kharkiv, in spite of the situation. A lot of people are killed, many people are wounded. My hospital is full of wounded civilians ... a lot of buildings are destroyed partly and some completely.”
Dr. Smirnov said that he and his colleagues “find the way to overcome the fear ... in constant work. Part of the work is online consulting assistance for routine patients. ... But the main time now is dedicated to providing the diabetes patients with insulin. This is a heavy job to do indeed.”
Dr. Mankovsky, who practices adult diabetology and endocrinology in Kyiv, continues to manage patients, but mostly remotely. “Practice is severely disrupted. I’m willing to see patients but it’s extremely difficult and dangerous for them and probably not possible to travel to see me. So that’s why all our communications now is distant, through phone or internet. ... We can communicate and I’m able to provide some recommendations for changes in treatment or some corrections in insulin therapy.”
Despite the Russians closing in on Kyiv, Dr. Mankovsky said, “I’ve decided to stay as long as possible. Then, nobody knows of course but I think I have to. ... We hear explosions every day. ... I’m in the center of the city and the streets are empty. It’s heartbreaking.”
Supplies are reaching refugees
Dr. Mankovsky said: “Now we have huge movement of refugees. Among them are a lot of people with diabetes who moved out of their place and nobody knows where they are. It’s really a huge disruption.”
According to the type 1 diabetes advocacy organization JDRF, many men with diabetes aged 18-60 are remaining in Ukraine to fight, despite the increased risk with the disease. But an estimated 15,000 children with type 1 diabetes and their families are attempting to escape the conflict by moving to the western regions of the country or over the borders.
“Those who make it to Hungary, Moldova, Poland, or Romania are being received with wonderful generosity. We have heard stories ranging from governments making it possible to pick up insulin free without a script to individuals emptying their cupboards of insulin for those whose need is urgent,” JDRF said in a statement on March 2.
For its part, Novo Nordisk has donated 55 million Danish kroner (about 7.3 million Euros, or $8.2 million U.S. dollars) to support international relief organizations in assisting refugees.
Ivan Tkac, MD, PhD, professor of medicine at Safárik University in Kosice, Slovakia, is assisting refugees, including those with diabetes. Slovakia is predominantly a transit country for refugees from Ukraine, he said in an interview.
“However, in solidarity with Ukraine, we are providing emergency medical care for both Ukrainians and refugees from third countries leaving Ukraine,” he said, noting that those individuals are primarily foreign students who had been studying there.
“Asylum seekers receive full medical insurance paid by the government of the Slovak Republic. As part of this care, the refugees are provided with the necessary amount of insulin and other antidiabetic drugs, as well as medical devices needed for the treatment of diabetes. The European Commission has pledged to supply Slovakia with the necessary quantities of medicines for the treatment of diabetes in the coming weeks as part of its assistance to the countries bordering Ukraine. In addition, some humanitarian organizations are organizing supplies of insulin and other medicines for soldiers fighting in the Ukrainian army,” Dr. Tkac said.
How you can help
A number of organizations are providing assistance specifically to people with diabetes, as well as broader medical assistance to people remaining in Ukraine and to refugees.
A collaboration between the Ministry of Health of Ukraine, the humanitarian agency Direct Relief, and the International Diabetes Federation is working to determine where supplies are short, to secure donations within Europe, and to open up “green corridors” within Ukraine to deliver them quickly to where they’re needed. They have asked those who wish to help to donate to Direct Relief and direct donations to the “Ukraine crisis.”
Another effort organized by IDF Europe is Connect Solidarity, a program that “aims to facilitate support from IDF Europe member associations across Europe wishing to help other national diabetes associations in Ukraine’s neighboring countries, provide advice, medicines, and supplies to Ukrainian refugees.”
IDF President Andrew Boulton, MD, told this news organization that he has been in almost daily contact with senior colleagues working in diabetes in Ukraine, and that he is working with the organization’s affiliated charity Life for a Child in addition to the other charitable agencies. “We will continue to do our utmost best to help those with diabetes living in Ukraine. However, this is, of course, very challenging, and we hope that we are doing the best we can in such a difficult situation. We all hope and pray that this situation is soon resolved.”
The European Association for the Study of Diabetes is taking a somewhat different approach, by encouraging its members to “support people with ill health, including diabetes, with donations through established [nongovernmental organizations] that have the capacity to help on site, such as United Nations High Commissioner for Refugees or International Committee of the Red Cross.”
Dr. Mankovsky told this news organization that he is very grateful for all the support from around the world: “Just thanks. I’ve got so much support, so many phone calls, so many letters ... not just me, all of us. People wise and friendship wise we feel support. It’s really important, emotionally and with insulin supply and other medications. Without that, it would be much more difficult.”
Pandemic-prompted changes enable wartime diabetes care
Dramatic changes in diabetes care delivery in Ukraine necessitated by the COVID-19 pandemic have proved indispensable during the Russian invasion.
In a piece published May 29, 2020, in the Journal of Diabetes Science and Technology, Dr. Mankovsky described how the pandemic hit just as Ukraine’s health system was pivoting from government controlled to insurance based.
Prior to the pandemic, patients with both type 1 and type 2 diabetes were regularly admitted to hospital for routine checkups, insulin dose management, and other treatments, a “remnant of the Soviet-era medical practice, which emphasized heavily on hospital admissions,” Dr. Mankovsky said in an interview.
This was the case, he wrote in the article, “despite the common understanding that such a system was a waste of resources ... this policy was changing much slower than we wanted.”
But the COVID-19 pandemic changed that practice “abruptly and dramatically,” so that all hospitalizations for patients with diabetes were stopped unless there was a real metabolic emergency.
Subsequently, Dr. Mankovsky wrote, “almost every health professional recognizes the particular importance of the new ways of communications with patients and with other colleagues.”
Indeed, in his email to this news organization, Dr. Smirnov mentioned that the routine diabetes management work he is still able to do remotely despite the extreme disruption in his region “is easy because of long-term COVID-period experience.”
Also because of the pandemic, insulin prescriptions were switched from traditional paper to electronic transfer, so that patients could easily pick them up at the pharmacy. “This new ... system proved to be not just very convenient for all parties involved, but in the current situation, it allowed us to prevent so many medically unnecessary visits to the clinics, which otherwise would have presented the real threat to the patients’ health and risk to get them infected,” Dr. Mankovsky wrote in 2020.
Now with the new danger, he said, “the inability to see patients is probably the least of our problems.”
A version of this article first appeared on Medscape.com.
Can a tool help overcome barriers to diabetes medication cost?
The resource, “Having Healthcare Cost Conversations to Improve Patient Outcomes: A Practical Guide,” was jointly developed by the Association of Diabetes Care & Education Specialists and Beyond Type 1, the nonprofit patient advocacy organization.
Indeed, the guide appeared as President Biden discussed his proposal to cap insulin costs at $35 per insulin vial during the State of the Union address, during which he introduced a young boy with type 1 diabetes in the guest box, as reported by this news organization. On March 3, Civica, a nonprofit coalition of health systems and philanthropies, announced it plans to manufacture generic insulin at a deeply discounted price, as reported by this news organization.
“Just to see diabetes front and center at the State of the Union followed by these announcements is certainly reflective of our own advocacy effort to make sure that people have affordable options for insulin, diabetes medications, services,” Kate Thomas, ADCES chief advocacy and external affairs officer, said in an interview. She added that ADCES has also pushed for legislation in Congress that would expand access to diabetes self-management training under the Medicare program.
The guide includes advice about overcoming barriers to discussing treatment costs with patients, suggested questions to ask patients about specific costs, and determinants of health and conversational approaches. Links are provided to resources for obtaining affordable insulin, other diabetes medications, and continuous glucose monitoring and insulin pump equipment.
“We know that, especially during primary care visits, there is limited time along with numbers of issues to talk about, so I think our challenge is how do we prioritize these conversations with something that can lead to action, not just saying you should do this but how do you actually do it,” Ms. Thomas said.
The introduction summarizes results from a 2021 Beyond Type 1 survey confirming prior findings reported by this news organization that cost is a frequent barrier for many individuals living with diabetes. “Especially right now where we are in terms of the impact of the pandemic and with peoples’ job statuses changing, I think it’s worthwhile to raise this in patient encounters,” Ms. Thomas said.
Overcoming conversational barriers
The first of three tables in the guide provides a list of “barriers to having a cost conversation” in the first column and “suggested solutions” in the second. For example, for the barrier, “You have insufficient time and/or knowledge about cost,” the suggestion is, “request and share available faculty and resources, including benefits coordinators, social workers, and community-based organizations. Work with the pharmacists and other members of the diabetes care team to identify resources that lower cost of medications.”
And for another barrier, “patients are often embarrassed or ashamed to initiate discussions of affordability,” the suggested solution is: “Normalize the issue of cost of care barriers for patients.”
A second table offers specific questions to ask patients about costs of medications and care, determinants of health, and financial barriers. These include: “What are some challenges you’ve had to accessing your medications or taking them as prescribed? What are some out-of-pocket health care costs you need help with? What challenges do you have accessing healthy food for you and your family?”
A link to a screening tool for social determinants of health is also included.
Language suggestions include talking about “cost of care” rather than “money,” asking patients if they’ve understood everything correctly by repeating back what they’ve said, and asking for confirmation and discussing follow-up.
Overall, the tool is designed to be a “broad conversation starter,” and not just about medications, Ms. Thomas said. “This is for all audiences and it’s meant to be something that the provider can tailor depending on who they’re speaking to. ... It’s about medications, but also the entire cost of care, including services and devices, transportation to appointments, access to food. ... Diabetes care isn’t just taking medication. It’s so many more factors.”
Ms. Thomas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The resource, “Having Healthcare Cost Conversations to Improve Patient Outcomes: A Practical Guide,” was jointly developed by the Association of Diabetes Care & Education Specialists and Beyond Type 1, the nonprofit patient advocacy organization.
Indeed, the guide appeared as President Biden discussed his proposal to cap insulin costs at $35 per insulin vial during the State of the Union address, during which he introduced a young boy with type 1 diabetes in the guest box, as reported by this news organization. On March 3, Civica, a nonprofit coalition of health systems and philanthropies, announced it plans to manufacture generic insulin at a deeply discounted price, as reported by this news organization.
“Just to see diabetes front and center at the State of the Union followed by these announcements is certainly reflective of our own advocacy effort to make sure that people have affordable options for insulin, diabetes medications, services,” Kate Thomas, ADCES chief advocacy and external affairs officer, said in an interview. She added that ADCES has also pushed for legislation in Congress that would expand access to diabetes self-management training under the Medicare program.
The guide includes advice about overcoming barriers to discussing treatment costs with patients, suggested questions to ask patients about specific costs, and determinants of health and conversational approaches. Links are provided to resources for obtaining affordable insulin, other diabetes medications, and continuous glucose monitoring and insulin pump equipment.
“We know that, especially during primary care visits, there is limited time along with numbers of issues to talk about, so I think our challenge is how do we prioritize these conversations with something that can lead to action, not just saying you should do this but how do you actually do it,” Ms. Thomas said.
The introduction summarizes results from a 2021 Beyond Type 1 survey confirming prior findings reported by this news organization that cost is a frequent barrier for many individuals living with diabetes. “Especially right now where we are in terms of the impact of the pandemic and with peoples’ job statuses changing, I think it’s worthwhile to raise this in patient encounters,” Ms. Thomas said.
Overcoming conversational barriers
The first of three tables in the guide provides a list of “barriers to having a cost conversation” in the first column and “suggested solutions” in the second. For example, for the barrier, “You have insufficient time and/or knowledge about cost,” the suggestion is, “request and share available faculty and resources, including benefits coordinators, social workers, and community-based organizations. Work with the pharmacists and other members of the diabetes care team to identify resources that lower cost of medications.”
And for another barrier, “patients are often embarrassed or ashamed to initiate discussions of affordability,” the suggested solution is: “Normalize the issue of cost of care barriers for patients.”
A second table offers specific questions to ask patients about costs of medications and care, determinants of health, and financial barriers. These include: “What are some challenges you’ve had to accessing your medications or taking them as prescribed? What are some out-of-pocket health care costs you need help with? What challenges do you have accessing healthy food for you and your family?”
A link to a screening tool for social determinants of health is also included.
Language suggestions include talking about “cost of care” rather than “money,” asking patients if they’ve understood everything correctly by repeating back what they’ve said, and asking for confirmation and discussing follow-up.
Overall, the tool is designed to be a “broad conversation starter,” and not just about medications, Ms. Thomas said. “This is for all audiences and it’s meant to be something that the provider can tailor depending on who they’re speaking to. ... It’s about medications, but also the entire cost of care, including services and devices, transportation to appointments, access to food. ... Diabetes care isn’t just taking medication. It’s so many more factors.”
Ms. Thomas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The resource, “Having Healthcare Cost Conversations to Improve Patient Outcomes: A Practical Guide,” was jointly developed by the Association of Diabetes Care & Education Specialists and Beyond Type 1, the nonprofit patient advocacy organization.
Indeed, the guide appeared as President Biden discussed his proposal to cap insulin costs at $35 per insulin vial during the State of the Union address, during which he introduced a young boy with type 1 diabetes in the guest box, as reported by this news organization. On March 3, Civica, a nonprofit coalition of health systems and philanthropies, announced it plans to manufacture generic insulin at a deeply discounted price, as reported by this news organization.
“Just to see diabetes front and center at the State of the Union followed by these announcements is certainly reflective of our own advocacy effort to make sure that people have affordable options for insulin, diabetes medications, services,” Kate Thomas, ADCES chief advocacy and external affairs officer, said in an interview. She added that ADCES has also pushed for legislation in Congress that would expand access to diabetes self-management training under the Medicare program.
The guide includes advice about overcoming barriers to discussing treatment costs with patients, suggested questions to ask patients about specific costs, and determinants of health and conversational approaches. Links are provided to resources for obtaining affordable insulin, other diabetes medications, and continuous glucose monitoring and insulin pump equipment.
“We know that, especially during primary care visits, there is limited time along with numbers of issues to talk about, so I think our challenge is how do we prioritize these conversations with something that can lead to action, not just saying you should do this but how do you actually do it,” Ms. Thomas said.
The introduction summarizes results from a 2021 Beyond Type 1 survey confirming prior findings reported by this news organization that cost is a frequent barrier for many individuals living with diabetes. “Especially right now where we are in terms of the impact of the pandemic and with peoples’ job statuses changing, I think it’s worthwhile to raise this in patient encounters,” Ms. Thomas said.
Overcoming conversational barriers
The first of three tables in the guide provides a list of “barriers to having a cost conversation” in the first column and “suggested solutions” in the second. For example, for the barrier, “You have insufficient time and/or knowledge about cost,” the suggestion is, “request and share available faculty and resources, including benefits coordinators, social workers, and community-based organizations. Work with the pharmacists and other members of the diabetes care team to identify resources that lower cost of medications.”
And for another barrier, “patients are often embarrassed or ashamed to initiate discussions of affordability,” the suggested solution is: “Normalize the issue of cost of care barriers for patients.”
A second table offers specific questions to ask patients about costs of medications and care, determinants of health, and financial barriers. These include: “What are some challenges you’ve had to accessing your medications or taking them as prescribed? What are some out-of-pocket health care costs you need help with? What challenges do you have accessing healthy food for you and your family?”
A link to a screening tool for social determinants of health is also included.
Language suggestions include talking about “cost of care” rather than “money,” asking patients if they’ve understood everything correctly by repeating back what they’ve said, and asking for confirmation and discussing follow-up.
Overall, the tool is designed to be a “broad conversation starter,” and not just about medications, Ms. Thomas said. “This is for all audiences and it’s meant to be something that the provider can tailor depending on who they’re speaking to. ... It’s about medications, but also the entire cost of care, including services and devices, transportation to appointments, access to food. ... Diabetes care isn’t just taking medication. It’s so many more factors.”
Ms. Thomas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gut microbiome species predict type 2 diabetes
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
according to results from a 15-year follow-up study of more than 5,000 people in Finland.
“We are not aware of previous long-term prospective studies of the associations between type 2 diabetes and the gut microbiome similar to the current study,” stated the authors of the study, published online Jan. 31, 2022, in Diabetes Care.
Though requiring further validation, the results “build on and extend previous mainly cross-sectional evidence and further support links between dietary habits, metabolic diseases, and type 2 diabetes that are modulated by the gut microbiome,” the authors wrote.
The findings are from a prospective study of data on fecal samples from 5,572 people in Finland in 2002 in the FINRISK 2002 population cohort. In 2017, the samples were sent for sequencing as follow-up.
Of note, the study excluded people with prevalent diabetes at baseline, including those being treated with antidiabetic drugs such as metformin.
Four species, two clusters associated with type 2 diabetes development
Over a median follow-up of 15.8 years, 432 (7.8%) participants went on to have a diagnosis of type 2 diabetes, and the presence of four species and two clusters at baseline were significantly associated with the development of type 2 diabetes.
The four species include Clostridium citroniae (hazard ratio, 1.21; unadjusted P = .02), C. bolteae (HR, 1.20; unadjusted P = .01), Tyzzerella nexilis (HR, 1.17; unadjusted P = .03), and Ruminococcus gnavus (HR, 1.17; P = .04).
And the two positively associated clusters mostly consisted of the same species (both HR, 1.18).
Importantly, the associations were nearly the same among participants in eastern and western Finland, which are known for having unique genetic as well as lifestyle differences that impact morbidity and mortality.
“Three of these taxa could be clustered together by proportional abundance in both geographic areas, and combined abundance of the four taxa was also predictive of incident type 2 diabetes,” the authors wrote.
They noted that the identified species have been previously associated with type 2 diabetes and appear to be linked in some ways to the quality of diet and with other metabolic diseases, such as fatty liver disease.
C. citroniae, for instance, has been associated with trimethylamine N-oxide (TMAO), a compound likely linked to the intake of red meat, and the authors noted that a direct association between red meat intake and type 2 diabetes risk has been known for more than 15 years.
TMAO has also been associated with adipose tissue inflammation and impeded hepatic insulin signaling, which are all involved in increased insulin resistance, high blood glucose levels, and type 2 diabetes, the authors explained.
R. gnavus has been previously associated with obesity in humans and animals. And the bacterial species is also “potentially related to glucose metabolism regulation and linked to increases in inflammatory cytokines, both of which are related to type 2 diabetes pathophysiology,” the authors reported.
Stepping stone toward improved prediction
Coauthor Teemu J. Niiranen, MD, PhD, of the division of medicine, Turku (Finland) University Hospital, noted that, while prior studies have linked type 2 diabetes with distinctive characteristics of gut microbiome composition, most studies have not included prospective data, and long-term studies have been lacking.
Furthermore, many of the studies could have been confounded by the use of antidiabetic drugs that could influence gut microbiome composition, including metformin, which was excluded in the current study.
“We avoid several of the biases related to cross-sectional studies, such as the confounding effects of diabetes medications,” Dr. Niiranen said in an interview.
“We also know the temporal sequence of the exposure and the outcome, and that the changes in the gut microbiome preceded the development of diabetes,” he said. “All in all, a cohort study like this provides a much greater level of evidence than cross-sectional studies.”
Dr. Niiranen noted, however, that “although we demonstrate that certain gut microbiome changes are associated with greater risk of future diabetes, we are still quite far from clinical use.”
In addition to needing to replicate the results in other ethnic groups and locations, “we would need to find optimal clinical cutoffs for clinical decision-making and demonstrate the amount increase in predictive ability, compared with conventional diabetes risk factors,” he said.
The study nevertheless “serves as a stepping stone toward the goal of improved prediction and the development of effective treatments for type 2 diabetes through modification of the gut microbiome,” the authors wrote.
Other research has shed light on gut bacteria that appear to be linked to the prevention rather than the development of diabetes, identifying species that help produce butyrate, a short-chain fatty acid that may in fact provide protection against type 2 diabetes.
And additional research does suggest potential clinical implications. Efforts to improve insulin sensitivity via the gut through fecal microbial transplantation are also making headway, with an oral capsule formulation showing benefit among patients with severe obesity.
The research was funded in part by grants from the Finnish Cultural Foundation, the Finnish Foundation for Cardiovascular Research, the Emil Aaltonen Foundation, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, and the Academy of Finland.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Past spontaneous abortion raises risk for gestational diabetes
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
Pregnant women with a history of spontaneous abortion had a significantly increased risk of gestational diabetes in subsequent pregnancies, based on data from more than 100,000 women.
Gestational diabetes is associated not only with adverse perinatal outcomes, but also with an increased risk of long-term cardiovascular and metabolic health issues in mothers and children, wrote Yan Zhao, PhD, of Tongji University, Shanghai, and colleagues.
Previous studies also have shown that spontaneous abortion (SAB) is associated with later maternal risk of cardiovascular disease and venous thromboembolism, the researchers said. The same mechanisms might contribute to the development of gestational diabetes, but the association between abortion history and gestational diabetes risk in subsequent pregnancies remains unclear, they added.
In a study published in JAMA Network Open, the researchers identified 102,259 pregnant women seen for routine prenatal care at a single hospital in Shanghai between January 2014 and December 2019. The mean age of the women was 29.8 years.
During the study period, 14,579 women experienced SAB (14.3%), 17,935 experienced induced abortion (17.5%), and 4,017 experienced both (11.9%).
In all, 12,153 cases of gestational diabetes were identified, for a prevalence of 11.9%. The relative risk of gestational diabetes was 1.25 for women who experienced SAB and 1.15 for those who experienced both SAB and induced abortion, and the association between SAB and gestational diabetes increased in a number-dependent manner, the researchers said. The increase in relative risk for gestational diabetes in pregnant women with one SAB, two SABs, and three or more SABs was 18%, 41%, and 43%, compared to pregnant women with no SAB history.
However, no association appeared between a history of induced abortion and gestational diabetes, the researchers said. “To date, no study has reported the association of prior induced abortion with gestational diabetes,” they wrote.
The study findings were limited by several factors including the reliance on self-reports for history of SAB and therefore possible underreporting, the researchers noted. Other limitations included the lack of data on the timing of SABs; therefore, the time between SAB and gestational diabetes diagnosis could not be included in the analysis, they said. Unknown variables and the inclusion only of women from a single city in China might limit the generalizability of the results, they added.
More research is needed to understand the biological mechanisms behind the association between SAB and gestational diabetes, an association that has potential public health implications, they noted. However, the results suggest that “pregnant women with a history of SAB, especially those with a history of recurrent SAB, should attend more antenatal visits to monitor their blood glucose and implement early prevention and intervention,” such as healthful eating and regular exercise, they wrote.
Findings confirm, not surprise
The diagnosis of gestational diabetes in the current study “was made with a slightly different test than we typically use in the United States – a 1-hour nonfasting glucola followed by a confirmatory 3-hour fasting glucola,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. The current study of both SAB and gestational diabetes is important because both conditions are very common and have been the focus of increased attention in the popular media and in scientific study, she said.
Dr. Prager said she was not surprised by the findings of a link between a history of gestational diabetes and a history of SAB, “but the association is likely that people at risk for gestational diabetes or who have undiagnosed diabetes/glucose intolerance are more likely to experience SAB,” she noted. “I would be surprised if the direction of the association is that SAB puts people at risk for gestational diabetes; more likely undiagnosed diabetes is a risk factor for SAB,” she added. “Perhaps we should be screening for glucose intolerance and other metabolic disorders more frequently in people who have especially recurrent SAB, as the more miscarriages someone had, the more likely they were in this study to be diagnosed with gestational diabetes;” or perhaps those with a history of SAB/recurrent SAB should be screened closer to 24 weeks’ than 28 weeks’ gestation to enable earlier intervention in those more likely to have gestational diabetes, Dr. Prager said.
The study was supported by the Key Program of the National Natural Science Foundation of China, the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Shanghai Municipal Medical and Health Discipline Construction Projects, and the Shanghai Rising-Star Program. The researchers and Dr. Prager had no financial conflicts to disclose. Dr. Prager serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
Gestational diabetes: Optimizing Dx and management in primary care
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24
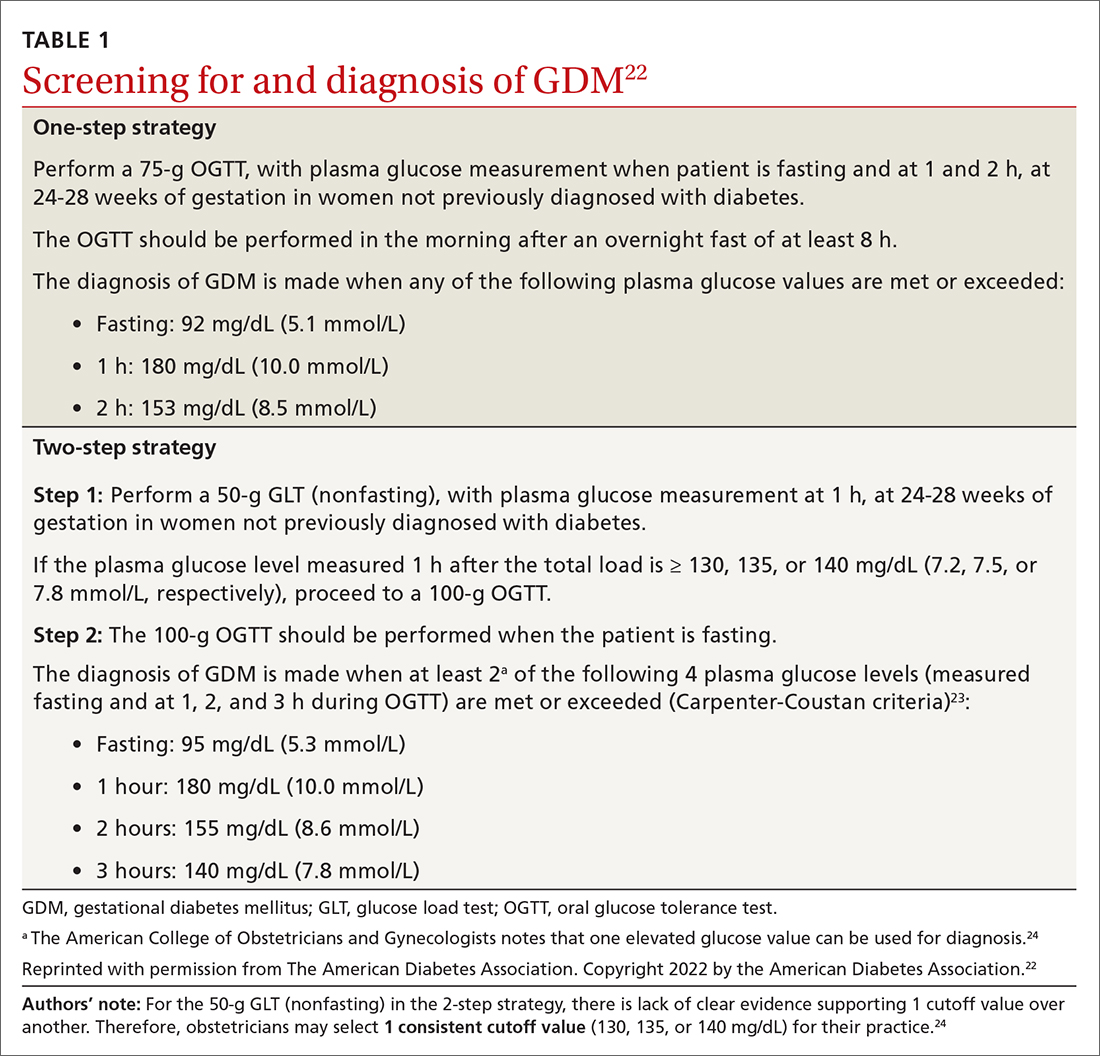
Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
Gestational diabetes mellitus (GDM), defined as new-onset hyperglycemia detected in a pregnant woman after 24 weeks of gestation, affects 4% to 10% of pregnancies in the United States annually1 and is a major challenge for health care professionals.2 During pregnancy, the body’s physiologic responses are altered to support the growing fetus. One of these changes is an increase in insulin resistance, which suggests that pregnancy alone increases the patient’s risk for type 2 diabetes (T2D). However, several other factors also increase this risk, including maternal age, social barriers to care, obesity, poor weight control, and family history.

If not controlled, GDM results in poor health outcomes for the mother, such as preeclampsia, preterm labor, and maternal T2D.3-5 For the infant, intrauterine exposure to persistent hyperglycemia is correlated with neonatal macrosomia, hypoglycemia, perinatal complications (eg, preterm delivery, fetal demise), and obesity and insulin resistance later in life.4
Primary care physicians (PCPs) are the patient’s main point of contact prior to pregnancy. This relationship makes PCPs a resource for the patient and specialists during and after pregnancy. In this article, we discuss risk factors and how to screen for GDM, provide an update on practice recommendations for treatment and management of GDM in primary care, and describe the effects of uncontrolled GDM.
Know the key risk factors
Prevention begins with identifying the major risk factors that contribute to the development of GDM. These include maternal age, social barriers to care, family history of prediabetes, and obesity and poor weight control.
Older age. A meta-analysis of 24 studies noted strong positive correlation between GDM risk and maternal age.6 One of the population-based cohort studies in the meta-analysis examined relationships between maternal age and pregnancy outcomes in women living in British Columbia, Canada (n = 203,414). Data suggested that the relative risk of GDM increased linearly with maternal age to 3.2, 4.2, and 4.4 among women ages ≥ 35, ≥ 40, and ≥ 45 years, respectively.7
Social barriers to care. Although the prevalence of GDM has increased over the past few decades,1 from 2011 to 2019 the increase in GDM in individuals at first live birth was significantly higher in non-Hispanic Asian and Hispanic/Latina women than in non-Hispanic White women.8 Data from the Centers for Disease Control and Prevention further suggest that diabetes was more prevalent among individuals with a lower socioeconomic status as indicated by their level of education.9 Ogunwole et al10 suggest that racism is the root cause of these disparities and leads to long-term barriers to care (eg, socioeconomic deprivation, lack of health insurance, limited access to care, and poor health literacy), which ultimately contribute to the development of GDM and progression of diabetes. It is important for PCPs and all health professionals to be aware of these barriers so that they may practice mindfulness and deliver culturally sensitive care to patients from marginalized communities.
Family history of prediabetes. In a population-based cohort study (n = 7020), women with prediabetes (A1C, 5.7%-6.4%) were 2.8 times more likely to develop GDM compared with women with normal A1C (< 5.7%).11 Similar results were seen in a retrospective cohort study (n = 2812), in which women with prediabetes were more likely than women with a normal first trimester A1C to have GDM (29.1% vs 13.7%, respectively; adjusted relative risk = 1.48; 95% CI, 1.15-1.89).12 In both studies, prediabetes was not associated with a higher risk for adverse maternal or neonatal outcomes.11,12
Continue to: While there are no current...
While there are no current guidelines for treating prediabetes in pregnancy, women diagnosed with prediabetes in 1 study were found to have significantly less weight gain during pregnancy compared with patients with normal A1C,12 suggesting there may be a benefit in early identification and intervention, although further research is needed.11 In a separate case-control study (n = 345 women with GDM; n = 800 control), high rates of gestational weight gain (> 0.41 kg/wk) were associated with an increased risk of GDM (odds ratio [OR] = 1.74; 95% CI, 1.16-2.60) compared with women with the lowest rate of gestational weight gain (0.27-0.4 kg/wk [OR = 1.43; 95% CI, 0.96-2.14]).13 Thus, it is helpful to have proactive conversations about family planning and adequate weight and glycemic control with high-risk patients to prepare for a healthy pregnancy.
Obesity and weight management. Patients who are overweight (body mass index [BMI], 25-29.9) or obese (BMI > 30) have a substantially increased risk of GDM (adjusted OR = 1.44; 95% CI, 1.04-1.81), as seen in a retrospective cohort study of 1951 pregnant Malaysian women.14 Several factors have been found to contribute to successful weight control, including calorie prescription, a structured meal plan, high physical activity goals (60-90 min/d), daily weighing and monitoring of food intake, behavior therapy, and continued patient–provider contact.15
The safety, efficacy, and sustainability of weight loss with various dietary plans have been studied in individuals who are overweight and obese.16 Ultimately, energy expenditure must be greater than energy intake to promote weight loss. Conventional diets with continuous energy restriction (ie, low-fat, low-carbohydrate, and high-protein diets) have proven to be effective for short-term weight loss but data on long-term weight maintenance are limited.16 The Mediterranean diet, which is comprised mostly of vegetables, fruits, legumes, fish, and grains—with a lower intake of meat and dairy—may reduce gestational weight gain and risk of GDM as suggested by a randomized controlled trial (RCT; n = 1252).17 Although the choice of diet is up to the patient, it is important to be aware of different diets or refer the patient to a registered dietician who can help the patient if needed.
Reduce risk with adequate weight and glycemic control
Prevention of GDM during pregnancy should focus on weight maintenance and optimal glycemic control. Two systematic reviews, one with 8 RCTs (n = 1792) and another with 5 studies (n = 539), assessed the efficacy and safety of energy-restricted dietary intervention on GDM prevention.18 The first review found a significant reduction in gestational weight gain and improved glycemic control without increased risk of adverse maternal and fetal outcomes.18 The second review showed no clear difference between energy-restricted and non–energy-restricted diets on outcomes such as preeclampsia, gestational weight gain, large for gestational age, and macrosomia.18 These data suggest that while energy-restricted dietary interventions made no difference on maternal and fetal complications, they may still be safely used in pregnancy to reduce gestational weight gain and improve glycemic control.18
Once a woman is pregnant, it becomes difficult to lose weight because additional calories are needed to support a growing fetus. It is recommended that patients with healthy pregestational BMI consume an extra 200 to 300 calories/d after the first trimester. However, extra caloric intake in a woman with obesity who is pregnant leads to metabolic impairment and increased risk of diabetes for both the mother and fetus.19 Therefore, it is recommended that patients with obese pregestational BMI not consume additional calories because excess maternal fat is sufficient to support the energy needs of the growing fetus.19
Continue to: Ultimately, earlier intervention...
Ultimately, earlier intervention—prior to conception—helps patients prepare for a healthier pregnancy, resulting in better long-term outcomes. It is helpful to be familiar with the advantages and disadvantages of common approaches to weight management and to be able to refer patients to nutritionists for optimal planning. When establishing a dietary plan, consider patient-specific factors, such as cultural diets, financial and time constraints, and the patient’s readiness to make and maintain these changes. Consistent follow-up and behavioral therapy are necessary to maintain successful weight control.
There are many screening tools, but 1 is preferred in pregnancy
There are several ways to diagnose diabetes in patients who are not pregnant, including A1C, a fasting glucose test, an oral glucose tolerance test (OGTT), or random glucose testing (plus symptoms). However, the preferred method for diagnosing GDM is OGTT because it has a higher sensitivity.20 A1C, while a good measure of hyperglycemic stability, does not register hyperglycemia early enough to diagnose GDM and fasting glucose testing is less sensitive because for most women with GDM, that abnormal postprandial glucose level is the first glycemic abnormality.21
When to screen. Blood glucose levels should be checked in all pregnant women as part of their metabolic panel at the first prenatal visit. A reflex A1C for high glucose levels can be ordered based on the physician’s preference. This may help you to identify patients with prediabetes who are at risk for GDM and implement early behavioral and lifestyle changes. However, further research is needed to determine if intervention early in pregnancy can truly reduce the risk of GDM.11
Screening for GDM should be completed at 24 to 28 weeks of gestation20 because it is likely that this is when the hormonal effects of the placenta that contribute to insulin resistance set the woman up for postprandial hyperglycemia. Currently, there are no evidence-based guidelines for the use of continuous glucose monitoring prior to 24 weeks of gestation to identify GDM.20 If persistent hyperglycemia is present before 24 weeks of gestation, it is considered evidence of a pre-existing metabolic abnormality and is diagnosed as “pregestational diabetes.” Treatment should follow guidelines established for women who had diabetes prior to pregnancy.
How to screen? There is ongoing discussion about what is the optimal screening method for GDM: a 1-step strategy with a fasting 75-g OGTT only, or a 2-step strategy with a 50-g non-fasting glucose load test followed by a fasting 100-g OGTT in women who do not meet the plasma glucose cutoff (TABLE 1).22-24 Hillier et al25 compared the effectiveness of these strategies in diagnosing GDM and identifying pregnancy complications for the mother and infant. They found that while the 1-step strategy resulted in a 2-fold increase in the diagnosis of GDM, it did not lead to better outcomes for mothers and infants when compared with the 2-step method.25 Currently, the majority of obstetricians (95%) prefer to use the 2-step method.24

Continue to: Manage lifestyle, monitor glucose
Manage lifestyle, monitor glucose
Management of GDM in most women starts with diabetes self-management education and support for therapeutic lifestyle changes, such as nutritional interventions that reduce hyperglycemia and contribute to healthy weight gain during pregnancy.20 This may include medical nutrition therapy that focuses on adequate nutrition for the mother and fetus. Currently, the recommended dietary intake for women who are pregnant (regardless of diabetes) includes a minimum of 175 g of carbohydrates, 71 g of daily protein, and at least 28 g of fiber. Further refinement of dietary intake, including carbohydrate restriction, should be done with guidance from a registered dietitian.20 If the obstetrics team does not include a registered dietitian, a referral to one may be necessary. Regular physical activity should be continued throughout pregnancy as tolerated. Social support, stress reduction, and good sleep hygiene should be encouraged as much as possible.
For successful outcomes, therapeutic lifestyle changes should be coupled with glucose monitoring. The Fifth International Workshop-Conference on Gestational Diabetes Mellitus recommends that women with GDM monitor fasting blood glucose and typically 1-hour postprandial glucose. The glucose goals in GDM are as follows26:
- Fasting glucose < 95 mg/dL (5.3 mmol/L), and either
- 1-hour postprandial glucose < 140 mg/dL (7.8 mmol/L), or
- 2-hour postprandial glucose < 120 mg/dL (6.7 mmol/L).
Importantly, in the second and third trimester, the A1C goal for women with GDM is 6.0%. This is lower than the more traditional A1C goal for 2 reasons: (1) increases in A1C, even within the normal range, increase adverse outcomes; and (2) pregnant women will have an increased red blood cell count turnover, which can lower the A1C.27 In a historical cohort study (n = 27,213), Abell et al28 found that women who have an A1C < 6.0% in the second and third trimester have the lowest risk of giving birth to large-for-gestational-age infants and for having preeclampsia.
Add insulin if glucose targets are not met
Most women who engage in therapeutic lifestyle change (70%-85%) can achieve an A1C < 6% and will not need to take medication to manage GDM.29 If pharmacotherapy is needed to manage glucose, insulin is the preferred treatment for all women with GDM.20 Treatment should be individualized based on the glucose trends the woman is experiencing. Common treatments include bedtime NPH if fasting hyperglycemia is most prominent and analogue insulin at mealtimes for women with prominent postprandial hyperglycemia.
Noninsulin agents such as metformin and sulfonylureas are not currently recommended by the American College of Obstetricians and Gynecologists or the American Diabetes Association for use in GDM.20,24 Despite being used for years in women with pregestational diabetes, metabolic syndrome, and polycystic ovary syndrome, there is evidence that metformin crosses the placenta and fetal safety has not yet been established in RCTs. The Metformin in Gestational Diabetes: The Offspring Follow-Up (MiG TOFU) study was a longitudinal follow-up study that evaluated body composition and metabolic outcomes in children (ages 7-9 years) of women with GDM who had received metformin or insulin while pregnant.30 At age 9 years, children who were exposed to metformin weighed more and had a higher waist-to-height ratio and waist circumference than those exposed to insulin.30
Continue to: Sulfonylureas are no longer recommended...
Sulfonylureas are no longer recommended because of the risk of maternal and fetal hypoglycemia and concerns about this medication crossing the placenta.24,31,32 Specifically, in a 2015 meta-analysis and systematic review of 15 articles (n = 2509), glyburide had a higher risk of neonatal hypoglycemia and macrosomia than insulin or metformin.33 For women who cannot manage their glucose with therapeutic lifestyle changes and cannot take insulin, oral therapies may be considered if the risk-benefit ratio is balanced for that person.34
Watch for effects of poor glycemic control on mother, infant
Preeclampsia is defined as new-onset hypertension and proteinuria after 20 weeks of gestation. The correlation between GDM and preeclampsia has partly been explained by their shared overlapping risk factors, including maternal obesity, excessive gestational weight gain, and persistent hyperglycemia.35 On a biochemical level, these risk factors contribute to oxidative stress and systemic vascular dysfunction, which have been hypothesized as the underlying pathophysiology for the development of preeclampsia.35
Neonatal macrosomia, defined as a birth weight ≥ 4000 g, is a common complication that develops in 15% to 45% of infants of mothers with GDM.36 Placental transfer of glucose in mothers with hyperglycemia stimulates the secretion of neonatal insulin and the ultimate storage of the excess glucose as body fat. After delivery, the abrupt discontinuation of placental transfer of glucose to an infant who is actively secreting insulin leads to neonatal hypoglycemia, which if not detected or managed, can lead to long-term neurologic deficits, including recurrent seizures and developmental delays.37 Therefore, it is essential to screen for neonatal hypoglycemia immediately after birth and serially up to 12 hours.38
Postpartum T2D. Poor glycemic control increases the risk of increasing insulin resistance developing into T2D postpartum for mothers.39 It also increases the risk of obesity and insulin resistance later in life for the infant.40 A retrospective cohort study (n = 461) found a positive correlation between exposure to maternal GDM and elevated BMI in children ages 6 to 13 years.41 Kamana et al36 further discussed this correlation and suggested that exposure to maternal hyperglycemia in utero contributes to fetal programming of later adipose deposition. Children may develop without a notable increase in BMI until after puberty.42
Partner with specialists to improve outcomes
Although most women with GDM are managed by specialists (obstetricians, endocrinologists, and maternal-fetal medicine specialists),43 these patients are still seeking care from their family physicians for other complaints. These visits provide key touchpoints during pregnancy and are opportunities for PCPs to identify a pregnancy-related complication or provide additional education or referral to the obstetrician.
Continue to: Also, if you work in an area...
Also, if you work in an area where specialists are less accessible, you may be the clinician providing the majority of care to a patient with GDM. If this is the case, you’ll want to watch for the following risk factors, which should prompt a referral to specialty care:
- a previous pregnancy with GDM20
- a previous birth of an infant weighing > 4000 g44
- baseline history of hypertension45
- evidence of insulin resistance or polycystic ovary syndrome46,47
- a history of cardiovascular disease20
- a need to treat GDM with pharmacotherapy.48
Ensuring a smooth transition after the birth
Optimal communication and hand-offs throughout pregnancy and after delivery will benefit everyone. When the pregnant patient’s care has been managed by an obstetrician, it is important to address the following issues during the hand-off:
- baseline medical problems
- medical screenings and treatments in pregnancy (retinopathy and nephropathy screening)
- aspirin initiation, if indicated
- management of thyroid abnormalities
- management of mental health conditions
- postpartum glucose management and T2D screening postpartum
- management of complications identified during pregnancy (retinopathy and nephropathy).
Timing and other elements of postpartum care. The first postpartum screen should occur at 4 to 12 weeks postpartum. OGTT is recommended instead of A1C at this time because A1C may still be lowered by the increased red blood cell turnover related to pregnancy and blood loss at delivery. Because women with GDM have a 50% to 75% lifetime risk of T2D,20 patients with normal test results should be re-tested every 1 to 3 years using any of the standard screening methods (A1C, fasting glucose, or OGTT).20
After delivery it may be difficult for women to follow-up with their own personal health care because they are focused on the care of their baby. The increased use of telehealth may make postpartum follow-up visits easier to attend.
Visits present opportunities. Postpartum visits present another opportunity for PCPs to screen for diabetes and other postpartum complications, including depression and thyroid abnormalities. Visits are also an opportunity to discuss timely contraception so as to prevent an early, unplanned pregnancy. Other important aspects of postpartum care are outlined in TABLE 2.20,49

CORRESPONDENCE
Connie L. Ha, BS, OMS IV, Department of Primary Care, 1310 Club Drive, Touro University California, Vallejo, CA 94592; [email protected]
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
1. Sheiner E. Gestational diabetes mellitus: long-term consequences for the mother and child grand challenge: how to move on towards secondary prevention? Front Clin Diabetes Healthc. 2020. doi: 10.3389/fcdhc.2020.546256
2. Angueira AR, Ludvik AE, Reddy TE, et al. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327-334. doi: 10.2337/db14-0877
3. Shou C, Wei Y-M, Wang C, et al. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Maternal-Fetal Med. 2019;1:91-94. doi: 10.1097/FM9.0000000000000019
4. Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342. doi: 10.3390/ijms19113342
5. Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J Pak Med Assoc. 2016;66(9 suppl 1):S74-S77.
6. Li Y, Ren X, He L, et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. doi: 10.1016/j.diabres.2020.108044
7. Schummers L, Hutcheon JA, Hacker MR, et al. Absolute risks of obstetric outcomes by maternal age at first birth: a population-based cohort. Epidemiology. 2018;29:379-387. doi: 10.1097/EDE.0000000000000818
8. Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326:660-669. doi: 10.1001/jama.2021.7217
9. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. Accessed February 2, 2022. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
10. Ogunwole SM, Golden SH. Social determinants of health and structural inequities—root causes of diabetes disparities. Diabetes Care. 2021;44:11-13. doi: 10.2337/dci20-0060
11. Chen L, Pocobelli G, Yu O, et al. Early pregnancy hemoglobin A1C and pregnancy outcomes: a population-based study. Am J Perinatol. 2019;36:1045-1053. doi: 10.1055/s-0038-1675619
12. Osmundson S, Zhao BS, Kunz L, et al. First trimester hemoglobin A1C prediction of gestational diabetes. Am J Perinatol. 2016;33:977-982. doi: 10.1055/s-0036-1581055
13. Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus [published correction appears in Obstet Gynecol. 2010;115:1092]. Obstet Gynecol. 2010;115:597-604. doi: 10.1097/AOG.0b013e3181cfce4f
14. Yong HY, Mohd Shariff Z, Mohd Yusof BN, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. doi: 10.1038/s41598-020-65251-2
15. Phelan S. Windows of opportunity for lifestyle interventions to prevent gestational diabetes mellitus. Am J Perinatol. 2016;33:1291-1299. doi: 10.1055/s-0036-1586504
16. Koliaki C, Spinos T, Spinou M, et al. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare (Basel). 2018;6:73. doi: 10.3390/healthcare6030073
17. Al Wattar BH, Dodds J, Placzek A, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. doi: 10.1371/journal.pmed.1002857
18. Zarogiannis S. Are novel lifestyle approaches to management of type 2 diabetes applicable to prevention and treatment of women with gestational diabetes mellitus? Global Diabetes Open Access J. 2019;1:1-14.
19. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest. 2019;129:4682-4690. doi: 10.1172/JCI130341
20. American Diabetes Association. 14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S200-S210. doi: 10.2337/dc21-S014
21. McIntyre HD, Sacks DA, Barbour LA, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2016;39:53-54. doi: 10.2337/dc15-1887
22. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768-773. doi: 10.1016/0002-9378(82)90349-0
24. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49-e64. doi: 10.1097/AOG.0000000000002501
25. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895-904. doi: 10.1056/NEJMoa2026028
26. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260. doi: 10.2337/dc07-s225
27. Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200-1201. doi: 10.2337/diacare.27.5.1200
28. Abell SK, Boyle JA, de Courten B, et al. Impact of type 2 diabetes, obesity and glycaemic control on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2017;57:308-314. doi: 10.1111/ajo.12521
29. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345-3355. doi: 10.2337/dc14-1530
30. Rowan JA, Rush EC, Plank LD, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018;6:e000456. doi: 10.1136/bmjdrc-2017-000456
31. Hebert MF, Ma X, Naraharisetti SB, et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin Pharmacol Ther. 2009;85:607-614. doi: 10.1038/clpt.2009.5
32. Malek R, Davis SN. Pharmacokinetics, efficacy and safety of glyburide for treatment of gestational diabetes mellitus. Expert Opin Drug Metab Toxicol. 2016;12:691-699. doi: 10.1080/17425255.2016.1187131
33. Balsells M, García-Patterson A, Solà I, et al. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015;350:h102. doi: 10.1136/bmj.h102
34. Kavitha N, De S, Kanagasabai S. Oral hypoglycemic agents in pregnancy: an update. J Obstet Gynaecol India. 2013;63:82-87. doi: 10.1007/s13224-012-0312-z
35. Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep. 2015;15:9. doi: 10.1007/s11892-015-0579-4
36. Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;66(suppl 2):14-20. doi: 10.1159/000371628
37. Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734-743. doi: 10.4239/wjd.v6.i5.734
38. Stanescu A, Stoicescu SM. Neonatal hypoglycemia screening in newborns from diabetic mothers—arguments and controversies. J Med Life. 2014;7(spec iss 3):51-52.
39. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31:292-301. doi: 10.1111/dme.12382
40. Stewart A, Malhotra A. Gestational diabetes and the neonate: challenges and solutions. Res Rep Neonatol. 2015;5:31-39. doi: 10.2147/RRN.S30971
41. Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87-92. doi: 10.1007/s00125-010-1925-3
42. Crume TL, Ogden L, Daniels S, et al. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941-946. doi: 10.1016/j.jpeds.2010.12.007
43. Levels of maternal care. Obstetric Care Consensus No. 9. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2019;134:e41-e55. doi: 10.1097/AOG.0000000000003383
44. Caughey AB, Cheng YW, Stotland NE, et al. Maternal and paternal race/ethnicity are both associated with gestational diabetes. Am J Obstet Gynecol. 2010;202:616.e1-e5. doi: 10.1016/j.ajog.2010.01.082
45. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. doi: 10.1016/j.ajog.2004.03.074
46. Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;5:CD011970. doi: 10.1002/14651858.CD011970.pub2
47. Ceysens G, Rouiller D, Boulvain M. Exercise for the diabetic pregnant woman. Cochrane Database Syst Rev. 2006;3:CD004225. doi: 10.1002/14651858.CD004225.pub2
48. Chawla R, Mukherjee JJ, Chawla M, et al. Expert group recommendations on the effective use of bolus insulin in the management of type 2 diabetes mellitus. Med Sci (Basel). 2021;9:38. doi: 10.3390/medsci9020038
49. American Diabetes Association. Introduction: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
PRACTICE RECOMMENDATIONS
› Manage gestational diabetes mellitus (GDM) with lifestyle behavior changes first and add insulin as a secondary treatment only if glycemic targets are not being met. A
› Treat hyperglycemia in GDM with insulin, not metformin or glyburide; these agents cross the placenta to the fetus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Lawsuit: 18-inch sponge left in stomach for 5 years; migrates internally
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
What is the psychological impact of type 1 diabetes?
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.
“Living with diabetes is not smooth sailing…From the onset of the disease in a child or adolescent through all the days that follow, there is nothing ordinary about it,” according to Aide aux Jeunes Diabétiques (AJD), a French association providing support for children and adolescents with diabetes. What is the psychological impact of the disease on patients and their loved ones? When we look at the life of a person with diabetes, are there key stages that call for more focused attention?
Nadine Hoffmeister, a psychologist at AJD, offers support to patients with diabetes and their parents as they navigate and deal with in-patient treatment for the disease. She recently spoke with this news organization.
Q: Are psychological issues more prevalent in patients with type 1 diabetes (T1D) than in the general population?
Dr. Hoffmeister: Having a chronic disease is not something that should be viewed as automatically making the person more susceptible to psychological issues. When we think about kids with T1D, it’s important to keep in mind that the risk for depression and the risk for eating disorders are, in general, higher in adolescence.
Of course, Clearly, the risk for eating disorders is there, given the constant focus on managing one’s diet. And there’s a greater risk for depression, because life with diabetes can really be trying. That said, how much impact the disease has depends in large part on the environment, the monitoring, and the collaboration of everyone involved.
Q: Are there key stages in the life of patients with T1D that call for targeted psychological support?
Dr. Hoffmeister: The thing about T1D is that it can affect anyone at any age – a small child, a teenager, a young adult. So, in that sense, all ‘firsts’ are key stages. They start, of course, with the first ‘first’: diagnosis. For children diagnosed at an early age, there’s the first day of nursery school or kindergarten, the first piece of birthday cake. Then we get to kids starting middle school and high school, places where they’re now left to their own devices. This is when, for the first time, they’ll have an opportunity to take a trip without their parents and siblings, to go to a party.
And then, there’s the first time using a particular treatment. For example, switching from injections to a pump requires not only an adjustment in terms of physically operating a new device, but a reorientation in terms of mentally settling into a new routine, a new way of administering medication, and so on. They have to learn how to get along with this machine that’s attached to them all the time. They have to view it as being a part of them, view it as a partner, a teammate, a friend. It’s not that easy.
Later on, one of the major stages is, of course, adolescence. Critical developments in the separation–individuation process are taking place. They start to feel the need to break free, to become autonomous, as they seek to fully come to terms with their disease.
Parents usually worry about this stage, adolescence. They’re scared that their child won’t be as vigilant, that they’ll be scatterbrained or careless when it comes to staying on top of all those things that need to be done to keep T1D under control. Most of the time, this stage goes better than they thought. Still, the fact remains that it’s difficult to find a happy medium between adolescence and diabetes. Indeed, there’s a bit of a paradox here. On the one hand, we have adolescence which, by definition, is a time of spontaneity, independence, of trying new things. On the other hand, we have diabetes and its limits and constraints, its care and treatment, day in and day out. We have to pay close attention to how the child navigates and makes their way through this stage of their life.
During adolescence, there’s also a heightened awareness and concern about how others look at you, see you – everywhere, not only in classrooms and hallways. If the way someone looks at them seems aggressive or intrusive, the child may start to feel scared. The risk then becomes that they’ll start feeling awkward or ashamed or embarrassed. We have to keep this in mind and help lead the child away from those feelings. Otherwise, they can end up with low self-esteem, they can start to withdraw.
It can sometimes get to the point where they choose to neglect their treatment so as to conform to the way others see them. Adults can easily lose sight of these kinds of things. So, it’s imperative that we talk to the child. If they’re having trouble following their treatment plan, maybe there’s something going on at school. So, let’s ask them: “How do you like your classes and teachers?” “How are you doing with your injections? Are you finding that they’re getting easier and easier to do?” And always keeping in mind the real possibility that the child may be feeling awkward, ashamed, embarrassed.
Q: Is enough being done to pick up on and address these children’s needs?
Dr. Hoffmeister: I think that these efforts are becoming more and more widespread. Still, there are disparities. When it comes to patients with chronic diseases, it’s not always easy to implement mental health care into the treatment plan. In some cases, there might not be a hospital nearby. And as we know, there are no spots available in medical and psychiatric centers. Of course, outside of hospital settings, we’re seeing the unfortunate situation of fewer and fewer middle schools and high schools having nurses on site.
And then, what options there are for getting support vary greatly from hospital to hospital. Some don’t have psychologists. Others have full schedules and not enough staff. That said, more and more teams are trying to set up regular appointments right from the time of diagnosis. This is a really good approach to take, even though the circumstances may not be ideal. After all, the person has just been told that they have diabetes; they’re not really in the best state of mind to have any kind of discussion.
Q: And so, it makes sense that AJD would offer the kind of mental health support that you’re now providing there.
Dr. Hoffmeister: Exactly. My position was created 4 years ago. I’m not at the hospital. I’m an external. The goal is to be able to offer this psychological support to everyone. I do consultations over the phone so that no matter where a person is in France, they’ll have access to this support. There’s great demand, and the requests are only increasing. I think this has to do with the fact that people are being diagnosed younger and younger. It’s a very complicated situation for the parents. No matter how young their child is, they want to get that support underway as soon as possible.
Q: You speak about the patients getting support. But doesn’t some kind of help have to be given to their parents and loved ones as well?
Dr. Hoffmeister: Yes. I’d say that 60% to 70% of the work I do at AJD is for parents. I also have some older adolescents and some younger kids whom I call to keep up with. But children aren’t very interested in discussing plans over the phone. For parents, the thing about diabetes is that they find themselves in these situations where their child is in the hospital for, say, a week, then is discharged, and all of a sudden, they find themselves at home as the ones in charge of their child’s treatment.
When it’s a little kid, the parents are the ones who are taking care of all the steps, the injections, the pumps. They’re dealing with the distress of a child going through episodes of nocturnal hypoglycemia. They’re experiencing varying degrees of anxiety in carrying out all of these responsibilities and, at the same time, the bond they have with their child is becoming stronger and stronger. So, there’s that anxiety. In this situation, parents may also feel a need for control. And they’re also feeling exhausted; the mental load of dealing with diabetes is very, very intense. To work through all this, many parents reach out for psychological support.
Then later on, when the child has gotten a little older, the parents find it difficult to get to the point of being able to just let go. But once the parents get to know their child better, get to know how their child experiences diabetes, they’ll get to that point. What they come to learn is that the child can take care of things, the child can feel what’s going on in their body, the child can be trusted.
Q: How can we help and support children with diabetes?
Dr. Hoffmeister: One of the most important things is to teach the child to come to terms with the disease and how it affects their body. In other words, the idea here is to adapt diabetes to one’s life, not the other way around. The goal is to not let diabetes take over.
When faced with standardized medical protocols, during a session with a psychologist, the child can talk about their life, give an idea of what a day in their life looks like. For example, the school cafeteria is a place where children get the opportunity to socialize and interact with their peers. We want to have that lunch period be as normal as possible for the child with diabetes. In some schools, lunchtime becomes a challenge. So, not seeing any other solution, mom stops working so the child can come home to eat. These are the kinds of situations where efforts to make the child feel included have failed. They’re tough to deal with, all around. And so this is why we do all we can to keep things as normal as possible for these children.
Q: What would you say is the one initiative out there that’s giving young patients with T1D the most help and support?
Dr. Hoffmeister: AJD offers stays at Care Management and Rehabilitation (SSR) sites. For kids and teenagers with diabetes, these places are like summer camps where every aspect of treatment is taken care of.
There’s a medical team monitoring their disease and a team of counselors always on hand. It’s a time when children may very well bring up things that are on their mind. All in all, the children have a safe and welcoming environment where treatment is provided and they can feel free to open up and talk.
If a problem crops up, I’m always on call to jump online. And throughout the stay, the medical team is keeping in touch to discuss the child’s care.
AJD is also an interdisciplinary association. We regularly organize practice exchange groups that bring together health care professionals and families from all over France. In this way, we’re able to collaborate and come up with resources, such as information packets and kits – for the newly diagnosed, for those starting intensive insulin therapy, and so on. These resources take into account medical protocols related to diabetes. They’re also designed with family life in mind. And having this set of resources works toward standardizing treatments.
A version of this article first appeared on Medscape.com.