User login
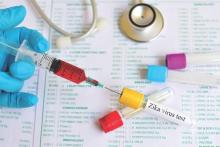
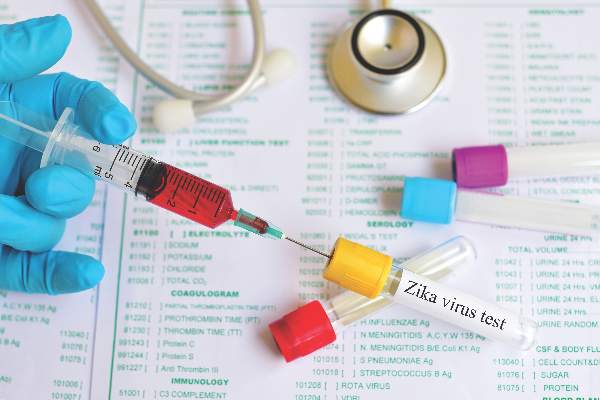
Donor blood testing highlights increasing Zika risk in Puerto Rico
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
An increasing prevalence of Zika virus infection detected among blood donors in Puerto Rico using a highly sensitive, investigational nucleic acid test likely reflects an overall increase in the incidence of infection in the population at large, according to the Centers for Disease Control and Prevention.
“The implications and importance of this information are that in the coming months, it is possible that thousands of women in Puerto Rico could become infected with Zika. This could lead to dozens or hundreds of infants being born with microcephaly in the coming year,” CDC Director Dr. Tom Frieden said in a telebriefing on June 17.
“For the thousands of other infants born to women infected with Zika who don’t have microcephaly, we simply don’t know, and might not know for years, if there will be long-term consequences on brain development,” Dr. Frieden added.
The incidence of infection among blood donors in Puerto Rico the week of June 5-11 was 1.1%. This is the highest weekly incidence since testing using a newly developed nucleic acid test authorized by the Food and Drug Administration under an investigational new drug application that was implemented in Puerto Rico in April.
Between April 3 and June 11, 2016, a total of 68 presumptive viremic donors were identified from 12,777 donations tested. The incidence of positive findings has increased steadily over time (MMWR. 2016 Jun 17. doi: 10.15585/mmwr.mm6524e2).
In the wake of the finding, which was reported June 17 in an early release of the Morbidity and Mortality Weekly Report, Dr. Frieden and Dr. Matthew J. Kuehnert, director of the CDC Office of Blood, Organ, and Other Tissue Safety sought to allay concerns regarding transmission of the virus via blood transfusion.
“The test being used to test blood in Puerto Rico is extremely accurate,” Dr. Kuehnert said, noting the importance of this testing, as several mosquito-borne illnesses are know to be transmissible by blood transfusion and many infected individuals are asymptomatic.
Further, at least one case of transfusion-transmitted Zika infection has been reported in Brazil, Dr. Kuehnert said.
The blood supply in Puerto Rico is being protected by laboratory testing, and in most other areas – including the continental United States – it is being protected by deferral of people who report travel to areas with such transmission. Some centers are electing to implement blood testing, but this is not currently a requirement, he said.
All donations that test positive for Zika are removed from the blood supply, and donors who test positive are provided with information on how to prevent spreading the virus to others.
The larger concern is the increasing prevalence of infections, as the nucleic acid test results “may be the most accurate, real time, leading indicator of Zika activity in Puerto Rico,” Dr. Frieden said.
The 1% prevalence of infection suggests substantial ongoing community transmission, given that viral nucleic acid can be detected for only 7-10 days after acute infection, and it translates to a greater than 1% rate of infection each month in the community, he noted.
“Our concern here is about protecting pregnant women, and with this rate of infection the possibility that there could be thousands of pregnant women affected, leading to dozens to hundreds of affected babies, is what’s of most concern,” Dr. Frieden said, adding that efforts are underway to reduce risk.
“We’re working intensively, in addition to keeping the blood supply safe, with the Puerto Rico health department, the government, communities, and people throughout Puerto Rico, to provide services for pregnant women – DEET, long sleeves, measures in their homes to reduce mosquito exposure that might reduce their risk of getting infected, as well as to control mosquitoes,” he said.
Dr. Frieden explained that controlling the Aedes aegypti mosquito is very difficult, and that it requires the effort of the entire community working together to protect a pregnant woman.
“We can’t make the risk zero … but even if we can reduce it by 10%, 30%, or 50%, that is a significant number of tragedies that we can prevent, and we’re doing everything that we can to do that … so that we don’t look back in 3, 6, or 12 months and say we wish we had done more back in June,” he said.
FROM MMWR
Three U.S. infants born with birth defects linked to Zika virus
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
There have been three infants born with birth defects and three pregnancy losses as a result of likely maternal Zika virus infection among U.S. women, according to figures released by the Centers for Disease Control and Prevention.
The figures, posted by the CDC on June 16, reflect reporting to the U.S. Zika Pregnancy Registry as of June 9. This is not real-time data and reflects only pregnancy outcomes for women with any laboratory evidence of possible Zika virus infection, though it is not known if Zika virus was the cause of the poor outcomes. The numbers also do not reflect outcomes among ongoing pregnancies.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The current numbers include outcomes reported in U.S. states and the District of Columbia. The CDC will begin reporting outcomes in U.S. territories in the coming weeks.
CDC officials plan to update the pregnancy outcome data every Thursday at http://www.cdc.gov/zika/geo/pregnancy-outcomes.html.
On Twitter @maryellenny
CDC updates guidelines for Zika virus testing, interpretation of results
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
Patients with suspected Zika virus infection who have negative real-time reverse transcription–polymerase chain reaction (rRT-PCR) test results are not automatically in the clear, officials at the Centers for Disease Control and Prevention warned in new guidelines on the interpretation of Zika virus antibody test results.
The CDC cautions that while a positive rRT-PCR test confirms a Zika virus infection, a negative test cannot exclude infection, because of Zika’s viremic similarity to other mosquito-borne flaviviruses such as dengue, West Nile, Japanese encephalitis, and yellow fever. In cases of a negative rRT-PCR results, patients should undergo immunoglobulin M (IgM) antibody testing; IgM testing should be done on any patient with a negative rRT-PCR test, regardless of the day on which the sample was originally collected. For confirmation of a positive, equivocal, or inconclusive result on the IgM test, patients will require a plaque reduction neutralization test (PRNT) as well.
“Recent evidence suggests that a 4-fold higher titer by PRNT might not discriminate between anti-Zika virus antibodies and crossreacting antibodies in all persons who have been previously infected with or vaccinated against a related flavivirus. Thus, a more conservative approach to interpreting PRNT results is now recommended to reduce the possibility of missing the diagnosis of either Zika or dengue virus infection,” CDC officials wrote. The report is in the May 31 issue of Morbidity and Mortality Weekly Report (doi: http://dx.doi.org/10.15585/mmwr.mm6521e1).
Any PRNT titer greater than 10 should be interpreted as proof of infection with the specific tested flavivirus, if the titer is less than 10 for other tested flaviviruses. If multiple flaviviruses are tested and all result in a titer of 10 or greater, than the result is proof of a recent infection with a flavivirus, not Zika specifically. And if the PRNT titer is less than 10 for a specific flavivirus, then that should be interpreted as there being no evidence of infection with that virus.
The CDC urges health care providers who have patients suspected of Zika virus infection to consult with their local and state health agencies to properly interpret any test results. Any pregnant women who have a confirmed Zika virus infection should be reported to the U.S. Zika Pregnancy Registry or the Puerto Rico Zika Active Pregnancy Surveillance System, with continued monitoring for any possible adverse effects to the pregnancy.
“If serologic testing indicates recent flavivirus infection that could be caused by either Zika or dengue virus, patients should be clinically managed for both infections because they might have been infected with either virus,” the CDC cautioned.
FROM MMWR
CDC monitoring 279 pregnant women in U.S. with Zika infection
More pregnant women in the United States and its territories may be at risk for Zika virus–related birth complications than previously reported, according to the Centers for Disease Control and Prevention.
As of May 12, CDC officials were monitoring 279 pregnant women with laboratory evidence of possible Zika virus infection – 157 in U.S. states and 122 in U.S. territories, according to a report published in the Morbidity and Mortality Weekly Report (2016 May 20. doi: 10.15585/mmwr.mm6520e1).
“We’ve learned a lot in the last 4 months, and now we know of reports of asymptomatic Zika infections linked to microcephaly, brain defects, and miscarriage,” Margaret Honein, Ph.D., chief of the birth defects branch at the CDC’s National Center for Birth Defects and Developmental Disabilities, said during a May 20 media briefing to announce enhanced tracking of the virus.
Recently published data indicating congenital defects in children born to mothers who had no recollection of ever having Zika-like symptoms spurred the CDC to widen its scope of data capture, Dr. Honein said.
Since February 2016, the CDC has been relying on data from the national arboviral disease surveillance system (ArboNET) to track Zika in pregnant women with symptoms of the Zika virus infection, or those with pregnancy complications consistent with the virus. Although ArboNET describes a subset of pregnancies at risk from Zika virus, it does not track actual congenital outcomes.
Now, federal health officials have created two registries solely dedicated to collecting data on birth outcomes among both symptomatic and asymptomatic women with any laboratory evidence of Zika virus infections. In the continental United States, there is the U.S. Zika Pregnancy Registry (USZPR). In Puerto Rico, there is the Zika Active Pregnancy Surveillance System (ZAPSS).
The 279 pregnant women currently being monitored reflect a broader group of pregnant women than numbers previously reported since it includes pregnant women who have any laboratory evidence of possible Zika virus infection, regardless of them being asymptomatic or whether they recalled ever having symptoms.
New figures on Zika infection in pregnancy will be posted on the CDC website each Thursday at noon EDT, and will reflect the previous week’s numbers.
“This reporting is in line with our recommendations for ongoing monitoring of pregnancies at risk for poor outcomes associated with Zika, based on our current understanding [of the virus],” Dr. Honein said. She recommended that pregnant women be tested for the virus at the start of prenatal care and again during the third trimester.The registry data will be used to determine those most at risk, characterize adverse Zika-related outcomes, and help officials plan for services needed by affected families, said Dr. Honein, encouraging all jurisdictions covered by the surveillance systems to participate in the data collection as quickly as possible.
Meanwhile, Dr. Honein said that the CDC is withholding specific information on known adverse birth outcomes in order to respect the affected families’ privacy, but did say that as of May 20, the CDC was aware of “less than a dozen adverse outcomes, including miscarriages and birth defects.”
Dr. Honein also declined to say how many of the cases reported to the registry involve sexual transmission of the virus versus actual contact with the Aedes aegypti mosquito.
On Twitter @whitneymcknight
More pregnant women in the United States and its territories may be at risk for Zika virus–related birth complications than previously reported, according to the Centers for Disease Control and Prevention.
As of May 12, CDC officials were monitoring 279 pregnant women with laboratory evidence of possible Zika virus infection – 157 in U.S. states and 122 in U.S. territories, according to a report published in the Morbidity and Mortality Weekly Report (2016 May 20. doi: 10.15585/mmwr.mm6520e1).
“We’ve learned a lot in the last 4 months, and now we know of reports of asymptomatic Zika infections linked to microcephaly, brain defects, and miscarriage,” Margaret Honein, Ph.D., chief of the birth defects branch at the CDC’s National Center for Birth Defects and Developmental Disabilities, said during a May 20 media briefing to announce enhanced tracking of the virus.
Recently published data indicating congenital defects in children born to mothers who had no recollection of ever having Zika-like symptoms spurred the CDC to widen its scope of data capture, Dr. Honein said.
Since February 2016, the CDC has been relying on data from the national arboviral disease surveillance system (ArboNET) to track Zika in pregnant women with symptoms of the Zika virus infection, or those with pregnancy complications consistent with the virus. Although ArboNET describes a subset of pregnancies at risk from Zika virus, it does not track actual congenital outcomes.
Now, federal health officials have created two registries solely dedicated to collecting data on birth outcomes among both symptomatic and asymptomatic women with any laboratory evidence of Zika virus infections. In the continental United States, there is the U.S. Zika Pregnancy Registry (USZPR). In Puerto Rico, there is the Zika Active Pregnancy Surveillance System (ZAPSS).
The 279 pregnant women currently being monitored reflect a broader group of pregnant women than numbers previously reported since it includes pregnant women who have any laboratory evidence of possible Zika virus infection, regardless of them being asymptomatic or whether they recalled ever having symptoms.
New figures on Zika infection in pregnancy will be posted on the CDC website each Thursday at noon EDT, and will reflect the previous week’s numbers.
“This reporting is in line with our recommendations for ongoing monitoring of pregnancies at risk for poor outcomes associated with Zika, based on our current understanding [of the virus],” Dr. Honein said. She recommended that pregnant women be tested for the virus at the start of prenatal care and again during the third trimester.The registry data will be used to determine those most at risk, characterize adverse Zika-related outcomes, and help officials plan for services needed by affected families, said Dr. Honein, encouraging all jurisdictions covered by the surveillance systems to participate in the data collection as quickly as possible.
Meanwhile, Dr. Honein said that the CDC is withholding specific information on known adverse birth outcomes in order to respect the affected families’ privacy, but did say that as of May 20, the CDC was aware of “less than a dozen adverse outcomes, including miscarriages and birth defects.”
Dr. Honein also declined to say how many of the cases reported to the registry involve sexual transmission of the virus versus actual contact with the Aedes aegypti mosquito.
On Twitter @whitneymcknight
More pregnant women in the United States and its territories may be at risk for Zika virus–related birth complications than previously reported, according to the Centers for Disease Control and Prevention.
As of May 12, CDC officials were monitoring 279 pregnant women with laboratory evidence of possible Zika virus infection – 157 in U.S. states and 122 in U.S. territories, according to a report published in the Morbidity and Mortality Weekly Report (2016 May 20. doi: 10.15585/mmwr.mm6520e1).
“We’ve learned a lot in the last 4 months, and now we know of reports of asymptomatic Zika infections linked to microcephaly, brain defects, and miscarriage,” Margaret Honein, Ph.D., chief of the birth defects branch at the CDC’s National Center for Birth Defects and Developmental Disabilities, said during a May 20 media briefing to announce enhanced tracking of the virus.
Recently published data indicating congenital defects in children born to mothers who had no recollection of ever having Zika-like symptoms spurred the CDC to widen its scope of data capture, Dr. Honein said.
Since February 2016, the CDC has been relying on data from the national arboviral disease surveillance system (ArboNET) to track Zika in pregnant women with symptoms of the Zika virus infection, or those with pregnancy complications consistent with the virus. Although ArboNET describes a subset of pregnancies at risk from Zika virus, it does not track actual congenital outcomes.
Now, federal health officials have created two registries solely dedicated to collecting data on birth outcomes among both symptomatic and asymptomatic women with any laboratory evidence of Zika virus infections. In the continental United States, there is the U.S. Zika Pregnancy Registry (USZPR). In Puerto Rico, there is the Zika Active Pregnancy Surveillance System (ZAPSS).
The 279 pregnant women currently being monitored reflect a broader group of pregnant women than numbers previously reported since it includes pregnant women who have any laboratory evidence of possible Zika virus infection, regardless of them being asymptomatic or whether they recalled ever having symptoms.
New figures on Zika infection in pregnancy will be posted on the CDC website each Thursday at noon EDT, and will reflect the previous week’s numbers.
“This reporting is in line with our recommendations for ongoing monitoring of pregnancies at risk for poor outcomes associated with Zika, based on our current understanding [of the virus],” Dr. Honein said. She recommended that pregnant women be tested for the virus at the start of prenatal care and again during the third trimester.The registry data will be used to determine those most at risk, characterize adverse Zika-related outcomes, and help officials plan for services needed by affected families, said Dr. Honein, encouraging all jurisdictions covered by the surveillance systems to participate in the data collection as quickly as possible.
Meanwhile, Dr. Honein said that the CDC is withholding specific information on known adverse birth outcomes in order to respect the affected families’ privacy, but did say that as of May 20, the CDC was aware of “less than a dozen adverse outcomes, including miscarriages and birth defects.”
Dr. Honein also declined to say how many of the cases reported to the registry involve sexual transmission of the virus versus actual contact with the Aedes aegypti mosquito.
On Twitter @whitneymcknight
Malaria vaccine provides long-term protection in phase I trial
A new malaria vaccine successfully protected a group of healthy United States adults from malaria for more than a year, according to Dr. Andrew Ishizuka and his associates.
In a phase I trial, 57 malaria-naive people received the PfSPZ Vaccine, which contains live but weakened Plasmodium falciparum sporozoites. Participants received either three or four intravenous doses, or four intramuscular injections with a dosage strength 10 times that of the IV immunizations, and were exposed to malaria 3 weeks after completing immunization. Participants who received the four-dose IV immunization had the highest rate of protection at 78%.
To assess long-term effectiveness of the vaccine, a group of five participants who had previously been exposed to malaria at 3 and 21 weeks post-immunization were exposed again after 59 weeks. No P. falciparum was found in the blood of these participants, while a group of six unvaccinated controls who were exposed to the parasites developed malaria.
“Malaria remains one of the most devastating diseases in the world, especially among young children in Africa. A malaria vaccine that provides long-term protection is urgently needed to reduce mortality and eliminate transmission. This study is an encouraging step forward in our goal to control and ultimately eradicate malaria,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a press release.
Find the study in Nature Medicine (2016 May. doi: 10.1038/nm.4110).
A new malaria vaccine successfully protected a group of healthy United States adults from malaria for more than a year, according to Dr. Andrew Ishizuka and his associates.
In a phase I trial, 57 malaria-naive people received the PfSPZ Vaccine, which contains live but weakened Plasmodium falciparum sporozoites. Participants received either three or four intravenous doses, or four intramuscular injections with a dosage strength 10 times that of the IV immunizations, and were exposed to malaria 3 weeks after completing immunization. Participants who received the four-dose IV immunization had the highest rate of protection at 78%.
To assess long-term effectiveness of the vaccine, a group of five participants who had previously been exposed to malaria at 3 and 21 weeks post-immunization were exposed again after 59 weeks. No P. falciparum was found in the blood of these participants, while a group of six unvaccinated controls who were exposed to the parasites developed malaria.
“Malaria remains one of the most devastating diseases in the world, especially among young children in Africa. A malaria vaccine that provides long-term protection is urgently needed to reduce mortality and eliminate transmission. This study is an encouraging step forward in our goal to control and ultimately eradicate malaria,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a press release.
Find the study in Nature Medicine (2016 May. doi: 10.1038/nm.4110).
A new malaria vaccine successfully protected a group of healthy United States adults from malaria for more than a year, according to Dr. Andrew Ishizuka and his associates.
In a phase I trial, 57 malaria-naive people received the PfSPZ Vaccine, which contains live but weakened Plasmodium falciparum sporozoites. Participants received either three or four intravenous doses, or four intramuscular injections with a dosage strength 10 times that of the IV immunizations, and were exposed to malaria 3 weeks after completing immunization. Participants who received the four-dose IV immunization had the highest rate of protection at 78%.
To assess long-term effectiveness of the vaccine, a group of five participants who had previously been exposed to malaria at 3 and 21 weeks post-immunization were exposed again after 59 weeks. No P. falciparum was found in the blood of these participants, while a group of six unvaccinated controls who were exposed to the parasites developed malaria.
“Malaria remains one of the most devastating diseases in the world, especially among young children in Africa. A malaria vaccine that provides long-term protection is urgently needed to reduce mortality and eliminate transmission. This study is an encouraging step forward in our goal to control and ultimately eradicate malaria,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a press release.
Find the study in Nature Medicine (2016 May. doi: 10.1038/nm.4110).
FROM NATURE MEDICINE
VIDEO: Delays in receiving Zika test results reported
WASHINGTON – The Centers for Disease Control and Prevention is recommending blood or urine testing for all pregnant women with possible Zika exposure through travel or because they live in an endemic area, regardless of whether they are symptomatic, Dr. Denise J. Jamieson said at the annual meeting of the American College of Obstetricians and Gynecologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Couples interested in conceiving who live in or have traveled to areas of active transmission should be alert for any signs of infection, no matter how slight; if they notice any, they should be tested for exposure before attempting conception, Dr. Jamieson, a medical officer with the CDC’s division of reproductive medicine, said in a video interview.
Unfortunately, she said at an update on the Zika situation, labs that process Zika blood and urine samples are backed up, and test results are being “unacceptably delayed.”
During a discussion period, some physicians in the audience complained of waiting up to 6 weeks for results. One said a local health department official told him that the CDC had a backlog of “a million tests.”
“We do not have a 1-million test backup,” Dr. Jamieson said. “But in some places there are still unacceptably long delays. This is not a test you can just check off on a form and send to a lab.”
Many of the tests are being funneled to the CDC’s Division of Vector-Borne Diseases in Ft. Collins, Colo.; that facility is experiencing a long turnaround time. Other samples are being handled in private labs with CDC contracts, but Dr. Jamieson said there’s an urgent need to expand the testing to many more commercial labs. There is no concrete plan for how or when this expansion will happen, however, she added.
Dr. Jamieson also discussed the CDC’s recommendation for managing pregnant women who have a positive serology or urine. These women should have serial ultrasounds every 3-4 weeks to track fetal brain development. Amniocentesis can positively confirm fetal exposure. A maternal-fetal medicine specialist should be on board if the fetus tests positive or if any concerning signs appear on imaging. Newborns can also be tested for exposure, as can placental and cord tissue.
On May 16, the World Health Organization released a comprehensive document describing a management algorithm for pregnant women who live in or travel to Zika-endemic areas.
Women who haven’t experienced signs and symptoms of an infection can proceed with routine care, and, if possible, an ultrasound before 18 weeks’ gestation and another at 28-30 weeks.
Women who have had signs of an infection should be tested for exposure. If negative, routine care with an early ultrasound and one at 28-30 weeks is indicated. If the results are positive, or if an early scan is concerning, patients should have a diagnostic ultrasound and amniocentesis, with referral to specialty care.
In the case of confirmed maternal exposure but normal early ultrasound, serial scans are indicated every month until delivery.
As a federal employee, Dr. Jamieson has no financial disclosures.
WASHINGTON – The Centers for Disease Control and Prevention is recommending blood or urine testing for all pregnant women with possible Zika exposure through travel or because they live in an endemic area, regardless of whether they are symptomatic, Dr. Denise J. Jamieson said at the annual meeting of the American College of Obstetricians and Gynecologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Couples interested in conceiving who live in or have traveled to areas of active transmission should be alert for any signs of infection, no matter how slight; if they notice any, they should be tested for exposure before attempting conception, Dr. Jamieson, a medical officer with the CDC’s division of reproductive medicine, said in a video interview.
Unfortunately, she said at an update on the Zika situation, labs that process Zika blood and urine samples are backed up, and test results are being “unacceptably delayed.”
During a discussion period, some physicians in the audience complained of waiting up to 6 weeks for results. One said a local health department official told him that the CDC had a backlog of “a million tests.”
“We do not have a 1-million test backup,” Dr. Jamieson said. “But in some places there are still unacceptably long delays. This is not a test you can just check off on a form and send to a lab.”
Many of the tests are being funneled to the CDC’s Division of Vector-Borne Diseases in Ft. Collins, Colo.; that facility is experiencing a long turnaround time. Other samples are being handled in private labs with CDC contracts, but Dr. Jamieson said there’s an urgent need to expand the testing to many more commercial labs. There is no concrete plan for how or when this expansion will happen, however, she added.
Dr. Jamieson also discussed the CDC’s recommendation for managing pregnant women who have a positive serology or urine. These women should have serial ultrasounds every 3-4 weeks to track fetal brain development. Amniocentesis can positively confirm fetal exposure. A maternal-fetal medicine specialist should be on board if the fetus tests positive or if any concerning signs appear on imaging. Newborns can also be tested for exposure, as can placental and cord tissue.
On May 16, the World Health Organization released a comprehensive document describing a management algorithm for pregnant women who live in or travel to Zika-endemic areas.
Women who haven’t experienced signs and symptoms of an infection can proceed with routine care, and, if possible, an ultrasound before 18 weeks’ gestation and another at 28-30 weeks.
Women who have had signs of an infection should be tested for exposure. If negative, routine care with an early ultrasound and one at 28-30 weeks is indicated. If the results are positive, or if an early scan is concerning, patients should have a diagnostic ultrasound and amniocentesis, with referral to specialty care.
In the case of confirmed maternal exposure but normal early ultrasound, serial scans are indicated every month until delivery.
As a federal employee, Dr. Jamieson has no financial disclosures.
WASHINGTON – The Centers for Disease Control and Prevention is recommending blood or urine testing for all pregnant women with possible Zika exposure through travel or because they live in an endemic area, regardless of whether they are symptomatic, Dr. Denise J. Jamieson said at the annual meeting of the American College of Obstetricians and Gynecologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Couples interested in conceiving who live in or have traveled to areas of active transmission should be alert for any signs of infection, no matter how slight; if they notice any, they should be tested for exposure before attempting conception, Dr. Jamieson, a medical officer with the CDC’s division of reproductive medicine, said in a video interview.
Unfortunately, she said at an update on the Zika situation, labs that process Zika blood and urine samples are backed up, and test results are being “unacceptably delayed.”
During a discussion period, some physicians in the audience complained of waiting up to 6 weeks for results. One said a local health department official told him that the CDC had a backlog of “a million tests.”
“We do not have a 1-million test backup,” Dr. Jamieson said. “But in some places there are still unacceptably long delays. This is not a test you can just check off on a form and send to a lab.”
Many of the tests are being funneled to the CDC’s Division of Vector-Borne Diseases in Ft. Collins, Colo.; that facility is experiencing a long turnaround time. Other samples are being handled in private labs with CDC contracts, but Dr. Jamieson said there’s an urgent need to expand the testing to many more commercial labs. There is no concrete plan for how or when this expansion will happen, however, she added.
Dr. Jamieson also discussed the CDC’s recommendation for managing pregnant women who have a positive serology or urine. These women should have serial ultrasounds every 3-4 weeks to track fetal brain development. Amniocentesis can positively confirm fetal exposure. A maternal-fetal medicine specialist should be on board if the fetus tests positive or if any concerning signs appear on imaging. Newborns can also be tested for exposure, as can placental and cord tissue.
On May 16, the World Health Organization released a comprehensive document describing a management algorithm for pregnant women who live in or travel to Zika-endemic areas.
Women who haven’t experienced signs and symptoms of an infection can proceed with routine care, and, if possible, an ultrasound before 18 weeks’ gestation and another at 28-30 weeks.
Women who have had signs of an infection should be tested for exposure. If negative, routine care with an early ultrasound and one at 28-30 weeks is indicated. If the results are positive, or if an early scan is concerning, patients should have a diagnostic ultrasound and amniocentesis, with referral to specialty care.
In the case of confirmed maternal exposure but normal early ultrasound, serial scans are indicated every month until delivery.
As a federal employee, Dr. Jamieson has no financial disclosures.
EXPERT ANALYSIS FROM ACOG 2016
Astrovirus MLB2 may cause CNS infection in immunocompromised patients
The astrovirus MLB2, normally prevalent in feces, can spread outside the digestive system and cause central nervous system infection in immunocompromised patients, according to Dr. Samuel Cordey and his associates.
The initial case-patient was a 21-year-old woman who was admitted because of an unusually severe headache and a fever. She was diagnosed with acute meningitis. Next-generation sequencing (NGS) identified 155 reads of astrovirus MLB2 in the patient’s cerebrospinal fluid, 9,340 in an anal swab, 18 in the patient’s plasma, and 16 in the urine. NGS did not identify any other RNA viruses, and no viral or bacterial pathogens were found in DNA sequencing.
In a subsequent pilot study, 943 stool samples from 615 patients and 424 CSF samples from 404 patients were tested for astrovirus MLB2 via reverse transcription PCR. Stool samples were positive for MLB2 in six patients, five of whom were severely immunocompromised. CSF samples were positive for MLB2 in one patient, a recipient of a hematopoietic stem cell transplant.
The study findings “could place astrovirus MLB2 in the differential diagnosis not only of diarrhea but also of aseptic meningitis and protracted infection in highly immunocompromised hosts. Potential determinants of extraintestinal dissemination, such as viral load kinetic, immune response, and host and viral genetic factors, require further characterization,” the investigators said.
Find the study in Emerging Infectious Diseases (doi: 10.3201/eid2205.151807).
The astrovirus MLB2, normally prevalent in feces, can spread outside the digestive system and cause central nervous system infection in immunocompromised patients, according to Dr. Samuel Cordey and his associates.
The initial case-patient was a 21-year-old woman who was admitted because of an unusually severe headache and a fever. She was diagnosed with acute meningitis. Next-generation sequencing (NGS) identified 155 reads of astrovirus MLB2 in the patient’s cerebrospinal fluid, 9,340 in an anal swab, 18 in the patient’s plasma, and 16 in the urine. NGS did not identify any other RNA viruses, and no viral or bacterial pathogens were found in DNA sequencing.
In a subsequent pilot study, 943 stool samples from 615 patients and 424 CSF samples from 404 patients were tested for astrovirus MLB2 via reverse transcription PCR. Stool samples were positive for MLB2 in six patients, five of whom were severely immunocompromised. CSF samples were positive for MLB2 in one patient, a recipient of a hematopoietic stem cell transplant.
The study findings “could place astrovirus MLB2 in the differential diagnosis not only of diarrhea but also of aseptic meningitis and protracted infection in highly immunocompromised hosts. Potential determinants of extraintestinal dissemination, such as viral load kinetic, immune response, and host and viral genetic factors, require further characterization,” the investigators said.
Find the study in Emerging Infectious Diseases (doi: 10.3201/eid2205.151807).
The astrovirus MLB2, normally prevalent in feces, can spread outside the digestive system and cause central nervous system infection in immunocompromised patients, according to Dr. Samuel Cordey and his associates.
The initial case-patient was a 21-year-old woman who was admitted because of an unusually severe headache and a fever. She was diagnosed with acute meningitis. Next-generation sequencing (NGS) identified 155 reads of astrovirus MLB2 in the patient’s cerebrospinal fluid, 9,340 in an anal swab, 18 in the patient’s plasma, and 16 in the urine. NGS did not identify any other RNA viruses, and no viral or bacterial pathogens were found in DNA sequencing.
In a subsequent pilot study, 943 stool samples from 615 patients and 424 CSF samples from 404 patients were tested for astrovirus MLB2 via reverse transcription PCR. Stool samples were positive for MLB2 in six patients, five of whom were severely immunocompromised. CSF samples were positive for MLB2 in one patient, a recipient of a hematopoietic stem cell transplant.
The study findings “could place astrovirus MLB2 in the differential diagnosis not only of diarrhea but also of aseptic meningitis and protracted infection in highly immunocompromised hosts. Potential determinants of extraintestinal dissemination, such as viral load kinetic, immune response, and host and viral genetic factors, require further characterization,” the investigators said.
Find the study in Emerging Infectious Diseases (doi: 10.3201/eid2205.151807).
FROM EMERGING INFECTIOUS DISEASES
CDC: Zika virus urine testing preferable to serum
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
New data showing that Zika virus can be found at higher levels or for longer duration in urine than serum, has prompted the Centers for Disease Control and Prevention to update its interim diagnostic testing guidance for the virus in public health laboratories.
The CDC now recommends that Zika virus real-time reverse transcription–polymerase chain reaction (rRT-PCR) be performed on urine collected less than 14 days after the onset of symptoms in patients with suspected Zika virus disease. The rRT-PCR is the preferred test for Zika infection because it can be performed rapidly and is highly specific, the CDC notes, and currently, the CDC Trioplex rRT-PCR assay is the only diagnostic tool authorized by the Food and Drug Administration for Zika virus testing of urine (MMWR. 2016 May 10. doi:10.15585/mmwr.mm6518e1).
In most patients, Zika virus RNA is unlikely to be detected in serum after the first week of illness, the CDC said, but adaptations of previously published diagnostic methods suggest that Zika virus RNA can be detected in urine for at least 2 weeks after onset of symptoms. However, the CDC affirmed that Zika virus rRT-PCR testing of urine should be performed in conjunction with serum testing if using specimens collected less than 7 days after symptom onset, and a positive result in either specimen type provides evidence of Zika virus infection.
The CDC added that, because viremia decreases over time and dates of illness onset may not be accurately reported, a negative rRT-PCR does not exclude Zika virus infection, and IgM antibody testing should be performed. The agency also said other laboratory-developed tests will need in-house validations to adequately characterize the performance of the assay and meet Clinical Laboratory Improvement Amendments requirements.
Read the full report here.
On Twitter @richpizzi
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
U.S. official raises concerns over Zika readiness
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
The ability of the United States to respond to a potential spike in Zika virus infection rates is a cause for concern, according to a top federal health official.
“The big question is will we get local transmission, and my response to that is very likely we will,” Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters during a joint media briefing with the Pan American Health Organization (PAHO) on May 3.
As many as 500 million people in the Americas are at risk for being infected by the Zika virus, PAHO’s Zika incident manager, Dr. Sylvain Aldighieri, said during the briefing.
In the continental United States to date, there have been about 400 travel-related cases of infection. In Puerto Rico, there have been nearly 700 locally reported cases, and one Zika-related death.
Countries at highest risk for Zika include those that have experienced any outbreaks of dengue fever or chikungunya in the past 15 years, Dr. Aldighieri said. Hawaii and U.S. territories in the Caribbean have experienced local dengue outbreaks during that time. Florida has had local outbreaks of both illnesses.
In the United States, Zika is poised to gain a stronger foothold even as funding for the study and prevention of the virus remains stalled in Congress, and a lack of cohesive public health messaging leaves the public vulnerable to misunderstanding the potential threat of the disease, according to Dr. Fauci.
A vaccine to fight Zika virus is currently under development. “Don’t confuse that with readiness,” Dr. Fauci cautioned.
Dr. Fauci said he believes the disbursement by Congress of President Obama’s requested $1.9 billion in Zika-related funds would facilitate a more comprehensive approach to preventing and treating the virus’s spread, but so far, the funding remains stalled.
As a result, Dr. Fauci said he has reallocated funds intended for other infectious disease research needs to cover Zika-related costs, but is concerned that continued congressional inaction could mean he is left with holes across many budgets. “That 1.9 billion dollars is essential,” he said.
Vaccine progress
In April, $589 millionin funds primarily earmarked for the Ebola crisis were redirected by the Obama administration to fight the Zika virus. That money is now being used in part to fund development of a vaccine that is expected to be ready for a phase I study of 80 people by September 2016. If successful, a phase II-b efficacy study of the vaccine would be conducted in the first quarter of 2017 in a country or region that has a high rate of infection.
Dr. Fauci said that although the study is not be as high-powered as would be ideal, researchers might be able to determine the vaccine’s efficacy with several thousand volunteers, taking into consideration that during the 1-3 years needed to gather conclusive data, herd immunity could skew rates of infection downward, bringing into question the vaccine’s actual efficacy.
“That’s just something we have to deal with,” Dr. Fauci said, saying that fewer people being infected is a good thing, either way.
Research gaps
Other pressing Zika research needs to include learning more about the virus’s impact on a developing fetus.
“We don’t know exactly what the percentage is of [infants born with] microcephaly,” Dr. Fauci said. “We don’t know beyond microcephaly what the long-range effects are on babies that look like they were born [without microcephaly] but might have other defects that are more subtle.”
Dr. Fauci said current data are unhelpful in that they show anywhere from 1% to 29% of infected mothers will give birth to children with congenital defects. However, he said that a coalition of nations affected by the virus is currently enrolling thousands of pregnant women in a cohort study to determine risk ratios.
“When we get the data from that study, we will be able to answer precisely what the percentage is, but today in May 2016, we don’t know the answer,” he said.
Predicting which infants are most susceptible, and at what point in utero abnormalities develop, are questions still under investigation, although a study published earlier this year supports the theory that infection during the first trimester poses the highest risk to a developing fetus.
Communicating risk
Another problem facing health officials is how to communicate the potential seriousness of an illness that, if it presents at all, does so only mildly, Dr. Fauci said. “In general, it’s a disease in which 80% of people don’t have any symptoms.”
The World Health Organization advises physicians to suspect Zika – particularly if a person has been in Zika-affected regions – if clinical symptoms include rash, fever, or both, plus at least one of these: arthralgia, arthritis, or conjunctivitis. Aside from bed rest, hydration, and over-the-counter analgesics, there are no specific treatments for the virus.
How to counsel women about avoiding pregnancy where Zika is a concern also poses challenges, particularly if the pregnancy is unintended, as about half of all American pregnancies are, or if, as Dr. Fauci told reporters, pregnancy is “guided by laws and religion.”
Although federal policy has not been to advise persons about whether to delay pregnancy, Dr. Fauci said U.S. officials are unwilling to contradict authorities in local regions such as Puerto Rico where such statements have been issued.
On April 28, the Food and Drug Administration authorized the emergency use of a commercial in vitro diagnostic test for use in individuals with symptoms of the virus, or those who have traveled to affected regions. Earlier this year, the FDA granted emergency authorization for use of a single test that can detect Zika, dengue, and chikungunya. Still, serology tests for Zika are often inconclusive, since the virus can mimic dengue or chikungunya, according to Dr. Aldighieri. “It can be complex to know if there is a Zika or dengue or chikungunya outbreak,” he said.
On Twitter @whitneymcknight
CDC reports hundreds of Zika virus cases in Puerto Rico
Since the beginning of the Zika virus outbreak in November 2015, Puerto Rico has had 683 cases of virus that have been laboratory-confirmed or presumed positive, according to new data from the Centers for Disease Control and Prevention.
Of the 683 Zika virus cases, 64% were found in women, with 65 cases occurring in pregnant women. The median age of patients was 34 years old. The most common symptoms were rash, myalgia, headache, fever, and arthralgia, all seen in more than 60% of patients. Hospitalization was required for 17 patients, and 1 death occurred due to complications from a severe thrombocytopenia. The data covers the time period of Nov. 1, 2015 to April 14, 2016.
Cases of Zika virus were negligible until the final week of November 2015, and rose slowly until spiking dramatically at the beginning of February 2016. Since early February, incidence of Zika has not fallen below 40 cases a week. Between Nov. 1, 2015 and April 14, 2016, there have been 110 cases of dengue and 61 cases of chikungunya reported, and neither had an incidence greater than 25 cases a week.
“Although Zika virus–associated deaths are rare, the first identified death in Puerto Rico highlights the possibility of severe cases, as well as the need for continued outreach to raise health care providers’ awareness of complications that might lead to severe disease or death,” the CDC investigators wrote. “To ensure continued blood safety, blood collection resumed with a donor screening program for Zika virus infection, and all units screened positive are removed.”
Find the full report in the CDC’s Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6517e2)
Since the beginning of the Zika virus outbreak in November 2015, Puerto Rico has had 683 cases of virus that have been laboratory-confirmed or presumed positive, according to new data from the Centers for Disease Control and Prevention.
Of the 683 Zika virus cases, 64% were found in women, with 65 cases occurring in pregnant women. The median age of patients was 34 years old. The most common symptoms were rash, myalgia, headache, fever, and arthralgia, all seen in more than 60% of patients. Hospitalization was required for 17 patients, and 1 death occurred due to complications from a severe thrombocytopenia. The data covers the time period of Nov. 1, 2015 to April 14, 2016.
Cases of Zika virus were negligible until the final week of November 2015, and rose slowly until spiking dramatically at the beginning of February 2016. Since early February, incidence of Zika has not fallen below 40 cases a week. Between Nov. 1, 2015 and April 14, 2016, there have been 110 cases of dengue and 61 cases of chikungunya reported, and neither had an incidence greater than 25 cases a week.
“Although Zika virus–associated deaths are rare, the first identified death in Puerto Rico highlights the possibility of severe cases, as well as the need for continued outreach to raise health care providers’ awareness of complications that might lead to severe disease or death,” the CDC investigators wrote. “To ensure continued blood safety, blood collection resumed with a donor screening program for Zika virus infection, and all units screened positive are removed.”
Find the full report in the CDC’s Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6517e2)
Since the beginning of the Zika virus outbreak in November 2015, Puerto Rico has had 683 cases of virus that have been laboratory-confirmed or presumed positive, according to new data from the Centers for Disease Control and Prevention.
Of the 683 Zika virus cases, 64% were found in women, with 65 cases occurring in pregnant women. The median age of patients was 34 years old. The most common symptoms were rash, myalgia, headache, fever, and arthralgia, all seen in more than 60% of patients. Hospitalization was required for 17 patients, and 1 death occurred due to complications from a severe thrombocytopenia. The data covers the time period of Nov. 1, 2015 to April 14, 2016.
Cases of Zika virus were negligible until the final week of November 2015, and rose slowly until spiking dramatically at the beginning of February 2016. Since early February, incidence of Zika has not fallen below 40 cases a week. Between Nov. 1, 2015 and April 14, 2016, there have been 110 cases of dengue and 61 cases of chikungunya reported, and neither had an incidence greater than 25 cases a week.
“Although Zika virus–associated deaths are rare, the first identified death in Puerto Rico highlights the possibility of severe cases, as well as the need for continued outreach to raise health care providers’ awareness of complications that might lead to severe disease or death,” the CDC investigators wrote. “To ensure continued blood safety, blood collection resumed with a donor screening program for Zika virus infection, and all units screened positive are removed.”
Find the full report in the CDC’s Morbidity and Mortality Weekly Report (doi: 10.15585/mmwr.mm6517e2)
FROM MMWR