User login
Fast foods contain endocrine-disrupting chemicals
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, such as chicken nuggets, hamburgers, and cheese pizza, new research suggests.
The first-of-its-kind study, which measured concentrations of chemicals such as phthalates in foods and gloves from U.S. fast food chains, is also the first to detect the plasticizer DEHT in fast foods.
“We knew from prior research that fast food consumption is linked to higher levels of phthalates in people’s bodies, but our study was novel because we actually collected these food items from fast food places and measured them,” said study author Lariah Edwards, PhD, a postdoctoral research scientist at the Milken Institute School of Public Health, George Washington University, Washington.
“Our research added an additional piece of information to the puzzle,” Dr. Edwards said in an interview.
A class of chemicals used in food packaging and food processing equipment, phthalates such as DEHP and DnBP, can leach out of these items and interfere with hormone production, Dr. Edwards said. They are linked with a wide variety of reproductive, developmental, brain, and immune effects, as well as with childhood obesity, asthma, cancer, and cardiovascular problems.
Meanwhile, nonphthalate or replacement plasticizers have been used in place of phthalates, some of which have been banned in certain products. But these plasticizers aren’t well studied, Dr. Edwards said, making the detection of DEHT in fast foods particularly concerning.
“There’s very limited research out there to understand the human health effects” of DEHT in food, she said, “so we’re being exposed before we understand what it’s doing to our health. It’s almost like we’re setting ourselves up for a big experiment.”
The study was recently published in the Journal of Exposure Science & Environmental Epidemiology .
Fast foods containing meat had highest concentrations of chemicals
Dr. Edwards and colleagues obtained 64 food samples, including hamburgers, fries, chicken nuggets, chicken burritos, and cheese pizza, as well as three pairs of unused gloves from six different fast food restaurants in San Antonio.
Using gas chromatography–mass spectrometry, they analyzed the samples for 11 chemicals, including eight phthalates and three replacement plasticizers.
The researchers detected 10 of the 11 chemicals in fast food samples: 81% of foods contained DnBP (di-n-butyl phthalate), and 70% contained DEHP (di(2-ethylhexyl phthalate)). Meanwhile 86% of samples contained replacement plasticizer DEHT (di(2-ethylhexyl terephthalate)).
Overall, fast food samples containing meat — including chicken nuggets, chicken burritos, and hamburgers — contained higher levels of these chemicals, Dr. Edwards noted.
“We know fast food is not the most nutritious, and now we’re seeing these chemicals in it we shouldn’t be exposed to,” she said.
The results also create implications for health equity, Dr. Edwards said, as Black people in the United States report eating more fast foods than other racial and ethnic groups for many reasons, such as longstanding residential segregation.
Many advocacy groups are pushing for stronger regulations on phthalates in foods, she said, and the study can be used to fuel those efforts.
“We’re hoping our findings help people understand what they’re eating and what’s in food,” Dr. Edwards said. “If they want to reduce exposure to phthalates in fast food, they can choose foods without meat in them. But not everyone has the option of reducing fast food consumption — personal choice is important, but policy is what’s going to protect us.”
Dr. Edwards noted that the research was limited by small sample sizes gathered in one U.S. city. Limitations in extraction methods also meant the researchers were able to detect chemicals in gloves only at high concentrations.
“That being said, I do think our results are fairly generalizable,” she added, “because the way fast foods are prepared at these restaurants is fairly consistent.”
The study was funded by the Passport Foundation, Forsythia Foundation, and Marisla Foundation. Dr. Edwards has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF EXPOSURE SCIENCE & ENVIRONMENTAL EPIDEMIOLOGY
New study ‘changes understanding of bone loss after menopause’
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the longest study of bone loss in postmenopausal women to date, on average, bone mineral density (BMD) at the femoral neck (the most common location for a hip fracture) had dropped by 10% in 25 years – less than expected based on shorter studies.
Specifically, average BMD loss at the femoral neck was 0.4% per year during 25 years in this new study from Finland, compared with a drop of 1.6% per year over 15 years reported in other cohorts.
Five-year BMD change appeared to predict long-term bone loss. However, certain women had faster bone loss, indicating that they should be followed more closely.
“Although the average bone loss was 10.1% ... there is a significant variation in the bone loss rate” among women in the study, senior author Joonas Sirola, MD, PhD, associate professor, University of Eastern Finland, and coauthor Heikki Kröger, MD, PhD, a professor at the same university, explained to this news organization in an email, so “women with fast bone loss should receive special attention.
The findings from the Kuopio Osteoporosis Risk Factor and Prevention study by Anna Moilanen and colleagues were published online October 19 in the Journal of Bone and Mineral Research.
Several factors might explain the lower than expected drop in femoral neck BMD (the site that is used to diagnose osteoporosis), Dr. Sirola and Dr. Kröger said. BMD depends on a person’s age, race, sex, and genes. And compared with other countries, people in Finland consume more dairy products, and more postmenopausal women there take hormone replacement therapy (HRT).
“If otherwise indicated, HRT seemed to effectively protect from bone loss,” the researchers noted.
Also, the number of women who smoked or used corticosteroids was low, so bone loss in other populations may be higher. Moreover, the women who completed the study may have been healthier to start with, so the results should be interpreted with caution, they urge.
Nevertheless, the study sheds light on long-term changes in BMD in postmenopausal women and “stresses the importance of high peak bone mass before menopause and keeping a healthy weight” during aging to protect bone health, they say.
Indeed the work “changes our understanding of bone loss in older women,” said Dr. Kröger in a press release from the university.
Check BMD every 5 years after menopause
Invited to comment, American Society of Bone and Mineral Research President Peter R. Ebeling, MD, who was not involved with the research, noted key findings are that the rate of femoral neck bone loss after perimenopause was far less than previously expected, and 5-year BMD change appeared to predict long-term bone loss in postmenopausal women.
“We know bone loss begins 1 year before menopause and accelerates over the next 5 years,” Dr. Ebeling, from Monash University, Melbourne, added in an email. “This study indicates some stabilization of bone loss thereafter with lesser effects of low estrogen levels on bone.”
“It probably means bone density does not need to be measured as frequently following the menopause transition and could be every 5 years, rather than every 2 years, if there was concern about continuing bone loss.”
Baseline risk factors and long-term changes in BMD
For the study, researchers examined the association between risk factors for bone loss and long-term changes in femoral neck BMD in 2,695 women living in Kuopio who were 47 to 56 years old in 1989. The women were a mean age of 53 years, and 62% were postmenopausal.
They answered questionnaires and had femoral neck BMD measured by DEXA every 5 years.
A total of 2,695, 2,583, 2,482, 2,135, 1,305, and 686 women were assessed at baseline and 5-, 10-, 15-, 20- and 25-year follow-ups, respectively, indicating significant study drop-out by 25 years.
By then, 17% of patients had died, 9% needed long-term care, some were unwilling to continue in the study, and others had factors that would have resulted in DEXA measurement errors (for example, hip implants, spine degeneration).
Researchers divided participants into quartiles of mean initial femoral neck BMD: 1.09 g/cm2, 0.97 g/cm2, 0.89 g/cm2, and 0.79 g/cm2, corresponding with quartiles 1 to 4 respectively (where quartile 1 had the highest initial femoral BMD and quartile 4 the lowest).
At 25 years, the mean femoral BMD had dropped to 0.97 g/cm2, 0.87 g/cm2, 0.80 g/cm2, and 0.73 g/cm2 in these respective quartiles.
Women lost 0.9%, 0.5%, 3.0%, and 1.0% of their initial BMD each year in quartiles 1 to 4, respectively.
And at 25 years, the women had lost 22.5%, 12.5%, 7.5%, and 2.5% of their initial BMD in the four quartiles, respectively.
Women in quartile 1 had the greatest drop in femoral BMD at 25 years, although their mean BMD at 25 years was higher than the mean initial BMD of the other women.
The prevalence of bone-affecting diseases, smoking, and use of vitamin D/calcium supplementation, corticosteroids, or alcohol was similar in the four quartiles and was not associated with significant differences in annual bone loss.
The most important protective factor was HRT
However, body mass index (BMI) and HRT were significantly different in the four quartiles.
On average, women in quartile 1 had a mean BMI of 26.7 kg/m2 at baseline and 27.8 kg/m2 at 25 years. Women in quartile 4 (lowest initial BMD and lowest drop in BMD) had a mean BMI of 24.9 kg/m2 at baseline and 28.4 kg/m2 at 25 years.
Women in quartile 4 (lowest initial BMD and lowest drop in BMD) were more likely to take HRT than women in quartile 1 (highest initial BMD and highest drop in BMD), at 41% versus 26%, respectively.
“The average decrease in bone mineral density was lower than has been assumed on the basis of earlier, shorter follow-ups where the bone loss rate at the femoral neck has been estimated to be even more than 20%,” Dr. Sirola commented in the press release.
“There were also surprisingly few risk factors affecting bone mineral density. The most significant factor protecting against bone loss was hormone replacement therapy. Weight gain during the follow-up also protected against bone loss,” Dr. Sirola added.
The study was funded by the Academy of Finland, Finnish Ministry of Education and Culture, and the Päivikki and Sakari Sohlberg Foundation. The authors and Dr. Ebeling have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nondiabetes hospitalization is wrong time to up diabetes meds
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
FROM JAMA NETWORK OPEN
Free vitamin D no better at predicting death in men than standard testing
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
In the clinical assessment of vitamin D concentrations, free 25-hydroxyvitamin D shows little added benefit to the current standard of total 25(OH)D, with deficiencies in each associated with at least a twofold risk of all-cause mortality, new research shows.
“In this prospective, population-based study of middle-aged and older European men, total 25(OH)D levels below 20 mcg/L were independently associated with a twofold increased all-cause mortality,” the researchers reported.
“Lower concentrations of free 25(OH)D were also predictive of mortality, but did not provide any additional information,” they noted. “The data do not support routine measurement of free 25(OH)D or 1,25(OH)2D [1,25-dihydroxyvitamin D] over total 25(OH)D levels.”
Despite vitamin D deficiency being well established as playing a role in a wide range of adverse health effects, including cardiovascular disease and mortality, there has been a lack of consensus on the optimal concentration of total 25(OH)D, with studies showing inconsistent levels to define insufficiency and deficiency.
One aspect of the debate has focused on precisely how to measure the concentrations, with some evidence supporting the “free hormone hypothesis,” which suggests that free 25(OH)D could represent a better indicator than the standard total 25(OH)D of functional availability of vitamin D, and have stronger clinical utility.
To investigate both issues, Marian Dejaeger, MD, PhD, and colleagues evaluated prospective data on 1,915 men recruited from eight centers around Europe in the European Male Aging Study in a report published in the Journal of Clinical Endocrinology & Metabolism
The men, who were aged between 40 and 79 years, had a mean follow-up of 12.3 years; during that time, about a quarter (23.5%) of them died.
In addition to other factors, including being older, having a higher body mass index, and having at least two comorbidities, men who died had significantly lower levels of total 25(OH)D, total 1,25(OH)2D, free 25(OH)D, and free 1,25(OH)2D, as well as higher parathyroid hormone and creatinine values.
After adjustment for key confounders, including body mass index, smoking, alcohol consumption, kidney function, number of comorbidities at baseline and other factors, men with a total 25(OH)D below 20 mcg/L had a significantly increased risk of mortality, compared with those who had normal levels of vitamin D, defined as above 30 mcg/L (hazard ratio, 2.03; P < .001).
In terms of free 25(OH)D, the lowest three free 25(OH)D quintiles (under 4.43 ng/L) similarly had a significantly higher mortality risk, compared with the highest quintile (HR, 2.09; P < .01) after adjustment for the confounders.
Further observations of all quintiles of other measures of 1,25(OH)2D and vitamin D binding protein (DBP) showed no associations with mortality after adjusting for confounders.
Methods of measurement
An important caveat of the study is the type of method used to measure free 25(OH)D. The authors calculated free 25(OH)D using a formula, as opposed to the alternative of direct measurement with an enzyme-linked immunosorbent assay kit, and there can be important differences between the two approaches, said Daniel Bikle, MD, PhD, a professor of medicine and dermatology at the San Francisco Veterans Affairs Medical Center and University of California, San Francisco, in a comment on the research.
“The biggest problem is that calculating free 25(OH)D does not give an accurate estimate of the real free level, so making conclusions regarding its role in clinical situations is subject to error,” said Dr. Bikle, who recently authored a review of the free hormone hypothesis.
A calculation approach “depends heavily on the total 25(OH)D level, so in a population with reasonably normal DBP and albumin levels, the correlation with total 25(OH)D is very high, so I am not surprised by the results showing no additional value,” he said in an interview.
The authors addressed their use of the calculation over the direct measurement in the study, noting that there is a “high correlation between both methods.”
But they added that, “as no equilibrium analysis method is available for free 25(OH)D, nor for free 1,25(OH)2D, no method can be considered superior.”
Dr. Dejaeger, of the department of public health and primary care, Katholieke Universiteit Leuven (Belgium), added that she agreed that high or low DBP could potentially shift some correlations, but noted that other research has shown calculated and direct measures to match relatively well.
“So we partly agree [with Dr. Bikle] not being surprised that we did not find an added value because we also found little variation in DBP, but we are not convinced that a different measurement method could make the difference here.”
Another caveat of the study is that, despite half of the measurements being taken in the summer, more than 90% of subjects in the study’s cohort had vitamin D insufficiency, defined in the study as total 25(OH)D levels below 30 mcg/L, and as many as 70% had deficiency, with levels below 20 mcg/L.
Therefore, “as the number of participants with high levels of total 25(OH)D in our study is small, a true threshold concentration for optimal vitamin D status cannot be defined on basis of our data,” the authors noted.
Under current recommendations, the Endocrine Society indicates that concentrations below 30 mcg/L are insufficient, while other groups, including the Institute of Medicine, suggest concentrations of 20 mcg/L or above are adequate.
Free hormone hypothesis
Under the free hormone hypothesis, which is observed with thyroid hormones and sex steroids, the very small fraction of free hormones that are not bound to protein carriers can enter cells and help facilitate biologic activity.
The hypothesis of a role of free 25(OH)D in mortality was supported by a recent study, in which free 25(OH)D levels – but not total 25(OH)D levels, were found to be independently associated with an increased risk of all-cause and cardiovascular mortality among patients with coronary artery disease.
However, two other studies are more consistent with the new findings, including one study showing no added value of free 25(OH)D as a marker for bone mineral density in older women, and another study showing no value as a marker of metabolic variables in healthy children.
“Currently, there are no hard data to support routine measurements of free 25(OH)D or 1,25(OH)2D over total 25(OH)D, the current standard of assessing vitamin D status, as stated in guidelines from different scientific bodies,” Dr. Dejaeger said in an interview.
The study received support from Versus Arthritis and the National Institute for Health Research Manchester Biomedical Research Centre. Dr. Dejaeger and Dr. Bikle had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Opioid-induced adrenal insufficiency for the hospitalist
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4
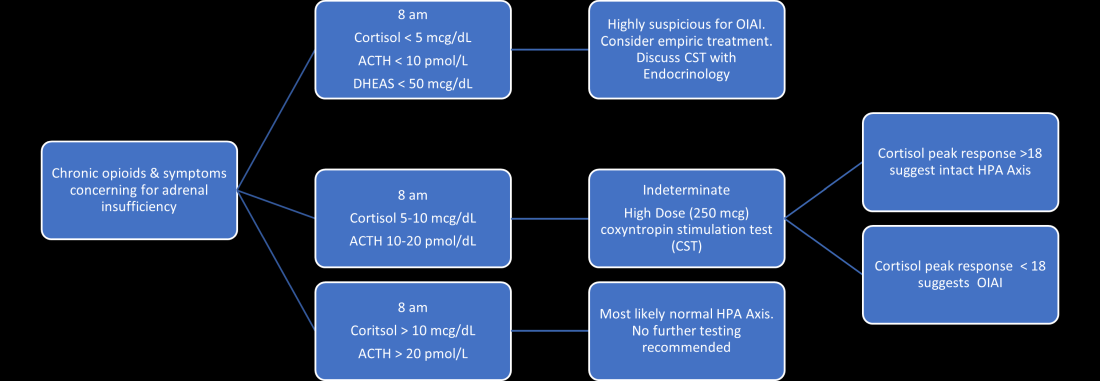
The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.
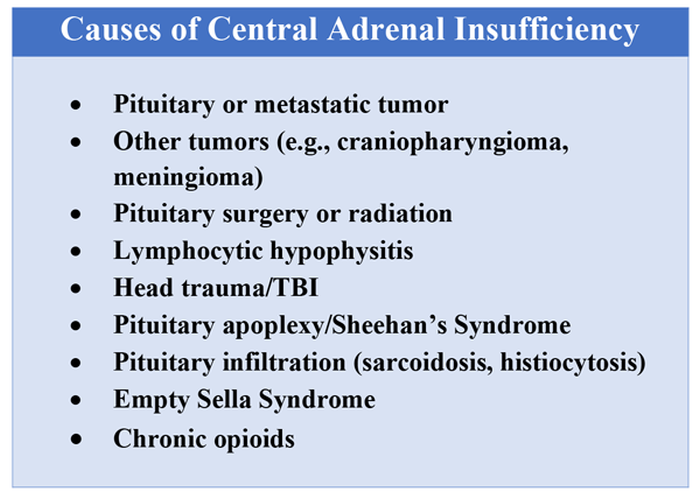
To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.
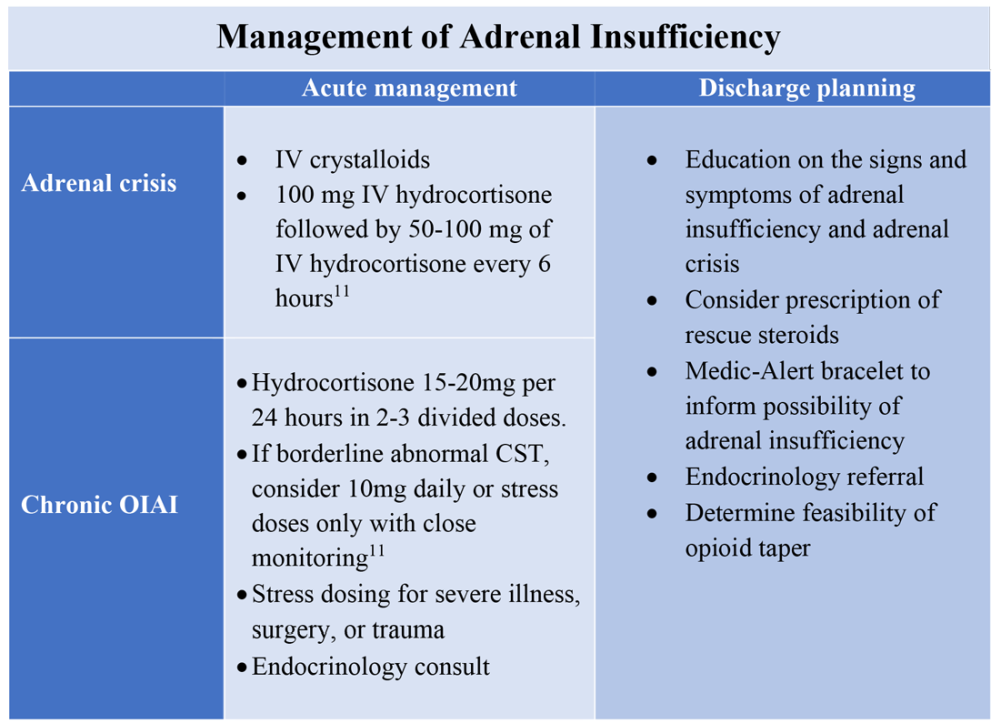
All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Consider OIAI, even among patients with common infections
Consider OIAI, even among patients with common infections
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
Case
A 60-year-old woman with metastatic breast cancer using morphine extended release 30 mg twice daily and as-needed oxycodone for cancer-related pain presents with fever, dyspnea, and productive cough for 2 days. She also notes several weeks of fatigue, nausea, weight loss, and orthostatic lightheadedness. She is found to have pneumonia and is admitted for intravenous antibiotics. She remains borderline hypotensive after intravenous fluids and the hospitalist suspects opioid-induced adrenal insufficiency (OIAI).
How is OIAI diagnosed and managed?
Brief overview of issue
In the United States, 5.4% of the population is currently using long-term opioids.1 Patients using high doses of opioids for greater than 3 months are 40%-50% more likely to be hospitalized than those on a lower dose or no opioids.2 Hospitalists frequently encounter common opioid side effects such as constipation, nausea, and drowsiness, but may be less familiar with their effects on the endocrine system. Chronic, high-dose opioids can suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause secondary, or central, adrenal insufficiency (AI).1
Recognition of OIAI is critical given the current opioid epidemic and life-threatening consequences of AI in systemically ill patients. While high-dose opioids may acutely suppress the HPA axis,3 OIAI is more commonly associated with long-term opioid use.4 The prevalence of OIAI among patients receiving long-term opioids ranges from 8.3% to 29%. This range reflects variations in opioid dose, duration of use, and different methods of assessing the HPA axis.1,4 When screening for HPA axis suppression in subjects taking chronic opioids, Lamprecht and colleagues found a prevalence of 22.5%.5 In comparison, Gibb and colleagues found the prevalence of secondary AI to be 8.3% in patients enrolled in a chronic pain clinic.6 Despite the high prevalence on biochemical screening, the clinical significance of OIAI is less clear. Clinical AI and adrenal crisis among patients on opioids are less frequent and mostly limited to case reports.7,8 In one retrospective cohort, one in 40 patients with OIAI presented with adrenal crisis during a hospitalization for viral gastroenteritis.9
With this prevalence, one would expect to diagnose OIAI more commonly in hospitalized patients. A concerning possibility is that this diagnosis is underrecognized because of either a lack of knowledge of the disease or the clinical overlap between the nonspecific symptoms of AI and other diagnoses. In patients reporting symptoms suggestive of OIAI, the diagnosis was delayed by a median of 12 months.9 The challenge for the hospitalist is to consider OIAI, even among patients with common infections such as pneumonia, viral gastroenteritis, or endocarditis who present with these nonspecific symptoms, while also avoiding unnecessary testing and treatment with glucocorticoids.
Overview of the data
Opiates and opioids exert their physiologic effect through activation of the mu, kappa, and delta receptors. These receptors are located throughout the body, including the hypothalamus and pituitary gland.4 Activation of these receptors results in tonic inhibition of the HPA axis and results in central AI.4 Central AI is characterized by a low a.m. cortisol, low adrenocorticotropic hormone (ACTH), and low dehydroepiandrosterone sulfate (DHEAS) levels.1,4 The low ACTH is indicative of central etiology. This effect of opioids is likely dose dependent with patients using more than 60 morphine-equivalent daily dose at greater risk.1,5
Unexplained or unresolved fatigue, musculoskeletal pain, nausea, vomiting, anorexia, abdominal pain, and orthostatic hypotension in a patient on chronic opioids should prompt consideration of OIAI.9 Once suspected, an 8 a.m. cortisol, ACTH level, and DHEAS level should be ordered. Because of the diurnal variation of cortisol levels, 8 a.m. values are best validated for diagnosis.10 While cutoffs differ, an 8 a.m. cortisol less than 5 mcg/dL combined with ACTH less than 10 pmol/L, and DHEAS less than 50 mcg/dL are highly suggestive of OIAI. Low or indeterminate baseline a.m. cortisol levels warrant confirmatory testing.4,10 While the insulin tolerance test is considered the gold standard, the high dose (250 mcg) cosyntropin stimulation test (CST) is the more commonly used test to diagnose and confirm AI. A CST peak response greater than 18-20 mcg/dL suggests an intact HPA axis (see Figure 1).10 This testing will diagnose central AI, but is not specific for OIAI. Other causes of central AI such as exogenous steroid use, pituitary pathology, and head trauma should be considered before attributing AI to opioids (see Table 1).4

The abnormal CST in central AI is from chronic ACTH deficiency and lack of adrenal stimulation resulting in adrenal atrophy. Adrenal atrophy leaves the adrenal glands incapable of responding to exogenous ACTH. This process takes several weeks; therefore, those with ACTH suppression caused by recent high-dose opioid use or subacute pituitary injury may have an indeterminate or normal cortisol response to high-dose exogenous ACTH.4 Even in the setting of a normal CST, there may remain uncertainty in the diagnosis of OIAI. When evaluating for central AI, the sensitivity and negative likelihood ratio of the CST are only 0.64 and 0.39, respectively.4 In the same cohort of 40 patients with OIAI, 11 patients had a normal CST.9 The low-dose (1 mcg) CST may increase the sensitivity, but the use of this test is limited because of technical challenges.1 Endocrinology consultation can assist when the initial diagnostic and clinical presentation is unclear.

To manage a patient on opioid therapy who has laboratory data consistent with central AI, the clinician must weigh the severity of symptoms, probability of opioid weaning, and risks associated with glucocorticoid treatment. Patients presenting with acute adrenal crisis, hypotension, or critical illness should be managed with intravenous steroid replacement per existing guideline recommendations.10,11
Patients with mild symptoms of nausea, vomiting, or orthostatic symptoms that resolve with treatment of their admitting diagnosis but who have evidence of an abnormal HPA axis should be considered for weaning opioid therapy. Evidence suggests that OIAI is reversible with reduction and cessation of chronic opioid use.4,9 These patients may not need chronic steroid replacement; however, they should receive education on the symptoms of AI and potentially rescue steroids for home use in the setting of severe illness. Patients with OIAI admitted for surgical procedures should be managed in accordance with existing guidelines for perioperative stress dosing of glucocorticoids for AI.
Those with persisting symptoms of OIAI and an abnormal HPA axis require endocrinology consultation and glucocorticoid replacement. There is limited evidence that suggests low dose steroid replacement in patients with OIAI can improve subjective perception of bodily pain, activity level, and mood in chronic opioid users.9 Li and colleagues found that 16 of 23 patients experienced improvement of symptoms on glucocorticoids, and 15 were able to discontinue opioids completely.9 The authors speculated that the improvement in fatigue and musculoskeletal pain after steroid replacement is what allowed for successful opioid weaning. Seven of 10 of these patients with available follow-up had recovery of the HPA axis during the follow-up period.9 In central AI, doses as low as 10-20 mg/day of hydrocortisone have been used.10,11 Hospitalists should educate patients on recognizing symptoms of AI, as this low dose may not be sufficient to prevent adrenal crisis.

All patients with evidence of abnormalities in the HPA axis should receive a Medic-Alert bracelet to inform other providers of the possibility of adrenal crisis should a major trauma or critical illness render them unconscious.4,10 Since OIAI is a form of central AI, mineralocorticoid replacement is not generally necessary.11 Endocrinology follow-up can help wean steroids as the HPA axis recovers after weaning opioid therapy. Recognizing and diagnosing OIAI can identify patients with untreated symptoms who are at risk for adrenal crisis, improve communication with patients on benefits of weaning opioids, and provide valuable patient education and safe transition of care.
Application of the data to the original case
To make the diagnosis of OIAI, 8 a.m. cortisol, ACTH, and DHEAS should be obtained. Her cortisol was less than 5 mcg/dL, ACTH was 6 pmol/L and DHEAS was 30 mcg/dL. A high dose CST was performed with 30-minute and 60-minute cortisol values of 6 mcg/dL and 9 mcg/dL, respectively. The abnormal CST and low ACTH indicate central AI. She should undergo testing for other etiologies of central AI, such as a brain MRI and pituitary hormone testing, before confirming the diagnosis of OIAI.
The insufficient adrenal response to ACTH in the setting of infection and hypotension should prompt glucocorticoid replacement. Tapering opioids could result in recovery of the HPA axis, though may not be realistic in this patient with chronic cancer-related pain. If the patient is at high risk for adverse effects of glucocorticoids, repeat testing of the HPA axis in the outpatient setting can assess if the patient truly needs steroid replacement daily rather than only during physiologic stress. The patient should be given a Medic-Alert bracelet and instructions on symptoms of AI and stress dosing upon discharge.
Bottom line
OIAI is underrecognized because of central adrenal insufficiency. Knowing its clinical characteristics, diagnostic pathways, and treatment options aids in recognition and management.
Dr. Cunningham, Dr. Munoa, and Dr. Indovina are based in the division of hospital medicine at Denver Health and Hospital Authority.
References
1. Donegan D. Opioid induced adrenal insufficiency: What is new? Curr Opin Endocrinol Diabetes Obes. 2019 Jun;26(3):133-8. doi: 10.1097/MED.0000000000000474.
2. Liang Y and Turner BJ. Opioid risk measure for hospitalization. J Hosp Med. 2015 July;10(7):425-31. doi: 10.1002/jhm.2350.
3. Policola C et al. Adrenal insufficiency in acute oral opiate therapy. Endocrinol Diabetes Metab Case Rep. 2014;2014:130071. doi: 10.1530/EDM-13-0071.
4. Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
5. Lamprecht A et al. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur J Endocrinol. 2018 Dec 1;179(6):353-62. doi: 10.1530/EJE-18-0530.
6. Gibb FW et al. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol (Oxf). 2016 June;85(6):831-5. doi:10.1111/cen.13125.
7. Abs R et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000 June;85(6):2215-22. doi: 10.1210/jcem.85.6.6615.
8. Tabet EJ et al. Opioid-induced hypoadrenalism resulting in fasting hypoglycaemia. BMJ Case Rep. 2019 Dec 11;12(12):e230551. doi: 10.1136/bcr-2019-230551.
9. Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-1297. doi: 10.4158/EP-2020-0297.
10. Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
11. Charmandari E et al. Adrenal insufficiency. Lancet. 2014 June 21;383(9935):2152-67. doi: 10.1016/S0140-6736(13)61684-0.
Key points
- Opioids can cause central adrenal insufficiency because of tonic suppression of the HPA axis. This effect is likely dose dependent, and reversible upon tapering or withdrawal of opioids.
- The prevalence of biochemical OIAI in chronic opioid users of 8%-29% clinical AI is less frequent but may be underrecognized in hospitalized patients leading to delayed diagnosis.
- Diagnosis of central adrenal insufficiency is based upon low 8 a.m. cortisol and ACTH levels and/or an abnormal CST. OIAI is the likely etiology in patients on chronic opioids for whom other causes of central adrenal insufficiency have been ruled out.
- Management with glucocorticoid replacement is variable depending on clinical presentation, severity of HPA axis suppression, and ability to wean opioid therapy. Patient education regarding symptoms of AI and stress dosing is essential.
Additional reading
Grossman AB. Clinical Review: The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010 Nov;95(11):4855-63. doi: 10.1210/jc.2010-0982.
Donegan D and Bancos I. Opioid-induced adrenal insufficiency. Mayo Clin Proc. 2018 July;93(7):937-44. doi: 10.1016/j.mayocp.2018.04.010.
Li T et al. Clinical presentation and outcomes of opioid induced adrenal insufficiency. Endocr Pract. 2020 Nov;26(11):1291-7. doi: 10.4158/EP-2020-0297.
Quiz
A 55-year-old man with chronic back pain, for which he takes a total of 90 mg of oral morphine daily, is admitted for pyelonephritis with fever, nausea, vomiting, dysuria, and abdominal pain. He is febrile and tachycardic on presentation, but his vitals quickly normalize after hydration and antibiotics. About 48 hours into his hospitalization his fevers, dysuria, and abdominal pain have resolved, but he has persistent nausea and headaches. On further questioning, he also reports weight loss and fatigue over the past 3 weeks. He is found to have a morning cortisol level less than 5 mcg/dL, as well as low levels of ACTH and DHEAS. OIAI is suspected.
Which of the following is true about management?
A. Glucocorticoid replacement therapy with oral hydrocortisone should be considered to improve his symptoms.
B. Tapering off opioids is unlikely to resolve his adrenal insufficiency.
C. Stress dose steroids should be started immediately with high-dose intravenous hydrocortisone.
D. Given high clinical suspicion for OIAI, further testing for other etiologies of central adrenal insufficiency is not recommended.
Explanation of correct answer
The correct answer is A. This patient’s ongoing nonspecific symptoms that have persisted despite treatment of his acute pyelonephritis are likely caused by adrenal insufficiency. In a symptomatic patient with OIAI, treatment with oral hydrocortisone should be considered to control symptoms and facilitate tapering opioids. Tapering and stopping opioids often leads to recovery of the HPA axis and resolution of the OIAI. Tapering opioids should be considered a mainstay of therapy for OIAI when clinically appropriate, as in this patient with chronic benign pain. Stress dose steroids are not indicated in the absence of critical illness, adrenal crisis, or major surgery. OIAI is a diagnosis of exclusion, and patients should undergo workup for other causes of secondary adrenal insufficiency.
What are the cardiorenal differences between type 1 and type 2 diabetes?
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
FROM EASD 2021
Bone risk: Is time since menopause a better predictor than age?
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
Although early menopause is linked to increased risks in bone loss and fracture, new research indicates that, even among the majority of women who have menopause after age 45, the time since the final menstrual period can be a stronger predictor than chronological age for key risks in bone health and fracture.
In a large longitudinal cohort, the number of years since a woman’s final menstrual period specifically showed a stronger association with femoral neck bone mineral density (BMD) than chronological age, while an earlier age at menopause – even among those over 45 years, was linked to an increased risk of fracture.
“Most of our clinical tools to predict osteoporosis-related outcomes use chronological age,” first author Albert Shieh, MD, told this news organization.
“Our findings suggest that more research should be done to examine whether ovarian age (time since final menstrual period) should be used in these tools as well.”
An increased focus on the significance of age at the time of the final menstrual period, compared with chronological age, has gained interest in risk assessment because of the known acceleration in the decline of BMD that occurs 1 year prior to the final menstrual period and continues at a rapid pace for 3 years afterwards before slowing.
To further investigate the association with BMD, Dr. Shieh, an endocrinologist specializing in osteoporosis at the University of California, Los Angeles, and his colleagues turned to data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of ambulatory women with pre- or early perimenopausal baseline data and 15 annual follow-up assessments.
Outcomes regarding postmenopausal lumbar spine (LS) or femoral neck (FN) BMD were evaluated in 1,038 women, while the time to fracture in relation to the final menstrual period was separately evaluated in 1,554 women.
In both cohorts, the women had a known final menstrual period at age 45 or older, and on average, their final menstrual period occurred at age 52.
After a multivariate adjustment for age, body mass index, and various other factors, they found that each additional year after a woman’s final menstrual period was associated with a significant (0.006 g/cm2) reduction in postmenopausal lumbar spine BMD and a 0.004 g/cm2 reduction femoral neck BMD (both P < .0001).
Conversely, chronological age was not associated with a change in femoral neck BMD when evaluated independently of years since the final menstrual period, the researchers reported in the Journal of Clinical Endocrinology and Metabolism.
Regarding lumbar spine BMD, chronological age was unexpectedly associated not just with change, but in fact with increases in lumbar spine BMD (P < .0001 per year). However, the authors speculate the change “is likely a reflection of age-associated degenerative changes causing false elevations in BMD measured by dual-energy x-ray absorptiometry.”
Fracture risk with earlier menopause
In terms of the fracture risk analysis, despite the women all being aged 45 or older, earlier age at menopause was still tied to an increased risk of incident fracture, with a 5% increase in risk for each earlier year in age at the time of the final menstrual period (P = .02).
Compared with women who had their final menstrual period at age 55, for instance, those who finished menstruating at age 47 had a 6.3% greater 20-year cumulative fracture risk, the authors note.
While previous findings from the Malmo Perimenopausal Study showed menopause prior to the age of 47 to be associated with an 83% and 59% greater risk of densitometric osteoporosis and fracture, respectively, by age 77, the authors note that the new study is unique in including only women who had a final menstrual period over the age of 45, therefore reducing the potential confounding of data on women under 45.
The new results “add to a growing body of literature suggesting that the endocrine changes that occur during the menopause transition trigger a pathophysiologic cascade that leads to organ dysfunction,” the authors note.
In terms of implications in risk assessment, “future studies should examine whether years since the final menstrual period predicts major osteoporotic fractures and hip fractures, specifically, and, if so, whether replacing chronological age with years since the final menstrual period improves the performance of clinical prediction tools, such as FRAX [Fracture Risk Assessment Tool],” they add.
Addition to guidelines?
Commenting on the findings, Peter Ebeling, MD, the current president of the American Society of Bone and Mineral Research, noted that the study importantly “confirms what we had previously anticipated, that in women with menopause who are 45 years of age or older a lower age of final menstrual period is associated with lower spine and hip BMD and more fractures.”
“We had already known this for women with premature ovarian insufficiency or an early menopause, and this extends the observation to the vast majority of women – more than 90% – with a normal menopause age,” said Dr. Ebeling, professor of medicine at Monash Health, Monash University, in Melbourne.
Despite the known importance of the time since final menstrual period, guidelines still focus on age in terms of chronology, rather than biology, emphasizing the risk among women over 50, in general, rather than the time since the last menstrual period, he noted.
“There is an important difference [between those two], as shown by this study,” he said. “Guidelines could be easily adapted to reflect this.”
Specifically, the association between lower age of final menstrual period and lower spine and hip BMD and more fractures requires “more formal assessment to determine whether adding age of final menstrual period to existing fracture risk calculator tools, like FRAX, can improve absolute fracture risk prediction,” Dr. Ebeling noted.
The authors and Dr. Ebeling had no disclosures to report.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Guidelines for managing hypo- and hyperparathyroidism
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large international team of experts has developed two comprehensive guidelines for diagnosing, evaluating, and managing hypoparathyroidism and hyperparathyroidism, which replace guidelines issued 5 and 7 years ago.
Aliya A. Khan, MD, presented an overview of the hypoparathyroidism guidelines and John P. Bilezikian, MD, presented key aspects of the hyperparathyroidism guidelines at the American Society of Bone and Mineral Research (ASBMR) 2021 Annual Meeting.
The guidelines will be published as 17 articles in two issues of the society’s Journal of Bone and Mineral Research in 2022 – one on hypoparathyroidism and the other on hyperparathyroidism.
The work represents an “unprecedented effort” by more than 100 experts from 16 countries (United States, Canada, Australia, Brazil, China, Denmark, France, Germany, India, Italy, Israel, Lebanon, Singapore, Spain, Sweden, and the United Kingdom), Dr. Bilezikian told this news organization in an interview.
More than 100 international and national endocrine and osteoporosis organizations, societies, and patient advocacy groups from more than 50 countries have expressed interest in endorsing the guidelines.
Management of hypoparathyroidism
The new guidelines on hypoparathyroidism replace the guidelines issued in 2016 that were developed at the First International Conference on the Management of Hypoparathyroidism, Dr. Khan, from McMaster University, Hamilton, Ont., said in an email.
There was a need for new hypoparathyroidism guidelines, she explained, because of the better understanding of associated complications, how to predict who will develop hypoparathyroidism postoperatively (and how to prevent this), how and when to investigate a genetic cause further, when to consider parathyroid hormone (PTH) replacement therapy (and the benefits of the various molecules available today as well as those being evaluated in clinical research), and how to diagnose and manage hypoparathyroidism during pregnancy and lactation.
The experts in hypoparathyroidism were divided into four task forces that covered epidemiology and financial burden, etiology and pathophysiology, genetics and diagnosis, and patient evaluation and management.
The guidelines, developed over the past 18 months, provide detailed evidence-based graded (strong to weak) as well as ungraded (current practice) recommendations.
Summarizing a few key takeaways, Dr. Khan noted the guidelines recommend that clinicians treating patients with hypoparathyroidism should:
- Diagnose hypoparathyroidism if serum calcium corrected for albumin is low in the presence of a low or inappropriately normal PTH confirmed on two occasions 2 weeks apart (which may be supported by other specified abnormalities).
- Determine the cause for the hypoparathyroidism (which includes postsurgery, genetic variant, autoimmune, radiation, or idiopathic causes).
- Evaluate target organ damage.
- Try to achieve treatment goals and minimize risks for long-term complications.
- Consider PTH replacement therapy if patients have inadequate control, with symptoms of hypocalcemia or hypercalcemia, high phosphate, kidney disease, or high urine calcium, or poor quality of life.
The guideline strongly recommends using PTH measurements after total thyroidectomy to try to predict which patients will develop permanent postsurgical hypoparathyroidism.
It provides a clinical approach for establishing the genetic etiology of hypoparathyroidism.
A meta-analysis of 81 studies identified that the most common symptoms/complications of chronic hypoparathyroidism were, in descending order, cataract (24%), infection (18%), nephrolithiasis, renal insufficiency, seizures, depression, ischemic heart disease, and arrhythmias.
Based on the best available evidence, the guideline advises that “clinicians need to carefully determine why a patient has hypoparathyroidism and develop an individualized treatment plan with conventional therapy consisting of calcium, active vitamin D, hydrochlorothiazide, and plain vitamin D,” Dr. Khan continued.
“If a patient has poorly controlled hypoparathyroidism with many symptoms or is not doing well, then clinicians must consider PTH replacement therapy, since this will replace the missing hormone, lower the urine calcium losses, bring the serum calcium back up to the normal reference range, and lower phosphate (which appears to be associated with kidney calcification and may also contribute to basal ganglia calcification and calcium deposits in the eye),” she noted.
The guideline also discusses the optimal way to monitor and treat patients during pregnancy, delivery, and breastfeeding to optimize outcomes for mother and baby. The key points are closer patient monitoring with normalization of calcium, urine calcium, phosphate, and vitamin D.
Management of primary hyperparathyroidism
There was a need to update the previous 2014 guidelines developed at the Fourth International Workshop on the Management of Primary Hyperparathyroidism because, among other things, recent studies have provided new evidence about the different clinical phenotypes of primary hyperparathyroidism and ways the disease affects the skeleton and kidneys, Dr. Bilezikian, from the College of Physicians and Surgeons, Columbia University, New York, explained.
The experts in hyperparathyroidism were divided into four task forces that covered epidemiology, pathophysiology and genetics; classical and nonclassical disease manifestations; surgical aspects; and patient evaluation and management.
As part of these topics, the experts reviewed biochemical, skeletal, and renal findings, nonclassical features (such as neurocognitive complaints), nutritional and pharmacologic approaches, and disease course with or without surgical or medical intervention.
They made recommendations for diagnosis of hypercalcemic and normocalcemic phenotypes, differential diagnosis, evaluation of the skeleton and the kidney, indications for surgery, role of parathyroid imaging, indications for pharmacologic intervention, and monitoring.
“Consider the way this disease has appeared to change in the last 50 years,” said Dr. Bilezikian. In the 1940s, 50s, and 60s, patients with hyperparathyroidism were really sick and had severe bone disease and kidney disease. Then in the 70s, 80s, and 90s, the disease was more often discovered because of a screening test; high serum calcium was a hallmark of finding asymptomatic hyperparathyroidism.
In recent years, hyperparathyroidism is often discovered incidentally, when examining the skeleton or kidneys, he continued.
Primary hyperparathyroidism can now be subdivided into three types: patients who have target organ (kidney, bone) involvement, patients who don’t have this, and patients who have normocalcemic primary hyperparathyroidism.
The guideline discusses new medications that have become available for hyperparathyroidism, as well as surgery (the only cure), including how preoperative imaging can identify the overactive parathyroid gland, and the guidelines go into detail about how to monitor a patient and why a clinician would or would not recommend surgery, Dr. Bilezikian explained.
In the end, treatment is tailored to the individual.
Last, the guideline identifies eight areas where more research is needed.
The guidelines were funded by unrestricted educational grants from Amolyt, Ascendis, Calcilytix, and Takeda. Dr. Khan has reported participating on advisory boards for Alexion, Amgen, Amolyt, and Takeda, being a consultant for Amgen, receiving grants from Alexion, Amgen, Takeda, and Ascendis, being an investigator for Alexion, Amgen, Takeda, Ascendis, and Chugai, and being a speaker for Alexion, Amgen, Takeda, and Ultragenyx. Dr. Bilezikian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Expensive insulins, pen devices dominate U.S. diabetes care
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
Despite the extensive recent focus on its cost, insulin use in the United States remains dominated by insulin glargine and other analogs, as well as pen devices for delivery, new research shows.
The findings come from a nationally representative audit of outpatient care with input from nearly 5,000 physicians who prescribed insulin to patients with type 2 diabetes in 2016-2020.
The dramatic rise in the price of insulin in the United States has been extensively discussed in recent years, particularly with the newer analogs as compared with older human insulins.
Few studies indicate analog insulins better than human insulins
“Our findings suggest that even with increased public scrutiny for insulin products ... [the market is] dominated by the use of insulin analogs and insulin pen delivery devices, with persistent uptake of newer products as they are approved,” lead author Rita R. Kalyani, MD, told this news organization.
“Though newer insulins offer potentially greater flexibility with reduced hypoglycemia for many patients, they are also much more costly, with minimal to no head-to-head studies suggesting significant differences in glucose-lowering efficacy when compared to human insulins,” she stressed.
“We found it surprising that, despite the much-publicized concerns regarding insulin costs, analog insulins continue to represent more than 80% of insulin visits in the U.S.” added Dr. Kalyani, of the Division of Endocrinology, Diabetes & Metabolism at Johns Hopkins University School of Medicine, Baltimore.
However, as expected, the study also revealed a gradual increased uptake in the use of biosimilar insulins as more have been introduced to the market.
Dr. Kalyani advised, “Clinicians should be aware of their individual prescribing patterns for insulin and consider the affordability of insulin for patients as part of shared decision-making during clinic visits, particularly given the greater financial strain that many patients have faced during the ongoing COVID-19 pandemic and the rising societal costs for diabetes care.”
The research was published online October 12 in JAMA Network Open by Dr. Kalyani and colleagues.
Analogs prevailed, while biosimilar use rose
The data come from the Health National Disease and Therapeutic Index, a quarterly sampling of approximately 4,800 physicians that provides nationally representative diagnostic and prescribing information on patients treated by office-based physicians in the United States.
Overall, there were 27,860,691 insulin treatment visits for type 2 diabetes in 2016-2020. Of those, long-acting analog insulins (glargine [Lantus], detemir [Levemir], and degludec [Tresiba]) accounted for 67.3% of treatment visits in 2016 and 74.8% of treatment visits in 2020.
Rapid-acting insulin analogs (lispro [Humalog], aspart [Novolog], faster aspart [Fiasp], and glulisine [Apidra]) accounted for about 21.2% of visits in 2016 and about 16.5% in 2020.
On the other hand, intermediate- and short-acting human insulins (NPH and regular) accounted for just 3.7% of visits in 2016 and 2.6% in 2020.
Grouped together, the long- and short-acting analogs accounted for 92.7% of visits in 2016 and 86.3% in 2020, while the human insulins represented just 7.3% of visits in 2016 and 5.5% in 2020.
The biosimilar analog insulins (glargine and lispro) first appeared in the database in 2017, accounting for 2.6% of visits that year and 8.2% by 2020.
Overall, the number of visits for insulin treatment declined by 18% between 2016 and 2020, from 6.0 million to 4.9 million. That drop may be due to multiple factors, Dr. Kalyani said.
“Recently updated clinical practice guidelines from professional societies such as the American Diabetes Association recommend the use of glucagon-like peptide-1 (GLP-1) receptor agonists prior to insulin when injectable medications are being considered [for type 2 diabetes],” she noted.
“In addition, during the pandemic, patients may not have been seeing their health care providers for routine diabetes care as often as before ... These and other factors may have contributed to the decrease in insulin visits that we observed.”
By specific insulins, glargine has topped the list all along, accounting for about half of all treatment visits, at 52.6% in 2020. Degludec came in second, at 17.4%, and lispro third, at 9.5%.
Use of pen devices also increased
The proportion of treatment visits for insulin vials/syringes declined from 63.9% in 2016 to 41.1% in 2020, while visits for insulin pens rose from 36.1% to 58.7%.
“Many pens are more costly compared to vials of the same insulin product. Interestingly, some studies have found that use of insulin pens may promote greater patient adherence to insulin and, as a result, more broadly decrease health care costs associated with diabetes. However, we did not specifically investigate the cost of insulin in our study,” Dr. Kalyani noted.
The proportion of visits for “newer” insulins, defined as those approved in 2010 or later, rose from 18.1% in 2016 to 40.9% in 2020, while the concurrent drop for insulins approved prior to 2010 was from 81.9% to 59.1%.
“The findings of our study provide insight into potential drivers of insulin costs in the U.S. and may inform health policy,” the researchers conclude.
Funded in part by the National Heart, Lung, and Blood Institute. Dr. Kalyani currently serves on the Endocrinologic and Metabolic Drugs Advisory Committee of the U.S. Food and Drug Administration.
A version of this article first appeared on Medscape.com.
White House unveils plan to combat endocrine-disrupting PFAS pollution
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.
The federal government is stepping up actions to protect Americans from per- and polyfluoroalkyl substances that continue to threaten health through pollution in the air, water, and foods, according to a statement from the White House on Oct. 18.
The comprehensive plan includes efforts to prevent per- and polyfluoroalkyl substances (PFAS) from being released into the air, drinking and ground water, and the food supply chain, according to the statement. Other efforts will expand cleanup and remediation of the impact of PFAS already present in the environment.
PFAS are a category of endocrine-disrupting chemicals (EDCs) that have been used for decades in a range of consumer products including cookware, stain-resistant clothes, fast food wrappers, treatments for carpets and furniture, and firefighting foams. PFAS can be released into the air, and also into surface water, drinking water, and ground water, because of how they are disposed, according to a 2020 report from the Endocrine Society and the International Pollutants Elimination Network. The report suggested that creation of more plastic products will likely increase exposure to PFAS and other EDCs.
The Environmental Protection Agency will take the lead on the Biden administration’s PFAS reduction efforts. The agency announced a PFAS Roadmap, which outlines actions to control PFAS over the next 3 years. The Roadmap’s goals include keeping PFAS out of the environment, holding polluters accountable for their actions, investing in scientific research to learn more about the impact of PFAS on human health, and prioritizing protection for disadvantaged communities. The EPA described its approach to PFAS as three pronged (Research, Restrict, Remediate). Planned actions noted on the EPA website include publication of a national PFAS testing strategy, establishing an improved review process for new PFAS, reviewing existing PFAS, and enhancing reporting to track sources and quantities of PFAS.
White House statement noted that other agencies committed to controlling PFAS include the Department of Defense, which will conduct cleanups and assessments at DOD and National Guard locations; the Food and Drug Administration, which will to expand its food supply testing to estimate dietary exposure to PFAS; and the Department of Agriculture, which is investigating causes and impacts of PFAS in the food system, and supporting research on environmental contaminants including PFAS.
The Department of Homeland Security has conducted an inventory of PFAS use, notably the use of PFAS in firefighting foams, and established an Emerging Contaminants Working Group to remediate PFAS and other contaminants. In addition, the Department of Health & Human Services monitors the evolving science on human health and PFAS and anticipates a report by the Centers for Disease Control and Prevention on the health effects of PFAS exposure, with data from eight states.
The American Chemistry Council (ACC), a trade association for American chemistry companies, issued a statement in response to the EPA’s PFAS Strategic Roadmap in which they supported the value of science-based regulation, but emphasized that PFAS are distinct from one another, and should not be grouped together for regulation purposes.
“According to EPA, approximately 600 PFAS substances are manufactured or in use today, each with its own unique properties and uses, from cellphones to solar panels, for which alternatives are not always available,” according to the ACC statement. “EPA’s Roadmap reinforces the differences between these chemistries and that they should not all be grouped together.” The newly formed Interagency Policy Committee on PFAS will coordinate PFAS response efforts across agencies and “help develop new policy strategies to support research, remediation, and removal of PFAS in communities across the country,” according to the White House statement.















