User login
MD-IQ only
When surgery is the next step in treating endometriosis—know your patient’s priorities and how to optimize long-term pain relief
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Treating endometriosis: maximizing all options for medical management, from hormones to new medical therapies
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Stephanie J. Estes, MD is a board certified Obstetrician/Gynecologist and Professor of Reproductive Endocrinology and Infertility at the Penn State Hershey Medical center in Hershey, Pennsylvania. As a subspecialist, she has a focus on endometriosis and fibroid research and also advances the care of women through advanced reproductive surgery techniques and robotic surgery.
Q: At what point do you consider medical therapies in your approach to a patient with endometriosis?
Dr. Estes: I consider medical therapies for every patient that I see. There are 3 categories that I think about:
- Are they a candidate for medical therapy?
- Are they a candidate for surgical therapy?
- Lastly, are they a candidate for fertility treatment?
The difference between the plans is if the patient desires fertility. Next, I consider the advantages and disadvantages of medical therapy. The pros of medical therapy are avoiding the risks of surgery, with known and unknown complications or adhesions. And the cons are side effects of medical therapy. Also, medical therapy does not address treating hydrosalpinges, endometriomas, or other deep infiltrating nodules and, clearly, it also inhibits fertility treatment. So, my overall process is looking at the patient’s goal of symptom management and how best to limit their number of surgical procedures. My approach spans many options, and I look at all of those to make an appropriate decision for each patient.
Q: How do you determine what options would be best suited for the patient?
Dr. Estes: The first line of symptom treatment best suited for the patient is always NSAIDs, which are nonsteroidal anti-inflammatory drugs. These are an appropriate first start in managing the dysmenorrhea and pelvic pain that can be associated with endometriosis. If there are no contraindications, the next most common way to manage endometriosis symptoms is combined oral contraceptive pills, transdermal patches, or rings. A key principle, especially when selecting a pill, is to look at the type of estrogen and progestin you’re choosing. Some practitioners may see these pills as equals, but there are differences. I always select a 20-µm ethinyl estradiol pill for my endometriosis patients. Then, I select a progestin, such as norethindrone or levonorgestrel, which provide good suppressive treatment.
The preferred hormonal therapy for endometriosis symptoms not only should be easy for the patient to use but also accomplish management of the symptoms that they are coming in for. Hormonal therapies have a low side-effect profile, which allows the patient to feel well for a long time. We know that endometriosis is a chronic disease, so this is something that the patient is going to need to manage for a long time. I really like to help my patients because they have other things to do in life. They want to take care of their kids, or they’re in school, or have other goals. Patients want to feel well while doing all these activities of life, and so an individualized approach is important. Some of my patients love taking pills, and they are perfectly happy to do so. Other people would prefer a more long-acting treatment so that they do not have to deal with remembering to take a medication every day.
Q: Explain how you would apply the use of an intrauterine device (IUD) to manage endometriosis.
Dr. Estes: I use many IUDs with progestin because evidence has shown them to be effective in managing endometriosis symptoms, specifically by decreasing dysmenorrhea. I will usually insert them during a patient’s surgery in the operating room. If I identify endometriosis and can place the IUD at the same time of the surgery, this see-and-treat philosophy maximizes the efficiency of patient care—and the patient avoids the discomfort of an in-office insertion. During the patient’s post-operative check, I evaluate how they feel and how the IUD is working for them. I think many patients are candidates for progestin IUDs, especially those who cannot take an estrogen-containing compound.
Q: Where do newly available therapies for endometriosis fit into your overall management approach?
Dr. Estes: Norethindrone 5 mg is an effective treatment for endometriosis and it is US Food and Drug Administration (FDA)‒approved for that cause. And then, the other medications that Penn State Hershey was part of the clinical trials for include Gonadotropin-releasing hormone (GnRH) antagonists such as elagolix. I saw the improvement in symptoms not only in the trial but also in continued follow-up of patients. GnRH antagonists are a group of medications that have a very good side-effect profile. Patients typically do not have significant side effects, however, hot flashes can me common. Hot flashes that can be ameliorated with some add back hormone therapy. The other drugs in the pipeline are relugolix and linzagolix. Relugolix is FDA approved for prostate cancer treatment. Endometriosis trials are expensive, and they are long and hard to do because of the pain factor—people do not want to stop their other medications to do the trial; however, the use of these medications will continue to be studied, and I look further to continuing to fine tune treatment options
Q: Is cost to the patient a consideration during management counseling, and should it be?
Dr. Estes: Absolutely. It comes up in every conversation because endometriosis is a long-term disease process that needs to be managed throughout the life cycle of a woman; you cannot have something that is going to be so expensive that will have to be taken for years and years or is not going to be continued. Because cost is critical, I use Lupron Depot as well as letrozole, and goserelin implants are also approved for endometriosis treatment. I also occasionally use danazol, which is a very different mechanism of action in select patients, so multiple options are all present. We have streamlined our pre-approval process for the GnRH antagonists to make it fairly easy.
It used to be a little bit harder, but now, if a patient has found that other medications did not offer relief for her endometriosis, then GnRH antagonists are much easier to obtain.
Q: Is there anything else you’d like to add?
Dr. Estes: Our patient involvement in clinical trials is just so valuable for endometriosis disease research. So, anyone out there living with endometriosis who would like to help medical science—wherever you live, wherever you are—get involved because this can help not only you but also the next person who comes after you. We are currently participating in a study right now on quinagolide. It is a vaginal ring that is not hormonal.
Again, we want to keep all options open and see what works and does not work. Science is so fantastic, and it is one of those summary points to note that we always need more information in terms of endometriosis. We really are aspiring to develop and apply treatment options that are effective throughout the lifespan of those affected by endometriosis.
Multidisciplinary management of endometriosis-associated pain
Andrea Rapkin, MD, is Board Certified by the American College of Obstetricians and Gynecologists (of which she is also a fellow). After obtaining her MD, she completed her residency in OBGYN at UCLA then joined the faculty at UCLA and is a Professor of Obstetrics and Gynecology. She was one of the first Obstetrician-Gynecologists to adapt the multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain.
You are the founder and director of a clinic focused on a multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain. How did you identify such a clinic as a therapeutic need for patients?
Dr. Rapkin: The short answer is that a significant proportion of women were not experiencing pain relief or had incomplete relief with traditional medical or surgical therapy. At the time, we also identified various red flags for traditional treatment failures. These red flags included the following: pain of greater than 6 months duration, pain out of proportion to pathology found on examination, multiple visceral and somatic complaints, and psychosocial abnormalities. We now understand more about the neurobiology underpinning these red flags.
With the widespread availability of laparoscopy in the late 70s and early 80s, many studies investigated the relationship between endometriosis lesions and pain. The general consensus is that there is no relationship between the location or severity of the endometriosis lesions or the disease stage (American Society for Reproductive Medicine staging) with type of symptoms, symptom severity, treatment response, recurrence, or even prognosis. In fact, pain recurrence after adequate surgical treatment is unrelated to the presence of endometriosis lesions found at the time of repeat laparoscopy. This lack of association between pain and presence of visible disease was supported by a recent New England Journal of Medicine article by Zondervan and colleagues demonstrating that up to 30% of women with chronic pelvic pain, present after excision of endometriotic lesions, become unresponsive to conventional treatment.
The neurobiological responses in an individual with chronic pain are more complicated than those seen in the setting of acute pain. Chronic pain may be triggered or maintained by an inflammatory process such as endometriosis but, over time, altered neural processing and psychosocial maladaptation can occur. The altered processing consists of both peripheral and central sensitization which change the way sensory information from the pelvic viscera and surrounding somatic structures in the periphery is transmitted and interpreted in the central nervous system (spinal cord and brain). Visceral pelvic pain can emanate from the uterus, ovaries and fallopian tubes, the urinary bladder, and the bowel, while the somatic sources include the surrounding abdominal wall, low back and pelvic floor muscles, and fascia, and bones. Signal amplification or peripheral sensitization in the pelvic region in women with endometriosis starts with localized inflammation, neovascularization, invasion and innervation of endometriotic implants. As the pelvic organs share thoracolumbar and sacral autonomic neural pathways, inflammation or dysfunction in one organ or tissue, such as the uterus, over time can sensitize or lead to dysfunction in other pelvic organs, such as the bladder or bowel (called viscero-visceral cross sensitization). Finally, somatic structures sharing intervention with the pelvic viscera, such as the fascia and muscles of the lower abdomen, pelvic floor lower back also become sources of pain because of a process called viscero-somatic sensitization. Women who have endometriosis are therefore more likely to experience IBS, bladder pain syndrome/interstitial cystitis and vulvodynia, and up to 80% of individuals can develop myofascial pain related to trigger points and muscle dysfunction. Up to 50% of women with bladder pain have endometriosis. Those with endometriosis or bladder pain are 2.5 times more likely to also have IBS.
Over time, other visceral and somatic structures innervated by higher levels of the spinal cord can be affected, leading to more widespread pain. Central sensitization manifests as an amplification of pain in the spinal cord and brain. The presence of more than two chronic “unexplained” pain conditions, such as chronic pelvic pain, vulvodynia, myofascial pain, headache, etc suggest the presence of central sensitization. Anxiety, depression, and maladaptive coping strategies often ensue. Functional MRI studies have documented altered central processing in the brain in many chronic pain states including endometriosis. Interdisciplinary therapy including physical therapy, mental health, and pain management/anesthesiology is more effective compared with medical and or surgical therapy alone for endometriosis-related pain in the setting of peripheral and central sensitization.
What should clinicians look for, or what stands out to them, to confirm the endometriosis diagnosis when pain is the presenting symptom?
Dr. Rapkin: There are no pathognomonic symptoms or biomarkers for endometriosis; however, the following historical features have been shown to be linked with a greater likelihood of finding endometriosis:
- persistent dysmenorrhea (menstrual pain) despite NSAID and hormonal treatment
- cyclical pain that is premenstrual and menstrual that progresses to chronic pain or is accompanied by abnormal or heavy menstrual bleeding
- deep dyspareunia
- dyschezia (pain with bowel movements), and sometimes bloating.
An individual with menstrual pain since menarche can have up to a 5% increased risk of endometriosis. Endometriosis in a first-degree relative elevates the risk for endometriosis by 7% to 10%.
Given the complexity of chronic pain, it is important not to assume endometriosis is the only source of pain. All the pelvic visceral and somatic structures should be evaluated. A thorough history addresses all the patient’s symptoms, including vaginal, gastrointestinal and genitourinary. Aggravating factors such as menstrual cycle, bowel and bladder functioning, physical activity, sexual intercourse and stress should be queried. In addition, assessment of mood, anxiety or depression, sleep disturbance and effect of pain on daily functioning are relevant as is history of abuse or trauma (physical, sexual or emotional). This history can be lengthy, so a detailed pain questionnaire is helpful. (See the pelvicpain.org website for a user-friendly pain questionnaire).
With the previously mentioned risk factors in mind, and after a thorough history has been obtained, a pain-localizing exam should be conducted including the abdominal wall, pelvic floor, and then the bimanual and rectovaginal exams for the abdominal wall myofascial/neuropathic pain assessment for which Carnett’s test can be very useful—tender points on the abdominal wall are palpitated and the patient is asked to give a numerical rating of the pain (1-10/10) and marked with a pen. The patient is then asked to either perform a bilateral straight leg raise or an abdominal crunch, and the areas are re-palpitated. If the marked areas are more painful to palpation during the abdominal crunch or the bilateral straight leg raise, it suggests an abdominal wall pain (myofascial or neuropathic) or component of the pain. Similarly, pelvic floor muscles should be assessed after the abdominal wall exam is completed. This is best accomplished with a unit-digital exam with palpation of pelvic muscles for tenderness and hypertonia on exam. These myofascial findings are often present in the setting of endometriosis, but they can be primary-unrelated to presence or absence of endometriosis.
What are your focused disciplines for approaching endometriosis-associated pain? How do you recommend these clinicians or specialists come together to effectively manage a patient’s conditions?
Dr. Rapkin: The gynecologist or primary care provider can address the chronic inflammatory, estrogen-dependent aspect of endometriosis. Begin with nonsteroidal, anti-inflammatory medication and combined estrogen-progestin or high-dose progestin-alone hormonal therapy to lower estrogenic stimulation of lesions and decidualize those progestin-sensitive lesions. For menstrual cycle related pain (luteal periovulatory or menstrual phase) cyclical exacerbation of other chronic pain conditions, early intervention is recommended. Adequately dosed preemptive nonsteroidal inflammatory agents and, if not tolerated or effective, begin combined hormonal contraceptives or intrauterine or higher dose progestins menstrual suppression, with either continuous monophasic hormonal contraceptives or progestins, is very important for pain that is cyclical or exacerbated in a cyclical fashion. Progestins can be administered orally, such as norethindrone acetate; intramuscularly or subdermally (depot medroxyprogesterone acetate or etonogestrel implant); or intrauterine (which does not lower estrogen levels but can be therapeutic for suppression of menses and local treatment of endometriosis). Failure of hormonal therapy and management of other co-occurring pain conditions warrants trial of a second-line medical therapy such as gonadotropin-releasing hormone antagonist or agonist or surgery for definitive diagnosis and surgical ablation or excision of endometriosis lesions.
I would suggest that gynecologists who treat women with endometriosis and chronic pain try to build a team in their geographic area. Relevant specialists for an interdisciplinary approach include:
- Pelvic floor physical therapist to evaluate and address myofascial dysfunction and pain and voiding abnormalities, such as urinary urgency or frequency and constipation
- Gastroenterologist for evaluation and treatment of irritable bowel or functional abdominal pain and bloating syndrome or inflammatory bowel disease. Urologist or urogynecologist to assess and treat bladder pain syndrome/interstitial cystitis
- Primary care evaluation for diffuse myofascial pain, fibromyalgia, arthralgias, and other inflammatory conditions, and for management of headache and migraine. Rheumatology and neurology specialists may be needed
- Mental health providers for treatment of anxiety, depression, or PTSD and to address stress management, coping skills and provide cognitive behavioral therapy
- Interventional pain management specialist such as physical medicine and rehabilitation (PM and R), pain anesthiologist, neurologist or interventional radiologist to provide relevant nerve blocks, trigger point injections, or botulinum toxin injection.
- Gynecologists experienced in the management of chronic pelvic pain also provide nerve blocks, trigger point and botulinum toxin injections.
Andrea Rapkin, MD, is Board Certified by the American College of Obstetricians and Gynecologists (of which she is also a fellow). After obtaining her MD, she completed her residency in OBGYN at UCLA then joined the faculty at UCLA and is a Professor of Obstetrics and Gynecology. She was one of the first Obstetrician-Gynecologists to adapt the multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain.
You are the founder and director of a clinic focused on a multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain. How did you identify such a clinic as a therapeutic need for patients?
Dr. Rapkin: The short answer is that a significant proportion of women were not experiencing pain relief or had incomplete relief with traditional medical or surgical therapy. At the time, we also identified various red flags for traditional treatment failures. These red flags included the following: pain of greater than 6 months duration, pain out of proportion to pathology found on examination, multiple visceral and somatic complaints, and psychosocial abnormalities. We now understand more about the neurobiology underpinning these red flags.
With the widespread availability of laparoscopy in the late 70s and early 80s, many studies investigated the relationship between endometriosis lesions and pain. The general consensus is that there is no relationship between the location or severity of the endometriosis lesions or the disease stage (American Society for Reproductive Medicine staging) with type of symptoms, symptom severity, treatment response, recurrence, or even prognosis. In fact, pain recurrence after adequate surgical treatment is unrelated to the presence of endometriosis lesions found at the time of repeat laparoscopy. This lack of association between pain and presence of visible disease was supported by a recent New England Journal of Medicine article by Zondervan and colleagues demonstrating that up to 30% of women with chronic pelvic pain, present after excision of endometriotic lesions, become unresponsive to conventional treatment.
The neurobiological responses in an individual with chronic pain are more complicated than those seen in the setting of acute pain. Chronic pain may be triggered or maintained by an inflammatory process such as endometriosis but, over time, altered neural processing and psychosocial maladaptation can occur. The altered processing consists of both peripheral and central sensitization which change the way sensory information from the pelvic viscera and surrounding somatic structures in the periphery is transmitted and interpreted in the central nervous system (spinal cord and brain). Visceral pelvic pain can emanate from the uterus, ovaries and fallopian tubes, the urinary bladder, and the bowel, while the somatic sources include the surrounding abdominal wall, low back and pelvic floor muscles, and fascia, and bones. Signal amplification or peripheral sensitization in the pelvic region in women with endometriosis starts with localized inflammation, neovascularization, invasion and innervation of endometriotic implants. As the pelvic organs share thoracolumbar and sacral autonomic neural pathways, inflammation or dysfunction in one organ or tissue, such as the uterus, over time can sensitize or lead to dysfunction in other pelvic organs, such as the bladder or bowel (called viscero-visceral cross sensitization). Finally, somatic structures sharing intervention with the pelvic viscera, such as the fascia and muscles of the lower abdomen, pelvic floor lower back also become sources of pain because of a process called viscero-somatic sensitization. Women who have endometriosis are therefore more likely to experience IBS, bladder pain syndrome/interstitial cystitis and vulvodynia, and up to 80% of individuals can develop myofascial pain related to trigger points and muscle dysfunction. Up to 50% of women with bladder pain have endometriosis. Those with endometriosis or bladder pain are 2.5 times more likely to also have IBS.
Over time, other visceral and somatic structures innervated by higher levels of the spinal cord can be affected, leading to more widespread pain. Central sensitization manifests as an amplification of pain in the spinal cord and brain. The presence of more than two chronic “unexplained” pain conditions, such as chronic pelvic pain, vulvodynia, myofascial pain, headache, etc suggest the presence of central sensitization. Anxiety, depression, and maladaptive coping strategies often ensue. Functional MRI studies have documented altered central processing in the brain in many chronic pain states including endometriosis. Interdisciplinary therapy including physical therapy, mental health, and pain management/anesthesiology is more effective compared with medical and or surgical therapy alone for endometriosis-related pain in the setting of peripheral and central sensitization.
What should clinicians look for, or what stands out to them, to confirm the endometriosis diagnosis when pain is the presenting symptom?
Dr. Rapkin: There are no pathognomonic symptoms or biomarkers for endometriosis; however, the following historical features have been shown to be linked with a greater likelihood of finding endometriosis:
- persistent dysmenorrhea (menstrual pain) despite NSAID and hormonal treatment
- cyclical pain that is premenstrual and menstrual that progresses to chronic pain or is accompanied by abnormal or heavy menstrual bleeding
- deep dyspareunia
- dyschezia (pain with bowel movements), and sometimes bloating.
An individual with menstrual pain since menarche can have up to a 5% increased risk of endometriosis. Endometriosis in a first-degree relative elevates the risk for endometriosis by 7% to 10%.
Given the complexity of chronic pain, it is important not to assume endometriosis is the only source of pain. All the pelvic visceral and somatic structures should be evaluated. A thorough history addresses all the patient’s symptoms, including vaginal, gastrointestinal and genitourinary. Aggravating factors such as menstrual cycle, bowel and bladder functioning, physical activity, sexual intercourse and stress should be queried. In addition, assessment of mood, anxiety or depression, sleep disturbance and effect of pain on daily functioning are relevant as is history of abuse or trauma (physical, sexual or emotional). This history can be lengthy, so a detailed pain questionnaire is helpful. (See the pelvicpain.org website for a user-friendly pain questionnaire).
With the previously mentioned risk factors in mind, and after a thorough history has been obtained, a pain-localizing exam should be conducted including the abdominal wall, pelvic floor, and then the bimanual and rectovaginal exams for the abdominal wall myofascial/neuropathic pain assessment for which Carnett’s test can be very useful—tender points on the abdominal wall are palpitated and the patient is asked to give a numerical rating of the pain (1-10/10) and marked with a pen. The patient is then asked to either perform a bilateral straight leg raise or an abdominal crunch, and the areas are re-palpitated. If the marked areas are more painful to palpation during the abdominal crunch or the bilateral straight leg raise, it suggests an abdominal wall pain (myofascial or neuropathic) or component of the pain. Similarly, pelvic floor muscles should be assessed after the abdominal wall exam is completed. This is best accomplished with a unit-digital exam with palpation of pelvic muscles for tenderness and hypertonia on exam. These myofascial findings are often present in the setting of endometriosis, but they can be primary-unrelated to presence or absence of endometriosis.
What are your focused disciplines for approaching endometriosis-associated pain? How do you recommend these clinicians or specialists come together to effectively manage a patient’s conditions?
Dr. Rapkin: The gynecologist or primary care provider can address the chronic inflammatory, estrogen-dependent aspect of endometriosis. Begin with nonsteroidal, anti-inflammatory medication and combined estrogen-progestin or high-dose progestin-alone hormonal therapy to lower estrogenic stimulation of lesions and decidualize those progestin-sensitive lesions. For menstrual cycle related pain (luteal periovulatory or menstrual phase) cyclical exacerbation of other chronic pain conditions, early intervention is recommended. Adequately dosed preemptive nonsteroidal inflammatory agents and, if not tolerated or effective, begin combined hormonal contraceptives or intrauterine or higher dose progestins menstrual suppression, with either continuous monophasic hormonal contraceptives or progestins, is very important for pain that is cyclical or exacerbated in a cyclical fashion. Progestins can be administered orally, such as norethindrone acetate; intramuscularly or subdermally (depot medroxyprogesterone acetate or etonogestrel implant); or intrauterine (which does not lower estrogen levels but can be therapeutic for suppression of menses and local treatment of endometriosis). Failure of hormonal therapy and management of other co-occurring pain conditions warrants trial of a second-line medical therapy such as gonadotropin-releasing hormone antagonist or agonist or surgery for definitive diagnosis and surgical ablation or excision of endometriosis lesions.
I would suggest that gynecologists who treat women with endometriosis and chronic pain try to build a team in their geographic area. Relevant specialists for an interdisciplinary approach include:
- Pelvic floor physical therapist to evaluate and address myofascial dysfunction and pain and voiding abnormalities, such as urinary urgency or frequency and constipation
- Gastroenterologist for evaluation and treatment of irritable bowel or functional abdominal pain and bloating syndrome or inflammatory bowel disease. Urologist or urogynecologist to assess and treat bladder pain syndrome/interstitial cystitis
- Primary care evaluation for diffuse myofascial pain, fibromyalgia, arthralgias, and other inflammatory conditions, and for management of headache and migraine. Rheumatology and neurology specialists may be needed
- Mental health providers for treatment of anxiety, depression, or PTSD and to address stress management, coping skills and provide cognitive behavioral therapy
- Interventional pain management specialist such as physical medicine and rehabilitation (PM and R), pain anesthiologist, neurologist or interventional radiologist to provide relevant nerve blocks, trigger point injections, or botulinum toxin injection.
- Gynecologists experienced in the management of chronic pelvic pain also provide nerve blocks, trigger point and botulinum toxin injections.
Andrea Rapkin, MD, is Board Certified by the American College of Obstetricians and Gynecologists (of which she is also a fellow). After obtaining her MD, she completed her residency in OBGYN at UCLA then joined the faculty at UCLA and is a Professor of Obstetrics and Gynecology. She was one of the first Obstetrician-Gynecologists to adapt the multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain.
You are the founder and director of a clinic focused on a multidisciplinary pain management approach to the evaluation and treatment of women with pelvic and vulvar pain. How did you identify such a clinic as a therapeutic need for patients?
Dr. Rapkin: The short answer is that a significant proportion of women were not experiencing pain relief or had incomplete relief with traditional medical or surgical therapy. At the time, we also identified various red flags for traditional treatment failures. These red flags included the following: pain of greater than 6 months duration, pain out of proportion to pathology found on examination, multiple visceral and somatic complaints, and psychosocial abnormalities. We now understand more about the neurobiology underpinning these red flags.
With the widespread availability of laparoscopy in the late 70s and early 80s, many studies investigated the relationship between endometriosis lesions and pain. The general consensus is that there is no relationship between the location or severity of the endometriosis lesions or the disease stage (American Society for Reproductive Medicine staging) with type of symptoms, symptom severity, treatment response, recurrence, or even prognosis. In fact, pain recurrence after adequate surgical treatment is unrelated to the presence of endometriosis lesions found at the time of repeat laparoscopy. This lack of association between pain and presence of visible disease was supported by a recent New England Journal of Medicine article by Zondervan and colleagues demonstrating that up to 30% of women with chronic pelvic pain, present after excision of endometriotic lesions, become unresponsive to conventional treatment.
The neurobiological responses in an individual with chronic pain are more complicated than those seen in the setting of acute pain. Chronic pain may be triggered or maintained by an inflammatory process such as endometriosis but, over time, altered neural processing and psychosocial maladaptation can occur. The altered processing consists of both peripheral and central sensitization which change the way sensory information from the pelvic viscera and surrounding somatic structures in the periphery is transmitted and interpreted in the central nervous system (spinal cord and brain). Visceral pelvic pain can emanate from the uterus, ovaries and fallopian tubes, the urinary bladder, and the bowel, while the somatic sources include the surrounding abdominal wall, low back and pelvic floor muscles, and fascia, and bones. Signal amplification or peripheral sensitization in the pelvic region in women with endometriosis starts with localized inflammation, neovascularization, invasion and innervation of endometriotic implants. As the pelvic organs share thoracolumbar and sacral autonomic neural pathways, inflammation or dysfunction in one organ or tissue, such as the uterus, over time can sensitize or lead to dysfunction in other pelvic organs, such as the bladder or bowel (called viscero-visceral cross sensitization). Finally, somatic structures sharing intervention with the pelvic viscera, such as the fascia and muscles of the lower abdomen, pelvic floor lower back also become sources of pain because of a process called viscero-somatic sensitization. Women who have endometriosis are therefore more likely to experience IBS, bladder pain syndrome/interstitial cystitis and vulvodynia, and up to 80% of individuals can develop myofascial pain related to trigger points and muscle dysfunction. Up to 50% of women with bladder pain have endometriosis. Those with endometriosis or bladder pain are 2.5 times more likely to also have IBS.
Over time, other visceral and somatic structures innervated by higher levels of the spinal cord can be affected, leading to more widespread pain. Central sensitization manifests as an amplification of pain in the spinal cord and brain. The presence of more than two chronic “unexplained” pain conditions, such as chronic pelvic pain, vulvodynia, myofascial pain, headache, etc suggest the presence of central sensitization. Anxiety, depression, and maladaptive coping strategies often ensue. Functional MRI studies have documented altered central processing in the brain in many chronic pain states including endometriosis. Interdisciplinary therapy including physical therapy, mental health, and pain management/anesthesiology is more effective compared with medical and or surgical therapy alone for endometriosis-related pain in the setting of peripheral and central sensitization.
What should clinicians look for, or what stands out to them, to confirm the endometriosis diagnosis when pain is the presenting symptom?
Dr. Rapkin: There are no pathognomonic symptoms or biomarkers for endometriosis; however, the following historical features have been shown to be linked with a greater likelihood of finding endometriosis:
- persistent dysmenorrhea (menstrual pain) despite NSAID and hormonal treatment
- cyclical pain that is premenstrual and menstrual that progresses to chronic pain or is accompanied by abnormal or heavy menstrual bleeding
- deep dyspareunia
- dyschezia (pain with bowel movements), and sometimes bloating.
An individual with menstrual pain since menarche can have up to a 5% increased risk of endometriosis. Endometriosis in a first-degree relative elevates the risk for endometriosis by 7% to 10%.
Given the complexity of chronic pain, it is important not to assume endometriosis is the only source of pain. All the pelvic visceral and somatic structures should be evaluated. A thorough history addresses all the patient’s symptoms, including vaginal, gastrointestinal and genitourinary. Aggravating factors such as menstrual cycle, bowel and bladder functioning, physical activity, sexual intercourse and stress should be queried. In addition, assessment of mood, anxiety or depression, sleep disturbance and effect of pain on daily functioning are relevant as is history of abuse or trauma (physical, sexual or emotional). This history can be lengthy, so a detailed pain questionnaire is helpful. (See the pelvicpain.org website for a user-friendly pain questionnaire).
With the previously mentioned risk factors in mind, and after a thorough history has been obtained, a pain-localizing exam should be conducted including the abdominal wall, pelvic floor, and then the bimanual and rectovaginal exams for the abdominal wall myofascial/neuropathic pain assessment for which Carnett’s test can be very useful—tender points on the abdominal wall are palpitated and the patient is asked to give a numerical rating of the pain (1-10/10) and marked with a pen. The patient is then asked to either perform a bilateral straight leg raise or an abdominal crunch, and the areas are re-palpitated. If the marked areas are more painful to palpation during the abdominal crunch or the bilateral straight leg raise, it suggests an abdominal wall pain (myofascial or neuropathic) or component of the pain. Similarly, pelvic floor muscles should be assessed after the abdominal wall exam is completed. This is best accomplished with a unit-digital exam with palpation of pelvic muscles for tenderness and hypertonia on exam. These myofascial findings are often present in the setting of endometriosis, but they can be primary-unrelated to presence or absence of endometriosis.
What are your focused disciplines for approaching endometriosis-associated pain? How do you recommend these clinicians or specialists come together to effectively manage a patient’s conditions?
Dr. Rapkin: The gynecologist or primary care provider can address the chronic inflammatory, estrogen-dependent aspect of endometriosis. Begin with nonsteroidal, anti-inflammatory medication and combined estrogen-progestin or high-dose progestin-alone hormonal therapy to lower estrogenic stimulation of lesions and decidualize those progestin-sensitive lesions. For menstrual cycle related pain (luteal periovulatory or menstrual phase) cyclical exacerbation of other chronic pain conditions, early intervention is recommended. Adequately dosed preemptive nonsteroidal inflammatory agents and, if not tolerated or effective, begin combined hormonal contraceptives or intrauterine or higher dose progestins menstrual suppression, with either continuous monophasic hormonal contraceptives or progestins, is very important for pain that is cyclical or exacerbated in a cyclical fashion. Progestins can be administered orally, such as norethindrone acetate; intramuscularly or subdermally (depot medroxyprogesterone acetate or etonogestrel implant); or intrauterine (which does not lower estrogen levels but can be therapeutic for suppression of menses and local treatment of endometriosis). Failure of hormonal therapy and management of other co-occurring pain conditions warrants trial of a second-line medical therapy such as gonadotropin-releasing hormone antagonist or agonist or surgery for definitive diagnosis and surgical ablation or excision of endometriosis lesions.
I would suggest that gynecologists who treat women with endometriosis and chronic pain try to build a team in their geographic area. Relevant specialists for an interdisciplinary approach include:
- Pelvic floor physical therapist to evaluate and address myofascial dysfunction and pain and voiding abnormalities, such as urinary urgency or frequency and constipation
- Gastroenterologist for evaluation and treatment of irritable bowel or functional abdominal pain and bloating syndrome or inflammatory bowel disease. Urologist or urogynecologist to assess and treat bladder pain syndrome/interstitial cystitis
- Primary care evaluation for diffuse myofascial pain, fibromyalgia, arthralgias, and other inflammatory conditions, and for management of headache and migraine. Rheumatology and neurology specialists may be needed
- Mental health providers for treatment of anxiety, depression, or PTSD and to address stress management, coping skills and provide cognitive behavioral therapy
- Interventional pain management specialist such as physical medicine and rehabilitation (PM and R), pain anesthiologist, neurologist or interventional radiologist to provide relevant nerve blocks, trigger point injections, or botulinum toxin injection.
- Gynecologists experienced in the management of chronic pelvic pain also provide nerve blocks, trigger point and botulinum toxin injections.
Step-wise medical therapy is cost effective for endometriosis
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
For patients with endometriosis-related dysmenorrhea, it is cost effective to use medical therapy before surgery, according to investigators.
A stepwise strategy involving two medications, then surgery, was associated with the lowest cost per quality-adjusted life-years (QALYs), reported lead author, Jacqueline A. Bohn, MD, of Oregon Health & Science University, Portland, and colleagues.
“In 2009, the medical costs associated with endometriosis in the United States were estimated at $69.4 billion annually,” the investigators wrote in Obstetrics and Gynecology. “Despite the recognized cost burden of this disease, cost-effectiveness data on the various treatment strategies is limited. Previous studies have investigated the direct and indirect costs regarding endometriosis; however, there are no prior studies that evaluate the cost-effectiveness of a stepwise regimen to guide management.”
To fill this knowledge gap, Dr. Bohn and colleagues created a cost-effectiveness model comparing four treatment strategies:
NSAIDs, then surgery
NSAIDs, then short-acting reversible contraceptives or long-acting reversible contraceptives (LARCs), then surgery
NSAIDs, then a short-acting reversible contraceptive or a LARC, then a LARC or gonadotropin-releasing hormone (GnRH) modulator, then surgery
Surgery alone
The analysis, which compared costs, QALYs, and incremental cost-effectiveness ratios, involved a theoretical cohort of 4,817,894 women aged 18-45 years, representing the estimated number of reproductive-age women in the United States with endometriosis-related dysmenorrhea. Costs were determined from published literature and inflated to 2019 dollars. Medical treatments were theoretically given for 6 months each, and the cost of laparoscopic surgery incorporated 12 months of postoperative care.
Of the four strategies, the two-medication approach was most cost effective, with a cost per QALY of $1,158. This was followed closely by the three-medication regimen, at $1,158, the single-medication regimen, at $2,108, and finally, surgery alone, at $4,338.
“We found that, although cost effective, requiring trial of a third medication offered little comparative advantage before proceeding directly to surgery after the second therapy fails,” the investigators wrote. “Yet, for the woman who is anxious about surgical intervention, or when a prolonged wait for a surgical specialist occurs, trial of a GnRH modulator may be worthwhile.”
Compared with surgery alone, each regimen starting with medical therapy remained below the standard willingness-to-pay threshold of $100,000 per QALY; however, the investigators recommend against trying more than three medications.
“Delaying surgical management in a woman with pain refractory to more than three medications may decrease quality of life and further increase cost,” they wrote.
To make surgery alone the most cost-effective option, surgery success would need to exceed 83%, Dr. Bohn and colleagues concluded.
According to Hugh Taylor, MD, of Yale University, New Haven, Conn., it’s unlikely that this surgery success threshold will be met, since surgery alone typically leads to recurrence.
“We know there’s a very high relapse rate after surgery,” Dr. Taylor said in an interview. “Even if the surgery may be initially successful, there’s roughly a 50% recurrence rate after about 2 years. So, finding the right medical therapy will give you more chance for long-term success.”
Dr. Taylor said it’s “really nice” that Dr. Bohn and colleagues conducted a sequential analysis because the findings support the most common approach in real-world practice.
“It confirms that starting with a medical therapy prior to surgery is an appropriate, successful treatment for endometriosis, which is something that many, many people in the community do, but we haven’t had a real trial to show that,” he said.
Dr. Taylor offered two areas of improvement for similar studies in the future: First, he suggested separating LARCs from oral contraceptives because LARCs may be less effective for some patients with endometriosis; and second, he suggested that limiting the third medication to a GnRH antagonist would be more applicable to real-world practice than using the broader category of GnRH modulators.
Although the three-medication approach involving a GnRH modulator was slightly more expensive than the two-medication approach, Dr. Taylor said the costs were so similar that a three-medication approach is “still reasonable,” particularly because it could spare patients from surgery.
Dr. Taylor also speculated that trying a GnRH antagonist could become more cost effective soon. Although only one GnRH antagonist is currently on the market, he noted that a second agent is poised for Food and Drug Administration approval, while a third is in the pipeline, and this competition may decrease drug prices.
The investigators disclosed support from the National Institutes of Health, Arnold Ventures, the World Health Organization, Merck, and others. Dr. Taylor reported that Yale University receives funding for endometriosis biomarker research from AbbVie.
FROM OBSTETRICS & GYNECOLOGY
Sacral nerve root endometriosis

Additional videos from SGS are available here, including these recent offerings:

Additional videos from SGS are available here, including these recent offerings:

Additional videos from SGS are available here, including these recent offerings:
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.
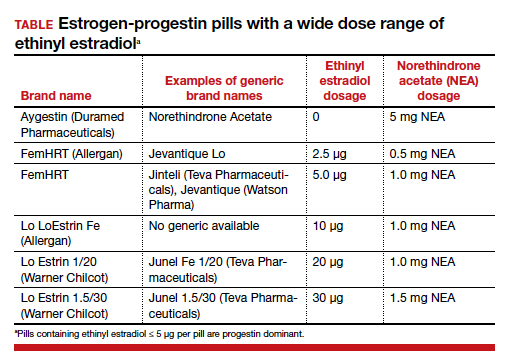
CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
Diaphragmatic endometriosis diagnosed many years after symptom onset
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Diaphragmatic endometriosis is often diagnosed several years after the start of symptoms – mainly moderate to severe pain – and this is potentially because of general lack of awareness of diaphragmatic endometriosis among the general population and medical professionals.
Findings of the international survey that explored the diagnosis and treatment of diaphragmatic endometriosis were presented at this year’s Royal College of Obstetricians and Gynecologists 2021 Virtual World Congress by medical student Rachel Piccus, MSc, based at the University of Birmingham (England). Robert Sutcliffe, MD, consultant in hepatobiliary and pancreatic surgery, at Queen Elizabeth Hospital Birmingham was senior author. Results were also published in the May 2021 issue of the European Journal of Obstetrics and Gynaecology and Reproductive Biology.
The study found that it took an average of five visits to a primary physician before referral to a gynecologist.
“Late diagnosis could also be due to the idea that diaphragmatic endometriosis symptoms often present before pelvic symptoms and therefore the site of pain is considered atypical for pelvic endometriosis,” Ms. Piccus said, adding that “clinicians are screening for cyclical pain, which is typical of endometriosis, but our study has shown that pain can in fact be more frequent – daily and weekly.”
These significant diagnostic delays, seen from the time of the initial primary care and gynecology consultation has the potential to significantly affect quality of life as seen in pelvic endometriosis, said Ms. Piccus. “These delays are partly due to a lack of awareness among gynecologists, but could also be due to pelvic laparoscopy being insufficient to examine the diaphragm behind the liver.”
Justin Clark, MD, consultant gynaecologist, Birmingham (England) Women’s and Children Hospital, moderated the session and agreed that the study highlights the need for greater awareness of this variant of endometriosis. “Whilst endometriosis affecting the diaphragm, subdiaphragm, and thorax is rare, the condition causes substantial morbidity.”
“Greater knowledge of thoracic endometriosis amongst clinicians in both primary and secondary care is needed to ensure accurate and timely diagnosis,” he added.
Diaphragmatic endometriosis is estimated to affect 1%-1.5% of all endometriosis patients and presents as cyclical pain in the chest, abdomen, and shoulder tip, as well as other respiratory symptoms such as catamenial pneumothorax and difficulty breathing.
“Cross-sectional imaging has shown low sensitivity so upper abdominal laparoscopy is the gold standard; however, this has implications for diagnostic delay because a strong clinical suspicion is required to refer for this invasive procedure,” explained Ms. Piccus referring to one of the reasons underpinning the need for the study.
When successfully diagnosed, treatment requires excision or ablation surgery and studies show symptomatic relief in 75%-100% of cases.
To gauge the extent of delayed diagnosis as well as treatment outcomes from a patient perspective, Ms. Piccus circulated an anonymous online survey among women with a previous history of surgery for diaphragmatic endometriosis.
Diaphragmatic endometriosis pain – daily and weekly as well as cyclical
A total of 137 participants responded to the survey, with a median age of 34 years (range, 19-53). Median age of diaphragmatic endometriosis onset was 27 years (range, 11-50), and importantly, diaphragmatic endometriosis symptoms started before pelvic symptoms in 90 respondents (66%).
The dominant symptom was pain. A total of 38% reported cyclical pain (related to endometrial shedding during menstruation), 15% weekly pain, and 47% daily pain, both of which were worse during the menstrual cycle. Furthermore, 14% reported other symptoms including catamenial pneumothorax, difficulty breathing, and hemoptysis.
“Whilst this cyclical pain is typical of endometriosis, we see that diagnostic delays may be due to misdiagnosis because clinicians are screening for this cyclical pain whilst our study has shown that pain can in fact be more frequent, being daily and weekly,” noted Ms. Piccus. Moderate to severe pain was reported in 67% of respondents and moderate in 31%, only 2% reported pain as mild.
Location of pain comprised moderate to severe pain in the upper abdomen (68%), chest (64%) and shoulder (54%). Pain was right-sided in 54%, left-sided in 11% and bilateral in 35%. Upper back and neck were also reported as sites of pain.
Indirectly providing a measure of the lack of awareness of diaphragmatic endometriosis on behalf of primary care, 122 participants reported initially visiting their primary care physician for help and 65 were given a diagnosis – in only 14 cases was that diaphragmatic endometriosis. There were a range of other gynecologic (e.g. ovarian cyst, two), respiratory (spontaneous pneumothorax, seven), gastrointestinal (gastritis/reflux, eight), musculoskeletal (six), and psychological (anxiety/stress, four) diagnoses.
A median of 5 primary care consultations (range, 1-100) were required before referral to a gynecologist, with 30% seeing a primary care physician over 10 times. A further 14 patients self-referred to gynecologist.
“These findings have implications for diagnostic delay, added Ms. Piccus. “While the majority of respondents were diagnosed less than a year from the first GP visit, the median delay was 2 years, with 31% diagnosed after 5 or more years. One took 23 years for an initial diagnosis.”
Most cases were diagnosed at the time of surgery – 93%, with 52% at pelvic laparoscopy, 35% upper abdominal laparoscopy, with 30% requiring two or more laparoscopies before they were diagnosed with diaphragmatic endometriosis. A total of 7% were diagnosed via cross-sectional imaging prior to surgery.
Treatment outcomes for diaphragmatic endometriosis
Reflecting the literature, surgery to remove the endometriosis lesions was mainly laparoscopic with 47% abdominal excisions, and 29% abdominal ablations; 6% received open abdominal procedures, and 18% received open thoracotomy or video-assisted thoracoscopic surgery.
The survey asked about postoperative symptoms 6 months after surgery and at the time of survey. Symptoms at 6 months post surgery had completely resolved in 18%, shown significant improvement in 48%, and no improvement in 20%. Worsening of symptoms was seen in 14%. Long-term pain was reported by 21% as severe, 27% as moderate, 35% as mild, and 17% had no symptoms.
Further findings included that 23% underwent additional procedures to treat their diaphragmatic endometriosis, and that there was no significant difference between excision and ablation, nor between age of onset of symptoms or length of diagnostic delay.
“Surgical treatment to remove these extra pelvic deposits of endometriosis will depend upon the type and distribution of thoracic endometriosis and a variety of surgical specialties may need to be involved including gynecologists, cardiothoracic, and upper gastrointestinal/liver surgeons,” Dr. Clark said.
He added that familiar hormonal medical treatments for more typical pelvic endometriosis should also be considered for primary and maintenance treatment. “These data suggest a high symptomatic recurrence rate after surgical treatment and so medical treatments should be considered to try and minimize the risks of endometriosis symptoms returning.”
Dr. Clark also pointed out that multidisciplinary clinical teams should be established in specialized centers to plan surgical and medical management to enhance clinical outcomes and collect data to better understand this enigmatic condition.
Ms. Piccus and Dr. Clark have no relevant conflicts of interest.
Managing deep infiltrating endometriosis
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
Deep endometriosis, including bowel endometriosis, happens in more than 10% of all women with endometriosis. Because it can be debilitating and difficult to diagnose, how is it identified?
Dr. Kho: Diagnosing deep infiltrating endometriosis (DIE) is one of the biggest challenges in the field. Currently, the gold standard is still histologic confirmation of endometriosis lesions, but I think the paradigm is shifting significantly. Advanced centers are now moving towards the use of imaging to visualize suspected endometriosis better. In skilled hands, ultrasonography and MRI can accurately detect DIE. The advantage of visualizing suspected deep endometriosis before surgery is that we can offer better counseling to patients and offer medical or surgical treatments or a combination of both. When the patient chooses surgery, we can be better prepared with a multidisciplinary team to remove the lesions completely.
The treatment of deep endometriosis can be challenging because it does not always respond to medical therapy, such as oral contraceptive pills or GnRH agonists. What treatment options have shown effectiveness?
Dr. Kho: It should be understood that we need to manage the symptoms and not the lesions. Just because there are endometriosis lesions identified does NOT mean that they need to be removed. The decision should be based on what the treatment goals are. A good discussion with the patient is important to determine what the treatment objectives are. Are we managing pain? Are we surgically treating infertility? Are we treating something else, such as bowel obstruction or blood in the urine? These questions and their answers should be first and foremost in the clinician’s mind.
Different medical treatment therapies are available and have been shown to be effective in managing endometriosis symptoms without having to immediately resort to surgery as with certain cases of suspected DIE. These medical management options include combined estrogen and progesterone contraceptive formulations, progesterone-only formulations, and GnRH agonists and antagonists. Patients should be counseled about the different options and their associated risks. Often, patients need to switch from one form to another when side effects encumber them.
If imaging or an exam reveals incidental findings of suspected deep endometriosis, it does not mean that treatment is required because some patients can be without symptoms or have no issues. But when the indication for pain or desire to achieve a pregnancy is clear, and the decision is to proceed with treatment, there are some guidelines:
- If the pain is severe and affects the patient’s daily quality of life, for example, with large, deep lesions, surgical excision appears to be effective in improving the patient’s quality of life.
- If infertility is the main indication, then other factors need to be considered. Has she had previous surgeries? What is her age? What is her ovarian reserve? What is the condition of the tubes? Is the use of artificial reproductive technology, or ART, an option for the couple? What is her cancer risk?
- Lastly, what is the extent of the lesion(s)? Both medical and surgical approaches for treatment carry risks. For surgery, complications can be severe with long-term consequences. Therefore, it is important to discuss all of this in detail with the patient.
When we understand what the main goal of the treatment is for endometriosis—such as either to treat pain or to achieve pregnancy, or both—we can tailor our surgical approach to achieve the goal(s). For example, in a patient who wants to achieve a pregnancy in the future, we may opt to do only what is necessary to remove endometriosis and relieve pain without impairing the patient’s fertility potential and overall quality of life.
Post-operatively, if the patient does not desire to become pregnant, medications to suppress the ovaries are an option to reduce the chance of disease recurrence.
What pearls can you provide when it comes to surgical techniques available and benefits for short- and long-term risks and limitations?
Dr. Kho: The surgeon should tailor his or her level of radicality to excise endometriosis to achieve the treatment goals that are mutually agreed on with the patient. For the surgeon bringing the patient into surgery, go in prepared. I strongly believe in preoperative imaging to provide the best mapping of the disease and in having other specialists or surgeons available—whether it be a urologist, a colorectal surgeon, a thoracic surgeon, a vascular surgeon, or an orthopedic surgeon (if needed)—depending on where the lesions are located.
Because we know that the best surgical outcomes come from surgeons with the highest volume, providers should be willing to refer patients with advanced disease to centers that specialize in the treatment of endometriosis. Many providers with this expertise undergo additional training to advance their skills. Advanced centers in endometriosis also provide patients with a multidisciplinary team to provide the best care.
What is the most optimal setup for a multidisciplinary team treating deep infiltrating endometriosis?
Dr. Kho: You will find that advanced centers for endometriosis will have in place a multidisciplinary team that includes a pain specialist, a surgical specialist, a pain psychologist, physical therapy, urologist, colorectal surgeon, and other sub-specialty surgeons to be able to look at the patients from all the different aspects. Often a reproductive endocrinologist or REI specialist would also be part of the group.
At our center, we have what we call a “benign tumor board,” where we discuss complex cases with representatives from different areas. This allows us to discuss the case, review what is known in the literature, and collectively develop a treatment plan.
Is there anything that was not covered in this discussion that you would like to mention?
Dr. Kho: Something that should be mentioned is that ovarian endometriosis is often associated with advanced endometriosis, often Stage 3 or 4. Clinicians should be aware that when imaging suggests the presence of ovarian endometriosis, studies show that the disease is isolated in the ovary in only 15% of cases—meaning that there is an 85% chance that endometriosis is present elsewhere in the pelvis. Therefore, the surgeon should be prepared for more extensive dissection when they bring the patient into surgery.
Lesions in pelvis may be ‘tip of the iceberg’ in endometriosis
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
Recognizing the systemic effects of endometriosis may help doctors better understand the experiences of patients with the disease and guide the approach to diagnosis and treatment, according to the president of the American Society for Reproductive Medicine (ASRM).
, Hugh S. Taylor, MD, said at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Its systemic manifestations may explain why women with endometriosis tend to have a lower body mass index, compared with women without the disease, Dr. Taylor said.
“Stem cells, microRNAs, and generalized inflammation are some of the mechanisms that mediate these long-range effects on distant organ systems,” he said.
Studies have indicated that lesions in the pelvis do not fully explain the disease, and investigators continue to elucidate how “endometriosis that we see in the pelvis is really just the tip of the iceberg,” said Dr. Taylor, chair of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn.
Pain, including dysmenorrhea, pelvic pain, and dyspareunia, “can be just as bad with ... stage 1 disease as it can be with stage 4 disease,” he said.
Some patients may not have pain, but have infertility. Other women are asymptomatic, and doctors find endometriosis incidentally.
One common definition of endometriosis – ectopic endometrial glands and stroma predominantly caused by retrograde menstruation – “probably overly simplifies this complex disease,” said Dr. Taylor, who reviewed the current understanding of endometriosis in an article in The Lancet. “The lesions in the pelvis are important. We see them. We treat them. But endometriosis has ... effects throughout the body.”
Dr. Taylor’s research group has shown that stem cells are a potential source of endometriosis. “There are cells from the endometriosis that can be found traveling in the circulation,” but their effects are unclear, he said.
Levels of several microRNAs may be increased or decreased in women with endometriosis, and these altered levels may induce the production of inflammatory cytokines. They also may serve as the basis of a blood test for endometriosis that could be ready for clinical use soon, Dr. Taylor said.
In a mouse model of endometriosis, the disease changes the electrophysiology of the brain and behavior. “We see changes in anxiety induced by endometriosis. We see changes in pain sensitivity induced by endometriosis. And we also see an increase in depression induced by endometriosis in this animal model,” Dr. Taylor said.
Although surgical therapy treats local disease, medical therapy may be needed to treat the systemic manifestations.
During a question-and-answer period after the presentation, Marcelle I. Cedars, MD, asked whether analgesic and hormonal management may be sufficient when a woman has suspected or laparoscopically diagnosed endometriosis and pain is the primary complaint.
“Given the understanding of endometriosis, how would you suggest approaching treatment?” asked Dr. Cedars, president elect of the ASRM and director of the division of reproductive endocrinology and infertility at the University of California, San Francisco.
Analgesic and hormonal therapies remain “the best treatments we have,” Dr. Taylor said. He starts treatment with an oral contraceptive and a nonsteroidal anti-inflammatory medication – “not only for pain relief but to tamp some of the inflammation associated with endometriosis,” he said. If an oral contraceptive does not work, a gonadotropin-releasing hormone antagonist typically is the next step.
Dr. Taylor has disclosed ties to Dot Lab and AbbVie. Dr. Cedars had no disclosures.
FROM ACOG 2021
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.