User login
DDW: Intragastric balloon eyed for primary obesity intervention
WASHINGTON – Obese patients implanted with an intragastric balloon lost significantly more weight than those following a behavioral modification program in a randomized, nonblinded trial.
Moreover, weight loss was preserved even after device removal, study author Dr. Barham Abu Dayyeh said at the annual Digestive Disease Week.
The Orbera intragastric balloon (Apollo Endosurgery) could fill a gap in the United States between obesity lifestyle interventions that are minimally effective and a range of bariatric surgical interventions that are effective, but come at a cost of increased complications and health care costs, he said. Moreover, only 1% of qualified patients actually end up having bariatric surgery.
The silicone, saline-filled intragastric balloon (IGB) has been widely used outside the U.S. for more than 17 years in more than 200,000 patients, added Dr. Abu Dayyeh* of Mayo Clinic in Rochester, Minn.
The multicenter trial was designed for premarketing approval in the U.S. of the Orbera IGB and randomly assigned 273 adults with a body mass index (BMI) of 30-40 kg/m2 for more than 2 years to a 12-month behavioral modification program with or without endoscopic placement of the IGB filled to 500-600 cc. The balloon was removed at month 6, with regular office visits through 1 year.
Eighteen patients withdrew before treatment; 215 patients were evaluable at 6 months, 206 at 9 months, and 191 at 12 months. The mean baseline BMI was 35 kg/m2 and 90% of patients were female.
At 6 months, the mean percent total body weight loss was greater in the IGB group than the control group (about 10% vs. 4%; P < .001), Dr. Abu Dayyeh said, noting that total body weight loss was significantly higher in the balloon group at each time point: 3, 6, 9, and 12 months.
Similarly, the mean percent of excess weight loss at 6 months was better in the balloon group than in the control group (about 40% vs. 13%; P < .001). The majority of excess weight loss achieved at 6 months was also maintained at 12 months, he said.
At 9 months (3 months after device removal), 45.6% of patients in the IGB group had an excess weight loss at least 15% higher than patients in the control group, which exceeded the 30% threshold set as a primary study outcome, he said.
The mean percent excess weight loss was 26.5% at 9 months in the balloon group, which also exceeded the 25% threshold set as a second primary outcome.
This IGB system “appears to meet the thresholds set forth by the ASGE/ASMBS PIVI for endoscopic bariatric therapies intended as a primary obesity intervention,” Dr. Abu Dayyeh said.
The American Society for Gastrointestinal Endoscopy/American Society for Metabolic and Bariatric Surgery PIVI (Preservation and Incorporation of Valuable endoscopic Innovations) recommends that endoscopic bariatric therapies intended as a primary obesity intervention achieve a mean minimum threshold of 25% excess weight loss at 12 months.
At 52 weeks, both groups had an improvement from baseline in diabetes, hypertension, and lipids, but the improvement was greater with the IGB, he said.
Beck Depression Scores and quality of life also improved in both groups, with the improvement again greater with the IGB.
Serious adverse events were reported by 7% of controls and 9.6% of the balloon group including 8 early removals for intolerance, 1 gastric outlet obstruction, 1 laryngospasm during placement, 1 case of severe abdominal cramping, and 1 case of severe dehydration.
Early device removals occurred in 22% of patients, 15 for symptoms and 13 at subject request, Dr. Abu Dayyeh said. No deaths occurred in the trial.
*Changed on July 8, 2015.
On Twitter @pwendl
WASHINGTON – Obese patients implanted with an intragastric balloon lost significantly more weight than those following a behavioral modification program in a randomized, nonblinded trial.
Moreover, weight loss was preserved even after device removal, study author Dr. Barham Abu Dayyeh said at the annual Digestive Disease Week.
The Orbera intragastric balloon (Apollo Endosurgery) could fill a gap in the United States between obesity lifestyle interventions that are minimally effective and a range of bariatric surgical interventions that are effective, but come at a cost of increased complications and health care costs, he said. Moreover, only 1% of qualified patients actually end up having bariatric surgery.
The silicone, saline-filled intragastric balloon (IGB) has been widely used outside the U.S. for more than 17 years in more than 200,000 patients, added Dr. Abu Dayyeh* of Mayo Clinic in Rochester, Minn.
The multicenter trial was designed for premarketing approval in the U.S. of the Orbera IGB and randomly assigned 273 adults with a body mass index (BMI) of 30-40 kg/m2 for more than 2 years to a 12-month behavioral modification program with or without endoscopic placement of the IGB filled to 500-600 cc. The balloon was removed at month 6, with regular office visits through 1 year.
Eighteen patients withdrew before treatment; 215 patients were evaluable at 6 months, 206 at 9 months, and 191 at 12 months. The mean baseline BMI was 35 kg/m2 and 90% of patients were female.
At 6 months, the mean percent total body weight loss was greater in the IGB group than the control group (about 10% vs. 4%; P < .001), Dr. Abu Dayyeh said, noting that total body weight loss was significantly higher in the balloon group at each time point: 3, 6, 9, and 12 months.
Similarly, the mean percent of excess weight loss at 6 months was better in the balloon group than in the control group (about 40% vs. 13%; P < .001). The majority of excess weight loss achieved at 6 months was also maintained at 12 months, he said.
At 9 months (3 months after device removal), 45.6% of patients in the IGB group had an excess weight loss at least 15% higher than patients in the control group, which exceeded the 30% threshold set as a primary study outcome, he said.
The mean percent excess weight loss was 26.5% at 9 months in the balloon group, which also exceeded the 25% threshold set as a second primary outcome.
This IGB system “appears to meet the thresholds set forth by the ASGE/ASMBS PIVI for endoscopic bariatric therapies intended as a primary obesity intervention,” Dr. Abu Dayyeh said.
The American Society for Gastrointestinal Endoscopy/American Society for Metabolic and Bariatric Surgery PIVI (Preservation and Incorporation of Valuable endoscopic Innovations) recommends that endoscopic bariatric therapies intended as a primary obesity intervention achieve a mean minimum threshold of 25% excess weight loss at 12 months.
At 52 weeks, both groups had an improvement from baseline in diabetes, hypertension, and lipids, but the improvement was greater with the IGB, he said.
Beck Depression Scores and quality of life also improved in both groups, with the improvement again greater with the IGB.
Serious adverse events were reported by 7% of controls and 9.6% of the balloon group including 8 early removals for intolerance, 1 gastric outlet obstruction, 1 laryngospasm during placement, 1 case of severe abdominal cramping, and 1 case of severe dehydration.
Early device removals occurred in 22% of patients, 15 for symptoms and 13 at subject request, Dr. Abu Dayyeh said. No deaths occurred in the trial.
*Changed on July 8, 2015.
On Twitter @pwendl
WASHINGTON – Obese patients implanted with an intragastric balloon lost significantly more weight than those following a behavioral modification program in a randomized, nonblinded trial.
Moreover, weight loss was preserved even after device removal, study author Dr. Barham Abu Dayyeh said at the annual Digestive Disease Week.
The Orbera intragastric balloon (Apollo Endosurgery) could fill a gap in the United States between obesity lifestyle interventions that are minimally effective and a range of bariatric surgical interventions that are effective, but come at a cost of increased complications and health care costs, he said. Moreover, only 1% of qualified patients actually end up having bariatric surgery.
The silicone, saline-filled intragastric balloon (IGB) has been widely used outside the U.S. for more than 17 years in more than 200,000 patients, added Dr. Abu Dayyeh* of Mayo Clinic in Rochester, Minn.
The multicenter trial was designed for premarketing approval in the U.S. of the Orbera IGB and randomly assigned 273 adults with a body mass index (BMI) of 30-40 kg/m2 for more than 2 years to a 12-month behavioral modification program with or without endoscopic placement of the IGB filled to 500-600 cc. The balloon was removed at month 6, with regular office visits through 1 year.
Eighteen patients withdrew before treatment; 215 patients were evaluable at 6 months, 206 at 9 months, and 191 at 12 months. The mean baseline BMI was 35 kg/m2 and 90% of patients were female.
At 6 months, the mean percent total body weight loss was greater in the IGB group than the control group (about 10% vs. 4%; P < .001), Dr. Abu Dayyeh said, noting that total body weight loss was significantly higher in the balloon group at each time point: 3, 6, 9, and 12 months.
Similarly, the mean percent of excess weight loss at 6 months was better in the balloon group than in the control group (about 40% vs. 13%; P < .001). The majority of excess weight loss achieved at 6 months was also maintained at 12 months, he said.
At 9 months (3 months after device removal), 45.6% of patients in the IGB group had an excess weight loss at least 15% higher than patients in the control group, which exceeded the 30% threshold set as a primary study outcome, he said.
The mean percent excess weight loss was 26.5% at 9 months in the balloon group, which also exceeded the 25% threshold set as a second primary outcome.
This IGB system “appears to meet the thresholds set forth by the ASGE/ASMBS PIVI for endoscopic bariatric therapies intended as a primary obesity intervention,” Dr. Abu Dayyeh said.
The American Society for Gastrointestinal Endoscopy/American Society for Metabolic and Bariatric Surgery PIVI (Preservation and Incorporation of Valuable endoscopic Innovations) recommends that endoscopic bariatric therapies intended as a primary obesity intervention achieve a mean minimum threshold of 25% excess weight loss at 12 months.
At 52 weeks, both groups had an improvement from baseline in diabetes, hypertension, and lipids, but the improvement was greater with the IGB, he said.
Beck Depression Scores and quality of life also improved in both groups, with the improvement again greater with the IGB.
Serious adverse events were reported by 7% of controls and 9.6% of the balloon group including 8 early removals for intolerance, 1 gastric outlet obstruction, 1 laryngospasm during placement, 1 case of severe abdominal cramping, and 1 case of severe dehydration.
Early device removals occurred in 22% of patients, 15 for symptoms and 13 at subject request, Dr. Abu Dayyeh said. No deaths occurred in the trial.
*Changed on July 8, 2015.
On Twitter @pwendl
AT DDW® 2015
Key clinical point: An intragastric balloon system is an effective adjunct to lifestyle intervention for weight loss in obese patients with a BMI of 30-40 kg/m2.
Major finding: Mean percent excess weight loss at 6 months was about 40% for the intragastric balloon group vs. 13% for controls (P < .001).
Data source: Prospective, randomized, nonblinded study in 273 obese patients with a BMI of 30-40 kg/m2.
Disclosures: Apollo Endosurgery sponsored the study. Dr. Dayyeh reported financial relationships with Apollo Endosurgery, Aspire Bariatrics, and GI Dynamics.
DDW: Urinary enzymes hint at gastric cancer
WASHINGTON – A simple urine test could detect gastric cancer even at an early stage, the test’s developers say.
The test, which looks for the presence of two metalloprotease enzymes labeled ADAM 12 and MMP-9/NGAL had 77.1% sensitivity and 82.9% specificity for gastric cancer when tested in 35 patients with the malignancy and an equal number of healthy controls, reported Dr. Takaya Shimura from the department of surgery at Boston Children’s Hospital and Harvard Medical School in Boston.
“This study represents the first demonstration of the presence of ADAM 12 and MMO-9/NGAL complex in the urine of gastric cancer patients,” he said at the annual Digestive Disease Week.
Dr. Stephen J. Meltzer of Johns Hopkins University, Baltimore, commented in an interview that the findings are convincing but preliminary.
A randomized clinical trial enrolling a larger number of patients and controls would be required before he would consider screening patients for the enzymes, said Dr. Meltzer, who was not involved in the study and comoderated the meeting session where the results were presented.
ADAM 12 (a disintegrin and metalloprotease 12) and MMP-9 (matrix metalloprotease 9) are both members of a family of enzymes involved in cellular adhesion, invasion, growth, and angiogenesis, Dr. Shimura explained. MMP-9, when complexed with NGAL (neutrophil gelatinase associated lipocalin) is protected from autodegradation.
The investigators, from the lab of Dr. Marsha A. Moses at Boston Children’s Hospital, and their collaborators in Japan had previously reported that MMPs in urine were independent predictors of both organ-confined and metastatic cancer.
Urinary assays are noninvasive, using easily accessed tissues that can be handled simply and inexpensively, making them ideal for cancer detection, Dr, Shimura said.
Current tests for gastric cancer, such as carcinoembryonic antigen (CEA) and cancer antigens (CA) 19-9 and 72-4, have poor sensitivity for detecting advanced disease, and are even worse at spotting early disease, he noted.
To see whether they could improve on the current lot of tests, the investigators enrolled 106 patients in a case-control study, settling eventually, after age and sex matching, on a cohort of 70 patients: 35 with primarily early-stage gastric cancer, and 35 healthy controls.
After screening the urine of participants for about 50 different antigenic proteins, they found that the patients with gastric cancer had significantly higher levels in their urine of both ADAM 12 (P < .001) and the MMP-9/NGAL complex (P = .020).
In a multivariate analysis, they showed that both enzymes were strong, independent predictors of gastric cancer, with an odds ratio for urinary MMO-9/NGAL of 6.71 (P = .002), and an OR of 15.4 for ADAM 12 (P = .002). In contrast, Helicobacter pylori infection was associated with a nonsignificant OR of 2.54.
In a receiver operating characteristic (ROC) analysis, they also found that MMP-9/NGAL was associated with an area-under-the curve (AUC) of 0.657 (P = .024), ADAM 12 was associated with an AUC of 0.757 (P < .001), and that the two combined had an AUC of 0.825 (P < .001).
As noted before, the sensitivity of the combined enzymes was 77%, and the specificity was 83%.
Finally, using immunohistochemical analysis, the investigators were able to show that gastric cancer tissues had high levels of coexpression of MMP-9 and NGAL (P <.001) and high expression levels of ADAM 12 (P < .001), compared with adjacent normal tissues.
WASHINGTON – A simple urine test could detect gastric cancer even at an early stage, the test’s developers say.
The test, which looks for the presence of two metalloprotease enzymes labeled ADAM 12 and MMP-9/NGAL had 77.1% sensitivity and 82.9% specificity for gastric cancer when tested in 35 patients with the malignancy and an equal number of healthy controls, reported Dr. Takaya Shimura from the department of surgery at Boston Children’s Hospital and Harvard Medical School in Boston.
“This study represents the first demonstration of the presence of ADAM 12 and MMO-9/NGAL complex in the urine of gastric cancer patients,” he said at the annual Digestive Disease Week.
Dr. Stephen J. Meltzer of Johns Hopkins University, Baltimore, commented in an interview that the findings are convincing but preliminary.
A randomized clinical trial enrolling a larger number of patients and controls would be required before he would consider screening patients for the enzymes, said Dr. Meltzer, who was not involved in the study and comoderated the meeting session where the results were presented.
ADAM 12 (a disintegrin and metalloprotease 12) and MMP-9 (matrix metalloprotease 9) are both members of a family of enzymes involved in cellular adhesion, invasion, growth, and angiogenesis, Dr. Shimura explained. MMP-9, when complexed with NGAL (neutrophil gelatinase associated lipocalin) is protected from autodegradation.
The investigators, from the lab of Dr. Marsha A. Moses at Boston Children’s Hospital, and their collaborators in Japan had previously reported that MMPs in urine were independent predictors of both organ-confined and metastatic cancer.
Urinary assays are noninvasive, using easily accessed tissues that can be handled simply and inexpensively, making them ideal for cancer detection, Dr, Shimura said.
Current tests for gastric cancer, such as carcinoembryonic antigen (CEA) and cancer antigens (CA) 19-9 and 72-4, have poor sensitivity for detecting advanced disease, and are even worse at spotting early disease, he noted.
To see whether they could improve on the current lot of tests, the investigators enrolled 106 patients in a case-control study, settling eventually, after age and sex matching, on a cohort of 70 patients: 35 with primarily early-stage gastric cancer, and 35 healthy controls.
After screening the urine of participants for about 50 different antigenic proteins, they found that the patients with gastric cancer had significantly higher levels in their urine of both ADAM 12 (P < .001) and the MMP-9/NGAL complex (P = .020).
In a multivariate analysis, they showed that both enzymes were strong, independent predictors of gastric cancer, with an odds ratio for urinary MMO-9/NGAL of 6.71 (P = .002), and an OR of 15.4 for ADAM 12 (P = .002). In contrast, Helicobacter pylori infection was associated with a nonsignificant OR of 2.54.
In a receiver operating characteristic (ROC) analysis, they also found that MMP-9/NGAL was associated with an area-under-the curve (AUC) of 0.657 (P = .024), ADAM 12 was associated with an AUC of 0.757 (P < .001), and that the two combined had an AUC of 0.825 (P < .001).
As noted before, the sensitivity of the combined enzymes was 77%, and the specificity was 83%.
Finally, using immunohistochemical analysis, the investigators were able to show that gastric cancer tissues had high levels of coexpression of MMP-9 and NGAL (P <.001) and high expression levels of ADAM 12 (P < .001), compared with adjacent normal tissues.
WASHINGTON – A simple urine test could detect gastric cancer even at an early stage, the test’s developers say.
The test, which looks for the presence of two metalloprotease enzymes labeled ADAM 12 and MMP-9/NGAL had 77.1% sensitivity and 82.9% specificity for gastric cancer when tested in 35 patients with the malignancy and an equal number of healthy controls, reported Dr. Takaya Shimura from the department of surgery at Boston Children’s Hospital and Harvard Medical School in Boston.
“This study represents the first demonstration of the presence of ADAM 12 and MMO-9/NGAL complex in the urine of gastric cancer patients,” he said at the annual Digestive Disease Week.
Dr. Stephen J. Meltzer of Johns Hopkins University, Baltimore, commented in an interview that the findings are convincing but preliminary.
A randomized clinical trial enrolling a larger number of patients and controls would be required before he would consider screening patients for the enzymes, said Dr. Meltzer, who was not involved in the study and comoderated the meeting session where the results were presented.
ADAM 12 (a disintegrin and metalloprotease 12) and MMP-9 (matrix metalloprotease 9) are both members of a family of enzymes involved in cellular adhesion, invasion, growth, and angiogenesis, Dr. Shimura explained. MMP-9, when complexed with NGAL (neutrophil gelatinase associated lipocalin) is protected from autodegradation.
The investigators, from the lab of Dr. Marsha A. Moses at Boston Children’s Hospital, and their collaborators in Japan had previously reported that MMPs in urine were independent predictors of both organ-confined and metastatic cancer.
Urinary assays are noninvasive, using easily accessed tissues that can be handled simply and inexpensively, making them ideal for cancer detection, Dr, Shimura said.
Current tests for gastric cancer, such as carcinoembryonic antigen (CEA) and cancer antigens (CA) 19-9 and 72-4, have poor sensitivity for detecting advanced disease, and are even worse at spotting early disease, he noted.
To see whether they could improve on the current lot of tests, the investigators enrolled 106 patients in a case-control study, settling eventually, after age and sex matching, on a cohort of 70 patients: 35 with primarily early-stage gastric cancer, and 35 healthy controls.
After screening the urine of participants for about 50 different antigenic proteins, they found that the patients with gastric cancer had significantly higher levels in their urine of both ADAM 12 (P < .001) and the MMP-9/NGAL complex (P = .020).
In a multivariate analysis, they showed that both enzymes were strong, independent predictors of gastric cancer, with an odds ratio for urinary MMO-9/NGAL of 6.71 (P = .002), and an OR of 15.4 for ADAM 12 (P = .002). In contrast, Helicobacter pylori infection was associated with a nonsignificant OR of 2.54.
In a receiver operating characteristic (ROC) analysis, they also found that MMP-9/NGAL was associated with an area-under-the curve (AUC) of 0.657 (P = .024), ADAM 12 was associated with an AUC of 0.757 (P < .001), and that the two combined had an AUC of 0.825 (P < .001).
As noted before, the sensitivity of the combined enzymes was 77%, and the specificity was 83%.
Finally, using immunohistochemical analysis, the investigators were able to show that gastric cancer tissues had high levels of coexpression of MMP-9 and NGAL (P <.001) and high expression levels of ADAM 12 (P < .001), compared with adjacent normal tissues.
AT DDW 2015
Key clinical point: Urinary levels of two metalloproteases were significantly elevated in the urine of patients with gastric cancer, compared with controls.
Major finding: High expression of ADAM 12 and MMP-9/NGAL complex had a 77% sensitivity and 83% specificity for gastric cancer.
Data source: Case-control study of 35 patients with gastric cancer and 35 controls.
Disclosures: The study was supported by the Advanced Medical Research Foundation in the United States and the Research Fellowship of the Uehara Memorial Foundation, Japan. Dr. Shimura reported having no conflicts of interest.
DDW: Scheduled for a colonoscopy? Pass the pretzels!
WASHINGTON – A novel, edible colon preparation could make obsolete the fasting and large volume of salty liquid cleansing that keep many a patient from completing their colonoscopies.
A pilot study showed that all 10 patients who ate a series of nutritionally balanced meals, drinks, and snacks such as pretzels and pudding had a successful colon cleansing according to the endoscopist at the time of the procedure, Dr. L. Campbell Levy of Dartmouth-Hitchcock Medical Center in Lebanon, N.H., reported at the annual Digestive Disease Week.
The preparation, which is blended with polyethylene glycol 3350, sorbitol, and ascorbic acid, did not produce any significant changes in electrolytes or creatinine.
There were no adverse events and, equally important, all 10 patients said that they would follow the edible bowel regimen again for a subsequent procedure.
In a video interview, he discussed the small study’s results and the plans for larger, randomized studies.
Dr. Levy reported no relevant conflicts. The inventor of the diet and the founder of Colonary Concepts were involved in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – A novel, edible colon preparation could make obsolete the fasting and large volume of salty liquid cleansing that keep many a patient from completing their colonoscopies.
A pilot study showed that all 10 patients who ate a series of nutritionally balanced meals, drinks, and snacks such as pretzels and pudding had a successful colon cleansing according to the endoscopist at the time of the procedure, Dr. L. Campbell Levy of Dartmouth-Hitchcock Medical Center in Lebanon, N.H., reported at the annual Digestive Disease Week.
The preparation, which is blended with polyethylene glycol 3350, sorbitol, and ascorbic acid, did not produce any significant changes in electrolytes or creatinine.
There were no adverse events and, equally important, all 10 patients said that they would follow the edible bowel regimen again for a subsequent procedure.
In a video interview, he discussed the small study’s results and the plans for larger, randomized studies.
Dr. Levy reported no relevant conflicts. The inventor of the diet and the founder of Colonary Concepts were involved in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – A novel, edible colon preparation could make obsolete the fasting and large volume of salty liquid cleansing that keep many a patient from completing their colonoscopies.
A pilot study showed that all 10 patients who ate a series of nutritionally balanced meals, drinks, and snacks such as pretzels and pudding had a successful colon cleansing according to the endoscopist at the time of the procedure, Dr. L. Campbell Levy of Dartmouth-Hitchcock Medical Center in Lebanon, N.H., reported at the annual Digestive Disease Week.
The preparation, which is blended with polyethylene glycol 3350, sorbitol, and ascorbic acid, did not produce any significant changes in electrolytes or creatinine.
There were no adverse events and, equally important, all 10 patients said that they would follow the edible bowel regimen again for a subsequent procedure.
In a video interview, he discussed the small study’s results and the plans for larger, randomized studies.
Dr. Levy reported no relevant conflicts. The inventor of the diet and the founder of Colonary Concepts were involved in the study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
AT DDW® 2015
Manufacturer releases new reprocessing instructions for TJF-Q180V duodenoscope
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
Olympus, the manufacturer of the TJF-Q180V duodenoscope, has issued new, validated instructions for reprocessing this particular model, as part of the response to recent reports of a possible association between multidrug-resistant bacterial infections and improperly processed duodenoscopes, according to the Food and Drug Administration.
The new instructions, which replace the manual reprocessing instructions included in the original labeling, and validation data have been reviewed by the FDA as part of its ongoing review of the device. The agency “recommends that any facilities that are using Olympus’ TJF-Q180V duodenoscope train staff on the new instructions and implement them as soon as possible,” according to an FDA statement. The instructions are provided in letters sent by Olympus to health care and other facilities that use this particular model.
“Key changes” have been made to the procedures for precleaning, manual cleaning, and manual high-level disinfection reprocessing procedures, the FDA said.
The TJF-Q180 V duodenoscope was the model used in four patients who had undergone an endoscopic retrograde cholangiopancreatography (ERCP) procedure between August 2014 and January 2015 with the same duodenoscope at Cedars-Sinai Medical Center in Los Angeles and had been infected with carbapenem-resistant Enterobacteriaceae (CRE). This outbreak was announced by the medical center in early March in a statement that said the infections occurred “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes” recommended in instructions provided by Olympus and the FDA.
In February, the FDA first announced that the agency had received reports of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures with duodenoscopes, despite proper cleaning and disinfection of the devices and that the “complex design of ERCP endoscopes (also called duodenoscopes) may impede effective reprocessing.”
In the latest statement, the FDA said it “is closely monitoring the possible association between reprocessed duodenoscopes and the transmission of infectious agents,” including multidrug-resistant bacterial infections caused by CRE. If they are not properly reprocessed, the statement adds, “residual body fluids and organic debris may remain in microscopic crevices of the device following an attempted cleaning and high-level disinfection. If these residual fluids contain microbial contamination, subsequent patients may be exposed to serious infections.”
Adverse events associated with duodenoscopes should be reported to the FDA’s MedWatch Program at 800-332-1088 or www.accessdata.fda.gov/scripts/medwatch.
AGA Resource
Read more about AGA’s efforts and recommendations to stop duodenoscope infections here.
AGA statement: How to stop duodenoscope infections
SAN FRANCISCO — A growing number of antibiotic-resistant infections have been reported following use of duodenoscopes, which are used in the advanced procedure endoscopic retrograde cholangiopancreatography, or ERCP. The American Gastroenterological Association (AGA) Center for GI Innovation and Technology convened a meeting on March 23, 2015, “Getting to Zero,” with experts in gastroenterology, epidemiology and infectious disease; the endoscope manufacturers Fuji and Pentax; and representatives from the U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC) and ECRI Institute to discuss how to prevent these infections.
“More than 500,000 ERCPs are performed each year throughout the U.S., saving the lives of hundreds of thousands of patients with very serious illnesses,” said AGA President Dr. John I. Allen, MBA, AGAF. “The value of these procedures can not be understated. AGA is committed to finding a path forward to remove the risk of device-transmitted infections and ensure safe patient care.”
“We must stop device-associated infections. It’s a complex issue without an easy solution, but first we need to protect our patients,” added Dr. Michael Kochman, AGAF, chair of the AGA Center for GI Innovation and Technology, explaining this means educating patients, redesigning the endoscopes, and finding new ways to clean them.The problem of this infection transmission lies in the complex design of the elevator channel in duodenoscopes, which can allow bacteria to remain after cleansing, even if reprocessing follows currently accepted procedures developed and approved by the manufacturers and FDA. Meeting participants also acknowledged other potential sites of failure in the design.
AGA supports current FDA and CDC guidance and suggests the following additional recommendations to improve patient safety.
Short-term recommendations for physicians
1. Treat all elevator-channel endoscopes the same, including both FNA echoendoscopes (EUS) and duodenoscopes.
2. Continue to follow the recently enhanced manufacturer reprocessing guidelines. Currently, FDA is working with each endoscope manufacturer to validate their enhanced reprocessing protocols.
3. Elevator-channel endoscopes should be tracked by patient and by device serial number to facilitate retrospective identification in case of infection.
4. Establish a two-phase infection surveillance program: a) track all patients who have had a procedure with an elevator-channel endoscope and b) periodically collect culture surveillance of all elevator-channel endoscopes.
5. Baseline culturing of all elevator-channel endoscopes is prudent; the sensitivity is unknown at this time. Importantly, a positive culture should trigger a thorough review of your reprocessing technique.
6. Develop a standard device reprocessing training program and ensure reprocessing staff demonstrate competency every 6 months, as well as with the introduction of new model endoscopes.
7. If you suspect a breach or infection, contact CDC immediately to aid in investigation.
Long-term recommendation: Device redesign
Further study of the failure modes resulting in the transmission of infection will inform the necessary components of a device redesign. FDA has committed to expeditiously review validation data for alternative scope designs that mitigate the risk of transmitting infection.
What Patients Need to Know
Most people will never have an ERCP. But for patients who need it, ERCP is a critical and life-saving procedure. ERCP is the least invasive way for doctors to diagnose and treat problems in the bile duct and pancreatic ducts, including infections, stones, tumors and blockages. Experts all agree that there is no demonstrable infection transmission risk related to upper endoscopy or colonoscopy. Patients should not defer or avoid these procedures, which include colon cancer screening tests.
To put the issue in perspective, the infectious complication rate for theses specific types of infections is about 150 out of more than 1 million procedures over the last two years. The therapeutic benefits of this procedure far outweigh the potential low risk of infection.
For more information about these bacteria, visit the CDC website.
AGA appreciates the involvement of CDC, FDA, Fuji, Pentax, ECRI Institute, and the GI and infectious disease experts in developing these recommendations and for their commitment to “Getting to Zero.”
SAN FRANCISCO — A growing number of antibiotic-resistant infections have been reported following use of duodenoscopes, which are used in the advanced procedure endoscopic retrograde cholangiopancreatography, or ERCP. The American Gastroenterological Association (AGA) Center for GI Innovation and Technology convened a meeting on March 23, 2015, “Getting to Zero,” with experts in gastroenterology, epidemiology and infectious disease; the endoscope manufacturers Fuji and Pentax; and representatives from the U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC) and ECRI Institute to discuss how to prevent these infections.
“More than 500,000 ERCPs are performed each year throughout the U.S., saving the lives of hundreds of thousands of patients with very serious illnesses,” said AGA President Dr. John I. Allen, MBA, AGAF. “The value of these procedures can not be understated. AGA is committed to finding a path forward to remove the risk of device-transmitted infections and ensure safe patient care.”
“We must stop device-associated infections. It’s a complex issue without an easy solution, but first we need to protect our patients,” added Dr. Michael Kochman, AGAF, chair of the AGA Center for GI Innovation and Technology, explaining this means educating patients, redesigning the endoscopes, and finding new ways to clean them.The problem of this infection transmission lies in the complex design of the elevator channel in duodenoscopes, which can allow bacteria to remain after cleansing, even if reprocessing follows currently accepted procedures developed and approved by the manufacturers and FDA. Meeting participants also acknowledged other potential sites of failure in the design.
AGA supports current FDA and CDC guidance and suggests the following additional recommendations to improve patient safety.
Short-term recommendations for physicians
1. Treat all elevator-channel endoscopes the same, including both FNA echoendoscopes (EUS) and duodenoscopes.
2. Continue to follow the recently enhanced manufacturer reprocessing guidelines. Currently, FDA is working with each endoscope manufacturer to validate their enhanced reprocessing protocols.
3. Elevator-channel endoscopes should be tracked by patient and by device serial number to facilitate retrospective identification in case of infection.
4. Establish a two-phase infection surveillance program: a) track all patients who have had a procedure with an elevator-channel endoscope and b) periodically collect culture surveillance of all elevator-channel endoscopes.
5. Baseline culturing of all elevator-channel endoscopes is prudent; the sensitivity is unknown at this time. Importantly, a positive culture should trigger a thorough review of your reprocessing technique.
6. Develop a standard device reprocessing training program and ensure reprocessing staff demonstrate competency every 6 months, as well as with the introduction of new model endoscopes.
7. If you suspect a breach or infection, contact CDC immediately to aid in investigation.
Long-term recommendation: Device redesign
Further study of the failure modes resulting in the transmission of infection will inform the necessary components of a device redesign. FDA has committed to expeditiously review validation data for alternative scope designs that mitigate the risk of transmitting infection.
What Patients Need to Know
Most people will never have an ERCP. But for patients who need it, ERCP is a critical and life-saving procedure. ERCP is the least invasive way for doctors to diagnose and treat problems in the bile duct and pancreatic ducts, including infections, stones, tumors and blockages. Experts all agree that there is no demonstrable infection transmission risk related to upper endoscopy or colonoscopy. Patients should not defer or avoid these procedures, which include colon cancer screening tests.
To put the issue in perspective, the infectious complication rate for theses specific types of infections is about 150 out of more than 1 million procedures over the last two years. The therapeutic benefits of this procedure far outweigh the potential low risk of infection.
For more information about these bacteria, visit the CDC website.
AGA appreciates the involvement of CDC, FDA, Fuji, Pentax, ECRI Institute, and the GI and infectious disease experts in developing these recommendations and for their commitment to “Getting to Zero.”
SAN FRANCISCO — A growing number of antibiotic-resistant infections have been reported following use of duodenoscopes, which are used in the advanced procedure endoscopic retrograde cholangiopancreatography, or ERCP. The American Gastroenterological Association (AGA) Center for GI Innovation and Technology convened a meeting on March 23, 2015, “Getting to Zero,” with experts in gastroenterology, epidemiology and infectious disease; the endoscope manufacturers Fuji and Pentax; and representatives from the U.S. Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC) and ECRI Institute to discuss how to prevent these infections.
“More than 500,000 ERCPs are performed each year throughout the U.S., saving the lives of hundreds of thousands of patients with very serious illnesses,” said AGA President Dr. John I. Allen, MBA, AGAF. “The value of these procedures can not be understated. AGA is committed to finding a path forward to remove the risk of device-transmitted infections and ensure safe patient care.”
“We must stop device-associated infections. It’s a complex issue without an easy solution, but first we need to protect our patients,” added Dr. Michael Kochman, AGAF, chair of the AGA Center for GI Innovation and Technology, explaining this means educating patients, redesigning the endoscopes, and finding new ways to clean them.The problem of this infection transmission lies in the complex design of the elevator channel in duodenoscopes, which can allow bacteria to remain after cleansing, even if reprocessing follows currently accepted procedures developed and approved by the manufacturers and FDA. Meeting participants also acknowledged other potential sites of failure in the design.
AGA supports current FDA and CDC guidance and suggests the following additional recommendations to improve patient safety.
Short-term recommendations for physicians
1. Treat all elevator-channel endoscopes the same, including both FNA echoendoscopes (EUS) and duodenoscopes.
2. Continue to follow the recently enhanced manufacturer reprocessing guidelines. Currently, FDA is working with each endoscope manufacturer to validate their enhanced reprocessing protocols.
3. Elevator-channel endoscopes should be tracked by patient and by device serial number to facilitate retrospective identification in case of infection.
4. Establish a two-phase infection surveillance program: a) track all patients who have had a procedure with an elevator-channel endoscope and b) periodically collect culture surveillance of all elevator-channel endoscopes.
5. Baseline culturing of all elevator-channel endoscopes is prudent; the sensitivity is unknown at this time. Importantly, a positive culture should trigger a thorough review of your reprocessing technique.
6. Develop a standard device reprocessing training program and ensure reprocessing staff demonstrate competency every 6 months, as well as with the introduction of new model endoscopes.
7. If you suspect a breach or infection, contact CDC immediately to aid in investigation.
Long-term recommendation: Device redesign
Further study of the failure modes resulting in the transmission of infection will inform the necessary components of a device redesign. FDA has committed to expeditiously review validation data for alternative scope designs that mitigate the risk of transmitting infection.
What Patients Need to Know
Most people will never have an ERCP. But for patients who need it, ERCP is a critical and life-saving procedure. ERCP is the least invasive way for doctors to diagnose and treat problems in the bile duct and pancreatic ducts, including infections, stones, tumors and blockages. Experts all agree that there is no demonstrable infection transmission risk related to upper endoscopy or colonoscopy. Patients should not defer or avoid these procedures, which include colon cancer screening tests.
To put the issue in perspective, the infectious complication rate for theses specific types of infections is about 150 out of more than 1 million procedures over the last two years. The therapeutic benefits of this procedure far outweigh the potential low risk of infection.
For more information about these bacteria, visit the CDC website.
AGA appreciates the involvement of CDC, FDA, Fuji, Pentax, ECRI Institute, and the GI and infectious disease experts in developing these recommendations and for their commitment to “Getting to Zero.”
RECOMMENDATIONS FROM 'GETTING TO ZERO:' FIRST MEETING OF REGULATORS, ENDOSCOPE MANUFACTURERS & GASTROENTEROLOGISTS
FDA guidance focuses on infections with reusable devices, including duodenoscopes
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Recommendations to manufacturers about improving the safety of reusable medical devices and an upcoming advisory committee meeting on duodenoscope-associated infections are two efforts recently announced by the Food and Drug Administration that address the risks associated with reusable devices.
A final guidance document for industry on reprocessing reusable medical devices includes recommendations “aimed at helping device manufacturers develop safer reusable devices, especially those devices that pose a greater risk of infection,” according to the March 12 announcement. Also included in the guidance are criteria that should be met in instructions for reprocessing reusable devices, “to ensure users understand and correctly follow the reprocessing instructions,” and recommendations that manufacturers should consider “reprocessing challenges” at the early stages of the design of such devices.
The same announcement said that in mid-May, the FDA was convening a 2-day meeting of the agency’s Gastroenterology and Urology Devices Panel to discuss the recent reports of infections associated with the use of duodenoscopes in endoscopic retrograde cholangiopancreatography (ERCP) procedures in U.S. hospitals.
The announcement was issued less than a month after the agency alerted health care professionals and the public about the association with duodenoscopes and the transmission of multidrug-resistant bacterial infections in patients who had undergone ERCP procedures, despite proper cleaning and disinfection of the devices. Between January 2013 and December 2014, the agency received 75 medical device adverse event reports for about 135 patients in the United States “relating to possible microbial transmission from reprocessed duodenoscopes,” according to the safety communication issued by the FDA on Feb. 19.
These reports and cases described in the medical literature have occurred even when manufacturer instructions for cleaning and sterilization were followed.
“Although the complex design of duodenoscopes improves the efficiency and effectiveness of ERCP, it causes challenges for cleaning and high-level disinfection,” according to the statement, which pointed out that it can be difficult to access some parts of the duodenoscopes when they are cleaned. Problems include the “elevator” mechanism at the tip of the duodenoscope, which should be manually brushed, but a brush may not be able to reach microscopic crevices in this mechanism and “residual body fluids and organic debris may remain in these crevices after cleaning and disinfection,” possibly exposing patients to serious infections if the fluids are contaminated with microbes.
The infections reported include carbapenem-resistant Enterobacteriaceae (CRE), according to the first FDA statement, which did not mention whether any of the reports were fatal.
But on Feb. 18, the UCLA Health System announced that CRE may have been transmitted to seven patients during ERCP procedures, and may have contributed to the death of two of the patients. The two devices implicated in these cases are no longer used and the medical center has started to use a decontamination process “that goes above and beyond manufacturer and national standards” for the devices, the statement said. More than 100 patients who had an ERCP between October 2014 and January 2015 at UCLA have been notified they may have been infected with CRE.
The FDA statement includes recommendations for facilities and staff that reprocess duodenoscopes, for patients, and for health care professionals. One recommendation is to take a duodenoscope out of service if there is any suspicion it may be linked to a multidrug-resistant infection in a patient who has undergone ERCP.
In early March, another outbreak was reported at Cedars-Sinai Medical Center in Los Angeles, which announced that four patients who had undergone an ERCP procedure between August 2014 and January 2015 with the same duodenoscope had been infected with CRE, “despite the fact that Cedars-Sinai meticulously followed the disinfection procedure for duodenoscopes recommended in instructions provided by the manufacturer (Olympus Corporation) and the FDA.” This duodenoscope was the TJF-Q180 V model, a Cedars-Sinai spokesperson confirmed.
This particular duodenoscope has not yet been cleared for marketing, but has been used commercially, according to an FDA statement March 4 updating the duodenoscope-associated infection issue. The statement said that there was “no evidence” that the lack of clearance was associated with infections, and that the reported infections were associated with duodenoscopes from all three manufacturers of the devices used in the United States. In addition, the FDA statement noted that if the TJF-Q180 V duodenoscope was removed from the market, there may not be enough duodenoscopes to meet “the clinical demand in the United States of approximately 500,000 procedures per year.”
At the advisory panel meeting May 14-15, the FDA will ask the expert panel to discuss and make recommendations on various issues, including approaches that ensure patient safety during ERCP procedures and the effectiveness of the cleaning, disinfection, and sterilization procedures for duodenoscopes.
The FDA is asking health care professionals to report any infections possibly related to ERCP duodenoscopes to the manufacturers and the FDA’s MedWatch program.
The Centers for Disease Control and Prevention has provided an interim protocol for health care facilities, with information on monitoring for bacterial contamination of duodenoscopes after reprocessing and other reprocessing issues.
Several factors predict postbariatric surgery readmission
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
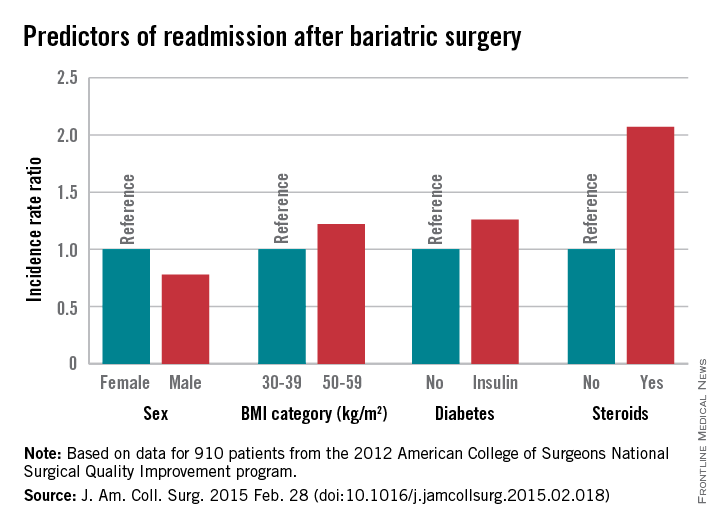
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.
“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.

“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
Bariatric surgery is generally safe and readmissions are rare, but prolonged operative time, operation complexity, and major postoperative complications are among several risk factors for readmission identified in a large retrospective cohort.
Of 18,186 patients from the 2012 American College of Surgeons National Surgical Quality Improvement program (ACS NSQIP) database who had bariatric surgery as a primary procedure, 5% were readmitted. Of 815 patients with any major complication, 31% were readmitted. Factors found on multivariate analysis to significantly predict readmission within 30 days were age, sex, body mass index, American Society of Anesthesiology (ASA) risk class, diabetes status, hypertension, and steroid use, Dr. Christa R. Abraham of Albany (N.Y.) Medical College and her colleagues reported online in the Journal of the American College of Surgeons.
Further, all major postoperative complications were significant predictors of readmission, including bleeding requiring transfusion, urinary tract infections, and superficial surgical site infection (SSI). Other significant predictors were deep SSI, organ space SSI, wound disruption, pneumonia, unplanned intubation, mechanical ventilation for more than 48 hours, pulmonary embolism, deep vein thrombosis, and sepsis, the investigators said (J. Am. Coll. Surg. 2015 [doi:10.1016/j.jamcollsurg.2015.02.018]).
Of the patients included in the study, 1,819 had a laparoscopic gastric band, 9,613 had laparoscopic Roux-en-Y gastric bypass, 6,439 had gastroplasties, and 315 had open Roux-en-Y gastric bypass. All had a BMI of at least 30 kg/m2, and had a postsurgery length of stay of 14 days or fewer. Most were ASA risk class 3 or lower, and most were functionally independent.
Complications were more common with laparoscopic and open Roux-en-y gastric bypass (5.5% and 11.8%, respectively) rather than with gastroplasty and sleeve (3.4%) and laparoscopic banding (1.4%).
The findings are of value, because while bariatric surgery is a low-risk procedure, and it is extremely common; in 2013 there were 179,000 such surgeries performed in the United States.

“Bariatric surgery is one of the fastest-growing surgical interest areas, making analysis of patient outcomes and reasons for readmission important,” the investigators explained.
The ability to identify high-risk patients could allow for targeted interventions to prevent readmission, they said.
For example, steroid use, which was identified as a risk factor in the current study, is modifiable.
“In our practice, steroids are discontinued for 6 weeks prior to bariatric surgery and patients who are steroid dependent are unlikely to undergo bariatric surgery,” they said.
Additionally, they “try to minimize readmission for patients with infections by treating with antibiotics following operation and continuing antibiotics at discharge.”
The investigators noted that the ACS NSQIP MORBPROB (estimated probability of morbidity) tool is a good tool for predicting readmission among prospective bariatric patients, although it may not fully capture the effect of preexisting conditions.
“These data led us to change our own practice by risk-stratifying patients with higher ASA and BMI to consider surgical options, and to begin early surveillance soon after discharge,” they said.
The authors reported having no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Knowing risk factors for readmission after bariatric surgery can allow for targeted interventions.
Major finding: Steroid use is among several risk factors for readmission following bariatric surgery (incidence rate ratio, 2.07)
Data source: A retrospective cohort study involving 18,186 patients.
Disclosures: The authors reported having no disclosures.
AGA guideline addresses asymptomatic neoplastic pancreatic cysts
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
For asymptomatic neoplastic pancreatic cysts discovered incidentally on abdominal imaging, surgery is warranted only if both a solid component and a dilated pancreatic duct are shown and/or if endoscopic* ultrasound with or without fine-needle aspiration has detected “concerning features,” according to a clinical practice guideline published in the April issue of Gastroenterology (doi:10.1053/j.gastro.2015.01.015).
Even then, patients should be referred for the procedure only to centers that perform high volumes of pancreatic surgery, so as to minimize the relatively high rates of morbidity and mortality associated with these invasive, expensive, and potentially harmful surgeries.
These are 2 of the 10 recommendations and “suggestions” in the American Gastroenterological Association guideline, which is the first such guideline to be based on a systematic evaluation of the available evidence, said Dr. Santhi Swaroop Vege of the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minn., and his associates.
Incidental discovery of asymptomatic pancreatic cysts is common with the increasing use of sophisticated abdominal imaging techniques. For example, approximately 15% of patients undergoing abdominal MRI for other indications are found to have them. Clinical management is very difficult because only a small fraction of these lesions prove to be malignant, and the data to guide diagnostic and treatment decisions are sparse and of very low quality, based almost entirely on retrospective case series. Nevertheless, Dr. Vege and his associates developed the guideline from the limited evidence that is available, because of the seriousness of the outcomes for that minority of cancers and the complexity of management strategies.
“These recommendations may result in significant controversy, as they advocate less frequent follow-up and a higher threshold before offering endoscopic ultrasound and/or surgery. However, consistent utilization should decrease inadvertent harm to patients and reduce the costs of health care delivery,” they noted.
After reviewing the literature, the investigators estimated that an asymptomatic cyst found incidentally on MRI has only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer. The guideline therefore suggests that surveillance is sufficient for asymptomatic pancreatic cysts smaller than 3 cm that don’t have a solid component or a dilated pancreatic duct. The preferred imaging modality is MRI, and the preferred surveillance interval is at 1 year after discovery. If no change is noted, surveillance every 2 years for a total of 5 years should be sufficient.
The risk of malignant transformation is estimated to be only 0.24% per year, and is even lower among cysts that show no changes over time. “The small risk of malignant progression in stable cysts is likely outweighed by the costs of surveillance and the risks of surgery,” so the guideline suggests that surveillance can be discontinued if no change has occurred after 5 years or if the patient is no longer a candidate for surgery. However, some patients, such as those with a family history of pancreatic cancer, may opt to continue surveillance.
In contrast, asymptomatic pancreatic cysts that have at least two high-risk features should be assessed using endoscopic ultrasound, with or without fine-needle aspiration. If these procedures reveal “concerning features,” the benefits of surgery probably outweigh the risks, and surgical excision/resection is conditionally recommended. However, even in these “suspect” lesions only an estimated 17% are found to harbor high-grade dysplasia. Any benefit ascribed to surgery must be balanced against “an overall postoperative mortality of 2% and major morbidity of 30% from our review of the literature,” Dr. Vege and his associates said.
In contrast to its suggestions and conditional recommendations, the AGA guideline strongly recommends that if surgery is being considered, patients be referred to “a center with demonstrated expertise in pancreatic surgery.” Their investigation showed that in the U.S. overall, all pancreatic surgeries carry a postoperative mortality of 6.6%, while in centers of excellence, the postoperative mortality is only 2%.
The guideline conditionally suggests that patients found to have invasive cancer or dysplasia in a resected cyst can undergo MRI surveillance of any remaining pancreas every 2 years, for as long as the patient remains a good candidate for further surgery.
Another recommendation is that patients be given a clear understanding of the benefits and risks of any surveillance program, because surveillance may not be appropriate for some. Certain patients have a high tolerance for risk and may decide against surveillance once the small risk of malignancy is explained to them. Others have a limited life expectancy and are unlikely to benefit from surveillance or surgery, and still others who are poor surgical candidates because of age or comorbidities shouldn’t be subjected to surveillance.
Finally, this AGA guideline pertains only to asymptomatic neoplastic pancreatic cysts. It doesn’t address lesions such as solid papillary neoplasms, cystic degeneration of adenocarcinomas, neuroendocrine tumors, or main duct intraductal papillary mucinous neoplasms without side-branch involvement, because identification of these lesions is more straightforward and the accepted management approach is surgical resection, Dr. Vege and his associates added.
*A correction was made on April 29, 2015.
Key clinical point: A new AGA clinical practice guideline suggests surgery is warranted only if asymptomatic neoplastic pancreatic cysts have both a solid component and a dilated pancreatic duct and/or concerning features on endoscopic ultrasound with or without fine-needle aspiration.
Major finding: An asymptomatic pancreatic cyst found incidentally on MRI is estimated to have only a 10 in 100,000 chance of being a mucinous invasive malignancy and a 17 in 100,000 chance of being a ductal cancer.
Data source: A review and summary of the available evidence regarding management of asymptomatic neoplastic pancreatic cysts, and a compilation of recommendations for clinicians.
Disclosures: Dr. Vege and his associates’ disclosures are available at the American Gastroenterological Association, Bethesda, Md.
Studies confirm genetic links to obesity
Two new genetic studies provide insights into the biology of obesity, according to findings reported in the journal Nature.
In a study of waist-to-hip ratios in more than 244,000 individuals, Dr. Dmitry Shungin of Umea University in Sweden and his colleagues identified 49 genetic locations associated with fat deposits, 19 of which had a stronger effect on women. In a simultaneously published study of body mass index (BMI) in nearly 340,000 patients, investigators identified 97 genetic locations associated with BMI, reported Dr. Adam E. Locke and colleagues at the University of Michigan, Ann Arbor.
“This work is the first step toward finding individual genes that play key roles in body shape and size,” said a University of Michigan statement. “The proteins these genes help produce could become targets for future drug development.”
Read the full article by Dr. Shungin here and the full article by Dr. Locke here.
Two new genetic studies provide insights into the biology of obesity, according to findings reported in the journal Nature.
In a study of waist-to-hip ratios in more than 244,000 individuals, Dr. Dmitry Shungin of Umea University in Sweden and his colleagues identified 49 genetic locations associated with fat deposits, 19 of which had a stronger effect on women. In a simultaneously published study of body mass index (BMI) in nearly 340,000 patients, investigators identified 97 genetic locations associated with BMI, reported Dr. Adam E. Locke and colleagues at the University of Michigan, Ann Arbor.
“This work is the first step toward finding individual genes that play key roles in body shape and size,” said a University of Michigan statement. “The proteins these genes help produce could become targets for future drug development.”
Read the full article by Dr. Shungin here and the full article by Dr. Locke here.
Two new genetic studies provide insights into the biology of obesity, according to findings reported in the journal Nature.
In a study of waist-to-hip ratios in more than 244,000 individuals, Dr. Dmitry Shungin of Umea University in Sweden and his colleagues identified 49 genetic locations associated with fat deposits, 19 of which had a stronger effect on women. In a simultaneously published study of body mass index (BMI) in nearly 340,000 patients, investigators identified 97 genetic locations associated with BMI, reported Dr. Adam E. Locke and colleagues at the University of Michigan, Ann Arbor.
“This work is the first step toward finding individual genes that play key roles in body shape and size,” said a University of Michigan statement. “The proteins these genes help produce could become targets for future drug development.”
Read the full article by Dr. Shungin here and the full article by Dr. Locke here.
No increased pancreatitis risk found with incretin therapy
No association between an increased risk of acute pancreatitis and treatment with incretin therapy for type 2 diabetes was found in a large case control-study.
To evaluate whether the risk of acute pancreatitis was increased among patients treated with incretin-based drugs – glucagonlike peptide–1 (GLP-1) receptor agonists and dipeptidyl peptidase–4 (DPP-4) inhibitors – the investigators compared the risks of acute pancreatitis associated with different antihyperglycemic drugs in 12,868 people hospitalized for the first time for acute pancreatitis, and in 12,680 controls matched for birth year, sex, and region of residence in Denmark.
The study addressed evidence indicating that incretins may cause pancreatitis and pancreatic cancer in humans, which includes adverse event reports suggesting a signal of pancreatitis, but the association between incretins and acute pancreatitis is controversial and is an issue that “remains under debate,” they noted.
The data used were from three Danish databases, including one of reimbursed prescriptions. After adjustment for comorbidities and drugs associated with pancreatitis, including alcoholism and obesity, the risk of acute pancreatitis was not increased among those who had ever used an incretin, with an odds ratio of 0.95, according to Dr. Reimar Thomsen of the department of clinical epidemiology, Institute of Clinical Medicine, Aarhus (Denmark) University Hospital, and his associates (Diabetes Care 2015 [doi:10.2337/dc13-2983])
Risk was also not increased among those who had been ever treated with a DPP-4 inhibitor (OR, 1.04), which included an OR of 1.06 for sitagliptin, or among those who had been treated with a GLP-1 receptor agonist (OR, 0.82), which included an OR of 0.75 for liraglutide. Nor was risk elevated among those treated with nonincretin antihyperglycemic drugs (OR, 1.05).
In addition, the adjusted odds ratio comparing the risk of acute pancreatitis associated with incretin treatments and other antihyperglycemics was not increased, approaching 1.
The crude odds ratios, before adjustment for confounding factors, was 1.36 in patients who had been treated with incretins and 1.44 among those who had been treated with other antihyperglycemic drugs. “The fact that crude ORs were increased to very similar levels for all antihyperglycemic drugs – given their different modes of action – points to a general underlying diabetes effect on pancreatitis risk, rather than a specific drug effect,” they commented. In the crude analyses, they determined that obesity, gallstones, and other diabetes-related risk factors “may explain much of the apparent risk increase,” the investigators added.
Other findings included an increased risk of acute pancreatitis among those who had recently started treatment with a DPP-4 inhibitor and in those who had recently started treatment with several other antihyperglycemic drugs, including sulfonylureas and insulin. “This lack of specificity suggests that either the increased pancreatitis risk is related to newly diagnosed and drug-treated type 2 diabetes per se, with a possibility of reverse causality due to pancreatogenic diabetes,” or that starting therapy with sulfonylureas and insulin “also causes acute pancreatitis, which should be further investigated,” they said.
Incretin-based drugs include injectable incretin mimetic agents (GLP-1 receptor agonists) such as liraglutide; and incretin enhancers (DPP-4) inhibitors, such as sitagliptin.
The study was supported by the Clinical Epidemiological Research Foundation in Denmark. One of the authors, from the Danish Center for Strategic Research in Type 2 Diabetes, at Odense University Hospital, disclosed having received research grants form Novo-Nordisk. Of the six authors, four are from the Aarhus University Hospital clinical epidemiology department, a member of the center, which receives funding that includes an unrestricted donation from Novo-Nordisk.
No association between an increased risk of acute pancreatitis and treatment with incretin therapy for type 2 diabetes was found in a large case control-study.
To evaluate whether the risk of acute pancreatitis was increased among patients treated with incretin-based drugs – glucagonlike peptide–1 (GLP-1) receptor agonists and dipeptidyl peptidase–4 (DPP-4) inhibitors – the investigators compared the risks of acute pancreatitis associated with different antihyperglycemic drugs in 12,868 people hospitalized for the first time for acute pancreatitis, and in 12,680 controls matched for birth year, sex, and region of residence in Denmark.
The study addressed evidence indicating that incretins may cause pancreatitis and pancreatic cancer in humans, which includes adverse event reports suggesting a signal of pancreatitis, but the association between incretins and acute pancreatitis is controversial and is an issue that “remains under debate,” they noted.
The data used were from three Danish databases, including one of reimbursed prescriptions. After adjustment for comorbidities and drugs associated with pancreatitis, including alcoholism and obesity, the risk of acute pancreatitis was not increased among those who had ever used an incretin, with an odds ratio of 0.95, according to Dr. Reimar Thomsen of the department of clinical epidemiology, Institute of Clinical Medicine, Aarhus (Denmark) University Hospital, and his associates (Diabetes Care 2015 [doi:10.2337/dc13-2983])
Risk was also not increased among those who had been ever treated with a DPP-4 inhibitor (OR, 1.04), which included an OR of 1.06 for sitagliptin, or among those who had been treated with a GLP-1 receptor agonist (OR, 0.82), which included an OR of 0.75 for liraglutide. Nor was risk elevated among those treated with nonincretin antihyperglycemic drugs (OR, 1.05).
In addition, the adjusted odds ratio comparing the risk of acute pancreatitis associated with incretin treatments and other antihyperglycemics was not increased, approaching 1.
The crude odds ratios, before adjustment for confounding factors, was 1.36 in patients who had been treated with incretins and 1.44 among those who had been treated with other antihyperglycemic drugs. “The fact that crude ORs were increased to very similar levels for all antihyperglycemic drugs – given their different modes of action – points to a general underlying diabetes effect on pancreatitis risk, rather than a specific drug effect,” they commented. In the crude analyses, they determined that obesity, gallstones, and other diabetes-related risk factors “may explain much of the apparent risk increase,” the investigators added.
Other findings included an increased risk of acute pancreatitis among those who had recently started treatment with a DPP-4 inhibitor and in those who had recently started treatment with several other antihyperglycemic drugs, including sulfonylureas and insulin. “This lack of specificity suggests that either the increased pancreatitis risk is related to newly diagnosed and drug-treated type 2 diabetes per se, with a possibility of reverse causality due to pancreatogenic diabetes,” or that starting therapy with sulfonylureas and insulin “also causes acute pancreatitis, which should be further investigated,” they said.
Incretin-based drugs include injectable incretin mimetic agents (GLP-1 receptor agonists) such as liraglutide; and incretin enhancers (DPP-4) inhibitors, such as sitagliptin.
The study was supported by the Clinical Epidemiological Research Foundation in Denmark. One of the authors, from the Danish Center for Strategic Research in Type 2 Diabetes, at Odense University Hospital, disclosed having received research grants form Novo-Nordisk. Of the six authors, four are from the Aarhus University Hospital clinical epidemiology department, a member of the center, which receives funding that includes an unrestricted donation from Novo-Nordisk.
No association between an increased risk of acute pancreatitis and treatment with incretin therapy for type 2 diabetes was found in a large case control-study.
To evaluate whether the risk of acute pancreatitis was increased among patients treated with incretin-based drugs – glucagonlike peptide–1 (GLP-1) receptor agonists and dipeptidyl peptidase–4 (DPP-4) inhibitors – the investigators compared the risks of acute pancreatitis associated with different antihyperglycemic drugs in 12,868 people hospitalized for the first time for acute pancreatitis, and in 12,680 controls matched for birth year, sex, and region of residence in Denmark.
The study addressed evidence indicating that incretins may cause pancreatitis and pancreatic cancer in humans, which includes adverse event reports suggesting a signal of pancreatitis, but the association between incretins and acute pancreatitis is controversial and is an issue that “remains under debate,” they noted.
The data used were from three Danish databases, including one of reimbursed prescriptions. After adjustment for comorbidities and drugs associated with pancreatitis, including alcoholism and obesity, the risk of acute pancreatitis was not increased among those who had ever used an incretin, with an odds ratio of 0.95, according to Dr. Reimar Thomsen of the department of clinical epidemiology, Institute of Clinical Medicine, Aarhus (Denmark) University Hospital, and his associates (Diabetes Care 2015 [doi:10.2337/dc13-2983])
Risk was also not increased among those who had been ever treated with a DPP-4 inhibitor (OR, 1.04), which included an OR of 1.06 for sitagliptin, or among those who had been treated with a GLP-1 receptor agonist (OR, 0.82), which included an OR of 0.75 for liraglutide. Nor was risk elevated among those treated with nonincretin antihyperglycemic drugs (OR, 1.05).
In addition, the adjusted odds ratio comparing the risk of acute pancreatitis associated with incretin treatments and other antihyperglycemics was not increased, approaching 1.
The crude odds ratios, before adjustment for confounding factors, was 1.36 in patients who had been treated with incretins and 1.44 among those who had been treated with other antihyperglycemic drugs. “The fact that crude ORs were increased to very similar levels for all antihyperglycemic drugs – given their different modes of action – points to a general underlying diabetes effect on pancreatitis risk, rather than a specific drug effect,” they commented. In the crude analyses, they determined that obesity, gallstones, and other diabetes-related risk factors “may explain much of the apparent risk increase,” the investigators added.
Other findings included an increased risk of acute pancreatitis among those who had recently started treatment with a DPP-4 inhibitor and in those who had recently started treatment with several other antihyperglycemic drugs, including sulfonylureas and insulin. “This lack of specificity suggests that either the increased pancreatitis risk is related to newly diagnosed and drug-treated type 2 diabetes per se, with a possibility of reverse causality due to pancreatogenic diabetes,” or that starting therapy with sulfonylureas and insulin “also causes acute pancreatitis, which should be further investigated,” they said.
Incretin-based drugs include injectable incretin mimetic agents (GLP-1 receptor agonists) such as liraglutide; and incretin enhancers (DPP-4) inhibitors, such as sitagliptin.
The study was supported by the Clinical Epidemiological Research Foundation in Denmark. One of the authors, from the Danish Center for Strategic Research in Type 2 Diabetes, at Odense University Hospital, disclosed having received research grants form Novo-Nordisk. Of the six authors, four are from the Aarhus University Hospital clinical epidemiology department, a member of the center, which receives funding that includes an unrestricted donation from Novo-Nordisk.
Key clinical point: Neither GLP-1 receptor agonists nor DPP-4 inhibitors increase the risk of acute pancreatitis in type 2 diabetes patients.
Major finding: The risk of acute pancreatitis was not increased among Danes who had been treated with an incretin drug (odds ratio, 0.95), after adjustment for comorbidities and medications that can increase risk.
Data source: A population-based case-control study in 12,868 patients hospitalized for the first time with acute pancreatitis between 2005 and 2012, matched with 128,680 controls, from national medical databases.
Disclosures: The study was supported by the Clinical Epidemiological Research Foundation in Denmark. Most of the authors are at Danish institutions that receive funding and grants from Novo-Nordisk.