User login
Assessing and treating sexual function after vaginal surgery
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
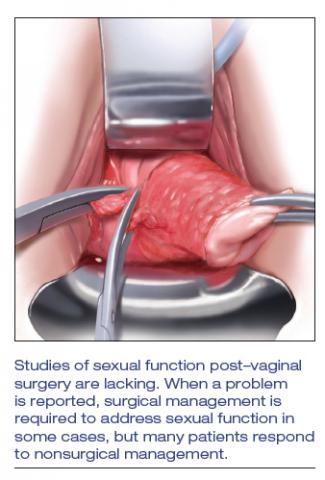
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
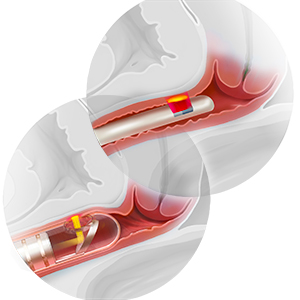
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
Sexual dysfunction is challenging for patients and clinicians. Just as sexual function is multidimensional—with physical and psychosocial elements—sexual dysfunction can likewise have multiple contributing factors, and is often divided into dysfunction of desire, arousal, orgasm, and sex-related pain. Addressing each of these dimensions of sexual dysfunction in relationship to pelvic reconstructive surgery is beyond the scope of this article. Here, we focus on aspects of sexual dysfunction most likely to be reported by patients after surgery for pelvic organ prolapse (POP) or urinary incontinence, or for both. We discuss what is known about why sexual dysfunction develops after these procedures; how to assess symptoms when sexual dysfunction occurs; and how best to treat these difficult problems.
CASE Postoperative sexual concerns
Your 62-year-old patient presents 2 weeks after vaginal hysterectomy, uterosacral vault suspension, anterior and posterior colporrhaphy, and retropubic midurethral polypropylene sling placement. She reports feeling tired but otherwise doing well.
The patient returns 8 weeks postoperatively, having just resumed her customary exercise routine, and reports that she is feeling well. Upon questioning, she says that she has not yet attempted to have sexual intercourse with her 70-year-old husband.
The patient returns 6 months later and reports that, although she is doing well overall, she is unable to have sexual intercourse.
How can you help this patient? What next steps in evaluation are indicated? Then, with an understanding of her problem in hand, what treatment options can you offer to her?
Surgery for pelvic-floor disorders and sexual function
The impact of surgery on sexual function is important to discuss with patients preoperatively and postoperatively. Because patients with POP and urinary incontinence have a higher rate of sexual dysfunction at baseline, it is important to know how surgery to correct these conditions can affect sexual function.1 Regrettably, many studies of surgical procedures for POP and urinary incontinence either do not include sexual function outcomes or are not powered to detect differences in these outcomes.
Native-tissue repair. A 2015 systematic review looked at studies of women undergoing native-tissue repair for POP without mesh placement of any kind, including a midurethral sling.2 Based on 9 studies that reported validated sexual function questionnaire scores, investigators determined that sexual function scores generally improved following surgery. Collectively, for studies included in this review that specifically reported the rate of dyspareunia before and after surgery, 47% of women reported improvement in dyspareunia; 39% reported no change; 18% reported deterioration in dyspareunia; and only 4% had de novo dyspareunia.
Colporrhaphy. Posterior colporrhaphy, commonly performed to correct posterior vaginal prolapse, can narrow vaginal caliber and the introitus, potentially causing dyspareunia. Early description of posterior colporrhaphy technique included plication of the levator ani muscles, which was associated with significant risk of dyspareunia postoperatively.3 However, posterior colporrhaphy that involves standard plication of the rectovaginal muscularis or site-specific repair has been reported to have a dyspareunia rate from 7% to 20%.4,5 It is generally recommended, therefore, that levator muscle plication during colporrhaphy be avoided in sexually active women.
Continue to: Vaginal mesh...
Vaginal mesh. Mesh has been used in various surgical procedures to correct pelvic floor disorders. Numerous randomized trials have comparatively evaluated the use of transvaginal polypropylene mesh and native tissue for POP repair, and many of these studies have assessed postoperative sexual function. In a 2013 systematic review on sexual function after POP repair, the authors found no significant difference in postoperative sexual function scores or the dyspareunia rate after vaginal mesh repair (14%) and after native-tissue repair (12%).6
Ask; then ask again
· Talk about sexual function before and after surgery
Remember the basics
· A thorough history and physical exam are paramount
Ask in a different way
· Any of several validated questionnaires can be a valuable adjunct to the history and physical exam
Individualize treatment
· Many patients respond to nonsurgical treatment, but surgical management is necessary in some cases
Studies of postsurgical sexual function are lacking
Important aspects of sexual function—orgasm, arousal, desire, lubrication, sexual satisfaction, effects on the partner—lack studies. A study of 71 sexually active couples assessed sexual function with questionnaires before and after vaginal native-tissue repair and found that, except for orgasm, all domains improved in female questionnaires. In male partners, interest, sexual drive, and overall satisfaction improved, whereas erection, ejaculation, and orgasm remained unchanged.7
Urinary incontinence during sexual intercourse affects approximately 30% of women with overactive bladder or stress incontinence.8 Several reviews have analyzed data on overall sexual function following urinary incontinence surgery:
- After stress incontinence surgery, the rate of coital incontinence was found to be significantly lower (odds ratio, 0.11).9 In this review, 18 studies, comprising more than 1,500 women, were analyzed, with most participants having undergone insertion of a midurethral mesh sling. Most women (55%) reported no change in overall sexual function, based on validated sexual questionnaire scores; 32% reported improvement; and 13% had deterioration in sexual function.
- As for type of midurethral sling, 2 reviews concluded that there is no difference in sexual function outcomes between retropubic and trans‑obturator sling routes.9,10
Although most studies that have looked at POP and incontinence surgeries show either improvement or no change in sexual function, we stress that sexual function is a secondary outcome in most of those studies, which might not be appropriately powered to detect differences in outcomes. Furthermore, although studies describe dyspareunia and overall sexual function in validated questionnaire scores, most do not evaluate other specific domains of sexual function. It remains unclear, therefore, how POP and incontinence surgeries affect orgasm, desire, arousal, satisfaction, and partner sexual domains; more studies are needed to focus on these areas of female sexual function.
How do we assess these patients?
We do know that sexual function is important to women undergoing gynecologic surgery: In a recent qualitative study of women undergoing pelvic reconstruction, patients rated lack of improvement in sexual function following surgery a “very severe” adverse event.11 Unfortunately, however, sexual activity and function is not always measured before gynecologic surgery. Although specific reporting guidelines do not exist for routine gynecologic surgery, a terminology report from the International Urogynecologic Association/International Continence Society (IUGA/ICS) recommends that sexual activity and partner status be evaluated prior to and following surgical treatment as essential outcomes.12 In addition, the report recommends that sexual pain be assessed prior to and following surgical procedures.12
Ascertain sexual health. First, asking your patients simple questions about sexual function, pain, and bother before and after surgery opens the door to dialogue that allows them, and their partner, to express concerns to you in a safe environment. It also allows you to better understand the significant impact of your surgical interventions on their sexual health.
Questionnaires. Objective measures of vaginal blood flow and engorgement exist, but assessment of sexual activity in the clinical setting is largely limited to self-assessment with questionnaires. Incorporating simple questions, such as “Are you sexually active?,” “Do you have any problems with sexual activity?,” and “Do you have pain with activity?” are likely to be as effective as a more detailed interview and can identify women with sexual concerns.13 Many clinicians are put at a disadvantage, however, because they are faced with the difficult situation of addressing postoperative sexual problems without knowing whether the patient had such reports prior to surgery.
Continue to: Aside from simple screening tools...
Aside from simple screening tools, a number of sexual function questionnaires have been developed. Some are generic, and others are condition-specific:
- Generic questionnaires are typically designed to address the function of a range of women. For example, the Female Sexual Function Index comprises 19 questions. Domains include orgasm, desire, arousal, lubrication, pain and satisfaction.14
- Condition-specific questionnaires of sexual function each have been validated in their target population so that they measure nuances in sexual health relevant to that population. The Pelvic Organ Prolapse/Incontinence Sexual Questionnaire—IUGA-Revised includes questions about the domains listed for the generic Index (above) plus questions about the impact of coital incontinence or bulge symptoms on sexual function.12
History-taking. If a woman identifies a problem with sexual function, a thorough history helps elicit whether the condition is lifelong or acquired, situational or general, and, most important, whether or not it is bothersome to her.14,15 It is important not to make assumptions when pursuing this part of the history, and to encourage patients to be candid about how they have sex and with whom.
Physical examination. The patient should undergo a complete physical exam, including 1) a detailed pelvic exam assessing the vulva, vagina, and pelvic-floor musculature, and 2) estrogenization of the tissue.
Partner concerns. For women who have a partner, addressing the concerns of that partner following gynecologic surgery can be useful to the couple: The partner might be concerned about inflicting pain or doing damage during sex after gynecologic surgery.
CASE Informative discussion
While ascertaining her sexual symptoms, your patient reveals to you that she has attempted sexual intercourse on 3 occasions; each time, penetration was too painful to continue. She tells you she did not have this problem before surgery.
The patient says that she has tried water-based lubricants and is using vaginal estrogen 3 times per week, but “nothing helps.” She reports that she is arousable and has been able to achieve orgasm with clitoral stimulation, but would like to have vaginal intercourse. Her husband does have erectile dysfunction, which, she tells you, can make penetration difficult.
On physical examination, you detect mild atrophy. Vaginal length is 9 cm; no narrowing or scarring of the vaginal introitus or canal is seen. No mesh is visible or palpable. The paths of the midurethral sling arms are nontender. However, levator muscles are tender and tense bilaterally.
Given these findings on examination, what steps can you take to relieve your patient’s pain?
What can we offer these patients?
Treating sexual dysfunction after pelvic reconstructive surgery must, as emphasized earlier, be guided by a careful history and physical exam. Doing so is critical to determining the underlying cause. Whenever feasible, offer the least invasive treatment.
The IUGA/ICS terminology report describes several symptoms of postoperative sexual dysfunction12:
- de novo sexual dysfunction
- de novo dyspareunia
- shortened vagina
- tight vagina (introital or vaginal narrowing, or both)
- scarred vagina (including mesh-related problems)
- hispareunia (pain experienced by a male partner after intercourse).
Of course, any one or combination of these symptoms can be present in a given patient. Furthermore, de novo sexual dysfunction, de novo dyspareunia, and hispareunia can have various underlying causes—again, underscoring the importance of the history and exam in determining treatment.
Continue to: Nonsurgical treatment...
Nonsurgical treatment
Nonhormonal vaginal lubricants and moisturizers; vaginal estrogen therapy. Although, in older women, vaginal atrophy is often not a new diagnosis postsurgically, the condition might have been untreated preoperatively and might therefore come into play in sexual dysfunction postoperatively. If a patient reports vaginal dryness or pain upon penetration, assess for vaginal atrophy and, if present, treat accordingly.
Vaginal dilation and physical therapy. A shortened, tight, or scarred vagina might be amenable to therapy with vaginal dilators and physical therapy, but might ultimately require surgery.
Pelvic-floor myalgia or spasm can develop after surgery or, as with atrophy, might have existed preoperatively but was left untreated. Pelvic-floor myalgia should be suspected if the patient describes difficult penetration or a feeling of tightness, even though scarring or constriction of the vagina is not seen on examination. Physical therapy with a specialist in pelvic floor treatment is a first-line treatment for pelvic-floor myalgia,16 and is likely to be a helpful adjunct in many situations, including mesh-related sexual problems.17
Oral or vaginal medications to relax pelvic-floor muscle spasm are an option, although efficacy data are limited. If pain is of longstanding duration and is thought to have a neuropathic component, successful use of tricyclic antidepressants, neuroleptics, and serotonin–norepinephrine reuptake inhibitors has been reported.18
Surgery
Data are sparse regarding surgical treatment of female sexual dysfunction after pelvic reconstructive surgery. Again, it is clear, however, that the key is carefully assessing each patient and then individualizing treatment. Patients can have any type of dysfunction that a patient who hasn’t had surgery can—but is also at risk of conditions directly related to surgery.
In any patient who has had mesh placed as part of surgery, thorough examination is necessary to determine whether or not the implant is involved in sexual dysfunction. If the dysfunction is an apparent result of surgery performed by another surgeon, make every effort to review the operative report to determine which material was implanted and how it was placed.
Trigger-point injection can be attempted in a patient who has site-specific tenderness that is not clearly associated with tissue obstruction of the vagina or mesh erosion.12,19 Even in areas of apparent banding or scarring related to mesh, trigger-point injection can be attempted to alleviate pain. How often trigger-point injections should be performed is understudied.
If, on examination, tenderness that replicates the dyspareunia is elicited when palpating the levator or obturator internus muscle, pelvic-floor muscle trigger-point injection can be offered (although physical therapy is first-line treatment). Trigger-point injection also can be a useful adjunct in women who have another identified cause of pain but also have developed pelvic-floor muscle spasm.
Not addressing concomitant pelvic-floor myalgia could prevent successful treatment of pain. Inclusion of a pudendal block also might help to alleviate pain.
Continue to: Surgical resection...
Surgical resection. If a skin bridge is clearly observed at the introitus, or if the introitus has been overly narrowed by perineorrhaphy but the remainder of the vagina has adequate length and caliber, surgical resection of the skin bridge might relieve symptoms of difficult penetration. In the case of obstructive perineorrhaphy, an attempt at reversal can be made by incising the perineum vertically but then reapproximating the edges transversely—sometimes referred to as reverse perineorrhaphy.
If scar tissue found elsewhere in the vagina might obstruct penetration, this condition might also be amenable to resection. When scarring is annular, relaxing incisions can be made bilaterally to relieve tension on that tissue; alternatively, it might be necessary to perform a Z-plasty. Nearly always, severe scarring is accompanied by levator myalgia, and a combined approach of surgery and physical therapy is necessary.
Neovagina. It is possible to find vaginal stenosis or shortening, to a varying degree, after surgical prolapse repair, with or without mesh or graft. As discussed, vaginal dilation should be offered but, if this is ineffective, the patient might be a candidate for surgical creation of a neovagina. Numerous techniques have been described for patients with congenital vaginal agenesis, with a few reports of similar techniques used to treat iatrogenic vaginal stenosis or obliteration.
The general principle of all neovagina procedures is to create a space between bladder and rectum of adequate caliber and length for desired sexual function. Reported techniques include a thigh or buttock skin graft, use of bowel or peritoneum, and, recently, a buccal mucosa graft.20,21
Resection or excision of mesh. In patients who develop sexual dysfunction after mesh placement, the problem can be caused by exposure of the mesh in the vagina or erosion into another organ, but can also arise in the absence of exposure or erosion. Patients might have tenderness to palpation at points where the mesh is palpable through the mucosa but not exposed.
Again, complete investigation is necessary to look for mesh involvement in the vagina and, depending on the type of implant, other adjacent organs. Assessing partner symptoms, such as pain and scratches, also can be telling.
If there is palpable tenderness on vaginal examination of the mesh, resection of the vaginal portion might be an option.17 Complete excision of mesh implants can be morbid, however, and might not provide a better outcome than partial excision. The risk of morbidity from complete mesh excision must be weighed against the likelihood that partial excision will not resolve pain and that the patient will require further excision subsequently.17,22 Excising fragmented mesh can be difficult; making every attempt to understand the contribution of mesh to sexual dysfunction is therefore critical to determining how, and how much of, the mesh comes out at the first attempt.
Last, for any woman who opts for surgical intervention to treat pain, you should engage in a discussion to emphasize the multidimensional nature of sexual function and the fact that any surgical intervention might not completely resolve her dysfunction.
Continue to: CASE Discussing options...
CASE Discussing options, choosing an intervention
You discuss the examination findings (no shortening or narrowing of the vagina) with the patient. She is relieved but puzzled as to why she cannot have intercourse. You discuss the tension and t
At 3-month follow-up, she reports great improvement. She is able to have intercourse, although she says she still has discomfort sometimes. She continues to work with the pelvic floor physical therapist and feels optimistic. You plan to see her in 6 months but counsel her to call if symptoms are not improving or are worsening.
It is difficult to counsel patients about sexual function after pelvic reconstructive surgery because data that could guide identification of problems (and how to treat them) are incomplete. Assessingsexual function preoperatively and having an open conversation about risks and benefits of surgery, with specific mention of its impact on sexual health, are critical (see “Key touchpoints in managing sexual dysfunction after pelvic reconstructive surgery”).
It is also crucial to assess sexual function postoperatively as a matter of routine. Validated questionnaires can be a useful adjunct to a thorough history and physical exam, and can help guide your discussions.
Treatment of postop sexual dysfunction must, first, account for the complex nature of sexual function and, second, be individualized, starting with the least invasive options, when feasible.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
- Rogers RG. Sexual function in women with pelvic floor disorders. Can Urol Assoc J. 2013;7:S199-S201.
- Jha S, Gray T. A systematic review and meta-analysis of the impact of native tissue repair for pelvic organ prolapse on sexual function. Int Urogynecol J. 2015;26:321-327.
- Thompson JC, Rogers RG. Surgical management for pelvic organ prolapse and its impact on sexual function. Sex Med Rev. 2016;4:213-220.
- Sung VW, Rardin CR, Raker CA, et al. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstet Gynecol. 2012;119:125-133.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
- Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24:1853-1857.
- Kuhn A, Brunnmayr G, Stadlmayr W, et al. Male and female sexual function after surgical repair of female organ prolapse. J Sex Med. 2009;6:1324-1334.
- Gray T, Li W, Campbell P, et al. Evaluation of coital incontinence by electronic questionnaire: prevalence, associations and outcomes in women attending a urogynaecology clinic. Int Urogynecol J. 2018;29:969-978.
- Jha S, Ammenbal M, Metwally M. Impact of incontinence surgery on sexual function: a systematic review and meta-analysis. J Sex Med. 2012;9:34-43.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-e71.e27.
- Dunivan GC, Sussman AL, Jelovsek JE, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Gaining the patient perspective on pelvic floor disorders’ surgical adverse events. Am J Obstet Gynecol. 2019;220:185.e1-e185.e10.
- Rogers RG, Pauls RN, Thakar R, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction. Int Urogynecol J. 2018;29:647-666.
- Plouffe L Jr. Screening for sexual problems through a simple questionnaire. Am J Obstet Gynecol. 1985;151:166-169.
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7:337-348.
- McCabe MP, Sharlip ID, Atalla E, et al. Definition of sexual dysfunctions in women and men: a consensus statement from the Fourth International Consultation of Sexual Medicine 2015. J Sex Med. 2015;13:135-143.
- Berghmans B. Physiotherapy for pelvic pain and female sexual dysfunction: an untapped resource. Int Urogynecol J. 2018;29:631-638.
- Cundiff GW, Quinlan DJ, van Rensburg JA, et al. Foundation for an evidence-informed algorithm for treating pelvic floor mesh complications: a review. BJOG. 2018;125:1026-1037.
- Steege JF, Siedhoff MT. Chronic pelvic pain. Obstet Gynecol. 2014;124:616-629.
- Wehbe SA, Whitmore K, Kellogg-Spadt S. Urogenital complaints and female sexual dysfunction (part 1). J Sex Med. 2010;7:1704-1713.
- Grimsby GM, Bradshaw K, Baker LA. Autologous buccal mucosa graft augmentation for foreshortened vagina. Obstet Gynecol. 2014;123:947-950.
- Morley GW, DeLancey JO. Full-thickness skin graft vaginoplasty for treatment of the stenotic or foreshortened vagina. Obstet Gynecol. 1991;77:485-489.
- Pickett SD, Barenberg B, Quiroz LH, et al. The significant morbidity of removing pelvic mesh from multiple vaginal compartments. Obstet Gynecol. 2015;125:1418-1422.
FDA modifies safety label for Addyi
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
The Food and Drug Administration has issued a safety labeling change for flibanserin (Addyi), a treatment for premenopausal women with acquired, generalized hypoactive sexual desire disorder, according to a press release issued April 11 by the agency. Previously, the warning said women should abstain from alcohol entirely.
According to the release, the manufacturer, Sprout, had hoped the FDA would remove the boxed warning and contraindication entirely. However, based on a review of two postmarket research studies, the agency chose to order these modifications to the warnings instead.
The first postmarket study was missing information related to participants’ blood pressure, which FDA officials thought was critical in determining risk; it appeared that this resulted from safety precautions built into the trial. The concern was that not only did this absent information provide further evidence of an interaction but that women at home would not have the benefit of these safety precautions and could suffer serious outcomes, including falls, accidents, and bodily harm. The other postmarketing trial showed that delaying administration of flibanserin until at least 2 hours after consuming alcohol reduced the risk of serious hypotension and syncope.
It is recommended that flibanserin be taken at bedtime because of risks associated with hypotension and syncope, as well as risks associated with central nervous system depression (such as sleepiness). Furthermore, patients are encouraged to discontinue treatment with flibanserin if their hypoactive sexual desire disorder does not improve after 8 weeks. The most common adverse reactions include dizziness, sleepiness, nausea, fatigue, insomnia, and dry mouth.
Full prescribing information is available on the FDA website, as is the full release regarding these safety label modifications.
Stress incontinence surgery improves sexual dysfunction
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
TUCSON, ARIZ. –
The finding comes from a secondary analysis of two randomized, controlled trials comparing Burch colposuspension, autologous fascial slings, retropubic midurethral polypropylene slings, and transobturator midurethral polypropylene slings. The analysis looked at outcomes at 24 months after surgery. Stephanie Glass Clark, MD, a resident at Virginia Commonwealth University, Richmond, presented the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
In the secondary analysis of the Stress Incontinence Surgical Treatment Efficacy Trial (SISTEr) and the Trial of Midurethral Slings (TOMUS) trials, Dr. Clark and her fellow researchers looked at the effect of surgical failure on sexual dysfunction outcomes. Subjective failure was defined as self-reported SUI symptoms or self-reported leakage by 3-day voiding diary beyond 3 months after the surgery. Objective failure was defined as any treatment for SUI after the surgery or a positive stress test or pad test beyond 3 months after the surgery.
Participants were excluded from the two studies if they were sexually inactive in the previous 6 months at baseline, at 12 months post baseline, or at 24 months. The studies employed the short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12), which had 12 questions with scores ranging from 0 to 4. The secondary analysis sample included 488 women from SISTEr and 436 women from TOMUS.
There were some baseline differences among groups between the two trials, including vaginal deliveries, race/ethnicity, stage of prolapse, and concomitant surgeries performed at time of the anti-incontinence procedure.
All four surgeries were associated with improvements in sexual function, with no statistically significant between-group differences. Mean PISQ-12 scores improved from a range of 31-33 to a range of 36-38 at 24 months. Although there is no published minimum important difference for PISQ-12 scores, an improvement of at least one-half of a standard deviation is generally accepted as clinically meaningful. “In this case, the standard deviation at baseline was just under 3 and so the improvement of each treatment group by more than 1.5 is a clinically meaningful improvement in their sexual function,” Dr. Clark said.
“Sexual dysfunction is a much more common problem than we previously thought, so we’ve been trying to figure out if patients with pelvic floor disorders like stress incontinence are going to have any improvement in sexual dysfunction by surgically treating their stress incontinence. Previously published data had been pretty conflicting,” Dr. Clark added in an interview.
That previous research was mostly retrospective and could have been impacted by patient selection bias. By analyzing clinical trials, the researchers hoped to test their idea that the pelvic floor symptoms themselves may be key to sexual dysfunction and that treating it surgically would improve matters.
The positive result is encouraging, but it still leaves unanswered questions about the mechanism behind the relationship. Dr. Clark wondered whether leaking urine leakage during sex might be the culprit, or whether it is fear or shame associated with the condition.
The answer may come from further analysis of women who were sexually inactive at baseline, but became sexually active over the course of the studies. “I think looking at that patient population in particular is going to be an interesting area of research. Is it that it was completely related to their pelvic floor disorder, and then we fixed it [so] they could have a more fulfilling sexual life?” speculated Dr. Clark.
The study received some funding from the National Institutes of Health. Dr. Clark reported no relevant financial disclosures.
SOURCE: Clark SG et al. SGS 2019, Oral Presentation 11.
REPORTING FROM SGS 2019
Following pelvic floor surgery, patients value functional goals
TUCSON, ARIZ. – according to results of a new study. Such negative reactions occur more frequently as time passes and may be related to incongruent patient expectations, which may in turn affect physician-patient communication.
“We must bridge the gap between expectations and the occurrence of an unanticipated problem. What this study highlights is a need for counseling beyond the traditional complications, and more discussion about the possibility of failure in terms of the things that the patients identify as important,” Brenna McGuire, MD, a resident at the University of New Mexico, Albuquerque, said while presenting the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
The work highlights the need to look at outcomes in a different way, said Vivian Sung, MD, who was not involved in the research and was a discussant following the presentation. “Most of our studies are designed with methodology to emphasize efficacy and often secondary outcomes to capture complications and adverse events. But there is a gray area. It’s something that’s evolving, and we’re getting better at,” Dr. Sung, professor of obstetrics and gynecology at Brown University and a urogynecologist at Women and Infants Hospital, both in Providence, R.I., said in an interview.
The success of a procedure is typically evaluated by determining incontinence during an office visit, but the problem may not be occurring at that particular moment, and the patient may not be happy with the overall outcome. “Sometimes you can fix one problem, and the other problems become more prominent, or new problems develop. [Incontinence alone is] not a perfect picture or what the patient was envisioning her outcome to be,” Dr. Sung said.
Expectations can potentially be better managed through better patient counseling, but that’s not a simple fix either, she noted. Most surgeons counsel patients on negative outcomes, but adverse events with a 5%-10% probability may fail to make an impression. “Really, the rate is zero or 100%. It’s not that it doesn’t seem like a meaningful complication, it’s just that it doesn’t seem like it will happen to you. And then when it does, it can be very devastating depending on what it is and what your expectation was.”
Dr. McGuire and her associates followed 20 women (mean age, 55 years; 50% non-Hispanic white, 25% Hispanic, 25% Native American) at a single institution in New Mexico who underwent surgeries for pelvic floor disorders. They interviewed each participant before and after surgery, at 4-6 weeks, and 6 months after surgery, asking them to rank adverse events at each time point.
Before surgery, patients expressed concerns about postoperative pain, injury, and catheter issues. At 6-8 weeks, the chief concerns were daily activities, sexual activity, and symptom reduction. At 6 months, incontinence, sexual dysfunction, and mental health issues predominated. In other words, concerns migrated from traditional complications to functional outcomes over time.
At the 6-8 week interview, a representative quote was: “It’s the fact that it didn’t work. It’s the fact that I’m still suffering from all the same symptoms.” At 6 months, another quote was: “I hate this so much. It really does impact my life negatively. It affects my work, it affects everything, and makes me very angry.”
Traditional adverse events such as pain and infection dropped in frequency between the preoperative interview and the 6-month interview from 7.5%-10.0% to 2.5%-5.0% by 6 months. However, functional outcomes were a different matter: Concerns about a failed surgery increased from 10% to 25%, sexual dysfunction from 4% to 8%, and effect on daily function from 4% to 11%.
The study was funded by the University of New Mexico. Dr. McGuire and Dr. Sung reported no relevant financial disclosures.
SOURCE: McGuire B et al. SGS 2019, Abstract 01.
TUCSON, ARIZ. – according to results of a new study. Such negative reactions occur more frequently as time passes and may be related to incongruent patient expectations, which may in turn affect physician-patient communication.
“We must bridge the gap between expectations and the occurrence of an unanticipated problem. What this study highlights is a need for counseling beyond the traditional complications, and more discussion about the possibility of failure in terms of the things that the patients identify as important,” Brenna McGuire, MD, a resident at the University of New Mexico, Albuquerque, said while presenting the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
The work highlights the need to look at outcomes in a different way, said Vivian Sung, MD, who was not involved in the research and was a discussant following the presentation. “Most of our studies are designed with methodology to emphasize efficacy and often secondary outcomes to capture complications and adverse events. But there is a gray area. It’s something that’s evolving, and we’re getting better at,” Dr. Sung, professor of obstetrics and gynecology at Brown University and a urogynecologist at Women and Infants Hospital, both in Providence, R.I., said in an interview.
The success of a procedure is typically evaluated by determining incontinence during an office visit, but the problem may not be occurring at that particular moment, and the patient may not be happy with the overall outcome. “Sometimes you can fix one problem, and the other problems become more prominent, or new problems develop. [Incontinence alone is] not a perfect picture or what the patient was envisioning her outcome to be,” Dr. Sung said.
Expectations can potentially be better managed through better patient counseling, but that’s not a simple fix either, she noted. Most surgeons counsel patients on negative outcomes, but adverse events with a 5%-10% probability may fail to make an impression. “Really, the rate is zero or 100%. It’s not that it doesn’t seem like a meaningful complication, it’s just that it doesn’t seem like it will happen to you. And then when it does, it can be very devastating depending on what it is and what your expectation was.”
Dr. McGuire and her associates followed 20 women (mean age, 55 years; 50% non-Hispanic white, 25% Hispanic, 25% Native American) at a single institution in New Mexico who underwent surgeries for pelvic floor disorders. They interviewed each participant before and after surgery, at 4-6 weeks, and 6 months after surgery, asking them to rank adverse events at each time point.
Before surgery, patients expressed concerns about postoperative pain, injury, and catheter issues. At 6-8 weeks, the chief concerns were daily activities, sexual activity, and symptom reduction. At 6 months, incontinence, sexual dysfunction, and mental health issues predominated. In other words, concerns migrated from traditional complications to functional outcomes over time.
At the 6-8 week interview, a representative quote was: “It’s the fact that it didn’t work. It’s the fact that I’m still suffering from all the same symptoms.” At 6 months, another quote was: “I hate this so much. It really does impact my life negatively. It affects my work, it affects everything, and makes me very angry.”
Traditional adverse events such as pain and infection dropped in frequency between the preoperative interview and the 6-month interview from 7.5%-10.0% to 2.5%-5.0% by 6 months. However, functional outcomes were a different matter: Concerns about a failed surgery increased from 10% to 25%, sexual dysfunction from 4% to 8%, and effect on daily function from 4% to 11%.
The study was funded by the University of New Mexico. Dr. McGuire and Dr. Sung reported no relevant financial disclosures.
SOURCE: McGuire B et al. SGS 2019, Abstract 01.
TUCSON, ARIZ. – according to results of a new study. Such negative reactions occur more frequently as time passes and may be related to incongruent patient expectations, which may in turn affect physician-patient communication.
“We must bridge the gap between expectations and the occurrence of an unanticipated problem. What this study highlights is a need for counseling beyond the traditional complications, and more discussion about the possibility of failure in terms of the things that the patients identify as important,” Brenna McGuire, MD, a resident at the University of New Mexico, Albuquerque, said while presenting the results at the annual scientific meeting of the Society of Gynecologic Surgeons.
The work highlights the need to look at outcomes in a different way, said Vivian Sung, MD, who was not involved in the research and was a discussant following the presentation. “Most of our studies are designed with methodology to emphasize efficacy and often secondary outcomes to capture complications and adverse events. But there is a gray area. It’s something that’s evolving, and we’re getting better at,” Dr. Sung, professor of obstetrics and gynecology at Brown University and a urogynecologist at Women and Infants Hospital, both in Providence, R.I., said in an interview.
The success of a procedure is typically evaluated by determining incontinence during an office visit, but the problem may not be occurring at that particular moment, and the patient may not be happy with the overall outcome. “Sometimes you can fix one problem, and the other problems become more prominent, or new problems develop. [Incontinence alone is] not a perfect picture or what the patient was envisioning her outcome to be,” Dr. Sung said.
Expectations can potentially be better managed through better patient counseling, but that’s not a simple fix either, she noted. Most surgeons counsel patients on negative outcomes, but adverse events with a 5%-10% probability may fail to make an impression. “Really, the rate is zero or 100%. It’s not that it doesn’t seem like a meaningful complication, it’s just that it doesn’t seem like it will happen to you. And then when it does, it can be very devastating depending on what it is and what your expectation was.”
Dr. McGuire and her associates followed 20 women (mean age, 55 years; 50% non-Hispanic white, 25% Hispanic, 25% Native American) at a single institution in New Mexico who underwent surgeries for pelvic floor disorders. They interviewed each participant before and after surgery, at 4-6 weeks, and 6 months after surgery, asking them to rank adverse events at each time point.
Before surgery, patients expressed concerns about postoperative pain, injury, and catheter issues. At 6-8 weeks, the chief concerns were daily activities, sexual activity, and symptom reduction. At 6 months, incontinence, sexual dysfunction, and mental health issues predominated. In other words, concerns migrated from traditional complications to functional outcomes over time.
At the 6-8 week interview, a representative quote was: “It’s the fact that it didn’t work. It’s the fact that I’m still suffering from all the same symptoms.” At 6 months, another quote was: “I hate this so much. It really does impact my life negatively. It affects my work, it affects everything, and makes me very angry.”
Traditional adverse events such as pain and infection dropped in frequency between the preoperative interview and the 6-month interview from 7.5%-10.0% to 2.5%-5.0% by 6 months. However, functional outcomes were a different matter: Concerns about a failed surgery increased from 10% to 25%, sexual dysfunction from 4% to 8%, and effect on daily function from 4% to 11%.
The study was funded by the University of New Mexico. Dr. McGuire and Dr. Sung reported no relevant financial disclosures.
SOURCE: McGuire B et al. SGS 2019, Abstract 01.
REPORTING FROM SGS 2019
Low sexual desire: Appropriate use of testosterone in menopausal women
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).
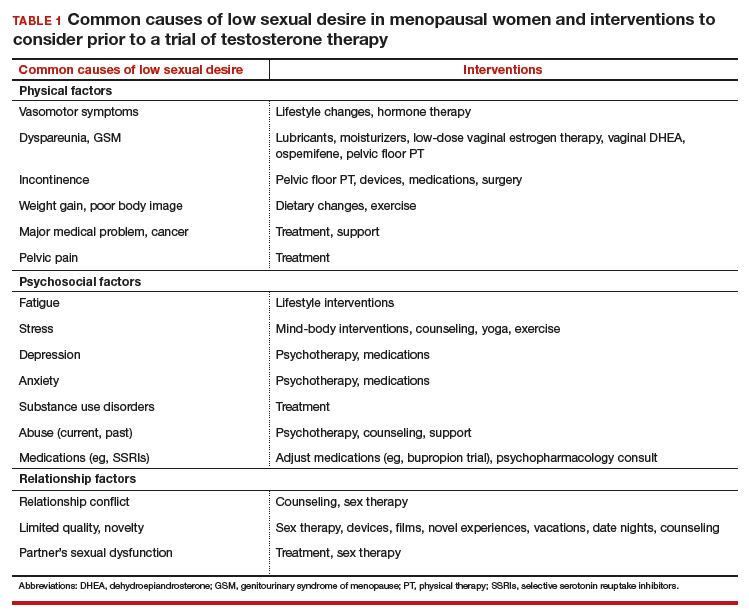
Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.
Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Midlife woman with low libido causing distress
At her annual gynecologic visit, a 55-year-old woman notes that she has almost no interest in sex. In the past, her libido was good and relations were pleasurable. Since her mid-40s, she has noticed a gradual decline in libido and orgasmic response. Sexual frequency has declined from once or twice weekly to just a few times per month. She has been married for 25 years and describes the relationship as caring and strong. Her husband is healthy with a good libido; his intermittent erectile dysfunction is treated with a phosphodiesterase-5 inhibitor. The patient’s low libido is distressing, as the decline in sexual frequency is causing some conflict for the couple. She requests that her testosterone level be checked because she heard that treatment with testosterone cream will solve this problem.
Evaluating and treating low libido in menopausal women
Low libido is a very common sexual problem for women. When sexual problems are accompanied by distress, they are classified as sexual dysfunctions. Although ObGyns should discuss sexual concerns at every comprehensive visit, if the patient has no associated distress, treatment is not necessarily indicated. A woman with low libido or anorgasmia who is satisfied with her sex life and is not bothered by these issues does not require any intervention.
Currently, the only indication for testosterone therapy that is supported by clinical trial evidence is low sexual desire with associated distress, known as hypoactive sexual desire disorder (HSDD). Although other sexual problems also commonly occur in menopausal women, such as disorders of orgasm and pain, testosterone is not recommended for these problems. In addition, testosterone is not approved by the US Food and Drug Administration (FDA) for the treatment of female sexual dysfunction.
Routinely inquire about sexual functioning
Ask your patients about sexual concerns at every comprehensive visit. You can easily incorporate into the review of systems a general question, such as, “Do you have any sexual concerns?” If the patient does mention a sexual problem, schedule a separate visit (given appointment time constraints) to address it. History and physical examination information you gather during the comprehensive visit will be helpful in the subsequent problem-focused visit.
Taking a thorough history is key when addressing a patient’s sexual problems, since identifying possible etiologies guides treatment. Often, the cause of female sexual dysfunction is multifactorial and includes physiologic, psychologic, and relationship issues.
- Evidence supports low-dose transdermal testosterone in carefully selected menopausal women with HSDD and no other identifiable reason for the sexual dysfunction
- Inform women considering testosterone for HSDD of the limited effectiveness and high placebo responses seen in clinical trials
- Women also must be informed that treatment is off-label (no testosterone formulations are FDA approved for women)
- Review with patients the limitations of compounded medications, and discuss possible adverse effects of androgens. Long-term safety is unknown and, as androgens are converted to estrogens
Explore potential causes, recommend standard therapies
Common causes of low libido in menopausal women include vasomotor symptoms, insomnia, urinary incontinence, cancer or another major medical problem, weight gain, poor body image, genitourinary syndrome of menopause (GSM) with dyspareunia, fatigue, stress, aging, relationship duration, lack of novelty, relationship conflict, and a partner’s sexual problems. Other common etiologies include depression, anxiety, and substance use disorders, as well as medications used to treat these disorders, including selective serotonin reuptake inhibitors (SSRIs).
Continue to: There are many effective therapies...
There are many effective therapies for low sexual desire to consider prior to initiating a trial of testosterone, which should be considered for HSDD only if the disorder persists after addressing all other possible contributing factors (TABLE 1).

Sex therapy, for example, provides information on sexual functioning and helps improve communication and mutual pleasure and satisfaction. Strongly encourage—if not require—a consultation with a sex therapist before prescribing testosterone for low libido. Any testosterone-derived improvement in sexual functioning will be enhanced by improved communication and additional strategies to achieve mutual pleasure.
Hormone therapy. Vasomotor symptoms, with their associated sleep disruption, fatigue, and reduced quality of life (QOL), often adversely impact sexual desire. Estrogen therapy does not appear to improve libido in otherwise asymptomatic women; however, in women with bothersome vasomotor symptoms treated with estrogen, sexual interest may increase as a result of improved sleep, fatigue, and overall QOL. The benefits of systemic hormone therapy generally outweigh its risks for most healthy women younger than age 60 who have bothersome hot flashes and night sweats.1
Nonhormonal and other therapies. GSM with dyspareunia is a principal cause of sexual dysfunction in older women.2 Many safe and effective treatments are available, including low-dose vaginal estrogen therapy, nonhormonal moisturizers and lubricants, ospemifene, vaginal dehydroepiandrosterone, and pelvic floor physical therapy.3 Urinary incontinence commonly occurs in midlife women and contributes to low libido.4
Lifestyle approaches. Address fatigue and stress by having the patient adjust her work and sleep schedules, obtain help with housework and meals, and engage in mind-body interventions, counseling, or yoga. Sexual function may benefit from yoga practice, likely as a result of the patient experiencing reduced stress and enhanced body image. Improving overall health and body image with regular exercise, optimal diet, and weight management may contribute to a more satisfying sex life after the onset of menopause.
Relationship refresh. Women’s sexual interest often declines with relationship duration, and both men and women who are in new relationships generally have increased libido, affirming the importance of novelty over the long term. Couples will benefit from “date nights,” weekends away from home, and trying novel positions, locations, and times for sex. Couple’s counseling may address relationship conflict.
Expert referral. Depression, anxiety, and substance use disorders are prevalent in menopausal women and contribute to sexual dysfunction. Effective therapy is available, although some pharmacologic treatments (including SSRIs) may be an additional cause of sexual dysfunction. In addition to recommending appropriate counseling and support, referring the patient to a psychopharmacologist with expertise in managing sexual adverse effects of medications may optimize care.
Continue to: Sexual function improves, but patient still wants to try testosterone
CASE Sexual function improves, but patient still wants to try testosterone
The patient returns for follow-up visits scheduled specifically to address her sexual concerns. Sex is more comfortable and pleasurable since initiating low-dose vaginal estrogen therapy. Having been on an SSRI since her mid-40s for mild depression, the patient switched to bupropion and notes improved libido and orgasmic response. She is exercising more regularly and working with a nutritionist to address a 15-lb weight gain after menopause. The couple saw a sex therapist and is communicating better about sex with more novelty in their repertoire. They are enjoying a regular date night. Although the patient’s sex life has improved with these interventions, she is still very interested in trying testosterone.
Testosterone’s effects on HSDD in menopausal women
After addressing the many factors that contribute to sexual disinterest, a trial of testosterone may be appropriate for a menopausal woman who continues to experience low libido with associated distress.
Testosterone levels decrease with aging in both men and women. Although testosterone levels decline by approximately 50% with bilateral oophorectomy, there is no decline in androgen levels with natural menopause.5 Testosterone circulates tightly bound to sex hormone–binding globulin (SHBG), so free or active testosterone will be reduced by oral estrogens, which increase SHBG levels.6 As most menopausal women will have a low testosterone level due to aging, measuring the testosterone level does not provide information about the etiology of the sexual problem.
Although some studies have identified an association between endogenous androgen levels and sexual function, the associations are modest and are of uncertain clinical significance.7-9 Not surprisingly, other factors, such as physical and psychologic health and the quality of the relationship, often are reported as more important predictors of sexual satisfaction than androgen levels.10
While endogenous testosterone levels may not correlate with sexual function, clinical trials of carefully selected menopausal women with HSDD have shown that androgen treatment generally results in improved sexual function.11 Studies demonstrate substantial improvements in sexual desire, orgasmic response, and frequency in menopausal women treated with high doses of intramuscular testosterone, which result in supraphysiologic androgen levels.12,13 While it is interesting that women with testosterone levels in the male low range have sizeable increases in sexual desire and response, long-term use of high-dose testosterone would result in unacceptable androgenic adverse effects and risks.
Continue to: Testosterone in low doses...
Testosterone in low doses. It is more relevant to consider the impact on female sexual function of low doses of testosterone, which raise the reduced testosterone levels seen in older women to the higher levels seen in reproductive-aged women.
A series of double-blind, multicenter, randomized, placebo-controlled trials in menopausal women with HSDD examined the impact on sexual function of a transdermal testosterone patch (300 μg) that increased blood testosterone levels to the upper limit of normal for young women.14-17 In these studies, compared with placebo, women using testosterone reported significant improvements in sexual desire, arousal, orgasmic response, frequency, and sexually related distress. Findings were consistent in surgically and naturally menopausal women, with and without the use of concurrent estrogen therapy. Improvements were clinically limited, however. On average, testosterone-treated women experienced 1 to 1.5 additional satisfying sexual events in a 4-week period compared with those treated with placebo. The percentage of women reporting a clinically meaningful benefit from treatment was significantly greater in women treated with testosterone (52%) compared with the placebo-treated women (31%).18 An appreciable placebo response was seen, typical of most studies of therapies for sexual dysfunction.

Safety concerns

Potential risks of testosterone treatment include acne, hirsutism, irreversible deepening of the voice, and adverse changes in lipids and liver function (TABLE 2).19 Adverse effects are dose dependent and are unlikely with physiologically dosed testosterone.
A 1-year study of testosterone patches in approximately 800 menopausal women with HSDD (with a subgroup of women followed for an additional year) provides the most comprehensive safety data available.17 Unwanted hair growth occurred more often in women receiving testosterone, without significant differences in blood biochemistry,hematologic parameters, carbohydrate metabolism, or lipids. Breast cancer was diagnosed in more women receiving testosterone than placebo. Although this finding may have been due to chance, the investigators concluded that long-term effects of testosterone treatment remain uncertain.
The FDA reviewed the data from the testosterone patch studies and determined that testosterone patches were effective for the treatment of HSDD in menopausal women, but more information was needed on long-term safety before approval could be granted. Another company then developed a testosterone gel product that produced similar blood levels as the testosterone patch. It was presumed that there would be similar efficacy; the principal goal of these studies was to examine long-term safety, particularly with respect to breast cancer and cardiovascular disease. Unexpectedly, although it raised testosterone blood levels to the upper limit of normal for young women, the testosterone gel product was no more effective than placebo.20 The clinical trial was ended, with safety data never published.
Continue to: Availability of testosterone formulations
Availability of testosterone formulations
Currently, no androgen therapies are FDA approved for the treatment of female sexual dysfunction. Although the best evidence regarding testosterone efficacy and safety involves the use of testosterone patches (300 μg), appropriately dosed for women, these patches are not currently available. FDA-approved testosterone patches are approved for the treatment of male hypogonadism, but use of these patches in women is not recommended since they would result in very high circulating testosterone levels.
Testosterone subcutaneous implants, pellets, and intramuscular injections also are not recommended for women because of the risk of excessive dosing. Small trials of menopausal women taking oral estrogen with low sexual desire found that oral formulations of testosterone improved libido in this study population.21 The combination of esterified estrogens (0.625 mg) and methyltestosterone (1.25 mg) is available as a compounded, non-FDA approved product. Oral androgen formulations generally are not advised, due to potential adverse effects on lipids and liver function.22
Compounded testosterone products. Ointments and creams may be compounded by prescription (TABLE 3). Product purity, dose, bioavailability, and quality typically are untested, and substantial variability exists between formulations and batches.23 Applying 1% testosterone cream or gel (0.5 g/day) topically to the thigh or lower abdomen should increase the low testosterone levels typically seen in menopausal women to the higher levels seen in younger women.24,25 Application to the vulva or vagina is not advised, as it may cause local irritation and is unpredictably absorbed.

Adapting male testosterone products. High-quality FDA-approved testosterone gel formulations are available for male hypogonadism. However, since women have approximately one-tenth the circulating testosterone levels of men, supraphysiologic dosing is a risk when these products are prescribed for women. Most testosterone products approved for men are provided in pumps or packets, and they are difficult to dose-adjust for women. Applying one-tenth the male dose of 1% testosterone gel (Testim), which comes in a resealable unit-dose tube, is an alternative to compounding. For men, the dose is 1 tube per day, so women should make 1 tube last for 10 days by using 3 to 4 drops of testosterone gel per day. Close physical contact must be avoided immediately after application, as topical hormone creams and gels are easily transferred to others. The safety and efficacy of compounded or dose-adjusted male testosterone products used in women are unknown.
Follow treated women closely. Women who elect to use transdermal testosterone therapy should be seen at 8 to 12 weeks to assess treatment response. Regular follow-up visits are required to assess response, satisfaction, and adverse effects, including acne and hirsutism. Since there may be little correlation between serum testosterone levels and the prescribed dose of a compounded testosterone product, testosterone levels should be measured regularly as a safety measure. The goal is to keep serum testosterone concentrations within the normal range for reproductive-aged women to reduce the likelihood of adverse effects. Testosterone levels should not be tested as an efficacy measure, however, as there is no testosterone level that will assure a satisfactory sex life.
CASE Conclusion
After a thorough discussion of high placebo response rates, potential adverse effects, unknown long-term risks, and off-label nature of testosterone use, the patient elects a trial of compounded 1% testosterone cream. Her clinician informs her of the limitations of compounded formulations and the need for regular testing of testosterone levels to prevent supraphysiologic dosing. At a follow-up visit 8 weeks later, she reports improved sexual desire and elects to continue treatment and monitoring. After using testosterone for 2 years, the patient is uncertain that she still is experiencing a significant benefit, stops testosterone treatment, and remains satisfied with her sex life.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
- The North American Menopause Society Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Simon JA, Nappi RE, Kingsberg SA, et al. Clarifying Vaginal Atrophy's Impact on Sex and Relationships (CLOSER) survey: emotional and physical impact of vaginal discomfort on North American postmenopausal women and their partners. Menopause. 2014;21:137-142.
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888-902.
- Shifren J, Monz B, Russo P, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
- Davison S, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847-3853.
- Shifren JL, Desindes S, McIlwain M, et al. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985-994.
- Davis SR, Davison SL, Donath S, et al. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294:91-96.
- Wahlin-Jacobsen S, Pedersen AT, Kristensen E, et al. Is there a correlation between androgens and sexual desire in women? J Sex Med. 2015;12:358-373.
- Randolph JF Jr, Zheng H, Avis NE, et al. Masturbation frequency and sexual function domains are associated with serum reproductive hormone levels across the menopausal transition. J Clin Endocrinol Metab. 2015;100:258-266.
- Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril. 2005;84:174-180.
- Shifren JL, Davis SR. Androgens in postmenopausal women: a review. Menopause. 2017;24:970-979.
- Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47:339-351.
- Huang G, Basaria S, Travison TG, et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause. 2014;21:612-623.
- Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
- Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab. 2005;90:5226-5233.
- Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 study. Menopause. 2006;13:770-779.
- Davis SR, Moreau M, Kroll R, et al; APHRODITE Study Team. Testosterone for low libido in postmenopausal women not taking estrogen. N Engl J Med. 2008;359:2005-2017.
- Kingsberg S, Shifren J, Wekselman K, et al. Evaluation of the clinical relevance of benefits associated with transdermal testosterone treatment in postmenopausal women with hypoactive sexual desire disorder. J Sex Med. 2007;4:1001-1008.
- Wierman ME, Arlt W, Basson R, et al. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3489-3510.
- Snabes M, Zborowski J, Simes S. Libigel (testosterone gel) does not differentiate from placebo therapy in the treatment of hypoactive sexual desire in postmenopausal women (abstract). J Sex Med. 2012;9(suppl 3):171.
- Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
- Somboonporn W, Davis S, Seif M, et al. Testsoterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;19:CD004509.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and American Society for Reproductive Medicine. Committee opinion 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol. 2012;(2 pt 1):411-415.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Shifren JL. Testosterone for midlife women: the hormone of desire? Menopause. 2015;22:1147-1149.
New and promising GSM treatments, more clinical takeaways from NAMS 2018
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
Learn more about NAMS: http://www.menopause.org/home
No signal for CV, breast effects with bioidentical vaginal estrogen for dyspareunia
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
that would suggest significant systemic absorption.
The lack of sex hormone binding globulin (SHBG) changes in the subset of women who received this test bolsters support for low systemic absorption from the low-dose vaginal softgel, Lisa Larkin, MD, said at the annual meeting of the North American Menopause Society in Orlando.
These safety data show that the vaginal route for this hormone is meeting a treatment goal for many menopausal women: “One goal of vaginal estrogen is to minimize systemic absorption and potentially reduce related side effects,” Dr. Larkin said.
TX-004HR (Imvexxy) delivers bioidentical solubilized 17 beta-estradiol (E2) via a softgel vaginal insert. It is Food and Drug Administration approved in 4-mcg and 10-mcg doses for the treatment of moderate to severe dyspareunia associated with menopause.
The phase 3 clinical trial (REJOICE) of TX-004HR met the coprimary endpoints of improving vaginal physiology, lowering vaginal pH, and decreasing the severity of dyspareunia at both the 4- and 10-mcg doses, said Dr. Larkin, an internal medicine physician in private practice in Mariemont, Ohio.
Serum estradiol levels for REJOICE participants were “similar to placebo and baseline, and generally within the postmenopausal range,” she said.
The randomized, double-blind, placebo-controlled trial tested 4-, 10-, and 25-mcg doses of TX-004HR. The self-administered vaginal inserts were used once daily for 2 weeks, then twice weekly for an additional 10 weeks.
In looking at treatment emergent adverse events (TEAEs), the REJOICE investigators were particularly interested in tracking cardiovascular and breast events, Dr. Larkin said. Participants received ECGs and clinical breast exams at baseline, and at study week 12. In addition, 72 of the women had SHBG measured at baseline and at weeks 2 and 12. The trial had a high completion rate of 94% at 12 weeks. The mean age of the women was 59 years, and the mean body mass index was 26.7 kg/m2. African American women made up 12% of the study; the remainder of the women were white.
In the end, 784 menopausal women with moderate to severe dyspareunia were randomized 1:1:1:1 to placebo or to receive one of the three dose levels of TX-004HR. Overall, “no clinically significant differences in adverse events were observed between treatment and placebo groups,” Dr. Larkin said. Only headache, vaginal discharge, and vulvovaginal pruritus occurred in at least 3% of the women in any treatment arm, with no differences between those taking TX-004HR and placebo. There were no malignancies or endometrial hyperplasia among the REJOICE participants: “There was no signal of estrogenic stimulation of the endometrium,” she said.
Looking at cardiovascular-related TEAEs, the five events that occurred were judged to be mild, and mostly not related to treatment. One case of first degree atrioventricular block and one case of sinus bradycardia were reported by the same woman, who was taking the 4-mcg dose of TX-004HR. One additional woman on that dose experienced palpitations, as did one woman taking placebo. “No coronary heart disease, venous thromboembolism, or other thrombotic episodes were noted” during the REJOICE trial, Dr. Larkin said. There were no clinically significant ECG changes during the study period that were judged related to treatment. Blood pressure was mildly increased in three women, one each in the 4-mcg, 10-mcg, and placebo study arms. The elevation was considered possibly related to the study in the 4-mcg and placebo takers. Two other women in the 4-mcg group experienced mild incident hypertension, with one woman’s hypertension judged possibly related to treatment.
Blood chemistry showed incident hypercholesterolemia for one woman in the 4-mcg group and one in the placebo group, and one woman taking the 10-mcg TX-0400HR dose and two taking placebo had increases in serum triglycerides.
Seven women reported breast-related TEAEs, with five of these considered possibly or probably treatment related. One woman on the 10-mcg dose had breast tenderness; all other events were among placebo takers.
Finally, among the subset of women whose SHBG levels were tested, “no dose-related pattern was apparent, and changes with TX-004HR were comparable to changes with placebo,” said Dr. Larkin, noting that there was no suggestion of significant systemic absorption.
“These safety data, in conjunction with the improved moderate to severe dyspareunia efficacy data and minimal estradiol absorption, support a local effect of the TX-004HR vaginal insert,” she said.
The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin disclosed that she is an advisory board member and on the speaker’s bureau for Valeant pharmaceuticals, is a consultant for TherapeuticsMD, and is an advisory board member for AMAG and Palatin Technologies.
SOURCE: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
FROM NAMS 2018
Key clinical point: Safety data from clinical trials of a bioidentical vaginal estrogen for dyspareunia in menopausal women showed no signs of CV or breast risks.
Major finding: There were no cardiovascular events or thrombotic episodes among menopausal women with dyspareunia treated with TX-004HR.
Study details: Randomized, double-blind, placebo-controlled trial of 784 menopausal women with moderate to severe dyspareunia.
Disclosures: The study was sponsored by TherapeuticsMD, the manufacturer of TH-004HR. Dr. Larkin reported financial relationships with several pharmaceutical companies, including TherapeuticsMD.
Source: Larkin L et al. NAMS 2018, Thursday concurrent session 1.
For dyspareunia, intravaginal prasterone may work best soon after menopause
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
Neither age nor previous hormone therapy had statistically significant associations with the effect of intravaginal prasterone on dyspareunia severity, according to a new subgroup analysis of clinical trial data. In a trend that did not reach statistical significance, though, said David F. Archer, MD.
“This was an unexpected finding,” he said in an interview.
In a subgroup analysis of data from two clinical trials of intravaginal prasterone (Intrarosa), Dr. Archer and his colleagues sought to investigate whether age, time since menopause, or any previous use of hormone replacement therapy influenced prasterone’s efficacy in treating dyspareunia.
Dr. Archer and his collaborators pooled data from two prospective, randomized, double-blind, placebo-controlled trials (NCT02013544 and NCT01256684) of intravaginal prasterone dosed at 0.50%, 6.5 mg once daily for 12 weeks; he presented the subgroup analyses at the annual meeting of the North American Menopause Society in San Diego.
For each subgroup, Dr. Archer, a professor of obstetrics and gynecology at Eastern Virginia Medical School, Norfolk, and his coinvestigators compared the mean differences in dyspareunia severity score of women who received prasterone and those who received placebo.
All subgroup analyses used the endpoint of improvement in moderate to severe dyspareunia or whether dyspareunia was the most bothersome symptoms for the women participating in the study. The investigators began by looking at the subgroup of 460 women who were 56 years and older at baseline and compared them with the 180 younger participants.
The 283 older participants who received prasterone saw a decrease of 0.36 points in a dyspareunia severity score versus a 0.44 point decrease for the 123 women aged 55 and younger who received prasterone, a nonsignificant difference between subgroups. The decrease compared with placebo-takers was significant in both cases, however (P = .0003 and P =.0031, respectively).
Looking at time since menopause, Dr. Archer and his collaborators divided participants into 33 individuals who were 1 or 2 years post menopause, 86 women who were 3-5 years post menopause, and 521 women who had experienced menopause at least 6 years before study baseline.
In this analysis, 22 of the earliest postmenopause women received prasterone, seeing a 1.59 point drop in dyspareunia severity. For the 59 women in the prasterone study arms who were 3-5 year past menopause, the decrease from baseline was 0.59 points. Finally, among the 325 women who received prasterone and experienced menopause 6 or more years ago, the decrease was 0.27 points.
Although there was a numeric difference in the change in dyspareunia score severity among these groups, the differences were not statistically significant, said Dr. Archer. Again, though, those who took prasterone had a significant reduction in dyspareunia severity scores when compared with those taking placebo (P less than .0001, P = .0136, and P = .0024, respectively).
In the prasterone study arms, 184 had previously used hormone therapy, and 222 had not. After 12 weeks of intravaginal prasterone, there was no statistically significant difference between the two subgroups, with a decreases in dyspareunia severity scores of 0.45 and 0.32, respectively. The decreases in severity scores when compared with those among women who took placebo were again statistically significant for both subgroups, however (P = .0002 and P = .0057, respectively).
Prasterone is a steroid that is also known as dehydroepiandrosterone (DHEA) and is an endogenous hormone that is a precursor for estrogens and androgens. Prasterone’s mechanism of action to reduce vulvar and vaginal atrophy is not completely understood, according to the Food and Drug Administration.
“The nonstatistically significant smaller effect on dyspareunia observed when treatment is initiated after a longer period after menopause suggests that a longer treatment period could be needed to achieve optimal benefit and that treatment of dyspareunia should be initiated as early as possible after menopause,” said Dr. Archer.
Dr. Archer reported grant support from and consulting relationships with several pharmaceutical companies, including Endoceutics, the producer of Intrarosa intravaginal prasterone.
FROM NAMS 2018
Key clinical point: Dyspareunia improvement was numerically, but not statistically, better soon after menopause.
Major finding: Dyspareunia scores dropped 1.59 points for those within 2 years of menopause, and 0.27 points for those 6 or more years post menopause.
Study details: Subgroup analysis of 640 postmenopausal women taking part in two clinical trials.
Disclosures: Dr. Archer reported receiving support from several pharmaceutical companies, including Endoceutics, the manufacturer of Intrarosa intravaginal prasterone.
Addressing your patient's sexual function after cancer
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recommended Resources:
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The techno vagina: The laser and radiofrequency device boom in gynecology
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In recent years, an increasing number of laser and radiofrequency device outpatient treatments have been heralded as safe and effective interventions for various gynecologic conditions. Laser devices and radiofrequency technology rapidly have been incorporated into certain clinical settings, including medical practices specializing in dermatology, plastic surgery, and gynecology. While this developing technology has excellent promise, many clinical and research questions remain unanswered.

Concerns about energy-based vaginal treatments
Although marketing material often suggests otherwise, most laser and radiofrequency devices are cleared by the US Food and Drug Administration (FDA) only for nonspecific gynecologic and hematologic interventions. However, both laser and radiofrequency device treatments, performed as outpatient procedures, have been touted as appropriate interventions for many conditions, including female sexual dysfunction, arousal and orgasmic concerns, vaginal laxity, vaginismus, lichen sclerosus, urinary incontinence, and vulvar vestibulitis.
Well-designed studies are needed. Prospective, randomized sham-controlled trials of energy-based devices are rare, and most data in the public domain are derived from case series. Many studies are of short duration with limited follow-up. Randomized controlled trials therefore are warranted and should have stringent inclusion and exclusion criteria. Body dysmorphic syndrome, for example, should be a trial exclusion. Study design for research should include the use of standardized, validated scales and long-term follow-up of participants.
Which specialists have the expertise to offer treatment? Important ethical and medical concerns regarding the technology need to be addressed. A prime concern is determining which health care professional specialist is best qualified to assess and treat underlying gynecologic conditions. It is not uncommon to see internists, emergency medicine providers, family physicians, plastic surgeons, psychiatrists, and dermatologists self-proclaiming their gynecologic “vaginal rejuvenation” expertise.
In my experience, some ObGyns have voiced concern about the diverse medical specialties involved in performing these procedures. Currently, no standard level of training is required to perform them. In addition, those providers lack the training needed to adequately and accurately assess the potential for confounding, underlying gynecologic pathology, and they are inadequately trained to offer patients the full gamut of therapeutic interventions. Many may be unfamiliar with female pelvic anatomy and sexual function and a multidisciplinary treatment paradigm.
We need established standards. A common vernacular, nosology, classification, and decision-tree assessment paradigm for genitopelvic laxity (related to the condition of the pelvic floor and not simply a loose feeling in the vagina) is lacking, which may make research and peer-to-peer discussions difficult.
Which patients are appropriate candidates? Proper patient selection criteria for energy-based vaginal treatment have not been standardized, yet this remains a paramount need. A comprehensive patient evaluation should be performed and include a discussion on the difference between an aesthetic complaint and a functional medical problem. Assessment should include the patient’s level of concern or distress and the impact of her symptoms on her overall quality of life. Patients should be evaluated for body dysmorphic syndrome and relationship discord. A complete physical examination, including a detailed pelvic assessment, often is indicated. A treatment algorithm that incorporates conservative therapies coupled with medical, technologic, and psychologic interventions also should be developed.
Various energy-based devices are available for outpatient procedures
Although the number of procedures performed (such as vaginal rejuvenation, labiaplasty, vulvar liposculpturing, hymenoplasty, G-spot amplification, and O-Shot treatment) for both cosmetic and functional problems has increased, the published scientific data on the procedures’ short- and long-term efficacy and safety are limited. The American College of Obstetricians and Gynecologists (ACOG) published a committee opinion stating that many of these procedures, including “vaginal rejuvenation,” may not be considered medically indicated and may lack scientific merit or ample supportive data to confirm their efficacy and safety.1 ObGyns should proceed with caution before incorporating these technologic treatments into their medical practice.
Much diversity exists within the device-technology space. The underpinnings of each device vary regarding their proposed mechanism of action and theoretical therapeutic and tissue effect. In device marketing materials, many devices have been claimed to have effects on multiple tissue types (for example, both vaginal mucosa and vulvar tissue), whereas others are said to have more focal and localized effects (that is, targeted behind the hymenal ring). Some are marketed as a one-time treatment, while others require multiple repeated treatments over an extended period. When it comes to published data, adverse effect reporting remains limited and follow-up data often are short term.
Radiofrequency and laser devices are separate and very distinct technologies with similar and differing proposed utilizations. Combining radiofrequency and laser treatments in tandem or sequentially may have clinical utility, but long-term safety may be a concern for lasers.
Radiofrequency-based devices
Typically, radiofrequency device treatments:
- are used for outpatient procedures
- do not require topical anesthesia
- are constructed to emit focused electromagnetic waves
- are applied to vaginal, vulvar, or vaginal introital or vestibular tissue
- deliver energy to the deeper connective tissue of the vaginal wall architecture.
Radiofrequency device energy can be monopolar, unipolar, bipolar, or multipolar depending on design. Design also dictates current and the number of electrodes that pass from the device to the grounding pad. Monopolar is the only type of radiofrequency that has a grounding pad; bipolar and multipolar energy returns to the treatment tip.
Radiofrequency devices typically are FDA 510(k)-cleared devices for nonspecific electrocoagulation and hemostasis for surgical procedures. None are currently FDA cleared in the United States for the treatment of vaginal or vulvar laxity or genitourinary syndrome of menopause (GSM). These energy-based devices aim to induce collagen contraction, neocollagenesis, vascularization, and growth factor infiltration to restore the elasticity and moisture of the underlying vaginal mucosa. Heat shock protein activation and inflammation activation are thought to be the underlying mechanisms of action.2–5
Treatment outcomes with 2 radiofrequency devices
Multiple prospective small case series studies have reported outcomes of women treated with the ThermiVa (ThermiAesthetics LLC) radiofrequency system.3,4 Typically, 3 treatments (with a between-treatment interval of 4 to 6 weeks) were applied. The clinical end point temperature had a range of 40°C to 45°C, which was maintained for 3 to 5 minutes per treated zone during 30 minutes’ total treatment time.
Some participants self-reported improvement in vaginal laxity symptoms with the 3 treatments. In addition, women reported subjective improvements in both vaginal atrophy symptoms and sexual function, including positive effect in multiple domains. No serious adverse events were reported in these case series. However, there was no placebo-controlled arm, and validated questionnaires were not used in much of this research.3,4
In another trial, the ThermiVa system was studied in a cohort of 25 sexually active women with self-reported anorgasmia or increased latency to orgasmic response.6 Participants received 3 treatments 4 weeks apart. Approximately three-quarters of the participants reported improved orgasmic responsivity, vaginal lubrication, and clitoral sensitivity. Notably, this research did not use validated questionnaires or a placebo or sham-controlled design. The authors suggested sustained treatment benefits at 9 to 12 months. While repeat treatment was advocated, data were lacking to support the optimal time for repeat treatment efficacy.6
A cryogen-cooled monopolar radiofrequency device, the Viveve system (Viveve Medical, Inc) differs from other radiofrequency procedures because it systematically cryogen cools and protects the surface of the vaginal mucosal tissue while heating the underlying structures.
The Viveve system was evaluated in 2 small pilot studies (24 and 30 participants) and in a large, randomized, sham-controlled, prospective trial that included 108 participants (VIVEVE I trial).5,7,8 Results from both preliminary small studies indicated that participants experienced significant improvement in overall sexual function at 6 months. In one of the small studies (in Japanese women), sustained efficacy at 12 months posttreatment was reported.7 Neither small study included a placebo-control arm, but they did include the use of validated questionnaires.
In the VIVEVE I trial (a multicenter international study), treatment in the active group consisted of a single, 30-minute outpatient procedure that delivered 90 J/cm2 of radiofrequency energy at the level just behind the hymenal ring behind the vaginal introitus. The sham-treated group received ≤1 J/cm2 of energy with a similar machine tip.8
Statistically significant improvements were reported in the arousal and orgasm domains of the validated Female Sexual Function Index (FSFI) for the active-treatment group compared with the sham-treated group. In addition, there were statistically significant differences in the FSFI and the Female Sexual Distress Scale–Revised total scores in favor of active treatment. Participants in the active-treatment arm reported statistically significant improvement in overall sexual satisfaction coupled with lowered overall sexual distress.8
These data are provocative, since the Viveve treatment demonstrated superior efficacy compared with the sham treatment, and prior evidence demonstrated that medical device trials employing a sham arm often demonstrate particularly large placebo/sham effects.9 A confirmatory randomized, sham-controlled multicenter US-based trial is currently underway. At present, the VIVEVE I trial remains the only published, large-scale, randomized, sham-controlled, blinded study of a radiofrequency-based treatment.
New emerging data support the efficacy and safety of this specific radiofrequency treatment in patients with mild to moderate urinary stress incontinence; further studies confirming these outcomes are anticipated. The Viveve system is approved in many countries for various conditions, including urinary incontinence (1 country), sexual function (17 countries), vaginal laxity (41 countries), and electrocoagulation and hemostasis (4 countries, including the United States).
Laser technology devices
Laser (Light Amplification by Stimulated Emission of Radiation) therapy, which uses a carbon dioxide (CO2), argon, YAG, or erbium energy source, also is currently marketed as a method to improve various gynecologic conditions, including genital pelvic relaxation syndrome, vaginal laxity, GSM, lichen sclerosus, and sexual problems such as dyspareunia and arousal or orgasmic disorders.
The CO2 laser therapy device, such as the MonaLisa Touch (DEKA Laser), appears to be very popular and widely available. It delivers fractional CO2 laser energy to the vaginal wall, creating sequential micro traumas that subsequently undergo a healing reaction; the newly healed area has an improved underlying tissue architecture (but at a superficial level). The laser’s proposed mechanism of action is that it ablates only a minute fraction of the superficial lamina propria; it acts primarily to stimulate rapid healing of the tissue, creating new collagen and elastic fibers. There is no evidence of scarring.10
Treatment outcomes with laser device therapy
Authors of a 2017 study series of CO2 laser treatments in women with moderate to severe GSM found that 84% of participants experienced significant improvement in sexual function, dyspareunia, and otherwise unspecified sexual issues from pretreatment to 12 to 24 months posttreatment.11 These findings are consistent with several other case series and provide supportive evidence for the efficacy and safety of CO2 laser therapy. This technology may be appropriate for the treatment of GSM.
Laser technology shows excellent promise for the treatment of GSM symptoms by virtue of its superficial mechanism of action. In addition, several trials have demonstrated efficacy and safety in breast cancer patient populations.12 This is particularly interesting since breast cancer treatments, such as aromatase inhibitors (considered a mainstay of cancer treatment), can cause severe atrophic vaginitis. Breast cancer survivors often avoid minimally absorbed local vaginal hormonal products, and over-the-counter products (moisturizers and lubricants) are not widely accepted. Hence, a nonhormonal treatment for distressing GSM symptoms is welcomed in this population.
Pagano and colleagues recently studied 82 breast cancer survivors in whom treatment with vaginal moisturizers and lubricants failed.12 Participants underwent 3 laser treatment cycles approximately 30 to 40 days apart; they demonstrated improvements in vaginal dryness, vaginal itchiness, stinging, dyspareunia, and reduced sensitivity.
Microablative fractional CO2 laser may help reestablish a normative vaginal microbiome by altering the prevalence of lactobacillus species and reestablishing a normative postmenopausal vaginal flora.13
The tracking and reporting of adverse events associated with laser procedures has been less than optimal. In my personal clinical experience, consequences from both short- and long-term laser treatments have included vaginal canal agglutination, worsening dyspareunia, and constricture causing vaginal hemorrhage.
Cruz and colleagues recently conducted a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy of fractional CO2 laser compared with topical estriol and laser plus estriol for the treatment of vaginal atrophy in 45 postmenopausal women.14 They found statistically significant differences in dyspareunia, dryness, and burning compared with baseline levels in all 3 treatment groups. Results with the fractional CO2 laser treatment were deemed to be similar to those of the topical estriol and the combined therapy.14
By contrast, an erbium (Er):YAG laser, such as the IntimaLase (Fotona, LLC) laser, functions by heating the pelvic tissue and collagen within the introitus and vaginal canal.15,16 When the underlying collagen is heated, the fibers are thought to thicken and shorten, which may result in immediate contracture of the treated tissue. Additionally, this process stimulates the existing collagen to undergo remodeling and it also may cause neocollagen deposition.15 In a general review of gynecologic procedures, after 1 to 4 treatment sessions (depending on the study), most patients reported improved sexual satisfaction or vaginal tightness.15
Although trials have included small numbers of patients, early evidence suggests some lasers with reportedly deeper penetration may be useful for treatment of vaginal laxity, but further studies are needed. In smaller studies, the Er:YAG laser has shown efficacy and safety in the treatment of stress urinary incontinence and improved lower urinary tract symptoms, quality of life, and sexual function.16,17
Insurance does not cover energy-based treatment costs
Currently, both laser and radiofrequency device treatments are considered fee-for-service interventions. Radiofrequency and laser treatments for gynecologic conditions are not covered by health insurance, and treatment costs can be prohibitive for many patients. In addition, the long-term safety of these treatments remains to be studied further, and the optimal time for a repeat procedure has yet to be elucidated.
The FDA cautions against energy-based procedures
In July 2018, the FDA released a statement of concern reiterating the need for research and randomized clinical trials before energy-based device treatments can be widely accepted, and that they are currently cleared only for general gynecologic indications and not for disorders and symptoms related to menopause, urinary incontinence, or sexual function.18
The FDA stated that “we have not cleared or approved for marketing any energy-based devices to treat these symptoms or conditions [vaginal laxity; vaginal atrophy, dryness, or itching; pain during sexual intercourse; pain during urination; decreased sexual sensation], or any symptoms related to menopause, urinary incontinence, or sexual function.” The FDA noted that serious complications have been reported, including vaginal burns, scarring, pain during sexual intercourse, and recurring, chronic pain. The FDA issued letters to 7 companies regarding concerns about the marketing of their devices for off-label use and promotion.
Several societies have responded. ACOG reaffirmed its 2016 position statement on fractional laser treatment of vulvovaginal atrophy.19 JoAnn Pinkerton, MD, Executive Director of The North American Menopause Society (NAMS), and Sheryl Kingsberg, PhD, President of NAMS, alerted their members that both health care professionals and consumers should tread cautiously, and they encouraged scrutiny of existing evidence as all energy-based treatments are not created equal.20 They noted that some research does exist and cited 2 randomized, sham-controlled clinical trials that have been published.
Looking forward
Various novel technologic therapies are entering the gynecologic market. ObGyns must critically evaluate these emerging technologies with a keen understanding of their underlying mechanism of action, the level of scientific evidence, and the treatment’s proposed therapeutic value.
Radiofrequency energy devices appear to be better positioned to treat urinary incontinence and vaginal relaxation syndrome because of their capability for deep tissue penetration. Current data show that laser technology has excellent promise for the treatment and management of GSM. Both technologies warrant further investigation in long-term randomized, sham-controlled trials that assess efficacy and safety with validated instruments over an extended period. In addition, should these technologies prove useful in the overall treatment armamentarium for gynecologic conditions, the question of affordability and insurance coverage needs to be addressed.
ObGyns must advocate for female sexual wellness and encourage a comprehensive multidisciplinary team approach for offering various therapies. Ultimately, responsible use of evidence-based innovative technology should be incorporated into the treatment paradigm.
Despite recent technologic advancements and applications in gynecologic care, minimally absorbed local vaginal hormonal products (creams, rings, intravaginal tablets) and estrogen agonists/antagonists remain the mainstay and frontline treatment for moderate to severe dyspareunia, a symptom of vulvovaginal atrophy due to menopause. Newer medications, such as intravaginal steroids1 and the recently approved bioidentical estradiol nonapplicator vaginal inserts,2 also offer excellent efficacy and safety in the treatment of this condition. These medications now are included under expanded insurance coverage, and they offer safe, simple, and cost-effective treatments for this underdiagnosed condition.
References
- Intrarosa [package insert]. Waltham, MA: AMAG Pharmaceuticals Inc; February 2018.
- Imvexxy [package insert]. Boca Raton, FL: TherapeuticsMD; 2018.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.
- ACOG Committee on Gynecologic Practice. ACOG Committee Opinion No. 378: Vaginal "rejuvenation" and cosmetic vaginal procedures. Obstet Gynecol. 2007;110(3):737-738.
- Dunbar SW, Goldberg DJ. Radiofrequency in cosmetic dermatology: an update. J Drugs Dermatol. 2015;14(11):1229-1238.
- Leibaschoff G, Izasa PG, Cardona JL, Miklos JR, Moore RD. Transcutaneous temperature-controlled radiofrequency (TTCRF) for the treatment of menopausal vaginal/genitourinary symptoms. Surg Technol Int. 2016;29:149-159.
- Alinsod RM. Temperature controlled radiofrequency for vulvovaginal laxity. Prime J. July 23, 2015. https://www.prime-journal.com/temperature-controlled-radiofrequency-for-vulvovaginal-laxity/. Accessed August 15, 2018.
- Millheiser LS, Pauls RN, Herbst SJ, Chen BH. Radiofrequency treatment of vaginal laxity after vaginal delivery: nonsurgical vaginal tightening. J Sex Med. 2010;7(9):3088-3095.
- Alinsod RM. Transcutaneous temperature controlled radiofrequency for orgasmic dysfunction. Lasers Surg Med. 2016;48(7):641-645.
- Sekiguchi Y, Utsugisawa Y, Azekosi Y, et al. Laxity of the vaginal introitus after childbirth: nonsurgical outpatient procedure for vaginal tissue restoration and improved sexual satisfaction using low-energy radiofrequency thermal therapy. J Womens Health (Larchmt). 2013;22 (9):775-781 .
- Krychman M, Rowan CG, Allan BB, et al. Effect of single-treatment, surface-cooled radiofrequency therapy on vaginal laxity and female sexual function: the VIVEVE I randomized controlled trial. J Sex Med. 2017;14(2):215-225.
- Kaptchuk TJ, Goldman P, Stone DA, Statson WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol. 2000;53(8): 786-792.
- Gotkin RH, Sarnoff SD, Cannarozzo G, Sadick NS, Alexiades-Armenakas M. Ablative skin resurfacing with a novel microablative CO2 laser. J Drugs Dermatol. 2009;8(2):138-144.
- Behnia-Willison F, Sarraf S, Miller J, et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. Eur J Obstet Gynecol Reprod Biol. 2017;213:39-44.
- Pagano T, De Rosa P, Vallone R, et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments, a retrospective study. Menopause. 2018;25(6):657-662.
- Anthanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climateric. 2016;19(5):512-518.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25(1): 21-28.
- Vizintin Z, Rivera M, Fistonic I, et al. Novel minimally invasive VSP Er:YAG laser treatments in gynecology. J Laser Health Acad. 2012;2012(1):46-58.
- Tien YM, Hsio SM, Lee CN, Lin HH. Effects of laser procedure for female urodynamic stress incontinence on pad weight, urodynamics, and sexual function. Int Urogynecol J. 2017;28(3):469-476.
- Oginc UB, Sencar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Laser Surg Med. 2015;47(9):689-697.
- US Food and Drug Administration. FDA warns against use of energy based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. July 30, 2018. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Accessed August 16, 2018.
- The American College of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and US Food and Drug Administration clearance: position statement. May 2016. https://www.acog.org/Clinical-Guidance-and-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Accessed August 16, 2018.
- The North American Menopause Society. FDA mandating vaginal laser manufacturers present valid data before marketing. August 1, 2018. https://www.menopause.org/docs/default-source/default-document-library/nams-responds-to-fda-mandate-on-vaginal-laser-manufacturers-08-01-2018.pdf. Accessed August 16, 2018.