User login
Inhibitor exhibits activity against hematologic malignancies
A dual kinase inhibitor is active against a range of hematologic malignancies, according to preclinical research.
Investigators found that ASN002, a SYK/JAK inhibitor, exhibited “potent” antiproliferative activity in leukemia, lymphoma, and myeloma cell lines.
ASN002 also inhibited tumor growth in mouse models of these malignancies and proved active against ibrutinib-resistant diffuse large B-cell lymphoma (DLBCL).
The investigators presented these results at the AACR Annual Meeting 2017 (abstract 4204).
The work was conducted by employees of Asana BioSciences, the company developing ASN002.
The investigators tested ASN002 in 178 cell lines and found the drug exhibited “strong antiproliferative activity” in a range of hematologic cancer cell lines, including:
- Leukemia—HEL31, HL60, Jurkat, MOLM-13, and MOLM-16
- Lymphoma—KARPAS-299, OCI-LY10, OCI-LY-19, Pfeiffer, Raji, Ramos, SU-DHL-1, SU-DHL-6, and SU-DHL-10
- Myeloma—H929, JJN3, OPM2, and U266.
In addition, ASN002 was active against ibrutinib-resistant clones derived from the DLBCL cell line SU-DHL-6.
In a SU-DHL-6 xenograft model, the combination of ASN002 and ibrutinib was more effective than either compound alone.
The investigators also found that ASN002 alone demonstrated “strong tumor growth inhibition” in mouse models of DLBCL (Pfeiffer and SU-DHL-6), myeloma (H929), and erythroleukemia (HEL).
The team pointed out that ASN002 is currently under investigation in a phase 1/2 study of patients with B-cell lymphomas (DLBCL, mantle cell lymphoma, and follicular lymphoma) as well as solid tumors.
The investigators said that, to date, ASN002 has been well tolerated and has shown encouraging early evidence of clinical activity and symptomatic benefit in the lymphoma patients. ![]()
A dual kinase inhibitor is active against a range of hematologic malignancies, according to preclinical research.
Investigators found that ASN002, a SYK/JAK inhibitor, exhibited “potent” antiproliferative activity in leukemia, lymphoma, and myeloma cell lines.
ASN002 also inhibited tumor growth in mouse models of these malignancies and proved active against ibrutinib-resistant diffuse large B-cell lymphoma (DLBCL).
The investigators presented these results at the AACR Annual Meeting 2017 (abstract 4204).
The work was conducted by employees of Asana BioSciences, the company developing ASN002.
The investigators tested ASN002 in 178 cell lines and found the drug exhibited “strong antiproliferative activity” in a range of hematologic cancer cell lines, including:
- Leukemia—HEL31, HL60, Jurkat, MOLM-13, and MOLM-16
- Lymphoma—KARPAS-299, OCI-LY10, OCI-LY-19, Pfeiffer, Raji, Ramos, SU-DHL-1, SU-DHL-6, and SU-DHL-10
- Myeloma—H929, JJN3, OPM2, and U266.
In addition, ASN002 was active against ibrutinib-resistant clones derived from the DLBCL cell line SU-DHL-6.
In a SU-DHL-6 xenograft model, the combination of ASN002 and ibrutinib was more effective than either compound alone.
The investigators also found that ASN002 alone demonstrated “strong tumor growth inhibition” in mouse models of DLBCL (Pfeiffer and SU-DHL-6), myeloma (H929), and erythroleukemia (HEL).
The team pointed out that ASN002 is currently under investigation in a phase 1/2 study of patients with B-cell lymphomas (DLBCL, mantle cell lymphoma, and follicular lymphoma) as well as solid tumors.
The investigators said that, to date, ASN002 has been well tolerated and has shown encouraging early evidence of clinical activity and symptomatic benefit in the lymphoma patients. ![]()
A dual kinase inhibitor is active against a range of hematologic malignancies, according to preclinical research.
Investigators found that ASN002, a SYK/JAK inhibitor, exhibited “potent” antiproliferative activity in leukemia, lymphoma, and myeloma cell lines.
ASN002 also inhibited tumor growth in mouse models of these malignancies and proved active against ibrutinib-resistant diffuse large B-cell lymphoma (DLBCL).
The investigators presented these results at the AACR Annual Meeting 2017 (abstract 4204).
The work was conducted by employees of Asana BioSciences, the company developing ASN002.
The investigators tested ASN002 in 178 cell lines and found the drug exhibited “strong antiproliferative activity” in a range of hematologic cancer cell lines, including:
- Leukemia—HEL31, HL60, Jurkat, MOLM-13, and MOLM-16
- Lymphoma—KARPAS-299, OCI-LY10, OCI-LY-19, Pfeiffer, Raji, Ramos, SU-DHL-1, SU-DHL-6, and SU-DHL-10
- Myeloma—H929, JJN3, OPM2, and U266.
In addition, ASN002 was active against ibrutinib-resistant clones derived from the DLBCL cell line SU-DHL-6.
In a SU-DHL-6 xenograft model, the combination of ASN002 and ibrutinib was more effective than either compound alone.
The investigators also found that ASN002 alone demonstrated “strong tumor growth inhibition” in mouse models of DLBCL (Pfeiffer and SU-DHL-6), myeloma (H929), and erythroleukemia (HEL).
The team pointed out that ASN002 is currently under investigation in a phase 1/2 study of patients with B-cell lymphomas (DLBCL, mantle cell lymphoma, and follicular lymphoma) as well as solid tumors.
The investigators said that, to date, ASN002 has been well tolerated and has shown encouraging early evidence of clinical activity and symptomatic benefit in the lymphoma patients. ![]()
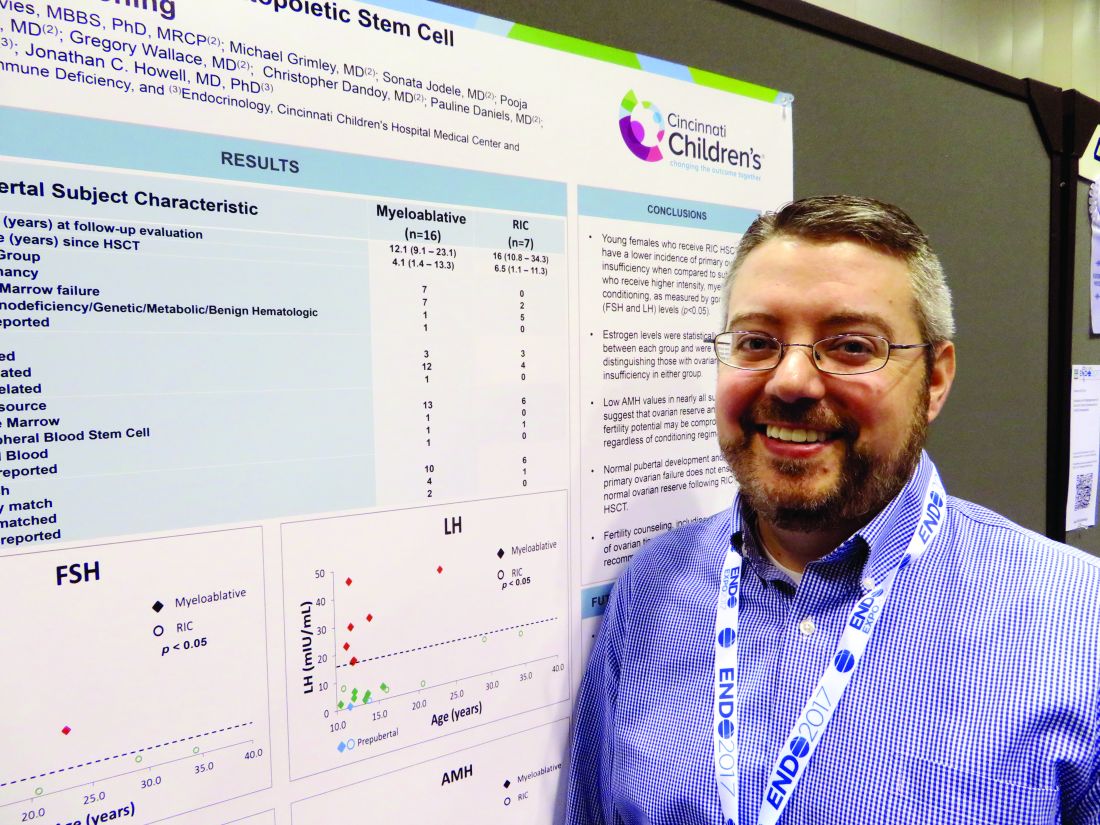
Reduced-intensity conditioning may not preserve fertility in young girls after bone marrow transplant
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Anti-Müllerian hormone was abnormally low or absent in all treated girls, whether they had reduced-intensity or high-intensity conditioning.
Data source: The prospective study is following 49 females aged 1-40 years.
Disclosures: Neither Dr. Howell nor any of his colleagues had any financial disclosures.
MAGNIFY in relapsed/refractory mantle cell lymphoma
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
MAGNIFY is a phase IIIB randomized trial actively recruiting patients with relapsed/refractory mantle cell lymphoma, based on studies posted at ClinicalTrials.gov.
MAGNIFY (NCT01996865) is a study of lenalidomide (CC-5013) plus rituximab maintenance therapy, followed by lenalidomide single-agent maintenance therapy, versus rituximab. Sponsored by Celgene, the maker of lenalidomide (Revlimid), the trial’s primary outcome measure is progression-free survival at up to 8 years.
The MAGNIFY trial includes patients with grades 1-3b or transformed follicular lymphoma, marginal zone lymphoma, or mantle cell lymphoma who had received at least one prior therapy and had stage I-IV measurable disease. About 500 patients are planned to enroll in 12 cycles of R2 induction, and slightly more than 300 patients are projected to be randomized after induction to the two maintenance arms. Induction includes oral lenalidomide 20 mg/day, days 1-21 per 28-day cycle; plus intravenous rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 3, 5, 7, 9, and 11 (28-day cycles).
Patients will then be randomized to maintenance lenalidomide 10 mg/day, given on days 1-21 per 28-day cycle for cycles 13-30; plus rituximab 375 mg/m2, given on day 1 of cycles 13, 15, 17, 19, 21, 23, 25, 27, and 29 (R2, Arm A), or rituximab alone (same schedule, Arm B). Patients receiving R2 maintenance after 18 cycles may continue maintenance lenalidomide monotherapy at 10 mg/day, days 1-21 per 28-day cycle, at the discretion of the patient and/or investigator, until disease progression as tolerated.
The primary endpoint is progression-free survival. Secondary endpoints include safety, overall survival, response rates, duration of response, and quality of life. Patients will be followed for at least 5 years after the last patient-initiated induction therapy. Enrollment in MAGNIFY began in March 2014; as of January 2016, 133 patients are enrolled, according to the study page at ClinicalTrials.gov.
Inhibitor produces durable responses in rel/ref iNHL
WASHINGTON, DC—An investigational drug can produce durable responses and has a manageable safety profile in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL), according to researchers.
The drug is copanlisib, an intravenous pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor.
In the phase 2 CHRONOS-1 trial, copanlisib produced an objective response rate (ORR) of 59.2%, with a complete response (CR) rate of 12%, in patients with relapsed/refractory iNHL.
The median duration of response exceeded 98 weeks.
There were 3 deaths considered related to copanlisib, and the most common treatment-related adverse events (AEs) were transient hyperglycemia and hypertension.
These results were presented at the AACR Annual Meeting 2017 (abstract CT149). The study is supported by Bayer, the company developing copanlisib.
CHRONOS-1 included 141 patients with iNHL. Most (n=104) had follicular lymphoma (FL), 23 had marginal zone lymphoma (MZL), 8 had small lymphocytic lymphoma, and 6 had lymphoplasmacytoid/Waldenstrӧm’s macroglobulinemia.
All patients had relapsed after or were refractory to at least 2 prior lines of therapy, which included both rituximab and an alkylating agent.
For this study, the patients received 60 mg of intravenous copanlisib intermittently on days 1, 8, and 15 of a 28-day cycle.
At the time of analysis, the median duration of treatment was 22 weeks (range, 1-105), and 46 patients were still receiving copanlisib.
Efficacy
For the entire cohort, the ORR was 59.2%. Twelve percent of patients achieved a CR, 47.2% had a partial response (PR), 29.6% had stable disease, and 2.1% had progressive disease.
Among patients with FL, the ORR was 58.7%, the CR rate was 14.4%, and the PR rate was 44.2%.
Among patients with MZL, the ORR was 69.6%, with 8.7% of patients achieving a CR and 60.9% achieving a PR.
For the entire cohort, the estimated median duration of response was 687 days (range, 0-687). For patients with FL, it was 370 days (range, 0-687).
The estimated median progression-free survival was 340 days (range, 0-736), and the median overall survival had not been reached at the time of analysis.
Safety
The most common treatment-related AEs were transient hyperglycemia (all grades, 49%/grade 3+, 40%) and hypertension (all grades, 29%/grade 3+, 23%).
The researchers said other AEs of interest were neutropenia (all grades, 25%/grade 3+, 19%), diarrhea (all grades, 18%/grade 3+, 4%), lung infection (all grades, 14%/grade 3+, 11%), pneumonitis (all grades, 7%/grade 3+, 1.4%), and colitis (0.7%, all grade 3+).
Laboratory AEs of interest were alanine aminotransferase increase (all grades, 23%/grade 1, 19%) and aspartate aminotransferase increase (all grades, 28%/grade 1, 25%).
There were 2 non-fatal opportunistic infections.
There were 6 deaths, and 3 of them were considered related to copanlisib. These 3 deaths were due to lung infection, respiratory failure, and a thromboembolic event.
“[I]nhibition of the PI3K pathway has been shown to be an effective therapeutic strategy in treating indolent lymphomas . . .,” said study investigator Martin Dreyling, MD, of Klinikum der Universität München-Grosshadern in Munich, Germany.
“However, concerns exist about the safety of available oral PI3K inhibitors . . . . The results of CHRONOS-1 demonstrate that intermittent intravenous administration of copanlisib achieved durable efficacy with a manageable safety profile in this difficult-to-treat patient population.” ![]()
WASHINGTON, DC—An investigational drug can produce durable responses and has a manageable safety profile in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL), according to researchers.
The drug is copanlisib, an intravenous pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor.
In the phase 2 CHRONOS-1 trial, copanlisib produced an objective response rate (ORR) of 59.2%, with a complete response (CR) rate of 12%, in patients with relapsed/refractory iNHL.
The median duration of response exceeded 98 weeks.
There were 3 deaths considered related to copanlisib, and the most common treatment-related adverse events (AEs) were transient hyperglycemia and hypertension.
These results were presented at the AACR Annual Meeting 2017 (abstract CT149). The study is supported by Bayer, the company developing copanlisib.
CHRONOS-1 included 141 patients with iNHL. Most (n=104) had follicular lymphoma (FL), 23 had marginal zone lymphoma (MZL), 8 had small lymphocytic lymphoma, and 6 had lymphoplasmacytoid/Waldenstrӧm’s macroglobulinemia.
All patients had relapsed after or were refractory to at least 2 prior lines of therapy, which included both rituximab and an alkylating agent.
For this study, the patients received 60 mg of intravenous copanlisib intermittently on days 1, 8, and 15 of a 28-day cycle.
At the time of analysis, the median duration of treatment was 22 weeks (range, 1-105), and 46 patients were still receiving copanlisib.
Efficacy
For the entire cohort, the ORR was 59.2%. Twelve percent of patients achieved a CR, 47.2% had a partial response (PR), 29.6% had stable disease, and 2.1% had progressive disease.
Among patients with FL, the ORR was 58.7%, the CR rate was 14.4%, and the PR rate was 44.2%.
Among patients with MZL, the ORR was 69.6%, with 8.7% of patients achieving a CR and 60.9% achieving a PR.
For the entire cohort, the estimated median duration of response was 687 days (range, 0-687). For patients with FL, it was 370 days (range, 0-687).
The estimated median progression-free survival was 340 days (range, 0-736), and the median overall survival had not been reached at the time of analysis.
Safety
The most common treatment-related AEs were transient hyperglycemia (all grades, 49%/grade 3+, 40%) and hypertension (all grades, 29%/grade 3+, 23%).
The researchers said other AEs of interest were neutropenia (all grades, 25%/grade 3+, 19%), diarrhea (all grades, 18%/grade 3+, 4%), lung infection (all grades, 14%/grade 3+, 11%), pneumonitis (all grades, 7%/grade 3+, 1.4%), and colitis (0.7%, all grade 3+).
Laboratory AEs of interest were alanine aminotransferase increase (all grades, 23%/grade 1, 19%) and aspartate aminotransferase increase (all grades, 28%/grade 1, 25%).
There were 2 non-fatal opportunistic infections.
There were 6 deaths, and 3 of them were considered related to copanlisib. These 3 deaths were due to lung infection, respiratory failure, and a thromboembolic event.
“[I]nhibition of the PI3K pathway has been shown to be an effective therapeutic strategy in treating indolent lymphomas . . .,” said study investigator Martin Dreyling, MD, of Klinikum der Universität München-Grosshadern in Munich, Germany.
“However, concerns exist about the safety of available oral PI3K inhibitors . . . . The results of CHRONOS-1 demonstrate that intermittent intravenous administration of copanlisib achieved durable efficacy with a manageable safety profile in this difficult-to-treat patient population.” ![]()
WASHINGTON, DC—An investigational drug can produce durable responses and has a manageable safety profile in patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL), according to researchers.
The drug is copanlisib, an intravenous pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor.
In the phase 2 CHRONOS-1 trial, copanlisib produced an objective response rate (ORR) of 59.2%, with a complete response (CR) rate of 12%, in patients with relapsed/refractory iNHL.
The median duration of response exceeded 98 weeks.
There were 3 deaths considered related to copanlisib, and the most common treatment-related adverse events (AEs) were transient hyperglycemia and hypertension.
These results were presented at the AACR Annual Meeting 2017 (abstract CT149). The study is supported by Bayer, the company developing copanlisib.
CHRONOS-1 included 141 patients with iNHL. Most (n=104) had follicular lymphoma (FL), 23 had marginal zone lymphoma (MZL), 8 had small lymphocytic lymphoma, and 6 had lymphoplasmacytoid/Waldenstrӧm’s macroglobulinemia.
All patients had relapsed after or were refractory to at least 2 prior lines of therapy, which included both rituximab and an alkylating agent.
For this study, the patients received 60 mg of intravenous copanlisib intermittently on days 1, 8, and 15 of a 28-day cycle.
At the time of analysis, the median duration of treatment was 22 weeks (range, 1-105), and 46 patients were still receiving copanlisib.
Efficacy
For the entire cohort, the ORR was 59.2%. Twelve percent of patients achieved a CR, 47.2% had a partial response (PR), 29.6% had stable disease, and 2.1% had progressive disease.
Among patients with FL, the ORR was 58.7%, the CR rate was 14.4%, and the PR rate was 44.2%.
Among patients with MZL, the ORR was 69.6%, with 8.7% of patients achieving a CR and 60.9% achieving a PR.
For the entire cohort, the estimated median duration of response was 687 days (range, 0-687). For patients with FL, it was 370 days (range, 0-687).
The estimated median progression-free survival was 340 days (range, 0-736), and the median overall survival had not been reached at the time of analysis.
Safety
The most common treatment-related AEs were transient hyperglycemia (all grades, 49%/grade 3+, 40%) and hypertension (all grades, 29%/grade 3+, 23%).
The researchers said other AEs of interest were neutropenia (all grades, 25%/grade 3+, 19%), diarrhea (all grades, 18%/grade 3+, 4%), lung infection (all grades, 14%/grade 3+, 11%), pneumonitis (all grades, 7%/grade 3+, 1.4%), and colitis (0.7%, all grade 3+).
Laboratory AEs of interest were alanine aminotransferase increase (all grades, 23%/grade 1, 19%) and aspartate aminotransferase increase (all grades, 28%/grade 1, 25%).
There were 2 non-fatal opportunistic infections.
There were 6 deaths, and 3 of them were considered related to copanlisib. These 3 deaths were due to lung infection, respiratory failure, and a thromboembolic event.
“[I]nhibition of the PI3K pathway has been shown to be an effective therapeutic strategy in treating indolent lymphomas . . .,” said study investigator Martin Dreyling, MD, of Klinikum der Universität München-Grosshadern in Munich, Germany.
“However, concerns exist about the safety of available oral PI3K inhibitors . . . . The results of CHRONOS-1 demonstrate that intermittent intravenous administration of copanlisib achieved durable efficacy with a manageable safety profile in this difficult-to-treat patient population.” ![]()
Half of patients retain response to CAR T-cell therapy
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
WASHINGTON, DC—Roughly half of patients who responded to chimeric antigen receptor (CAR) T-cell therapy in the ZUMA-1 trial have retained that response at a median follow-up exceeding 8 months.
The CAR T-cell therapy, axicabtagene ciloleucel (formerly KTE-C19), initially produced an objective response rate (ORR) of 82% in this trial of patients with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL).
At a median follow-up of 8.7 months, 44% of all patients (53% of responders) are still in response, and 39% are in complete response (CR).
Thirteen percent of patients had grade 3 or higher cytokine release syndrome (CRS), and 28% had neurologic events.
There were 2 deaths related to axicabtagene ciloleucel.
Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa, Florida, presented these updated results from ZUMA-1 at the AACR Annual Meeting 2017 (abstract CT019).
ZUMA-1 is sponsored by Kite Pharma but is also funded, in part, by the Leukemia and Lymphoma Society Therapy Acceleration Program.
Patients and treatment
The trial enrolled 111 patients, 101 of whom were successfully treated with axicabtagene ciloleucel. Seven patients could not be treated due to serious adverse events, 1 due to unavailable product, and 2 due to non-measurable disease.
Seventy-seven of the patients had diffuse large B-cell lymphoma (DLBCL), and 24 had transformed follicular lymphoma (TFL) or primary mediastinal B-cell lymphoma (PMBCL). Eighty-five percent of patients had stage III/IV disease.
Seventy-nine percent were refractory to chemotherapy and did not have a prior autologous stem cell transplant (auto-SCT). Twenty-one percent did undergo auto-SCT and relapsed within 12 months of the procedure.
Sixty-nine percent of patients had received 3 or more lines of prior therapy, and 54% were refractory to 2 consecutive lines of prior therapy.
For this study, the patients received a conditioning regimen of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) for 3 days.
Two days after the conditioning regimen was completed, patients received axicabtagene ciloleucel at a target dose of 2 × 106 CAR T cells/kg.
Efficacy
The following table shows overall response data, response data at 6 months, and ongoing responses at the primary analysis data cut-off.
| DLBCL (n=77) | TFL/PMBCL (n=24) | Combined (n=101) | ||||
| ORR (%) | CR (%) | ORR (%) | CR (%) | ORR (%) | CR (%) | |
| ORR | 82 | 49 | 83 | 71 | 82 | 54 |
| Month 6 | 36 | 31 | 54 | 50 | 41 | 36 |
| Ongoing | 36 | 31 | 67 | 63 | 44 | 39 |
The researchers said the ORR was generally consistent in key subgroups. The ORR was 83% in patients who were refractory to their second or greater line of therapy and 76% in patients who relapsed within 12 months of auto-SCT.
Overall, the median duration of response was 8.2 months. However, the median duration of response has not been reached for patients with a CR.
At a median follow-up of 8.7 months, the median overall survival has not been reached.
Safety
The most common grade 3 or higher adverse events included anemia (43%), neutropenia (39%), decreased neutrophil count (32%), febrile neutropenia (31%), decreased white blood cell count (29%), thrombocytopenia (24%), encephalopathy (21%), and decreased lymphocyte count (20%).
The incidence of grade 3 or higher CRS was 13%, and the incidence of neurologic events was 28%. These represent decreases from the interim analysis of ZUMA-1, when the rate of grade 3+ CRS was 18%, and the rate of neurological events was 34%.
“We believe the rates of CRS and neurologic events decreased over the course of the study as clinicians gained experience in the management of adverse events,” said Jeff Wiezorek, MD, senior vice-president of clinical development at Kite Pharma.
There were 3 deaths throughout the course of the trial that were not a result of disease progression.
Two deaths were deemed related to axicabtagene ciloleucel. One was a case of hemophagocytic lymphohistiocytosis. The other was cardiac arrest in the setting of CRS.
The third death was the result of a pulmonary embolism and was considered unrelated to axicabtagene ciloleucel. ![]()
Demystifying the diagnosis and classification of lymphoma: a guide to the hematopathologist’s galaxy
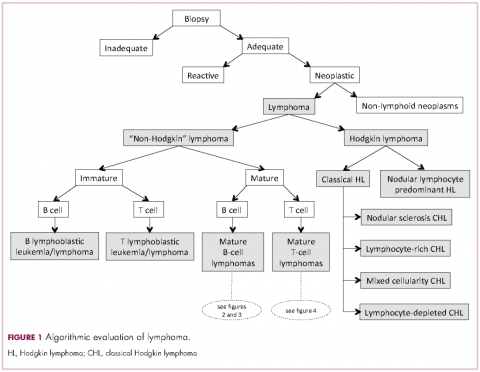
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
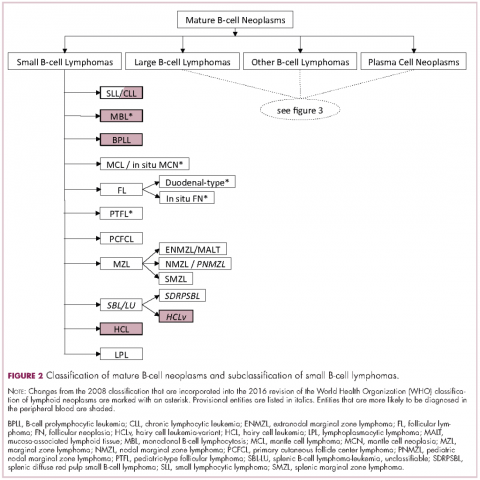
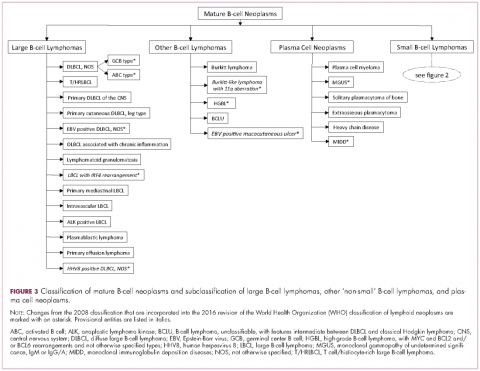
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
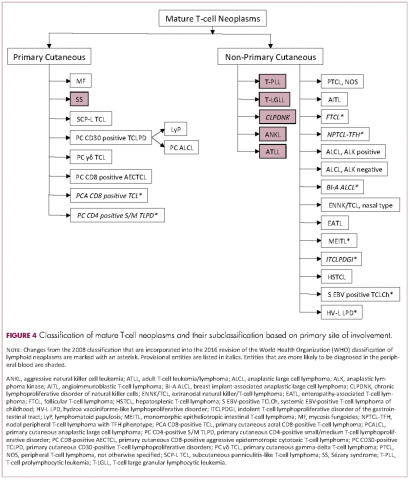
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
Phase 2 study of daratumumab in NHL won’t proceed
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
Therapy can produce durable CRs in NHL
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
When given after low-dose chemotherapy, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy can produce durable complete responses (CRs) in patients with relapsed/refractory non-Hodgkin lymphoma (NHL), according to research published in the Journal of Clinical Oncology.
In this phase 1 study, the overall response rate was 73%, and 50% of patients had an ongoing CR at last follow-up.
Fifty-five percent of patients experienced grade 3/4 neurologic toxicities, though these events eventually resolved.
This research was conducted under a cooperative research and development agreement between the National Cancer Institute and Kite Pharma, Inc.
Kite is developing the CAR T-cell therapy axicabtagene ciloleucel (formerly known as KTE-C19), and the therapy tested in this trial has the same CAR construct as axicabtagene ciloleucel.
Results from this study (NCT00924326) were previously published in the Journal of Clinical Oncology in 2014.
The current report included 22 patients with relapsed/refractory NHL. Seventeen patients had diffuse large B-cell lymphoma (DLBCL), 2 had primary mediastinal B-cell lymphoma (PMBCL), 2 had follicular lymphoma (FL), and 1 had mantle cell lymphoma (MCL).
Patients received a single dose of CAR T cells 2 days after a low-dose chemotherapy conditioning regimen consisting of cyclophosphamide and fludarabine.
Response
The overall response rate was 73% (16/22), with a CR rate of 55% (n=12) and a partial response (PR) rate of 18% (n=4).
Among patients with DLBCL, there were 9 CRs, 4 PRs, 1 patient with stable disease, and 3 patients with progressive disease.
Both FL patients achieved a CR, as did the patient with MCL. One patient with PMBCL had stable disease, and the other progressed.
Eleven of the 12 CRs are ongoing, with durations ranging from more than 7 months to more than 24 months. The median duration of CR is 12.5 months.
The researchers found that serum IL-15 levels and CAR T-cell expansion correlated with treatment response (CR or PR).
The median peak blood CAR+ cell level was 98/μL in patients who achieved a response and 15/μL in those who did not (P=0.027).
High serum IL-15 levels were significantly associated with high peak blood CAR+ cell levels (P=0.001) and response (P<0.001).
Toxicity
Fifty-five percent of patients had grade 3 or 4 neurologic toxicities, the most common of which were dysphasia (n=9) and confusion (n=8).
The researchers said all acute toxicities resolved completely, and none of the patients died as a result of toxicity.
One patient experienced vision loss 3 months after receiving CAR T-cell therapy. The researchers said they could not confirm the cause of the vision loss, but it is consistent with fludarabine toxicity.
One patient developed myelodysplastic syndrome, which was thought to be related to prior therapy.
The researchers noted that patients who experienced grade 3/4 neurologic toxicity had significantly higher levels of blood CAR+ cells than patients who had neurologic toxicities of a lower grade (P=0.003).
In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3/4 neurologic toxicities (P=0.006 and 0.014, respectively). ![]()
Pathogenesis of breast-implant-associated ALCL
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
SAN FRANCISCO—A small study suggests an abnormal immune response characterized by the production of interleukin-13 (IL-13) underlies the pathogenesis of breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
The immune response appears to be directed toward bacterial antigens on the surface of the breast implants.
Marshall E. Kadin, MD, of Roger Williams Medical Center in Providence, Rhode Island, presented these findings at the 9th Annual T-cell Lymphoma Forum.
Dr Kadin noted that BIA-ALCL is a rare type of CD30+ T-cell ALCL that has been reported in more than 200 women worldwide.
Although a cause-and-effect relationship between breast implants and BIA-ALCL has been suggested, the underlying pathogenesis of this malignancy is unclear.
A bacterial biofilm containing gram-negative bacilli has been detected in breast implants from patients with BIA-ALCL.
Therefore, Dr Kadin and his colleagues hypothesized that an immune response toward the bacterial antigens may mediate the pathogenesis of BIA-ALCL.
The researchers studied 13 clinical samples of breast implant capsules and regional lymph nodes from 4 patients with BIA-ALCL, 7 patients with systemic ALCL, and 1 patient with peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS).
Immunohistochemistry was used to determine the presence of IL-13, IL-4, GATA3, and immunoglobulin E (IgE) in these samples.
All clinical samples of anaplastic cells from breast implant capsules tested positive for the presence of IL-13 (13/13). GATA3 was expressed in most anaplastic cell samples (12/13), and IL-4 expression was found in some anaplastic cell samples (6/13). IL-13 and GATA3 expression were observed in some intra-capsular small lymphocytes.
While IL-13 was also detected in BIA-ALCL cell lines, it was not found in 4 of the 7 systemic ALCL cases or the PTCL-NOS case.
Dr Kadin said the lack of IL-13 receptor expression in BIA-ALCL cell lines suggests that IL-13 is not an autocrine growth factor for BIA-ALCL, and its expression is most likely associated with an allergic immune response.
IL-13 is known to induce immunoglobulin class switching in plasma cells to produce IgE. H&E and Giemsa staining of the BIA-ALCL tumor tissue and involved regional lymph nodes revealed IgE and eosinophils on the surface of mast cells and follicular dendritic cells.
Taken together, these data point to an allergic reaction in breast implant capsules of BIA-ALCL.
Dr Kadin was hopeful that these findings could be extrapolated to prevent BIA-ALCL by identifying individuals at higher risk for developing the disease.
The next step for this research is to decipher the role of bacterial antigens in mediating the immune response and whether women who develop BIA-ALCL have a significant increase in other atopic conditions. ![]()
CAR designers report high B-cell cancer response rates
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
ORLANDO – Patients with advanced hematologic malignancies of B-cell lineage had robust immune responses following infusion of a chimeric antigen receptor (CAR)–T-cell construct designed to deliver a specific balance of antigens, investigators reported.
Adults with relapsed or refractory B-lineage acute myeloid leukemia (ALL), non–Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL) who received a CAR-T cell construct consisting of autologous CD4-positive and CD-8-positive T cells that were transduced separately, recombined, and then delivered in a single infusion had comparatively high overall response and complete response rates, reported Cameron Turtle, MBBS, PhD, from the Fred Hutchinson Cancer Research Center in Seattle.
“We know that patients have a highly variable CD4 to CD8 ratio, so by actually controlling this and separately transducing, expanding, and then reformulating in this defined composition, we’re able to eliminate one source of variability in CAR-T cell products,” Dr. Turtle said at the ASCO-SITC Clinical Immuno-Oncology Symposium.
In preclinical studies, an even balance of CD4-positive and CD8-positive central memory T cells or naive T cells evoked more potent immune responses against B-cell malignancies in mice than CD19-positive cells, he explained
To see whether this would also hold true in humans, the investigators enrolled into a phase I/II trial adults with relapsed/refractory B-cell malignancies, including ALL (36 patients), NHL (41), and CLL (24). No patients were excluded on the basis of either absolute lymphocyte, circulating tumors cells, history of stem cell transplant, or results of in vitro test expansions.
All patients underwent leukapheresis for harvesting of T-cells, and populations of CD4- and CD8-positive cells were separated and transduced with a lentiviral vector to express a CD19 CAR and a truncated human epidermal growth factor receptor that allowed tracing of the transduced cells via flow cytometry. The patients underwent lymphodepleting chemotherapy with cyclophosphamide (for the earliest patients), or cyclophosphamide plus fludarabine. Fifteen days after leukapheresis, the separated, transduced, and expanded cells were combined and delivered back to patients in a single infusion at one of three dose levels: 2 x 105, 2 x 106, or 2 x 107 CAR-T cells/kg.
ALL results
Two of the 36 patients with ALL died from complications of the CAR-T cell infusion process prior to evaluation. The 34 remaining patients all had morphologic bone marrow complete responses (CR). Of this group, 32 also had bone marrow CR on flow cytometry.
Using immunoglobulin H (IgH) deep sequencing in a subset of 20 patients 3 weeks after CAR-T cell infusion, the investigators could not detect the malignant IgH index clone in 13 of the patients, and found fewer than 10 copies in the bone marrow of 5 patients.
Six of seven patients with extramedullary disease at baseline had a complete response. The remaining patient in this group had an equivocal PET scan result, and experienced a relapse 2 months after assessment.
The investigators also determined that the lymphodepletion regimen may affect overall results, based on the finding that 10 of 12 patients who received cyclophosphamide alone achieved a CR, but seven of these 10 patients had a relapse within a few months. Of these seven patients. five received a second T-cell infusion, but none had significant T-cell expansion. The investigators traced the failure of the second attempt to a CD8-mediated transgene immune response to a murine single-chain variable fragment used in the construct.
For subsequent patients, they altered the lymphodepletion regimen to include fludarabine to prevent priming of the anti-CAR transgenic immune response. This modification resulted in improved progression-free survival and overall survival for subsequent patients receiving a second infusion, Dr. Turtle said.
NHL results
Of the 41 patients with NHL, 30 (73%) had aggressive histologies, including diffuse large B-cell lymphoma, primary mediastinal large B-cell lymphoma, T-cell/histiocyte-rich large B-cell, and Burkitt lymphomas, and 11 (27%) had indolent histologies, including mantle cell and follicular lymphomas. Most of the patients had received multiple prior lines of therapy, and 19 (46%) had undergone either an autologous or allogeneic stem cell transplant.
Of the 39 evaluable patients who completed therapy, the overall response rate was 67%, including 13 (39%) with CR. Dr. Turtle noted that the CR rate was substantially higher among patients who received cyclophosphamide and fludarabine lymphodepletion, compared with cyclophosphamide alone.
There were also a few responses, including two CRs, among patients with indolent histologies, he said.
CLL, safety results
All 24 patients with CLL had previously received ibrutinib (Imbruvica). Of this group, 19 either had no significant responses to the drug, inactivating mutations, or intolerable toxicities. All but 1 of the 24 patients also had high-risk cytogenetics.
Of the 16 ibrutinib-refractory patients who were evaluable for restaging, 14 had no evidence of disease in bone marrow by flow cytometry at 4 weeks. The overall response rate in this group was 69%, which included four CRs.
Among a majority of all patients, toxicity with the CAR-T cell therapy was mild to moderate. Early cytokine changes appeared to be predictive of serious adverse events such as the cytokine release syndrome, a finding that may allow clinicians to intervene early to prevent complications, Dr. Turtle said.
In the CAR-T cell therapy, “multiple things affect the response and toxicity, including CAR T-cell dose, disease burden, the anti-CAR transgene immune response and the lymphodepletion regimen, not to mention other patient factors that we’re still sorting out,” he commented.
The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.
AT THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point: A defined CAR-T cell construct was associated with high response rates in patients with B-cell malignancies.
Major finding: The overall response rate among patients with ibrutinib-refractory chronic lymphocytic leukemia was 69%, including four complete responses.
Data source: Phase I/II dose-finding, safety and efficacy study in patients with B-lineage hematologic malignancies
Disclosures: The trial was funded by the National Institutes of Health, Life Science Development Fund, Juno Therapeutics and the Bezos Family Foundation. Dr. Turtle disclosed consultancy, honoraria, and/or research funding from Juno Therapeutics and Seattle Genetics.