User login
Smoking-cessation interest and success vary by race, ethnicity
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).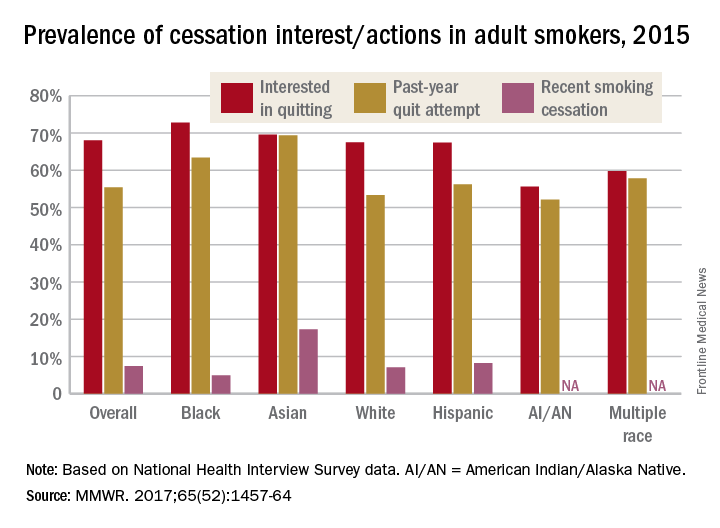
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Just over 55% of adult cigarette smokers made an attempt to quit in the past year, and 7.4% said that they recently quit, according to investigators from the Centers or Disease Control and Prevention.
Data from the 2015 National Health Interview Survey (NHIS) show that 68% of cigarette smokers were interested in quitting, with considerable variation seen according to race and ethnicity (MMWR. 2017;65[52]:1457-64).
American Indian/Alaska Native smokers were the least likely to be interested in quitting (55.6%) and to have attempted to quit (52.1%), but the sample size was too small to report a reliable quit rate. The amount of survey participants of multiple races was also too small to report a reliable quit rate. Among that group, 59.8% were interested in quitting and 57.8% had attempted to quit in the past year, the NHIS data showed.
The sizes of surveyed populations for individual races and ethnicities were not reported, but the total sample size for the 2015 NHIS was 33,672.
Most cigarette smokers attempt to quit without evidence-based techniques
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).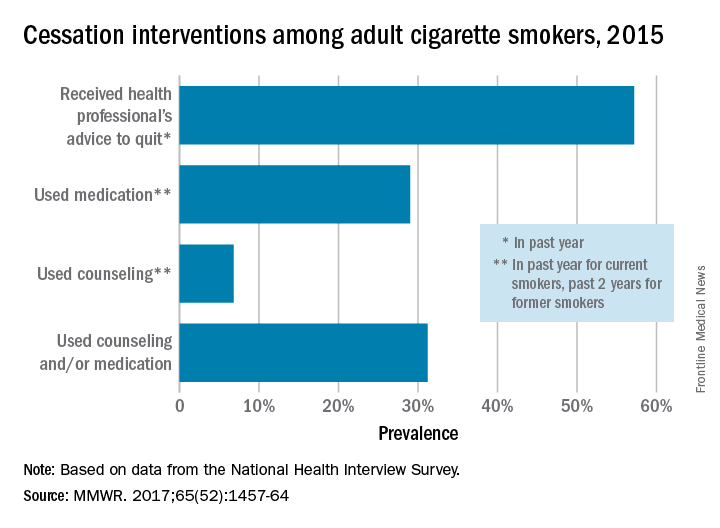
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
More than half of cigarette smokers have received advice to quit from a health care professional, but less than a third used medication or counseling in their cessation attempt, according to investigators from the Centers for Disease Control and Prevention.
In 2015, just over 57% of adult smokers said that a health care professional had advised them to quit in the past year. Of those who tried to quit, 29% used medication such as nicotine patches or gum, varenicline, or bupropion; 7% used counseling (including a stop-smoking clinic, class, or support group and a telephone help line); and 31% used counseling and/or medication, the investigators reported (MMWR 2017;65[52]:1457-64).
With the overall cessation rate at less than 10%, “it is critical for health care providers to consistently identify smokers, advise them to quit, and offer evidence-based cessation treatments, and for insurers to cover and promote the use of these treatments and remove barriers to accessing them,” the investigators wrote.
FROM MMWR
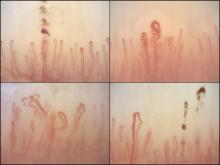
Nailfold analysis can predict cardiopulmonary complications in systemic sclerosis
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: Across the major autoantibody subtypes seen in an SSc cohort, NVC pattern showed a stable association with presence of interstitial lung disease (OR, 1.3-1.4) or elevated systolic pulmonary artery pressure (OR, 2.2-2.4).
Data source: A cross-section of 287 patients in a Dutch SSc cohort.
Disclosures: The study was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
In NSCLC, delayed chemo yields survival benefit comparable to early chemo
Patients with non–small-cell lung cancer (NSCLC) for whom adjuvant chemotherapy must be delayed for as long as 18 weeks have mortality outcomes that are no worse than those of patients who start chemotherapy soon after surgery, and those who undergo delayed chemotherapy have a significantly lower risk for death than patients who have no chemotherapy at all, investigators report.
A retrospective review of data on 12,473 patients with previously untreated NSCLC showed that there were no significant differences in 5-year overall survival (OS) estimates among patients who started multi-agent chemotherapy at 18-38 days postoperatively, from 39 to 56 days after surgery (the reference interval), or from 57 to 127 days after surgery, reported Daniel J. Boffa, MD, of Yale University, New Haven, Conn., and his colleagues.
In addition, when they used propensity score matching to pair patients who received chemotherapy with patients who did not undergo chemotherapy, they found that even late chemotherapy was associated with a significantly lower risk for death.
“Clinicians should still consider chemotherapy in appropriately selected patients that are healthy enough to tolerate it, up to 4 months after NSCLC resection. Further study is warranted to confirm these findings,” the investigators concluded (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5829).
In the retrospective review of records from the National Cancer Database, the investigators limited the study to patients for whom chemotherapy is typically prescribed: those with lymph node metastases, tumors 4 cm or larger, and/or local extension of disease. They looked at the association between the time to initiation of adjuvant chemotherapy and survival using Cox modeling with restricted cubic splines, a validated statistical method for evaluating links between survival and independent variables.
Dr. Boffa and his associates found that the unadjusted Kaplan-Meier 5-year OS estimates did not differ between the groups, at 53% for the early chemotherapy group (hazard ratio [HR] vs. the reference group, 1.009, P = .79), 55% for the reference group, and 53% for the later chemotherapy group (HR 1.037, P = .27).
Comparing adjuvant chemotherapy timing on the efficacy of surgery alone in patients matched by tumor stage and other features, the researchers found that chemotherapy started during any of the three intervals was associated with an approximately 34% reduction in risk of death compared with no chemotherapy (HR for the respective time intervals 0.672, 0.645, and 0.664; P less than .001 for each comparison).
The study helps to clarify for clinicians the benefits of adjuvant chemotherapy in select patients with NSCLC in a real-world setting, Howard (Jack) West, MD, of the Swedish Cancer Institute, Seattle, said in an accompanying editorial (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5798).
“While retrospective data cannot define the benefit of delayed adjuvant chemotherapy with the clarity of a prospective randomized trial, we must remember that in the land of the blind, the one-eyed man is king; these limited data inject an evidence-based answer for a very common clinical question for which we have been forced by necessity to rely only on our best judgments,” he wrote.
The study was internally supported. The authors and Dr. West reported no conflict of interest disclosures.
Patients with non–small-cell lung cancer (NSCLC) for whom adjuvant chemotherapy must be delayed for as long as 18 weeks have mortality outcomes that are no worse than those of patients who start chemotherapy soon after surgery, and those who undergo delayed chemotherapy have a significantly lower risk for death than patients who have no chemotherapy at all, investigators report.
A retrospective review of data on 12,473 patients with previously untreated NSCLC showed that there were no significant differences in 5-year overall survival (OS) estimates among patients who started multi-agent chemotherapy at 18-38 days postoperatively, from 39 to 56 days after surgery (the reference interval), or from 57 to 127 days after surgery, reported Daniel J. Boffa, MD, of Yale University, New Haven, Conn., and his colleagues.
In addition, when they used propensity score matching to pair patients who received chemotherapy with patients who did not undergo chemotherapy, they found that even late chemotherapy was associated with a significantly lower risk for death.
“Clinicians should still consider chemotherapy in appropriately selected patients that are healthy enough to tolerate it, up to 4 months after NSCLC resection. Further study is warranted to confirm these findings,” the investigators concluded (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5829).
In the retrospective review of records from the National Cancer Database, the investigators limited the study to patients for whom chemotherapy is typically prescribed: those with lymph node metastases, tumors 4 cm or larger, and/or local extension of disease. They looked at the association between the time to initiation of adjuvant chemotherapy and survival using Cox modeling with restricted cubic splines, a validated statistical method for evaluating links between survival and independent variables.
Dr. Boffa and his associates found that the unadjusted Kaplan-Meier 5-year OS estimates did not differ between the groups, at 53% for the early chemotherapy group (hazard ratio [HR] vs. the reference group, 1.009, P = .79), 55% for the reference group, and 53% for the later chemotherapy group (HR 1.037, P = .27).
Comparing adjuvant chemotherapy timing on the efficacy of surgery alone in patients matched by tumor stage and other features, the researchers found that chemotherapy started during any of the three intervals was associated with an approximately 34% reduction in risk of death compared with no chemotherapy (HR for the respective time intervals 0.672, 0.645, and 0.664; P less than .001 for each comparison).
The study helps to clarify for clinicians the benefits of adjuvant chemotherapy in select patients with NSCLC in a real-world setting, Howard (Jack) West, MD, of the Swedish Cancer Institute, Seattle, said in an accompanying editorial (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5798).
“While retrospective data cannot define the benefit of delayed adjuvant chemotherapy with the clarity of a prospective randomized trial, we must remember that in the land of the blind, the one-eyed man is king; these limited data inject an evidence-based answer for a very common clinical question for which we have been forced by necessity to rely only on our best judgments,” he wrote.
The study was internally supported. The authors and Dr. West reported no conflict of interest disclosures.
Patients with non–small-cell lung cancer (NSCLC) for whom adjuvant chemotherapy must be delayed for as long as 18 weeks have mortality outcomes that are no worse than those of patients who start chemotherapy soon after surgery, and those who undergo delayed chemotherapy have a significantly lower risk for death than patients who have no chemotherapy at all, investigators report.
A retrospective review of data on 12,473 patients with previously untreated NSCLC showed that there were no significant differences in 5-year overall survival (OS) estimates among patients who started multi-agent chemotherapy at 18-38 days postoperatively, from 39 to 56 days after surgery (the reference interval), or from 57 to 127 days after surgery, reported Daniel J. Boffa, MD, of Yale University, New Haven, Conn., and his colleagues.
In addition, when they used propensity score matching to pair patients who received chemotherapy with patients who did not undergo chemotherapy, they found that even late chemotherapy was associated with a significantly lower risk for death.
“Clinicians should still consider chemotherapy in appropriately selected patients that are healthy enough to tolerate it, up to 4 months after NSCLC resection. Further study is warranted to confirm these findings,” the investigators concluded (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5829).
In the retrospective review of records from the National Cancer Database, the investigators limited the study to patients for whom chemotherapy is typically prescribed: those with lymph node metastases, tumors 4 cm or larger, and/or local extension of disease. They looked at the association between the time to initiation of adjuvant chemotherapy and survival using Cox modeling with restricted cubic splines, a validated statistical method for evaluating links between survival and independent variables.
Dr. Boffa and his associates found that the unadjusted Kaplan-Meier 5-year OS estimates did not differ between the groups, at 53% for the early chemotherapy group (hazard ratio [HR] vs. the reference group, 1.009, P = .79), 55% for the reference group, and 53% for the later chemotherapy group (HR 1.037, P = .27).
Comparing adjuvant chemotherapy timing on the efficacy of surgery alone in patients matched by tumor stage and other features, the researchers found that chemotherapy started during any of the three intervals was associated with an approximately 34% reduction in risk of death compared with no chemotherapy (HR for the respective time intervals 0.672, 0.645, and 0.664; P less than .001 for each comparison).
The study helps to clarify for clinicians the benefits of adjuvant chemotherapy in select patients with NSCLC in a real-world setting, Howard (Jack) West, MD, of the Swedish Cancer Institute, Seattle, said in an accompanying editorial (JAMA Oncol. 2017 Jan. 5 doi: 10.1001/jamaoncol.2016.5798).
“While retrospective data cannot define the benefit of delayed adjuvant chemotherapy with the clarity of a prospective randomized trial, we must remember that in the land of the blind, the one-eyed man is king; these limited data inject an evidence-based answer for a very common clinical question for which we have been forced by necessity to rely only on our best judgments,” he wrote.
The study was internally supported. The authors and Dr. West reported no conflict of interest disclosures.
FROM JAMA ONCOLOGY
Key clinical point: Chemotherapy delayed for up to 18 weeks after surgery offers survival benefits comparable to those of earlier chemotherapy in non–small-cell lung cancer.
Major finding: There were no statistical differences in 5-year survival of patients with NSCLC started on chemotherapy either 18-38, 39-56, or 57-127 days after surgery.
Data source: Retrospective observational study of 12,473 patients with untreated NSCLC in the National Cancer Database.
Disclosures: The study was internally supported. The authors and Dr. West reported no conflict of interest disclosures.
Confirmation CT prevents unnecessary pulmonary nodule bronchoscopy
It’s probably a good idea to do a repeat CT the morning of a scheduled bronchoscopy to make sure the pulmonary nodule is still there, according to investigators from Johns Hopkins University, Baltimore.
From Jan. 2015 to June 2016, 116 patients there were scheduled for navigational bronchoscopy to diagnose pulmonary lesions found on screening CTs. Eight (6.9%) – four men, four women, with an average age of 50 years – had a decrease in size or resolution of their lesion on confirmatory CT, leading to cancellations of their procedure. The number needed to screen to prevent one unnecessary procedure was 15. For canceled cases, the average time from screening CT to scheduled bronchoscopy was 53 days; for patients who underwent a bronchoscopy, it was 50 days (Ann Am Thorac Soc. 2016 Dec;13[12]:2223-8).
It can take months to schedule a bronchoscopy after a pulmonary nodule is found on CT screening. Once in a while, the investigators and others have found, even suspicious nodules resolve on their own, and patients end up having a bronchoscopy they don’t need.
“If there is a significant delay from the initial imaging, practitioners should consider repeat studies before proceeding with the scheduled procedure ... Same-day imaging may decrease unnecessary procedural risk ... The optimal time that should be allowed to pass is difficult to ascertain,” said investigators led by Roy Semaan, MD, of the division of pulmonary and critical care medicine at Hopkins.
The team used a newer version of electromagnetic navigation bronchoscopy (Veran Medical Technologies, St. Louis), which requires expiratory and inspiratory CTs the morning of the procedure so software can build a virtual airway model to localize the nodule.
In addition to nodule resolution, same-day CTs might identify disease progression that alters the diagnostic plan of care.
“The most obvious risk associated with repeat CT imaging is the increased radiation exposure to the patient. Patients in our study who received inspiratory and expiratory CT scans ... had a mean exposure of 9.485 mSv, which is not “negligible, but one-time doses at this range are generally considered to be low risk for contributing to the future development of a malignancy,” the team said.
The extra cost of a same-day noncontrast chest CT – about $300, the authors said – is more than offset if it cancels “an unnecessary procedure with its associated risks,” they said.
Dr. Semaan had no disclosures. Three investigators reported grants and personal fees from Veran.
It’s probably a good idea to do a repeat CT the morning of a scheduled bronchoscopy to make sure the pulmonary nodule is still there, according to investigators from Johns Hopkins University, Baltimore.
From Jan. 2015 to June 2016, 116 patients there were scheduled for navigational bronchoscopy to diagnose pulmonary lesions found on screening CTs. Eight (6.9%) – four men, four women, with an average age of 50 years – had a decrease in size or resolution of their lesion on confirmatory CT, leading to cancellations of their procedure. The number needed to screen to prevent one unnecessary procedure was 15. For canceled cases, the average time from screening CT to scheduled bronchoscopy was 53 days; for patients who underwent a bronchoscopy, it was 50 days (Ann Am Thorac Soc. 2016 Dec;13[12]:2223-8).
It can take months to schedule a bronchoscopy after a pulmonary nodule is found on CT screening. Once in a while, the investigators and others have found, even suspicious nodules resolve on their own, and patients end up having a bronchoscopy they don’t need.
“If there is a significant delay from the initial imaging, practitioners should consider repeat studies before proceeding with the scheduled procedure ... Same-day imaging may decrease unnecessary procedural risk ... The optimal time that should be allowed to pass is difficult to ascertain,” said investigators led by Roy Semaan, MD, of the division of pulmonary and critical care medicine at Hopkins.
The team used a newer version of electromagnetic navigation bronchoscopy (Veran Medical Technologies, St. Louis), which requires expiratory and inspiratory CTs the morning of the procedure so software can build a virtual airway model to localize the nodule.
In addition to nodule resolution, same-day CTs might identify disease progression that alters the diagnostic plan of care.
“The most obvious risk associated with repeat CT imaging is the increased radiation exposure to the patient. Patients in our study who received inspiratory and expiratory CT scans ... had a mean exposure of 9.485 mSv, which is not “negligible, but one-time doses at this range are generally considered to be low risk for contributing to the future development of a malignancy,” the team said.
The extra cost of a same-day noncontrast chest CT – about $300, the authors said – is more than offset if it cancels “an unnecessary procedure with its associated risks,” they said.
Dr. Semaan had no disclosures. Three investigators reported grants and personal fees from Veran.
It’s probably a good idea to do a repeat CT the morning of a scheduled bronchoscopy to make sure the pulmonary nodule is still there, according to investigators from Johns Hopkins University, Baltimore.
From Jan. 2015 to June 2016, 116 patients there were scheduled for navigational bronchoscopy to diagnose pulmonary lesions found on screening CTs. Eight (6.9%) – four men, four women, with an average age of 50 years – had a decrease in size or resolution of their lesion on confirmatory CT, leading to cancellations of their procedure. The number needed to screen to prevent one unnecessary procedure was 15. For canceled cases, the average time from screening CT to scheduled bronchoscopy was 53 days; for patients who underwent a bronchoscopy, it was 50 days (Ann Am Thorac Soc. 2016 Dec;13[12]:2223-8).
It can take months to schedule a bronchoscopy after a pulmonary nodule is found on CT screening. Once in a while, the investigators and others have found, even suspicious nodules resolve on their own, and patients end up having a bronchoscopy they don’t need.
“If there is a significant delay from the initial imaging, practitioners should consider repeat studies before proceeding with the scheduled procedure ... Same-day imaging may decrease unnecessary procedural risk ... The optimal time that should be allowed to pass is difficult to ascertain,” said investigators led by Roy Semaan, MD, of the division of pulmonary and critical care medicine at Hopkins.
The team used a newer version of electromagnetic navigation bronchoscopy (Veran Medical Technologies, St. Louis), which requires expiratory and inspiratory CTs the morning of the procedure so software can build a virtual airway model to localize the nodule.
In addition to nodule resolution, same-day CTs might identify disease progression that alters the diagnostic plan of care.
“The most obvious risk associated with repeat CT imaging is the increased radiation exposure to the patient. Patients in our study who received inspiratory and expiratory CT scans ... had a mean exposure of 9.485 mSv, which is not “negligible, but one-time doses at this range are generally considered to be low risk for contributing to the future development of a malignancy,” the team said.
The extra cost of a same-day noncontrast chest CT – about $300, the authors said – is more than offset if it cancels “an unnecessary procedure with its associated risks,” they said.
Dr. Semaan had no disclosures. Three investigators reported grants and personal fees from Veran.
Key clinical point:
Major finding: Of 116 patients, eight (6.9%) – four men, four women, average age 50 years – had a decrease in size or resolution of their lesion on confirmatory CT, leading to cancellation of their procedure.
Data source: Prospective series from Johns Hopkins University.
Disclosures: Three investigators reported grants and personal fees from Veran.
FDA eases mental health warnings in smoking cessation drugs’ labels
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Labels on two smoking cessation treatments will offer less severe warnings for mental health risk potentials in people with no history of psychiatric disorders, the Food and Drug Administration has announced.
Varenicline (Chantix) will no longer include a boxed warning for serious mental health side effects. The label for bupropion (Zyban) will still include a boxed warning, but language describing the potential for serious psychiatric adverse events will no longer appear within it. Updates will also be made to both labels to describe side effects on mood, behavior, or thinking.
In addition, varenicline’s label will reflect trial data showing its superior efficacy, compared with oral bupropion or nicotine patch. Although a patient medication guide will still be included with each prescription, the risk evaluation and mitigation strategy that prompted the guide will no longer be in place.
Earlier this year, two FDA advisory committees voted in favor of updating varenicline’s label, based on data from a randomized, controlled trial of more than 8,000 smokers, half of whom had a history of psychiatric disorders.
The trial showed no clinically significant difference in risk of adverse events across the smoking cessation treatments varenicline, bupropion, nicotine patch, or placebo study arms, although the risk was higher in the psychiatric cohorts in each.
Overall, 2% of those without a history of mental illness experienced neuropsychiatric adverse events, compared with between 5% and 7% of those with such a history.
The trial was cosponsored by Pfizer, maker of Chantix, and GlaxoSmithKline, maker of Zyban.
The FDA approved varenicline for smoking cessation in 2006 and approved bupropion, which also is indicated to treat depression and seasonal affective disorder, in 1997. After numerous postmarketing reports of increased incidents of psychiatric disorders occurring in smokers who used either drug, the agency added the boxed warning to each in 2009.
FDA officials advised clinicians to guard against changes in mental health status in smokers using these therapies. However, “the results of the trial confirm that the benefits of stopping smoking outweigh the risks of these medicines,” they noted.
On Twitter @whitneymcknight
Severe postoperative pain following thoracotomy predicts persistent pain months later
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
Patients who suffer from severe pain in the days immediately following an open thoracotomy are significantly more likely to still be experiencing pain from the procedure 6 months later, according to a study published in the Journal of Clinical Anesthesia.
“A recognized cause of persistent postsurgical pain is poorly controlled immediate postoperative pain,” wrote the authors, led by Gopinath Niraj, MD, of the University Hospitals of Leicester (England) NHS Trust. “Open thoracotomy can induce significant pain during the immediate postoperative period. Patients undergoing thoracotomy also have one of the greatest incidences of chronic postoperative pain and disability among all the surgical procedures.”
Dr. Niraj and his coinvestigators conducted an audit on 504 patients who underwent open thoracotomy at a single center between May 2010 and April 2012. The audit consisted of a questionnaire composed of 15 questions, which asked yes/no questions about the existence of and location of postoperative pain, and numerical questions regarding the severity of pain. Scores of 7 or higher on a 10-point scale indicated “severe pain,” according to the investigators (J Clin Anesth. 2017;36:174-7). Subjects were evaluated at 72 hours and at 6 months after the operation.
Of the 504 patients, there were 364 survivors, of which 306 received questionnaires. Of those 306, 133 (43%) reported at least five incidents of severe pain within 72 hours of undergoing the operation. Within this group, 109 (82%) reported feeling some amount of persistent pain 6 months later. Chronic post-thoracotomy pain was considered severe in 10% of those subjects, while 24% reported it as moderate and 48% said it was mild.
A total of 289 of the 306 subjects (95%) received an epidural analgesic in the 72 hours after thoracotomy. In terms of satisfaction with pain management, patients were overall positive; 36.3% rated it “excellent,” 43.8% called it “good,” while only 15.8% said it was “fair” and 3.8% said it was “poor.”
“Our audit has some limitations,” the authors noted. “The retrospective project relied on patient self-report and recall.”
Dr. Niraj and his coauthors did not report any financial conflicts. No funding sources for this study were disclosed.
FROM THE JOURNAL OF CLINICAL ANESTHESIA
Key clinical point:
Major finding: 133 of 306 patients were in severe pain 72 hours after thoracotomy; of these, 109 (82%) still had pain 6 months later.
Data source: Retrospective, single-center study of 504 thoracotomy patients between May 2010 and April 2012.
Disclosures: Authors reported no financial disclosures nor funding source.
Noncancerous disease has a significant impact on lung cancer surgery survival
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
Every surgeon performing lung resection comes across elderly patients who are at a higher risk than usual for a formal lung resection. In this era of screening and the abundant use of CT scans, this is increasingly common. Selection of the optimal treatment approach is often done intuitively, balancing the increased risk of surgery vs. the improved cancer-specific survival and the baseline life expectancy of the patient. This manuscript provides more quantitative estimates of this balance and draws attention, through a competing risks analysis, to the importance of non–cancer-related mortality in elderly patients.
The authors point out that non–cancer-related mortality is more common than cancer-related mortality for up to 2.5 years after surgery in patients greater than 75 years of age. This way of examining a situation is different from the usual emphasis on 30-day (and more recently the 90-day) perioperative mortality. The manuscript significantly adds to the decision-making framework of this increasingly important population and is a useful read for all lung cancer surgeons.
Sai Yendamuri, MD, is an attending surgeon in the department of thoracic surgery, the director, Thoracic Surgery Research Laboratory, and associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is associate medical editor for Thoracic Surgery News.
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
After older patients undergo lung resection for stage I non–small-cell lung cancer, they are actually at greater risk of death from something other than lung cancer for up to 2.5 years, according to researchers at Memorial Sloan Kettering Cancer Center, New York. The findings were published online in the Journal of Clinical Oncology (2016;34: doi: 10.1200/JCO.2016.69.0834).
“As age increases, the risk of competing events increases, such as death from noncancer diseases,” wrote Takashi Eguchi, MD, and coauthors. “In this era of personalized cancer therapy, important to the stratification of individualized treatments is the determination of how both cancer and noncancer risk factors – specifically, comorbidities associated with increasing age – contribute to the risk of death.”
The researchers examined outcomes in three different age groups: younger than 65, 65-74, and 75 and older. The study focused on 2,186 patients with pathologic stage I non–small-cell lung cancer (NSCLC) among a population of 5,371 consecutive patients who had resection for primary lung cancer from 2000 to 2011. Seventy percent of patients in the study group were 65 and older, and 29.2% were 75 and older.
In all age groups, the calculated 5-year cumulative incidence of death (CID) for lung cancer–specific causes exceeded that for noncancer causes, but at significant intervals the 65-and-over groups were more likely to die from the latter. For the overall study group, noncancer-specific causes accounted for a higher CID through 18 months after surgery, when the CID for both cancer and noncancer causes crossed at around 2.9. At 5 years, the overall lung cancer–specific CID was 10.4 vs. 5.3 for noncancer specific causes.
However, in the older age groups, those trends were more pronounced. In those aged 65-74, CID for both causes met at around 3.15 at 18 months (10.7 for lung cancer–specific and 4.9 for noncancer specific at 5 years), whereas for those 75 and older, CID for noncancer causes exceeded that for lung cancer–related causes for 2.5 years, when both were around 6; reaching 13.2 for lung cancer–specific and 9 for noncancer-specific at 5 years.
In the 65-and-younger group, lung cancer– and noncancer-specific CIDs were equal for about 3 months after surgery, when the lung cancer deaths tracked upward and the trends diverged (at 5 years, CID was 7.5 for lung cancer–specific and 1 for noncancer specific).
“We have shown that in patients with stage I NSCLC, the majority of postoperative severe morbidity, 1-year mortality, and 5-year noncancer-specific mortality were attributable to cardiorespiratory diseases,” Dr. Eguchi and colleagues said.
“We have also shown that short-term mortality is primarily attributable to noncancer-specific diseases.” The findings underscore the importance of screening older patients for noncancer-specific diseases that could alter outcomes, the researchers said.
Of the 2,186 stage I NSCLC patients in the study, 167 developed severe morbidities after surgery; 68.3% developed respiratory problems and 18.6% went on to develop cardiovascular problems. Patients who had lobectomy were more likely to develop respiratory problems than were those who had sublobar resection, Dr. Eguchi and coauthors said.
Respiratory and cardiovascular diseases were the most frequent causes of death early after surgery. At 30 days, respiratory disease accounted for 5 deaths and cardiovascular disease 7 of 15 total deaths at 30 days; and at 90 days, 11 and 7, respectively, of 27 overall deaths. Even at 1 year, noncancer issues were the leading cause of death (50%), followed by lung cancer–specific causes (27.8%) and other cancer specific disease (13.3%).
“Noncancer-specific mortality represents a significant competing event for lung cancer–specific mortality, with an increasing impact as age increases,” Dr. Eguchi and coauthors said. “These findings can provide patients with more accurate information on survivorship on the basis of their individual preoperative status and help determine patients’ optimal treatment options.”
The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Risk of non-cancer death after curative resection of stage 1 non–small-call lung cancer (NSCLC) exceeded that of lung-cancer deaths 1.5 to 2.5 years after surgery in older patients.
Major finding: In patients aged 75 and older the risk of non–lung-cancer–related death exceeded the risk of death from lung cancer for 2.5 years after surgery, whereas in patients 65 and younger the risk of non–lung cancer death exceeded that of lung-cancer death for 3 months after surgery.
Data Source: Single-center analysis of 5,371 consecutive patients who had curative lung cancer resection from 2000 to 2011, 2,186 of whom had stage 1 NSCLC.
Disclosures: The study received financial support from coauthor Prasad S. Adusumilli, MD. Dr. Eguchi and Dr. Adusumilli and the other coauthors had no relevant financial relationships to disclose.
VIDEO: Improved QOL an added benefit of pembrolizumab for NSCLC patients
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
This is a very nice analysis using a well-validated group of instruments to assess quality of life. The researchers also achieved a high level of compliance, with 79%-85% of patients completing the quality-of life questionnaire at 15 weeks, when the primary measure of health status occurred.
The mean difference of the weighted global health status score of 7.8 points between the pembrolizumab and chemotherapy patients was a little less than a minimally important difference, but in the context of a randomized, controlled trial this difference probably tells us that there is health status improvement in the pembrolizumab patients. In addition, the individual symptom and function domains showed that in general pembrolizumab performed better than chemotherapy.
Michael Boyer, MD , is professor of medicine at the University of Sydney and a thoracic oncologist and chief clinical officer of the Chris O’Brien Lifehouse in Sydney. He has received research support from Merck and from Pfizer, Roche, Eli Lilly, BMS, AstraZeneca, and Clovis. He made these comments as designated discussant for the report.
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
VIENNA – Patients with metastatic non–small-cell lung cancer with high levels of PD-L1 who received first-line pembrolizumab treatment had clinically meaningful improvement in their quality of life, compared with patients randomized to chemotherapy, in a prespecified secondary analysis of data from the drug’s pivotal trial.
This boost in quality of life as well as other measures of health status add to the pivotal trial’s primary finding of significantly increased progression-free survival compared with chemotherapy, as well as previously-reported secondary findings of superior overall survival, objective response rate, and safety with pembrolizumab compared with chemotherapy (N Engl J Med. 2016 Nov 10;375[19]:1823-33), Julie R. Brahmer, MD, said at the World Conference on Lung Cancer, sponsored by the International Association for the Study of Lung Cancer.
The primary endpoint of the Study of Pembrolizumab Compared to Platinum-Based Chemotherapies in Participants With Metastatic Non–Small Cell Lung Cancer (KEYNOTE-024) showed an average 4.3-month increase in progression-free survival with pembrolizumab immunotherapy, compared with a standard chemotherapy regimen.
Improved quality of life on top of improved efficacy and safety is an important added benefit from pembrolizumab that should further spur its widespread adoption as first-line treatment for approved patients, Dr. Brahmer said in a video interview.
“When you talk about improving efficacy by months, patients and physicians want to also see improved quality of life,” said Dr. Brahmer, director of thoracic oncology at the Johns Hopkins Kimmel Cancer Center in Baltimore. “If symptoms are not improved or there are a ton of side effects with the treatment then use might be low.”
Based on its performance in KEYNOTE-024, pembrolizumab (Keytruda) received Food and Drug Administration approval on Oct. 24, 2016, as first-line treatment for patients with metastatic non–small-cell lung cancer that has a tumor proportion score of at least 50% for programmed death ligand 1 (PD-L1). Pembrolizumab is a monoclonal antibody that binds and blocks PD-1, the immune-cell receptor that tumor-cell PD-L1 binds to make immune cells less active. Other new immune checkpoint inhibitor drugs that act by blocking PD-1 or PD-L1 have shown similar quality of life benefits, she noted.
Routine availability of pembrolizumab as initial treatment for patients who have tumors with this level of PD-L1 expression (and also have no EGFR or ALK genomic aberrations) is shifting practice, Dr. Brahmer said.
“It’s catching on. The limitation right now is making sure patients get tested” for their PD-L1 tumor proportion score at the time they are first diagnosed. “Medical oncologists need to educate pathologists that we need this testing automatically, upfront. It’s not there yet,” she said.
Patients also are enthused. “There is a lot of chemo-exhaustion among patients. They are looking for something different, and something that uses their immune system makes sense.” But only about one quarter of patients have tumors with this level of PD-L1 expression; the others must start chemotherapy first before trying immunotherapy, unless they have an EGFR mutation. Out-of-pocket cost for pembrolizumab is also a major issues for many patients, she said.
KEYNOTE-024 randomized 305 patients at 102 international sites and followed patients for a median of 11 months. Dr. Brahmer and her associates made two primary analyses of patient-reported outcomes. One was measurement of global health status at 15 weeks after the start of treatment using the European Organization for the Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire designed to assess quality of life. Weighted averaging of the EORTC QCQ-C30 scores showed a mean improvement of 7.8 points (P = .002) in the pembrolizumab patients compared with the chemotherapy patients, a difference Dr. Brahmer called “clinically meaningful” as well as statistically significant.
A second analysis of patient-reported outcomes used a second EORTC instrument, the QLC-LC13, which combines assessment of cough, chest pain, and dyspnea. Treatment with pembrolizumab significantly reduced the time to deterioration as measured by this questionnaire by a relative 34%, (P = .029).
A third analysis looked at 15 individual function or symptom domains that make up the QLQ-C30. In general, these showed more improvements with pembrolizumab than with chemotherapy. One notable subcategory was fatigue, which showed significant improvement with pembrolizumab compared with a small worsening with chemotherapy.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
AT WCLC 2016
Key clinical point:
Major finding: The weighted average change from baseline in QLQ-C30 was 7.8 points higher in pembrolizumab patients compared with chemotherapy patients.
Data source: KEYNOTE-024, a multicenter, international randomized trial comprising 305 patients.
Disclosures: Merck, which markets pembrolizumab (Keytruda), sponsored KEYNOTE-024. Dr. Brahmer has served on an advisory board for Merck.
Adding respiratory rate to triage criteria improves accurate staging of chest trauma patients
WASHINGTON – Adding respiratory rate and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr. Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr. Yonge, a surgical fellow at Oregon Health & Science University, Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
• Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
• Level II activations capture moderate to severe injuries; trauma surgeon and respiratory therapist presence is mandated.
• Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the emergency department or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs. 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr. Yonge then looked for clinical measures that would improve triage. Tachypnea (respiratory rate of more than 20 breaths per minute) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal respiratory rate. Tachypnea was significantly associated with a diagnosis of flail chest, emergency department intubation, and chest tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury – tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiologic marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr. Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr. Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing respiratory rate as part of field triage, it often isn’t recorded or is only estimated, Dr. Yonge said. That’s one reason he used the 20-breaths-per-minute cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Neither he nor Dr. Schreiber had any financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Adding respiratory rate and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr. Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr. Yonge, a surgical fellow at Oregon Health & Science University, Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
• Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
• Level II activations capture moderate to severe injuries; trauma surgeon and respiratory therapist presence is mandated.
• Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the emergency department or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs. 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr. Yonge then looked for clinical measures that would improve triage. Tachypnea (respiratory rate of more than 20 breaths per minute) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal respiratory rate. Tachypnea was significantly associated with a diagnosis of flail chest, emergency department intubation, and chest tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury – tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiologic marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr. Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr. Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing respiratory rate as part of field triage, it often isn’t recorded or is only estimated, Dr. Yonge said. That’s one reason he used the 20-breaths-per-minute cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Neither he nor Dr. Schreiber had any financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Adding respiratory rate and suspected blunt chest injury to a trauma assessment in the field significantly improved the appropriate triaging of level III trauma patients.
When the assessment specifically evaluated for tachypnea in the setting of blunt chest injury, undertriaging improved by 1.2%, John Yonge, MD, said at the annual clinical congress of the American College of Surgeons.
“When we applied this new criteria to our 10-year study, we identified 661 patients who should have been activated as a level I or level II,” but instead were assessed as less critically injured, Dr. Yonge said in an interview. This initial misstep significantly extended the time before patients could have critical surgical procedures and was related to higher mortality among them.
Dr. Yonge, a surgical fellow at Oregon Health & Science University, Portland, and his mentor Martin Schreiber, MD, conducted the retrospective study of 7,880 trauma patients admitted at level III activation from 2004 to 2014. The OHSU trauma system has three activation levels.
• Level I activations are reserved for the most critically injured patients; attending trauma surgeon and anesthesiologist presence is mandatory.
• Level II activations capture moderate to severe injuries; trauma surgeon and respiratory therapist presence is mandated.
• Level III activations are designed to capture patients who do not require an immediate lifesaving intervention; the presence of the trauma surgery chief resident and attending emergency medicine physician is mandatory.
Patients were considered undertriaged if they were admitted as level III activations, but then required a critical intervention (chest tube placement, intubation, needle thoracostomy, or intracranial pressure monitoring) in the emergency department or ultimately met level I or II activation criteria.
Among all the level III patients, 466 (6%) were undertriaged: 390 were undertriaged based on the existing level I or II activation criteria, and 76 were considered undertriaged based on the need for a critical intervention.
Most of the undertriaged patients (65%) met criteria for level I activation; the rest should have been triaged as level II patients. Compared with appropriately staged level III patients, mortality among the undertriaged patients was significantly higher (3.2% vs. 0.6%). Undertriaged patients also experienced longer delays before initiation of major emergency surgery: a mean of 147 minutes, compared with 106 minutes for appropriately triaged level I patients and 62 minutes for appropriately triaged level II patients.
Dr. Yonge then looked for clinical measures that would improve triage. Tachypnea (respiratory rate of more than 20 breaths per minute) in the field stood out as a significant factor. Tachypneic patients who had a suspected chest injury were 70% more likely to be undertriaged than were those with a normal respiratory rate. Tachypnea was significantly associated with a diagnosis of flail chest, emergency department intubation, and chest tube placement.
The team then constructed a new triage criterion for patients with suspected chest injury – tachypnea combined with suspected blunt thoracic injury. By applying that model to their study population of level III patients, they determined that the level III undertriage rate would be reduced by 1.2%.
Tying the physiologic marker of tachypnea to a suspected clinical diagnosis is a key factor, Dr. Yonge noted. “Just adding tachypnea doesn’t help us. In fact, it would overwhelm us, because a trauma patient could very well be tachypneic because he’s experiencing panic. But tying it to a suspected clinical diagnosis gives us a meaningful result.”
He confirmed this linkage with an additional analysis. “We looked to see how severely injured these patients were and found that 71% of them had an Abbreviated Injury Score (AIS) to the chest of 3 or more, indicating a severe chest injury. Only 29% had an AIS of 2 or less. So this proves that respiratory rate is a valid triage criterion and can be used to identify patients who need a higher level of trauma care.”
The challenge now, Dr. Yonge said, is incorporating the marker into clinical practice. “It doesn’t matter how many statistics you do, if you can’t educate the prehospital providers in this, it’s useless. They are the crux of the trauma system.”
Although national guidelines do recommend assessing respiratory rate as part of field triage, it often isn’t recorded or is only estimated, Dr. Yonge said. That’s one reason he used the 20-breaths-per-minute cutoff rate. “It doesn’t even take a full minute to assess this, but it can make a big improvement in care.”
Neither he nor Dr. Schreiber had any financial disclosures.
[email protected]
On Twitter @alz_gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Adding a triage assessment criterion of tachypnea plus suspected chest injury improved undertriage of chest trauma patients by 1.2%.
Data source: The retrospective database study comprising 7,880 patients.
Disclosures: Neither Dr. Yonge nor Dr. Schreiber had any financial disclosures.