User login
VIDEO: Identifying preexisting conditions crucial before pneumonectomy, even for benign disease
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When performing pneumonectomy on patients with benign disease, it is important to be aware of specific preexisting conditions that could complicate surgery before bringing patients into the operating room.
“Sometimes the usual, standard operative procedure is not appropriate, given the circumstances of a particular patient, [and] typically, these pneumonectomies for benign disease are very challenging operations because the inflamed lung is usually quite densely adherent to the inside of the chest cavity,” explained Dr. G. Alex Patterson of Washington University in St. Louis.
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting sponsored by the American Association for Thoracic Surgeons, Dr. Patterson talked about the challenges associated with pneumonectomies for benign disease and how surgeons can safely navigate them.
Dr. Patterson had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AATS FOCUS ON THORACIC SURGERY
VIDEO: Complications during thoracoscopic lobectomy are surmountable
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – When it comes to intraoperative complications during thoracoscopic lobectomy, the mantra for success is to always have a preoperative plan, but be flexible enough to improvise should anything out of the norm arise.
“Many surgeons, when they ask [me] about this specific topic, ask what specific tricks [I] have, but I don’t like to use the word ‘trick’ [because] it’s not something we can do that other people can’t,” explained Dr. Thomas A. D’Amico, chief of general thoracic surgery at Duke University in Durham, North Carolina.
“It’s really just about strategy – how you start an operation, what the conduct of it should be, and when you see things that are less common or more difficult cases, how you think about those and manage those.”
In an interview at the Focus on Thoracic Surgery: Technical Challenges and Complications meeting held by the American Association for Thoracic Surgeons, Dr. D’Amico talked about why surgeons around the world are apprehensive about thoracoscopic lobectomy and why it’s important to begin training residents on how to properly perform the procedure as soon as possible, as it helps mitigate uncertainty while giving them valuable experience to solve any issues that may come up during an operation.
Dr. D’Amico disclosed that he is a consultant for Scanlan, but that it is not relevant to this discussion.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AATS FOCUS ON THORACIC SURGERY
Is Skip N2 metastasis its own category?
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
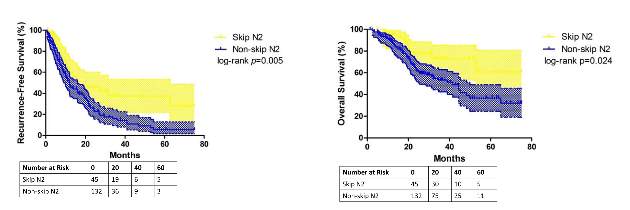
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
Key clinical point: Skip N2 metastases in resectable lung cancer have distinct histological characteristics from non-skip N2 disease.
Major finding: A subset of patients with skip N2 metastasis had higher rates of acinar adenocarcinoma subtype and right-lung disease.
Data source: Retrospective analysis of 177 patients with lung adenocarcinoma and N2 metastasis
Disclosures: The study received funding from the government of China and Shanghai Municipality as well as Shanghai Hospital Development Center. The authors have no relationships to disclose.
Point/Counterpoint: Does surgery play a role in N2 disease treatment following induction therapy?
POINT: Surgery has its uses for some
BY DR. STEPHEN G. SWISHER
When talking about the role of surgery after induction therapy with persistent N2 disease, one must acknowledge that this is such a heterogeneous disease. You can have single-station N2; resectable, bulky multistation N2; and so on. Then there’s unresectable stage IIIA, but let’s focus mainly on resectable stage IIIA disease.
I can’t tell you how many audiences I’ve faced that absolutely believe the myth that surgery plays no role in Stage IIIA non–small cell lung cancer based on data from stage IIIA disease patients randomized to chemoradiation followed by surgery. The problem with these study results is the high mortality in the pneumonectomy subset. There’s no difference in the overall survival of the two groups, but that doesn’t mean that everyone in that group wouldn’t benefit from surgery.
The curve showed that pneumonectomy did not benefit after chemoradiation in a non–high-volume center. You can see a steep drop in the mortality early on, but it catches up again at the end. If you look at the overall 5-year survival rate, even in the pneumonectomy subset, you’re looking at 22% vs. 24%.
But in the lobectomy set, you see something completely different. You’ve got a doubling of survivors and no mortality early on, and a doubling of 5-year survival from 18% to 36%.
And yet, people continue saying that there’s no role for surgery. Well, I think there is a role for surgery, and there are subsets of N2 for which surgery can be particularly beneficial. We have to move more toward what the medical oncologists do, which is personalize therapy and look at subsets of N2 disease so that we know which patients we can benefit and which ones we can’t.
Moving on to the second myth: Surgery plays no role in N2 residual disease after induction therapy. This myth is based on the results of a couple of prospective studies in the 1980s and ’90s that showed residual N2 disease after chemo- and radiotherapy leads to survival of 16-35 months in most cases. I’d say that those results are true, but it’s not to say there aren’t subsets within these populations that benefited. With preoperative chemo and radiation, it’s basically the same thing – poor prognoses in patients with N2 or N3 disease, so the standard becomes never to operate on these individuals.
A European study prospectively took 30 patients and treated them with induction chemotherapy. They saw a 5-year survival rate of 62% if a patient downgrades to lymph node–negative disease and the positron-emission tomographic (PET) findings were good. But they also saw a subset with a small amount of disease within the lymph nodes at the N2 stage and a poor response on PET; Their 5-year survival rate was around 19%. So I’d argue that PET response and the number of lymph nodes involved are the key criteria, and you shouldn’t routinely deny surgery to these patients.
Our experience at MD Anderson Cancer Center over the last 10 years has been to treat N2 and N3 admissions, with surgery, followed by postoperative radiation of 50 Gy. We’re able to achieve very-low morbidity with this regimen, and no mortality after 30 and 90 days. Just to show the heterogeneity: Single-station, microscopic N2 disease should really be resected.
You just can’t lump together everyone with residual N2 after induction therapy, since PET-CTs and most other diagnostic procedures have high false-negative rates. And like I’ve said, it doesn’t matter because N2 disease is really a subset disease. Microscopic N2 disease behaves in a completely different way than does macroscopic, multiple-level N2 disease. And even more important is how the patient’s primary tumor responds to the chemotherapy or chemoradiation; that will tell you how well they’re going to do even if they have a small amount of residual disease in the lymph nodes.
Dr. Swisher is at the University of Texas MD Anderson Cancer Center in Houston. He disclosed that he is a consultant/advisory board member for GlaxoSmithKline.
COUNTERPOINT: Surgery seems to have little value, adds risk.
BY DR. SCOTT J. SWANSON
Dr. Swisher and I probably agree more than we disagree, but I’m going to start by saying that N2 disease is bad, and most of these populations are heterogeneous. But if you feel that a curve toward the bottom of a graph is good, then you should think twice. Anywhere from a 15%-30% survival rate is not great and shows that we have a long way to go. The overall impression among oncologists in several countries is that it’s not really clear whether surgery adds value. Even in very good centers like MD Anderson where there is minimal risk, surgery inherently still involves some risk.
So then, what do we do with persistent N2 disease? I’d say that most of the time, it should be treated with chemotherapy or chemotherapy and radiation. In some cases, N2 disease can be treated with a creative, mutation-driven immunotherapy approach. Most of the time though, surgery is just not a good idea.
Interestingly, about one-third of lung cancer patients present with stage IIIA disease, so it’s important that we as a medical community sort out these treatment options. I think we’re all in agreement that single-station, microscopic, PET-negative/CT-negative disease is not the same as extranodal or multistation PET-positive disease, so we’ve got to begin to substratify N2 disease.
In an intergroup trial of about 200 patients per arm published in the Lancet, patients went to surgery if they didn’t regress after evaluation. The progression-free survival rate did seem to favor surgery; and, if you look at the lobectomy subset, the results are certainly strong. But again, we’re dealing with curves that are pretty low. The pneumonectomy subset drops off and then starts to catch up, but clearly pneumonectomy was a problem in this multicenter trial. The most important graph to this debate shows subjects that persistently had nodal disease had very poor survival. It’s hard to argue for surgery when results show that only about 24% of them are going to be alive down the road. If oncologists across most of the United States say they believe that surgery isn’t a good idea, we’re not going to use this graph to change their minds; we need to change our way of thinking.
So the conclusion to take away from that presentation is that N0 status at surgery significantly predicts greater 5-year survival. So, conversely, surgery is not helpful for the patient with node-positive disease. Surgical resection should only be considered if lobectomy is the operation in question. Pneumonectomy carries risk, and surgery has no beneficial value, compared with chemotherapy unless the patient has been downstaged.
Our experience at Brigham and Women’s Hospital in Boston is similar to Dr. Swisher’s at MD Anderson. During the first 8 years of our thoracic division, we looked at 103 patients who had surgery after induction therapy for N2 disease. The induction plan in those patients was chemotherapy only for 75, radiation only for 18, and chemoradiation for 10. Almost 40% of patients had pneumonectomies, and the rest had lobectomies.
Mortality was 3.9%, major morbidity was 7%, and about 30% were downstaged. Those 5-year survival rates were about 36%. Persistent nodal disease, either N1 or N2, was seen in about 75%, and most of them were N2; the 5-year survival rate there was about 9% with a median of about 15.9 months. Beauty is in the eye of the beholder, so you may look at that and say that 15.9 months isn’t bad, but there’s still a huge subgroup that’s node positive, so here I’d say that pushing surgery is not the best strategy. We also found in this group that adenocarcinomas were much harder to clear.
We’re on very-safe ground to push surgery in the node-negative group, but you’ve got to be careful in the node-positive group.
Survival is relatively limited in N2 disease in general. Surgery may be of value if you downstage the patient, if you’re doing a lobectomy or if you see squamous cell carcinoma. Going forward, we really ought to focus our attention on identifying responders more reliably and improving downstaging – with different or individualized chemotherapy, or perhaps even immunologic therapy.
For the present, we can talk about radiation dosing. High-dose radiation is clearly a viable option for some patients. In addition, we can improve identification of N2 subgroups. Not all N2 disease is the same, so it should not be treated the same across the board.
Dr. Swanson is at Brigham and Women’s Hospital in Boston. He disclosed that he is a consultant/advisory board member for Covidien and Ethicon Endo-Surgery.
This article grew out of the debate by Dr. Swisher and Dr. Swanson at the annual meeting of the Society of Thoracic Surgeons.
POINT: Surgery has its uses for some
BY DR. STEPHEN G. SWISHER
When talking about the role of surgery after induction therapy with persistent N2 disease, one must acknowledge that this is such a heterogeneous disease. You can have single-station N2; resectable, bulky multistation N2; and so on. Then there’s unresectable stage IIIA, but let’s focus mainly on resectable stage IIIA disease.
I can’t tell you how many audiences I’ve faced that absolutely believe the myth that surgery plays no role in Stage IIIA non–small cell lung cancer based on data from stage IIIA disease patients randomized to chemoradiation followed by surgery. The problem with these study results is the high mortality in the pneumonectomy subset. There’s no difference in the overall survival of the two groups, but that doesn’t mean that everyone in that group wouldn’t benefit from surgery.
The curve showed that pneumonectomy did not benefit after chemoradiation in a non–high-volume center. You can see a steep drop in the mortality early on, but it catches up again at the end. If you look at the overall 5-year survival rate, even in the pneumonectomy subset, you’re looking at 22% vs. 24%.
But in the lobectomy set, you see something completely different. You’ve got a doubling of survivors and no mortality early on, and a doubling of 5-year survival from 18% to 36%.
And yet, people continue saying that there’s no role for surgery. Well, I think there is a role for surgery, and there are subsets of N2 for which surgery can be particularly beneficial. We have to move more toward what the medical oncologists do, which is personalize therapy and look at subsets of N2 disease so that we know which patients we can benefit and which ones we can’t.
Moving on to the second myth: Surgery plays no role in N2 residual disease after induction therapy. This myth is based on the results of a couple of prospective studies in the 1980s and ’90s that showed residual N2 disease after chemo- and radiotherapy leads to survival of 16-35 months in most cases. I’d say that those results are true, but it’s not to say there aren’t subsets within these populations that benefited. With preoperative chemo and radiation, it’s basically the same thing – poor prognoses in patients with N2 or N3 disease, so the standard becomes never to operate on these individuals.
A European study prospectively took 30 patients and treated them with induction chemotherapy. They saw a 5-year survival rate of 62% if a patient downgrades to lymph node–negative disease and the positron-emission tomographic (PET) findings were good. But they also saw a subset with a small amount of disease within the lymph nodes at the N2 stage and a poor response on PET; Their 5-year survival rate was around 19%. So I’d argue that PET response and the number of lymph nodes involved are the key criteria, and you shouldn’t routinely deny surgery to these patients.
Our experience at MD Anderson Cancer Center over the last 10 years has been to treat N2 and N3 admissions, with surgery, followed by postoperative radiation of 50 Gy. We’re able to achieve very-low morbidity with this regimen, and no mortality after 30 and 90 days. Just to show the heterogeneity: Single-station, microscopic N2 disease should really be resected.
You just can’t lump together everyone with residual N2 after induction therapy, since PET-CTs and most other diagnostic procedures have high false-negative rates. And like I’ve said, it doesn’t matter because N2 disease is really a subset disease. Microscopic N2 disease behaves in a completely different way than does macroscopic, multiple-level N2 disease. And even more important is how the patient’s primary tumor responds to the chemotherapy or chemoradiation; that will tell you how well they’re going to do even if they have a small amount of residual disease in the lymph nodes.
Dr. Swisher is at the University of Texas MD Anderson Cancer Center in Houston. He disclosed that he is a consultant/advisory board member for GlaxoSmithKline.
COUNTERPOINT: Surgery seems to have little value, adds risk.
BY DR. SCOTT J. SWANSON
Dr. Swisher and I probably agree more than we disagree, but I’m going to start by saying that N2 disease is bad, and most of these populations are heterogeneous. But if you feel that a curve toward the bottom of a graph is good, then you should think twice. Anywhere from a 15%-30% survival rate is not great and shows that we have a long way to go. The overall impression among oncologists in several countries is that it’s not really clear whether surgery adds value. Even in very good centers like MD Anderson where there is minimal risk, surgery inherently still involves some risk.
So then, what do we do with persistent N2 disease? I’d say that most of the time, it should be treated with chemotherapy or chemotherapy and radiation. In some cases, N2 disease can be treated with a creative, mutation-driven immunotherapy approach. Most of the time though, surgery is just not a good idea.
Interestingly, about one-third of lung cancer patients present with stage IIIA disease, so it’s important that we as a medical community sort out these treatment options. I think we’re all in agreement that single-station, microscopic, PET-negative/CT-negative disease is not the same as extranodal or multistation PET-positive disease, so we’ve got to begin to substratify N2 disease.
In an intergroup trial of about 200 patients per arm published in the Lancet, patients went to surgery if they didn’t regress after evaluation. The progression-free survival rate did seem to favor surgery; and, if you look at the lobectomy subset, the results are certainly strong. But again, we’re dealing with curves that are pretty low. The pneumonectomy subset drops off and then starts to catch up, but clearly pneumonectomy was a problem in this multicenter trial. The most important graph to this debate shows subjects that persistently had nodal disease had very poor survival. It’s hard to argue for surgery when results show that only about 24% of them are going to be alive down the road. If oncologists across most of the United States say they believe that surgery isn’t a good idea, we’re not going to use this graph to change their minds; we need to change our way of thinking.
So the conclusion to take away from that presentation is that N0 status at surgery significantly predicts greater 5-year survival. So, conversely, surgery is not helpful for the patient with node-positive disease. Surgical resection should only be considered if lobectomy is the operation in question. Pneumonectomy carries risk, and surgery has no beneficial value, compared with chemotherapy unless the patient has been downstaged.
Our experience at Brigham and Women’s Hospital in Boston is similar to Dr. Swisher’s at MD Anderson. During the first 8 years of our thoracic division, we looked at 103 patients who had surgery after induction therapy for N2 disease. The induction plan in those patients was chemotherapy only for 75, radiation only for 18, and chemoradiation for 10. Almost 40% of patients had pneumonectomies, and the rest had lobectomies.
Mortality was 3.9%, major morbidity was 7%, and about 30% were downstaged. Those 5-year survival rates were about 36%. Persistent nodal disease, either N1 or N2, was seen in about 75%, and most of them were N2; the 5-year survival rate there was about 9% with a median of about 15.9 months. Beauty is in the eye of the beholder, so you may look at that and say that 15.9 months isn’t bad, but there’s still a huge subgroup that’s node positive, so here I’d say that pushing surgery is not the best strategy. We also found in this group that adenocarcinomas were much harder to clear.
We’re on very-safe ground to push surgery in the node-negative group, but you’ve got to be careful in the node-positive group.
Survival is relatively limited in N2 disease in general. Surgery may be of value if you downstage the patient, if you’re doing a lobectomy or if you see squamous cell carcinoma. Going forward, we really ought to focus our attention on identifying responders more reliably and improving downstaging – with different or individualized chemotherapy, or perhaps even immunologic therapy.
For the present, we can talk about radiation dosing. High-dose radiation is clearly a viable option for some patients. In addition, we can improve identification of N2 subgroups. Not all N2 disease is the same, so it should not be treated the same across the board.
Dr. Swanson is at Brigham and Women’s Hospital in Boston. He disclosed that he is a consultant/advisory board member for Covidien and Ethicon Endo-Surgery.
This article grew out of the debate by Dr. Swisher and Dr. Swanson at the annual meeting of the Society of Thoracic Surgeons.
POINT: Surgery has its uses for some
BY DR. STEPHEN G. SWISHER
When talking about the role of surgery after induction therapy with persistent N2 disease, one must acknowledge that this is such a heterogeneous disease. You can have single-station N2; resectable, bulky multistation N2; and so on. Then there’s unresectable stage IIIA, but let’s focus mainly on resectable stage IIIA disease.
I can’t tell you how many audiences I’ve faced that absolutely believe the myth that surgery plays no role in Stage IIIA non–small cell lung cancer based on data from stage IIIA disease patients randomized to chemoradiation followed by surgery. The problem with these study results is the high mortality in the pneumonectomy subset. There’s no difference in the overall survival of the two groups, but that doesn’t mean that everyone in that group wouldn’t benefit from surgery.
The curve showed that pneumonectomy did not benefit after chemoradiation in a non–high-volume center. You can see a steep drop in the mortality early on, but it catches up again at the end. If you look at the overall 5-year survival rate, even in the pneumonectomy subset, you’re looking at 22% vs. 24%.
But in the lobectomy set, you see something completely different. You’ve got a doubling of survivors and no mortality early on, and a doubling of 5-year survival from 18% to 36%.
And yet, people continue saying that there’s no role for surgery. Well, I think there is a role for surgery, and there are subsets of N2 for which surgery can be particularly beneficial. We have to move more toward what the medical oncologists do, which is personalize therapy and look at subsets of N2 disease so that we know which patients we can benefit and which ones we can’t.
Moving on to the second myth: Surgery plays no role in N2 residual disease after induction therapy. This myth is based on the results of a couple of prospective studies in the 1980s and ’90s that showed residual N2 disease after chemo- and radiotherapy leads to survival of 16-35 months in most cases. I’d say that those results are true, but it’s not to say there aren’t subsets within these populations that benefited. With preoperative chemo and radiation, it’s basically the same thing – poor prognoses in patients with N2 or N3 disease, so the standard becomes never to operate on these individuals.
A European study prospectively took 30 patients and treated them with induction chemotherapy. They saw a 5-year survival rate of 62% if a patient downgrades to lymph node–negative disease and the positron-emission tomographic (PET) findings were good. But they also saw a subset with a small amount of disease within the lymph nodes at the N2 stage and a poor response on PET; Their 5-year survival rate was around 19%. So I’d argue that PET response and the number of lymph nodes involved are the key criteria, and you shouldn’t routinely deny surgery to these patients.
Our experience at MD Anderson Cancer Center over the last 10 years has been to treat N2 and N3 admissions, with surgery, followed by postoperative radiation of 50 Gy. We’re able to achieve very-low morbidity with this regimen, and no mortality after 30 and 90 days. Just to show the heterogeneity: Single-station, microscopic N2 disease should really be resected.
You just can’t lump together everyone with residual N2 after induction therapy, since PET-CTs and most other diagnostic procedures have high false-negative rates. And like I’ve said, it doesn’t matter because N2 disease is really a subset disease. Microscopic N2 disease behaves in a completely different way than does macroscopic, multiple-level N2 disease. And even more important is how the patient’s primary tumor responds to the chemotherapy or chemoradiation; that will tell you how well they’re going to do even if they have a small amount of residual disease in the lymph nodes.
Dr. Swisher is at the University of Texas MD Anderson Cancer Center in Houston. He disclosed that he is a consultant/advisory board member for GlaxoSmithKline.
COUNTERPOINT: Surgery seems to have little value, adds risk.
BY DR. SCOTT J. SWANSON
Dr. Swisher and I probably agree more than we disagree, but I’m going to start by saying that N2 disease is bad, and most of these populations are heterogeneous. But if you feel that a curve toward the bottom of a graph is good, then you should think twice. Anywhere from a 15%-30% survival rate is not great and shows that we have a long way to go. The overall impression among oncologists in several countries is that it’s not really clear whether surgery adds value. Even in very good centers like MD Anderson where there is minimal risk, surgery inherently still involves some risk.
So then, what do we do with persistent N2 disease? I’d say that most of the time, it should be treated with chemotherapy or chemotherapy and radiation. In some cases, N2 disease can be treated with a creative, mutation-driven immunotherapy approach. Most of the time though, surgery is just not a good idea.
Interestingly, about one-third of lung cancer patients present with stage IIIA disease, so it’s important that we as a medical community sort out these treatment options. I think we’re all in agreement that single-station, microscopic, PET-negative/CT-negative disease is not the same as extranodal or multistation PET-positive disease, so we’ve got to begin to substratify N2 disease.
In an intergroup trial of about 200 patients per arm published in the Lancet, patients went to surgery if they didn’t regress after evaluation. The progression-free survival rate did seem to favor surgery; and, if you look at the lobectomy subset, the results are certainly strong. But again, we’re dealing with curves that are pretty low. The pneumonectomy subset drops off and then starts to catch up, but clearly pneumonectomy was a problem in this multicenter trial. The most important graph to this debate shows subjects that persistently had nodal disease had very poor survival. It’s hard to argue for surgery when results show that only about 24% of them are going to be alive down the road. If oncologists across most of the United States say they believe that surgery isn’t a good idea, we’re not going to use this graph to change their minds; we need to change our way of thinking.
So the conclusion to take away from that presentation is that N0 status at surgery significantly predicts greater 5-year survival. So, conversely, surgery is not helpful for the patient with node-positive disease. Surgical resection should only be considered if lobectomy is the operation in question. Pneumonectomy carries risk, and surgery has no beneficial value, compared with chemotherapy unless the patient has been downstaged.
Our experience at Brigham and Women’s Hospital in Boston is similar to Dr. Swisher’s at MD Anderson. During the first 8 years of our thoracic division, we looked at 103 patients who had surgery after induction therapy for N2 disease. The induction plan in those patients was chemotherapy only for 75, radiation only for 18, and chemoradiation for 10. Almost 40% of patients had pneumonectomies, and the rest had lobectomies.
Mortality was 3.9%, major morbidity was 7%, and about 30% were downstaged. Those 5-year survival rates were about 36%. Persistent nodal disease, either N1 or N2, was seen in about 75%, and most of them were N2; the 5-year survival rate there was about 9% with a median of about 15.9 months. Beauty is in the eye of the beholder, so you may look at that and say that 15.9 months isn’t bad, but there’s still a huge subgroup that’s node positive, so here I’d say that pushing surgery is not the best strategy. We also found in this group that adenocarcinomas were much harder to clear.
We’re on very-safe ground to push surgery in the node-negative group, but you’ve got to be careful in the node-positive group.
Survival is relatively limited in N2 disease in general. Surgery may be of value if you downstage the patient, if you’re doing a lobectomy or if you see squamous cell carcinoma. Going forward, we really ought to focus our attention on identifying responders more reliably and improving downstaging – with different or individualized chemotherapy, or perhaps even immunologic therapy.
For the present, we can talk about radiation dosing. High-dose radiation is clearly a viable option for some patients. In addition, we can improve identification of N2 subgroups. Not all N2 disease is the same, so it should not be treated the same across the board.
Dr. Swanson is at Brigham and Women’s Hospital in Boston. He disclosed that he is a consultant/advisory board member for Covidien and Ethicon Endo-Surgery.
This article grew out of the debate by Dr. Swisher and Dr. Swanson at the annual meeting of the Society of Thoracic Surgeons.
Clinical advances drive lung cancer staging, classification changes
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
AT THE IASLC WORLD CONFERENCE
Key clinical point: Improved understanding of lung cancer over the last decade has prompted updates to international tumor staging and classification systems.
Major finding: The WHO 2015 Classification of Lung Tumors is expected to aid clinical practice.
Data source: Conference presentation of key changes to the WHO Classification and TNM staging system.
Disclosures: Dr. Travis and Dr. Rami-Porta reported having no disclosures relevant to their presentations.
Assessing progression, impact of radiofrequency ablation in Barrett’s esophagus
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
These two studies highlight two different hot topics in the management of patients with a Barrett’s esophagus. The first is the low rate of neoplastic progression in patients undergoing surveillance for nondysplastic BE. The second relates to the management of patients with low-grade dysplasia (LG

|
| Dr. Jacques Bergman |
Population-based BE surveillance studies have shown lower progression rates than have prospective surveillance studies. The biggest difference between these two is that not all patients in population-based studies actually undergo subsequent surveillance endoscopies and/or surveillance is carried out less rigorously than in prospective surveillance studies. Patients who have undergone a baseline endoscopy showing no neoplasia first need to develop early neoplasia (which is generally asymptomatic) that then needs to progress to a symptomatic stage before they are diagnosed. During this interval they may die from other causes or may be lost to follow-up. Patients in strict surveillance programs will be diagnosed at an earlier stage and at a higher rate. This is especially true in the first years of follow-up, when the initial screening endoscopy has its largest effect. Over time, the difference then fades away as suggested by the 9% progression rate of both types of studies at 20 years of follow-up. Both perspectives are relevant for patients. For elderly patients with significant comorbidity, the 5-year data from population-based studies reassure them not to undergo surveillance endoscopies because even when an early cancer develops it is unlikely to bear any clinical relevance, whereas for patients with a long life expectancy, the 9% cancer risk at 20 years and the dismal prognosis of a symptomatic Barrett’s cancer may be strong arguments for participating in a surveillance program.
For patients with LGD, the situation is different: The rate of progression is much higher than that reported for nondysplastic BE, and with radiofrequency ablation (RFA), an effective and safe tool is at hand to significantly reduce this rate of neoplastic progression. Small et al. reported that only three patients need to be treated with RFA to prevent one patient from progressing to high-grade dysplasia or cancer. These data are in agreement with data from a prospective randomized study on the use of RFA for patients with a confirmed diagnosis of LGD. Most societies therefore consider a confirmed histologic diagnosis of LGD a justified indication for prophylactic ablation with RFA.
However, this does not imply that all patients with LGD should be ablated. First, only patients in whom the histologic diagnosis of LGD is confirmed by an expert BE pathologist should be considered for RFA. In approximately 75% of patients, the LGD diagnosis will be downstaged to nondysplastic BE upon expert review. Second, the lessons learned from the Kroep study also apply here: For an elderly LGD patient with or without significant comorbidity, the decision to proceed to RFA is different from the decision for patients with a longer life expectancy, especially if an intermediate solution – to continue endoscopic surveillance and proceed to endoscopic management in case neoplasia is diagnosed – is also considered.
Jacques Bergman, M.D., Ph.D., is professor of gastrointestinal endoscopy, director of endoscopy, at the Academic Medical Center, Amsterdam. He received research support for clinical studies and consulted for Covidien/Medtronic GI solutions.
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
Patients with Barrett’s esophagus have about a 0.2% annual chance of developing esophageal adenocarcinoma in the 5 years after initial diagnosis, but the likelihood then rises so that about 9% of all patients will develop cancer by 20 years out, according to a study in the September issue of Gastroenterology.
The modeled rates of progression for the early years after diagnosis are substantially lower than are those reported by prospective studies, which involve more intensive surveillance and therefore suffer from detection bias, said Dr. Sonja Kroep of Erasmus Medical Center, Rotterdam, the Netherlands, and her associates. “Clinicians informing their patients about their cancer risk can best use this clinical progression rate, which is not influenced by surveillance-detected cancers,” they wrote.
Past analyses have yielded varying results for the rate at which Barrett’s esophagus with low-grade dysplasia progresses to high-grade dysplasia and esophageal carcinoma. For their study, Dr. Kroep and her associates calibrated a model based on the annual rate of 0.18% reported by population-level studies, and used it to simulate prospective studies and to predict results from both population-based and prospective studies for various follow-up periods (Gastroenterology 2015 Apr 29. pii: S0016-5085(15)00601-0).
For the first 5 years of follow-up, the model predicted a 0.19% annual rate of transformation to esophageal adenocarcinoma for population-based studies and a 0.36% annual rate for prospective studies, the researchers reported. At 20 years, these rates rose to 0.63% and 0.65% annually, for a cumulative incidence rate of 9.1% to 9.5%. Between the 5-year and 20-year thresholds, the gap between rates of progression for the two types of studies narrowed from 91% to 5%. Taken together, the findings suggest that for the first 5 years after a diagnosis of Barrett’s esophagus, rates of progression to esophageal adenocarcinoma reflect those from population-level studies instead of surveillance-based prospective studies, the investigators said. “Clinicians should use this information to explain to patients their short-term and long-term risks if no action is taken, and then discuss the risks and benefits of surveillance,” they added.
In a separate retrospective study, radiofrequency ablation of low-grade esophageal dysplasia was linked to substantially lower rates of progression compared with watchful waiting in the form of endoscopic surveillance, said Dr. Aaron Small of the University of Pennsylvania, Philadelphia, and his associates. Their study included 125 patients with Barrett’s esophagus and low-grade dysplasia who underwent surveillance only, and 45 patients who underwent radiofrequency ablation at three university medical centers.
Over median follow-up periods of more than 2 years, the risk of progression with radiofrequency ablation was significantly lower than with endoscopic surveillance only, even after the researchers controlled for year of diagnosis (adjusted hazard ratio, 0.06; 95% confidence interval, 0.008-0.48; P = .008). The ablation group also had fewer visible macroscopic lesions, although the difference was not significant. “We estimate that for every three patients treated with radiofrequency ablation, one additional patient with low-grade dysplasia will avoid progression to high-grade dysplasia or esophageal adenocarcinoma within 3 years,” the researchers wrote. “Although selection bias cannot be excluded, these findings provide additional evidence for the use of endoscopic ablation therapy for low-grade dysplasia” (Gastroenterology 2015 Apr 24. pii: S0016-5085(15)00569-7).
The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest. The study by Dr. Small and his associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest, but seven coauthors reported ties with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Barrett’s esophagus with low-grade dysplasia had a lower rate of progression to cancer than that suggested by prospective surveillance studies, but radiofrequency ablation might further cut the risk.
Major finding: About 0.2% of cases progress during the 5 years after diagnosis, and RFA might significantly decrease risk of progression (adjusted hazard ratio, 0.06).
Data source: A model of rates of progression based on population-level studies, and a multicenter retrospective study of 170 patients with Barrett’s esophagus and low-grade dysplasia.
Disclosures: The study by Dr. Small and associates was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and by institutional funds. Dr. Small reported no conflicts of interest; seven coauthors reported ties with a number of pharmaceutical companies. The study by Dr. Kroep and her associates was funded by grant U01 CA152926, and the investigators reported having no conflicts of interest.
Adjuvant erlotinib showed no benefit in NSCLC patients
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Among patients with completely resected non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR), disease-free survival (DFS) rates were similar between erlotinib and placebo groups, according to results from the RADIANT phase III trial.
EGFR-activating mutations (EGFRm) were observed in a subgroup of patients (16.5%, 89 with del19 and 72 with L858R mutations) but these patients were not stratified by mutation status, which limited interpretation of the results. For the erlotinib vs. placebo arms of the EGFRm-positive subgroup, median DFS was 46.4 and 28.5 months, respectively, with 2-year DFS rates of 75% and 54%. The results were not statistically significant due to hierarchical testing. There were between-arm imbalances of disease characteristics, and the placebo arm of the EGFRm-positive subgroup had substantially worse DFS than the intention-to-treat population.
“The trend toward improvement in DFS with erlotinib in the EGFRm-positive subgroup warrants further evaluation,” wrote Dr. Karen Kelly of UC Davis Comprehensive Cancer Center, Sacramento, California, and colleagues (J Clin Oncol. 2015 Aug 31. doi:10.1200/JCO.2015.61.8918).
The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry (IHC) and EGFR high copy number or amplification by fluorescence in situ hybridization (FISH). Previous studies indicated that EGFR protein expression detected by IHC and FISH may be predictive of prolonged survival with EGFR tyrosine kinase inhibitors (TKIs) such as erlotinib. However, subsequent to activation of the RADIANT trial, two phase III studies failed to show that IHC and FISH results were predictive of EGFR-TKI efficacy. Emerging data show that EGFRm-positive status is the strongest predictor of EFGR-TKI sensitivity.
The duration of treatment in the RADIANT trial was 2 years, but the authors speculate that, based on results from the recent SELECT trial in which only four patients experienced relapse while taking erlotinib, a longer treatment duration “may be needed to achieve the goal of increasing the cure rate of early-stage NSCLC in an EGFRm-positive population.”
KRAS mutation status, tested in 828 patient samples, was not prognostic and did not predict erlotinib benefit.
The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several of her coauthors were employed by or reported consulting or advisory roles with industry sources.
Key clinical point: Adjuvant erlotinib demonstrated no DFS benefit in patients with non–small-cell lung cancer (NSCLC) whose tumors expressed epidermal growth factor receptor (EGFR).
Major finding: After a median follow up of 47 months, the median DFS for patients who received erlotinib was 50.5 months compared with 48.2 months for placebo.
Data source: The randomized, double-blind, placebo-controlled RADIANT trial included 973 patients with completely resected stage IB to IIIA NSCLC with EGFR-expressing tumors by immunohistochemistry and EGFR high copy number or amplification by fluorescence in situ hybridization.
Disclosures: The study was supported by Astellas Pharma Global Development, F. Hoffmann-La Roche, and Genentech. Dr. Kelly reported having no disclosures. Several coauthors were employed by or reported consulting or advisory roles with industry sources.
3-D–printed devices mitigate pediatric tracheobronchomalacia
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The use of 3-D–printed airway implants mitigated life-threatening tracheobronchomalacia (TBM) in three infants.
Major finding: Three infants with a terminal form of TBM ceased exhibiting life-threatening airway disease and showed continued growth of pulmonary airways after 3-D tracheal implants.
Data source: A study performed at the University of Michigan, Ann Arbor, of three infants with terminal TBM who received a medical device emergency use exemption for a 3-D tracheal implant.
Disclosures: The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
Surgeon volume may affect choice of surgery type for NSCLC
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
Because the McMaster University study derived the reported outcomes from registry data, determining the reasons that influenced surgeons’ choices of lung resection is impossible, Dr. Eric Lim of the Imperial College of Medicine, London, said in his invited commentary (J Thorac Cardiovasc Surg. 2015 May 21 [doi:10.1016/j.jtcvs.2015.05.048]).
The study authors noted that lower-volume surgeons were more inclined to perform pneumonectomy, and, Dr. Lim noted, previous studies have found that higher-volume centers tended to see more patients with advanced-stage cancers and increased morbidities. “An alternative explanation is that higher-volume surgeons have better skill sets to undertake procedures such as sleeve lobectomies that would lower the pneumonectomy rates and possibly more segmentectomies to lower the wedge-resection rate,” Dr. Lim said.
Until better evidence exists on what procedure is best for central and peripheral tumors, “surgeons can argue either way,” Dr. Lim said. The questions that follow from the study should concentrate on the relative harm of each procedure and the level of practice variation that’s unacceptable.
“As a surgical community, it is incumbent on us to continue to evaluate surgical treatments generating the highest levels of evidence possible (randomized trials) and have sufficient humility to cross refer to colleagues when appropriate to ensure the best care for our patients,” Dr. Lim concluded.
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
A surgeon’s comfort level with a favored operation for non–small cell lung cancer can strongly influence whether the patient will have that operation, which, in turn, can affect the patient’s outcome and long-term survival, according to an analysis of a population-linked database. For patients whose surgeons have lower levels of experience, that could mean a greater chance they will have more invasive total lung removal rather than more difficult operations that spare part of the affected lung, according to investigators at McMaster University in Hamilton, Ontario.
“If a surgeon with high surgical volumes is less likely to perform higher-risk pneumonectomy procedures than one with lower volumes, this may translate to a significant reduction in adverse events,” said lead author Dr. Christian Finley and coauthors online in the Journal of Thoracic and Cardiovascular Surgery (2015 Jun 30 [doi: 10.1016/j.jtcvs.2015.04.060]). “Surgeon volume should be considered an important component in how care is delivered in this population.”
The McMaster investigators evaluated 8,070 patients in an Ontario population-based linked database who underwent surgical resection for non–small cell lung cancer during 2004-2011, including pneumonectomy, or total lung removal (842 patients), lobectomy (6,212 patients), and wedge resection (1,002 patients). Over the years of the study, the proportion of patients who underwent pneumonectomy fell by more than half, from 14.8% in 2004 to 7.6% in 2011.
Of the three procedures, pneumonectomy carries a threefold greater mortality and while the procedure is often avoidable, there may be cases where it’s necessary because of the location of the tumor, Dr. Finley and his colleagues said. Lobectomy is desirable because it spares the parenchyma and has lower recurrence rates than laser resections.
The study investigators aimed to explore the hypothesis that surgeons with less expertise are more inclined to perform the higher-risk pneumonectomy or sublobar resections such as a segmentectomy or a wedge resection than a lobectomy, the rationale being that these procedures can be less challenging than a standard or sleeve lobectomy. The study analyzed results from 124 different physicians at 45 institutions in Ontario.
Data analysis showed that physician volume, age, year of procedure, sex, and comorbidities were predictive of the surgeon performing a pneumonectomy. “Adjusting for these variables, the results indicated that for each 10-unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%,” Dr. Finley and his colleagues wrote. They also found no significant difference in stage distribution among low-, medium-, and high-volume surgeons.
“This is meaningful as pneumonectomy is known to have the highest mortality rate of lung cancer resection, found in this study to be 12.6%, demonstrating a potentially large impact on patient survival,” Dr. Finley and his colleagues said.
This analysis cites an earlier study that surgeon volume for many procedures was a key determinant in the link between hospital volume and operative mortality (N Engl J Med. 2003 Nov 27;349[2]:2117-27.). “This study suggests that a patient may improve their chance of survival substantially, even at high-volume institutions, by selecting surgeons who perform operations more frequently,” Dr. Finley and his colleagues said.
They said that despite their study’s limitations, the findings on how surgeon experience can influence the choice of lung resection for cancer warrant further study.
McMaster University, Division of Thoracic Surgery, provided funding for the study. The study authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Surgeon volume is a predictor of procedure selection for lung cancer surgery and has implications on outcomes.
Major finding: For each 10 unit increase in physician volume, the relative risk of performing a pneumonectomy decreased by 9.1%.
Data source: Dataset of 8,070 patients constructed from Ontario population-based linked databases accessed via the Institute for Clinical Evaluate Sciences.
Disclosures: McMaster University, Division of Thoracic Surgery, provided study funding. The authors had no disclosures.
How patient-reported outcomes may alter care
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
“Whether ‘subjective’ or not, the measurements of five common postoperative symptoms appear to reflect the real world experience of many,” Dr. Robert B. Cameron of the University of California, Los Angeles, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.06.017]).
What’s more, because the study investigators collected patient data after discharge, they eliminated opportunities to “game the system,” in Dr. Cameron’s words. One example of gaming the outcome measures would be to discharge patients early after surgery with a Heimlich chest tube, subjecting them to problems at home and requiring physicians visits and even emergency department care that can go unrecorded.
“Subjective” patient experiences are “real issues,” Dr. Cameron said. “After all, patients are the customers that the health care system serves.”
Patient-reported outcome measures could dramatically influence how physicians and health systems report patient outcomes. “While length of stay (LOS) and hospital complication rates appear to be valid outcome measures to report, in reality, they are outcomes only in a world when hospitals are the ‘customers,’ ” Dr. Cameron said. “In a patient-centric system, when patient outcomes matter, [patient-reported outcomes], such as those proposed by the MD Anderson investigators, are what truly matter.”
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
As Medicare and commercial payers rely increasingly on quality outcomes to determine reimbursement levels, clinicians focused on traditional clinical outcomes like length of hospital stay and readmission may be missing the point if they don’t take into consideration more patient-centric outcome. A team of investigators at MD Anderson Cancer Center in Houston recently evaluated that institution’s tool for measuring patient-reported outcomes in tracking results after thoracic surgery for lung cancer.
Christopher P. Fagundes, Ph.D., led the research team that reported their findings in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2015 [doi:10.1016/j.jtcvs.2015.05.057]).
“We used the MD Anderson Symptom Inventory (MDASI) to elicit patient reports of the worst symptoms experienced after thoracic surgery,” Dr. Fagundes and his colleagues said in what may be the first study to use patient-reported outcomes to chart a recovery course after surgery. “This study demonstrates that the MDASI is a sensitive tool for detecting symptomatic recovery with an expected relationship among surgery type, preoperative performance status, and comorbid conditions,” the authors stated.
The MDASI measures the severity of 13 common cancer-related symptoms over the previous 24 hours and rates each on a scale from 0-10, with 10 as most severe. Patients in the study completed a written MDASI questionnaire, 60 upon enrollment, and 77 patients 3 and 5 days after surgery in the hospital. After discharge and for 3 months after surgery, patients received weekly calls from a computer/telephone interactive voice-response system that asked them to rate their symptoms.
All patients were newly diagnosed and treatment naive with early-stage non–small cell lung cancer (NSCLC) who had either standard open thoracotomy or video-assisted thoracoscopic surgery (VATS) lobectomy.
The investigators used a two-pronged approach to define recovery after surgery: symptoms returning to their baseline levels or to a mild level of severity. In the first week after surgery, pain, fatigue, and shortness of breath were “highly prevalent,” perhaps a combined effect from surgical insult and care during and after surgery. After a month, ratings for most symptoms returned to baseline, but fatigue remained the most-persistent symptom during the 3-month study.
NSCLC patients typically have a host of symptoms after major surgery, including inflammation, organ stress, and reaction to medications. In addition, up to a quarter have postsurgical complications that can amplify their symptoms and even delay their course of cancer treatment. The five most-severe postoperative symptoms patients in the study reported were fatigue, pain, shortness of breath, disturbed sleep, and drowsiness.
The median time to return to mild symptom severity for these five symptoms was shorter than return to baseline severity, with fatigue taking longer. Pain recovered significantly faster for patients who underwent VATS lobectomy vs. standard open thoracotomy (8 vs. 18 days, respectively; P = .022), according to the researchers. In addition, the researchers found that patients who had poor preoperative performance status or comorbidities reported significantly higher postoperative pain (P less than .05).
Having the ability to measure patient-reported outcomes allows physicians to identify patients at greatest risk for symptoms after surgery and to help caregivers develop pathways to speed up recovery and get patients to chemotherapy or other cancer treatments on schedule, Dr. Fagundes and his coauthors said.
“Using a straightforward, concise tool like the MDASI to obtain the patient’s perspective on how well he or she is recovering is a clinically relevant and user-friendly method for optimizing perioperative care,” the authors added.
Minimally invasive surgical techniques along with opioid-sparing analgesia have been incorporated into enhanced recovery programs to aim for better objective postoperative outcomes like fewer complications and shorter hospital stays. “Missing from these metrics is the voice of the patient, who is arguably the best source of information about what ‘recovery’ from surgery means,” Dr. Fagundes and colleagues said. “Lack of research on how to define and measure symptomatic and functional recovery after major cancer surgery from the patent’s perspective is an important gap in comprehensive postoperative care; it also compromises any comparison of ERP [enhanced recovery program] innovations against standard care.”
Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Using the MD Anderson Symptom Inventory to elicit patient-reported symptom burden is a simple, clinically relevant way to optimize care after thoracic surgery for lung cancer.
Major finding: Assessing symptoms from the patient’s perspective throughout the postoperative recovery period is an effective strategy for evaluating perioperative care.
Data source: Sixty newly diagnosed patients with early-stage non–small cell lung cancer scheduled for thoracic surgery prospectively were recruited for evaluation.
Disclosures: Study coauthors received funding from the National Cancer Institute of the National Institutes of Health, the MD Anderson Cancer Center Support Grant program, and the American Cancer Society Research Scholar Grant program. The coauthors reported no conflicts of interest in this work.