User login
ARIEL3 analysis: Rucaparib PFS benefit in recurrent OC occurs across age groups
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
HONOLULU – The improved progression-free survival and reduced risk of disease progression seen with maintenance rucaparib versus placebo in women with recurrent ovarian cancer in the pivotal ARIEL3 study occurred across age subgroups, according to a post hoc exploratory analysis of data from the phase 3 study.
In general, the safety profile of the first-in-class poly (ADP-ribose) polymerase (PARP) inhibitor was consistent across age groups, as well, but rates of dose modifications and treatment discontinuations varied slightly by age subgroup in the rucaparib (Rubraca)and placebo arms, Jonathan A. Ledermann, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
No clear trend emerged with respect to those differences, said Dr. Ledermann, a professor of medical oncology at University College of London Cancer Institute and UCL Hospitals.
Initial results from the randomized, placebo-controlled study were published in 2017 and showed a significant progression-free survival (PFS) benefit with 600 mg of maintenance rucaparib vs. placebo (10.8 vs. 5.4 months, respectively, in the intention-to-treat population). For the current analysis, outcomes were assessed for the ITT population across three baseline age-based subgroups: those under age 65 years, those aged 65-74 years, and those 75 years or older.
Investigator-assessed PFS – the primary endpoint of ARIEL3 – for the rucaparib vs. placebo groups was 11.1 vs. 5.4 months among 237 and 117 patients under age 65 years in the groups, respectively (hazard ratio, 0.33); 8.3 vs. 5.3 for 113 and 64 patients aged 65-74 years, respectively (HR, 0.43); and 9.2 and 5.5 months in 25 and 8 patients aged 75 and older (HR, 0.47), he said.
“The hazard ratios are comparable across all three age cohorts,” Dr. Ledermann said.
Treatment-emergent adverse events (TEAEs) of any grade occurring in at least 35% of patients included nausea, asthenia, vomiting, dysgeusia, constipation, anemia, ALT/AST increase, diarrhea, abdominal pain, thrombocytopenia, and pruritus.
The rates of these events were similar across age groups, although slight, nonsignificant numerical increases in grade 3 toxicities occurred with increasing age, he noted.
“If we look at dose interruption and dose reduction rates – again, broadly similar, [but with] a slight trend toward an increase in older age groups,” he said.
Dose modifications related to TEAEs, however, occurred in 65.5% of rucaparib and 9.4% of placebo patients aged under 65 years, compared with 82.3% and 12.5% of those aged 65-74, and 83.3% and 12.5% of those aged 75 or older, respectively. Discontinuations because of TEAEs occurred in 13.6% and 1.7% in those under age 65 years vs. 19.5% and 3.1% in those aged 65-74 years, and 20.8% and 0% of those aged 75 years or older in the groups, respectively.
Of note, the age subgroups and the treatment and placebo groups were well balanced except more of those under age 65 years had a deleterious germline or somatic BRCA mutation (96 and 49 in the rucaparib and placebo arms, respectively) than did patients aged 65-74 years (29 and 15, respectively), and patients aged 75 years and older (5 and 2, respectively).
ARIEL3 was funded by Clovis Oncology. Dr. Ledermann reported financial relationships (consulting, grant receipt, honorarial reimbursement, and/or speakers bureau fees) with AstraZeneca, Clovis Oncology, Pfizer, Seattle Genetics, Roche, and MSD/Merck.
SOURCE: Ledermann J et al. SGO 2019, Abstract 4.
REPORTING FROM SGO 2019
Can prophylactic salpingectomies be achieved with the vaginal approach?
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique
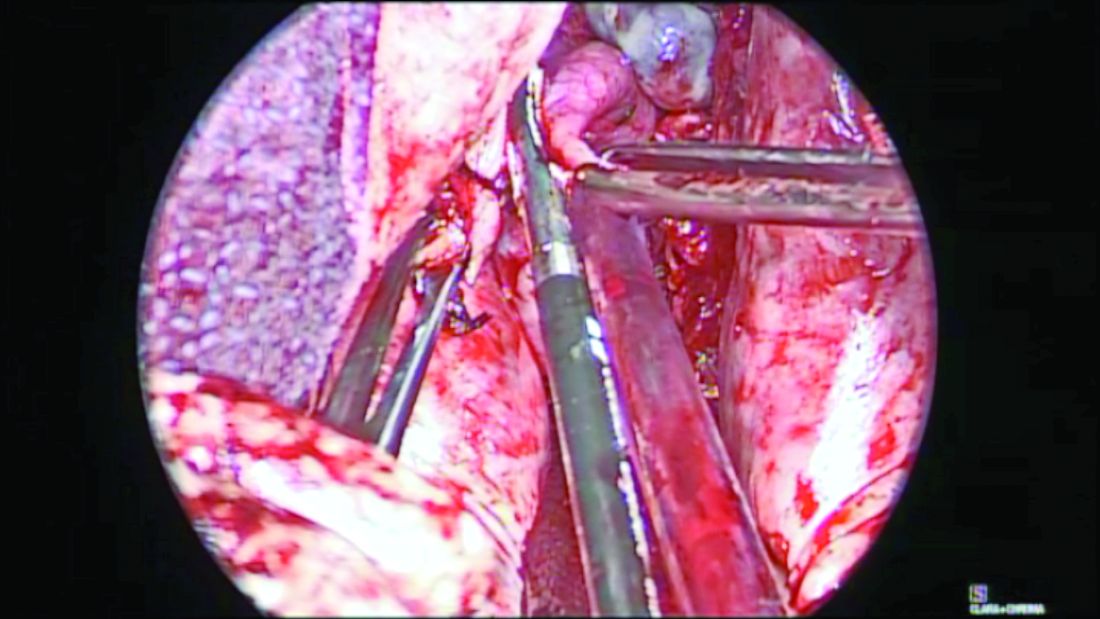
Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique
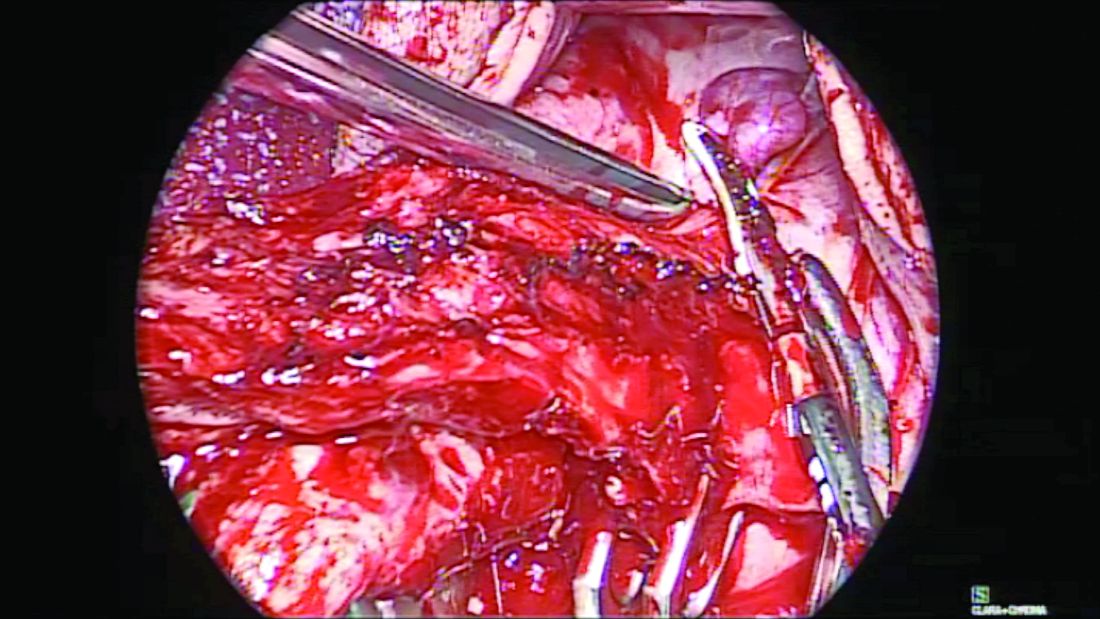
When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
In the last decade, there has been a major shift in our understanding of the pathogenesis of ovarian cancers. Current literature suggests that many high-grade serous carcinomas develop from the distal aspect of the fallopian tube and that serous tubal intraepithelial carcinoma is likely the precursor. The critical role that the fallopian tubes play as the likely origin of many serous ovarian and pelvic cancers has resulted in a shift from prophylactic salpingo-oophorectomy, which may increase risk for cardiovascular disease, to prophylactic bilateral salpingectomy (PBS) at the time of hysterectomy.
It is important that this shift occur with vaginal hysterectomy (VH) and not only with other surgical approaches. It is known that PBS is performed more commonly during laparoscopic or abdominal hysterectomy, and it’s possible that the need for adnexal surgery may further contribute to the decline in the rate of VH performed in the United States. This is despite evidence that the vaginal approach is preferred for benign hysterectomy even in patients with a nonprolapsed and large fibroid uterus, obesity, or previous pelvic surgery. Current American College of Obstetricians and Gynecologists’ guidelines also state that the need to perform adnexal surgery is not a contraindication to the vaginal approach.
So that more women may attain the benefits and advantages of VH, we need more effective teaching programs for vaginal surgery in residency training programs, hospitals, and community surgical centers. Moreover, we must appreciate that PBS with VH is safe and feasible. There are multiple techniques and tools available to facilitate the successful removal of the tubes, particularly in difficult cases.
The benefit and safety of PBS
Is PBS really effective in decreasing the incidence and mortality of ovarian cancer? A proposed randomized trial in Sweden with a target accrual of 4,400 patients – the Hysterectomy and Opportunistic Salpingectromy Study (HOPPSA, NCT03045965) – will evaluate the risk of ovarian cancer over a 10- to 30-year follow-up period in patients undergoing hysterectomy through all routes. While we wait for these prospective results, an elegant decision-model analysis suggests that routine PBS during VH would eliminate one diagnosis of ovarian cancer for every 225 women undergoing hysterectomy (reducing the risk from 0.956% to 0.511%) and would prevent one death for every 450 women (reducing the risk from 0.478% to 0.256%). The analysis, which drew upon published literature, Medicare reimbursement data, and the National Surgical Quality Improvement Program database, also found that PBS with VH is a less expensive strategy than VH alone because of an increased risk of future adnexal surgery in women retaining their tubes.1

The question of whether PBS places a woman at risk for early menopause is a relevant one. A study following women for 3-5 years after surgery showed that the addition of PBS to total laparoscopic hysterectomy in women of reproductive age does not appear to modify ovarian function.2 However, a recently published retrospective study from the Swedish National Registry showed that women who underwent PBS with abdominal or laparoscopic benign hysterectomy had an increased risk of menopausal symptoms 1 year after surgery.3 Women between the ages of 45-49 years were at highest risk, suggesting increased vulnerability to possible vascular effects of PBS. A longer follow-up period may be necessary to assess younger age groups.
In a multicenter, prospective and observational trial involving 69 patients undergoing VH, PBS was feasible in 75% (a majority of whom [78%] had pelvic organ prolapse) and increased operating time by 11 minutes with no additional complications noted. The surgeons in this study, primarily urogynecologists, utilized a clamp or double-clamp technique to remove the fimbriae.4
The decision-model analysis mentioned above found that PBS would involve slightly more complications than VH alone (7.95% vs. 7.68%),1 and a systematic review that I coauthored of PBS in low-risk women found a small to no increase in operative time and no additional estimated blood loss, hospital stay, or complications for PBS.5
Tools and techniques
Vaginal PBS can be accomplished easily with traditional clamp-cut-tie technique in cases where the fallopian tubes are accessible, such as in patients with uterine prolapse. Generally, most surgeons perform a distal fimbriectomy only for risk-reduction purposes because this is where precursor lesions known as serous tubal intraepithelial cancer (STIC) reside.
To perform a fimbriectomy in cases where the distal portion of the tube is easily accessible, a Kelly clamp is placed across the mesosalpinx, and a fine tie is used for ligature. In more challenging hysterectomy cases, such as in lack of uterine prolapse, large fibroid uterus, morbid obesity, and in patients with previous tubal ligation, the fallopian tubes can be more difficult to access. In these cases, I prefer the use of the vessel-sealing device to seal and divide the mesosalpinx.
Here I describe three specific techniques that can facilitate the removal of the fallopian tubes in more challenging cases. In each technique, the entire fallopian tubes are removed – without leaving behind the proximal stump. The residual stump has the potential of developing into a hydrosalpinx that may necessitate another procedure in the future for the patient.
Separate the fallopian tube before clamping the ‘utero-ovarian ligament’ technique

Before completion of the hysterectomy and clamping of the round ligament/fallopian tube/utero-ovarian ligament (RFUO) complex (commonly referred as the “utero-ovarian ligament”), I recommend first identifying the proximal portion of the fallopian tube. The isthmus is sealed and divided from its attachment to the uterine cornua, and a clamp is placed on the remaining round ligament/utero-ovarian ligament complex. The pedicle is then cut and tied. (Figure 1.) After removal of the uterus, the fallopian tube is ready to be grasped with an Allis clamp or Babcock forceps, and the remaining mesosalpinx is sealed and divided all the way to the distal portion/fimbriae.
Round ligament–mesosalpinx technique

When the uterus is large or lacks prolapse, the fallopian tubes can be difficult to visualize. In such cases, I recommend the use of the round ligament–mesosalpinx technique. After completion of the hysterectomy and ligation of the RFUO complex, a long and moist vaginal pack (I prefer the 4” x 36” cotton vaginal pack by Dukal) is used to push the bowels back and expose the adnexae. The round ligament is identified within the RFUO complex and transected using a monopolar instrument. This step that separates the round ligament from the RFUO complex successfully releases the adnexae from the pelvic sidewall, making it easier to access the fallopian tubes (and the ovaries, when needed). A window is created in the mesosalpinx, and a curved clamp is placed on the ovarian vessels. Using sharp scissors, the proximal portion of the fallopian tube contained within the RFUO complex is separated, and the mesosalpinx is sealed and divided all the way to the distal end using the vessel-sealing device. (Figure 2.)
vNOTES (transvaginal Natural Orifice Translumenal Endoscopic Surgery) salpingectomy technique

When the adnexae is noted to be high in the pelvis or when it is adherent to the pelvic sidewall, I recommend the vNOTES technique. It involves insertion of a mini-gel port into the vaginal opening. (Figure 3.) A 5-mm or 10-mm scope is inserted through this port for visualization. The fallopian tube can be grasped with a laparoscopic grasper and the mesosalpinx sealed and divided using a vessel-sealing device. (Figure 4.) Often, because the bowel is already retracted up with the vaginal pack, insufflation is not necessary with this procedure.

The change in our understanding of the etiology of ovarian cancer calls for salpingectomy during hysterectomy. With such tools, devices, and techniques that facilitate the vaginal removal of the fallopian tubes, the need for prophylactic salpingectomy should not be a deterrent to pursuing a hysterectomy vaginally.
Dr. Kho is head of the section of benign gynecology at the Cleveland Clinic.
References
1. Am J Obstet Gynecol. 2017;217(5):503-4.
2. J Minim Invasive Gynecol. 2017 Jan 1;24(1):145-50.
3. Am J Obstet Gynecol. 2019;220:85.e1-10.
4. Am J Obstet Gynecol. 2017;217:605.e1-5.
5. J Minim Invasive Gynecol. 2017 Feb;24(2):218-29.
Neratinib shows promise in HER2-mutant cervical cancer
HONOLULU – Treatment with the pan-HER tyrosine kinase inhibitor neratinib leads to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, preliminary efficacy results from the phase 2 SUMMIT basket trial suggest.
Of 11 trial subjects evaluable for efficacy, 3 (27%) had objective confirmed responses and 6 others had stable disease for at least 16 weeks (clinical benefit rate, 55%). Median progression-free survival was 7 months, Anishka D’Souza, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Study subjects included HER2-mutant metastatic cervical cancer patients. Most (89%) had adenocarcinoma and 11% had squamous cell carcinoma, said Dr. D’Souza of the University of Southern California, Los Angeles.
The median number of total prior regimens was two, with a range of one to four, she said. Patients received oral neratinib at a dose of 240 mg once daily.
The treatment was generally well tolerated; the most common adverse event was diarrhea, with only one grade 3/4 case occurring, she said, noting that high-dose loperamide prophylaxis was mandatory during cycle 1 because of the high incidence of diarrhea.
Though preliminary, the findings are notable as somatic HER2 (ERBB2) mutations, observed in about 5% of metastatic cervical cancer cases, are associated with poor prognosis, she explained, noting that there are limited treatment options and few long-term durable responses.
“There is a great need for additional treatment options,” she said.
Neratinib has been shown to have single-agent clinical activity in multiple HER2‑mutant cancers, so Dr. D’Souza and her colleagues are assessing its safety and efficacy as either monotherapy or in combination with other treatments across multiple tumor types. The current analysis focuses on the cohort of patients who received neratinib monotherapy.
Neratinib use in this cohort led to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, she said, adding that “this is an ongoing study and we are continuing to enroll patients to expand our dataset.”
Dr. D’Souza reported having no financial disclosures.
SOURCE: D’Souza A et al. SGO 2019, Abstract 18.
HONOLULU – Treatment with the pan-HER tyrosine kinase inhibitor neratinib leads to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, preliminary efficacy results from the phase 2 SUMMIT basket trial suggest.
Of 11 trial subjects evaluable for efficacy, 3 (27%) had objective confirmed responses and 6 others had stable disease for at least 16 weeks (clinical benefit rate, 55%). Median progression-free survival was 7 months, Anishka D’Souza, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Study subjects included HER2-mutant metastatic cervical cancer patients. Most (89%) had adenocarcinoma and 11% had squamous cell carcinoma, said Dr. D’Souza of the University of Southern California, Los Angeles.
The median number of total prior regimens was two, with a range of one to four, she said. Patients received oral neratinib at a dose of 240 mg once daily.
The treatment was generally well tolerated; the most common adverse event was diarrhea, with only one grade 3/4 case occurring, she said, noting that high-dose loperamide prophylaxis was mandatory during cycle 1 because of the high incidence of diarrhea.
Though preliminary, the findings are notable as somatic HER2 (ERBB2) mutations, observed in about 5% of metastatic cervical cancer cases, are associated with poor prognosis, she explained, noting that there are limited treatment options and few long-term durable responses.
“There is a great need for additional treatment options,” she said.
Neratinib has been shown to have single-agent clinical activity in multiple HER2‑mutant cancers, so Dr. D’Souza and her colleagues are assessing its safety and efficacy as either monotherapy or in combination with other treatments across multiple tumor types. The current analysis focuses on the cohort of patients who received neratinib monotherapy.
Neratinib use in this cohort led to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, she said, adding that “this is an ongoing study and we are continuing to enroll patients to expand our dataset.”
Dr. D’Souza reported having no financial disclosures.
SOURCE: D’Souza A et al. SGO 2019, Abstract 18.
HONOLULU – Treatment with the pan-HER tyrosine kinase inhibitor neratinib leads to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, preliminary efficacy results from the phase 2 SUMMIT basket trial suggest.
Of 11 trial subjects evaluable for efficacy, 3 (27%) had objective confirmed responses and 6 others had stable disease for at least 16 weeks (clinical benefit rate, 55%). Median progression-free survival was 7 months, Anishka D’Souza, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Study subjects included HER2-mutant metastatic cervical cancer patients. Most (89%) had adenocarcinoma and 11% had squamous cell carcinoma, said Dr. D’Souza of the University of Southern California, Los Angeles.
The median number of total prior regimens was two, with a range of one to four, she said. Patients received oral neratinib at a dose of 240 mg once daily.
The treatment was generally well tolerated; the most common adverse event was diarrhea, with only one grade 3/4 case occurring, she said, noting that high-dose loperamide prophylaxis was mandatory during cycle 1 because of the high incidence of diarrhea.
Though preliminary, the findings are notable as somatic HER2 (ERBB2) mutations, observed in about 5% of metastatic cervical cancer cases, are associated with poor prognosis, she explained, noting that there are limited treatment options and few long-term durable responses.
“There is a great need for additional treatment options,” she said.
Neratinib has been shown to have single-agent clinical activity in multiple HER2‑mutant cancers, so Dr. D’Souza and her colleagues are assessing its safety and efficacy as either monotherapy or in combination with other treatments across multiple tumor types. The current analysis focuses on the cohort of patients who received neratinib monotherapy.
Neratinib use in this cohort led to durable responses and disease control in heavily pretreated metastatic patients with HER2-mutant cervical cancer, she said, adding that “this is an ongoing study and we are continuing to enroll patients to expand our dataset.”
Dr. D’Souza reported having no financial disclosures.
SOURCE: D’Souza A et al. SGO 2019, Abstract 18.
REPORTING FROM SGO 2019
Perceived cancer risk may improve access to HPV vaccine
HONOLULU – The perceived risk of HPV-related cancer appears to overcome variables that typically impede access to HPV vaccination, according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
An analysis showed that adolescents in Alabama are more likely to receive the HPV vaccine if they live below the poverty line and reside in rural areas. The study also revealed a positive association between vaccine uptake and the incidence of HPV-related cancer by county.
These results suggest the perceived risk of HPV-related cancer may outweigh rurality and poverty—factors that might otherwise hinder access to health care, according to Jennifer Y. Pierce, MD, of Mitchell Cancer Institute in Mobile, Ala.
She discussed this idea when presenting the study results at the meeting.
“There are 39,000 preventable cases of HPV-related cancer in the United States,” Dr. Pierce said. “In Alabama, we are [ranked] third for cervical cancer incidence and first for cervical cancer mortality. When we look at vaccination rates in Alabama, unfortunately, we have the opposite problem. We are 45th for HPV vaccination.”
Dr. Pierce also noted that, nationally, adolescents in rural areas are 11% less likely to be vaccinated than their peers in urban areas.
“When we looked in Alabama, that did not exist,” Dr. Pierce said. “So we wanted to know, ‘What are the factors associated with HPV vaccination rate, by county, in the state of Alabama?’ because we had widely disparate rates by county.”
Dr. Pierce and her colleagues looked at data from the U.S. census, county health rankings for Alabama, the Alabama state cancer registry, and other sources. The researchers wanted to determine rates of HPV vaccination in 13- to 17-year-olds as well as rates of HPV-related cancers and variables associated with HPV vaccination by county.
Dr. Pierce said that, of the 67 counties in Alabama, 50%-70% of them are rural. Forty of them have a higher percent poverty level than the state mean. Twenty-three counties have no pediatrician, and four counties have no vaccine provider other than the health department.
By county, cancer rates were positively associated with HPV vaccination in both sexes. Higher cervical cancer rates correlated with higher HPV vaccination rates in females (r = .49; P = .011) and males (r = .46; P = .017). Higher HPV-related cancer rates in males correlated with higher HPV vaccination rates in females (r = .49; P = .001) and males (r = .46; P = .001).
The researchers found no significant association between vaccine uptake and the primary care provider ratio or the number of pediatricians per county. However, private insurance (r = –.40; P = .001) and higher median household income (r = –.40; P = .0007) were associated with lower HPV vaccine uptake. Rurality (r = .27; P = .025) and having a higher percentage of people below the poverty line (r = .39; P = .0011) were associated with higher vaccine uptake.
“How do we explain this paradox?” Dr. Pierce asked. “I think, really, it speaks to the strong commitment of our county public health departments who have, for a long time, been pushing the HPV vaccine and are doing a fairly good job of vaccinating. But I think, even more so, we need to focus on this question of perceived risk.”
“Our poor, rural adolescents in Alabama are being vaccinated at a higher rate than their more affluent peers, and those HPV vaccination rates appear to be directly linked to the cancer incidence rates in those counties.”
Dr. Pierce had no disclosures.
SOURCE: Pierce JY et al. SGO 2019, Abstract 13.
HONOLULU – The perceived risk of HPV-related cancer appears to overcome variables that typically impede access to HPV vaccination, according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
An analysis showed that adolescents in Alabama are more likely to receive the HPV vaccine if they live below the poverty line and reside in rural areas. The study also revealed a positive association between vaccine uptake and the incidence of HPV-related cancer by county.
These results suggest the perceived risk of HPV-related cancer may outweigh rurality and poverty—factors that might otherwise hinder access to health care, according to Jennifer Y. Pierce, MD, of Mitchell Cancer Institute in Mobile, Ala.
She discussed this idea when presenting the study results at the meeting.
“There are 39,000 preventable cases of HPV-related cancer in the United States,” Dr. Pierce said. “In Alabama, we are [ranked] third for cervical cancer incidence and first for cervical cancer mortality. When we look at vaccination rates in Alabama, unfortunately, we have the opposite problem. We are 45th for HPV vaccination.”
Dr. Pierce also noted that, nationally, adolescents in rural areas are 11% less likely to be vaccinated than their peers in urban areas.
“When we looked in Alabama, that did not exist,” Dr. Pierce said. “So we wanted to know, ‘What are the factors associated with HPV vaccination rate, by county, in the state of Alabama?’ because we had widely disparate rates by county.”
Dr. Pierce and her colleagues looked at data from the U.S. census, county health rankings for Alabama, the Alabama state cancer registry, and other sources. The researchers wanted to determine rates of HPV vaccination in 13- to 17-year-olds as well as rates of HPV-related cancers and variables associated with HPV vaccination by county.
Dr. Pierce said that, of the 67 counties in Alabama, 50%-70% of them are rural. Forty of them have a higher percent poverty level than the state mean. Twenty-three counties have no pediatrician, and four counties have no vaccine provider other than the health department.
By county, cancer rates were positively associated with HPV vaccination in both sexes. Higher cervical cancer rates correlated with higher HPV vaccination rates in females (r = .49; P = .011) and males (r = .46; P = .017). Higher HPV-related cancer rates in males correlated with higher HPV vaccination rates in females (r = .49; P = .001) and males (r = .46; P = .001).
The researchers found no significant association between vaccine uptake and the primary care provider ratio or the number of pediatricians per county. However, private insurance (r = –.40; P = .001) and higher median household income (r = –.40; P = .0007) were associated with lower HPV vaccine uptake. Rurality (r = .27; P = .025) and having a higher percentage of people below the poverty line (r = .39; P = .0011) were associated with higher vaccine uptake.
“How do we explain this paradox?” Dr. Pierce asked. “I think, really, it speaks to the strong commitment of our county public health departments who have, for a long time, been pushing the HPV vaccine and are doing a fairly good job of vaccinating. But I think, even more so, we need to focus on this question of perceived risk.”
“Our poor, rural adolescents in Alabama are being vaccinated at a higher rate than their more affluent peers, and those HPV vaccination rates appear to be directly linked to the cancer incidence rates in those counties.”
Dr. Pierce had no disclosures.
SOURCE: Pierce JY et al. SGO 2019, Abstract 13.
HONOLULU – The perceived risk of HPV-related cancer appears to overcome variables that typically impede access to HPV vaccination, according to a speaker at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
An analysis showed that adolescents in Alabama are more likely to receive the HPV vaccine if they live below the poverty line and reside in rural areas. The study also revealed a positive association between vaccine uptake and the incidence of HPV-related cancer by county.
These results suggest the perceived risk of HPV-related cancer may outweigh rurality and poverty—factors that might otherwise hinder access to health care, according to Jennifer Y. Pierce, MD, of Mitchell Cancer Institute in Mobile, Ala.
She discussed this idea when presenting the study results at the meeting.
“There are 39,000 preventable cases of HPV-related cancer in the United States,” Dr. Pierce said. “In Alabama, we are [ranked] third for cervical cancer incidence and first for cervical cancer mortality. When we look at vaccination rates in Alabama, unfortunately, we have the opposite problem. We are 45th for HPV vaccination.”
Dr. Pierce also noted that, nationally, adolescents in rural areas are 11% less likely to be vaccinated than their peers in urban areas.
“When we looked in Alabama, that did not exist,” Dr. Pierce said. “So we wanted to know, ‘What are the factors associated with HPV vaccination rate, by county, in the state of Alabama?’ because we had widely disparate rates by county.”
Dr. Pierce and her colleagues looked at data from the U.S. census, county health rankings for Alabama, the Alabama state cancer registry, and other sources. The researchers wanted to determine rates of HPV vaccination in 13- to 17-year-olds as well as rates of HPV-related cancers and variables associated with HPV vaccination by county.
Dr. Pierce said that, of the 67 counties in Alabama, 50%-70% of them are rural. Forty of them have a higher percent poverty level than the state mean. Twenty-three counties have no pediatrician, and four counties have no vaccine provider other than the health department.
By county, cancer rates were positively associated with HPV vaccination in both sexes. Higher cervical cancer rates correlated with higher HPV vaccination rates in females (r = .49; P = .011) and males (r = .46; P = .017). Higher HPV-related cancer rates in males correlated with higher HPV vaccination rates in females (r = .49; P = .001) and males (r = .46; P = .001).
The researchers found no significant association between vaccine uptake and the primary care provider ratio or the number of pediatricians per county. However, private insurance (r = –.40; P = .001) and higher median household income (r = –.40; P = .0007) were associated with lower HPV vaccine uptake. Rurality (r = .27; P = .025) and having a higher percentage of people below the poverty line (r = .39; P = .0011) were associated with higher vaccine uptake.
“How do we explain this paradox?” Dr. Pierce asked. “I think, really, it speaks to the strong commitment of our county public health departments who have, for a long time, been pushing the HPV vaccine and are doing a fairly good job of vaccinating. But I think, even more so, we need to focus on this question of perceived risk.”
“Our poor, rural adolescents in Alabama are being vaccinated at a higher rate than their more affluent peers, and those HPV vaccination rates appear to be directly linked to the cancer incidence rates in those counties.”
Dr. Pierce had no disclosures.
SOURCE: Pierce JY et al. SGO 2019, Abstract 13.
REPORTING FROM SGO 2019
WISP: Early data provide further support for ISDO in women at high OC risk
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
HONOLULU – Women at high risk for ovarian cancer who undergo risk-reducing salpingo-oophorectomy (RRSO) or interval salpingectomy with delayed oophorectomy (ISDO) experience significant reductions in cancer distress, according to preliminary findings from the WISP (Women Choosing Surgical Prevention) study.
However, those who choose RRSO experience worse menopausal symptoms and higher rates of regret, Karen H. Lu, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. Of 183 women enrolled to date in the prospective, nonrandomized, multicenter study, 91 underwent RRSO and 92 underwent ISDO at a median age of 40.9 and 37.5 years, respectively. Significant decreases in cancer-related distress as measured using the Impact of Event scale were seen in both groups at 6-month follow-up after surgery: The mean scores in the RRSO arm were 19.6 at baseline and 10.9 at 6 months, and in the ISDO arm they were 20.8 and 14.0, respectively.
The decrease in scores at 6 months did not differ significantly between study arms, but women in the RRSO arm had a significantly increased incidence of hot flashes, night sweats, vaginal dryness, and weight gain after surgery, said Dr. Lu, professor and chair in the department of gynecologic oncology and reproductive medicine and the J. Taylor Wharton Distinguished Chair in Gynecologic Oncology at the University of Texas MD Anderson Cancer Center, Houston.
“There was a significant difference in quality of life physical scores in RRSO versus the salpingectomy arm,” she said, adding that no difference was seen between the groups in quality of life mental scores.
The level of decision regret was significantly higher in the women who underwent RRSO (scores,14.1 vs. 8.7 on the 100-point scale), she noted.
“We hypothesized that use of HRT [hormone replacement therapy] after RRSO may have accounted for this difference, and looked at the two subsets,” she said. “Within the RRSO group, 25% did not go on HRT, and while higher than ISDO scores, these scores did not account for the large difference in decision regret.”
About half of the women had a history of breast cancer.
“Clinically, this agrees with what we see in that women with a prior history of breast cancer have less angst about undergoing RRSO,” she said, adding that 75% of women in the RRSO arm did go on HRT, and “these women represent the majority of our ‘previvors’ – healthy women with no history of cancer who struggle with the RRSO decision and who are able to take HRT.”
“Interestingly, compared to ISDO, RRSO women on HRT had the highest decisional regret,” she said.
An analysis of numerous possible predictors of decisional regret showed that only baseline depression score was a significant independent predictor, she noted.
Study participants are premenopausal women aged 30-50 years who have a high risk of ovarian cancer because of a pathogenic germline mutation in an ovarian cancer predisposition gene, and a normal CA125 and transvaginal ultrasound within 6 months before enrollment. All receive scripted counseling about the recommended age for oophorectomy and can choose their study arm. Planned enrollment at nine participating U.S. centers is 270 women.
The RRSO and ISDO arms at current enrollment are statistically similar with respect to mutation type, education level, breast cancer history, and first/second-degree ovarian cancer, but significantly more women in the ISDO arm had undergone risk-reducing mastectomy (51% vs. 35%), Dr. Lu noted, adding that to date no ovarian cancers have been identified at initial surgery, between surgeries, or at the time of completion oophorectomy in the ISDO patients – although only two women in that arm have undergone completion oophorectomy.
In the RRSO arm, one high-grade serous tubal intraepithelial neoplasia (STIC) was found at the time of surgery in a PALB2 mutation carrier.
“We believe this is the first report of a STIC in a PALB2 carrier. This woman was 39 years old with a strong family history of breast and ovarian cancer,” she said.
The findings suggest that “for these high-risk women who underwent ISDO ... the option encouraged them to undergo limited but potentially lifesaving surgical prophylaxis at an earlier age rather than wait to undergo full surgical prophylaxis at the upper-accepted age limit due to fear of early menopause,” Dr. Lu said.
Safety continues to be closely monitored in this study and the use of ISDO outside of the clinical trial setting is not recommended, she added, noting that an expansion of both WISP and the TUBA trial, for which preliminary results showing similar outcomes were also presented at the SGO meeting, is planned.
Dr. Lu reported having no disclosures.
SOURCE: Lu KH et al. SGO 2019, Late-Breaking Abstract 3.
REPORTING FROM SGO 2019
Triplet appears safe, effective for gynecologic cancers
HONOLULU – Pembrolizumab plus bevacizumab and oral metronomic cyclophosphamide can be effective in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, a phase 2 trial suggests.
The tumor response rate observed with the three-drug regimen (40%) was better than response rates previously reported for pembrolizumab monotherapy (8%) and bevacizumab plus cyclophosphamide (24%), according to Emese Zsiros, MD, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
The combination proved “very safe” and was associated with “excellent quality of life,” she said at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Dr. Zsiros and her colleagues enrolled 40 patients on a phase 2 trial (NCT02853318) of pembrolizumab, bevacizumab, and oral cyclophosphamide in recurrent, advanced-stage epithelial ovarian, fallopian tube, or primary peritoneal cancer. The trial had a safety lead-in cohort of five patients.
At baseline, the mean patient age was 62.2 years (range, 44.9-88.7 years). Most patients (82.5%, n = 33) had high-grade serous histology. The patients had received a mean of 3.2 (range, 1-12) prior lines of chemotherapy. Most patients (75%, n = 30) were platinum resistant, but 10 patients (25%) were platinum sensitive and declined platinum-based therapy.
Study treatment consisted of IV pembrolizumab at 200 mg plus IV bevacizumab at 15 mg/kg every 3 weeks and oral cyclophosphamide at 50 mg every day. Patients were treated until disease progression or unacceptable toxicity.
Results
“The triple regimen was, overall, really well tolerated,” Dr. Zsiros said.
The most common grade 1/2 treatment-related adverse events (AEs) were fatigue (n = 14), diarrhea (n = 13), nausea (n = 9), hypertension (n =7), white blood cell decrease (n = 6), and arthralgia (n = 6).
Grade 3 related AEs included hypertension (n = 5), lymphocyte count decrease (n = 3), and white blood cell decrease (n = 1). There was one grade 4 drug-related AE of decreased lymphocyte count.
The overall response rate was 40%, with all 16 responders having a partial response. The rate of stable disease was 55% (n = 22).
“Only 2 patients out of the 40 progressed after initiation of the treatment, and I would like to point out that both of these patients had a very large disease burden,” Dr. Zsiros said.
She also noted that more than 77% of patients had a decrease in tumor size from baseline.
The disease control rate (partial response plus stable disease) was 95.0% (n = 38) initially and 62.5% (n = 25) at 6 months. However, three patients had not yet reached 6 months follow-up at the data cutoff.
“I would like to point out that 30% of the patients [n = 12] derived an especially long-term clinical benefit over 12 months and 12 cycles of treatment,” Dr. Zsiros said.
She added that quality of life assessment revealed “high physical, emotional, cognitive, and social functioning throughout the clinical trial.” The researchers also observed significantly improved body image from baseline (P less than .002).
Dr. Zsiros reported relationships with Iovance Biotherapeutics and AstraZeneca. The trial was sponsored by Roswell Park Cancer Institute in collaboration with the National Cancer Institute.
SOURCE: Zsiros E et al. SGO 2019, Abstract LBA4.
HONOLULU – Pembrolizumab plus bevacizumab and oral metronomic cyclophosphamide can be effective in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, a phase 2 trial suggests.
The tumor response rate observed with the three-drug regimen (40%) was better than response rates previously reported for pembrolizumab monotherapy (8%) and bevacizumab plus cyclophosphamide (24%), according to Emese Zsiros, MD, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
The combination proved “very safe” and was associated with “excellent quality of life,” she said at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Dr. Zsiros and her colleagues enrolled 40 patients on a phase 2 trial (NCT02853318) of pembrolizumab, bevacizumab, and oral cyclophosphamide in recurrent, advanced-stage epithelial ovarian, fallopian tube, or primary peritoneal cancer. The trial had a safety lead-in cohort of five patients.
At baseline, the mean patient age was 62.2 years (range, 44.9-88.7 years). Most patients (82.5%, n = 33) had high-grade serous histology. The patients had received a mean of 3.2 (range, 1-12) prior lines of chemotherapy. Most patients (75%, n = 30) were platinum resistant, but 10 patients (25%) were platinum sensitive and declined platinum-based therapy.
Study treatment consisted of IV pembrolizumab at 200 mg plus IV bevacizumab at 15 mg/kg every 3 weeks and oral cyclophosphamide at 50 mg every day. Patients were treated until disease progression or unacceptable toxicity.
Results
“The triple regimen was, overall, really well tolerated,” Dr. Zsiros said.
The most common grade 1/2 treatment-related adverse events (AEs) were fatigue (n = 14), diarrhea (n = 13), nausea (n = 9), hypertension (n =7), white blood cell decrease (n = 6), and arthralgia (n = 6).
Grade 3 related AEs included hypertension (n = 5), lymphocyte count decrease (n = 3), and white blood cell decrease (n = 1). There was one grade 4 drug-related AE of decreased lymphocyte count.
The overall response rate was 40%, with all 16 responders having a partial response. The rate of stable disease was 55% (n = 22).
“Only 2 patients out of the 40 progressed after initiation of the treatment, and I would like to point out that both of these patients had a very large disease burden,” Dr. Zsiros said.
She also noted that more than 77% of patients had a decrease in tumor size from baseline.
The disease control rate (partial response plus stable disease) was 95.0% (n = 38) initially and 62.5% (n = 25) at 6 months. However, three patients had not yet reached 6 months follow-up at the data cutoff.
“I would like to point out that 30% of the patients [n = 12] derived an especially long-term clinical benefit over 12 months and 12 cycles of treatment,” Dr. Zsiros said.
She added that quality of life assessment revealed “high physical, emotional, cognitive, and social functioning throughout the clinical trial.” The researchers also observed significantly improved body image from baseline (P less than .002).
Dr. Zsiros reported relationships with Iovance Biotherapeutics and AstraZeneca. The trial was sponsored by Roswell Park Cancer Institute in collaboration with the National Cancer Institute.
SOURCE: Zsiros E et al. SGO 2019, Abstract LBA4.
HONOLULU – Pembrolizumab plus bevacizumab and oral metronomic cyclophosphamide can be effective in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, a phase 2 trial suggests.
The tumor response rate observed with the three-drug regimen (40%) was better than response rates previously reported for pembrolizumab monotherapy (8%) and bevacizumab plus cyclophosphamide (24%), according to Emese Zsiros, MD, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y.
The combination proved “very safe” and was associated with “excellent quality of life,” she said at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
Dr. Zsiros and her colleagues enrolled 40 patients on a phase 2 trial (NCT02853318) of pembrolizumab, bevacizumab, and oral cyclophosphamide in recurrent, advanced-stage epithelial ovarian, fallopian tube, or primary peritoneal cancer. The trial had a safety lead-in cohort of five patients.
At baseline, the mean patient age was 62.2 years (range, 44.9-88.7 years). Most patients (82.5%, n = 33) had high-grade serous histology. The patients had received a mean of 3.2 (range, 1-12) prior lines of chemotherapy. Most patients (75%, n = 30) were platinum resistant, but 10 patients (25%) were platinum sensitive and declined platinum-based therapy.
Study treatment consisted of IV pembrolizumab at 200 mg plus IV bevacizumab at 15 mg/kg every 3 weeks and oral cyclophosphamide at 50 mg every day. Patients were treated until disease progression or unacceptable toxicity.
Results
“The triple regimen was, overall, really well tolerated,” Dr. Zsiros said.
The most common grade 1/2 treatment-related adverse events (AEs) were fatigue (n = 14), diarrhea (n = 13), nausea (n = 9), hypertension (n =7), white blood cell decrease (n = 6), and arthralgia (n = 6).
Grade 3 related AEs included hypertension (n = 5), lymphocyte count decrease (n = 3), and white blood cell decrease (n = 1). There was one grade 4 drug-related AE of decreased lymphocyte count.
The overall response rate was 40%, with all 16 responders having a partial response. The rate of stable disease was 55% (n = 22).
“Only 2 patients out of the 40 progressed after initiation of the treatment, and I would like to point out that both of these patients had a very large disease burden,” Dr. Zsiros said.
She also noted that more than 77% of patients had a decrease in tumor size from baseline.
The disease control rate (partial response plus stable disease) was 95.0% (n = 38) initially and 62.5% (n = 25) at 6 months. However, three patients had not yet reached 6 months follow-up at the data cutoff.
“I would like to point out that 30% of the patients [n = 12] derived an especially long-term clinical benefit over 12 months and 12 cycles of treatment,” Dr. Zsiros said.
She added that quality of life assessment revealed “high physical, emotional, cognitive, and social functioning throughout the clinical trial.” The researchers also observed significantly improved body image from baseline (P less than .002).
Dr. Zsiros reported relationships with Iovance Biotherapeutics and AstraZeneca. The trial was sponsored by Roswell Park Cancer Institute in collaboration with the National Cancer Institute.
SOURCE: Zsiros E et al. SGO 2019, Abstract LBA4.
REPORTING FROM SGO 2019
Combo may improve PFS, OS for certain ovarian cancer patients
HONOLULU – Combining avelumab with pegylated liposomal doxorubicin (PLD) may provide a survival benefit in certain patients with platinum-resistant or refractory epithelial ovarian cancer, a phase 3 trial suggests.
In the overall study population, progression-free survival (PFS) and overall survival (OS) rates were not significantly different for patients who received avelumab plus PLD and those who received avelumab or PLD alone.
However, some subgroups did experience survival benefits with the combination, including patients who were positive for programmed death–ligand 1 (PD-L1) and those who had received two or three prior lines of therapy.
Eric Pujade-Lauraine, MD, PhD, of ARCAGY-GINECO in Paris, presented these results from the JAVELIN Ovarian 200 trial at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial enrolled 566 patients with platinum-resistant or refractory epithelial ovarian cancer. They were not preselected for PD-L1 expression.
Patients were randomized 1:1:1 to receive avelumab at 10 mg/kg every 2 weeks (n = 188), avelumab plus PLD at 40 mg/m2 every 4 weeks (n = 188), or PLD (n = 190).
Baseline characteristics were similar across the treatment arms. The median age was 61 in the avelumab arm and 60 in the other two arms (range, 26-86 years). In each arm, about 37% of patients had bulky disease, and all but two patients (both in the avelumab arm) had an Eastern Cooperative Oncology Group performance status of 0 or 1.
Nearly half of patients had received one line of prior therapy, and the rest had received two or three prior lines of therapy. About 75% of patients had platinum-resistant disease, and 25% were platinum refractory.
The median duration of study treatment was 10.1 weeks in the avelumab arm and 16.0 weeks in the pegylated liposomal doxorubicin (PLD) arm. In the combination arm, the median treatment duration was 16.9 weeks for avelumab and 16.3 weeks for PLD. In each arm, the most common reason for treatment discontinuation was disease progression.
Safety
Dr. Pujade-Lauraine said no new safety signals were observed with avelumab alone or in combination.
Serious treatment-related adverse events (AEs) occurred in 7.5% of patients in the avelumab arm, 17.6% of patients in the combination arm, and 10.7% of patients in the PLD arm. Discontinuation because of a treatment-related AE occurred in 6.4%, 4.4%, and 7.3% of patients, respectively.
There was one treatment-related AE leading to death in the avelumab arm and one in the PLD arm.
AEs that were more common in the combination arm than in the avelumab and PLD arms (respectively) were fatigue/asthenia (42.3%, 26.7%, and 28.8%), rash (34.1%, 8.0%, and 16.9%), stomatitis (28.0%, 2.1%, and 20.3%), and palmar-plantar erythrodysesthesia syndrome (33.0%, 0.5%, and 22.6%).
Response
The objective response rate was 3.7% in the avelumab arm, 13.3% in the combination arm, and 4.2% in the PLD arm. There were two complete responses; both occurred in the combination arm.
The response rate was significantly higher in the combination arm (odds ratio, 3.458, P = .0018) than in the PLD arm, but there was no significant difference in response rate between the avelumab arm and the PLD arm (OR, 0.890, P = .8280).
The median duration of response was 9.2 months in the avelumab arm, 8.5 months in the combination arm, and 13.1 months in the PLD arm.
Survival
There was a trend toward improved PFS with avelumab plus PLD, but the significance threshold was not met.
The median PFS was 1.9 months in the avelumab arm, 3.7 months in the combination arm, and 3.5 months in the PLD arm. With the PLD arm as a reference, the stratified hazard ratio was 1.68 for the avelumab arm (P greater than .999) and 0.78 for the combination arm (P = .0301).
The median OS was 11.8 months in the avelumab arm, 15.7 months in the combination arm, and 13.1 months in the PLD arm. The HR was 1.14 for the avelumab arm (P = .8253) and 0.89 for the combination arm (P = .2082).
“[A]velumab plus PLD showed clinical activity, but, in this unselected population, the trial did not meet the primary objective [of improving PFS or OS compared to PLD alone],” Dr. Pujade-Lauraine noted. “However, prespecified analyses indicate a potential role of PD-L1 expression as a predictor of clinical benefit.”
When Dr. Pujade-Lauraine and his colleagues looked at patients who were positive for PD-L1 (57%), the researchers found a significant improvement in PFS, but not OS, with avelumab plus PLD.
The median PFS was 1.9 months in the avelumab arm (HR,1.45, P = .0303), 3.7 months in the combination arm (HR, 0.65, P = .0143), and 3.0 months in the PLD arm.
The median OS was 13.7 months in the avelumab arm (HR, 0.83, P = .3580), 17.7 months in the combination arm (HR, 0.72, P = .0842), and 13.1 months in the PLD arm.
The researchers also found that PFS and OS were better with avelumab plus PLD versus PLD alone among patients who had received two or three prior treatment regimens at baseline. The HR was 0.62 for PFS and 0.64 for OS.
Dr. Pujade-Lauraine said further subgroup analyses are ongoing.
This trial was sponsored by Pfizer. Dr. Pujade-Lauraine reported relationships with AstraZeneca, Clovis Oncology, Incyte, Pfizer, Roche, and Tesaro.
SOURCE: Pujade-Lauraine E et al. SGO 2019, Abstract LBA1.
HONOLULU – Combining avelumab with pegylated liposomal doxorubicin (PLD) may provide a survival benefit in certain patients with platinum-resistant or refractory epithelial ovarian cancer, a phase 3 trial suggests.
In the overall study population, progression-free survival (PFS) and overall survival (OS) rates were not significantly different for patients who received avelumab plus PLD and those who received avelumab or PLD alone.
However, some subgroups did experience survival benefits with the combination, including patients who were positive for programmed death–ligand 1 (PD-L1) and those who had received two or three prior lines of therapy.
Eric Pujade-Lauraine, MD, PhD, of ARCAGY-GINECO in Paris, presented these results from the JAVELIN Ovarian 200 trial at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial enrolled 566 patients with platinum-resistant or refractory epithelial ovarian cancer. They were not preselected for PD-L1 expression.
Patients were randomized 1:1:1 to receive avelumab at 10 mg/kg every 2 weeks (n = 188), avelumab plus PLD at 40 mg/m2 every 4 weeks (n = 188), or PLD (n = 190).
Baseline characteristics were similar across the treatment arms. The median age was 61 in the avelumab arm and 60 in the other two arms (range, 26-86 years). In each arm, about 37% of patients had bulky disease, and all but two patients (both in the avelumab arm) had an Eastern Cooperative Oncology Group performance status of 0 or 1.
Nearly half of patients had received one line of prior therapy, and the rest had received two or three prior lines of therapy. About 75% of patients had platinum-resistant disease, and 25% were platinum refractory.
The median duration of study treatment was 10.1 weeks in the avelumab arm and 16.0 weeks in the pegylated liposomal doxorubicin (PLD) arm. In the combination arm, the median treatment duration was 16.9 weeks for avelumab and 16.3 weeks for PLD. In each arm, the most common reason for treatment discontinuation was disease progression.
Safety
Dr. Pujade-Lauraine said no new safety signals were observed with avelumab alone or in combination.
Serious treatment-related adverse events (AEs) occurred in 7.5% of patients in the avelumab arm, 17.6% of patients in the combination arm, and 10.7% of patients in the PLD arm. Discontinuation because of a treatment-related AE occurred in 6.4%, 4.4%, and 7.3% of patients, respectively.
There was one treatment-related AE leading to death in the avelumab arm and one in the PLD arm.
AEs that were more common in the combination arm than in the avelumab and PLD arms (respectively) were fatigue/asthenia (42.3%, 26.7%, and 28.8%), rash (34.1%, 8.0%, and 16.9%), stomatitis (28.0%, 2.1%, and 20.3%), and palmar-plantar erythrodysesthesia syndrome (33.0%, 0.5%, and 22.6%).
Response
The objective response rate was 3.7% in the avelumab arm, 13.3% in the combination arm, and 4.2% in the PLD arm. There were two complete responses; both occurred in the combination arm.
The response rate was significantly higher in the combination arm (odds ratio, 3.458, P = .0018) than in the PLD arm, but there was no significant difference in response rate between the avelumab arm and the PLD arm (OR, 0.890, P = .8280).
The median duration of response was 9.2 months in the avelumab arm, 8.5 months in the combination arm, and 13.1 months in the PLD arm.
Survival
There was a trend toward improved PFS with avelumab plus PLD, but the significance threshold was not met.
The median PFS was 1.9 months in the avelumab arm, 3.7 months in the combination arm, and 3.5 months in the PLD arm. With the PLD arm as a reference, the stratified hazard ratio was 1.68 for the avelumab arm (P greater than .999) and 0.78 for the combination arm (P = .0301).
The median OS was 11.8 months in the avelumab arm, 15.7 months in the combination arm, and 13.1 months in the PLD arm. The HR was 1.14 for the avelumab arm (P = .8253) and 0.89 for the combination arm (P = .2082).
“[A]velumab plus PLD showed clinical activity, but, in this unselected population, the trial did not meet the primary objective [of improving PFS or OS compared to PLD alone],” Dr. Pujade-Lauraine noted. “However, prespecified analyses indicate a potential role of PD-L1 expression as a predictor of clinical benefit.”
When Dr. Pujade-Lauraine and his colleagues looked at patients who were positive for PD-L1 (57%), the researchers found a significant improvement in PFS, but not OS, with avelumab plus PLD.
The median PFS was 1.9 months in the avelumab arm (HR,1.45, P = .0303), 3.7 months in the combination arm (HR, 0.65, P = .0143), and 3.0 months in the PLD arm.
The median OS was 13.7 months in the avelumab arm (HR, 0.83, P = .3580), 17.7 months in the combination arm (HR, 0.72, P = .0842), and 13.1 months in the PLD arm.
The researchers also found that PFS and OS were better with avelumab plus PLD versus PLD alone among patients who had received two or three prior treatment regimens at baseline. The HR was 0.62 for PFS and 0.64 for OS.
Dr. Pujade-Lauraine said further subgroup analyses are ongoing.
This trial was sponsored by Pfizer. Dr. Pujade-Lauraine reported relationships with AstraZeneca, Clovis Oncology, Incyte, Pfizer, Roche, and Tesaro.
SOURCE: Pujade-Lauraine E et al. SGO 2019, Abstract LBA1.
HONOLULU – Combining avelumab with pegylated liposomal doxorubicin (PLD) may provide a survival benefit in certain patients with platinum-resistant or refractory epithelial ovarian cancer, a phase 3 trial suggests.
In the overall study population, progression-free survival (PFS) and overall survival (OS) rates were not significantly different for patients who received avelumab plus PLD and those who received avelumab or PLD alone.
However, some subgroups did experience survival benefits with the combination, including patients who were positive for programmed death–ligand 1 (PD-L1) and those who had received two or three prior lines of therapy.
Eric Pujade-Lauraine, MD, PhD, of ARCAGY-GINECO in Paris, presented these results from the JAVELIN Ovarian 200 trial at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The trial enrolled 566 patients with platinum-resistant or refractory epithelial ovarian cancer. They were not preselected for PD-L1 expression.
Patients were randomized 1:1:1 to receive avelumab at 10 mg/kg every 2 weeks (n = 188), avelumab plus PLD at 40 mg/m2 every 4 weeks (n = 188), or PLD (n = 190).
Baseline characteristics were similar across the treatment arms. The median age was 61 in the avelumab arm and 60 in the other two arms (range, 26-86 years). In each arm, about 37% of patients had bulky disease, and all but two patients (both in the avelumab arm) had an Eastern Cooperative Oncology Group performance status of 0 or 1.
Nearly half of patients had received one line of prior therapy, and the rest had received two or three prior lines of therapy. About 75% of patients had platinum-resistant disease, and 25% were platinum refractory.
The median duration of study treatment was 10.1 weeks in the avelumab arm and 16.0 weeks in the pegylated liposomal doxorubicin (PLD) arm. In the combination arm, the median treatment duration was 16.9 weeks for avelumab and 16.3 weeks for PLD. In each arm, the most common reason for treatment discontinuation was disease progression.
Safety
Dr. Pujade-Lauraine said no new safety signals were observed with avelumab alone or in combination.
Serious treatment-related adverse events (AEs) occurred in 7.5% of patients in the avelumab arm, 17.6% of patients in the combination arm, and 10.7% of patients in the PLD arm. Discontinuation because of a treatment-related AE occurred in 6.4%, 4.4%, and 7.3% of patients, respectively.
There was one treatment-related AE leading to death in the avelumab arm and one in the PLD arm.
AEs that were more common in the combination arm than in the avelumab and PLD arms (respectively) were fatigue/asthenia (42.3%, 26.7%, and 28.8%), rash (34.1%, 8.0%, and 16.9%), stomatitis (28.0%, 2.1%, and 20.3%), and palmar-plantar erythrodysesthesia syndrome (33.0%, 0.5%, and 22.6%).
Response
The objective response rate was 3.7% in the avelumab arm, 13.3% in the combination arm, and 4.2% in the PLD arm. There were two complete responses; both occurred in the combination arm.
The response rate was significantly higher in the combination arm (odds ratio, 3.458, P = .0018) than in the PLD arm, but there was no significant difference in response rate between the avelumab arm and the PLD arm (OR, 0.890, P = .8280).
The median duration of response was 9.2 months in the avelumab arm, 8.5 months in the combination arm, and 13.1 months in the PLD arm.
Survival
There was a trend toward improved PFS with avelumab plus PLD, but the significance threshold was not met.
The median PFS was 1.9 months in the avelumab arm, 3.7 months in the combination arm, and 3.5 months in the PLD arm. With the PLD arm as a reference, the stratified hazard ratio was 1.68 for the avelumab arm (P greater than .999) and 0.78 for the combination arm (P = .0301).
The median OS was 11.8 months in the avelumab arm, 15.7 months in the combination arm, and 13.1 months in the PLD arm. The HR was 1.14 for the avelumab arm (P = .8253) and 0.89 for the combination arm (P = .2082).
“[A]velumab plus PLD showed clinical activity, but, in this unselected population, the trial did not meet the primary objective [of improving PFS or OS compared to PLD alone],” Dr. Pujade-Lauraine noted. “However, prespecified analyses indicate a potential role of PD-L1 expression as a predictor of clinical benefit.”
When Dr. Pujade-Lauraine and his colleagues looked at patients who were positive for PD-L1 (57%), the researchers found a significant improvement in PFS, but not OS, with avelumab plus PLD.
The median PFS was 1.9 months in the avelumab arm (HR,1.45, P = .0303), 3.7 months in the combination arm (HR, 0.65, P = .0143), and 3.0 months in the PLD arm.
The median OS was 13.7 months in the avelumab arm (HR, 0.83, P = .3580), 17.7 months in the combination arm (HR, 0.72, P = .0842), and 13.1 months in the PLD arm.
The researchers also found that PFS and OS were better with avelumab plus PLD versus PLD alone among patients who had received two or three prior treatment regimens at baseline. The HR was 0.62 for PFS and 0.64 for OS.
Dr. Pujade-Lauraine said further subgroup analyses are ongoing.
This trial was sponsored by Pfizer. Dr. Pujade-Lauraine reported relationships with AstraZeneca, Clovis Oncology, Incyte, Pfizer, Roche, and Tesaro.
SOURCE: Pujade-Lauraine E et al. SGO 2019, Abstract LBA1.
REPORTING FROM SGO 2019
Preliminary data show similar declines in worry, low regret with RRSO and RRS/RRO
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
HONOLULU – Women with BRCA1/2 mutations who undergo risk-reducing salpingo-oophorectomy (RRSO) or risk-reducing salpingectomy followed by delayed risk-reducing oophorectomy (RRS/RRO) experience reduced cancer-related worry after surgery and low levels of decision regret, according to preliminary findings from the Dutch multicenter TUBA trial.
Of 384 women included to date in the ongoing trial, 51% carried a BRCA1 mutation and 49% carried a BRCA2 mutation; 72% chose RRS/RRO. A total of 289 completed the 3-month follow-up and 197 completed 1-year follow-up. At 3 and 12 months the decline on the Cancer Worry Scale was similar in the groups after adjusting for age and other baseline characteristics, with 1.9- and 1.4-point declines from baseline scores of 14.4 and 14.0 at 3 months, and 2.2- and 1.0-point declines at 12 months in the RRSO and RRS/RRO groups, respectively, Miranda P. Steenbeek, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
The mean scores on the 100-point Decision Regret Scale at 1-year follow-up were low at 13.4 and 13.0 after RRSO and RRS, respectively.
“But it is interesting that there is one group of women who underwent standard salpingo-oophorectomy, but weren’t using hormone replacement therapy [HRT], as these women had a higher level of decision regret [mean score, 18.8],” said Dr. Steenbeek of Radboud University Nijmegen, the Netherlands. “It is interesting to see how these results will develop over time.”
Current recommendations for preventive surgery in woman at increased risk because of BRCA1/2 mutations call for RRSO around age 40 years. However, an innovative approach using RRS followed by RRO has emerged as recent data indicate the fallopian tube, rather than the ovary, as the origin of high-grade serous ovarian cancer, she explained.
The TUBA trial was launched at 13 Dutch oncology centers in 2015, and plans to enroll 510 BRCA1/2 mutation carriers. Participants choose either standard RRSO within current guidelines (at age 35-40 years for BRCA1 carriers, age 40-45 years for BRCA2 carriers) or the innovative RRS approach followed by RRO at up to 5 years after the current guideline age (at age 40-45 years for BRCA1 carriers, age 45-50 years for BRCA2 carriers), she said.
These early findings show that baseline levels of cancer worry are higher among women choosing RRSO, which might explain the slightly larger postsurgery decline in worry in the RRSO group, Dr. Steenbeek said, noting that the higher level of regret with RRSO without HRT might be related to more severe menopausal complaints in that subset of patients.
“Based on these results we cannot conclude whether or not salpingectomy is a safe alternative to for salpingo-oophorectomy, and therefore it is very important to not perform salpingectomy with delayed oophorectomy outside the safety of a clinical and control of a clinical trial,” she said, adding that a report on the quality of life data from the study is expected in 2020.
Dr. Steenbeek reported having no disclosures.
SOURCE: Steenbeek MP et al. SGO 2019, Abstract LB2.
REPORTING FROM SGO 2019
Financial toxicity may be common in gynecologic cancer patients
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
SOURCE: Liang M et al. SGO 2019. Abstract 8.
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
SOURCE: Liang M et al. SGO 2019. Abstract 8.
HONOLULU – Financial toxicity may be common among gynecologic cancer patients starting a new line of treatment, based on the results of a survey presented at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
More than half of the 121 patients surveyed reported mild, moderate, or severe financial toxicity, Margaret Liang, MD, of University of Alabama at Birmingham, said in presenting the findings.
Younger age and lower income were both risk factors for financial toxicity, and having insurance did not protect patients from financial toxicity. Dr. Liang noted that insurance coverage “was not protective for financial toxicity, as the majority of those who screened positive for financial toxicity were insured.” Specifically, 89% of patients with financial toxicity and 98% of patients without it were insured (P = .07).
“This finding supports financial toxicity screening regardless of insurance status and may favor universal screening,” Dr. Liang said.
She and her colleagues conducted the survey of 121 gynecologic cancer patients who had started a new line of systemic therapy in the previous 8 weeks.
The patients’ mean age was 59 years (range, 33-80), and 58% were starting their first line of systemic therapy. Half of the patients had a household income below $40,000, and about one-third of the patients were employed. Most had private (74%) or public (20%) insurance, and 7% of patients were uninsured.
To assess financial toxicity, the researchers used the Comprehensive Score for Financial Toxicity (COST). A score of less than 26 was used as a threshold of financial toxicity. The severity of financial toxicity was graded on a scale of 1 to 4, with a lower score indicating worse toxicity.
Patients were most concerned about having enough money to cover the cost of treatment. Patients reported the lowest mean score—0.97—in response to the statement, “I know that I have enough money in savings, retirement, or assets to cover the costs of my treatment.”
Patients were least concerned about losing their job or income. They reported the highest mean score—3.29—in response to the statement, “I am concerned about keeping my job and income, including working at home.” However, as Dr. Liang pointed out, only one-third of patients were employed at baseline.
In all, 54% of patients reported financial toxicity—37% mild, 16% moderate, and 1% severe.
Dr. Liang and her colleagues found that younger age and lower income were associated with an increased risk for financial toxicity. The mean age was 57 years in patients with financial toxicity and 62 years in patients without it (P = .02). Household incomes were below $40,000 in 63% of patients with financial toxicity and 34% of those without it (P less than .01).
The researchers also found that patients with financial toxicity were significantly more likely to say their cancer diagnosis resulted in lost wages, borrowed money, altered spending habits, the need to sacrifice other things, and not paying bills on time (P less than .01 for all).
On the other hand, patients with financial toxicity were not significantly more likely to become unemployed, file for bankruptcy, sell their house, or get a second job due to their cancer diagnosis.
However, it’s important to note that these data were collected within 8 weeks of patients starting their new line of therapy. The final survey results will include follow-up at 3 months and 6 months.
Dr. Liang said the fact that more than half of patients reported financial toxicity within 8 weeks of starting a new line of therapy suggests early interventions are needed to prevent or reduce financial toxicity. This may include counseling patients on anticipated costs of care, screening for financial toxicity, and linking patients to available financial resources.
Dr. Liang had no financial disclosures.
SOURCE: Liang M et al. SGO 2019. Abstract 8.
REPORTING FROM SGO 2019
Tumor testing cost-effective for triage to germline testing in HGSOC patients
HONOLULU – Tumor testing for the triage of women with high-grade serous ovarian cancer to confirmatory genetic testing for BRCA mutations appears feasible, according to a cost-effectiveness analysis.
In fact, based on a Markov Monte Carlo simulation model developed to compare a tumor-testing approach with a universal germline testing approach, tumor testing yields an incremental cost-effectiveness ratio (ICER) of $127,000 per year of life gained, Janice S. Kwon, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“This is well in excess of [the $50,000 to $100,000 that] would be considered an acceptable threshold in the United States,” said Dr. Kwon of the University of British Columbia, Vancouver, Canada.
“We predict that tumor testing will be a cost-effective method of triage in women with high-grade serous ovarian cancer for confirmatory genetic testing to identify BRCA mutation carriers, assuming high sensitivity and acceptable cost of tumor testing,” she said.
In many areas around the world, germline testing is recommended for all women with high-grade serous ovarian cancer (HGSOC) because they have a 20% chance of carrying a BRCA 1 or 2 mutation.
“However, we all know that the referral rate for genetic testing is far from optimal, and furthermore, there are costs incurred to the healthcare system for resources utilized for genetic counseling and testing,” she said.
Tumor testing for triage is an alternative approach.
“If you consider 100 women with high-grade serous ovarian cancer and follow them through the conventional pathway in which all of them are referred for germline testing, you would expect to find 20 mutation carriers. If you take those same 100 women and apply tumor testing first, then 25 are expected to have a mutation in the tumor, Dr. Kwon said.
“If [all 25] are referred for germline testing, you would expect to find the same number of BRCA mutation carriers but with far less resource utilization.”
The remaining 75 are not expected to have a mutation in the tumor, and they may not need to be referred for confirmatory genetic testing unless there is a compelling family history or panel testing reveals a concerning mutation, she explained.
Since a randomized trial to compare these two strategies is not feasible, Dr. Kwon and her colleagues performed the current cost-effectiveness analysis.
The Markov simulation model was used to estimate the number of BRCA mutation carriers from index cases and their first degree relatives, and the number of cancer cases averted among first degree relatives, assuming they would undergo risk-reducing surgery.
“We conducted extensive sensitivity analyses to account for uncertainly around various parameters and we modeled a time horizon of 50 years,” Dr. Kwon noted. “We know that there are approximately 10,000 new [HGSOC] cases diagnosed in the United States every year, and we assumed that for every woman with [HGSOC], there was at least 1 female first-degree relative who would benefit from genetic testing.”
The model showed that applying tumor testing first would lead to a substantial reduction in the number of women undergoing germline mutation testing, but the number of BRCA mutation carriers identified would be comparable with the two strategies – assuming that the sensitivity of tumor testing is less than 100%, she said.
“As expected, the average lifetime costs associated with germline testing would be less than that for tumor testing, and even though you would expect that more first-degree relatives would be identified as BRCA mutation carriers after universal germline testing for index cases, the life expectancy gain for those first-degree relatives is averaged over the entire cohort at risk, and therefore the average incremental gain or benefit was actually quite small,” she said, noting that this yielded the ICER of $127,000 per year of life gained.
Based on this finding, tumor testing would be the preferred strategy, she added.
Sensitivity analysis around the sensitivity and specificity of tumor testing showed that tumor testing would be cost effective if its sensitivity is above 97%, and that tumor testing is cost-effective as long as it costs less than a third of the cost of germline testing – including genetic counseling.
Dr. Kwon has received research funding from AstraZeneca.
SOURCE: Kwon J et al., SGO 2019: Abstract 5.
HONOLULU – Tumor testing for the triage of women with high-grade serous ovarian cancer to confirmatory genetic testing for BRCA mutations appears feasible, according to a cost-effectiveness analysis.
In fact, based on a Markov Monte Carlo simulation model developed to compare a tumor-testing approach with a universal germline testing approach, tumor testing yields an incremental cost-effectiveness ratio (ICER) of $127,000 per year of life gained, Janice S. Kwon, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“This is well in excess of [the $50,000 to $100,000 that] would be considered an acceptable threshold in the United States,” said Dr. Kwon of the University of British Columbia, Vancouver, Canada.
“We predict that tumor testing will be a cost-effective method of triage in women with high-grade serous ovarian cancer for confirmatory genetic testing to identify BRCA mutation carriers, assuming high sensitivity and acceptable cost of tumor testing,” she said.
In many areas around the world, germline testing is recommended for all women with high-grade serous ovarian cancer (HGSOC) because they have a 20% chance of carrying a BRCA 1 or 2 mutation.
“However, we all know that the referral rate for genetic testing is far from optimal, and furthermore, there are costs incurred to the healthcare system for resources utilized for genetic counseling and testing,” she said.
Tumor testing for triage is an alternative approach.
“If you consider 100 women with high-grade serous ovarian cancer and follow them through the conventional pathway in which all of them are referred for germline testing, you would expect to find 20 mutation carriers. If you take those same 100 women and apply tumor testing first, then 25 are expected to have a mutation in the tumor, Dr. Kwon said.
“If [all 25] are referred for germline testing, you would expect to find the same number of BRCA mutation carriers but with far less resource utilization.”
The remaining 75 are not expected to have a mutation in the tumor, and they may not need to be referred for confirmatory genetic testing unless there is a compelling family history or panel testing reveals a concerning mutation, she explained.
Since a randomized trial to compare these two strategies is not feasible, Dr. Kwon and her colleagues performed the current cost-effectiveness analysis.
The Markov simulation model was used to estimate the number of BRCA mutation carriers from index cases and their first degree relatives, and the number of cancer cases averted among first degree relatives, assuming they would undergo risk-reducing surgery.
“We conducted extensive sensitivity analyses to account for uncertainly around various parameters and we modeled a time horizon of 50 years,” Dr. Kwon noted. “We know that there are approximately 10,000 new [HGSOC] cases diagnosed in the United States every year, and we assumed that for every woman with [HGSOC], there was at least 1 female first-degree relative who would benefit from genetic testing.”
The model showed that applying tumor testing first would lead to a substantial reduction in the number of women undergoing germline mutation testing, but the number of BRCA mutation carriers identified would be comparable with the two strategies – assuming that the sensitivity of tumor testing is less than 100%, she said.
“As expected, the average lifetime costs associated with germline testing would be less than that for tumor testing, and even though you would expect that more first-degree relatives would be identified as BRCA mutation carriers after universal germline testing for index cases, the life expectancy gain for those first-degree relatives is averaged over the entire cohort at risk, and therefore the average incremental gain or benefit was actually quite small,” she said, noting that this yielded the ICER of $127,000 per year of life gained.
Based on this finding, tumor testing would be the preferred strategy, she added.
Sensitivity analysis around the sensitivity and specificity of tumor testing showed that tumor testing would be cost effective if its sensitivity is above 97%, and that tumor testing is cost-effective as long as it costs less than a third of the cost of germline testing – including genetic counseling.
Dr. Kwon has received research funding from AstraZeneca.
SOURCE: Kwon J et al., SGO 2019: Abstract 5.
HONOLULU – Tumor testing for the triage of women with high-grade serous ovarian cancer to confirmatory genetic testing for BRCA mutations appears feasible, according to a cost-effectiveness analysis.
In fact, based on a Markov Monte Carlo simulation model developed to compare a tumor-testing approach with a universal germline testing approach, tumor testing yields an incremental cost-effectiveness ratio (ICER) of $127,000 per year of life gained, Janice S. Kwon, MD, reported at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer.
“This is well in excess of [the $50,000 to $100,000 that] would be considered an acceptable threshold in the United States,” said Dr. Kwon of the University of British Columbia, Vancouver, Canada.
“We predict that tumor testing will be a cost-effective method of triage in women with high-grade serous ovarian cancer for confirmatory genetic testing to identify BRCA mutation carriers, assuming high sensitivity and acceptable cost of tumor testing,” she said.
In many areas around the world, germline testing is recommended for all women with high-grade serous ovarian cancer (HGSOC) because they have a 20% chance of carrying a BRCA 1 or 2 mutation.
“However, we all know that the referral rate for genetic testing is far from optimal, and furthermore, there are costs incurred to the healthcare system for resources utilized for genetic counseling and testing,” she said.
Tumor testing for triage is an alternative approach.
“If you consider 100 women with high-grade serous ovarian cancer and follow them through the conventional pathway in which all of them are referred for germline testing, you would expect to find 20 mutation carriers. If you take those same 100 women and apply tumor testing first, then 25 are expected to have a mutation in the tumor, Dr. Kwon said.
“If [all 25] are referred for germline testing, you would expect to find the same number of BRCA mutation carriers but with far less resource utilization.”
The remaining 75 are not expected to have a mutation in the tumor, and they may not need to be referred for confirmatory genetic testing unless there is a compelling family history or panel testing reveals a concerning mutation, she explained.
Since a randomized trial to compare these two strategies is not feasible, Dr. Kwon and her colleagues performed the current cost-effectiveness analysis.
The Markov simulation model was used to estimate the number of BRCA mutation carriers from index cases and their first degree relatives, and the number of cancer cases averted among first degree relatives, assuming they would undergo risk-reducing surgery.
“We conducted extensive sensitivity analyses to account for uncertainly around various parameters and we modeled a time horizon of 50 years,” Dr. Kwon noted. “We know that there are approximately 10,000 new [HGSOC] cases diagnosed in the United States every year, and we assumed that for every woman with [HGSOC], there was at least 1 female first-degree relative who would benefit from genetic testing.”
The model showed that applying tumor testing first would lead to a substantial reduction in the number of women undergoing germline mutation testing, but the number of BRCA mutation carriers identified would be comparable with the two strategies – assuming that the sensitivity of tumor testing is less than 100%, she said.
“As expected, the average lifetime costs associated with germline testing would be less than that for tumor testing, and even though you would expect that more first-degree relatives would be identified as BRCA mutation carriers after universal germline testing for index cases, the life expectancy gain for those first-degree relatives is averaged over the entire cohort at risk, and therefore the average incremental gain or benefit was actually quite small,” she said, noting that this yielded the ICER of $127,000 per year of life gained.
Based on this finding, tumor testing would be the preferred strategy, she added.
Sensitivity analysis around the sensitivity and specificity of tumor testing showed that tumor testing would be cost effective if its sensitivity is above 97%, and that tumor testing is cost-effective as long as it costs less than a third of the cost of germline testing – including genetic counseling.
Dr. Kwon has received research funding from AstraZeneca.
SOURCE: Kwon J et al., SGO 2019: Abstract 5.
REPORTING FROM SGO 2019
















